Abstract
Background and Aims
The shoot apical meristem (SAM) is the key organizing element in the plant body and is responsible for the core of plant body organization and shape. Surprisingly, there are almost no comparative data that would show links between parameters of the SAM and whole-plant traits as drivers of the plant’s response to the environment.
Methods
Interspecific differences in SAM anatomy were examined in 104 perennial herbaceous angiosperms.
Key Results
There were differences in SAM parameters among individual species, their phylogenetic patterns, and how their variation is linked to variation in plant above-ground organs and hence species’ environmental niches. SAM parameters were correlated with the size-related traits of leaf area, seed mass and stem diameter. Of the two key SAM parameters (cell size and number), variation in all organ traits was linked more strongly to cell number, with cell size being important only for seed mass. Some of these correlations were due to shared phylogenetic history (e.g. SAM diameter versus stem diameter), whereas others were due to parallel evolution (e.g. SAM cell size and seed mass).
Conclusion
These findings show that SAM parameters provide a functional link among sizes and numbers of plant organs, constituting species’ environmental responses.
Keywords: Corner’s rule, cell number, cell size, phylogenetic analysis, genome size
INTRODUCTION
The impressive richness of plant body forms arises from different combinations of numbers and shapes of leaves, branches and buds. Biomass partitioning into these organs determines the shape of the plant and its potential to respond to environmental stimuli such as injury or changes in resource availability. One of the key developmental decisions that plants face is whether, for a given body part, to have fewer and larger such parts versus more numerous but smaller ones. A plant individual has to make this decision for various organs, as leaves, twigs/branches, flowers and seeds all involve a similar kind of size versus number trade-off (Harper et al., 1970; Ackerly and Donoghue, 1998; Moles et al., 2004; Yang et al., 2008).
Importantly, however, sizes of individual plant organs are not independent of each other. In trees, researchers long ago formulated ‘Corner’s rule’, stating that leaf size is positively correlated with the diameter of the previous year’s twig and negatively with both the number of leaves per twig and the degree of tree branching intensity (Corner, 1949; Ackerly and Donoghue, 1998; Brouat et al., 1998; Westoby and Wright, 2003). Similar correlations between leaf size, leaf number and shoot size are known from herbs (Yang et al., 2008; Whitman and Aarssen, 2010; Yan et al., 2012). Such correlations extend to generative organs such as seed mass (and hence seed number), which is known to be correlated with branch diameter and leaf size (Cornelissen, 1999; Leslie et al., 2014). These correlations are due to either biomechanical or developmental constraints (Niklas, 1994). They may also arise because the genes responsible for the size of one organ can also be responsible for sizes of other organs and consequently for the size of the whole plant (Guo and Simons, 2011).
Differences in organ sizes are governed by differences in cell number and in cell size, which correspond to the two main processes that determine transition from the primordium to the fully formed organ, namely cell multiplication and cell expansion (Guo and Simmons, 2011; Gonzalez et al., 2012). Differences in organ size among species are primarily governed by cell number (Gonzalez et al., 2010; Brodribb et al. 2013). In contrast, cell size is less flexible both among and within species and is constrained by its strong relationship to nuclear genome size (Knight and Beaulieu, 2008; Niklas, 2015), although it can be involved in plant responses to environmental factors such as shade or drought (Huber et al., 2014; Carins Murphy et al., 2016).
Despite the current knowledge base regarding both the cellular nature of organ development and organ correlations and trade-offs (Powell and Lenhard, 2012; Holt et al., 2014; Sluis and Hake, 2015), we lack a more detailed understanding of links between sizes of individual organs and their components, and between organ sizes and whole-plant traits. The key component of such links is the size and structure of the stem apical meristem (SAM; Carraro et al., 2006; Hamant and Traas, 2010), which is the source of undifferentiated cells whose multiplication directly affects stem growth, leaf primordia formation and, after the change to the generative mode, flower primordia formation (Barlow, 1989; Bäurle and Laux, 2003; Sluis and Hake, 2015). The diameter of the SAM has been shown to be the key determinant of stem size and leaf primordia (Mauseth, 2004), implying that its size, structure and shape can provide the missing link between various organ structures in plants. Consequently, we can hypothesize that plant organs and whole-body measures have their counterpart in the structure of their apical meristems (Green, 1999; Tsukaya, 2014).
Surprisingly, in spite of the strong support of Corner’s rule or similar constraints in several plant groups, we lack data about relationships between SAM parameters (e.g. cell size, cell number and SAM size) and the size of organs derived from it (Gonzalez et al., 2012). Thus, although it is known that SAM size changes during plant development (Abbe, 1941; Sharma and Sharma, 1989; Medford, 1992; Fletcher, 2002; Hamant and Traas, 2010), there have been only a few detailed interspecific comparative studies of SAM anatomy (Laufs et al., 1998; Kwiatkowska and Dumais, 2003; Mauseth, 2004; Bonser and Aarssen, 2006; Classen-Bockhoff, 2016; Ronse De Craene, 2016) that would permit linking the size and shape of the SAM with the sizes and shapes of organs that develop from it.
Therefore, we aim here to examine relationships between the anatomical parameters of the SAM and the sizes of organs derived from it across a large set of herbaceous angiosperms. We are primarily interested in herbaceous species because the absence of secondary thickening means that differentiation at the apex, and hence functioning of the SAM, is the only driver of the final plant’s shape. We first examine how apical meristems differ among individual species and identify descriptive parameters that can be used to capture this variation. Second, we examine phylogenetic patterns of these parameters and ask to what extent they are phylogenetically conservative. Third, we examine how this variation is linked to variation in plant above-ground organs such as leaf area, stem thickness, height at maturity and shoot longevity. We pay specific attention to meristem overall diameter as it may be the key determinant of the potential of a herbaceous plant to form large above-ground structures. Because meristem size has two key components, viz. number of cells and mean cell size, we also examine these components separately and identify their independent effects on size of plant organs. We also take account of the fact that meristematic cell size is tightly linked to cellular genome content (Price et al. 1973) and examine genome sizes as potential constraints on meristem parameters. Finally, we ask which correlations between meristem parameters and organ parameters are due to shared phylogenetic history and which are due to parallel evolution.
MATERIALS AND METHODS
Preparation and evaluation of SAM
Our study encompassed 104 Central European perennial herbaceous plant species from 38 genera. These genera were selected to span major angiosperm clades, while species within these groups were selected based on differences in their major organ parameters to enable representative sampling of variation in organ parameters within these groups.
Species were collected in the field, with rare aquatic plants coming from the collection of aquatic and wetland plants of the Institute of Botany of the Czech Academy of Sciences in Třeboň (Czech Republic, http://www.wetcol.butbn.cas.cz). Several plant species were provided by the botanical garden of Charles University in Prague (Czech Republic, www.bz-uk.cz) and Planta Naturalis (Markvartice, Czech Republic, www.plantanaturalis.cz). All plants were grown under field conditions in the experimental garden of the Institute of Botany of the Czech Academy of Sciences in Průhonice (Czech Republic). Growing the plants in appropriate pots sunk completely in the soil facilitated access to the underground buds in the non-vegetative season. Only well-developed adult plants were used for meristem sampling.
