Abstract
The prognosis is extremely poor for patients with brain metastases in recursive partitioning analysis (RPA) class 3. It is not clear whether dose elevation for brain lesions in addition to whole-brain radiotherapy could improve survival for those patients. This study aimed to assess the efficacy and safety of dose elevation with intensity-modulated radiation therapy (IMRT) for patients with 1 to 3 brain metastases in RPA class 3.
From January 2013 to December 2015, 24 patients with 1 to 3 brain metastases in RPA class 3 were included in this study. The median age was 60 (range 41–85) years and the mean graded prognostic assessment (GPA) score was 1.25 (range 0.5–2). Whole-brain radiotherapy (30 Gy) with a simultaneous integrated boost (SIB) to the brain metastases (totaling 40 Gy) was delivered in 10 fractions using IMRT technique. Survival times and overall safety were assessed. The significance of prognostic variables on survival was assessed by both univariate and multivariate analyses.
All of the patients completed the planned SIB schedule. The overall response rate was 66.7%. The median survival time (MST) was 8 months for the entire group of patients. The MST was 5 months for patients with a GPA score of 0.5 to 1 (n = 11 patients) and 12 months with a GPA score of 1.5 to 2 (n = 13 patients). No acute or late toxicities greater than grade 2 were detected. Age and subsequent chemotherapy were significantly associated with MST on univariate and multivariate analyses.
It is feasible to elevate radiation doses to 40 Gy using the IMRT technique in RPA class 3 patients with 1 to 3 brain metastases without serious toxicities. The preliminary results are encouraging and further studies with larger cohorts are warranted.
Keywords: brain metastases, graded prognostic assessment, intensity-modulated radiation therapy, recursive partitioning analysis
1. Introduction
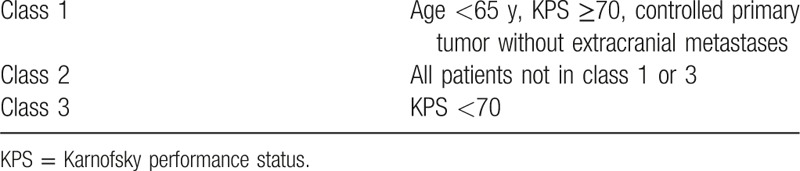
Brain metastases are common in patients with various cancers, and the incidence is still rising while the overall prognosis remains poor in patients with brain metastases.[1,2] Recursive partitioning analysis (RPA) has been widely used to classify patients with brain metastases into 3 different prognostic classes (detailed in Table 1).[3] All patients with a Karnofsky Performance Status (KPS) of <70 were included in RPA class 3 and the prognosis remains extremely dismal with a median survival time (MST) of only 2.3 months.[3] Although RPA class 3 patients are common in patients with brain metastases, little attention has been paid to them in recent studies. Whole-brain radiation therapy (WBRT) and supportive care are the main treatments for patients with brain metastases in RPA class 3.
Table 1.
Recursive partitioning analysis.
A survival benefit for patients with a single unresectable brain metastasis and an improved quality of life for all patients with brain metastases have been defined in those receiving WBRT combined with stereotactic radiosurgery (SRS) boost, compared to those receiving WBRT alone.[4] Other reports suggest that a combination of WBRT and SRS for patients with limited brain metastases significantly improves the local control of brain lesions.[5,6] Interestingly, Agboola et al[7] treated patients with brain metastases by resection and WBRT, and discovered that the MST was significantly longer in RPA class 3, as well as in RPA class 1 and RPA class 2, compared with the radiation therapy oncology group (RTOG) data. Their study indicates that even patients with limited brain metastases in RPA class 3 might benefit from more aggressive treatment strategies. However, the role of dose escalation to brain lesions during WBRT has not been evaluated for patients with limited brain metastases in RPA class 3.
However, SRS is unavailable in most radiation centers. Intensity-modulated radiation therapy (IMRT) allows dose escalation to brain metastases as a simultaneous integrated boost (SIB) during WBRT. Lagerwaard et al[8] validated that delivery of an SIB to brain metastases is feasible with RapidArc. Edwards et al[9] treated 11 patients with bulky brain metastases with an SIB during WBRT and found good early results of local control (no progression in brain during median follow-up of 4 months) without serious adverse effects. Thus, SIB to brain metastases could provide the advantages of SRS during WBRT without the need for an extra procedure. In the present study, we investigated the efficacy and safety of SIB to brain lesions during WBRT in RPA class 3 patients with 1 to 3 brain metastases using an IMRT technique.
