ABSTRACT
The carcinoembryonic antigen glypican-3 (GPC3) is a good target of anticancer immunotherapy against pediatric solid tumors expressing GPC3. In this non-randomized, open-label, phase I clinical trial, we analyzed the safety and efficacy of GPC3-peptide vaccination in patients with pediatric solid tumors. Eighteen patients with pediatric solid tumors expressing GPC3 underwent GPC3-peptide vaccination (intradermal injections every 2 weeks), with the primary endpoint being the safety of GPC3-peptide vaccination and the secondary endpoints being immune response, as measured by interferon (IFN)-γ enzyme-linked immunospot assay and Dextramer staining, and the clinical outcomes of tumor response, progression free survival (PFS), and overall survival (OS). Our findings indicated that GPC3 vaccination was well tolerated. We observed disease-control rates [complete response (CR)+partial response+stable disease] of 66.7% after 2 months, and although patients in the progression group unable to induce GPC3-peptide-specific cytotoxic T lymphocytes (CTLs) received poor prognoses, patients in the partial-remission and remission groups or those with hepatoblastoma received good prognoses. The GPC3-peptide vaccine induced a GPC3-specific CTL response in seven patients, with PFS and OS significantly longer in patients with high GPC3-specific CTL frequencies than in those with low frequencies. Furthermore, we established GPC3-peptide-specific CTL clones from a resected-recurrent tumor from one patient, with these cells exhibiting GPC3-peptide-specific cytokine secretion. The results of this trial demonstrated that the GPC3-peptide-specific CTLs induced by the GPC3-peptide vaccine infiltrated tumor tissue, and use of the GPC3-peptide vaccine might prevent the recurrence of pediatric solid tumors, especially hepatoblastomas, after a second CR.
KEYWORDS: peptide vaccine, glypican-3 (GPC3), CTL, pediatric solid tumors, phase I
Introduction
Pediatric solid tumors are relatively rare, with an estimated incidence of 73 to 148 per 1,000,000 people.1 In recent decades, multidisciplinary approaches to and progress in specialized care using a combination of chemotherapy, surgery, and radiotherapy have improved the treatment of pediatric solid tumors, with current overall survival rates from 75% to 90% associated with non-metastatic tumors.2 However, of the 10% to 30% of pediatric patients with metastatic or refractory solid tumors, 15% to 20% experience a relapse at distant sites.3 Additionally, various critical, late complications continue to occur. Therefore, the development of novel, effective therapies is urgently required to prolong survival in patients with refractory pediatric solid tumors accompanied by good quality of life (QOL) while minimizing the risk of adverse reactions.
Immunotherapy is a potentially attractive option for pediatric solid tumors. Many tumor antigens such as disialoganglioside, glycoprotein B7 homolog three protein, glycoprotein non-metastatic B, and Wilms-tumor antigen, identified in pediatric solid tumors, represent potential antigens for peptide vaccines.4-9 Additionally, immunotherapeutic methods using tumor-antigen-derived peptide vaccines demonstrated certain degrees of antitumor efficacy in clinical trials in patients with refractory pediatric solid tumors.10,11 To improve treatment outcomes and survival responses associated with tumor-antigen-derived peptide vaccines, more effective tumor antigens are needed.
Carcinoembryonic antigen glypican-3 (GPC3) is a cell-surface heparan sulfate proteoglycan linked to the extracytoplasmic cell-surface membrane by a glycosylphosphatidylinositol anchor.12 GPC3 is associated with cell growth, development, and responses to various growth factors13 and represents a good target for anticancer immunotherapy against pediatric solid tumors owing to its overexpression in many pediatric solid tumors, especially yolk sac tumors (90.0%) and hepatoblastomas (60.0%).14 Clinical trials of a GPC3-derived peptide vaccine conducted in adult patients with hepatocellular carcinoma (HCC) and ovarian clear-cell carcinoma confirmed its safety and indicated correlations between GPC3-specific cytotoxic T lymphocyte (CTL) frequency and overall survival (OS).15-18 On the basis of these results, we conducted a phase I clinical trial of a GPC3-derived peptide vaccine to evaluate its efficacy and safety in patients with refractory pediatric malignant tumors. We used an HLA-A*24:02-restricted GPC3298–306 peptide (EYILSLEEL) and an HLA-A*02:01-restricted GPC3144–152 peptide (FVGEFFTDV), which cover almost all common HLA class I types in Japan and were used in previous adult studies. The primary endpoints of this phase I study (UMIN-CTR number: 000006357) were vaccine safety, tolerability, and recommended phase II dosage, and immunological and clinical responses in this trial were assessed as secondary endpoints.
Results
Patient characteristics
Eighteen patients were enrolled in this study (Table 1), with a median follow-up period of 23.2 months (range: 1–56 months). None of the patients dropped out because of adverse events caused by peptide vaccination, and all patients received adequate follow-up to monitor toxicity. Average patient age was 17.5 years (range: 2–24 years), and the rates of male and female patients were equal. Six, four, and eight patients were diagnosed with tumor progression, partial remission, and remission, respectively, and grouped accordingly. Seven patients received the HLA-A2-restricted GPC3144–152 peptide (FVGEFFTDV), and 11 patients received the HLA-A24-restricted GPC3298–306 peptide (EYILSLEEL), with these 11 and seven patients injected with 1.5 mg and 3.0 mg doses of the GPC3-peptide vaccine, respectively. All 18 patients had undergone conventional chemotherapy, surgery, and radiation therapy prior to receiving GPC3-peptide vaccine therapy; however, all of them showed progression of the disease prior to enrollment in this study. We evaluated the GPC3 and HLA class I expression in the primary tumors from all patients (Table 2), with GPC3 expression detected in all of the patients, except one (case 3) who could not be evaluated. Specifically, patients with hepatoblastoma exhibited strong GPC3 expression (degree of staining: 2+), and cell-membrane expression of HLA class I was evident in seven of 18 patients (39%).
Table 1.
Patient characteristics.
| No. | Age | Sex | Clinical diagnosisa | Groupb | HLA-A | Body weight | Dose of peptide | Prior therapyc |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | M | hepatoblastoma | progression | 02 | 30.0 | 3.0 mg | Ope, CITA, ITEC |
| 2 | 2 | M | germ cell tumor | partial remission | 24 | 10.6 | 1.5 mg | JEB, Ope, IE, 05A1, 05A3, RT |
| 3 | 3 | F | hepatoblastoma | remission | 24 | 12.6 | 1.5 mg | CDDP, CITA, ITEC, CPT-11 |
| 4 | 11 | F | hepatoblastoma | remission | 24 | 38.2 | 3.0 mg | Ope, CITA, ITEC, Topo/CPA |
| 5 | 16 | M | hepatoblastoma | progression | 24 | 41.0 | 3.0 mg | Ope, CITA, ITEC, WT1 |
| 6 | 5 | F | hepatoblastoma | remission | 02 | 17.5 | 1.5 mg | Ope, CITA, Topo/CPA, ITEC |
| 7 | 4 | M | hepatoblastoma | remission | 24 | 15.4 | 1.5 mg | CITA, ITEC, TACE, Ope, BU/Mel, CPT-11 |
| 8 | 14 | F | rhabdomyosarcoma | remission | 24 | 45.6 | 3.0 mg | VAIA, VCR/CPT-11, RT |
| 9 | 4 | M | rhabdomyosarcoma | partial remission | 24 | 16.0 | 1.5 mg | Ope, VAC, VIE, RT |
| 10 | 8 | F | CNS tumor | progression | 02 | 19.5 | 1.5 mg | MTX, VCR/CPM, VCR/IFO/ActD, MTX, RT, TMZ/VP, TMZ/CPT-11, IFNβ |
| 11 | 3 | F | rhabdomyosarcoma | progression | 02 | 18.4 | 1.5 mg | Ope, RT, VAC, TMZ/CPT-11, GEM/DTX, VDC/IE, vazopanib |
| 12 | 10 | M | CNS tumor | progression | 24 | 19.6 | 1.5 mg | VCR/CPA/CDDP, TMZ, ETP, VCR/IFO/ActD, VNR |
| 13 | 2 | F | hepatoblastoma | remission | 24 | 11.0 | 1.5 mg | CITA, ITEC |
| 14 | 22 | M | rhabdomyosarcoma | partial remission | 02 | 71.6 | 3.0 mg | VAC, RT |
| 15 | 19 | M | CNS tumor | progression | 24 | 76.0 | 3.0 mg | Ope, Temozolimide, CARE, PE/CDDP, TMZ/ETP, RT |
| 16 | 3 | F | Wilms tumor | remission | 02 | 11.2 | 1.5 mg | DD-4A, Ope, RT |
| 17 | 5 | M | MRT | remission | 24 | 14.5 | 1.5 mg | Ope, RT, VAIA |
| 18 | 24 | F | pancreatoblastoma | partial remission | 02 | 76.7 | 3.0 mg | CDDP/ADR, Ope |
Clinical diagnosis. CNS, central nervous system; MRT, malignant rhabdoid tumor.