Well-developed renewal buds were collected late in the autumn or very early in the spring. SAMs were carefully excised from freshly removed renewal plant buds under a dissecting microscope. The dissected tissue (thick longitudinal medial section) was immediately placed in FPA fixative (5 mL of formaldehyde, 5 mL of propionic acid, 90 mL of 70 % ethanol) for at least 24 h. Before staining with propidium iodide (PI), the tissue was gradually dehydrated and placed for 12 h in 100 % ethanol to remove all chlorophyll. After gradual rehydration, the tissue samples were stained with PI (5 mg/mL) in 0.1 m l-arginine, pH 12.4, following the method of Clark et al. (1993) for 5 d. After 2 d of washing the samples in 0.1 m l-arginine, pH 8, SAMs were viewed with a Zeiss confocal LSM5 microscope equipped with an argon laser (excitation 514 nm).
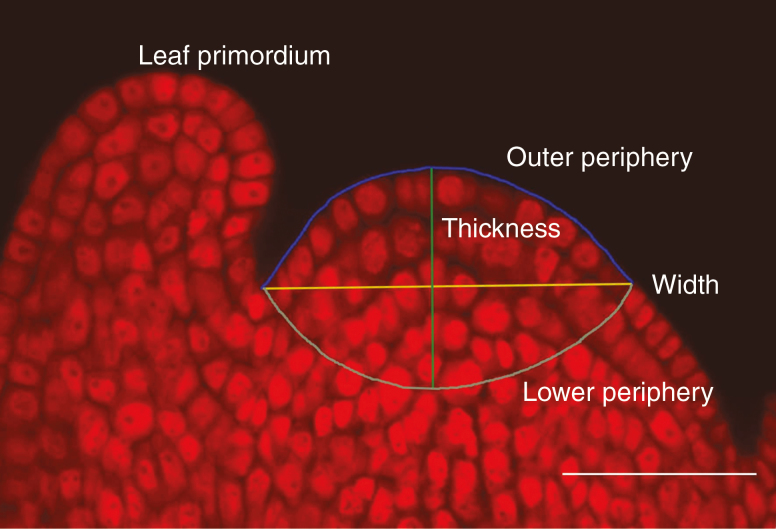
The SAM photographs were analysed in the LSM Image Browser (Zeiss, ver. 4.0.0.157). The SAM was defined as the part of the shoot tip above the youngest leaf primordia; its boundary was recognized based on the cell arrangement and inner structure of meristematic cells (Romberger, 1963) so that the measurements would be comparable among all species. We measured the length and the number of cells along four main lines of the medial cross-section of the SAM. These were width, thickness, periphery and inner periphery (Fig. 1). The periphery of the SAM was defined as the perimeter of the SAM sector limited by the two last developing leaf primordia; width corresponded to the distance of the SAM part between leaf primordia; the inner periphery was measured as the inner perimeter of the meristematic cells (defined as small cells with relatively large nuclei arranged in rows) in the sector between newly developing leaf primordia. Height was defined as the distance between the most distant parts of the periphery and inner periphery. Multiple SAMs were evaluated for each species (average = 13.0 SAMs analysed per species, s.d. = 7.4). Altogether, 1359 SAMs were measured.
Fig. 1.
Measured parameters of the SAM, i.e. width, thickness, inner (=lower) periphery and outer periphery, are shown on a longitudinal medial cross-section of Centaurea phrygia meristem stained with PI and viewed through the confocal microscope. For definitions of measured parameters see the Materials and methods section. Scale bar = 50 µm.
Care was taken to collect meristems from comparable buds and shoots. Because this was not always possible, we classified SAMs into three developmental stages to make the stages fully comparable. Stage 1 was characterized as a well-developed renewal bud. Stage 2 referred to a more developed SAM, which in most plants corresponded to young offshoots. Stage 3 comprised SAMs in the last vegetative stage shortly before transition to inflorescence. The majority of measurements were taken of specimens in stages 1 or 2; only for a few species (Achillea nobilis, Poa nemoralis, Poa pratensis, Rumex acetosella) were larger proportions of measurements done on meristems in stage 3.
Additional data sources
Data on plant height at maturity were taken from Kubát et al. (2002), data on seed mass, leaf area and specific leaf area from the LEDA Traitbase (Kleyer et al., 2008). If leaf area information was unavailable from LEDA, it was measured in studied plants using the LEDA protocol (Knevel et al., 2005) in ImageJ software (https://imagej.nih.gov/ij/). Data on shoot lifespan (cyclicity) were taken from the CLO-PLA database version 3.2 (Klimešová et al., 2017). Plant stem diameter was measured on five to seven fully developed experimental plants immediately above the ground. All these parameters are further referred to as plant traits.
Estimates of 2C DNA values were compiled from the Plant DNA C-values database maintained at the Royal Botanic Gardens, Kew (prime estimates; Bennett and Leitch, 2005). Species missing from the database were measured at the Laboratory of Flow Cytometry of the Institute of Botany, Czech Academy of Science at Průhonice. A two-step procedure as described in Doležel et al. (2007) was adopted to estimate genome sizes of selected plant species. The method described by Chumová et al. (2015) was used with the standard plants Pisum sativum ‘Ctirad’, 2C = 8.76 pg (Doležel et al., 1998); Bellis perennis, 2C = 3.38 pg (Schönswetter et al., 2007); and Solanum pseudocapsicum, 2C = 2.59 pg (Temsch et al., 2010; see Supplementary Data S1 for standards used for individual plant species). Phylogenetic data were taken from Durka and Michalski (2012), which contains a tree with dated branch lengths and contains all but one species from our species list.
Data analysis
We used principal components analysis (PCA) on standardized values of variables measured on meristems to identify major directions of their variation. We used the package vegan (Oksanen et al., 2013). The PCA was run on the means of measurements of individual species to avoid biasing the outcome due to different numbers of measurements on individual species. Based on the outcome of the PCA, we defined four summary variables expressing meristem size, shape and structure: (1) overall meristem size (area at a cross-section), defined as , where w is meristem width and t is meristem thickness; (2) number of cells, defined as the number of cells at the outer periphery; (3) cell size, defined as residuals from linear regression of outer periphery length on the number of cells at the outer periphery; and (4) meristem shape, defined as residuals from the regression of log(cell number at the inner periphery/cell number of thickness) on number of cells at the outer periphery and length of the outer periphery. We further refer to these variables as meristem size, number of cells, cell size and meristem shape. Although meristem size is essentially a combination of number of cells and cell size, in many cases it is useful to express these components both separately and in combination.
We assessed relative contributions of individual levels (genus, species, ontogenetic stage) to overall variation in the data set by fitting a linear mixed model with genus, species (nested in genus) and ontogeny (nested in species) as random factors. The model was fitted using the function lmer from the package lme4 (Bates et al., 2015). Phylogenetic signals of meristem summary variables and plant traits were assessed using Pagel’s λ (Freckleton et al., 2002). We fitted λ using a maximum likelihood approach employing the pgls function (with no predictor to get λ for the summary variable proper) from the R package caper (Orme, 2012), calculated the upper and lower confidence limits and tested the significances of the differences of λ from 0 and 1. Mapping of trait values on the phylogenetic tree was done using the function contMap from the package phytools (Revell, 2012, 2014).
Relationships between meristem summary variables and plant traits were analysed using simple linear correlations. All quantitative plant traits were log-transformed before analysis. To address possible phylogenetic dependence of trait values among species in correlation analyses, we used the approach of Diniz-Filho et al. (1998; see also Desdevises et al., 2003). We summarized the matrix of phylogenetic distances using non-standardized principal coordinates analysis (PCoA) using the function dudi.pco from the ade4 package for R (Dray and Dufour, 2007). The first 14 axes from this PCoA accounted for 90 % of the total phylogenetic variation. These axes were used as covariates to get phylogenetic correlations. To determine the scaling relationship between meristem cell size and nuclear genome size, we used standard major axis (SMA) regression (R language, package lmodel2; Legendre, 2014). The scaling exponent for the relationship between genome size and cell size was calculated as the slope of the SMA regression after logarithmic transformation of both variables. We calculated confidence intervals of scaling exponents to assess their differences from slopes theoretically derived from geometrical arguments.
