Abstract
Background
Full length Tissue factor (flTF) is a key player in hemostasis and also likely contributes to venous thromboembolism (VTE), the third most common cardiovascular disease. flTF and its minimally coagulant isoform, alternatively spliced TF (asTF), have been detected in thrombi, suggesting participation of both isoforms in thrombogenesis, but data on participation of asTF in hemostasis is lacking. Therefore, we assessed the role of asTF in flTF cofactor activity modulation, using a co-expression system.
Objective
To investigate the interplay between flTF and asTF in hemostasis on endothelial cell surface.
Methods
Immortalized endothelial (ECRF) cells were adenovirally transduced to express asTF and flTF, after which flTF cofactor activity was measured on cells and microvesicles (MVs). To study co-localization of flTF/asTF proteins, confocal microscopy was performed. Finally, intracellular distribution of flTF was studied in the presence or absence of heightened asTF levels.
Results
Levels of flTF antigen and cofactor activity were not affected by asTF co-expression. asTF and flTF were found to localize in distinct subcellular compartments. Only upon heightened overexpression of asTF, lower flTF protein levels and cofactor activity were observed. Heightened asTF levels also induced a shift of flTF from non-raft to lipid raft plasma membrane fractions, and triggered the expression of ER stress marker BiP. Proteasome inhibition resulted in increased asTF – but not flTF – protein expression.
Conclusion
At moderate levels, asTF appears to have negligible impact on flTF cofactor activity on endothelial cells and MVs; however, at supra-physiological levels, asTF is able to reduce the levels of flTF protein and cofactor activity.
Keywords: Alternatively spliced Tissue Factor, Full length Tissue Factor, Hemostasis, Coagulation
1. Introduction
Venous thromboembolism (VTE) belongs to the top three most common cardiovascular diseases in industrialized nations [1]. VTE mainly comprises deep vein thrombosis and pulmonary embolism, which occurs with an incidence rate of approximately 1–2 events per 1000 individuals per year. Thrombosis is initiated after changes in blood flow, composition of blood, and/or damage to the vessel wall, also known as the triad of Virchow [2]. Hypercoagulability may be caused by increased microvesicle (MV) levels in blood and is associated with VTE in cancer patients, as reviewed elsewhere [3,4].
MVs can be shed via e.g. blebbing of the cell membrane and fall within a size range of 10–1000 nm [5]. In numerous disease settings these MVs can be shed from platelets, monocytes, and/or endothelial cells, and are believed to contribute to pathological processes such as cancer and VTE [6]. To date, it is unclear whether MVs are a cause or a consequence of VTE. Depending on the origin of the cell, these MVs may contain negatively charged phosphatidylserine (PS) and/or Tissue Factor full length form (TF/flTF) in the outside leaflet of their lipid bilayer, which increases the procoagulant potential of these vesicles [7].
flTF, a glycosylated transmembrane protein, is the only initiator of the extrinsic coagulation cascade in vivo [8], and a key player in thrombosis [3,9]. flTF is also required for vessel formation and maturation, as flTF deficiency results in the abrogation of mouse embryonic development between days 8.5 and 10.5 due to defects in the yolk sac vasculature [10,11]. In 2003, the structure of an alternative isoform of TF termed alternatively spliced Tissue Factor (asTF), was reported [12]. During TF pre-mRNA processing, exon 5 is spliced out, resulting in a frameshift that yields a soluble TF isoform with a unique C-terminal domain. We have previously shown that recombinant asTF induces angiogenesis in an integrin-dependent manner. asTF binding to αvβ3-integrins promotes endothelial cell migration, while capillary formation is induced by asTF via α6β1-integrin activation [13]. In cancer, asTF induces tumor growth and metastasis in a β1-integrin dependent manner, and recruits monocytes to the tumor stroma [14–16]. asTF also increases the coagulant potential of pancreatic ductal adenocarcinoma cells and MVs via an indirect mechanism [17].