Group. progression, patient in refractory, recurrent, or progressive status; partial remission, patient in partial remission or stable disease; remission, patient in remission without chance of cure.
Prior therapy. ActD, actinomycin-D; ADR, adriamycin; BU, busulfan; Ope, surgery; CDDP, cisplatin ; CITA, CDDP-pirarubicin ; CPA, cyclophosphamide; CPM, cyclophosphamide; CPT-11, irinotecan; DD-4A, dactinomycin-VCR; DTX, docetaxel; ETP, etoposide; IFO, ifosfamide; IFNβ, interferon-beta; ITEC, Ifosfamide-pirarubicin-ETP-carboplatin; JEB, carboplatin-ETP-bleomycin; IE IFO-ETP ; Mel, melphalan; PE, CDDP-ETP; TACE, transarterial chemoembolization ; Topo, topoisomerase inhibitors; RT, radiotherapy; WT1, WT1 peptide vaccine; MTX, methotrexate; TMZ, temozolomide; GEM, gemcitabine; VAC, VCR-ADR-CPM VAIA, VCR-ActD- IFO-doxorubicin; VCR, vincristine; VDC, VCR-doxorubicin- CPA; VIE, vincristine-IFO-ETP; VNR, vinorelbine; VP, VNR –CDDP; 05A1 and 05A3, CPA-VCR-pirarubicin-CDDP.
Table 2.
Patient clinical response and GPC3 specific CTL response.
| The spot number of GPC3 specific CTLd |
Expression in the primary tumore |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Tumor responsea | PFSb (months) | OSc (months) | Pre vaccine | Post vaccine | increased CTL | GPC3 | HLA class I | |
| 1 | NE | 1 | 1 | 3 | 0 | – | 2+ | 1+ | |
| 2 | SD | 19 | 56 | 0 | 72 | + | 1+ | 2+ | |
| 3 | CR | 55 | 55 | 2 | 17 | + | NA | NA | |
| 4 | CR | 45 | 45 | 9 | 30 | + | 2+ | 2+ | |
| 5 | NE | 2 | 2 | 1 | 1 | – | 2+ | + | |
| 6 | CR | 44 | 44 | 0 | 641 | + | 2+ | – | |
| 7 | CR | 44 | 44 | 3 | 103 | + | 2+ | 2+ | |
| 8 | PD | 3 | 22 | 0 | 6 | – | 1+ | 1+ | |
| 9 | CR | 4 | 15 | 0 | 6 | – | 1+ | – | |
| 10 | PD | 0 | 1 | NA | NA | NA | 1+ | – | |
| 11 | PR | 4 | 9 | 0 | 6 | – | 1+ | – | |
| 12 | PD | 2 | 6 | 0 | 2 | – | 2+ | 1+ | |
| 13 | CR | 27 | 27 | 0 | 37 | + | 2+ | 2+ | |
| 14 | SD | 4 | 16 | 0 | 5 | – | 1+ | – | |
| 15 | SD | 4 | 5 | 4 | 2 | – | 2+ | – | |
| 16 | PD | 1 | 23 | 0 | 2 | – | 1+ | – | |
| 17 | CR | 22 | 23 | 0 | 2 | – | 2+ | – | |
| 18 | SD | 3 | 23 | 0 | 13 | + | 1+ | – | |
Tumor response. Tumor responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) guideline assessment.
PFS, progression free survival.
OS, overall survival.
Number of GPC3-specific CTL spots. The number of GPC3 peptide-specific CTL spots (post-vaccination) was the maximum number of spots in an ex vivo IFN-γ ELISPOT assay for GPC3 peptide, performed after vaccination and using 5 × 105 PBMCs. -, the spot number of GPC3 specific CTL increased < 10 after vaccine. +, The spot number of GPC3 specific CTL increased > 10 after vaccine.
Expression in the primary tumor. Expression of GPC3 and HLA class I was determined by immunohistochemistry. Degree of staining of tumor cells for GPC3: -, no reactive; 1+, weak reactive; 2+, strong reactive; NA, not analyzed. Degree of staining of tumor cells for HLA class I: -, no membranous reactive; 1+, weak membranous reactive; 2+, strong membranous reactive; NA, not analyzed.
Toxicity
The adverse events observed in this trial are listed in Table 3. No dose-limiting toxicity (DLT) or dose-specific adverse events were observed. Grade 3 or 4 adverse events correlated with receipt of GPC3-peptide vaccine therapy were not observed in any patients during the follow-up period. Almost all of the adverse events were judged as grade 1, except for three grade 2 adverse events (cases 6 and 7: drug fever; case 6: upper respiratory infection). Although grade 3 adverse events (case 7: drug fever; case 10: epilepsy and depressed level of consciousness; case 15: fever, spasticity, and increased aspartate aminotransferase levels) were observed, the effect and safety evaluation committee, including the external members, judged these events unrelated to the treatment, but rather to disease progression. Thirteen patients experienced grade 1 or 2 transient immune-related events, including local skin reactions at the injection site, drug fever, and flushing. These results suggested that the GPC3-peptide vaccine therapy was well tolerated.
Table 3.
The incidence of adverse events relation to the GPC3 vaccine.
| Adverse event | Grade I(%) | Grade II(%) |
|---|---|---|
| Injection site reaction | 81 (37.0) | 0 |
| Drug fever | 3 (1.4) | 2 (0.9) |
| fatigue | 2 (0.9) | 0 |
| Head ache | 1 (0.5) | 0 |
| Upper respiratory infection | 0 | 1 (1.3) |
| Muscle pain | 1 (0.5) | 0 |
Clinical responses
Two patients (cases 1 and 5) were not able to undergo a computed tomography (CT) scan 2 months after the first vaccination owing to tumor progression. These patients were judged to have disease progression, but were not removed from the analyses based on advice from the effect and safety evaluation committee, including the external members. Among the 18 patients, one and six patients were judged to have a complete response (CR) in partial remission (n = 4) and remission (n = 8) group, respectively, after 2 months, according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria. In the progression group (n = 6), only one patient had SD, with no patient judged as exhibiting a CR. The disease-control rate [CR+partial response (PR)+SD] was 66.7% after 2 months, with the median time to tumor progression at 4 months. As for the comparison in remission and partial-remission (not advanced group) vs progression (advanced group) patients, the not advanced group showed better progression-free survival (PFS) [hazard ratio (HR) = 7.6; p < 0.01] and better overall survival (OS) (HR = ∞; p < 0.001) than advanced group (Fig. 1A).
Figure 1.
