Abstract
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide. Although surgery is known as the most promising radical treatment, a high recurrent or metastatic rate after surgery has limited its clinical efficacy. Sorafenib, a target agent, has seemed to be the only option for metastatic HCC patients to date, but none of clinical trials showed it could prolong the overall survival (OS) of advanced HCC to 1 year. How to prolong the OS and improve cure rate of HCC patients is still beset with difficulties. This report presents a rare case of recurrent HCC patient with complete regression of target lesion with 2 years of Chinese herbal treatment. A 64-year-old Chinese man with hepatitis B virus–associated chronic hepatitis presented HCC has been clinically diagnosed tumor relapse and omentum metastasis with computed tomography and α-fetoprotein blood test 4 months after surgery. It was decided the patient would receive traditional Chinese medicine treatment because of poor prognosis. After approximately 2 years of treatment, recurrent hepatic tumor and omentum metastasis have been found in complete regression. The patient remains alive over 31 months after relapse.
Keywords: recurrent hepatocellular carcinoma, complete remission, traditional Chinese medicine, spontaneous regression, activities
Hepatocellular carcinoma (HCC) is the most frequent histological type of primary liver cancer and the second most common cause of cancer-related mortality in males worldwide.1 Although many treatments including transarterial chemoembolization, chemotherapy, radiotherapy, and target agents such as sorafenib are exploited, the prognosis of patients with advanced HCC still remains so poor that the majority of them survive less than 12 months.2 Traditional Chinese medicine (TCM), an alternative usually applied to the control of side effects caused by chemical and radiotherapy, has been rarely reported to have reputable efficacy when used for advanced HCC alone. The present study presents a case of omentum metastasis of recurrent HCC with complete regression of target lesion after treatment with TCM, as revealed by image studies and blood test. The patient provided written informed consent.
Case Report
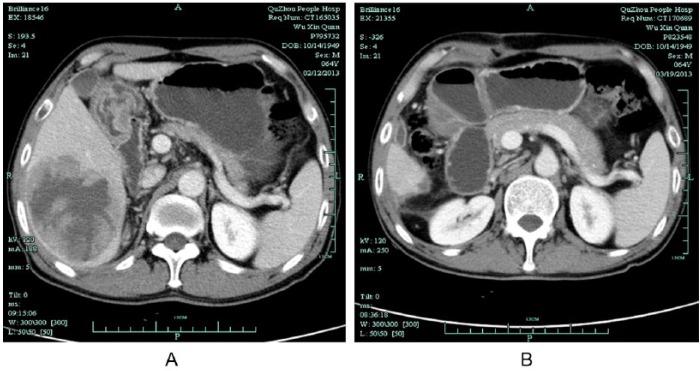
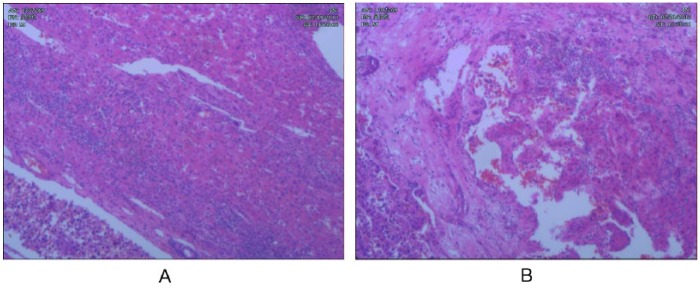
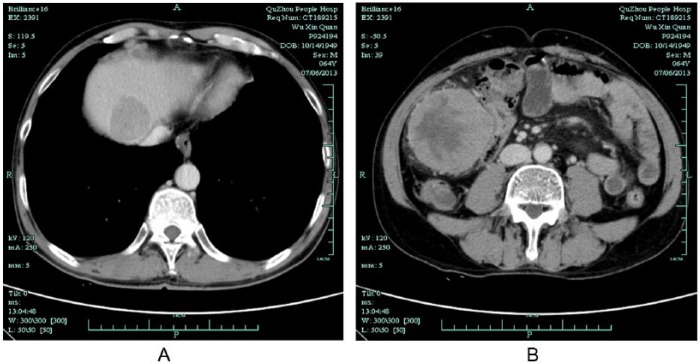
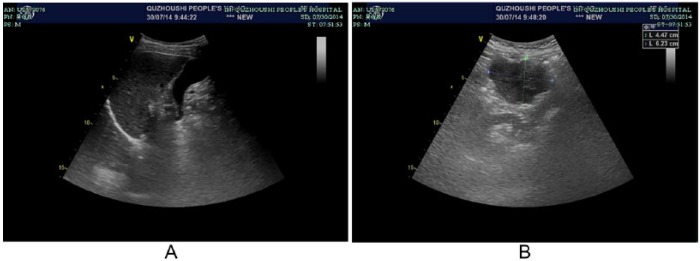
A 64-year-old Chinese man came to our hospital with right upper quadrant abdominal pain that had lasted 10 days. His medical history included chronic viral hepatitis B infection. Physical examination revealed mild tenderness and percussion pain on the hepatic region. Ultrasound evaluation showed an enlarged solid mass in the right lobe of the liver. Computed tomography (CT) scan of the whole abdomen confirmed a single low-density mass measuring 9.2 × 7.5 cm with central necrosis (Figure 1A), and absence of abdominal lymph node enlargement or any distant metastasis. Blood tests showed