ABSTRACT
Shewanella spp. constitute a reservoir of antibiotic resistance determinants. In a bile sample, we identified three extended-spectrum-β-lactamase (ESBL)-producing bacteria (Escherichia coli, Klebsiella pneumoniae, and Shewanella sp. strain JAB-1) isolated from a child suffering from cholangitis. Our objectives were to characterize the genome and the resistome of the first ESBL-producing isolate of the genus Shewanella and determine whether plasmidic exchange occurred between the three bacterial species. Bacterial isolates were characterized using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), standard biochemical tools, and antimicrobial susceptibility testing. Shewanella sp. JAB-1 and ESBL gene-encoding plasmids were characterized using PacBio and Illumina whole-genome sequencing, respectively. The Shewanella sp. JAB-1 chromosome-encoded OXA-48 variant was cloned and functionally characterized. Whole-genome sequencing (WGS) of the Shewanella sp. clinical isolate JAB-1 revealed the presence of a 193-kb plasmid belonging to the IncA/C incompatibility group and harboring two ESBL genes, blaCTX-M-15 and blaSHV-2a. blaCTX-M-15 gene-carrying plasmids belonging to the IncY and IncR incompatibility groups were also found in the E. coli and K. pneumoniae isolates from the same patient, respectively. A comparison of the blaCTX-M-15 genetic environment indicated the independent origin of these plasmids and dismissed in vivo transfers. Furthermore, characterization of the resistome of Shewanella sp. JAB-1 revealed the presence of a chromosome-carried blaOXA-535 gene, likely the progenitor of the plasmid-carried blaOXA-436 gene, a novel blaOXA-48-like gene. The expression of blaOXA-535 in E. coli showed the carbapenem-hydrolyzing activity of OXA-535. The production of OXA-535 in Shewanella sp. JAB-1 could be evidenced using molecular and immunoenzymatic tests, but not with biochemical tests that monitor carbapenem hydrolysis. In this study, we have identified a CTX-M-15-producing Shewanella species that was responsible for a hepatobiliary infection and that is likely the progenitor of OXA-436, a novel plasmid-encoded OXA-48-like class D carbapenemase.
KEYWORDS: CTX-M-15, OXA carbapenemase, WGS, plasmids, progenitor
INTRODUCTION
Shewanella spp. are nonfermentative Gram-negative bacilli that are widely distributed throughout the world. They are found mainly in seawater in areas with warm climates (1, 2). Although Shewanella spp. are an unusual cause of infections in humans, the number of cases reported is increasing (1, 3–5). In most cases, this organism is cultured from samples of immunocompromised patients suffering from soft tissue infections after seawater exposure (3, 6). More recently, several cases of Shewanella infections have been reported in patients suffering from hepatobiliary diseases (3–5, 7). Human infections are most often caused by Shewanella algae and Shewanella putrefaciens (3). However, it is often difficult to identify the bacteria at the species level using conventional culture-based and biochemical tests. 16S rRNA gene sequencing improved the identification of Shewanella species as pathogenic organisms (3, 5).
Most Shewanella spp. are susceptible to piperacillin-tazobactam, ceftazidime, cefepime, imipenem, meropenem, gentamicin, tobramycin, amikacin, and ciprofloxacin, but susceptibility to penicillins is more varied (3, 7). Several naturally occurring β-lactamases have been identified in Shewanella spp., especially class D β-lactamases (8–11), also known as oxacillinases. For instance, Shewanella oneidensis strain MR-1 was found to naturally harbor the blaOXA-54 gene, which is related to the blaOXA-48 gene, a plasmid-mediated carbapenem-hydrolyzing class D β-lactamase (CHDL) gene involved in carbapenem resistance in Enterobacteriaceae (10). Moreover, OXA-55 was identified in S. algae and possesses biochemical properties similar to those of OXA-54, in particular the ability to hydrolyze carbapenems (11). Shewanella xiamenensis has been identified as the source of the blaOXA-181 gene encoding another OXA-48-like variant with carbapenemase activity that is highly prevalent on the Indian subcontinent and increasingly reported in France (9, 12). Recently, next-generation sequencing enabled the identification of several blaOXA-48-like genes, such as blaOXA-199, blaOXA-252, blaOXA-514, and blaOXA-515, in different Shewanella species isolates, in food-producing animals, and in water samples (2). Furthermore, analysis of publicly available Shewanella species genomes revealed the presence of several uncharacterized blaOXA-48 like genes, confirming the importance of Shewanella spp. as a OXA-48-like class D β-lactamase reservoir (13, 14).
Extended-spectrum β-lactamases (ESBLs) are being increasingly reported worldwide in Enterobacteriaceae (15) but have never been described in Shewanella species. ESBLs belong to the Ambler class A β-lactamases (16). ESBLs are usually described as acquired β-lactamases that are encoded mostly by plasmid-located genes. There are three major types of ESBLs: TEM, SHV, and CTX-M (16). CTX-Ms are now the most prevalent ESBLs worldwide (17). CTX-M-producing Enterobacteriaceae are not only responsible for nosocomial infections and hospital outbreaks, but they are now also considered to be true community pathogens (15).
In this study, we have characterized three CTX-M15-producing bacteria, including a Shewanella sp. isolate, obtained from a child suffering from cholangitis. Using antimicrobial susceptibility testing and whole-genome sequencing, we have characterized this Shewanella strain, investigated its resistome, and characterized the plasmids carrying the CTX-M15 determinant. Furthermore, we have cloned and expressed the naturally carried OXA-48-like gene (blaOXA-535) and demonstrated its carbapenemase activity.
RESULTS
Case report.
