Abstract
We used randomizations to analyse patterns of co-occurrence of sexual and apomictic (asexual) members of the North American Crepis agamic complex (Asteraceae). We expect strong asymmetry in reproductive interactions in Crepis: apomicts produce clonal seeds with no need for pollination and are not subject to reproductive interference from co-occurring relatives. However, because they still produce some viable pollen, apomicts can reduce reproductive success of nearby sexual relatives, potentially leading to eventual local exclusion of sexuals. Consistent with this, randomizations reveal that sexuals are over-represented in isolated sites, while apomicts freely co-occur. Incorporation of taxonomic and phylogenetic evidence indicates that this pattern is not driven by local origins of asexuals. Our evidence that patterns of local co-occurrence are structured by reproductive interference suggests an underappreciated role for these interactions in community assembly, and highlights the need for explicit tests of the relative contributions of ecological and reproductive interactions in generating patterns of limiting similarity.
Keywords: apomixis, asymmetric gene flow, competitive exclusion, limiting similarity, reproductive exclusion, reproductive interference
1. Introduction
The failure of close relatives to co-occur is most often attributed to the short- or long-term outcomes of ecological processes such as competition [1–4]. This framework traces back at least to Darwin [5, chapter 3, p. 74], who noted ‘we can dimly see why the competition should be most severe between allied forms, which fill nearly the same place in the economy of nature’. The expectation that recent shared ancestry imparts similar traits and ecological requirements [6–8] suggests that if close relatives co-occur, competition for a shared limiting resource could lead to the exclusion of weaker competitors [2]. Exceptional cases of co-occurring close relatives are then variously taken as evidence of habitat filtering, transient neutral dynamics, identical niche requirements or environmental heterogeneity with cryptic niche divergence [1,4,8]. Despite a long tradition of interpreting phylogenetic limiting similarity as evidence for the importance of ecological processes in structuring communities, there is increasing recognition that the resemblance of close relatives extends beyond shared resource acquisition traits, notably to reproductive traits, and that these similarities may also impact the likelihood of co-occurrence [1,9].
As predicted for competitive interactions, reproductive interactions are also expected to be stronger between close relatives (e.g. congeners and sister taxa), because shared recent ancestry can also yield shared reproductive traits, including similarities in the timing of reproduction, mate recognition, pollination system and gamete recognition [9]. Until recently, reproductive interference, the negative consequences of reproductive interactions between species [10], has less often been considered a potential force in generating patterns of co-occurrence. Given that interspecific reproductive interactions usually reduce fitness relative to conspecific interactions [11], reproductive interference provides an alternative (or additional) explanation for the tendency of close relatives to avoid co-occurrence [1,9]. The costs of interspecific reproductive interactions can lead to local reproductive exclusion, in which the species most negatively impacted by reproductive interactions is eventually extirpated [9], and may also promote spatial separation of species through ecological character displacement or niche divergence [12]. Alternatively, if reproductive interference is a primary force limiting co-occurrence, then the existence of weaker reproductive interactions should favour co-occurrence of close relatives. Studies that can isolate the impact of one or the other mechanism and thus disentangle their contributions to coexistence are thus of special importance.
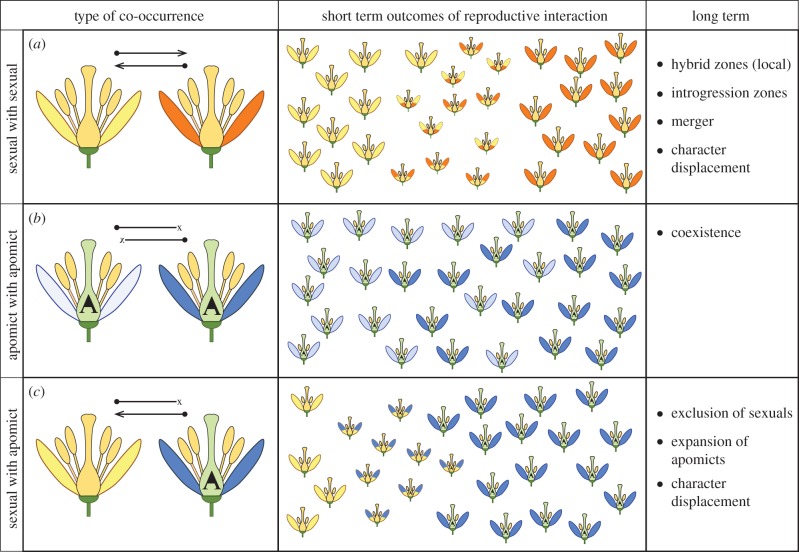
Sexual–asexual complexes provide a unique window on co-occurrence because closely related sexuals and asexuals differ in their vulnerability to reproductive interactions. In effect, the polymorphism in the reproductive system allows us to test predictions about co-occurrence of sexuals and asexuals that rest solely on their reproductive interactions. Our study focuses on agamic complexes in plants, which comprise closely related sexual and apomictic lineages. Sexuals are usually outcrossing, hermaphroditic diploids [13], while apomicts (plants that produce clonal seeds by various developmental pathways; cf. gametophytic apomicts) are typically polyploids [14]. Many apomicts produce at least some viable pollen [15], probably as a vestige of their recent sexual past [16]. In these groups, three types of reproductive interactions—sexual–sexual, asexual–asexual and sexual–asexual—can occur, yielding different predictions about short- and long-term co-occurrence (figure 1). For example, two related sexuals that co-occur (figure 1a) may produce hybrids of varying fitness, impacting co-occurrence in multiple ways: asymmetric costs can lead to local exclusion of the most negatively impacted species [17], or to ecological character displacement, niche divergence and eventual spatial separation. Alternatively, reproductive character displacement could permit stable co-occurrence of sympatric sexuals [18]. These diverse outcomes make it difficult to derive expectations about how frequently we expect co-occurrence of sexuals to occur, or how long we expect it to persist. Reproductive interactions between asexuals, and between asexuals and sexuals yield clearer expectations of co-occurrence (figure 1b,c), which we focus on here. When two asexuals co-occur (figure 1b), the absence of reproductive interactions (i.e. presuming nearly obligate or obligate asexuality) predicts stable co-occurrence (assuming that reproductive interactions are the primary limit to co-occurrence). When a sexual and an asexual co-occur (figure 1c), asymmetric or unidirectional (if asexuality is obligate) reproductive interactions through interspecific pollen transfer from apomicts to co-occurring sexuals may either reduce seed set, yield hybrid offspring or both. Hybrids of intermediate ploidy are commonly produced via natural and experimental crosses between apomicts and sexuals [19], but unless they are apomictic, interploidy hybrids are often of lower viability or fertility [20] than non-hybrid offspring. While transfer of apomixis to hybrids can occur and yield viable and fertile progeny (e.g. [21]), it removes those offspring from the sexual population. We emphasize that in terms of the fates of co-occurring sexuals and asexuals, the key point is that the presence of apomicts generates a recruitment cost for the sexuals relative to the apomicts; when they co-occur, we expect female function and seed set of the apomict to be unaffected, while the sexual suffers greater negative impacts via reduced recruitment of sexual diploid offspring (through reduced seed set, or by the production of interploidy offspring that are either of low fertility (if sexual) or apomictic). These predicted outcomes for co-occurrence of sexuals and apomicts form the basis of our test: if reproductive interactions are the primary determiners of co-occurrence of close relatives, then we predict a relationship between reproductive mode and patterns of co-occurrence; if co-occurrence is limited primarily by resource competition, we expect no such relationship.
