Abstract
Ocean acidification (OA) is a pressing threat to reef-building corals, but it remains poorly understood how coral calcification is inhibited by OA and whether corals could acclimatize and/or adapt to OA. Using a novel geochemical approach, we reconstructed the carbonate chemistry of the calcifying fluid in two coral species using both a pH and dissolved inorganic carbon (DIC) proxy (δ11B and B/Ca, respectively). To address the potential for adaptive responses, both species were collected from two sites spanning a natural gradient in seawater pH and temperature, and then subjected to three pHT levels (8.04, 7.88, 7.71) crossed by two temperatures (control, +1.5°C) for 14 weeks. Corals from the site with naturally lower seawater pH calcified faster and maintained growth better under simulated OA than corals from the higher-pH site. This ability was consistently linked to higher pH yet lower DIC values in the calcifying fluid, suggesting that these differences are the result of long-term acclimatization and/or local adaptation to naturally lower seawater pH. Nevertheless, all corals elevated both pH and DIC significantly over seawater values, even under OA. This implies that high pH upregulation combined with moderate levels of DIC upregulation promote resistance and adaptive responses of coral calcification to OA.
Keywords: Hawai‘i, Montipora capitata, Porites compressa, calcifying fluid, adaptive capacity, pH upregulation
1. Introduction
Rising sea surface temperatures and ocean acidification (OA) are among the most serious threats facing coral reefs today [1]. Consequently, the many ecosystem services provided by coral reefs, such as coastal protection and the income generated via tourism, fisheries and other resources [2], are also at risk. As atmospheric CO2 concentrations continue to increase, it is therefore critical to understand if and how tropical reef corals may be able to acclimatize and/or adapt to ocean acidification and warming.
In some locations, coral reefs already naturally experience lower pH levels and/or warmer seawater temperatures that are not expected to occur elsewhere until the middle or end of this century [3–6]. Such environments represent important analogues for future ocean conditions, and are well suited to assess the capacity of reef corals to acclimatize and/or adapt to warmer, more acidic conditions in their natural environment, as well as over more realistic time scales. One such location is Hawai'i's Kāne‘ohe Bay where seawater already has a lower pH throughout the year (−0.1 to −0.2 pH units), and is warmer by +1–2°C in summer, compared to present-day offshore waters, or conditions on some other nearby reefs [7–9]. Conditions similar to those in Kāne‘ohe Bay are not expected to occur on many other Hawaiian reefs until at least the middle of the century under high CO2 emissions, or the end of the century under moderate CO2 emissions [10]. Nevertheless, abundant coral reefs not only exist within Kāne‘ohe Bay, but they have recovered from catastrophic human disturbance which reduced coral cover to only a few percent by the late 1970s [9], and did so under conditions of low pH and high temperature. Thus, the environmental history of Kāne‘ohe Bay provides a unique opportunity to study whether decade-long exposure to low-pH and high-temperature conditions has influenced the ability of corals to cope with the chronic and even more extreme ocean acidification and warming that is expected by the end of this century under high CO2 emissions.
OA is the decrease of seawater pH, carbonate ion concentration and saturation state of aragonite (Ωarag) due to uptake of atmospheric CO2 by the surface ocean. In tropical reef corals, OA often results in lower calcification rates [11–13], although this is not always the case [12,14,15]. Despite many years of research, however, it remains poorly understood why coral calcification often decreases in response to OA, and why some corals are more resistant to OA than others. Deciphering the mechanisms underlying differential OA sensitivity is challenging, in part because corals precipitate their aragonite skeleton from an extracellular, semi-isolated space between their tissue and skeleton (the calcifying fluid) that is extremely difficult to access. Using techniques such as geochemical tracers (e.g. the boron isotope pH proxy), microsensors and pH-sensitive dyes, it is now well established that corals exert significant control over the chemical composition of their calcifying fluid. By exchanging H+ for Ca2+ ions (or possibly other cations), corals significantly elevate the pH of their calcifying fluid (pHcf) over the external seawater pH [16–20]. This capacity to upregulate pHcf is maintained even under OA conditions, although pHcf typically decreases under acidification as compared to ambient seawater pH levels [16,20,21]. However, pHcf not only responds to changes in seawater pH, but also to total alkalinity and dissolved inorganic carbon (DIC) [22]. These workers showed that the chemistry in the calcifying fluid is influenced by more than one component of the carbonate system, and suggested that corals may regulate the exchange of multiple chemical species in order to maintain calcification [22]. Interestingly, some coral species upregulate pHcf more than others [17,23] or even maintain high pHcf independent of changes in external seawater pH [24,25]. Although high pHcf is not always linked to greater resistance of calcification to OA [21], strong control over pHcf generally appears to be a critical mechanism involved in coral calcification, and varying capacity to upregulate pHcf may underlie differential resistance of coral calcification to OA.
Although characterization of internal pH upregulation has significantly advanced our understanding of coral calcification, a second parameter of the carbonate system is needed to calculate the saturation state of the calcifying fluid (Ωcf), which almost certainly plays a critical role in the rate of precipitation of the coral skeleton. It is often assumed that corals also elevate DIC inside the calcifying fluid (DICcf) above seawater DIC values [17,26], and when combined with pH upregulation, this results in high Ωarag values (approx. 10–25) that promote the relatively rapid calcification rates typical of tropical corals. This view has been supported by studies using skeletal B/Ca as a proxy for internal carbonate ion concentration ([carb]cf) [21,27–29], but the ubiquity of elevated DICcf and [carb]cf was challenged by the first direct measurements of [carb]cf using microsensors [18]. Cai et al. [18] suggested that high Ωcf can also be achieved without significantly elevating DICcf above seawater values, if pHcf is also upregulated to a greater degree than reported in some previous studies. Such a strategy may be advantageous to cope with OA because fewer protons need to be transported to achieve a given pH level due to the lower buffer capacity of the calcifying fluid [18]. This discrepancy highlights the need to better understand DIC dynamics inside the calcifying fluid under both ambient and low pH conditions, and to examine the potential role of DIC upregulation as a mechanism that could confer resistance to OA in tropical corals.