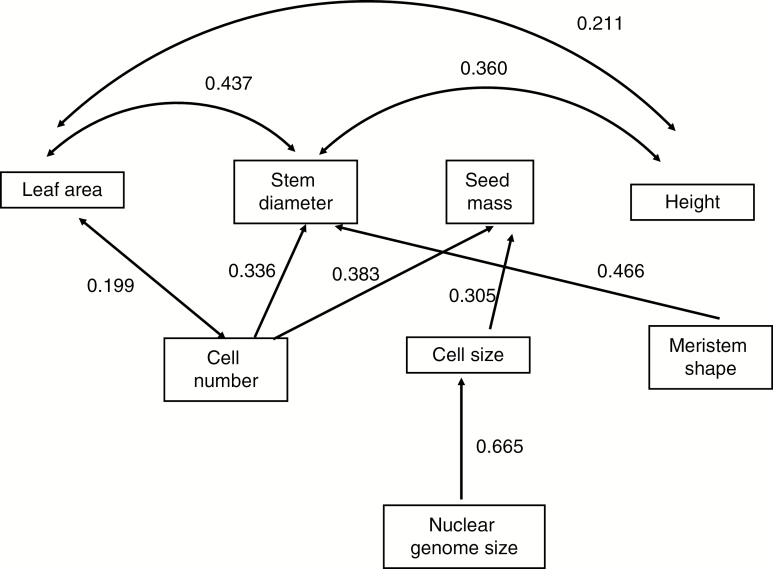
To take into account potential correlations between plant traits, we used path analysis (Grace, 2006). We assumed genome size to be an exogenous variable (i.e. not affected by any of the variables in the data set), whereas all other variables were assumed to be endogenous (i.e. affected by some variables and potentially affecting some other variables). We further assumed that three meristem summary variables (excluding meristem size, which is a combination of cell number and cell size) were potentially affected by genome size, and that they could affect plant traits (leaf area, plant height, stem diameter and seed mass). We assumed independent covariation among error components of the plant traits, but did not assume any covariation among the meristem summary variables as these were constructed to be independent of each other. We then searched for the best model in terms of good fit of the data assessed by the χ2 test. In addition, we also examined path coefficients and covariations in an identical model using residualized values of the variables, i.e. with phylogenetic effect removed by the approach of Diniz-Filho et al. (1998) The path models were fitted using the function sem from the package sem (Fox et al., 2016).
RESULTS
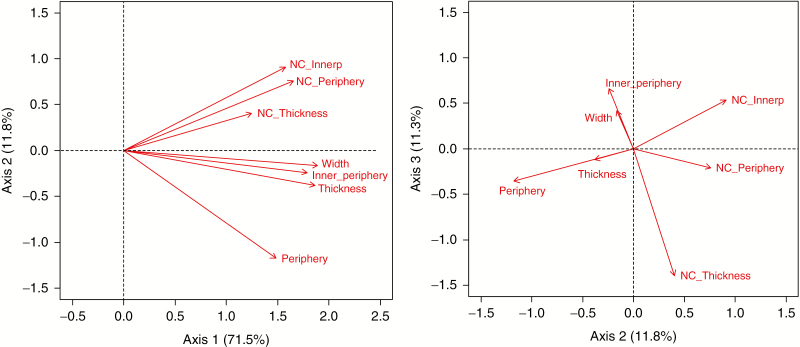
Measured mean meristem parameters of species were fairly strongly intercorrelated. The first three axes of the PCA of seven meristem parameters explained 95 % of the total variation (the first three axes explained 75 %, 12 % and 11 % of the total variation; Fig. 2), indicating that the data set was essentially three-dimensional. The first dimension expressed primarily meristem size; the second axis expressed cell size; the third axis expressed meristem shape, namely large versus small values of inner periphery relative to thickness and outer periphery (positive versus negative scores, respectively). these three axes were highly correlated with the summary variables: the first axis with meristem size and cell number, the second with cell size and the third with meristem shape (Supplementary Data S2).
Fig. 2.
First three axes of the principal components analysis of meristem parameters based on species means. NC_Innerp, number of cells at the Inner Periphery; NC_Thickness, number of cells along the meristem thickness (longest vertical dimension); NC_periphery, number of cells along the (outer) periphery. For definitions of these morphological terms see Fig. 1.
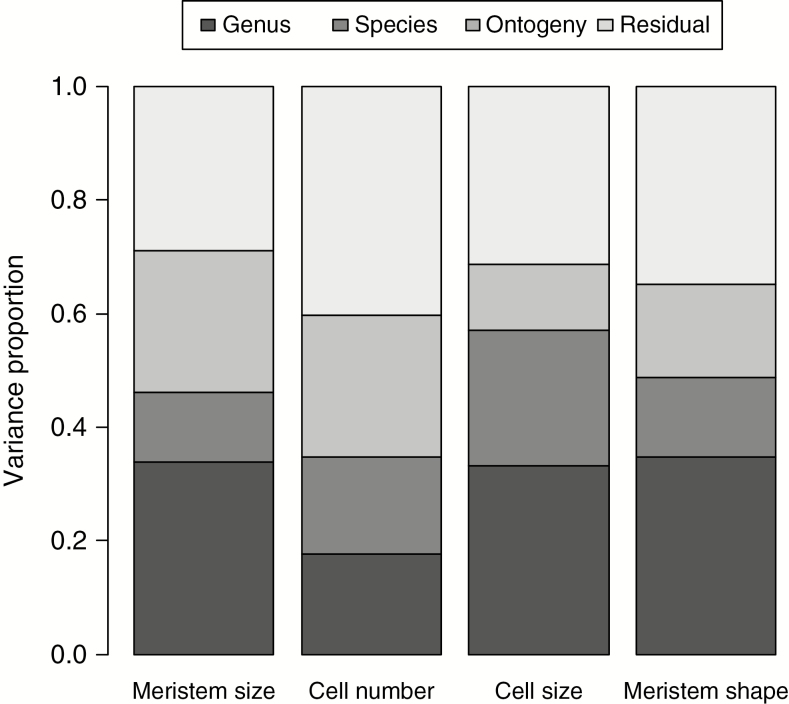
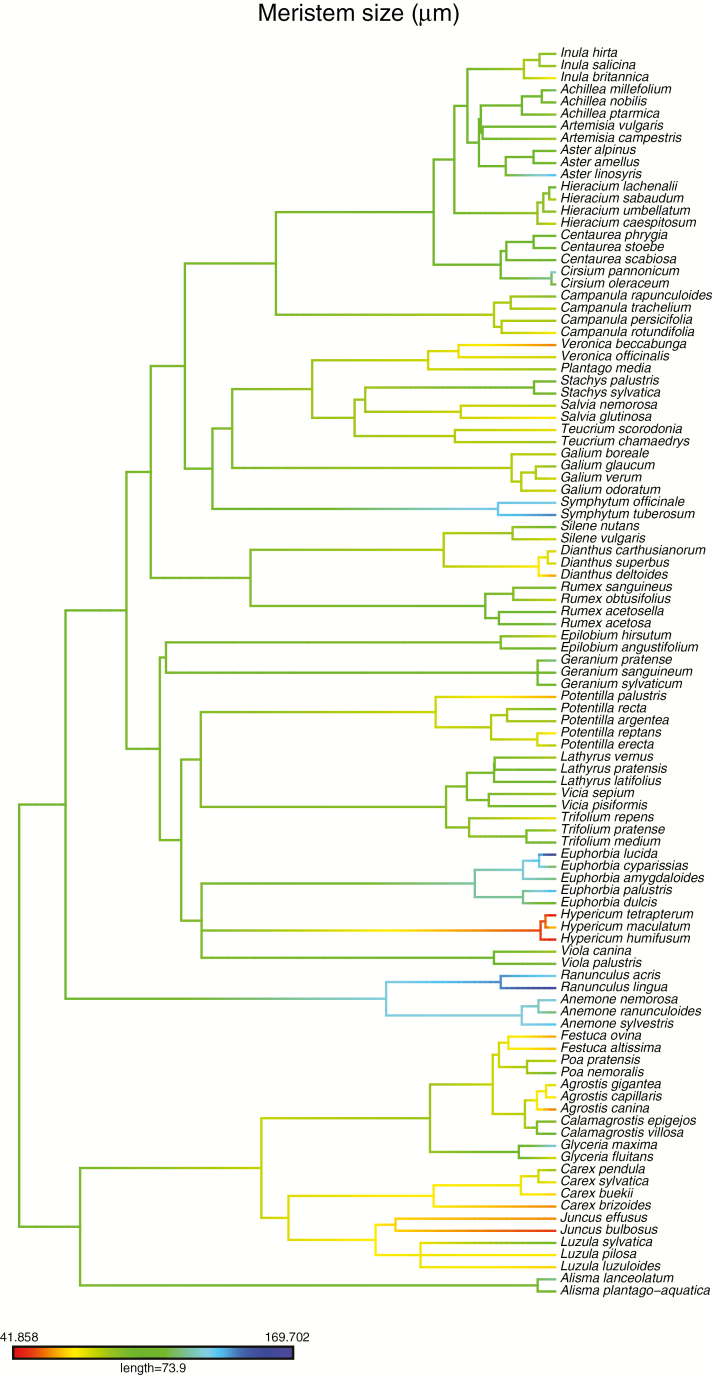
The meristem summary variables varied during SAM ontogeny, but all of them showed considerable species-level and genus-level variation (Fig. 3); ontogeny was most important for number of cells (and meristem size), whereas it contributed only weakly to cell size. Meristem shape also differed only weakly among the ontogenetic stages. Three summary variables, viz. meristem size, cell size and meristem shape, showed fairly strong phylogenetic signals in the data (Table 1, Fig. 4, Supplementary Data S2 and S3). Meristem diameter was large primarily in Euphorbiaceae and Ranunculaceae, whereas it was small in Hypericum and almost all species from the commelinid clade (Fig. 5). Meristem shape was bulging, i.e. thicker relative to the periphery and width, mainly in Poaceae (Fig. 5), whereas it was flatter relative to the periphery in campanulids and Epilobium (Fig. 5). In contrast, cell number did not show any statistically significant phylogenetic signal. Of the plant traits, seed size, nuclear genome size and stem diameter had fairly high phylogenetic signals; the λ value of seed mass was not significantly different from 1 (Table 1). In contrast, height at maturity and leaf area displayed much weaker signals, with the signal in height not significantly different from 0 (Table 1).