2. Methods
2.1. Inclusion criteria
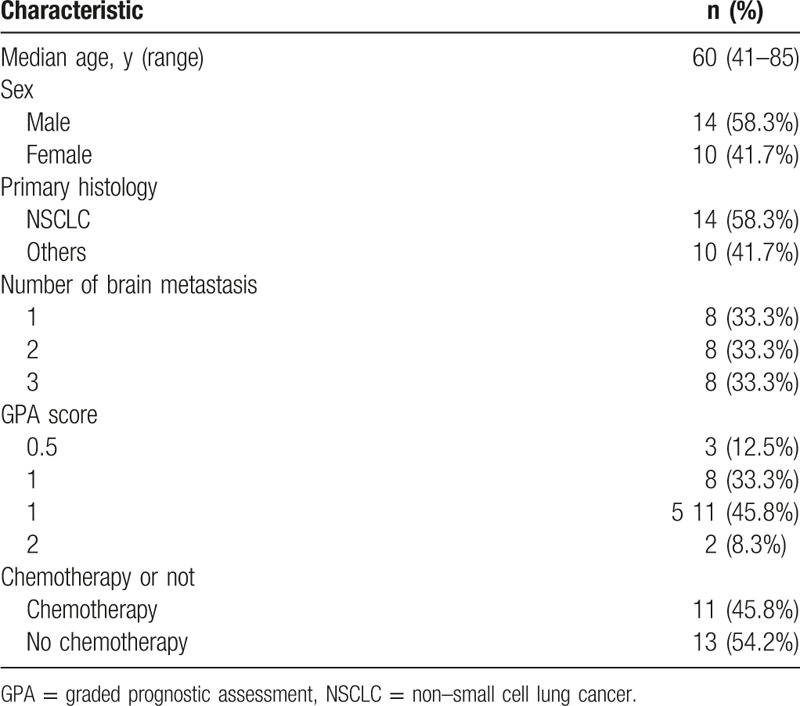
This study was approved by the Research Ethics Board of Zhejiang Provincial People's Hospital and written consent was obtained from all the patients included in this study. Patient eligibility for the study was as follows: histologically proven cancer; imaging findings confirmed 1 to 3 brain metastases on pretreatment contrast-enhanced magnetic resonance imaging (MRI); KPS < 70; and no previous cranial radiation therapy. Exclusion criteria were as follows: metastases close to (within 5 mm) the brainstem or optic apparatus, cytological, or imaging-based evidence of leptomeningeal metastases, with other histologic confirmation of malignancy, and/or a lack of informed consent. Twenty-four RPA class 3 patients from January 2013 to December 2015 with 1 to 3 brain metastases were included in this study. The clinical characteristics of the 24 patients included in this study are shown in Table 2.
Table 2.
The clinical baseline characteristics of all patients.
2.2. Performance status assessment, RPA, and GPA
Performance status was assessed using the standard KPS scale. RPA and GPA score was assessed and documented prospectively before the start of radiotherapy.[10]
2.3. Treatment plan
All patients had a custom head thermoplastic mask constructed for both simulation and radiotherapy. A planning computed tomography (CT) scan through the whole head was obtained with 2.5-mm slice thickness. The PTVboost was created by adding a 3-mm margin to the visible metastases (GTVboost). The PTVwbrt was derived from the whole brain plus the addition of a 3-mm margin. An IMRT plan was generated for every patient on the Pinnacle 3 Treatment Planning System using the SIB technique; the prescribed dose was 30 Gy in 10 separate fractions to 95% volume of the PTVwbrt, with 40 Gy to 95% volume of the PTVboost simultaneously. A maximum dose of 35 Gy in 10 fractions to the brainstem and optic chiasm was permitted. The dose constraint for the lens was 5 Gy. For all patients, treatment plans were generated with 6-MV photons, by use of multileaf collimation with a leaf width of 10 mm (Siemens Oncor Impression Plus accelerator), with 5 to 7 coplanar photon beams designed. Beam weight and direction were inversely optimized until all of the criteria were met. The dose rate for treatment delivery was 300 monitor units per minute. All plans were delivered on a Siemens Oncor Impression Plus accelerator and verified in a plastic water phantom by using the 2D ion chamber array detector MatriXX (IBA, Schwarzenbruck, Germany) before radiotherapy. The measured dose distribution in the MatriXX device was compared with that calculated on the treatment planning system on the same plane. The use of steroids, mannitol, and other medicine for symptom alleviation was decided by the attending oncologist.