Even though asTF promotes cancer progression non-proteolytically, coagulant properties of asTF and thus its involvement in thrombosis and/or hemostasis are controversial [18–20]. Both flTF and asTF accumulate in occlusive thrombi [21], suggesting a role for asTF in thrombus formation. asTF may in principle influence coagulation as it retains the first 166 residues of flTF critical to forming a complex with FVII(a), including the 165–166 lysine doublet involved in the binding of FVII(a) and FX [22,23], but asTF lacks a complete binding site for the macromolecular substrates FIX and FX [12,24]. Initial functional studies showed that high concentrations of recombinant asTF shorten clotting times in the presence of PS-containing phospholipid vesicles [12], but these studies did not uncover how and whether asTF influences flTF-dependent clotting. In arterial lipid-rich plaques, the functional activity of asTF likely contributes to thrombus formation in a slow and long-term manner; however, in these settings asTF more likely serves to recruit monocytes that destabilize the plaque (reviewed in [25]). Another study showed that coagulant activity in supernatants from cytokine-stimulated endothelial cells decreased upon asTF depletion [20], but again this study did not explore whether asTF and flTF may synergize, or alternatively, have opposing functions in coagulation initiation. Finally, a study by Böing and colleagues found that asTF expressed in flTF-null HEK293 cells did not influence coagulation initiation, but again, this study did not evaluate the possible effects of asTF on flTF function [18].
While the above studies investigated TF isoforms in isolation, in vivo, F3 expression results in simultaneous biosynthesis of flTF and asTF. Although the relative abundance of asTF can vary widely, asTF is never exclusively expressed [25]. The lack of functional data on TF isoform-dependent coagulation activation prompted us to study the effects of flTF/asTF co-expression on TF cofactor activity of endothelial cells and endothelial cell-derived MVs.
2. Materials and methods
2.1. Reagents
Full-length Tissue Factor (flTF)-specific mouse monoclonal antibody mAb TF9-10H10 and alternatively spliced Tissue Factor (asTF)-specific rabbit monoclonal antibody RabMab1 were described previously [14]. Anti-BiP (C50B12) and anti-GAPDH (14C10) rabbit monoclonal antibodies were purchased from Cell Signaling. B-actin (N-21)-specific rabbit polyclonal antibody was purchased from Santa Cruz Biotechnology.
2.2. Cell culture and adenoviral transductions
The immortalized human endothelial cell line ECRF was cultured in medium 199 (Life Technologies), supplemented with 20% FCS (PAA), 0,5% bovine pituitary extract (Gibco), 1 ml heparin Na LEO 5000 IE/ml (LEO Pharma Inc.) and Penicillin/Streptomycin in a 5% CO2 incubator at 37 °C. ECRF cells were transduced with GFP (mock), flTF (NM_001993) and/or asTF (NM_001178096) adenoviral constructs as previously described [26]. Briefly, ECRF cells were transduced, and the experiments were performed 2 days later. To induce MP shedding, cells were serum-starved [27] in M199 medium for 3 h at 37 °C, after which conditioned media was harvested and centrifuged for 5 min at 1,000 ×g to remove cell debris. To pellet MVs, the supernatant was spun at 20,000 ×g for 1 h as described [28].
2.3. Adenovirus production
Adenoviral constructs encoding asTF or flTF were generated using AdenoX Expression system (Clontech). For large-scale adenovirus production, HEK293 cells were transduced with adenoviral constructs for 3–4 days at 37 °C. Cells were freeze-thawed three times, after which supernatant was collected and stored at −80 °C. Numbers of viral particles were determined via QuickTiter Adenovirus Titer ELISA kit (Cell biolabs).
2.4. Reverse transcriptase-PCR analysis
Total cellular RNA was isolated with TRIzol (Life Technologies) according to the manufacturer’s instructions. 1 μg RNA was converted to first strand cDNA using Super Script II kit (Life Technologies). PCR amplifications for β-actin and flTF were performed at 95 °C for 5 min, 95 °C for 30 s, 56 °C for 30 s, 72 °C for 1 min (25 cycles for β-actin; 29 cycles for flTF), 72 °C for 10 min. PCR conditions for asTF were performed at 95 °C for 5 min, 95 °C for 1 min, 61 °C for 1 min, 72 °C for 1 min (40 cycles), 72 °C for 5 min. A common asTF/flTF forward primer was used: forward, 5′-TTACACACAGACACAGAGTGTGA-3′, asTF reverse primer, 5′-GAATATTTCTTTCTTTCCTGAACTTGAAG-3′, flTF reverse primer, 5′-TTGAACA CTGAAACAGTAGTTTTCTCC-3′. The β-actin primers were: forward, 5′-AAGAGATGGCCCACGGCTGCT-3′, and reverse, 5′-CCTTCTGCATCCTGT CGGCA-3′.