α-fetoprotein 24627.50 ng/mL (normal range = 0-13.4 ng/mL). The preoperative liver function was evaluated as Child A. Hepatic resection was performed for a hepatic tumor measuring 9.5 × 8.5 × 5.0 cm (Figure 1B). Pathologic examination (1302269) confirmed HCC with intravascular cancer emboli (Figure 2). After 4 months, CT reexamination revealed recurrent hepatic carcinoma in the right lobe of the liver with omentum metastasis measuring 7.4 × 7.2 cm (Figure 3). Blood tests showed α-fetoprotein elevating from 458.54 ng/mL to 9021.55 ng/mL. Clinical diagnosis of stage IVB recurrent HCC with omentum metastasis was made. The patient was categorized as having a poor prognosis because of the cancer relapse with potential metastasis. With the consideration of the poor prognosis of recurrent HCC and financial difficulties of the patient himself, conservative therapy with TCM was finally chosen. The composition of the formula with doses of herbs listed in Table 1 was used for 1 day. The solution was mixed equitably after being boiled twice, then taken at 9:00 to 10:00 am and 15:00 to 16:00 pm each day. During the TCM treatment, herbs were supplied every 2 weeks in our hospital. α-Fetoprotein variations were detected every 6 months while image examination was recommended every 6 to 12 months. During 2 years of treatment with Chinese herbs and antiviral treatment without any other therapy, the changes of recurrent hepatic carcinoma and omentum mass by CT scans and ultrasonography presented (Figures 4-6) with few toxicities of the herbal formula observed. The variations of the tumor measurements with the reports of ultrasonography and CT scans are listed in Table 2. Blood surveillance showed α-fetoprotein level range of 2766.67 to 3869.90 ng/mL (Table 3). The patient has been alive over 31 months since HCC relapse.
Figure 1.
Computed tomography findings before and after surgery. (A) An enlarged solid mass in right lobe of liver on February 11, 2013. (B) CT scan after tumor resection on March 18, 2013.
Figure 2.
Pathological findings of tumor by hepatic resection. (A) Hepatocellular carcinoma cells with HE staining. (B) Intravascular cancer emboli.
Figure 3.
Computed tomography scan of recurrent liver tumor and omentum metastasis on July 5, 2013. (A) Recurrent tumor in right lobe of liver. (B) Omentum metastasis in right abdomen.
Table 1.
Herbs and Dose in the Formula Used in the Case.
| Herbs | Dose (g) | Herbs | Dose (g) |
|---|---|---|---|
| Radix Bupleuri | 6 | Radix Paeoniae Alba | 15 |
| Angelica Sinensis | 10 | Radix Curcumae | 10 |
| Radix Salviae Miltiorrhizae | 20 | Ligusticum Wallichii | 15 |
| Actinidia valvata Dunn | 30 | Melia Toosendan | 10 |
| Fructus Aurantii | 10 | Artemisia carvifolia | 10 |
| Caulis Spatholobi | 20 | Chinese Lobelia | 15 |
| Sedum Sarmentosum Bunge | 30 | Squama Manis | 3 |
| Radix Ranunculi Ternati | 10 | Salvia Chinensis | 30 |
| Hedyotis Diffusa | 20 | Liquidambar Formosana Hance | 15 |
| Centipede | 3 |
Figure 4.