Figure 1.
Schematic of expected reproductive interactions (left column) and their short- (centre column) and long-term (right column) outcomes under three scenarios. (a) Two closely related sexual lineages (each species indicated by different-coloured petals). Arrows above the flowers indicate the direction of potential interspecific pollen flow. The smaller size of offspring suggests lower fitness than their parents. (b) Two (obligate) apomictic lineages (designated with a stylized ‘A’ in the ovary), indicating no pollen flow, no hybrid formation and stable co-occurrence. (c) Reproductive interactions of a sexual and an apomictic lineage, showing unidirectional pollen flow from the apomict to the sexual recipient. (Online version in colour.)
Here, we test the predicted outcomes of reproductive interactions between sexuals and asexuals using patterns of co-occurrence in the North American Crepis agamic complex. This group, broadly distributed in the sagebrush-steppe zone of western North America, comprises eight taxonomic species (some of which are further divided into subspecies, for a total of 17 taxa). Species can include diploid sexual outcrossing lineages and an array of derivative polyploid apomictic lineages that reproduce via clonal seeds [22,23]. The taxonomy of the complex is therefore somewhat artificial, given that many of the polyploid apomictic lineages are allopolyploids and that differences in ploidy and the reproductive mode (sexual versus apomictic) contribute to isolating barriers between apomicts and sexuals [22,23]. A notable feature of the complex is that local sites can comprise one to many species and one to many ploidy levels within taxonomic species [22,24]. Within these sites (which have the spatial properties typical of populations of a single species), individuals of Crepis are typically more or less continuously interspersed at low-to-moderate densities within the sagebrush matrix. Apomixis in Crepis is autonomous (pollen is not required for embryo initiation or formation [25]), but apomicts almost always produce some viable pollen (see the electronic supplementary material, table S1 for available pollen data for a subset of our samples). Apomicts can serve as pollen donors where they co-occur with sexuals, yielding interploidy hybrids [19]. In addition, apomictic embryos in North American Crepis are known to develop precociously (they are often initiated before flowers open), and therefore, their ovules are probably unavailable for fertilization [25], emphasizing that these apomicts should be impervious to reproductive interference.
We hypothesized that if reproductive interference is key in limiting coexistence, sexual cytotypes should tend to occur in isolation because of the reproductive costs of co-occurrence that arise from pollen flow from asexual counterparts. By contrast, distinct apomicts should freely co-occur because they are immune to pollen flow from other asexuals or from sexuals, and therefore experience no reproductive cost of co-occurrence. In addition, where sexuals do co-occur with apomicts, we expect to find evidence of reproductive interference, through the presence of hybrid lineages of intermediate ploidy. We test our hypotheses about the expected frequencies of co-occurrence of sexuals and asexuals, and explore alternative explanations for our findings, using randomizations.
2. Material and methods
(a). Data sources
We use data on patterns of distribution from 589 individuals at 121 sites, surveyed for a phylogenetic study of the North American Crepis agamic complex [22]. These sites are scattered throughout the core range of the complex (northern California, eastern Oregon and central Washington), which includes all sexual diploids, and the bulk of the diversity of polyploid apomicts [22,23]. Over five field seasons, we targeted collecting efforts to fill in coverage of regions with ecological or taxonomic diversity, based on prior examination of herbarium specimens and the monograph of Babcock and Stebbins. We sampled sites as encountered, with no a priori knowledge of whether they included one or more species or ploidy level; all sites are comparable in scale to populations, whether one or more Crepis species was present. At each site, we sampled at least one individual of each distinct species and morphotype. We could not distinguish sexuals from apomicts in the field.
Taxonomic keys were used to determine species, and flow cytometry used to infer ploidy [22]. In this paper, ‘ploidy’ refers to the assigned DNA–ploidy from estimates of DNA content [26]. As previously reported [22], we assigned each sample to a ‘cyto-taxon’ — a particular ploidy level of a particular species or subspecies (e.g. 4× Crepis atribarba subsp. originalis is counted as distinct from 5× of the same subspecies, and from 4× C. atribarba subsp. atribarba), and treated each cyto-taxon as a distinct sexual or asexual lineage. Although it was uncommon for multiple samples of the same morphotype from the same site to differ in ploidy, it remains possible that we under-sampled cryptic ploidy variation within morphotypes.