2.5. Western blotting
For western blotting, cells and MVs were lysed in 2× sample buffer (Life Technologies), proteins were denatured at 95 °C for 5 min and cell lysates were sonicated for 10 s. For lipid raft collection, cells were lysed in Brij58 buffer (50 mM Tris, 150 mM CaCl, 1 mM CaCl2, 1 mM MgCl2, 1% Brij58), centrifuged for 10 min at 800 ×g to pellet cytoskeletal fractions and nuclei, and the supernatant was spun at 16,000 ×g for 30 min to pellet lipid rafts. Lysates were loaded on 4–12% Bis-Tris PLUS Gels (Thermo Fischer Scientific) and transferred to 0.2 μm pore size PVDF membranes. Membranes were blocked in 5% milk/TBST and incubated with flTF- or asTF-specific primary antibodies O/N in blocking buffer; mAb TF9-10H10 (flTF-speciic), RabMab1 (asTF-speciic) [14]. After multiple TBST washing steps, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Bands were visualized with Western lightning Plus ECL (PerkinElmer) on X-ray film (Santa Cruz).
2.6. FXa generation
TF-dependent coagulant activity on the surface of ECRF cells and MVs were performed in HBS with 1.5 mM CaCl2. The reaction was started by the addition of 1 nM FVIIa (Novo Nordisk) and 50 nM FX (Stago). After 30 min at 37 °C the reaction was quenched, after which the generated FXa was measured using the kinetic chromogenic substrate Spectrozyme FXa (Sekisui) as described before [26].
2.7. Immunofluorescence
ECRF cells were grown on glass coverslips, transduced with flTF or asTF adenovirus and serum starved, as described above. The cells were washed and fixed with 2% formaldehyde in PBS for 20 min at RT. In selected experiments, cells were subsequently permeabilized with 0.1% Triton-X100 for 5 min and blocked with 5% BSA/PBS for 30 min. Primary custom rabbit polyclonal antibody specific for human asTF (pAb-1979) [14] was applied at 5 μg/ml in the blocking buffer and incubated O/N at 4 °C. The following day, cells were incubated with goat anti-rabbit-Alexa488 and Alexa594-conjugated 10H10 for 1 h in the dark. Cover-slips were mounted with ProlongGold containing DAPI (Thermo Fischer Scientific) for nuclear staining. Images of immunofluorescently labeled cells were captured using a Leica SP5 confocal microscope.
3. Results
3.1. asTF exhibits minimal coagulant activity when expressed in human en-dothelial cells
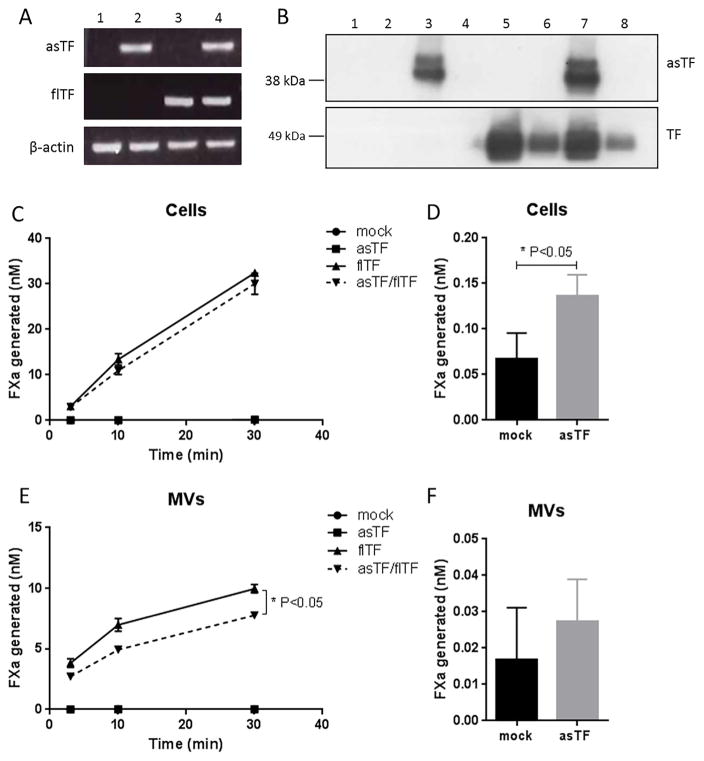
The role of asTF in coagulation initiation has previously been assessed, but the interplay between flTF and asTF has never been elucidated. To gain insight into such an interplay, we studied asTF’s effect on TF cofactor function when expressed in endothelial cells. In our in vitro model, asTF and flTF antigen levels were much higher compared to those under TNFα and LPS stimulation conditions (data not shown). Immortalized endothelial ECRF cells were transduced to express asTF and/or flTF and, as expected, expression