Abstract
Fresh collections, type studies and molecular phylogenetic analyses of a multigene matrix of partial nuSSU-ITS-LSU rDNA, rpb2, tef1 and tub2 sequences were used to evaluate the boundaries of Cucurbitaria in a strict sense and of several related genera of the Cucurbitariaceae. Two species are recognised in Cucurbitaria and 19 in Neocucurbitaria. The monotypic genera Astragalicola, Cucitella, Parafenestella, Protofenestella, and Seltsamia are described as new. Fenestella is here included as its generic type F. fenestrata (= F. princeps), which is lecto- and epitypified. Fenestella mackenzei and F. ostryae are combined in Parafenestella. Asexual morphs of Cucurbitariaceae, where known, are all pyrenochaeta- or phoma-like. Comparison of the phylogenetic analyses of the ITS-LSU and combined matrices demonstrate that at least rpb2 sequences should be added whenever possible to improve phylogenetic resolution of the tree backbone; in addition, the tef1 introns should be added as well to improve delimitation of closely related species.
Key words: Ascomycota, Dothideomycetes, new taxa, Phoma, phylogenetic analysis, Pleosporales, Pyrenochaeta, pyrenomycetes
Taxonomic novelties: New genera: Astragalicola Jaklitsch & Voglmayr, Cucitella Jaklitsch & Voglmayr, Parafenestella Jaklitsch & Voglmayr, Protofenestella Jaklitsch & Voglmayr, Seltsamia Jaklitsch & Voglmayr
New species: Astragalicola amorpha Jaklitsch & Voglmayr, Cucitella opali Jaklitsch & Voglmayr, Cucurbitaria oromediterranea Jaklitsch & Voglmayr, Neocucurbitaria acanthocladae Jaklitsch & Voglmayr, N. aetnensis Jaklitsch & Voglmayr, N. cinereae Jaklitsch & Voglmayr, N. cisticola Jaklitsch & Voglmayr, N. juglandicola Jaklitsch & Voglmayr, N. populi Jaklitsch & Voglmayr, N. rhamnicola Jaklitsch & Voglmayr, N. rhamnioides Jaklitsch & Voglmayr, N. ribicola Jaklitsch & Voglmayr, N. vachelliae Jaklitsch & Voglmayr, Parafenestella pseudoplatani Jaklitsch & Voglmayr, Protofenestella ulmi Jaklitsch & Voglmayr, Seltsamia ulmi Jaklitsch & Voglmayr
New combinations: Neocucurbitaria rhamni (Nees : Fr.) Jaklitsch & Voglmayr, Parafenestella mackenziei (Wanas. et al.) Jaklitsch & Voglmayr, Parafenestella ostryae (Wanas. et al.) Jaklitsch & Voglmayr
Epitypifications (basionyms): Sphaeria rhamni Nees, Fenestella princeps Tul. & C. Tul., Valsa fenestrata Berk. & Broome
Introduction
The family Cucurbitariaceae was described by Winter (1885; as Cucurbitarieae), who listed Cucurbitaria, Gibbera, Gibberidea, Nitschkia and Otthia as members of this family. He used the family for non-stromatic pyrenomycetes forming ascomata “in lawns”, i.e., more or less grouped and superficial on the substrate or on a hypostroma when present. Arx & Müller (1975) incorporated the family in the Pleosporaceae. Over the years the family was reduced to Cucurbitaria, while Barr (1987) included also Cucurbidothis, Otthia, Rhytidiella and Syncarpella. This concept (excluding Otthia) was presented by Doilom et al. (2013), who also included Pyrenochaeta and Pyrenochaetopsis following earlier phylogenetic analyses (Aveskamp et al., 2010, de Gruyter et al., 2010, de Gruyter et al., 2012). They also epitypified the generic type of Cucurbitaria, C. berberidis, using material collected in Austria. However, Cucurbidothis pityophila does not belong to the Cucurbitariaceae. It has a putative coniothyrium-like asexual morph intimately associated with ascomata. According to Valenzuela-Lopez et al. (2018) this species (represented by strain CBS 149.32) is a member of the Didymosphaeriaceae, albeit with a very long branch in their phylogenetic tree. Cucurbidothis was often treated as a synonym of Curreya (Von Arx and Müller, 1975, Von Arx and van der Aa, 1983). The generic type of the latter, C. conorum, has not been collected recently. Also this fungus may not be a member of the Cucurbitariaceae, judging from, e.g., the biseriate arrangement of ascospores in clavate asci and some stromatic tissues surrounding the ascomata. Barr (1981) had even combined C. conorum in Pleospora. Other species assigned to Curreya, C. acaciae, C. austroafricana, C. grandicipis and C. proteae belong to Teichospora in the Teichosporaceae (Jaklitsch et al. 2016). Rhytidiella and Syncarpella differ from all fungi identified in the Cucurbitariaceae by cylindrical to vermiform phragmospores (see Doilom et al. 2013) and ecologically by inducing cankers (Barr and Boise, 1989, Zalasky, 1975). No DNA data are available for these genera.
Cucurbitaria is one of the oldest genera of ascomycetes separated from Sphaeria. The genus, as defined by its type species, C. berberidis, is characterised by tuberculate perithecioid ascomata with basally thickened and elongated peridium sitting on a common subiculum often termed hypostroma and erumpent from bark in groups, by cylindrical fissitunicate asci with uniseriate arrangement of the brown muriform ascospores, and a pyrenochaeta- or, more generalised, phoma-like asexual morph. This and other species of Cucurbitaria are usually regarded as saprotrophs or necrotrophs (Doilom et al., 2013, Mirza, 1968).
A vague original definition of the genus Cucurbitaria led to misuse of the generic name for many unrelated genera of pyrenomycetes. Therefore, Index Fungorum (June 2017) lists 465 epithets including 34 infraspecific taxa, of which at least 340 do not belong to the Cucurbitariaceae. Owing to Kuntze (1898) more than 220 combinations in Cucurbitaria represent nectria-like fungi (Hypocreales); others are homonyms, illegitimate names or erratic entries, many others belong to different genera. Welch (1926) studied morphologically type materials present in American herbaria, commented on many taxa and accepted only five species in Cucurbitaria (C. arizonica, C. berberidis, C. caraganae, C. elongata and C. laburni). He synonymised many names, excluded others from the genus and determined that type material of most species was inadequate for unequivocal interpretation. Barr (1990a) accepted 11 species for North America. The latest comprehensive monographic study of the genus was performed by Mirza (1968) in the pre-molecular period. He studied 28 species, of which he described six new ones, creating two homonyms, cultured eight species and reported that six asexual genera, Camarosporium, Coniothyrium, Hendersonia, Leptophoma, Phoma and Pyrenochaeta, were associated with sexual morphs of this genus. In pure culture he found several developmental conidial stages including diplodia-like morphs. In recent years the connection of asexual morphs to their sexual morphs has proven to be phylogenetically informative at the generic to even ordinal level in the Dothideomycetes (Crous, 2009, Crous et al., 2009, Slippers et al., 2013, Hyde et al., 2013, Jaklitsch and Voglmayr, 2016). However, the respective genera are often polyphyletic, mainly because morphological delimitation of similar genera offering few easily recognisable and little varying features or which are incompletely studied regarding their life cycles, is difficult or sometimes impossible, and therefore unrelated fungi are subsumed under a common generic name (Crous et al. 2009). For example, phoma-like genera such as Pleurostromella (Petrak 1922), Pleurophoma, Pleurophomella, Pyrenochaeta and others are morphological variants for the same asexual morphs that have been associated with the Cucurbitariaceae, but they can be also found in many other families of the Pleosporales (de Gruyter et al., 2012, Jaklitsch and Voglmayr, 2016).
For many Cucurbitaria species a camarosporium-like asexual morph was determined by morphology and culture studies (Mirza, 1968, Sivanesan, 1984). However, Camarosporium appears to be unavailable for these fungi after the epitypification of its type species, C. quaternatum by Crous & Groenewald (2017). Recently, Camarosporium s. lat. was treated by Wanasinghe et al. (2017a), who combined some of the most common species, particularly those on fabaceous hosts such as Cucurbitaria caraganae, C. elongata and C. laburni, in their new genus Camarosporidiella (Camarosporidiellaceae).
A few species once in Cucurbitaria have recently been identified as belonging to different genera, e.g., Cucurbitaria bicolor, which is a synonym of Thyronectria rhodochlora in the Nectriaceae, Hypocreales (Checa et al. 2015), while Cucurbitaria obducens, C. piceae and C. rhododendri belong to the Melanommataceae (Jaklitsch & Voglmayr 2017).
The phylogenetic studies cited above suggest that only few taxa remain in Cucurbitariaceae s. str. Pyrenochaeta has been attributed to Cucurbitariaceae, as Cucurbitaria berberidis produces a pyrenochaeta-like asexual morph, but also other Pyrenochaeta spp., e.g. P. cava and P. nobilis, and Pyrenochaetopsis spp. were identified as members of the Cucurbitariaceae (Chen et al., 2015, de Gruyter et al., 2010, de Gruyter et al., 2012). Recently, Wanasinghe et al. (2017b) placed two of them (P. quercina, P. unguis-hominis) in their new genus Neocucurbitaria. Most recently, Valenzuela-Lopez et al. (2018) performed an extensive study of phoma- and pyrenochaeta-like coelomycetes, studying more than 350 strains mostly from the CBS and the UTHSC, including many new isolates from medical environments. They established several new families and genera, recognised many Phoma spp. in various genera of the Didymellaceae, as had been partly also shown in earlier works (see, e.g., Chen et al. 2015). In the Cucurbitariaceae Valenzuela-Lopez et al. (2018) combined Pyrenochaeta cava, P. hakeae and P. keratinophila in Neocucurbitaria, clarified the concept of and epitypified Pyrenochaeta quercina, the basionym of N. quercina, and described the new species Neocucurbitaria aquatica and N. irregularis. They also described the new monotypic genus Allocucurbitaria, and for Plenodomus corni, earlier also known as Pyrenochaeta corni (Boerema et al. 1996) and for the new species P. italica, based on a strain previously identified as Pyrenochaeta corni, they described the new genus Paracucurbitaria. Valenzuela-Lopez et al. (2018) excluded all other species of Pyrenochaeta that had been recognised by Wanasinghe et al. (2017b) as belonging to the Cucurbitariaceae from the family erecting several new genera and families. They also excluded Pyrenochaeta s. str. from the Cucurbitariaceae and erected a new family for Pyrenochaetopsis.
In our present work we include the genera Allocucurbitaria, Cucurbitaria, Neocucurbitaria, Paracucurbitaria, and the five new monotypic genera Astragalicola, Cucitella, Parafenestella, Protofenestella, and Seltsamia. The genus Fenestella is included as its generic type F. fenestrata (= F. princeps), which is lecto- and epitypified in order to stabilize its name and phylogenetic position.
Materials and methods
Isolates and specimens
All isolates used in this study originated from ascospores or conidia (where noted) of fresh specimens. Strain identifiers including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the Westerdijk Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS culture collection). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. The following cultures were sequenced but not further treated here: Phaeosphaeria (Amarenomyces) ammophilae: Sweden, Halland: Varberg, Apelviken, sandy beach, from old leaves of Ammophila arenaria, 31 Oct. 2015, S. Lund, det. and comm. O. Eriksson (WU 36958; culture AA); Plenodomus hendersoniae: Austria, Steiermark, Deutschlandsberg, Koralmgebiet, forest road to Grünangerhütte from the north, before the wooden bridge over the Schwarze Sulm, on Salix appendiculata, 16 May 2015, G. Friebes (WU 36959; culture LTO). Herbarium acronyms are according to Thiers (2017). Freshly collected specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses. Isolates/sequences in bold were isolated/sequenced in the present study.
Ex-epitype of Alternaria tenuis Nees.
Sequence retrieved from genome deposited at JGI-DOE (http://genome.jgi.doe.gov/).
Culture preparation and phenotype analysis
Cultures were prepared and maintained as described previously (Jaklitsch 2009) except that CMD (CMA: Sigma, St Louis, Missouri; supplemented with 2 % (w/v) D(+)-glucose-monohydrate) or 2 % malt extract agar (MEA; 2 % w/v malt extract, 2 % w/v agar-agar; Merck, Darmstadt, Germany) was used as the isolation medium. Cultures used for the study of asexual morph micro-morphology were grown on CMD or MEA at 22 ± 3 °C, rarely SNA (Nirenberg 1976) for conidiation assessment, in darkness. Microscopic observations were made in tap water except where noted. Morphological analyses of microscopic characters were carried out as described by Jaklitsch (2009). Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 and Nomarski differential interference contrast (DIC) using the compound microscopes Nikon Eclipse E600 or Zeiss Axio Imager.A1 equipped with a Zeiss Axiocam 506 colour digital camera. Images and data were gathered using a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured by the NIS-Elements D v. 3.0 or 3.22.15 or Zeiss ZEN Blue Edition software. For certain images of ascomata the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch, 2011, Jaklitsch et al., 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or the modified CTAB method of Riethmüller et al. (2002).
The following loci were amplified and sequenced: the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a ca. 900 bp fragment of the large subunit nuclear ribosomal DNA (nLSU rDNA) as a single fragment with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a ca. 1.0–1.4 kb fragment of the small subunit nuclear ribosomal DNA (nSSU rDNA) with primers SL1 (Landvik et al. 1997) and NSSU1088 (Kauff & Lutzoni 2002); a ca. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) gene with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999) or dRPB2-5f and dRPB2-7r (Voglmayr et al. 2016a); a ca. 1.2–1.3 kb fragment of the translation elongation factor 1-alpha (tef1) gene with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005) or EF1-2218R (Rehner & Buckley 2005); and a ca. 0.7 kb fragment of the beta tubulin (tub2) gene with primers T1 (O'Donnell & Cigelnik 1997) or T1HV (Voglmayr et al. 2016b) and BtHV2r (Voglmayr et al., 2016b, Voglmayr et al., 2017). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, U.K.) with the same primers as in PCR; in addition, primers ITS4 (White et al. 1990), and LR3 (Vilgalys & Hester 1990) were used for the ITS-LSU region. In some cases the tef1 was cycle-sequenced with internal primers TEF1_INTF (forward; Jaklitsch 2009) and TEF1_INT2 (reverse; Voglmayr & Jaklitsch 2017). Sequencing was performed on an automated DNA sequencer (3730xl Genetic Analyzer, Applied Biosystems).
Analysis of sequence data
For the phylogenetic analyses, combined matrices of ITS-LSU, SSU, rpb2, tef1 and tub2 sequences were produced. GenBank sequences of selected families of Pleosporales from the suborder Pleosporineae were selected according to Hyde et al. (2013) and recent additions (Crous and Groenewald, 2017, Valenzuela-Lopez et al., 2018, Wanasinghe et al., 2017b) and supplemented with GenBank nucleotide sequences of some additional taxa. For some strains for which the whole genome data are available, sequences were retrieved from JGI-DOE (http://genome.jgi.doe.gov/). Two representative taxa (Massarina eburnea, Trematosphaeria pertusa) from the suborder Massarineae were selected as outgroup (Tanaka et al. 2015). All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit v. 7.0.9.0 (Hall 1999). Due to alignment problems, the nucleotide characters at the very 5′ end of the ITS1 were excluded for all taxa outside Cucurbitariaceae, Pyrenochaetopsidaceae and Pyrenochaeta nobilis. For phylogenetic analyses, two matrices were produced, one comprising only ITS-LSU sequences and a second combined matrix of ITS-LSU, SSU, rpb2, tef1 and tub2, containing only accessions for which, in addition to the LSU, at least rpb2 or tef1 were available. The ITS-LSU matrix contained 1 649 nucleotide characters and the combined matrix 6 058 nucleotide characters: 1 697 from the ITS-LSU, 1 002 from the SSU, 1 070 from rpb2, 1 453 from tef1, and 836 from tub2. Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localised incongruence among the markers used for the combined analysis, using the level of bootstrap support (Sung et al. 2007) as described in Jaklitsch & Voglmayr (2014). For this, the 70 % maximum parsimony (MP) bootstrap consensus trees from 100 bootstrap replicates calculated for each individual partition, with the same parameters given below and with each replicate limited to 1 million rearrangements, were compared. These bootstrap trees were also used for an evaluation of the phylogenetic resolution of the individual markers; but for this the 50 % bootstrap support was implemented.
Maximum parsimony (MP) analysis of the combined matrices was performed using a parsimony ratchet approach. For this, a nexus file was prepared using PRAP v. 2.0b3 (Müller 2004), implementing 1 000 ratchet replicates with 25 % of randomly chosen positions upweighted to 2, which was then run with PAUP v. 4.0a156 (Swofford 2002). The resulting best trees were then loaded in PAUP and subjected to heuristic search with TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analysis with 1 000 replicates was performed using 5 (ITS-LSU matrix) or 10 (combined multigene matrix) rounds of replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 1 million rearrangements in the ITS-LSU matrix. In all MP analyses molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to minbrlen.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMA substitution model with 1 000 bootstrap replicates. The matrix was partitioned for the individual gene regions, and substitution model parameters were calculated separately for them.
For evaluation and discussion of bootstrap support, values below 70 % were considered low, between 70 and 90 % medium/moderate, and above 90 % high.
Results
Molecular phylogeny
Test for localised incongruence among the markers
In the MP bootstrap tree of tub2, Neocucurbitaria rhamnioides C118 was placed basal to the N. rhamnioides - N. rhamnicola clade with high support, which was in conflict with the bootstrap trees of all other markers; in the combined analyses the tub2 was therefore excluded for N. rhamnioides C118. No additional significant topological conflicts were observed between the bootstrap trees of the various genes, indicating the absence of significant incongruence and combinability of the loci (Wiens 1998).
Phylogenetic analyses
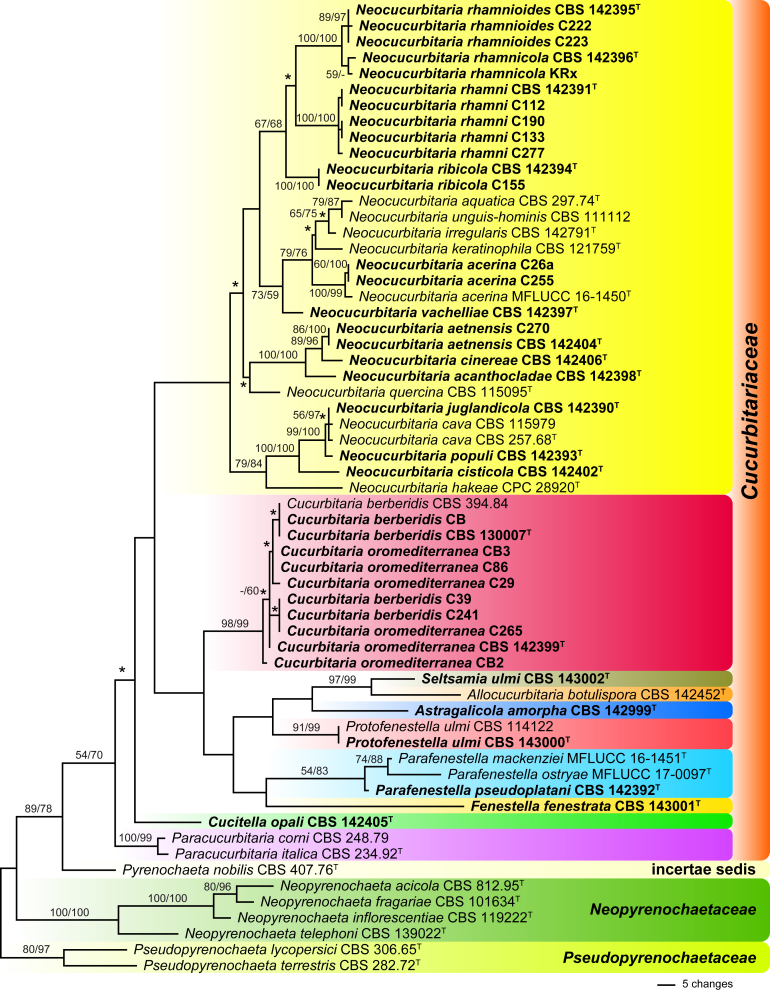
Of the 1 649 nucleotide characters of the ITS-LSU matrix, 211 were parsimony informative. Maximum parsimony analyses revealed 2 720 MP trees 843 steps long, one of which is shown in Fig. 1. The MP trees were identical in the deeper nodes, but within Cucurbitariaceae several nodes especially within Cucurbitaria and Neocucurbitaria collapsed to a polytomy in the strict consensus tree (marked by asterisks in Fig. 1).
Fig. 1.
Phylogram of one of 2720 MP trees 843 steps long (CI = 0.518, RI = 0.750), obtained by PAUP from an analysis of the ITS-LSU matrix of Cucurbitariaceae, Neopyrenochaetaceae and Pseudopyrenochaetaceae, with the latter selected as outgroup according to Fig. 2. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. Strains formatted in bold were isolated and sequenced in the current study; ex-type strains are indicated by a superscript T. Nodes that collapsed in the strict consensus of all 2 720 MP trees are marked by an asterisk (*). Note the lack of internal support for most backbone nodes, and the lack of resolution for closely related taxa.
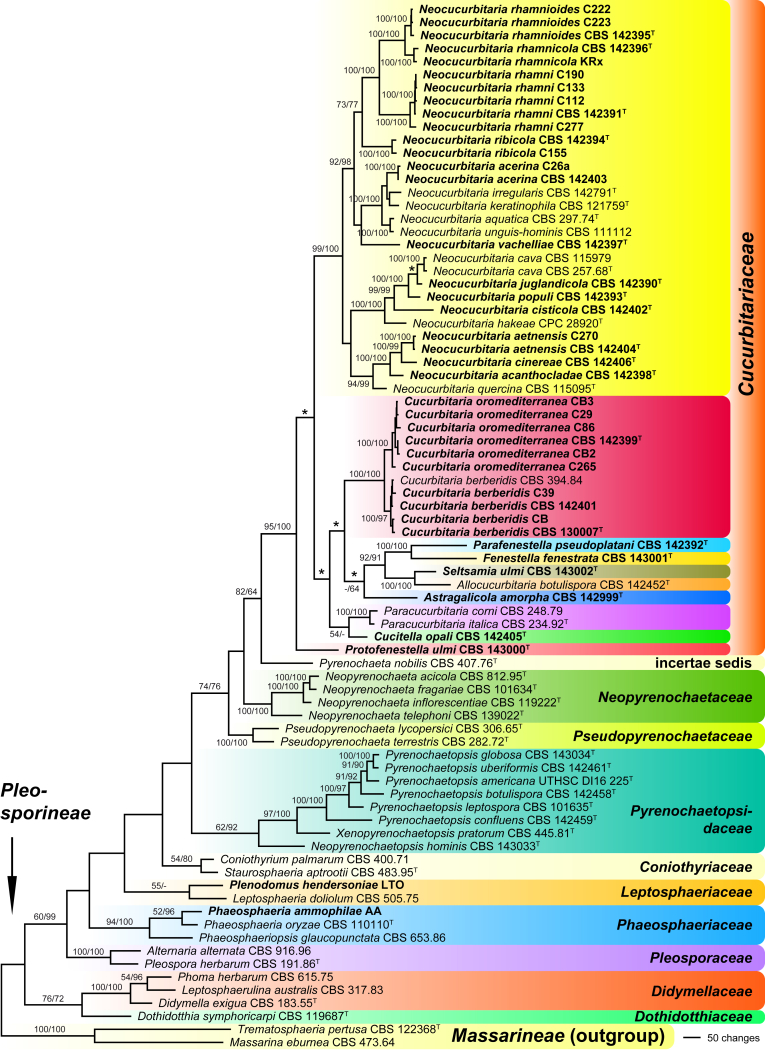
Of the 6 058 nucleotide characters of the combined matrix, 1 471 were parsimony informative (319 of ITS-LSU, 44 of SSU, 506 of rpb2, 345 of tef1, and 257 of tub2). Maximum parsimony analyses revealed 64 MP trees 9 817 steps long, one of which is shown as Fig. 2. Topologies of the MP trees were identical except for a few deeper nodes in Cucurbitariaceae and a polytomy of Neocucurbitaria cava, N. populi and N. juglandicola (marked by asterisks in Fig. 2).
