Significance
During communication, social ostensive signals (like gaze) are exchanged in a temporally contingent manner. Synchronized behavior creates social connectedness within human dyads, and even infants synchronize behaviorally with adults. However, the neural mechanisms that support infant–adult synchronization are unknown. Here, we provide evidence that infants up-regulate neural synchronization with adult partners when offered direct ostensive gaze, as compared with gaze aversion. Gaze therefore brings infant–adult neural activity into mutual alignment, creating a joint-networked state that may facilitate communicative success. Further, infants’ own communicative attempts were positively associated with adults’ neural synchronization to them, indicating mutual regulation of synchronization within infant–adult dyads. Thus, interpersonal neural synchronization may provide a mechanism by which infants construct their own earliest social networks.
Keywords: neural synchronization, dyadic interaction, mutual gaze, ostensive signals, intention
Abstract
When infants and adults communicate, they exchange social signals of availability and communicative intention such as eye gaze. Previous research indicates that when communication is successful, close temporal dependencies arise between adult speakers’ and listeners’ neural activity. However, it is not known whether similar neural contingencies exist within adult–infant dyads. Here, we used dual-electroencephalography to assess whether direct gaze increases neural coupling between adults and infants during screen-based and live interactions. In experiment 1 (n = 17), infants viewed videos of an adult who was singing nursery rhymes with (i) direct gaze (looking forward), (ii) indirect gaze (head and eyes averted by 20°), or (iii) direct-oblique gaze (head averted but eyes orientated forward). In experiment 2 (n = 19), infants viewed the same adult in a live context, singing with direct or indirect gaze. Gaze-related changes in adult–infant neural network connectivity were measured using partial directed coherence. Across both experiments, the adult had a significant (Granger) causal influence on infants’ neural activity, which was stronger during direct and direct-oblique gaze relative to indirect gaze. During live interactions, infants also influenced the adult more during direct than indirect gaze. Further, infants vocalized more frequently during live direct gaze, and individual infants who vocalized longer also elicited stronger synchronization from the adult. These results demonstrate that direct gaze strengthens bidirectional adult–infant neural connectivity during communication. Thus, ostensive social signals could act to bring brains into mutual temporal alignment, creating a joint-networked state that is structured to facilitate information transfer during early communication and learning.
Temporally contingent social interactions between adults and infants play a vital role in supporting early learning across multiple domains of language, cognition, and socioemotional development (1, 2). Infants rely heavily on the temporal dynamics of facial cues such as eye contact and gaze direction to infer intention, meaning, and causality (3–5), which is unsurprising given that infants’ early visual experience is heavily composed of faces (6). Of all cues, direct gaze is thought to be one of the most salient ostensive signals in human communication for conveying communicative intent (4). Gaze also acts to release and reinforce infants’ own social responses such as smiling and vocalization (7, 8). From birth, infants prefer to look at pictures of faces with direct gaze over averted gaze (9). By 4 mo, direct gaze elicits a larger amplitude in the face-sensitive N170 event-related potential (ERP) relative to averted gaze (10), which suggests that gaze also enhances infants’ neural processing of face-related information.
Social Synchronization Through Gaze in Communication
According to the social brain hypothesis, human brains have fundamentally evolved for group living (11). Social connectedness is created when group members act jointly (e.g., synchronously) or contingently (e.g., turn-taking) with each other (12). Even infants show synchronization with their adult caregivers, and adult–infant temporal contingencies have long been observed in behavioral and physiological domains. For example, patterns of temporally synchronous activity between parent and child during social interaction have been noted for gaze (13), vocalizations (14), affect (15), autonomic arousal (16, 17), and hormones (18). The synchronization of gaze (through mutual gaze and gaze-following) is thought to foster social connectedness between infants and adults (19). Previous research has also suggested that infants, like adults (20), show neural synchronization (or phase-locking) of cortical oscillatory activity to temporal structures in auditory signals (21). However, adult–infant behavioral and physiological synchronization is typically observed over much slower timescales (e.g., minutes or seconds) than neural synchronization (tens or hundreds of milliseconds). Thus, it remains to be seen whether neural synchronization also develops between infants and adults during social interaction and if/how such neural coupling is related to social synchronizing signals like gaze.
Recently, researchers have begun to examine the neural mechanisms that support the contingency (temporal dependency) of one partner’s neural activity with respect to the other during social interactions (see refs. 22 and 23 for reviews). This work has revealed that during verbal communication (especially face-to-face communication, which permits mutual gaze), adult speaker–listener pairs develop synchronous patterns of activity between brain regions such as the inferior frontal gyrus, prefrontal, and parietal cortices (24, 25). Further, the strength of speaker–listener neural synchronization predicts communication success (26). Thus, in adults, effective communication involves the mutual alignment of brain activity, as well as the temporal alignment of behavior (e.g., conversational turn-taking and mutual gaze). However, to our knowledge, no previous research has yet investigated whether infants’ neural activity also shows contingency on an adult partner’s neural activity and whether gaze acts as a neural synchronization cue during adult–infant communication.