Some corals have considerable capacity to acclimatize and/or adapt to OA (C.P.J. & R.J.T. 2017, unpublished data). Specifically, individuals of the reef-building Hawaiian coral Porites compressa from Kāne‘ohe Bay, where seawater pH is 0.1–0.2 units lower compared to offshore, were significantly less sensitive to acidification and calcified significantly more under simulated OA conditions than conspecifics from a nearby high-pH site where the chemistry is more representative of present-day offshore waters [30]. In contrast, Montipora capitata corals from the same two locations had similar pH tolerance under OA, but generally calcified faster than P. compressa corals [30]. Although these findings highlight the adaptive capacity of reef corals, the specific calcification mechanisms that underlie greater resistance to OA and drive such adaptive responses remain unknown. Given the clearly demonstrated role of internal pH upregulation in facilitating coral calcification, and the potential importance of DIC upregulation for calcification as well, differing capacities to upregulate pH or DIC may play critical roles in facilitating coral acclimatization and/or adaptation to OA.
To elucidate the calcification mechanisms underlying the adaptive capacity of Hawaiian corals, we analysed coral skeletons from a combined ocean acidification and warming experiment to investigate internal pH and DIC upregulation (via skeletal boron isotopes and B/Ca ratios) in P. compressa and M. capitata corals collected from the low-pH site, Kāne‘ohe Bay, and the nearby high-pH site, Waimānalo Bay. This is the first time that these novel geochemical techniques [29] have been applied to understand whether coral can improve their tolerance to OA and climate change based on their environmental history. These corals were maintained at three pH levels (8.04, 7.88, 7.71 ± 0.02; reported on the total hydrogen ion scale, pHT) in combination with two temperature levels (ambient, +1.5°C ± 0.1°C). We hypothesized that corals with higher OA tolerance (i.e. M. capitata from both sites and P. compressa from Kāne‘ohe Bay) upregulate internal pH and/or DIC more than corals with lower OA tolerance (i.e. P. compressa from Waimānalo Bay), enabling them to calcify faster and to better resist OA than the more sensitive corals.
2. Methods
(a). Coral collection sites
Coral branches from 11 to 12 colonies of M. capitata and P. compressa were collected from each of two different sites (spanning natural gradients in seawater chemistry and temperature) on O'ahu Island, Hawai'i, in October 2011. Kāne‘ohe Bay corals were collected from reefs adjacent to the Hawai'i Institute of Marine Biology (HIMB) in Kāne‘ohe Bay (21°26′6″ N, 157°47′12″ W). This site represents future ocean conditions due to naturally low pH (−0.15 to −0.20 pH units below offshore; mean pHT approx. 7.85–7.90) and elevated temperatures (+1–2°C above offshore during the May–October warm season; mean monthly maximum temperature is 28.0°C versus 26.4–27.0°C in offshore waters, or on many Hawaiian reefs) [7–9,30]. Low pH at this site is the result of net calcification and net heterotrophy within Kāne‘ohe Bay, whereas elevated temperature is the result of summer heating along with shallow water depth and long residence times [7–9,30]. Waimānalo Bay corals were collected offshore Kaiona Beach Park (21°19′36″ N, 157°40′54″ W; 18 km to the southeast of Kāne‘ohe Bay). This site experiences higher pH and lower temperature than at HIMB, and these values average close to present-day offshore conditions (mean pHT approx. 8.05; mean monthly maximum temperature approx. 27.0°C) [31]. Daily variability in seawater temperature, pHT and DIC is similar at the two sites, with a mean temperature range of approximately 2°C, a mean pHT range of approximately 0.2 pH units and a mean DIC range of approximately 200 µmol kg−1, respectively [32]. Seawater DIC and total alkalinity, however, are both slightly lower at the Kāne‘ohe site (approx. 1940 µmol kg−1 and approx. 2150 µeq kg−1, respectively) than at the Waimānalo site (approx. 2000 µmol kg−1 and approx. 2300 µeq kg−1, respectively) due to high rates of net calcification within Kāne‘ohe Bay [7,31].
(b). Experimental protocol
After collection, corals were allowed to recover and acclimate in an indoor, flow-through aquarium system at HIMB for 2.5 months prior to the start of the experiment in December 2011. During this time, they were maintained at 26–27°C and received seawater from Kāne‘ohe Bay. This acclimation period in a common garden allowed us to exclude short-term environmental history as a factor in the observed responses to experimental treatments. Corals were maintained under experimental treatment conditions representing three pHT levels (8.04, 7.88, 7.71 ± 0.02) crossed by two temperature levels (present-day offshore, +1.5°C ± 0.1°C) for a total of 14 weeks. During the first five weeks, corals were kept at seasonal maximum temperatures (control = 26.8°C, control +1.5°C = 28.3°C), followed by nine weeks at mean annual temperatures, which were 1.5°C lower than during the first temperature phase (control = 25.3°C, control +1.5°C = 26.8°C). Further details can be found in the electronic supplementary material. Electronic supplementary material, table S1 summarizes the environmental conditions of each treatment.
(c). Physiological analyses
Calcification rates were determined individually for each temperature phase using the buoyant weight technique [33] and normalized to weight at the beginning of the corresponding temperature phase. These phase-specific rates guided the sampling of skeletal material for geochemical analyses, and are used here to interpret the geochemical results because only the most recently deposited skeleton was targeted for geochemical sampling (see details below). However, normalizing calcification to weight rather than surface area does not affect the interpretation of our data since the choice of normalization had little impact on the calcification rate results. Therefore, the calcification rates shown here represent growth during the second temperature phase, where corals were maintained at either 25.3 or 26.8°C for nine weeks. These calcification rates are presented here for (i) all corals used in the experiment (n = typically 11–12 colonies per treatment) and (ii) only the subset of corals that were used for geochemical analyses (n = 2–9 colonies per treatment, though typically 5–6). Please see electronic supplementary material, table S1 for details on sample size per treatment.