A female child was directly transferred from a hospital in central Africa to a hospital pediatric department in France for biliary surgery. During her previous hospitalization, she underwent a cholecystectomy that was complicated with a leak from the common bile duct forming an abdominal effusion. A drain was then introduced to constitute an external bypass. Upon admission at the hospital, she had fever with abdominal pain, and cholangitis was diagnosed. The liquid collected from the drain was addressed to the microbiology laboratory, and antibiotic treatment with piperacillin and tazobactam was started. The culture revealed a polymicrobial infection due to numerous Gram-negative bacteria (Pseudomonas putida, Citrobacter freundii, Klebsiella pneumoniae, Escherichia coli, Stenotrophomonas maltophilia, and a Shewanella sp.) and to Enterococcus avium. Antimicrobial susceptibility testing revealed the presence of extended-spectrum β-lactamases (ESBL) in Shewanella sp., E. coli, and K. pneumoniae isolates. One week after admission, the patient underwent a laparotomy to perform a Roux-en-Y biliary bypass procedure and to introduce an internal-external biliary drain to protect the anastomosis. Five days after the surgery, the patient became feverish. A blood sample and a bile sample collected from the drain revealed the presence of an ESBL-producing K. pneumoniae isolate. Antibiotic therapy was switched to imipenem for 15 days. The biliary drain was withdrawn 1 month after the surgery, and no other complications occurred.
Identification of Shewanella sp. JAB-1.
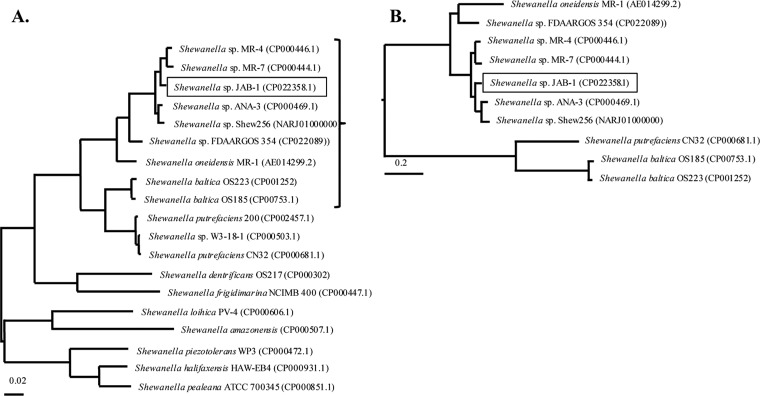
After 24 h of growth on a blood agar plate, the Shewanella sp. JAB-1 colonies were small, smooth, and oxidase and catalase positive. The API32GN system was unable to identify Shewanella sp. even at the genus level. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was used for species identification, yielding S. putrefaciens, with a best score value of 2.0, Shewanella profunda with a score of 1.7, and Shewanella baltica with a score of 1.5. Since MALDI-TOF MS discrepancies for the identification of Shewanella spp. are known, sequencing of 16S rRNA gene was performed. The 1,406-bp 16S rRNA gene sequence was 98% identical to Shewanella seohaensis S7-3T GU944672, making this strain the closest relative based on patristic distances (18). As the 16S rRNA gene sequence may not be discriminatory enough to differentiate closely related Shewanella species (9), we used whole-genome sequencing (WGS) data to dive deeper into the identification of this isolate. Genomes of representative Shewanella species were collected, and rpoB-gyrB concatemer sequences were extracted for phylogenetic analysis (Fig. 1A). The analysis revealed that Shewanella sp. JAB-1 did not belong to any known Shewanella species. Phylogeny based on the whole genome revealed that Shewanella sp. JAB-1 was closely related to four other isolates, namely, MR-4, MR-7, ANA-3, and Shew256, with average nucleotide identity (ANI) values of 94.6%, 94.35%, 94.32% and 93.90%, respectively (Fig. 1B).
FIG 1.
Phylogenetic analysis of representative Shewanella spp. (A) Unrooted phylogenetic tree based on gyrB and rpoB genes. The tree was constructed by maximum likelihood method with Tamura-Nei model using MEGA program (MEGA7.0). The tree is drawn to scale, with branch lengths representing the evolutionary distances. (B) A focus on Shewanella spp. close to Shewanella sp. JAB-1 was made, and the phylogenetic tree was based on whole-genome sequences. It was constructed by maximum likelihood method with Jukes-Cantor model, using the Parsnp software. The tree is drawn to scale based on the single nucleotide polymorphisms (SNPs) of the alignment. Accession numbers are shown in parentheses next to each organism name.
Genomic characteristics of Shewanella sp. JAB-1.
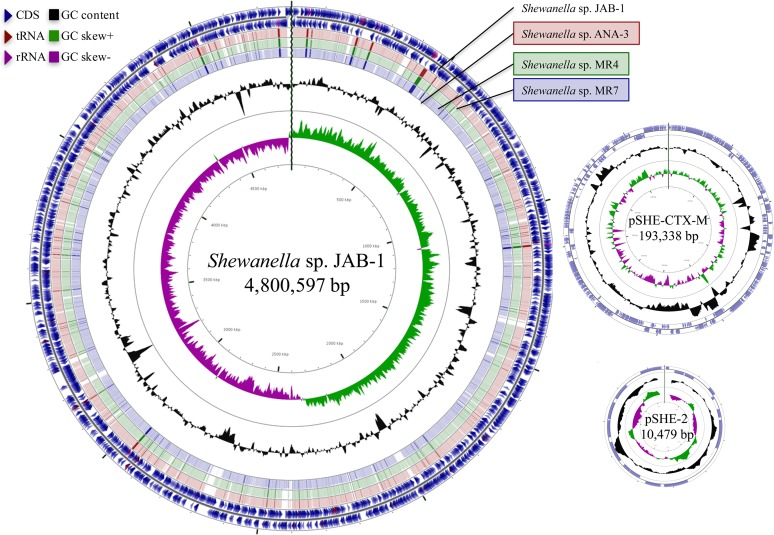
To reconstruct the genome of Shewanella sp. JAB-1, long-read PacBio-based sequencing was performed. The genome was 4,800,597 bp, with an average G+C content of 48%. It was composed of 4,161 coding sequences (CDS), 9 copies of rRNA operons, and 102 tRNA genes (Fig. 2). PacBio assembly revealed the presence of two plasmids of 10.5 and 193 kb in size (Fig. 2).
FIG 2.
Genome representation of Shewanella sp. JAB-1 and its plasmids. The representation was performed using the CGview server. The outermost two rings show features extracted from the Shewanella sp. JAB-1 genome. The next three rings show the positions of BLAST hits detected through BLASTn comparisons of Shewanella sp. JAB-1 genome against the three closest genomes of Shewanella (MR-4, MR-7, and ANA-3, represented by red, green, and blue circles, respectively). Darker arc indicates high percent identity of the hit. The black circle displays the GC content, and inner circles display GC skew.