Gaze-Cuing of Interpersonal Neural Synchronization
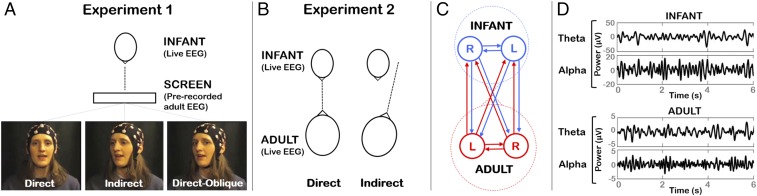
Here, we assessed whether the temporal dependency (synchronization) between adult and infant neural signals differed between direct and indirect gaze. Two experiments were performed to assess gaze-cuing of interpersonal synchronization in video and live modalities, respectively. In experiment 1, infants watched a prerecorded video of an experimenter singing nursery rhymes. Patterns of temporal dependency were assessed between infants’ neural activity recorded “live” and adult’s prerecorded neural activity (Fig. 1). We manipulated the adult speaker’s gaze to be either direct to the infant, indirect (head averted at a 20° angle), or direct-oblique (head averted but eyes toward the infant). The direct-oblique condition was included to control for the side view of the face that was presented during indirect gaze and to preclude the possibility that infants were responding to superficial visual differences between stimuli. In experiment 2, which used an entirely separate cohort, infants listened live to an adult reciting nursery rhymes while she presented direct or indirect gaze to the infant. Partial directed coherence (27), a statistical measure of Granger causality (28), was used to measure gaze-related changes in interpersonal neural synchronization within the adult–infant dyadic social network.
Fig. 1.
Illustration of experimental protocols and connectivity analysis. (A) In experiment 1, infants viewed a video screen showing an experimenter reciting nursery rhymes. Three gaze conditions were presented interleaved: direct, indirect (head averted by 20°), and direct-oblique (head averted by 20°, direct gaze). The infant’s live EEG was compared with the adult’s prerecorded EEG. (B) In experiment 2, infant and adult sat opposite each other. Direct and indirect gaze (head averted by 20°) conditions were presented. (C) The adult–infant network comprised left (L) and right (R) electrodes each from the infant and adult. Interpersonal neural connectivity was assessed across all pairwise connections between electrodes using partial directed coherence. (D) Examples of infant and adult EEG data, which were analyzed within Theta (3–6 Hz) and Alpha (6–9 Hz) bands.
Predictions
In terms of affect and physiological changes, research has shown that the influence of infants and parents on one another is bidirectional (29, 30). Accordingly, we predicted that (i) significant neural coupling would exist between adults and infants during social interaction, (ii) direct (and direct-oblique) gaze would both be associated with higher interpersonal neural connectivity than indirect gaze, and (iii) in experiment 1 (video), only unidirectional [adult-to-infant (A → I)] coupling would be observed, but in experiment 2 (live), bidirectional [adult-to-infant (A → I) and infant-to-adult (I → A)] coupling would be observed. Further, as temporally contingent social interactions with adults are known to facilitate infants’ own vocalizations (8, 31), we predicted that infants’ vocalization efforts would be greater during direct than indirect gaze.
Results
Gaze Modulation of Interpersonal Neural Connectivity.
General Partial Directed Coherence (GPDC) measures the degree of influence that each electrode channel directly has on every other electrode channel in the network (27). Here, GPDC values were computed for real and surrogate (shuffled) data, for all nonself channel pairs (connections), for each participant dyad, for each gaze condition, and in Theta and Alpha EEG bands (Fig. 1 C and D). In the subsequent network diagrams (Figs. 2 and 3), only connections whose GPDC values significantly exceeded their surrogate threshold are plotted. A breakdown of GPDC values for each neural connection is provided in SI Appendix, section 1 (SI Appendix, Tables S1 and S2). Here we focus our analysis on mean A → I and I → A connectivity.
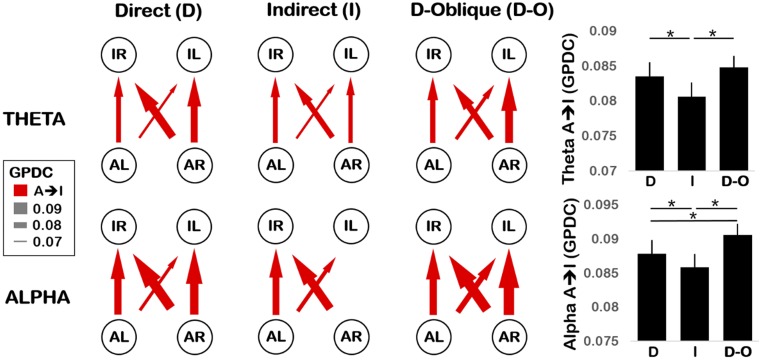
Fig. 2.