Ultrasonography of liver and omentum metastasis with TCM treatment on July 30, 2014. (A) Recurrent tumor in liver disappeared. (B) Omentum metastasis measuring 6.2 cm × 4.5 cm.
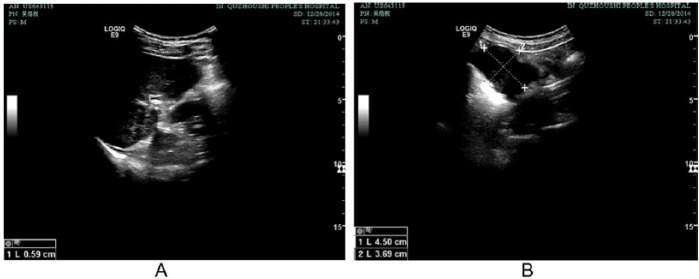
Figure 5.
Ultrasonography of liver and omentum metastasis with TCM treatment on December 28, 2014. (A) Recurrent tumor in liver disappeared. (B) Omentum metastasis measuring 4.5 cm × 3.7 cm.
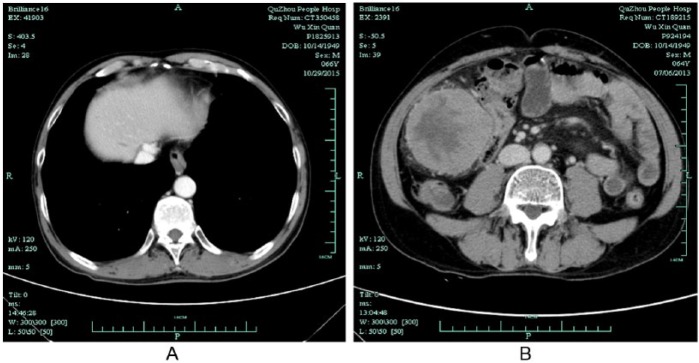
Figure 6.
Computed tomography scan of liver and abdomen with 2 years of TCM treatment on October 29, 2015. (A) CT showed tumor in the liver disappeared. (B) Omentum metastasis in the abdomen vanished.
Table 2.
Progress of the Disappearance of Recurrent Hepatic Carcinoma and Omentum Metastasis.
| Date | Measurements of the Tumor With Imaging |
|
|---|---|---|
| Liver (LD × SD) | Omentum (LD × SD) | |
| February 11, 2013 | 9.2 cm × 7.5 cm | N/A |
| July 5, 2013 | 3.9 cm × 3.7 cm | 7.4 cm × 7.2 cm |
| July 30, 2014 | N/A | 6.2 cm × 4.5 cm |
| December 28, 2014 | N/A | 4.5 cm × 3.7 cm |
| October 29, 2015 | N/A | N/A |
Abbreviations: LD, longest diameter; SD, shortest diameter.
Table 3.
Progress of α-Fetoprotein (AFP) Throughout the Course of the Case.
| Date | AFP (0-13.4 ng/mL) |
|---|---|
| February 11, 2013 | 24627.50 |
| February 19, 2013 | 20076.10 |
| February 21, 2013 | 8794.96 |
| March 4, 2013 | 2627.80 |
| March 18, 2013 | 458.54 |
| July 2, 2013 | 9021.55 |
| December 19, 2013 | 2515.58 |
| July 30, 2014 | 3485.91 |
| December 29, 2014 | 2766.67 |
| May 24, 2015 | 3294.28 |
| October 28, 2015 | 3869.90 |
Discussion
Hepatocarcinoma, one of the most common solid tumors, is a minimally curable disease even with surgery, target therapy, locoregional therapy, stereotactic body radiation therapy, and chemotherapy. Clinical studies evaluating the use of cytotoxic chemotherapy in the treatment of patients with advanced HCC have typically reported low response rates, and evidence for a favorable impact of chemotherapy on overall survival in patients with HCC is lacking.3-5 For target therapy, there have been 2 randomized, placebo-controlled, phase III trials for assessment of sorafenib in the treatment of patients with advanced or metastatic HCC so far (SHARP and Asian-Pacific trial), neither of which has shown sorafenib to prolong overall survival of patients with advanced HCC to 1 year.5,6 Therefore, most patients with advanced or metastatic HCC are not eligible for potential curative therapies.
