ABSTRACT
Polymyxins are a last line of defense against multidrug-resistant Gram-negative pathogens. Recent pharmacological data show that intravenous polymyxins can cause nephrotoxicity in up to 60% of patients, and the plasma concentrations of polymyxins achieved with the currently recommended dosage regimens are suboptimal in a large proportion of patients. Simply increasing the daily dose of polymyxins is not possible due to nephrotoxicity. This study aimed to examine the protective effect of methionine against polymyxin-induced nephrotoxicity. Methionine (400 mg/kg of body weight), polymyxin B (35 mg/kg), a combination of methionine (100 or 400 mg/kg) and polymyxin B, and saline were administered to mice twice daily over 3.5 days. Kidneys were collected immediately at the end of the experiment for histological examination. The effect of methionine on the pharmacokinetics of polymyxin B was investigated in rats. The attenuation of polymyxin B (0.75 mM)-induced mitochondrial superoxide production by methionine (10.0 mM) was examined in rat kidney (NRK-52E) cells. Histological results revealed that the polymyxin-induced nephrotoxicity in mice was ameliorated by methionine in a dose-dependent manner. The methionine doses were well tolerated in the mice and rats, and the pharmacokinetics of polymyxin B in rats were not affected by methionine. In the group receiving polymyxin B-methionine, the total body clearance of polymyxin B was very similar to that in the group receiving polymyxin B alone (3.71 ± 0.57 versus 3.12 ± 1.66 ml/min/kg, P > 0.05). A substantial attenuation of polymyxin-induced mitochondrial superoxide production in NRK-52E cells was observed following pretreatment with methionine. Our results demonstrate that coadministration of methionine significantly ameliorated polymyxin-induced nephrotoxicity and decreased mitochondrial superoxide production in renal tubular cells.
KEYWORDS: methionine, nephrotoxicity, oxidative stress, polymyxins
INTRODUCTION
Over the last 2 decades there has been a significant increase in the incidence of infections caused by multidrug-resistant (MDR) Gram-negative bacteria (1). Even worse, a scarcity of novel antibiotics in the development pipeline leads the world to a state in which it is vulnerable to these difficult-to-treat infections (1). Polymyxins B and E (the latter of which is also known as colistin) are increasingly used as a last line of defense against Gram-negative superbugs (2). Polymyxins became available in the clinic in the late 1950s and were never subjected to contemporary drug development procedures. There was a significant lack of pharmacological information about polymyxins, until the past decade (3, 4). The clinical use of intravenous polymyxins has been limited by the potential nephrotoxicity, a dose-limiting factor which can occur in up to 60% of patients (5, 6). Therefore, there is an urgency to develop novel approaches to minimize polymyxin-induced nephrotoxicity.
Both megalin and PEPT2 are involved in the renal uptake of polymyxins (7, 8); however, the precise mechanism of polymyxin-induced nephrotoxicity remains unknown. The renal handling of polymyxins almost certainly contributes to their propensity for causing kidney tubular cell damage (9, 10). Following intravenous administration of colistin and polymyxin B, only a very small fraction of the dose is excreted in urine after filtration by glomeruli (11, 12). The majority of the filtered polymyxin molecules undergo extensive tubular reabsorption, leading to the very significant accumulation of polymyxin in tubular cells, which causes renal tubular damage (9, 11–13). Our recent studies demonstrated that polymyxin-induced nephrotoxicity involves apoptosis in kidney tubular cells, which appears to be partially mediated by the generation of reactive oxygen species (ROS) and caspase activation (14–16). It has been demonstrated that several antioxidants can attenuate polymyxin-induced nephrotoxicity in animals (16–19).
Methionine, one of the most easily oxidizable amino acids, and its derivatives have strong antioxidant activity that allows them to scavenge free radicals that originate from both ROS and reactive nitrogen species (RNS) (20–22). In rodent models, the coadministration of methionine attenuates the nephrotoxicity induced by nephrotoxic agents, such as cisplatin and dl-serine (23, 24). In the present study, the nephroprotective effect of methionine on polymyxin-induced nephrotoxicity was examined in a mouse model. The impact of coadministered methionine on the pharmacokinetics of polymyxin B in rats and its effect on mitochondrial superoxide production in a renal tubular cell culture model were also investigated. Our findings indicate the potential use of methionine as a nephroprotective agent to ameliorate polymyxin-induced nephrotoxicity.
RESULTS
Histopathological assessment of polymyxin B-induced nephrotoxicity.