2.4. Radiation-induced toxicity
Acute toxicity (≤3 month follow-up) and late toxicity (>3 month follow-up) were scored according to the RTOG scoring system.[11]
2.5. Follow-up
Patients were followed up regularly at 1 month after the end of radiotherapy and every 3 months thereafter until death. Contrast CT or MRI was recommended when patient's symptoms were exacerbated or when the patient presented with new symptoms.
2.6. Statistical analysis
Survival time was calculated from the start of radiotherapy to patient death or the last date of follow-up using the Kaplan-Meier method. Recorded events were death (all causes of death were included). Patients who were alive were censored using the date of last follow-up. Univariate analysis and multivariate analysis were used to assess the prognostic variables for survival. All analyses were performed using the SPSS statistical package (SPSS 17.0, Chicago, IL).
3. Results
The size of brain metastases varied from 0.6 to 5.9 cm (2.93 ± 1.67) and a size bigger than 4 cm was found in 6 (25%) of the 24 patients. Follow-up ranged between 2 and 25 months. Twenty-three out of the 24 patients had died during the follow-up, and 1 patient was lost after follow-up of 14 months after radiotherapy. Twenty-one patients died of progressive disease and 2 patients died of pneumonia. Among the 21 patients who died because of disease progression, 2 patients died of intracranial progression, 17 patients died of extracranial progression, and 2 patients died of both intracranial progression and extracranial progression.
One patient (4.2%) achieved a complete response (CR) and 15 patients (62.5%) achieved partial responses (PRs) after radiotherapy. The overall response rate (CR + PR) was 66.7%. The MST was 8 months for the entire group of patients for this study (Fig. 1). Age and subsequent (postradiotherapy) chemotherapy were significantly associated with overall survival (OS) upon both univariate (Table 3) and multivariate analyses (Table 4). However, other possible prognostic factors were not shown to be related with OS, including sex, GPA score, histology, and extracranial metastasis after either univariate (Table 3) or multivariate analysis (Table 4). The MST was 5 months for patients with a GPA score of 0.5 to 1 (11 patients) and 12 months with a GPA score of 1.5 to 2 (13 patients) (P = .181, Fig. 2).
Figure 1.
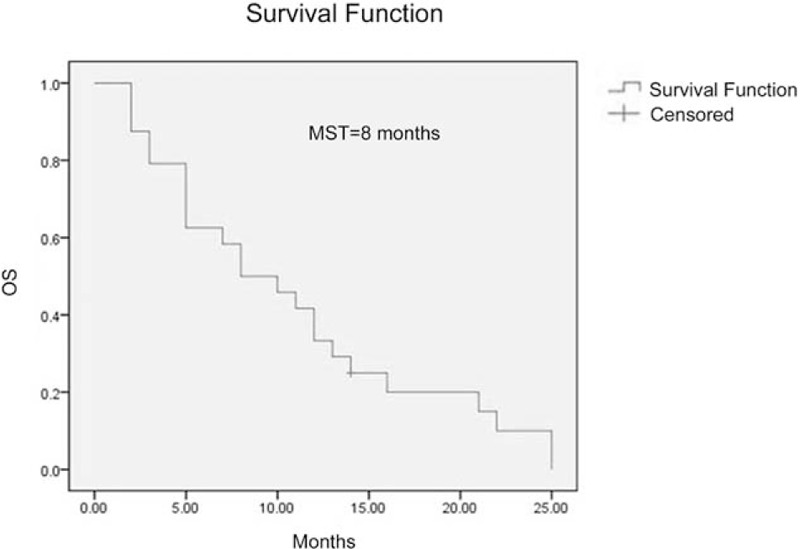
Overall survival in recursive partitioning analysis (RPA) class 3 patients. , MST = median survival time.