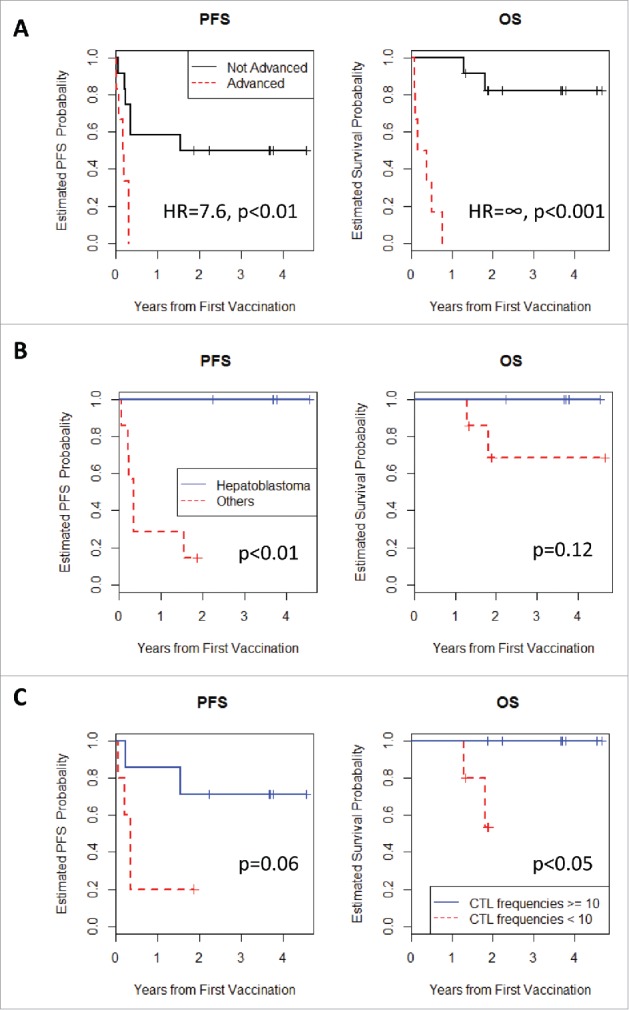
(A) Kaplan-Meier curves for PFS and OS. Patients in the partial-remission and remission groups (not advanced) exhibited longer PFS and OS than those in the progression group (advanced) (p < 0.01 and p < 0.001, respectively). (B) Kaplan-Meier curves for PFS and OS. Hepatoblastoma patients in the partial-remission group exhibited longer PFS and OS than those harboring other pediatric solid tumors. (C) Kaplan-Meier curves for PFS and OS. Patients with GPC3-specfic CTL frequencies ≥10 exhibited longer PFS and OS than those with GPC3-specfic CTL frequencies <10 (p = 0.06 and p < 0.05, respectively).
Given that patients with hepatoblastoma exhibited strong GPC3 expression in the primary tumor (Table 2), we compared their PFS and OS with those with other cancers, except for patients in the progression group. Our results indicated surprisingly that none of the hepatoblastoma patients showed disease progression or died during the follow-up period (Fig. 1B). As for the difference in PFS, it was statistically significant (p<0.01). These results suggested that patients in remission and harboring a hepatoblastoma without the chance of cure or SD might benefit from GPC3-peptide vaccine therapy.
We evaluated the level of circulating GPC3 before and after vaccinations to assess their utility in GPC3-peptide vaccine therapy (Supplementary Fig. 1). GPC3 levels were not detectable in the plasma of patients with central nervous system tumors (cases 10, 12, and 15), malignant rhabdoid tumors (case 17), or pancreatoblastomas (case 18) during the follow-up period. Two patients (cases 2 and 13) exhibiting decreased and extended maintenance of low GPC3 levels presented comparatively long PFS with good QOL. The hepatoblastoma patients (cases 4, 6, and 7) in the remission group and who had maintained low GPC3 levels exhibited no recurrence. Patients (cases 1, 5, and 16) judged as progressive disease exhibited increased GPC3 levels.
PFS and OS rates correlate with GPC3-specific CTL frequency
In cancer immunotherapy, CTLs are often the final effectors of immune-mediated cancer regression. Therefore, peripheral blood mononuclear cells (PBMCs) obtained from all patients before and after vaccination were examined by ex vivo interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay to determine whether the GPC3-peptide vaccine was capable of inducing a specific CTL response. As a representative data, the raw data were shown in Fig. 2. In order to eliminate the reaction to impurities contained in the peptide, the difference from the spot number against HIV peptide was taken as the number of GPC3 peptide specific spot. During vaccination, one patient (case six: HLA-A2) in whom GPC3 expression was diffusely positive (Supplementary Fig. 2) maintained increasing numbers of GPC3-peptide-specific CTLs in 5 × 105 PBMCs (Fig. 2A). In addition, similar results were obtained from ex vivo Dextramer analysis that is less sensitive to the impurities (Fig. 2B). As shown in Fig. 3 and Table 2, we found that the GPC3-peptide vaccine induced a GPC3-specific CTL response in seven of the 18 patients (39%), and almost all of the patients showing increased GPC3-specific CTL frequency were in remission and had diagnosed hepatoblastoma (71%). By contrast, GPC3-specific CTL frequency never increased in the progression group. GPC3-specific CTLs were directly detected ex vivo without in vitro peptide stimulation in almost all patients following GPC3-peptide vaccination. This was consistent with a previous clinical study involving adults, which showed that GPC3-specific CTL frequency after vaccination correlated with OS.15 Here, we compared the PFS and OS between patients with GPC3-specific CTL frequencies ≥10 (n = 7) and those with GPC3-specific CTL frequencies <10 (n = 10), finding that the patients of CTL frequencies ≥10 showed better PFS (p = 0.06) and also better OS (p<0.05). Furthermore, no patients with GPC3-specific CTL frequencies ≥10 died during the course of the study (Fig. 1C).
Figure 2.
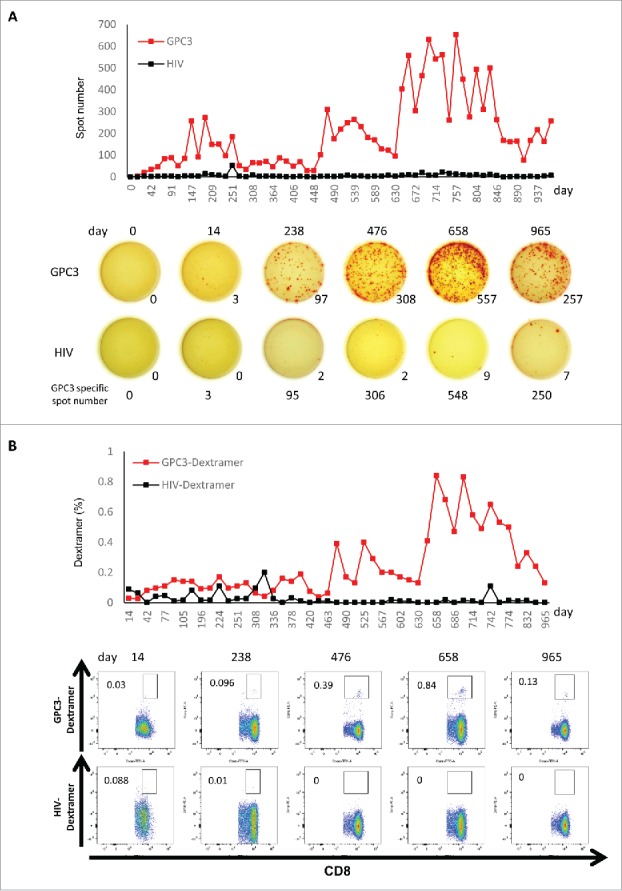
Ex vivo IFN-γ ELISPOT assay (A) and ex vivo Dextramer assay (B) were performed using PBMCs obtained from case 6 before the first vaccination and 2 weeks after each vaccination. The raw data are shown. The GPC3 specific spot number indicates the number of GPC3 peptide-specific spot calculated by subtracting the spot number in a well of HIV peptide.
Figure 3.
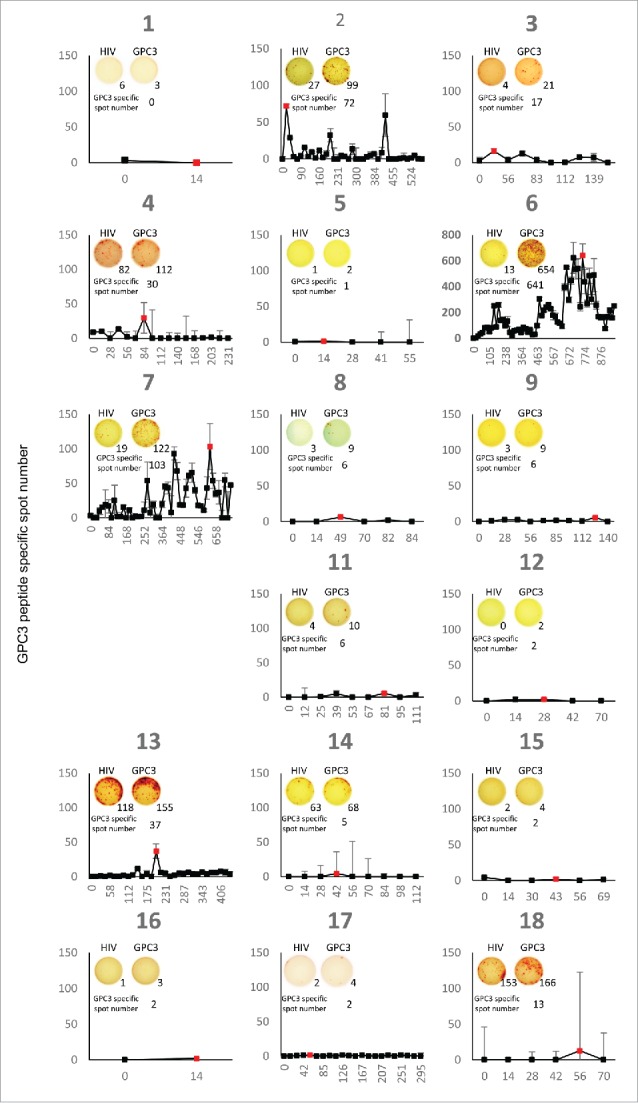
PBMCs were obtained from each patient before the first vaccination and 2 weeks after each vaccination. Ex vivo IFN-γ ELISPOT assay were performed for all patients. The GPC3 specific spot number indicates the number of GPC3 peptide-specific spot calculated by subtracting the spot number in a well of HIV peptide. The red point indicates the maximum spot number of each patient after vaccinations. The picture is raw data of the maximum spot of each patient.