(d). Geochemical analyses
Corals were stained with alizarin red (15 mg l−1 for 7 h) before the start of the experiment but not again at the beginning of the second temperature phase. Therefore, only the most recently deposited skeleton from this second temperature phase was targeted for geochemical sampling. This phase was much longer (nine versus five weeks), providing plenty of skeletal material that could be confidently sampled (guided by known calcification rates, see the electronic supplementary material for more details). This is an established sampling approach (e.g. [23,34]) that has previously been applied successfully to the same Hawaiian coral species as in this study [34]. The uppermost layer of the bleached and dried branch tips was then gently shaved with a diamond-tipped Dremel tool [23]. Boron isotopes (δ11B) and B/Ca ratios were analysed following the method of McCulloch et al. [35]. Further details regarding the geochemical analyses can be found in the electronic supplementary material.
(e). Carbonate chemistry of the calcifying fluid
The pH of the calcifying fluid was estimated using the coral skeletal boron isotopic composition following established methods [17,36]. Biological pH regulation (ΔpH) was then calculated as the difference between pHcf and seawater pH [36]. Recently, it has also been shown that the coral skeletal B/Ca concentration can be used to constrain the DIC concentration of the coral calcifying fluid (DICcf) [29,37]. We therefore estimated the carbonate ion concentration within the calcifying fluid 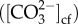 from measurements of both pHcf and coral skeletal B/Ca [29]. Biological DIC-upregulation within the calcifying fluid was calculated as the ratio of DICcf and seawater DIC (DICcf/DICsw). Further details are given in the electronic supplementary material.
from measurements of both pHcf and coral skeletal B/Ca [29]. Biological DIC-upregulation within the calcifying fluid was calculated as the ratio of DICcf and seawater DIC (DICcf/DICsw). Further details are given in the electronic supplementary material.
(f). Statistical analyses
Generalized linear mixed model (GLMM) analysis was used to test for the effects of species, site, pH and temperature on coral δ11B, pHcf, ΔpH, B/Ca, DICcf, DICcf/DICsw, 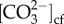 , Ωcf and calcification rate of the two coral species. Parent colony was a random factor nested within both species and site. Tukey adjusted p-values were used for post hoc tests when main effects were significant. When a significant interaction was observed, multiple pair-wise comparisons were conducted using Tukey adjusted p-values. p-values less than 0.05 were considered significant. GLMM analyses were performed using SAS software, version 9.3 of the SAS System for Windows. Linear regressions were calculated for the relationship between pHcf and seawater pH as well as DICcf using SigmaPlot version 12.5.
, Ωcf and calcification rate of the two coral species. Parent colony was a random factor nested within both species and site. Tukey adjusted p-values were used for post hoc tests when main effects were significant. When a significant interaction was observed, multiple pair-wise comparisons were conducted using Tukey adjusted p-values. p-values less than 0.05 were considered significant. GLMM analyses were performed using SAS software, version 9.3 of the SAS System for Windows. Linear regressions were calculated for the relationship between pHcf and seawater pH as well as DICcf using SigmaPlot version 12.5.
3. Results
(a). Internal pH and DIC upregulation
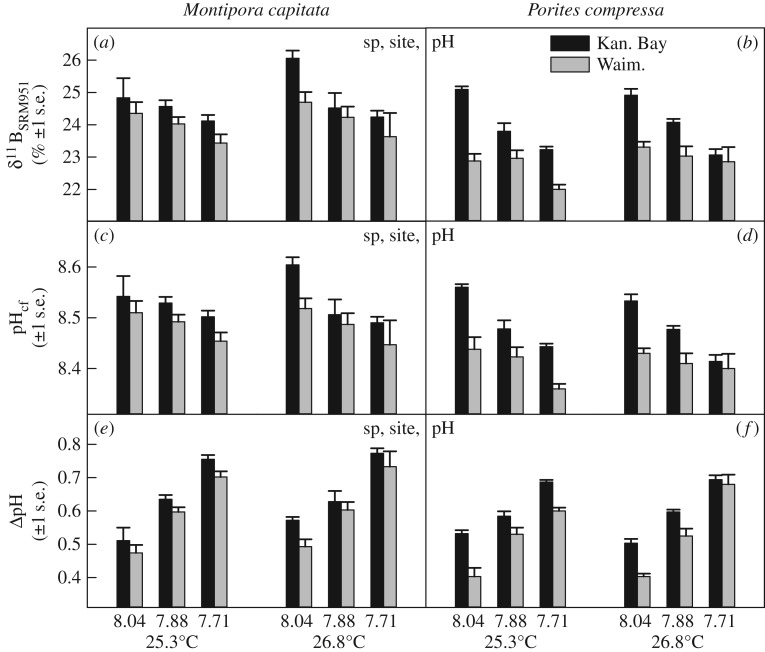
Using skeletal boron isotopes (δ11B) as pH proxies, corals from both species and sites elevated the pH of their calcifying fluid (pHcf) above seawater pH levels (by up to 0.77 pH units), even under future ocean acidification scenarios (figure 1a–f). We found that δ11B and pHcf were nevertheless highly correlated with seawater pH (electronic supplementary material, figure S1a), but biological pH upregulation resulted in internal pH changes that were only 20–40% as large as the changes in external seawater pH (electronic supplementary material, figure S1b). However, δ11B and consequently also pHcf and biological pH upregulation (ΔpH) differed significantly between species, sites and pH treatments (figure 1a–f; electronic supplementary material, table S3). Higher pHcf values were generally found for M. capitata compared with P. compressa, and Kāne‘ohe Bay corals of both species upregulated pHcf more than Waimānalo Bay corals (figure 1c–f). Furthermore, pHcf was lowest at the lowest seawater pH (figure 1c,d), yet corals upregulated pHcf the most at these lower seawater pH levels (figure 1e,f).
Figure 1.
Coral skeletal boron isotopes (δ11B) (a,b), calcifying fluid pH (pHcf) (c,d) and biological pH upregulation (ΔpH) (e,f) in M. capitata and P. compressa from Kāne‘ohe Bay and Waimānalo Bay, Hawai'i. Mean ± 1 s.e. are shown. Significant species (sp), site, pH and temperature (temp) effects are indicated when present (electronic supplementary material, table S3).