Marked histopathological alterations (P < 0.0001) were manifested in the kidneys of mice in the group treated with polymyxin B (cumulative dose, 245 mg/kg of body weight). These alterations were absent in the groups treated with the saline control, methionine alone, and polymyxin B plus methionine at 400 mg/kg (Table 1 and Fig. 1A to D). In the group treated with polymyxin B, tubular damage with focal necrosis of tubular epithelial cells and numerous casts was evident (Fig. 1B). The protective effect of methionine appeared to be dose dependent, as the tubular damage caused by polymyxin B was significantly reduced in the group of mice treated with 400 mg/kg methionine (Fig. 1D) but slightly less in the group treated with 100 mg/kg methionine (Fig. 1C). Both methionine doses (100 and 400 mg/kg) were well tolerated by the mice. According to the overall scores and the semiquantitative score (SQS) of kidney damage, moderate kidney lesions were observed in the polymyxin B-treated group, and these were prevented by coadministration of methionine (Table 1).
TABLE 1.
Histological results for kidneys collected from mice in each groupa
| Treatment | Overall kidney damage score | SQS for kidney damageb |
|---|---|---|
| Polymyxin B | 5, 4, 0, 24, 0, 3 | +1, +2, 0, +2, 0, +1 |
| Polymyxin B + methionine 100 | 0, 0, 3, 2, 2, 0 | 0, 0, +1, +1, +1, 0 |
| Polymyxin B + methionine 400 | 0, 0, 0, 0, 0, 0 | 0, 0, 0, 0, 0, 0 |
In the groups treated with the saline control and methionine alone, no kidney damage was observed, and therefore, data for these groups are not included here.
The extent of kidney lesions was graded using the SQS, where 0, +1, and +2 correspond to no change, mild damage, and mild to moderate damage, respectively (16).
FIG 1.
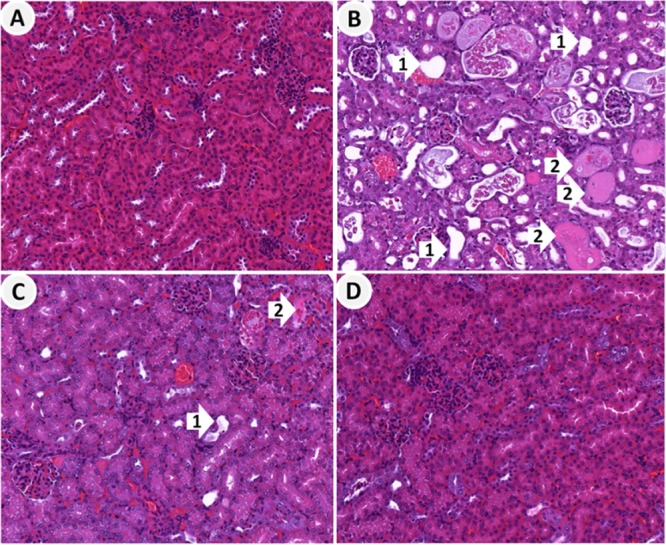
Representative histological images of hematoxylin-eosin-stained kidney sections from mice in the control and treatment groups. (A) Kidney sections from saline-treated control mice showing normal glomeruli and renal tubules; (B) kidney sections from mice treated with polymyxin B showing severely damaged and dilated tubules (arrow 1), degeneration and necrosis of tubular epithelial cells, and tubular casts (arrow 2); (C) kidney sections from mice treated with polymyxin B plus methionine at 100 mg/kg showing damaged tubules with mild tubular dilation (arrow 1) and degeneration and tubular casts (arrow 2); (D) kidney sections from mice treated with polymyxin B plus methionine at 400 mg/kg showing normal glomeruli and renal tubules without significant lesions.
Effect of methionine on the pharmacokinetics of polymyxin B.
The mean plasma concentration-time profiles of polymyxin B in rats following a single intravenous bolus of polymyxin B (1 mg/kg) alone and following a single intravenous bolus of polymyxin B 15 min after the administration of methionine (200 mg/kg) are presented in Fig. 2. The mean plasma concentration-time profiles (Fig. 2) and urinary recoveries (Table 2) were very similar between the two groups, and there were no significant differences (P > 0.05) in the pharmacokinetic parameters of polymyxin B between the two groups (Table 2). The urinary recovery of polymyxin B was very low in both the polymyxin B and the polymyxin B plus methionine groups, and <1.5% of the administered dose was excreted in urine as unchanged polymyxin B. Very similar renal clearances of polymyxin B were observed with or without the coadministration of methionine (Table 2).
FIG 2.
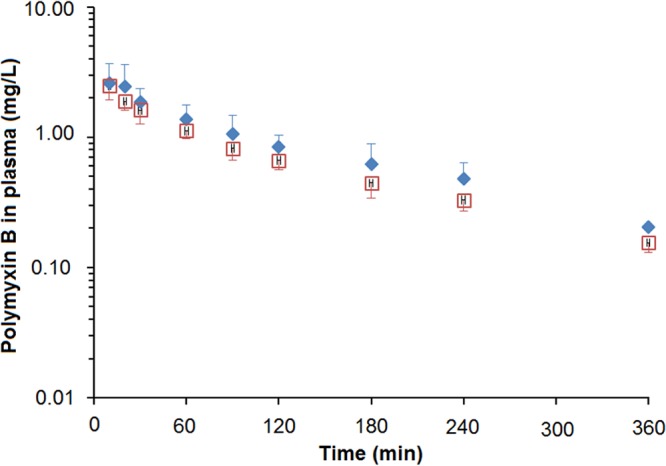
Plasma concentration-time profiles of polymyxin B after intravenous administration of polymyxin B alone (1 mg/kg) (◆) and after coadministration of polymyxin B (1 mg/kg) with methionine at 200 mg/kg (□) (n = 4 in each group; mean ± standard deviation [SD]).
