Abstract
Background
Nivolumab, a monoclonal antibody of immune checkpoint programmed death 1 on T cells (PD-1), combined with ipilimumab, an immune checkpoint cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitor, as combination therapy on the one hand and nivolumab as monotherapy on the other, have both demonstrated improved efficacy compared with ipilimumab alone in the CheckMate 067 study. However, the combination resulted in a higher frequency of grade 3/4 adverse events (AEs), which could result in diminished health-related quality of life (HRQoL). Here we report analyses of HRQoL for patients with advanced melanoma in clinical trial CheckMate 067.
Patients and methods
HRQoL was assessed at weeks 1 and 5 per 6-week cycle for the first 6 months, once every 6 weeks thereafter, and at two follow-up visits using the European Organization for Research and Treatment of Care Core Quality of Life Questionnaire and the EuroQoL Five Dimensions Questionnaire. In addition to the randomised population, patient subgroups, including BRAF mutation status, partial or complete response, treatment-related AEs of grade 3/4, and those who discontinued due to any reason and due to an AE, were investigated.
Results
Nivolumab and ipilimumab combination and nivolumab alone both maintained HRQoL, and no clinically meaningful deterioration was observed over time compared with ipilimumab. In addition, similar results were observed across patient subgroups, and no clinically meaningful changes in HRQoL were observed during follow-up visits for patients who discontinued due to any cause.
Conclusion
These results further support the clinical benefit of nivolumab monotherapy and nivolumab and ipilimumab combination therapy in patients with advanced melanoma. The finding that the difference in grade 3/4 AEs between the arms did not translate into clinically meaningful differences in the reported HRQoL may be relevant in the clinical setting.
Study number
Keywords: Health-related quality of life, Checkpoint inhibitors, Advanced melanoma, Nivolumab, Ipilimumab
1. Introduction
The treatment of advanced melanoma has progressed considerably with the introduction of T-cell checkpoint inhibitors, including inhibitors of cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death 1 (PD-1) [1–4]. An antibody to CTLA-4, ipilimumab, improved the overall survival (OS) of patients with advanced melanoma in two phase III, randomised controlled trials [1,5]. The PD-1 inhibitors nivolumab and pembrolizumab have shown improved progression-free survival (PFS) and OS compared with ipilimumab [3,4,6]; in addition, nivolumab has shown improved PFS and OS compared with chemotherapy [2].
Combination treatment with nivolumab plus ipilimumab has shown considerable efficacy across clinical trials [4,6–9], including the phase II CheckMate 069 study and the phase III CheckMate 067 study in patients with untreated advanced melanoma, with objective response rates of 59% and 58%, respectively, for the combination and 11% and 19%, respectively, for ipilimumab alone [4,8]. In addition, 2-year OS for the combination compared with ipilimumab alone was 64% versus 54% [9] and 64% versus 45% [6].
The greater efficacy of combined checkpoint inhibitors is accompanied by higher incidence of grade 3 and 4 adverse events (AEs) versus either agent alone. In CheckMate 069, treatment-related grade 3/4 AEs were reported in 54% of patients who received combination and 20% of patients who received ipilimumab alone [9]; for CheckMate 067, the percentages were 55% and 27%, respectively [4]. This toxicity profile might diminish health-related quality of life (HRQoL). Patient-reported outcomes (PROs), such as symptoms, HRQoL, and patient-perceived health status supplement clinical data and are now more important during decision-making in oncology because they provide a holistic understanding of patient experience and treatment effectiveness [10,11]. Ipilimumab monotherapy did not have a significant negative HRQoL impact in patients with stage III or IV melanoma [12], and nivolumab monotherapy maintained baseline HRQoL levels compared with dacarbazine in patients with advanced melanoma [13]. Here we report analyses of HRQoL for patients with advanced melanoma treated with nivolumab plus ipilimumab, nivolumab alone, or ipilimumab alone in CheckMate 067. In addition to the full HRQoL patient population, we examined subgroups including patients with BRAF mutation status, patients with a partial or complete response, patients with treatment-related AEs of grade 3 or 4, and patients who discontinued due to any reason and those who discontinued due to an AE.
2. Patients and methods
2.1. Study design
This study presents 12-month HRQoL data from the 067 CheckMate double-blind, phase III study; study details have been published [4]. Briefly, patients aged ≥ 18 years with histologically confirmed stage III (unresectable) or stage IV melanoma with no prior systemic treatment for advanced disease were randomised 1:1:1 and stratified by programmed cell death ligand 1 (PD-L1) status, BRAF status, and metastatic stage. Patients received one of the following by intravenous infusion, with the appropriate placebo: nivolumab 3 mg/kg every 2 weeks (Q2W); nivolumab 1 mg/kg Q3W plus ipilimumab 3 mg/kg Q3W for 4 doses, followed by nivolumab 3 mg/kg Q2W; or ipilimumab 3 mg/kg Q3W for 4 doses. Treatment was continued until disease progression, development of unacceptable toxic events, or withdrawal of consent. Per investigator, patients with clinical benefit and without substantial AEs could be treated beyond progression.
