Abstract
Infants exposed in utero to opioids will demonstrate a withdrawal syndrome known as neonatal abstinence syndrome (NAS). Buprenorphine is a long-acting opioid with therapeutic use in medication-assisted treatment of opioid dependency in adults and adolescents. Emerging data from clinical trials and treatment cohorts demonstrate the efficacy and safety of sublingual buprenorphine for those infants with NAS who require pharmacologic treatment. Pharmacometric modeling will assist in defining the exposure–response relationships and facilitate dose optimization.
NEONATAL ABSTINENCE SYNDROME
The placental transfer of drugs from the mother to the fetus is well described. The developmental kinetics of drug permeability at the fetal “blood–brain barrier” in humans is not fully defined, but is clearly immature early in pregnancy.1 Many prescribed medications or drugs of abuse can impact postnatal outcomes, particularly with chronic use during pregnancy. Some substances cause neonatal symptomatology that is a direct effect of maternal transfer of the xenobiotic to the neonate. This is characteristic of a toxidrome. For example, intrauterine cocaine exposure is associated with tremors, high-pitched cry, and autonomic instability in the infant that is self-limited and improves as the drug is cleared from the system.2 Other psychoactive agents are associated with symptoms that emerge as the drug concentration falls within the neonate, indicating a withdrawal syndrome. Opioids, serotonin reuptake inhibitors,3 nicotine,4 and antipsychotics5 all can cause withdrawal or adaptation symptoms. The term “neonatal abstinence syndrome” is nonspecific. While the bulk of morbidity and symptoms are due to withdrawal from opioids, concomitant maternal use of other drugs associated with withdrawal symptoms is common. These exposures typically will worsen NAS symptoms and duration, but exposures are difficult to quantify in practice and ultimately do not impact management decisions. Others, including the US Food and Drug Administration (FDA), advocate the use of the term “neonatal opioid withdrawal syndrome” (NOWS) to more specifically link symptoms to opioid exposure.6 For the purposes of this review, the more commonly and general term NAS will be used to implicitly describe withdrawal symptoms driven primarily by in utero opioid exposure.
Physician prescription of opioids occurs in 14–37% of pregnancies,7 but the majority are for a short duration and not associated with neonatal withdrawal. Prolonged in utero exposure to opioids is requisite for a withdrawal syndrome, although a threshold exposure has not been established and maternal methadone dose is only weakly associated with neonatal symptoms.8 Cardinal manifestations of neonatal opioid withdrawal are grouped into central nervous system (CNS), autonomic, and gastrointestinal domains. Seizures are of greatest concern, but are uncommon in the current era of earlier recognition and treatment. NAS symptoms have clinical impact mainly on feeding, with attendant negative effects on growth and development. Nonpharmacologic treatments should be used in all infants with in utero exposure to opioids. There is no universal definition of what constitutes a nonpharmacologic treatment, but common measures include the infant rooming with the mother,9 encouraging breast feeding,10 multiple small feedings, swaddling,11 and minimization of stimuli. Conceptually, all infants should be considered to be along a spectrum of symptom severity. Even with nonpharmacological interventions, over half of infants with signs of withdrawal will require pharmacologic treatment.
CURRENT PHARMACOLOGIC APPROACHES TO NAS
The need for pharmacologic treatment, and titration of dose, is guided by a symptom score. The Finnegan scoring instrument12 is the most widely used, but local variations are common. The MOTHER NAS score,13 a modification of the Finnegan instrument, is standardized and is the most commonly reported in clinical trials. An opioid is the primary therapy,14 using a strategy of titrating doses to control symptoms and slowly weaning down. Morphine is used at 80% of centers in the US, with methadone being used in the remainder.15 Phenobarbital or clonidine are used as adjunctive therapies in conjunction with an opioid when either maximum opioid dose has been reached, or in an initial coadministration with the goal of reducing opioid exposure. There is significant site-to-site heterogeneity in pharmacologic regimens, including starting and maximum dose, uptitration, and weaning parameters.16 There is a lack of definitive trials to guide therapy, although results of a multicenter trial comparing methadone and morphine (NCT01958476) will provide some guidance between these two drugs. There is similarly no consensus of the role of adjunctive therapy. Differing regimens involve the addition of clonidine or phenobarbital at maximum dose of opioid, the use of either agent in parallel with the opioid, or as an opioid-free monotherapy. Phenobarbital is a more nonspecific CNS depressant and is believed to be particularly useful in poly-substance exposures, although there are theoretical concerns about neurotoxicity.17 Clonidine has a mechanism more specific to opioid withdrawal and has a better evidence base of randomized trials, but it is used in less than 10% of hospitals treating NAS.15
IMPLICATIONS OF ADULT MEDICALLY ASSISTED THERAPY TO NEONATAL THERAPIES
Methadone and buprenorphine are the two primary treatments for opioid use disorder. Short-acting opioids such as morphine are not used in adults as maintenance replacement. The opioid use disorder is defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as a series of maladaptive behaviors prompted by physical tolerance and withdrawal symptoms. These behaviors are associated with significant morbidity and mortality over age-matched cohorts, primarily due to accidents, overdose, infection, and violence. Treatment with methadone or buprenorphine compared to no long-acting opioid reduces all-cause mortality by ~30%.18 Substitution therapy has been examined primarily with illicit opioids, but appears effective in prescription opioid dependency as well.19 Cochrane reviews suggest that, in the aggregate, methadone has an efficacy advantage over buprenorphine in retaining patients in maintenance opioid replacement therapy.20 However, at medium- or high-dose ranges, efficacy appeared similar. Infants with NAS have physical dependency, but none of the maladaptive behaviors associated with addiction. As such, extrapolation from adult therapeutics is somewhat limited. Relief from withdrawal symptoms is a more analogous pharmacodynamic endpoint in neonates than retention in a drug treatment program or urine drug screen results. For the acute control of withdrawal symptoms in adults, buprenorphine has improved efficacy relative to clonidine, and possibly to methadone.21 Anecdotal clinician observations suggest buprenorphine may have an easier transition to the final weaning doses than methadone.