(Left) Network depiction of experiment 1 Theta (3–6 Hz, Top) and Alpha (6–9 Hz, Bottom) connectivity, plotting GPDC values for direct (Left), indirect (Middle), and direct-oblique gaze (Right) conditions. Nodes represent C3 (L) and C4 (R) electrodes for adult (A) and infant (I). Arrows indicate the direction and strength of connectivity (higher GPDC value, thicker arrow). Connections that do not significantly exceed the surrogate threshold are excluded. (Right) Grand mean GPDC values averaged across all adult-to-infant (A → I) connections for Theta (Top) and Alpha (Bottom) in direct (D), indirect (I), and direct-oblique (D-O) gaze conditions. Error bars show the SEM. *P < 0.05.
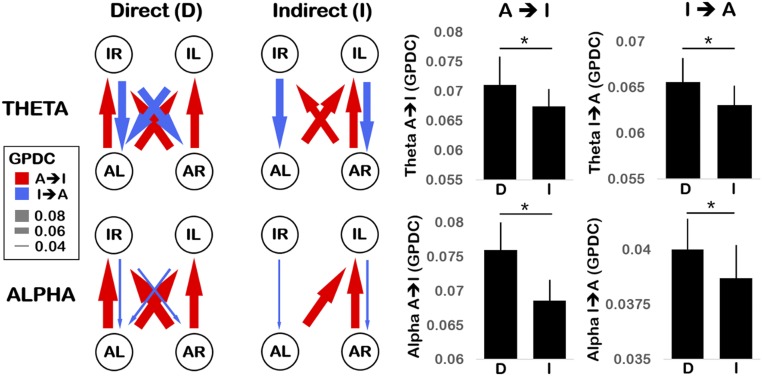
Fig. 3.
(Left) Network depiction of experiment 2 Theta (3–6 Hz, Top) and Alpha (6–9 Hz, Bottom) connectivity, plotting GPDC values for direct (Left) and indirect (Right) gaze conditions. Nodes represent C3 (L) and C4 (R) electrodes for adult (A) and infant (I). Arrows indicate the direction and strength of connectivity (higher GPDC value, thicker arrow). Connections that do not significantly exceed the surrogate threshold are excluded. (Right) Grand mean GPDC values averaged across all adult-to-infant (A → I, Left) and infant-to-adult (I → A, Right) connections for Theta (Top) and Alpha (Bottom) in direct (D) and indirect (I) gaze conditions. Error bars show the SEM. *P < 0.05.
Experiment 1: Video.
Only unidirectional A → I connectivity was observed in experiment 1; no significant I → A connectivity was detected (Fig. 2). This confirmed the validity of the GPDC measure as infants could not have affected the adult’s prerecorded neural activity. Dunnett’s tests revealed that, as predicted, A → I connectivity was (i) significantly stronger for direct > indirect gaze in both Theta and Alpha bands (P < 0.01 and P < 0.05, respectively, one-tailed) and (ii) significantly stronger for direct-oblique > indirect gaze in both Theta and Alpha bands (P < 0.0001 for both, one-tailed). However, while connectivity in the direct and direct-oblique conditions was not significantly different in the Theta band (P = 0.30) as predicted, for the Alpha band a significant difference between these conditions was observed (direct-oblique > direct, P < 0.01).
Experiment 2: Live.
During the live experiment, bidirectional connectivity was observed with significant A → I as well as I → A influences (Fig. 3).
Regarding A → I connectivity, consistent with experiment 1, Dunnett’s tests revealed that the adult's influence on infants was significantly stronger for direct > indirect gaze in both Theta and Alpha bands (P < 0.05 and P < 0.0001, respectively, one-tailed).
For I → A connectivity, Dunnett's tests indicated that infants' influence on the adult was likewise significantly stronger for direct > indirect gaze in both Theta and Alpha bands (P < 0.01 and P < 0.05, respectively, one-tailed).
Infant Vocalization Analysis.
For experiment 1 (video), there was no difference in the number of infant vocalizations (summed over all categories) between gaze conditions (means: direct = 8.2 per infant, indirect = 7.4, direct-oblique = 7.1), F(2, 32) = 0.29, P = 0.75, η2p = 0.02. There was also no difference in the duration of vocalizations across gaze conditions (means: direct = 0.69 s per utterance, indirect = 0.82 s, direct-oblique = 0.70 s), F(2, 24) = 0.37, P = 0.70, η2p = 0.03. However, for experiment 2 (live), we observed a significantly higher number of vocalizations during direct gaze (mean 6.3 per infant) than indirect gaze (mean 5.0 per infant), t(18) = 2.41, P < 0.05, but no difference in the duration of vocalizations (mean: direct = 0.80 s per utterance, indirect = 0.85 s), t(15) = −0.79, P = 0.44.