2.2. Assessments
HRQoL was collected, as available, in all randomised patients and assessed at weeks 1 and 5 of each 6-week cycle for the first 6 months and then once every 6 weeks thereafter as well as at two visits in the follow-up period (Fig. A.1). Secondary end-point assessment was European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 Questionnaire Version 3 [14,15]; European Quality of Life-5 Dimensions (EQ-5D) Summary Index and Visual Analogue Scale (VAS) [16,17] and the Work Productivity and Activity Impairment: General Health (WPAI:GH) were exploratory end-points [18]. WPAI:GH analyses were not included in this report because they only include patients in the workforce and the number of patients was too low for adequate analysis.
2.3. Statistical analyses
Analyses were performed on all randomised patients with both a baseline and ≥ 1 post-baseline assessment. Analyses were also performed on subgroups including patients with BRAF mutation status, patients with a partial or complete response, and patients with AEs of grade 3 or 4. In addition, follow-up data were analysed for patients who discontinued due to any reason and those who discontinued due to an AE. For each instrument, the questionnaire completion rate was defined as the proportion of patients who completed the questionnaire at the indicated time point using the number of patients in the study at the respective time point as the denominator. It was predetermined that conclusions would only be drawn from time points for which ≥ 30 patients completed assessments.
Continuous data were described using descriptive statistics, and categorical data were summarised using counts and percentages. Mean changes from baseline at each time point were reported and assessed according to minimally important difference (MID) values, with statistical significance assessed at P ≤ 0.05. The EORTC QLQ-C30 is a 30-item, self-administered, multidimensional, cancer-specific, HRQoL PRO questionnaire, with a difference of 10 points on a 100-point scale considered clinically meaningful [19]. The EQ-5D-3L descriptive system is composed of the five dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression with a utility index score difference of 0.08 considered clinically meaningful; the EQ-5D VAS records the respondent's self-rated health on a vertical VAS with a score of 7 considered clinically meaningful [20].
To assess longitudinal changes from baseline within and between each treatment, modelling was conducted using all observed data through week 55 via a mixed-effects model for repeated measures (MMRM), including baseline PRO score and stratification factors as covariates. MMRM can give unbiased estimates with certain missing data contexts and can be more powerful than a two-sample t-test [21]. Least squares (LS) means and standard error (SE) as well as LS means difference with 95% confidence interval (CI) were calculated for nivolumab versus ipilimumab and for nivolumab + ipilimumab combination versus ipilimumab alone. Estimates were based on a mixed model with treatment and time point as fixed effects, study day as a random effect, and time point as a repeated measure.
Values were imputed for missing items for the EORTC QLQ-C30 by using values equal to the average of the non-missing items for scales in which at least half of the items were completed. A scale in which fewer than half of the items were completed was treated as missing and, once the instrument was scored, no imputation was used to handle missing data. No adjustment was made for missing data when scoring the EQ-5D index or the EQ-5D VAS and no imputation was used to handle missing data for the longitudinal analysis.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Baseline characteristics and questionnaire completion rates
The study conducted at 137 sites in 21 countries had an enrolment period from June 2013 to March 2014 [4]. Of the 1296 patients enrolled, 945 were randomised: 316 to nivolumab (313 treated), 314 to nivolumab + ipilimumab (313 treated), and 315 to ipilimumab (311 treated). HRQoL outcomes were performed on all 803 randomised patients with both a baseline and at least one post-baseline assessment (HRQoL randomised population; Table 1). Baseline demographics and disease characteristics were similar among the treatment groups and to the main study all-randomised-patients group (Table 1) [4].
Table 1.
Characteristics of patients at baseline.
| Patient-reported outcome population | Overall study [4] | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Nivolumab (n = 270) | Nivolumab + ipilimumab (n = 274) | Ipilimumab (n = 259) | Overalla (n = 803) | Total (N = 945) | |
| Age | |||||
| Mean (SD) | 58.9 (13.2) | 59.2 (14.0) | 60.7 (13.2) | 59.6 (13.5) | 60 (13.7) |
| Sex, n (%) | |||||
| Male | 173 (64.1) | 183 (66.8) | 167 (64.5) | 523 (65.1) | 610 (64.6) |
| Female | 97 (35.9) | 91 (33.2) | 92 (35.5) | 280 (34.9) | 335 (35.4) |
| ECOG performance status score, n (%) | |||||
| 0 | 209 (77.4) | 207 (75.5) | 186 (71.8) | 602 (75.0) | 692 (73.2) |
| ≥1 | 61 (22.6) | 67 (24.5) | 73 (28.2) | 201 (25.0) | 252 (26.7) |
| Metastasis stage, n (%) | |||||
| M0/M1a/M1b | 114 (42.2) | 116 (42.3) | 111 (42.9) | 341 (42.5) | 397 (42.0) |
| M1c | 156 (57.8) | 158 (57.7) | 148 (57.1) | 462 (57.5) | 548 (58.0) |
| Lactate dehydrogenase status, n (%) | |||||
| ≤ULN | 172 (63.7) | 181 (66.1) | 167 (64.5) | 520 (64.8) | 589 (62.3) |
| >ULN | 94 (34.8) | 93 (33.9) | 90 (34.7) | 277 (34.5) | 341 (36.1) |
| Missing | 4 (1.5) | 0 | 2 (0.8) | 6 (0.7) | 15 (1.6) |
| Brain metastases, n (%) | |||||
| Yes | 6 (2.2) | 9 (3.3) | 9 (3.5) | 24 (3.0) | 34 (3.6) |
| No | 264 (97.8) | 265 (96.7) | 250 (96.5) | 779 (97.0) | 911 (96.4) |
| BRAF mutation status, n (%) | |||||
| Mutation | 83 (30.7) | 93 (33.9) | 84 (32.4) | 260 (32.4) | 298 (31.5) |
| Wild type | 187 (69.3) | 181 (66.1) | 175 (67.6) | 543 (67.6) | 647 (68.5) |
| PD-L1 status, n (%) | |||||
| Positive | 126 (46.7) | 126 (46.0) | 120 (46.3) | 372 (46.3) | 223 (23.6) |
| Negative | 144 (53.3) | 148 (54.0) | 139 (53.7) | 431 (53.7) | 620 (65.6) |
Based on patients who had both baseline and at least one post-baseline HRQoL assessment.