BUPRENORPHINE IN PREGNANCY
There is high-quality evidence demonstrating improved neonatal outcomes when mothers are maintained on opioid replacement therapy throughout pregnancy. Optimal therapeutic approaches are not solely pharmacologic, but combine pharmacologic opioid replacement with intensive counseling. The risk of relapse and patient substitution with illicit opioids following withdrawal of opioid replacement is high, and is an option only in highly selected cases with intense supervision.22 Methadone has been the standard replacement opioid during pregnancy, with a recent increase in buprenorphine as the primary opioid. Based on three randomized controlled trials and 15 cohort trials, buprenorphine has been associated with lower risk of preterm birth and improved in utero growth parameters compared to methadone maintenance, without evidence of increased harms.23 While in the landmark MOTHER trial, maternal buprenorphine did not significantly reduce the number of infants who required pharmacologic treatment for NAS, it did reduce the duration of therapy and total morphine dose compared to methadone.13 The dose of buprenorphine transferred during breastfeeding is low,24 and the practice is encouraged in most stable mothers not actively abusing medications.25,26 There is no evidence that maternal treatment with buprenorphine or methadone should drive the subsequent choice of pharmacologic treatment of NAS. The time to emergence of NAS symptoms following buprenorphine is similar to methadone exposure at 36–60 h in most infants, although there are occasional cases of more prolonged emergence of symptoms. Anxiety and depression incidence are higher in women with opioid use disorders,27 so concomitant benzodiazepine28 or antidepressant exposure29 are common and can prolong the emergence time of symptoms.
BUPRENORPHINE PHARMACOKINETIC (PK)
Buprenorphine has agonism at delta and opioid receptor-like (ORL1) receptors and antagonism at the kappa receptor. However, it is agonism at the mu-opioid receptor that is thought to be the primary mode of action in reducing withdrawal symptoms and decreasing the effect of other exogenous opioids. Oral administration is not feasible due to a large first-pass metabolism. The high degree of lipophilicity allows effective sublingual dosing. Ethanolic solutions are absorbed from the sublingual mucosa in adults with an absolute bioavailability of 28–51%.30,31 This occurs in 2–4 min, and longer retention times are not associated with increased systemic exposure.30,32 Early publications and the product label of intravenous (i.v.) buprenorphine list a short elimination half-life. This is artifactual, due to lower sensitivity and nonspecificity of earlier radioimmune assays, along with a low therapeutic serum concentration due to the potency of buprenorphine.33 Mean terminal elimination in adults after i.v. dosing of 2–16 mg with a sensitive assay was 25 ± 1 h with a volume of distribution of ~800 L.34 Administration by the sublingual compared to i.v. administration increases volume of distribution, with the oral mucosa acting as a reservoir, which accounts for a prolonged elimination half-life up to 24–42 h. The typical sublingual tablet dose range used in adults is between 4–24 mg/day, with the majority of patients controlled with 16 mg or less. Two mg of buprenorphine causes occupancy of 40% of mu-opioid receptors, while at 16 or 32 mg >80% of receptors are occupied.35,36 Peak serum concentration is 0.5–1, 5–6, and 13–14 ng/mL for 2, 16, and 32 mg sublingual tablets, respectively.16 Buprenorphine PK predicts receptor occupancy reasonably well, and by linking to pharmacodynamic (PD) response, ~50–60% mu-opioid receptor occupancy provides relief of withdrawal symptoms.37 This degree of occupancy correlates with a serum concentration of 1.0 ng/mL, consistent with an estimate of 0.7 ng/mL from another investigation in opioid-experienced subjects.38 The concentration needed to suppress reinforcing and subjective effects of abused opioids, which is less relevant in NAS, is estimated to be >3 ng/mL.37 While these data are useful, caution should be used in extrapolation to the neonatal population. Opioid exposure may upregulate mu-opioid receptor density compared to healthy volunteers.35 Differences in body water and fat composition between neonates and adults may impact the distributive characteristics. Lastly, while there are parallels in the pathophysiology of withdrawal from opioids between adults and neonates, the biology in newborns is not as well characterized and more complicated due to the neurodevelopmental trajectory in newborns.
BUPRENORPHINE CLINICAL STUDIES IN NAS
An open-label, active control, phase I investigation was the first use of sublingual buprenorphine in NAS.39 In the first cohort of 26 term infants without exposure to benzodiazepines, buprenorphine demonstrated a significant reduction in length of treatment and stay compared to standard of care oral morphine (Table 1). The treatment protocol for both arms used escalating doses until symptom control, with phenobarbital added when a maximum dose was achieved. Phenobarbital was discontinued before weaning took place. A second cohort of 24 infants was enrolled with the same design, but modifications included higher initial and maximum dose, and an increased rate of uptitration of dose (25% vs. 20%).40 Both this and a subsequent double-blind, double-dummy, single-site, randomized controlled trial (BBORN: Blinded Buprenorphine OR Neonatal morphine solution) demonstrated significant reductions in length of stay and length of hospitalization.41 The BBORN treatment regimen differed from the open-label cohorts in a more aggressive morphine uptitration (20% vs. 10%) and a parallel weaning of phenobarbital and opioid in both arms. Across all published trials, 11 buprenorphine and nine morphine-treated infants required phenobarbital adjunctive therapy due to inadequate control of symptoms with opioid alone. A small unpublished cohort of 11 infants with concomitant benzodiazepine exposure has enrolled using the same regimen as the BBORN trial.