GPC3 peptide-specific CTLs could recognize pediatric tumor cell lines expressing naturally GPC3
To investigate the ability of GPC3 peptide-specific CTLs induced by peptide vaccination, we established the GPC3 peptide-specific CTL clones from PBMCs of case 6 (HLA-A2). After in vitro stimulation, GPC3-Dextramer+ cells (1.95% of CD8+ cells) were sorted to a single cell. The established CTL clone was CD8+ GPC3 Dextramer+ cells (99.8%) (Fig. 4A). The CTL clone had GPC3 peptide-specific cytotoxic activity (Fig. 4B). Next, we assessed GPC3 expression of HLA-A*02:01+ pediatric tumor cell lines (Fig. 4C, D). HepG2, G-401 and HuH-6 cells expressed naturally GPC3 but not SK-N-DZ cells. The levels of GPC3 expression of SK-Hep-1/hGPC3 was higher than that of pediatric tumor cell lines, because it was stable transfectant of full-length GPC3 gene. The CTL clone could recognize SK-Hep-1/hGPC3, HepG2, G-401 and HuH-6, and secrete IFN-γ (Fig. 4E). In an HLA blocking experiment, the IFN-γ production of the CTL clone against tumor cell lines expressing GPC3 was markedly inhibited by anti-HLA class I mAb as compared with that by IgG2a isotype control (Fig. 4F). These results indicate that the CTL clone recognized pediatric tumor cell lines in an HLA-class I-restricted manner. By using siRNA, we confirmed GPC3 specificity in antigen recognition of the CTL clone. The IFN-γ production of the CTL clone against pediatric tumor cell lines pretreated with GPC3 siRNA was significantly decreased (Fig. 4G). These results clearly indicate that the CTL clone could recognize the GPC3 peptide when it is naturally processed by an HLA-matched pediatric tumor cell lines.
Figure 4.
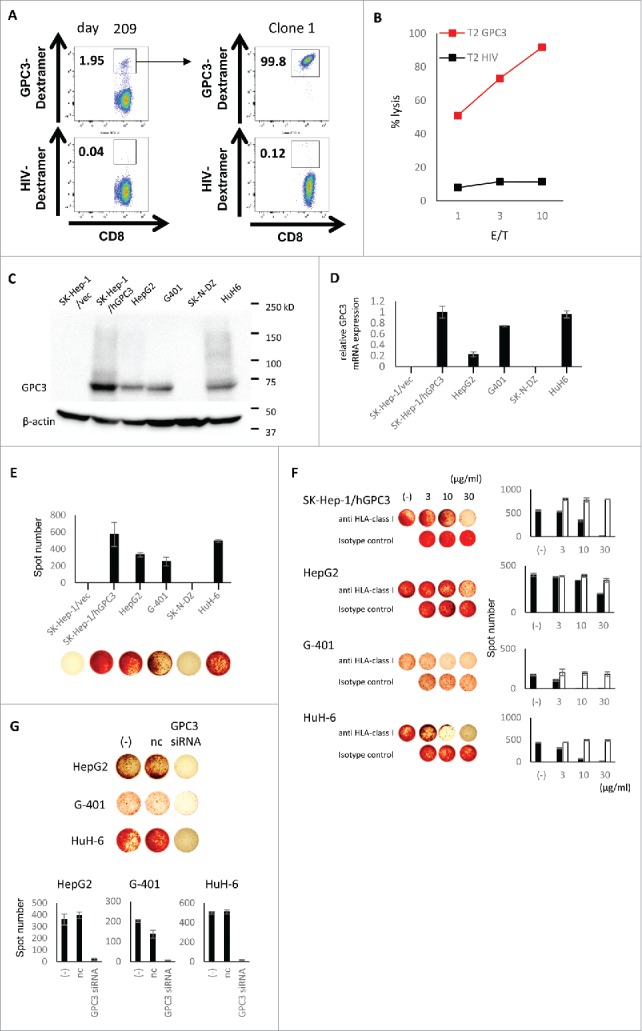
Analysis of GPC3144-152 peptide specific CTL clone. (A) CD8+ GPC3-Dextramer+ cells in PBMCs of case 6 following peptide stimulation were sorted to a single cell. GPC3144-152 peptide specific CTL clone was successfully established. (B) Cytotoxicity assay of the CTL clone against GPC3144-152 or HIV19-27 peptide pulsed T2 cells. Effector/target (E/T) ratio is 1, 3, 10. (C, D) The GPC3 expression of cancer cell lines were assessed by western blot (C) and quantitative real-time PCR (D). The relative GPC3 mRNA expression (ratio to SK-Hep-1/hGPC3) are shown. (E) The reactivity of CTL clone against cancer cell lines. The CTL clone were co-cultured with cancer cell lines for 20 hour (E/T = 2). The production of IFN-γ were detected by ELISPOT assay. Data are expressed as the mean ± SD. (F) Inhibition of IFN-γ production by anti-HLA class I mAb. GPC3 expressing cancer cell lines used as target cells (E /T = 2). The IFN-γ production of the CTL clone was markedly inhibited by anti-HLA class I mAb as compared with that by IgG2a isotype control. The black bar indicates anti HLA-class I. The white bar indicates isotype control. The antibodies were used at the concentration of 3, 10, 30 μg/ml. Data are expressed as the mean ± SD. (G) The IFN-γ production of the CTL clone against cancer cell lines pretreated with GPC3 siRNA (E/T = 2). The IFN-γ production of the CTL clone was decreased by GPC3 siRNA. Data are expressed as the mean ± SD. nc, negative control.
Tumor infiltration of GPC3-peptide-specific CTLs following peptide vaccination
To confirm whether tumor-infiltrating lymphocytes were GPC3-peptide-specific CTLs, we attempted to detect GPC3-peptide-specific CTLs in tumor-resected specimens from vaccinated patients. Although one patient (case 2: HLA-A24) induced high GPC3-peptide-specific CTLs (Fig. 5A) with high HLA class I expression, the patient also exhibited small solitary recurrence in the lung. A lung metastatic tumor-resected specimen was obtained from this patient following GPC3-peptide vaccination and after receiving informed consent. We analyzed the GPC3-specific CTL frequency of this resected specimen by flow cytometry, using the GPC3 peptide Dextramer. We assessed GPC3-specific CTL frequency as the percentage of both Dextramer- and CD8-positive cells, with our results showing that the frequency of GPC3-peptide-specific CTLs from the resected specimen was 0.03% following vaccination (Fig. 5B). Moreover, we established GPC3-peptide-specific CTL clones (TIL clone 1, 2) from the specimen by single-cell sorting, using GPC3-Dextramer variants (Fig. 5C). The GPC3-Dextramer+ TIL clones exhibited IFN-γ secretion against SK-Hep-1/hGPC3 that transfected full-length GPC3 gene or GPC3298-306 peptide pulsed T2A24 cells, but not against SK-Hep-1/vec that transfected empty vector or HIV583–591-pulsed T2A24 cells (Fig. 5D). We also detected CD107a molecule on the surface of the TIL clones co-cultured with GPC3298-306 peptide pulsed T2A24 cells, which could be used as a surrogate marker to identify antigen-specific CTLs that degranulate against target cells (Fig. 5E). Furthermore, the TIL clone 1 exhibited GPC3-peptide-specific TNF-α secretion (Fig. 5F). These results suggested that GPC3-peptide-specific CTLs induced by GPC3-peptide vaccination infiltrated into the tumor tissue and prevented tumor progression.
