Abstract
Gestational diabetes mellitus (GDM) is defined as any degree of hyperglycaemia that is recognized for the first time during pregnancy. This definition includes cases of undiagnosed type 2 diabetes mellitus (T2DM) identified early in pregnancy and true GDM which develops later. GDM constitutes a greater impact on diabetes epidemic as it carries a major risk of developing T2DM to the mother and foetus later in life. In addition, GDM has also been linked with cardiometabolic risk factors such as lipid abnormalities, hypertensive disorders and hyperinsulinemia. These might result in later development of cardiovascular disease and metabolic syndrome. The understanding of the different risk factors, the pathophysiological mechanisms and the genetic factors of GDM, will help us to identify the women at risk, to develop effective preventive measures and to provide adequate management of the disease. Clinical trials have shown that T2DM can be prevented in women with prior GDM, by intensive lifestyle modification and by using pioglitazone and metformin. However, a matter of controversy surrounding both screening and management of GDM continues to emerge, despite several recent well-designed clinical trials tackling these issues. The aim of this manuscript is to critically review GDM in a detailed and comprehensive manner, in order to provide a scientific analysis and updated write-up of different related aspects.
Keywords: Diabetes in pregnancy, Diagnostic criteria for gestational diabetes mellitus, Gestational diabetes mellitus-related comorbidities, Genetics of gestational diabetes mellitus, Gestational diabetes mellitus, Lipids abnormalities in gestational diabetes mellitus, Management of gestational diabetes mellitus, Medical nutrition therapy, Pathophysiology of gestational diabetes mellitus, Risk factors for gestational diabetes mellitus
Core tip: Gestational diabetes mellitus (GDM) constitutes a greater impact on the overwhelming diabetes epidemic. The recent IADPSG revised criteria are considered landmark and evidence based approach in the evolution of screening and diagnosis of GDM. However, there is, still, no consensus on its application, mainly due to concerns related to the benefit of treatment in the additionally diagnosed women and the increased cost. Herein, the authors discuss screening and diagnostic criteria, risk factors, etiology and pathophysiology of GDM along with standard management in antenatal period and during labor.
INTRODUCTION
From a historical perspective, diabetes in pregnancy was considered as a fatal condition to both mother and foetus prior to the discovery of insulin in 1921[1]. In 1950 Hoet et al[2] described the neonatal and obstetric complications of hyperglycaemia in pregnancy. They reported that the “milieu interieur” at the time of foetal life was related with the characteristic features of the infant born. According to this theory , this milieu predisposes the child to obesity, hyperglycaemia and ultimately diabetes. Hoet et al[2] emphasized on the need to correct the “transitory hyperglycaemia of pregnancy” by insulin, to prevent “meta-gestational diabetes” in mother and metabolic consequences in the infant[2]. Even earlier than Hoet et al[2], Jorgen Pedersen reported that the maternal metabolic milieu of hyperglycaemia, increases the foetal blood glucose level and results in pancreatic islet hypertrophy, which in turn increases insulin secretion, consequently increasing also the glucose consumption by the foetus[3]. The Hyperglycemia and Adverse Pregnancy Outcome Study (HAPO), demonstrated that the increase in maternal glucose level was associated with increased umbilical C-peptide and infant’s body weight at birth[4].
DEFINITION AND CLASSIFICATION OF DIABETES IN PREGNANCY
The classification of abnormalities of glucose intolerance recognized in pregnancy, is necessary for both epidemiological and clinical purposes. The World Health Organization (WHO) in previous reports defined gestational diabetes mellitus (GDM) as either diabetes or glucose intolerance that is primarily detected during pregnancy. This loose definition of GDM includes a category of “severe hyperglycaemia” lacks a strong evidence basis, from randomized controlled clinical trials (RCTs)[5]. Several trials investigated the association between the glycaemic status of the mother and the outcome in both mother and foetus. However, these trials did not include the category of “severe hyperglycaemia” in their design. For instance, in HAPO study mothers with fasting blood glucose > 5.8 mmol/L and 2-h post oral glucose load > 11.1 mmol/L were excluded[4]. The Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS), excluded mothers with fasting blood glucose ≥ 7.0 mmol/L and 2-h post oral glucose load > 11.0 mmol/L[6]. Moreover, in a study from Landon et al[7], mothers with fasting blood glucose ≥ 5.3 mmol/L were also excluded from the trial. The term “overt diabetes” has been used by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) to describe the category of severe hyperglycaemia that was mimicking pre-existing diabetes (PED)[8].
The classic classification system of diabetes in pregnancy was initially developed by Dr. Priscilla White in 1949 and referred currently as the White’s classification. On the basis of age at onset, diabetes duration, metabolic, and vascular complications, Dr. White divided diabetes in pregnancy in classes from “A” (more favourable) to “F” (less favourable). The original White’s classification underwent multiple modifications, until 1980[9]. The first revision was done in 1965 by shifting vascular complications to “D” and adding class “R” which denotes the presence of proliferative retinopathy. In 1972 a further update was made in which, GDM was included in class “A” and class “D” was subdivided into five categories. The latest modification applied to the White’s classification includes addition of GDM as a distinct separate class and deletion of class “E” and “G”[9]. The American College of Obstetricians and Gynecologists (ACOG) proposed another classification for GDM, adding a note for the presence or absence of metabolic complications, doubting the usefulness of the White’s classification in clinical practice[10].
Currently, the term diabetes in pregnancy has been suggested to include all cases of hyperglycaemia observed during pregnancy comprising GDM and PED. The latter include pre-gestational type 2 diabetes mellitus (T2DM) and type 1 diabetes mellitus (T1DM)[11], and GDM is defined as any degree of hyperglycaemia that is recognized for the first time during pregnancy. This definition of GDM should be understood as to include cases of undiagnosed T2DM “overt diabetes” identified early in pregnancy and true GDM which develops later in pregnancy[4,7,11] (Figure 1).
Figure 1.
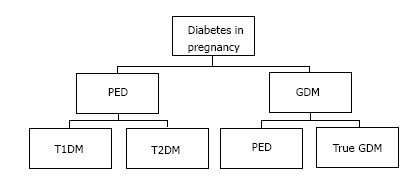
Classification of diabetes in pregnancy[4,7,11]. GDM: Gestational diabetes mellitus; PED: Pre-existing diabetes; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus.
UNIVERSAL VS SELECTIVE APPROACH OF GDM SCREENING
The screening for GDM, that was established 50 years ago, demonstrates the increased risk of hyperglycaemia during pregnancy[4], and the evidence supporting that effective treatment may reduce hyperglycemia related adverse pregnancy outcomes[6-8]. However, the decision of whether the screening for GDM should be performed in all pregnant women or selectively in women at high risk of developing T2DM was controversial.
Early screening for GDM is of particular importance, especially in women from population endemic in T2DM. However, early screening for GDM and increased rate of diagnosis is expected to increase psychological stress[11].
In the first antenatal visit, IADPSG recommends either universal or selective screening for women at high risk, to identify women with overt diabetes. In the second phase at 24-28 wk’ gestation the IADPSG recommends screening for GDM for all women, i.e., universal screening using 2-h 75g OGTT. Performing selective screening, as recommended by the IADPSG, in early pregnancy is uncontroversial among various expert groups. However, universal screening for all women, using a 75-g OGTT in late pregnancy, remains controversial[8]. The ADA[12] and the ADIPS[13] support universal screening, while the National institute for health and Clinical excellence (NICE)[14] and the Scottish Intercollegiate Guidelines Network (SIGN)[15], recommend selective screening for women with risk factors. Moreover, NICE recommends early screening with a 75-g OGTT in women with previous history of GDM, and at 24-28 wk’ gestation for those with risk factors[14].
Current guidelines suggest selective screening during pregnancy based on the presence of risk factors. However, there is no international agreement about which factors will best identify GDM risk, while some of them are defined differently. For instance, BMI > 30 kg/m2 is suggested by the NICE and SIGN[14,15], as a risk factor for GDM, while ADIPS suggest a BMI > 35 kg/m2[13], and ADA > 25 kg/m2[12]. Maternal age was only used by the ADIPS to identify women for selective screening but not by NICE, SIGN or ADA (Table 1)[12-15]. A retrospective observational study designed to assess the selective risk factors recommended by the NICE, ADIPS and ADA was undertaken in women who were screened for GDM using 2-h 75 g OGTT. The sensitivity and specificity of each screening guideline to identify GDM based on these predictors was calculated. The study reported that the most sensitive risk factors for GDM development were the increased maternal age, the increased body weight and past history of GDM. The ADA factors were found to have a sensitivity of 100% and specificity of 3.9%, whereas, both NICE and ADIPS factors had a superior specificity compared to ADA (NICE: 32.4%; ADIPS: 13.7%), but lower sensitivity (NICE: 92.7%; ADIPS: 98.6%). It is obvious that the number of women who would be screened for GDM is higher with the use of the ADA proposed risk factors compared to those proposed by the NICE and the ADIPS, therefor fewer women were exempted from screening, almost similar to the universal screening. On the other hand, less women would be targeted for screening with the use of NICE and ADIPS guidelines, thus, more women with GDM would be missed[16].
Table 1.
| NICE[14] and SIGN[15] |
| BMI > 30 kg/m2 |
| Previous history of macrosomic baby ≥ 4.5 kg |
| Previous history of GDM |
| Family history of diabetes (first-degree family member with diabetes) |
| Ethnic backgrounds |
| South Asian (India, Pakistan or Bangladesh) |
| Black Caribbean |
| Middle Eastern (Saudi Arabia, United Arab Emirates, Iraq, Jordan, Syria, Oman, Qatar, Kuwait, Lebanon or Egypt) |
| ADIPS[13] |
| Moderate risk factors for GDM |
| Ethnic backgrounds: Asian, Indian, Aboriginal, Torres Strait Islander, Pacific Islander, Maori, Middle Eastern, and non-White African |
| BMI 25-35 kg/m2 |
| High risk factors for GDM |
| Previous history of GDM |
| Previous history of high blood glucose |
| Age ≥ 40 yr |
| Family history of diabetes (1st degree relation with diabetes or a sister with GDM) |
| BMI > 35 kg/m2 |
| Previous history of macrosomic child ≥ 4.5 kg |
| PCOS |
| Medications: Corticosteroids, antipsychotics |
| ADA[12] |
| BMI > 25 kg/m2 |
| No physical activity |
| 1st degree relation with diabetes |
| Ethnic backgrounds (African-American, Latino, Native-American, Asian-American, Pacific Islander) |
| Previous macrosomic child > 9 lb |
| Previous history of GDM |
| Hypertension |
| HDL-C < 0.90 mmol/L and/or triglyceride > 2.82 mmol/L |
| PCOS |
| HbA1c ≥ 5.7% and previous IGT or IFG |
| Signs of insulin resistance such as acanthosis nigricans |
| History of CVD |
ADA: American Diabetes Association; HDL-C: High-density lipoprotein cholesterol; IFG: Impaired fasting glycaemia; IGT: Impaired glucose tolerance; NICE: National Institute for Health and Clinical Excellence; PCOS: Polycystic ovarian syndrome; SIGN: Scottish Intercollegiate Guidelines Network; ADIPS: Australasian Diabetes in Pregnancy Society; BMI: Body mass index; CVD: Cardiovascular disease; GDM: Gestational diabetes mellitus; HbA1c: Glycosylated haemoglobin.
RISK FACTORS FOR GDM
Common risk factors
Several risk factors have been implicated in the development of GDM. In general, these are similar to the factors associated with overt diabetes and include increased maternal age, obesity, ethnic background, family history of T2DM and a previous history of GDM. In addition, other risk factors include previous history of a macrocosmic baby, previous adverse pregnancy outcome, glycosuria, polyhydramios or large foetus in present pregnancy[16]. Among these risk factors, increased maternal weight is the most commonly evaluated reversible risk factor. In a nested case-control study, women who presented an increasing weight at a rate of 2.3-10.0 kg/year had a 2.5-times increased risk for GDM[17]. In a population-based study, women with PCOS had 2.4-times increased odds for GDM compared with women without PCOS[18]. Some medications used to treat other conditions, may also affect glucose intolerance increasing the risk for GDM[19,20]. Other reported risk factors include essential hypertension or gestational hypertension and multiple pregnancies[21].
Dietary risk factors
Several cross-sectional and retrospective studies have shown that consumption of macronutrient constituents of the diet during pregnancy may predict development of GDM[21]. Wang et al[22] demonstrated an independent significant relationship between reduced intake of polyunsaturated fat and development of GDM. In another study evaluating the effect of lifestyle behavior in white women, revealed a significant correlation of high consumption of saturated fat consumption and risk of GDM, whereas high consumption of polyunsaturated fat was associated with decreased risk for GDM[23]. Moreover, the prospective Nurses’ Health Study II (NHS-II) provided data for several dietary factors among female nurses in the United States in relation to risk for different diseases. In this study the “prudent dietary pattern” included an increased consumption of fruit, green leafy vegetables, poultry, and fish, in contrast to the “Western pattern” which included an increased consumption of red meat, processed meat, refined grain products, sweets, French fries, and pizza. Analyzing the dietary patterns and their association with the risk of GDM development in NHS-II trial, it was found a significant relative risk (RR) for GDM development with the increased intake of the Western diet and the decreased intake of prudent diet[24]. Other types of diet that influence the risk of GDM include high glycemic load and a low cereal-fiber diet[21,25].
Micronutrients have also been found to influence glucose tolerance. Zhang et al[26] studied the effect of ascorbic acid using data from the prospective OMEGA study. In this study a maternal level of ascorbic acid ≤ 55.9 μmol/L was found to be associated with a 3.1-times increased risk for GDM compared with maternal level of ≥ 74.6 μmol/L. Women whose vitamin C intake was < 70 mg per day were found to be associated with a 1.8-times increased risk for GDM compared with higher intakes[26]. The effect of vitamin D status on the risk of GDM was assessed in a nested case-control study from a prospective cohort of pregnant women. A maternal plasma level of 25-hydroxyvitamin D < 20 ng/mL was found in 33% of women diagnosed with GDM compared to 14% in the control group. After adjustment for other confounding factors, a maternal level of 25-hydroxyvitamin D < 20 ng/mL was found to be associated with a 2.66-times increased risk for GDM compared with the control group[27].