Fig. 3.
Components of variance of meristem parameters (normalized to sum to 1, residual variation not included). For additional details see the Materials and methods section.
Table 1.
Phylogenetic signals of meristem summary variables and plant traits as assessed by Pagel’s λ. Non-significant differences from endpoint values are indicated in bold. A λ value of 0 means no phylogenetic conservatism in the trait; a value of 1 means Brownian motion evolution of the trait
| Estimated λ | Lower 95% CI | Upper 95% CI | Significance of difference from 0 | Significance of difference from 1 | |
|---|---|---|---|---|---|
| Meristem traits | |||||
| Size | 0.739 | 0.528 | 0.877 | <0.001 | <0.001 |
| Cell number | 0.291 | 0.000 | 0.618 | 0.155 | <0.001 |
| Cell size | 0.662 | 0.429 | 0.829 | <0.001 | <0.001 |
| Shape | 0.621 | 0.358 | 0.835 | <0.001 | <0.001 |
| Plant traits | |||||
| Height | 0.205 | 0.000 | 0.626 | 0.230 | <0.001 |
| Stem diameter | 0.786 | 0.495 | 0.921 | <0.001 | <0.001 |
| Leaf area | 0.530 | 0.138 | 0.795 | 0.016 | <0.001 |
| Seed mass | 0.982 | 0.910 | 1.000 | <0.001 | 0.389 |
| Genome size | 0.921 | 0.804 | 0.982 | <0.001 | 0.003 |
CI, confidence interval.
Fig. 4.
Mapping of meristem size on the phylogenetic tree from the Daphne phylogeny.
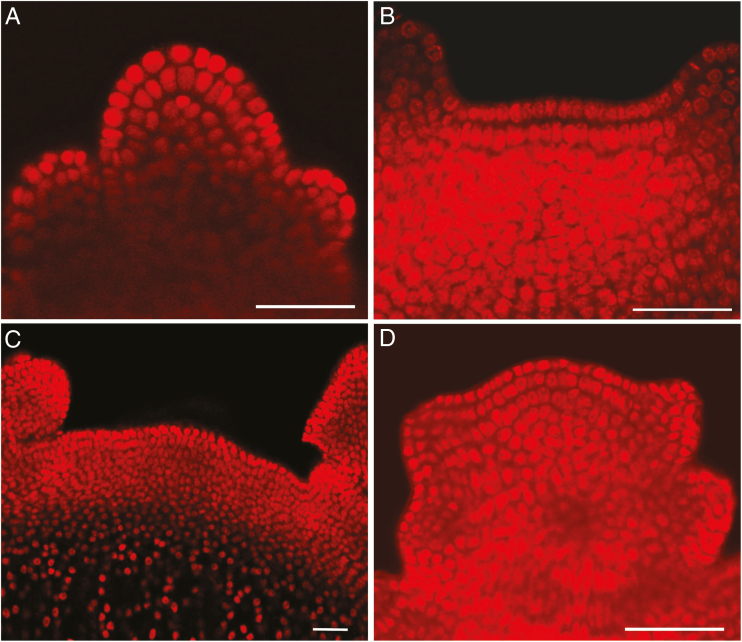
Fig. 5.
Longitudinal medial cross-sections of SAMs stained by PI and viewed through the confocal microscope. The image shows a bulgy SAM of Poa pratensis (A), a flat SAM of Epilobium hirsutum (B), a large SAM of Euphorbia lucida (C) and a small SAM of Hypericum maculatum (D). Scale bar (A–D) = 50 µm.
All meristem summary variables showed correlations with plant traits, although strengths and types of these correlations differed among the summary variables (Supplementary Data S4). Meristem size was positively correlated with a number of size-related plant traits, namely stem diameter and leaf area, and further with seed mass (Fig. 6). Its first component, cell number, was related mainly to stem diameter; the second component, cell size, to seed mass (Fig. 7). Meristem shape was correlated with stem diameter and leaf area, and, to a lesser degree, with shoot lifespan (short-lived shoots have thickness greater relative to inner periphery). In general, stem diameter and seed mass were strongly predicted by meristem summary variables, whereas leaf area was predicted much less by them; plant height was almost completely uncorrelated with any of the meristem parameters. Some of the correlations became weaker when phylogeny was taken into account (Supplementary Data S4). Cell size correlations remained high, whereas cell number correlations often became weaker (e.g. with seed mass). Variation in stem diameter can be decomposed into two essentially uncorrelated components due to independent correlations with meristem size and plant height (Table 2). While the component attributable to meristem size is due to shared phylogenetic history and disappears if phylogeny is taken into account, the latter effect is independent of phylogeny (Table 2).