Table 1.
Buprenorphine clinical experience in NAS
| Study | Publication | Design | Buprenorphine | Comparator (N) | Reduction in length of treatment (days buprenorphine vs. comparator) |
||
|---|---|---|---|---|---|---|---|
| N | Initial dose (µg/kg/day) |
Maximum dose (µg/kg/day) |
|||||
| Kraft39 | 2008 | Open label, randomized | 13 | 13.2 | 39 | Morphine (13) | 32% (22 vs. 32) |
| Kraft40 | 2011 | Open label, randomized | 12 | 15.3 | 60 | Morphine (12) | 39% (23 vs. 38) |
| Hall42 | 2016 | Retrospective cohort | 38 | 13.2 | 39 | Methadone (163) | 33% (9.3 vs. 14) |
| Hall43 | 2017 | Retrospective cohort | 174 | 13.5 | 22.5 | Methadone or Morphine (186) | 29% (7.4 vs. 10.4) |
| Kraft41 | 2017 | Blinded, randomized | 30 | 15.3 | 60 | Morphine (33) | 46% (15 vs. 28) |
| MOP Plus (NCT01671410) | Unpublished | Open label, randomized | 6 | 15.3 | 60 | Morphine (5) | N.A. |
Hall et al. reported results from a cohort of buprenorphine-treated infants compared to standard of care methadone.42 Phenobarbital was added if a maximum dose of buprenorphine was reached or if weaning was not achieved after 24–48 h. Phenobarbital was continued after the opioid was weaned off, and some infants were discharged to home on phenobarbital. The weaning protocol also differed from that in the clinical trials described above (Table 2). Although treatment selection (buprenorphine or methadone) was not randomized, the demographic characteristics of the two groups of infants were similar. Opioid treatment duration and length of stay was less than was seen in the clinical trial cohorts described by Kraft et al.,39–41 but the magnitude of the reduction on each measure was similar. A large follow-up cohort from Hall using a slightly modified buprenorphine and adjunct regimen demonstrated a similar reduction in length of opioid treatment.43
Table 2.
Dose regimens of buprenorphine in NAS
| Kraft41 | Hall42 | |
|---|---|---|
| Weight used for calculation of dose throughout treatment period | At time of drug initiation | Birth |
| Initial unit dose (µg/kg q8 hours) | 5.3 | 4.4 |
| Maximum unit dose (µg/kg q8 hours) | 20 | 13 |
| Up-titration rate | 25% | 0.8 µg/kg increments |
| Maximum # of up-titrations | 6 | 11 |
| Weaning rate | 10% | 0.8 µg/kg increments until 4.4 µg/kg, then fixed reduction in dose and interval |
| Cessation dose | Within 10% of starting dose | 1.7 µg/kg q24 |
| Add phenobarbital | At maximum buprenorphine dose | At maximum buprenorphine dose or unable to wean after 24–48 hr |
| Phenobarbital weaning | 5 mg/kg reduced to 2.5 mg/kg after buprenorphine 50% of maximum dose; phenobarbital cessation after three additional down titration steps | Phenobarbital continued after buprenorphine stopped; dose weaned as an outpatient |
| Inpatient observation following cessation of last scheduled dose (hrs) | 48 | 72 |
BUPRENORPHINE SAFETY
Buprenorphine has a favorable safety profile in adults for respiratory depression compared to other full mu-opioid agonists. Deaths from buprenorphine are uncommon in adults, unless there is concomitant use of alcohol, benzodiazepine, or other hypnotics. Possibly due to partial agonism, with increasing doses there is a ceiling phenomenon after which there is minimal change in PD effects. This favorable effect on respiratory depression explains the 3-fold lower rate of overdose while on methadone treatment compared to buprenorphine.44 Although generally responding well with overdose, children under 3 years appear more susceptible to respiratory depression than adults.45,46 The buprenorphine exposure-response relationship varies with the specific PD endpoint measured, but for respiratory rate depression the plateau occurs at 16 mg of sublingual solution in adult, opioid-naïve subjects. This dose is associated with a Cmax of ~10 ng/mL.47 Recent investigations confirm that the ceiling effect is pharmacodynamic and not pharmacokinetic, as there is a linear dose-to-exposure relationship, with serum concentrations of >170 ng/mL well tolerated in opioid-experienced volunteers.34 In adults, the arrhythmogenicity of methadone also likely increases morbidity relative to buprenorphine. In adult population studies, buprenorphine has a 10-fold lower incidence of arrhythmia than methadone,48 likely due to less of a propensity for QT prolongation.49 This favorable profile is maintained in adolescents.50 No arrhythmia adverse event reports for patients under age 18 have been submitted to the FDA.51