SCREENING AND DIAGNOSTIC CRITERIA FOR GDM
From a historical perspective, O'Sullivan et al[28,29] first to provide an evidence for the benefits of screening glycaemic abnormalities during pregnancy in women without history of diabetes. They suggested diagnostic criteria for GDM on the basis of a 3-h 100 g oral glucose tolerance test (OGTT) that have been justified to predict later development of diabetes and the risk of increased perinatal morbidity and mortality in pregnant women with GDM[28,29].
A significant debate has been shown to surround the issue of defining glucose abnormalities in pregnancy during the 50 years following the O’Sullivan and Mahan’s criteria. The major reason for this dispute in diagnosis is the existence of several diagnostic criteria and glycaemic cut-offs for detection of GDM.
O’Sullivan and Mahan diagnostic criteria for GDM
These were obtained from the results of a study carried out by O’Sullivan et al[28] in 1964 and included 752 pregnant women who screened for GDM using 3-h 100 g OGTT. Whole venous blood glucose rather than plasma glucose was estimated using Somogyi-Nelson measurements[30] in four samples (Table 2). Consequently, O’Sullivan et al[28,31] was able to predict the development of diabetes over a period of 7-8 years in 29% of women whose whole blood glucose values were more than two standard deviations above the mean. Accordingly, the cut-off values for diagnosis of GDM were estimated based on the mean plus two standard deviations rounded to the nearest 5 mg/dL. These two cut-off values were required to make the diagnosis (Table 2)[28,31].
Table 2.
Various diagnostic criteria for gestational diabetes mellitus and cut-off values
| Diagnostic criteria | Sample | WVB (mg/dL) | VP |
| O’Sullivan and Mahan (Women screened using 3-h 100 g OGTT and two cut-off values are required to diagnose GDM)[28,31] | Fasting | 90 | 90 mg/dL |
| 1 h | 165 | 165 mg/dL | |
| 2 h | 143 | 145 mg/dL | |
| 3 h | 127 | 125 mg/dL | |
| NDDG criteria (Women screened using 3-h 100 g OGTT and two cut-off values are required to diagnose GDM)[32] | Fasting | 90 | 105 mg/dL |
| 1 h | 170 | 190 mg/dL | |
| 2 h | 145 | 165 mg/dL | |
| 3 h | 125 | 145 mg/dL | |
| Carpenter and coustan criteria (Women screened using 3-h 100 g OGTT and two cut-off values are required to diagnose GDM)[33] | Fasting | 90 | 95 mg/dL |
| 1 h | 165 | 180 mg/dL | |
| 2 h | 143 | 155 mg/dL | |
| 3 h | 127 | 140 mg/dL | |
| WHO 1999 criteria (Women screened using 2-h 75 g OGTT and one cut-off value is required to diagnose GDM)[5] | Fasting | 126 mg/dL | |
| 2 h | 140 mg/dL | ||
| Recent IADPSG criteria (GDM) (Women screened using 2-h 75 g OGTT and one cut-off value is required to diagnose GDM)[8] | Fasting | 92 mg/dL | |
| 1 h | 180 mg/dL | ||
| 2 h | 153 mg/dL | ||
| Recent IADPSG criteria (Overt diabetes) (Women screened using 2-h 75 g OGTT and one cut-off value is required to diagnose GDM)[8] | Fasting | 126 mg/dL | |
| HbA1c | ≥ 6.5% | ||
| RPG | 200 mg/dL |
GDM: Gestational diabetes mellitus; RPG: Random plasma glucose; VP: Venous blood; WHO: World Health Organization; WVB: Whole venous blood; HbA1c: Glycosylated haemoglobin; IADPSG: International Association of Diabetes and Pregnancy Study Groups; NDDG: National Diabetes Data Group; OGTT: Oral Glucose Tolerance Test.
NDDG criteria
Following the O’Sullivan and Mahan study, glucose levels were measured in plasma rather than whole blood venous samples. Accordingly, the NDDG proposed cut-off values of glucose for GDM diagnosis were the same with the ones proposed by O’Sullivan and Mahan, converted from whole blood to plasma values (Table 2). In NDDG criteria, however, the 1-h blood glucose value changed from 165 mg/dL (O’Sullivan’s criteria) to 170 mg/dL without any clarification[32].
Carpenter and Coustan criteria
A further modification was also made to the original
O’Sullivan and Mahan diagnostic criteria by Carpenter and Coustan[33] in 1982. This modification was based on the fact that the whole blood glucose determined by the non-specific Somogyi-Nelson technique measures both glucose and other reducing constituents. In the late 1970s, glucose levels were measured using glucose oxidase technique. With this method glucose levels were approximately 5 mg/dL lower compared to Somogyi-Nelson technique[34]. Accordingly, Carpenter and Coustan[33] used the original O’Sullivan and Mahan values by subtracting 5 mg/dL from the blood glucose values to offset the difference in the analytic method used, and added 14% to offset the variation of changing from whole blood to plasma values[30,34] (Table 2).
The glucose cut-off values in the Carpenter and Coustan criteria were lower than that in O’Sullivan and the NDDG diagnostic criteria. This may explain, in part, the increasing prevalence of GDM in the years followed. Ferrara et al[35] demonstrated this theory in a cohort of multi-ethnic Northern California women who were not known to have diabetes before. The population was screened for GDM using 1-h 50 g OGTT and those who had a plasma glucose ≥ 140 mg/dL underwent further 3-h 100 g OGTT. In this cohort the prevalence of GDM appeared to be significantly increased when applying Carpenter and Coustan criteria compared to NDDG criteria[35].
WHO 1999 criteria
The WHO criteria include a 2-h 75 g OGTT test. This test was first introduced in the 1980s for type 2 diabetes and glucose abnormalities diagnosis. In particular, a 75 g of glucose were administrated orally to a pregnant woman following by an overnight fast of 8 to 14 h. Plasma glucose was measured in the fasting state, and 2 h later. Unlike the 3-h 100 g OGTT, 1 h and 3 h measurements were not required. Only one cut point was sufficient to diagnose GDM (Table 2)[5]. Compared to the O’Sullivan and Mahan diagnostic criteria, the WHO 1999, criteria were not evidence-based, as their cut-off values were selected arbitrary according to expert opinion and consensus. However, the validity of this test as screening tool was only evidenced recently after being used in the HAPO study[4].
Recent IADPSG criteria
In 1998, IADPSG organization was established. The main goal of this organization was to enable cooperation between different national and international societies with principal interest on diabetes in pregnancy. The evidence provided by the HAPO study, formed the basis of the recent IADPSG criteria for screening and diagnosis of GDM[8]. HAPO study included 25505 pregnant women from a diverse, heterogeneous, multinational population from 15 centers, designed to evaluate the risk of adverse outcomes related to the maternal glycaemic values that previously were considered normal. GDM was screened with a 2-h 75 g OGTT at 24- to 32-wk gestation[4]. Unlike previous studies, HAPO study was designed to evaluate the development of adverse pregnancy outcomes rather than future development of T2DM[4]. In 2008 the IADPSG consensus panel decided to set the level of glucose thresholds for GDM diagnosis based on the odds ratio of 1.75 relative to the mean for specific adverse outcomes. Accordingly, the glucose thresholds for diagnosis of GDM were calculated as the average glucose levels at which odds ratios for adverse outcomes have attained 1.75 times the estimated odds ratios for their development. The Consensus Panel members of the IADPSG also reviewed the data surrounding the issue of overt diabetes detected during pregnancy. Due to lack of evidence from well-designed RCTs they proposed diagnostic criteria based on expert opinion. The IADPSG recommended screening for the presence of this category during the first antenatal visit by performing FPG, random plasma glucose (RPG) or glycosylated haemoglobin (HbA1c). Only one abnormal value was proposed to be sufficient to diagnose overt diabetes (Table 2). A 2-h 75 g OGTT earlier than 24-28 wk of gestation was not routinely advised. However, it was proposed that all pregnant women should undertake this test at 24-28 wk of pregnancy excluding those with diagnostic results for overt diabetes or GDM at first antenatal visit. Upon performing 2-h 75 g OGTT at 24-28 wk gestation, GDM is recognized based on the cut-off values of FPG, 1-h plasma glucose, or 2-h plasma glucose (Table 2). It is noteworthy that, only one abnormal glucose value is sufficient to diagnose GDM, while a FPG value of more than 92 mg/dL in early pregnancy is sufficient to diagnose GDM[8].
Falavigna et al[36] reported significant benefits of screening and subsequent treatment of GDM with the use of IADPSG criteria compared to the previous WHO 1999 criteria. Consequently, the 2011 ADA guidelines recommended IADPSG criteria for screening and diagnosis of GDM[37]. However, three years later (2014) ADA guidelines, recommended either the “one-step” approach performed using 2-h 75-g OGTT, or the previous Carpenter and Coustan “two-step” approach using 1-h 50-g OGTT screening followed by 3-h 100-g OGTT[12]. The reason behind this change in ADA guidelines was addressed by the National Institutes of Health (NIH) as a reason for the increasing prevalence of GDM noting also the uncertainty related to benefit of treatment in the additionally diagnosed women as a result of the new criteria[38].
Cost-effectiveness of detecting GDM using the new criteria
Obviously the increased prevalence of GDM upon using the Carpenter and Coustan criteria would lead to greater health care cost. Studies demonstrated that women with GDM diagnosed by the Carpenter and Coustan criteria but not identified by the NDDG criteria had increased risk of adverse outcomes such as macrosomia, neonatal hypoglycaemia, and hyperbilirubinemia[35,39].
The IADPSG criteria have been adopted by various expert groups including the ADA[40], the WHO[5] and the Australasian Diabetes in Pregnancy Society (ADIPS)[13]. However, the concerns related to the benefit of treatment in the additionally diagnosed women and the increased cost of the health care services impedes its wide use. The recently conducted prospective St. Carlos Gestational Diabetes Study was designed to assess the cost-effectiveness of the “one-step” IADPSG, compared with the “two-step” Carpenter and Coustan criteria. The prevalence of GDM in the population evaluated by Carpenter and Coustan criteria was 10.6% and reached 35.5% when using the IADPSG criteria. This study demonstrated a reduction in the rate of gestational hypertension, prematurity, need for cesarean delivery, small for gestational age (SGA), Large for Gestational Age (LGA) and admission to Neonatal Intensive Care Unit (NICU) with the use of the new criteria. In addition, a total of €14358.06 per hundred women would be saved if IADPSG criteria were applied instead of Carpenter and Coustan criteria[41]. Therefore this recent evidence demonstrates improved pregnancy outcomes together with cost-effectiveness despite the rise in prevalence which may supports the use of the IADPSG criteria as an international standard approach.
EPIDEMIOLOGY
Epidemiology data are useful in both health care planning and cost savings. The prevalence of GDM has been progressively increasing and it reflects the background prevalence of obesity and T2DM in general population[42,43]. Higher rates of GDM were found to raise in parallel with higher rates of T2DM. This may be related to the common risk factors including obesity, physical inactivity, ethnic background and urbanization[44]. In a cohort of 123040 Northern Californian pregnant women without pre-existing diabetes, Hedderson et al[45] reported that the prevalence of GDM was low among non-Hispanic white women and African Americans, and high in Asians and Filipinas (Figure 2). Interestingly higher rates of GDM were demonstrated among those with lowest BMI (Asians and Filipinas) and lower rates were found in those with highest BMI (non-Hispanic white women and African Americans)[45].
Figure 2.
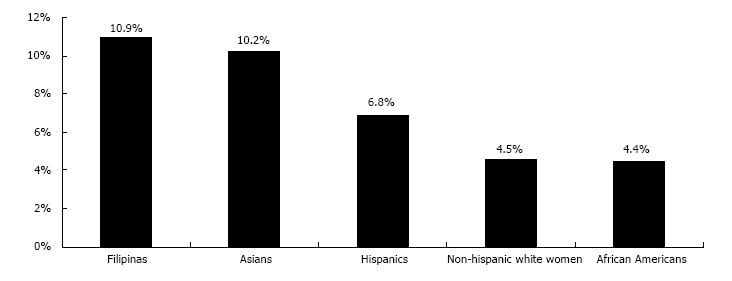
Ethnic variations in the prevalence of gestational diabetes mellitus[45].
The exact prevalence rate of GDM remains unknown and may differ widely based on the diagnostic criteria used for screening. In recent years the diagnostic criteria have changed considerably and there has been no agreement about which criteria to use. In the new IADPSG criteria only one value is sufficient to confirm the diagnosis and this may increase the prevalence of GDM to rates as high as 15%-20%[8].
Variation in prevalence rates could also be related to diversity of the populations being studied. In populations at low risk for GDM, such as in Sweden, the prevalence is less than 2%, while in those at high risk, such as the Indigenous American, Northern Californian Hispanics and Northern Californian Asians, the prevalence ranges from 4.9% to 12.8%[46]. Also higher rates were observed in Middle East countries such as in United Arab Emirates (20.6%), Qatar (16.3%), Bahrain (13.5%) and Saudi Arabia (12.5%). Some developed countries have also higher prevalence rates such as Canada (17.8%) and France (12.1%), but lower rates were observed in Australia (9.5%) and 4.8% in United States[46].
ETIOLOGY AND PATHOPHYSIOLOGY OF GDM
Pregnancy represents a complex metabolic and physiological condition that can be considered as a status of biological tolerance test which has the ability to detect insulin resistance earlier[11]. Insulin resistance in pregnancy could be the result of maternal obesity with varying degree of adipocytokine production, or increased production of diabetogenic placental hormones. In addition to insulin resistance, pancreatic β-cell dysfunction might also play a role in the pathophysiology of GDM (Table 3).
Table 3.