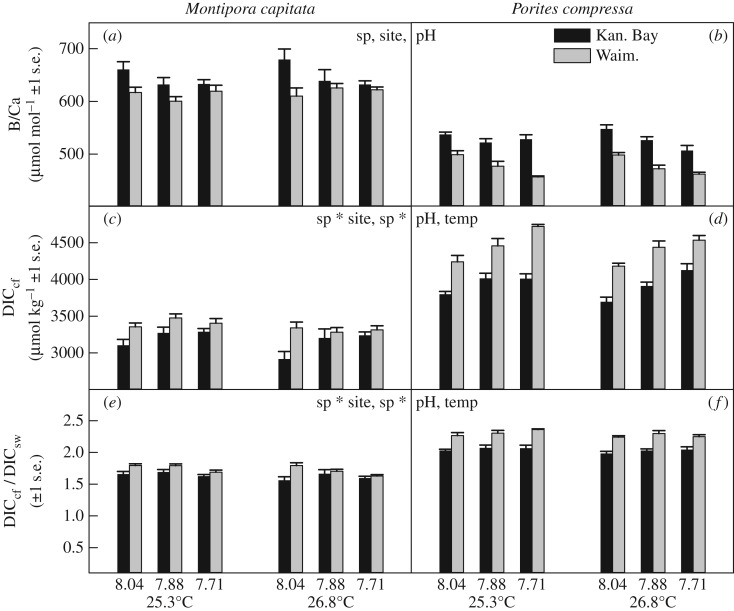
Coral skeletal B/Ca ratios also differed significantly between species and sites, and among pH treatments (figure 2a,b; electronic supplementary material, table S4). Again, M. capitata had generally higher B/Ca than P. compressa, and Kāne‘ohe Bay corals from both species had higher B/Ca than Waimānalo Bay corals. The lowest B/Ca values were observed at medium and low pH treatments. When calcifying fluid DIC concentrations (DICcf) and internal DIC upregulation (DICcf/DICsw) were estimated from B/Ca and pHcf, all corals were found to significantly upregulate DICcf above seawater DIC values (by a factor of 1.6–2.1×) (figure 2c–f).
Figure 2.
Coral skeletal B/Ca ratios (a,b), calcifying fluid DIC concentration (DICcf) (c,d) and biological DIC upregulation (DICcf/DICsw) (e,f) in M. capitata and P. compressa from Kāne‘ohe Bay and Waimānalo Bay, Hawai'i. Mean ± 1 s.e. are shown. Significant species (sp), site, pH and temperature (temp) effects are indicated when present (electronic supplementary material, table S4).
However, for both DICcf and DICcf/DICsw, significant interactive effects between species and site as well as species and pH were observed (figure 2c–f; electronic supplementary material, table S4). Porites compressa from Waimānalo Bay had higher DICcf and thus higher DICcf/DICsw than conspecifics from Kāne‘ohe Bay, which in turn had higher DICcf and DICcf/DICsw than M. capitata from Waimānalo Bay. The lowest DICcf and DICcf/DICsw values were observed in M. capitata from Kāne‘ohe Bay. Regarding interactive effects between species and pH, P. compressa at low and medium seawater pH had the highest DICcf and DICcf/DICsw values, respectively, whereas M. capitata at high and low seawater pH had the lowest such values. Additionally, DICcf and DICcf/DICsw were also significantly affected by temperature, with generally greater values observed at 25.3 than at 26.8°C (electronic supplementary material, table S4). The pHcf values were inversely correlated with DICcf values for all species and sites, although P. compressa had much higher R2 values than M. capitata (electronic supplementary material, figure S2).
(b). Calcifying fluid saturation state and calcification rates
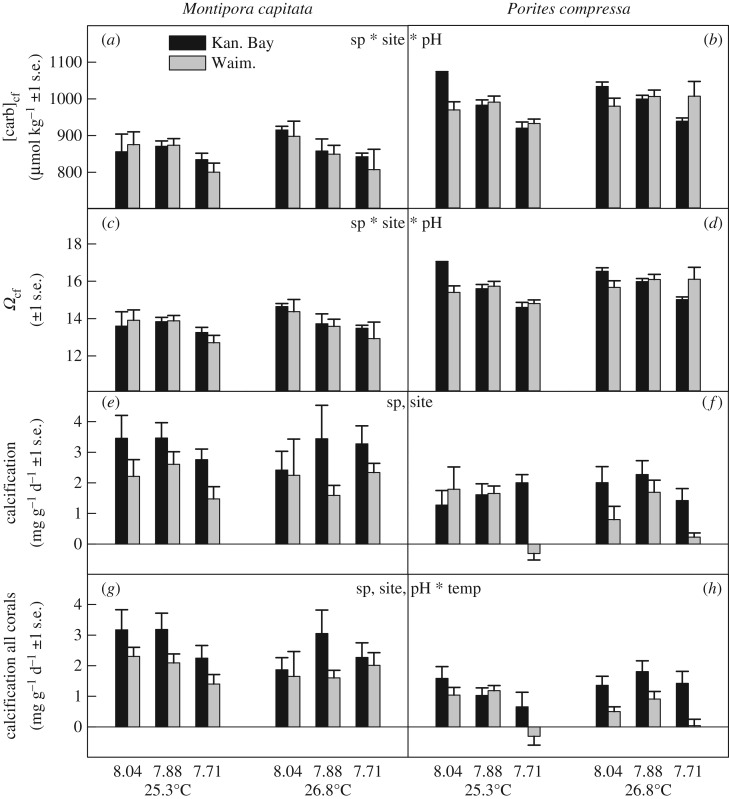
Since all corals elevated DICcf significantly above seawater values, the carbonate ion concentration 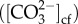 and aragonite saturation state of the calcifying fluid (Ωcf) were also elevated significantly above seawater values (figure 3a–d), with Ωcf reaching values between 13.3 and 17.0. For both
and aragonite saturation state of the calcifying fluid (Ωcf) were also elevated significantly above seawater values (figure 3a–d), with Ωcf reaching values between 13.3 and 17.0. For both 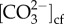 and Ωcf, a significant interaction between species, site and pH was observed (electronic supplementary material, table S5): P. compressa corals from Kāne‘ohe Bay at high seawater pH had the highest values, whereas M. capitata from Waimānalo Bay at low seawater pH had the lowest values (figure 3a–d).
and Ωcf, a significant interaction between species, site and pH was observed (electronic supplementary material, table S5): P. compressa corals from Kāne‘ohe Bay at high seawater pH had the highest values, whereas M. capitata from Waimānalo Bay at low seawater pH had the lowest values (figure 3a–d).