Fig. 2.
Phylogram of one of 64 MP trees 9 817 steps long (CI = 0.345, RI = 0.625), obtained by PAUP from an analysis of the combined matrix (SSU-ITS-LSU, rpb2, tef1, tub2) of Cucurbitariaceae and selected Pleosporales. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches. Strains formatted in bold were isolated and sequenced in the current study; ex-type strains are indicated by a superscript T. Nodes that collapsed in the strict consensus of the 64 MP trees are marked by an asterisk (*).
Comparison of the phylogenetic analyses of the ITS-LSU matrix with the combined matrix shows a significant increase of resolution within Cucurbitariaceae in the latter. While the closely related Cucurbitaria berberidis and C. oromediterranea were not resolved in the ITS-LSU tree (Fig. 1), they received high to maximum support in the combined analyses (Fig. 2); likewise, the weakly (59 % MP) to unsupported (ML) Neocucurbitaria rhamnicola (Fig. 1) received maximum support (Fig. 2). In addition, internal support of many other nodes increased substantially; most notably to mention the high (MP) to maximum (ML) support for Cucurbitariaceae and the genus Neocucurbitaria which had low and no significant support, respectively, in the ITS-LSU tree. Also many nodes of the backbone within Neocucurbitaria received medium to high support in the combined analyses, which were unsupported in the ITS-LSU analyses; for instance, the monophyly of the three species on Rhamnus (Fig. 2).
Comparison of the phylogenetic resolution of the individual markers
Comparison of the bootstrap trees of the individual markers used for evaluation of localised incongruence revealed also highly interesting insights into their phylogenetic resolution and support. Only the most relevant outcomes with respect to Cucurbitariaceae are discussed here (data not shown). As expected, the SSU has too little phylogenetic information and almost all nodes within Pleosporineae lack significant support; it is therefore not further discussed here. With the other markers, the Cucurbitariaceae are only resolved by rpb2 and ITS-LSU, receiving low (64 %) and medium (83 %) support, respectively. Backbone support within Cucurbitariaceae is generally highest with rpb2, followed by ITS-LSU and tub2; however, the deeper nodes within Cucurbitariaceae are unsupported with all markers. The genus Neocucurbitaria receives medium support by the tub2 (72 %) and the ITS-LSU (82 %) analyses, and high support (99 %) by the rpb2 analyses. Within Neocucurbitaria, rpb2 consistently revealed a high support for all main clades and is superior to ITS-LSU and tub2, where many of these clades received only low to medium support. The genus Cucurbitaria received medium support (88 %) in tub2, high support (98 %) in ITS-LSU and maximum support in rpb2. However, with these markers the two closely related Cucurbitaria species remained unresolved in ITS-LSU and were only partially resolved in rpb2 (C. berberidis, 95 %), but fully resolved in tub2 (C. berberidis, 54 %; C. oromediterranea, 99 %). Neocucurbitaria rhamnicola received low (64 %), medium (83 %) and maximum support by ITS-LSU, tub2 and rpb2, respectively, and N. rhamnioides received high support by ITS-LSU (90 %) and rpb2 (92 %). In the tef1 analyses all deeper nodes of the tree were unsupported, but it is the best marker for resolution of closely related species; it is the only marker where the species pairs Cucurbitaria berberidis/C. oromediterranea and Neocucurbitaria rhamnicola/N. rhamnioides were resolved with maximum support.
Morphology
It is noted that most representatives of the Cucurbitariaceae studied here have true paraphyses. Hamathecial threads with free apices among immature asci are necessary to assess this feature. However, in the materials of several species no immature asci were present, therefore we term the hamathecial threads ?paraphyses due to uncertainty.
Taxonomy
Cucurbitariaceae G. Winter [as Cucurbitarieae], Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 1.2: 308. 1885.
Ascomata immersed in bark, erumpent, often becoming superficial, scattered or gregarious in or on a subiculum or in a valsoid pseudostroma, perithecioid, globose, subglobose, turbinate, lenticular or pyriform, brown to black; surface verruculose to coarsely tubercular. Ostioles inconspicuous or papillate to cylindrical, ostiolar canal periphysate. Peridium pseudoparenchymatous, usually brown. Hamathecium comprising numerous hyaline, filiform, septate and often anastomosing paraphyses, sometimes possibly pseudoparaphyses. Asci cylindrical to oblong, bitunicate, fissitunicate, with an ocular chamber and typically with a short stipe, containing 4–8 ascospores in uni- to partly biseriate arrangement. Ascospores ellipsoid, fusoid or oblong, brown, muriform, rarely with a gelatinous sheath, sometimes with appendage cells. Asexual morphs coelomycetous, phoma- or pyrenochaeta-like.
Saprobic on wood, bark and leaves or fungicolous, sometimes pathogenic on humans, also isolated from soil, possibly endophytic in plants.
Type genus. – Cucurbitaria.
Notes: In most taxa, particularly of Cucitella, Fenestella, Neocucurbitaria and Parafenestella, where the study of the hamathecium was possible, we detected paraphyses with free apices among immature asci. Cucurbitaria may have pseudoparaphyses, but this has not been reassessed.
Cucurbitaria Gray, Nat. Arr. Brit. Pl. (London) 1: 519. 1821.
Synonym: Crotonocarpia Fuckel, Jb. nassau. Ver. Naturk. 23–24: 163. 1870 (1869–1870).
Ascomata erumpent from bark, scattered or aggregated in clusters on a subiculum, globose to turbinate, brown to black; apex obtuse, surface usually coarsely warted. Ostiolar openings inconspicuous, central, sunken, sometimes visible as a minute, light-coloured areas. Peridium firm and thick, pseudoparenchymatous, brown to black outside, lighter-coloured to the inside, typically thickened and often distinctly elongated basally. Hamathecium of branched ?paraphyses. Asci cylindrical, bitunicate, fissitunicate, with a short stipe, a simple or knob-like base, and a distinct ocular chamber; containing 8 ascospores in uniseriate arrangement. Ascospores ellipsoid, straight, muriform, slightly constricted at the median primary septum, golden-, reddish- to dark brown, smooth.
Asexual morph pyrenochaeta-like. Pycnidia on natural hosts and in artificial culture with apical setae, superficial (or immersed in agar), more or less globose, dark brown to black. Peridium thin, pseudoparenchymatous, brown. Conidiogenous cells phialidic, cylindrical to lageniform, formed on simple or basally branched conidiophores and on basal hyaline cells in nature and in artificial culture. Conidia produced acropleurogenously, i.e. at one side of the conidiophore on phialides or pegs, and terminally. Conidia 1-celled, oblong, cylindrical or ellipsoid, straight or curved, hyaline to pale brownish, guttulate.
Type species: Cucurbitaria berberidis (Pers.) Gray.
Notes: Most of the generic synonyms of Cucurbitaria listed in Species Fungorum and by Doilom et al. (2013) are different fungi or require reassessment, therefore we list only Crotonocarpia. For Gemmamyces and Megaloseptoria see Jaklitsch & Voglmayr (2017); also Gibberidea does not belong here (unpubl. results).
Cucurbitaria berberidis (Pers.) Gray, Nat. Arr. Brit. Pl. (London) 1: 519. 1821.
Basionym: Sphaeria berberidis Pers., Neues Mag. Bot. 1: 83. 1794. : Fr.: Syst. Mycol. 2 (2): 415. 1823.
Synonyms: Hypoxylon berberidis (Pers. : Fr.) J. Kickx f., Rech. Serv. Fl. Crypt. Fland. 1: 18. 1841.
Crotonocarpia moriformis Fuckel, Jb. nassau. Ver. Naturk. 23–24: 163. 1870 (1869–1870).
Phoma berberidis Sacc., Michelia 1(no. 2): 259. 1878.
Pyrenochaeta berberidis (Sacc.) Brunaud, Act. Soc. linn. Bordeaux, Trois. sér. 40: 83. 1886.
Gibberidea berberidis (Pers. : Fr.) Rabenh. ex Kuntze, Revis. gen. pl. (Leipzig) 3(2): 481. 1898.
Cucurbitaria moriformis (Fuckel) M. E. Barr, Mycotaxon 29: 503. 1987.
See Doilom et al. (2013) for description and typification.
Material examined: Austria, Kärnten, St. Margareten im Rosental, Drau-Auen, grid square 9452/1, on branches of Berberis vulgaris, 31 Dec. 2002, W. Jaklitsch W.J. 2043 (WU 39966); Wograda, grid square 9452/3, on Berberis vulgaris, 14 Apr. 2006, W. Jaklitsch W.J. 2901 (WU 35985); ibid., 30 Apr. 2011, W. Jaklitsch (WU 31405 epitype; ex-epitype-culture CBS 130007 = CB1, second isolate CB); Vienna, 3rd district, Botanical Garden, on branches of Berberis sp., 14 Mar. 2016, W. Jaklitsch & H. Voglmayr (WU 35987; culture C241 = CBS 142401). Belgium, Sint-Huibrechts-Lille, Neerpelt, on branch of Berberis vulgaris ssp. atropurpurea, 28 Jan. 2014, P. Bormans (WU 35986; culture C39).
Notes: Cucurbitaria ephedricola sensu Ariyawansa et al. (2015), discussed in Wanasinghe et al. (2017b), is clearly C. berberidis. The authors only produced SSU and LSU (accessions KT313005, KT313007) of their single isolate HA 42, and they are identical in composition and length with those of C. berberidis strain CBS 394. 84. Their specimen was collected in the Italian region Emilia Romagna and neither the collector nor the authors were obviously able to identify the host. According to Flora Italiana (http://luirig.altervista.org/flora/taxa/floraindice.php) no Ephedra occurs in Emilia Romagna. There is no basis to select arbitrarily a name out of numerous Cucurbitaria names (see e.g. Mirza 1968) without knowing the host. Wanasinghe et al. (2017b) argued that their specimen differed in morphology from C. berberidis. However, it may be poorly developed, but otherwise we do not see much difference. We suggest that the authors re-check the morphology of their specimen.
Cucurbitaria oromediterranea Jaklitsch & Voglmayr, sp. nov., MycoBank MB822999. Fig. 3.
Fig. 3.
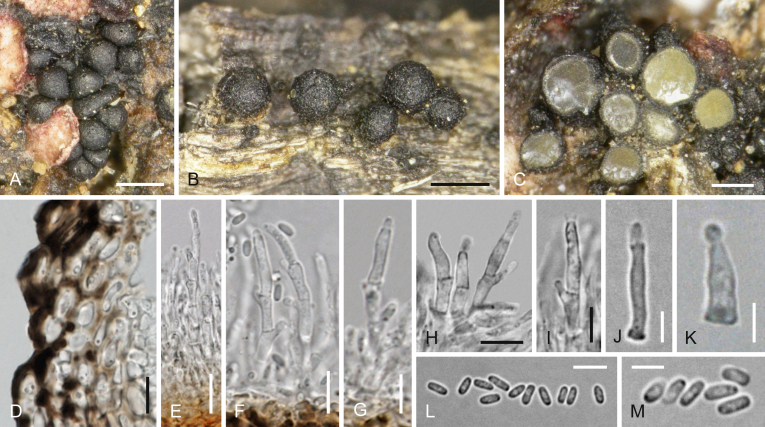
A–I, K–W, Y–F1.Cucurbitaria oromediterranea. J, X.C. berberidis (WU 35987). A, B. Ascomata in face view. C, D. Ascoma in vertical section showing thickened basal peridium. E, F. Peridium in vertical section, showing opaque warts in E. G. Subicular hyphae. H–J. Asci. K, L. Ascus tips showing ocular chamber. M. Hamathecium. N–X. Ascospores. Y. Pycnidium on natural substrate. Z–F1. Asexual morph on CMD at 22 °C after 2–3 wk. Z. Pycnidium. A1. Setae. B1–D1. Conidiophores and phialides. E1, F1. Conidia. A, C–G, L, M, Q–T, W. WU 35988; B, N–P, U, V, Y–F1. WU 35991/C86; H, I. WU 35989; K. WU 35990. Scale bars: A, B = 0.5 mm; C, D = 150 μm; E, Y, Z = 50 μm; F, M–X, C1 = 10 μm; G, K, L, B1 = 7 μm; H–J, A1 = 20 μm; D1–F1 = 5 μm.
Etymology: Referring to its occurrence in oromediterranean regions.
Ascomata (300–)430–620(–750) μm (n = 67) diam, (300–)500–630(–650) μm (n = 15) high, scattered or aggregated in small groups, erumpent-superficial, globose, subglobose to turbinate or pulvinate, often collapsing from above, with sunken centre, sometimes laterally fused, black, with surface coarsely cracked into plates; seated on a subiculum of thick-walled, dark brown 2.5–6 μm wide hyphae continuing in the wood. Ostioles (59–)80–120(–135) μm (n = 15) long, (55–)71–108(–115) μm (n = 15) wide at the apex, usually indistinct at the surface, sometimes indistinctly papillate, sometimes with reddish-brown centre. Peridium (77–)114–233(–268) μm (n = 15) wide at base, (53–)63–127(–150) μm (n = 15) at the sides; outer layer narrow, dark brown to black, opaque, coarsely warted, of thick-walled cells immersed in a dark amorphous resinous mass; inner layer thick, particularly at the base, consisting of brown, thin-walled, pseudoparenchymatous cells (4.5–)6.5–18(–26.5) × (3.5–)5.5–11(–15) μm (n = 30). Hamathecium of moderately branched, 1–3.5(–4) μm wide ?paraphyses. Asci (144–)165–225(–260) × (14–)16–20(–22) μm (n = 35), cylindrical, bitunicate, fissitunicate, with a short stipe and a simple or knob-like base, narrow walls with endotunica thickened at the apex and a distinct ocular chamber; containing 8 ascospores in uniseriate arrangement. Ascospores (22–)25–33(–40) × (9.3–)11.3–14.5(–17.2) μm, l/w (1.9–)2.1–2.5(–2.8) (n = 76), ellipsoid, straight, with (6–)7–8(–12) transverse and 2–4 longitudinal septa, slightly constricted at the median primary septum, ends rounded to subacute, first hyaline to yellowish, turning golden-, reddish- to dark brown, in 3 % KOH dark brown to nearly black, smooth. Pycnidia on natural hosts (73–)110–235(–330) μm (n = 43) diam, scattered, superficial, globose, dark brown to black, with apical setae.
Cultures and asexual morph: A 90 mm Petri dish containing CMD, inoculated at the side and incubated in the dark at 22 °C entirely covered by mycelium after 4–6 wk. Colony dark olive brown to nearly black, margin hyaline, not or indistinctly zonate, odour indistinct. Pycnidia (70–)90–125 μm (n = 17) diam, usually numerous, formed within 2 wk in the centre, in a concentric zone or scattered over the whole colony, superficial or immersed in agar, first hyaline to greyish, turning black, globose with a small papilla or pyriform, with protruding cells and setae at the surface; extruded conidial drops hyaline to greyish brown. Setae concentrated at the apex, up to 60 μm long and 7 μm thick at their bases, greyish to dark brown, with rounded ends. Peridium thin, of thin-walled pale brown cells (4.7–)6.5–10(–15) μm diam (n = 25) forming a t. angularis, outside darker, thicker-walled and more rounded, inside lined by a layer of angular hyaline cells giving rise to conidiophores or phialides. Conidiophores hyaline, simple or branched near the base into two or several branches, each with lateral pegs or unicellular branches forming solitary terminal phialides. Phialides (5.3–)7.0–9.8(–10.7) × (1.6–)1.7–2.2(–2.5) (n = 23), cylindrical, sinuous or lageniform. Conidia (2.5–)3.3–4.0(–4.5) × (0.9–)1.1–1.7(–2.1) μm, l/w (1.6–)2.2–3.3(–4.8) (n = 64), 1-celled, oblong, cylindrical or ellipsoid, straight or slightly curved, often attenuated toward one end, hyaline to pale brownish, containing two subterminal guttules.
Habitat: On wood and bark of Berberis spp., known from B. aetnensis, B. cretica and B. hispanica.
Distribution: At elevations from above ca. 900 m in the Mediterranean.
Holotype: Greece, Crete, Omalos plain, heading to Seliniotikos Giros, N 35 19 20 E 23 54 22, elev. 1 120 m, on twigs of Berberis cretica, 5 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 35988; ex-holotype culture CBS 142399 = C229).
Other material examined: Greece, Crete, Omalos, on twigs of Berberis cretica, 26 Jun. 2013, W. Jaklitsch (WU 35989; culture CB2); path to a waste dump off the road to Omalos, on Berberis cretica, soc. Thyronectria caudata, Thyridium sp., 28 Nov. 2011, W. Jaklitsch (WU 32130). Italy, Sicily, Etna, south side, elev. ca. 1 900 m, roadside, on twigs of Berberis aetnensis, 17 Jun. 2016, W. Jaklitsch & H. Voglmayr (WU 35993; culture C265). Spain, Andalusia, Granada, La Zubia, Cerro del Trevenque, above the Jardín Botanico de la Cortijuela, above 1 600 m, on twigs of Berberis hispanica, 14 May 2014, W. Jaklitsch & S. Tello (WU 35992; culture CB3); same data, elev. ca. 1 700 m, soc. Thyronectria caudata, S. Tello & W. Jaklitsch (WU 33428); ca. 1 600 m, on twigs of Berberis hispanica, 14 May 2014, W. Jaklitsch & S. Tello (WU 35991; culture C86); Jaén, Jaén, La Pandera, N 37°37′54″ W 3°46′34.4″, elev. 1 800 m, on Berberis hispanica, 12 May 2014, S. Tello, W. Jaklitsch, D. Extrada & D. Merino (WU 33429); Málaga, Sierra de las Nieves, Natural Park, Parauta Pinsapar de la Escalereta, elev. 1 150 m, on Berberis hispanica, 26 Dec. 2013, M. Becerra (WU 35990; culture C29).
Notes: Cucurbitaria oromediterranea is virtually indistinguishable from C. berberidis in its sexual and asexual morphs and cultures, only ascospores are on average more reddish brown in C. oromediterranea and ascomatal pustules are often less conspicuous due to a lower number of ascomata per group. However, multigene phylogeny clearly separates it from C. berberidis (Fig. 2), and both species can be reliably distinguished by tef1 and tub2 sequences where they differ in 26 (including 3 gaps) and 12 fixed nucleotide substitutions, respectively. Cucurbitaria oromediterranea is the specific host of Thyronectria caudata (Jaklitsch & Voglmayr 2014) and, as inferred from the distribution of the latter, it occurs at high elevations in the African, Asian and European Mediterranean region. Thus, Cucurbitaria berberidis and C. oromediterranea have clearly different ecological requirements. The hamathecial threads have free rounded ends, but their development is unclear, therefore we term them ?paraphyses. In culture on CMD we found conidiophores only in entirely mature pycnidia, not in young ones.
Astragalicola Jaklitsch & Voglmayr, gen. nov., MycoBank MB823000.
Etymology: Referring to its occurrence on Astragalus.
Only known as asexual morph. Pycnidia scattered or aggregated in groups on wood and bark, more or less superficial, globose, non-papillate, black; contents with a waxy to gelatinous consistency; ostiolar opening apical; peridium pseudoparenchymatous. Phialides and conidiophores densely aggregated at inner side. Conidiophores simple or branched once. Phialides lageniform to cylindrical or sigmoid. Conidia formed on phialides, their base cells or lateral pegs on conidiophores, oblong or narrowly ellipsoid, straight or curved, 1-celled, hyaline, guttulate.
Type species: Astragalicola amorpha Jaklitsch & Voglmayr.
Notes: The genus Astragalicola differs from Phoma by the presence of conidiophores and from Pyrenochaeta by the lack of setae. Erection of a new genus was necessary following phylogenetic analysis, as it forms a separate clade.
Astragalicola amorpha Jaklitsch & Voglmayr, sp. nov., MycoBank MB823001. Fig. 4.
Fig. 4.
Astragalicola amorpha (WU 35994). A, B. Pycnidia in face view on the natural substrate. C. Sectioned pycnidia showing olivaceous interior. D. Peridium in vertical section. E–K. Conidiophores and phialides. L, M. Conidia. D, J, L, M. in 3 % KOH. Scale bars: A, B = 300 μm; C = 150 μm; D, F, H = 7 μm; E = 10 μm; G, I, L = 5 μm; J, K, M = 3 μm.
Etymology: Referring to its sole occurrence as asexual morph.
Pycnidia (140–)195–281(–336) μm diam (n = 34), aggregated in groups on bark or scattered on wood, superficial with bases often immersed, sometimes laterally fused, globose, non-papillate, with a minute apical ostiolar opening, more or less smooth, black, contents olivaceous, with a waxy to gelatinous consistency; peridium ca. 20–70 μm thick, outer layer unevenly dark brown pigmented, of thick-walled cells (4–)5.5–10(–16.5) μm diam (n = 30) forming t. angularis to globulosa, inner layer pseudoparenchymatous, hyaline, giving rise to densely aggregated phialides and conidiophores. Conidiophores up to ca. 45 μm long, simple or branched once. Phialides (5.3–)6.2–9.3(–11) × (1.4–)1.8–2.3(–2.5) μm, l/w (2.1–)2.8–4.9(–6) (n = 30), lageniform to cylindrical, often curved or sigmoid. Conidia formed on phialides and laterally on their base cells or lateral pegs on conidiophores, (2.3–)2.5–3.0(–3.5) × (1.0–)1.2–1.4(–1.6) μm, l/w (1.7–)1.9–2.4(–2.8) (n = 83), oblong or narrowly ellipsoid, straight or slightly curved, 1-celled, hyaline, with 1–2 or more minute guttules, sometimes attenuated toward one end.
Culture: Colony radius 35 mm on CMD after 15 d at 22 °C; colony circular, olivaceous with pale margin, radial structure, few pycnidia forming at the near margin; odour indistinct.
Habitat: on stems of Astragalus angustifolius.
Distribution: Greece (Crete), only known from the holotype location.
Holotype: Greece, Crete, Psiloritis, at the margin of the Nida plateau, on basal stem parts of Astragalus angustifolius, intimately associated with a reddish Fusarium sp. (holomorph; reddish-violaceous ascomata containing bicellular ascospores); soc. Camarosporium sp. (C227, C227b), a member of the Lophiostomataceae (C227c) and a Scopinella sp., 8 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 35994; ex-holotype culture CBS 142999 = C227a, from conidia).
