Abstract
γ-aminobutyric acid (GABA) begins as the key excitatory neurotransmitter in newly forming circuits, with chloride efflux from GABA type A receptors (GABAARs) producing membrane depolarization, which promotes calcium entry, dendritic outgrowth and synaptogenesis. As development proceeds, GABAergic signaling switches to inhibitory hyperpolarizing neurotransmission. Despite the evidence of impaired GABAergic neurotransmission in neurodevelopmental disorders, little is understood on how agonist-dependent GABAAR activation controls the formation and plasticity of GABAergic synapses. We have identified a weakly depolarizing and inhibitory GABAAR response in cortical neurons that occurs during the transition period from GABAAR depolarizing excitation to hyperpolarizing inhibitory activity. We show here that treatment with the GABAAR agonist muscimol mediates structural changes that diminish GABAergic synapse strength through postsynaptic and presynaptic plasticity via intracellular Ca2+ stores, ERK and BDNF/TrkB signaling. Muscimol decreases synaptic localization of surface γ2 GABAARs and gephyrin postsynaptic scaffold while β2/3 non-γ2 GABAARs accumulate in the synapse. Concurrent with this structural plasticity, muscimol treatment decreases synaptic currents while enhancing the γ2 containing benzodiazepine sensitive GABAAR tonic current in an ERK dependent manner. We further demonstrate that GABAAR activation leads to a decrease in presynaptic GAD65 levels via BDNF/TrkB signaling. Together these data reveal a novel mechanism for agonist induced GABAergic synapse plasticity that can occur on the timescale of minutes, contributing to rapid modification of synaptic and circuit function.
Keywords: GABA type A receptors, Inhibition, Neuronal development, Synaptic plasticity, Muscimol, ERK, BDNF, Calcium
1. Introduction
Synaptic development occurs first with the emergence of excitatory GABAergic signals that drive the subsequent establishment of glutamatergic responses (Ben-Ari et al., 2007; Deng et al., 2007). At this time, GABA depolarizes neurons due to a high intracellular chloride level ([Cl−]i) maintained by the cation-chloride importer NKCC1. This depolarizing response relies on chloride efflux via GABAARs and leads to an increase in intracellular calcium ([Ca2+]i) through activation of voltage gated calcium channels and removal of magnesium block from NMDA receptors. The transition from a depolarizing to hyperpolarizing response occurs due to a rise in expression of the K-Cl extruder KCC2 that reduces [Cl−]i (Kaila et al., 2014), with in vitro cultured neuron and brain slice studies reporting the switch occurs in most brain areas (cerebellum, hypothalamus, cortex, hippocampus) during the second week of development (Ganguly et al., 2001; He et al., 2014; Mueller et al., 1984; Obrietan and van den Pol, 1995; Owens et al., 1996; Succol et al., 2012; Tyzio et al., 2007). Depolarizing GABAAR responses during development and in the adult brain can either promote excitation or generate shunting inhibition, depending on the magnitude of depolarization (Chiang et al., 2012; Khalilov et al., 2015; Staley and Mody, 1992). Importantly, two recent in vivo studies demonstrated that GABA primarily depolarizes and inhibits network activity in the intact neonatal cortex around P3–P9, although the timing of the developmental switch to a hyperpolarizing response in vivo was not identified (Kirmse et al., 2015; Valeeva et al., 2016).
GABAARs are heteropentamers typically composed of two α (α1–6), two β (β1–3), and either a γ (γ1–3) or a δ subunit (Olsen and Sieghart, 2008). γ2 subunits are expressed in 90% of GABAARs in the CNS (Lee and Maguire, 2014), with the major subtype being α1β2γ2, followed by the abundant α3β3γ2 and α2β3γ2 subtypes (Nutt, 2006). Despite the great diversity of GABAAR subunits and possible configurations, these receptors produce two types of currents: synaptic (phasic) and tonic. Presynaptic terminal release of GABA onto postsynaptically clustered GABAARs triggers fast, transient synaptic currents, while ambient GABA that “spills over” results in activation of extrasynaptic receptors that generate a persistent tonic current. Subunit composition determines receptor cell surface localization as well as physiological and pharmacological properties. Receptors containing α1–α3 subunits are primarily located at GABAergic synapses via direct association with gephyrin (Mukherjee et al., 2011; Tretter et al., 2008, 2011), the key GABAergic and glycinergic postsynaptic scaffolding protein, while the γ2 subunit is required for maintenance of postsynaptic receptors and gephyrin (Essrich et al., 1998). While predominantly clustered at synapses, γ2 containing receptors are also found at extrasynaptic sites and contribute to tonic current that is sensitive to positive allosteric modulation by the benzodiazepine drug class (Prenosil et al., 2006). Extrasynaptic γ2 GABAARs play a significant role in tonic inhibition, with benzodiazepines able to enhance the level of the tonic current by approximately two fold (Korol et al., 2015). Knockout of the γ2 subunit removes 94% of the benzodiazepine binding sites in the brain and benzodiazepine potentiation of GABA currents (Gunther et al., 1995). Although synaptic and extrasynaptic receptors are often described as distinct entities, single molecule tracking, electrophysiological, biochemical and other imaging based methods have revealed that receptors in both populations diffuse continually in the plasma membrane, with enhanced accumulation of synaptic receptors depending on interactions between gephyrin and GABAAR.
Many studies have improved our understanding of the formation of GABAergic synapses, however little is known about GABAAR agonist induced synaptic plasticity mechanisms. The aim of this study was to investigate the role of GABAAR activation in modulation of GABAergic synaptic plasticity after synapse establishment. Here we reveal that agonist induced GABAergic synaptic plasticity can occur on the timescale of minutes, contributing to rapid modification of synaptic and circuit function. Importantly, this depolarizing and inhibitory GABAAR response results in structural and functional synaptic changes that rely on release of intracellular Ca2+ stores, ERK and BDNF/TrkB signaling.
2. Materials and methods
2.1. Neuron culture and transfection
All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Cortical neurons were prepared from embryonic day 18 rats and nucleofected (Lonza) at plating as needed (Jacob et al., 2005).
2.2. Immunofluorescence, imaging and analysis of cells
Surface GABAAR γ2 (Rbt, 1:1000, Synaptic Systems, #224003) or β2/3 (Mouse, 1:200, Millipore, MAB341) was done under non-permeabilized conditions, followed by permeabilization and staining for endogenous gephyrin (Mouse, 1:300, Synaptic Systems, #147011 mAb7A), VIATT (Rbt 1:1000, Synaptic Systems, #131002), or GAD65 (Guinea pig, 1:500, Synaptic Systems, #198104) as previously described (Jacob et al., 2005). Total and phospho ERK immunofluorescence studies used ERK antibody (Rabbit, 1:2000, Cell Signaling #9107) and phospho ERK antibody (Rabbit, 1:200, Cell Signaling #9102). Drug treatments were performed as described in the Drug treatments and Western Blots section of the methods. Images were taken on a Nikon A1 Confocal microscope using a 60× oil immersion objective (NA 1.49) at a 2× zoom. Data were analyzed using NIS Elements software (Nikon, NY). In all immunofluorescence studies, 20 µm length regions of interest (ROIs) from two to three proximal dendrites per neuron were used for analysis. For ERK and phospho ERK measurements, the sum intensity was quantified for each ROI. For synaptic GABAAR and gephyrin measurements, a 1-bit binary image was made of the presynaptic GABAergic terminal marker (GAD65 or VIAAT) and only surface GABAAR or gephyrin fluorescence that colocalized with the synaptic binary were measured. For the total dendrite measurements, surface GABAAR, gephyrin, VIAAT or GAD65 fluorescence was quantified for the entire dendritic ROI. The value quantified is the sum fluorescence intensity: pixel intensity values within a given ROI or synaptic region are added, thus containing a combined measurement of area and intensity changes. For neuron immunofluorescence analysis and calcium imaging, 3–5 independent neuron cultures were used for each experiment. Finally, image acquisition and analysis was performed blind to experimental condition and laser settings for acquisition and thresholding values for analysis were kept constant between cultures.
2.3. Drug treatments and western blots
For muscimol only drug treatments, cortical neuronal cultures were treated for either 0 or 30 min in HEPES-buffered imaging solution (HBS) containing 135 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 10 mM HEPES, 2.5 mM CaCl2, 11 mM glucose, pH 7.4. Other drug treatments were performed as follows: 5 min pretreatment with 1 µM TTX or HBS control, then 0 or 30 min treatment with 50 µM muscimol; 30 min pretreatment with 1 µM U0126 or DMSO in HBS control, then 0 or 30 min treatment with 50 µM muscimol; 1 h pretreatment with 200 nM thapsigargin and 50 µM BAPTA-AM (TGBA) or DMSO in HBS control, then 0 or 30 min treatment with 50 µM muscimol; 30 min pretreatment with 10 µM Nimodipine or DMSO in HBS control, then 0 or 30 min treatment with 50 µM muscimol; 10 min pretreatment with 20 µM bicuculline or DMSO in HBS control; 30 min pretreatment with 10 µM ANA-12 or DMSO in HBS control, then 0 or 30 min treatment with 50 µM muscimol. For muscimol treatments with and without extracellular calcium, cells were pretreated for 30 min either in normal HBS or HBS −Ca2+ (0 mM CaCl2 + 1 mM EGTA), then treated with 50 µM muscimol for 0 or 30 min. For synaptic stimulation by KCl (50 mM KCl in HBS(equimolar substitution of KCl for NaCl: 89.7 mM NaCl, 50 mM KCl, 1.2 mM MgCl2, 10 mM HEPES, 2.5 mM CaCl2, 11 mM glucose, pH 7.4)), cells were washed once with HBS or HBS −Ca2+ and then were treated for 3 min with either HBS, HBS −Ca2+, or KCl ± Ca2+ (0 mM CaCl2 + 1 mM EGTA) solution. At the end of treatment, cells were lysed in RIPA buffer containing 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 1 mM EDTA, 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 2 mM sodium orthovanadate, and protease inhibitor cocktail (Sigma, St. Louis, MO), solubilized for 20–30 min at 4 °C, and spun at 15000 × g for 15 min. For acute cortical slice experiments, slices were incubated in artificial cerebrospinal fluid (ACSF: 124 mM NaCl, 3 mM KCl, 25 mM NaHCO3, 2 mM CaCl2, 1.1 mM NaH2PO4, 2 mM MgSO4, 10 mM d-glucose) at 37 °C for 1 h for recovery before experimentation. Slices from the same animal were then treated for either 0, 5 or 30 min in ACSF containing 50 µM muscimol, followed by lysis in homogenization buffer (20 mM Tris 7.4, 1 mM EDTA, 320 mM sucrose, 20 mM Na pyrophosphate, 10 mM NaF, 20 mM β-glycerophosphate) and centrifugation (1000 rcf for 10 min) to isolate the supernatant. A BCA protein assay was performed on the supernatant, and equivalent amounts of protein were loaded onto 8% Tris gels. After electrophoresis and transfer to nitrocellulose, samples were probed with primary antibody overnight [pERK 1:2000 (Rbt, Cell Signaling, #4370), total ERK 1:2000 (Mouse, Cell Signaling, #9107)], followed by the appropriate secondary antibody. If for presentation purposes, lanes from the same western blot were spliced together, the splice site is indicated by a dashed vertical line. For each sample lane, pERK levels were normalized to total ERK levels. For analysis, two replicates of each sample were loaded and averaged, and all samples were normalized to the averaged control values for the t0 muscimol treatment (or the no drug pretreatment t0 muscimol values) for each blot. For western blot analysis of in vitro neuronal cultures 3–9 independent cultures were used for each experiment.