Figure 3.
Calcifying fluid carbonate ion concentration ([carb]cf) (a,b), calcifying fluid aragonite saturation state (Ωcf) (c,d), calcification rates for corals used for geochemical analyses (e,f) and calcification rates for all corals in the experiment (g,h) in M. capitata and P. compressa from Kāne‘ohe Bay and Waimānalo Bay, Hawai'i. Mean ± 1 s.e. are shown. Significant species (sp), site, pH and temperature (temp) effects are indicated when present (electronic supplementary material, table S5).
Calcification rates obtained using the buoyant weight method differed significantly between species and sites, but not among pH or temperature treatments (figure 3e,f; electronic supplementary material, table S5) for the subset of corals examined for geochemistry. Montipora capitata generally calcified more than P. compressa, and Kāne‘ohe Bay corals calcified more than corals from Waimānalo Bay. When calcification rates were analysed for all corals in the experiment, rather than just the subset analysed for geochemistry, a significant interactive effect between pH and temperature was also observed (in addition to the same species and site effects as above): corals calcified fastest at high seawater pH and 25.3°C, and slowest at the same temperature combined with low seawater pH (figure 3g,h; electronic supplementary material, table S5). Notably, only the 25.3°C/pH = 7.71 treatment resulted in negative calcification rates, or net dissolution, in P. compressa corals from Waimānalo Bay (figure 3f,h).
4. Discussion
(a). Drivers of long-term acclimatization and/or adaptation to ocean acidification
Corals that originated from Kāne‘ohe Bay (where seawater is 1–2°C warmer in summer and pH is 0.1–0.2 units lower compared with offshore) calcified faster and significantly more under simulated OA conditions than conspecifics from Waimānalo Bay, a nearby high-pH site where water chemistry and temperature are more similar to present-day offshore waters (figure 3). Interestingly, this resistance to acidification was consistently linked to a combination of higher pHcf and lower DICcf in Kāne‘ohe Bay corals compared to Waimānalo Bay corals (figures 1 and 2). This pattern was particularly evident regarding differences in DICcf, as demonstrated by the significant interactive effects between species and pH, and species and site (electronic supplementary material, table S4). Although similar interactive effects were not observed for calcification rates, they nevertheless mirrored trends in DICcf, with highest rates being correlated with lowest DICcf (figures 2c,d and 3e–h).
These observations suggest that combining high pHcf and moderately elevated DICcf is a critical calcification mechanism underlying the potential for corals to mount adaptive responses under OA and warming. By modulating the interactive dynamics of pH and DIC upregulation within their calcifying fluid, these corals have a significant ability to acclimatize and/or adapt to OA by modifying the physiological processes involved in calcification. Since all corals were given 2.5 months to acclimate in a common garden prior to the start of the experiment, any fast-acting mechanisms of pH acclimatization should have been exhausted before the start of the experiment, leaving long-term acclimatization (i.e. physiological changes which operate over a time scale of greater than 2.5 months) or local adaptation (i.e. fixed, heritable differences among coral genotypes) as possible explanations for the distinct responses to acidification we observed. Although rapid mechanisms involved in coral temperature acclimation can occur within a few weeks [38,39], previous studies have failed to find any evidence that corals acclimatize to acidification even after 1 year of exposure [13,20,40], suggesting that local adaptation may be the primary driver of the differences in OA tolerance shown here. It is also important to consider that low pH may or may not be the ultimate driving force behind the differential calcification rates and OA resistance we observed. Instead, it is possible that separate environmental factors, such as elevated temperature, have selected for fast-growing, pH-tolerant corals in Kāne‘ohe Bay, and that their enhanced OA resistance is a by-product of selection due to other factors. Whether or not low pH is the primary agent leading to increased OA resistance among these corals, this study is the first to detail mechanisms which allow corals to mount adaptive responses to acidification.
Despite clear site-specific differences in pH and DIC upregulation, both coral species significantly elevated their internal pHcf and DICcf above seawater values (figures 1 and 2; electronic supplementary material, figure S1). pHcf values were elevated above seawater pH by up to 0.77 pH units, supporting the idea that biological pH upregulation is a universal mechanism employed for calcification in scleractinian corals [16–20,24,25,36]. Similarly, our study confirms that internal pHcf typically decreases linearly under OA, albeit with a much lower sensitivity (20–40%) because pH upregulation increases under such conditions (figure 1e,f; electronic supplementary material, figure S1) [16,17,20]. Remarkably, this relationship holds true for most coral species studied to date, except for Porites corals from a highly variable reef flat and natural CO2 vents [24,25].
Our study is among the first to infer internal DICcf concentrations for tropical reef corals, and the first to assess how long-term acclimatization and/or adaptation to naturally lower seawater pH and higher summer temperatures modulate the response of DICcf to OA. In M. capitata and P. compressa, DICcf concentrations ranged from approximately 3300–4200 µmol kg−1 and were thus approximately 1.6–2.2 times higher than seawater DIC, respectively (figure 2e,f). These results are generally consistent with other boron-based studies showing that corals significantly elevate DICcf above seawater concentrations [21,28,29]. However, our findings contradict recent microsensor measurements of the calcifying fluid showing carbonate ion concentrations of 600–1500 µmol kg−1 and DICcf values close to seawater [18]; in addition, our estimates give much narrower species-specific values that co-vary (figures 2 and 3). Given the spatially dependent sensitivity of microsensor studies and the inability to conduct pH and carbonate ion microsensor profiles simultaneously within the same pocket of calcifying fluid [18], direct comparisons between microsensor and stable isotope studies are challenging, at best. Importantly, our findings are in strong contrast to the study of Allison et al. [27], which estimated that DICcf would be less than half that of seawater DIC if borate is assumed to substitute for carbonate ions during mineral precipitation. Instead, the authors argued that in order to make sense of their geochemical data, borate must substitute for bicarbonate ions or a combination of bicarbonate and carbonate ions in the crystal lattice, which is then followed by recrystallization of the calcium bicarbonate into aragonite, but without boron exchange occurring during this process. A more parsimonious explanation is that borate substitutes for carbonate ions alone during mineral precipitation, as we assume here, and that the discrepancy between these two studies can now be explained by the order of magnitude difference between the experimentally established partitioning coefficient used in our study and the one used by Allison et al. [27].