Figure 5.
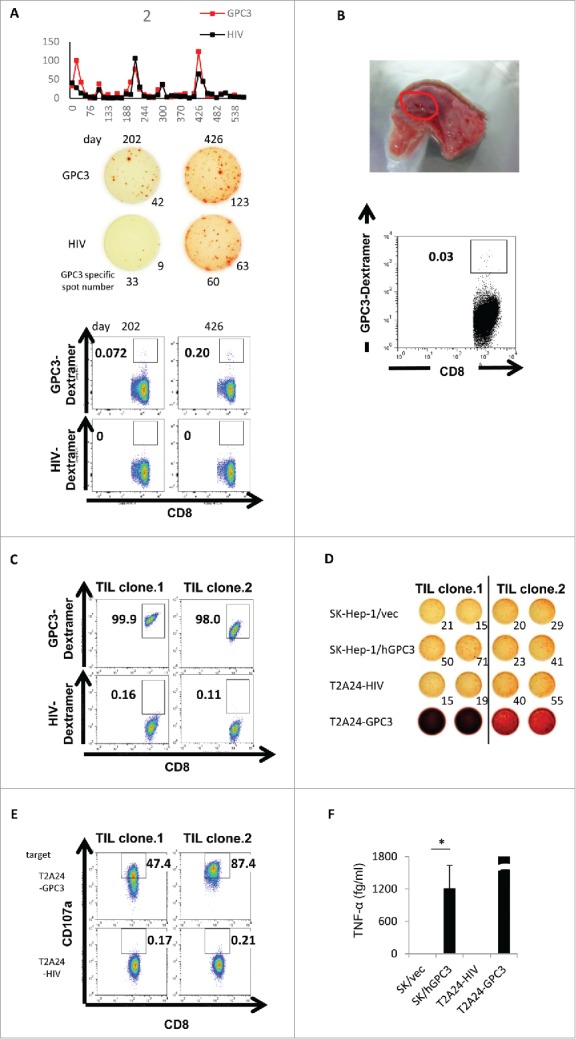
Immunological-response assessment in case 2. (A) The raw data of ex vivo IFN-γ ELISPOT assay using PBMCs of case 2. Dextramer assay using PBMCs of case 2 were performed after in vitro peptide stimulation. (B) Ex vivo GPC3-Dextramer staining after vaccination. GPC3-peptide-specific CTL frequency is indicated as the percentage of Dextramer-positive CTLs to CD8-positive cells in the tumor-resected specimen (red circle). (C) Dextramer analysis of the establishment of the GPC3-peptide-specific CTL clones in the tumor-biopsy specimen. (D) IFN-γ ELISPOT assay against SK-Hep-1/vec, SK-Hep-1/hGPC3, and peptide-pulsed T2A24. Effector / target (E / T) ratio = 0.2. (E) Externalized CD107a analysis of the establishment of the GPC3-peptide-specific CTL clones in the tumor-biopsy specimen. T2A24 pulsed with GPC3298–306 or HIV583–591 peptide were used as target cells. (F) TNF-α levels in the CTL clone (TIL clone.1) (1.0 × 105 cells/well) after a 24-h co-culture with the indicated target cells (5 × 104 cells/well). Data represent the mean ± standard deviation of triplicate cultures. *p < 0.05.
Discussion
Since we did not observe DLT in this study, the incremental doses of peptide per patient body weight were adequate. Adverse events related to this therapy indicated that this therapy was also well tolerated (grade 1: 40.3%; grade 2: 2.2%), although recent phase I clinical trials related to pediatric solid tumors showed DLT and grade 3 or 4 adverse events.19,20 Given our results indicating the adequate induction of GPC3-specific CTLs, the peptide doses used in this study are recommended for future clinical trials.
Previous studies showed that GPC3 is overexpressed in many pediatric and adult malignant tumors, including Wilms tumors, hepatoblastomas, melanomas, ovarian clear-cell carcinomas, and lung squamous-cell carcinomas.21-24 Our findings following evaluation of GPC3 expression in the primary tumors of 18 patients by immunohistochemistry indicated consistent GPC3 expression in various pediatric solid tumors. Moreover, all hepatoblastomas exhibited strong GPC3 expression (degree of staining: 2+), and a previous study examining 65 hepatoblastoma patients by immunohistochemistry reported that all subjects exhibited GPC3-positive results.25 These findings suggested that the rate of GPC3 expression in hepatoblastomas might be higher than that in HCC in adults (72–81%).26-29 GPC3 was originally identified as an oncofetal protein, with fetal liver tissues found to be GPC3-positive.28 Therefore, GPC3 was considered more closely associated with tumorigenesis in pediatric solid tumors as compared with adult tumors. Here, although all primary tumors from patients exhibited GPC3 expression, we did not observe significant differences in PFS and OS rates between patients exhibiting weak GPC3 expression (degree of staining: 1+) and those exhibiting strong GPC3 expression (degree of staining: 2+). Therefore, regardless of the degree of GPC3 expression, our findings indicated that all patients might benefit from GPC3-peptide vaccine therapy.
While hepatoblastoma patients whose tumors are unresectable but without overt metastases have event free survival (EFS) of 60–70%, children with metastatic disease at presentation fare poorly with approximately 20–50% EFS.30 For the relapsed patients, International Childhood Liver Tumor Strategy Group showed that 52% of the patients achieved a second CR, and that three-year EFS and overall survival for the relapsed patients were 34% and 43%, respectively.31 Data analysis of malignant extracranial pediatric germ cell tumors trials from Children's Oncology Group (United States) and the Children's Cancer and Leukemia Group (United Kingdom) showed long-term disease-free survival (LTDFS) in subgroups according to age, tumor site, stage, etc, which LTDFS for stage IV extragonadal tumors with age below 11 years was 79%.32 Although the number of our patient is small, it should be emphasized that our patients with advanced cancer are surviving after the current vaccine therapy, compared to historical controls, especially, patients with hepatoblastoma.
The primary endpoint of this study was assessment of vaccination safety; however, we also showed that tumor-antigen-specific CTLs played a critical role in GPC3-targeted immunotherapy. Although GPC3-specific CTL frequency was significantly correlated with PFS and OS in this study, significant correlations between immune responses and OS were not reported in other peptide-vaccine-therapy trials involving pediatric solid tumors.11,33 We found that patients with GPC3-specific CTL frequencies ≥10 exhibited longer survival duration than those with GPC3-specific CTL frequencies <10. The GPC3-peptide vaccine induced GPC3-specific CTL responses in seven of 18 patients, and of those seven patients, five (71%) were diagnosed with hepatoblastoma, with all patients exhibiting induced GPC3-specific CTL responses also receiving a good prognosis. Although complete resection is a critical component associated with curing patients with hepatoblastoma, 60% to 80% of patient tumors are unresectable at diagnosis depending on the surgical guidelines used.34,35 In the case of chemotherapy, event-free survival in patients with hepatoblastoma that was not resected at diagnosis generally approaches at least ∼65% to ∼70%.34,36,37 Although this varies depending on additional prognostic factors, as well as the different staging systems, this result suggests that GPC3-peptide vaccine therapy would be an attractive form of immunotherapy for patients with hepatoblastoma. However, GPC3-specific CTL responses were not observed in patients with undetectable plasma GPC3 levels before and after vaccination. Specifically, six patients in the progression group received a poor prognosis in the absence of GPC3-specific CTL responses. This highlighted the difficulty in treating advanced pediatric solid tumors solely with GPC3-peptide vaccine therapy, and to the best of our knowledge, there are no effective immunotherapies against advanced pediatric tumors. However, novel immunotherapies, including those using immunomodulatory antibodies and chimeric antigen-receptor T cells, are expected to improve survival rates.38,39
Validated biomarkers are not currently available for the prediction of efficacy and the selection of patients that might benefit from GPC3-peptide vaccines to treat pediatric solid tumors. It was recently reported that GPC3-expression level serves as a predictive marker and improves diagnostic efficacy when used with other tumor markers in adults with HCC.40,41 In this study, GPC3 levels decreased temporarily at least once in cases 2, 3, 5, 6, 7, 11, and 13 during the follow-up period. Furthermore, two patients (cases 2 and 13) who maintained low GPC3 levels received comparatively good prognoses, suggesting that circulating GPC3 level might also serve as a predictive marker of GPC3-peptide-vaccine efficacy for the treatment of pediatric solid tumors. Future clinical trials should incorporate the investigation of biomarkers that could be potentially predictive of treatment response.