ECOG, Eastern Cooperative Oncology Group; SD, standard deviation; ULN, upper limit of normal.
Questionnaires were completed over a maximum of 79 weeks as well as two follow-up visits following treatment discontinuation (Table 2). Completion rates for the EORTC QLQ-C30 at baseline plus ≥1 visit were 85.1% for nivolumab, 87.3% for nivolumab + ipilimumab, and 82.2% for ipilimumab. Completion rates for the EQ-5D-3L at baseline plus ≥1 visit were 84.5% for nivolumab, 87.3% for nivolumab + ipilimumab, and 81.9% for ipilimumab. At each time point through week 67, completion rates remained ≥50% for both questionnaires. Of the 383 patients who progressed in the first 4 months, 69 (54%) in the nivolumab group, 38 (46%) in the nivolumab + ipilimumab group, and 97 (56%) in the ipilimumab group had follow-up PRO data. Analysis conclusions were drawn from time points for which ≥30 patients completed assessments. Although overall completion rates for WPAI:GH were similar to EORTC QLQ-C30 and EQ-5D-3L, ranging from 80.6% to 86.3% at baseline, completion rates for individual subsections at baseline were very low, including percentage of work time missed (28.9–30.4%), percentage of impairment while working (21.9–24.0%), and percentage of overall work impairment (21.9–23.7%).
Table 2.
Patient-reported outcome completion rates.a
| Patients, n/N (%) | Nivolumab (n = 316) | Nivolumab + ipilimumab (n = 314) | Ipilimumab (n = 315) |
|---|---|---|---|
| EORTC QLQ-C30 | |||
| Baselineb | 284/316 (89.9) | 290/314 (92.4) | 279/315 (88.6) |
| Baseline plus ≥1 | 269/316 (85.1) | 274/314 (87.3) | 259/315 (82.2) |
| Week 5 | 228/302 (75.5) | 182/293 (62.1) | 220/300 (73.3) |
| Week 7 | 237/291 (81.4) | 182/276 (65.9) | 217/291 (74.6) |
| Week 11 | 198/271 (73.1) | 113/226 (50.0) | 163/244 (66.8) |
| Week 13 | 193/249 (77.5) | 106/201 (52.7) | 129/205 (62.9) |
| Week 17 | 156/220 (70.9) | 83/164 (50.6) | 103/158 (65.2) |
| Week 19 | 164/205 (80.0) | 97/155 (62.6) | 98/144 (68.1) |
| Week 23 | 133/188 (70.7) | 87/137 (63.5) | 76/118 (64.4) |
| Week 25 | 142/182 (78.0) | 97/130 (74.6) | 74/111 (66.7) |
| Week 31 | 121/164 (73.8) | 92/125 (73.6) | 50/87 (57.5) |
| Week 37 | 112/152 (73.7) | 90/119 (75.6) | 48/75 (64.0) |
| Week 43 | 100/145 (69.0) | 74/114 (64.9) | 44/65 (67.7) |
| Week 49 | 92/128 (71.9) | 65/97 (67.0) | 40/58 (69.0) |
| Week 55 | 63/100 (63.0) | 48/72 (66.7) | 24/43 (55.8) |
| Week 61 | 38/58 (65.5) | 29/42 (69.0) | 17/28 (60.7) |
| Week 67 | 13/21 (61.9) | 16/22 (72.7) | 9/13 (69.2) |
| Week 73 | 3/4 (75.0) | 6/9 (66.7) | 0/2 (0) |
| Week 79 | – | 2/2 (100.0) | 1/2 (50.0) |
| Follow-up 1c | 96/104 (92.3) | 105/108 (97.2) | 124/136 (91.2) |
| Follow-up 2c | 58/62 (93.5) | 96/99 (97.0) | 97/103 (94.2) |
| EQ-5D | |||
| Baselineb | 282/316 (89.2) | 290/314 (92.4) | 278/315 (88.3) |
| Baseline plus ≥1 | 267/316 (84.5) | 274/314 (87.3) | 258/315 (81.9) |
| Week 5 | 227/302 (75.2) | 180/293 (61.4) | 218/300 (72.7) |
| Week 7 | 237/291 (81.4) | 182/276 (65.9) | 216/291 (74.2) |