Starting with the range-wide ploidy determinations in Sears & Whitton [22], we double-checked the assignment of individuals of the same species at the same site that had been assigned consecutive ploidy levels above 4× (e.g. 5× and 6× C. atribarba). The ranges of DNA content values are wider at these ploidy levels, so we grouped such individuals under a single ploidy level when their genome sizes differed by less than 10%. In our subsequent analyses, our pool of ‘closely related species’ is, in fact, a pool of all of the distinct cyto-taxa that we detected in our sampling. Across the 17 species and subspecies in the complex, we identified a total of 47 distinct cyto-taxa. We treated all diploid cyto-taxa as sexual and all polyploids as apomictic, based on previous characterization of this system [25].
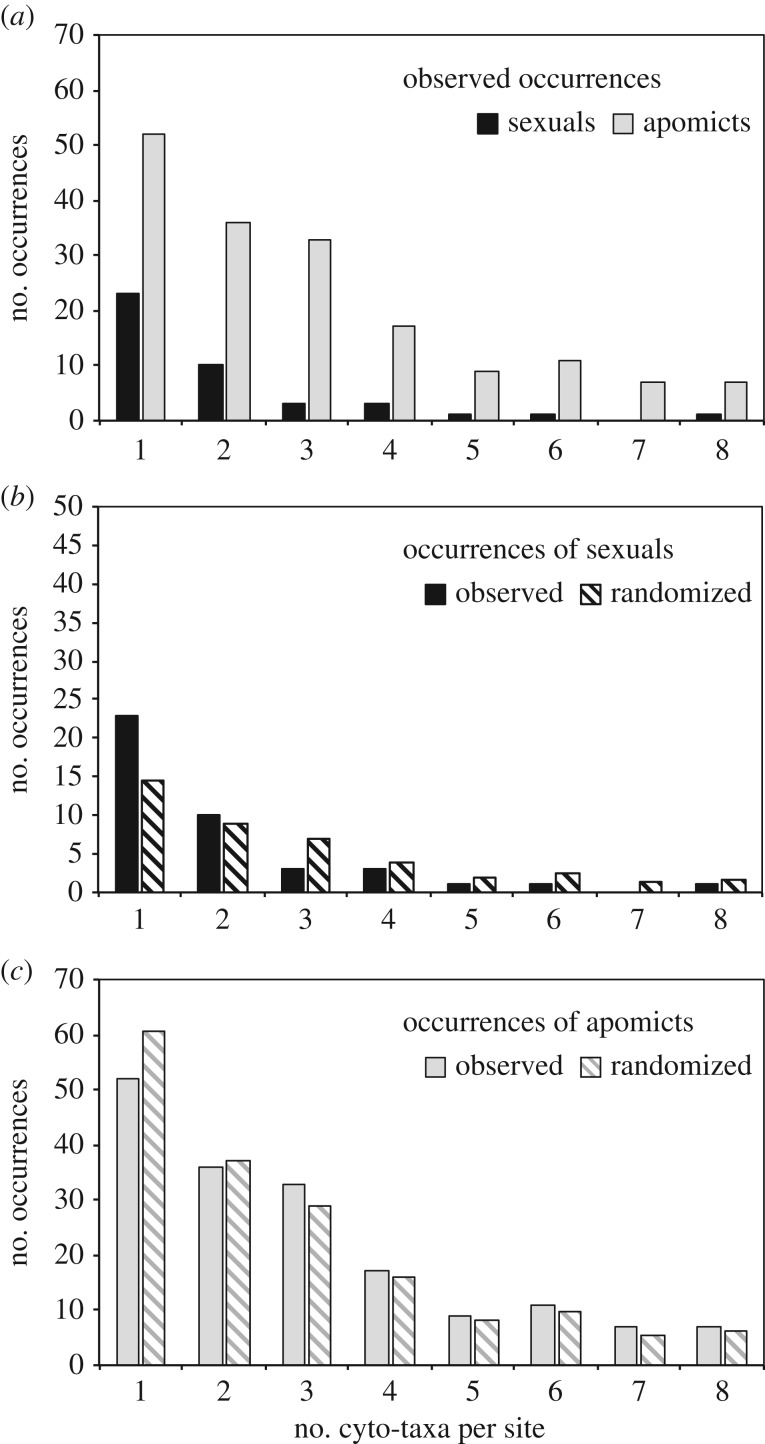
We found a total of 214 occurrences of cyto-taxa in the 121 surveyed sites, with sites comprising one to eight cyto-taxa (figure 2a; see the electronic supplementary material, table S1 for the list of sites and cyto-taxa and electronic supplementary material table S2 for the randomization data). Among sites, there were 42 total observations of diploid and 172 of polyploid cyto-taxa. In the randomizations, we refer to each detection of a cyto-taxon at a site as a distinct cyto-taxon occurrence. For example, there are six occurrences of the cyto-taxon ‘tetraploid Crepis pleurocarpa’ across the 121 sites; site 5014 includes a total of three cyto-taxon occurrences, including diploid and tetraploid C. pleurocarpa and tetraploid Crepis occidentalis.
Figure 2.
Observed and random expectations for the number of cyto-taxa per site in the North American Crepis. (a) Histogram of observed complexity at 121 sites, broken down separately for apomicts and sexuals (e.g. of the 46 cyto-taxon observations representing the 23 sites with two cyto-taxa, there are 10 observations of diploids (sexuals) and 36 observations of polyploids (apomicts)). (b,c) Histograms comparing observed and expected (based on randomizations) occurrences of sexuals (b) and apomicts (c).
(b). Randomizations
To test the hypothesis that sexual diploids are more likely to occur on their own than the apomictic polyploids, we asked whether the pattern of occurrences of each ploidy level is distinct from what we would expect if they co-occurred randomly across our 121 sites. Each of the 1 million randomizations reshuffled the cyto-taxon occurrences across the 121 sites, maintaining the number of observations of each cyto-taxon and the number of distinct cyto-taxa at each site. Thus, a cyto-taxon observed at 10 sites would have a particular null expectation of how many of those occurrences would be in a site alone.