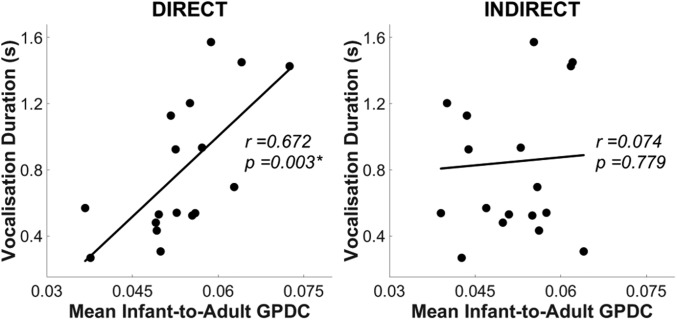
Further, during experiment 2 (live), individual differences in infants’ vocalization durations were significantly associated with their I → A GPDC values [r = 0.67, P < 0.05, Benjamini–Hochberg false discovery rate (FDR) corrected] (32) (see Fig. 4). However, this correlation only emerged during direct gaze and was absent for indirect gaze (r = 0.07, P = 0.78). Therefore, infants who produced longer vocalizations also influenced the adult more strongly—but only when she offered direct gaze. SI Appendix, section 2 provides further analyses of infants’ vocalizations.
Fig. 4.
Scatterplots showing the correlation between (n = 19) individual infants’ mean infant-to-adult GPDC value (averaged across Theta and Alpha bands, x axis), and their vocalization duration (y axis) in experiment 2. Left and Right show direct and indirect gaze conditions, respectively. *P < 0.05 (Benjamini–Hochberg FDR corrected).
Discussion
Temporally contingent social interactions between adults and infants scaffold early learning and development. Here, we tested the hypothesis that gaze acts as an interpersonal neural synchronization cue between dyadic (adult–infant) partners. Two experiments were performed to assess the effect of direct speaker gaze on interpersonal synchronization using video (experiment 1) and live (experiment 2) modalities. Across both experiments, significant neural coupling between infants and adults was observed during social interaction, relative to rigorous control analyses that accounted for nonspecific neural coupling. Adult–infant neural coupling was observed consistently across video and live presentation formats, using two separate cohorts of infants. Further, during unidirectional interactions in experiment 1 (i.e., infants watching a prerecorded adult speaker), the adult had a significant influence on infants’ neural activity, but (as expected) infants had no influence on the adult’s neural activity. Conversely, during live (bidirectional) social interactions (experiment 2), there were significant and bidirectional patterns of influence between adult and infant.
Across both experiments, we consistently observed that direct gaze produced higher interpersonal neural synchronization than indirect gaze in both Theta and Alpha frequency bands. Further, in experiment 2 (live), the synchronizing effect of gaze was observed bidirectionally: During direct gaze, the adult had a stronger influence on the infant, and the infant also had a stronger influence on the adult. This gaze-related increase in synchronization was not due to power differences in the EEG spectra, nor was it a metaphenomenon of changes in basic sensory processing of the speech signal (which remained unchanged across gaze conditions). In experiment 1, we further showed that the gaze effect was not driven by superficial visual differences in the stimuli, since direct-oblique stimuli were visually similar to indirect stimuli but produced greater synchronization. It was also not the case that infants were more inattentive during indirect gaze, as infants looked just as long at indirect and direct-oblique stimuli in experiment 1 and at indirect and direct stimuli in experiment 2. Therefore, the increased interpersonal neural synchronization produced by direct gaze appears to reflect stronger mutual oscillatory phase alignment between adult and infant.
A Mechanism for Interpersonal Neural Synchronization.
One mechanism that might mediate this effect is mutual phase resetting in response to salient social signals. The phase of cortical oscillations (the neural feature used in GPDC computations) reflects the excitability of underlying neuronal populations to incoming sensory stimulation (33). Sensory information arriving during high-receptivity periods is more likely to be encoded than information arriving during low-receptivity periods. Consequently, neuronal oscillations have been proposed to be a mechanism for temporal sampling of the environment (20). Specifically, salient events are thought to reset the phase of ongoing neuronal oscillations to match the temporal structure of these events and optimize their encoding (33). Consequently, interpersonal neural synchronization could increase within a dyad during the course of social interaction because each partner is continuously producing salient social signals (such as gaze, gestures, or vocalizations) that act as synchronization triggers to reset the phase of his or her partner’s ongoing oscillations. As a result, infants’ most receptive periods become well-aligned to adults’ speech temporal patterns (e.g., prosodic stress and syllable patterns) (34), optimizing communicative efficiency. This mechanism could also allow slow-varying behavioral synchronization signals (like gaze) to hierarchically control fast-varying neural synchronization between partners (33).
Direct Gaze Supports Communication Through Synchronization.