of the two TF isoforms was observed in these cells, both on mRNA (Fig. 1a) and antigen (Fig. 1b) levels. We found that flTF expression levels did not significantly alter asTF expression levels, and vice versa. Antigen analysis on MVs indicated the presence of flTF, but not asTF, suggesting that asTF does not associate with endothelial MVs at appreciable levels (Fig. 1b; upper panel). A chromogenic FXa generation assay was performed to address whether asTF influences cofactor activity of flTF in an endothelial setting. Mock transduced ECRF cells generated <1 nM FXa, while asTF expression led to a very minor yet statistically significant increase in TF activity (Fig. 1d). flTF-transduced ECRF generated 30 nM FXa after 30 min (Fig. 1c). In cells co-expressing asTF and flTF, asTF did not influence flTF cofactor activity. Similar results were obtained with MVs shed from these cells, but the levels of TF cofactor activity and flTF antigen were lower than those observed in ECRF cells (Fig. 1e). We conclude that asTF – by itself and without exogenously added phospholipids –has minimal coagulant activity, which is consistent with prior reports on endothelial human asTF [17]; when co-expressed with flTF, asTF does not significantly alter TF cofactor activity of human endothelial cells – neither on cell surfaces, nor on MVs derived from them.
Fig. 1.
Characterization of asTF and flTF in ECRF cells after adenoviral transduction. A) mRNA levels of asTF and flTF were determined in mock-transduced (1), asTF-transduced (2), flTF-transduced (3) and asTF/flTF transduced cells (4) via RT-PCR. B) asTF and flTF protein expression in cell lysates and MVs assessed using western blot. Odd numbers represent total lysates, and even numbers – MVs. C) and E) FXa generation on cells and MVs in the presence of 1 nM FVIIa and 50 nM FX. D) and F) Bar graphs represent FXa generation on mock (black) versus asTF (grey) ECRF cells and MVs. (*p < 0.05).
3.2. asTF and flTF proteins do not co-localize in human endothelial cells
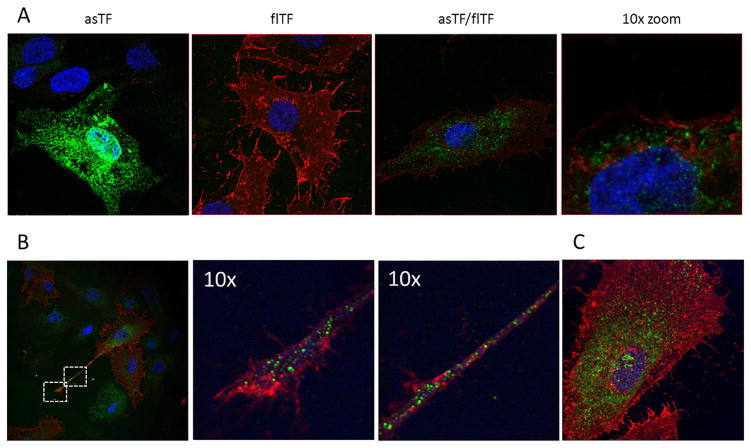
It is unknown whether asTF and flTF can physically interact. Therefore, we co-expressed asTF and flTF in ECRF cells, and immunofluorescence staining was performed. The majority of the flTF pool was found on the surface of plasma membrane, while asTF predominantly homed to intracellular ECRF compartments (Fig. 2a). Confocal analysis of multiple z-stacks confirmed that asTF and flTF proteins do not co-localize in ECRF cells (Supplementary Fig. 1). Similarly, asTF and flTF did not co-localize in cell protrusions where MV shedding takes place (Fig. 2b). Lack of co-localization was also observed in HUVEC cells when both TF isoforms were co-expressed (Fig. 2c). We conclude that, in endothelial cells, asTF and flTF proteins home to distinct sub-compartments and are thus not in close proximity to each other.