| Week 11 | 198/271 (73.1) | 113/226 (50.0) | 163/244 (66.8) |
| Week 13 | 193/249 (77.5) | 106/201 (52.7) | 129/205 (62.9) |
| Week 17 | 156/220 (70.9) | 83/164 (50.6) | 103/158 (65.2) |
| Week 19 | 163/205 (79.5) | 97/155 (62.6) | 97/144 (67.4) |
| Week 23 | 133/188 (70.7) | 87/137 (63.5) | 76/118 (64.4) |
| Week 25 | 142/182 (78.0) | 97/130 (74.6) | 74/111 (66.7) |
| Week 31 | 121/164 (73.8) | 92/125 (73.6) | 50/87 (57.5) |
| Week 37 | 112/152 (73.7) | 89/119 (74.8) | 48/75 (64.0) |
| Week 43 | 100/145 (69.0) | 74/114 (64.9) | 44/65 (67.7) |
| Week 49 | 92/128 (71.9) | 65/97 (67.0) | 40/58 (69.0) |
| Week 55 | 63/100 (63.0) | 48/72 (66.7) | 24/43 (55.8) |
| Week 61 | 38/58 (65.5) | 29/42 (69.0) | 17/28 (60.7) |
| Week 67 | 13/21 (61.9) | 16/22 (72.7) | 9/13 (69.2) |
| Week 73 | 3/4 (75.0) | 6/9 (66.7) | 0/2 (0) |
| Week 79 | – | 2/2 (100.0) | 1/2 (50.0) |
| Follow-up 1c | 96/104 (92.3) | 105/108 (97.2) | 124/136 (91.2) |
| Follow-up 2c | 58/62 (93.5) | 96/99 (97.0) | 97/103 (94.2) |
Completion rate is calculated using the number of patients with non-missing PRO data at baseline and data from ≥ 1 post-baseline visit, divided by the number of patients in the study at each respective time point.
Baseline completion rate based on subjects having any baseline data with no post-baseline data requirement.
Follow-up visit 1 = 30 d from the last dose (± 7 d) or coinciding with the date of discontinuation (±7 d) if date of discontinuation is greater than 37 d after last dose; follow-up visit 2 = 84 d (± 7 d) from follow-up visit 1.
3.2. HRQoL randomised population analyses
Changes from baseline for EORTC QLQ-C30 are shown in Fig. 1A. Slight deterioration from baseline started at week 5 and showed an overall trend toward stabilization from week 25. No clinically meaningful changes were observed in any treatment group while on treatment. Clinically meaningful deterioration was observed at week 7 for nivolumab + ipilimumab for role functioning as well as the fatigue and appetite loss symptom scales (data not shown).
Fig. 1.
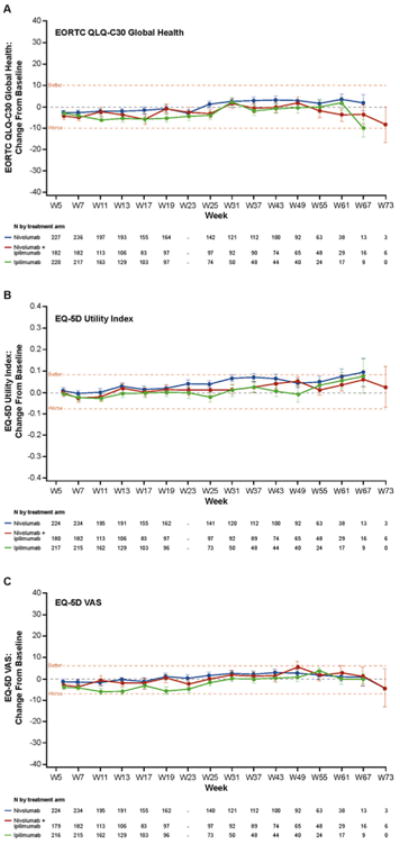
Change in baseline in HRQoL for total quality-of-life population. Mean (SD) EORTC QLQ-C30 global health score (A) at baseline was 74.7 (19.4) for nivolumab, 70.7 (22.3) for nivolumab + ipilimumab, and 73.5 (20.5) for ipilimumab; mean (SD) EQ-5D utility index score (B) at baseline was 0.803 (0.219) for nivolumab, 0.779 (0.234) for nivolumab + ipilimumab, and 0.791 (0.226) for ipilimumab; mean (SD) EQ-5D VAS score (C) at baseline was 75.9 (18.5) for nivolumab, 74.0 (19.9) for nivolumab + ipilimumab, and 75.8 (18.3) for ipilimumab. Clinical significance (denoted by the horizontal dashed line at these points) was determined by the MID value for each test, which was 10 points for EORTC QLQ-C30, 0.8 points for EQ-5D utility index, and 7 points for EQ-5D VAS. SD, standard deviation.
Changes from baseline for EQ-5D utility index are shown in Fig. 1B and for EQ-5D VAS in Fig. 1C. No clinically meaningful changes were observed in any group while on treatment in any time point.