2.4. Ca2+ imaging and analysis
Primary rat cortical neurons were incubated in Fura 2-AM (3 µM; Molecular Probes, Eugene, OR, USA) for 60 min at 37 °C in 5% CO2. Fura 2-AM was prepared in HBS containing bovine serum albumin (5 mg/ml: Sigma Aldrich) to promote dye loading. Calcium imaging was performed as described previously (Zhang et al., 2012). Briefly, loaded coverslips were placed on an inverted epifluorescence microscope chamber (IX70; Olympus, Tokyo, Japan) and constantly perfused (2 ml/min) with saline by a gravity-driven Y-system. Fura-2 was excited by 340 nm and 380 nm UV light and fluorescence emission at 510 nM was captured using a monochromator. Images were taken at 20× magnification every 3s, 10s or 30s by use of illumination lasting between 250 and 450 ms. Drugs were prepared in HEPES buffered saline from concentrated stock solutions and applied via perfusion during times indicated in figures. For calcium imaging experiments, 10 µM Nimodipine was applied for 5 min prior to muscimol application. Images were captured and analyzed using the program C-imaging (Compix, Cranberry Township, PA). A region of interest (ROI) was made around each cell and the average value of fluorescent intensity was calculated. Background fluorescence was subtracted from images to minimize autofluorescence and dark noise. Changes in [Ca+2]i were determined by the ratio of 340/380 nm fluorescent signal measured in a cell body ROI. Prior to treatment, all experiments were allowed to perfuse 5 min in saline to determine baseline [Ca+2]i. Magnitude of [Ca+2]i in response to treatment was calculated from the difference in the peak change in amplitude from baseline. Only cells which had amplitudes 5% > than baseline and responded to a brief 80 mM KCl exposure were used for calculations. Data analysis was performed using a custom analysis program created in MatLab (TheMathWorks).
2.5. Electrophysiology
Cortical neurons were continuously perfused with saline solution containing the following (in mM) 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.5 CaCl2, 11 d-Glucose and 10 HEPES, adjusted to pH 7.4 with 1 M NaOH. For perforated patch experiments, the recordings were performed at room temperature with gramicidin (20 µg/ml). The recording electrodes contained the following (in mM) 140 KCl and 10 HEPES, adjusted to 7.4 with 1 M KOH. To determine the reversal potential of GABAA receptor-mediated currents (EGABA), we voltage-clamped neurons at specified holding potentials (10 mV increments in either direction beginning at −60 mV) before and after the application of GABAAR agonist muscimol. Experiments were performed with bath application of TTX (1 µM), DNQX (10 µM) and AP5 (40 µM) to block voltage-gated sodium channels, AMPA receptors and NMDA receptors, respectively. Muscimol (10 µM) was applied through a pico-spritzer electrode placed 50–100 µm away from the neuron of interest. Recordings were started after the establishment of an access resistance below 30 MΩ, usually about 10 min after seal formation. For each cell, PSC-amplitudes were measured and plotted against holding potential. For miniature inhibitory postsynaptic current (mIPSC) data, the recordings were performed at 32 °C. The recording electrodes contained the following (in mM) 140 CsCl, 0.1 CaCl2, 2 MgCl2, 2.5 Phosphocreatine, 1.1 EGTA, 2 ATP-Mg, 1 GTP-Na and 10 HEPES, adjusted to 7.2 with 1 M CsOH. Cells were held at −60 mV. mIPSCs were recorded in the presence of TTX (1 µM), DNQX (10 µM) and AP5 (40 µM) to block voltage-gated sodium channels, AMPA receptors and NMDA receptors, respectively. To detect mIPSCs, obtained from 2-min-long continuous recordings, the threshold for event detection was set at 3 × root mean square noise level. Software-detected events were visually verified, and their frequency, amplitude, 10–90% rise time and decay time were measured. The decay phase of averaged mIPSCs were fitted to a mono exponential decay function in Mini Analysis Program. In a subset of recordings, neurons were pre-incubated for 10 min in MEK inhibitor U0126 (1 µM), followed by 30 min in saline containing muscimol (50 µM). For tonic inhibition, the positive allosteric GABAAR modulator flurazepam (3 µM) was applied along with the above blockers and later bicuculline (50 µM) was applied through a pico-spritzer electrode. For tonic current measurements, an all-points histogram was plotted for approximately a 10-s period immediately before and during application of bicuculline (50 µM). Tonic currents are represented as the change in baseline amplitude, normalized to capacitance. We obtained all data using an Axon Multiclamp 700 B amplifier (Molecular devices) and Signal software (CED), filtered at 1–2 kHz and digitized at 10–20 kHz and stored digitally. Series resistance (RS) was not compensated and experiments were discarded if RS changed by more than 20% during the recordings. Data processing was performed using Clampfit 10.3 (Molecular Devices), and Mini Analysis Program 6 (Synaptosoft) software.
2.6. Statistical analysis
For all studies, statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Either unpaired t-tests, one way ANOVA, or two way ANOVA statistical tests with post hoc t-tests were used where appropriate, and are indicated in the text. All data are reported as mean ± SEM unless otherwise indicated.
3. Results
3.1. Identification of a depolarizing and inhibitory GABAAR response after the major phases of dendritic outgrowth and synaptogenesis
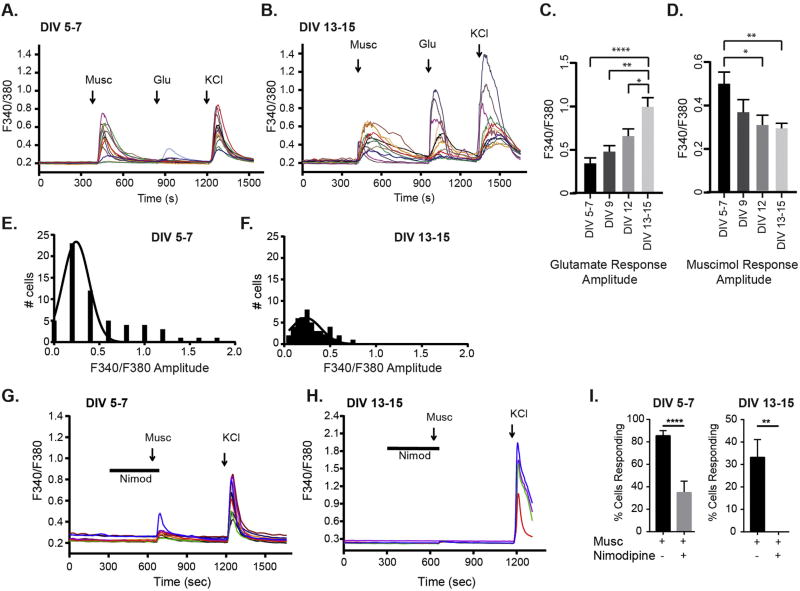
To identify the transition period from depolarizing excitation driven by GABAAR activation to when glutamatergic responses predominate, we performed calcium imaging experiments using the ratiometric dye Fura-2 AM. We compared [Ca2+]i responses to glutamate and the GABAAR agonist muscimol from the period of GABAergic synaptogenesis (days in vitro DIV 5–7) to when these synapses have matured and glutamatergic synapses are established (DIV 13–15) (Craig et al., 1996; Danglot et al., 2003). All experiments concluded with a final depolarizing KCl stimulus as a positive control for cell viability. Glutamate (20 µM) evoked a small [Ca2+]i rise at DIV 5–7, with amplitudes and responses increasing by DIV 13–15 (Fig. 1A, B, C), in line with normal glutamatergic synapse development. Muscimol (50 µM) evoked a large [Ca2+]i rise early in development (Fig. 1A, B, D). As expected with early excitatory GABAergic signaling, this [Ca2+]i rise was completely blocked by the competitive GABAAR antagonist bicuculline (% cells responding: Musc 79 ± 4, Musc + bicuculline = 0 ± 0, mean ± SEM, n = 76–103 neurons per treatment, t-test, ****p < 0.0001). Neurons at DIV 5–7 showed the highest [Ca2+]i rise with muscimol treatment; as development proceeded, the response significantly diminished (Fig. 1A,B, D). The overall decrease in muscimol evoked [Ca2+]i response in older neurons is due to both smaller response amplitudes and a decrease in the number of events (Fig. 1E, F). The muscimol evoked rise in [Ca2+]i occurs primarily via L-type voltage gated calcium channels (VGCCs) as the L-type VGCC blocker nimodipine blocked the response in neurons of both ages (Fig. 1 G, H, I: % cells responding at DIV 5 Musc = 86 ± 4, Musc + nimodipine = 35 ± 10, n = 71–169 neurons per treatment, t-test, ****p < 0.0001; % cells responding at DIV 13 Musc = 33 ± 8%, Musc + nimodipine = 0 ± 0%, n = 56–93 neurons, t-test, **p < 0.01).
Fig. 1.