TABLE 2.
Impact of methionine on the pharmacokinetics of polymyxin Ba in rats
| Treatment | Vol of distribution (ml/kg) | Clearance (ml/min/kg) | Half-life (min) | Urinary recovery over 24 h (%) |
|---|---|---|---|---|
| Polymyxin B | 421 ± 201 | 3.12 ± 1.66 | 108 ± 28.9 | 0.70 ± 0.32 |
| Polymyxin B + methionine 200 | 574 ± 129 | 3.71 ± 0.57 | 123 ± 26.9 | 0.77 ± 0.57 |
| P value | 0.15 | 0.53 | 0.46 | 0.84 |
Polymyxin B was administered to the rats (n = 4) at 1 mg/kg as an intravenous bolus.
Attenuation of mitochondrial oxidative stress by methionine.
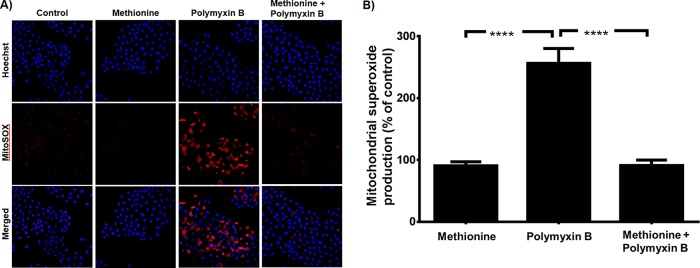
In the in vitro study with NRK-52E cells, the MitoSOX red dye-derived fluorescence in the cells treated with polymyxin B alone (0.75 mM) was >2-fold higher (P < 0.0001) than that in the control cells and those treated with 10 mM methionine in the absence of polymyxin B (Fig. 3A and B). In cells treated with polymyxin B in the presence of methionine, the MitoSOX red dye-derived fluorescence was similar to that in the group treated with methionine alone but substantially lower (P < 0.0001) than that in cells treated with polymyxin B alone (Fig. 3).
FIG 3.
Mitochondrial superoxide production in NRK-52E cells treated with 0.75 mM polymyxin B in the absence and presence of 10 mM methionine. (A) Detection of cellular mitochondrial superoxide using MitoSOX red dye. (B) Measurements of mitochondrial superoxide production presented as mean ± SD (n = 3). ****, P < 0.0001 (compared to control samples).
DISCUSSION
Possibly due to suboptimal use, resistance to the last-line polymyxins (25) in Gram-negative superbugs is increasingly reported worldwide (3, 26, 27). Resistance to polymyxins virtually means the total lack of treatment options against these problematic Gram-negative bacterial infections. Unfortunately, the administration of higher doses of polymyxins is limited by the high prevalence of the dose-limiting nephrotoxicity (which occurs in about one in every two patients) (5, 28, 29). The attenuation of polymyxin-induced nephrotoxicity will allow widening of the therapeutic window of polymyxins and the administration of higher daily doses for optimum pharmacodynamics in patients (16–18). In view of the fact that a significant role of oxidative stress in polymyxin-induced nephrotoxicity has recently been reported in vitro and in vivo, we hypothesized that the coadministration of an antioxidant with polymyxins may attenuate the nephrotoxicity (14, 16–19, 30). Methionine has been show to provide protection against oxidative stress in kidney tubular cells and kidney toxicity due to nephrotoxic compounds (31–33). The present study demonstrated that the coadministration of methionine significantly attenuated the nephrotoxicity induced by polymyxin B both in vitro and in vivo.
Both colistin methanesulfonate (CMS; an inactive prodrug of colistin) (34) and polymyxin B are used for intravenous administration in patients. CMS was not employed in the present study due to its complex chemistry and conversion to colistin in vivo (35, 36). The protective effect of methionine was first examined in our mouse nephrotoxicity model (37) using two different dosage regimens of methionine (Fig. 1). In a recent clinical trial, escalating supplemental oral doses (9.2, 22.5, 46.3, and 91 mg/kg/day) of methionine were administered for 4 weeks to healthy humans (38). The tolerable dose of methionine was 91 mg/kg/day, although an increase in the plasma homocysteine concentration was observed at that dose level (38). A typical loading dose of 100 mg/kg/day methionine was suggested to be tested in humans without any association with serious complications (39). The highest methionine dose of 800 mg/kg/day in mice in the current study was based on animal scaling (in which the 800-mg/kg/day dose in mice is ∼12 times higher than the equivalent mg/kg dose in humans) (40) and information in the literature, where a comparable animal-scaled dose showed significant protective effects against oxidative stress-mediated toxicities in rats or guinea pigs following coadministration with other drugs, such as gentamicin and cisplatin (33, 41, 42). In considering the relative magnitude of the doses used, it is also important to recognize that the respective bioavailability following oral administration in humans and parenteral administration in the present and earlier animal studies is not known. Methionine was administered prior to the administration of polymyxin B to avoid a possible interaction between the drugs during administration.