3.3. MMRM results for benefit assessment
Longitudinal modelling was performed using all observed data for patients remaining in the study through week 55 including baseline PRO and stratification factors as covariates. Table A.1 shows the LS means and SE for nivolumab versus ipilimumab and for nivolumab versus nivolumab + ipilimumab across the longitudinal model through week 55, as well as the difference in LS means between treatment arms with 95% CIs. No clinically meaningful changes were observed based on MID within any treatment arm for EORTC QLQ-C30 or EQ-5D.
3.4. Subgroup analyses
Fig. 2 shows changes from baseline for all 3 HRQoL outcomes for randomised patients with BRAF mutation (left) and BRAF wild type (right). A clinically meaningful decline was observed in the BRAF mutation subgroup with ipilimumab treatment at weeks 11 and 13 in the EQ-5D VAS and a clinically meaningful improvement in the BRAF wild-type subgroup for nivolumab at weeks 31–37. No other clinically meaningful changes were observed at any other time point.
Fig. 2.
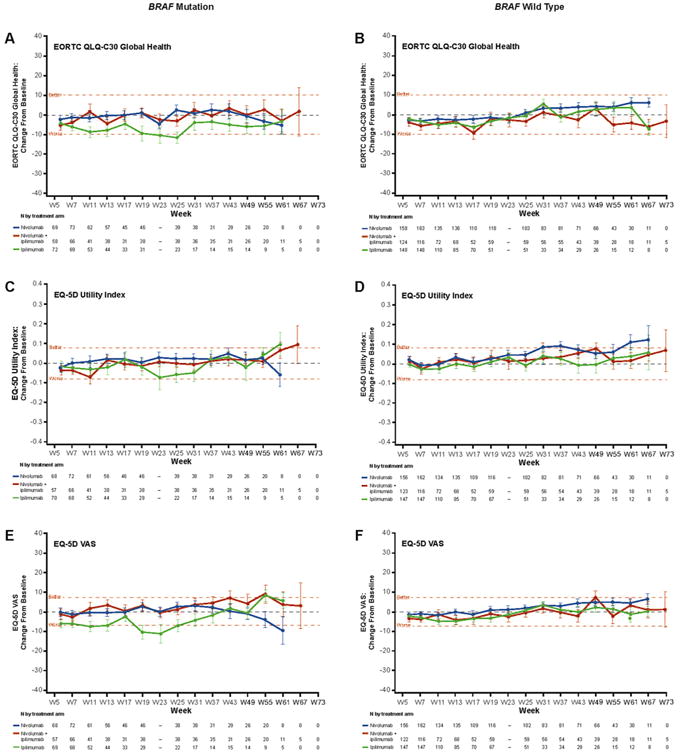
Change in baseline in HRQoL for BRAF mutation status subgroup. Clinical significance (denoted by the horizontal dashed line at these points) was determined by the MID value for each test, which was 10 points for EORTC QLQ-C30 (A, B), 0.8 points for EQ-5D utility index (C, D), and 7 points for EQ-5D VAS (E, F).
Changes from baseline for all three HRQoL outcomes for randomised patients with either complete or partial response are shown in Fig. 3 (left). A clinically meaningful decline was observed with the ipilimumab treatment group for the EQ-5D VAS at week 19. No other clinically meaningful changes were observed.
Fig. 3.
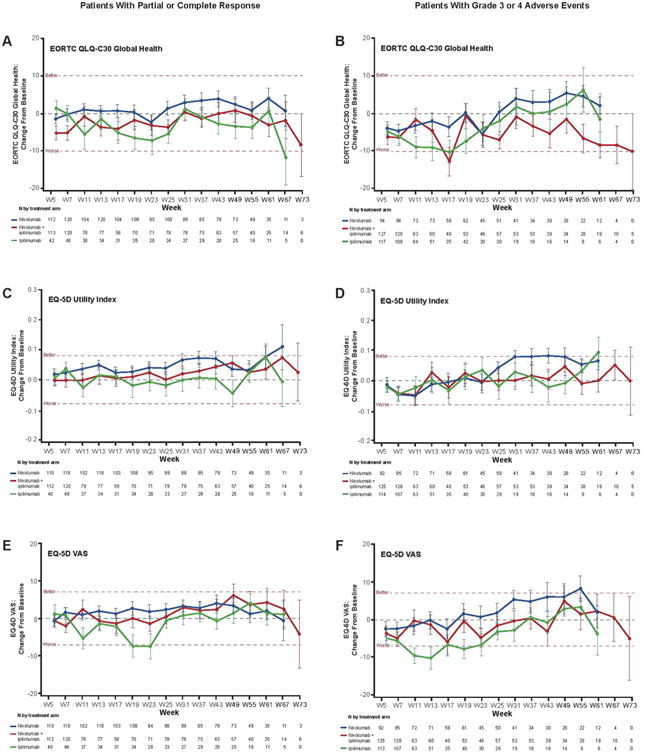
Change in baseline in HRQoL for patient response and AEs subgroups. Clinical significance (denoted by the horizontal dashed line at these points) was determined by the MID value for each test, which was 10 points for EORTC QLQ-C30 (A, B), 0.8 points for EQ-5D utility index (C, D), and 7 points for EQ-5D VAS (E, F).