Our findings suggest that direct gaze from the adult may reset the phase of infants’ oscillations to align with that of the adults’, thereby increasing mutual synchronization (i.e., stronger A → I connectivity). One aspect of our results was, however, unpredicted. In experiment 1, we had predicted an equal effect for direct and direct-oblique gaze, yet we found that Alpha neural synchrony was higher for direct-oblique than direct gaze. One possible explanation for this is that infants are less frequently exposed to direct eye contact when the speaker’s head is averted, which could therefore present greater novelty. However, infants did not look for longer at the speaker during the direct-oblique condition relative to the direct gaze condition, which is inconsistent with this explanation. A second potential explanation is that the direct-oblique condition provided a stronger intentional ostensive cue because the speaker’s gaze was intentionally forward while her face and body were averted. This predicts that social cues that are perceived as the most intentional will produce the strongest increases in interpersonal connectivity. Further, since phase resetting optimizes information transfer between dyadic partners (33), stronger intentional signals could produce more effective phase resetting, which would increase the potential for mutual communication and learning within the dyad. Future work should investigate this hypothesis in more detail.
As observed in previous studies (8), we also found that infants vocalized more frequently toward the adult during live direct gaze (when interpersonal synchronization was higher) than indirect gaze. Further, individual infants who vocalized for longer under live direct gaze also had stronger neural connectivity with their adult partner (i.e., stronger I → A connectivity), even during segments when no vocalizations were occurring. One possible reason for this could be that infants’ vocalizations (which were communicative signals to the adult and could potentially trigger phase resetting) acted as a social feedback mechanism to positively reinforce and sustain dyadic synchronicity (8, 31, 35).
Our present findings may offer the potential for integrating three separate strands of research into early learning: first, research that has pointed to the importance of eye gaze as an ostensive cue during learning (3); second, research into the importance of contingent social feedback, which is thought to energize early learning (31); and third, research into the role of bidirectional parent–child synchrony in structuring and scaffolding learning experiences (36). Phase resetting due to synchronization triggers that are more prevalent during mutual than indirect gaze may, potentially, offer the means for providing contingent feedback (in which the child responds to the parent, and vice versa) within the framework of the periodic oscillatory activity that structures and scaffolds early learning (36). Over longer time frames, infants’ neural synchrony with adults may also offer an implicit mechanism for learning adult-like response patterns via entrainment.
Limitations and Conclusion
Our results converge with previous dual functional near-infrared spectroscopy (fNIRS) studies (24, 37) where greater frontal neural synchronization between adults was observed during eye contact. However, one limitation of the current work is that due to the adult’s speech production artifacts, only two EEG channels, C3 and C4, could be analyzed from each individual. Thus, unlike the fNIRS studies, we were unable to make inferences about the potential neural sources of these effects. A second limitation of the current work is that, by excluding a large proportion of infants’ “active” data by technical necessity, this could present a selective view of the neural dynamics underlying adult–infant engagement. Nonetheless, the current data are still valuable in providing insight into adult–infant neural coupling during social communication.
The current study demonstrates that adults and infants show significant mutual neural coupling during social interactions and that direct gaze strengthens adult–infant neural connectivity in both directions during communication. Further, live gaze appeared to stimulate infants’ own communicative efforts, which could help to reinforce dyadic synchronization. Thus, gaze and speech act as cues for interpersonal synchronization. The contingent exchange of these social signals acts to bring adults’ and infants’ brains into temporal alignment, creating a joint-networked state that is structured to optimize information transfer during communication and learning.
Methods
Participants.
Experiments 1 and 2 involved separate infant cohorts—experiment 1: 19 infants (13 male, 6 female), median age 8.2 m (SE, 0.26 m), and experiment 2: 29 infants (15 male, 14 female), median age 8.3 m (SE, 0.44 m). Infants’ mothers were native English speakers, and all infants had no neurological problems as assessed by maternal report. The same female adult experimenter participated in both experiments with all infants. The study received ethical approval from the Cambridge Psychology Research Ethics Committee. Parents provided written informed consent on behalf of their infants.
Materials.
For both experiments, seven familiar nursery rhymes were used as sung stimuli (SI Appendix, section 3). Sung nursery rhymes were used because these are integral to play and caretaking routines with infants, such as during feeding and putting to sleep (38). Infants are equally or more behaviorally responsive to sung compared with spoken language (39); thus, sung speech is likely to evoke a robust neural response from infants. In experiment 1, prerecorded video stimuli were used with mean pitch, pitch variability, duration, and loudness matched across gaze conditions (SI Appendix, Table S5). For experiment 2 (live), the experimenter was recorded during each session to ensure acoustic consistency across gaze conditions (SI Appendix, Table S6). Paired t tests indicated no significant differences between conditions for all acoustic parameters. The experimenter was instructed to maintain a neutral facial expression across all gaze conditions, varying only her gaze direction.
Protocol.
Experiment 1.
Infants sat upright in a high chair 70 cm from a display monitor (90 cm width × 60 cm height), showing a life-sized image of a female experimenter’s head against a black background. Each nursery rhyme was presented in three gaze conditions (Fig. 1): direct, indirect (head averted by 20°), and direct-oblique (head averted by 20°, but direct gaze). The direct-oblique condition was included to control for the side view of the face that was presented during indirect gaze. During stimulus recording, the experimenter gaze-fixated on a life-sized picture of an infant to standardize her visual input across conditions. Each nursery rhyme was presented six times (twice per gaze condition, order counterbalanced).