Pharmacologic treatment of NAS with morphine or methadone in a monitored inpatient setting is safe and well tolerated. Clinical experience with buprenorphine has been similarly favorable. In published clinical trials of NAS, there have been three serious adverse events. The second patient randomized to buprenorphine developed generalized seizures 78 h after the initial dose, but there was no causal link of undertreatment of withdrawal or a dose-dependent effect of buprenorphine. Postevent evaluation revealed normal serum laboratory and lumbar puncture indices, and negative cultures. An interictal EEG was negative and magnetic resonance imaging (MRI) of the brain revealed a small amount of dependent subdural hemorrhage within the posterior fossa, likely related to the birthing process and deemed unlikely to be pathogenic, with no parenchymal abnormalities. At 1-year follow-up, the child was developmentally normal and seizure-free. Other serious adverse events in the clinical trials included cytomegalovirus (CMV) infection and supraglottoplasty associated with Pierre Robin syndrome, both of which were extant prior to buprenorphine exposure. Hall et al. did not identify any safety issues associated with buprenorphine in their cohort of use in a treatment paradigm.42 Elevated transaminases have been noted in adult cohorts treated with buprenorphine. Liver functions were monitored during NAS clinical trials without any elevations, consistent with a lack of signal in adolescents52 and pediatric overdose patients.46
BUPRENORPHINE PK/PD IN NAS
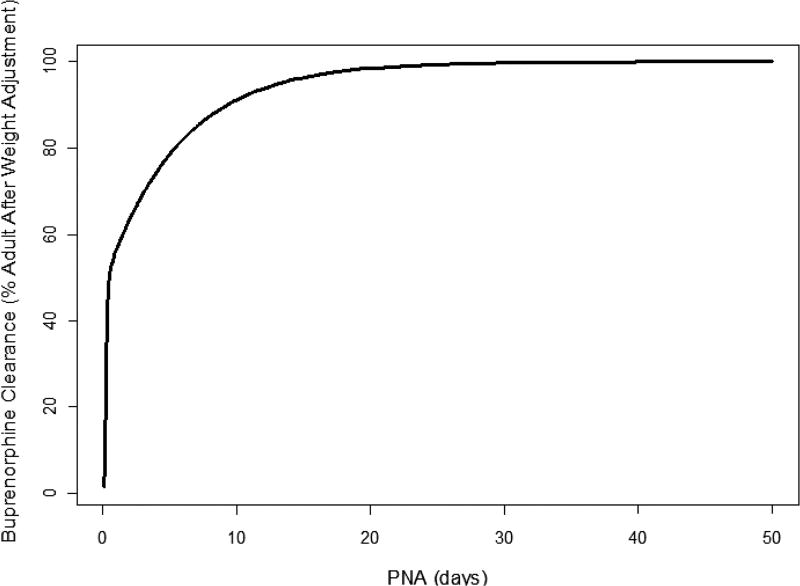
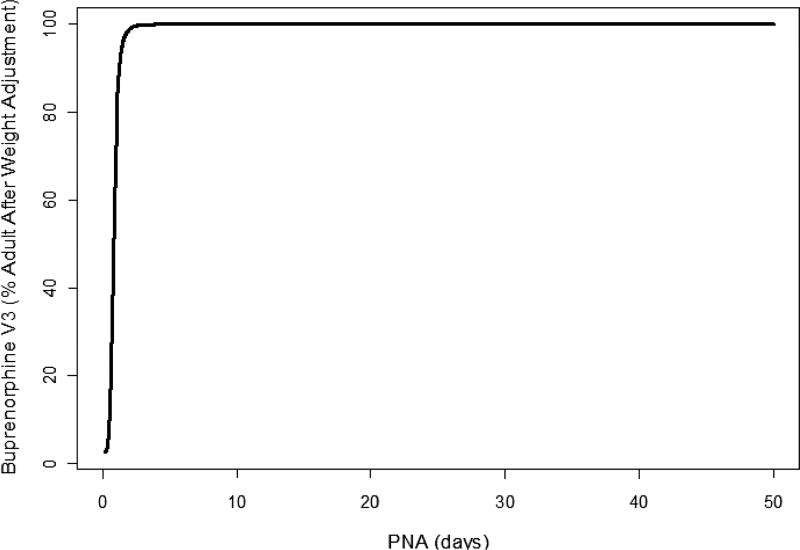
The first published description of buprenorphine in neonates consisted of a single intravenous study by Barrett et al. in critically ill premature infants requiring pain control.53 This trial used a radioimmune assay, which differed from subsequent studies. Mean concentration at steady state was 4.3 ng/mL. Clearance and Vd were low, and postinfusion terminal sampling demonstrated a half-life of 20 h (Table 3). Phase I and III clinical trials in NAS sublingual administration had a collection of PK samples assayed using liquid chromatography–tandem mass spectrometry optimized for small sample volume,54 from which a population PK model was generated.55 Initial attempts using only the phase I infant data did not construct a reasonable model, and thus rich adult volunteer data were used to supplement the model with a final use of (n = 209) from 24 neonates and from five healthy male volunteers (n = 94). The model identified weight and postnatal age as the most important covariates in determining apparent clearance of buprenorphine. These, however, only explained 5% of intraindividual variability of clearance. Mean clearance in the studied population (mean weight 3 kg and age of 5 days) was 3.5 L/hr/kg, which is consistent the adult range of 1.3–3.2 L/hr/kg. There were significant developmental effects, with a model-predicted clearance that rose quickly with age, reaching 50% of adult values at 0.5 days and 90% by 10 days (Figures 1, 2). Concomitant phenobarbital dosing did not impact buprenorphine PK, but the number of infants driving this observation was small. Data from the phase III BBORN trial based on this structural model revealed similar results to that seen in the phase I trial.56 Kamatkar et al. described the PK of buprenorphine in 63 samples from a cohort of 20 NAS infants treated at the University of Cincinnati in an open-label approach. Published parameters were used in a one-compartment model with Bayesian estimates of individual PK parameters.57 In all published models of buprenorphine in NAS, interindividual variability was high. The lack of dense sampling close to a dose and lack of concomitant i.v. administration limit full characterization of adoption kinetics. A possible source of variability is the relative fraction of dose absorbed through the sublingual route. In adults, the bioavailability of a swallowed dose is low due to high first-pass metabolism. The hepatic extraction ratio approaches 1 in adults.33 It is unknown if this applies in neonates for a number of reasons. Buprenorphine is metabolized primarily by CYP 3A4.58 Total 3A4 activity, as measured by cisapride probes, is substantially reduced in neonates.59 The duodenal expression of 3A4 protein and metabolic activity, as measured by 6β-hydroxytestosterone formation, are markedly decreased in the neonatal population.60 Both CYP 3A4 and p-glycoprotein expression are highly variable between individual children of the same age.61 In addition, gut 3A4 expression or activity are poor predictors of systemic exposure of 3A4 substrates in pediatric patients. Fetal CYP 3A7 declines soon after birth, but is