In a secondary analysis, we repeated the randomizations after grouping together all polyploid occurrences of the same taxonomic species at each site, therefore considering sites to have multiple polyploids only if these were identified as different species. This was done to explore whether patterns of co-occurrence could result from frequent local origins and establishment of apomicts via, e.g. occasional crossing to produce a new apomictic lineage in situ. Grouping all polyploid cytotype occurrences of the same taxonomic species at the same site in this manner only reduced the number of occurrences of polyploid cyto-taxa from 172 to 152 (i.e. in most cases, co-occurring cyto-taxa were not identified as the same species).
As described below (Results), we found support for an overrepresentation of sites in which sexual diploids occur in isolation, consistent with a role for reproductive interference in limiting co-occurrence. Given this, we examined the cases in which diploids co-occur with apomicts to see whether there was evidence of hybridization between them (which we would expect if the pollen from apomicts is effective in fertilizing sexual ovules, as predicted; figure 1c). We focused on sites at which sexual diploids co-occur with at least one tetraploid cyto-taxon (tetraploids are the most common polyploids), and the expectation that these would probably also include a suitable triploid (i.e. identified as the same species as either the co-occurring diploid or tetraploid), plausibly generated by hybridization. In the observed data, we counted the number of sites at which a diploid and tetraploid co-occur, and then counted the number of those sites in which a suitable triploid also occurs. To derive the null expectation for the proportion of such diploid–triploid–tetraploid co-occurrences, our randomization of 1 million replicates held the data as observed constant, except for the triploid occurrences. The triploid occurrences were removed and then randomly assigned back to sites, regardless of whether the site originally held a triploid or not, constrained only to avoid placing two occurrences of the same cyto-taxon at the same site (which would have reduced them to a single occurrence) (see the electronic supplementary material for the code used in randomizations).
3. Results
(a). Are diploids over-represented in isolated sites?
Comparison of observed frequencies of isolated and co-occurring cyto-taxa (figure 2a) against a null expectation from 1 million randomizations of cyto-taxa among simulated sites indicates that sexual cyto-taxa occur alone far more frequently than expected based on their observed frequency in our sample (figure 2b; p < 0.002, frequency of randomizations in which diploids were alone in as many or more sites as observed). The pattern of co-occurrence of apomictic cyto-taxa matches a random expectation (figure 2c) for ploidies 3 through to 9 (p = 0.96, 0.97, 0.63, 0.87, 0.14, 0.71, 0.34).
Our results were unchanged when we repeated the randomizations after reclassifying all co-occurring polyploids of the same species at each site as one cyto-taxon: apomicts still matched random expectation of co-occurrence (p = 0.99) and sexuals remained over-represented in isolated sites relative to the null expectation (p = 0.02).
(b). Is there evidence that diploids and tetraploids have hybridized where they co-occur?
We observed 21 occurrences of a diploid (sexual) co-occurring with tetraploid, and in 11 of these cases, a suitable triploid (assigned to the same species as the co-occurring diploid or tetraploid) was also present at the site. In a number of cases, a diploid co-occurred with more than one tetraploid, and in such cases, it could be argued that each diploid–tetraploid pair should not be counted as an independent opportunity for hybridization. Accordingly, we reduced the data to count each site with diploid–triploid–tetraploid co-occurrences only once, yielding a count of 11 sites with a diploid and at least one tetraploid present, four of which also include at least one suitable triploid. Comparing our observations of diploid–triploid–tetraploid co-occurrences against expectations generated by randomizing triploids among sites indicates that suitable triploids occur at sites with diploids and tetraploids more often than expected, whether we assess their occurrence relative to the 11 sites (p < 0.002) or 21 occurrences of diploid–tetraploid sets (p < 0.001).
4. Discussion
We detected frequent co-occurrence of apomicts contrasted against a significantly stronger tendency for sexuals to occur in isolation in the North American Crepis agamic complex. This pattern is consistent with the action of asymmetric reproductive interference, in which pollen of apomicts reduces the reproductive success of co-occurring sexuals, leading to their eventual local exclusion. Previous findings in Crepis [19] and other agamic complexes document asymmetric reproductive interactions, providing support for the mechanism that we invoke, while alternative explanations, discussed below, are limited in their ability to account for observed patterns of co-occurrence. We wish to underscore the potential to use such sexual–asexual systems (and other systems with asymmetric reproductive interactions, e.g. between selfers and outcrossers [27,28]) to explore the relative role of reproductive versus ecological interactions in limiting co-occurrence.
(a). Asymmetric reproductive interference as a threat to the persistence of sexuals
Our previous studies of this complex support the view that the persistence of sexuals can be threatened by reproductive interference from nearby apomicts. Hersh et al. [19] conducted crosses using pollen from apomictic polyploid (8×) Crepis barbigera to fertilize ovules of sexual diploids of C. atribarba in one of our sites (no. 4011, electronic supplementary material, table S1). Relative to the success of diploid–diploid crosses, they found no decline in seed set, but seeds from crosses with apomicts were mainly hybrids of intermediate (approx. 5×) ploidy [19], indicating that diploid recruitment can be reduced by the presence of nearby apomicts. Indeed, individuals of intermediate ploidy were also detected among open pollinated seeds and the standing population [19]. Most apomictic Crepis that we have surveyed produce some viable pollen (see the electronic supplementary material, table S1), and evidence of ongoing local hybridization has previously been found [13,24], suggesting that the potential for reproductive interference is widespread in this system. Furthermore, it is worth noting that even if pollen from apomicts fails to produce viable offspring, recruitment of sexual diploids can still be reduced through stigma clogging, or embryo or seedling abortion [10]. We also found indirect support for the ability of pollen from apomicts to reduce recruitment of sexual diploids, as we interpret the tendency of triploids to occur where diploids and tetraploids are also present as evidence of hybridization.