Notes: Cucurbitaria astragali P. Karst. & Har. is no probable candidate for an earlier name of this fungus, as it was described from Astragalus monspessulanus, a herbaceous host, and its putative asexual morph was identified as a Hendersonia with large, 4-septate conidia (Karsten & Hariot 1890). Phoma astragali Ellis & Kellerm. is a nomen nudum fide Boerema et al. (2004; p. 61); Phoma astragali Cooke & Harkn. is now known as Stagonosporopsis astragali (Cooke & Harkn.) Aveskamp et al. (Aveskamp et al. 2010). Phoma astragalicola Hollós has much larger conidia (6–8 × 2.5–3 μm) than Astragalicola amorpha and occurs in leaves (Saccardo 1913). Phoma astragalina (Gonz. Frag.) Boerema & Kesteren differs from Astragalicola amorpha by narrower conidia and occurrence on various herbaceous hosts in south-western Asia (Boerema and van Kesteren, 1981, Boerema et al., 1994).
Cucitella Jaklitsch & Voglmayr, gen. nov., MycoBank MB 823002.
Etymology: Based on a combination of the generic names Cucurbitaria (Cuci) and Fenestella (tella).
Ascomata depressed subglobose to pyriform, black, immersed in bark on subiculum, erumpent through cracks in the periderm, aggregated or laterally fused in clusters forming compact pustules causing small bumps on the bark surface. Ascomatal apices often papillate, black, rounded or apically flattened, with circular or angular outline. Peridium pseudoparenchymatous, thickest around the ostioles. Hamathecium of numerous branched paraphyses with free ends among immature asci. Asci cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 2–6 obliquely uniseriately arranged ascospores. Ascospores ellipsoid, with thick wall, several transverse and longitudinal septa, with three main transverse septa thicker than others, slightly to distinctly constricted at the median primary septum, dark brown when mature, often with lighter ends.
Pycnidia occurring in association with ascomata on natural substrate, aggregated, globose, collapsing cupulate, shiny black. Peridium pseudoparenchymatous, inner side lined by densely stacked simple or basally branched conidiophores with lateral pegs and solitary terminal, narrowly lageniform to subcylindrical phialides. Conidia formed on pegs and phialides, 1-celled, cylindrical to allantoid, hyaline, smooth. In pycnidia formed in culture conidia were only produced on sessile phialides.
Type species: Cucitella opali Jaklitsch & Voglmayr.
Note: Compact pustules and ascospores with a relatively large number of septa and lighter ends suggest a generic affiliation with Fenestella, but the multigene phylogeny disproves this hypothesis.
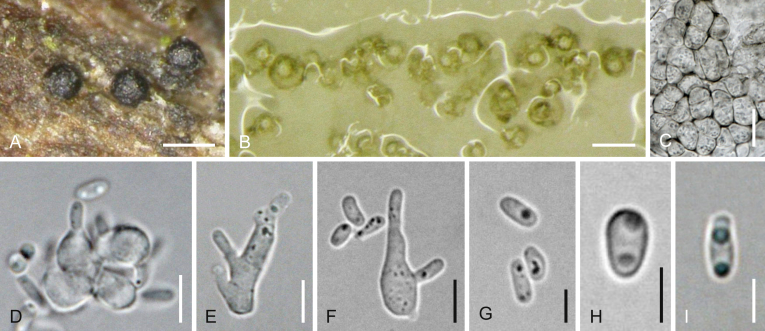
Cucitella opali Jaklitsch & Voglmayr, sp. nov., MycoBank MB823003. Fig. 5.
Fig. 5.
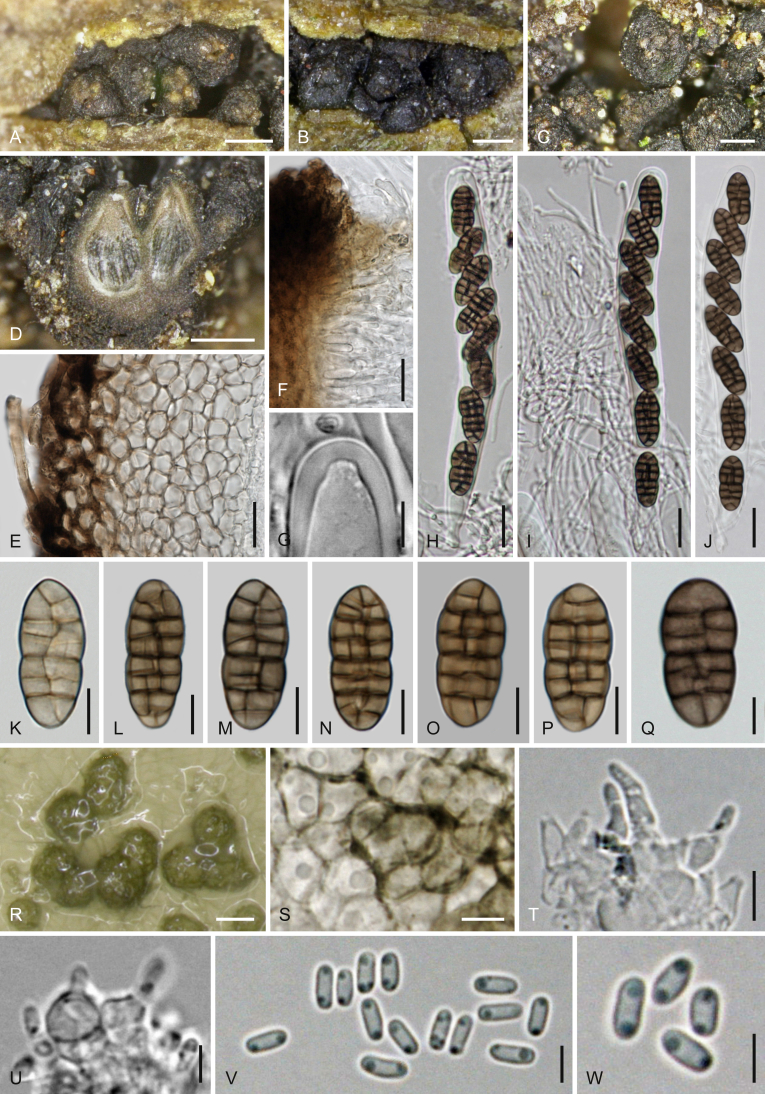
Cucitella opali (WU 35995/CBS 142405). A–O. Sexual morph (WU 35995). A. Pustule of ascomata in bark with two ascomata in vertical section. B. Peridium in vertical section. C. Subicular hyphae. D. Ascus apex. E–H. Asci (exotunica broken in G). I–O. Ascospores (I. young). P–S. Asexual morph on natural substrate. P, Q. Pycnidia. R. Cluster of short conidiophores with phialides. S. Conidia. T–Z. Asexual morph in culture (CBS 142405 on CMD after 5–6 d at 22 °C). T. Pycnidia. U. Pycnidial wall. V, W. Conidiogenous cells. X–Z. Conidia. D, F, I, O. in 3 % KOH. Scale bars: A, P = 150 μm; B–D, J–O, U = 10 μm; E–H = 25 μm; I, R = 7 μm; Q = 250 μm; S, V–Z = 3 μm; T = 70 μm.
Etymology: Due to its occurrence on Acer opalus.
Ascomata (230–)305–537(–600) μm (n = 14) diam, ca. 160–430 μm high, depressed subglobose, globose to pyriform, black, immersed in bark on a brownish subiculum, erumpent through cracks in the periderm, tightly aggregated or laterally fused in clusters of up to ca. 10, forming compact pustules causing small bumps on the bark surface. Ascomatal apices often papillate, (44–)65–105(–115) μm (n = 16) diam, black, rounded or apically flattened, with circular or angular outline. Subiculum consisting of 2–6 μm wide, thick-walled, hyaline, greyish brown to medium brown hyphae. Peridium 15–55 μm thick, thin at the base, thickest around the ostioles, consisting of a thick-walled t. angularis, dark brown outside becoming lighter brown to yellowish or hyaline toward the inner side, formed by cells (3.5–)5.2–9.5(–13.7) μm (n = 60) diam. Hamathecium consisting of numerous branched, 1–3(–4) μm wide paraphyses with free ends among immature asci. Asci (183–)189–205(–216) × (21.2–)22.3–27.2(–29.2) μm (n = 12), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing (2–)4–6 obliquely uniseriately arranged ascospores. Ascospores (30–)32–40.5(–47) × (13.7–)15.5–19.3(–23.7) μm, l/w (1.8–)1.9–2.2(–2.6) (n = 48), ellipsoid, with wider upper part, thick wall, (9–)10–14(–16) transverse and 3–5 longitudinal septa, three main transverse septa thicker than others, slightly to distinctly constricted at the median primary septum, first hyaline, turning yellow, yellowish brown, finally dark brown, usually with lighter ends, smooth; slightly to distinctly darker in 3 % KOH when mature.
Asexual morph on natural substrate: Pycnidia 150–340 μm diam, occurring in association with ascomata, aggregated in small groups in bark cracks, globose, collapsing cupulate, shiny black. Peridium pseudoparenchymatous, consisting of dark brown thick-walled cells, inside lined by densely stacked conidiophores, the latter up to ca. 50 μm long, simple or branched at the base, with lateral pegs and terminal solitary, narrowly lageniform to subcylindrical phialides 4.5–7(–7.8) × (1.5–)1.7–2.5(–2.8) μm (n = 10). Conidia formed on pegs and phialides, (3–)3.4–4.2(–5) × (0.9–)1.1–1.3(–1.5) μm, l/w (2.3–)2.7–3.8(–4.5) (n = 27), 1-celled, cylindrical to allantoid, hyaline, smooth.
Cultures and asexual morph: Colony radius on CMD 23–26 mm after 20 d at 22 °C; colony first hyaline, turning olivaceous to nearly black, margin hyaline, aerial hyphae inconspicuous, odour indistinct to slightly unpleasant. Pycnidia (after 5–6 d) 40–120 μm diam, forming within a few days in large numbers, immersed to superficial, evenly scattered or aggregated in small groups, globose to pyriform, papillate, first hyaline, slowly turning olivaceous. Peridium consisting of a thin, moderately thick-walled, olivaceous brown t. globulosa-angularis of (4–)5.7–10(–13.5) μm (n = 45) wide cells; inner side lined by hyaline cells giving rise to phialides. Phialides (3.8–)4.3–6(–7) × (2.7–)3–4.4(–5.4) μm (n = 16), sessile, crowded, mostly subglobose, less commonly lageniform or conical. Conidia (2.8–)3.3–4.6(–6.3) × (1–)1.1–1.3(–1.5) μm, l/w (2.2–)2.7–4(–5.6) (n = 70), 1-celled, allantoid, cylindrical to sigmoid, hyaline, containing 0–2 small guttules, smooth.
Habitat: In bark of Acer opalus.
Distribution: Europe, only known from the type location.
Holotype: France, Rougon, Gorges du Verdon, at the tunnels, on a twig of Acer opalus, 29 Jul. 2011, H. Voglmayr (WU 35995; ex-holotype culture CBS 142405 = FV).
Notes: Ascospores of this species resemble those of Parafenestella pseudoplatani but are distinctly smaller. In contrast to the latter, C. opali forms compact pustules on the natural host.
Fenestella Tul. & C. Tul., Selecta Fungorum Carpologia: Xylariei- Valsei- Spaeriei 2: 207. 1863, emend. Jaklitsch & Voglmayr.
Ascomata immersed in bark, aggregated on a subiculum and sometimes on a crumbly stromatic crust, forming a pustular pseudostroma appearing as bumps, causing irregular ruptures of the host periderm; upper surface filled by a brown to black crumbly disc with inconspicuous sunken or slightly projecting papillate ostioles or lacking a disc and then filled by more or less convergent papillate to cylindrical ostiolar necks; as a final stage of development sometimes entire pseudostroma becoming superficial on inner bark or wood. Ascomata depressed subglobose to pyriform or distorted by mutual pressure, often obliquely oriented toward a common centre. Peridium pseudoparenchymatous. Hamathecium consisting of narrow, branched and anastomosing ?paraphyses. Asci cylindrical to oblong, bi- and fissitunicate, containing 4–8 mostly uniseriate ascospores. Ascospores fusoid to ellipsoid or oblong, brown with lighter end cells, with or without hyaline appendage cells. Asexual morphs: phoma-like where known; none detected in the generic type.
Type species: Fenestella fenestrata (Berk. & Broome) J. Schröt.
Notes: Huhndorf & Glawe (1990) obtained a pycnidial asexual morph in culture, which was produced from ascospores of a putative Fenestella from an American Acer sp., but did not name it. The phylogenetic relationship of that fungus with Fenestella s. str. is however unclear.
Pending further studies, the genus Fenestella is here only included with its type species in order to fix its name by lecto- and epitypification and to clarify the positions of some other morphologically similar fungi, which phylogenetically do not belong to the genus.
Fenestella bipapillata, a species included by Phookamsak & Hyde (2015), was relegated to Dictyoporthe by Jaklitsch & Barr (1997).
Fenestella fenestrata (Berk. & Broome) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2(4): 435. 1897(1908). Fig. 6.
Fig. 6.
Fenestella fenestrata. A, B. Pseudostroma pustules in face view (B. vertically cut half pustule). C. Valsoid group of exposed ascomata in remnants of subiculum. D, E. Sectioned pseudostromata in (oblique) side view showing ascomata and eroded ostioles (shiny patches in E are due to a glue previously used for attachment to a sheet). F–H. Ascus apices (F. immature). I–K. Peridium (I, J. in face view; K. in vertical section). L–O. Asci. P–A1. Ascospores (in R, S, V with hamathecial threads in the background and free end in S; in X without appendage cells). K, S, W, Y, Z. in 3 % KOH. A, D, H, J, K, N, O, V–A1. lectotype PC 0084495; B, I, P–S. epitype WU 35996; C, E–G, L, T, U. PC 0084493; M. PC 0084496. Scale bars: A, E = 500 μm; B–D = 300 μm; F–H, K, P–W, Y–A1 = 15 μm; I, J, X = 10 μm; L–O = 30 μm.
Basionym: Valsa fenestrata Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3 3: 366. 1859.
Synonym: Fenestella princeps Tul. & C. Tul., Selecta Fung. Carpol. 2: 207. 1863.
Pseudostromata (0.9–)1.3–2.2(–2.9) mm (n = 66), up to 0.7, rarely 0.9 mm high, subglobose, flat conical to pulvinate with flat base, immersed beneath the periderm of the host forming up to 0.5 mm high bumps or, after removal of the periderm more or less superficial on the inner bark or wood, with circular to more or less elliptic outline, sometimes confluent with one or two other stromata, containing 2–10(–12) ascomata, depending on development conditions, surrounded by subicular hyphae and sometimes by a thin dark crumbly stromatic crust; central upper disc flat and sunken in fissures of the periderm, brown to black, sometimes with lighter spots, smooth or tuberculate, usually without clearly discernible ostiolar openings, but the latter sometimes appearing as flat brown or black discs ca. 180–330 μm diam, often becoming exposed by erosion of the uppermost layer, consisting of a narrow brown wall, whitish internal tissue of the ostiolar canal and a dark mass of ascospores or empty canal in their centre. Ascomata (390–)520–830(–1 140) μm (n = 68) diam, depressed subglobose to pyriform or distorted by mutual pressure, often obliquely oriented toward a common centre, collapsing from the sides. Ostioles eccentric, vertical or oblique and convergent. Peridium ca. 15–50 μm thick, pseudoparenchymatous; cells (5–)6.5–11(–15.5) μm (n = 94) diam in section and in surface view, thick-walled, dark brown outside to nearly hyaline inside, and with subicular hyphae originating on its surface. Subicular hyphae 2–6 μm wide, sometimes inflated to 8 μm, pale brown, darker toward the peridium, thick-walled. Hamathecium consisting of numerous branched and anastomosing, 1–4 μm wide ?paraphyses with free ends. Asci (256–)290–352(–377) × (22.3–)24–30.8(–36) μm (n = 22), cylindrical to oblong, bitunicate, fissitunicate, with an ocular chamber, a short and narrow stipe and simple base, containing (4–)8 mostly uniseriate ascospores. Ascospores (36.5–)49.3–65(–73) × (14.2–)18–25(–31) μm, l/w (2.1–)2.4–2.9(–3.7) (n = 116) including the hyaline cellular appendages, first hyaline, with a conspicuously thick wall, a median primary septum and a canal through the wall of each end, developing 2–4 additional main septa, turning pale or yellowish brown, when mature fusoid to ellipsoid or oblong, symmetric or slightly inequilateral, slightly constricted at the primary septum, dark to blackish brown, with 13–20 transverse and 4–6(–7) longitudinal septa. Terminal cells often paler, with or without a hyaline, ca. 2–7 μm long, rounded or longish, rarely acute appendage cell. Ascospores turning blackish brown to black in 3 % KOH and slightly to strongly swelling with septa often becoming indistinct, appendages remaining hyaline. Asexual morph not observed; none detected in culture.
Typification and other comments: Lectotype of Valsa fenestrata and Fenestella princeps here designated: UK, England, Wiltshire, Spye Park, on twigs of Alnus glutinosa, Mar. 1859, C.E. Broome (PC 0084495!; MBT378881, MBT378882). Epitype of Valsa fenestrata and Fenestella princeps, here designated: Austria, Oberösterreich, Raab, Wetzlbach, on twigs of Alnus glutinosa, soc. effete Diaporthe sp., 29 Apr. 2017, H. Voglmayr (WU 35996; MBT378883, MBT378884; ex-epitype culture CBS 143001 = FP9).
Background: Tulasne & C. Tulasne (1863) based their new genus Fenestella on Valsa fenestrata and used the new species epithet princeps for it, apparently because they disliked having the same word in both the genus and species name. Berkeley & Broome (1859) had described Valsa fenestrata citing two specimens, one from Quercus in Orton Wood, Leicestershire, leg. A. Bloxam, and one from Alnus glutinosa in Spye Park, Wiltshire, leg. C. E. Broome, March 1859. The Quercus specimen is extant in K as K(M) 233193. Barr (1990b) argued that Valsa fenestrata is a nomen dubium, because obtuse ascospores illustrated by Berkeley & Broome (1859) for the Quercus specimen indicate that two species were present. However, Tulasne & C. Tulasne (1863) explicitly referred to the Alnus specimen from Spye Park, which they received from Broome. In conclusion, F. fenestrata and F. princeps are the same species and its host is Alnus glutinosa. Broome's specimen from Spye Park was obviously separated into the two fragments PC 0084494 and PC 0084495 (both extant in PC). PC 0084494 only contains the sexual morph of a Cytospora with ascospores (11.8–)12.5–15(–16.2) × (3–)3.5–4(–4.5) μm, l/w (2.8–)3.2–4.1(–4.7) (n = 30), while PC 0084495 contains F. fenestrata. Therefore, we here designate the latter as lectotype of Valsa fenestrata and Fenestella princeps and epitypify these names with a fresh specimen from Alnus glutinosa. Nomenclaturally F. princeps is the generic type, but the species epithet fenestrata is older and has therefore priority. Tulasne & C. Tulasne (1863) cited also own material, which they collected in April 1860 on Alnus glutinosa in France near Paris (PC 0084493); this specimen corroborates the link to Berkeley & Broome's (1859) protologue plus specimen and thus conspecificity. They also noted that they did not see Berkeley's Quercus specimen and did not find the fungus on Quercus in France, but argued that there is apparently no difference to the Alnus material. Even if the fungus on Quercus was a different species, lectotypification makes this irrelevant. Tulasne & C. Tulasne (1863) also subsumed a specimen from Otth, provisionally named Valsa macrospora by him (PC 0084496) from Alnus glutinosa, under F. princeps. All cited materials were deposited in PC by Tulasne & C. Tulasne in 1873.
Phookamsak & Hyde (2015) examined and presented Otth's material (PC 0084496) as typical for F. princeps and differentiated it from F. fenestrata, which they based on two specimens from GZU, by “multiloculate stromata” and smaller ascospores and asci in the former. It is not clear, whether PC 0084496 is conspecific with F. fenestrata, because the “multiloculate stromata” seem to be those of an effete Melanconis sp. (with white to yellowish ectostromatic discs and ca. 3–10 discrete circinate black ostioles) colonised by a Fenestella with narrowly fusoid ascospores, which, on the other hand, considerably overlap in size with those of other material cited above; our measurements of this material correspond well to those of Phookamsak & Hyde (2015). In any case, in none of the other specimens including type material ascomata were found to be immersed in a Melanconis pseudostroma. The epitype from Austria corresponds well to the British and French materials, and the culture derived from it is used to infer the phylogenetic position of F. fenestrata.
Other specimens examined: France, SW Paris, Chaville, on dead twigs of Alnus glutinosa, Apr. 1860, L. R. Tulasne (PC 0084493). Switzerland, on dead branches of Alnus sp., autumn 1861, G. Otth (PC 0084496; as Valsa macrospora, cited in Delectum Otthianum Fungorum Thunensium, no. 33 fide Tul. & C. Tul. 1863).
Notes: In all available type and authentic materials (PC 008493, PC 008495, WU 35996) asci are only partially developed and the interior of ascomata appears white and few asci with ascospores are present, or they are over-mature and empty. Hamathecial threads with free ends were seen in all specimens, but their development is unknown, therefore it is not clear whether the term paraphyses or pseudoparaphyses applies. Direct association with Diaporthe and the thin stromatic encasement of ascomata, which is often present, may suggest that Fenestella fenestrata is fungicolous on Diaporthe. If the material of PC 0084496 is conspecific, then Melanconis is another host genus of the fungus. Study of the materials cited above has made clear that the number of ascomata per pseudostroma but also ascospore size are no good criteria to distinguish among species. We found the following variation in ascospore size (measurements include appendage cells): In the lectotype (PC 0084495) ascospores vary between (36.5–)41.5–58.5(–63.7) × (14.8–)16.4–21.7(–22.8) μm and (45.7–)53.5–67(–73) × (15.3–)19.2–25.7(–30) μm among two ascomata. In Tulasne's material (PC 0084493) ascospores vary between (37.8–)45.7–66.7(–69) × (14.2–)16.5–25.9(–27.2) μm and (45.5–)51–66(–66.5) × (18.3–)20.3–28.2(–31) μm among two ascomata. Summarized ascospore measurements of the epitype are (47.5–)53.2–63(–67.5) × (16–)19–23.3(–25) μm. As some spores may be slightly compressed in mounts and some may not be entirely mature or aberrant, the typical ascospore size is interpolated as (42–)45–65 × 17–25 μm. Appendage cells are sometimes distinctly elongated, suggesting that germination occurs preferentially at these loci. Ascospore colour is strongly dependent on development, age of the specimen, mounting medium and method of microscopy.
Neocucurbitaria Wanas. et al., Mycosphere 8(4): 408. 2017, emend. Jaklitsch & Voglmayr.
Ascomata immersed in and erumpent from bark or superficial in bark fissures on inner bark or wood, scattered or aggregated in varying groups, sometimes confluent in masses, globose, subglobose, pyriform or collapsing-discoid or turbinate, sometimes deeply cupulate, brown to black, disposed on or surrounded by subiculum; surface verruculose, warted or irregularly or radially cracked. Ascomatal apices highly variable, even within species, rounded or flat, papillate or non-papillate, sometimes with radial cracks, furrowed, stellate or irregularly tuberculate, brown, black, reddish or yellow, containing a minute central ostiolar opening. Ostiolar canal periphysate. Subiculum consisting of 2–7 μm wide, thick-walled, hyaline, greyish to dark brown hyphae usually forming loose mats, sometimes forming compact masses agglutinating ascomata. Peridium pseudoparenchymatous, usually less than 100(–120) μm thick, consisting of encrusted thick-walled, pale to blackish brown cells with encrusted pigment at the outer side, often intermingled with subicular hyphae, becoming lighter to hyaline and thinner-walled towards the inner side; sometimes ostiolar region fortified by a hyaline layer. Hamathecium formed by numerous branched, 1–3(–4) μm wide paraphyses with free ends. Asci cylindrical, oblong or subclavate, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 4–8 ascospores in uniseriate, sometimes partly biseriate arrangement. Ascospores ellipsoid, fusoid, oblong to subclavate, usually slightly constricted at the median primary septum with upper half not to distinctly enlarged, first hyaline to yellowish, pale or yellow-brown, turning dark to blackish brown at maturity, darkening in 3 % KOH, with several transverse and 1 to few longitudinal septa, end cells sometimes slightly lighter, smooth.