Experiment 2.
Infants sat upright in a high chair facing the female experimenter at a distance of 70 cm. Each nursery rhyme was presented in two gaze conditions. In the direct condition, the experimenter looked directly at the infant while singing; in the indirect condition, she fixated at a target 20° to the left or right side of the infant (see Fig. 1 and SI Appendix, section 4 for the experimenter’s view). Each nursery rhyme was presented four times (twice direct, twice indirect, order counterbalanced).
EEG Acquisition.
In experiment 1, EEG was recorded separately from infants (during testing) and from the female adult experimenter (during stimulus recording) from 32 electrodes according to the international 10–20 placement system. In experiment 2, EEG was recorded simultaneously from the infant and the adult experimenter from two central electrodes (C3 and C4), referenced to the vertex (Cz). Further details of EEG acquisition are given in SI Appendix, section 5.
EEG Artifact Rejection and Preprocessing.
To ensure that the analyzed EEG data reflected only attentive and movement-free neural activity, a two-stage artifact rejection procedure was applied. First, session videos were manually reviewed to select only periods when infants were still and looking directly at the experimenter. Next, manual artifact rejection was performed to further exclude segments where the EEG amplitude exceeded +100 μV. Full descriptions of the artifact rejection procedures and inclusion rates following artifact rejection are given in SI Appendix, section 6. Data were then downsampled to 200 Hz, low-pass filtered <45 Hz to suppress electrical line noise, and segmented into 1.0-s epochs for connectivity analysis.
EEG Analyses: Speech Artifacts, Power Spectrum, and GPDC Network Connectivity.
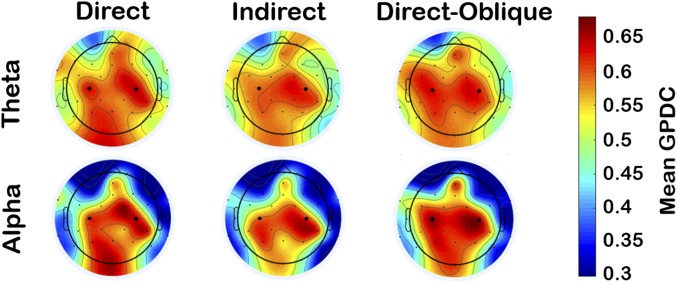
Speech production artifacts were present in the EEG signal of the adult speaker. To assess the topography and spectral profile of these artifacts, we compared the adult’s EEG during speech production relative to resting state (SI Appendix, section 7). Despite rigorous analyses, we were able to identify no evidence of EEG signal distortion by speech artifacts in the central region (e.g., C3/C4) in Theta and Alpha bands, although evidence of artifacts at other frequency bands and for more peripheral electrode positions was clearly present. Therefore, to avoid spurious results arising from speech artifacts, the connectivity analysis used only Theta and Alpha bands for C3 and C4 electrodes for both adult and infant. To confirm the representativeness of this region of analysis for the infant, we assessed infants’ whole-head (32-channel) connectivity to adults’ C3 and C4 electrodes (Fig. 5 and SI Appendix, section 12). Across gaze conditions, the strongest connectivity between infant and adult was topographically observed over infants’ central and posterior regions (including C3 and C4) for both Theta and Alpha bands. Therefore, C3 and C4 were indeed representative regions of analysis for the infant.
Fig. 5.
Experiment 1 infant scalp topography of the mean adult (C3/C4)-to-infant GPDC values for direct gaze (Left), indirect gaze (Middle), and direct-oblique gaze (Right) conditions, for Theta (Top) and Alpha (Bottom) frequency bands. Electrodes C3 and C4 are enlarged for ease of reference. For each subplot, a top–down view of the scalp is shown where left/right map congruently to left/right sides of the infant’s head, respectively.
A detailed description of EEG analysis methods is given in SI Appendix, sections 8 and 9. Briefly, first the EEG power spectra of infant and adult signals were assessed for each experimental condition to confirm that the gaze manipulation did not generate any detectable power changes that might systematically bias the connectivity analysis. Second, to assess network connectivity in each gaze condition, GPDC—a directional causal measure of direct information flow between channels in a network—was computed (27). GPDC measures the degree of influence that channel i directly has on channel j with respect to the total influence of i on all channels in the network. Here, each electrode [infant left (IL), infant right (IR), adult left (AL), adult right (AR)] was one channel (Fig. 1C).
Control Analyses.
The first control analysis established a threshold for nonspecific connectivity between brains that was unrelated to the experimental task (SI Appendix, section 10). A surrogate dataset was generated for each participant pair where the fine-grained temporal correspondence between adult and infant neural signals was disrupted by randomly pairing adult and infant epochs from different timepoints within the same experimental session (i.e., shuffling). An identical connectivity analysis was then performed on this surrogate dataset. For each participant pair, neural connection, and frequency band, a threshold value was computed by taking the average surrogate value across all gaze conditions. Paired t tests [Benjamini–Hochberg FDR-corrected at P < 0.05 (32), one-tailed] were then used to assess whether the real data significantly exceeded their respective threshold values.