While limited crossing data are available from the Crepis agamic complex, the potential for asymmetric reproductive interference driven by male function of apomicts has been documented in multiple additional systems. Evidence ranges from quantifying the amount of viable pollen in apomicts [15], to showing the transmission of apomixis to the offspring of apomicts and sexuals [29], and demonstrating that hybridization between apomicts and sexuals yields new apomicts [30]. Support for the ability of apomictic pollen to threaten the persistence of sexuals also comes from the triploid apomictic common dandelion, Taraxacum officinale. Brock [31] found that pollen moves freely between non-native apomictic T. officinale and the native sexual diploid Taraxacum ceratophorum. Although hybrids are rare, hybrid seeds are produced in crosses with mixed pollen loads, and rates of hybridization probably depend on the relative abundance of the two species [32]. In Japan, the native sexual diploid Taraxacum japonicum is declining in response to expansion of the exotic apomictic T. officinale [33]. In this case, the decline results from reduced seed set of the native species owing to pollinator preference for the abundant apomict. In Taraxacum, many of the hybrids between apomicts and sexuals are weak or infertile, but the fertile offspring are typically apomictic [29]. The work in Taraxacum supports asymmetric reproductive interference from apomicts as a mechanism that threatens recruitment and eventual persistence of co-occurring sexuals.
Theoretical models of the longer-term impacts of reproductive interactions between sexuals and apomicts [34–37] generally find little support for stable coexistence, although some (e.g. [38]) predict long periods (thousands of generations) of co-occurrence. These models assume that sexuals have a fitness advantage (through survival), and therefore when apomixis dominates, it is because pollen transfer from apomicts reduces the reproductive fitness of sexuals sufficiently to outweigh their survival advantage.
(b). Alternative explanations for spatial isolation of sexuals
We considered two alternatives to reproductive interference that could generate a tendency for spatially isolated sexuals: frequent local origin of apomicts and ecological divergence between sexuals and apomicts.
(i). Local origins
Our more conservative randomizations, in which we pooled different ploidy levels within apomictic lineages, suggest that local origins are not the primary drivers of patterns of co-occurrence, but we also consider here the inferences of local origins from plastid DNA sequence data in Sears & Whitton [22]. Because plastid DNA is usually maternal inherited in flowering plants, co-occurrence of phylogenetically distinct haplotypes can be taken as evidence of establishment via seed dispersal (and not local origins), as previously inferred in this [24,39] and other groups [40–42]. Haplotype data are available for a total of 50 cyto-taxa co-occurring in 21 sites in the online supplementary data in [22]. Among these, 40 cases involve co-occurring cyto-taxa with distinct haplotypes (i.e. that differ by more than a single mutation in figure 4 of [22]). Although we expect local origins to add to the establishment of novel cyto-taxa in Crepis over time, combining information on haplotype co-occurrence with the results of the conservative randomizations, we are confident in asserting that the patterns of co-occurrence of apomicts that we observe are not driven predominatly driven by local origins.
(ii). Ecological divergence
Ecological divergence of sexuals and related apomictic polyploids could also contribute to the greater tendency of sexual diploids to be found in isolation from apomicts, for example, if niche divergence between lineages of different reproductive types is sufficient to preclude co-occurrence. There are no data available to directly assess niche divergence among diverse lineages of sexuals and apomicts of the North American Crepis. We note, however, that while our previous sampling prioritized the collection of sexual diploids for phylogenetic studies, over several field seasons we have not detected obvious distinctions between the places where sexuals and apomicts grow. Our best strategy for finding sexual diploids continues to be to go to the places where these were previously found [23,43] and confirm ploidy using flow cytometry. In addition, over the 18 sites where we found co-occurring sexuals and apomicts, there are examples of each of the eight sexual diploid species, suggesting that ecological divergence does not consistently preclude co-occurrence of sexuals and apomicts (see the electronic supplementary material, table S1). Also, regional taxonomic treatments of the complex (e.g. the recent flora of California [44]) do not distinguish sexuals from apomicts. We also note that sexual diploids have ranges that are fully overlapping with the ranges of multiple apomicts (maps in [23,43]). While these are admittedly anecdotal arguments, we believe they support the view that, over broad areas and timescales, apomicts and sexuals are capable of colonizing the same sites, suggesting that additional factors (i.e. reproductive interference) contribute to observed patterns of co-occurrence.
(c). Longer-term outcomes of reproductive interactions
The types of reproductive interactions that we highlight in Crepis have the potential to contribute to niche divergence via broader-scale eco-evolutionary dynamics [45]. For example, if reproductive interference consistently reduces the fitness of sexual diploids (e.g. [38]), selection could favour traits in the sexuals that reduce the potential for reproductive interference via maladaptive hybridization, leading to ecological or reproductive character displacement [46]. Under such a scenario, ecological character displacement may contribute to generating distinct distributions of sexuals and asexuals [47], as postulated by Lynch [46]. However, depending on the fitness consequences of hybridization, the life histories of organisms and the colonization history of sites (e.g. whether sexuals or apomicts have precedence at a site), long periods of co-occurrence of sexuals and asexuals could be part of the normal cycles of sexual–asexual complexes [38]. Nonetheless, over the long term, in systems where male function of asexuals is maintained, the persistence of sexuals may rest upon their ability to avoid extinction by hybridization [17], and their chances of persisting would be greatly enhanced by niche divergence that prevents or reduces co-occurrence with apomicts.