Asexual morph on natural hosts: Pycnidia superficial on inner bark or wood on variably developed subiculum, often scattered among ascomata or erumpent in dense fascicles through bark, globose, subglobose to collapsing discoid to cupulate, often with a minute apical papilla; peridium pseudoparenchymatous, dark brown. Surface smooth and glabrous or bearing some hyphal outgrowths. Inner side lined with hyaline cells giving rise to densely arranged phialides and simple conidiophores. Conidiophores simple, bearing lateral pegs and solitary terminal phialides. Phialides lageniform to cylindrical, straight or curved. Conidia formed on phialides and on lateral pegs of conidiophores, oblong, allantoid or ellipsoid, sometimes attenuated toward one end, 1-celled, hyaline, with 1–2 subterminal guttules, smooth.
Asexual morph in CMD culture: Pycnidia superficial on or immersed in agar, globose, conical to pulvinate or nearly cylindrical, with light rounded papilla, first usually olivaceous, darkening with time, with a light, often eccentric opening. Pycnidial wall formed by a thin t. angularis-globulosa of rather thin- to thick-walled subhyaline, olivaceous to brown cells; surface sometimes with brown hyphal appendages, lacking setae. Inner side of the peridium lined by globose to angular hyaline cells giving rise to phialides and sometimes short, simple or basally branched conidiophores. Phialides sessile and crowded on base cells or terminally on conidiophores, subglobose, broadly conical or lageniform to cylindrical, straight, curved or sigmoid. Conidia formed on phialides and pegs on the sides of conidiophores, oblong, allantoid to ellipsoid or drop-like, straight or slightly curved, 1-celled, hyaline, sometimes dilute brownish in age, typically containing 0–2, sometimes more, subterminal guttules, smooth. Probably saprotrophic on wood and bark of trees and shrubs, sometimes parasitic on human skin.
Type species: Neocucurbitaria unguis-hominis (Punith. & M.P. English) Wanas. et al.
Note: The generic description is here enlarged in order to represent the whole morphological variation shown by the members studied here.
Neocucurbitaria acanthocladae Jaklitsch & Voglmayr, sp. nov., MycoBank MB823004. Fig. 7.
Fig. 7.
Neocucurbitaria acanthocladae. A–R. Sexual morph (WU 35997). A–C. Ascomata in face view (C. ostiolar area). D. Ascoma with black furrowed ostiole. E. Obliquely disposed ascoma with yellow-brown subiculum and yellow apex. F. Ostiolar area with yellow-brown surrounding wall. G. Peridium in vertical section. H. Subicular hypha. I. Hamathecium. J–L. Asci. M. Ascus apex. N–R. Ascospores (N, R. young). S–W. Asexual morph in culture (CBS 142398 on CMD after 7–10 d at 22 °C). S. Pycnidial wall. T, U. Conidiogenous cells. V, W. Conidia. F, I–R. in 3 % KOH. Scale bars: A, B = 200 μm; C = 70 μm; D, E = 100 μm; F = 30 μm; G, J–L = 20 μm; H, I, S = 10 μm; M–R = 5 μm; T–W = 3 μm.
Etymology: For its occurrence on Genista acanthoclada.
Ascomata (157–)284–443(–471) μm (n = 26) diam, immersed in and erumpent from bark in variable groups of up to 10 individuals, very variable in size and shape, more or less globose or pyriform, brown, surrounded by cream, pale brown or yellowish to yellow-brown subiculum. Ascomatal apices (88–)115–182(–235) μm (n = 32) diam, highly variable, convex or rounded or more commonly flat and furrowed or stellate, brown, black or yellow. Subiculum sometimes forming compact masses agglutinating ascomata, consisting of ca. 2–7 μm wide, thick-walled, hyaline to pale brown hyphae encrusted by yellow particles dissolving in 3 % KOH. Peridium 20–65 μm thick, pseudoparenchymatous, consisting of thick-walled medium brown to olivaceous, (4–)6–10(–12.5) μm (n = 34) wide cells becoming lighter and thinner-walled towards the inner side; particularly in the ostiolar region often forming a golden yellow outer and a brown inner layer. Hamathecium formed by numerous branched, 1–3 μm wide paraphyses with free ends. Asci (135–)153–183(–191) × (13.2–)13.4–17.3(–19.5) μm (n = 24), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 6–8 (obliquely) uniseriately arranged ascospores. Ascospores (21–)22–27(–30.5) × (9.0–)9.8–12.2(–14.4) μm, l/w (1.9–)2.1–2.4(–2.6) (n = 51), ellipsoid, slightly constricted at the median primary septum, upper half not or scarcely enlarged, first pale to medium brown, turning dark brown, with 7–10(–11) transverse and 2–3 longitudinal septa, smooth.
Cultures and asexual morph: colony radius on CMD at 22 °C ca. 6 mm after 1 wk, 26 mm after 55 d; colony olivaceous, grey-brown to dark grey, with radial rays; odour sour-yeasty. Pycnidia 45–118 μm diam, forming after ca. 1 wk in small numbers, mostly immersed in agar, solitary, irregularly disposed; pycnidial peridium thin, formed by thick-walled, greyish-olivaceous, angular cells (4–)6.5–10.8(–12.8) μm (n = 27) diam; inner side lined by hyaline cells giving rise to conidiogenous cells. Conidiogenous cells (4.5–)5.0–6.5(–7.5) × (2.2–)2.7–4.3(–4.9) μm (n = 25), sessile, crowded, phialidic, lageniform to subglobose, often with elongated neck. Conidia (2.8–)3.3–3.8(–3.9) × (1.2–)1.3–1.5(–1.7) μm, l/w (2–)2.3–2.8(–2.9) (n = 32), 1-celled, oblong to allantoid, hyaline, containing 0–2 subterminal guttules, smooth.
Habitat: on wood and bark of Genista acanthoclada.
Distribution: Southern Europe, only known from the type location in Crete.
Holotype: Greece, Crete, Agios Ioannis, heading to Zoniana, 35° 19′ 24.7N 24° 46′ 47.2E, elev. 465 m, on branch of Genista acanthoclada, soc. Platystomum sp., 8 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 35997; ex-holotype culture CBS 142398 = C225).
Notes: Unlike any other species of Neocucurbitaria, N. acanthocladae is characterised by a light coloured peridium and yellow stellate ostioles. The latter are to some extent shared with N. aetnensis, which differs by host, black ascomata tending to be more erumpent and ascospores with different septation. The phylogenetically related N. cinereae has also different ascospore characters. These three species occur on Genista spp.
Neocucurbitaria acerina Wanas. et al., Mycosphere 8(4): 410. 2017. Fig. 8.
Fig. 8.
Neocucurbitaria acerina. A–N. Sexual morph. A–C. Ascomata in face view. D. Peridium in vertical section. E. Ascus apex. F–K. Ascospores. L–N. Asci. O–T. Asexual morph in culture (CMD, after 4–7 d at 22 °C). O. Pycnidia. P. Pycnidial wall. Q. Pycnidial appendages. R. Conidiogenous cells. S, T. Conidia. D–G, L–N, P–R. in 3 % KOH. A–N, P, Q, S, T. WU 35998/CBS 142403; O, R. C26a. Scale bars: A, B = 150 μm; C = 100 μm; D, L–N = 10 μm; E, G, P, R = 5 μm; F, H–K, Q = 7 μm; O = 100 μm; S, T = 3 μm.
Ascomata (177–)202–338(–410) μm (n = 20) diam and high, scattered or variably aggregated in small groups, immersed below bark epidermis, becoming free upon shredding of the bark, with bases usually immersed, sitting on an inconspicuous subiculum of ca. 2–5 μm wide, thick-walled brown hyphae also originating at ascomatal sides, becoming seta-like near the ostiolum; black, more or less globose with rounded apical papilla (43–)53–95(–110) μm diam (n = 15), collapsing from the sides or base; surface verruculose to nearly smooth. Peridium ca. 20–60 μm thick, of a thick-walled dark brown t. angularis becoming lighter and thinner-walled towards inner side, formed by (3.5–)5–9.5(–12) μm (n = 47) long cells; pigment in outer cell layers coarsely encrusted; near the ostiolum inside fortified by a hyaline t. angularis. Hamathecium formed by a dense tissue of numerous richly branched, 1–3.5 μm wide paraphyses with free ends. Asci (95–)103–136(–157) × (10.5–)11.7–13.5(–14.7) μm (n = 32), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 8 uni- partly biseriately arranged ascospores. Ascospores (14–)17.5–22.4(–25.8) × (5.6–)6.5–7.7(–8.7) μm, l/w (2.2–)2.6–3.1(–3.6) (n = 64), fusoid-oblong-subclavate, slightly constricted at the median primary septum, upper half slightly enlarged, first yellow-brown, soon turning dark brown, with (5–)6–8(–9) transverse and 1–2 longitudinal septa, smooth.
Pycnidia scattered on bark, 60–150 μm diam, subglobose to collapsing discoid.
Cultures and asexual morph in culture (on CMD after 4–7 d at 22 °C): colony radius 8–11 mm after 1 wk, 14–18 mm after 2 wk; mycelium dense, first hyaline, turning greyish-olivaceous or dark grey-brown, finally black; long aerial hyphae forming strands; odour indistinct to slightly unpleasant. Pycnidia (43–)53–115(–141) μm (n = 20) diam, formed after a few days, numerous, scattered to aggregated, globose to pulvinate, olivaceous, turning black within a week. Pycnidial wall formed by a coarse t. angularis of rather thin-walled, (3.5–)5–10(–12.5) μm (n = 30) long cells; surface with brown hyphal appendages; inner side lined by hyaline cells giving rise to conidiogenous cells. Conidiogenous cells sessile, crowded, phialidic, very variable, lageniform to subglobose, (4.0–)5.0–7.5(–9.5) × (1.6–)1.7–3.9(–5.3) μm. Conidia (2.2–)2.5–3.3(–4.0) × (0.9–)1.1–1.5(–1.9) μm, l/w (1.6–)2–2.6(–3) (n = 84), 1-celled, oblong to narrowly ellipsoid, straight or slightly curved, hyaline, typically containing 2 subterminal guttules, smooth.
Habitat: on wood and bark of Acer spp., known from A. campestre and A. pseudoplatanus.
Distribution: Europe (Austria, Italy).
Material examined: Austria, Niederösterreich, Lunz-Mittersee, on dead bark of a standing trunk of Acer pseudoplatanus, 10 May 2016, H. Voglmayr (WU 35998; culture CBS 142403 = C255). Vienna, Strebersdorf, Krottenhofgasse, on a dead, partly decorticated branch of Acer pseudoplatanus, asexual morph, soc. Parafenestella pseudoplatani, 17 Nov. 2013, W. Jaklitsch (WU 35999; culture C26a from conidia).
Notes: Apparently there is no old name of Cucurbitaria or Fenestella described from Acer that may be used for this species. The following types were examined or information was gathered: In the holotype of Cucurbitaria acerina Fuckel (G 00266375(!), from Herbier Fuckel 1894 in Herbier Barbey Boissier), collected by Fuckel on Acer campestre in Germany, ascomata are immersed in rows and erumpent from bark, they are depressed subglobose, non-papillate, contain hamathecium of branched 1–3 μm wide threads, 6–8-spored, cylindrical, fissitunicate asci ca. 154 × 15 μm, with spores uniseriate, partly biseriate in the middle. Ascospores are (18–)21–25(–26.5) × (8–)8.7–10.2(–11.3) μm, l/w (1.9–)2.2–2.7(–3.2) (n = 41), narrowly ellipsoid to fusoid, pale to medium brown, with 3–7 transverse and 1(–2) longitudinal septa; in 3 % KOH slightly darker, greyish brown, with more distinct, dark, conspicuous septa, distinctly constricted at the median septum, upper part slightly wider. Ascospore characteristics like shape, light colour, usually less and more distantly set septa, which are darker and thicker than the wall, suggest that C. acerina is different from N. acerina.
Cucurbitaria protracta Fuckel (holotype G 00266414!, from Herbier Fuckel 1894), collected from thin twigs of Acer campestre, is very similar to C. acerina and possibly only a form of that species. It differs from the latter by slightly smaller ascospores, (15.4–)19–23(–24) × (6.5–)7.7–9.7(–10.6) μm, l/w (2–)2.2–2.7(–3.1) (n = 47), which have usually only 3 transverse septa and one longitudinal septum, but may have up to 6 transverse septa; they are light to medium brown, with thick and dark septa.
Cucurbitaria homalea (Fr.) Sacc., basionym Melogramma homaleum Fr., described from Acer pseudoplatanus, is a thyridaria-like fungus, according to our examination of type material in UPS.
No type material of Cucurbitaria negundinis G. Winter, described from Acer negundo, has been located. According to Mirza (1968) the species is characterised by larger, esp. broader ascospores (26–37 × 9–12 μm or 20–26 × 9–11 μm, from 2 specimens) having 3–7 (mostly 3, 4 or 5) transverse and 1 longitudinal septa, and camarosporium-like conidia associated on the natural host may point to a position outside the Cucurbitariaceae.
Fenestella frit (Fr.) Sacc., based on Sphaeria frit Fr., also described from Acer negundo: Fries (1823) cited the specimen Scleromyc. Suec. Exs. 227 labelled Sphaeria coronata in UPS in the protologue of Sphaeria frit. It contains empty perithecia with cylindrical necks. It is thus no cucurbitariaceous fungus, and no further interpretation is possible. Another collection labelled as Sphaeria frit by Fries is extant in UPS, but it is likely a later collection, i.e., no type material. According to the description by Currey (1859), Fenestella mougeotii (Pers. ex Curr.) Sacc., described from Acer pseudoplatanus in France, may match N. acerina, but the name is illegitimate due to the sanctioned name Sphaeria mougeotii Fr., the basionym of Sphaeronaemella mougeotii (Fr.) Sacc.
Neocucurbitaria aetnensis Jaklitsch & Voglmayr, sp. nov., MycoBank MB823005. Fig. 9.
Fig. 9.
Neocucurbitaria aetnensis. A–Q. Sexual morph. A–C. Ascomata in face view. D. Ascomata in vertical section. E. Peridium and a subicular hypha in vertical section. F. Part of ostiole with periphyses. G. Ascus apex. H–J. Asci. K–Q. Ascospores (K. young). R–W. Asexual morph in culture (CMD, after 6–7 d at 22 °C). R. Pycnidia. S. Pycnidial wall. T, U. Conidiogenous cells. V, W. Conidia. H–Q. in 3 % KOH. A, B, D–P, T. WU 36929/CBS 142404; C, Q–S, U–W. WU 36930/C270. Scale bars: A–D = 200 μm; E, H–J = 15 μm; F = 10 μm; G, Q, S, T = 5 μm; K–P = 7 μm; R = 70 μm; U–W = 3 μm.
Etymology: As it has been only found on the volcano Etna, on Genista aetnensis.
Ascomata (195–)240–393(–513) μm (n = 41) diam, immersed in bark, erumpent through fissures, crowded in small or large groups, or solitary, globose, subglobose or pyriform, sometimes collapsing at the sides, with rounded, furrowed or tuberculate, yellow, reddish or black apical papilla (62–)99–186(–212) μm (n = 30) diam, or with radial cracks or non-papillate; ostiole periphysate. Surface grey to black, verruculose. Peridium 22–120 μm thick, pseudoparenchymatous, composed of 2–3 layers of (4–)6–10.5(–15.3) μm (n = 41) long cells, hyaline and thin-walled in a narrow inner layer, pale (greyish-) brown to subhyaline and thin- to moderately thick-walled in a broad middle layer and dark brown to opaque, thick-walled and intermingled with subicular hyphae in narrow outer layer partly tending to be thicker toward the base; pigment encrusted. Basal and lateral subiculum consisting of 2–7 μm wide, thick-walled dark brown hyphae. Hamathecium consisting of numerous branched, 1–3 μm wide paraphyses with free ends. Asci (114–)125–154(–182) × (13.7–)14.5–17.5(–19) μm (n = 19), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 6–8 ascospores in (obliquely) uniseriate arrangement. Ascospores (19.3–)20.3–23.6(–26.3) × (8.3–)9–10.5(–11) μm, l/w (1.9–)2.1–2.4(–2.5) (n = 50), ellipsoid with broadly rounded ends, slightly constricted at the median primary septum, upper half sometimes slightly enlarged, medium to dark brown, with 5–7 transverse and 1–2 longitudinal septa, smooth.
Pycnidia co-occurring with ascomata, 45–105 μm diam, globose to collapsing-cupulate, shiny black, finely papillate.
Cultures and asexual morph: Colony radius 20 mm on CMD after 1 mo at 22 °C; colony dense, first greyish, turning dark olivaceous brown to nearly black with hyaline or olivaceous margin, more or less zonate, with radial grooves, sometimes nearly stellate, white crystals forming on the surface; odour indistinct. Pycnidia (after 6 d) 38–147 μm diam, forming within a few days in the centre and/or at the colony margin, immersed to nearly superficial, densely but singly disposed or in small groups, more or less globose, olivaceous to nearly black, usually surrounded by brown, often submoniliform hyphae; conidia emerging in whitish drops. Peridium consisting of a thin t. angularis-globulosa of moderately thick-walled, olivaceous to subhyaline, guttulate cells (4–)5–9(–11) μm (n = 50) diam; inner side lined by hyaline cells giving rise to phialides. Phialides (3.2–)3.8–6.2(–7.7) × (1.9–)2.1–4(–5.7) μm (n = 36), sessile, crowded, lageniform to globose. Conidia (2.8–)3–3.7(–4.2) × (1.1–)1.3–1.7(–2.1) μm, l/w (1.7–)1.9–2.6(–3) (n = 58), 1-celled, oblong, straight or slightly curved, hyaline, partly becoming dilute brownish when aged, containing 0–2, sometimes more, subterminal guttules, smooth.
Habitat: On wood and bark of Genista aetnensis.
Distribution: Europe, Italy, only known from the type location.
Holotype: Italy, Sicily, Etna, east side, near Zafferana Etnea, on corticated, 1.5–2.5 cm thick branches of Genista aetnensis, 17 Jun. 2016, W. Jaklitsch & H. Voglmayr (WU 36929; ex-holotype culture CBS 142404 = C261).
Other material examined: Italy, Sicily, Etna, north side, on branch of Genista aetnensis, 18 Jun. 2016, W. Jaklitsch & H. Voglmayr (WU 36930; culture C270).
Notes: For distinction from N. acanthocladae see notes under this species. Neocucurbitaria cinereae differs, e.g., by smaller ascospores from N. aetnensis.
Neocucurbitaria cinereae Jaklitsch & Voglmayr, sp. nov., MycoBank MB823006. Fig. 10.
Fig. 10.
Neocucurbitaria cinereae. A–P. Sexual morph (WU 36931). A, B. Ascomata in face view. C. Ascoma in vertical section. D. Peridium in vertical section. E. Apex of immature ascus. F. Hamathecium. G–J. Asci (G. immature). K–P. Ascospores (K, L. young). Q–U. Asexual morph in culture (CBS 142406 on CMD after 5–6 d at 22 °C). Q. Pycnidia. R. Pycnidial wall. S. Conidiogenous cells. T, U. Conidia. E–J, L–P. in 3 % KOH. Scale bars: A, B, Q = 250 μm; C = 100 μm; D, F, R–T = 7 μm; E, U = 3 μm; G–J = 15 μm; K–P = 5 μm.
Etymology: For its occurrence on Genista cinerea.
Ascomata (162–)195–305(–338) μm (n = 23) diam, 160–440 μm high, erumpent from bark, aggregated in large groups on a subiculum or scattered, globose, subglobose or pyriform, sometimes vertically elongated, black, with verruculose surface; apex usually distinctly papillate, (58–)68–106(–132) μm (n = 20) diam, rounded to irregularly warted, reddish, dark brown or black, sometimes finely cracked; ostiole periphysate. Peridium 25–85 μm thick, consisting of a thick-walled, dark brown and heavily encrusted outer t. angularis and a rather thin-walled hyaline inner layer, formed by cells (2.8–)4.8–9.3(–12.5) μm (n = 75) diam. Basal subiculum consisting of 3–6 μm wide, thick-walled, light to dark brown hyphae. Hamathecium consisting of numerous, branched and anastomosing, 1–3(–4) μm wide paraphyses. Asci (115–)118–130(–135) × (11.5–)12–14(–14.8) μm (n = 16), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 8, rarely 4 ascospores in (obliquely) uniseriate arrangement. Ascospores (17–)17.8–19.3(–20.5) × (7.2–)8–9(–10) μm, l/w (2–)2.1–2.3(–2.6) (n = 47), ellipsoid, slightly constricted at the median primary septum, upper half slightly enlarged, first hyaline with 1–3 septa, turning pale brown and eventually dark brown, with (3–)5–7 transverse and 1–2 longitudinal septa, smooth.
Pycnidia co-occurring with ascomata, 44–103 μm diam, globose, shiny black, finely papillate.
Cultures and asexual morph: Colony radius 9 mm after 5 d on CMD at 22 °C; mycelium first colourless, turning olivaceous to greyish brown, later dark olivaceous to nearly black with radially disposed spots; odour slightly unpleasant. Pycnidia 54–141 μm diam, formed within a few days, aggregated in groups of up to 10, turning dark olivaceous to nearly black, more or less globose, fusing laterally, papillate. Pycnidial wall thin, formed by thick-walled, olivaceous brown, angular to globose or ellipsoid cells (3.7–)5.5–9.5(–11.5) μm (n = 37) diam; inner side lined by hyaline cells giving rise to conidiogenous cells. Conidiogenous cells (3–)4–6.6(–7.8) × (2–)2.3–4(–4.6) μm (n = 21), sessile, crowded, phialidic, broadly conical, subglobose or lageniform. Conidia (3–)3.5–4.3(–4.8) × (1.4–)1.6–2(–2.5) μm, l/w (1.6–)1.9–2.5(–2.8) (n = 48), 1-celled, oblong, sometimes attenuated toward one end, hyaline, containing 2 or more minute guttules, smooth.
Habitat: In bark of Genista cinerea.
Distribution: Europe, Spain, only known from the type locality.
Holotype: Spain, Andalusia, Granada, near Montefrío, 37°20′54″ N, 4°1′8″ W, elev. 785 m, on partly decorticated twig of Genista cinerea, soc. Diaporthe sp., 11 May 2014, W. Jaklitsch (WU 36931; ex-holotype culture CBS 142406 = KU9).
Notes: Neocucurbitaria cinereae, the third species of this genus described from a Genista, differs from N. acanthocladae and N. aetnensis by smaller ascospores and from the former also by ascospore septation. Ascomata of N. cinereae tend to occur in large groups and they only rarely have yellowish apices, which are not stellate.
Neocucurbitaria cisticola Jaklitsch & Voglmayr, sp. nov., MycoBank MB823047. Fig. 11.
Fig. 11.