A function-based comparison using the RAST server revealed unique features in Shewanella sp. JAB-1, with 90 and 100 unique CDS with known functions compared to MR-4 and MR-7, respectively. These CDS include widely distributed functions notably involved in amino acid and carbohydrate metabolism, gene regulation, and resistance to antibiotic compounds (Fig. 2).
Susceptibility testing and detection of resistance genes.
E. coli, K. pneumoniae, and Shewanella sp. JAB-1 shared the same phenotype regarding susceptibility to β-lactams. They were resistant to all penicillins tested, and association with clavulanic acid did not fully restore penicillin activity. They were resistant to expanded-spectrum cephalosporins but susceptible to cefoxitin and to a piperacillin-tazobactam combination. Finally, double-disk synergy tests revealed ESBL activity in the three strains.
In agreement with this phenotype, we identified in the genome of Shewanella sp. four β-lactamase genes: two ESBL genes, blaCTX-M-15 and blaSHV-2a; the narrow-spectrum oxacillinase gene blaOXA-1; and a novel blaOXA-535 gene. The blaOXA-535 gene codes for an oxacillinase that is 91.3% identical to the carbapenem-hydrolyzing class D (CHDL) β-lactamase OXA-48, 98% identical to OXA-436 and OXA-48-like of Shewanella sp. strain ANA-3 (3 amino acids difference), and 99% to OXA-48-like in Shewanella sp. strains MR-4 and MR-7 (1 and 2 amino acids difference, respectively). OXA-436 is a plasmid-encoded OXA-48 variant detected in several species responsible for an outbreak in Denmark (O. Samuelsen, F. Hansen, B. Aasnaes, L. Jakobsen, P. Littauer, L. M. Soes, B. J. Holzknecht, L. P. Andersen, M. Stegger, A. S. Paal, and A. M. Hammerum, unpublished data; GenBank BioProject PRJNA297498). This outbreak involved OXA-436-producing Citrobacter freundii, K. pneumoniae, and Enterobacter asburiae.
Shewanella sp. JAB-1 is resistant to aminoglycosides (gentamicin, tobramycin, and netilmicin) due to the production of two acetyltransferase genes [aac(6′)-Ib-cr and aac(3)-IIa] and two phosphotransferase genes [aph(3″)-Ib and aph(6)-Id]. The resistance to ciprofloxacin is likely due to the acquisition of the aac(6′)-Ib-cr, known to confer reduced susceptibility to this fluoroquinolone by N-acetylation of its piperazinyl amine (19). Amikacin and co-trimoxazole are two antibiotics that remained active in vitro.
Plasmid characterization and genetic context of blaCTX-M-15 genes in the three ESBL-producing isolates.
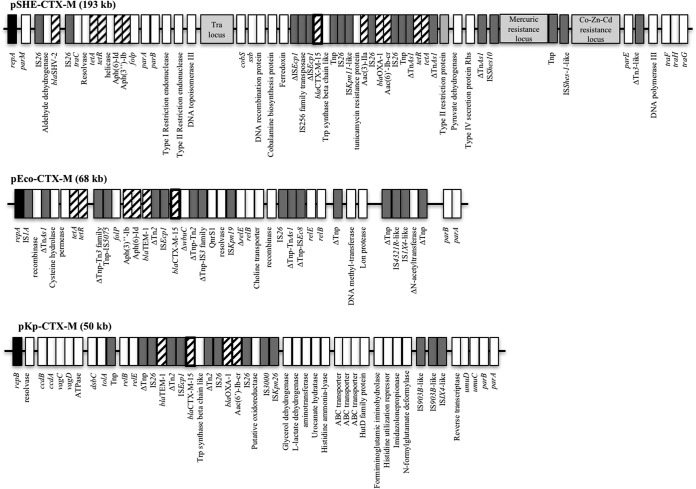
Plasmid extraction of Shewanella sp. JAB-1 and subsequent electroporation and mating-out assay into E. coli TOP10 conferred resistance to penicillin and expanded-spectrum cephalosporins, with double-disk synergy images consistent with an ESBL phenotype. Amplification of the blaCTX-M-15 gene on the E. coli transformants and transconjugants, as well as coresistance to aminoglycosides, indicated that both the blaCTX-M-15 gene and aac(6′)-Ib-cr gene were located on the largest plasmid, namely, pSHE-CTX-M, of ca. 193 kb. pSHE-CTX-M was fully sequenced using PacBio technologies. The overall structure of the plasmid indicated that it belonged to the IncA/C incompatibility group (Fig. 3). The plasmid backbone shares features of IncA/C-type plasmid, i.e., partitioning, replication, and conjugation apparatus. In the variable region, several antibiotic resistance genes have been identified in addition to blaCTX-M-15, blaSHV-2a, and blaOXA-1 β-lactamase genes, the four aminoglycoside-modifying enzyme genes [aph(3″)-Ib, aph(6)-Id, aac(6′)-Ib-cr, and aac(3)-IIa], the tetracycline resistance gene (tetA), the sulfonamide resistance gene (sul2), and the chloramphenicol resistance gene (catB3).
FIG 3.
Schematic representation of the three ESBL-encoding plasmids. Main features are represented. Antimicrobial resistance-associated genes are indicated by hatched boxes. Transposon-related genes and insertion sequences are indicated by dark grey boxes. Replicase genes are indicated by black boxes. tra locus and heavy-metal resistance loci are indicated with light grey boxes. △ indicates a partial protein. Tnp, transposase.