We clearly demonstrated the presence of GPC3-peptide-specific CTLs in PBMCs, as well as the infiltration of these cells into tumor tissue. This evidence serves as a proof-of-concept for immunotherapy using tumor-antigen-specific CTLs. Moreover, we were able to isolate a high-affinity GPC3-specific T cell clone from the infiltrated tumors, and are currently developing more effective immunotherapies, including T cell receptor (TCR)-engineered T cell therapy using TCRs from these GPC3-peptide-specific CTL clones.
To date, there have been no reports indicating adequate antitumor efficacy of peptide vaccines in clinical trials involving patients with pediatric solid tumors. One explanation is that a sole tumor-associated antigen is insufficient to induce antitumor responses in pediatric refractory solid tumors and would also not be capable of escaping immunosurveillance. Therefore, this study aimed to improve vaccine therapies against pediatric solid tumors by performing a phase I clinical trial involving a peptide vaccine. Although our results did not show the desired efficacy against advanced pediatric solid tumors, we observed that the peptide vaccine might be effective as an adjuvant therapy for patients with advanced pediatric solid tumors.
In conclusion, this phase I clinical trial of a GPC3-derived peptide vaccine confirmed its safety and revealed its ability to induce a plethora of immunological responses in GPC3-expressing pediatric solid tumors. We also showed that GPC3-specific CTL frequency correlated with PFS and OS in patients with pediatric solid tumors who had received the GPC3-peptide vaccine. Our results indicated that the GPC3-peptide vaccine could be beneficial for patients with relapsed or refractory pediatric solid tumors and those experiencing high rates of GPC3-overexpressing hepatoblastoma recurrence after a second CR.
Materials and methods
Patient eligibility
This phase I clinical trial was approved by the Ethics Committee of the National Cancer Center, Japan (Tokyo, Japan), and was carried out from September 2011 to June 2016. Patients with refractory pediatric tumors without leukemia were enrolled after providing informed written consent. The patients were divided into three groups (progression group: patient in refractory, recurrent, or progressive status; partial-remission group: patient in partial remission or SD; and the remission group: patient in remission without chance of cure). The following eligibility criteria were employed: 1) patients with histological confirmation of GPC3 expression in tumor cells; 2) no expectation of response to other therapies; 3) age between 1 and 40 years; 4) an Eastern Cooperative Oncology Group performance status of 0 to 2; 5) no prior therapy within 4 weeks; 6) life expectancy of ≥3 months; 7) HLA-A24- or HLA-A2-positive status as determined using commercially available genomic DNA typing tests (Mitsubishi Chemical Medicine, Tokyo, Japan); 8) confirmation of the following laboratory results within 14 days (absolute neutrophil count ≥1000/μL; hemoglobin ≥8.0 g/dL; platelets ≥75,000/μL; serum creatinine adjusted according to age as follows: ≤0.8 mg/dL (<5 years), ≤1.2 mg/dL (<5–9 years), and ≤1.5 mg/dL (<10–19 years); total bilirubin ≤1.5mg/dL; aspartate aminotransferase ≤165 IU/L; and alanine aminotransferase ≤200 IU/L); and 9) patients aged ≤15 years required written informed consent from legal guardian, and those aged from 16 to 19 years required written informed consent from both the patient and the legal guardian The following exclusion criteria were applied: 1) pleural effusion or ascites requiring removal by puncture; 2) active concurrent cancer or secondary cancer within 5 disease-free years of primary cancer; 3) active infection requiring systemic medication; 4) active gastrointestinal bleeding; 5) severe complications, including cardiac failure, renal failure, liver failure, active gastro-duodenal ulcer, ileus, or uncontrolled diabetes mellitus; 6) severe psychiatric disorder; 7) past history of severe drug allergy; 8) currently taking systemic steroids or immunosuppressant medication; 9) judged inappropriate for the trial by a responsible researcher; and 10) unsuitability for the trial based on clinical judgment.
Study design and endpoints
This study was a non-randomized, open-label, uncontrolled phase I clinical trial to evaluate the efficacy and safety of the GPC3 peptides in pediatric patients with refractory malignant tumors. HLA-A*24:02-restricted GPC3298–306 peptide (EYILSLEEL) (American Peptide Company, Sunnyvale, CA, USA) was used in HLA-A24-positive patients, and HLA-A*02:01-restricted GPC3144–152 peptide (FVGEFFTDV) (American Peptide Company) was used in HLA-A2-positive patients. Peptides were administered in liquid form, emulsified with incomplete Freund's adjuvant (IFA) (Montanide ISA-51VG; SEPPIC, Paris, France), by intradermal injection every 2 weeks until disease progression or recurrence. The peptides and IFA were synthesized according to Good Manufacturing Practice guidelines. Administration of two incremental doses of peptide per patient body weight (<20kg: 1.5mg; >20kg: 3.0mg) was planned. The primary endpoint was the safety of peptide vaccination. The secondary endpoints were clinical outcomes, including PFS, OS, and GPC3-specific immune responses to GPC3 vaccination. This study was approved by the Ethics Committee of the National Cancer Center, Japan and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The trial has been registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR number: 000006357).
Evaluation of toxicity and clinical response
Patients were examined for signs of toxicity during and after vaccination. Adverse events were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Hematological examinations were conducted prior to each vaccination. The tumor size was evaluated by CT or magnetic resonance imaging before vaccination, and then every 8 to 12 weeks after the first vaccination. Tumor responses were evaluated according to RECIST guidelines (version 1.1).42
Cell lines
The HLA-A*02:01 human cancer cell lines, hepatocellular carcinoma HepG2, wilm's tumor G-401, neuroblastoma SK-N-DZ and hepatoblastoma HuH-6 were used as target cells. The human liver-cancer cell line SK-Hep-1 (GPC3−, HLA-A*02:01 /A*24:02) were stably transfected with a human full-length GPC3 gene (SK-Hep-1/hGPC3; GPC3+, HLA-A*02:01 /A*24:02) or empty vector (SK-Hep-1/vec; GPC3−, HLA-A*02:01 /A*24:02). T2 (HLA-A*02:01, TAP−) cells and T2A24 (HLA-A*24:02, TAP−) cells were pulsed with GPC3144–152 or HIV19-27 peptide and GPC3298–306 or HIV583–591 peptide, respectively (ProImmune, Oxford, UK) at room temperature for 1h. These cells were maintained in RPMI 1640 or DMEM medium (Sigma, St Louis, MO, USA) supplemented with 10% FCS, penicillin (100 U/ ml) and streptomycin (100 μg / mL) at 37°C in a humidified atmosphere containing 5% CO2.
Ex vivo IFN-γ ELISPOT assay
An ex vivo IFN-γ ELISPOT assay was performed to measure the antigen-specific CTL response, as described previously.43 Briefly, peripheral blood (10mL) was obtained from each patient before the first vaccination and 2 weeks after each vaccination and centrifuged with a Ficoll-Paque gradient. PBMCs were frozen prior to immunological analysis, and all PBMCs obtained from an individual patient were incubated in the same plate and analyzed by ex vivo IFN-γ ELISPOT assay at the same time. Non-cultured PBMCs (5 × 105/well) were added to plates in the presence of peptide antigens (10µg/mL) and incubated for 20 h at 37°C in 5% CO2. The GPC3 antigen used was the HLA-A2-restricted GPC3144–152 (FVGEFFTDV) peptide or the HLA-A*24:02-restricted GPC3298–306 peptide (EYILSLEEL). PBMCs plus HLA-A2-restricted HIV19–27 (TLNAWVKVV) peptide (ProImmune) or HLA-A*24:02-restricted HIV583-591 (RYLKDQQLL) (ProImmune) were used as negative controls. The assays were performed in duplicate. The GPC3 specific spot number indicates the number of GPC3 peptide-specific spot calculated by subtracting the spot number in a well of HIV peptide.