Persistent depolarizing GABAAR response across development. A,B, [Ca2+]i responses to muscimol and glutamate at DIV 5–7 and DIV 13–15. Colored traces represent different neuron responses from the same coverslip in a single experiment. C, The average amplitude response to glutamate increased over time. D, The average [Ca2+]i amplitude response to muscimol decreased across development. (C,D: Mean ± SEM, n = 13–57 neurons per DIV period, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, one-way ANOVA followed by Tukey's post hoc test). E, F. Amplitude histograms showing muscimol response range at DIV 5–7 (E) and DIV 13–15 (F). G–I. L-type VGCC blocker nimodipine (Nimod) block the muscimol responses in neurons of both ages: % cells responding at DIV 5–7 Musc = 86 ± 4, Musc + nimodipine = 35 ± 10, n = 71–169 neurons per treatment, t-test, ****p < 0.0001; % cells responding at DIV 13–15 Musc = 33 ± 8%, Musc + nimodipine = 0 ± 0%, n = 56–93 neurons, t-test, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
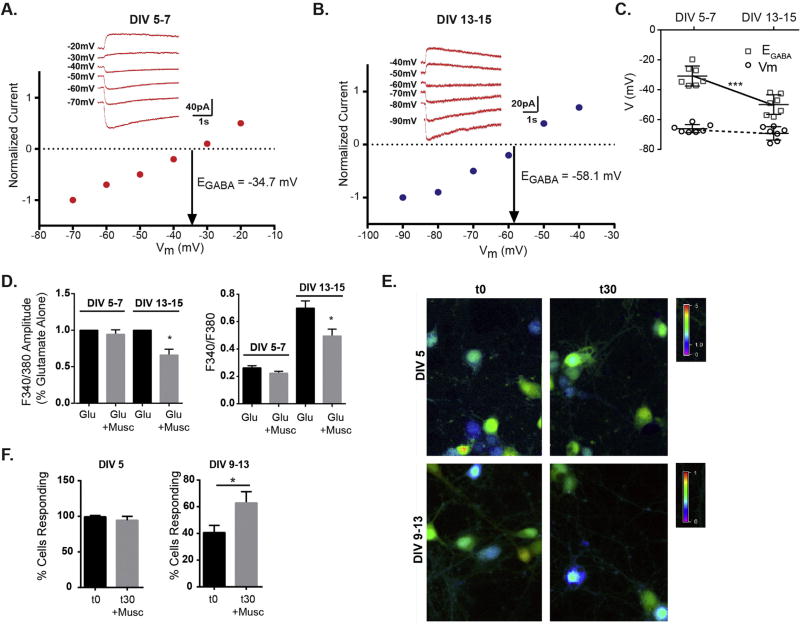
The muscimol evoked modest [Ca2+]i rise observed in DIV 13–15 neurons suggests that GABAAR activity is still depolarizing, despite the initial expectation that GABAAR activity would have switched to a hyperpolarizing response and not result in a [Ca2+]i rise. To unequivocally define the GABAAR response, we used gramicidin-perforated patch recordings, a method that preserves endogenous intracellular Cl− concentration, to determine the reversal potential for GABAAR (EGABA) and the resting membrane potential (Vm) at DIV 5–7 and DIV 13–15. We found that EGABA was depolarized relative to the resting membrane potential in neurons of both age ranges, indicating a depolarizing response (Fig. 2A, B). However, younger neurons exhibited more positive EGABA values than older neurons while the resting membrane potential was equivalent between the two ages of neurons (Fig. 2C). These data indicate that young neurons are more depolarized by GABAAR activity, and that in older neurons, EGABA is still positive to Vm producing a weak depolarization that is likely to shunt membrane conductance and inhibit overall excitability. This data is consistent with the diminished muscimol evoked [Ca2+]i rise at DIV 13–15 seen in Fig. 1. To test the functional effect of GABAAR activation on coincident glutamatergic activity we compared the glutamate induced [Ca2+]i rise to the combined treatment of glutamate and muscimol in DIV 13–15 neurons and DIV 5–7 neurons. Muscimol cotreatment decreased the average glutamate response amplitude in older neurons, and had no effect on younger neurons (Fig. 2D). The lack of an additive effect of glutamate and muscimol depolarizing effects in young neurons likely results from maximal saturation of the Fura signal with the agonist concentrations used. Finally, to confirm that prolonged muscimol treatment did not deplete intracellular chloride levels ([Cl−]i), produce chloride unloading and abolish the depolarizing response, particularly in young neurons where the driving force on chloride is greater, calcium imaging experiments were performed after 30 min muscimol exposure, followed by a brief 2 min washout period. Comparison of t0 or t30 muscimol treatment (Fig. 2 E,F), revealed that after 30 min muscimol exposure, the GABAAR response remains depolarizing and the percentage of cells responding to muscimol does not decrease, both in younger neurons where the majority of cells are depolarized and in older neurons where fewer are depolarized. Thus, cellular mechanisms are in place that block significant chloride depletion and maintain the existing driving force for Cl− at each age. Interestingly, older neurons exhibited a slight increase in the percentage of cells responding, suggesting some increase in GABAAR response or downstream positive modulation of VGCC. Together this data suggests that weak depolarizing GABAAR currents in older neurons produce shunting effects, inhibiting cell excitability and diminishing [Ca2+]i produced by glutamate receptor activity.
Fig. 2.
Mild GABAAR induced depolarization inhibits neuron excitability. A,B, Representative gramicidin-perforated patch clamp recordings and I–V plots of normalized muscimol-activated currents from neurons in voltage clamp at DIV 5–7 and DIV 13–15. C. The reversal potential for muscimol elicited currents (EGABA) in DIV 13–15 neurons was more negative compared to DIV 5–7 (DIV 5–7 = −30.9 ± 6.7 mV, DIV 13–15 = −50.0 ± 6.6 mV), while resting membrane potential (Vm) was unchanged (DIV 5–7 Vm = −66.1 ± 2.7 mV, DIV 13–15 Vm = −69.2 ± 4.5 mV). Mean ± SD, n = 7 neurons for each age, ***p < 0.001, unpaired t-test. D. Muscimol inhibits the average glutamate response amplitude only in DIV13–15 neurons. Left graph shows response values normalized to glutamate alone response. Right graph gives absolute F 340/F380 values: DIV 5–7 neurons glutamate = 0.26 ± 0.02 vs glutamate + muscimol = 0.22 ± 0.014; DIV 13–15 neurons glutamate = 0.70 ± 0.05 vs glutamate + muscimol = 0.50 ± 0.05. (Mean ± SEM, n = 37–84 neurons, *p < 0.05). E. Representative calcium imaging data at t0 and t30 (after 30 min muscimol treatment). F. The % cells responding at DIV 5 were unchanged by muscimol treatment (n = 89–126 neurons) and the % cells responding increased slightly with muscimol treatment in DIV 9–13 neurons: t0 = 40.6 ± 5, t30 = 62.8 ± 8 (Mean ± SEM, n = 42–82 neurons, *p < 0.05).
3.2. Depolarizing and inhibitory GABAAR activity leads to delayed ERK activation
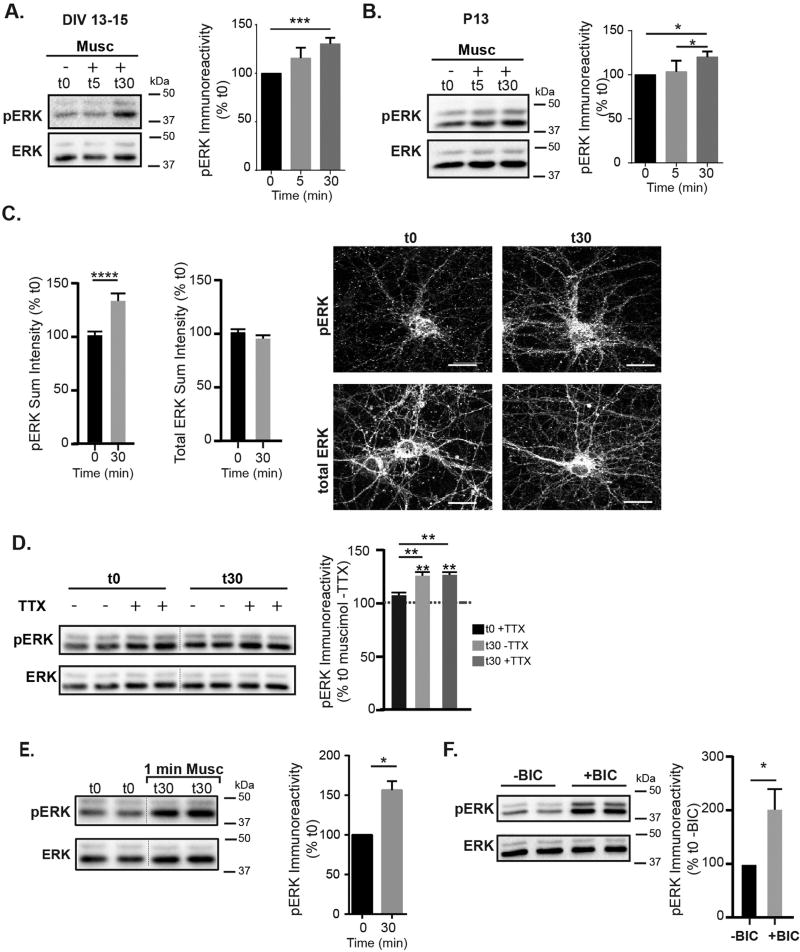
Very early in development, GABAAR agonist application activates receptors tonically in young neurons (DIV 2–5) to produce excitation, a rise in [Ca2+]i and rapid activation of ERK within minutes, a key step in circuit development (Obrietan et al., 2002). Due to the muscimol induced low rise in [Ca2+]i that persists after GABAAR and glutamatergic synapse establishment (Fig. 1), we examined the effect of GABAAR signaling on ERK activation in neurons with established GABAergic synapses (DIV 13–15). With muscimol treatment, at t5 min, pERK levels were unchanged; however, at t30 min, there was a significant increase in pERK activation while total ERK levels were unchanged (Fig. 3A). We observed a similar effect of muscimol treatment on acute cortical slices from postnatal day 13 rats (P13) (Fig. 3B). Quantification of pERK and total ERK levels in cortical neurons using immunofluorescence also showed a significant increase in pERK at t30 with no difference in total ERK levels (Fig. 3C). To confirm that the increase in pERK levels in older neurons was solely due to GABAAR signaling and not overall changes in network signaling, we pretreated cortical neuron cultures with 1 µM TTX for 5 min, then examined muscimol induced changes in pERK levels at t0 and t30 min. TTX pretreatment did not block the GABAAR dependent pERK increase (Fig. 3D), indicating that enhanced ERK phosphorylation was due to GABAAR activation and not overall network activity. In addition, shorter muscimol treatment produced a similar level of ERK activation at t30: a brief 1 min muscimol treatment was followed by washout and evaluation of total and phospho ERK levels at t30 (Fig. 3E). Finally, consistent with the calcium imaging data indicating a shunting inhibitory GABAAR response that dampens coincident glutamatergic activity (Fig. 2), receptor blockade with the GABAAR antagonist bicuculline led to rapid and robust ERK activation (Fig. 3F) similar to glutamate treatment alone (data not shown). This inhibitory role for GABAAR is well documented, as antagonist treatment (bicuculline or picrotoxin) is a standard means of robustly increasing excitatory synaptic activity and activating ERK in a glutamate receptor dependent manner (Bateup et al., 2013; Ivanov et al., 2006; Wiegert et al., 2007; Zhou et al., 2009, 2013). Together with the calcium imaging and electrophysiological results, this biochemical data indicates that well after GABAergic synapse maturation, agonist application produces a depolarizing GABAAR response that leads to delayed ERK activation and minimizes overall cell excitability.
Fig. 3.
