Abstract
Background and Purpose
GPR35 has long been considered an orphan GPCR, because no endogenous ligand of GPR35 has been discovered. CXCL17 (a chemokine) has been reported to be an endogenous ligand of GPR35, and it has even been suggested that it be called CXCR8. However, at present there is no supporting evidence that CXCL17 does interact with GPR35.
Experimental Approach
We applied two assay systems to explore the relationship between CXCL17 and GPR35. An AP‐TGF‐α shedding assay in GPR35 over‐expressing HEK293 cells was used as a gain‐of‐function assay. GPR35 knock‐down by siRNA transfection was performed in endogenously GPR35‐expressing THP‐1 cells.
Key Results
In the AP‐TGF‐α shedding assay, lodoxamide, a well‐known synthetic GPR35 agonist, was confirmed to be the most potent agonist among other reported agonists. However, neither human nor mouse CXCL17 had an effect on GPR35. Consistent with previous findings, G proteins Gαi/o and Gα12/13 were found to couple with GPR35. Furthermore, lodoxamide‐induced activation of GPR35 was concentration‐dependently inhibited by CID2745687 (a selective GPR35 antagonist). In endogenously GPR35‐expressing THP‐1 cells, lodoxamide concentration‐dependently inhibited migration and this inhibitory effect was blocked by CID2745687 treatment or GPR35 siRNA transfection. However, even though CXCL17 stimulated the migration of THP‐1 cells, which is consistent with a previous report, this stimulatory effect of CXCL17 was not blocked by CID2745687 or GPR35 siRNA.
Conclusions and Implications
The present findings suggest that GPR35 functions as a migration inhibitory receptor, but CXCL17‐stimulated migration of THP‐1 cells is not dependent on GPR35.
Abbreviations
- AP‐TGF‐α
alkaline phosphatase fusion protein of TGF‐α
- CXCL17
Chemokine (C‐X‐C motif) ligand 17
- GPR35
G protein coupled receptor 35
- LPA
lysophosphatidic acid
- PGE2
prostaglandin E2
Introduction
Human GPR35 was first described as an intronless GPCR gene encoding 309 amino acids (O'Dowd et al., 1998), and subsequently, it was reported to be expressed in lungs, stomach, small intestine, colon, spleen and immune cells (including mast cells, basophils, eosinophils and invariant natural killer‐like T cells) in man (Wang et al., 2006; Fallarini et al., 2010; Yang et al., 2010). For a considerable time, GPR35 was considered an orphan receptor as no endogenous ligand had been discovered. Several endogenous molecules, such as lysophosphatidic acid, kynurenic acid, cGMP and reverse T3, were proposed as ligands (Wang et al., 2006; Oka et al., 2010; Jenkins et al., 2011; Deng et al., 2012; Southern et al., 2013), but their potencies were too low (in the μM range) to support their roles as GPR35 ligands (Divorty et al., 2015). Synthetic surrogate agonists, such as zaprinast, pamoic acid, compound 1, PSB‐13253 and lodoxamide, have been successfully identified or developed (Taniguchi et al., 2006; Jenkins et al., 2010; Zhao et al., 2010; Funke et al., 2013; Neetoo‐Isseljee et al., 2013; Thimm et al., 2013; MacKenzie et al., 2014), and of these lodoxamide most potently interacts with human and rodent GPR35 (MacKenzie et al., 2014). Selective synthetic antagonists for GPR35 were also developed, that is, CID‐2745687 (also known as SPB05142 and ML194) and CID‐2786812 (ML145) (Zhao et al., 2010; Heynen‐Genel et al., 2010a,b; Jenkins et al., 2012). GPR35 has been reported to couple to both Gαi/o and Gα13 subunits and to recruit β‐arrestin‐2 (Wang et al., 2006; Guo et al., 2008; Ohshiro et al., 2008; Jenkins et al., 2010, 2011). Recently, CXCL17 (a chemokine) was reported to be an endogenous ligand of GPR35, and GPR35 was proposed to be CXCR8 (Maravillas‐Montero et al., 2015). Furthermore, it was reported that CXCL17 increases the intracellular Ca2+ concentration in THP‐1 cells (a human monocytic cell line) to promote chemotaxis at a concentration of 100 nM (Maravillas‐Montero et al., 2015). However, no supporting evidence has since been obtained with respect to the relationship between CXCL17 and GPR35, despite the fact that GPR35 is an emerging target in several pathological conditions, including inflammatory and cardiovascular diseases (Divorty et al., 2015). The results presented by Maravillas‐Montero et al. (2015) are intriguing but conflict with previous findings (Divorty et al., 2015). Accordingly, verification of this ligand–receptor interaction by another laboratory or using another assay system is required (Im, 2004). Therefore, we used two assay systems to verify the matching of CXCL17 and GPR35. An AP‐TGF‐α shedding assay in GPR35 overexpressing HEK293 cells was used as a gain‐of‐function assay, and a GPR35 knock‐down system, obtained by siRNA transfection, was applied to endogenously GPR35‐expressing THP‐1 cells as a loss‐of‐function assay.