Changes from baseline for all three HRQoL outcomes for randomised patients with a grade 3 or 4 AE are shown in Fig. 3 (right panels). Clinically meaningful declines were observed in the early weeks with ipilimumab in the EQ-5D VAS outcome and with nivolumab + ipilimumab in the EORTC QLQ-C30 at week 17. No other clinically meaningful changes were observed, although the nivolumab group had borderline improvement in the EQ-5D utility index from weeks 37–43.
Fig. 4 shows changes from baseline (last measurement before discontinuation) to follow-up visits 1 and 2 for all three HRQoL outcomes for patients who discontinued for any reason (left) or due to an AE (right). No clinically meaningful changes were observed in any of the three assessments for patients who discontinued for any reason. Due to low patient numbers in other groups of patients who discontinued due to an AE, conclusions can only be drawn for the nivolumab + ipilimumab group. For this group, a clinically meaningful decline was observed only inthe EQ-5D VAS assessment at follow-up visits 1 and 2.
Fig. 4.
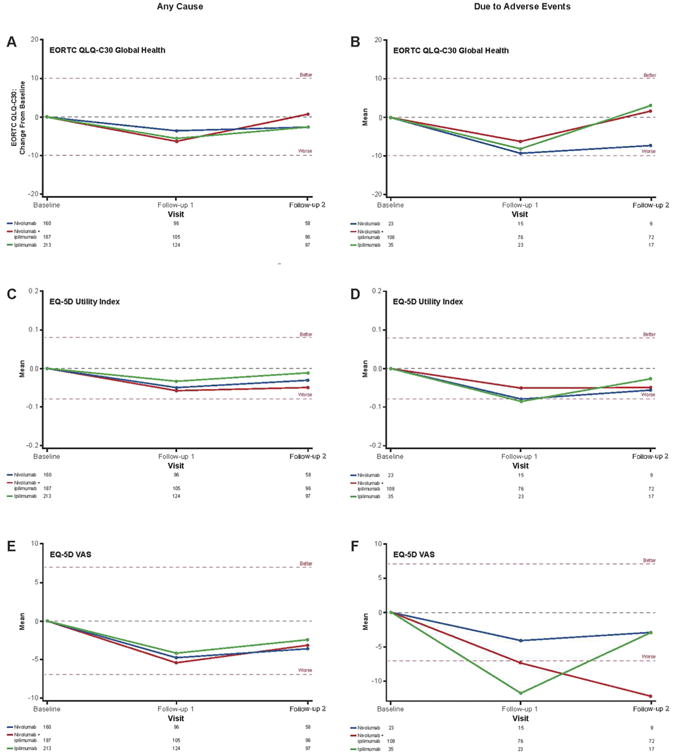
Change in baseline in HRQoL for patients who discontinued therapy for any cause or due to an AE. Clinical significance (denoted by the horizontal dashed line at these points) was determined by the MID value for each test, which was 10 points for EORTC QLQ-C30 (A, B), 0.8 points for EQ-5D utility index (C, D), and 7 points for EQ-5D VAS (E, F).
3.5. HRQoL combined with response rate and grade 3/4 AEs
To observe response rate and grade 3/4 AEs with regard to HRQoL, we created an overlay of the randomised population EORTC QLQ-C30 with best overall response (BOR) at 6 months and 12 months for the 3 treatment groups, along with percentage of grade 3/4 AEs at 11 weeks, 6 months, and 12 months for the three treatment groups (Fig. A.2). Differences in BOR and grade 3/4 AEs between the arms do not seem to correlate with EORTC QLQ-C30.
4. Discussion
Nivolumab alone and combined with ipilimumab have both demonstrated improved efficacy compared with ipilimumab alone in the CheckMate 067 study, although the combination resulted in a higher frequency of grade 3/4 AEs [4]. Here we show that nivolumab + ipilimumab and nivolumab alone maintained HRQoL and no deterioration was observed over time compared with ipilimumab. In addition, similar results were observed across patient subgroups including BRAF mutation status, patients with complete and partial responses, and patients with grade 3 or 4 AEs over time. In the main scale analyses, no clinically meaningful changes were detected for either nivolumab or nivolumab + ipilimumab around the week 12 time point (first staging for these patients). In addition, no changes in HRQoL were observed during follow-up visits for patients who discontinued due to any cause; for patients who discontinued due to an AE, clinically meaningful declines were only observed for one of the three assessments.
Patient-focussed care is becoming a more critical component of quality health care [22]. Specifically, PROs are more prominent and were used in 27% of clinical trials registered from November 2007 to December 2013 [11]. Moreover, an initiative has been established to provide recommendations on analysis standardization of HRQoL and other PROs in randomised cancer trials [10].