GABAAR activity regulates ERK activation. A. Representative immunoblot and analysis of time course for ERK phosphorylation following muscimol treatment at 13–15 DIV. pERK immunoreactivity at t30 was increased compared to t0 (t0 = 100%, t5 = 115 ± 9%, t30 = 130.4 ± 7%, mean ± SEM, n = 6–9 cultures, t30 compared to t0 *p < 0.05, one way ANOVA, Tukey's multiple comparisons test). Total ERK levels were unchanged (t0 = 100%, t5 = 100 ± 12%, t30 = 106 ± 7%, mean ± SEM, n = 6–9 cultures) B. pERK levels were similarly increased in postnatal day 13 rat acute cortical slices: pERK t0 = 100%, t5 = 103 ± 6%, t30 = 120 ± 3%. Total ERK levels were unchanged (t0 = 100%, t5 = 112 ± 8%, t30 = 104 ± 9%. mean ± SEM, n = 4 independent animals, *p < 0.05, one way ANOVA, Tukey's multiple comparisons test. C. Representative immunostaining for pERK and total ERK at t0 and t30 muscimol treatment. Scale bars are 20 µm. Quantification of sum fluorescence intensities, normalized to t0. pERK values increased: t0 = 100 ± 2%, t30 = 121 ± 3 (Mean ± SEM, n = 59–61 neurons, t0 v t30 ****p < 0.0001, t-test). D. TTX does not block the muscimol-dependent pERK increase in DIV 13–15 neurons. Neurons were pre-treated with 1 µM TTX, and then treated for either 0 or 30 min with muscimol. t0 −TTX = 100%, t0 +TTX = 108 ± 3, t30 −TTX = 126 ± 4, t30 + TTX = 126 ± 3 (mean ± SEM, n = 3 cultures, **p < 0.01, two-way ANOVA and post hoc t-tests). E. 1 min muscimol treatment followed by washout produced similar ERK activation to 30 min muscimol treatment: t0 = 100%, t30 (1 min Musc + 29 min washout) = 156.3 ± 11 ((Mean ± SEM, n = 3 cultures,*p < 0.05, unpaired t-test). F. Bicuculline rapidly increases pERK immunoreactivity in DIV 13–15 neurons: t0−BIC = 100.0%, t0+BIC = 201 ± 39 (Mean ± SEM, n = 6–9 cultures, *p < 0.05, unpaired t-test).
3.3. Sustained agonist exposure induces structural plasticity at GABAergic synapses
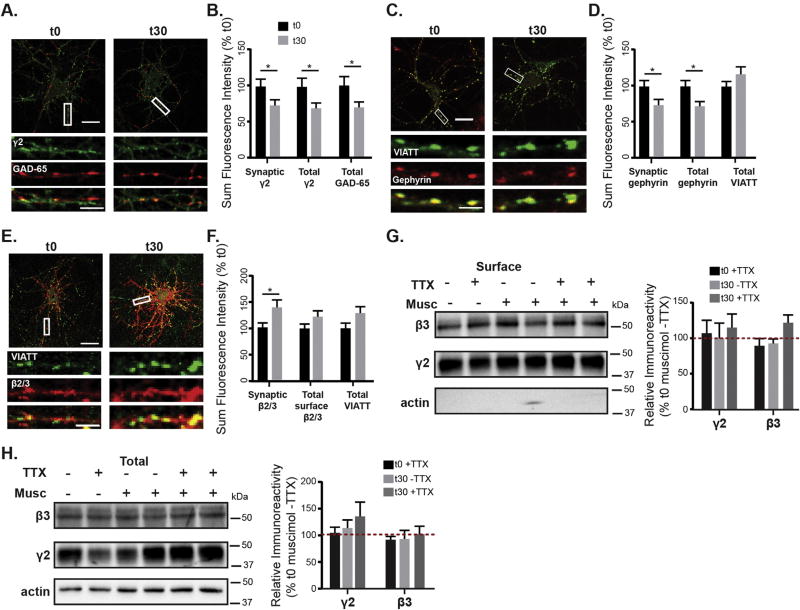
To assess the effects of muscimol treatment on GABAAR synaptic plasticity, we used immunofluorescence and confocal microscopy to examine key components of the GABAergic synapse: surface γ2-containing GABAARs, the gephyrin synaptic scaffold and the presynaptic GABAergic terminal marker glutamic acid decarboxylase 65 which is responsible for local GABA synthesis (GAD65). Neurons undergoing either 0 or 30 min muscimol treatment were fixed and stained under non-permeabilizing conditions for surface γ2 GABAAR, then permeabilized and stained for GAD65 (Fig. 4A). Muscimol treated neurons exhibited a significant decrease in synaptic localization of surface γ2 GABAAR, as determined by colocalization with GAD65 (Fig. 4B). As the majority of γ2 GABAARs detectable by immunofluorescence are clustered at synapses, this resulted in a decrease in total dendritic surface γ2 GABAAR (Fig. 4B). There was also a significant decrease in presynaptic GAD65 (Fig. 4B), indicating that muscimol exposure initiates presynaptic plasticity. In contrast to the GAD isoform GAD67, which is diffusely present throughout the neuron and is required for basal GABA synthesis, GAD65 is synaptically localized and has been shown to modulate GABAergic neurotransmission in response to changes in demand (Tian et al., 1999).
Fig. 4.
Muscimol treatment results in GABAergic synaptic plasticity. A. DIV 13–15 neurons treated with muscimol for 0 or 30 min, fixed, stained for surface γ2, then permeabilized and stained for GAD65. B. Quantification of sum fluorescence intensity of synaptic surface γ2 levels (t0 = 100 ± 10%, t30 = 70 ± 7%), surface total γ2 (t0 = 100 ± 12%, t30 = 67 ± 7%), and total levels of GAD65 (t0 = 100 ± 8%, t30 = 76 ± 8%). n = 40–42 neurons, *p < 0.05, t-test. C. mCherry-gephyrin neurons treated with muscimol for 0 or 30 min, then fixed, permeabilized, and stained for gephyrin and VIATT. D. Muscimol treatment decreased the sum fluorescence intensity of synaptic gephyrin clusters (synaptic fluorescence t0 = 100 ± 8%, t30 = 71 ± 8%, *p < 0.05) and total gephyrin clusters (total fluorescence t0 = 100 ± 8%, t30 = 72 ± 6%,*p < 0.05, n = 41–42 neurons, t-test), with no changes in total VIATT. E. DIV 13–15 neurons treated with muscimol for either 0 or 30 min, fixed, stained for surface β2/3, then permeabilized and stained for VIATT. A,C,E: scale bars 20 µm, and 2 µm on enlargements. F. Muscimol treatment induced an increase in synaptic β2/3 sum fluorescence intensity (t0 = 100.0 ± 8%, t30 = 137.1 ± 13%, *p < 0.05, n = 44–45 neurons, t-test), with no changes in total β2/3 or VIATT sum intensities. G, H. Neurons were surface biotinylated and lysed in RIPA. Immunoblots show that surface and total protein levels of γ2 and β3 subunits, either with or without TTX were unchanged after muscimol (n = 3–6 cultures, ns, two-way ANOVA).
Next we examined if muscimol exposure altered the gephyrin postsynaptic scaffold. mCherry-tagged gephyrin expressing neurons (Brady and Jacob, 2015) were used in fixed immunofluorescence experiments with presynaptic GABAergic terminals immunolabeled with the vesicular inhibitory amino acid transporter (VIAAT − GABA/glycine transporter). Muscimol treatment decreased the synaptic and total dendritic sum fluorescence intensity of gephyrin clusters (Fig. 4D). In addition, the density of gephyrin clusters decreased with muscimol exposure (t0 = 3.18 ± 0.18 clusters/10 µm, t30 = 2.56 ± 0.17 clusters/10 µm, n = 42–43 neurons, *p < 0.05, t-test). VIAAT levels were unaltered (Fig. 4D) in contrast with GAD65 (Fig. 4A), suggesting that 30 min of muscimol treatment selectively affects GAD65, potentially decreasing GABA synthesis or the strength of GAD65 positive presynaptic terminals.
In order to readily measure overall changes in surface GABAAR including both diffusely localized extrasynaptic and synaptically clustered receptors, we performed additional fixed immunofluorescence experiments staining for almost all GABAAR with a surface β2/3 subunit antibody. Measurements of total dendritic surface β2/3 showed no significant change (Fig. 4F). Consistent with the earlier analysis (Fig. 4D), VIAAT levels were unchanged (Fig. 4E, F). Interestingly, the sum fluorescence intensity of synaptic β2/3 increased (Fig. 4F), suggesting increased synaptic levels of another receptor subtype that is non γ2 subunit containing. This indication of GABAAR exchange at synapses is consistent with single particle tracking experiments showing increased movement of receptors between extrasynaptic and synaptic domains with decreased gephyrin scaffolding (Petrini et al., 2014; Renner et al., 2012). As an alternative method for measuring surface total GABAAR changes, we examined the levels of surface γ2 and β GABAAR subunits using cell-surface biotinylation. TTX treatments were additionally included in these experiments to show the specific requirement for GABAAR activation, rather than a change in general network activity. Cell-surface biotinylation experiments did not detect any significant changes in surface levels of either γ2 or β3 subunits after 30 min of muscimol treatment (Fig. 4G). Furthermore, analysis of neuron lysates by western blot showed no changes in total protein levels of γ2 or β3 (Fig. 4H). Together these data indicate that during muscimol exposure there is a decrease in synaptic gephyrin and a shift in surface localization of GABAAR subtypes, with γ2 GABAARs moving out of synapses and movement of β2/3 containing non-γ2 GABAARs into the synapse.