In our mouse model, the kidneys of the mice in the polymyxin B-treated group had notable histological damage (Fig. 1), including acute cortical and tubular necrosis, which indicates the possibility of ischemia in the etiology of the lesions. Acute cortical and tubular necrosis is reversible, but these lesions potentially signal the onset of chronic renal failure (43). Although no thrombosis or vascular injury was evident from the histological observations in the present study, the interplay of pathophysiological pathways, such as tubular injury, oxidative stress, inflammation, and vascular injury in the kidney, has recently been implicated in cisplatin- and aminoglycoside-induced nephrotoxicity (44, 45). Similar to the findings for the mice in the control group, no histological kidney damage was observed in mice treated with only methionine at 400 mg/kg (data not shown). The nephroprotective effect of methionine was demonstrated by histopathological examination of the mouse kidneys, showing a marked amelioration of tubular necrosis and a lower SQS in the group treated with polymyxin B plus methionine compared to the findings for the group treated with polymyxin B (Fig. 1 and Table 1). Clearly, the higher dose of methionine showed much better nephroprotection against polymyxin B-induced nephrotoxicity in mice than the lower dose. Examination of intermediate doses of methionine would assist in defining the dose-response relationship for nephroprotection and identify the lowest dose needed to afford essentially complete protection.
The pharmacokinetic parameters of polymyxin B following administration of an intravenous bolus (1.0 mg/kg) in rats (Table 2) were comparable to those reported recently (9, 46). The coadministration of methionine did not significantly alter the pharmacokinetics of polymyxin B in rats (Fig. 2). The percentages of polymyxin B recovered unchanged in urine over 24 h were less than 1% in the absence and presence of methionine treatment. Following filtration through glomeruli, polymyxin B is subject to very extensive renal tubular reabsorption by carrier-mediated pathways (e.g., megalin and PEPT2) (7, 8, 10–12). This highly efficient reabsorption plays a critical role in the remarkable accumulation of polymyxins within the kidney (17, 47). The coadministration of methionine did not change the renal clearance or the overall renal handling of polymyxin B, suggesting that the uptake of methionine by renal tubular cells did not compete with the carrier-mediated reabsorption of polymyxin B. We have recently discovered that the renal toxicity of polymyxin is related to the extraordinary intracellular accumulation and activation of apoptosis in renal proximal tubular cells (13, 15). As methionine also undergoes extensive tubular reabsorption (48, 49), our results suggest that the intracellular trafficking or accumulation of methionine in renal tubular cells is crucial for its protective role against polymyxin B-induced nephrotoxicity by neutralizing cellular oxidative stress.
A potential limitation of our animal study is that the nephrotoxicity experiment was conducted in mice (Fig. 1; Table 1), while the pharmacokinetic experiment was performed in rats (Fig. 2; Table 2). Rat models for pharmacokinetic studies allow the collection of adequate blood samples from each animal at multiple time points. The dose of 1 mg/kg polymyxin B used in rats was lower than the dose administered to mice, even after allometric scaling between these species, albeit similar doses have previously been used to investigate the pharmacokinetic parameters of polymyxins in rats (12, 46). Notwithstanding this, the dose administered in mice was selected in order to reproducibly induce nephrotoxicity, and this mouse nephrotoxicity model has been used in our toxicological studies of polymyxins (12, 46, 47).
The exact mechanism of nephrotoxicity induced by polymyxins is not known; however, our recent studies have indicated that it is closely associated with the accumulation in the kidney and the generation of oxidative stress (15). Polymyxin B exposure in renal proximal tubular cells causes the generation of ROS and leads to mitochondrial dysfunction and cell death (15). As mitochondria play an important role in the generation of ROS, in the present study, polymyxin B-induced oxidative stress was examined in NRK-52E cells. The plasma concentrations of polymyxin B after intravenous administration of the currently recommended dosage regimens in critically ill patients are usually less than 10 μg/ml (11). However, in cell culture studies, the polymyxin B in kidney tubular cells can accumulate to concentrations on the order of 100 mM (13). Therefore, 0.9 mg/ml (i.e., 0.75 mM) polymyxin B was chosen for use in the cell culture study in the current study in order to induce significant oxidative stress while maintaining cell viability sufficient for the protection experiment (15). A significant increase in the level of mitochondrial superoxide production due to polymyxin B treatment was evident in the present study, while the mitochondrial oxidative stress was minimal in methionine-treated cells. Interestingly, the coadministration of methionine with polymyxin B significantly reduced the level of ROS production in mitochondria in NRK-52E cells to that seen in the control cells (Fig. 3). Methionine and its metabolites have been revealed to be competent scavengers of free radicals and to detoxify the oxidants by several mechanisms, such as through the transfer of electrons and hydrogen and radical adduct formation (20, 21). In addition to ROS/RNS scavenging, methionine plays a key role in the cellular antioxidant defense system (50) and restores mitochondrial homeostasis (51). Hence, the protective effect of methionine against polymyxin-induced nephrotoxicity very likely relates to its nucleophilic antioxidant activity that allows it to scavenge oxygen free radicals (52–54). The detailed mechanism of attenuation of polymyxin-induced nephrotoxicity by methionine is not clear and is under investigation in our laboratory.