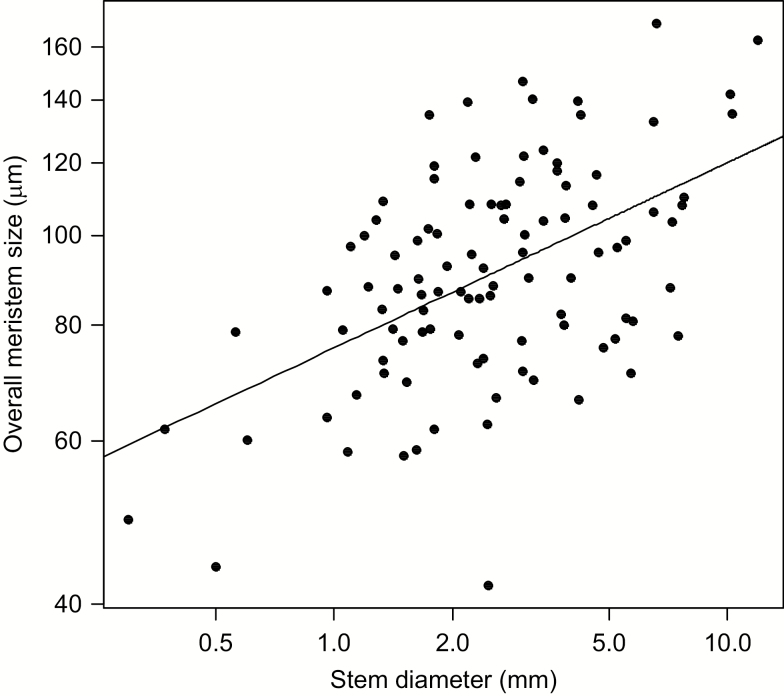
Fig. 6.
Relationship between stem diameter and overall meristem size (area of the cross-section) based on species means. The line is the ordinary least squares regression line. R2 = 0.261.
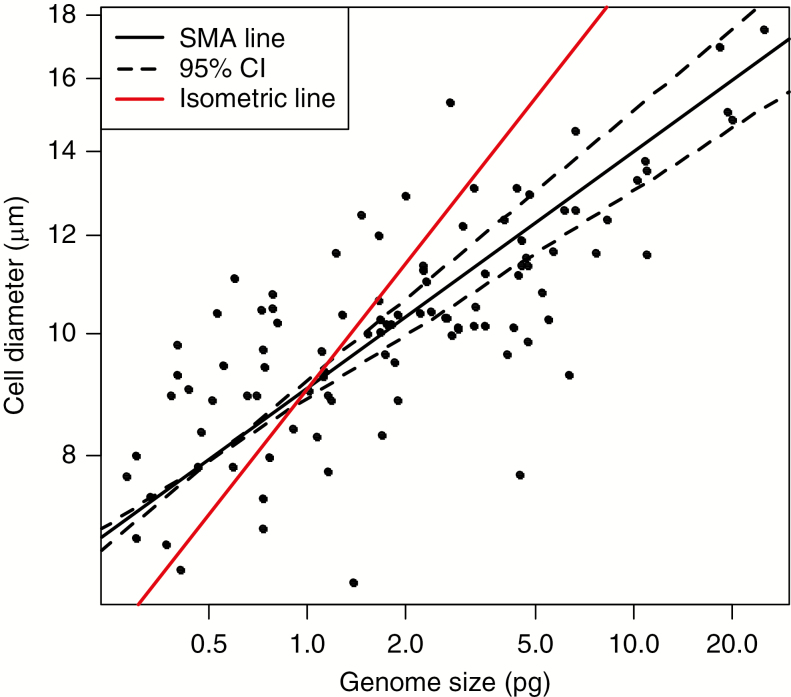
Fig. 7.
Relationship between cell size at the outer periphery and nuclear genome size. The continuous line is the standard major axis regression (SMA) line, dashed lines show the 95 % confidence interval (CI) for this line, and the red line is the expected isometric relationship (slope 1/3). R2 = 0.568 (ordinary least squares).
Table 2.
Analysis of variation in stem diameter. Values in the table are adjusted R2
| Source of variation1 | Non-phylogenetic | Phylogenetic | |
|---|---|---|---|
| Height | Total | 0.186 | 0.246 |
| Net | 0.122 | 0.201 | |
| Meristem size | Total | 0.260 | 0.072 |
| Net | 0.196 | 0.025 | |
| Shared effect | Net | 0.057 | 0.037 |
1Total refers to variation explained by a linear model with the source variable as the only predictor; Net refers to variation explained by a partial model with the effect of the other variable removed (i.e. total variation minus variation due to the shared effect of both variables, corrected to get adjusted values).
Effects strongly influenced by shared phylogenetic history are shown in bold.
Nuclear genome size was correlated both with meristem size and cell size; the latter remained high also in phylogenetic analysis. Correlation of meristem shape with nuclear genome size increased in phylogenetic analysis. The slope of the relationship between cell size at the periphery and nuclear genome size was strongly non-isometric (Fig. 7). The scaling exponent estimated by SMA regression was 0.189 (95 % confidence interval for the slope was 0.16–0.216).
The best path model showed a strong effect of nuclear genome size on cell size, but no effect on either cell number or meristem shape (Fig. 8). Cell size in turn affected seed mass, but did not affect any other plant trait. In contrast, cell number affected leaf area, stem diameter and seed mass; meristem shape affected stem diameter. Plant height showed covariation with stem diameter, but was not affected by cell number or stem diameter. In a path analysis with residualized variables (i.e. with phylogenetic effect removed), there were only two effects of meristem variables remaining: cell size affecting seed mass, and cell number affecting stem diameter (data not shown).
Fig. 8.
Path analysis of relationship between meristem summary variables, nuclear genome size and plant traits. χ2 = 10.16, d.f. = 8, P = 0.253. R2 for individual plant traits: leaf area, 0.088; stem diameter, 0.347; seed mass, 0.232; height, 0.014. For additional details see the Materials and methods section.
DISCUSSION
Using comparative data from 104 perennial herbaceous angiosperms, we were able to show that parameters of the shoot apical meristem (overall size, cell size and number and shape) were correlated with size-related traits in several plant organs, namely leaf area, seed mass and stem diameter. Although the correlative nature of the study does not permit more definitive statements about the causes of such relationships, SAM parameters clearly provide a functionally interpretable link among sizes of these plant organs. The SAM parameters showed strong covariation with stem diameter and seed mass, weaker covariation with leaf area, and no relationship with plant height. Some of these patterns are due to shared phylogenetic history (e.g. between meristem size and stem diameter), whereas other relationships remained strong even in phylogenetic analyses and are thus likely due to parallel evolution (e.g. meristem cell size and seed mass).
Meristem cell size and number
Our data demonstrate that both ontogenetic and phylogenetic variation in meristem cell number were much higher than that of cell size (e.g. Abbe et al., 1941; Sharma and Sharma, 1989; Bäurle and Laux, 2003; Gonzalez et al. 2012; Brodribb et al. 2013). Cell number changes during SAM ontogeny, meaning that the plant can change the numbers of cells in different apices and thus influence the numbers of cells in the primordia that are formed from them. Cell number also showed much weaker phylogenetic patterns, with closely related species differing significantly in this parameter. This indicates that changes in molecular and biochemical processes that determine changes in cell numbers are much easier than in those that determine cell sizes (Guo and Simmons, 2011).