The observed correlation between high calcification rates, high pHcf and relatively low DICcf in Kāne‘ohe Bay corals provides novel insights into the calcification mechanisms underlying coral resistance to acidification and informs our understanding of the adaptive capacities of corals under OA and climate change. Clearly, maintaining high pHcf is favourable for calcification as it promotes the conversion of bicarbonate ions and carbon dioxide to carbonate ions, resulting in a high saturation state within the calcifying fluid. The advantages of maintaining only moderately elevated DICcf under acidification, however, are less clear because higher DICcf levels would result in a proportional increase in saturation state at a given pH. However, if moderately elevated DICcf is combined with higher pHcf, as observed in M. capitata, this also results in high saturation states. Such a strategy can be advantageous because compared with a high DICcf /low pHcf scenario, it is less energetically expensive to maintain a given pHcf at lower DICcf because the lower buffer capacity of the fluid requires the removal of fewer protons to achieve that pH. Consequently, a low DICcf/high pHcf scenario may have allowed Kāne'ohe corals to grow faster than Waimānalo Bay corals, especially if the energy savings were invested in other key processes, such as the production of organic matrix or proton excretion from the coelenteron. Cai et al. [18] already pointed out that a lower DICcf scenario may be particularly advantageous under OA if proton removal from the coelenteron becomes increasingly difficult due to rising seawater proton concentration [41]. Interestingly, lower sensitivity of calcification to OA was also linked to lower DICcf values in Pocillopora damicornis from subtropical Western Australia, though this species also maintained lower pHcf than OA-sensitive Acropora yongei from the same location [21]. The low DICcf/high pHcf scenario could also have drawbacks because the lower buffering capacity in this scenario also means that pH upregulation is more vulnerable to changes in the physiological and biochemical conditions that control proton pumping [18]. Alternatively, it is also possible that DIC transport is rate limited, such that lower DICcf may be a by-product of higher calcification rates, rather than a driver of them. Consequently, fast calcification rates would result in low DICcf due to the more rapid depletion of DIC within the calcifying fluid. While our findings show that the low DICcf/high pHcf scenario is clearly linked to adaptive responses and resistance to OA, the underlying reasons remain currently poorly understood. Further research into the sources, pathways and energetic requirements of DICcf transport is required to resolve this.
(b). Drivers of species-specific calcification resistance to ocean acidification
A growing body of literature shows that some coral species are naturally more resistant to OA than others [12,15]. The mechanisms underlying resistance to OA in these species, however, are poorly understood, although factors such as calcification rate or growth form may play a role [42]. In our study, branching M. capitata generally calcified faster and also maintained growth better under OA conditions than branching P. compressa (figure 3). As already observed for Kāne‘ohe Bay corals, the high calcification rates of M. capitata were linked to high pHcf and moderately elevated DICcf, whereas the lower calcification rates of P. compressa were linked to lower pHcf but higher DICcf compared with M. capitata (figures 1–3). This suggests again that high pH in combination with moderate DIC upregulation promotes faster growth and resistance to OA.
It should be noted that Ωcf was somewhat higher in P. compressa (approx. 15–17) than in M. capitata (approx. 13–15), yet the latter species calcified faster. The higher calcification rates observed under lower Ωcf are counterintuitive, given that the rate of abiotic mineral precipitation is proportional to the saturation state. Even more counterintuitive is the finding that P. compressa corals from Waimānalo had negative calcification rates at 25.3°C and a pH of 7.71 (figure 3f,h), yet nevertheless maintained high Ωcf values of approximately 15 (figure 3d). Similar maintenance of high Ωcf despite impaired growth rates has also been observed in severely heat-stressed corals [28]. The apparent decoupling between calcification rates and Ωcf in more than just this treatment points to the importance of additional, biological factors for mediating coral calcification. This includes the production and delivery of organic matrix molecules [43], which can induce aragonite precipitation in vitro at Ωarag ∼1.3 [44]. Particularly in OA-sensitive species such as P. compressa, it may be that low pH impairs calcification primarily via disruption of processes such as organic matrix production, while maintenance of high Ωcf appears unaffected. Alternatively, OA may reduce net coral calcification indirectly by increasing rates of carbonate dissolution in previously deposited skeleton. Indeed, many P. compressa from Waimānalo showed axial growth well beyond the alizarin stain line under the lowest pH, allowing us to sample these corals for geochemical analyses, and yet they also experienced a net decrease in skeletal mass over time.
Our study confirms previous findings that corals with high resistance to OA also have high pHcf levels, and that pHcf is only moderately responsive to external seawater pH [24,25]. For example, massive Porites corals exposed to a natural gradient of seawater pH at the CO2 seeps in Papua New Guinea maintained high internal pHcf down to seawater pH levels expected by the end of this century, and pHcf decreased slightly only when seawater pH declined further [25]. Calcification estimates derived from coring these Porites, as well as a calcification model, suggest that their growth will not be affected by OA until at least end-of-century conditions [3,25]. Similarly, Porites cylindrica from the highly dynamic Heron Island reef flat maintained high pHcf and skeletal growth independent of large fluctuations in external seawater pH or superimposed OA treatments [24]. Although these studies highlight the importance of biological pH upregulation, they were not able to fully constrain the carbonate chemistry of the calcifying fluid using a second parameter, as we do here with DICcf. It is unknown if these other, acidification-resistant coral species also maintain moderately elevated DICcf concentrations. Our study is therefore among the first to demonstrate that coral resistance to OA is a complex interplay between pH and DIC upregulation.