Fig. 2.
asTF and flTF do not co-localize when concomitantly expressed. A) After transduction, ECRF cells were fixed and stained with pAb-1979 (asTF, green) and 10H10 (flTF, red). A 10-fold magnified image (right panel) shows asTF and flTF in different cellular sub-compartments. B) Localization of asTF/flTF in cellular protrusions. C) asTF and flTF localization in HUVEC cells.
3.3. Heightened overexpression of asTF reduces flTF expression levels
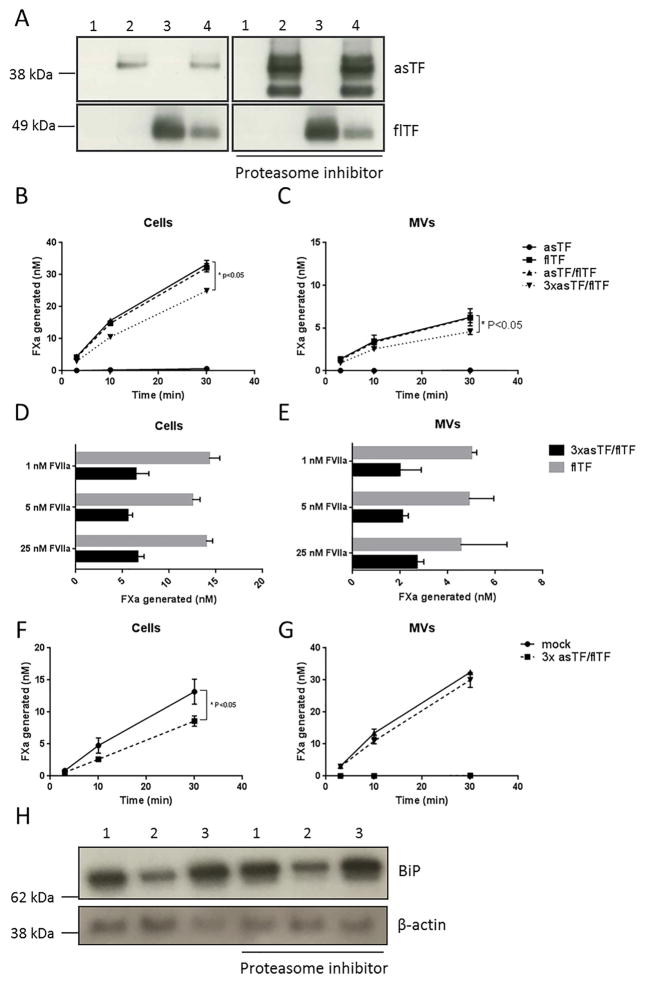
As we did not observe asTF/flTF protein co-localization, we then studied whether supra-physiological asTF levels may impact TF cofactor activity. A 3-fold higher dose of asTF adenovirus hampered flTF adeno-virus expression when co-transduced with asTF (Fig. 3a. left panel). As expected, this decrease in flTF protein levels also yielded a significantly lower FXa conversion rate on endothelial cells and MVs (Fig. 3b and c, and Supplementary Fig. 2). Cofactor activity and/or protein levels of flTF were not affected by increasing GFP expression (Supplementary Fig. 3), demonstrating that this effect was not due to high protein expression and the resultant loading of the ER. To study if FVIIa is sequestered by binding to asTF, increasing amounts of recombinant FVIIa were added to the cells and MV; higher FVIIa concentrations did not restore TF cofactor activity (Fig. 3d and e). In untransformed HUVECs, supra-physiological levels of asTF also lowered flTF-dependent FXa generation (Fig. 3f and g). Because of the unaltered asTF protein levels after escalating virus doses, we hypothesized that excess asTF protein might be targeted to the proteasome. Inhibition of the proteasome showed increased asTF protein levels, while flTF protein levels remained unaffected (Fig. 3h). As high asTF levels were cleared by the cells, we gathered that high asTF levels were likely to induce stress within the endoplasmic reticulum (ER). Indeed, BiP, a marker for ER stress, was upregulated as a consequence of high asTF (co-) expression (Fig. 3h). We conclude that heightened overexpression of asTF leads to proteosomal degradation of asTF protein, yet not flTF protein, and concomitant ER stress.
Fig. 3.