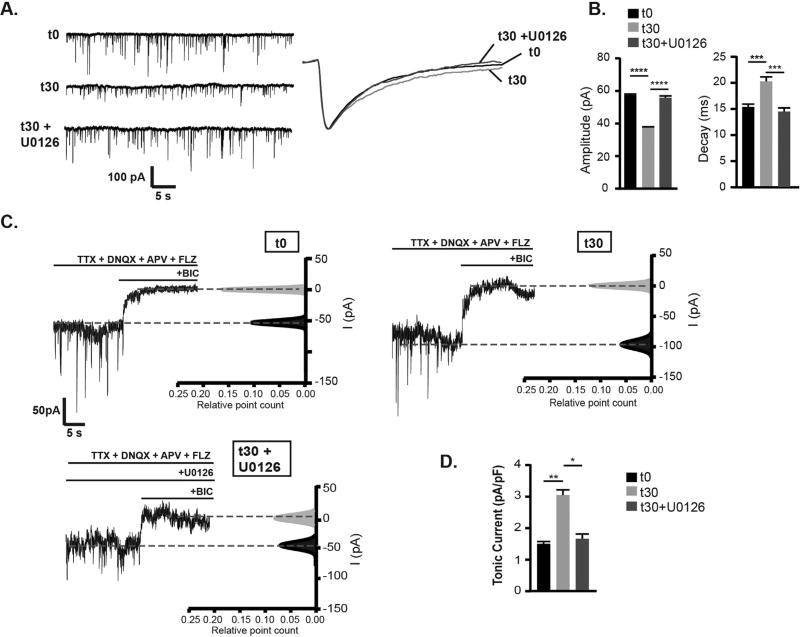
3.4. Muscimol treatment decreases synaptic currents while enhancing GABAAR tonic current in an ERK-dependent manner
Given the robust effect of GABAAR agonist treatment on structural synaptic plasticity, the effect of muscimol on the efficacy of synaptic inhibition was determined. Analysis of miniature inhibitory postsynaptic currents (mIPSCs) in cultured cortical neurons was performed following muscimol treatment using whole cell patch-clamp recordings. To evaluate the role of muscimol evoked ERK activation in modulating GABAergic neurotransmission, additional experiments were conducted with the MEK/ERK inhibitor U0126. Muscimol exposure significantly decreased mIPSC amplitude, and U0126 blocked this decrease (Fig. 5A,B). Muscimol treatment also prolonged the decay of mIPSCs in an ERK dependent manner (Fig. 5A,B). Both the decrease in mIPSC amplitude and increase in decay are consistent with loss of synaptic γ2 GABAAR as seen in the immunofluorescence data presented here (Fig. 4) and reported with genetic knockout or knockdown of γ2 or gephyrin (Essrich et al., 1998; Kerti-Szigeti et al., 2014). Neither muscimol treatment nor ERK inhibition altered mIPSC frequency, suggesting presynaptic release of GABA containing vesicles is unaltered (mIPSC frequency: t0 = 2.8 ± 0.4 Hz, t30 = 2.7 ± 0.4 Hz, t30 + U0126 = 2.8 ± 0.4 Hz). The lack of change in frequency suggests that the GAD65 decrease does not dramatically or immediately impact GABA release levels when assessed by mIPSCs. Similarly, neuron cultures from GAD65 knockout animals do not show basal spontaneous IPSC frequency or amplitude deficits, however GABA release is reduced under conditions of sustained synaptic activation (Tian et al., 1999).
Fig. 5.
Muscimol treatment decreases synaptic currents and concurrently increases the tonic current in an ERK- dependent manner. A. Whole-cell recordings of mIPSCs from DIV 13–15 neurons. Neurons were incubated in standard extracellular saline containing TTX, DNQX, and APV to isolate GABAAR currents for t = 0 min (t0), in saline containing muscimol for 30 min (t30), or pretreated with 1 µM U0126 for 10 min then treated with muscimol for 30 min(t30 + U0126), then mIPSC recordings were performed in saline. B. Muscimol treatment decreased mIPSC amplitudes which was blocked by U0126 pretreament: t0 = 57.9 ± 0.6 pA, t30 = 37.2 ± 0.4 pA, t30 + U0126 = 55.8 ± 1.0 pA. Muscimol treatment slowing of mIPSC decay was blocked by U0126: t0 = 15.5 ± 0.6 ms, t30 = 20.5 ± 0.9 ms, t30 + U0126 = 14.4 ± 0.7 ms (Mean ± SEM, n = 7 neurons per condition, one way ANOVA, Tukey's multiple comparisons test). C. Tonic currents recorded from neurons treated as in A. with the addition of flurazepam (FLZ) to amplify GABAAR tonic currents. To identify the tonic current, bicuculline (BIC) was added. D. Muscimol treatment increased tonic current, which was blocked by U0126 pretreatment: in pA/pF t0 = 1.5 ± 0.1, t30 = 3.0 ± 0.4, t30 + U0126 = 1.6 ± 0.4 (Mean ± SEM, n = 6–7 neurons per condition, one way ANOVA, Tukey's multiple comparisons test). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Due to the possibility of agonist induced changes in GABAAR localization also altering the tonic current, whole-cell patch clamp recordings were next used to assess changes in extrasynaptic receptor activity. Holding current was measured with the addition of the benzodiazepine flurazepam to enhance tonic current detection. Bath application of the GABAAR competitive antagonist bicuculline dramatically decreased the whole-cell holding current in neurons treated with muscimol, compared to control and U0126 pretreated neurons (Fig. 5C,D). Whole-cell current change, measured as a difference between baseline before and after bicuculline application was 1.5 ± 0.1 pA/pF for control t0, 3.0 ± 0.4 pA/pF for t30 min muscimol, and 1.6 ± 0.4 pA/pF for t30 min muscimol + U0126 (Fig. 5C,D). This data revealed that muscimol treatment enhanced the overall tonic current in an ERK dependent manner.
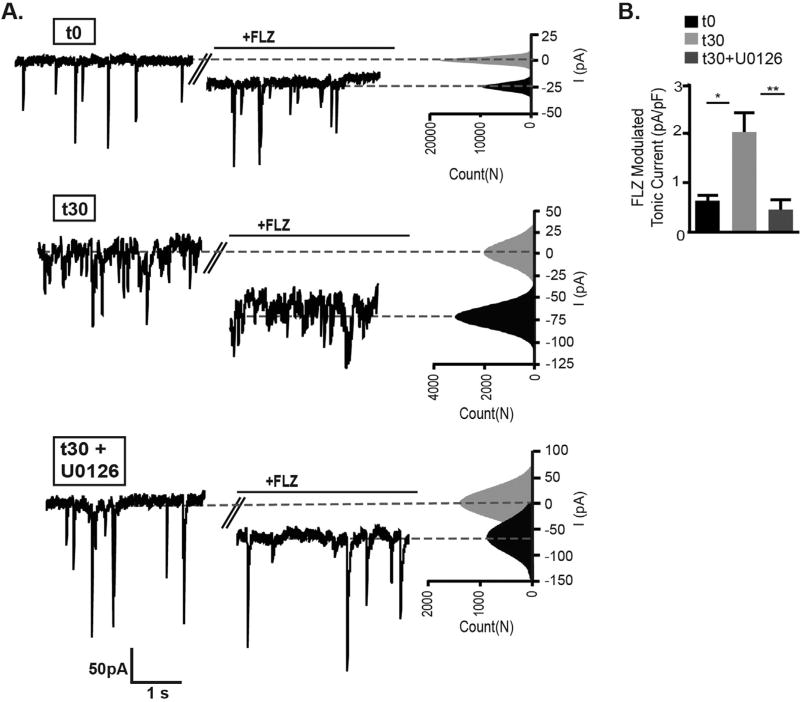
As the decrease in synaptic γ2 and gephyrin levels suggests movement of γ2 GABAAR into extrasynaptic areas (Fig. 4), we next separately examined the γ2 containing component of the tonic current (Fig. 6). In addition to their predominant role in synaptic GABAAR neurotransmission, γ2 containing receptors are also found at lower levels at extrasynaptic sites and contribute to tonic current that is sensitive to positive allosteric modulation by the benzodiazepine drug class (Brady and Jacob, 2015; Nusser et al., 1998; Somogyi et al., 1996). We used the benzodiazepine flurazepam (3 µM) which binds at the interface of γ2 with α1, 2, 3, or 5 subunits to specifically detect enhancement of γ2 GABAAR tonic current. In agreement with a higher level of γ2 GABAAR in extrasynaptic regions, flurazepam significantly potentiated the holding current in muscimol treated neurons (Fig. 6A,B). In neurons pretreated with U0126, flurazepam showed a lower potentiation of holding current that was equivalent to control neurons (t0 = 0.61 ± 0.14 pA/pF, t30 = 2.04 ± 0.39 pA/pF, t30 + U0126 = 0.46 ± 0.21 pA/pF). In summary, these functional data combined with biochemical and imaging results indicate that muscimol treatment via ERK activation induces GABAergic postsynaptic plasticity that diminishes γ2 GABAAR and gephyrin synaptic localization, resulting in decreased synaptic currents while concurrently increasing γ2 GABAAR tonic current.
Fig. 6.
Benzodiazepine sensitive γ2 containing GABAAR tonic current is enhanced by muscimol treatment. Neurons were incubated in standard extracellular saline containing for t = 0 min (t0), in saline containing muscimol for 30 min (t30), or pretreated with 1 µM U0126 for 10 min then treated with muscimol for 30 min (t30 + U0126). A. Baseline currents were recorded in extracellular saline containing TTX, DNQX, and APV, followed by perfusion of flurazepam (FLZ). Benzodiazepine sensitive tonic current was expressed as the difference in baseline current before and after application of FLZ (3 µM). After subtracting baseline from the current evoked by FLZ, the tonic current was normalized to cell size. Representative traces with corresponding all-point histograms fitted with single Gaussian functions are shown. B. Muscimol treatment increased the γ2 containing GABAAR contribution to tonic current, which was blocked by U0126 pretreatment: in pA/pF t0 = 0.61 ± 0.14, t30 = 2.04 ± 0.39, t30 + U0126 = 0.46 ± 0.21 (Mean ± SEM, n = 6–7 neurons per condition, one way ANOVA, Tukey's multiple comparisons test). *p < 0.05, **p < 0.01.