The unusual features of the North American Crepis agamic complex provided us with a unique window onto the role of reproductive interactions in co-occurrence of close relatives, but studies of other congeners that occur in sympatry, and of species assemblages that share reproductive resources (e.g. plants that share pollinators) have also highlighted the role of divergence in reproductive traits in permitting co-occurrence [48–50]. For example, Eaton et al. [48] showed that among a set of 116 species of Pedicularis that co-occur in various combinations on the Tibetan Plateau, there was greater divergence and lower variance in floral traits in sympatry. They inferred that lability in pollinator-related floral traits facilitates co-occurrence by reducing reproductive interference. More broadly, plant–pollinator interactions are increasingly recognized as contributing to community assembly [50], primarily through their impact on pollination efficiency, which reduces reproductive interference and thus increases reproductive success of individual species. Following these and other authors, our work suggests that reduced reproductive interference can favour co-occurrence.
(d). The relative importance of reproductive and competitive exclusion
We noted that a key challenge to teasing apart the relative importance of competitive and reproductive interactions in limiting co-occurrence is that both types of forces are predicted to be strongest between close relatives, i.e. recent shared evolutionary history increases the likelihood of shared traits related to resource use and reproduction. It follows that tests of community structure that include or focus on close relatives and are based simply on patterns of co-occurrence will confound the effects of reproductive and ecological interactions. Patterns of species co-occurrence fall squarely in the realm of community ecology, so it is understandable that competition and other ecological processes have served as focal mechanisms for probing community assembly. Given that reproductive interactions are the traditional domain of studies of speciation and reproductive isolation, the lack of emphasis on their role in explaining community assembly also makes some sense. It is notable that studies of plant–pollinator interactions, which bridge the divide between reproductive isolation and community dynamics, have a stronger, but still limited track record of incorporating reproductive and ecological interactions in analysis of community assembly [49–52].
While there are few studies that consider the separate and joint roles of reproductive interference and resource competition, the models of Kuno [53], and Kishi & Nakazawa [54] predict that, for a given strength of interaction, reproductive interference can more readily prevent coexistence than resource competition. Furthermore, Kishi & Nakazawa [54] show that the addition of reproductive interactions restricts conditions for coexistence, i.e. conditions that predicted coexistence under resource competition alone lead to exclusion when reproductive interference is added.
Differences in the expected spatial reaches of reproductive versus resource interactions further support our argument that the former may dominate when gametes disperse over greater distances than the individuals that produce them [52], as is expected for most plants. In accordance with this expectation, Takakura et al. [55] found negative effects of interspecific pollen transfer from non-native apomictic dandelion, T. officinale, on seed set of the native T. japonicum at scales of 2–5 m, well beyond the mean distance between nearest neighbours of the two species. Under these sorts of spatial conditions, we envisage that competitive interactions may be too weak to lead to competitive exclusion, and thus the ecological conditions that permit coexistence may be relatively common, which could tip the balance towards a greater role for reproductive interactions.
5. Conclusion
While there is evidence that close relatives share traits that influence resource acquisition, there is also an emerging realization that competitive hierarchies may fail to predict community composition owing to a diversity of factors. Our study highlights a different set of interactions — reproductive interactions — that are probably at play in determining coexistence, especially among sister species and close congeners, and that are typically not considered part of the remit of studies of community assembly. Given that these two sets of interactions can contribute to setting the conditions that permit co-occurrence or drive ecological divergence, clarifying their relative roles is important to our understanding of the forces that govern the distribution of related species. Furthermore, while we focus here on close relatives in which evidence of reproductive interference can be readily described, we note that the range of mechanisms of reproductive interference extends to interactions among more distantly related species [10], and we therefore urge a more careful and explicit consideration of the potential impacts of these interactions on community dynamics.
Supplementary Material
Supplementary Material
Acknowledgements
Our manuscript was improved through critical and constructive feedback on an early draft from Amy Angert's laboratory group at UBC, and insightful comments on a later version provided by Rachel Germain, Evan Hersh, Loren Rieseberg, and two anonymous reviewers. Erica Li-Leger carefully gathered pollen data. Andy Johnson's support with flow cytometer use in the UBC Biomedical Research Centre is gratefully acknowledged, as is the professional support of Linda Jennings and Danielle An in managing vouchers and loans at the UBC Herbarium.
Data accessibility
The data on DNA content and ploidy for samples at each site, as well as the code and input data used in randomizations are available, are in the electronic supplementary material. The site data are also available on Dryad, https://dx.doi.org/10.5061/dryad.76gp5 [56].
Author contributions
J.W. conceived the study and co-designed the randomization tests, and wrote the manuscript. C.J.S. conducted fieldwork, identified plants, gathered and analysed flow cytometry and DNA sequence data, and commented on the manuscript. W.P.M. co-designed the randomization tests, wrote the code and implemented the analyses, and commented on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by NSERC Canada Discovery Grants to J.W. and W.P.M.