Etiology and pathophysiology of gestational diabetes mellitus
| Insulin resistance |
| Pregnancy and obesity as states of low grade inflammation[48,49] |
| Adipocytokines |
| ↓ Adiponectin[51-53] |
| ↑ TNF-α[55-58] |
| ↑ IL-6[56,62-64] |
| ↑ Leptin[56,58,65,66] |
| ↑ AFABP[79] |
| ↑ RBP-4[69] |
| ?↑ Resistin[54,62,72] |
| ?↑ Visfatin[76,77] |
| ? Novel adipocytokines (Vaspin, Apelin and Omentin)[50] |
| Endothelial function and angiogenic growth factors[74,80,81] |
| ↓ EPC |
| ↓ SOD |
| ↑ eNOS |
| ↑ PAI-1 |
| ↑ sEng |
| ↑ sICAM-1 |
| ↑ sVCAM-1 |
| ↑ t-PA |
| ↑ PLGF |
| ↑ sFlt-1 |
| Proteomics biomarkers |
| Haptoglobin, protein SMG8 and apoptosis inducing factor-1[83] |
| Apolipoprotein CIII[84] |
| Peptides precursors of clusterin, isoform 1 of fibrinogen alpha chain and apolipoprotein CII[85] |
| Glycosylated fibronectin[86] |
| Transthyretin–retinol binding protein-retinol complex[87] |
| Pancreatic β-cell dysfunction |
| Autoimmunity[92] |
| Glucokinase gene defect[93] |
AFABP: Adipocyte fatty acid-binding protein; eNOS: Endothelial nitric oxide synthase; sEng: Soluble endoglin; sFlt-1: Soluble fms-like tyrosine kinase-1; sICAM-1: Soluble intercellular adhesion molecule-1; sVCAM-1: Soluble vascular cell adhesion molecule-1; SOD: Superoxide dismutase; t-PA: Tissue plasminogen activator; TNF-α: Tumor necrosis factor-α; RBP-4: Retinol-binding protein-4; EPC: Endothelial progenitor cells; hPGH: Human placental growth hormone; hPL: Human placental lactogen; IL-6: Interleukin-6; PLGF: Placental growth factor; PAI-1: Plasminogen Activator Inhibitor type-1.
Obesity, pregnancy and inflammatory status
Obesity and overweight are nearly frequent findings among women in their childbearing years. In the United Kingdom 32% of women whose age ranges between 35-64 years old are overweight and 21% of them are obese[47]. Obesity is considered a state of chronic inflammation in which inflammatory markers are produced in excess to systemic circulation. These inflammatory markers influence alterations in post-receptor insulin signaling resulting in increased insulin resistance[48]. Moreover, pregnancy per se is an additional inflammatory condition in which there is physiological adaptation of the innate immune system to prevent rejection of the growing foetus. In normal pregnancy, the cytokines produced by humoral immunity significantly predominate in activity over that produced by cell-mediated immunity. This strong shifting towards humoral immunity has a beneficial role in maintaining a good relationship between mother and foetus at the expense of creating an inflammatory milieu that increases insulin resistance[49].
New potential mediators of insulin resistance
The adipose tissue is an endocrine organ that produces adipocytokines including pro- and anti-inflammatory mediators such as leptin, adiponectin, and resistin. Obesity is associated with an alteration in adipocytokines production from both adipocytes and macrophages. These inflammatory mediators may act locally to aggravate inflammation in adipose tissue, and increases peripheral insulin resistance. Altered adipocytokines production can also act centrally on the hypothalamus, promoting increased food intake and hyperglycemia. During pregnancy, adipocytokines have been shown to influence glucose tolerance via mechanisms interfering with regulation of insulin secretion and insulin receptor signaling and it explains, in part, the development of insulin resistance[50].
Adiponectin is an adipocytokine polypeptide that has anti-inflammatory properties and insulin sensitizing action. Williams et al[51] reported that a maternal level of adiponectin < 6.4 μg/mL was associated with a 4.6-times increased risk for GDM compared with control group. Ranheim et al[52] has also demonstrated reduced adiponectin mRNA levels in biopsies from abdominal subcutaneous adipose tissues and reduced plasma level in women with GDM, independently of obesity. A meta-analysis of 25 prospective studies reported that a level of adiponectin < 2.25 μg/mL in early pregnancy, was associated with significant risk for GDM compared with normal pregnant women[53].
Tumor necrosis factor-α (TNF-α) interferes with insulin receptor signaling and β-cell function having a greater influence in hyperglycaemia[50,54]. Three observational studies and one meta-analysis found that women with GDM had significantly higher levels of TNF-α compared with normal glycaemic pregnant women[55-58]. However, conflicting results have been reported in other studies[59-61]. This could be related to the smaller population sizes and the diversity in matching and adjustment for the confounding variables.
Interleukin-6 (IL-6); an inflammatory marker that has been found to be significantly higher in women with GDM, compared with normal women, independent of adiposity[56,62,63]. Similarly, a recent cross-sectional trial showed that IL-6 could be independently used to predict development of GDM when assessed in the first trimester[64].
Leptin is a protein hormone related to the bulk of fat stores[54] that has been reported significantly elevated in women with GDM compared with controls[56,58,65,66]. In a predictive risk model it was proposed that each 10 ng/mL increase of leptin levels, was associated with a 20% increase risk for GDM[66]. However, some trials reported conflicting results[67,68].
Retinol-binding protein-4 (RBP-4) a carrier for retinol (vitamin A) that has been demonstrated significantly increased in women with GDM compared with controls[69], while other studies found no significant differences[70,71]. These conflicting results may be related to the design of the studies, which were mostly cross-sectional, limiting their ability to detect a causal relationship.
Resistin is a peptide hormone related to energy homeostasis and has been shown to be elevated in women with GDM compared to normal pregnancies[54,62,72]. However, Megia et al[73] reported lower resistin levels in women with GDM compared with euglycaemic women while other studies reported no difference[74,75].
Visfatin is a protein related to glucose homeostasis that has been demonstrated to be significantly higher in women with GDM compared with controls[76,77]. However Chan et al[78] reported decreased serum visfatin in Chinese women with GDM.
Adipocyte Fatty Acid-Binding Protein (AFABP) has been shown to be significantly elevated in a cross-sectional study in women with GDM compared with controls[79].
Vaspin, apelin and omentin are novel adipokines with a controversial role in the pathogenesis of GDM. Some studies demonstrated increased levels, while others report unchanged or even decreased levels. Most of these trials were cross-sectional in design. The lack of controlled prospective trials are limiting further conclusions on their role on GDM[50].
Endothelial functions and angiogenic growth factors
Several studies have shown that endothelial function and angiogenic growth factors were altered in women with GDM. For instance, a case-control study demonstrated decreased total endothelial progenitor cells (EPC), decreased expression of superoxide dismutase (SOD), increased levels of soluble adhesion molecules (both sICAM-1and sVCAM-1) and increased endothelial nitric oxide synthase (eNOS) expression in women with GDM compared with controls. These alterations in endothelial function were also present in fetuses of GDM mothers which might infer an increased risk of future development of T2DM and CVD[80]. In one cross-sectional study, tissue plasminogen activator (t-PA) (reflect endothelial activation) was found significantly elevated in women with GDM[81]. Increased levels of Plasminogen Activator Inhibitor type-1 (PAI-1) have been also reported to be associated with increasing glucose levels in a subgroup of pregnant women from the HAPO study[74].
Proteomics and metabolomics for early prediction of GDM
Proteomics is a wide-ranging analysis of samples produced in humans using mass spectrometry, capable of generating huge data about proteins produced. In a recent review by Singh et al[82], several technologies were developed to identify protein biomarkers that serve as early predictors of GDM development. Wang et al[83] used Sodium Dodecyl Sulfate-Polyacrylamide Gel (SDS PAGE) and mass spectrometry (MS) tool to separate proteins from sera of women with GDM and hypertensive disorder. They identified haptoglobin, protein SMG8 and apoptosis inducing factor-1 as potential markers for GDM development. Kim et al[84] used surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) and MS to analyze serum samples from healthy women at 16-20 wk gestation. They demonstrated that apolipoprotein CIII was significantly higher in women who later developed GDM compared with controls, but there was no difference between the two groups when apolipoprotein AII level was investigated. Ai et al[85] used Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) followed by weak cation exchange magnetic bead (WCX-MB) to identify lower molecular weight peptides in healthy women at their 20 wk of gestation. Subsequently, they showed that three peptides were significantly different in women who later developed GDM compared with controls. These three peptides were demonstrated to be precursors of clusterin, isoform 1 of fibrinogen alpha chain and apolipoprotein CII[85]. Similarly, other studies using these novel techniques, have shown that glycosylated fibronectin[86] and the transthyretin-retinol binding protein-retinol complex[87] were early predictors of GDM, even before the onset of glucose intolerance.
Diabetogenic placental hormones
Ryan et al[88] provided evidence for the role of placental hormones in the induction of insulin resistance in pregnant rats. They suggested that increasing levels of progesterone, cortisol, prolactin and human placental lactogen (hPL) play a causal role in the insulin resistance during pregnancy, but whether they have the same effect in human pregnancy remains to be elucidated. hPL has been suggested to be a major contributory factor of insulin resistance in humans[89].
Pancreatic β-cell dysfunction
Another etiology for GDM was thought to be related to β-cell dysfunction that occurs on the setting of insulin resistance state[90]. Xiang et al[91] have shown a reduction of pancreatic β-cell function by 67% in women with GDM compared with normal glucose tolerance controls. This impairment of β-cell function was thought to be attributed to an autoimmune process[92]. However, Molęda et al[93] excluded autoimmunity as a cause of GDM based on negative anti-glutamic acid decarboxylase (anti-GAD) antibodies test and suggested a genetic defect which might be responsible for the β-cell secretory dysfunction.
GENETICS OF GDM
Studies demonstrated increased risk of GDM in women with family history of T2DM compared with those of matched controls[94]. Insulin resistance and insulin insufficiency were characteristic features of GDM, and both were partly shown hereditary in a recent study in twins[95].
GDM could be considered a polygenic, heterogeneous disease similar to T2DM in which multiple factors act together to cause the condition. The genes studied in relation to GDM are categorized as those related to insulin secretion, insulin and insulin receptors, insulin resistance and energy metabolism, human leukocyte antigen (HLA) and others (Table 4).
Table 4.
Genetic variants studied in relation to gestational diabetes mellitus
| Gene | Location | Variant | Association |
| Genes related to insulin secretion | |||
| ABCC8 | 11p15.1 | tagGCC allele of exon 16 and the AGG allele of the R1273R | Significant[97] |
| KCNJ11 | 11p15.1 | E23K | Significant[98] |
| UCP-2 | 11q13 | UCP2-866G> A | Controversial[98] |
| MT-ND1 | mtDNA | T3398C mutation | Significant[100] |
| TCF7L2 | 10q25.3 | rs7903146 | Significant[101,102] |
| GCK | 7p15.3-p15.1 | rs1799884 (-30G/A) | Significant[105-107] |
| HNF4A | 20q13.12 | rs2144908, rs2425637 and rs1885088 | No association[105] |
| HNF1A | 12q24.2 | rs1169288, rs1800574 | No association[105,108] |
| Genes of insulin and insulin receptors | |||
| INS | 11p15.5 | INS-VNTR class-III allele | Controversial[110,111] |
| INSR | 19p13.3-p13.2 | INSR allele-1 Kpn I RFLP | Significant[112] |
| IGF2 | 11p15.5 | IGF2 Bam HI RFLP | Significant[112] |
| IGF2BP2 | 3q27.2 | rs4402960 | Significant[113-115] |
| IRS1 | 2q36 | rs1801278 (Gly972Arg) | Controversial[98,107] |
| Genes of insulin resistance | |||
| PPARG | 3p25 | rs1801282 | No association[107] |
| PPARGC1A | 4p15.1 | rs8192678 | No association[101,118] |
| ADRB3 | 8p11.23 | rs4994 (Trp64Arg) | Controversial[101,194,119-121] |
| SLC2A1 | 1p34.2 | SLC2A1 Xba I RFLP | No association[112] |
| ADIPOQ | 3q27 | rs1501299 | No association[101] |
| FOXC2 | 16q24.1 | -512C allele | No association[101] |
| HLA genes | |||
| HLA | 6p21 | DR3 and DR4 | Controversial[122,123,125] |
| HLA | 6p21 | DR3-DQ2/X, DR4-DQ8/X with positive autoantibodies | Associated[124] |
| HLA | 6p21 | DR7-DQ2/X, DR9-DQ9/X and DR14-DQ5/X | Associated[124] |
| HLA | 6p21 | DQB1 alleles | Associated[111] |
| Other genes | |||
| CAPN10 | 2q37.3 | SNP-19, SNP-43, SNP-44, SNP-63) | No association[98,101] |
| HFE | 6p21.3 | C282Y in Northern and Central European women | Associated[127] |
| HFE | 6p21.3 | H63D | No association[127] |
| MBL2 | 10q11.2 | rs1800450 (Gly54Asp) | Significant[128] |
| MBL2 | 10q11.2 | rs5030737 (Arg52Cys) | No association[128] |
| SERPINE1 | 7q22.1 | -675 4G/5G | Could be associated[130] |
ABCC8: ATP-binding cassette transporter sub-family C member 8; ADIPOQ: Adiponectin ADRB3 adrenergic receptor β3; CAPN10: Calpaine 10; FOXC2: Forkhead box C2; GCK: Glucokinase; HFE: Haemochromatosis; HLA: Human leukocyte antigen; HNF4A: Hepatocyte nuclear factor 4 alpha; HNF1A: Hepatocyte nuclear factor 1 alpha; IGF2BP2: Insulin-like growth factor-2 mRNA-binding protein-2; IGF2: Insulin-like growth factor 2; IRS1: Insulin receptor substrate 1; INS: Insulin; INSR: Insulin receptor; KCNJ11: Potassium channel inwardly rectifying subfamily J member 11; MBL2: Mannose binding lectin 2; MT-ND1: Mitochondrial NADH dehydrogenase-1; PPARG: Peroxisome proliferator-activated receptor gamma; PPARGCIA: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; RFLP: Restriction fragment length polymorphism; SERPINE1: Serpin peptidase inhibitor, clade E, member 1; SLC2A1: Solute carrier family 2 (facilitated glucose transporter), member 1; SNP: Single nucleotide polymorphism; TCF7L2: Transcription factor 7-like 2; UCP-2: Uncoupling protein-2; VNTR: Variable number of tandem repeats.
Genes related to insulin secretion
KCNJ11 and ABCC8 genes: Pancreatic β-cell insulin secretion is dependent on the functional integrity of the K-ATP channel which is composed of eight subunits; four Kir6.2 subunits encoded by potassium channel inwardly rectifying subfamily J member 11 (KCNJ11) and four sulfonylurea receptor-1 (SUR1) subunits encoded by ATP-binding cassette transporter sub-family C member 8 (ABCC8)[96]. One study demonstrated that two variants of the ABCC8 gene; the tagGCC allele of exon 16 and the AGG allele of the R1273R were significantly associated with GDM compared with controls[97]. In a large case-control study, Shaat et al[98] reported a significant association between E23K genetic variant within the KCNJ11 gene and GDM compared with controls.