In summary, our study is the first to demonstrate that methionine is protective against polymyxin-induced nephrotoxicity and indicates the critical involvement of oxygen free radicals. Our results may have an important potential to optimize polymyxin use in the clinic by increasing its therapeutic index by the coadministration of an appropriate nephroprotectant, thereby achieving desirable pharmacodynamics while reducing toxicities. Importantly, the mechanistic insights into polymyxin-induced nephrotoxicity and the protection provided by methionine provide valuable information for designing novel, safer polymyxin-like lipopeptide antibiotics.
MATERIALS AND METHODS
Chemicals and reagents.
Polymyxin B sulfate (catalog number P0972-10MU, batch number BCBF8382V, USP purity), 10% neutral buffered formalin, and methionine (catalog number M9500, lot number MKBJ7665V) were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Fresh stock solutions of polymyxin B (10 mg base/ml) and methionine (20 mg/ml) were prepared in sterile 0.9% saline for animal studies. All other chemicals were of analytical grade.
Animals.
This study was approved by the Animal Ethics Committee of the Monash Institute of Pharmaceutical Sciences, Monash University (Parkville, Victoria, Australia). All experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Swiss mice (female; age, 6 weeks; body weight, 20 to 25 g) and Sprague-Dawley rats (male; body weight, 300 to 380 g) were from the Monash Animal Research Platform (Clayton, Victoria, Australia). The temperature and humidity of the facility, which had a 12-h light and 12-h dark cycle, were controlled. The animals were housed individually in metabolic cages and had free access to food and water. Following a 2-day acclimation, cannulae were inserted in the jugular vein and the carotid artery in each rat in the pharmacokinetic study.
Effect of methionine on polymyxin B-induced nephrotoxicity.
Five groups of mice (n = 6 mice in each group) were employed to examine the effect of methionine on polymyxin B-induced nephrotoxicity. Polymyxin B (which was administered subcutaneously) and methionine (which was administered intraperitoneally) were simultaneously administered twice daily (8 h apart during daylight hours) over 3.5 days. The groups were treated with the following: (i) 0.9% saline (the control group), (ii) polymyxin B at 35 mg base/kg (in which polymyxin B in saline was administered for a total of 7 doses for a cumulative dose of polymyxin B of 245 base mg/kg), (iii) methionine at 400 mg/kg (in which methionine was administered in saline for a cumulative methionine dose of 2,800 mg/kg), (iv) polymyxin B at 35 mg/kg plus methionine at 100 mg/kg (in which the cumulative polymyxin B dose was 245 base mg/kg and the cumulative methionine dose was 700 mg/kg), and (v) polymyxin B at 35 mg/kg plus methionine at 400 mg/kg (in which the cumulative polymyxin B dose was 245 base mg/kg and the cumulative methionine dose was 2,800 mg/kg). At 4 h after the last dose on day 4, blood was collected via cardiac puncture and centrifuged at 4°C and 5,000 × g for 10 min, and the plasma was stored at −80°C prior to analysis for polymyxin B as described below. Immediately after blood sampling, the kidneys from each mouse were collected and placed in 10% neutral buffered formalin for histopathological examination.
Histopathological assessment of polymyxin B-induced nephrotoxicity.