References
- 1.Weber MG, Strauss SY. 2016. Coexistence in close relatives: beyond competition and reproductive isolation in sister taxa. Ann. Rev. Ecol. Evol. Syst. 47, 359–381. ( 10.1146/annurev-ecolsys-112414-054048) [DOI] [Google Scholar]
- 2.Gause GF. 1934. The struggle for existence. Baltimore, MD: Williams and Wilkins. [Google Scholar]
- 3.McPeek MA. 2014. Limiting factors, competitive exclusion, and a more expansive view of species coexistence. Am. Nat. 183, iii–iv. ( 10.1086/675305) [DOI] [PubMed] [Google Scholar]
- 4.Silvertown J. 2004. Plant coexistence and the niche. Trends Ecol. Evol. 19, 605–611. ( 10.1016/j.tree.2004.09.003) [DOI] [Google Scholar]
- 5.Darwin C. 1859. The origin of species. London, UK: J. Murray. [Google Scholar]
- 6.Burns JH, Strauss SY. 2011. More closely related species are more ecologically similar in an experimental test. Proc. Nat Acad. Sci. USA 108, 5302–5307. ( 10.1146/annurev.ecolsys.33.010802.150448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505. ( 10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 8.Cavender-Bares J, Ackerly DD, Baum DA. 2004. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 163, 823–843. ( 10.1086/386375) [DOI] [PubMed] [Google Scholar]
- 9.Pfennig KS, Pfennig DW. 2009. Character displacement: ecological and reproductive responses to a common evolutionary problem. Q Rev. Biol. 84, 253–276. ( 10.1086/605079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochkirch A, Gröning J, Bücker A. 2007. Sympatry with the devil: reproductive interference could hamper species coexistence. J. Anim. Ecol. 76, 633–642. ( 10.1111/j.1365-2656.2007.01241.x) [DOI] [PubMed] [Google Scholar]
- 11.Vallejo-Marin M, Hiscock SJ. 2016. Hybridization and hybrid speciation under global change. New Phytol. 211, 1170–1187. ( 10.1111/nph.14004) [DOI] [PubMed] [Google Scholar]
- 12.Kishi S, Tsubaki Y. 2014. Avoidance of reproductive interference causes resource partitioning in bean beetle females. Popul. Ecol. 56, 73–80. ( 10.1007/s10144-013-0390-5) [DOI] [Google Scholar]
- 13.Whitton J, Sears CJ, Baack EJ, Otto SP. 2008. The dynamic nature of apomixis in the angiosperms. Int. J. Plant. Sci. 169, 169–182. ( 10.1086/523369) [DOI] [Google Scholar]
- 14.Richards AJ. 1997. Plant breeding systems. New York, NY: Garland Science. [Google Scholar]
- 15.Meirmans PG, Den Nijs H(J)CM, Van Tienderen PH. 2006. Male sterility in triploid dandelions: asexual females vs asexual hermaphrodites. Heredity 96, 45–52. ( 10.1038/sj.hdy.6800750) [DOI] [PubMed] [Google Scholar]
- 16.Maynard SJ. 1978. The evolution of sex. Cambridge, UK: Cambridge University. [Google Scholar]
- 17.Todesco M, et al. 2016. Hybridization and extinction. Evol. Appl. 9, 892–908. ( 10.1111/eva.12367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins R. 2013. Reinforcement in plants. New Phytol. 197, 1095–1103. ( 10.1111/nph.12119) [DOI] [PubMed] [Google Scholar]
- 19.Hersh E, Grimm J, Whitton J.. 2016. Attack of the clones: reproductive interference between sexuals and asexuals in the Crepis agamic complex. Ecol. Evol. 6, 6473–6483. ( 10.1002/ece3.2353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husband BC. 2004. The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol. J. Linn. Soc. 82, 537–546. ( 10.1111/j.1095-8312.2004.00339.x) [DOI] [Google Scholar]
- 21.Noyes RD. 2000. Diplospory and parthenogenesis in sexual× agamospermous (apomictic) Erigeron (Asteraceae) hybrids. Int. J. Plant. Sci. 161, 1–12. ( 10.1086/314238) [DOI] [PubMed] [Google Scholar]
- 22.Sears CJ, Whitton J. 2016. A reexamination of the North American Crepis agamic complex and comparison with the findings of Babcock and Stebbins' classic biosystematic monograph. Am. J. Bot. 103, 1289–1299. ( 10.3732/ajb.1600057) [DOI] [PubMed] [Google Scholar]
- 23.Babcock EB, Stebbins GL. 1938. The American species of Crepis: their interrelationships and distribution as affected by polyploidy and apomixis. Carnegie Inst. Wash. Publ. 504, 1–199. [Google Scholar]
- 24.Whitton J, Dlugosch KM, Sears CJ. 2008. Molecular and morphological evidence for and against gene flow in sympatric apomicts of the North American Crepis agamic complex (Asteraceae). Botany 86, 877–885. ( 10.1139/B08-071) [DOI] [Google Scholar]
- 25.Stebbins GL, Jenkins JA. 1939. Aposporic development in the North American species of Crepis. Genetica 21, 191–224. ( 10.1007/BF01508152) [DOI] [Google Scholar]
- 26.Suda J, Krahulcová A, Trávníek P, Krahulec F. 2006. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon 55, 447–450. ( 10.2307/25065591) [DOI] [Google Scholar]
- 27.Fishman L, Wyatt R. 1999. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 53, 1723–1733. ( 10.2307/2640435) [DOI] [PubMed] [Google Scholar]
- 28.Grossenbacher DL, Whittall JB. 2011. Increased floral divergence in sympatric monkeyflowers. Evolution 65, 2712–2718. ( 10.1111/j.1558-5646.2011.01306.x) [DOI] [PubMed] [Google Scholar]
- 29.Tas ICQ, van Dijk PJ. 1999. Crosses between sexual and apomictic dandelions (Taraxacum). I: the inheritance of apomixis. Heredity 83, 707–714. ( 10.1046/j.1365-2540.1999.00619.x) [DOI] [PubMed] [Google Scholar]
- 30.Sochor M, Vašut RJ, Sharbel TF, Trávníček B. 2015. How just a few makes a lot: speciation via reticulation and apomixis on example of European brambles (Rubus subgen. Rubus, Rosaceae). Mol. Phylogenet. Evol. 89, 13–27. ( 10.1016/j.ympev.2015.04.007) [DOI] [PubMed] [Google Scholar]
- 31.Brock MT. 2004. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Am. J. Bot. 91, 656–663. ( 10.3732/ajb.91.5.656) [DOI] [PubMed] [Google Scholar]