expressed during the first month of life during treatment for NAS. However, 3A7 has substantially less metabolic activity than 3A4/5 in the metabolism of buprenorphine.62 Taken together, the observations suggest a larger fraction of orally administered drug administered in neonates reaching the systemic circulation than in adults, which represents another potential source of inter-individual variability. Intraindividual variability may be impacted by sublingual dose administration. The mode of administration was a solution under the tongue, followed by insertion of a pacifier. For volumes greater than 0.5 mL, the dose was split into two separated by 2 min. Assuming that infants have sizable first-pass metabolism, variability in the relative fraction that is swallowed vs. being retained in the sublingual fossa for at least 2 min could account for some dose-to-dose variability. The use of a q8 rather than a longer interval dosing regimen was employed in anticipation of this potential source of variability, as well as providing greater ability to tailor individual dose changes based on symptomatology.
Table 3.
Pharmacokinetic parameters of buprenorphine in NAS
| Model compartments |
No. of infants |
No. of samples |
Route | Indication | Gestational age range (weeks) |
Clearance (L/hr) |
Vd (L) | Ka (hr−1) | T1/2 (hr) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Barrett et al.53 | One | 12 | N.A. | IV | Pain in prematurity | 27–32 | 1.5 | 6.2 | 0.177 | 20 |
| Ng et al.55 | Two | 24 | 209 | SL | NAS | 37–46 | 7.8 | 147 | 0.416 | 11 |
| Kamatkar et al.57 | One | 20 | 63 | SL | NAS | 37–42 | 2.0 | 12.9 | 0.64 | 4.4 |
| Moore et al.56 | Two | 28 | 172 | SL | NAS | 37–42 | 7.61 | 160 | 0.416 | 10 |
Ng et al. and Moore et al. values are based upon an adult model structure and simulated for a 3-kg, 5-day-old infant. Vd represents a pseudovolume (V2 + V3).
Figure 1.
Model-based developmental trajectory of neonatal postnatal age compared to adult clearance of buprenorphine.55 PNA, postnatal age.
Figure 2.
Model-based developmental trajectory of neonatal postnatal age compared to adult peripheral volume (V3) of buprenorphine.55 PNA, postnatal age.
Initial exposure–response analysis suggests that buprenorphine concentration is the primary driver of control of withdrawal symptoms. This is supported by observations of 1) increased clearance associated with worse NAS symptoms, 2) more severe NAS generally requiring a higher AUC to control symptoms, and 3) that higher average concentrations of buprenorphine were correlated with faster time to stabilization.56 Major covariates that drive this variability in drug exposure and clearance have not been identified. However, the linkage between exposure and response when combined with disease state models will allow future simulations to identify regimens more likely to control symptoms quickly and minimize duration of pharmacologic treatment.
FORMULATION
Current use of buprenorphine has employed a 30% ethanolic solution, which was based on early work in adults. This was chosen primarily to mirror adult formulations, for which there was extant absorption data. This formulation is stable at room temperature for at least 30 days and at 7 days in polypropylene-dispensing syringes.63 Additional advantages included microbiologic asepsis and simplicity of preparation in the context of a clinical trial. Ethanol does not decrease opioid withdrawal and is not expected to have provided any of the efficacy seen in NAS clinical trials. While ethanol is a common excipient in neonatal medications, including phenobarbital, formulations in neonatology should be in alcohol-free vehicles where feasible. Consistent with prior observations that neonates clear ethanol faster than adults,64 infants receiving therapeutic buprenorphine had a rapid fall in alcohol concentration between doses.65 No values were above 70 mg/L, which is less than the American Academy of Pediatrics suggested limit of <250 mg/L after a single dose.66 Approximately a third of infants had a concentration above the European Medicines Agency suggested lower limit of 10 mg/L listed in the 2014 guidance, which is still in draft form. The model-derived estimates are of a 7% bioavailability using this formulation in NAS patients.55 At the current time, there is no published ethanol-free formulation of buprenorphine. In addition to demonstration of shelf stability, an investigation of relative bioavailability would be needed to define comparative absorptive kinetics between a new and reference formulation before widespread use on NAS patients could be undertaken.