Neocucurbitaria cisticola. A–L. Sexual morph (WU 36932). A, B. Ascomata in face view. C. Peridium in vertical section. D. Ascoma in vertical section. E. Apex of young ascus. F–I. Ascospores. J–L. Asci. M–S. Asexual morph in culture (CBS 142402 on CMD after 5 d at 22 °C). M. Pycnidia with conidial drops. N. Pycnidial wall. O, P. Conidiogenous cells. Q–S. Conidia. E–L, Q. in 3 % KOH. Scale bars: A, B = 100 μm; C = 10 μm; D, M = 70 μm; E–I, N–P = 5 μm; J–L = 15 μm; Q–S = 3 μm.
Etymology: For its occurrence on Cistus.
Ascomata (147–)195–310(–372) μm (n = 24) diam, scattered or aggregated in small groups below the host epidermis on a subiculum, erumpent through bark fissures, subglobose to pyriform, collapsing at the sides, with a distinct shiny apical papilla (44–)52–83(–88) μm (n = 16) diam, the latter flattened at the top and circular or angular in outline. Surface grey to black, verrucose. Subiculum consisting of 2–7 μm wide, thick-walled, dark brown hyphae. Peridium 15–55 μm thick, consisting of a thick-walled t. angularis, dark brown to opaque outside, becoming lighter (to subhyaline in the ostiolar region) and thinner-walled towards inner side, formed by cells (4–)5–9(–12) μm (n = 30) diam. Hamathecium formed by numerous, richly branched, 1–2.5(–3) μm wide paraphyses with numerous free ends. Asci (115–)130–159(–166) × (14.3–)15–17(–18) μm (n = 18), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 8 obliquely uniseriately arranged ascospores. Ascospores (20–)21.3–24.3(–26.3) × (9–)9.7–10.8(–11.4) μm, l/w (2–)2.1–2.4(–2.7) (n = 45), fusoid to ellipsoid, often with pointed and paler ends, constricted at the median primary septum, upper half slightly enlarged, pale brown when young, turning dark (reddish) brown, with 6–7 transverse and 1–3 longitudinal septa, smooth.
Cultures and asexual morph: Colony radius on CMD 26 mm after 24 d at 22 °C; colony circular, first hyaline, turning pale dull brown with greyish dots in the centre, odour indistinct. Pycnidia (after 5 d) 58–147 μm diam, forming within a few days, scattered, more or less globose, first hyaline, turning olivaceous; conidia emerging in whitish drops. Peridium consisting of a thin t. globulosa-angularis of moderately thick-walled, (4.3–)6.8–11.8(–14.2) μm (n = 34) wide cells; inner side lined by hyaline cells giving rise to phialides and short 1–2-celled conidiophores. Phialides (4.5–)5.2–8.2(–10) × (1.8–)2.1–4.3(–6.3) μm (n = 30), sessile, crowded, or solitary terminally on conidiophores, lageniform to subglobose. Conidia formed on phialides and lateral pegs on conidiophores, (2.9–)3.2–4(–4.5) × (1.1–)1.3–1.6(–1.9) μm, l/w (2–)2.3–2.9(–3.6) (n = 40), 1-celled, oblong, straight or slightly curved, hyaline, containing 0–2 subterminal guttules, smooth.
Habitat: On wood and bark of Cistus monspeliensis.
Distribution: Spain, La Gomera, only known from the type location.
Holotype: Spain, La Gomera, SE Vallehermoso, at the Mirador de Alojera, on a twig of Cistus monspeliensis, 23 Mar. 2016, H. Voglmayr (WU 36932; ex-holotype culture CBS 142402 = C244).
Notes: No Cucurbitaria or Pyrenochaeta has been described from Cistus. Phoma cisti Brunaud, described from Cistus salvifolius in France, has minute subovoid conidia to 3 μm long (Saccardo 1892, p. 153), while conidia of P. cistina, described from Cistus laurifolius in Kew, England, are larger, 6–7 × 2.5 μm. The latter was combined in Phomopsis by Grove (1917).
Neocucurbitaria juglandicola Jaklitsch & Voglmayr, sp. nov., MycoBank MB823007. Fig. 12.
Fig. 12.
Neocucurbitaria juglandicola (A–S) and Cucurbitaria juglandis (T). A–J. Sexual morph (WU 36933). A. Ascomata in face and lateral view. B–G. Ascospores. H–J. Asci. K–N. Asexual morph on natural substrate (WU 36933). K. Pycnidia in face view. L. Peridium. M. Conidiophores and phialides. N. Conidia. O–S. Asexual morph in culture (CBS 142390 on CMD after 4–6 d at 22 °C). O. Pycnidia. P. Peridium. Q. Conidiogenous cells. R, S. Conidia. T. Conidia from Cucurbitaria juglandis type material G00266380 (in 3 % KOH). Scale bars: A, K, O = 100 μm; B–G, L, P = 7 μm; H–J, M = 10 μm; N, R–T = 3 μm; Q = 5 μm.
Etymology: For its occurrence on Juglans.
Ascomata (177–)180–260(–320) μm diam (n = 12), (177–)192–246(–265) μm high (n = 8), immersed in bark, becoming visible in bark fissures, scattered or aggregated in small groups, depressed globose to pyriform, with or without a rounded apical papilla, black, with verruculose to nearly smooth surface, basally and laterally surrounded by subiculum of ca. 2–5 μm wide, thick-walled brown hyphae. Peridium to ca. 60 μm thick, consisting of a thick-walled dark brown t. angularis becoming lighter and thinner-walled towards inner side, formed by (4–)5–9.5(–11) μm (n = 20) long cells. Hamathecium formed by branched, 1–3 μm wide paraphyses. Asci (79–)95–124(–133) × (11.7–)13–16.7(–17) μm (n = 14), oblong, bitunicate, fissitunicate, with a distinct ocular chamber, short stipe and a simple or knob-like base, containing 8 uni- partly biseriately arranged ascospores. Ascospores (15.5–)17.7–21.5(–26.8) × (8.0–)8.8–10.2(–11.0) μm, l/w (1.8–)1.9–2.2(–2.6) (n = 61), ellipsoid, straight or slightly curved, slightly constricted at the median primary septum, upper half often slightly enlarged, first pale brown, turning medium to dark brown, with 5–7(–8) transverse and 1–2 longitudinal septa, smooth.
Asexual morph on the natural host: Pycnidia (53–)64–115(–142) μm diam (n = 16), scattered below the host epidermis on subicular hyphae, also in association with ascomata, globose, sometimes with a blunt rounded apical papilla. Peridium consisting of a thin brown t. angularis of (3–)4.5–7(–7.5) μm (n = 30) long cells, lined at the interior with a layer of hyaline angular to rounded cells. Phialides lageniform to cylindrical, (4.3–)5.0–6.8(–7.2) × (1.4–)1.7–2.3(–2.5) μm (n = 14), formed on the hyaline inner cells or singly terminally on short, to ca. 30 μm long, simple, 1–3 celled conidiophores. Conidia formed on phialides and on lateral pegs on conidiophores, (2.2–)2.5–3.0(–3.2) × (1.3–)1.4–1.5(–1.6) μm, l/w (1.5–)1.6–2(–2.2) (n = 12), 1-celled, oblong to ellipsoid, hyaline, smooth.
Cultures and asexual morph in culture: Colony radius on CMD at 22 °C 27 mm after 1 mo, mycelium zonate, dense, first pale greyish, dull dark brown with numerous pycnidial dots in the centre, margin uneven; odour indistinct. Pycnidia 40–90 μm diam, forming in the centre within a few days, immersed in agar or superficial, scattered or aggregated in small numbers, green-olivaceous to black, globose to nearly cylindrical, with a light, often eccentric opening; conidial drops white to greyish-brown. Pycnidial peridium thin, consisting of a thick-walled t. angularis-prismatica of (4–)6–11(–14.5) μm (n = 32) long cells with encrusted olivaceous pigment; inner side lined by hyaline cells giving rise to conidiogenous cells. Conidiogenous cells sessile, crowded, mostly subglobose, also lageniform, (3.5–)4.5–6.2(–6.5) × (1.8–)2–4(–6) μm (n = 18). Conidia (2.5–)3.3–4.1(–4.5) × (1.1–)1.2–1.5(–2.2) μm, l/w (1.9–)2.4–3.1(–3.6) (n = 45), 1-celled, oblong to allantoid or sigmoid, hyaline, containing 0–2 subterminal guttules, smooth.
Habitat: On bark of Juglans regia.
Distribution: Only known from the type location in Vienna, Austria.
Holotype: Austria, Vienna 22nd district, at AGES, Spargelfeldstraße 191, on twig of Juglans regia, soc. Cytospora sp., 13 Feb. 2015, R. Moosbeckhofer, comm. B. Wergen (WU 36933; ex-holotype culture CBS 142390 = BW6).
Notes: This species is apparently different from Cucurbitaria juglandis. Type material in G (G00266380) does not contain any sexual morph but plenty of two fungi, which Fuckel regarded as asexual states of C. juglandis: many conspicuous pustules of a Diplodia, and a fungus with 1-celled hyaline conidia. The latter are produced on phialides (5.7–)6.5–10(–10.8) × 2–3.5 μm (n = 6) and measure (3.5–)4–5(–6) × (2–)2.2–2.7(–3.1) μm, l/w (1.5–)1.7–2.2(–2.5) (n = 24) and thus differ from conidia of N. juglandicola by shape and size. On the herbarium label a nearly ellipsoid ascus with biseriate spore arrangement and ascospores are described, which are pale yellow, measure 26–28 × 8–10 μm, have 6–8 transverse septa and are muriform, which agrees with Fuckel (1871) but does not suggest that the fungus belongs to Cucurbitaria. Phylogenetically N. juglandicola is close to but distinct from N. cava (cf. Fig. 1, Fig. 2 and Valenzuela-Lopez et al. 2018). It should be noted that branch length of N. cava in Fig. 2 is certainly significantly underestimated due to the lack of the phylogenetically highly informative tef1 and much shorter tub2 sequences (333 vs. 673 bp) available for N. cava.
Neocucurbitaria populi Jaklitsch & Voglmayr, sp. nov., MycoBank MB823008. Fig. 13.
Fig. 13.
Neocucurbitaria populi. A–Q. Sexual morph (WU 36934). A, B. Ascomata in face view. C. Ascoma in vertical section. D. Peridium in vertical section. E. Ascus apex. F. Subiculum. G, L–Q. Ascospores. H–K. Asci. R–Z. Asexual morph in culture (CBS 142393 on CMD after 5 d at 22 °C). R. Pycnidia (left with conidial drop). S, W–Z. Conidia. T. Pycnidial wall and conidiogenous cells. U, V. Conidiogenous cells. F–Q. in 3 % KOH. Scale bars: A, B = 500 μm; C, R = 100 μm; D, F = 10 μm; E, G, U, Y, Z = 5 μm; H–K = 15 μm; L–Q, S, T, V–X = 7 μm.
Etymology: For its occurrence on Populus.
Ascomata (200–)215–347(–405) μm (n = 20) diam, ca. 250–350 μm high, densely aggregated in numbers of up to ca. 30 in pustules erumpent from bark, on ample subiculum between and below them, more or less globose to pyriform, sometimes laterally collapsing, later with a distinct apical papilla (44–)58–101(–133) μm (n = 24) diam. Surface black, verruculose to nearly smooth. Subiculum consisting of ca. 2–6 μm wide, thick-walled olive-brown hyphae penetrating bark and wood. Peridium 20–60 μm thick, consisting of a thick-walled dark brown t. angularis becoming lighter and thinner-walled towards inner side, formed by cells (3.5–)5–9.5(–12) μm (n = 35) diam. Hamathecium formed by numerous branched, 1–3 μm wide paraphyses. Asci (120–)130–159(–178) × (15.8–)16.5–19.5(–22.2) μm (n = 24), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 8 (obliquely) uniseriately arranged ascospores. Ascospores (21.4–)22.3–28.5(–32.2) × (9.5–)10.5–13.5(–15.5) μm, l/w (1.8–)1.9–2.3(–2.6) (n = 37), ellipsoid, slightly constricted at the median primary septum, upper half often slightly enlarged, first yellow- to golden brown, soon turning dark to blackish brown, with (5–)7–8(–10) transverse and 2–4 longitudinal septa, smooth.
Cultures and asexual morph: Colonies on CMD at 22 °C reaching a growth radius of ca. 15 mm after 1 mo, mycelium soon turning brown, finally black, odour indistinct. Pycnidia ca. 73–205 μm diam, forming after a few days, partly immersed in agar, dark olivaceous to black, globose, longish, sometimes fusing, with central ostiole, papillate or not, often covered by aerial hyphae. Pycnidial peridium thin, consisting of a coarse, thick-walled, dark brown t. angularis of (4–)5.5–10(–11.5) μm (n = 20) long cells; inner side lined by hyaline cells giving rise to conidiogenous cells. Conidiogenous cells sessile, crowded, phialidic, lageniform to subglobose, (3.2–)4.3–7.2(–8.2) × (1.7–)2.3–4(–5) μm (n = 34). Conidia (2.8–)3.0–3.8(–4.8) × (1.1–)1.3–1.6(–1.9) μm, l/w (1.8–)2.1–2.8(–3.2) (n = 45), 1-celled, oblong to allantoid, hyaline, containing 0–2 subterminal guttules, smooth.
Habitat: On wood and bark of Populus sp.
Distribution: Northern Europe, only known from the type location in Sweden.
Holotype: Sweden, Skåne, Åhus, Ripa, Motocrossbanan, on branch of cultivated Populus sp., 3 Mar. 2013, S.-Å. Hanson Herb SÅH 2013-020 (WU 36934; ex-holotype culture CBS 142393 = C28).
Notes: Yellow particles on ascomata in Fig. 13A, B are parts of a lichen. Two species of Cucurbitaria have been described from Populus. One is C. populina (Bacc. & P. Avetta) Rehm from Populus nigra in Italy (Rehm in Schnabl 1892). This is a younger homonym of C. populina (Pers.) Quél. (Quélet 1883) and therefore illegitimate and unavailable. The latter belongs to Cytospora according to Species Fungorum. The second one is C. staphula Dearn. ex R.H. Arnold & R.C. Russell from Populus balsamifera, P. trichocarpa and P. tremuloides in North America. Cucurbitaria staphula differs from Neocucurbitaria populi by larger, biseriately arranged ascospores of different shape (lower end extended and often pointed), a different asexual morph (Macrophoma tumefaciens) and by occurrence on galls (Arnold and Russell, 1960, Arnold, 1974). The phylogenetically closely related N. juglandicola differs from N. populi in smaller ascospores having only 1–2 longitudinal septa, and by 33 and 31 nucleotide substitutions in tef1 and rpb2, respectively.
Neocucurbitaria rhamni (Nees : Fr.) Jaklitsch & Voglmayr, comb. nov., MycoBank MB823009. Fig. 14.
Fig. 14.
Neocucurbitaria rhamni. A–D. Ascomata in face view. E, F. Peridium in vertical section (inner layer in F). G, H. Asci. I. Ascus apex showing ocular chamber. J–P. Ascospores. Q–Z. Asexual morph on the natural hosts. Q, R. Pycnidia. S. Pycnidial wall in section. T–X. Conidiophores and phialides. Y, Z. Conidia. A1–F1. Asexual morph in culture at 22 °C (A1–E1. after 7 d on SNA; F1. after 20 d on MEA). A1–C1. Conidiophores and phialides. D1–F1. Conidia. M–P, S–F1. in 3 % KOH. A, D, A1–F1. WU 36936/C112; B, Q, Y, Z. WU 36944; C, M. Lectotype B 700016439; E–L. WU 36941; N–P, R–V. WU 36935; W, X. WU 36939. Scale bars: A, B = 300 μm; C, D, Q, R = 150 μm; E–H, S = 15 μm; I, U, V, X, Y, A1–C1 = 5 μm; J–P, T = 7 μm; W, Z, D1–F1 = 3 μm.
Basionym: Sphaeria rhamni Nees, Syst. Pilze (Würzburg): 299. 1817.
Synonyms: Sphaeria rhamni Nees : Fr., Syst. Myc. 2: 417. 1823.
Cucurbitaria rhamni (Nees : Fr.) Fuckel, Jb. nassau. Ver. Naturk. 23–24: 174. 1870(1869–1870).
Ascomata (200–)268–432(–676) μm diam (n = 149), (90–)120–222(–333) μm high (n = 82), aggregated in highly variable groups of 2 to more than 200, or scattered singly on an inconspicuous to well-developed subiculum of olivaceous- to dark-brown, thick-walled, 2–6.5 μm wide hyphae, erumpent to superficial on wood and bark, mostly becoming visible in bark fissures, first subglobose, soon collapsing at the top to become turbinate or discoid, shiny black, carbonaceous, surface irregularly and coarsely tubercular. Ostiolar openings usually inconspicuous, whitish, or surrounded by a reddish to blackish papilla (39–)57–83(–92) μm diam (n = 18) delimited by a narrow concentric depression. Peridium (38–)49–83(–110) μm thick (n = 46), pseudoparenchymatous, consisting of a dark brown to nearly black outer layer of thick-walled incrusted cells and a broad pale brown to hyaline thinner-walled inner layer; peridial cells (3–)5–9.5(–15.5) μm diam (n = 106). Hamathecium of 1.5–4.5 wide, branched ?paraphyses. Asci (115–)118–152(–162) × (12.0–)12.3–14.8(–16.1) μm (n = 15), cylindrical to subclavate, bitunicate, fissitunicate, with a short stipe and a knob-like base, narrow walls with endotunica thickened at the apex and a distinct ocular chamber, containing 4–8 uniseriately arranged ascospores. Ascospores (15.7–)18–22(–24.8) × (7.2–)8.2–9.8(–12) μm, l/w (1.7–)2.0–2.5(–2.8) (n = 87), fusoid to ellipsoid, straight, with obtuse to subacute ends, upper part often broader, with 3–7 transverse and 1(–2) longitudinal septa, golden to dark brown, end cells often paler, constricted at the primary septum, less at other septa, smooth.
Asexual morph on the natural host: Pycnidia (50–)69–122(–157) μm diam (n = 56), scattered on variably developed subiculum of 2.5–6 μm wide brown hyphae on wood, accompanying ascomata, or erumpent in dense fascicles through bark, globose, sometimes collapsing becoming cupulate, with a small papilla, black, smooth and glabrous. Peridium dark brown, consisting of cells (4–)6–10(–12) μm diam (n = 30) forming t. angularis to t. prismatica, lined inside by a layer of hyaline cells giving rise to densely arranged conidiophores or phialides. Conidiophores simple, up to ca. 35 μm long, 1–3-celled, with solitary terminal phialides and lateral pegs typically present at the upper end of conidiophore cells. Phialides (4.8–)5.5–8.0(–9.5) × (2.0–)2.1–2.7(–3.2) μm, l/w (2.2–)2.3–3.5(–4.5) (n = 28), lageniform to subcylindrical. Conidia (2.5–)2.8–3.3(–3.5) × (1.3–)1.4–1.7(–2.1) μm, l/w (1.4–)1.7–2.2(–2.5) (n = 65), ellipsoid, oblong, often attenuated toward one end (drop-like), 1-celled, hyaline, containing 1–2 guttules, smooth.
Cultures and asexual morph in culture: Colony radius 33–45 mm after 1 mo on CMD at 22 °C; colony dense, dark olivaceous brown, usually with conspicuous radial texture, aerial hyphae and odour indistinct. Pycnidia mainly formed in the centre. On SNA after 1 wk at 22 °C pycnidia ca. 100–160 μm diam, more or less superficial on agar concentrated in the colony centre; peridium thin, forming a t. angularis of (olivaceous) brown cells; setae lacking. Phialides formed on conidiophores arising from an inner layer of hyaline pseudoparenchymatous cells or formed directly on the latter. Conidiophores simple or with sparse single branches. Phialides (4.5–)5.8–8.4(–8.7) × (1.7–)2.0–2.6(–2.8) μm, l/w (2.0–)2.3–4.2(–5.1) (n = 12), lageniform to cylindrical. Conidia (2.5–)2.7–3.3(–3.7) × 1.5–1.7(–1.9) μm, l/w (1.5–)1.7–2.1(–2.3) (n = 31), oblong or ellipsoid, often attenuated toward one end. On MEA after 3 wk conidia becoming slightly larger and inflated, (2.5–)3.0–4.0(–5.0) × (1.7–)2.0–2.5(–2.9) μm, l/w (1.2–)1.3–1.9(–2.4) (n = 21); also phialides becoming inflated to subglobose.
Hosts: On dead attached or broken branches of Rhamnus frangula, R. cathartica, and R. saxatilis, typically near attachment areas of thin dead branches or twigs; also reported from R. alpina (Mirza 1968).
Distribution: Europe, also reported from Canada on Rhamnus cathartica (Barr 1990a).
Typification: In the herbarium B two specimens are extant, which have the same handwriting of T.F.L. Nees von Esenbeck, but do not bear any collection data. Sphaeria rhamni B 70 0016438! contains a few stromata of Diaporthe fibrosa on Rhamnus cathartica. However, B 70 0016439! bears the script “Sphaeria rhamni mihi” as given in the protologue, indicating that this is the material, which Nees von Esenbeck himself identified as his taxon Sphaeria rhamni and likely used as the original material for the description of his taxon. We therefore here designate B 70 0016439 as lectotype of Sphaeria rhamni (MBT378885). The material agrees with the current concept of Cucurbitaria rhamni, as it contains coarsely tuberculate, more or less discoid ascomata both scattered and in loose or dense groups; more than 100 ascomata are present on the smaller piece of the specimen. As asci are only present in fragments and there are more than one species on Rhamnus spp., we here epitypify Sphaeria rhamni. Epitype, here designated: Austria, Kärnten, St. Margareten im Rosental, Dullach, Drau-Auen, grid square 9452/1, on twigs of Rhamnus frangula, 11 Jul. 2013, W. Jaklitsch (WU 36935; MBT378886; ex-epitype culture CBS 142391 = C1).
Other material examined: Austria, Kärnten, St. Margareten im Rosental, alluvial forest at the brook Tumpfi, grid square 9452/4, on twigs of Rhamnus frangula, 7 Jun. 2014, W. Jaklitsch (WU 36936; culture C112 from conidia); shrubs in village area, grid square 9452/4, on twigs of R. frangula, 28 Oct. 1995, W. Jaklitsch W.J. 773 (WU 36937); ibidem, on decorticated wood of Rhamnus cathartica, 2 Dec. 1995 (WU 36938); Zabrde, grid square 9452/4, 7 Sep. 2014, W. Jaklitsch (WU 36939; culture C130). Niederösterreich, Engelhartstetten, at Schloß Hof, on a partly decorticated branch of Rhamnus cathartica, 17 Jun. 2017, H. Voglmayr & I. Greilhuber (WU 36940); Klausen-Leopoldsdorf, near Ranzenbach, on twigs of R. frangula, 28 Mar. 2016, W. Jaklitsch (WU 36941; culture C242); Mauerbach, near the cemetery, grid square 7763/1, on twigs of R. frangula, 25 Aug. 2001, W. Jaklitsch W.J. 1777 (WU 36942); Mödling, Eichkogel, on thin twigs of Rhamnus saxatilis, 12 Nov. 2016, H. Voglmayr & I. Greilhuber (WU 36943; culture C277). Norway, Aust-Agden, Froland kommune, Ytre Lauvrak, on twigs of R. frangula, 3 Oct. 2014, W. Jaklitsch (WU 36944; culture C133). Spain, Madrid, Somosierra, Dehesa de Somosierra, at the Arroyo de la Dehesa, N 41°7′29.55″ W 3°34′44.8″, elev. 1400 m, on twigs of R. frangula, 13 Apr. 2015, W. Jaklitsch, J. Checa, M. Blanco, Á. López & F. J. Rejos (WU 36945; culture C190).