Methods
AP‐TGF‐α shedding assay
HEK‐293 cells (ATCC, Manassas, VA, USA) were cultured at 37°C in a 5% CO2 humidified incubator and maintained in high glucose DMEM, containing 10% (v.v‐1) heat‐inactivated FBS, 100 U·mL−1 penicillin, 50 μg·mL−1 streptomycin, 2 mM glutamine and 1 mM sodium pyruvate.
HEK‐293 cells were seeded at a density of 2.0 × 105 cells·mL−1 in a 12‐well plate and, 16 h later, transfected with plasmids (an alkaline phosphatase fusion protein of TGF‐α, human GPR35 or Gα proteins) for 24 h using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. The next day, transfected HEK‐293 cells were re‐seeded in a 96‐well plate, ligands were added in a concentration‐dependent manner, and the plate was placed in an incubator for 1 h. Conditioned media were transferred into another empty 96‐well plate and then p‐NPP‐containing solution was added to the conditioned media plate and to the cell plate. Plates were measured for 405 nm at 0 and 1 h after treatment of p‐NPP‐containing solution. The ratio of the two absorbances was used as a measure of GPCR activation.
Culture of THP‐1 cells
Human THP‐1 cells (a human monocytic leukaemia cell line) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% heat‐inactivated FBS (HyClone, Logan, UT, USA), 100 U·mL−1 penicillin, 50 μg·mL−1 streptomycin, 2 mM glutamine, at 37°C in a humidified 5% CO2 incubator.
Migration assay
THP‐1 migration was measured using 24‐well Transwell inserts (6.5 mm) fitted with polycarbonate filters (5 μm pore size) (Corning Costar, Acton, MA, USA). THP‐1 cells (1 × 106 cells in 150 μL of RPMI 1640 medium) were added to the upper chamber, and 600 μL of RPMI 1640 medium containing the indicated concentrations of lodoxamide or human CXCL17 was added to the lower chamber. Plates were incubated at 37°C in 5% CO2 for 24 h, and the cells that migrated into the lower chamber were collected and counted using a haemocytometer.
Transfection
The following oligonucleotides were purchased from Bioneer (Korea); GPR35 siRNA sense 5′‐CCACAAAAGCCAGGACUCU(dTdT)‐3′, antisense 5′‐AGAGUCCUGGCUUUUGUGG(dTdT)‐3′. SN‐1003 from Bioneer was used as scrambled siRNA. Briefly, THP‐1 cells were seeded at a density of 1.0 × 105 cells in 12 well plates, and 24 h later, GPR35 siRNA (100 nM) was introduced to the cells using Lipofectamine LTX reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. Non‐silencing siRNA and siRNAs specific for GPR35 were transfected, and 48 h after transfection, target knock‐downs were confirmed by RT‐PCR and Western blotting.
Statistics
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Results are expressed as the means ± SEM for the number of determinations indicated. The statistical significances of differences were determined by ANOVA, and significance was accepted for P values <0.01. * Indicates significance compared to untreated controls and # compared to lodoxamide‐treated or negative control groups.