Uncoupling protein-2 gene: Uncoupling protein-2 (UCP-2) is a trans-membrane mitochondrial carrier protein which facilitates mitochondrial proton leak, thereby reducing ATP production resulting in inhibition of insulin secretion[99]. In a large case-control study, Shaat et al[98] showed no significant difference in the rate of the UCP2-866G>A variant within the UCP-2 gene in Scandinavian women with GDM compared with controls.
Mitochondrial NADH dehydrogenase-1 (MT-ND1) gene: In a case-control study, Chen et al[100] showed that the T3398C mutation within the MT-ND1 gene was significantly associated with GDM compared with normoglycaemic controls. This mutation may alter the function of NADH dehydrogenase resulting in impairment of the mitochondrial ETC with a subsequent reduction in insulin secretion.
Transcription factor 7-like2 (TCF7L2) gene: Two studies showed a significant association between the T allele of the rs7903146 genetic variant within the TCF7L2 gene and GDM compared with non-diabetic controls[101,102].
Glucokinase (GCK) (MODY2) gene: Two studies found no significant association between the rs1799884 (-30G/A) variant and risk of GDM[103,104]. However, two other recent large studies demonstrated a significant association[105,106]. Moreover, a meta-analysis including seven studies, also demonstrated a significant association between the rs1799884 variant and risk of GDM[107].
Hepatocyte nuclear factor 4 alpha (HNF4A, MODY1) gene: Three variants within the HNF4A, MODY1 gene (rs2144908, rs2425637 and rs1885088) were tested in a case-control study and were found not to be associated with risk of GDM[105].
Hepatocyte nuclear factor 1 alpha (HNF1A, MODY3) gene: Two variants within the (HNF1A, MODY3) gene were examined in two case-control studies. Shaat et al[105] found an association of the rs1169288 (Ile27Leu) variant and risk of GDM that was not statistically significant whereas Lauenborg et al[108] showed no association of the rs1800574 (Ala98Val) variant with GDM.
Genes of insulin and insulin receptors
Insulin (INS) gene: This gene contains a promoter region of variable number of tandem repeats (VNTR) with VNTR class-I allele occurs more frequently than VNTR class-III allele[109]. Litou et al[110] reported a significant association between the rate of VNTR class-III allele and risk for GDM. However Shaat et al[111] failed to demonstrate such an association.
Insulin receptor (INSR) gene: INSR gene restriction fragment length polymorphisms (RFLPs) was tested by Ober et al[112] and showed that, the INSR allele-1 Kpn I RFLP was significantly associated with GDM among black and Caucasian women. Interestingly, the influence of BMI on the risk of GDM was found to be significant only in individuals with positive INSR allele-1, which would suggest a possible role of the INSR allele-1 in obesity[112].
Insulin-like growth factor-2 (IGF2) gene: Ober et al[112] showed an increased risk of GDM in Caucasian women who are carriers of IGF2 Bam HI RFLP, but only in the presence of INSR allele-1.
Insulin-like growth factor-2 mRNA-binding protein-2 (IGF2BP2) gene: In a recent large study, Cho et al[113] showed a significant association between rs4402960; a genetic variant of the IGF2BP2 gene and risk of GDM in Korean women. Similarly, Wang et al[114] and Lauenborg et al[115] showed the same results among Chinese and Danish Caucasian women respectively.
Insulin receptor substrate 1 (IRS1) gene: Shaat et al[98] failed to demonstrate a significant association between the T-allele of the rs1801278 (Gly972Arg) variant and risk of GDM. However, in a meta-analysis of four studies, a significant association was demonstrated[107].
Genes of insulin resistance and energy metabolism
Peroxisome proliferator-activated receptor gamma (PPARG) gene: PPARG gene encodes production of a factor that is essential in the regulation of adipocytes, the differentiation and metabolism of lipids and glucose[116]. A meta-analysis of eight studies showed no association of PPARG/rs1801282 variant and risk for GDM compared with controls[107].
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) gene: PPARGC1A gene encodes production of a protein that plays a role in regulation of genes related to energy metabolic processes[117]. Two case-control studies among Scandinavian and Caucasian women demonstrated that the rs8192678 variant of the PPARGC1A gene is not associated with GDM[101,118].
Adrenergic receptor β3 (ADRB3) gene: One study showed a significant association between the rs4994 variant of the (ADRB3) gene and the risk of developing GDM among Caucasian women[119]. However, other studies failed to confirm this association among Scandinavian, Caucasian and Taiwanese women[101,120,121]. Similarly a meta-analysis of five studies also showed no association between the rs4994 variant and risk of GDM[107].
Solute carrier family 2 (facilitated glucose transporter), member 1 (SLC2A1) gene: Ober et al[112] failed to demonstrate an association between two fragments of the SLC2A1 gene Xba I RFLP and risk of GDM.
Adiponectin (ADIPOQ) gene and Forkhead box C2 (FOXC2) gene: Shaat et al[101] tested the rs1501299, a variant within ADIPOQ gene and -512C allele variant within FOXC2 gene in a large population of Scandinavian women and found no differences in variant rate among women with GDM and controls.
HLA genes
Freinkel et al[122] reported a significantly higher rate of HLA-DR3 and HLA-DR4 among black women with GDM. Similarly, Ferber et al[123] showed a significant association of the HLA-DR3 allele among GDM women who were positive to islet cell autoantibodies (ICA), and HLA-DR4 allele in those who were positive to glutamic acid decarboxylase autoantibodies (GAD65Ab). Another Swedish study showed increased frequency of HLA-DR3-DQ2/X or DR4-DQ8/X among women with GDM and positive autoantibodies, and HLA-DR7-DQ2/X, DR9-DQ9/X and DR14-DQ5/X among women with GDM and negative autoantibodies[124]. Moreover, Shaat et al[111] showed that HLA-DQB1 alleles were associated with increased risk of GDM among Scandinavian women. However Rubinstein et al[125] found no differences in variant rates of HLA-DR3 and HLA-DR4 among women with GDM and controls.
Other genes
Calpaine 10 gene: Two studies had tested the single nucleotide polymorphisms (SNPs) within the calpaine 10 (CAPN10) gene. Shaat et al[98] found no difference between the rate of SNP-43 and SNP-44 among women with GDM and controls and similar results were obtained by Leipold et al[126] when they tested SNP-43, SNP-19 and SNP-63 variants.
Haemochromatosis gene
In a case-control study, Cauza et al[127] tested both C282Y and H63D variants within haemochromatosis (HFE) gene in a large population of women. They showed an increased rate of C282Y but not H63D variant among Northern and Central European women with GDM compared with controls. However the rate of both variants did not differ between Southern and non-European women with GDM and controls[127].
Mannose-binding lectin 2 gene: The rate of Arg52 Cys variant within the mannose-binding lectin 2 (MBL2) gene in women with GDM does not differ from that in controls. However, the Gly54Asp variant was found significantly associated with increased risk of GDM compared with controls[128].
Serpin peptidase inhibitor, clade E, member 1 (SERPINE1) gene: The most common allele is in the promoter region at position -675 and has a run of 5 G's. The 4/G allele can result from deletion of one nucleotide and accordingly (4G/4G, 4G/5G, and 5G/5G) genotypes are recognized. The 5G/5G genotype is associated with decreased levels of PAI-1 activity[129]. Leipold et al[130] demonstrated a significantly higher rate of the 5G/5G genotype among women with normal fasting and glucose tolerance which may suggest a possible role of the -675 4G/5G variant in the increased risk of GDM.
ABNORMALITIES AND SIGNIFICANCE OF LIPIDS IN GDM
Brizzi et al[131] showed a significant increase in serum triglycerides, total cholesterol and LDL-C during normal pregnancy, with HDL-C level only slightly decreased. Metzger et al[132] showed an increase in serum triglyceride and plasma free fatty acids during the third trimester of pregnancy. However an elevation in serum cholesterol level did not show a significant difference. A meta-analysis of 60 studies showed significant elevation of triglycerides, non-HDL-C and decreased HDL-C level in women with GDM compared with control. Again, no difference was found in cholesterol or LDL-C levels between the two groups[133].
In addition, lipid abnormalities may also affect foetal growth similar to that of elevated plasma glucose. Kitajima et al[134] reported a significant association between maternal triglyceride levels at mid-term and the risk of foetal macrosomia. In a recent study, Son et al[135] related higher risk of developing large for gestational age (LGA) newborn to increased levels of maternal triglyceride. Moreover, in a prospective study of 150 pregnant women, maternal triglycerides and FFA levels were independent factors associated with LGA[136].
Lipid abnormalities might serve as predictors of later GDM development. In a cross-sectional study, Savvidou et al[81] showed that a decreased level of HDL-C at first trimester is predictive of later development of GDM.
GDM-RELATED COMORBIDITIES
Several studies demonstrated a clear association between maternal glucose levels and adverse consequences to both mother and foetus, and this continuous relationship has been also shown related to mild glucose levels[4,137]. These adverse consequences are independent of other factors such as BMI and increasing weight during pregnancy as shown in the HAPO study[4].
Maternal comorbidities
Hypertensive disorders: The reason behind development of hypertension in patients with diabetes is attributed to the effect of hyperinsulinemia on increasing weight, and renal sodium retention[138]. Hypertensive disorders during pregnancy are classified into three categories; chronic hypertension ,preeclampsia and gestational hypertension[139]. The HAPO study demonstrated that GDM women with the highest BMI had eight times the likelihood of developing preeclampsia as women with the lowest BMI[4]. Barden et al[140] showed increased risk of preeclampsia in women with GDM compared with controls. On the contrary, in a recent retrospective study, GDM and chronic hypertension were found protective against development of preeclampsia and gestational hypertension[141]. In the long-term hypertensive disorders carry the risk of developing T2DM, hypertension, metabolic syndrome and CVD[142].
Preterm birth: This is defined as infants born alive prior to 37 wk gestation[4,7]. In the HAPO study 6.9% of the total participants had preterm birth, but less frequently encountered than neonatal requirement for NICU (8.0%) and birth weight > 90th percentile (9.6%). Preterm birth was shown significantly associated with increased post OGTT maternal glucose but not fasting glucose levels[4].
Shoulder dystocia: Traumatic vaginal delivery resulting from delivery of large babies exposes the woman with GDM to operative procedures and episiotomies[143]. Shoulder dystocia may also occurs in infants weighing < 4.0 kg[138] and necessitates the use of operative procedures to deliver the shoulders[144]. In the HAPO study shoulder dystocia was less frequently encountered compared with other outcomes (1.3%) and was shown associated with increased post OGTT maternal glucose and also fasting glucose levels[4].
Caesarean delivery: Usually is required to overcome an adverse complication associated with GDM such as shoulder dystocia, and since it is a major surgical procedure, it carries the risk of complications such as infection, bleeding, thrombosis and wound dehiscence[144]. In the HAPO study 16.0% of the total participants had a primary Caesarean delivery and 7.7% had a repeated Caesarean delivery and both had been shown associated with increased post OGTT maternal glucose and fasting glucose levels[4]. The Toronto Trihospital study showed that Caesarean deliveries were still conducted despite reduced infant’s birth weights in treated women with GDM[39] which might suggest that GDM per se could also be an indicator for Caesarean delivery.
Long-term metabolic comorbidities in mothers: Uncontrolled hyperglycaemia in women with GDM constitutes a status of an increased risk of developing T2DM later in life[145,146]. This had been observed by O’Sullivan and Mahan when they set the first cut-off values for diagnosis of GDM based on the ability of these values to predict later development of T2DM[28,29]. In a recent meta-analysis, Bellamy et al[145] demonstrated that women with GDM had 7.43 times the likelihood of developing T2DM as pregnant women without GDM.
Neonatal comorbidities
Neonatal hypoglycaemia occurs as a result of foetal hyperinsulinaemia in response to exposure to high glucose levels from the mother[144]. It can occur even if mothers were not treated during pregnancy as was evident in the ACHOIS[6] and Landon et al[7] trials where insignificant difference between the occurrence of neonatal hypoglycaemia in women treated with insulin and in untreated women was reported. In the HAPO study neonatal hypoglycaemia occurred in 2.1% of the total participants and was associated with increased post OGTT maternal glucose but not with fasting glucose levels[4].
Hyperbilirubinemia is likely to be related with increased foetal red cell mass stimulated by decreased oxygen consumption as a result of maternal hyperglycaemia and subsequent foetal hyperinsulinaemia[144]. Hyperbilirubinemia occurred in 8.3% of the HAPO’s population but relatively less associated with maternal OGTT glucose levels[4].
Macrosomia, was hypothetically described by Pedersen almost half a century ago as a consequence of foetal, hyperinsulinaemia in response to high trans-placental flow of glucose from the mother[3]. This assumption was clearly demonstrated in the HAPO study where increasing maternal glucose level was associated with increased umbilical C-peptide and infant’s body weight at birth[4].
Neonatal hypocalcaemia has been reported in newborn of pregnant women with preexisting diabetes and is likely to be related to hypomagnesaemia[147]. However, its occurrence in neonates born by women with GDM remains infrequent and of little clinical importance[148]. The cause of hypocalcaemia in women with GDM may be related to low vitamin D status in mothers[27].
Respiratory distress syndrome may be the consequence of foetal hyperinsulinaemia interfering with the effect of cortisol on surfactant synthesis[149]. One study showed no difference in the incidence of respiratory distress syndrome in newborns of women with GDM and those born to normoglycaemic women[150].
Other neonatal comorbidities associated with GDM, but to a lesser degree than preexisting diabetes, include hypertrophic cardiomyopathy[151] and major congenital malformations[152].
Long-term metabolic comorbidities in the offspring: The Pedersen hypothesis forms the basis for the current understanding of the effects of intrauterine hyperglycemia on foetal β-cell hypertrophy and adipose tissue and late development of obesity and T2DM in the offspring[3,146]. Children born by women with GDM had an eight times increased risk of developing diabetes or prediabetes when they are 19-27 years old compared with children born from women without GDM[146].