Notes: Mirza (1968) listed Diplodia frangulae Fuckel, Camarosporium rhamni Allescher and Microdiplodia rhamni Fuckel as putative asexual morphs detected by association on natural hosts, but the true asexual morph is phoma-like with transition to pyrenochaeta-like, based on unicellular hyaline conidia (both morphs), absence of setae (phoma-like), short conidiophores (pyrenochaeta-like). The hamathecium consists probably of true paraphyses rather than pseudoparaphyses, as free rounded ends have been seen among young asci. On Rhamnus cathartica ostiolar apices of Diaporthe fibrosa sometimes mimic ascomata of C. rhamni. In the lectotype some aberrant ascospores up to 27.7 × 12.6 μm are present. For comparison among the three species recognized on Rhamnus spp. see notes under N. rhamnioides.
Neocucurbitaria rhamnicola Jaklitsch & Voglmayr, sp. nov., MycoBank MB823010. Fig. 15.
Fig. 15.
Neocucurbitaria rhamnicola. A–C. Ascomata in face view. D–G. Asci. H. Ascus apex showing ocular chamber. I. Hamathecium. J. Ascoma in vertical section. K–P. Ascospores. Q–W. Asexual morph in culture (on MEA after 8 d at 22 °C). Q. Pycnidia. R. Hyphal appendage on pycnidial wall. S. Pycnidial wall in face view. T–V. Conidiophores and conidiogenous cells. W. Conidia. R–W. in 3 % KOH. A, D, I–M, Q–W. WU 36946/CBS 142396. B, C, E–H, N–P. WU 36947. Scale bars: A, B = 300 μm; C = 100 μm; D = 15 μm; E–G = 10 μm; H, T, U = 5 μm; I, K–P, R, S = 7 μm; J = 50 μm; Q = 200 μm; V, W = 3 μm.
Etymology: For its occurrence on Rhamnus.
Ascomata (195–)256–375(–452) μm diam (n = 46), (91–)115–175(–215) μm high (n = 20), scattered or aggregated in small groups in bark fissures on some brown subiculum on inner bark layers and wood, subglobose with flattened top, collapsing-discoid with a central depression and reddish to black central papilla (33–)58–90(–97) μm diam (n = 33), sometimes deeply cupulate lacking a papilla, surface smooth to verruculose, black. Peridium (38–)41–76(–97) μm thick (n = 23), pseudoparenchymatous, outer layer dark brown, pigment incrusted, inner layer yellowish to hyaline; cells thick-walled, (4–)5–8(–12) μm (n = 47). Subiculum consisting of 2–6.5 μm wide brown, thick-walled hyphae. Hamathecium consisting of 1–3 μm wide branched ?paraphyses. Asci (98–)109–140(–150) × (11–)12–16(–18) μm (n = 25), cylindrical to narrowly clavate, bitunicate, fissitunicate, thick-walled, with a distinct ocular chamber, a short stipe and a simple to knob-like base, 8 (obliquely) uniseriately to biseriately arranged ascospores. Ascospores (15.5–)18–22(–26.5) × (7–)8.5–10.7(–11.7) μm, l/w (1.7–)1.9–2.3(–2.7) (n = 60), ellipsoid to fusoid, constricted at the median septum, upper part often slightly enlarged, lower often attenuated toward the base when 3-septate, with 3–7 transverse and 1(–2) longitudinal septa, medium to dark brown, darkening in 3 % KOH, smooth.
Cultures and asexual morph: colony radius 12 mm after 8 d, 45 mm after 37 d on CMD; colony dark olivaceous brown to nearly black, with radial texture; odour indistinct; pycnidia formed in small numbers. Colony diam 7 mm after 8 d on MEA at 22 °C, colony brown, turning nearly black, finely zonate, completely covered by pycnidia and white aerial hyphae; pycnidia more or less in radial rows, laterally fused, 50–210 μm diam, with hyaline to olivaceous conidial drops. Peridium thin, subhyaline, pale olivaceous to brownish, consisting of a t. angularis of thin-walled, angular to subglobose cells (6.0–)6.8–11(–15) × (5–)6–8.5(–9.5) μm (n = 30), with 2–4 celled, pale brown hyphal outgrowths to ca. 30 × 5–7 μm. Phialides (4.0–)5.0–7.3(–8.7) × (2.0–)2.5–4.3(–6.0) μm, l/w (1.1–)1.4–2.6(–3.4) (n = 39), lageniform, often basally inflated, arranged in dense clusters. Conidia (2.5–)2.8–3.7(–4.3) × (1.1–)1.4–1.8(–2) μm, l/w (1.3–)1.7–2.4(–2.6) (n = 46), 1-celled, hyaline, oblong or narrowly ellipsoid, straight or curved, also drop-like, often with 1–2 guttules, sometimes scar distinctly truncate.
Habitat: On wood and bark of Rhamnus spp., known from R. alaternus and R. lycioides.
Distribution: Southern Europe (Spain).
Holotype: Spain, Guadalajara, Chiloeches, Finca Roma, below El Viso, N 40°32′6.27″ W 3°13′4.26″, elev. 750 m, on branches of Rhamnus lycioides, 10 Apr. 2015, W. Jaklitsch, J. Checa, Á. López & F. J. Rejos (WU 36946; ex-holotype culture CBS 142396 = C185).
Other material examined: Spain, Andalusia, N Castellar, on a branch of Rhamnus alaternus, 5 Apr. 2014, W. Jaklitsch (WU 36947; cultures KRx, KRy).
Neocucurbitaria rhamnioides Jaklitsch & Voglmayr, sp. nov., MycoBank MB823011. Fig. 16.
Fig. 16.
Neocucurbitaria rhamnioides. A–C. Ascomata in face view. D. Peridium in vertical section. E. Subicular hyphae. F. Ascus apex after fissitunicate dehiscence. G. Ascus base. H–J. Asci. K–O. Ascospores. P–U. Asexual morph on the natural substrate. P. Pycnidium. Q, R. Conidiophores and phialides. S–U. Conidia. J, O, Q, R, U. in 3 % KOH. A, D, I, K, N, O, Q–U. WU 36948; B, E, J, L, M, P. WU 36949; C, F–H. WU 36950. Scale bars: A = 300 μm; B, C = 150 μm; D–F, H–J = 15 μm; G, K, M–O, Q = 7 μm; L, R–T = 5 μm; P = 50 μm; U = 2 μm.
Etymology: For its resemblance of Neocucurbitaria rhamni.
Ascomata (163–)255–360(–426) μm diam (n = 92), (97–)125–240(–390) μm high (n = 67), scattered or aggregated in groups of up to ca. 30 individuals, in bark fissures on inner bark layers and wood, also singly erumpent-superficial on bark, subglobose, collapsing-discoid or turbinate with a central papilla (33–)40–92(–157) μm diam (n = 36), sometimes deeply cupulate lacking papilla; surface finely verruculose, developing more or less radial cracks on the upper part of the ascoma, black. Peridium ca. 30–80 μm thick, pseudoparenchymatous, outer layer dark brown, pigment incrusted, inner layer yellowish to hyaline; cells thick-walled, (4–)4.5–8(–12) μm diam (n = 46). Basal subiculum consisting of 2–6.5 μm wide brown, thick-walled hyphae, sometimes ascending to the top of ascomata, but not originating from upper ascomatal parts. Hamathecium consisting of 1–3.5 μm wide branched ?paraphyses. Asci (92–)103–129(–146) × (11–)11.5–13(–14.5) μm (n = 20), cylindrical, bitunicate, fissitunicate, thick-walled, with a distinct ocular chamber, a short stipe and a simple to knob-like base, containing 8 ascospores in (obliquely) uniseriate arrangement. Ascospores (14–)17–22(–25.5) × (7–)7.5–10(–11) μm, l/w (1.8–)2.1–2.4(–2.7) (n = 70), ellipsoid to fusoid, constricted at the median septum, upper part often slightly enlarged, lower often attenuated toward the base when 3-septate, with 3–7 transverse and 1(–2) longitudinal septa, medium to dark brown, darkening in 3 % KOH, smooth.
Asexual morph on natural hosts: Pycnidia (43–)62–110(–141) μm diam (n = 30), superficial on inner bark or wood on usually inconspicuous subiculum, scattered among ascomata, globose, often with a minute apical papilla; peridium pseudoparenchymatous, dark brown, bearing some hyphal outgrowths on the outer side. Inner side lined with hyaline cells giving rise to phialides and simple, to ca. 25 μm long conidiophores. Conidiophores bearing lateral pegs and solitary phialides terminally. Phialides (4.3–)5.0–7.7(–8.7) × (1.5–)1.7–2.3(–2.7) (n = 18), lageniform to cylindrical, straight or curved. Conidia (2.5–)3.0–3.5(–3.8) × (1.0–)1.1–1.3(–1.5) μm, l/w (2.3–)2.4–2.9(–3.1) (n = 44), oblong to allantoid, 1-celled, hyaline, with 1–2 subterminal guttules.
Cultures on CMD at 22 °C: colony radius up to 28 mm after 3 wk; colony circular, dark olivaceous brown, dense, with radial texture, sometimes finely zonate; odour indistinct.
Habitat: on wood and bark of Rhamnus spp., known from R. myrtifolius and R. saxatilis.
Distribution: Mediterranean Europe.
Holotype: Spain, Córdoba, Las Lagunillas, La Tiñosa, 37°22′56″N, 4°15′5.8″W, 1 360 m, on twigs of Rhamnus myrtifolius, 17 May 2014, S. Tello (WU 36948; ex-holotype culture CBS 142395 = C118).
Other material examined: Greece, Crete, Omalos, on twigs of Rhamnus saxatilis ssp. prunifolius, 5 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 36949; culture C222); near Askifou, on twigs of Rhamnus saxatilis ssp. prunifolius, 6 Jun. 2015, W. Jaklitsch & H. Voglmayr (WU 36950; culture C223).
Notes: Neocucurbitaria rhamnioides differs from N. rhamni in the geographical distribution and lack of conspicuous tubercles on ascomata. A distinction from N. rhamnicola is difficult, but the former tends to develop fine cracks in the central ascomatal surface and occurs on other species of Rhamnus. Ascospore shape and septation depend on the specimen, as in one specimen ascospores with 3 transverse septa and a strongly attenuated lower part may be common, and in another little-varying, 7-septate ascospores may be common. Ascomata on bark are often associated with lichens and may be mistaken for lichen apothecia at first sight. Species recognition is primarily based on the statistical support of the monophyletic lineages, but hosts and geography help to distinguish among them. Both species differ by 17 and 20 fixed nucleotide substitutions in tef1 and rpb2, respectively.
Neocucurbitaria ribicola Jaklitsch & Voglmayr, sp. nov., MycoBank MB823012. Fig. 17, Fig. 18.
Fig. 17.
Neocucurbitaria ribicola, sexual morph. A–E. Ascomata in face view (emphasizing furrowed apices in C–E). F. Ascoma in vertical section. G. Peridium in vertical section. H. Subicular hyphae. I. Ascomatal seta. J, K. Asci. L. Upper part of ascus. M, N. Ascus apices (immature in M). O–W. Ascospores (young in P). H–O, Q–V. in 3 % KOH. A, C, D–I, L, P, S, T, V, W. WU 36952; B, J, K, M–O, Q, R, U. WU 36951. Scale bars: A–C, F = 200 μm; D, E = 100 μm; G, J, K = 15 μm; H, L–N = 10 μm; I, O–W = 7 μm.
Fig. 18.
Neocucurbitaria ribicola, asexual morph. A. Pycnidia on the natural substrate (WU 36951). B–I. Asexual morph in culture (CMD, after 5–10 d at 22 °C). B. Pycnidia. C. Pycnidial wall. D–F. Conidiogenous cells and short conidiophore (in E). G–I. Conidia. C–I. in 3 % KOH. B–H. CBS 142394; I. C155. Scale bars: A = 100 μm; B = 75 μm; C = 10 μm; D–F = 5 μm; G–I = 3 μm.
Etymology: For its occurrence on Ribes.
Ascomata (250–)335–542(–628) μm diam (n = 41), (220–)295–456(–516) μm high (n = 24), scattered or aggregated in small groups or fused into amorphous masses in bark fissures on inner bark layers or wood, sometimes surrounding the whole twig in groups of more than 50 individuals, arranged vertically, obliquely or parallel to the host surface, globose, subglobose to pyriform, collapsing from the side when old. Surface olivaceous to dull brown, covered by subicular hyphae except for the apical papilla, rarely glabrous and then verruculose. Apical papillae (44–)125–210(–265) μm diam (n = 29), often convergent, black, usually stout, rounded in section or more often sulcate, consisting of several radial lobes, or irregularly cracked. Peridium 40–90 μm thick, usually thickened around the papilla to ca. 130 μm, pseudoparenchymatous, consisting of a broad dark brown to black outer layer consisting of thick-walled cells with walls incrusted by coarse amorphous pigment particles, and a narrow inner layer consisting of thinner-walled, brown to hyaline cells; cells (4–)6–11(–13) μm diam (n = 39). Subicular hyphae 2.5–6.5 μm wide, olivaceous to dark brown, thick-walled, short and seta-like around the ostiole, long and scarcely branched on other parts of the ascoma, penetrating into host tissues. Hamathecium consisting of branched, 1–2.5(–3) μm wide ?paraphyses. Asci (150–)158–198(–202) × 14.8–18.2(–20.2) μm (n = 11), cylindrical, bitunicate, fissitunicate, thick-walled, with a distinct ocular chamber often containing refractive bodies, a short stipe and simple or knob-like base, containing 6–8 ascospores in uniseriate arrangement. Ascospores (20–)21.5–26.5(–30) × (9–)9.8–11.7(–13) μm, l/w (1.8–)2.0–2.5(–2.7) (n = 71), broadly ellipsoid or broadly fusiform, with the upper part usually broader, with (3–)5–8(–9) transverse and 1–3 longitudinal septa, constricted at the median septum, yellowish brown when young, turning dark to blackish brown at maturity, smooth, containing large guttules when young and vital.
Pycnidia uncommon on the natural host, scattered among ascomata, globose, with a minute apical papilla, black, more or less smooth, ca. 70–100 μm diam.
Cultures and asexual morph in culture: Colony radius on CMD at 22 °C 21 mm after 18 d, 47 mm after 2 mo; 5–6 mm 7 d after reconstitution from −80 °C. Colony greyish olivaceous to brown with hyaline margin, finely zonate, odour indistinct to slightly unpleasant. Pycnidia numerous, (33–)38–54(–70) μm (n = 25) diam, globose, with large light rounded papilla 12–30 μm diam, pale olivaceous, darkening with time, scattered or densely disposed around the inoculation plug within a few days, later in additional zones and in radial rows, on the agar surface and immersed in the agar, producing whitish milky conidial drops. Peridium consisting of a thin olivaceous brown t. angularis of (3.5–)4.5–7.5(–10) μm (n = 43) long cells; surface with olivaceous hyphal outgrowths. Inner side of the peridium lined by globose to angular hyaline cells giving rise to conidia, lageniform, straight, curved or sigmoid phialides (5.7–)6–8(–9.2) × (2.1–)2.3–3(–3.3) μm (n = 15) and short, 1–3-celled, simple conidiophores. Conidia formed on hyaline base cells, sessile phialides, single terminal phialides and pegs on the sides of the conidiophores, (2.6–)3.2–4(–5) × (1.2–)1.4–1.8(–2.4) μm, l/w (1.4–)1.9–2.6(–3.3) (n = 120), 1-celled, hyaline, oblong to ellipsoid or drop-like, with 1–2 subterminal guttules, smooth. On MEA pycnidia becoming larger, up to ca. 130 μm diam, densely aggregated-confluent in the centre, superposed by long white aerial hyphae. After 10 d no conidiogenous cells detectable, and aberrant conidia up to 6 × 2 μm present.
Habitat: on partly decorticated twigs and branches of Ribes rubrum, sometimes associated with Thyronectria berolinensis.
Distribution: Central Europe, uncommon.
Holotype: Austria, Kärnten, St. Margareten im Rosental, village area, grid square 9452/4, on twigs of Ribes rubrum, 2 May 2014, W. Jaklitsch (WU 36951; ex-holotype culture CBS 142394 = C55).
Other material examined: Austria, Oberösterreich, Schärding, Enzenkirchen, Landersberg, grid square 7648/1, on twig of Ribes rubrum, partly covered by algae and ascomata of Thyronectria berolinensis, 21 Mar. 2015, H. Voglmayr (WU 36952; culture C155).
Notes: Neocucurbitaria ribicola is an uncommon species or easily overlooked due to its inconspicuous ascomata, which are covered by hyphae or other fungi. In addition, dead twigs of Ribes are usually inhabited by many other fungi. The holotype (WU 36951) contains less material than WU 36952, but is better developed, containing more intact asci.
Another species described from Ribes, Cucurbitaria ribis (Niessl 1872), is apparently a different fungus, as it differs from C. ribicola by centrally depressed, shiny black and glabrous ascomata, a conspicuously thick peridium, absence of a furrowed ostiolar region, and lighter coloured ascospores with fewer longitudinal septa. The latter were described by Niessl (1872) as smaller than in C. ribicola, but examination of the type shows a strongly overlapping size range. Mirza's (1968) description fits quite well for C. ribis, although he did not see the type. We have currently no fresh material that agrees with Niessl's fungus, and due to the morphological differences we describe a new species. Below we give a description of the holotype of C. ribis:
Cucurbitaria ribis Niessl, Verh. nat. Ver. Brünn 10: 198. 1872. Fig. 19.
Fig. 19.
Cucurbitaria ribis (holotype M-0281853). A. Ascomata in face view. B. Ascoma from the side, one sectioned. C–E. Asci. F. Peridium. G. Ascus apex. H–M. Ascospores. C–H, J–M. in 3 % KOH. Scale bars: A, B = 200 μm; C–F = 15 μm; G–M = 7 μm.
Ascomata (195–)265–502(–567) μm (n = 21) diam, (103–)138–284(–309) μm (n = 14) high, scattered or crowded in rather loose groups on inner bark layers, subglobose, depressed globose to more or less discoid, black, finely verruculose to nearly smooth; ostioles generally invisible, apex sometimes indistinctly papillate. Peridium ca. 60–165 μm thick, consisting of dark brown thick-walled angular cells (4–)5–11(–14) μm (n = 40) with encrusted pigment, opaque outside, becoming lighter and thinner-walled to the interior. Hamathecium consisting of numerous branched, 1–4 μm wide, apically free paraphyses. Asci (115–)125–149(–156) × (12.8–)16.2–19.7(–22) μm (n = 23), cylindrical to oblong, bitunicate, fissitunicate, with an ocular chamber, a short stipe and a simple to knob-like base, containing 8 obliquely uni- partly biseriate ascospores. Ascospores (17.7–)19.8–25(–28.5) × (8–)9.4–11.3(–13) μm, l/w (1.8–)2–2.3(–2.6) (n = 65), ellipsoid to oval, with 3–7 transverse and 1–2 longitudinal septa, constricted at the median primary septum, medium to dark brown, dark olivaceous brown in 3 % KOH, paler and narrower when young, smooth.
Habitat: On partly decorticated twigs and branches of Ribes.
Distribution: Central Europe, also reported from Pakistan by Mirza (1968), uncommon.
Holotype: Czech Republic, Moravia, near Brno (Brünn), on Ribes rubrum, G. v. Niessl, no date given (M-0281853!)
Notes: According to Niessl (1872) ascomata of Cucurbitaria ribis surround decorticated regions of twigs and form dense groups, but are not aggregated into dense clusters as in C. laburni, and their shape is typical for the genus Cucurbitaria. Inspection of the holotype revealed that the typical arrangement of ascospores in the asci is obliquely uniseriate, but also some biseriate arrangement can be noted. The ascospores are oval, with the upper part being broader than the lower and having typically 3, the lower 2 transverse septa. One longitudinal septum goes through the whole spore and branches sometimes at one end. The spore size given by Niessl (1872) was 18–20 × 7–8 μm. Although he depicted subglobose ascomata with distinct papillae, the ascomatal shape in the holotype is typically depressed globose lacking a papilla.
Neocucurbitaria vachelliae Jaklitsch & Voglmayr, sp. nov., MycoBank MB823013. Fig. 20.
Fig. 20.
Neocucurbitaria vachelliae. A–M. Sexual morph (WU 36953). A–C. Ascomata in face view. D. Peridium in vertical section. E. Ascus apex. F. Subiculum. G–J. Ascospores (young in G). K–M. Asci. N–S. Asexual morph in culture (CBS 142397 on CMD after 6–7 d at 22 °C). N. Pycnidia with conidial drops. O. Pycnidial wall. P, Q. Conidiophores and phialides. R, S. Conidia. E, G–J, L, M. in 3 % KOH. Scale bars: A, B = 200 μm; C, N = 100 μm; D, K = 15 μm; E, O, P = 7 μm; F, L, M = 10 μm. G–J, Q–S = 5 μm.
Etymology: For its occurrence on Vachellia.
Ascomata (195–)215–375(–478) μm diam (n = 21), (120–)150–310(–360) μm high (n = 25), scattered or aggregated in variable groups below the host epidermis on ample subiculum of branched, 2–6.5 wide brown thick-walled hyphae, erumpent, often only upper part visible, more or less globose or collapsing-discoid or turbinate, only rarely with a distinct rounded papilla. Surface black or grey, verruculose, irregularly cracked when old. Peridium 15–70 μm thick, of equal thickness or thickened around the ostiole, consisting of a thick-walled dark brown t. angularis becoming lighter and thinner-walled towards the inner side, formed by cells (4–)4.5–9(–12.5) μm (n = 30) diam, and an inner layer of thick-walled glassy subhyaline cells. Hamathecium forming a dense reticulum of richly branched, 1–4 μm wide paraphyses with free apical ends. Asci (74–)84–108(–121) × (12.3–)13–16(–17) μm (n = 22), oblong to narrowly clavate, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing 4–6–8 ascospores in (obliquely) uniseriate or, particularly in the upper part, biseriate arrangement. Ascospores (15.8–)18.0–22.2(–26.2) × (6.8–)7.5–9.3(–10.7) μm, l/w (1.9–)2.2–2.6(–3.2) (n = 40), ellipsoid to fusoid with rounded ends or clavate, slightly constricted at the median primary septum, upper half usually slightly enlarged, with (4–)6–7 transverse and 1–2 longitudinal septa, first pale brown, soon turning dark brown, end cells sometimes slightly lighter, smooth.
Colonies on CMD at 22 °C reaching a growth radius of ca. 9 mm after 10 d, ca. 19 mm after 22 d, mycelium dense, colourless, turning olivaceous from the centre, aerial hyphae often numerous, spreading from the centre; odour indistinct. Pycnidia forming within a few days, (53–)70–146(–195) μm (n = 25) diam, numerous, partly immersed in agar, scattered to aggregated, more or less globose, with central papilla, sometimes laterally fused, pale olive when young, soon turning dark olivaceous to black; conidia emerging in whitish to olivaceous drops. Peridium consisting of a thin t. prismatica-angularis of thick-walled cells (2.5–)5–10(–14) μm (n = 43) diam; inner side lined by hyaline cells giving rise to conidiophores and phialides. Conidiophores simple or basally divided into several nearly parallel branches with lateral pegs and solitary terminal phialides. Phialides (4.8–)6.7–10.0(–10.5) × (1.8–)2.0–2.5(–2.8) μm, l/w (2.1–)3.1–4.6(–5.3) (n = 25), lageniform. Conidia (2.6–)3.4–4.5(–5.1) × (1.2–)1.3–1.7(–2.1) μm, l/w (2.1–)2.3–2.9(–3.5) (n = 49), oblong to allantoid, sometimes pinched, 1-celled, hyaline, containing 0–2 subterminal guttules, smooth.