Spontaneous regression of cancer was defined as partial or complete disappearance of malignant tumor without any anticancer therapy.7 Kinds of malignant tumors including colon cancer, breast cancer, renal cell carcinoma, neuroblastoma, and choriocarcinoma have been reported to convert to spontaneous regression in a PubMed search.8-12 However, spontaneous regression of HCC still remains a rare event.13 Because of the antitumor treatment with Chinese herbs for 2 years in this case, spontaneous regression of HCC has not been taken into consideration.
Traditional Chinese medicine, which has been observed to be effective and used in China for more than a thousand years, was widely exploited in diseases including malignancy. According to recent research, traditional Chinese herbal extracts seem to be emerging as a novel antitumor selection in the treatment of cancers including nasopharyngeal carcinoma, bladder carcinoma, and HCC.14-17 The formula in the case, made in our hospital, boiled by the patient himself, mainly contains crude of 19 herbs listed with doses in Table 1. The patient denied any changes in lifestyle or diet that he started along with the Chinese herbs.
All herbs in the formula have been searched for the possible antitumor activities with PubMed. According to the result, the antitumor activities of herbs in the formula have been identified (Table 4), some of which including Actinidia valvata Dunn, Toosendanin, Radix Curcumae, and Artemisia carvifolia involved in the formula have been verified for antitumor activity on HCC. Saponin extracted from the root of Actinidia valvata has been reported to have anti-HCC activity in vitro with HCC cells in cell lines BEL-7402, HepG2, PLC, SMMC-7721, MHCC-97-H, and MHCC-97-L.18 The extract could restrain adhesion, invasion, mobility, and migration abilities of BEL-7402 and MHCC-97-H cells in vitro.18 Toosendanin extract has potent anti-HCC effects via suppressing proliferation and inducing apoptosis of cancer cells in vitro with HCC cell lines SMMC-7721 and Hep3B and in vivo with BALB/c mice. The mechanism of apoptosis involves the mitochondrial pathway and death receptor pathway.19 Curcumin extracted from Radix Curcumae has demonstrated a synergistic effect with bevacizumab on the inhibition of the effects of the VEGF signaling pathways in HCC progression.20 Beta-elemene, well known for its antitumor activity, capable of sensitizing HCC cells to oxaliplatin, could also be extracted from Radix Curcumae.21 Despite all these findings, changes caused by chemical reactions when herbs are mixed and boiled together still remain unknown. Therefore, more research on the possible activities of herbs might be indeed necessary to lead to the discovery of new antitumor drugs.
Table 4.
Reported Potential Antitumor Activities of Herbs Involved in the Formula.