The formalin-fixed kidneys from the mice in each group (described above) were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin-eosin. A semiquantitative evaluation of the degree of renal tissue injury was carried out by the Histopathology and Organ Pathology Service of the Australian Phenomics Network (Department of Anatomy and Neuroscience, The University of Melbourne, Parkville, Victoria, Australia) using a semiquantitative scoring system (16). The histopathologist was blind in regard to the group allocation of the mouse kidneys. Lesion severity was graded as follows: grade 1, mild acute tubular damage with tubular dilation, prominent nuclei, and a few pale tubular casts; grade 2, severe acute tubular damage with necrosis of tubular epithelial cells and numerous tubular casts; and grade 3, acute cortical necrosis/infarction of tubules and glomeruli with or without papillary necrosis. These grades were scored as follows: grade 1 was given a score of 1, grade 2 was given a score of 4, and grade 3 was given a score of 10. The percentage of the kidney slices affected following treatment was scored as follows: <1% was given a score of 0, 1 to <5% was given a score of 1, 5 to <10% was given a score of 2, 10 to <20% was given a score of 3, 20 to <30% was given a score of 4, 30 to <40% was given a score of 5, and ≥40% was given a score of 6. Overall scores were calculated as the product of the percentage score and the grade score. Finally, semiquantitative scores (SQS) were assigned for kidney histological results as follows: an SQS of 0 indicated no significant change (overall score, <1), an SQS of +1 indicated mild damage (overall score, 1 to <15), an SQS of +2 indicated mild to moderate damage (overall score, 15 to <30), an SQS of +3 indicated moderate damage (overall score, 30 to <45) an SQS of +4 indicated moderate to severe damage (overall score, 45 to <60), and an SQS of +5 indicated severe damage (overall score, 60).
Effect of methionine on the pharmacokinetics of polymyxin B.
Rats were divided into two groups (n = 4 each) and received the following bolus doses via the jugular vein cannula: (i) 0.9% saline 15 min prior to the administration of 1 mg/kg polymyxin B (the polymyxin B group) and (ii) 200 mg/kg methionine 15 min prior to the administration of 1 mg/kg polymyxin B (the polymyxin B plus methionine group). Blood samples (0.2 ml) were collected from the carotid artery prior to and at 10, 20, 30, 60, 90, 120, 180, 240, and 360 min after administration of polymyxin B in both groups. All urine voided over the intervals from 0 to 6 h and 6 to 24 h postdosing was collected, and the volume of each collection was recorded before the samples were stored for later analysis. The concentrations of polymyxin B in plasma and urine were measured by high-performance liquid chromatography (HPLC), and the pharmacokinetic parameters of polymyxin B were determined as described below (55).
HPLC analysis of polymyxin B.
HPLC was utilized to determine the polymyxin B concentrations in plasma and urine. For both matrices (plasma and urine), calibration curves were constructed, with the polymyxin B concentrations ranging from 0.10 to 5.00 μg/ml. For quality control samples containing 0.50 and 4.00 μg/ml (n = 6), the accuracy and reproducibility were 0.49 ± 0.02 μg/ml and 4.04 ± 0.06 μg/ml, respectively, for plasma samples, and 0.51 ± 0.03 μg/ml and 3.92 ± 0.04 μg/ml, respectively, for urine samples. Appropriate dilutions were applied for analyzing the samples with concentrations above the calibration range, and the appropriate quality control samples were included. The limit of quantitation for polymyxin B in plasma and urine samples was 0.10 μg/ml.
Attenuation of mitochondrial oxidative stress by methionine.
Rat kidney proximal tubular (NRK-52E) cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5% CO2. NRK-52E cells were incubated without (control) or with 0.75 mM polymyxin B (equivalent to ∼0.90 mg/ml of polymyxin B) in the absence and presence of methionine (10 mM) for 24 h in DMEM with 0.1% FBS. At the end of the treatments, the cells were washed with phosphate-buffered saline and incubated with 5.0 μM MitoSOX red dye (excitation and emission wavelengths, 514 and 531 to 622 nm, respectively) for 45 min (at 37°C in 5% CO2) for measurement of mitochondrial ROS production. Nuclei were stained with 5 μg/ml of Hoechst 33342 (excitation and emission wavelengths, 405 and 410 to 551 nm, respectively), and the number of cells was counted. The fluorescence intensity was quantified by laser scanning microscopy using a Zeiss LSM 700 confocal microscope with ZEN imaging software to determine the number of cells and the level of production of mitochondrial ROS (14). All the images were processed using NIH ImageJ software (v1.48k, 2013). All experiments were conducted with three replicates.
Data analyses.
Data from the mouse, rat, and cell culture studies were subjected to the Shapiro-Wilk test and Levene's test for the homogeneity of variance among groups to examine the normal distribution for all continuous variables. For the histopathological scores, Kruskal-Wallis one-way analysis of variance (ANOVA) by ranks was conducted. The pharmacokinetic data from rats were analyzed using WinNonlin software (noncompartmental model 201, version 5.2; Pharsight Corp., Cary, NC) (17). An unpaired t test was employed to compare the pharmacokinetic parameters between the polymyxin B group and the polymyxin B plus methionine group. For mitochondrial superoxide production, comparisons among the treatment groups were performed using ANOVA and Tukey's test, with the significance level being a P value of <0.05.
ACKNOWLEDGMENTS
This study is supported by a grant (APP1085637) from the Australian National Health and Medical Research Council (NHMRC). J.L. and T.V. are also supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI111965).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
T.V. is an Australian NHMRC Industry Career Development Research Fellow. J.L. is an Australian NHMRC Senior Research Fellow.