Dextramer staining and flow-cytometry analysis
PBMCs were stained with HLA-A*02:01 Dextramer-RPE [GPC3144–152 (FVGEFFTDV) or HIV19–27 (TLNAWVKVV); Immudex, Copenhagen, Denmark] and HLA-A*24:02 Dextramer-RPE [GPC3298–306 (EYILSLEEL) or HIV583–591 (RYLKDQQLL); Immudex] for 10 min at room temperature and with anti-CD8-FITC (ProImmune) for 20 min at 4°C. Flow cytometry was performed using a FACSAria cell sorter (BD Biosciences, San Jose, CA, USA) as described previously.43
CD107a staining and flow-cytometry analysis
The CTL clones were incubated with T2A24 pulsed with GPC3298–306 or HIV583–591 peptide at a 2:1 ratio for 3.5 h at 37°C. CD107a-specific antibodies (BD Biosciences) were included during the incubation period.
Response of CTL clones against cancer cell lines
The CTL clones were cocultured with each cancer cell line as a target cell at the indicated effector / target (E / T) ratio, and cytotoxicity assay or IFN-γ ELISPOT assay was carried out. Blocking of HLA-class I was carried out using anti HLA-ABC antibody (clone W6/32, BioLegend, San Diego, CA, USA) and IgG2a Isotype control.
Measurement of plasma GPC3 concentrations
Plasma GPC3 concentrations were measured using a fully automated assay kit provided by Sysmex Corporation (Kobe, Japan). Briefly, a biotinylated monoclonal antibody reagent was employed to capture GPC3 from clinical plasma samples from patients, and streptavidin-coated magnetic beads were used to capture the immune complexes. Following magnetic separation and washing for bound/free fraction (B/F) separation, a second monoclonal antibody labeled with alkaline phosphatase was reacted with the immune complex. After a second round of B/F separation, the immune complex was quantified using the HISCL chemiluminescent reagent (Sysmex Corporation). All reactions were performed at 42°C in the HISCL-800 fully automated immunoassay system (Sysmex Corporation) within 17 min.
Cytokine measurements
TNF-α levels in the culture supernatants were evaluated using Cytometric bead array flex sets (BD Biosciences) according to manufacturer protocol. The resulting data were analyzed using FCAP Array Software (v3.0; BD Biosciences).
Immunohistochemical analysis
Biopsy specimens were taken from all the vaccinated patients, each of whom provided informed written consent. Specimens were stained with hematoxylin and eosin or monoclonal antibodies against GPC3 (clone 1G12; dilution 1:300; BioMosaics, Burlington, VT, USA) or HLA class I (clone EMR8/5; dilution 1:2500; Hokudo, Sapporo, Japan) according to manufacturer protocol.
Western blot
The cell lysate were obtained from cancer cell lines. Western blot was performed using anti GPC3 antibody (clone 1G12, BioMosaics) or anti β-actin antibody (clone AC-15, SIGMA).
Real-time PCR
RNA preparation and quantitative real-time PCR (qRT-PCR). Total RNA was isolated using RNeasy Mini kit (QIAGEN, Valencia, CA, USA) following the manufacturer's instructions. GPC3 gene expression levels were analyzed by qRT-PCR assays using the following primers generated according to the indicated reference sequences: sense, 5′-GAGCCAGTGGTCAGTCAAAT-3′ and antisense, 5′-CTTCATCATCACCGCAGTC-3′. Amplification reactions were carried out in 96-well plates in 25 μL reaction volume using the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). All reactions were performed in technical triplicate using an ABI 7500 Fast Real-Time PCR System. Relative expression of the GPC3 gene to the endogenous control gene, β-actin, was calculated using the comparative CT method. β-actin qRT-PCR primer sequences were: sense, 5′-TCCATCATGAAGTGTGACGT-3′ and antisense, 5′-GAGCAATGATCTTGATCTTCAT-3′.
RNA interference
Small interfering RNAs specific for human GPC3 were chemically synthesized double-strand RNAs (Invitrogen, Carlsbad, CA, USA). A non-silencing siRNA, AllStras Neg. Control siRNA, was obtained from Qiagen (Valencia, CA, USA). The GPC3-specific siRNA sequence used in this study was: 5′-GGAGGCUCUGGUGAUGGAAUGAUAA-3′. Synthetic siRNA duplexes were transfected using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocols.
Generation of CTL clones
CD3+CD8+ GPC3 Dextramer+ cells from PBMCs and enzyme-treated tumors were sorted using a FACSAria cell sorter (BD Biosciences), seeded into a 96-well plate (1 cell/well), and stimulated by the addition of irradiated (100Gy) allogeneic PBMCs (8 × 104 cells/well) as feeder cells in AIM-V medium supplemented with 10% human AB serum, interleukin-2 (200 U/ mL), and phytohemagglutinin-P (5µg/mL) for 14 to 21 days as described previously.43
Statistical analysis
All statistical analyses were performed using R software packages (R Foundation for Statistical Computing; http://www.r-project.org). Survival rates were analyzed by the R package survfit (Kaplan-Meier method). HRs and significance levels were analyzed by the R package coxph (Cox proportional hazard analysis). Statistical significance was defined as a p < 0.05.
Supplementary Material
Disclosure of conflicts of interest
T. N. is a scientific advisor for Ono Pharmaceutical Co., Ltd. T.N., T.Y. and K.S. are supported by fundamental research funding from Takeda Pharmaceutical Co., Ltd. The other authors have no potential conflicts of interest to declare with regard to this study.
Acknowledgments
This work was supported in part by the National Cancer Center Research and Development Fund (25-A-7) and (28-A-8), as well as Health and Labor Science Research Grants for Clinical Research on Applying Health Technology and Research for Promotion of Cancer Control Programmes, Japan and joint research funding from Takeda Pharmaceutical Co, Ltd.
Funding
This work was supported in part by the National Cancer Center Research and Development Fund (25-A-7 and 28-A-8), as well as Health and Labor Science Research Grants for Clinical Research on Applying Health Technology and Research for Promotion of Cancer Control Programmes, Japan.
Abbreviations
- B/F
bound/free fraction
- CI
confidence interval
- CR
complete response
- CTL
cytotoxic T lymphocyte
- DLT
dose-limiting toxicity
- ELISPOT
enzyme-linked immunospot
- GPC3
glypican-3
- HCC
hepatocellular carcinoma
- HLA
human leukocyte antigen
- HR
hazard ratio
- IFA
incomplete Freund's adjuvant
- IFN
interferon
- MHC
major histocompatibility complex
- OS
overall survival
- PBMC
peripheral blood mononuclear cell
- PFS
progression-free survival
- PR
partial response
- QOL
quality of life
- RECIST
Response Evaluation Criteria in Solid Tumors
- SD
stable disease
- TCR
T cell receptor
- TNF-α
tumor necrosis factor-α
References
- 1.Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, Israels T, Jeha S, Harif M, Sullivan MJ, et al.. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. J Clin Oncol. 2015;33:3065-73. doi: 10.1200/JCO.2014.60.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson PC. Improving the outcome for children with cancer: Development of targeted new agents. CA Cancer J Clin. 2015;65:212-20. doi: 10.3322/caac.21273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs J, Seitz G, Handgretinger R, Schafer J, Warmann SW. Surgical treatment of lung metastases in patients with embryonal pediatric solid tumors: an update. Semin Pediatr Surg. 2012;21:79-87. doi: 10.1053/j.sempedsurg.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 4.Heiner JP, Miraldi F, Kallick S, Makley J, Neely J, Smith-Mensah WH, Cheung NK. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987;47:5377-81. [PubMed] [Google Scholar]
- 5.Roth M, Linkowski M, Tarim J, Piperdi S, Sowers R, Geller D, Gill J, Gorlick R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120:548-54. doi: 10.1002/cncr.28461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrenkov K, Ostrovnaya I, Gu J, Cheung IY, Cheung NK. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr Blood Cancer. 2016;63:1780-5. doi: 10.1002/pbc.26097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764-71. doi: 10.1002/ijc.28474 [DOI] [PubMed] [Google Scholar]
- 8.Zhou LT, Liu FY, Li Y, Peng YM, Liu YH, Li J. Gpnmb/osteoactivin, an attractive target in cancer immunotherapy. Neoplasma. 2012;59:1-5. doi: 10.4149/neo_2012_001 [DOI] [PubMed] [Google Scholar]