3.5. GABAAR dependent activation of ERK relies on MEK, intracellular calcium release, and BDNF
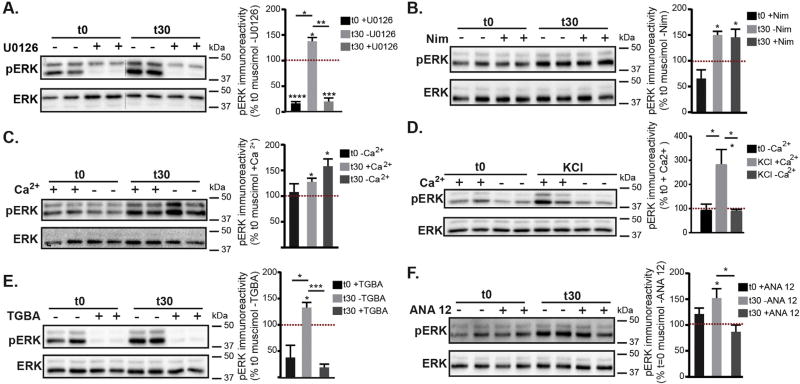
To investigate the mechanisms contributing to muscimol induced GABAergic synaptic plasticity we first used U0126 to confirm that muscimol treatment leads to MEK and ERK activation. Pretreatment with U0126 decreased basal ERK activation and completely blocked muscimol induced ERK activation (Fig. 7A). We next assessed the calcium dependence of muscimol induced ERK activation as GABAAR mediated depolarization could result in calcium influx via L-type VGCCs and calcium release from intracellular stores, activating Ras and leading to ERK phosphorylation (Rosen et al., 1994). A 10 min pretreatment with the L-type VGCCs blocker nimodipine did not block the muscimol dependent increase in pERK (Fig. 7B). As other VGCCs could be involved, we next excluded Ca2+ from the extracellular medium and examined ERK activation. Exclusion of extracellular Ca2+ did not prevent the muscimol induced increase in pERK levels (Fig. 7C). In contrast, a well characterized synaptic stimulation protocol using brief KCl depolarization required extracellular Ca2+ influx to activate ERK (Fig. 7D), as previously shown (Dolmetsch et al., 2001; Rosen et al., 1994). Therefore depolarization of the membrane without altering [Cl−]i results in an alternative mechanism of ERK activation. GABAAR dependent ERK activation shown here is also mechanistically distinct from AMPA receptor or NMDA receptor activation, which require extracellular calcium influx (typically via VGCC, NMDAR or calcium permeable AMPAR, reviewed in (Thomas and Huganir, 2004)) to stimulate Ca2+ release from stores and rapidly activate ERK (Perkinton et al., 1999; Tian and Feig, 2006; Zhou et al., 2009). Recent studies indicate that depolarization in the absence of extracellular Ca2+ can trigger internal stores Ca2+ release in a variety of neuron subtypes (Billups et al., 2006; De Crescenzo et al., 2006; Kim et al., 2007; Ryglewski et al., 2007; Sun et al., 2016). To determine whether depolarizing GABAAR stimulation was releasing intracellular Ca2+ stores and activating ERK, we depleted endoplasmic reticulum (ER) Ca2+ stores with a 60 min pretreatment of thapsigargin in combination with BAPTA-AM to chelate intracellular Ca2+. Depletion of intracellular Ca2+ stores (TGBA) prevented muscimol-dependent ERK activation (Fig. 7E). The intracellular calcium chelator BAPTA-AM does not affect extracellular Ca2+, as rapid glutamate-dependent ERK activation is not blocked with TGBA treatment (data not shown). Together, these data indicate that in neurons with established synapses and a depolarizing, inhibitory GABAAR response, delayed ERK activation relies on intracellular Ca2+ release from the ER that is not dependent on extracellular Ca2+ influx. We next examined the effects of BDNF activity on muscimol-dependent ERK activation, as GABAAR activity early in development can lead to BDNF release (Porcher et al., 2011) and exogenous BDNF can induce ERK activation (Obrietan et al., 2002; Tokuoka et al., 2000). Pretreatment with 10 µM ANA-12, a potent and specific TrkB inhibitor (Cazorla et al., 2011) did not alter basal pERK levels but significantly blocked muscimol-dependent ERK activation (Fig. 7F). In summary, our biochemical data show that GABAAR dependent ERK activation can be blocked with the MEK/ERK inhibitor U0126 and also by the TrkB inhibitor ANA-12 (Fig. 7), implicating these two signaling pathways. Furthermore, in contrast to KCl depolarization dependent activation of ERK which relies upon extracellular Ca2+ influx to release calcium stores (as also seen with AMPA and NMDA receptor signaling), GABAAR agonist activation of ERK via intracellular stores is independent of extracellular Ca2+.
Fig. 7.
Muscimol-dependent ERK activation relies on intracellular Ca2+ release and BDNF/TrkB pathways, in contrast with KCl depolarization. Neurons at DIV 13–15, all pretreatments were accompanied by matched vehicle controls (see Experimental Procedures) followed by 0 or 30 min muscimol treatment. A–E. Representative immunoblots with pERK immunoreactivity normalized to t0 with vehicle control. Mean ± SEM, n = 3–6 cultures, two way ANOVA and post hoc t-tests *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. A. U0126 pretreatment. B. Nimodipine pretreatment (Nim). C. Neurons were muscimol treated in the presence of either normal extracellular Ca2+ in HBS (+Ca2) or HBS containing 0 mM Ca2+ and 1 mM EGTA (−Ca2). D. Synaptic stimulation protocol, neurons were briefly treated with KCl in the presence of either normal extracellular Ca2+ in HBS (+Ca2) or HBS containing 0 mM Ca2+ and 1 mM EGTA (−Ca2). E. Thapsigargin and BAPTA-AM pretreatment (TGBA). F. ANA 12 TrkB inhibitor pretreatment.
3.6. ERK activation contributes to muscimol evoked post but not presynaptic GABAergic synapse plasticity
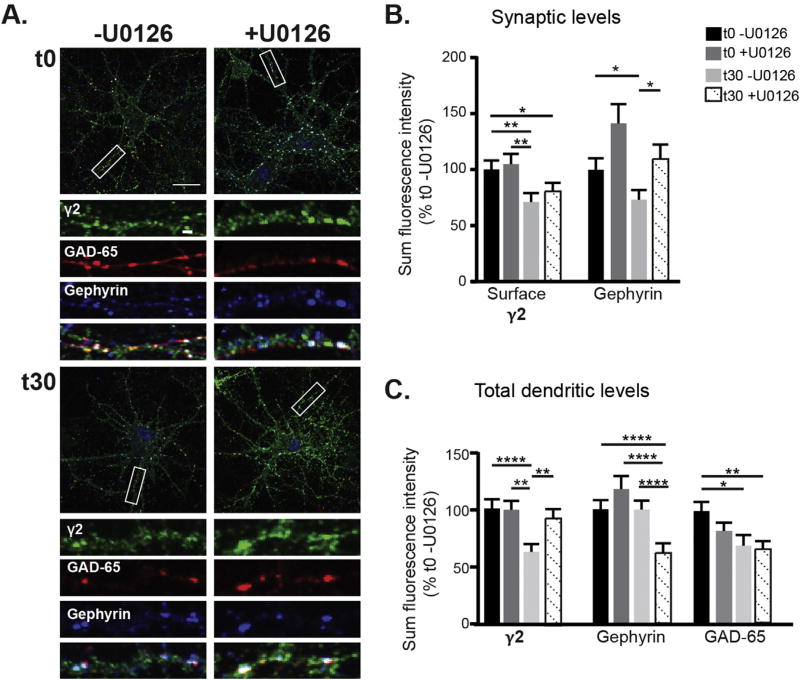
Thus far we have shown that muscimol treatment in neurons with established GABAergic synapses decreases synaptic GABAAR currents while enhancing γ2 tonic current in an ERK dependent manner. We have also observed muscimol induced structural plasticity at GABAAR synapses, leading to a decrease in γ2 and gephyrin synaptic clustering, and lowering of GAD65 levels. To further dissect the mechanisms contributing to this GABAergic synaptic plasticity we first evaluated the role of ERK using fixed immunofluorescence and confocal microscopy. After pretreating ± U0126 followed by muscimol treatment, neurons were immunostained for surface γ2 containing GABAAR, then permeabilized and stained for GAD65 and gephyrin (Fig. 8A). ERK inhibition with U0126 blocked the muscimol-induced decrease in total γ2 surface levels (Fig. 7C) but did not restore γ2 synaptic levels (Fig. 8B). This data suggests that ERK activation contributes to GABAAR activity dependent postsynaptic plasticity, along with additional pathways regulating γ2 synaptic clustering. Muscimol treatment significantly decreased endogenous synaptic gephyrin levels (Fig. 8B), consistent with earlier results using tagged gephyrin (Fig. 4C,D). The lack of a change in total gephyrin levels (Fig. 8C) suggests dispersal of gephyrin away from synapses and a more diffuse organization of the scaffolding, likely to have been undetected in experiments with tagged gephyrin. The loss of synaptic gephyrin was blocked by MEK/ERK inhibition with U0126, indicating that gephyrin reorganization occurs downstream of muscimol-dependent ERK activation (Fig. 8B). In contrast, U0126 treatment did not block the muscimol induced decrease in GAD65 levels (Fig. 8C). Together these data suggest that the effects of muscimol-dependent ERK activation are confined to postsynaptic changes leading to loss of synaptic gephyrin and surface γ2 clusters, with pre-synaptic changes occurring through a separate signaling mechanism.
Fig. 8.
Pretreatment with the ERK inhibitor U0126 blocks muscimol-induced postsynaptic plasticity events. A. DIV 13–15 neurons were pretreated with either U0126 or DMSO vehicle control then treated with muscimol. Neurons were fixed, stained for surface γ2 levels, then permeabilized and stained for intracellular gephyrin and GAD65. Scale bars 20 µm, and 2 µm on enlargements. B. Synaptic immunofluorescence analysis and C. Total dendritic immunofluorescence analysis. Mean ± SEM, n = 42–44 neurons, two-way ANOVA and post hoc t-tests. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
3.7. BDNF signaling supports agonist induced post and pre synaptic structural plasticity at GABAAR synapses
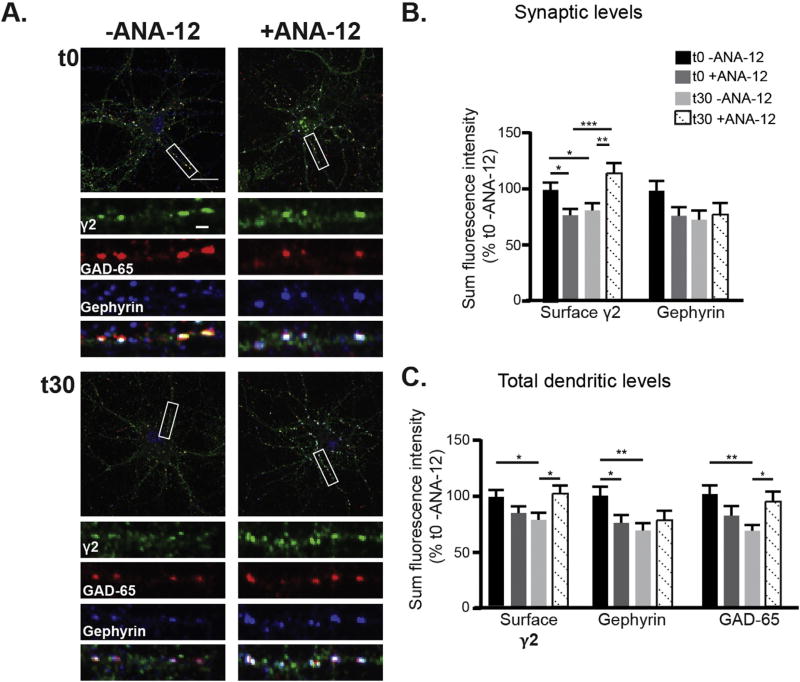
As we had observed that muscimol-dependent ERK activation could be blocked by an inhibitor of BDNF and TrkB signaling (Fig. 7E, ANA-12), we next assessed the contribution of BDNF signaling to GABAAR dependent synaptic plasticity. We pretreated neurons with the TrkB inhibitor ANA-12 for 30 min followed by muscimol exposure. As before, neurons were stained for surface γ2 subunit levels, then permeabilized and stained for GAD65 and gephyrin (Fig. 9A). TrkB inhibition blocked the decrease in synaptic and total surface levels of γ2 GABAAR (Fig. 9B–C). Total gephyrin levels were significantly decreased by muscimol treatment and synaptic levels trended towards a decrease, with TrkB inhibition resulting in no clear rescue of the loss of gephyrin (Fig. 9B–C). In contrast, TrkB inhibition completely restored GAD65 levels to control values (Fig. 9C). Together these data support a role for ERK and BDNF signaling in the dispersal of γ2 GABAAR from synapses, while gephyrin declustering is mediated by ERK alone. Furthermore, muscimol treatment induces BDNF signaling that triggers a decrease in presynaptic GAD65, likely through dendritic BDNF release acting onto presynaptic terminals (Singh et al., 2006). Consistent with this data, TrkB receptors are found at GABAergic synapses, displaying colocalization with GAD65 presynaptically and γ2 containing GABAAR postsynaptically (Swanwick et al., 2004).