Cell size was strongly correlated with nuclear genome size. This corresponds well to patterns known from other cell types (Knight and Beaulieu, 2008; Niklas, 2015). Surprisingly, the slope of the relationship was allometric, i.e. the slope parameter was much lower than the isometric value expected on geometrical grounds (1/3), as genome size scales with volume and hence with the third power of a linear measure such as cell diameter (Šímová and Herben 2012). The same was also true for cell size measured at the inner periphery (data not shown). This contrasts with the findings of Šímová and Herben (2012), who showed that, with the exception of stomata, relationships between cell sizes and genome sizes typically follow patterns expected by simple geometry. The negatively allometric relationship means that meristems actively manage their cell sizes to counter the isometric relationship forced onto them by the scaling law (Kondorosi et al., 2000; John et al., 2013). Interestingly, stomata are the only differentiated cells known to show this negative allometry (Šímová and Herben, 2012). This may be due to stomata development, as the cells giving rise to them retain ‘meristemoid’ division longer than other differentiated cells in the leaf (Gonzalez et al., 2012) and thus bear vestiges of the negative allometry of the apical meristem. Both these negative allometries are explained by the fact that cell numbers can to some degree be compensated for by changing cell size, either interspecifically or intraspecifically (Vernoux et al., 2000; Tsukaya, 2002; Huber et al., 2014; Carins Murphy et al., 2016).
Meristem parameters and links among individual organs
Most plant traits were primarily determined by meristem size, with different contributions of cell sizes and cell numbers. Variation in all examined plant traits was linked more strongly to cell number than to cell size. This is in accordance with, using a large comparative set, the patterns identified in cell size/cell number variation in individual species (e.g. Gonzalez et al., 2010). Stem diameter is the prime plant trait that is determined by meristem cell number. Stem diameter is the key parameter in the biomechanical body plan of plants and is known to scale with plant height in many plant groups (Niklas, 1994). Interestingly, we found that plant height is correlated with stem diameter, but this correlation does not seem to be mediated by any of the meristem parameters. It therefore does not seem to be derived only from meristem properties and may be, at least partly, constrained by specific anatomical properties (Schweingruber et al., 2014). Indeed, variation in stem diameter has two components: one of them is phylogenetically conservative and is tightly linked to meristem size, the other is linked to variation in plant height (which is only weakly phylogenetically conservative). The latter is clearly due to a biomechanical constraint (e.g. Niklas, 1994), whereas the former is primarily due to a developmental constraint with clear phylogenetic patterns. Hence plant height does not place a strong constraint on meristem size and therefore is uncoupled from variation in traits that are determined by meristem parameters, such as seed mass (Thompson and Rabinowitz, 1989; Moles et al., 2004; Rees and Venable, 2007).
The absence of a relationship of overall height with meristem parameters and its weak relationship with leaf size and stem diameter in perennial herbs clearly shows that overall height is a different kind of plant trait from the other traits that we are studying, at least in herbaceous plants. Leaf size, (relative) stem diameter and seed mass are parameters of plant body parts and are linked to each other by rules such as Corner’s rule (Cornelissen, 1999; Hodgson et al., 2017). Our data show that these rules have a common basis in the structure of their apical meristems and consequently in leaf and floral primordia. Phylogenetic conservatism of seed mass (Lord et al., 1996) and leaf area (McCarthy et al., 2007; Flores et al., 2014; Liu et al., 2015) is fairly strong, which is also the case for stem diameter (this paper). In contrast, plant height is an aggregate trait that can be achieved by varying these contributing attributes, and its relationships to the drivers of these attributes is consequently weaker.
Furthermore, meristem cell number determines, although more weakly than stem diameter, the leaf area of individual species. From a developmental point of view, leaf area is a composite variable, as leaves may strongly differ both in their shapes (whole versus divided leaves) and in the thicknesses (in cell numbers) that characterize them (Tsukaya, 2014; Dkhar and Pareek, 2014; Hodgson et al., 2017). Meristem size may affect these components differently. Still, we were not able to find any strong relationship to meristem summary variables that would involve leaf thickness (measured as specific leaf area) or overall leaf mass and be independent of leaf area. It is challenging to search for indirect processes by which meristem size can be linked to seed mass (Cornelissen, 1999, Hodgson et al., 2017). In principle, seed size may be constrained by the sizes of the cells that gave rise to it, but also may be constraining the meristem size in seedlings, with small seeds producing small seedlings, which are bound to have small meristems and consequently small cells to retain a reasonable cell number. At any rate, meristem parameters (namely cell number) constitute a proximate link between sizes of these two seemingly unrelated organs (see also Hodgson et al. 2017, Santini et al., 2017).
Meristem cell size does not seem to be an important driver of sizes of individual organs except for seed mass (which also has an independent contribution of cell number, in addition to cell size). Seed mass has been shown to be correlated with nuclear genome size (Thompson, 1990; Grotkopp et al., 2004; Knight et al., 2005; Knight and Beaulieu, 2008; for a deeper analysis see Beaulieu et al., 2007). Our current analysis shows that this correlation is mediated by meristem size (path analysis shows no detectable direct effect of genome size on seed mass that would be independent of meristem cell size).
Limitations of the study
As meristems are highly dynamic structures, their parameters change during their ontogeny (Abbe et al., 1941; Bäurle and Laux, 2003). This concerns mainly the number of cells, whereas cell size remains typically stable (Abbe et al., 1941). Meristems grow in size in developing buds during the vegetative season, and go through winter in a particular developmental phase. In the spring, the meristems attain their final size by increasing the number of cells (Bäurle and Laux, 2003). Even though we sampled meristems primarily on well-developed buds, we cannot exclude the possibility of sampling bias due to the potential for interspecific differences in phenologies and ontogenetic rates in different species to yield differences in the stages of renewal meristem. Therefore, measured meristem parameters are contingent on the ontogenetic stage of the plant when sampled, as demonstrated by the high proportion of variation in meristem parameters due to ontogenetic stage. Moreover, in some plant species nearly all buds are in the same stage of development (mainly plants with annual shoots only, e.g. Aster linosyris), whereas plant species with overwintering shoots (polycyclic species sensuKlimešová et al., 2016) typically possess buds of different ontogenetic stages at the same time.
Conclusions
The strong relationships between meristem parameters and several plant traits and the clear phylogenetic patterns of these parameters show that comparative study of them can shed light on a number of developmental correlations across plant species. In contrast to correlations among organs, such as those captured by Corner’s rule, correlations that involve the SAM make much better sense from the functional and developmental point of view, as the SAM is the ultimate source of the sizes and shapes of the individual plant organs. In addition, it could help to separate biomechanical correlations from developmental ones, and identify the source of the latter, as we did in the case of stem diameter.
AUTHOR CONTRIBUTIONS
J.K., R.S. and T.H. designed the research, R.S. collected plants and collected all anatomical data, T.H. and R.S. analysed the data, T.H. wrote the text with contributions by R.S. and J.K.
SUPPLEMENTARY DATA
Supplementary Data are available online at https://academic.oup.com/aob and consist of the following. S1: list of species used, sources of material and standards for genome size estimation. S2: correlation of PCA scores with the summary variables and mapping of summary variables on the PCA plot. S3: mapping all four summary variables onto the phylogenetic tree. S4: correlation matrix of mean-per-species meristem parameters and traits.