Materials
Lodoxamide was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada), and kynurenic acid was from Sigma‐Aldrich (St. Louis, MO, USA). Recombinant mouse CXCL17 and human CXCL17 were purchased from R&D system (Minneapolis, MN, USA), and zaprinast, CID‐2745687 and pamoic acid were from Tocris (Ellisville, Missouri, USA). Plasmids for the AP‐TGF‐α shedding assay, that is, an alkaline phosphatase fusion protein of TGF‐α, human GPR35 and eight Gα proteins, were kindly provided by Dr Junken Aoki at Tohoku University. The antibody for GPR35 (NBP2‐24640) was purchased from Novus Biologicals (Littleton, CO, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
Lodoxamide, but not CXCL17, activates GPR35 in HEK293 cells
As a gain‐of‐function assay, GPR35 was overexpressed in HEK293 cells and its activity was measured by AP‐TGF‐α shedding assay. AP‐TGF‐α shedding assay is generally considered the best system to test or verify functions of almost all GPCRs (Inoue et al., 2012). Lodoxamide (a synthetic GPR35 agonist) was found to be a more potent agonist than other reported agonists, such as pamoic acid, kynurenic acid and zaprinast (Figure 1A). EC50 values for lodoxamide, pamoic acid, zaprinast and kynurenic acid were 1 nM, 9 nM, 0.7 μM and 4.1 mM respectively. Furthermore, the lodoxamide‐induced activation of GPR35 was concentration‐dependently inhibited by CID2745687 (a selective GPR35 antagonist) (Figure 1B). AP‐TGF‐α shedding was observed when GPR35 was co‐transfected with Gαq/i1, Gαq/i3, Gαq/o, Gαq/12 or Gαq/13, but not with Gαq/s, Gαq/z or Gα16 (Figure 2), which suggests GPR35 can couple to Gαi1, Gαi3, Gαo, Gα12 or Gα13, which is consistent with the previously reported G protein coupling character of GPR35 to Gαi/o and Gα13 (Divorty et al., 2015). However, neither human nor mouse CXCL17 activated GPR35 (Figure 3), which contrasts with the previous report by Maravillas‐Montero et al. (2015).
Figure 1.
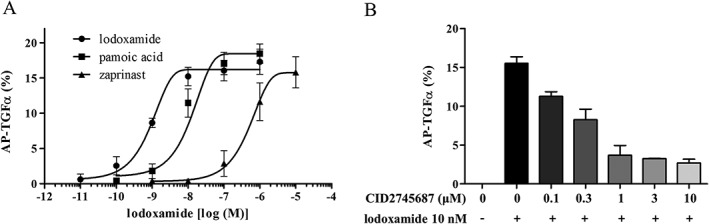
Lodoxamide induced the activation of GPR35, and this activation was inhibited by CID2745687 (a GPR35 selective antagonist). (A) Concentration–response curves for lodoxamide, pamoic acid and zaprinast as determined by the AP‐TGFα shedding assay. Agonism of GPR35 agonists was examined in GPR35 coexpressed with a mixture of eight Gα proteins in HEK‐293 cells. Results are presented as the means ± SEM of three individual experiments. (B) Inhibition of the lodoxamide‐induced GPR35 activation by CID2745687. Antagonism by CID2745687 was tested in the presence of 10 nM of lodoxamide. Results are presented as the means ± SEM of three individual experiments.
Figure 2.
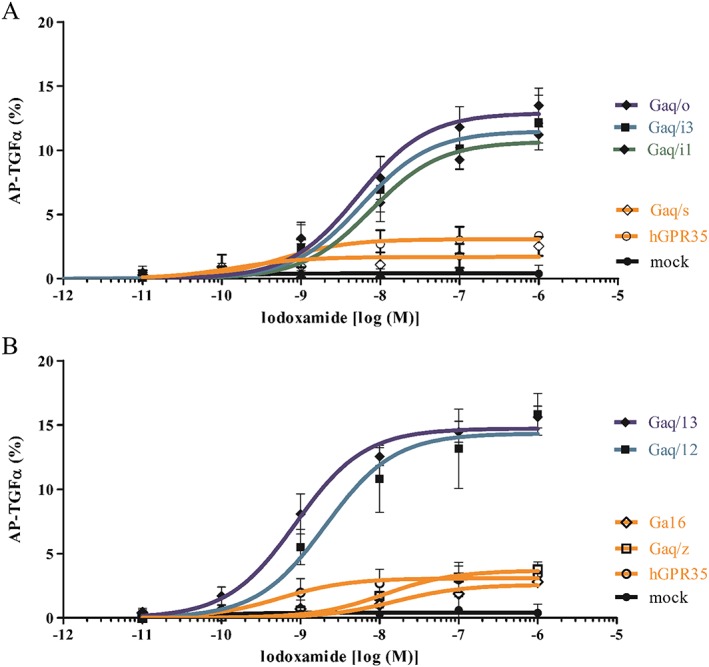
GPR35 coupling of G proteins. AP‐TGFα release responses were measured in human GPR35‐transfected HEK‐293 cells, in which eight Gα proteins were coexpressed individually. In Gαq/i1, Gαq/i3, Gαq/o, Gαq/12 or Gαq/13‐coexpressing cells, GPR35 induced potent AP‐TGFα shedding responses, whereas Gαq/s, Gαq/z or Gα16‐coexpressing cells did not. Results are presented as the means ± SEM of three individual experiments.