E. coli JAB-1 carried three plasmids, as observed following Kieser extraction (data not shown). Electroporation in E. coli TOP10 and selection on ticarcillin conferred an ESBL phenotype as well as resistance to co-trimoxazole. The transformant carried a plasmid of 68 kb, namely, pECO-CTX-M, which was fully sequenced. Several antibiotic resistance genes were found on this IncY plasmid (Fig. 3). The ESBL-encoding blaCTX-M-15 gene and the narrow-spectrum β-lactamase-encoding blaTEM-1 gene, dfrA14, encoding a trimethoprim-insensitive dihydrofolate reductase variant, sul2, encoding a dihydropteroate synthase that is not inhibited by sulfonamide, tetA (tetracycline efflux protein) and aminoglycoside resistance genes [aph(6)-Id and aph(3″)-Ib] were present on the plasmid. Finally, the plasmid-carried fluoroquinolone resistance gene qnrS1 was responsible for reduced susceptibility to ciprofloxacin.
Kieser extraction showed that K. pneumoniae JAB-1 carries three plasmids (data not shown). Electroporation into E. coli TOP10 and selection on ticarcillin conferred an ESBL phenotype, and the transformant carried a plasmid of 60 kb, called pKP-CTX-M, which belonged to the IncR incompatibility group and was fully sequenced (Fig. 3). We identified in this plasmid five resistance genes encoding three β-lactamases (blaCTX-M-15, blaTEM-1b, and blaOXA-1), an aminoglycoside acetyltransferase gene [aac(6′)-Ib-cr], and the chloramphenicol resistance gene catB3.
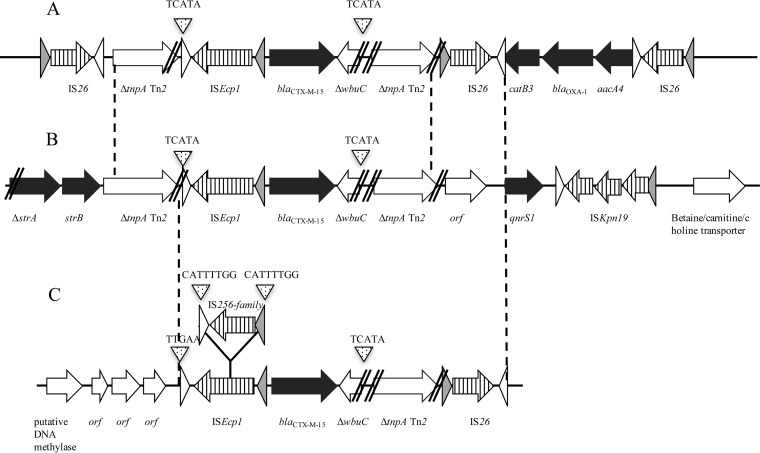
A comparison of the three blaCTX-M-15 gene-harboring plasmids revealed major differences in size, backbone, and incompatibility group, thus ruling out in vivo plasmid exchanges between these three species (Fig. 3). There was no plasmid deposited in the NCBI database that shared the same structure as pSHE-CTX-M and pKP-CTX-M, but we found one plasmid called tig00003056 (accession no. CP021681.1) very close to pECO-CTX-M (A. Jousset, personal communication). To gain further insights into the acquisition of the blaCTX-M-15 gene by these plasmids, we analyzed the genetic environment of the ESBL gene (Fig. 4). It revealed that the blaCTX-M-15 gene was part of an ISEcp1-borne transposon, as previously described (20). In pKP-CTX-M and pECO-CTX-M, the ISEcp1-borne transposon was inserted in the transposase tnpA gene of Tn2, generating the same target site duplication (TSD; TCACA; Fig. 4A and B). This is in favor of the idea of the acquisition of blaCTX-M-15 gene occurs by homologous recombination of this region rather than by a transposition event. This structure was also identified in other blaCTX-M-15 gene-harboring plasmids, such as the archetypal IncFII-type plasmid pC15-1A circulating in Canada in the early 2000s (21). In pSHE-CTX-M, ISEcp1-blaCTX-M-15 is also inserted within the Tn2 transposase gene, but this insertion differs by two different features: (i) the DNA fragment upstream of Tn2 transposase is missing and (ii) an insertion sequence (IS) belonging to the IS256 family with a TSD of 8 bp was identified (CATTTTGG) within the ISEcp1 transposase gene. Our data suggest a common ancestor for these structures, but they have evolved differently and are carried on different plasmids. Despite the insertion of an IS256-like gene in ISEcp1 in pSHE-CTX-M, the expression of CTX-M-15 did not seem to be impacted based on the MICs measured for third-generation cephalosporins (Table 1). Accordingly, the promoter sequences provided by ISEcp1 (−35 TTGAAA and −10 TACAAT) remained intact.
FIG 4.
Genetic environment of the blaCTX-M-15 gene of K. pneumoniae JAB-1 (A), E. coli JAB-1 (B), and Shewanella sp. JAB-1 (C). Antimicrobial resistance genes are indicated with black arrows. Open reading frames within insertion sequences (IS) are represented with striped arrows. Target site duplications are indicated with small dotted triangle. Inverted repeats IRR and IRL are represented by white and light gray arrows, respectively. Parallel lines indicate truncated genes.
TABLE 1.
MIC values of Shewanella spp. and transformants
| Antibiotic | MIC (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| S. putrefaciens (OXA-181 chr) | S. xiamenensis CIP 67.65 | S. algae CIP 103562 | Shewanella sp. JAB-1 (OXA-535 chr) | E. coli TOP10(pTOPO-OXA-535) | E. coli TOP10(pTOPO OXA-48) | E. coli TOP10 | |
| Temocillin | 0.38 | 0.75 | 0.19 | 0.25 | >1,024 | >1,024 | 4 |
| Cefotaxime | 0.094 | 0.064 | 0.032 | >32 | 0.064 | 0.75 | 0.06 |
| Ceftazidime | 0.125 | 0.125 | 0.064 | 1.5 | 0.19 | 0.19 | 0.12 |
| Imipenem | 4 | 0.75 | 0.5 | 0.38 | 1 | 0.75 | 0.25 |
| Ertapenem | 0.064 | 1 | 0.023 | 1 | 0.25 | 0.25 | 0.003 |
| Meropenem | 0.125 | 0.5 | 0.023 | 0.38 | 0.19 | 0.25 | 0.016 |
chr indicates the presence of a natural oxacillinase in the chromosome.
Characterization of OXA-535.