FUTURE DIRECTIONS
Mirroring the state of neonatal therapeutics for many classes of medications, dose regimens for NAS were largely empiric following the spread of pharmacologic treatment for NAS in the 1970s. Clonidine was the first medication with generation of rich PK data.67 While the PK of i.v. morphine in critically ill infants has been well characterized, similar data for oral administration data in NAS has only recently been described.68 Methadone PK in NAS has been described.69 The power of pharmacometric models is the ability to simulate dose regimens that can then be tested in patient populations. Model-based simulation does not guarantee determination of the ideal dose, but is a clear advantage of the empiric and intuition-based approaches of dose selection commonly used. An elegant demonstration of this approach was implemented by Hall et al.70 The methadone PK/PD model generated from observational data 69 was used to simulate a new dose regimen. This was tested in a prospective fashion against the existing standard of care, leading to a reduction in length of stay.
The original dose of buprenorphine used in investigations of NAS was informed only by i.v. data in preterm infants coupled with relatively rich adult PK data. After the first cohort in the phase I study, a modest dose adjustment was made in starting and maximum doses, as well as uptitration rate. Broadly, simulation can help in defining an exposure response that could identify a target effective concentration. In the NAS population this appears to be ~0.8 ng/mL, which is comparable to adult estimates. Specific areas that would benefit from dose optimization are time to stabilization of symptoms and the weaning regimen. It is not clear if obtaining control of symptoms earlier impacts the duration of weaning, but reaching stabilization early without overshooting the proper dose would reduce the duration of infant symptoms and reduce the length of treatment. The weaning phase makes up the majority of time of treatment, so optimization of dosing in this period has the larger potential impact for reducing duration of treatment. Given the half-life and excellent safety profile, changing the dosing interval after stabilization from q8 to q12 or even q24 could facilitate transition to an outpatient setting.
CONCLUSION
Buprenorphine has demonstrated an efficacy advantage over standard opioid replacement therapy for NAS in both controlled clinical trials and treatment settings. Although the total number of treated patients in these cohorts is modest, consistency in effect size in different populations lends external validity to the findings. Buprenorphine is safe in NAS, and sublingual dosing has been demonstrated to be feasible in the neonatal population. Validation and testing of an ethanol-free formulation may encourage wider use. Pharmacometric analyses under development hold promise in defining exposure–response relationships and optimization of dose regimens, including the potential for safely moving some of the weaning phases of treatment to the outpatient setting.
Acknowledgments
Walter K. Kraft was supported by National Institute on Drug Abuse, R01 DA029076.
Footnotes
CONFLICT OF INTEREST
Dr. Kraft has served as an unpaid consultant to Chiesi Farmaceutici S.p.A.
References
- 1.Goasdoue K, Miller SM, Colditz PB, Bjorkman ST. Review: the blood-brain barrier; protecting the developing fetal brain. Placenta. 2017;54:111–116. doi: 10.1016/j.placenta.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Bauer CR, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch. Pediatr. Adolesc. Med. 2005;159:824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 3.Salisbury AL, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am. J. Psychiatry. 2016;173:147–157. doi: 10.1176/appi.ajp.2015.14080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones HE, et al. Cigarette smoking in opioid-dependent pregnant women: Neonatal and maternal outcomes. Drug Alcohol Depend. 2013;131:271–277. doi: 10.1016/j.drugalcdep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. [Accessed 6 September 2017];FDA Drug Safety Communication: Antipsychotic drug labels updated on use during pregnancy and risk of abnormal muscle movements and withdrawal symptoms in newborns. 2011 < https://www.fda.gov/Drugs/DrugSafety/ucm243903.htm>.
- 6.Food and Drug Administration. [Accessed 6 September 2017];Neonatal opioid withdrawal syndrome and medication-assisted treatment with methadone and buprenorphine. 2016 < https://www.fda.gov/Drugs/DrugSafety/ucm503630.htm>.