It is important to note here that our findings are potentially biased towards more stress-resistant genotypes due to the strict criteria that we imposed while selecting corals for geochemical analyses (see electronic supplementary material, methods). These criteria were imposed as quality control during the sampling process but may also have resulted in selecting more stress-resistant genotypes. However, the same criteria were applied to all corals so that the observed differences between species, sites and treatments are nevertheless valid. Moreover, our robust samples sizes in most treatments (electronic supplementary material, table S1) ensured that intraspecific diversity was still present, with both more and less stress-resistant genotypes present in each treatment.
(c). Implications for the future of coral reefs
Our findings demonstrate that as oceans continue to warm and acidify, coral pH and DIC upregulation will largely be unaffected by small increases in temperature (within the seasonal range). However, both a priori resistance and long-term acclimatization and/or adaptation of coral calcification to OA seem to be facilitated by achieving high pHcf yet only moderately elevated DICcf. We demonstrate here that reef corals have a significant capacity to adjust their calcification strategies in response to long-term exposure to naturally lower seawater pH and warmer summer temperatures, as occurs naturally on the reefs of Kāne‘ohe Bay. Therefore, shifts to higher pHcf yet only moderately elevated DICcf are probably also possible in response to OA and warming occurring over the coming decades, potentially allowing at least some corals to develop resistance to OA and maintain calcification rates in the future.
Supplementary Material
Acknowledgements
We would like to thank K. Rankenburg for help in the laboratory, and C. Beltran, N. Silbiger, N. Ferguson, E. Brown, H. Putnam, E. DeCarlo, M. Donahue, P. Jokiel, K. Bahr and R. Gates for their discussions, assistance and advice.
Data accessibility
All data from this manuscript will be submitted to the Biological and Chemical Oceanography Data Management Office (BCO-DMO) of the U.S. National Science Foundation after publication.
Authors' contributions
C.P.J. and R.J.T. designed the experiment. C.P.J. conducted the experiment and physiological analyses. V.S. conducted the geochemical analyses and analysed the data. All authors were involved in the preparation of the paper.
Competing interests
We have no competing interests.
Funding
Funding was provided by the Australian Research Council Centre of Excellence for Coral Reef Studies and an ARC Laureate Fellowship to M.T.M. Funding for C.P.J. and R.J.T. was provided by a George Melendez Wright Climate Change Fellowship, Sea Grant Omnibus 2014–2016, Project ID#2180, Hawai'i Sea Grant Omnibus 2012–2014, Project ID# 1950, and the US National Science Foundation (OA#14-16889).
References
- 1.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 2.Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 29, 215–233. ( 10.1016/S0921-8009(99)00009-9) [DOI] [Google Scholar]
- 3.Fabricius KE, et al. 2011. Losers and winners on coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169. ( 10.1038/nclimate1122) [DOI] [Google Scholar]
- 4.Crook ED, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A. 2012. Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31, 239–245. ( 10.1007/s00338-011-0839-y) [DOI] [Google Scholar]
- 5.Schoepf V, Stat M, Falter JL, McCulloch MT. 2015. Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17639 ( 10.1038/srep17639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riegl B, Purkis SJ, Al-Cibahy AS, Al-Harthi S, Grandcourt E, Al-Sulaiti K, Baldwin J, Abdel-Moati MA. 2012. Coral bleaching and mortality thresholds in the SE Gulf: Highest in the world. In Coral reefs of the gulf: adaptation to climatic extremes (eds Riegl B, Purkis SJ), pp. 95–105. Berlin, Germany: Springer. [Google Scholar]
- 7.Shamberger KEF, Feely RA, Sabine CL, Atkinson MJ, DeCarlo EH, Mackenzie FT, Drupp PS, Butterfield DA. 2011. Calcification and organic production on a Hawaiian coral reef. Mar. Chem. 127, 64–75. ( 10.1016/j.marchem.2011.08.003) [DOI] [Google Scholar]
- 8.Guadayol Ò, Silbiger NJ, Donahue MJ, Thomas FIM. 2014. Patterns in temporal variability of temperature, oxygen and pH along an environmental gradient in a coral reef. PLoS ONE 9, e85213 ( 10.1371/journal.pone.0085213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahr KD, Jokiel PL, Toonen RJ. 2015. The unnatural history of Kāne‘ohe Bay: coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3, e950 ( 10.7717/peerj.950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeke RK, Jokiel PL, Buddemeier RW, Brainard RE. 2011. Projected changes to growth and mortality of Hawaiian corals over the next 100 years. PLoS ONE 6, e18038 ( 10.1371/journal.pone.0018038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2013. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398. ( 10.4319/lo.2013.58.1.0388) [DOI] [Google Scholar]
- 12.Schoepf V, et al. 2013. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 8, e75049 ( 10.71371/journal.pone.0075049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT.. 2008. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27, 473–483. ( 10.1007/s00338-008-0380-9) [DOI] [Google Scholar]
- 14.Jury CP, Whitehead RF, Szmant AM. 2010. Effects of variation in carbonate chemistry on the calcification rates of Madracis auretenra (Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644. ( 10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 15.Comeau S, Carpenter RC, Nojiri Y, Putnam HM, Sakai K, Edmunds PJ. 2014. Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc. R. Soc. B 281, 20141339 ( 10.1098/rspb.2014.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcomb M, Venn AA, Tambutte E, Tambutte S, Allemand D, Trotter J, McCulloch M. 2014. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 ( 10.1038/srep05207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulloch MT, Falter J, Trotter J, Montagna P. 2012. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2, 623–627. ( 10.1038/nclimate1473) [DOI] [Google Scholar]