| Activities | Source | Antitumor Spectrum | References |
|---|---|---|---|
| Saikosaponin | Radix Bupleuri | Breast, lung | 22, 23 |
| Paeoniflorin | Radix Paeoniae Alba | Gastric, lung, cervical, breast | 24-27 |
| Angelica | Angelica Sinensis | Colorectal | 28 |
| Curcumin | Radix Curcumae | Colorectal, breast, ovarian, lung, pancreatic, cervical, hepatocellular | 20, 29-34 |
| Curcumol | Radix Curcumae | Colorectal | 35 |
| Beta-Elemene | Radix Curcumae | Esophageal, ovarian, hepatocellular, kidney, lung | 21, 36-39 |
| Ligustrazine | Ligusticum Wallichii | Lung, breast | 40, 41 |
| Saponin | Actinidia valvata Dunn | Hepatocellular | 18 |
| Toosendanin | Melia Toosendan | Hepatocellular | 19 |
| Limonoids | Fructus Aurantii | Colon, breast, cervical | 42-44 |
| Artemisinin | Artemisia Carvifolia | Cervical, gastric, breast | 45-47 |
| Artesunate | Artemisia Carvifolia | Breast, hepatocellular, ovarian, gastric, esophageal, bladder, colorectal | 48-54 |
| Spatholobus suberectus | Caulis Spatholobi | Breast, lung | 55, 56 |
| Lobeline | Chinese Lobelia | Colon | 57 |
| Polysaccharides, Saponins | Radix Ranunculi Ternati | Gastric | 58 |
| Ursolic acid | Salvia Chinensis | Pancreatic, ovarian, gastric, lung, breast, prostate | 59-64 |
| Hedyotis diffusa water extract | Hedyotis diffusa | Breast, prostate, colorectal, bladder | 65-68 |
| Centipede extract | Centipede | Cervical | 69 |
In conclusion, complete regression of target lesion in recurrent HCC by TCM is an interesting phenomenon, the mechanism of which still remains unknown. Further discussion and deeper research on the anti-HCC activity of TCM will help in understanding this phenomenon and in curing malignancies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49(43):7-11. [PubMed] [Google Scholar]
- 3. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [DOI] [PubMed] [Google Scholar]
- 4. Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15:1008-1014. [DOI] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [DOI] [PubMed] [Google Scholar]
- 6. Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532-1538. [DOI] [PubMed] [Google Scholar]
- 7. Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maillet L, Chopin N, Treilleux I, et al. Spontaneous regression of breast cancer after biopsy. About two cases [in French]. Gynecol Obstet Fertil. 2014;42:269-272. [DOI] [PubMed] [Google Scholar]
- 9. Kihara K, Fujita S, Ohshiro T, Yamamoto S, Sekine S. Spontaneous regression of colon cancer. Jpn J Clin Oncol. 2015;45:111-114. [DOI] [PubMed] [Google Scholar]
- 10. Chan BP, Booth CM, Manduch M, Touma NJ. Spontaneous regression of metastatic pulmonary renal cell carcinoma in the setting of sarcomatoid differentiation of the primary tumour. Can Urol Assoc J. 2013;7:E587-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diede SJ. Spontaneous regression of metastatic cancer: learning from neuroblastoma. Nat Rev Cancer. 2014;14:71-72. [DOI] [PubMed] [Google Scholar]
- 12. Bakyalakshmi K, Bharathi R, Ponniah I. A regressing and metastasizing tumor—the choriocarcinoma. J Oral Maxillofac Surg. 2013;71:214-219. [DOI] [PubMed] [Google Scholar]
- 13. Chiesara F, Spagnolo A, Koch M, Moretti A. A case of hepatocellular carcinoma: spontaneous regression? Dig Liver Dis. 2014;46:659-660. [DOI] [PubMed] [Google Scholar]
- 14. Lu CC, Lin MY, Chen SY, et al. The investigation of a traditional Chinese medicine, Guizhi Fuling Wan (GFW) as an intravesical therapeutic agent for urothelial carcinoma of the bladder. BMC Complement Altern Med. 2013;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ting CT, Li WC, Chen CY, Tsai TH. Preventive and therapeutic role of traditional Chinese herbal medicine in hepatocellular carcinoma. J Chin Med Assoc. 2015;78:139-144. [DOI] [PubMed] [Google Scholar]