The second control analysis examined basic sensory processing of the speech stimulus, which could indirectly affect adult–infant neural coupling. Entrainment (oscillatory phase-locking) between the EEG signal and the speech amplitude envelope was measured in each gaze condition. As described in SI Appendix, section 11, no significant differences in neural entrainment to the speech signal between gaze conditions were found in either experiment.
Statistical Analysis of Gaze Effects on Interpersonal GPDC Connectivity.
We hypothesized that interpersonal neural connectivity would be higher during direct (and direct-oblique) gaze than indirect gaze (i.e., direct = direct-oblique > indirect). We also wished to assess whether the adult’s influence on the infant (i.e., A → I GPDC) and the infant’s influence on the adult (i.e., I → A GPDC) would show the same pattern of gaze modulation. As previous work with infants has not found hemispheric differences for gaze effects (9), interhemispheric connectivity patterns were not explored further. Accordingly, the four interhemispheric connections (L/R → L/R) were collapsed into one average each for A → I and I → A directional influences. These two directional indices were computed for each gaze condition, for Theta and Alpha bands. For experiment 1, only A → I connections were analyzed, as all I → A connections were not significantly above threshold (this was expected, as the adult’s EEG was prerecorded).
The effects of gaze on A → I and I → A connectivity were assessed using two statistical approaches. First, to assess overall patterns and interactions, repeated-measures ANOVAs were performed, taking frequency and gaze condition as within-subjects factors. Second, to assess specific contrasts between pairs of gaze conditions at each frequency, Dunnett’s multiple range t tests (40) were conducted, which independently control for the familywise error rate. For Theta and Alpha bands, the following pairwise tests were performed for experiment 1: (i) direct > indirect, (ii) direct-oblique > indirect, and (iii) direct = direct-oblique. For experiment 2, only the direct > indirect test was performed. Dunnett’s test results are reported in the main text, and ANOVA results are provided in SI Appendix, section 13. Separate analyses were also performed to examine infants’ looking times (SI Appendix, section 14) and the effects of infant age on neural connectivity (SI Appendix, section 15). Finally, a permutation analysis was performed (SI Appendix, section 16) to assess the internal reliability of the gaze findings, both within and across experiments. All statistical tests were two-tailed unless there were a priori directional hypotheses (i.e., Dunnett’s test for direct/direct-oblique > indirect; data > surrogate threshold), for which one-tailed tests were used.
Infant Vocalizations.
Infants’ vocalizations were coded from session videos according to Oller’s (41) infraphonological acoustic classification system (SI Appendix, section 2). Each infant’s (i) number and (ii) duration of vocalizations were computed during each gaze condition. To explore the relationship between neural coupling and infants’ communicative attempts, vocalization indices were correlated with A → I and I → A GPDC values for both experiments. Of note, the connectivity analyses only included segments of EEG data when no vocalizations were occurring.
Supplementary Material
Acknowledgments
This research was funded by a UK Economic and Social Research Council (ESRC) Transforming Social Sciences Grant ES/N006461/1 (to V.L. and S.W.), a Lucy Cavendish College Junior Research Fellowship (to V.L.), Nanyang Technological University start-up Grant M4081585.SS0 (to V.L.), and a British Academy Post-Doctoral Fellowship and ESRC Future Research Leaders Fellowship ES/N017560/1 (to S.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. U.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702493114/-/DCSupplemental.