- 9.Oue T, Uehara S, Yamanaka H, Takama Y, Oji Y, Fukuzawa M. Expression of Wilms tumor 1 gene in a variety of pediatric tumors. J Pediatr Surg. 2011;46:2233-8. doi: 10.1016/j.jpedsurg.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 10.Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung NK. Phase I trial of a bivalent gangliosides vaccine in combination with beta-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res. 2014;20:1375-82. doi: 10.1158/1078-0432.CCR-13-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashii Y, Sato E, Ohta H, Oka Y, Sugiyama H, Ozono K. WT1 peptide immunotherapy for cancer in children and young adults. Pediatr Blood Cancer. 2010;55:352-5. doi: 10.1002/pbc.22522 [DOI] [PubMed] [Google Scholar]
- 12.Filmus J. Glypicans in growth control and cancer. Glycobiology. 2001;11:19R-23R. doi: 10.1093/glycob/11.3.19R [DOI] [PubMed] [Google Scholar]
- 13.Song HH, Filmus J. The role of glypicans in mammalian development. Biochim Biophys Acta. 2002;1573:241-6. doi: 10.1016/S0304-4165(02)00390-2 [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita Y, Tanaka S, Souzaki R, Miyoshi K, Kohashi K, Oda Y, Nakatsura T, Taguchi T. Glypican 3 expression in pediatric malignant solid tumors. Eur J Pediatr Surg. 2015;25:138-44. [DOI] [PubMed] [Google Scholar]
- 15.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, et al.. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-96. doi: 10.1158/1078-0432.CCR-11-3044 [DOI] [PubMed] [Google Scholar]
- 16.Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, et al.. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. doi: 10.1080/2162402X.2015.1129483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki S, Sakata J, Utsumi F, Sekiya R, Kajiyama H, Shibata K, Kikkawa F, Nakatsura T. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology. 2016;5:e1238542. doi: 10.1080/2162402X.2016.1238542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, et al.. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;e1346764 in press. doi: 10.1080/2162402X.2017.1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson AD, Federico SM, Aerts I, Hargrave DR, DuBois SG, Iannone R, Geschwindt RD, Wang R, Haluska FG, Trippett TM, et al.. A phase 1 study of oral ridaforolimus in pediatric patients with advanced solid tumors. Oncotarget. 2016;7:84736-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vo KT, Karski EE, Nasholm NM, Allen S, Hollinger F, Gustafson WC, Long-Boyle JR, S3 Shiboski, Matthay KK, DuBois SG. Phase 1 study of sirolimus in combination with oral cyclophosphamide and topotecan in children and young adults with relapsed and refractory solid tumors. Oncotarget. 2017;8:23851-23861. doi: 10.18632/oncotarget.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saikali Z, Sinnett D. Expression of glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 2000;89:418-22. doi: 10.1002/1097-0215(20000920)89:5%3c418::AID-IJC4%3e3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 22.Toretsky JA, Zitomersky NL, Eskenazi AE, Voigt RW, Strauch ED, Sun CC, Huber R, Meltzer SJ, Schlessinger D. Glypican-3 expression in Wilms tumor and hepatoblastoma. J Pediatr Hematol Oncol. 2001;23:496-9. doi: 10.1097/00043426-200111000-00006 [DOI] [PubMed] [Google Scholar]
- 23.Maeda D, Ota S, Takazawa Y, Aburatani H, Nakagawa S, Yano T, Taketani Y, Kodama T, Fukayama M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod Pathol. 2009;22:824-32. [DOI] [PubMed] [Google Scholar]
- 24.Aviel-Ronen S, Lau SK, Pintilie M, Lau D, Liu N, Tsao MS, Jothy S. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol. 2008;21:817-25. doi: 10.1038/modpathol.2008.37 [DOI] [PubMed] [Google Scholar]
- 25.Zynger DL, Gupta A, Luan C, Chou PM, Yang GY, Yang XJ. Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum Pathol. 2008;39:224-30. doi: 10.1016/j.humpath.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, Hosaka S, Beppu T, Ishiko T, Kamohara H, et al.. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306:16-25. doi: 10.1016/S0006-291X(03)00908-2 [DOI] [PubMed] [Google Scholar]
- 27.Nakatsura T, Nishimura Y. Usefulness of the novel oncofetal antigen glypican-3 for diagnosis of hepatocellular carcinoma and melanoma. BioDrugs. 2005;19:71-7. doi: 10.2165/00063030-200519020-00001 [DOI] [PubMed] [Google Scholar]
- 28.Shirakawa H, Kuronuma T, Nishimura Y, Hasebe T, Nakano M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, et al.. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int J Oncol. 2009;34:649-56. [DOI] [PubMed] [Google Scholar]
- 29.Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T, et al.. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-7. doi: 10.1111/j.1349-7006.2009.01206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trobaugh-Lotrario AD, Katzenstein HM. Chemotherapeutic approaches for newly diagnosed hepatoblastoma: past, present, and future strategies. Pediatr Blood Cancer. 2012;59:809-12. doi: 10.1002/pbc.24219 [DOI] [PubMed] [Google Scholar]
- 31.Semeraro M, Branchereau S, Maibach R, Zsiros J, Casanova M, Brock P, Domerg C, Aronson DC, Zimmermann A, Laithier V, et al.. Relapses in hepatoblastoma patients: clinical characteristics and outcome–experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer. 2013;49:915-22. doi: 10.1016/j.ejca.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 32.Frazier AL, Hale JP, Rodriguez-Galindo C, Dang H, Olson T, Murray MJ, Amatruda JF, Thornton C, Arul GS, Billmire D, et al.. Revised risk classification for pediatric extracranial germ cell tumors based on 25 years of clinical trial data from the United Kingdom and United States. J Clin Oncol. 2015;33:195-201. doi: 10.1200/JCO.2014.58.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawada A, Inoue M, Kondo O, Yamada-Nakata K, Ishihara T, Kuwae Y, Nishikawa M, Ammori Y, Tsuboi A, Oji Y, et al.. Feasibility of Cancer Immunotherapy with WT1 Peptide Vaccination for Solid and Hematological Malignancies in Children. Pediatr Blood Cancer. 2016;63:234-41. doi: 10.1002/pbc.25792 [DOI] [PubMed] [Google Scholar]
- 34.Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, Haas JE, King DR, Liu-Mares W, Sensel MG, et al.. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children's Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665-75. doi: 10.1200/JCO.2000.18.14.2665 [DOI] [PubMed] [Google Scholar]
- 35.Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr. 2014;26:29-36. doi: 10.1097/MOP.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 36.Zsiros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V, et al.. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28:2584-90. doi: 10.1200/JCO.2009.22.4857 [DOI] [PubMed] [Google Scholar]
- 37.Zsiros J, Brugieres L, Brock P, Roebuck D, Maibach R, Zimmermann A, Childs M, Pariente D, Laithier V, Otte JB, et al.. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): a prospective, single-arm, feasibility study. Lancet Oncol. 2013;14:834-42. doi: 10.1016/S1470-2045(13)70272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heczey A, Louis CU. Advances in chimeric antigen receptor immunotherapy for neuroblastoma. Discovery medicine. 2013;16:287-94. [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp LM, Katsanis E. Targeted immunotherapy for pediatric solid tumors. Oncoimmunology. 2016;5:e1087637. doi: 10.1080/2162402X.2015.1087637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ofuji K, Saito K, Suzuki S, Shimomura M, Shirakawa H, Nobuoka D, Sawada Y, Yoshimura M, Tsuchiya N, Takahashi M, et al.. Perioperative plasma glypican-3 level may enable prediction of the risk of recurrence after surgery in patients with stage I hepatocellular carcinoma. Oncotarget. 2017;8:37835-37844. doi: 10.18632/oncotarget.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attallah AM, El-Far M, Omran MM, Abdelrazek MA, Attallah AA, Saeed AM, Farid K. GPC-HCC model: a combination of glybican-3 with other routine parameters improves the diagnostic efficacy in hepatocellular carcinoma. Tumour Biol. 2016;37:12571-7. doi: 10.1007/s13277-016-5127-6 [DOI] [PubMed] [Google Scholar]
- 42.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 43.Yoshikawa T, Nakatsugawa M, Suzuki S, Shirakawa H, Nobuoka D, Sakemura N, Motomura Y, Tanaka Y, Hayashi S, Nakatsura T. HLA-A2-restricted glypican-3 peptide-specific CTL clones induced by peptide vaccine show high avidity and antigen-specific killing activity against tumor cells. Cancer Sci. 2011;102:918-25. doi: 10.1111/j.1349-7006.2011.01896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