The effects of supra-physiological asTF levels on TF cofactor activity. A) Western blot analysis of total lysates of mock (1), asTF (2), flTF (3) and asTF/flTF transduced (4) ECRF cells. Cells were incubated for 12 h with 5 μM MG132, to inhibit the proteasome. B) and C) FXa generation on cells and MVs in the presence of 1 nM FVIIa and 50 nM FX. D) and E) FXa generation on flTF and asTF/flTF cells and their MVs in the presence of 1 nM, 5 nM or 25 nM FVIIa and 50 nM FX. F) and G) FXa generation on HUVEC cells and MVs with heightened overexpression of asTF, respectively. H) Western blot analysis for the ER-stress marker BiP in ECRF cells and asTF (1), flTF (2), and asTF/flTF (3) transduced cells.
3.4. asTF impacts distribution of flTF into non-lipid raft fractions of endothelial cell plasma membrane
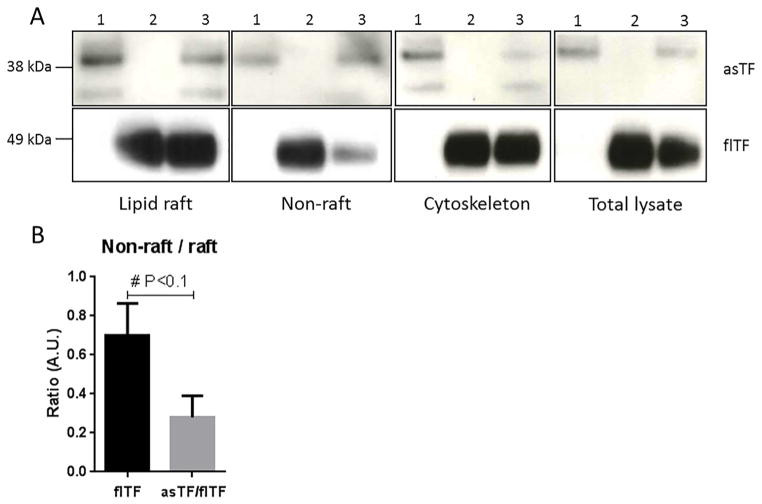
As flTF protein levels and TF coagulant activity are decreased due to heightened asTF, the question emerges as to whether asTF is able to induce changes in flTF sub-compartment distribution in the plasma membrane. Therefore, plasma membrane was fractionated into the material containing lipid rafts, and that free of lipid rafts. asTF levels were equally distributed between raft and non-raft fractions (Fig. 4a, upper panel). asTF localization patterns were not changed upon co-expression of flTF. The majority of flTF was found in the lipid raft fraction, yet a smaller fraction of the total flTF pool was still present in non-raft fractions (Fig. 4a, lower panel). Strikingly, flTF distribution was changed when asTF was co-expressed, showing a decrease of flTF protein present in the non-raft fraction (Fig. 4b). These results suggest that the decrease in flTF expression, when flTF is co-expressed with asTF, can be attributed to asTF-triggered downregulation of flTF present in non-raft fractions. Separately, these results suggest that coagulant-active flTF is mostly located in non-lipid raft plasma membrane fractions of human endothelial cells.
Fig. 4.
Effects of asTF expression on sub-cellular distribution of flTF, A) Protein levels of asTF (1), flTF (2) and asTF/flTF (3) transduced cells in lipid raft fraction, non-raft fraction, cytoskeleton, and total cell lysate. B) The flTF ratio in non-raft versus raft fractions in the absence and presence of asTF, respectively; analysed via ImageJ.
4. Discussion
In this study, we investigated the interplay between asTF and flTF in coagulation initiation. Our data indicate that simultaneous expression of asTF and flTF has no effect on TF cofactor activity, but heightened asTF overexpression modulates flTF expression in human endothelial cells. We base our conclusions on the following observations: i) low asTF levels did not alter flTF cofactor activity on cells and/or MVs; ii) asTF and flTF proteins did not co-localize in cellular sub-compartments; iii) heightened levels of asTF decreased the levels of flTF protein in non-raft plasma membrane fractions, thereby reducing TF cofactor activity.