Figure 3.
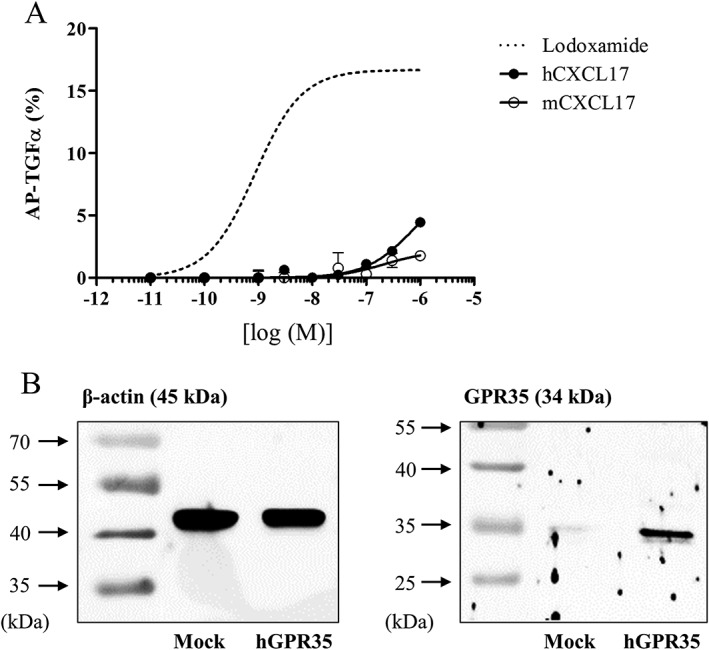
Human and mouse CXCL17 responses as determined by the AP‐TGF‐α shedding assay. (A) Effects of human and mouse CXCL17 in the AP‐TGFα shedding assay. Release of AP‐TGFα by both human and mouse CXCL17 was measured in HEK‐293 cells transfected with GPR35 and a mixture of eight Gα proteins. Results are presented as the means ± SEM of three individual experiments. Concentration–response curve for lodoxamide was duplicated from Figure 1 for a comparison. (B) HEK‐293 cells were transfected with plasmids of human GPR35 or mock plasmid along with AP‐TGF‐α and G proteins 24 h, and then, GPR35 proteins (B) were evaluated by Western blotting.
Lodoxamide inhibits the migration of THP‐1 cells through GPR35, but CXCL17‐stimulated migration is not dependent on GPR35
THP‐1 cells were used for the loss‐of‐function assay, because they express GPR35 endogenously and because its involvement in the action of CXCL17 action was observed only in these cells (Maravillas‐Montero et al., 2015). We observed that CXCL17 stimulated the migration of THP‐1 cells (Figure 4A), which is consistent with the previous findings (Maravillas‐Montero et al., 2015). However, the CXCL17‐induced simulation of migration was not blocked by treatment with the GPR35 antagonist CID2745687 (Figure 4B) or by GPR35 siRNA transfection (Figure 5C). In contrast, lodoxamide inhibited the migration of THP‐1 cells in a concentration‐dependent manner (Figure 4C), and this inhibition by lodoxamide was blocked by CID2745687 treatment (Figure 4D) or GPR35 siRNA transfection (Figure 5D). These findings suggest that GPR35 functions as a migration inhibitory receptor for lodoxamide in THP‐1 cells and that CXCL17 stimulates migration in a GPR35‐independent manner.
Figure 4.