PREVENTION OF T2DM IN WOMEN WITH PRIOR HISTORY OF GDM
Identifying women with GDM at high risk of progressing to T2DM is a key element of early prevention plan. The FPG levels during pregnancy were found to be an important predictor of early postpartum conversion to diabetes[153], and also, as shown by Kim et al[154] as the most common factor linked to greater risk of progression to T2DM in the long-term. Area under the OGTT curve, as well as, the degree of glucose level post OGTT, and earlier diagnosis of GDM were also found to be well associated with early postpartum conversion to diabetes[153,154].
The Diabetes Prevention Program (DPP) study showed that intensive lifestyle modification and metformin at a dose of 850 mg twice daily, reduced the incidence of T2DM by 58% and 31% respectively[155]. This beneficial effect has been further confirmed in the long-term follow-up of the original DPP study: DPP Outcomes Study (DPPOS)[156]. In the Troglitazone in Prevention of Diabetes (TRIPOD) study, administration of troglitazone was found to reduce the incidence of T2DM by more than 50% among high-risk Hispanic women with prior GDM. Following withdrawal of troglitazone from the market, the intervention arm was stopped, but the effect of protection from diabetes had persisted for further eight months[157]. In addition, Hispanic women with prior GDM who had finished the TRIPOD study were asked to take part in the Pioglitazone in Prevention of Diabetes (PIPOD) study. After three years, pioglitazone was shown to reduce the incidence of T2DM at a rate of 4.3%/year compared with the rate of 12.1%/year of the original TRIPOD control group[158].
PREVENTION OF GDM
Obese women have a greater risk of GDM than women with normal body weight[17] and it seems logical that behavioral intervention in the form of dietary modification and exercise may result in either reduced risk for GDM or at least halt obesity related gestational comorbidities. However, the current state of evidence does not support this assumption and results were conflicting. For instance, in the United Kingdom Pregnancies Better Eating and Activity Trial (UPBEAT), Poston et al[159] did not found behavioral intervention (combination of both healthy diet and physical activity) reducing GDM incidence in obese pregnant women compared with standard antenatal care. Similarly, in the pilot study of Vitamin D And Lifestyle Intervention for GDM prevention (DALI), Simmons et al[160] demonstrated a 33% reduction in GDM incidence among obese pregnant women on healthy diet compared with physical activity group. However, this reduction was not significantly different between the two groups[160]. By contrast, in the more recent Finnish Gestational Diabetes Prevention Study (RADIEL), Koivusalo et al[161] showed that combined physical activity and dietary modification in obese pregnant women, reduced the incidence of GDM by 39%. The conflicting results obtained could be related to recruitment to RADIEL study of obese women who were identified as high risk on the basis of BMI ≥ 30 kg/m2 and/or presence of history of GDM. Previous RCTs on T2DM prevention have shown that the effect of lifestyle intervention was more pronounced in the high risk groups[155]. Another explanation of the results obtained in this study is that, unlike UPBEAT, all individuals recruited were only white women. Moreover, women with history of GDM comprise one-third of total population recruited in the RADIEL study, whereas they form only less than one-tenth in the DALI or UPBEAT studies. This fact means that more women with possible β-cell dysfunction rather than insulin resistance were participated in the RADIEL study[162].
ANTENATAL MANAGEMENT OF GDM
Benefits of treatment
Identification of women with GDM is of utmost importance in order to be engaged in a management plan aiming to reduce both foetal and maternal comorbidities. The ACHOIS[6] and Landon et al[7] trials were large RCTs designed to assess benefits of treating GDM. Both studies showed that treatment of GDM reduced the risk of adverse complications including macrosomia, LGA, shoulder dystocia and hypertensive disorders. In a systematic review of 3157 women from seven studies including the ACHOIS and Landon, the benefit of treatment was assessed using lifestyle and if necessary insulin interventions. An overall significant reduction in macrosomia, LGA, shoulder dystocia, preeclampsia and hypertensive disorders was demonstrated. The risk for perinatal mortality, admission to NICU and birth trauma were also reduced but not statistically significant[163].
In a recent meta-analysis of five RCTs and six retrospective studies the treatment of GDM was found to significantly reduce the risk of preeclampsia, macrosomia and shoulder dystocia but did not present any significant reduction in other comorbidities[164].
Components of antenatal management
Management of women with GDM during antenatal period should consist of medical nutrition therapy (MNT) and weight management, exercise, self-monitoring of blood glucose (SMBG) and pharmacological therapy if required. This should be followed by management during labor and post natal period[144].
MNT and weight management
Women with GDM should be counseled by a dietitian once diagnosis is made to initiate MNT which is the mainstay of any management plan. The aim is to attain normal glycaemic control without ketosis and foetal compromise along with maintenance of adequate weight gain based on prenatal BMI[144]. In determining an appropriate dietary intake for women with GDM, several studies were conducted to compare the different types of diet. Limited caloric intake had been widely recommended for obese women with GDM. This approach was shown detrimental in a randomized prospective study wherein a reduced total caloric intake from 2400 kcal/d to 1200 kcal/d resulted in a significant ketosis among obese women with GDM compared with controls[165]. On the other hand, a caloric intake > 25 kcal/kg per day would prevent both ketosis and foetal growth compromise in obese women[166].
For this reason the ACOG recommends a reduction of the caloric intake to approximately 24 kcal/kg per day for pregnant women with > 120% of the normal body weight[167]. The caloric requirements, composition and distribution throughout the day were also defined by the ACOG (Table 5)[167]. Monitoring weight changes is important to ensure adequacy of dietary therapy and to maintain a weight gain within the recommended rates. The guidelines of the institute of medicine (IOM) in America regarding weight gain were released to assist in prevention of adverse pregnancy outcomes. The IOM recommended a weight gain during pregnancy of 12.5-18 kg for underweight (BMI < 19.8 kg/m2), 11.5-16 kg for healthy women (BMI 19.8-26.0 kg/m2), 7-11.5 kg for overweight (BMI 26.0-29.0 kg/m2), and at least 7 kg for obese (BMI ≥ 29.0 kg/m2)[168].
Table 5.
The ACOG recommendations of the caloric requirements, composition and distribution throughout the day in pregnant women with diabetes[167]
| Caloric requirements | ||
| Normal BMI | 30-35 kcal/kg per day | |
| < 90% of Normal BMI | 30-40 kcal/kg per day | |
| > 120% of Normal BMI | 24 kcal/kg per day | |
| Caloric composition | ||
| Complex, high-fiber CHO | 40%-50% | |
| Proteins | 20% | |
| Unsaturated fats | 30%-40% | |
| Caloric distribution | ||
| Breakfast | 10%-20% | |
| Lunch | 20%-30% | |
| Dinner | 30%-40% | |
| Snacks | Up to 30% |
BMI: Body mass index; CHO: Carbohydrates.
In Langford et al[169] study, overweight women who gained weight within the IOM recommendations had a reduced risk for preeclampsia, Cesarean delivery, and macrosomia compared with controls. Whereas those who gained weight over the IOM recommendations had significantly increased risk for preterm birth, macrosomia, and Cesarean delivery[170].
Role of exercise
Exercise is associated with improved insulin sensitivity which might improve both fasting and postprandial glucose levels avoiding the use of insulin in some women with GDM[171]. Exercise has been shown reduce the need of insulin in women with GDM compared with controls[172]. Using data from the Norwegian Mother and Child Cohort (MoBa) study, Magnus et al[173] found that exercise reduces the risk of preeclampsia in pregnant women. Likewise, a case-control study showed that in women who underwent regular physical activity, the risk reduction of preeclampsia was 35% compared with controls[174]. The ADA recommends moderate physical activity as part of any management plan, provided clearance from medical or obstetrical problem[175].
SMBG
After the diagnosis of GDM physical activity and MNT is recommended. Additionally frequent SMBG is required to monitor the glycaemic control of the pregnant woman and to determine whether it is adequately achieved or there is need of initiating a pharmacological therapy[10]. It has been reported that frequent SMBG is associated with reduced risk of adverse outcomes[176]. Frequent SMBG based on postprandial rather than preprandial monitoring, has shown to be superior in improving glycaemic control in insulin treated women[177]. Continuous glucose monitoring (CGM) is a novel technology allowing a 24-h assessment of glucose levels. A recent prospective study among Chinese women with GDM has shown a significant improvement of the glycaemic control and a decreased risk of adverse outcomes with the use of CGM technology compared with controls[178].
Pharmacological treatment
Women with GDM who fail to maintain glycaemic targets with nutritional therapy, should initiate pharmacological treatment. In most cases human insulin is the first choice, while some insulin analogues, and certain oral agents may also be used[179]. Although insulin is usually indicated when glycaemic targets are exceeded, some reports from randomized trials suggest initiating insulin based only on foetal ultrasonic parameters, such as increased foetal abdominal girth[180,181]. Recently, Balsells et al[182] reported in a meta-analysis that ultrasound-guided management could result in a significant reduction of LGA and foetal macrosomia. In addition, it has been reported that ultrasound-guided management reduces the need for insulin treatment when foetal growth is normal, limiting also the risk of SGA[181].
Insulin
Certain types of insulin are used to treat diabetes in pregnancy. The dose and regimen used is determined based on the severity of hyperglycaemia. Women who have fasting hyperglycaemia may require a single nocturnal injection of NPH-insulin started at a dose 0.2 units/kg initially, while other women may require only prandial insulin to control post-meal glucose elevations. If both fasting- and post-meal glucose levels are elevated, then a regimen consisting of NPH-insulin twice daily along with short-acting or rapid-acting insulin analogue administered just prior to meals can maintain euglycaemia. In such case, the total daily dose is usually 0.7-1.0 units/kg, divided equally between NPH-insulin and prandial-insulin[179]. Insulin doses are adjusted to attain glycaemic targets and to avoid the risk of hypoglycaemia. Types of insulin that can be used during pregnancy include human insulin both short-acting and NPH-insulin and rapid-acting analogues (lispro and aspart). Long-acting insulin analogues are not extensively studied for use during pregnancy[179,183]. However, a recent RCT showed that insulin detemir was not inferior to NPH-insulin in safety and efficacy[184].
Oral agents
Systematic reviews and meta-analysis of several studies testing oral agents or insulin treatment in GDM have shown that both strategies present comparable safety and efficacy[185-187]. However, the long-term safety of using oral agents in GDM remains obscure[180,184].
Metformin has been shown similar to insulin results in achieving satisfactory glucose control, with no difference in perinatal outcome[187]. When used alone, metformin was found to be associated with less maternal weight gain but with more risk of preterm birth compared with insulin treatment. Furthermore, compared with glyburide, metformin treatment was associated with less macrosomia and less maternal weight gain[186]. In one RCT, Niromanesh et al[188] demonstrated a significant reduction in maternal weight gain in women treated with metformin compared with women who received insulin (P < 0.001). In addition, a lower rate of birth weight was reported in the neonates born to women in metformin arm compared with women in insulin therapy, but the difference was not significant. In a meta-analysis of six studies involved 1420 women with GDM, Su et al[189] showed that treatment with metformin did not significantly increased the rate of neonatal and maternal comorbidities while it was associated with reduced weight gain and neonatal hypoglycaemia. In the same study, however, the use of metformin was associated with an increased risk of preterm birth[189].
Glyburide has also shown similar efficacy and outcomes with insulin[190,191]. However, a recent meta-analysis of 15 studies, demonstrated an increased risk of macrosomia and neonatal hypoglycaemia in women treated with glyburide compared to insulin[186]. Similarly, a recent retrospective study involved 110879 women with GDM, showed that neonatal comorbidities were more frequently encountered when women were treated with glyburide compared with women who received insulin[192]. In one RCT, Casey et al[193] demonstrated a significant reduction in fasting glycaemia of women with mild GDM who were treated with glyburide and nutritional therapy compared with placebo. It was also demonstrated that glyburide therapy in combination with diet did not show any benefit in reducing birth weight or improving maternal or neonatal comorbidities[193].
Recommendations of prominent professional bodies
The current ADA (2017)[194] and NICE (2015)[14] guidelines recommend that once GDM is diagnosed, treatment should start with MNT, exercise, weight gain monitoring and frequent SMBG. The glycaemic targets should be maintained at an FPG level of 95 mg/dL, 1-h postprandial of 140 mg/dL and 2-h postprandial of 120 mg/dL. If these targets are not achieved, a pharmacological intervention should be initiated. ADA recommends insulin treatment as the first-line pharmacologic therapy, while NICE suggests metformin in women with GDM who are not achieving glycaemic targets, provided that it is well tolerated and not contraindicated[14,194]. Although there is no clear guidance for their use, ADA recommends to consider both metformin and glyburide for the treatment of GDM, with concerns related to long-term safety as both medicines are crossing the placenta[194]. The NICE guidelines recommend insulin initiation as add-on therapy to the ongoing metformin and lifestyle measures if glycaemic targets were not met. However, insulin may be initiated with or without metformin and lifestyle; if an FPG ≥ 126 mg/dL, or an FPG 108-124 mg/dL is measured along with the presence of macrosomia or hydramnios. In addition, NICE suggests to consider glyburide in women who failed to achieve glycaemic targets with metformin and refusing to take insulin or in women intolerant to metformin[14]. ADA and NICE do not report any restriction on the type of insulin for use during pregnancy, while NICE prefer the use of NPH-insulin as the first choice for long-acting insulin and aspart or lispro for rapid-acting insulin analogues[14,194].
MANAGEMENT DURING LABOR AND POSTPARTUM
There is no general agreement on the timing and mode of delivery in women with GDM. However, induction of labor is beneficial in terms of avoiding late perinatal death and obstetric complications related to foetal overgrowth[195]. The ACOG recommend considering elective Caesarean delivery if the estimated foetal weight more than 4.5 kg to prevent birth trauma[167]. Insulin requirement during labor is generally decreased because of increased physical work and also because women may remain fasting for long time. Some women may also need glucose infusion to prevent ketosis[196]. Women who require pharmacological therapy during antenatal period may need insulin during labor to control hyperglycaemia and to check their blood glucose 2-hourly[144]. The Endocrine Society recommends maintaining glucose level in the range of 72-126 mg/dL during labor[197].
Following delivery, most women return back to their previous pre-gestational glycaemic levels soon afterwards. However, some women may continue with hyperglycaemia possibly representing the category of undiagnosed T2DM which is usually present early in pregnancy[144]. For this reason the Endocrine Society recommends to keep checking for glucose level until 72 h following delivery to rule out continuing hyperglycaemia. Treatment is then justified on individual basis if T2DM is diagnosed or, if not, it is recommended to perform a 2-h 75 g OGTT 6-12 wk following delivery to test for glucose intolerance or T2DM[197].