Investigating HRQoL is important for immune checkpoint inhibitors because of the associated toxicity profile. In this study, HRQoL stayed within ranges defined as MID in the EORTC QLQ-C30, EQ-5D utility index, and EQ-5D VAS assessments, including across various subgroups of patients. HRQoL has also been better maintained by pembrolizumab than by chemotherapy in a phase II study in patients with ipilimumab-refractory melanoma [23]. Combined targeted therapy of BRAF inhibitors and mitogen-activated protein kinase kinase enzymes MEK1 and/or MEK2 (MEK) inhibitors is another treatment for advanced melanoma. The addition of trametinib to dabrafenib in a phase III trial in patients with metastatic melanoma and the BRAF V600 mutation did not result in patient deterioration of HRQoL and, in fact, improved EORTC QLQ-C30 scores compared with dabrafenib mono-therapy [24]. Similar results were observed in a phase III open-label trial for the combination of dabrafenib plus trametinib compared with vemurafenib in patients with BRAF V600 metastatic melanoma [25].
Of note, the marked difference in the rate of grade 3/4 AEs observed in the three arms (nivolumab + ipilimumab [58.5%], nivolumab [20.8%], and ipilimumab [27.7%] [6]) did not translate into a clinically meaningful difference in HRQoL. This may be due to several factors, for example, HRQoL may be driven by anxiety and willingness to survive at treatment start, thereby neutralising the negative perception of AEs. Also, the instruments used to assess HRQoL are designed for patients treated with chemotherapy and may not detect the impact of the AEs observed with immunotherapy. Lastly, patient numbers were low in both the later weeks of analysis, and within some of the subgroups analysed, thus limiting the interpretation of the results. Using these results, one could conclude that nivolumab + ipilimumab does not seem to be limited by HRQoL considerations, however, trial populations may differ from the ‘real-world’ patient with melanoma in terms of motivation, the likelihood of PRO reporting, and the ability to withstand treatment-related AEs.
Along with OS and PFS, quality-of-life measures have been included for use in the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) [26]. This scale, proposed to be a standardised validated approach to stratify the magnitude of clinical benefit anticipated from anticancer therapies, will be used to emphasise treatments in the ESMO guidelines. The CheckMate 067 study demonstrated improved efficacy, and has now shown stable HRQoL for multiple assessments.
In conclusion, HRQoL was maintained in all treatment groups with little clinically meaningful changes noted in EORTC QLQ-C30, EQ-5D utility index, and EQ-5D VAS assessments. Similar results were observed across patient subgroups including BRAF mutation status, patients with complete and partial responses, and patients with grade 3 or 4 AEs over time, as well as during follow-up visits for patients who discontinued due to any cause. These results further support the clinical benefit of nivolumab monotherapy and nivolumab + ipilimumab combination therapy over ipilimumab monotherapy in patients with advanced melanoma.
Supplementary Material
Acknowledgments
We thank the patients and investigators who participated in the CheckMate 067 trial. Professional medical writing and editorial assistance were provided by Melissa Kirk, PhD, and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb.
Funding: This study was funded by Bristol-Myers Squibb (Princeton, NJ, USA).
Conflict of interest statement: DS declares honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Sysmex, Immunocore, Grünenthal Group, Merck Serono, Agenus, Array BioPharm, AstraZeneca, LEO Pharma, Incyte, Pfizer, Pierre Fabre, Philogen, and Regeneron; reports a consultant or advisory role for Roche/Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Sysmex, Amgen, Grünenthal Group, and Immunocore; and has received research funding from Bristol-Myers Squibb. JL reports research funding from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, and Pfizer and travel funding from Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Eisai, GlaxoSmithKline, and Roche. JW declares honoraria from EMD Serono and Janssen Oncology; a consultant or advisory role for Bristol-Myers Squibb, Medimmune, Ziophanr, Polynoma, Polaris, Jounce, and GlaxoSmithKline; travel funding from Bristol-Myers Squibb; research funding from Bristol-Myers Squibb, Merck, Medimune, and GlaxoSmithKline; and is a patent co-investor of DNA vaccine of cancer in companion animals. FSH declares research funding from Bristol-Myers Squibb, unpaid consultant role for Bristol-Myers Squibb, and pending institution patent for Immune Target. VCS reports speaker's bureau role for Bristol-Myers Squibb, Roche, and GlaxoSmithKline; consultant or advisory role for Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck Sharp & Dohme; and travel expenses from Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck Sharp & Dohme. RG has been an ad board participant for Bayer-Onyx, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Prometheus, and Roche; and has received research funding from Bayer-Onyx, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Prometheus, Roche, Eisai, Merck, Novartis, and Pfizer. PR reports honoraria from Bristol-Myers Squibb, Roche, Novartis, Merck Sharp & Dohme, and GlaxoSmithKline; speaker's bureau for Novartis, Merck Sharp & Dohme, and Pfizer; a consultant or advisory role for Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, and Amgen; research funding from Bristol-Myers Squibb; and travel funding from Novartis. JJG has been on a speaker's bureau for Bristol-Myers Squibb, GlaxoSmithKline, and Roche; has had a consultant or advisory role for Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Roche, Merck, and Amgen; and has received research funding from Bristol-Myers Squibb and Roche and travel funding from Roche. CLC has been a speaker for Bristol-Myers Squibb and Genentech; has had a