Table 3.
Univariate analysis: predictors for OS.
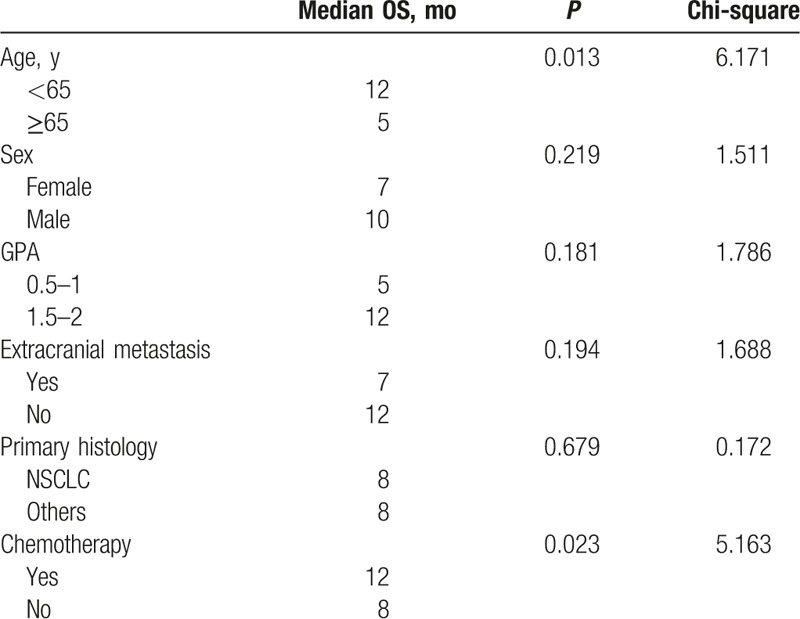
Table 4.
Multivariate Cox regression analysis: predictors for OS.
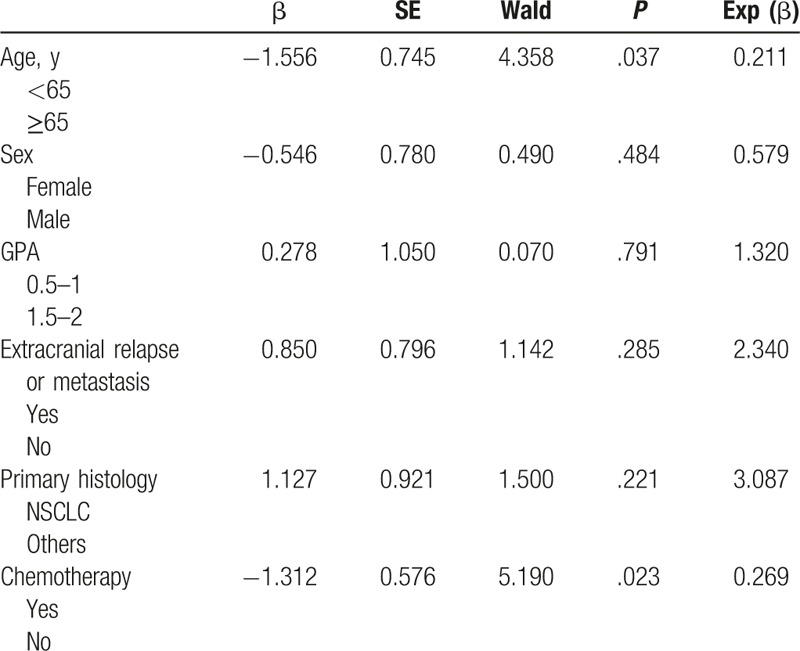
Figure 2.