- 16. Zeng X, Li X, Xue X, et al. Activation of apoptosis in hepatocellular carcinoma by the Chinese traditional medicine Hu Qisan. Exp Ther Med. 2013;5:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mao CG, Tao ZZ, Wan LJ, Han JB, Chen Z, Xiao BK. The efficacy of traditional Chinese medicine as an adjunctive therapy in nasopharyngeal carcinoma: a systematic review and meta-analysis. J BUON. 2014;19:540-548. [PubMed] [Google Scholar]
- 18. Zheng GY, Xin HL, Li B, Xu YF, Yi TJ, Ling CQ. Total saponin from root of Actinidia valvata Dunn prevents the metastasis of human hepatocellular carcinoma cells. Chin J Integr Med. 2012;18:197-202. [DOI] [PubMed] [Google Scholar]
- 19. Liu XL, Wang H, Zhang L, et al. Anticancer effects of crude extract from Melia toosendan Sieb. et Zucc on hepatocellular carcinoma in vitro and in vivo. Chin J Integr Med. 2016;22:362-369. [DOI] [PubMed] [Google Scholar]
- 20. Gao JZ, Du JL, Wang YL, Li J, Wei LX, Guo MZ. Synergistic effects of curcumin and bevacizumab on cell signaling pathways in hepatocellular carcinoma. Oncol Lett. 2015;9:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Lin Z, Zhang B, et al. Beta-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci Rep. 2016;6:21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen JC, Chang NW, Chung JG, Chen KC. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 2003;31:363-377. [DOI] [PubMed] [Google Scholar]
- 23. Hsu YL, Kuo PL, Lin CC. The proliferative inhibition and apoptotic mechanism of Saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 2004;75:1231-1242. [DOI] [PubMed] [Google Scholar]
- 24. Fang S, Zhu W, Zhang Y, Shu Y, Liu P. Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-kappaB activation. Mol Med Rep. 2012;5:351-356. [DOI] [PubMed] [Google Scholar]
- 25. Wu Q, Chen GL, Li YJ, Chen Y, Lin FZ. Paeoniflorin inhibits macrophage-mediated lung cancer metastasis. Chin J Nat Med. 2015;13:925-932. [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Zhang S. Modulating Bcl-2 family proteins and caspase-3 in induction of apoptosis by paeoniflorin in human cervical cancer cells. Phytother Res. 2011;25:1551-1557. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Yuan Y, Cui J, Xiao T, Jiang D. Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway. Biomed Pharmacother. 2016;78:197-203. [DOI] [PubMed] [Google Scholar]
- 28. An J, Li XN, Zhao BC, Wang Q, Lan Y, Wu Q. Chemo-preventive effect of Angelica sinensis’ supercritical extracts on AOM/DSS-induced mouse colorectal carcinoma associated with inflammation [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2014;39:1265-1269. [PubMed] [Google Scholar]
- 29. Zaman MS, Chauhan N, Yallapu MM, et al. Curcumin nanoformulation for cervical cancer treatment. Sci Rep. 2016;6:20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taurin S, Nimick M, Larsen L, Rosengren RJ. A novel curcumin derivative increases the cytotoxicity of raloxifene in estrogen receptor-negative breast cancer cell lines. Int J Oncol. 2016;48:385-398. [DOI] [PubMed] [Google Scholar]
- 31. Seo JA, Kim B, Dhanasekaran DN, Tsang BK, Song YS. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca(2+) ATPase activity in ovarian cancer cells. Cancer Lett. 2016;371:30-37. [DOI] [PubMed] [Google Scholar]
- 32. Sahebkar A. Curcumin: a natural multitarget treatment for pancreatic cancer. Integr Cancer Ther. 2016,15:333–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajitha B, Belalcazar A, Nagaraju GP, et al. Inhibition of NF-kappaB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016;373:227-233. [DOI] [PubMed] [Google Scholar]
- 34. Li S, Fang C, Zhang J, et al. Catanionic lipid nanosystems improve pharmacokinetics and anti-lung cancer activity of curcumin [published online March 16, 2016]. Nanomedicine. doi: 10.1016/j.nano.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Huang F, Bai Z, Chi B, Wu J, Chen X. Curcumol inhibits growth and induces apoptosis of colorectal cancer LoVo cell line via IGF-1R and p38 MAPK pathway. Int J Mol Sci. 2015;16:19851-19867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu Z, Wu H, Li Y, et al. beta-Elemene inhibits the proliferation of esophageal squamous cell carcinoma by regulating long noncoding RNA-mediated inhibition of hTERT expression. Anticancer Drugs. 2015;26:531-539. [DOI] [PubMed] [Google Scholar]