Previous studies of coagulant properties of asTF are fairly inconclusive [19,20]. This is the first study that reports on asTF expressed concomitantly with flTF in an endothelial cell setting, to study asTF’s possible function in hemostasis. While FXa generation on cells expressing only asTF was very low, a significantly higher FXa conversion rate was observed on asTF expressing cells compared to mock-transduced cells (Fig. 1c and d). Although Fig. 1d seems to demonstrate a lowered rate of FXa generation by asTF/flTF MVs compared to flTF MVs, the slope of the asTF/flTF MVs curve is identical to that of the flTF MVs curve, likely revealing an artifact during MV isolation and/or processing; we note that this artifact was not consistently observed (Fig. 3c). Western blot analysis on MVs and supernatants revealed that asTF is not secreted from non-activated endothelial cells (data not shown). Also, stimulation of HUVEC cells with IL-1α did not result in asTF secretion, as previously reported by Böing et al. [18]. However, other studies that utilized endothelial or malignant cell lines were able to demonstrate asTF in conditioned media and/or plasma of cancer patients [14,29]. Up till now, asTF cofactor activity was only observed in cell supernatants under inflammatory conditions or in a cancerous setting [17,20]. Thus, it might very well be that it is only in unique disease settings, e.g. cancer, that the soluble asTF protein is secreted by endothelial cells whereby it can modulate coagulation. Studies to measure the quantities of MVs and their phosphatidylserine content will provide insight as to how asTF might be involved in direct or indirect modulation of flTF-containing MV shedding/coagulant activity.
When asTF was co-expressed with flTF, asTF did not change total TF cofactor activity. When higher adenovirus titers were used, we hardly detected increases in asTF protein levels. Only after treatment with a proteasome inhibitor, we observed restored asTF levels after overexpression. asTF increased ER stress, as BiP levels were elevated (Fig. 3h). Interestingly, it has been shown that overexpression of BiP reduces cofactor activity of flTF [30,31]. However, this does not completely explain the reduced FXa conversion rates in asTF/flTF co-expressing cells, as we observe reduced flTF protein levels. BiP is an ER resident chaperone protein and plays a role in the quality control and folding of proteins. The two TF isoforms share the large N-terminal domain, but differ in their relatively small C-terminal domains, whereby the highly hydrophobic alpha-helical region is present only in flTF. Because asTF already increases ER stress, during protein synthesis BiP may also increasingly recognize flTF as an incorrectly folded protein and lock it in an unfolded state in the presence of asTF, and this might explain lower flTF expression [32]. These results indicate that, like many other alternatively spliced soluble proteins [33], asTF may be an unstable protein degraded via a proteasome-dependent pathway.
Our study has an important limitation. The asTF and flTF expression levels used in our study exceed those observed after stimulation with inflammatory agents such as TNFa and LPS (results not shown). Thus, our model may not fully mimic endothelial cell biology. Nevertheless, our intention was not to create a biologically faithful endothelial model with physiological asTF and flTF expression levels per se, but to investigate the effects of asTF/flTF co-expression on total TF cofactor activity. Our finding that supra-physiological asTF levels never observed in endothelial cells – even under inflammatory conditions – do not influence FXa generation, further supports our conclusion that asTF does not influence TF cofactor function in this cell type.
In conclusion, asTF appears to have a very limited role in normal hemostasis. It has minimal cofactor activity and does not alter the cofactor function of flTF expressed in human endothelial cells and/or TF+ MVs derived from them.
Supplementary Material
Acknowledgments
This work was supported by a VIDI fellowship (Netherlands Organisation for Scientific Research, grant 17.106.329) to H. H. Versteeg. V.Y. Bogdanov is partially supported by the NIH (grant R01 CA190717).
Abbreviations
- asTF
alternatively spliced Tissue Factor
- ECRF
immortalized human umbilical vein endothelial cells
- ER
endoplasmic reticulum
- FVIIa
activated coagulation factor VII
- FXa
activated coagulation factor X
- flTF
full length Tissue Factor
- HUVEC
human umbilical vein endothelial cells
- IL-1α
interleukin-α
- MVs
microvesicles
- PS
phosphatidylserine
- VTE
venous thromboembolism
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.thromres.2017.05.028.
Conflicts of interest
None.