Fig. 9.
Pretreatment with the BDNF/TrkB inhibitor ANA-12 prevents muscimol-induced decreases in synaptic γ2 GABAAR clustering and GAD65 levels. A. DIV 13–15 neurons were pretreated with either 10 µM ANA-12 or DMSO vehicle control then treated with muscimol. Neurons were fixed, stained for surface γ2 levels, then permeabilized and stained for gephyrin and GAD65. Scale bars 20 µm, and 2 µm on enlargements. B. Synaptic immunofluorescence analysis and C. total dendritic immunofluorescence analysis. Mean ± SEM, n = 56–58 neurons, two-way ANOVA and post hoc t-tests. *p < 0.05, **p < 0.01, and ***p < 0.001.
4. Discussion
Despite the critical role of GABA and activity dependent plasticity in neuronal circuit development, most studies have examined modulation of excitatory glutamatergic synapses on principal cells. However, recent reports have identified plasticity mechanisms at GABAergic synapses on pyramidal cells and interneurons, revealing that dynamic modulation of these synapses is of fundamental importance (Barberis and Bacci, 2015). The majority of investigations of GABAergic postsynaptic plasticity have focused on excitation driven changes. For example, NMDA receptor activation can bidirectionally modulate GABAergic postsynaptic strength and receptor clustering through regulation of phosphatase (Bannai et al., 2009) or CaMKII activity (Marsden et al., 2010; Petrini et al., 2014). In this study we have identified and provided mechanistic insight into GABAAR agonist dependent rapid pre and postsynaptic GABAergic plasticity, summarized in Model Fig. 10. First, we demonstrate that at DIV 13–15, well after the major phases of dendritic outgrowth and synaptogenesis, cortical neurons weakly depolarize in response to muscimol treatment, inhibiting cell excitability and leading to delayed ERK activation at 30 min. This cellular response is distinct from early excitatory depolarizing GABAAR activity that generates a large [Ca2+]i increase and rapidly activates ERK (Obrietan et al., 2002). Next, we show that muscimol exposure decreases synaptic localization of surface γ2 GABAARs and gephyrin while β2/3 non-γ2 GABAARs accumulate in the synapse. This structural plasticity is matched by an ERK dependent decrease in synaptic GABAAR currents and a concurrent enhancement in the tonic current from extrasynaptic γ2 containing benzodiazepine sensitive GABAAR. These results complement a recent single particle tracking study in mature hippocampal neurons that showed muscimol treatment enhanced GABAAR and gephyrin diffusion away from synapses, while antagonist treatment reduced diffusion (Gouzer et al., 2014). Furthermore, GABAAR diffusion was unaffected by both NMDA- and AMPA-type glutamate receptor blockade, and hence independent of excitatory glutamatergic activity. Together with our data these observations suggest that ligand binding induces changes in GABAAR conformation and receptor scaffold interactions that are a critical means for controlling GABAAR signaling.
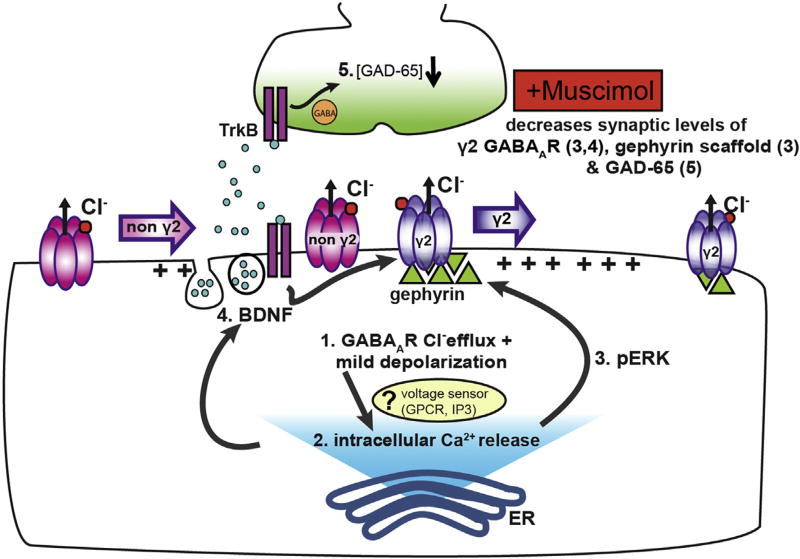
Fig. 10.
Model for muscimol induced GABAergic synaptic plasticity. Synaptic GABA release in neurons with established synapses produces mild depolarization via chloride efflux and low release of intracellular Ca2+ from ER stores, resulting in low pERK levels and minimal BDNF stimulation. Prolonged exposure to muscimol enhances depolarization (1) and increases Ca2+ store release (2), potentially via a Ca2+ influx independent voltage sensor. Ca2+ store release in turn activates ERK above baseline (3) and promotes release of dendritic BDNF (4). Enhanced pERK activity decreases synaptic γ2 GABAAR and gephyrin, leading to higher extrasynaptic γ2 GABAAR levels (3). BDNF signaling contributes to the decrease in synaptic γ2 GABAAR (4), as well as diminishing presynaptic GAD65 levels (5).
Mechanistically, we show that muscimol-dependent ERK activation is independent of extracellular calcium influx, but relies on release of intracellular calcium stores. This ERK activation contrasts that occurring with both synaptic stimulation protocols (brief KCl depolarization, shown here) and AMPAR or NMDAR activation which rely on extracellular calcium entry via NMDAR, VGCC or calcium permeable AMPAR to induced release of intracellular calcium stores and activate ERK. Recent studies during the last decade have identified that depolarization in the absence of extracellular Ca2+ can trigger internal stores Ca2+ release in a variety of neuron subtypes (Billups et al., 2006; De Crescenzo et al., 2006; Kim et al., 2007; Ryglewski et al., 2007; Sun et al., 2016). Mechanisms identified include voltage-induced Ca2+ release from internal stores via IP3 receptors (Ryglewski et al., 2007; Sun et al., 2016) and L-type VGCC direct gating of the ryanodine receptor in hippocampal (Kim et al., 2007) and hypothalamic neurons (De Crescenzo et al., 2006). As we show that GABAAR agonist activation of ERK is not blocked by nimodipine, this may rule out the latter mechanism, although incomplete blockade of VGCC by dihydropyridine antagonists could be a confound (Lipscombe et al., 2004; Xu and Lipscombe, 2001). A possibility for muscimol treatment induced intracellular calcium-release could be a membrane voltage sensor activating IP3 to induce internal store calcium-release (Fig. 10). Future experiments will need to be performed for more detailed identification of the specific stimuli responsible for GABAAR depolarization induced release of calcium stores.
Downstream of muscimol induced depolarization, biochemical and imaging experiments identified ERK and BDNF signaling as regulating the dispersal of γ2 GABAAR from synapses, while synaptic gephyrin declustering is mediated by ERK alone. In contrast to the agonist induced downregulation of GABAergic synapse strength we show here, muscimol stimulation in young neurons (DIV 6) with excitatory GABA polarity promotes BDNF release and insertion of GABAARs (Porcher et al., 2011). In addition to this postsynaptic plasticity, we show that muscimol induces a presynaptic decrease in GAD65 levels via BDNF/TrkB signaling, likely through dendritic BDNF release and retrograde signaling. Prior studies examining presynaptic plasticity have focused on long-term effects, with the predominant observation that reduced neuronal activity leads to a homeostatic decrease in GAD65: 1) modulation of neuronal activity over days regulates GAD expression bidirectionally in a homeostatic manner via both BDNF dependent and independent pathways (Hanno-Iijima et al., 2015), 2) in vivo experimental manipulations that decrease activity (visual deprivation) lead to decreased GAD65 puncta which can be rescued by activity restoration (re-exposure to light) (Kreczko et al., 2009), and 3) chronic activity blockade (24 – 48 h TTX treatment) homeostatically alters pre and postsynaptic GABAergic synapse plasticity, decreasing GABAAR synaptic clusters, mIPSC amplitude and frequency (Kilman et al., 2002). Importantly, we observe a muscimol dependent decrease in GAD65 levels within 30 min. It is likely that with the shorter timescale of our experiments, GAD65 levels are not sufficiently depleted to produce a change in mIPSC frequency. This is consistent with the lack of basal IPSC frequency deficits in neuron cultures from GAD65 knockout animals (Tian et al., 1999) in contrast to GAD67 knockouts that have a >90% reduction in basal GABA brain levels and exhibit neonatal lethality (Asada et al., 1997). In further support of our findings showing muscimol induced GABAergic synapse plasticity occurring via BDNF signaling, exogenous BDNF has also been shown to decrease presynaptic GABA release and postsynaptic GABAAR response (Brunig et al., 2001; Lemtiri-Chlieh and Levine, 2010).
In summary, this study identifies a key role for agonist induced plasticity in regulation of GABAergic synapse development and defines intracellular signaling pathways downstream of receptor activation that contribute to these events. These findings suggest that as neurons transition from depolarizing, excitatory GABAAR signaling towards an inhibitory, hyperpolarizing response, a key intermediate phase with depolarizing and inhibitory GABAAR activity prevents overexcitation while also restraining GABAergic synapse growth and strength to promote circuit development. This mechanism could be particularly relevant to early in vivo plasticity, as two recent studies have demonstrated that GABA primarily depolarizes and inhibits network activity in the intact neonatal cortex around P3–P9 (Kirmse et al., 2015; Valeeva et al., 2016). Interestingly, Kirmse et al. did not observe a hyperpolarizing response in vivo at P25–27, suggesting that shunting inhibition could be the main form of inhibition in the juvenile/adult cortex in vivo. Importantly, a recent report showed that in vivo muscimol treatment during early postnatal brain development led to increased anxiety- and depression-related behaviors in adult mice (Salari et al., 2015), hallmarks of impaired GABAergic signaling. Clearly future work investigating the mechanisms of GABAAR synaptic plasticity induced by agonist and other allosteric drugs is merited.