Supplementary Material
ACKNOWLEDGEMENTS
We thank František Krahulec for help with species collection and identification, Jana Vítová and Pavel Trávníček for genome size measurements and description of the methods used, Petr Šmarda for thoughtful comments on an earlier version of the paper and Jonathan Rosenthal for language correction. We also thank curators of several botanical gardens for providing species for this study. The research was supported by the Czech Science Foundation (Centre of Excellence PLADIAS, 14-36079G).
LITERATURE CITED
- Abbe EC, Randolph LF, Einset J. 1941. The developmental relationship between shoot apex and growth pattern of leaf blade in diploid maize. American Journal of Botany 28: 778–784. [Google Scholar]
- Ackerly DD, Donoghue MJ. 1998. Leaf size, sapling allometry, and Corner’s rules: phylogeny and correlated evolution in maples (Acer). American Naturalist 152: 767–791. [DOI] [PubMed] [Google Scholar]
- Barlow PW. 1989. Meristems, metamers and modules and the development of shoot and root systems. Botanical Journal of the Linnean Society 100: 255–279. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bäurle I, Laux T. 2003. Apical meristems: the plant’s fountain of youth. Bioessays 25: 961–970. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Moles AT, Leitch IJ, Bennett MD, Dickie JB, Knight CA. 2007. Correlated evolution of genome size and seed mass. New Phytologist 173: 422–437. [DOI] [PubMed] [Google Scholar]
- Bennett MD, Leitch IJ. 2005. Nuclear DNA amounts in angiosperms: progress, problems and prospects. Annals of Botany 95: 45–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser SP, Aarssen LW. 2006. Meristem allocation and life-history evolution in herbaceous plants. Canadian Journal of Botany 84: 143–150. [Google Scholar]
- Brodribb TJ, Jordan GJ, Carpenter RJ. 2013. Unified changes in cell size permit coordinated leaf evolution. New Phytologist 199: 559–570. [DOI] [PubMed] [Google Scholar]
- Brouat C, Gibernau M, Amsellem L, McKey D. 1998. Corner’s rules revisited: ontogenetic and interspecific patterns in leaf-stem allometry. New Phytologist 139: 459–470. [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ. 2016. Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Annals of Botany 118: 1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro N, Peaucelle A, Laufs P, Traas J. 2006. Cell differentiation and organ initiation at the shoot apical meristem. Plant Molecular Biology 60: 811–826. [DOI] [PubMed] [Google Scholar]
- Chumová Z. 2015. Evolutionary and taxonomic implications of variation in nuclear genome size: lesson from the grass genus Anthoxanthum (Poaceae). PLOS ONE 10: e0133748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1993. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119: 397–418. [DOI] [PubMed] [Google Scholar]
- Classen-Bockhoff R. 2016. The shoot concept of the flower: still up to date? Flora 221: 46–53. [Google Scholar]
- Cornelissen JHC. 1999. A triangular relationship between leaf size and seed size among woody species: allometry, ontogeny, ecology and taxonomy. Oecologia 118: 248–255. [DOI] [PubMed] [Google Scholar]
- Corner EJH. 1949. The annonaceous seed and its four integuments. New Phytologist 48: 332–364. [Google Scholar]
- Desdevises Y, Legendre P, Azouzi L, Morand S. 2003. Quantifying phylogenetically structured environmental variation. Evolution 57: 2647–2652. [DOI] [PubMed] [Google Scholar]
- Diniz-Filho JAF, de Sant’Ana CER, Bini LM. 1998. An eigenvector method for estimating phylogenetic inertia. Evolution 52: 1247–1262. [DOI] [PubMed] [Google Scholar]
- Dkhar J, Pareek A. 2014. What determines a leaf’s shape? EvoDevo 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucretti S et al. 1998. Plant genome size estimation by flow cytometry: Inter-laboratory comparison. Annals of Botany 82: 17–26. [Google Scholar]
- Doležel J, . 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233–2244. [DOI] [PubMed] [Google Scholar]
- Dray S, Dufour AB. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20. [Google Scholar]
- Durka W, Michalski SG. 2012. Daphne: a dated phylogeny of a large European flora for phylogenetically informed ecological analyses. Ecology 93: 2297–2297. [Google Scholar]
- Fletcher JC. 2002. Coordination of cell proliferation and cell fate decisions in the angiosperm shoot apical meristem. BioEssays 24: 27–37. [DOI] [PubMed] [Google Scholar]
- Flores O, Garnier E, Wright IJ et al. 2014. An evolutionary perspective on leaf economics: phylogenetics of leaf mass per area in vascular plants. Ecology and Evolution 4: 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Nie Y, Byrnes J. 2016. sem: structural equation models.R package version 3.1–8 https://CRAN.R-project.org/package=sem. [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. American Naturalist 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Gonzalez N, De Bodt S, Sulpice R et al. 2010. Increased leaf size: different means to an end. Plant Physiology 153: 1261–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Vanhaeren H, Inze D. 2012. Leaf size control: complex coordination of cell division and expansion. Trends in Plant Science 17: 332–340. [DOI] [PubMed] [Google Scholar]
- Grace JB. 2006. Structural equation modeling and natural systems.Cambridge, UK: Cambridge University Press. [Google Scholar]
- Green PB. 1999. Expression of pattern in plants: combining molecular and calculus-based biophysical paradigms. American Journal of Botany 86: 1059–1076. [PubMed] [Google Scholar]
- Grotkopp E, Rejmánek M, Sanderson MJ, Rost TL. 2004. Evolution of genome size in pines (Pinus) and its life-history correlates: supertree analyses. Evolution 58: 1705–1729. [DOI] [PubMed] [Google Scholar]
- Guo M, Simmons CR. 2011. Cell number counts – the fw2.2 and CNR genes and implications for controlling plant fruit and organ size. Plant Science 181: 1–7. [DOI] [PubMed] [Google Scholar]
- Hamant O, Traas J. 2010. The mechanics behind plant development. New Phytologist 185: 369–85. [DOI] [PubMed] [Google Scholar]
- Harper JL, Lovell PH, Moore KG. 1970. The shapes and sizes of seeds. Annual Review of Ecology and Systematics 1: 327–356. [Google Scholar]
- Hodgson JG, Santini BA, Marti GM et al. 2017. Trade-offs between seed and leaf size (seed–phytomer–leaf theory): functional glue linking regenerative with life history strategies … and taxonomy with ecology? Annals of Botany doi:10.1093/aob/mcx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AL, van Haperen JMA, Groot EP, Laux T. 2014. Signaling in shoot and flower meristems of Arabidopsis thaliana. Current Opinion in Plant Biology 17: 96–102 [DOI] [PubMed] [Google Scholar]
- Huber H, de Brouwer J, von Wettberg EJ, During HJ, Anten NPR. 2014. More cells, bigger cells or simply reorganization? Alternative mechanisms leading to changed internode architecture under contrasting stress regimes. New Phytologist 201: 193–204. [DOI] [PubMed] [Google Scholar]
- John GP, Scoffoni C, Sack L. 2013. Allometry of cells and tissues within leaves. American Journal of Botany 100: 1936–48. [DOI] [PubMed] [Google Scholar]
- Kleyer M, Bekker RM, Bakker J et al. 2008. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology 96: 1266–1274. [Google Scholar]
- Klimešová J. 2016. Links between shoot and plant longevity and plant economics spectrum: environmental and demographic implications. Perspectives in Plant Ecology, Evolution and Systematics 22:55–62. [Google Scholar]