Breast feeding improves weight and glucose tolerance and should be encouraged[198]. Women should also be counseled for the method of contraception and the choices which include hormonal therapy or copper-releasing intrauterine device are acceptable. Hormonal therapy does not appear to increase the risk of developing diabetes[199].
CONCLUSION
To conclude, in the short-term, several maternal and foetal comorbidities were found to be associated with GDM. In the long-term GDM carries a major risk of developing T2DM later in life for both the mother and the offspring. Therefore GDM could be considered as an important health issue. However, a matter of controversy surrounding both the screening and the management of GDM continues to emerge. This may necessitates the need for further studies to demonstrate the benefits of a universal screening and the effects of treatment in reducing the risk of long- and short-term complications. The studies conducted to evaluate the risk factors associated with GDM were observational, thus, and difficult to conclude about possible causality. This was also the case when reviewing the etiology and pathophysiology of GDM, therefore, large prospective clinical trials are needed to extensively study the risk and etiological factors of GDM. Heritability is another important factor influencing the development of GDM, that has not yet clearly elucidated. The conclusions drawn from most of the trials conducted are limited mainly due to the lack of statistical power and the controversial results obtained. Well-designed trials looking for specific genes linkage, expression and association along with functional studies might be needed in the future. Identifying abnormal genes would help in a better understanding of the pathophysiology of GDM and in developing the “keys” for intervention and prevention. Moreover, women with GDM have increased risk of developing T2DM, metabolic syndrome and CVD and therefore identification of women with GDM may trigger the onset of early prevention strategies. There is no universal consensus on how to monitor and treat GDM in terms of caloric control, glycaemic targets and specific pharmacological interventions. However, it is unquestioned that further well-designed RCTs are required in the future to ascertain optimal glycemic targets, and appropriate monitoring of women to assess their risk status for later development of T2DM and CVD.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest for this paper.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 12, 2017
First decision: May 16, 2017
Article in press: October 31, 2017
P- Reviewer: Hill DJ, Savona-Ventura C, Sahoo JP S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
Contributor Information
AbdelHameed Mirghani Dirar, Prince Abdel Aziz Bin Musaad Hospital, Diabetes and Endocrinology Center, Arar 91421, North Zone Province, Saudi Arabia.
John Doupis, Iatriko Paleou Falirou Medical Center, Division of Diabetes and Clinical Research Center, Athens 17562, Greece; Postgraduate Diabetes Education, Institute of Molecular and Experimental Medicine, Cardiff University School of Medicine, Cardiff CF14 4XN, United Kingdom. john.doupis@joslin.harvard.edu.
References
- 1.Ramírez-Torres MA. The importance of gestational diabetes beyond pregnancy. Nutr Rev. 2013;71 Suppl 1:S37–S41. doi: 10.1111/nure.12070. [DOI] [PubMed] [Google Scholar]
- 2.Hoet JP, Lukens FD. Carbohydrate metabolism during pregnancy. Diabetes. 1954;3:1–12. doi: 10.2337/diab.3.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. [Google Scholar]
- 5.WHO Guidelines Approved by the Guidelines Review Committee. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 6.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 7.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacks DA, Metzger BE. Classification of diabetes in pregnancy: time to reassess the alphabet. Obstet Gynecol. 2013;121:345–348. doi: 10.1097/AOG.0b013e31827f09b5. [DOI] [PubMed] [Google Scholar]
- 10.ACOG technical bulletin. Diabetes and pregnancy. Number 200-December 1994 (replaces No. 92, May 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1995;48:331–339. [PubMed] [Google Scholar]
- 11.Chamberlain C, McNamara B, Williams ED, Yore D, Oldenburg B, Oats J, Eades S. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States. Diabetes Metab Res Rev. 2013;29:241–256. doi: 10.1002/dmrr.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Nankervis A, McIntyre HD, Moses RG, Ross GP, Callaway LK. Testing for gestational diabetes mellitus in Australia. Diabetes Care. 2013;36:e64. doi: 10.2337/dc12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Collaborating Centre for Women’s and Children’s Health (UK) Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. London: National Institute for Health and Care Excellence (UK); 2015. [PubMed] [Google Scholar]
- 15.Scottish Intercollegiate Guidelines Network. National clinical guideline 116: Management of diabetes in pregnancy. Edinburgh: SIGN; 2010. Available from: http://www.sign.ac.uk/pdf/sign116.pdf. [Google Scholar]
- 16.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol. 2011;51:26–30. doi: 10.1111/j.1479-828X.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 17.Hedderson MM, Williams MA, Holt VL, Weiss NS, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2008;198:409.e1–409.e7. doi: 10.1016/j.ajog.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo JC, Feigenbaum SL, Escobar GJ, Yang J, Crites YM, Ferrara A. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care. 2006;29:1915–1917. doi: 10.2337/dc06-0877. [DOI] [PubMed] [Google Scholar]
- 19.Bodén R, Lundgren M, Brandt L, Reutfors J, Kieler H. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69:715–721. doi: 10.1001/archgenpsychiatry.2011.1870. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JE, Smith RS, Lagrandeur R, Lorenz RP. Gestational diabetes mellitus in women receiving beta-adrenergics and corticosteroids for threatened preterm delivery. Obstet Gynecol. 1997;90:880–883. doi: 10.1016/s0029-7844(97)00544-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94:1975S–1979S. doi: 10.3945/ajcn.110.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Storlien LH, Jenkins AB, Tapsell LC, Jin Y, Pan JF, Shao YF, Calvert GD, Moses RG, Shi HL, et al. Dietary variables and glucose tolerance in pregnancy. Diabetes Care. 2000;23:460–464. doi: 10.2337/diacare.23.4.460. [DOI] [PubMed] [Google Scholar]
- 23.Bo S, Menato G, Lezo A, Signorile A, Bardelli C, De Michieli F, Massobrio M, Pagano G. Dietary fat and gestational hyperglycaemia. Diabetologia. 2001;44:972–978. doi: 10.1007/s001250100590. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia. 2006;49:2604–2613. doi: 10.1007/s00125-006-0422-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C. Risk factors for gestation diabetes-from an epidemiological standpoint. In: Kim C, Ferrara A, editors. Gestational diabetes during and after pregancy. London, United Kingdom: Springer-Verlag London Limited; 2010. pp. 71–81. [Google Scholar]
- 26.Zhang C, Williams MA, Sorensen TK, King IB, Kestin MM, Thompson ML, Leisenring WM, Dashow EE, Luthy DA. Maternal plasma ascorbic Acid (vitamin C) and risk of gestational diabetes mellitus. Epidemiology. 2004;15:597–604. doi: 10.1097/01.ede.0000134864.90563.fa. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, Williams MA. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 29.O’Sullivan JB, Charles D, Mahan CM, Dandrow RV. Gestational diabetes and perinatal mortality rate. Am J Obstet Gynecol. 1973;116:901–904. doi: 10.1016/s0002-9378(16)33834-0. [DOI] [PubMed] [Google Scholar]
- 30.O’sullivan JB, Kantor N. Variability of blood sugar levels with an automated method. Public Health Rep. 1963;78:1023–1029. [PMC free article] [PubMed] [Google Scholar]
- 31.O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895–900. doi: 10.1016/s0002-9378(16)33833-9. [DOI] [PubMed] [Google Scholar]
- 32.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 34.Mager M, Farese G. What is “true” blood glucose?a comparison of three procedures. Am J Clin Pathol. 1965;44:104–108. [PubMed] [Google Scholar]
- 35.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care. 2002;25:1625–1630. doi: 10.2337/diacare.25.9.1625. [DOI] [PubMed] [Google Scholar]
- 36.Falavigna M, Prestes I, Schmidt MI, Duncan BB, Colagiuri S, Roglic G. Impact of gestational diabetes mellitus screening strategies on perinatal outcomes: a simulation study. Diabetes Res Clin Pract. 2013;99:358–365. doi: 10.1016/j.diabres.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 Suppl 1:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, Minkoff HL, Poindexter B, Prosser LA, Sawaya GF, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013;29:1–31. [PubMed] [Google Scholar]
- 39.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? Toronto Trihospital Gestational Diabetes Investigators. JAMA. 1996;275:1165–1170. [PubMed] [Google Scholar]
- 40.American Diabetes Association. Standards of Medical Care in Diabetes¬2015. Diabetes Care. 2015;38:S1–S94. doi: 10.2337/dc14-2142. Available from: http://www.diabetes.teithe.gr/UsersFiles/entypa/STANDARDS%20OF%20MEDICAL%20CARE%20IN%20DIABETES%202015.pdf. [DOI] [PubMed] [Google Scholar]
- 41.Duran A, Sáenz S, Torrejón MJ, Bordiú E, Del Valle L, Galindo M, Perez N, Herraiz MA, Izquierdo N, Rubio MA, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care. 2014;37:2442–2450. doi: 10.2337/dc14-0179. [DOI] [PubMed] [Google Scholar]
- 42.Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, Posner SF, Callaghan WM. Diabetes trends among delivery hospitalizations in the U.S., 1994-2004. Diabetes Care. 2010;33:768–773. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstet Gynecol Clin North Am. 2007;34:173–199, vii. doi: 10.1016/j.ogc.2007.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care. 2012;35:1492–1498. doi: 10.2337/dc11-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Womens Health. 2011;3:367–373. doi: 10.2147/IJWH.S26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu CK, Teoh TG, Robinson S. Obesity in pregnancy. BJOG. 2006;113:1117–1125. doi: 10.1111/j.1471-0528.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 48.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 49.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 50.Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int J Mol Sci. 2015;16:13442–13473. doi: 10.3390/ijms160613442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 52.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 53.Bao W, Baecker A, Song Y, Kiely M, Liu S, Zhang C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: A systematic review. Metabolism. 2015;64:756–764. doi: 10.1016/j.metabol.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16:921–937. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 55.Kinalski M, Telejko B, Kuźmicki M, Kretowski A, Kinalska I. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–454. doi: 10.1055/s-2005-870238. [DOI] [PubMed] [Google Scholar]
- 56.Atègbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, Miled A, Grissa A, Jerbi M, Tabka Z, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 57.López-Tinoco C, Roca M, Fernández-Deudero A, García-Valero A, Bugatto F, Aguilar-Diosdado M, Bartha JL. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine. 2012;58:14–19. doi: 10.1016/j.cyto.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Zhao YH, Chen YP, Yuan XL, Wang J, Zhu H, Lu CM. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: a systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:926932. doi: 10.1155/2014/926932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asemi Z, Jazayeri S, Najafi M, Samimi M, Shidfar F, Tabassi Z, Shahaboddin M, Esmaillzadeh A. Association between markers of systemic inflammation, oxidative stress, lipid profiles, and insulin resistance in pregnant women. ARYA Atheroscler. 2013;9:172–178. [PMC free article] [PubMed] [Google Scholar]
- 60.McIntyre HD, Chang AM, Callaway LK, Cowley DM, Dyer AR, Radaelli T, Farrell KA, Huston-Presley L, Amini SB, Kirwan JP, Catalano PM; Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. Hormonal and metabolic factors associated with variations in insulin sensitivity in human pregnancy. Diabetes Care. 2010;33:356–360. doi: 10.2337/dc09-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jahromi AS, Zareian P, Madani A. Association of Insulin Resistance with Serum Interleukin-6 and TNF-α Levels During Normal Pregnancy. Biomark Insights. 2011;6:1–6. doi: 10.4137/BMI.S6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258–263. doi: 10.1080/09513590802653825. [DOI] [PubMed] [Google Scholar]
- 63.Morisset AS, Dubé MC, Côté JA, Robitaille J, Weisnagel SJ, Tchernof A. Circulating interleukin-6 concentrations during and after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2011;90:524–530. doi: 10.1111/j.1600-0412.2011.01094.x. [DOI] [PubMed] [Google Scholar]
- 64.Hassiakos D, Eleftheriades M, Papastefanou I, Lambrinoudaki I, Kappou D, Lavranos D, Akalestos A, Aravantinos L, Pervanidou P, Chrousos G. Increased Maternal Serum Interleukin-6 Concentrations at 11 to 14 Weeks of Gestation in Low Risk Pregnancies Complicated with Gestational Diabetes Mellitus: Development of a Prediction Model. Horm Metab Res. 2016;48:35–41. doi: 10.1055/s-0034-1395659. [DOI] [PubMed] [Google Scholar]
- 65.Chen D, Xia G, Xu P, Dong M. Peripartum serum leptin and soluble leptin receptor levels in women with gestational diabetes. Acta Obstet Gynecol Scand. 2010;89:1595–1599. doi: 10.3109/00016349.2010.514040. [DOI] [PubMed] [Google Scholar]
- 66.Qiu C, Williams MA, Vadachkoria S, Frederick IO, Luthy DA. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet Gynecol. 2004;103:519–525. doi: 10.1097/01.AOG.0000113621.53602.7a. [DOI] [PubMed] [Google Scholar]
- 67.Simmons D, Breier BH. Fetal overnutrition in polynesian pregnancies and in gestational diabetes may lead to dysregulation of the adipoinsular axis in offspring. Diabetes Care. 2002;25:1539–1544. doi: 10.2337/diacare.25.9.1539. [DOI] [PubMed] [Google Scholar]