REFERENCES
- 1.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. 2013. 10×'20 progress—development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velkov T, Roberts KD, Nation RL, Thompson PE, Li J. 2013. Pharmacology of polymyxins: new insights into an ‘old’ class of antibiotics. Future Microbiol 8:711–724. doi: 10.2217/fmb.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kubin CJ, Ellman TM, Phadke V, Haynes LJ, Calfee DP, Yin MT. 2012. Incidence and predictors of acute kidney injury associated with intravenous polymyxin B therapy. J Infect 65:80–87. doi: 10.1016/j.jinf.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Kwon JA, Lee JE, Huh W, Peck KR, Kim YG, Kim DJ, Oh HY. 2010. Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents 35:473–477. doi: 10.1016/j.ijantimicag.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Yamaguchi H, Ogura J, Kobayashi M, Yamada T, Iseki K. 2013. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 57:6319–6324. doi: 10.1128/AAC.00254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu XX, Chan T, Xu CH, Zhu L, Zhou QT, Roberts KD, Chan HK, Li J, Zhou FF. 2016. Human oligopeptide transporter 2 (PEPT2) mediates cellular uptake of polymyxins. J Antimicrob Chemother 71:403–412. doi: 10.1093/jac/dkv340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelraouf K, Braggs KH, Yin T, Truong LD, Hu M, Tam VH. 2012. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 56:4625–4629. doi: 10.1128/AAC.00280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad MA, Roberts KD, Yu HH, Liu B, Schofield AV, James SA, Howard DL, Nation RL, Rogers K, de Jonge MD, Thompson PE, Fu J, Velkov T, Li J. 2015. Significant accumulation of polymyxin in single renal tubular cells: a medicinal chemistry and triple correlative microscopy approach. Anal Chem 87:1590–1595. doi: 10.1021/ac504516k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azad MA, Akter J, Rogers K, Nation RL, Velkov T, Li J. 2015. Major pathways of polymyxin-induced apoptosis in rat kidney proximal tubular cells. Antimicrob Agents Chemother 59:2136–2143. doi: 10.1128/AAC.04869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azad MA, Finnin BA, Poudyal A, Davis K, Li J, Hill PA, Nation RL, Velkov T, Li J. 2013. Polymyxin B induces apoptosis in kidney proximal tubular cells. Antimicrob Agents Chemother 57:4329–4335. doi: 10.1128/AAC.02587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2011. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother 55:4044–4049. doi: 10.1128/AAC.00328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousef JM, Chen G, Hill PA, Nation RL, Li J. 2012. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 67:452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezoti Fonseca C, Watanabe M, Vattimo MDF. 2012. Role of heme oxygenase-1 in polymyxin B-induced nephrotoxicity in rats. Antimicrob Agents Chemother 56:5082–5087. doi: 10.1128/AAC.00925-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozyilmaz E, Ebinc FA, Derici U, Gulbahar O, Goktas G, Elmas C, Oguzulgen IK, Sindel S. 2011. Could nephrotoxicity due to colistin be ameliorated with the use of N-acetylcysteine? Intensive Care Med 37:141–146. doi: 10.1007/s00134-010-2038-7. [DOI] [PubMed] [Google Scholar]
- 20.Njaa RL, Utne F, Braekkan OR. 1968. Antioxidant properties of methionine esters. Nature 218:571–572. doi: 10.1038/218571a0. [DOI] [PubMed] [Google Scholar]
- 21.Barelli S, Canellini G, Thadikkaran L, Crettaz D, Quadroni M, Rossier JS, Tissot JD, Lion N. 2008. Oxidation of proteins: basic principles and perspectives for blood proteomics. Proteomics Clin Appl 2:142–157. doi: 10.1002/prca.200780009. [DOI] [PubMed] [Google Scholar]
- 22.Pryor WA, Jin X, Squadrito GL. 1994. One- and two-electron oxidations of methionine by peroxynitrite. Proc Natl Acad Sci U S A 91:11173–11177. doi: 10.1073/pnas.91.23.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deegan PM, Pratt IS, Ryan MP. 1994. The nephrotoxicity, cytotoxicity and renal handling of a cisplatin-methionine complex in male Wistar rats. Toxicology 89:1–14. doi: 10.1016/0300-483X(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 24.Wachstein M. 1947. Influence of DL-methionine and other substances on the nephrotoxic action of DL-serine. Nature 159:236. [DOI] [PubMed] [Google Scholar]
- 25.Falagas ME, Bliziotis IA. 2007. Pandrug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era? Int J Antimicrob Agents 29:630–636. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Nation RL. 2006. Old polymyxins are back, is resistance close? Clin Infect Dis 43:663–664. doi: 10.1086/506571. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Rayner CR, Nation RL, Owen RJ, Tan KE, Spelman D. 2006. Hetero-resistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elias LS, Konzen D, Krebs JM, Zavascki AP. 2010. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother 65:2231–2237. doi: 10.1093/jac/dkq285. [DOI] [PubMed] [Google Scholar]