- 37. Li QQ, Lee RX, Liang H, et al. beta-Elemene enhances susceptibility to cisplatin in resistant ovarian carcinoma cells via downregulation of ERCC-1 and XIAP and inactivation of JNK. Int J Oncol. 2013;43:721-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhan YH, Liu J, Qu XJ, et al. beta-Elemene induces apoptosis in human renal-cell carcinoma 786-0 cells through inhibition of MAPK/ERK and PI3K/Akt/ mTOR signalling pathways. Asian Pac J Cancer Prev. 2012;13:2739-2744. [DOI] [PubMed] [Google Scholar]
- 39. Zhang F, Xu L, Qu X, et al. Synergistic antitumor effect of beta-elemene and etoposide is mediated via induction of cell apoptosis and cell cycle arrest in non-small cell lung carcinoma cells. Mol Med Rep. 2011;4:1189-1193. [DOI] [PubMed] [Google Scholar]
- 40. Ai Y, Zhu B, Ren C, et al. Discovery of new monocarbonyl ligustrazine-curcumin hybrids for intervention of drug-sensitive and drug-resistant lung cancer. J Med Chem. 2016;59:1747-1760. [DOI] [PubMed] [Google Scholar]
- 41. Chen J, Wang W, Wang H, Liu X, Guo X. Combination treatment of ligustrazine piperazine derivate DLJ14 and adriamycin inhibits progression of resistant breast cancer through inhibition of the EGFR/PI3K/Akt survival pathway and induction of apoptosis. Drug Discov Ther. 2014;8:33-41. [DOI] [PubMed] [Google Scholar]
- 42. Chidambara Murthy KN, Jayaprakasha GK, Patil BS. Citrus limonoids and curcumin additively inhibit human colon cancer cells. Food Funct. 2013;4:803-810. [DOI] [PubMed] [Google Scholar]
- 43. Kim J, Jayaprakasha GK, Patil BS. Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013;4:258-265. [DOI] [PubMed] [Google Scholar]
- 44. Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic Res. 2010;44:624-634. [DOI] [PubMed] [Google Scholar]
- 45. Mondal A, Chatterji U. Artemisinin represses telomerase subunits and induces apoptosis in HPV-39 infected human cervical cancer cells. J Cell Biochem. 2015;116:1968-1981. [DOI] [PubMed] [Google Scholar]
- 46. Zhang HT, Wang YL, Zhang J, Zhang QX. Artemisinin inhibits gastric cancer cell proliferation through upregulation of p53. Tumour Biol. 2014;35:1403-1409. [DOI] [PubMed] [Google Scholar]
- 47. Wang S, Sasaki T. Synthesis of artemisinin dimers using the Ugi reaction and their in vitro efficacy on breast cancer cells. Bioorg Med Chem Lett. 2013;23:4424-4427. [DOI] [PubMed] [Google Scholar]
- 48. Tran TH, Nguyen AN, Kim JO, Yong CS, Nguyen CN. Enhancing activity of artesunate against breast cancer cells via induced-apoptosis pathway by loading into lipid carriers [published online January 11, 2016]. Artif Cells Nanomed Biotechnol. doi: 10.3109/21691401.2015.1129616. [DOI] [PubMed] [Google Scholar]
- 49. Ilamathi M, Santhosh S, Sivaramakrishnan V. Artesunate as an anti-cancer agent targets Stat-3 and favorably suppresses hepatocellular carcinoma [published online February 12, 2016]. Curr Top Med Chem. [DOI] [PubMed] [Google Scholar]
- 50. Greenshields AL, Shepherd TG, Hoskin DW. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate [published online February 15, 2016]. Mol Carcinog. doi: 10.1002/mc.22474. [DOI] [PubMed] [Google Scholar]
- 51. Zhang P, Luo HS, Li M, Tan SY. Artesunate inhibits the growth and induces apoptosis of human gastric cancer cells by downregulating COX-2. Onco Targets Ther. 2015;8:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu L, Zuo LF, Zuo J, Wang J. Artesunate induces apoptosis and inhibits growth of Eca109 and Ec9706 human esophageal cancer cell lines in vitro and in vivo. Mol Med Rep. 2015;12:1465-1472. [DOI] [PubMed] [Google Scholar]