- 7.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet. Gynecol. 2014;123:997–1002. doi: 10.1097/AOG.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary BJ, et al. Methadone dose and neonatal abstinence syndrome-systematic review and meta-analysis. Addiction. 2010;105:2071–2084. doi: 10.1111/j.1360-0443.2010.03120.x. [DOI] [PubMed] [Google Scholar]
- 9.Holmes AV, et al. Rooming-in to treat neonatal abstinence syndrome: Improved family-centered care at lower cost. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2929. [DOI] [PubMed] [Google Scholar]
- 10.Short VL, Gannon M, Abatemarco DJ. The association between breastfeeding and length of hospital stay among infants diagnosed with neonatal abstinence syndrome: A population-based study of in-hospital births. Breastfeed. Med. 2016;11:343–349. doi: 10.1089/bfm.2016.0084. [DOI] [PubMed] [Google Scholar]
- 11.van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, Kuis W, Schulpen TW, L’Hoir MP. Swaddling: A systematic review. Pediatrics. 2007;120:e1097–106. doi: 10.1542/peds.2006-2083. [DOI] [PubMed] [Google Scholar]
- 12.Finnegan LP, Connaughton JF, Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: Assessment and management. Addict. Dis. 1975;2:141–158. [PubMed] [Google Scholar]
- 13.Jones HE, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst. Rev. 2010;(10):CD002059. doi: 10.1002/14651858.CD002059.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Patrick SW, et al. Improving care for neonatal abstinence syndrome. Pediatrics. 2016:137. doi: 10.1542/peds.2015-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones HE, Fielder A. Neonatal abstinence syndrome: Historical perspective, current focus, future directions. Prev. Med. 2015;80:12–17. doi: 10.1016/j.ypmed.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Kraft WK, Stover MW, Davis JM. Neonatal abstinence syndrome: Pharmacologic strategies for the mother and infant. Semin. Perinatol. 2016;40:203–212. doi: 10.1053/j.semperi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug Alcohol Depend. 2009;105:9–15. doi: 10.1016/j.drugalcdep.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Larance B, Degenhardt L, Gowing L, Kehler C, Lintzeris N. Opioid agonist treatment for pharmaceutical opioid dependent people. Cochrane Database Syst. Rev. 2016;5:CD011117. doi: 10.1002/14651858.CD011117.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014;2:CD002207. [Google Scholar]
- 21.Gowing L, Ali R, White JM, Mbewe D. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst. Rev. 2017;2:CD002025. doi: 10.1002/14651858.CD002025.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet. Gynecol. 2017;130:e81–e94. doi: 10.1097/AOG.0000000000002235. [DOI] [PubMed] [Google Scholar]
- 23.Zedler BK, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: A systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111:2115–2128. doi: 10.1111/add.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindemalm S, Nydert P, Svensson JO, Stahle L, Sarman I. Transfer of buprenorphine into breast milk and calculation of infant drug dose. J. Hum. Lact. 2009;25:199–205. doi: 10.1177/0890334408328295. [DOI] [PubMed] [Google Scholar]
- 25.Rosen-Carole C, Hartman S Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding Promotion in the Prenatal Setting, Revision 2015. Breastfeed. Med. 2015;10:451–457. doi: 10.1089/bfm.2015.29016.ros. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachs HC Committee on Drugs. The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics. 2013;132:e796–809. doi: 10.1542/peds.2013-1985. [DOI] [PubMed] [Google Scholar]
- 27.Patrick SW, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135:842–850. doi: 10.1542/peds.2014-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr. Serv. 2002;53:39–49. doi: 10.1176/appi.ps.53.1.39. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor AB, O’Brien L, Alto WA, Wong J. Does concurrent in utero exposure to buprenorphine and antidepressant medications influence the course of neonatal abstinence syndrome? J. Matern. Fetal. Neonatal Med. 2016;29:112–114. doi: 10.3109/14767058.2014.987750. [DOI] [PubMed] [Google Scholar]
- 30.Mendelson J, Upton RA, Everhart ET, Jacob P, Jones RT., 3rd Bioavailability of sublingual buprenorphine. J. Clin. Pharmacol. 1997;37:31–37. doi: 10.1177/009127009703700106. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlman JJ, Jr, Lalani S, Magluilo J, Jr, Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J. Anal. Toxicol. 1996;20:369–378. doi: 10.1093/jat/20.6.369. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg DS, et al. Sublingual absorption of selected opioid analgesics. Clin. Pharmacol. Ther. 1988;44:335–342. doi: 10.1038/clpt.1988.159. [DOI] [PubMed] [Google Scholar]
- 33.Elkader A, Sproule B. Buprenorphine: Clinical pharmacokinetics in the treatment of opioid dependence. Clin. Pharmacokinet. 2005;44:661–680. doi: 10.2165/00003088-200544070-00001. [DOI] [PubMed] [Google Scholar]
- 34.Huestis MA, Cone EJ, Pirnay SO, Umbricht A, Preston KL. Intravenous buprenorphine and norbuprenorphine pharmacokinetics in humans. Drug Alcohol Depend. 2013;131:258–262. doi: 10.1016/j.drugalcdep.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zubieta J, et al. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: A preliminary study. Neuropsychopharmacology. 2000;23:326–334. doi: 10.1016/S0893-133X(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 36.Greenwald MK, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- 37.Greenwald MK, Comer SD, Fiellin DA. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: Implications for clinical use and policy. Drug Alcohol Depend. 2014;144:1–11. doi: 10.1016/j.drugalcdep.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhlman JJ, Jr, Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–559. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- 39.Kraft WK, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: A randomized trial. Pediatrics. 2008;122:e601–607. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2010;106:574–580. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraft WK, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N. Engl. J. Med. 2017;376:2341–2348. doi: 10.1056/NEJMoa1614835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall ES, et al. A cohort comparison of buprenorphine versus methadone treatment for neonatal abstinence syndrome. J. Pediatr. 2016;170:39–44.e1. doi: 10.1016/j.jpeds.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Hall ES, Ward RR, Folger AT, Wexelblatt SL. Comparison of neonatal abstinence syndrome treatment with sublingual buprenorphine versus conventional opioids. Am J Perinatal. doi: 10.1055/s-0037-1608634. [E-pub ahead of print]. < https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0037-1608634>. [DOI] [PubMed]