consultant or advisory role for Bristol-Myers Squibb, Genentech, Merck, and GlaxoSmithKline; and has received research funding from Bristol-Myers Squibb, Genentech, Merck, and GlaxoSmithKline. CL reports no disclosures. JW declares honoraria from Bristol-Myers Squibb, Merck, Roche, Astellas, Pfizer, and Novartis; speaker's bureau participation for Bristol-Myers Squibb, Novartis, and Astellas; an advisory or consultant role for Bristol-Myers Squibb, Merck, Roche, Astellas, Pfizer, and Novartis; and has received travel funding from Bristol-Myers Squibb, Novartis, and Astellas. MKC reports a consultant or advisory role for AstraZeneca, research funding from Bristol-Myers Squibb, and has immediate family members who are employed by Bristol-Myers Squibb and Celgene. MAP reports attending an advisory board and receiving research funding from Bristol-Myers Squibb. MS declares honoraria from Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck; speaker's bureau participation for Bristol-Myers Squibb and Roche; and a consultant or advisory role for Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Merck. PFF reports honoraria from Delcath Systems and a consultant or advisory role, travel expenses, and expert testimony for GlaxoSmithKline, Roche, and Bristol-Myers Squibb. RD declares honoraria from Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, GlaxoSmithKline, Amgen, Takeda, Pierre Fabre; a consultant or advisory role for Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, GlaxoSmithKline, Amgen, Takeda, and Pierre Fabre; research funding from Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, and GlaxoSmithKline; and travel funding from Bristol-Myers Squibb. AH is an employee of Tasman Oncology and Tasman Oncology Research. FT is an employee of Adelphi Values and reports research funding from Bristol-Myers Squibb. JS is an employee of Bristol-Myers Squibb. DW is an employee of Bristol-Myers Squibb and has an immediate family member who holds stock in Antares Pharma. SK is an employee of Bristol-Myers Squibb. AA is employed by Flatiron Health, Inc., has received research funding from Bristol-Myers Squibb, Celgene, DARA Bio-sciences, Dendreon, GlaxoSmithKline, Helsinn, Kan-glaite, and Pfizer and honoraria from Bristol-Myers Squibb, Helsinn, and Merck; reports a consultant or advisory role for ACORN Research and Bristol-Myers Squibb; owns stock in Athenahealth, Inc. and holds a leadership role for Flatiron Health, Inc. and Athena-health, Inc. GL reports honoraria from Merck MSD, Roche, and Bristol-Myers Squibb and a consultant or advisory role for Merck MSD, Roche, Bristol-Myers Squibb, Amgen, Novartis and Pierre-Fabre.
Footnotes
Appendix A. Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejca.2017.05.031.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. For the KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or mono-therapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival (OS) results from a phase III trial of nivolumab (NIVO) combined with ipilimumab (IPI) in treatment- naïve patients with advanced melanoma (CheckMate 067). Presented at: American Association for Cancer Research Annual Meeting; April 1-5, 2017; Washington, DC. Abstract 9040. [Google Scholar]
- 7.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–68. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. 2016;17(11):e510–4. doi: 10.1016/S1470-2045(16)30510-1. [DOI] [PubMed] [Google Scholar]
- 11.Vodicka E, Kim K, Devine EB, Gnanasakthy A, Scoggins JF, Patrick DL. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007-2013) Contemp Clin Trials. 2015;43:1–9. doi: 10.1016/j.cct.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Revicki DA, van den Eertwegh AJ, Lorigan P, Lebbe C, Linette G, Ottensmeier CH, et al. Health related quality of life outcomes for unresectable stage III or IV melanoma patients receiving ipilimumab treatment. Health Qual Life Outcomes. 2012;10:66. doi: 10.1186/1477-7525-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, et al. Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: results from the phase III CheckMate 066 study. Ann Oncol. 2016;27(10):1940–6. doi: 10.1093/annonc/mdw265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 15.Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. EORTC QLQ-C30 reference values. [Last accessed January 19, 2017];2008 http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf.
- 16.EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.van Reenen M, Oppe M. EQ-5D-3L user guide. Version 5.1. http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-3L_UserGuide_2015.pdf.
- 18.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality of life scores. J Clin Oncol. 1998;16(1):139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashbeck EL, Bel ML. Single time point comparisons in longitudinal randomized controlled trials: power and bias in the presence of missing data. BMC Med Res Methodol. 2016;16:43. doi: 10.1186/s12874-016-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 23.Schadendorf D, Dummer R, Hauschild A, Robert C, Hamid O, Daud A, et al. Health-related quality of life in the randomized KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer. 2016;67:46–54. doi: 10.1016/j.ejca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Schadendorf D, Amonkar MM, Stroyakovskiy D, Levchenko E, Gogas H, de Braud F, et al. Health-related quality of life impact in a randomised phase III study of the combination of dabrafenib and trametinib versus dabrafenif monotherapy in patients with BRAF V600 metastatic melanoma. Eur J Cancer. 2015;51(7):833–40. doi: 10.1016/j.ejca.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Grob JJ, Amonkar MM, Karaszewska B, Schachter J, Dummer R, Mackiewicz A, et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomized trial. Lancet Oncol. 2015;16(13):1389–98. doi: 10.1016/S1470-2045(15)00087-X. [DOI] [PubMed] [Google Scholar]
- 26.Cherney NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26:1547–73. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