- 68.Festa A, Shnawa N, Krugluger W, Hopmeier P, Schernthaner G, Haffner SM. Relative hypoleptinaemia in women with mild gestational diabetes mellitus. Diabet Med. 1999;16:656–662. doi: 10.1046/j.1464-5491.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 69.Ueland T, Dalsoren T, Voldner N, Godang K, Henriksen T, Bollerslev J. Retinol-binding protein-4 is not strongly associated with insulin sensitivity in normal pregnancies. Eur J Endocrinol. 2008;159:49–54. doi: 10.1530/EJE-07-0682. [DOI] [PubMed] [Google Scholar]
- 70.Krzyzanowska K, Zemany L, Krugluger W, Schernthaner GH, Mittermayer F, Schnack C, Rahman R, Brix J, Kahn BB, Schernthaner G. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia. 2008;51:1115–1122. doi: 10.1007/s00125-008-1009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khovidhunkit W, Pruksakorn P, Plengpanich W, Tharavanij T. Retinol-binding protein 4 is not associated with insulin resistance in pregnancy. Metabolism. 2012;61:65–69. doi: 10.1016/j.metabol.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 72.Chen D, Fang Q, Chai Y, Wang H, Huang H, Dong M. Serum resistin in gestational diabetes mellitus and early postpartum. Clin Endocrinol (Oxf) 2007;67:208–211. doi: 10.1111/j.1365-2265.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 73.Megia A, Vendrell J, Gutierrez C, Sabaté M, Broch M, Fernández-Real JM, Simón I. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur J Endocrinol. 2008;158:173–178. doi: 10.1530/EJE-07-0671. [DOI] [PubMed] [Google Scholar]
- 74.Lowe LP, Metzger BE, Lowe WL Jr, Dyer AR, McDade TW, McIntyre HD; HAPO Study Cooperative Research Group. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95:5427–5434. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lobo TF, Torloni MR, Gueuvoghlanian-Silva BY, Mattar R, Daher S. Resistin concentration and gestational diabetes: a systematic review of the literature. J Reprod Immunol. 2013;97:120–127. doi: 10.1016/j.jri.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, Schernthaner G. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 77.Ferreira AF, Rezende JC, Vaikousi E, Akolekar R, Nicolaides KH. Maternal serum visfatin at 11-13 weeks of gestation in gestational diabetes mellitus. Clin Chem. 2011;57:609–613. doi: 10.1373/clinchem.2010.159806. [DOI] [PubMed] [Google Scholar]
- 78.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, Tsai EM. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Kralisch S, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M, Fasshauer M. Serum levels of adipocyte fatty acid binding protein are increased in gestational diabetes mellitus. Eur J Endocrinol. 2009;160:33–38. doi: 10.1530/EJE-08-0540. [DOI] [PubMed] [Google Scholar]
- 80.Mordwinkin NM, Ouzounian JG, Yedigarova L, Montoro MN, Louie SG, Rodgers KE. Alteration of endothelial function markers in women with gestational diabetes and their fetuses. J Matern Fetal Neonatal Med. 2013;26:507–512. doi: 10.3109/14767058.2012.736564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59:3017–3022. doi: 10.2337/db10-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh A, Subramani E, Datta Ray C, Rapole S, Chaudhury K. Proteomic-driven biomarker discovery in gestational diabetes mellitus: a review. J Proteomics. 2015;127:44–49. doi: 10.1016/j.jprot.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Wang SS, Hu SW, Zhong M. [Identification of protein markers for gestational diabetes mellitus complicated by pregnancy-induced hypertensive syndrome] Nanfang Yike Daxue Xuebao. 2011;31:1224–1227. [PubMed] [Google Scholar]
- 84.Kim SM, Park JS, Norwitz ER, Lee SM, Kim BJ, Park CW, Jun JK, Kim CW, Syn HC. Identification of proteomic biomarkers in maternal plasma in the early second trimester that predict the subsequent development of gestational diabetes. Reprod Sci. 2012;19:202–209. doi: 10.1177/1933719111417889. [DOI] [PubMed] [Google Scholar]
- 85.Ai T, Chen F, Zhou S, Zhang J, Zheng H, Zhou Y, Hu W, Liu X, Li L, Lin J. Magnetic Bead-Based Serum Peptidome Profiling in Patients with Gestational Diabetes Mellitus. Biomed Res Int. 2015;2015:586309. doi: 10.1155/2015/586309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagalla SR, Snyder CK, Michaels JE, Laughlin MJ, Roberts CT, Balaji M, Balaji V, Seshiah V, Rao PV. Maternal serum biomarkers for risk assessment in gestational diabetes. A potential universal screening test to predict GDM status. Indian J Endocrinol Metab. 2015;19:155–159. doi: 10.4103/2230-8210.140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fruscalzo A, Londero AP, Driul L, Henze A, Tonutti L, Ceraudo M, Zanotti G, Berni R, Schweigert FJ, Raila J. First trimester concentrations of the TTR-RBP4-retinol complex components as early markers of insulin-treated gestational diabetes mellitus. Clin Chem Lab Med. 2015;53:1643–1651. doi: 10.1515/cclm-2014-0929. [DOI] [PubMed] [Google Scholar]
- 88.Ryan EA, Enns L. Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab. 1988;67:341–347. doi: 10.1210/jcem-67-2-341. [DOI] [PubMed] [Google Scholar]
- 89.Handwerger S, Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. 2000;13:343–356. doi: 10.1515/jpem.2000.13.4.343. [DOI] [PubMed] [Google Scholar]
- 90.Kaaja R, Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. Rev Diabet Stud. 2008;5:194–202. doi: 10.1900/RDS.2008.5.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes. 1999;48:848–854. doi: 10.2337/diabetes.48.4.848. [DOI] [PubMed] [Google Scholar]
- 92.Singh SK, Rastogi A. Gestational diabetes mellitus. Diabetes and Metabolic Syndrome. Clinical Research and Reviews. 2008;2:227–234. [Google Scholar]
- 93.Molęda P, Fronczyk A, Safranow K, Majkowska L. Adipokines and β-cell dysfunction in normoglycemic women with previous gestational diabetes mellitus. Pol Arch Med Wewn. 2015;125:641–648. doi: 10.20452/pamw.3043. [DOI] [PubMed] [Google Scholar]
- 94.Williams MA, Qiu C, Dempsey JC, Luthy DA. Familial aggregation of type 2 diabetes and chronic hypertension in women with gestational diabetes mellitus. J Reprod Med. 2003;48:955–962. [PubMed] [Google Scholar]
- 95.Poulsen P, Levin K, Petersen I, Christensen K, Beck-Nielsen H, Vaag A. Heritability of insulin secretion, peripheral and hepatic insulin action, and intracellular glucose partitioning in young and old Danish twins. Diabetes. 2005;54:275–283. doi: 10.2337/diabetes.54.1.275. [DOI] [PubMed] [Google Scholar]
- 96.Gloyn AL, Siddiqui J, Ellard S. Mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2006;27:220–231. doi: 10.1002/humu.20292. [DOI] [PubMed] [Google Scholar]
- 97.Rissanen J, Markkanen A, Kärkkäinen P, Pihlajamäki J, Kekäläinen P, Mykkänen L, Kuusisto J, Karhapää P, Niskanen L, Laakso M. Sulfonylurea receptor 1 gene variants are associated with gestational diabetes and type 2 diabetes but not with altered secretion of insulin. Diabetes Care. 2000;23:70–73. doi: 10.2337/diacare.23.1.70. [DOI] [PubMed] [Google Scholar]
- 98.Shaat N, Ekelund M, Lernmark A, Ivarsson S, Almgren P, Berntorp K, Groop L. Association of the E23K polymorphism in the KCNJ11 gene with gestational diabetes mellitus. Diabetologia. 2005;48:2544–2551. doi: 10.1007/s00125-005-0035-0. [DOI] [PubMed] [Google Scholar]
- 99.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y, Liao WX, Roy AC, Loganath A, Ng SC. Mitochondrial gene mutations in gestational diabetes mellitus. Diabetes Res Clin Pract. 2000;48:29–35. doi: 10.1016/s0168-8227(99)00138-2. [DOI] [PubMed] [Google Scholar]
- 101.Shaat N, Lernmark A, Karlsson E, Ivarsson S, Parikh H, Berntorp K, Groop L. A variant in the transcription factor 7-like 2 (TCF7L2) gene is associated with an increased risk of gestational diabetes mellitus. Diabetologia. 2007;50:972–979. doi: 10.1007/s00125-007-0623-2. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe RM, Allayee H, Xiang AH, Trigo E, Hartiala J, Lawrence JM, Buchanan TA. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes. 2007;56:1481–1485. doi: 10.2337/db06-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chiu KC, Go RC, Aoki M, Riggs AC, Tanizawa Y, Acton RT, Bell DS, Goldenberg RL, Roseman JM, Permutt MA. Glucokinase gene in gestational diabetes mellitus: population association study and molecular scanning. Diabetologia. 1994;37:104–110. doi: 10.1007/BF00428785. [DOI] [PubMed] [Google Scholar]
- 104.Allan CJ, Argyropoulos G, Bowker M, Zhu J, Lin PM, Stiver K, Golichowski A, Garvey WT. Gestational diabetes mellitus and gene mutations which affect insulin secretion. Diabetes Res Clin Pract. 1997;36:135–141. doi: 10.1016/s0168-8227(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 105.Shaat N, Karlsson E, Lernmark A, Ivarsson S, Lynch K, Parikh H, Almgren P, Berntorp K, Groop L. Common variants in MODY genes increase the risk of gestational diabetes mellitus. Diabetologia. 2006;49:1545–1551. doi: 10.1007/s00125-006-0258-8. [DOI] [PubMed] [Google Scholar]
- 106.Freathy RM, Hayes MG, Urbanek M, Lowe LP, Lee H, Ackerman C, Frayling TM, Cox NJ, Dunger DB, Dyer AR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the International Association of Diabetes and Pregnancy Study Groups. Diabetes. 2010;59:2682–2689. doi: 10.2337/db10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang C, Bao W, Rong Y, Yang H, Bowers K, Yeung E, Kiely M. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum Reprod Update. 2013;19:376–390. doi: 10.1093/humupd/dmt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauenborg J, Damm P, Ek J, Glümer C, Jørgensen T, Borch-Johnsen K, Vestergaard H, Hornnes P, Pedersen O, Hansen T. Studies of the Ala/Val98 polymorphism of the hepatocyte nuclear factor-1alpha gene and the relationship to beta-cell function during an OGTT in glucose-tolerant women with and without previous gestational diabetes mellitus. Diabet Med. 2004;21:1310–1315. doi: 10.1111/j.1464-5491.2004.01343.x. [DOI] [PubMed] [Google Scholar]
- 109.Bennett ST, Todd JA. Human type 1 diabetes and the insulin gene: principles of mapping polygenes. Annu Rev Genet. 1996;30:343–370. doi: 10.1146/annurev.genet.30.1.343. [DOI] [PubMed] [Google Scholar]
- 110.Litou H, Anastasiou E, Thalassinou L, Sarika HL, Philippou G, Alevizaki M. Increased prevalence of VNTR III of the insulin gene in women with gestational diabetes mellitus (GDM) Diabetes Res Clin Pract. 2007;76:223–228. doi: 10.1016/j.diabres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 111.Shaat N, Ekelund M, Lernmark A, Ivarsson S, Nilsson A, Perfekt R, Berntorp K, Groop L. Genotypic and phenotypic differences between Arabian and Scandinavian women with gestational diabetes mellitus. Diabetologia. 2004;47:878–884. doi: 10.1007/s00125-004-1388-5. [DOI] [PubMed] [Google Scholar]
- 112.Ober C, Xiang KS, Thisted RA, Indovina KA, Wason CJ, Dooley S. Increased risk for gestational diabetes mellitus associated with insulin receptor and insulin-like growth factor II restriction fragment length polymorphisms. Genet Epidemiol. 1989;6:559–569. doi: 10.1002/gepi.1370060502. [DOI] [PubMed] [Google Scholar]
- 113.Cho YM, Kim TH, Lim S, Choi SH, Shin HD, Lee HK, Park KS, Jang HC. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia. 2009;52:253–261. doi: 10.1007/s00125-008-1196-4. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y, Nie M, Li W, Ping F, Hu Y, Ma L, Gao J, Liu J. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLoS One. 2011;6:e26953. doi: 10.1371/journal.pone.0026953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lauenborg J, Grarup N, Damm P, Borch-Johnsen K, Jørgensen T, Pedersen O, Hansen T. Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab. 2009;94:145–150. doi: 10.1210/jc.2008-1336. [DOI] [PubMed] [Google Scholar]
- 116.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 117.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leipold H, Knoefler M, Gruber C, Huber A, Haslinger P, Worda C. Peroxisome proliferator-activated receptor gamma coactivator-1alpha gene variations are not associated with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:104–107. doi: 10.1016/j.jsgi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 119.Festa A, Krugluger W, Shnawa N, Hopmeier P, Haffner SM, Schernthaner G. Trp64Arg polymorphism of the beta3-adrenergic receptor gene in pregnancy: association with mild gestational diabetes mellitus. J Clin Endocrinol Metab. 1999;84:1695–1699. doi: 10.1210/jcem.84.5.5650. [DOI] [PubMed] [Google Scholar]
- 120.Fallucca F, Dalfrà MG, Sciullo E, Masin M, Buongiorno AM, Napoli A, Fedele D, Lapolla A. Polymorphisms of insulin receptor substrate 1 and beta3-adrenergic receptor genes in gestational diabetes and normal pregnancy. Metabolism. 2006;55:1451–1456. doi: 10.1016/j.metabol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Tsai PJ, Ho SC, Tsai LP, Lee YH, Hsu SP, Yang SP, Chu CH, Yu CH. Lack of relationship between beta3-adrenergic receptor gene polymorphism and gestational diabetes mellitus in a Taiwanese population. Metabolism. 2004;53:1136–1139. doi: 10.1016/j.metabol.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Freinkel N, Metzger BE, Phelps RL, Dooley SL, Ogata ES, Radvany RM, Belton A. Gestational diabetes mellitus. Heterogeneity of maternal age, weight, insulin secretion, HLA antigens, and islet cell antibodies and the impact of maternal metabolism on pancreatic B-cell and somatic development in the offspring. Diabetes. 1985;34 Suppl 2:1–7. doi: 10.2337/diab.34.2.s1. [DOI] [PubMed] [Google Scholar]
- 123.Ferber KM, Keller E, Albert ED, Ziegler AG. Predictive value of human leukocyte antigen class II typing for the development of islet autoantibodies and insulin-dependent diabetes postpartum in women with gestational diabetes. J Clin Endocrinol Metab. 1999;84:2342–2348. doi: 10.1210/jcem.84.7.5813. [DOI] [PubMed] [Google Scholar]