- 18.Cai W-J, et al. 2016. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Comm. 7, 11144 ( 10.1038/ncomms11144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Horani FA, Al-Moghrabi SM, de Beer D. 2003. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426. ( 10.1007/s00227-002-0981-8) [DOI] [Google Scholar]
- 20.Venn AA, Tambutte E, Holcomb M, Laurent J, Allemand D, Tambutte S. 2013. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl Acad. Sci. USA 110, 1634–1639. ( 10.1073/pnas.1216153110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comeau S, Cornwall CE, McCulloch MT. 2017. Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 7, 7573 ( 10.1038/s41598-017-08003-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comeau S, Tambutte E, Carpenter RC, Edmunds PJ, Evensen NR, Allemand D, Ferrier-Pages C, Tambutte S, Venn A. 2017. Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. B 284, 20161669 ( 10.1098/rspb.2016.1669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoepf V, McCulloch MT, Warner ME, Levas SJ, Matsui Y, Aschaffenburg M, Grottoli AG. 2014. Short-term coral bleaching is not recorded by skeletal boron isotopes. PLoS ONE 9, e112011 ( 10.1371/journal.pone.0112011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiou L, Falter J, Trotter J, Kline DI, Holcomb M, Dove SG, Hoegh-Guldberg O, McCulloch M. 2015. pH homeostasis during coral calcification in a free ocean CO2 enrichment (FOCE) experiment, Heron Island reef flat, Great Barrier Reef. Proc. Natl Acad. Sci. USA 112, 13 219–13 224. ( 10.1073/pnas.1505586112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall M, Fietzke J, Schmidt GM, Fink A, Hofmann LC, de Beer D, Fabricius KE. 2016. Internal pH regulation facilitates in situ long-term acclimation of massive corals to end-of-century carbon dioxide conditions. Sci. Rep. 6, 30688 ( 10.1038/srep30688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holcomb M, Cohen AL, Gabitov RI, Hutter JL. 2009. Compositional and morphological features of aragonite precipitated experimentally from seawater and biogenically by corals. Geochim. Cosmochim. Acta 73, 4166–4179. ( 10.1016/j.gca.2009.04.015) [DOI] [Google Scholar]
- 27.Allison N, Cohen I, Finch AA, Erez J, Tudhope AW. 2014. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Comm. 5, 5741 ( 10.1038/ncomms6741) [DOI] [PubMed] [Google Scholar]
- 28.D'Olivo JP, McCulloch MT. 2017. Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci. Rep. 7, 2207 ( 10.1038/s41598-017-02306-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCulloch M, D'Olivo Cordero JP, Falter J, Holcomb M, Trotter J.. 2017. Coral calcification in a changing world and the interactive dynamics of pH and DIC up-regulation. Nat. Comm. 8, 15686 ( 10.1038/ncomms15686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drupp P, De Carlo EH, Mackenzie FT, Bienfang P, Sabine CL. 2011. Nutrient inputs, phytoplankton response, and CO2 variations in a semi-enclosed subtropical embayment, Kaneohe Bay, Hawaii. Aquat. Geochem. 17, 473–498. ( 10.1007/s10498-010-9115-y) [DOI] [Google Scholar]
- 31.Lantz CA, Atkinson MJ, Winn CW, Kahng SE. 2014. Dissolved inorganic carbon and total alkalinity of a Hawaiian fringing reef: chemical techniques for monitoring the effects of ocean acidification on coral reefs. Coral Reefs 33, 105–115. ( 10.1007/s00338-013-1082-5) [DOI] [Google Scholar]
- 32.Silbiger NJ, Guadayol Ò, Thomas FIM, Donahue MJ. 2014. Reefs shift from net accretion to net erosion along a natural environmental gradient. Mar. Ecol. Prog. Ser. 515, 33–44. ( 10.3354/meps10999) [DOI] [Google Scholar]
- 33.Jokiel PL, Maragos JE, Franzisket L. 1978. Coral growth: buoyant weight technique. In Coral reefs: research methods (eds Stoddart DR, Johannes RE), pp. 529–541. Paris, France: UNESCO. [Google Scholar]
- 34.Grottoli AG, Rodrigues LJ, Juarez C. 2004. Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar. Biol. 145, 621–631. ( 10.1007/s00227-004-1337-3) [DOI] [Google Scholar]
- 35.McCulloch MT, Holcomb M, Rankenburg K, Trotter J. 2014. Rapid, high-precision measurements of boron isotopic compositions in marine carbonates. Rapid Commun. Mass Spectrom. 28, 1–9. ( 10.1002/rcm.7065) [DOI] [PubMed] [Google Scholar]
- 36.Trotter J, et al. 2011. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet. Sci. Lett. 303, 163–173. ( 10.1016/j.epsl.2011.01.030) [DOI] [Google Scholar]
- 37.Holcomb M, DeCarlo TM, Gaetani GA, McCulloch M. 2016. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76. ( 10.1016/j.chemgeo.2016.05.007) [DOI] [Google Scholar]
- 38.Middlebrook R, Hoegh-Guldberg O, Leggat W. 2008. The effect of thermal history on the susceptibility of reef-building corals to thermal stress. J. Exp. Biol. 211, 1050–1056. ( 10.1242/jeb.013284) [DOI] [PubMed] [Google Scholar]
- 39.Bay RA, Palumbi SR. 2015. Rapid acclimation ability mediated by transcriptome changes in reef-building corals. Genome Biol. Evol. 7, 1602–1612. ( 10.1093/gbe/evv085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312. ( 10.1038/nclimate1200) [DOI] [Google Scholar]
- 41.Jokiel PL. 2011. Ocean acidification and control of reef coral calcification by boundary layer limitation of proton flux. Bull. Mar. Sci. 19, 639–657. ( 10.5343/bms.2010.1107) [DOI] [Google Scholar]
- 42.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC. 2014. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 59, 1081–1091. ( 10.4319/lo.2014.59.3.1081) [DOI] [Google Scholar]
- 43.Von Euw S, et al. 2017. Biological control of aragonite formation in stony corals. Science 356, 933–938. ( 10.1126/science.aam6371) [DOI] [PubMed] [Google Scholar]
- 44.Mass T, Drake Jeana L, Haramaty L, Kim JD, Zelzion E, Bhattacharya D, Falkowski Paul G. 2013. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 23, 1126–1131. ( 10.1016/j.cub.2013.05.007) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from this manuscript will be submitted to the Biological and Chemical Oceanography Data Management Office (BCO-DMO) of the U.S. National Science Foundation after publication.