- 44.Sordo L, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toce MS, Burns MM, O’Donnell KA. Clinical effects of unintentional pediatric buprenorphine exposures: Experience at a single tertiary care center. Clin. Toxicol. (Phila) 2017;55:12–17. doi: 10.1080/15563650.2016.1244337. [DOI] [PubMed] [Google Scholar]
- 46.Hayes BD, Klein-Schwartz W, Doyon S. Toxicity of buprenorphine overdoses in children. Pediatrics. 2008;121:e782–786. doi: 10.1542/peds.2007-1774. [DOI] [PubMed] [Google Scholar]
- 47.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin. Pharmacol. Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 48.Sessler NE, Walker E, Chickballapur H, Kacholakalayil J, Coplan PM. Disproportionality analysis of buprenorphine transdermal system and cardiac arrhythmia using FDA and WHO postmarketing reporting system data. Postgrad. Med. 2017;129:62–68. doi: 10.1080/00325481.2016.1271698. [DOI] [PubMed] [Google Scholar]
- 49.Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch. Intern. Med. 2007;167:2469–2475. doi: 10.1001/archinte.167.22.2469. [DOI] [PubMed] [Google Scholar]
- 50.Ciftci Demirci A, Gunes H, Adaletli H, Bulanik E, Erdogan A. Liver enzyme levels in adolescent patients treated with buprenorphine and additional psychotropic agents. Am. J. Drug Alcohol Abuse. 2015;41:107–113. doi: 10.3109/00952990.2014.983272. [DOI] [PubMed] [Google Scholar]
- 51.Kao DP, Haigney MC, Mehler PS, Krantz MJ. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468–1475. doi: 10.1111/add.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogenschutz MP, Abbott PJ, Kushner R, Tonigan JS, Woody GE. Effects of buprenorphine and hepatitis C on liver enzymes in adolescents and young adults. J. Addict. Med. 2010;4:211–216. doi: 10.1097/ADM.0b013e3181c4e27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett DA, Simpson J, Rutter N, Kurihara-Bergstrom T, Shaw PN, Davis SS. The pharmacokinetics and physiological effects of buprenorphine infusion in premature neonates. Br. J. Clin. Pharmacol. 1993;36:215–219. doi: 10.1111/j.1365-2125.1993.tb04220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Anal. Biochem. 2002;306:31–39. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 55.Ng CM, et al. Population pharmacokinetic model of sublingual buprenorphine in neonatal abstinence syndrome. Pharmacotherapy. 2015;35:670–680. doi: 10.1002/phar.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore J, Gastonguay M, Adeniyi-Jones S, Moody D, Kraft W. Population pharmacokinetic and pharmacodynamic analysis of buprenorphine for the treatment of neonatal abstinence syndrome. Clin. Pharmacol. Ther. 2017;101:PI-009. [Google Scholar]
- 57.Kamatkar S, Mizuno T, Ward L, Isemann B, Vinks A, Akinbi H. Pediatric Academic Societies. San Francisco, CA: 2017. Pharmacokinetics of buprenorphine in the neonatal abstinence syndrome. May 2017. [Google Scholar]
- 58.Kobayashi K, et al. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab. Dispos. 1998;26:818–821. [PubMed] [Google Scholar]
- 59.Kearns GL, et al. Cisapride disposition in neonates and infants: In vivo reflection of cytochrome P450 3A4 ontogeny. Clin. Pharmacol. Ther. 2003;74:312–325. doi: 10.1016/S0009-9236(03)00225-X. [DOI] [PubMed] [Google Scholar]
- 60.Johnson TN, Tanner MS, Taylor CJ, Tucker GT. Enterocytic CYP3A4 in a paediatric population: Developmental changes and the effect of coeliac disease and cystic fibrosis. Br. J. Clin. Pharmacol. 2001;51:451–460. doi: 10.1046/j.1365-2125.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fakhoury M, et al. Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age. Drug Metab. Dispos. 2005;33:1603–1607. doi: 10.1124/dmd.105.005611. [DOI] [PubMed] [Google Scholar]
- 62.Chang Y, Moody DE, McCance-Katz EF. Novel metabolites of buprenorphine detected in human liver microsomes and human urine. Drug Metab. Dispos. 2006;34:440–448. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 63.Anagnostis EA, Sadaka RE, Sailor LA, Moody DE, Dysart KC, Kraft WK. Formulation of buprenorphine for sublingual use in neonates. J. Pediatr. Pharmacol. Ther. 2011;16:281–284. doi: 10.5863/1551-6776-16.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marek E, Kraft WK. Ethanol pharmacokinetics in neonates and infants. Curr. Ther. Res. Clin. Exp. 2014;76:90–97. doi: 10.1016/j.curtheres.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marek E, et al. [Accessed 5 September 2017];Ethanol pharmacokinetics in neonates secondary to medication administration. 2015 < http://jdc.jefferson.edu/petposters/1/>.
- 66.American Academy of Pediatrics. Ethanol in liquid preparations intended for children. Pediatrics. 1984;73:405–407. [PubMed] [Google Scholar]
- 67.Xie HG, Cao YJ, Gauda EB, Agthe AG, Hendrix CW, Lee H. Clonidine clearance matures rapidly during the early postnatal period: A population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J. Clin. Pharmacol. 2011;51:502–511. doi: 10.1177/0091270010370587. [DOI] [PubMed] [Google Scholar]
- 68.Liu T, Lewis T, Gauda E, Gobburu J, Ivaturi V. Mechanistic population pharmacokinetics of morphine in neonates with abstinence syndrome after oral administration of diluted tincture of opium. J. Clin. Pharmacol. 2016;56:1009–1018. doi: 10.1002/jcph.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiles JR, et al. Pharmacokinetics of oral methadone in the treatment of neonatal abstinence syndrome: A pilot study. J. Pediatr. 2015;167:1214–1220.e3. doi: 10.1016/j.jpeds.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall ES, Meinzen-Derr J, Wexelblatt SL. Cohort analysis of a pharmacokinetic-modeled methadone weaning optimization for neonatal abstinence syndrome. J. Pediatr. 2015;167:1221–1225.e1. doi: 10.1016/j.jpeds.2015.09.038. [DOI] [PubMed] [Google Scholar]