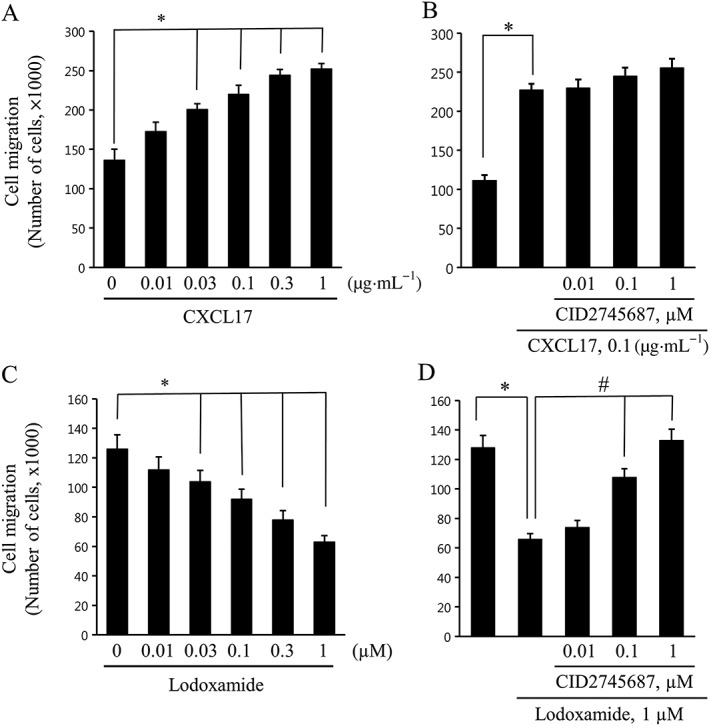
Migration responses to CXCL17 or lodoxamide in THP‐1 cells. (A and C) THP‐1 cells were added to upper Transwell chambers and allowed to migrate for 24 h toward lower chambers containing the indicated concentrations of CXCL17 or lodoxamide. (B and D) THP‐1 cells were pretreated with the indicated concentrations of CID2745687 for 1 h and then added to the upper chambers and allowed to migrate for 24 h toward lower chambers containing 0.1 μg·mL−1 CXCL17 or 1 μM lodoxamide. Results are presented as the means ± SEM of three or four individual experiments.
Figure 5.
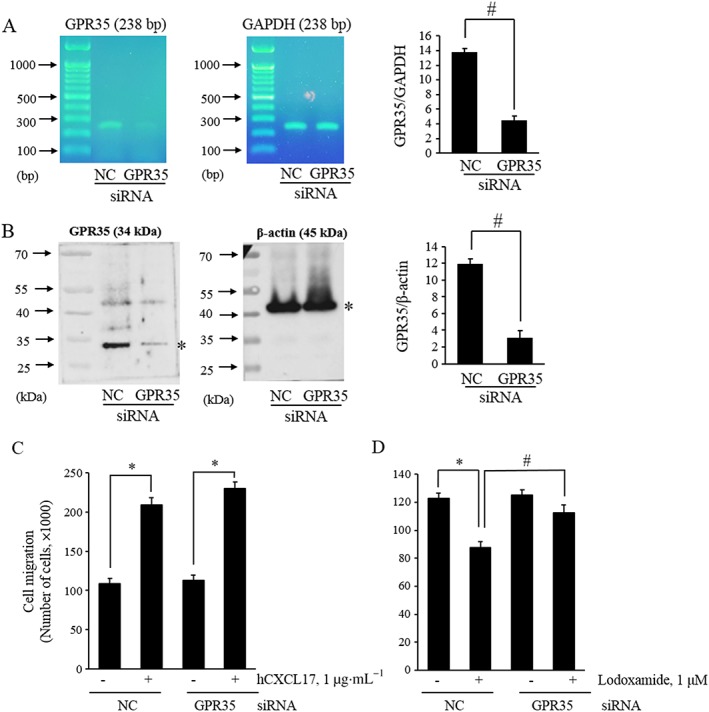
GPR35‐dependence of migration response to lodoxamide in THP‐1 cells. (A and B) THP‐1 cells were transfected with scrambled siRNA as negative control (NC) or GPR35 siRNA (GPR35) for 48 h, and then, the expressions of GPR35 mRNA (A) or protein (B) were evaluated by RT–PCR or Western blotting. (C and D) Transfected THP‐1 cells were added to the upper chambers and allowed to migrate for 24 h towards the lower chambers containing 0.1 μg·mL−1 CXCL17 or 1 μM lodoxamide. Results are presented as the means ± SEM of three individual experiments.
Discussion
Confirmation of ligand–receptor matching by another laboratory is important, because mismatching can cause considerable confusion, as exemplified by the mismatching of OGR‐1 and GPR4 with sphingosylphosphorylcholine and lysophosphatidylcholine respectively (Xu and Casey, 1996; Zhu et al., 2001, 2005; Bektas et al., 2003). However, when several assay systems support a ligand–receptor matching result, even in a single report, the result is usually reliable, for example, for the LPA‐GPR23 interaction (GPR23 was later renamed as the LPA4 receptor) (Noguchi et al., 2003; Im, 2004; Lee et al., 2007; Kihara et al., 2014).