To determine whether OXA-535 can hydrolyze carbapenems, the blaOXA-535 gene was cloned into pTOPO together with its surrounding regions. E. coli TOP10 expressing the recombinant plasmid (pTOPO-OXA-535) displayed a class D carbapenemase phenotype with resistance to temocillin, resistance to penicillins that was not restored by the addition of clavulanic acid, and susceptibility to third-generation cephalosporins (Table 1). In addition, E. coli TOP10(pTOPO-OXA-535) had reduced susceptibility to carbapenems compared to E. coli TOP10. The Carba NP test performed on the recombinant strain was positive, suggesting that the OXA-535 enzyme hydrolyzes carbapenems. Furthermore, specific activity (SA) measured with a culture extract of E. coli TOP10(pTOPO-preOXA-535) showed that this strain hydrolyzes imipenem significantly (SA = 270 mU · mg · 1iter of protein), similar to that of E. coli TOP10(pTOPO-OXA-48), which was used as a control (SA = 130 mU · mg · 1iter of protein). The MICs for β-lactams for OXA-535 and OXA-48 were similar, confirming that these enzymes possessed similar resistance patterns (Table 1).
Interestingly, when performed directly on the Shewanella sp. JAB-1, the Carba NP test and the β Carba test were negative. Since tests based on carbapenem hydrolysis can lack sensitivity to detect isolates expressing β-lactamase with low carbapenemase activity, such as OXA-48-like enzymes, an immunochromatographic test known to detect OXA-48-like enzymes with high sensitivity was performed (22). Indeed, the OXA-48 K-SeT gave a positive result on colonies of Shewanella. Therefore, it seems that the OXA-535 enzyme is expressed at a low level but sufficiently to be detected by the immunochromatographic test. At last, a molecular test routinely used in clinical microbiology lab, the Xpert Carba-R test, was performed directly on the Shewanella sp. JAB-1 colonies and yielded a positive PCR for OXA-48-like carbapenemase.
DISCUSSION
We report the case of a biliary infection that occurred in a child with a novel Shewanella sp. belonging to an uncharacterized species. MALDI-TOF MS identified this isolate at the genus level, whereas WGS provided high resolution to identify this isolate as closely related to Shewanella sp. MR-4, MR-7, ANA-3, and Shew256 (Fig. 2). Phylogenetic analyses revealed that these strains belong to a unique lineage that likely constitutes a novel species.
Shewanella spp. are increasingly reported as pathogens, especially in patients with underlying hepatobiliary diseases. In a case series performed by Chen et al., all patients with bacteremia had underlying hepatobiliary disorders (23). Here, bacterial culture from the bile of the patient identified in addition to the Shewanella sp. JAB-1 six other clinically relevant bacteria. In a case series performed by To et al., bile samples whose cultures were positive for Shewanella were also polymicrobial, along with other enteric bacteria (7). Since Shewanella spp. are often isolated with other pathogens in clinical specimens, their clinical significance might be difficult to assess (4).
Next-generation sequencing enabled us to study the complete resistome of the Shewanella sp. JAB-1 as well as the features of the three ESBL-encoding plasmids (pSHE-CTX-M, pECO-CTX-M, and pKP-CTX-M). Even if the three strains shared the same clinical environment, they did not share their plasmid, since they belong to different incompatibility groups. Plasmid continuous sequences allowed us to determine the genetic environment of blaCTX-M-15. Indeed, distinct episodes of homologous recombination and of transpositions seem to have occurred rather than direct plasmid conjugation between strains in the patient.
The blaCTX-M-15 and blaSHV-2a genes are two ESBL genes present on an IncA/C plasmid of 193 kb in Shewanella sp. JAB-1. ESBL production has, to date, never been reported in any Shewanella species. IncA/C plasmids are known to efficiently spread many resistance genes, in particular, blaCMY-like Ambler class C β-lactamase genes (24). They have been detected worldwide, and carbapenemase genes, such as blaNDM-1 and blaVIM-4, have been identified on that plasmid scaffold (25, 26). Recently, characterization of ESBL-encoding plasmids in clinical isolates of K. pneumoniae in Taiwan revealed that the most common replicon type was incompatibility group IncA/C (27). Coexpression of blaCTX-M and blaSHV is not rare on this scaffold (27). Therefore, IncA/C plasmids seem to efficiently spread ESBL genes among Enterobacteriaceae and non-Enterobacteriaceae.
By sequencing the Shewanella sp. JAB-1 genome, we identified a new oxacillinase variant called OXA-535. According to the enzymatic activities, OXA-535 can be considered a new carbapenem-hydrolyzing β-lactamase. OXA-535 presented only 3 amino acids difference with the plasmidic carbapenemase OXA-436. Moreover, we could identify a 7.3-kb fragment in the chromosome of Shewanella sp. JAB-1 including the blaOXA-535 gene, which presented 96% nucleotide identity with the plasmid-carried blaOXA-436 gene region. Considering the high similarities between these structures, we can speculate that this 7.3-kb fragment has been mobilized on a plasmid and that the blaOXA-535 gene is likely the precursor of the plasmid-carried blaOXA-436 gene.
Interestingly, antimicrobial susceptibility testing, the Carba NP test, the β Carba test, and OXA-48 K-SeT indicated that blaOXA-535 gene is barely expressed in Shewanella sp. JAB-1. Accordingly, we did not identify any insertion element (IS) upstream of the blaOXA-535 gene that could provide strong promoter sequences, unlike the plasmid-encoded OXA-181 and OXA-48, where ISEcp1 and IS1999, respectively, allow strong carbapenemase expression. Like in other sequenced Shewanella spp., we identify a gene coding for a peptidase C15 family upstream of blaOXA-535 and a lysR gene coding for a putative LysR transcriptional regulator downstream (13).
The Xpert Carba-R test performed on the colonies could amplify the blaOXA-535 gene (data not shown). We can speculate that if this test would have been performed directly on a rectal swab of this patient, as is recommended for screening of high-risk patients (28), it might have been falsely positive for the detection of a carbapenemase-producing Enterobacteriaceae. A false-positive molecular screening test has already been reported by Antonelli et al., likely due to the presence in the human gut of S. xiamenensis carrying blaOXA-416 in its chromosome (29). The rate of fecal carriage of Shewanella spp. in the general population is currently unknown, as well as the bacterial concentration sufficient to yield a positive molecular test result. Nevertheless, the analytical limit of detection of the Xpert Carba-R assay is quite low (ranging from 1.1 × 102 to 1.2 × 103 CFU/swab depending on the carbapenemase gene [30]). Given these data, false-positive molecular test results remain troublesome, since hepatobiliary diseases have been proposed as a risk factor for infections (or colonization) due to Shewanella spp. and can therefore contribute to regularly inoculate the gastrointestinal tract.