- 32.Brock MT. 2009. Prezygotic barriers to gene flow between Taraxacum ceratophorum and the invasive Taraxacum officinale (Asteraceae). Oecologia 161, 241–251. ( 10.1007/s00442-009-1383-0) [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Takakura K-I, Nishida T. 2009. Alien pollen grains interfere with the reproductive success of native congener. Biol. Invasions 12, 1617–1626. ( 10.1007/s10530-009-9574-5) [DOI] [Google Scholar]
- 34.Joshi A, Moody ME. 1995. Male gamete output of asexuals and the dynamics of populations polymorphic for reproductive mode. J. Theor. Biol. 174, 189–197. ( 10.1006/jtbi.1995.0091) [DOI] [PubMed] [Google Scholar]
- 35.Joshi A, Moody ME. 1998. The cost of sex revisited: effects of male gamete output of hermaphrodites that are asexual in their female capacity. J. Theor. Biol. 195, 533–542. ( 10.1006/jtbi.1998.0811) [DOI] [PubMed] [Google Scholar]
- 36.Carrillo C, Mogie M, Britton NF. 2002. Coexistence of sexual and asexual conspecifics: a cellular automaton model. J. Theor. Biol. 217, 275–285. ( 10.1006/jtbi.2002.3039) [DOI] [PubMed] [Google Scholar]
- 37.Britton NF, Mogie M. 2001. Poor male function favours the coexistence of sexual and asexual relatives. Ecol. Lett. 4, 116–121. ( 10.1046/j.1461-0248.2001.00201.x) [DOI] [Google Scholar]
- 38.Mogie M. 2011. Pollen profile, spatial structure, and access to sex in asexual hermaphrodites. Biol. J. Linn. Soc. 103, 954–966. ( 10.1111/j.1095-8312.2011.01667.x) [DOI] [Google Scholar]
- 39.Holsinger KE, Mason-Gamer RJ, Whitton J.. 1999. Genes, demes and plant conservation. In Genetics and the extinction of species (eds Landweber LF, Dobson AP), pp. 23–46. Princeton, NJ: Princeton University Press. [Google Scholar]
- 40.Thompson SL, Whitton J. 2006. Patterns of recurrent evolution and geographic parthenogenesis within apomictic polyploid Easter daises (Townsendia hookeri). Mol. Ecol. 15, 3389–3400. ( 10.1111/j.1365-294X.2006.03020.x) [DOI] [PubMed] [Google Scholar]
- 41.Petit RJ, Pineau E, Demesure B. 1997. Chloroplast DNA footprints of postglacial recolonization by oaks. Proc. Natl Acad. Sci. USA 94, 9996–10 001. ( 10.1073/pnas.94.18.9996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCauley DE. 1995. The use of chloroplast DNA polymorphism in studies of gene flow in plants. Trends Ecol. Evol. 10, 198–202. ( 10.1016/S0169-5347(00)89052-7) [DOI] [PubMed] [Google Scholar]
- 43.Sears CJ. 2011. Systematic investigations into the North American Crepis agamic complex. PhD thesis, The University of British Columbia, Vancouver, British Columbia, Canada. [Google Scholar]
- 44.Baldwin BG, Goldman DH, Keil DJ, Patterson RW, Rosatti TJ, Wilken DH (eds). 2012. The Jepson manual. Vascular plants of california, 2nd edn Berkeley, CA: University of California Press. [Google Scholar]
- 45.Pelletier F, Garant D, Hendry AP. 2009. Eco-evolutionary dynamics. Phil. Trans. R. Soc. B 364, 1483–1489. ( 10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lynch M. 1984. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q Rev. Biol. 59, 257–290. ( 10.2307/2827008) [DOI] [Google Scholar]
- 47.Vandel A. 1931. La parthénogenèse. Paris, France: Doin. [Google Scholar]
- 48.Eaton DAR, Fenster CB, Hereford J, Huang S-Q, Ree RH. 2012. Floral diversity and community structure in Pedicularis (Orobanchaceae). Ecology 93, S182–S194. ( 10.1890/11-0501.1) [DOI] [Google Scholar]
- 49.Armbruster WS. 1986. Reproductive interactions between sympatric Dalechampia species: are natural assemblages ‘random’ or ‘organized’? Ecology 67, 522–533. ( 10.2307/1938595) [DOI] [Google Scholar]
- 50.Sargent RD, Ackerly DD. 2008. Plant–pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130. ( 10.1016/j.tree.2007.11.003) [DOI] [PubMed] [Google Scholar]
- 51.Waser NM. 1978. Interspecific pollen transfer and competition between co-occurring plant species. Oecologia 36, 223–236. ( 10.1007/BF00349811) [DOI] [PubMed] [Google Scholar]
- 52.Armbruster WS. 1995. The origins and detection of plant community structure: reproductive versus vegetative processes. Folia Geobotanica 30, 483–497. ( 10.1007/BF02803978) [DOI] [Google Scholar]
- 53.Kuno E. 1992. Competitive exclusion through reproductive interference. Res. Popul. Ecol. 34, 275–284. ( 10.1007/BF02514797) [DOI] [Google Scholar]
- 54.Kishi S, Nakazawa T. 2013. Analysis of species coexistence co-mediated by resource competition and reproductive interference. Popul. Ecol. 55, 305–313. ( 10.1007/s10144-013-0369-2) [DOI] [Google Scholar]
- 55.Takakura KI, Matsumoto T, Nishida T. 2011. Effective range of reproductive interference exerted by an alien dandelion, Taraxacum officinale, on a native congener. J. Plant Res. 124, 269–276. ( 10.1007/s10265-010-0368-8) [DOI] [PubMed] [Google Scholar]
- 56.Whitton J, Sears CJ, Maddison WP. 2017. Data from: Co-occurrence of related asexual, but not sexual, lineages suggests that reproductive interference limits coexistence Dryad Digital Repository. ( 10.5061/dryad.76gp5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Whitton J, Sears CJ, Maddison WP. 2017. Data from: Co-occurrence of related asexual, but not sexual, lineages suggests that reproductive interference limits coexistence Dryad Digital Repository. ( 10.5061/dryad.76gp5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data on DNA content and ploidy for samples at each site, as well as the code and input data used in randomizations are available, are in the electronic supplementary material. The site data are also available on Dryad, https://dx.doi.org/10.5061/dryad.76gp5 [56].