Acknowledgments
This work was funded by the Whitehall Foundation (Grant # 2012-12-36) and Pharmacology and Chemical Biology Department Startup Funds. MLB is in part supported by NINDS T32 NS086749. We thank Stephanie L. Daugherty, Gregory Facchine, and Dr. William C. de Groat for advice on calcium imaging experiments and analysis. We thank Dr. Paul A. Davies and Dr. Tarek Z. Deeb for their advice regarding electrophysiological experiments and analysis.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
MLB and TCJ designed experiments. JMLG & SD performed and analyzed calcium imaging experiments. MLB performed and analyzed most of the biochemical experiments. MLB, CEM, NG, and TCJ performed immunofluorescence experiments and analyzed data. JP and TCJ designed and analyzed electrophysiology experiments. JP performed electrophysiology experiments. TCJ and MLB wrote the paper. MLB and TCJ thank all lab members for editorial comments on the manuscript.
References
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R-G, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid-decarboxylase. Proc. Natl. Acad. Sci. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Barberis A, Bacci A. Editorial: plasticity of GABAergic synapses. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H, Denefrio C, Johnson C, Saulnier J, Sabatini B. Temporal dynamics of a homeostatic pathway controlling neural network activity. Front. Mol. Neurosci. 2013;6 doi: 10.3389/fnmol.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Billups D, Billups B, Challiss RA, Nahorski SR. Modulation of Gq-protein-coupled inositol trisphosphate and Ca2+ signaling by the membrane potential. J. Neurosci. 2006;26:9983–9995. doi: 10.1523/JNEUROSCI.2773-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ML, Jacob TC. Synaptic localization of alpha5 GABA (A) receptors via gephyrin interaction regulates dendritic outgrowth and spine maturation. Dev. Neurobiol. 2015;75:1241–1251. doi: 10.1002/dneu.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur. J. Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PH, Wu PY, Kuo TW, Liu YC, Chan CF, Chien TC, Cheng JK, Huang YY, Chiu CD, Lien CC. GABA is depolarizing in hippocampal dentate granule cells of the adolescent and adult rats. J. Neurosci. 2012;32:62–67. doi: 10.1523/JNEUROSCI.3393-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J. Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol. Cell Neurosci. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- De Crescenzo V, Fogarty KE, Zhuge R, Tuft RA, Lifshitz LM, Carmichael J, Bellve KD, Baker SP, Zissimopoulos S, Lai FA, Lemos JR, Walsh JV., Jr Dihydropyridine receptors and type 1 ryanodine receptors constitute the molecular machinery for voltage-induced Ca2+ release in nerve terminals. J. Neurosci. 2006;26:7565–7574. doi: 10.1523/JNEUROSCI.1512-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Yao J, Fang C, Dong N, Luscher B, Chen G. Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures. J. Neurosci. 2007;27:10860–10869. doi: 10.1523/JNEUROSCI.2744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gouzer G, Specht CG, Allain L, Shinoe T, Triller A. Benzodiazepine-dependent stabilization of GABA(A) receptors at synapses. Mol. Cell Neurosci. 2014;63:101–113. doi: 10.1016/j.mcn.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanno-Iijima Y, Tanaka M, Iijima T. Activity-dependent bidirectional regulation of GAD expression in a homeostatic fashion is mediated by BDNF-dependent and independent pathways. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Nomura T, Xu J, Contractor A. The developmental switch in GABA polarity is delayed in fragile × mice. J. Neurosci. 2014;34:446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiology. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J. Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti-Szigeti K, Nusser Z, Eyre MD. Synaptic GABAA receptor clustering without the gamma2 subunit. J. Neurosci. 2014;34:10219–10233. doi: 10.1523/JNEUROSCI.1721-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Minlebaev M, Mukhtarov M, Khazipov R. Dynamic changes from depolarizing to hyperpolarizing GABAergic actions during giant depolarizing potentials in the neonatal rat Hippocampus. J. Neurosci. 2015;35:12635–12642. doi: 10.1523/JNEUROSCI.1922-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yun HM, Baik JH, Chung KC, Nah SY, Rhim H. Functional interaction of neuronal Cav1.3 L-type calcium channel with ryanodine receptor type 2 in the rat hippocampus. J. Biol. Chem. 2007;282:32877–32889. doi: 10.1074/jbc.M701418200. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Kummer M, Kovalchuk Y, Witte OW, Garaschuk O, Holthoff K. GABA depolarizes immature neurons and inhibits network activity in the neonatal neocortex in vivo. Nat. Commun. 2015;6:7750. doi: 10.1038/ncomms8750. [DOI] [PubMed] [Google Scholar]
- Korol SV, Jin Z, Birnir B. The GLP-1 Receptor Agonist Exendin-4 and Diazepam Differentially Regulate GABAA Receptor-Mediated Tonic Currents in Rat Hippocampal CA3 Pyramidal Neurons. PLoS One. 2015;10:e0124765. doi: 10.1371/journal.pone.0124765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczko A, Goel A, Song L, Lee HK. Visual deprivation decreases somatic GAD65 puncta number on layer 2/3 pyramidal neurons in mouse visual cortex. Neural Plast. 2009 doi: 10.1155/2009/415135. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V, Maguire J. The impact of tonic GABA(A) receptor-mediated inhibition on neuronal excitability varies across brain region and cell type. Front. Neural Circuits. 2014;8:3. doi: 10.3389/fncir.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J. Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J. Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20559–20564. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller AL, Taube JS, Schwartzkroin PA. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J. Neurosci. 1984;4:860–867. doi: 10.1523/JNEUROSCI.04-03-00860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J. Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D. GABAA receptors: subtypes, regional distribution, and function. J. Clin. Sleep. Med. 2006;2:S7–S11. [PubMed] [Google Scholar]
- Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism–a positive feedback circuit in developing neurons. J. Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J. Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkinton MS, Sihra TS, Williams RJ. Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J. Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita J-B, Jacob TC, Moss SJ, Benfenati F, Medini P, Kneussel M, Barberis A. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 2014;5 doi: 10.1038/ncomms4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcher C, Hatchett C, Longbottom RE, McAinch K, Sihra TS, Moss SJ, Thomson AM, Jovanovic JN. Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J. Biol. Chem. 2011;286:21667–21677. doi: 10.1074/jbc.M110.201582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy J-M, Vogt KE. Specific subtypes of GABA a receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J. Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Renner M, Schweizer C, Bannai H, Triller A, Lévi S. Diffusion barriers constrain receptors at synapses. PLoS One. 2012;7:e43032. doi: 10.1371/journal.pone.0043032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LB, Ginty DD, Weber MJ, Greenberg ME. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Ryglewski S, Pflueger HJ, Duch C. Expanding the Neuron's calcium signaling repertoire: intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 2007;5:e66. doi: 10.1371/journal.pbio.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari AA, Bakhtiari A, Homberg JR. Activation of GABA-A receptors during postnatal brain development increases anxiety- and depression-related behaviors in a time- and dose-dependent manner in adult mice. Eur. Neuropsychopharmacol. 2015;25:1260–1274. doi: 10.1016/j.euroneuro.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Singh B, Henneberger C, Betances D, Arevalo MA, Rodriguez-Tebar A, Meier JC, Grantyn R. Altered balance of glutamatergic/GABAergic synaptic input and associated changes in dendrite morphology after BDNF expression in BDNF-deficient hippocampal neurons. J. Neurosci. 2006;26:7189–7200. doi: 10.1523/JNEUROSCI.5474-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Succol F, Fiumelli H, Benfenati F, Cancedda L, Barberis A. Intracellular chloride concentration influences the GABAA receptor subunit composition. Nat. Commun. 2012;3:738. doi: 10.1038/ncomms1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H-L, Tsai W-C, Li B-Y, Tao W, Chen P-S, Rubart M. Voltage-induced Ca2+ release in postganglionic sympathetic neurons in adult mice. PLoS One. 2016;11:e0148962. doi: 10.1371/journal.pone.0148962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanwick CC, Harrison MB, Kapur J. Synaptic and extrasynaptic localization of brain-derived neurotrophic factor and the tyrosine kinase B receptor in cultured hippocampal neurons. J. Comp. Neurol. 2004;478:405–417. doi: 10.1002/cne.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Feig LA. Age-dependent participation of Ras-GRF proteins in coupling calcium-permeable AMPA glutamate receptors to Ras/Erk signaling in cortical neurons. J. Biol. Chem. 2006;281:7578–7582. doi: 10.1074/jbc.M512060200. [DOI] [PubMed] [Google Scholar]
- Tokuoka H, Saito T, Yorifuji H, Wei F, Kishimoto T, Hisanaga S. Brain-derived neurotrophic factor-induced phosphorylation of neurofilament-H subunit in primary cultures of embryo rat cortical neurons. J. Cell Sci. 2000;113:1059–1068. doi: 10.1242/jcs.113.6.1059. [DOI] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy J-M, Pangalos MN, Moss SJ. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha2 subunits to gephyrin. J. Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric H-M, Moss SJ, Schindelin H, Harvey RJ, Sieghart W, Harvey K. Molecular basis of the γ-aminobutyric acid a receptor α3 subunit interaction with the clustering protein gephyrin. J. Biol. Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia. 2007;48(5):96–105. doi: 10.1111/j.1528-1167.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- Valeeva G, Tressard T, Mukhtarov M, Baude A, Khazipov R. An optogenetic approach for investigation of excitatory and inhibitory network GABA actions in mice expressing Channelrhodopsin-2 in GABAergic neurons. J. Neurosci. 2016;36:5961–5973. doi: 10.1523/JNEUROSCI.3482-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Bengtson CP, Bading H. Diffusion and not active transport underlies and limits ERK1/2 synapse-to-nucleus signaling in hippocampal neurons. J. Biol. Chem. 2007;282:29621–29633. doi: 10.1074/jbc.M701448200. [DOI] [PubMed] [Google Scholar]
- Xu W, Lipscombe D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Pietra C, Lovati E, de Groat WC. Activation of neurokinin-1 receptors increases the excitability of Guinea pig dorsal root ganglion cells. J. Pharmacol. Exp. Ther. 2012;343:44–52. doi: 10.1124/jpet.112.196113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Hollern D, Liao J, Andrechek E, Wang H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013;4:e560. doi: 10.1038/cddis.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Moon C, Zheng F, Luo Y, Soellner D, Nuñez JL, Wang H. NMDA-stimulated ERK1/2 signaling and the transcriptional up-regulation of plasticity-related genes are developmentally regulated following in vitro neuronal maturation. J. Neurosci. Res. 2009;87:2632–2644. doi: 10.1002/jnr.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]