In the case of CXCL17‐GPR35 matching, Maravillas‐Montero et al. reported that CXCL17 promotes the chemotaxis of THP‐1 cells in a pertussis toxin‐sensitive manner and that chemotaxis was enhanced in PGE2‐treated THP‐1 cells (Maravillas‐Montero et al., 2015). These findings support the notion that CXCL17 can induce chemotaxis and that its receptor might be coupled to pertussis toxin‐sensitive Gαi/o proteins (Maravillas‐Montero et al., 2015). Our data support the stimulatory effect of CXCL‐17 on migration in THP‐1 cells. However, GPR35 was not found to be involved. Increased expression of GPR35 in PGE2‐treated THP‐1 cells and reduced expression of GPR35 in the lungs of CXCL17 knockout mice were suggested to be indirect evidence of CXCL17‐GPR35 matching (Maravillas‐Montero et al., 2015), but these increased or decreased expression levels of GPR35 might have occurred coincidentally due to variable responses to CXCL17 (Maravillas‐Montero et al., 2015).
Maravillas‐Montero et al. also reported that CXCL17 increases the intracellular Ca2+ concentration in human THP‐1 cells (Maravillas‐Montero et al., 2015). Furthermore, CXCL17‐induced intracellular Ca2+ increase was observed in GPR35‐transfected BA/F3 cells and HEK293 cells (Maravillas‐Montero et al., 2015). However, these cells were not treated with a GPR35 antagonist or subjected to GPR35 knock‐down to confirm that GPR35 mediated this CXCL17‐induced Ca2+ response (Maravillas‐Montero et al., 2015). The report of Maravillas‐Montero et al. contrasts with data from previous studies, in which GPR35‐activation by other ligands did not promote coupling to Gαq pathway (Jenkins et al., 2011; 2012; Mackenzie et al., 2011; Maravillas‐Montero et al., 2015). Similarly, it was reported that lysophosphatidic acid induced and increase in Ca2+ in GPR35‐expressing HEK293 cells (Oka et al., 2010), although this was contradicted by subsequent reports (Oka et al., 2010; Southern et al., 2013; Divorty et al., 2015). In addition, we did not observe a CXCL17‐induced increase in intracellular Ca2+ in THP‐1 cells, although we did observe that ATP increased Ca2+ in these cells (Supporting Information Figure S1).
As mentioned above, pharmacological evidence for CXCL17‐GPR35 matching was inadequate in the previous study and was not supported by our findings. To summarize, CXCL17 did not activate GPR35 in the AP‐TGF‐α shedding assay, which provided a means of validating the effects of other GPR35 activators. Furthermore, CXCL17‐promoted migration was not blocked by CID‐2745687 (a GPR35 selective antagonist) or by GPR35 knock‐down in THP‐1 cells. However, lodoxamide inhibited migration in these THP‐1 cells, and this inhibitory effect was blocked by CID‐2745687 and by GPR35 knock‐down. Therefore, our findings indicate that GPR35 functions as a migration inhibitory receptor and that CXCL17 stimulates migration in a GPR35‐independent manner in THP‐1 monocytic cells.
There are two isoforms of human GPR35, that is, the short GPR35a and long GPR35b (Okumura et al., 2004). As shown in Supporting Information Figure S2, GPR35a is mainly expressed in THP‐1 cells and we used GPR35a. Because lodoxamide has been used as a mast cell stabilizer, we tested the selectivity of lodoxamide for GPR35 by applying the AP‐TGF‐α shedding assay in H1 histamine or LPA1 lysophosphatidic acid receptor overexpressing HEK293 cells. As shown in Supporting Information Figure S3, the H1 and LPA1 receptors were confirmed to be functional, but lodoxamide did not activate either receptor.
Author contributions
S.‐J.P. conducted the experiments of Figures 1 and 2. S.‐J.L. conducted the experiments of Figures 3 and 4. S.‐J.P. and S.‐Y.N. conducted the experiments for the Supporting Information figures. D.‐S.I. planned the experiments, analyzed data and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Effects of lodoxamide, CXCL17, and APT on intracellular Ca2 + concentrations in THP‐1 cells. A) Representative [Ca2+]itraces of THP‐1 cells treated with lodoxamide(100 nMor 1 mM), hCXCL17 (100 nM), mCXCL17 (100 nM), or ATP (100 mM). Arrows indicate when compounds were added.B) Histogram of Ca2 + responses. Results are presented as the means ± SEs of three independent experiments.
Figure S2 THP‐1 cells express GPR35a. RT‐PCR was conducted to detect GPR35a and GPR35b. THP‐1 cells expressed mainly GPR35a and we used GPR35a. Primersequences are followings. hGPR35a Forward GTGTTCGTGGTCTGCTTCCT, hGPR35b Forward GTCCTTGCGTCTCTCTGACC, hGPR35 Reverse GAGAGTCCTGGCTTTTGTGG.