References
- 1.Rogoff B. Apprenticeship in Thinking: Cognitive Development in Social Context. Oxford Univ Press; New York: 1990. [Google Scholar]
- 2.Csibra G, Gergely G. The teleological origins of mentalistic action explanations: A developmental hypothesis. Dev Sci. 1998;1:255–259. [Google Scholar]
- 3.Brooks R, Meltzoff AN. Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: A longitudinal, growth curve modeling study. J Child Lang. 2008;35:207–220. doi: 10.1017/s030500090700829x. [DOI] [PubMed] [Google Scholar]
- 4.Csibra G, Gergely G. Natural pedagogy. Trends Cogn Sci. 2009;13:148–153. doi: 10.1016/j.tics.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Senju A, Csibra G. Gaze following in human infants depends on communicative signals. Curr Biol. 2008;18:668–671. doi: 10.1016/j.cub.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 6.Fausey CM, Jayaraman S, Smith LB. From faces to hands: Changing visual input in the first two years. Cognition. 2016;152:101–107. doi: 10.1016/j.cognition.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff P. Observations on the early development of smiling. In: Foss BM, editor. Determinants of Infant Behavior. Vol 2. Methuen; London: 1963. pp. 113–138. [Google Scholar]
- 8.Bloom K. Social elicitation of infant vocal behavior. J Exp Child Psychol. 1975;20:51–58. doi: 10.1016/0022-0965(75)90025-9. [DOI] [PubMed] [Google Scholar]
- 9.Farroni T, Csibra G, Simion F, Johnson MH. Eye contact detection in humans from birth. Proc Natl Acad Sci USA. 2002;99:9602–9605. doi: 10.1073/pnas.152159999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farroni T, Johnson MH, Csibra G. Mechanisms of eye gaze perception during infancy. J Cogn Neurosci. 2004;16:1320–1326. doi: 10.1162/0898929042304787. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 12.Marsh KL, Richardson MJ, Schmidt RC. Social connection through joint action and interpersonal coordination. Top Cogn Sci. 2009;1:320–339. doi: 10.1111/j.1756-8765.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaye K, Fogel A. The temporal structure of face-to-face communication between mothers and infants. Dev Psychol. 1980;16:454–464. [Google Scholar]
- 14.Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD. Rhythms of dialogue in infancy: Coordinated timing in development. Monogr Soc Res Child Dev. 2001;66:i–viii, 1–132. [PubMed] [Google Scholar]
- 15.Cohn JF, Tronick EZ. Mother-infant face-to-face interaction: Influence is bidirectional and unrelated to periodic cycles in either partner’s behavior. Dev Psychol. 1988;24:386–392. [Google Scholar]
- 16.Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav Dev. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Waters SF, West TV, Mendes WB. Stress contagion: Physiological covariation between mothers and infants. Psychol Sci. 2014;25:934–942. doi: 10.1177/0956797613518352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spangler G. The emergence of adrenocortical circadian function in newborns and infants and its relationship to sleep, feeding and maternal adrenocortical activity. Early Hum Dev. 1991;25:197–208. doi: 10.1016/0378-3782(91)90116-k. [DOI] [PubMed] [Google Scholar]
- 19.Brooks R, Meltzoff AN. Gaze following: A mechanism for building social connections between infants and adults. In: Mikulincer M, Shaver PR, editors. Mechanisms of Social Connection: From Brain to Group. American Psychological Association; Washington, DC: 2014. pp. 167–183. [Google Scholar]
- 20.Giraud AL, Poeppel D. Cortical oscillations and speech processing: Emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telkemeyer S, et al. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29:14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends Cogn Sci. 2012;16:114–121. doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hari R, Himberg T, Nummenmaa L, Hämäläinen M, Parkkonen L. Synchrony of brains and bodies during implicit interpersonal interaction. Trends Cogn Sci. 2013;17:105–106. doi: 10.1016/j.tics.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, et al. Neural synchronization during face-to-face communication. J Neurosci. 2012;32:16064–16069. doi: 10.1523/JNEUROSCI.2926-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, et al. Measuring speaker-listener neural coupling with functional near infrared spectroscopy. Sci Rep. 2017;7:43293. doi: 10.1038/srep43293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens GJ, Silbert LJ, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci USA. 2010;107:14425–14430. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baccalá LA, Sameshima K. Partial directed coherence: A new concept in neural structure determination. Biol Cybern. 2001;84:463–474. doi: 10.1007/PL00007990. [DOI] [PubMed] [Google Scholar]
- 28.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- 29.Atzaba-Poria N, Deater-Deckard K, Bell MA. Mother–child interaction: Links between mother and child frontal electroencephalograph asymmetry and negative behavior. Child Dev. 2017;88:544–554. doi: 10.1111/cdev.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tronick E. The Neurobehavioral and Social-Emotional Development of Infants and Children. WW Norton & Company; New York: 2007. [Google Scholar]
- 31.Goldstein MH, Schwade JA. Social feedback to infants’ babbling facilitates rapid phonological learning. Psychol Sci. 2008;19:515–523. doi: 10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 33.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong V, Goswami U. Acoustic-emergent phonology in the amplitude envelope of child-directed speech. PLoS One. 2015;10:e0144411. doi: 10.1371/journal.pone.0144411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray L, Trevarthen C. The infant’s role in mother-infant communications. J Child Lang. 1986;13:15–29. doi: 10.1017/s0305000900000271. [DOI] [PubMed] [Google Scholar]
- 36.Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry. 2007;48:329–354. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- 37.Saito DN, et al. “Stay tuned”: Inter-individual neural synchronization during mutual gaze and joint attention. Front Integr Neurosci. 2010;4:127. doi: 10.3389/fnint.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trehub SE, et al. Mothers’ and fathers’ singing to infants. Dev Psychol. 1997;33:500–507. doi: 10.1037//0012-1649.33.3.500. [DOI] [PubMed] [Google Scholar]
- 39.Corbeil M, Trehub SE, Peretz I. Speech vs. singing: Infants choose happier sounds. Front Psychol. 2013;4:372. doi: 10.3389/fpsyg.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 41.Oller DK. The Emergence of Speech Capacity. Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.