Habitat: On wood and bark of Vachellia gummifera.
Distribution: Morocco, only known from the type location
Holotype: Morocco, Agadir, Ait Melloul, behind the Institut Agronomique et Vétérinaire Hassan II (IAV), on branch of Vachellia (Acacia) gummifera, soc. Diaporthe sp., Eutypa sp., 6 May 2015, W. Jaklitsch, M. Mokhtari & M. Louay (WU 36953; ex-holotype culture CBS 142397 = C192).
Notes: Other Cucurbitaria species described from Acacia spp. comprise Cucurbitaria arizonica, described from Acacia greggii A. Gray (as “Acacia grayii”, now known as Senegalia greggii), also Cucurbitaria halimodendri (cf. Mirza 1968) and Cucurbitaria pakistanica (Petrak & Ahmad 1954) from Acacia modesta, now known as Senegalia modesta, in Pakistan. Ascospores of C. arizonica have only three transverse septa (Barr 1990a), C. halimodendri belongs to Camarosporidiella (Wanasinghe et al. 2017a), and C. pakistanica (Petrak & Ahmad 1954) is described with ascospores having 3–5 transverse septa and a single longitudinal septum. Type material of C. pakistanica from W (W 1980/07242!) contains mostly a Diplodia sp. and some black, verruculose, turbinate to collapsed discoid ascomata 175–355 μm diam, erumpent from bark in dense groups on brown subiculum, with a peridium of large (to 19 μm diam) dark brown, thick-walled cells forming t. angularis-globulosa, mostly immature cylindrical asci and numerous, to 4.5 μm thick hamathecial threads. Ascospores are uniseriately arranged, (18–)19–22(–22.8) × (8.7–)9.2–10.2(–10.8) μm, l/w (1.8–)2–2.3(–2.4) (n = 20), ellipsoid to oblong, with 3–5(–6) transverse and 1 longitudinal septa, pale to medium brown. Ascoma shape, peridium and hamathecium suggest an affinity to Camarosporium s. l. rather than to the Cucurbitariaceae.
Parafenestella Jaklitsch & Voglmayr, gen. nov., MycoBank MB823014.
Etymology: Owing to its phylogenetic neighbourhood to Fenestella.
Ascomata scattered or variably aggregated below the host epidermis becoming visible in bark fissures, subglobose, globose to pyriform, sometimes collapsing-discoid upon drying when immature or becoming vertically pinched, black, often apically white inside, usually surrounded by subicular hyphae. Apical papilla black, rounded or oblong in section, often flattened. Peridium pseudoparenchymatous. Hamathecium consisting of numerous branched and anastomosing paraphyses. Asci cylindrical, bitunicate, fissitunicate, thick-walled, with an ocular chamber, a short stipe and simple or knob-like base, containing 6–8 ascospores in (obliquely overlapping) uniseriate arrangement. Ascospores ellipsoid with upper part slightly wider, with often subacute and lighter ends, several transverse and longitudinal septa, constricted at the median primary septum, hyaline to pale or yellowish brown when young, turning greyish brown and finally dark to blackish brown at maturity, smooth.
Asexual morph in culture: Pycnidia more or less globose, olivaceous, green to black; surface often roughened by hyphal appendages. Peridium pseudoparenchymatous. Phialides formed on hyaline base cells or apically on short simple or basally branched conidiophores, lageniform to cylindrical or subglobose. Conidia 1-celled, oblong or allantoid, sometimes attenuated towards one end or pinched, hyaline, guttulate, smooth.
Ecology: Apparently fungicolous or saprobic in bark.
Type species: Parafenestella pseudoplatani Jaklitsch & Voglmayr.
Notes: The genus Parafenestella differs from Fenestella phylogenetically, by absence of well-delimited pseudostromata and ascospores, which show a transition from fenestella- to (neo)cucurbitaria-like.
Parafenestella pseudoplatani Jaklitsch & Voglmayr, sp. nov., MycoBank MB823015. Fig. 21.
Fig. 21.
Parafenestella pseudoplatani (WU 36954/CBS 142392). A–M. Sexual morph. A–C. Ascomata in face view. D. Peridium and subicular hyphae in vertical section. E. Ascus apex. F. Ascus stipe and base. G–J. Ascospores. K–M. Asci (young in K). N–U. Asexual morph. N. Pycnidia on natural substrate. O. Pycnidia. P. Pycnidial wall. Q, R. Phialides from natural substrate. S–U. Conidia. O, P, S–U. From culture (CMD, after 7 d at 22 °C). D–F, I–M, Q, R. in 3 % KOH. Scale bars: A–C = 200 μm; D, K–M = 20 μm; E, G–J, P–R = 7 μm; F = 10 μm. N = 100 μm. O = 70 μm. S, T = 5 μm; U = 3 μm.
Etymology: For its occurrence on Acer pseudoplatanus.
Ascomata (195–)230–340(–390) μm (n = 15) diam, scattered or variably aggregated in Diaporthe pseudostromata below the epidermis becoming visible in bark fissures, subglobose to pyriform, sometimes collapsing-discoid upon drying when immature or becoming vertically pinched, black, often apically white inside, usually nearly completely covered by thick-walled, dark brown, 2–6.5 μm wide subicular hyphae. Apical papilla (53–)87–137(–147) μm (n = 12) diam, black, rounded or oblong in section, often flattened. Peridium ca. 30–80 μm thick, black, sometimes slightly thickened at the sides, consisting of a thick-walled, dark brown to nearly black t. angularis becoming slightly lighter and thinner-walled towards inner side, formed by (4–)6–11(–15) μm (n = 40) long cells, at upper parts lined by hyaline cells inside. Hamathecium consisting of numerous branched and anastomosing, 1–3.5 μm wide paraphyses (free ends occurring with immature asci). Asci (139–)165–230 × (16.5–)17–19.5(–20) μm (n = 10), cylindrical, bitunicate, fissitunicate, thick-walled, with an ocular chamber, a short stipe and simple or knob-like base, containing 6–8 ascospores in (obliquely overlapping) uniseriate arrangement. Ascospores (21.8–)24.5–29.5(–31.5) × (11–)11.5–14(–16) μm, l/w (1.6–)1.9–2.3(–2.5) (n = 32), ellipsoid with upper part slightly wider, with often subacute and sometimes lighter ends, (7–)8–12(–13) transverse and 3–4 longitudinal septa, strongly constricted at the median primary septum, yellowish brown when young, turning greyish brown and finally blackish brown at maturity, smooth.
Pycnidia co-occurring with ascomata, crowded on a subiculum in Diaporthe pseudostromata, globose, black, 60–150 μm diam, variably collapsing.
Cultures and asexual morph in culture: Growth radius ca. 14 mm after 2 wk on CMD at 22 °C. Colony dense, without distinct odour, pale, mycelium remaining colourless but colony appearing greyish brown due to numerous pycnidia starting to form within 24 h after inoculation. Pycnidia 43–90 μm diam, concentrated and aggregated around the inoculation plug, more scattered with distance from the centre, more or less globose, first pale olivaceous, slowly turning green to nearly black, surface roughened by hyphal appendages, with colourless to pale olivaceous conidial drop. Peridium consisting of a green t. angularis to t. prismatica of moderately thick-walled, (3.5–)5–8(–10) μm (n = 41) long cells. Phialides formed on hyaline base cells or apically on short simple or basally branched, up to 30 μm long conidiophores, (4.3–)5.5–7.5(–8.8) × (1.5–)2–2.5(–3) μm (n = 39), lageniform to cylindrical or subglobose. Conidia (3.2–)3.5–4(–4.5) × (1.0–)1.2–1.5(–1.7) μm, l/w (2–)2.5–3.1(–3.4) (n = 59), 1-celled, oblong or allantoid, sometimes attenuated towards one end or pinched, hyaline, with 2 subterminal drops, smooth.
Habitat: In Diaporthe pseudostromata on dead branches of Acer pseudoplatanus.
Distribution: Central Europe (Austria); only known from the type locality.
Holotype: Austria, Vienna, Strebersdorf, Krottenhofgasse, on branch of Acer pseudoplatanus, 17 Nov. 2013, W. Jaklitsch (WU 36954; ex-holotype culture CBS 142392 = C26, C26T).
Notes: In the holotype the asexual morph of Neocucurbitaria acerina is also present. Due to minor differences it is difficult to differentiate between these two asexual morphs morphologically. Data referring to the natural substrate are recorded from pycnidia densely clustered on Diaporthe pseudostromata. Subglobose conidiogenous cells from pycnidia in culture were not included in measurements. For other species described in Cucurbitaria and Fenestella on Acer see notes under Neocucurbitaria acerina.
Parafenestella mackenziei (Wanas. et al.) Jaklitsch & Voglmayr, comb. nov., MycoBank MB823016.
Basionym: Fenestella mackenziei Wanas. et al., Mycosphere 8: 407. 2017.
Notes: This species was described as a new species in Fenestella by Wanasinghe et al. (2017a) from Rosa canina in Italy. It clusters with P. pseudoplatani rather than with Fenestella fenestrata (Fig. 1), therefore we combine it in Parafenestella.
Parafenestella ostryae (Wanas. et al.) Jaklitsch & Voglmayr, comb. nov., MycoBank MB823017.
Basionym: Fenestella ostryae Wanas. et al., Mycosphere 8: 404. 2017.
Note: This species was described from Ostrya carpinifolia in Italy; see Wanasinghe et al. (2017a) for descriptions, illustrations and additional data of this and the foregoing species.
Protofenestella Jaklitsch & Voglmayr, gen. nov., MycoBank MB823018.
Etymology: A primitive form of Fenestella, characterised by random and indefinite disposition of its ascomata on natural substrate.
Ascomata globose, pyriform, depressed subglobose to lenticular, immersed below the host epidermis, solitary or randomly disposed, loosely or densely aggregated in often large ill-defined groups, not forming defined pustules, inconspicuous at the bark surface, surrounded by subiculum. Ostioles inconspicuous, short and blunt conical. Peridium pseudoparenchymatous, covered with subicular hyphae outside, becoming lighter and thinner-walled toward inner side. Hamathecium formed by numerous richly branched ?paraphyses. Asci cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing (4–)8 (obliquely or partly overlapping) uniseriately arranged ascospores. Ascospores ellipsoid to fusoid, symmetric to slightly asymmetric, first hyaline to yellowish, with 3–4(–5) transverse septa, developing a longitudinal septum; wall distinctly thicker than septa except for the ends containing narrow canals; later brown to dark brown, with several transverse and longitudinal septa; not to distinctly constricted at the median to slightly eccentric primary septum; ends often lighter coloured, brownish to hyaline, often protruding and subacute, pierced by a pore and often with a hyaline roundish to cylindrical cellular appendage at one or both ends; turning blackish brown in 3 % KOH.
Asexual morph in culture: Pycnidia more or less globose, papillate, nearly black. Peridium thin pseudoparenchymatous. Phialides sessile, clustered in small groups or short chains, subglobose to lageniform. Conidia 1-celled, oblong to allantoid or ellipsoid, hyaline, guttulate, smooth.
Ecology: Probably saprobic in bark.
Type species: Protofenestella ulmi Jaklitsch & Voglmayr.
Note: Protofenestella differs from Fenestella phylogenetically and by non-clustered ascomata.
Protofenestella ulmi Jaklitsch & Voglmayr, sp. nov., MycoBank MB823019. Fig. 22.
Fig. 22.
Protofenestella ulmi. A–U. Sexual morph. A. Ostiole on bark surface and subiculum surrounding ascoma below bark surface. B. Ascoma in vertical section. C. Peridium and subicular hyphae in vertical section. D. Subiculum. E–H. Asci. I. Ascus apex (with immature 3-septate ascospore). J–U. Ascospores (J. immature, showing thick wall and terminal inner canals; K. in mature ascus apex). V–C1. Asexual morph in culture (CMD, after 6 d at 22 °C). V. Pycnidia. W. Pycnidial wall. X–Z. Conidiogenous cells. A1–C1. Conidia. D, K, O, U. in 3 % KOH. A–J, L–N, P–C1. WU 36955/CBS 143000; K. UPS F-178445; O. UPS F-178444. Scale bars: A, B = 150 μm; C = 50 μm; D, I, K, M–U, W = 10 μm; E–H = 25 μm; J, Z, A1 = 7 μm; L = 15 μm; V = 100 μm; X, Y, B1, C1 = 5 μm.
Etymology: For its occurrence on Ulmus.
Ascomata (390–)475–675(–780) μm (n = 41) diam, ca. 200–500 μm high, globose, pyriform, depressed subglobose to lenticular, immersed below the host epidermis, solitary or randomly disposed, loosely or densely aggregated in often large ill-defined groups, not forming defined pustules, inconspicuous at the bark surface, surrounded by ample subiculum consisting of thick-walled, hyaline, silvery to pale brown, 2–5 μm wide hyphae, tending to be darker brown toward the peridium. Ostioles inconspicuous, short and blunt conical, sometimes yellow inside, concealed beyond (100–)115–285(–500) μm (n = 21) wide, pale greyish to brown, circular to longish cracks at the bark surface, often darkened by ascospore deposits or when old. Peridium 15–45 μm thick, consisting of a t. angularis of (2.5–)4–8(–10) μm (n = 53) wide cells, thick-walled and dark brown with inhomogenously disposed pigment and densely covered with subicular hyphae outside, becoming lighter and thinner-walled toward inner side. Inner ascomatal surface appearing whitish when old and effete. Hamathecium formed by numerous richly branched, 1–3 μm wide ?paraphyses. Asci (184–)214–294(–325) × (20–)23.5–29.5(–32.5) μm (n = 27), cylindrical, bitunicate, fissitunicate, with an ocular chamber, short stipe and a simple or knob-like base, containing (4–)8 (obliquely) uniseriately arranged, sometimes partly overlapping ascospores. Ascospores (32–)38–52(–63) × (14.2–)16–21.3(–25) μm, l/w (1.8–)2.1–2.8(–3.3) (n = 117), ellipsoid to fusoid, symmetric to slightly asymmetric, first hyaline to yellowish, with 3–4(–5) transverse septa, developing a longitudinal septum; wall distinctly thicker than septa except for the ends containing narrow canals; later medium (olivaceous-)brown to dark brown, with 12–18(–20) transverse and 3–7 longitudinal septa, with a thin, rarely slightly swelling perispore, lacking a sheath; not to distinctly constricted at the median to slightly eccentric primary septum; ends often lighter coloured, brownish to hyaline, often protruding and subacute, pierced by a pore and often with a hyaline roundish to cylindrical cellular appendage up to 4 μm long at one or both ends; turning blackish brown in 3 % KOH.
Cultures and asexual morph: Colony radius on CMD 30 mm after 23 d at 22 °C; colony greyish olive, turning unevenly brown; aerial hyphae scant, odour indistinct. Pycnidia (after 6 d) 40–100 μm diam, forming within a few days in small numbers, scattered, more or less globose, papillate, nearly black. Peridium consisting of a thin, rather thin-walled, olivaceous brown t. angularis of (2.7–)5–8.5(–10) μm (n = 44) wide cells; inner side lined by hyaline cells giving rise to phialides. Phialides (4.3–)4.9–6.6(–8) × (2.2–)3–4.5(–5) μm (n = 17), sessile, clustered in small groups or short chains, subglobose to lageniform. Conidia (2.9–)3.5–4.5(–5.4) × (1.3–)1.6–2(–2.2) μm, l/w (1.7–)1.9–2.7(–3.5) (n = 46), 1-celled, oblong to allantoid or ellipsoid, hyaline, containing 2 or more small guttules, smooth.
Habitat: In bark of Ulmus spp.
Distribution: Europe (Austria, Sweden).
Holotype: Austria, Vienna 10th district, Unterlaa, on branches of Ulmus minor, soc. Diplodia sp., Thyronectria rhodochlora and Nigrograna sp., 14 Mar. 2015, R. Moosbeckhofer G00485, comm. B. Wergen/I. Greilhuber (WU 36955; ex-holotype culture CBS 143000 = FP5).
Other material examined: Austria, Vienna 21st district, Marchfeldkanalweg, near Felix Slavik Straße, on branches of Ulmus minor, soc. Diplodia sp., 10 May 2003, W. Jaklitsch W.J. 2115 (WU 36956). Sweden, Uppland: Balingsta par., Borås, ca. 50 m W of the old house, chopped off branches of Ulmus glabra, soc. Kirschsteiniothelia aethiops, Nectria sp., several coelomycetes, Hypoxylon and myxomycete spores, K. & L. Holm, 29 Mar. 1988 (UPS F-178445; as Fenestella fenestrata; culture UPSC 2554-55 = CBS 114122); Dalby par., Tuna, S of the western farm, at the feet of the cliff, on twig of Ulmus glabra, K. & L. Holm, 17 Apr. 1988 (UPS F-178444; as Fenestella fenestrata).
Notes: Phylogenetically, Protofenestella ulmi is unrelated to Fenestella (Fig. 1, Fig. 2). Although ascospore morphology agrees perfectly with Fenestella, P. ulmi differs morphologically from the latter genus in that ascomata do not form defined clusters. All available materials contain either scant or immature/overmature ascomata that are accompanied by several other fungi, some of which form erumpent clusters of grouped ascomata. Well-developed asci were only found in WU 36955, therefore this specimen serves as holotype. Ascospore measurements include appendages, the latter are in total up to 7 μm long. Pleomassaria ulmicola differs from P. ulmi by ascospores with fewer longitudinal septa and the presence of a swelling sheath. See also notes under Seltsamia ulmi.
Seltsamia Jaklitsch & Voglmayr, gen. nov., MycoBank MB823020.
Etymology: Based on the German word “seltsam” for strange, because the morphology of the fungus, esp. the ascospore sheath, is untypical for Cucurbitariaceae.
Ascomata pyriform, black, immersed singly or in valsoid groups beneath periderm above ascomata of its host, upright or oblique with convergent ostiolar necks, surrounded by subiculum, forming bumps, becoming visible through bark fissures. Ostiolar necks forming stout papillae. Peridium leathery, black, pseudoparenchymatous, 3-layered. Hamathecium consisting of branched ?paraphyses. Asci cylindrical, with a distinct ocular chamber, a slightly elongated stipe and a simple base, containing 8 uni- to partly biseriately arranged ascospores. Ascospores fusoid to subclavate, with the upper part slightly widened, first yellow, with 3 main septa, later brown, finally with numerous transverse and longitudinal septa, surrounded by a swelling sheath around each hemisphere.
Asexual morph unknown.
Habitat: Fungicolous, e.g., on Hapalocystis bicaudata on Ulmus glabra.
Type species: Seltsamia ulmi Jaklitsch & Voglmayr.
Seltsamia ulmi Jaklitsch & Voglmayr, sp. nov., MycoBank MB823021. Fig. 23.
Fig. 23.
Seltsamia ulmi (WU 36957). A. Horizontal section at the ostiolar level. B. Horizontal section at the ascomatal level. C, D. Ascoma in vertical section. E. Peridium in vertical section showing remnants of subicular hyphae. F–H. Asci (young in F). I. Ascus apex showing ocular chamber. J–Q. Ascospores (showing swelling sheath in J and K). E, J. in 3 % KOH. Scale bars: A–C = 200 μm; D = 100 μm; E–H, K = 25 μm; I = 10 μm; J, L–Q = 15 μm.
Etymology: For its occurrence on Ulmus.
Ascomata (480–)524–760(–870) μm diam (n = 14), pyriform, black, immersed singly or in valsoid groups of up to ca. 10 individuals, upright or oblique, usually with ostiolar necks convergent to a common centre, surrounded by subiculum, slightly lifting the bark forming bumps, becoming visible through bark fissures. Ostiolar necks forming more or less stout papillae 150–300 μm diam, when young whitish to yellowish inside. Peridium leathery, black, ca. 40–160 μm thick, consisting of a narrow opaque outer layer of dark brown thick-walled cells (4.8–)8–14.5(–17) μm diam (n = 60), a median brown t. angularis of thin-walled and similarly sized cells, and an inner layer of brown compressed cells. Subicular hyphae originating on the ascoma surface, densely woven, making measurement of peridial thickness difficult, dark brown, thick-walled, 3–8 μm wide. Hamathecium consisting of 1–3.5(–4) μm wide, agglutinated, branched ?paraphyses. Asci (310–)346–419(–457) × (30–)35–44(–52.5) μm (n = 32), cylindrical, with a distinct ocular chamber, a slightly elongated stipe and a simple base, containing 8 uni- to partly biseriately arranged ascospores. Ascospores (52.5–)58.5–67(–71.5) × (16–)18.8–22.5(–26) μm, l/w (2.5–)2.8–3.3(–3.7) (n = 85), fusoid to subclavate, with the upper part slightly widened, first yellow, with 3 main septa, later brown, finally with numerous, ca. 17–25 transverse and 3–7 longitudinal septa, surrounded by a sheath around each hemisphere, quickly swelling and losing contours in water.
Cultures: Mycelium filling a 90 mm Petri dish on MEA, when centrally inoculated, after 36 d at room temperature. Colony dense, dark olive-brown, odour indistinct, no asexual morph detectable.
Habitat: On Hapalocystis bicaudata on corticated Ulmus glabra.
Distribution: Only known from the holotype in Norway.
Holotype: Norway, Aust-Agder, Froland kommune, Ytre Lauvrak, associated with Hapalocystis bicaudata on corticated twigs of Ulmus glabra, soc. Stylonectria wegeliniana, 3 Oct. 2014, H. Voglmayr & W. Jaklitsch (WU 36957; ex-holotype culture CBS 143002 = L150).
Notes: This is an exceptional species. Its home in the Cucurbitariaceae as following from molecular phylogeny is unexpected. Morphologically, the swelling ascospore sheath would suggest a pleomassaria-like fungus, clustering of ascomata in association with other pyrenomycetes on the other hand, Fenestella. Pleomassaria ulmicola (basionym Cucurbitaria ulmicola Fuckel) has smaller ascospores and fewer septa, viz. 38.5–48.5 × 11–16.5 μm and 8–13 transverse, 2–3 longitudinal septa (cf. Barr 1982). Fuckel (1870) had described C. ulmicola with 8–10 septate muriform ascospores of 36 × 11 μm. Type material of C. ulmicola (from Ulmus minor in Schloßpark Reichartshausen at Oestrich-Winkl, Germany) was distributed as Fungi Rhenani 2170. All specimens from G (G 00127253, G 00127254, G 00127255) and W (W 2015-01917, W 1922-12005) examined contain overmature, effete, depauperate material. No ascospores as given above were found. In G 00127254 we found a few collapsed-discoid ascomata and few small brown spores with 3–4/1 septa, 14.4–18.6 × 6.4–8.7 μm and some Dothidotthia cf. ramulicola. Also G 00127255 contains a few ascomata of a Dothidotthia sp. In W 1922-12005 some Diplodia sp. is present.