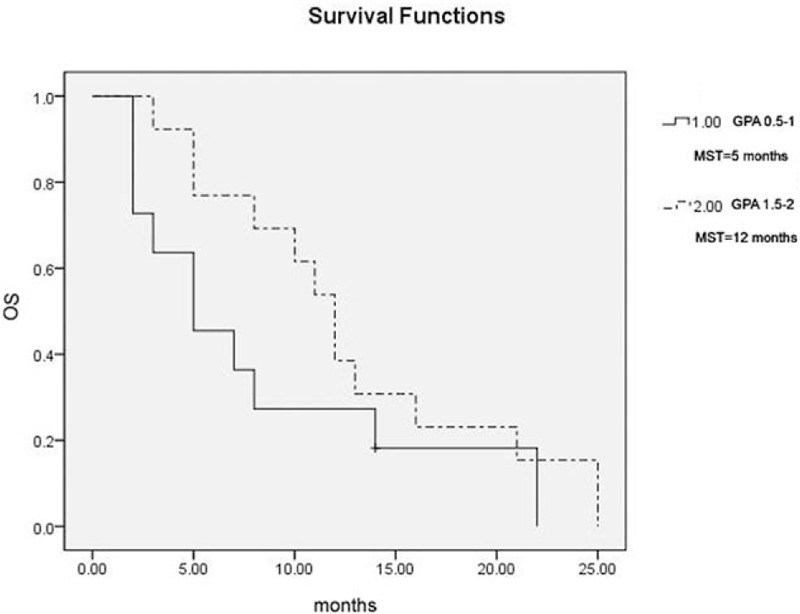
Overall survival stratified by GPA score. GPA = graded prognostic assessment, MST = median survival time.
All of the patients included in this study completed the planned IMRT schedule without interruption. RTOG grade 1 radiation-induced erythema was observed in 20.8% of patients. RTOG grade 1 and 2 leukopenia was observed in 33.3% of patients. Grade 1 and 2 acute central nervous system toxicity was observed in 16.7% of patients. No acute or late toxicity events greater than grade 2 were detected.
4. Discussion
Although patients in RPA class 3 are common, they are usually excluded from clinical trials because of extremely poor prognoses. To the best of our knowledge, this is the first study whose main aim was to investigate the efficacy and safety of IMRT using the SIB technique focused on RPA class 3 patients with 1 to 3 brain metastases, and the preliminary results are really promising. RPA class is the most widely used prognostic index for patients with brain metastases and Gaspar suggested that the use of RPA classification in clinical studies would allow comparison of new treatment techniques with others.[3] The MST of the entire group of patients was 8 months in this study, which is apparently longer compared to that of patients in RPA class 3 (2.3 months) in the RTOG RPA database who underwent WBRT alone.[3] Radiation dose elevation with SRS may benefit selected patients with brain metastases in RPA class 1 and 2, not only in local control but also in OS based on 2 randomized controlled trials.[4,5] In addition, Sanghavi et al[6] reported that SRS, in addition to WBRT, significantly improved the survival of patients in all 3 RPA classes compared with the patients who underwent WBRT alone in the RTOG RPA database. In that study, the survival gain was the greatest in the RPA class 3 patients.[6] Moreover, the MST was 8.3 months, which was comparable to that in this study. It could be suggested that radiation dose escalation with the SIB technique was comparable to WBRT combined with SRS in treating RPA class 3 patients with 1 to 3 brain metastases.
The prognosis for patients with 1 to 3 brain metastases appears to be better than those who have >3 metastases and these particular patients may benefit from more aggressive treatment.[12] However, the number of metastases is an important prognostic factor not included in the RPA classification. We included patients with 1 to 3 brain metastases only in this study, which partially accounts for the superior results. Lutterbach et al[13] classified RPA class 3 patients with brain metastases into class 3a, class 3b, and class 3c subgroups based on several prognostic factors including age, status of the primary tumor, and the number of brain metastases. The MST was 3.2, 1.9, and 1.2 months, respectively from class 3a to class 3c. Resection followed by WBRT improved median survival significantly compared with WBRT alone in RPA class 3 group patients (4.3 vs 1.6 months, P < .0001), in subgroup 3a (5.6 vs 1.8 months) and 3b (4 vs 1.6 months) patients. Even in the best prognostic subgroup class 3a, the median survival was only 1.8 months after WBRT alone. The median survival for the whole group of patients in RPA class 3 was 1.8 months, which correlated well with the RTOG database. The prognosis of the patients in the present study was no better than that of class 3a, whereas the MST in the present study was much longer than that in the subgroup treated with WBRT alone reported by Lutterbach et al,[13] indicating that radiation dose escalation may account for longer survival times. Sanghavi et al[6] also showed that the MST of patients in RPA class 3 treated with WBRT + SRS was longer than that of patients treated with WBRT alone. Patient suitable for SRS should have limited brain metastases and the maximum diameter of metastases should be no >4 cm, although the number of brain metastases or the maximum diameter was not mentioned in the report by Sanghavi et al.[6] Taken together, we suggest that RPA class 3 patients with limited brain metastases could benefit from radiation dose escalation in addition to WBRT.