Figure S3 Lodoxamide did not induce activation of H1 histamine or LPA1 lysophosphatidic acid receptor. Concentration‐response curves of histamine (A), LPA (B), and lodoxamide(C and D) as determined by the AP‐TGFα shedding assay in H1 or LPA1 coexpressed with a mixture of eight Gα proteins in HEK‐293 cells. Results are presented as the means ± SEs of 3 individual experiments.
Acknowledgements
This research was supported by a grant from the Basic Science Research Programme through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (#2016R1D1A1A009917086) and by a grant from the NRF funded by the Korean government (MSIP) (#2009‐0083538).
Park, S.‐J. , Lee, S.‐J. , Nam, S.‐Y. , and Im, D.‐S. (2018) GPR35 mediates lodoxamide‐induced migration inhibitory response but not CXCL17‐induced migration stimulatory response in THP‐1 cells; is GPR35 a receptor for CXCL17?. British Journal of Pharmacology, 175: 154–161. doi: 10.1111/bph.14082.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide To PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas M, Barak LS, Jolly PS, Liu H, Lynch KR, Lacana E et al (2003). The G protein‐coupled receptor GPR4 suppresses ERK activation in a ligand‐independent manner. Biochemistry 42 (12181–12191): 42. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Hu H, Fang Y (2012). Multiple tyrosine metabolites are GPR35 agonists. Sci Rep 2: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divorty N, Mackenzie AE, Nicklin SA, Milligan G (2015). G protein‐coupled receptor 35: an emerging target in inflammatory and cardiovascular disease. Front Pharmacol 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarini S, Magliulo L, Paoletti T, de Lalla C, Lombardi G (2010). Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun 398: 420–425. [DOI] [PubMed] [Google Scholar]
- Funke M, Thimm D, Schiedel AC, Muller CE (2013). 8‐Benzamidochromen‐4‐one‐2‐carboxylic acids: potent and selective agonists for the orphan G protein‐coupled receptor GPR35. J Med Chem 56: 5182–5197. [DOI] [PubMed] [Google Scholar]
- Guo J, Williams DJ, Puhl HL 3rd, Ikeda SR (2008). Inhibition of N‐type calcium channels by activation of GPR35, an orphan receptor, heterologously expressed in rat sympathetic neurons. J Pharmacol Exp Ther 324: 342–351. [DOI] [PubMed] [Google Scholar]
- Heynen‐Genel S, Dahl R, Shi S, Sauer M, Hariharan S, Sergienko E et al (2010a). Selective GPR35 antagonists – probes 1 & 2. In Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD). [PubMed]
- Heynen‐Genel S, Dahl R, Shi S, Sauer M, Hariharan S, Sergienko E et al (2010b). Selective GPR35 antagonists – probe 3. In Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD). [PubMed]
- Im DS (2004). Discovery of new G protein‐coupled receptors for lipid mediators. J Lipid Res 45: 410–418. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A et al (2012). TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat Methods 9: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Jenkins L, Brea J, Smith NJ, Hudson BD, Reilly G, Bryant NJ et al (2010). Identification of novel species‐selective agonists of the G‐protein‐coupled receptor GPR35 that promote recruitment of β‐arrestin‐2 and activate Gα13 . Biochem J 432: 451–459. [DOI] [PubMed] [Google Scholar]
- Jenkins L, Alvarez‐Curto E, Campbell K, de Munnik S, Canals M, Schlyer S et al (2011). Agonist activation of the G protein‐coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα13 and β‐arrestin‐2. Br J Pharmacol 162: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L, Harries N, Lappin JE, MacKenzie AE, Neetoo‐Isseljee Z, Southern C et al (2012). Antagonists of GPR35 display high species ortholog selectivity and varying modes of action. J Pharmacol Exp Ther 343: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Maceyka M, Spiegel S, Chun J (2014). Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br J Pharmacol 171: 3575–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Dubin AE, Chun J (2007). LPA4/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing Gs‐, Gq/Gi‐mediated calcium signaling and G12/13‐mediated Rho activation. J Biol Chem 282: 4310–4317. [DOI] [PubMed] [Google Scholar]