References
- 1.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122(7):2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naess IA, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–699. doi: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 3.van Es N, et al. Clinical significance of tissue factor-exposing microparticles in arterial and venous thrombosis. Semin Thromb Hemost. 2015;41(7):718–727. doi: 10.1055/s-0035-1556047. [DOI] [PubMed] [Google Scholar]
- 4.Unlu B, Versteeg HH. Effects of tumor-expressed coagulation factors on cancer progression and venous thrombosis: is there a key factor? Thromb Res. 2014;133(Suppl 2):S76–S84. doi: 10.1016/S0049-3848(14)50013-8. [DOI] [PubMed] [Google Scholar]
- 5.Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27(1):31–39. doi: 10.1016/j.blre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 6.van der Pol E, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 7.Diamant M, et al. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106(19):2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 8.Rao LV, Kothari H, Pendurthi UR. Tissue factor encryption and decryption: facts and controversies. Thromb Res. 2012;129(Suppl 2):S13–S17. doi: 10.1016/j.thromres.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol. 2011;73:515–525. doi: 10.1146/annurev-physiol-042210-121137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmeliet P, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383(6595):73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 11.Bugge TH, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93(13):6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdanov VY, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9(4):458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg YW, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106(46):19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocaturk B, et al. Alternatively spliced tissue factor promotes breast cancer growth in a beta1 integrin-dependent manner. Proc Natl Acad Sci U S A. 2013;110(28):11517–11522. doi: 10.1073/pnas.1307100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kocaturk B, et al. Alternatively spliced tissue factor synergizes with the estrogen receptor pathway in promoting breast cancer progression. J Thromb Haemost. 2015;13(9):1683–1693. doi: 10.1111/jth.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unruh D, et al. Antibody-based targeting of alternatively spliced tissue factor: a new approach to impede the primary growth and spread of pancreatic ductal adenocarcinoma. Oncotarget. 2016;7(18):25264–25275. doi: 10.18632/oncotarget.7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unruh D, et al. Alternatively spliced tissue factor contributes to tumor spread and activation of coagulation in pancreatic ductal adenocarcinoma. Int J Cancer. 2014;134(1):9–20. doi: 10.1002/ijc.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boing AN, et al. Human alternatively spliced tissue factor is not secreted and does not trigger coagulation. J Thromb Haemost. 2009;7(8):1423–1426. doi: 10.1111/j.1538-7836.2009.03521.x. [DOI] [PubMed] [Google Scholar]
- 19.Censarek P, et al. Alternatively spliced human tissue factor (asHTF) is not pro-coagulant. Thromb Haemost. 2007;97(1):11–14. [PubMed] [Google Scholar]
- 20.Szotowski B, et al. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96(12):1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 21.Bogdanov VY, et al. Identification and characterization of murine alternatively spliced tissue factor. J Thromb Haemost. 2006;4(1):158–167. doi: 10.1111/j.1538-7836.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhofer D, et al. Epitope location on tissue factor determines the anticoagulant potency of monoclonal anti-tissue factor antibodies. Thromb Haemost. 2000;84(6):1072–1081. [PubMed] [Google Scholar]
- 23.Ruf W, et al. Tissue factor residues 157–167 are required for efficient proteolytic activation of factor X and factor VII. J Biol Chem. 1992;267(31):22206–22210. [PubMed] [Google Scholar]
- 24.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125(Suppl 2):S36–S38. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogdanov VY, Versteeg HH. “Soluble tissue factor” in the 21st century: definitions, biochemistry, and pathophysiological role in thrombus formation. Semin Thromb Hemost. 2015;41(7):700–707. doi: 10.1055/s-0035-1556049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Versteeg HH, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111(1):190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ettelaie C, et al. Characterization of physical properties of tissue factor-containing microvesicles and a comparison of ultracentrifuge-based recovery procedures. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unruh D, et al. Levels of alternatively spliced tissue factor in the plasma of patients with pancreatic cancer may help predict aggressive tumor phenotype. Ann Surg Oncol. 2015;22(Suppl 3):S1206–S1211. doi: 10.1245/s10434-015-4592-2. [DOI] [PubMed] [Google Scholar]
- 30.Watson LM, et al. Overexpression of the 78-kDa glucose-regulated protein/immunoglobulin-binding protein (GRP78/BiP) inhibits tissue factor procoagulant activity. J Biol Chem. 2003;278(19):17438–17447. doi: 10.1074/jbc.M301006200. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharjee G, et al. Re’gulation of tissue factor—mediated initiation of the coagulation cascade by cell surface grp78. Arterioscler Thromb Vasc Biol. 2005;25(8):1737–1743. doi: 10.1161/01.ATV.0000173419.31242.56. [DOI] [PubMed] [Google Scholar]
- 32.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 33.Stamm S, et al. Function of alternative splicing. Gene. 2005;344:1–20. doi: 10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.