GPA is another useful prognostic index that has been widely used in recent years. The GPA scale is based on 4 factors: age, KPS, extracranial metastases (none or present), and number of metastases (1, 2–3, or >3). In the RTOG database, the MST according to GPA score was 2.6 months for GPA 0 to 1 and 3.8 months for GPA 1.5 to 2.5.[10]
We also prospectively stratified patients by the GPA scale. The GPA scores varied from 0.5 to 2 in this study. The MST was 5 months with a GPA score of 0.5 to 1 and 12 months with a GPA score of 1.5 to 2, which is much better than that in the RTOG database.[10] The MST was 3.1 months for patients with a GPA score of 0 to 1 and 5.4 months with a GPA score of 1.5 to 2.5 after treatments in a recent retrospective report, which was also inferior to our results in this study.[14] Therefore, we suggest that the MST could be improved by dose escalation using the SIB technique in patients with GPA score 0 to 2.
It has been reported that the primary cancer type, sex, age, extracranial metastasis, and subsequent chemotherapy were also important prognostic factors.[3–7]In this study, age and subsequent chemotherapy were found to be significantly associated with MST while primary cancer type, sex, histology, and extracranial metastasis were not. However, these results should be interpreted with caution. Most of the patients without subsequent chemotherapy had been heavily pretreated with chemotherapy (≥2 lines) while most of the patients with subsequent chemotherapy were chemotherapy-naive or had only received first-line chemotherapy at the time of enrollment. We considered it unsuitable to compare the effect of subsequent chemotherapy on survival time between the 2 groups in this study.
SRS is the most widely used technique for dose escalation combined with WBRT. However, SRS is unavailable in most radiotherapy departments, and is also time consuming with high costs. Several published articles have validated the feasibility of WBRT with an SIB by IMRT in patients with brain metastases.[8,9,15] Edwards et al[9] treated 11 patients with 1 to 4 brain metastases using SIB technology. The SIB technique deserves further study because it could be used to efficiently provide a boost to multiple brain metastases without the need for extra SRS procedures and some authors noted its advantage over WBRT plus SRS in dose conformation.[8]
Although SIB is an emerging technology, the best dose schedule has not been defined. The addition of a radiotherapy boost to brain lesions during WBRT appeared to increase the MST to 14.5 months in 1 report, which suggests that patients with 1 to 3 brain metastases may benefit from dose escalation.[16] It was furthermore found that a higher dose was associated with better MST significantly upon univariate analysis (>39 vs. ≤39 Gy; P < .01) in that study.[16] The biological effective dose (BED) is accepted as an effective tool to compare different radiation schedules and the BED can be calculated as: BED = nd [1 + d/(α/β)].[17] A systematic review showed the dose-effect relationship of stereotactic radiotherapy in brain metastases and concluded that a BED12 of at least 40 Gy should be applied to brain metastases in order to acquire a 1-year local control rate of at least 70%.[18] The BED12 is 53.3 Gy, which is high enough to achieve good local control in the present study. In addition, no serious radiotherapy-related side effects were found in this study, which corresponds to several published studies.[8,9,15] We considered 40 Gy in 10 fractions a reasonable SIB dose schedule during standard WBRT, whereas many patients in this study have bulk metastases (>4 cm).
The prognosis is extremely poor for patients with brain metastases in RPA class 3 in the literature; however, patients with 1 to 3 brain metastases may benefit from more aggressive treatments as we show in the present study. Meanwhile, the present study does have some limitations. First, it is a small-sized study and selection bias certainly may exist. Second, quality of life was not documented in this study. Third, patients with brain metastases from different primary cancer sites were included and their prognoses may differ significantly. Further studies with a larger patient pool are needed to assess the efficacy and safety of our treatment plan.