MATERIALS AND METHODS
Bacterial strains.
Shewanella sp. JAB-1, Escherichia coli JAB-1, and Klebsiella pneumoniae JAB-1 clinical isolates were from the Bicêtre Hospital, Le Kremlin-Bicêtre, France. They have been identified with MALDI-TOF MS using the Bruker MS system (Bruker Daltonics, Bremen, Germany), according to the manufacturer's instructions. In addition, sequencing of 16S rRNA using universal primers 16S 8-27 and 16S 1512-1491 was performed for the Shewanella isolate, as described previously (9). Biochemical features were studied using API32GN and API20E (bioMérieux, Marcy l'Etoile, France), according to the manufacturer's instructions. Electrocompetent E. coli TOP10 (Invitrogen, Éragny, France) was used as a recipient for electroporation experiments. E. coli J53 RIFr, which is resistant to azide and rifampin (RIFr), was used for conjugation experiments. E. coli 50192 was used as a reference strain for plasmid extraction (31). S. algae CIP 103562, S. putrefaciens CIP 67.65, and S. xiamenensis S12 (9) were used as references for susceptibility testing and determination of biochemical features.
Susceptibility testing and MIC determinations.
Susceptibility testing was performed by the disk diffusion method on Mueller-Hinton agar plates (Bio-Rad) incubated for 18 h at 37°C. There are no recognized Clinical and Laboratory Standards Institute (CLSI) MIC interpretative standards specified for the Shewanella genus. We used the MIC breakpoints of CLSI approved standard M100-S24 categories for other non-Enterobacteriaceae, in accordance with previous reports (3). The MICs of carbapenems were determined using the Etest (bioMérieux, La Balme-les-Grottes, France).
Resistance gene detection.
Genes coding for Ambler class A β-lactamases were sought by PCR using primers specific for the blaTEM, blaSHV, and blaCTX-M genes, as previously described (32). PCR products were purified using the QIAquick PCR purification kit (Qiagen, Courtabœuf, France) and sequenced on both strands with an automated sequencer (ABI 3100; Applied Biosystems, Foster City, CA).
Cloning experiments.
Genomic DNA of the Shewanella sp. JAB-1 isolate extracted using the Qiagen DNAamp kit (Qiagen) was used as the template for amplification of the blaOXA-535 gene with its surrounding region (290 nucleotides [nt] before the ATG and 181 nt after the stop codon) and was performed with pre-OXA-535-For (5′-CGTTTGGGTTTGCTTCAT-3′) and pre-OXA-535-Rev (5′-GACTAGGCTTTTTGCGTT-3′) primers, and the PCR product was inserted into pTOPO (Invitrogen, Éragny, France), resulting in pTOPO-preOXA-535. E. coli TOP10 carrying recombinant plasmid pTOPO-preOXA-535 was selected using a Trypticase soy (TS) agar plate containing ticarcillin (50 mg/liter) and kanamycin (50 mg/liter). The inserted DNA fragment was verified by Sanger sequencing.
Plasmid extraction, electroporation, and mating-out assay.
Natural plasmids were extracted using the Kieser extraction method (33) and subsequently analyzed by electrophoresis on a 0.7% agarose gel. ESBL-encoding plasmid DNA of Shewanella sp. JAB-1 (pSHE-CTX-M), E. coli (pECO-CTX-M), and K. pneumoniae (pKP-CTX-M) isolates were extracted using the Qiagen plasmid maxikit (Qiagen, Courtabœuf, France) and analyzed by agarose gel electrophoresis (Invitrogen, Paris, France).
Transfer of the β-lactam resistance markers from Shewanella sp. JAB-1 was performed by liquid mating-out assays at 37°C using E. coli J53 RIFr as the recipient strain (31).
Recombinant plasmids (pTOPO-preOXA-48 and pTOPO-preOXA-535) were introduced by electroporation into E. coli TOP10 using a Gene Pulser II (Bio-Rad Laboratories) (31).
Detection of carbapenemase presence.
Bacterial colonies of Shewanella sp. JAB-1 recovered from Trypticase soy agar were used to perform Xpert Carba-R test version 2 (Cepheid, Sunnyvale, CA, USA), OXA-48 K-SeT assay (Coris BioConcept, Gembloux, Belgium), the Carba NP test, and the β Carba test, according to the manufacturer's recommendations (22, 34, 35) or updated guidelines (36). The Carba NP test was also performed on recombinant E. coli TOP10 carrying pTOPO-preOXA-535.
Enzymatic activities.
The specific activities of the OXA-48 and OXA-535 β-lactamases were determined using the supernatant of a whole-cell crude extract obtained from an overnight culture of E. coli clones expressing those two β-lactamases (pTOPO-preOXA-48 and pTOPO-preOXA-535 in E. coli TOP10) with an Ultrospec 2000 UV spectrophotometer (Amersham Pharmacia Biotech), as previously described (37). Imipenem was used as the substrate at a concentration of 100 μM.
Whole-genome sequencing and bioinformatic analysis.
Total DNA of Shewanella sp. JAB-1 was extracted using the UltraClean microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA), according to the manufacturer's instructions. Total DNA and plasmid DNA (pSHE-CTX-M, pECO-CTX-M, and pKP-CTX-M) concentrations were measured using the Qubit double-stranded DNA (dsDNA) BR assay kit (Life Technologies, Carlsbad, CA, USA). The DNA libraries were prepared using the Nextera XT version 3 kit (Illumina, San Diego, CA, USA), according to the manufacturer's instructions, and then run on the HiSeq or MiSeq system (Illumina) to generate paired-end 150-bp reads. Total Shewanella DNA was also sequenced using PacBio long-read technology (Macrogen, Seoul, South Korea).