- Klimešová J, Danihelka J, Chrtek J, de Bello F, Herben T. 2017. CLO-PLA: a database of clonal and bud-bank traits of the Central European flora. Ecology 98:1179–1179. [DOI] [PubMed] [Google Scholar]
- Knevel IC, Bekker RM, Kunzmann D, Stadler M. Thompson K. 2005. The LEDA Traitbase collecting and measuring standards of life-history traits of the NW European flora.Groningen, The Netherlands: University of Groningen. [Google Scholar]
- Knight CA, Beaulieu JM. 2008. Genome size scaling through phenotype space. Annals of Botany 101: 759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, Molinari NA, Petrov DA. 2005. The large genome constraint hypothesis: evolution, ecology and phenotype. Annals of Botany 95: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E, Roudier F, Gendreau E. 2000. Plant cell-size control: growing by ploidy? Current Opinion in Plant Biology 3: 488–492. [DOI] [PubMed] [Google Scholar]
- Kubát K, Hrouda L, Chrtek J jun, Kaplan Z, Kirschner J, Štěpánek J. 2002. Klíč ke květeně České republiky. [Key to the Flora of the Czech Republic] Prague, Czech Republic: Academia. [Google Scholar]
- Kwiatkowska D, Dumais J. 2003. Growth and morphogenesis at the vegetative shoot apex of Anagallis arvensis L. Journal of Experimental Botany 54: 1585–1595. [DOI] [PubMed] [Google Scholar]
- Laufs P, Grandjean O, Jonak C, Kieu K, Traas J. 1998. Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell 10: 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P. 2014. lmodel2: Model II Regression.R package version 1.7–2. https://CRAN.R-project.org/package=lmodel2. [Google Scholar]
- Leslie AB, Beaulieu JM, Crane PR, Donoghue MJ. 2014. Cone size is related to branching architecture in conifers. New Phytologist 203: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu Q, He P, Santiago LS, Yang K, Ye Q. 2015. Strong phylogenetic signals and phylogenetic niche conservatism in ecophysiological traits across divergent lineages of Magnoliaceae. Nature Scientific Reports 5: 12246. doi:10.1038/srep12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J, Westoby M, Leishmann M. 1996. Seed size and phylogeny in six temperate floras: constraints, niche conservatism, and adaptation. American Naturalist 146: 349–364 [Google Scholar]
- Mauseth JD. 2004. Giant shoot apical meristems in cacti have ordinary leaf primordia but altered phyllotaxy and shoot diameter. Annals of Botany 94: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MC, Enquist BJ, Kerkhoff AJ. 2007. Organ partitioning and distribution across the seed plants: assessing the relative importance of phylogeny and function. International Journal of Plant Sciences 168: 751–761. [Google Scholar]
- Medford JI. 1992. Vegetative apical meristems. Plant Cell 4: 1029–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Falster DS, Leisnman MR, Westoby M. 2004. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. Journal of Ecology 92: 384–396. [Google Scholar]
- Niklas KJ. 1994. Plant allometry: the scaling of form and process.Chicago: The University of Chicago Press. [Google Scholar]
- Niklas KJ. 2015. A phyletic perspective on cell growth. Cold Spring Harbor Perspectives in Biology doi:10.1101/cshperspect. a019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB. 2013. Package ‘vegan’.Version 2.0–10 Vienna: R Foundation for Statistical Computing, Vienna; http://www.R-project.org. [Google Scholar]
- Orme D. 2012. The caper package: comparative analysis of phylogenetics and evolution in R. R Foundation for Statistical Computing, Vienna: Available at: http://cran.r-project.org/web/packages/caper/. [Google Scholar]
- Powell AE, Lenhard M. 2012. Control of organ size in plants. Current Biology 22: 360–367. [DOI] [PubMed] [Google Scholar]
- Price H, Sparrow A, Nauman A. 1973. Correlations between nuclear volume, cell volume and DNA content in meristematic cells of herbaceous angiosperms. Experientia 29: 1028–1029. [Google Scholar]
- Rees M, Venable DL. 2007. Why do big plants make big seeds? Journal of Ecology 95: 926–936. [Google Scholar]
- Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Revell LJ. 2014. Package ‘phytools’.https://cran.r-project.org/web/packages/phytools. [Google Scholar]
- Romberger JA. 1963. Meristems, growth, and development in woody plants; an analytical review of anatomical, physiological, and morphogenic aspects. Technical Bulletin (United States Department of Agriculture), No. 1293. [Google Scholar]
- Ronse De Craene LP. 2016. Meristic changes in flowering plants. How flowers play with numbers. Flora 221: 22–37. [Google Scholar]
- Santini BA, Hodgson JG, Thompson K et al. 2017. The triangular seed mass-leaf area relationship holds for annual plants and is determined by habitat productivity. Functional Ecology (in press). doi:10.1111/1365–2435.12870. [Google Scholar]
- Sharma M, Sharma KC. 1989. Developmental studies on shoot apical organization in Zinnia elegans Jacq. Botanical Bulletin of Academia Sinica 30: 1–7. [Google Scholar]
- Schönswetter P, Suda J, Popp M, Weiss-Schneeweiss H, Brochmann C. 2007. Circumpolar phylogeography of Juncus biglumis (Juncaceae) inferred from AFLP fingerprints, cpDNA sequences, nuclear DNA content and chromosome numbers. Molecular Phylogenetics and Evolution 42: 92–103. [DOI] [PubMed] [Google Scholar]
- Schweingruber FH, Říha P, Doležal J. 2014. Variation in stem anatomical characteristics of Campanuloideae species in relation to evolutionary history and ecological preferences. PloS ONE: e88199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluis A, Hake S. 2015. Organogenesis in plants: initiation and elaboration of leaves. Trends in Genetics 31: 300–306. [DOI] [PubMed] [Google Scholar]
- Šímová I, Herben T. 2012. Geometrical constraints in the scaling relationships between genome size, cell size and cell cycle length in herbaceous plants. Proceedings of the Royal Society B. Biological Sciences 7: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temsch EM, Greilhuber J, Krisai R. 2010. Genome size in liverworts. Preslia 82: 63–80. [Google Scholar]
- Thompson K. 1990. Genome size, seed size and germination temperature in herbaceous angiosperms. Evolutionary Trends in Plants 4: 113–116. [Google Scholar]
- Thompson K, Rabinowitz D. 1989. Do big plants have big seeds? American Naturalist 133: 722–728. [Google Scholar]
- Tsukaya H. 2002. Interpretation of mutants in leaf morphology: genetic evidence for a compensatory system in leaf morphogenesis that provides a new link between cell and organismal theory. International Review of Cytology 217: 1–39. [DOI] [PubMed] [Google Scholar]
- Tsukaya H. 2014. Comparative leaf development in angiosperms. Current Opinion in Plant Biology 17: 103–109. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Autran D, Traas J. 2000. Developmental control of cell division patterns in the shoot apex. Plant Molecular Biology 43: 569–581. [DOI] [PubMed] [Google Scholar]
- Westoby M, Wright I. J. 2003. The leaf size-twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135: 621–628. [DOI] [PubMed] [Google Scholar]
- Whitman T, Aarssen LW. 2010. The leaf size/number trade-off in herbaceous angiosperms. Journal of Ecology 3: 49–58. [Google Scholar]
- Yan E-R, Milla R, Aarssen LW, Wang X-H. 2012. Functional relationships of leafing intensity to plant height, growth form and leaf habit. Acta Oecologica 41: 20–29. [Google Scholar]
- Yang D, Li G, Sun S. 2008. The generality of leaf size versus number trade-off in temperate woody species. Annals of Botany 102: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.