- 124.Törn C, Gupta M, Sanjeevi CB, Aberg A, Frid A, Landin-Olsson M. Different HLA-DR-DQ and MHC class I chain-related gene A (MICA) genotypes in autoimmune and nonautoimmune gestational diabetes in a Swedish population. Hum Immunol. 2004;65:1443–1450. doi: 10.1016/j.humimm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Rubinstein P, Walker M, Krassner J, Carrier C, Carpenter C, Dobersen MJ, Notkins AL, Mark EM, Nechemias C, Hausknecht RU, et al. HLA antigens and islet cell antibodies in gestational diabetes. Hum Immunol. 1981;3:271–275. doi: 10.1016/0198-8859(81)90023-9. [DOI] [PubMed] [Google Scholar]
- 126.Leipold H, Knöfler M, Gruber C, Haslinger P, Bancher-Todesca D, Worda C. Calpain-10 haplotype combination and association with gestational diabetes mellitus. Obstet Gynecol. 2004;103:1235–1240. doi: 10.1097/01.AOG.0000127790.15556.3d. [DOI] [PubMed] [Google Scholar]
- 127.Cauza E, Hanusch-Enserer U, Bischof M, Spak M, Kostner K, Tammaa A, Dunky A, Ferenci P. Increased C282Y heterozygosity in gestational diabetes. Fetal Diagn Ther. 2005;20:349–354. doi: 10.1159/000086811. [DOI] [PubMed] [Google Scholar]
- 128.Megia A, Gallart L, Fernández-Real JM, Vendrell J, Simón I, Gutierrez C, Richart C. Mannose-binding lectin gene polymorphisms are associated with gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:5081–5087. doi: 10.1210/jc.2004-0211. [DOI] [PubMed] [Google Scholar]
- 129.Eriksson P, Kallin B, van ‘t Hooft FM, Båvenholm P, Hamsten A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci USA. 1995;92:1851–1855. doi: 10.1073/pnas.92.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leipold H, Knoefler M, Gruber C, Klein K, Haslinger P, Worda C. Plasminogen activator inhibitor 1 gene polymorphism and gestational diabetes mellitus. Obstet Gynecol. 2006;107:651–656. doi: 10.1097/01.AOG.0000199953.27961.f9. [DOI] [PubMed] [Google Scholar]
- 131.Brizzi P, Tonolo G, Esposito F, Puddu L, Dessole S, Maioli M, Milia S. Lipoprotein metabolism during normal pregnancy. Am J Obstet Gynecol. 1999;181:430–434. doi: 10.1016/s0002-9378(99)70574-0. [DOI] [PubMed] [Google Scholar]
- 132.Metzger BE, Phelps RL, Freinkel N, Navickas IA. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care. 1980;3:402–409. doi: 10.2337/diacare.3.3.402. [DOI] [PubMed] [Google Scholar]
- 133.Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122:643–651. doi: 10.1111/1471-0528.13261. [DOI] [PubMed] [Google Scholar]
- 134.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24--32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97:776–780. doi: 10.1016/s0029-7844(01)01328-x. [DOI] [PubMed] [Google Scholar]
- 135.Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2010;89:700–704. doi: 10.3109/00016341003605677. [DOI] [PubMed] [Google Scholar]
- 136.Schaefer-Graf UM, Graf K, Kulbacka I, Kjos SL, Dudenhausen J, Vetter K, Herrera E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care. 2008;31:1858–1863. doi: 10.2337/dc08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moses RG, Calvert D. Pregnancy outcomes in women without gestational diabetes mellitus related to the maternal glucose level. Is there a continuum of risk? Diabetes Care. 1995;18:1527–1533. doi: 10.2337/diacare.18.12.1527. [DOI] [PubMed] [Google Scholar]
- 138.Salzer L, Yogev Y. Complications of gestational diabetes. Chapter 5. In: Petry CJ, editor. Gestational diabetes: Origins, complications, and treatment. Boca Raton: CRC Press. USA: Taylor and Francis Group; 2014. pp. 95–109. [Google Scholar]
- 139.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 140.Barden A, Singh R, Walters BN, Ritchie J, Roberman B, Beilin LJ. Factors predisposing to pre-eclampsia in women with gestational diabetes. J Hypertens. 2004;22:2371–2378. doi: 10.1097/00004872-200412000-00020. [DOI] [PubMed] [Google Scholar]
- 141.Esakoff TF, Rad S, Burwick RM, Caughey AB. Predictors of eclampsia in California. J Matern Fetal Neonatal Med. 2016;29:1531–1535. doi: 10.3109/14767058.2015.1057489. [DOI] [PubMed] [Google Scholar]
- 142.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 143.Nassar AH, Usta IM, Khalil AM, Melhem ZI, Nakad TI, Abu Musa AA. Fetal macrosomia (> or =4500 g): perinatal outcome of 231 cases according to the mode of delivery. J Perinatol. 2003;23:136–141. doi: 10.1038/sj.jp.7210877. [DOI] [PubMed] [Google Scholar]
- 144.Kim C. Gestational diabetes: risks, management, and treatment options. Int J Womens Health. 2010;2:339–351. doi: 10.2147/IJWH.S13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 146.Damm P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int J Gynaecol Obstet. 2009;104 Suppl 1:S25–S26. doi: 10.1016/j.ijgo.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 147.Demarini S, Mimouni F, Tsang RC, Khoury J, Hertzberg V. Impact of metabolic control of diabetes during pregnancy on neonatal hypocalcemia: a randomized study. Obstet Gynecol. 1994;83:918–922. doi: 10.1097/00006250-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 148.Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med. 1998;152:249–254. doi: 10.1001/archpedi.152.3.249. [DOI] [PubMed] [Google Scholar]
- 149.Bourbon JR, Farrell PM. Fetal lung development in the diabetic pregnancy. Pediatr Res. 1985;19:253–267. doi: 10.1203/00006450-198503000-00001. [DOI] [PubMed] [Google Scholar]
- 150.Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28:122–127. doi: 10.1016/s1701-2163(16)32068-0. [DOI] [PubMed] [Google Scholar]
- 151.Ullmo S, Vial Y, Di Bernardo S, Roth-Kleiner M, Mivelaz Y, Sekarski N, Ruiz J, Meijboom EJ. Pathologic ventricular hypertrophy in the offspring of diabetic mothers: a retrospective study. Eur Heart J. 2007;28:1319–1325. doi: 10.1093/eurheartj/ehl416. [DOI] [PubMed] [Google Scholar]
- 152.Balsells M, García-Patterson A, Gich I, Corcoy R. Major congenital malformations in women with gestational diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:252–257. doi: 10.1002/dmrr.1304. [DOI] [PubMed] [Google Scholar]
- 153.Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol. 2002;186:751–756. doi: 10.1067/mob.2002.121895. [DOI] [PubMed] [Google Scholar]
- 154.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 155.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 158.Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 160.Simmons D, Jelsma JG, Galjaard S, Devlieger R, van Assche A, Jans G, Corcoy R, Adelantado JM, Dunne F, Desoye G, et al. Results From a European Multicenter Randomized Trial of Physical Activity and/or Healthy Eating to Reduce the Risk of Gestational Diabetes Mellitus: The DALI Lifestyle Pilot. Diabetes Care. 2015;38:1650–1656. doi: 10.2337/dc15-0360. [DOI] [PubMed] [Google Scholar]
- 161.Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, Kaaja RJ, Pöyhönen-Alho M, Tiitinen A, Huvinen E, et al. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care. 2016;39:24–30. doi: 10.2337/dc15-0511. [DOI] [PubMed] [Google Scholar]
- 162.Simmons D, van Poppel MN; DALI consortium. UPBEAT, RADIEL, and DALI: what’s the difference? Lancet Diabetes Endocrinol. 2015;3:761. doi: 10.1016/S2213-8587(15)00318-6. [DOI] [PubMed] [Google Scholar]
- 163.Falavigna M, Schmidt MI, Trujillo J, Alves LF, Wendland ER, Torloni MR, Colagiuri S, Duncan BB. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract. 2012;98:396–405. doi: 10.1016/j.diabres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159:123–129. doi: 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 165.Magee MS, Knopp RH, Benedetti TJ. Metabolic effects of 1200-kcal diet in obese pregnant women with gestational diabetes. Diabetes. 1990;39:234–240. doi: 10.2337/diab.39.2.234. [DOI] [PubMed] [Google Scholar]
- 166.Algert S, Shragg P, Hollingsworth DR. Moderate caloric restriction in obese women with gestational diabetes. Obstet Gynecol. 1985;65:487–491. [PubMed] [Google Scholar]
- 167.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675–685. doi: 10.1097/00006250-200503000-00049. [DOI] [PubMed] [Google Scholar]
- 168.Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Nutrition During Pregnancy: Part I Weight Gain: Part II Nutrient Supplements. Washington (DC): National Academies Press (US);; 1990. Available from: https://www.ncbi.nlm.nih.gov/books/NBK235235/ [PubMed] [Google Scholar]
- 169.Langford A, Joshu C, Chang JJ, Myles T, Leet T. Does gestational weight gain affect the risk of adverse maternal and infant outcomes in overweight women? Matern Child Health J. 2011;15:860–865. doi: 10.1007/s10995-008-0318-4. [DOI] [PubMed] [Google Scholar]
- 170.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008;112:1015–1022. doi: 10.1097/AOG.0b013e31818b5dd9. [DOI] [PubMed] [Google Scholar]
- 171.Horton ES. Exercise in the treatment of NIDDM. Applications for GDM? Diabetes. 1991;40 Suppl 2:175–178. doi: 10.2337/diab.40.2.s175. [DOI] [PubMed] [Google Scholar]
- 172.de Barros MC, Lopes MA, Francisco RP, Sapienza AD, Zugaib M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2010;203:556.e1–556.e6. doi: 10.1016/j.ajog.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 173.Magnus P, Trogstad L, Owe KM, Olsen SF, Nystad W. Recreational physical activity and the risk of preeclampsia: a prospective cohort of Norwegian women. Am J Epidemiol. 2008;168:952–957. doi: 10.1093/aje/kwn189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 175.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–e167. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hawkins JS, Casey BM, Lo JY, Moss K, McIntire DD, Leveno KJ. Weekly compared with daily blood glucose monitoring in women with diet-treated gestational diabetes. Obstet Gynecol. 2009;113:1307–1312. doi: 10.1097/AOG.0b013e3181a45a93. [DOI] [PubMed] [Google Scholar]
- 177.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, Evans AT. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 178.Yu F, Lv L, Liang Z, Wang Y, Wen J, Lin X, Zhou Y, Mai C, Niu J. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99:4674–4682. doi: 10.1210/jc.2013-4332. [DOI] [PubMed] [Google Scholar]
- 179.Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118:1379–1393. doi: 10.1097/AOG.0b013e31823974e2. [DOI] [PubMed] [Google Scholar]
- 180.Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, Nobile de Santis M, Radaelli T, Motta G, Costa M, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes Metab. 2004;30:237–244. doi: 10.1016/s1262-3636(07)70114-3. [DOI] [PubMed] [Google Scholar]
- 181.Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, Bryne JD, Sutherland C, Montoro MN, Buchanan TA. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care. 2001;24:1904–1910. doi: 10.2337/diacare.24.11.1904. [DOI] [PubMed] [Google Scholar]
- 182.Balsells M, García-Patterson A, Gich I, Corcoy R. Ultrasound-guided compared to conventional treatment in gestational diabetes leads to improved birthweight but more insulin treatment: systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2014;93:144–151. doi: 10.1111/aogs.12291. [DOI] [PubMed] [Google Scholar]
- 183.Cheung NW. The management of gestational diabetes. Vasc Health Risk Manag. 2009;5:153–164. doi: 10.2147/vhrm.s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mathiesen ER, Hod M, Ivanisevic M, Duran Garcia S, Brøndsted L, Jovanovic L, Damm P, McCance DR; Detemir in Pregnancy Study Group. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care. 2012;35:2012–2017. doi: 10.2337/dc11-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dhulkotia JS, Ola B, Fraser R, Farrell T. Oral hypoglycemic agents vs insulin in management of gestational diabetes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:457.e1–457.e9. doi: 10.1016/j.ajog.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 186.Balsells M, García-Patterson A, Solà I, Roqué M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. Obstet Gynecol Surv. 2015;70:305–307. doi: 10.1136/bmj.h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 188.Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract. 2012;98:422–429. doi: 10.1016/j.diabres.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 189.Su DF, Wang XY. Metformin vs insulin in the management of gestational diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;104:353–357. doi: 10.1016/j.diabres.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 190.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–1138. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 191.Moretti ME, Rezvani M, Koren G. Safety of glyburide for gestational diabetes: a meta-analysis of pregnancy outcomes. Ann Pharmacother. 2008;42:483–490. doi: 10.1345/aph.1K577. [DOI] [PubMed] [Google Scholar]
- 192.Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr, Jonsson Funk M. Association of Adverse Pregnancy Outcomes With Glyburide vs Insulin in Women With Gestational Diabetes. JAMA Pediatr. 2015;169:452–458. doi: 10.1001/jamapediatrics.2015.74. [DOI] [PubMed] [Google Scholar]
- 193.Casey BM, Duryea EL, Abbassi-Ghanavati M, Tudela CM, Shivvers SA, McIntire DD, Leveno KJ. Glyburide in Women With Mild Gestational Diabetes: A Randomized Controlled Trial. Obstet Gynecol. 2015;126:303–309. doi: 10.1097/AOG.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 194.American Diabetes Association. 13 Management of Diabetes in Pregnancy. Diabetes Care. 2017;40:S114–S119. doi: 10.2337/dc17-S016. [DOI] [PubMed] [Google Scholar]
- 195.Witkop CT, Neale D, Wilson LM, Bass EB, Nicholson WK. Active compared with expectant delivery management in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113:206–217. doi: 10.1097/AOG.0b013e31818db36f. [DOI] [PubMed] [Google Scholar]
- 196.Jovanovic L. Glucose and insulin requirements during labor and delivery: the case for normoglycemia in pregnancies complicated by diabetes. Endocr Pract. 2004;10 Suppl 2:40–45. doi: 10.4158/EP.10.S2.40. [DOI] [PubMed] [Google Scholar]
- 197.Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, Yogev Y. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yasuhi I, Soda T, Yamashita H, Urakawa A, Izumi M, Kugishima Y, Umezaki Y. The effect of high-intensity breastfeeding on postpartum glucose tolerance in women with recent gestational diabetes. Int Breastfeed J. 2017;12:32. doi: 10.1186/s13006-017-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database Syst Rev. 2012;(4):CD006133. doi: 10.1002/14651858.CD006133.pub4. [DOI] [PubMed] [Google Scholar]