Illumina read de novo assembly was performed using CLC Genomics Workbench 9.0, according to the manufacturer's recommendations (Qiagen, Courtabœuf, France). PacBio subreads were assembled with both Canu (38) and the RS_HGAP_Assembly.3 protocol from the SMRT Analysis toolkit version 2.3, while consensus accuracy was further polished using Quiver (39), as was previously described (40). PCR sequencing was used to complete the assembly of pECO-CTX-M and pKP-CTX-M plasmids. A phylogenetic tree based on the gyrB-rpoB concatemer was drawn with the MEGA7 software using the maximum likelihood method based on the Tamura-Nei model (41). Phylogenetic analysis based on whole-genome sequences of Shewanella spp. close to Shewanella sp. JAB-1 was done using the Parsnp program from the Harvest Suite (42). tRNAs were detected by ARAGORN (http://mbio-serv2.mbioekol.lu.se/ARAGORN/) (43). Circular representation was performed by CGview (44). Shewanella sp. JAB-1 was compared with three closely related strains: Shewanella MR-4 (accession no. CP000446.1), MR-7 (accession no. CP000444.1), and ANA-3 (accession no. CP000469.1).
Accession number(s).
The nucleotide sequence of the blaOXA-535 gene of the assembled chromosome sequence of Shewanella sp. JAB-1 and of its 10.5-kb plasmid, with no antibiotic resistance features, have been deposited at the EMBL/GenBank nucleotide sequence database under the accession numbers KX828709, CP022358, and CP022360, respectively. The three assembled ESBL-encoding plasmids (pSHE-CTX-M, pECO-CTX-M, and pKP-CTX-M) have been deposited at the EMBL/GenBank nucleotide sequence database under the accession numbers CP022359, MF510423, and MF510424, respectively.
ACKNOWLEDGMENTS
We thank the Institut Pasteur Collection (https://www.pasteur.fr/fr/crbip) for providing strains S. algae CIP 103562 and S. putrefaciens CIP 67.65.
This work was supported by the Assistance Publique-Hôpitaux de Paris, by a grant from the Université Paris Sud (EA 7361), and by the LabEx LERMIT supported by a grant from the French National Research Agency (ANR-10-LABX-33) and by the LabEx IBEID. This work was also funded in part by a grant from the Joint Programming Initiative on Antimicrobial Resistance (ANR-14-JAMR-0002).
L.D. is coinventor of the Carba NP test, the patent for which has been licensed to bioMérieux (La Balme-les-Grottes, France).
REFERENCES
- 1.Holt HM, Gahrn-Hansen B, Bruun B. 2005. Shewanella algae and Shewanella putrefaciens: clinical and microbiological characteristics. Clin Microbiol Infect 11:347–352. doi: 10.1111/j.1469-0691.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.Ceccarelli D, van Essen-Zandbergen A, Veldman KT, Tafro N, Haenen O, Mevius DJ. 2017. Chromosome-based blaOXA-48-like variants in Shewanella species isolates from food-producing animals, fish, and the aquatic environment. Antimicrob Agents Chemother 61:e01013-16. doi: 10.1128/AAC.01013-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P-Y, Lin C-F, Tung K-C, Shyu C-L, Wu M-J, Liu J-W, Chang C-S, Chan K-W, Huang J-A, Shi Z-Y. 2013. Clinical and microbiological features of Shewanella bacteremia in patients with hepatobiliary disease. Intern Med 52:431–438. doi: 10.2169/internalmedicine.52.8152. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka T, Noda T, Noguchi A, Nakamura H, Ibaraki K, Yamaoka K. 2007. Shewanella infection in decompensated liver disease: a septic case. J Gastroenterol 42:87–90. doi: 10.1007/s00535-006-1957-0. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Gallego I, Chaves F, Orellana MA. 2016. Epidemiological and clinical characteristics of Shewanella spp. infections in a tertiary hospital in Madrid. Infect Dis (Lond) 48:760–762. doi: 10.3109/23744235.2016.1169554. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez H, Vogel BF, Gram L, Hoffmann S, Schaebel S. 1996. Shewanella alga bacteremia in two patients with lower leg ulcers. Clin Infect Dis 22:1036–1039. doi: 10.1093/clinids/22.6.1036. [DOI] [PubMed] [Google Scholar]
- 7.To KKW, Wong SSY, Cheng VCC, Tang BSF, Li IWS, Chan JFW, Seto W-K, Tse H, Yuen K-Y. 2010. Epidemiology and clinical features of Shewanella infection over an eight-year period. Scand J Infect Dis 42:757–762. doi: 10.3109/00365548.2010.490562. [DOI] [PubMed] [Google Scholar]
- 8.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob Agents Chemother 54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potron A, Poirel L, Nordmann P. 2011. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis. Antimicrob Agents Chemother 55:4405–4407. doi: 10.1128/AAC.00681-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirel L, Héritier C, Nordmann P. 2004. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob Agents Chemother 48:348–351. doi: 10.1128/AAC.48.1.348-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Héritier C, Poirel L, Nordmann P. 2004. Genetic and Biochemical characterization of a chromosome-encoded carbapenem-hydrolyzing Ambler class D β-lactamase from Shewanella algae. Antimicrob Agents Chemother 48:1670–1675. doi: 10.1128/AAC.48.5.1670-1675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dortet L, Cuzon G, Ponties V, Nordmann P. 2017. Trends in carbapenemase-producing Enterobacteriaceae, France, 2012 to 2014. Euro Surveill 22:pii=30461. doi: 10.2807/1560-7917.ES.2017.22.6.30461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacão M, Araújo S, Vendas M, Alves A, Henriques I. 2017. Shewanella species as the origin of blaOXA-48 genes: insights into gene diversity, associated phenotypes and possible transfer mechanisms. Int J Antimicrob Agents 17:30222–30224. doi: 10.1016/j.ijantimicag.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Almuzara M, Montaña S, Lazzaro T, Uong S, Parmeciano Di Noto G, Traglia G, Bakai R, Centrón D, Iriarte A, Quiroga C, Ramirez MS. 2017. Genetic analysis of a PER-2-producing Shewanella sp. strain harbouring a variety of mobile genetic elements and antibiotic resistant determinants. J Glob Antimicrob Resist 11:81–86. doi: 10.1016/j.jgar.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Pitout JD, Laupland KB. 2008. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 16.Paterson DL, Bonomo RA. 2005. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev 18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez F, Endimiani A, Hujer KM, Bonomo RA. 2007. The continuing challenge of ESBLs. Curr Opin Pharmacol 7:459–469. doi: 10.1016/j.coph.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flandrois J-P, Perrière G, Gouy M. 2015. leBIBIQBPP: a set of databases and a webtool for automatic phylogenetic analysis of prokaryotic sequences. BMC Bioinformatics 16:251. doi: 10.1186/s12859-015-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Hye Park C, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 20.Poirel L, Bonnin RA, Nordmann P. 2012. Genetic support and diversity of acquired extended-spectrum β-lactamases in Gram-negative rods. Infect Genet Evol 12:883–893. [DOI] [PubMed] [Google Scholar]
- 21.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob Agents Chemother 48:3758–3764. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glupczynski Y, Evrard S, Ote I, Mertens P, Huang T-D, Leclipteux T, Bogaerts P. 2016. Evaluation of two new commercial immunochromatographic assays for the rapid detection of OXA-48 and KPC carbapenemases from cultured bacteria. J Antimicrob Chemother 71:1217–1222. doi: 10.1093/jac/dkv472. [DOI] [PubMed] [Google Scholar]
- 23.Chen YS, Liu YC, Yen MY, Wang JH, Wang JH, Wann SR, Cheng DL. 1997. Skin and soft-tissue manifestations of Shewanella putrefaciens infection. Clin Infect Dis 25:225–229. doi: 10.1086/514537. [DOI] [PubMed] [Google Scholar]
- 24.Call DR, Singer RS, Meng D, Broschat SL, Orfe LH, Anderson JM, Herndon DR, Kappmeyer LS, Daniels JB, Besser TE. 2010. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob Agents Chemother 54:590–596. doi: 10.1128/AAC.00055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potron A, Nordmann P, Poirel L. 2013. Characterization of OXA-204, a carbapenem-hydrolyzing class D lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 57:633–636. doi: 10.1128/AAC.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. 2012. Evolution of IncA/C blaCMY-2-carrying plasmids by acquisition of the blaNDM-1 Carbapenemase Gene Antimicrob Agents Chemother 56:783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang C-Y, Lin H-J, Chang L-L, Ma L, Siu LK, Tung Y-C, Lu P-L. 2017. Characterization of extended-spectrum β-lactamase-carrying plasmids on clinical isolates of Klebsiella pneumoniae from Taiwan. Microb Drug Resist 23:98–106. doi: 10.1089/mdr.2015.0212. [DOI] [PubMed] [Google Scholar]
- 28.Hoyos-Mallecot Y, Ouzani S, Dortet L, Fortineau N, Naas T. 2017. Performance of the Xpert Carba-R v2 in the daily workflow of a hygiene unit in a country with a low prevalence of carbapenemase-producing Enterobacteriaceae. Int J Antimicrob Agents 49:774–777. doi: 10.1016/j.ijantimicag.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Antonelli A, Di Palo DM, Galano A, Becciani S, Montagnani C, Pecile P, Galli L, Rossolini GM. 2015. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48–producing Enterobacteriaceae. Diagn Microbiol Infect Dis 82:1–3. doi: 10.1016/j.diagmicrobio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Tato M, Ruiz-Garbajosa P, Traczewski M, Dodgson A, McEwan A, Humphries R, Hindler J, Veltman J, Wang H, Cantón R. 2016. Multisite evaluation of Cepheid Xpert Carba-R assay for detection of carbapenemase-producing organisms in rectal swabs. J Clin Microbiol 54:1814–1819. doi: 10.1128/JCM.00341-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzon G, Naas T, Truong H, Villegas M-V, Wisell KT, Carmeli Y, Gales AC, Navon-Venezia S, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg Infect Dis 16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao V, Lambert T, Nhu DQ, Loan HK, Hoang NK, Arlet G, Courvalin P. 2002. Distribution of extended-spectrum β-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob Agents Chemother 46:3739–3743. doi: 10.1128/AAC.46.12.3739-3743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 34.Dortet L, Fusaro M, Naas T. 2016. Improvement of the Xpert Carba-R kit for the detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:3832–3837. doi: 10.1128/AAC.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernabeu S, Dortet L, Naas T. 2017. Evaluation of the β Carba test, a colorimetric test for the rapid detection of carbapenemase activity in Gram-negative bacilli. J Antimicrob Chemother 72:1646–1658. doi: 10.1093/jac/dkx061. [DOI] [PubMed] [Google Scholar]
- 36.Dortet L, Brechard L, Poirel L, Nordmann P. 2014. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol 63:772–776. doi: 10.1099/jmm.0.071340-0. [DOI] [PubMed] [Google Scholar]
- 37.Dortet L, Oueslati S, Jeannot K, Tandé D, Naas T, Nordmann P. 2015. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother 59:3823–3828. doi: 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlin K, Koren S, Chin C-S, Drake JP, Landolin JM, Phillippy AM. 2015. Assembling large genomes with single-molecule sequencing and locality-sensitive hashing. Nat Biotechnol 33:623–630. doi: 10.1038/nbt.3238. [DOI] [PubMed] [Google Scholar]
- 39.Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 40.Almeida A, Alves-Barroco C, Sauvage E, Bexiga R, Albuquerque P, Tavares F, Santos-Sanches I, Glaser P. 2016. Persistence of a dominant bovine lineage of group B Streptococcus reveals genomic signatures of host adaptation. Environ Microbiol 18:4216–4229. doi: 10.1111/1462-2920.13550. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treangen TJ, Ondov BD, Koren S, Phillippy AM. 2014. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 15:524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laslett D. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res 32:11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stothard P, Wishart DS. 2005. Circular genome visualization and exploration using CGView. Bioinformatics 21:537–539. doi: 10.1093/bioinformatics/bti054. [DOI] [PubMed] [Google Scholar]