- Mackenzie AE, Lappin JE, Taylor DL, Nicklin SA, Milligan G (2011). GPR35 as a novel therapeutic target. Front Endocrinol 2: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, Hudson BD et al (2014). The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35. Mol Pharmacol 85: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravillas‐Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A (2015). Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. J Immunol 194: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neetoo‐Isseljee Z, MacKenzie AE, Southern C, Jerman J, McIver EG, Harries N et al (2013). High‐throughput identification and characterization of novel, species‐selective GPR35 agonists. J Pharmacol Exp Ther 344: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T (2003). Identification of p2y9/GPR23 as a novel G protein‐coupled receptor for Lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem 278: 25600–25606. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH et al (1998). Discovery of three novel G‐protein‐coupled receptor genes. Genomics 47: 310–313. [DOI] [PubMed] [Google Scholar]
- Ohshiro H, Tonai‐Kachi H, Ichikawa K (2008). GPR35 is a functional receptor in rat dorsal root ganglion neurons. Biochem Biophys Res Commun 365: 344–348. [DOI] [PubMed] [Google Scholar]
- Oka S, Ota R, Shima M, Yamashita A, Sugiura T (2010). GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun 395: 232–237. [DOI] [PubMed] [Google Scholar]
- Okumura S, Baba H, Kumada T, Nanmoku K, Nakajima H, Nakane Y et al (2004). Cloning of a G‐protein‐coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer Sci 95: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (D1): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern C, Cook JM, Neetoo‐Isseljee Z, Taylor DL, Kettleborough CA, Merritt A et al (2013). Screening beta‐arrestin recruitment for the identification of natural ligands for orphan G‐protein‐coupled receptors. J Biomol Screen 18: 599–609. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Tonai‐Kachi H, Shinjo K (2006). Zaprinast, a well‐known cyclic guanosine monophosphate‐specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett 580: 5003–5008. [DOI] [PubMed] [Google Scholar]
- Thimm D, Funke M, Meyer A, Muller CE (2013). 6‐Bromo‐8‐(4‐[(3)H]methoxybenzamido)‐4‐oxo‐4H‐chromene‐2‐carboxylic Acid: a powerful tool for studying orphan G protein‐coupled receptor GPR35. J Med Chem 56: 7084–7099. [DOI] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H et al (2006). Kynurenic acid as a ligand for orphan G protein‐coupled receptor GPR35. J Biol Chem 281: 22021–22028. [DOI] [PubMed] [Google Scholar]
- Xu Y, Casey G (1996). Identification of human OGR1, a novel G protein‐coupled receptor that maps to chromosome 14. Genomics 35: 397–402. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu JY, Wu X, Summer S, Whoriskey J, Saris C et al (2010). G‐protein‐coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 86: 1–5. [DOI] [PubMed] [Google Scholar]
- Zhao P, Sharir H, Kapur A, Cowan A, Geller EB, Adler MW et al (2010). Targeting of the orphan receptor GPR35 by pamoic acid: a potent activator of extracellular signal‐regulated kinase and β‐arrestin2 with antinociceptive activity. Mol Pharmacol 78: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Baudhuin LM, Hong G, Williams FS, Cristina KL, Kabarowski JH et al (2001). Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein‐coupled receptor GPR4. J Biol Chem 276: 41325–41335. [DOI] [PubMed] [Google Scholar]
- Zhu K, Baudhuin LM, Hong G, Williams FS, Cristina KL, Kabarowski JH et al (2005). Retraction. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein‐coupled receptor GPR4. J Biol Chem 280: 43280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effects of lodoxamide, CXCL17, and APT on intracellular Ca2 + concentrations in THP‐1 cells. A) Representative [Ca2+]itraces of THP‐1 cells treated with lodoxamide(100 nMor 1 mM), hCXCL17 (100 nM), mCXCL17 (100 nM), or ATP (100 mM). Arrows indicate when compounds were added.B) Histogram of Ca2 + responses. Results are presented as the means ± SEs of three independent experiments.
Figure S2 THP‐1 cells express GPR35a. RT‐PCR was conducted to detect GPR35a and GPR35b. THP‐1 cells expressed mainly GPR35a and we used GPR35a. Primersequences are followings. hGPR35a Forward GTGTTCGTGGTCTGCTTCCT, hGPR35b Forward GTCCTTGCGTCTCTCTGACC, hGPR35 Reverse GAGAGTCCTGGCTTTTGTGG.
Figure S3 Lodoxamide did not induce activation of H1 histamine or LPA1 lysophosphatidic acid receptor. Concentration‐response curves of histamine (A), LPA (B), and lodoxamide(C and D) as determined by the AP‐TGFα shedding assay in H1 or LPA1 coexpressed with a mixture of eight Gα proteins in HEK‐293 cells. Results are presented as the means ± SEs of 3 individual experiments.