5. Conclusion
In conclusion, it is feasible to plan and deliver boost dose(s) during WBRT with IMRT in RPA class 3 patients with 1 to 3 brain metastases. Further studies are warranted to verify the observed promising efficacy and safety.
Footnotes
Abbreviations: BED = biological effective dose, CR = complete response, CT = computed tomography, GPA = graded prognostic assessment, IMRT = intensity-modulated radiation therapy, KPS = Karnofsky performance status, MRI = magnetic resonance imaging, MST = median survival time, PR = partial response, RPA = recursive partitioning analysis, RTOG = radiation therapy oncology group, SIB = simultaneous integrated boost, SRS = stereotactic radiosurgery, WBRT = whole-brain radiation therapy.
Jia Yang and Wenming Zhan have contributed equally to the article.
This study was supported in part by medical scientific research foundation of Zhejiang province (2014KYB201). The funders did not participate in the study design, data collection, data analysis, or preparation of the manuscript.
This study was conducted in accordance with the Helsinki Declaration II and was approved by the Institutional Review Boards of Zhejiang Provincial People's Hospital.
The authors report no conflicts of interest.
References
- [1].Sul J, Posner JB. Brain metastases: epidemiology and pathophysiology. Cancer Treat Res 2007;136:1–21. [DOI] [PubMed] [Google Scholar]
- [2].Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 2012;4:CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745–51. [DOI] [PubMed] [Google Scholar]
- [4].Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665–72. [DOI] [PubMed] [Google Scholar]
- [5].Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427–34. [DOI] [PubMed] [Google Scholar]
- [6].Sanghavi SN, Miranpuri SS, Chappell R, et al. Radiosurgery for patients with brain metastases: a multi-institutional analysis, stratified by the RTOG recursive partitioning analysis method. Int J Radiat Oncol Biol Phys 2001;51:426–34. [DOI] [PubMed] [Google Scholar]
- [7].Agboola O, Benoit B, Cross P, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys 1998;42:155–9. [DOI] [PubMed] [Google Scholar]
- [8].Lagerwaard FJ, Van der Hoorn EA, Verbakel WF, et al. Whole-brain radiotherapy with simultaneous integrated boost to multiple brain metastases using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2009;75:253–9. [DOI] [PubMed] [Google Scholar]
- [9].Edwards AA, Keggin E, Plowman PN. The developing role for intensity-modulated radiation therapy (IMRT) in the non-surgical treatment of brain metastases. Br J Radiol 2010;83:133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008;70:510–4. [DOI] [PubMed] [Google Scholar]
- [11].Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6. [DOI] [PubMed] [Google Scholar]
- [12].Nieder C, Nestle U, Motaref B, et al. Prognostic factors in brain metastases: should patients be selected for aggressive treatment according to recursive partitioning analysis (RPA) classes? Int J Radiat Oncol Biol Phys 2000;46:297–302. [DOI] [PubMed] [Google Scholar]
- [13].Lutterbach J, Bartelt S, Stancu E, et al. Patients with brain metastases: hope for recursive partitioning analysis (RPA) class 3. Radiother Oncol 2002;63:339–45. [DOI] [PubMed] [Google Scholar]
- [14].Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rodrigues G, Eppinga W, Lagerwaard F, et al. A pooled analysis of arc-based image-guided simultaneous integrated boost radiation therapy for oligometastatic brain metastases. Radiother Oncol 2012;102:180–6. [DOI] [PubMed] [Google Scholar]
- [16].Casanova N, Mazouni Z, Bieri S, et al. Whole brain radiotherapy with a conformational external beam radiation boost for lung cancer patients with 1-3 brain metastasis: a multi institutional study. Radiat Oncol 2010;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thames HD, Jr, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys 1982;8:219–26. [DOI] [PubMed] [Google Scholar]
- [18].Wiggenraad R, Verbeek-de Kanter A, Kal HB, et al. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol 2011;98:292–7. [DOI] [PubMed] [Google Scholar]