- 53. Zuo W, Wang ZZ, Xue J. Artesunate induces apoptosis of bladder cancer cells by miR-16 regulation of COX-2 expression. Int J Mol Sci. 2014;15:14298-14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krishna S, Ganapathi S, Ster IC, et al. A randomised, double blind, placebo-controlled pilot study of oral artesunate therapy for colorectal cancer. EBioMedicine. 2015;2:82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng F, Meng CW, Zhou QM, Chen JP, Xiong L. Cytotoxic evaluation against breast cancer cells of isoliquiritigenin analogues from Spatholobus suberectus and their synthetic derivatives. J Nat Prod. 2016;79:248-251. [DOI] [PubMed] [Google Scholar]
- 56. Tang Y, Fu Q, He W, Sun YK, Wang YZ, Wang XM. Non-apoptotic programmed cell death induced by extract of Spatholobus suberctus in human lung cancer A549 cells [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2008;33:2040-2044. [PubMed] [Google Scholar]
- 57. Ma Y, Wink M. Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine. 2008;15:754-758. [DOI] [PubMed] [Google Scholar]
- 58. Niu L, Zhou Y, Sun B, Hu J, Kong L, Duan S. Inhibitory effect of saponins and polysaccharides from Radix ranunculi ternati on human gastric cancer BGC823 cells. Afr J Tradit Complement Altern Med. 2013;10:561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prasad S, Yadav VR, Sung B, Gupta SC, Tyagi AK, Aggarwal BB. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget. 2016;7:13182-13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Wang W, Qian L, Zhang Q, Lai D, Qi C. Ursolic acid inhibits the proliferation of human ovarian cancer stem-like cells through epithelial-mesenchymal transition. Oncol Rep. 2015;34:2375-2384. [DOI] [PubMed] [Google Scholar]
- 61. Yang Y, Jiang M, Hu J, et al. Enhancement of radiation effects by ursolic acid in BGC-823 human adenocarcinoma gastric cancer cell line. PLoS One. 2015;10:e0133169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu J, Zhao S, Tang Q, et al. Activation of SAPK/JNK mediated the inhibition and reciprocal interaction of DNA methyltransferase 1 and EZH2 by ursolic acid in human lung cancer cells. J Exp Clin Cancer Res. 2015;34:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wen JH, Wei XH, Sheng XY, et al. Effect of ursolic acid on breast cancer resistance protein-mediated transport of rosuvastatin in vivo and vitro. Chin Med Sci J. 2015;30:218-225. [DOI] [PubMed] [Google Scholar]
- 64. Meng Y, Lin ZM, Ge N, Zhang DL, Huang J, Kong F. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway. Am J Chin Med. 2015;43:1471-1486. [DOI] [PubMed] [Google Scholar]
- 65. Pan LT, Sheung Y, Guo WP, Rong ZB, Cai ZM. Hedyotis diffusa plus Scutellaria barbata induce bladder cancer cell apoptosis by inhibiting Akt signaling pathway through downregulating miR-155 expression. Evid Based Complement Alternat Med. 2016;2016:9174903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu E, Wang D, Chen J, Tao X. Novel cyclotides from Hedyotis diffusa induce apoptosis and inhibit proliferation and migration of prostate cancer cells. Int J Clin Exp Med. 2015;8:4059-4065. [PMC free article] [PubMed] [Google Scholar]
- 67. Yeh YC, Chen HY, Yang SH, et al. Hedyotis diffusa combined with Scutellaria barbata are the core treatment of Chinese herbal medicine used for breast cancer patients: a population-based study. Evid Based Complement Alternat Med. 2014;2014:202378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin J, Wei L, Shen A, et al. Hedyotis diffusa Willd extract suppresses Sonic hedgehog signaling leading to the inhibition of colorectal cancer angiogenesis. Int J Oncol. 2013;42:651-656. [DOI] [PubMed] [Google Scholar]
- 69. Zhou YQ, Han L, Liu ZQ, Du KC, Li KY. Effect of centipede extract on cervical tumor of mice and its mechanism [in Chinese]. Zhong Yao Cai. 2011;34:859-864. [PubMed] [Google Scholar]