- 29.Pastewski AA, Caruso P, Parris AR, Dizon R, Kopec R, Sharma S, Mayer S, Ghitan M, Chapnick EK. 2008. Parenteral polymyxin B use in patients with multidrug-resistant Gram-negative bacteremia and urinary tract infections: a retrospective case series. Ann Pharmacother 42:1177–1187. doi: 10.1345/aph.1K346. [DOI] [PubMed] [Google Scholar]
- 30.Ozkan G, Ulusoy S, Orem A, Alkanat M, Mungan S, Yulug E, Yucesan FB. 2013. How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother 57:3463–3469. doi: 10.1128/AAC.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basinger MA, Jones MM, Holscher MA. 1990. l-Methionine antagonism of cis-platinum nephrotoxicity. Toxicol Appl Pharmacol 103:1–15. doi: 10.1016/0041-008X(90)90257-U. [DOI] [PubMed] [Google Scholar]
- 32.Short EI. 1952. Mechanism of methionine protection against the nephrotoxicity of polymyxin A. Br J Pharmacol Chemother 7:248–254. doi: 10.1111/j.1476-5381.1952.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derakhshanfar A, Bidadkosh A, Sadeghian MH. 2009. l-Methionine attenuates gentamicin nephrotoxicity in male Wistar rat: pathological and biochemical findings. Iran J Vet Res 10:323–328. [Google Scholar]
- 34.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2004. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother 53:837–840. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother 52:987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- 37.Roberts KD, Azad MAK, Wang JP, Horne AS, Thompson PE, Nation RL, Velkov T, Li J. 2015. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant Gram-negative bacteria. ACS Infect Dis 1:568–575. doi: 10.1021/acsinfecdis.5b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutz NEP, Simbo SY, Ligthart-Melis GC, Cynober L, Smriga M, Engelen MPKJ. 2017. Tolerance to increased supplemented dietary intakes of methionine in healthy older adults. Am J Clin Nutr 106:675–683. doi: 10.3945/ajcn.117.152520. [DOI] [PubMed] [Google Scholar]
- 39.Garlick PJ. 2006. Toxicity of methionine in humans. J Nutr 136(6 Suppl):1722S–1725S. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration. 2005. Guidance for industry: estimating the maximum safe starting dose in adult healthy volunteer. U.S. Food and Drug Administration, Rockville, MD. [Google Scholar]
- 41.Hinduja S, Kraus KS, Manohar S, Salvi RJ. 2015. d-Methionine protects against cisplatin-induced neurotoxicity in the hippocampus of the adult rat. Neurotox Res 27:199–204. doi: 10.1007/s12640-014-9503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha SH, Schacht J. 2000. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: d-methionine is a potential protectant. Hear Res 142:34–40. doi: 10.1016/S0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 43.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group. 2009. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 44.Quiros Y, Vicente-Vicente L, Morales AI, Lopez-Novoa JM, Lopez-Hernandez FJ. 2011. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci 119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI. 2011. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- 46.Sivanesan S, Roberts K, Wang JP, Chea SE, Thompson PE, Li J, Nation RL, Velkov T. 2017. Pharmacokinetics of the individual major components of polymyxin B and colistin in rats. J Nat Prod 80:225–229. doi: 10.1021/acs.jnatprod.6b01176. [DOI] [PubMed] [Google Scholar]
- 47.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, Chung DR, Peck KR, Song JH. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother 60:1163–1167. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 48.Storch KJ, Wagner DA, Burke JF, Young VR. 1990. [1-13C; methyl-2H3]methionine kinetics in humans: methionine conservation and cystine sparing. Am J Physiol 258:E790–E798. [DOI] [PubMed] [Google Scholar]
- 49.Storch KJ, Wagner DA, Burke JF, Young VR. 1988. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am J Physiol 255:E322–E331. [DOI] [PubMed] [Google Scholar]
- 50.Yang M, Vousden KH. 2016. Serine and one-carbon metabolism in cancer. Nat Rev Cancer 16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 51.Bender A, Hajieva P, Moosmann B. 2008. Adaptive antioxidant methionine accumulation in respiratory chain complexes explains the use of a deviant genetic code in mitochondria. Proc Natl Acad Sci U S A 105:16496–16501. doi: 10.1073/pnas.0802779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo S, Levine RL. 2009. Methionine in proteins defends against oxidative stress. FASEB J 23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine RL, Moskovitz J, Stadtman ER. 2000. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life 50:301–307. doi: 10.1080/15216540051081056. [DOI] [PubMed] [Google Scholar]
- 54.Levine RL, Mosoni L, Berlett BS, Stadtman ER. 1996. Methionine residues as endogenous antioxidants in proteins. Proc Natl Acad Sci U S A 93:15036–15040. doi: 10.1073/pnas.93.26.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. 2008. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 47:1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]