Petrak (1922) reported a specimen collected in Podhorn, Czech Republic from Ulmus sp., which he identified as Cucurbitaria ulmicola. He described ascospores as blackish brown with a very variable size, 30–60 × 14–24 μm and mostly 9–14/1–2 septa. By association on the natural host he described the putative asexual morph Pleurostromella ulmicola having conidia 2–3 × 0.75–1.2 μm, thus it is neither the asexual morph of Protofenestella ulmi (conidia ca. 3–5 × 1.3–2.2 μm) nor of S. ulmi, which did not produce an asexual morph in culture. All other species described from Ulmus under Cucurbitaria or Fenestella (C. naucosa (Fr.) Fuckel, C. ulmea P. Karst., Fenestella ulmicola Ellis & Everh.) were described with much smaller ascospores having much fewer septa than S. ulmi.
Discussion
Molecular Phylogeny
Although many earlier phylogenetic trees presented for the Pleosporales (e.g. Zhang et al., 2012, Hyde et al., 2013) pretended having been calculated using multigene matrices, they were basically constructed by using LSU (and SSU) sequences, as there is still lack of protein-coding markers for the vast majority of taxa. Even in most recent papers relevant to the topic (Crous and Groenewald, 2017, Wanasinghe et al., 2017b) primarily or exclusively ribosomal markers were used to construct trees, on which taxonomic conclusions were built. Although ITS, which was also used by these authors, improves resolution, it does not offer firm and reliable criteria to decide where to draw lines between families and genera. Resolution and statistical support of clades is also highly depending on the number and selection of taxa. Wanasinghe et al. (2017b) included in Cucurbitariaceae many Pyrenochaeta and Pyrenochaetopsis spp., which are shown by multigene analyses not to be part of the family (see below).
We performed an analysis based on ITS-LSU alone to elucidate the phylogenetic position of Neocucurbitaria acerina and of the new Fenestella spp. described by Wanasinghe et al. (2017b): as shown in Fig. 1, Parafenestella, with the three species P. mackenziei, P. ostryae and P. pseudoplatani, received low (54 % MP) to moderate (83 % ML) support. However, taxonomic conclusions based on ITS-LSU alone are in part ambiguous or problematic, as, e.g., Pyrenochaeta nobilis may be judged as belonging to the Cucurbitariaceae or not. Cucurbitaria berberidis and C. oromediterranea cannot be resolved with ITS-LSU, and also the genus Neocucurbitaria receives no support (Fig. 1).
Only inclusion of protein-coding phylogenetic markers considerably improves resolution and offers more stable and reliable topologies and hence bases for taxonomic inferences. Valenzuela-Lopez et al. (2018) included rpb2 and tub2 sequences for their phylogenetic work on phoma- and pyrenochaeta-like fungi, improving the framework for placing these coelomycetes in Cucurbitariaceae, Didymellaceae and other families and genera. Within Cucurbitariaceae, they added the two new genera Allocucurbitaria and Paracucurbitaria, recognized several additional species in Neocucurbitaria, and stabilised names such as N. cava (earlier known as Pyrenochaeta cava) by typification.
We included the taxa recognised by Valenzuela-Lopez et al. (2018) in Cucurbitariaceae in our multigene analyses represented by Fig. 2. As a result, Cucurbitariaceae are highly supported, and sister group relationship of the generic type Pyrenochaeta nobilis to all other Cucurbitariaceae receives low to moderate support. The family is subdivided into several well supported clades: In Cucurbitaria we presently recognise only two taxa from Berberis spp.: C. oromediterranea is phylogenetically clearly separated from C. berberidis, and can be molecularly distinguished by 26 and 12 diagnostic nucleotide substitutions in tef1 and tub2 sequences, respectively. Neocucurbitaria is the largest clade receiving high to maximum support in the multigene analysis. There are several highly supported clades in Neocucurbitaria. Two of them are host-specific, N. acanthocladae, N. aetnensis and N. cinereae on Genista spp. and N. rhamni, N. rhamnicola and N. rhamnioides on Rhamnus spp.; on the other hand the highly supported clade containing N. cisticola, N. juglandicola and N. populi among others consists of species occurring on unrelated hosts. There is also considerable morphological variation within Neocucurbitaria, but, e.g., the various shapes of ascomatal apices including ostiolar areas from inconspicuous and rounded to papillate or irregularly tubercular, furrowed or stellate, may occur within a single species (see, e.g., N. acanthocladae). Furthermore, furrowed or stellate ostiolar areas also occur in other subclades, e.g., N. ribicola.
A third major clade, the Fenestella clade (Fenestella and Parafenestella) is here only treated rudimentarily. Additional phylogenetic data to be included in a future work may show more clearly why erection of the genus Parafenestella is justified. Seltsamia is phylogenetically distinct from Allocucurbitaria, Astragalicola and the “Fenestella clade”. Clustering of Cucitella with Paracucurbitaria in the multigene tree is weakly supported (54 %) in MP and unsupported in ML analyses, i.e. the position of the former is uncertain within the Cucurbitariaceae, warranting its status as a distinct genus.
Comparison of the bootstrap trees of the individual markers show that especially the rpb2 gene contributes substantially to the phylogenetic resolution of many internal as well terminal nodes, whereas the tef1 especially contributes to resolution of closely related species. The same has also been observed in other lineages of Pleosporales, e.g. the Massarinaceae (Voglmayr & Jaklitsch 2017). This may be partly due to the high number of parsimony informative characters of rpb2 compared to the other markers, but also to the fact that rpb2 is a protein coding gene, which enables a highly reliable alignment compared to ITS and tef1 introns, which become difficult to align over a wider range of taxa due to frequent indels and subsequent genomic rearrangements, adding a substantial amount of homoplasy. In addition, the ITS-region, although established as the primary barcode of fungi for pragmatic reasons (Schoch et al. 2012), usually shows significantly less variation between closely related taxa than, e.g., the tef1 introns (e.g. Voglmayr et al. 2017), which therefore provide a far better resolution in case of closely related species. For these reasons, it is highly recommended that rpb2 and tef1 introns are sequenced and used in combined analyses with the ITS-LSU rDNA. However, as seen from the combined analyses (Fig. 2), additional suitable protein-coding genes will be necessary to further improve the resolution of many nodes.
Morphology
Although phylogenetic analyses are the major argument for generic delimitation, there are, however, also morphological features that characterize genera. We summarize these characters here:
Astragalicola: is only known from the asexual morph with rather large pycnidia having olivaceous contents with waxy to gelatinous consistency.
Cucurbitaria: produces conspicuously and coarsely warted ascomata with thick, basally thickened and elongated wall. Asexual morphs form setose pycnidia on the natural substrates and in artificial culture.
Cucitella: is fenestella-like, forms compact pustules and ascospores with several transverse and longitudinal septa, dark brown with lighter ends. It is therefore unfortunately only safely distinguishable from Fenestella phylogenetically.
Fenestella: forms pseudostromata; ascospores have many septa difficult to count. Peridium as in Neocucurbitaria.
Neocucurbitaria: ascomatal peridium thin, when thickened then only slightly in the upper part; pycnidia non-setose.
Parafenestella: ascomata aggregated or not, not forming well-delimited pseudostromata, papillate, ascospores fenestella- to (neo)cucurbitaria-like.
Protofenestella: like Fenestella, but ascomata immersed and evenly effused, not forming well-defined groups or pseudostromata.
Seltsamia: pleomassaria-like; ascospores with a bipartite swelling gelatinous sheath.
Asexual morphs in the Cucurbitariaceae
Since de Gruyter et al. (2010) Pyrenochaeta belongs to the Cucurbitariaceae. Pyrenochaeta differs from Phoma by setose pycnidia, while conidiophores commonly occur in Pyrenochaeta but rarely in Phoma (Sutton 1980). Phoma s. str. belongs to the Didymellaceae (de Gruyter et al. 2012). Schneider (1979) monographed Pyrenochaeta and neotypified its generic type, P. nobilis (ex-neotype culture CBS 407.76), collected in Italy on Laurus leaves. Its pycnidia are setose in nature and in artificial culture. Although P. berberidis, the asexual morph name and now a synonym of C. berberidis, has the same features, Schneider (1979) did not accept it in Pyrenochaeta, but included P. quercina and P. unguis-hominis, both of which are now in Neocucurbitaria (Wanasinghe et al., 2017b, Valenzuela-Lopez et al., 2018). Three other species, N. cava, N. hakeae and N. keratinophila, hitherto classified in Pyrenochaeta, were combined in Neocucurbitaria by Valenzuela-Lopez et al. (2018), who also placed Pyrenochaeta corni in their new genus Paracucurbitaria and excluded Pyrenochaeta nobilis from the Cucurbitariaceae.
Valenzuela-Lopez et al. (2018) reported abundant setae in the description of the family Cucurbitariaceae, the generic description of Neocucurbitaria and in N. quercina, citing an old description for that species. In culture of N. quercina, however, they found very short, thin-walled pycnidial setae rounded at the top, which may rather be interpreted as hyphal outgrowths. We found setose pycnidia only in asexual morphs of Cucurbitaria s. str. Asexual morphs of all other representatives of the Cucurbitariaceae we studied may be called phoma-like, because they produce pycnidia that may have hyphal appendages but lack setae. However, pycnidia in nature show a marked tendency to produce conidiophores with lateral pegs and lageniform phialides (acropleurogenous), but in culture conidiophores are often absent and only sessile, nearly globose conidiogenous cells are formed, i.e. the “two different ways of conidiation” as addressed, e.g., by de Gruyter et al. (2010), are in fact only dependent on external/environmental conditions for one and the same species. This fact has already been surmised for Nigrograna mackinnonii, which did not form conidiophores in culture (de Gruyter et al. 2012), while pycnidia of other Nigrograna species produced conidiophores on the natural substrate (Jaklitsch & Voglmayr 2016). Thus, formation of pycnidia in artificial culture represents an unnatural condition, and conidiophores may be produced or not. In conclusion, presence or absence of conidiophores in pycnidia formed in culture is no taxonomic criterion on the generic and even species level.
Hosts and Distribution
It is difficult to specifically collect fungi belonging to Cucurbitariaceae. Much more common are camarosporium-like fungi, which formerly belonged to Cucurbitaria, particularly Camarosporidiella, Camarosporium, Neocamarosporium, Paracamarosporium (incl. Pseudocamarosporium; see, e.g., Crous and Groenewald, 2017, Wanasinghe et al., 2017a), Staurosphaeria, or taxa of the Melanommataceae (Jaklitsch & Voglmayr 2017), are difficult to differentiate from the Cucurbitariaceae morphologically, and they may occur on the same hosts. As an example, we collected three phylogenetically different but morphologically similar fungi on Pyrus not included in this work. As a consequence, every single specimen has to be cultured and sequenced to be certain about phylogenetic relationships and generic affinities.
Sexual morphs of Cucurbitariaceae occur on wood and bark of various trees and shrubs and less commonly on tougher herbaceous plants with lignified tissues (Astragalus) and appear to be host-specific (Mirza 1968 pro parte and this work). Asexual morphs may occur on such substrates, but those for which no sexual morph is known, also occur on various other substrates, particularly on leaves but also in soil and in medical environments (Valenzuela-Lopez et al. 2018). Based on current data, Cucurbitariaceae occur in temperate and Mediterranean climates. More collecting is necessary to draw a more complete picture.
Acknowledgements
We thank the fungarium curators of B, G, K, M, PC, UPS, W for sending and W. Till (WU) for managing collections, A. Igersheim for information on specimens in W, M. Mokhtari for organizing excursions in Morocco, B. Norden for organizing an excursion in Norway, F. J. Rejos (AH) for organizing excursions in Guadalajara, M. Becerra, P. Bormans, O. Eriksson, G. Friebes, I. Greilhuber, S.-Å. Hanson, M. Louay, R. Moosbeckhofer and B. Wergen for fresh material or data. The financial support by the Austrian Science Fund (FWF; project P25870-B16) is gratefully acknowledged.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Ariyawansa H.A., Hyde K.D., Jayasiri S.C. Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity. 2015;75:27–274. [Google Scholar]
- Arnold R.H. Vol. 17. National Mycological Herbarium, Biosystematics Research Institute, Agriculture Canada; Ottawa, Ontario: 1974. (Cucurbitaria staphula. Fungi Canadenses). [Google Scholar]
- Arnold R.H., Russell R.C. Cucurbitaria staphula on Populus and its association with Macrophoma tumefaciens. Mycologia. 1960;52:499–512. [Google Scholar]
- Aveskamp M.M., de Gruyter J., Woudenberg J.H.C. Highlights of the Didymellaceae: a polyphasic approach to characterize Phoma and related pleosporalean genera. Studies in Mycology. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M.E. The genus Curreya: An example of taxonomic confusion in the ascomycetes. Mycologia. 1981;73:599–609. [Google Scholar]
- Barr M.E. On the Pleomassariaceae (Pleosporales) in North America. Mycotaxon. 1982;15:349–383. [Google Scholar]
- Barr M.E. University of Massachusetts; Amherst, Massachusetts: 1987. Prodromus to class Loculoascomycetes. [Google Scholar]
- Barr M.E. Some dictyosporous genera and species of Pleosporales in North America. Memoirs of the New York Botanical Garden. 1990;62:1–92. [Google Scholar]
- Barr M.E. Melanommatales (Loculoascomycetes) North American Flora. Series II. 1990;13:1–129. [Google Scholar]
- Barr M.E., Boise J.R. Syncarpella (Pleosporales, Cucurbitariaceae) Memoirs of the New York Botanical Garden. 1989;49:298–304. [Google Scholar]
- Berkeley M.J., Broome C.E. Notices of British Fungi. The Annals and Magazine of Natural History Including Zoology, Botany, and Geology, ser. 1859;3(3):356–377. [Google Scholar]
- Boerema G.H., de Gruyter J., van Kesteren H.A. Contributions towards a monograph of Phoma (Coelomycetes) — III. 1. Section Plenodomus: Taxa often with a Leptosphaeria teleomorph. Persoonia. 1994;15:431–487. [Google Scholar]
- Boerema G.H., de Gruyter J., Noordeloos M.E. CABI Publishing, CAB International; Wallingford, UK: 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. 448 pp. [Google Scholar]
- Boerema G.H., van Kesteren H.A. Nomenclatural notes on some species of Phoma sect. Plenodomus. Persoonia. 1981;11:317–331. [Google Scholar]
- Boerema G.H., Loerakker W.M., Hamers M.E.C. Contributions towards a monograph of Phoma (Coelomycetes) — III. 2. Misapplications of the type species name and the generic synonyms of section Plenodomus (Excluded species) Persoonia. 1996;16:141–190. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Checa J., Jaklitsch W.M., Blanco M.N. Two new species of Thyronectria from Mediterranean Europe. Mycologia. 2015;107:1314–1322. doi: 10.3852/15-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Jiang J.R., Zhang G.Z. Resolving the Phoma enigma. Studies in Mycology. 2015;82:137–217. doi: 10.1016/j.simyco.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W. Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Diversity. 2009;38:1–24. [Google Scholar]
- Crous P.W., Groenewald J.Z. The Genera of Fungi – G 4: Camarosporium and Dothiora. IMA Fungus. 2017;8:131–152. doi: 10.5598/imafungus.2017.08.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Carnegie A.J. Unravelling Mycosphaerella: do you believe in genera? Persoonia. 2009;23:99–118. doi: 10.3767/003158509X479487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey F. Synopsis of the fructification of the compound Sphaeriae of the Hookerian herbarium. The Transactions of the Linnean Society of London. 1859;22 257–287 + pl. 45–49. [Google Scholar]
- de Gruyter J.D., Woudenberg J.H.C., Aveskamp M.M. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia. 2010;102:1066–1081. doi: 10.3852/09-240. [DOI] [PubMed] [Google Scholar]
- de Gruyter J.D., Woudenberg J.H.C., Aveskamp M.M. Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology. 2012;75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog G.S., Gerrits van den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Doilom M., Liu J.K., Jaklitsch W.M. An outline of the family Cucurbitariaceae. Sydowia. 2013;65:167–192. [Google Scholar]
- Fries E. Sphaeria frit. Systema mycologicum (Lundae) 1823;2(2):395. [Google Scholar]
- Fuckel L. [1869–70]. Symbolae Mycologicae. Beiträge zur Kenntnis der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde. 1870;23–24:1–459. [Google Scholar]
- Fuckel L. [1871–72]. Symbolae Mycologicae. Beiträge zur Kenntnis der Rheinischen Pilze. Erster Nachtrag. Jahrbücher des Nassauischen Vereins für Naturkunde. 1871;25–26:287–346. [Google Scholar]
- Grove W.B. The British species of Phomopsis. Bulletin of Miscellaneous Information, Royal Botanic Gardens, Kew. 1917;2:49–73. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis, program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Huhndorf S.M., Glawe D.A. Pycnidial development from ascospores of Fenestella princeps. Mycologia. 1990;82:541–548. [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.-K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Jaklitsch W.M. European species of Hypocrea – Part I. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Barr M.E. Dictyoporthe bipapillata - a new combination and a key to the species of Dictyoporthe. Österreichische Zeitschrift für Pilzkunde. 1997;6:45–49. [Google Scholar]
- Jaklitsch W.M., Komon M., Kubicek C.P. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M., Olariaga I., Voglmayr H. Teichospora and the Teichosporaceae. Mycological Progress. 2016;15 doi: 10.1007/s11557-016-1171-2. 31 (1–20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Stadler M., Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology. 2016;85:35–64. doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Three former taxa of Cucurbitaria and considerations on Petrakia in the Melanommataceae. Sydowia. 2017;69:81–95. doi: 10.12905/0380.sydowia69-2017-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten P.A., Hariot P. Ascomycetes novi. Revue Mycologique Toulouse. 1890;12(48):169–173. [Google Scholar]
- Kauff F., Lutzoni F. Phylogeny of Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Kuntze O. Vol. 3. Arthur Felix; Germany, Leipzig: 1898. pp. 1–576. (Revisio generum plantarum). [Google Scholar]
- Landvik S., Egger K., Schumacher T. Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nordic Journal of Botany. 1997;17:403–418. [Google Scholar]
- Liu Y.L., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Mirza F. Taxonomic investigations on the ascomycetous genus Cucurbitaria S. F. Gray. Nova Hedwigia. 1968;16:161–213. [Google Scholar]
- Müller K. PRAP - calculation of Bremer support for large data sets. Molecular Phylogenetics and Evolution. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Nirenberg H.I. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem. 1976;169(i–v):1–117. [Google Scholar]
- O'Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Petrak F. Mykologische Notizen IV. Annales Mycologici. 1922;20:300–345. [Google Scholar]
- Petrak F., Ahmad S. Beiträge zur Pilzflora Pakistans. Sydowia. 1954;8:162–185. [Google Scholar]
- Phookamsak R., Hyde K.D. Fenestellaceae. Mycosphere. 2015;6:402–413. [Google Scholar]
- Quélet L. Quelques especes critiques ou nouvelles de la Flore Mycologique de France. Comptes Rendus de l'Association Française pour l'Avancement des Sciences. 1883;11:387–412. [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Riethmüller A., Voglmayr H., Göker M. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia. 2002;94:834–849. doi: 10.1080/15572536.2003.11833177. [DOI] [PubMed] [Google Scholar]
- Saccardo P.A. Sphaeropsideae, Sphaerioideae, Hyalosporae, Phoma. Sylloge Fungorum. 1892;10:153. [Google Scholar]
- Saccardo P.A. Deuteromycetae, Sphaerioidaceae, Phoma. Sylloge Fungorum. 1913;22:876. [Google Scholar]
- Schnabl J.N. Mykologische Beiträge zur Flora Bayerns. Berichte der Bayerischen Botanischen Gesellschaft. 1892;2:63–69. [Google Scholar]
- Schneider R. Die Gattung Pyrenochaeta De Notaris. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem. 1979;189:1–73. [Google Scholar]
- Schoch C.L., Seifert K.A., Huhndorf S. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D., Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution. 2012;12:335–337. [Google Scholar]
- Sivanesan A. J. Cramer; Vaduz: 1984. The bitunicate Ascomycetes and their anamorphs. [Google Scholar]
- Slippers B., Boissin E., Phillips A.J.L. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Studies in Mycology. 2013;76:31–49. doi: 10.3114/sim0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Sung G.H., Sung J.M., Hywel-Jones N.L. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localised incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sutton B.C. Commonwealth Mycological Institute; Kew: 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers B. New York Botanical Garden's Virtual Herbarium; 2017. Index Herbariorum: A global directory of public herbaria and associated staff.http://sweetgum.nybg.org/ih/ [Google Scholar]
- Tulasne L.R., Tulasne C. 1863. Selecta Fungorum Carpologia. 2. i–xix, 319 pp., 34 tabs. Paris. [Google Scholar]
- Valenzuela-Lopez N., Cano-Lira J.F., Guarro J. Contribution to the taxonomy of Dothideomycetes; the families Cucurbitariaceae and Didymellaceae. Studies in Mycology. 2018;90 doi: 10.1016/j.simyco.2017.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Akulov O.Y., Jaklitsch W.M. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycological Progress. 2016;15:921. doi: 10.1007/s11557-016-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Castlebury L.A., Jaklitsch W. Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales) Persoonia. 2017;38:136–155. doi: 10.3767/003158517X694768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Gardiennet A., Jaklitsch W.M. Asterodiscus and Stigmatodiscus, two new apothecial dothideomycete genera and the new order Stigmatodiscales. Fungal Diversity. 2016;80:271–284. doi: 10.1007/s13225-016-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Diversity. 2011;46:133–170. [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Corynespora, Exosporium and Helminthosporium revisited – new species and generic reclassification. Studies in Mycology. 2017;87:43–76. doi: 10.1016/j.simyco.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arx J.A., Müller E. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology. 1975;9:1–159. [Google Scholar]
- Von Arx J.A., van der Aa H.A. Notes on Curreya (Ascomycetes, Dothideales) Sydowia. 1983;36:1–5. [Google Scholar]
- Von Niessl G. Beiträge zur Kenntniss der Pilze. Verhandlungen des Naturforschenden Vereines in Brünn. 1872;10 153–217, pl. 3–7. [Google Scholar]
- Wanasinghe D.N., Hyde K.D., Jeewon R. Phylogenetic revision of Camarosporium (Pleosporineae, Dothideomycetes) and allied genera. Studies in Mycology. 2017;87:207–256. doi: 10.1016/j.simyco.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe D.N., Phookamsak R., Jeewon R. A family level rDNA based phylogeny of Cucurbitariaceae and Fenestellaceae with descriptions of new Fenestella species and Neocucurbitaria gen. nov. Mycosphere. 2017;8:397–414. [Google Scholar]
- Welch D.S. A monographic study of the genus Cucurbitaria in North America. Mycologia. 1926;18:51–86. [Google Scholar]
- Werle E., Schneider C., Renner M. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: A guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Wiens J.J. Combining datasets with different phylogenetic histories. Systematic Biology. 1998;47:568–581. doi: 10.1080/106351598260581. [DOI] [PubMed] [Google Scholar]
- Winter H.G. Pilze. II. Abtheilung. Ascomyceten: Gymnoasceen und Pyrenomyceten. In: Rabenhorst G.L., editor. 2nd edn. 1(2) Kummer; Leipzig: 1885. pp. 65–528. (Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz). [Google Scholar]
- Zalasky H. Cell deformities in bark and sapwood caused by Rhytidiella moriformis and Keissleriella emergens infections in poplar. Canadian Journal of Botany. 1975;53:780–783. [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L., Hyde K.D. Pleosporales. Fungal Diversity. 2012;53:1–221. doi: 10.1007/s13225-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]