Abstract
Background
To compare the efficacy of three antiseptic solutions [0.5%, and 1.0% alcohol/chlorhexidine gluconate (CHG), and 10% aqueous povidone-iodine (PVI)] for the prevention of intravascular catheter colonization, we conducted a randomized controlled trial in patients from 16 intensive care units in Japan.
Methods
Adult patients undergoing central venous or arterial catheter insertions were randomized to have one of three antiseptic solutions applied during catheter insertion and dressing changes. The primary endpoint was the incidence of catheter colonization, and the secondary endpoint was the incidence of catheter-related bloodstream infections (CRBSI).
Results
Of 1132 catheters randomized, 796 (70%) were included in the full analysis set. Catheter-tip colonization incidence was 3.7, 3.9, and 10.5 events per 1000 catheter-days in 0.5% CHG, 1% CHG, and PVI groups, respectively (p = 0.03). Pairwise comparisons of catheter colonization between groups showed a significantly higher catheter colonization risk in the PVI group (0.5% CHG vs. PVI: hazard ratio, HR 0.33 [95% confidence interval, CI 0.12–0.95], p = 0.04; 1.0% CHG vs. PVI: HR 0.35 [95% CI 0.13–0.93], p = 0.04). Sensitivity analyses including all patients by multiple imputations showed consistent quantitative conclusions (0.5% CHG vs. PVI: HR 0.34, p = 0.03; 1.0% CHG vs. PVI: HR 0.35, p = 0.04). No significant differences were observed in the incidence of CRBSI between groups.
Conclusions
Both 0.5% and 1.0% alcohol CHG are superior to 10% aqueous PVI for the prevention of intravascular catheter colonization.
Trial registration
Japanese Primary Registries Network; No.: UMIN000008725 Registered on 1 September 2012
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1890-z) contains supplementary material, which is available to authorized users.
Keywords: Chlorhexidine, Povidone-iodine, Catheter-related infections, Anti-infective agents, Local, Anti-bacterial agents, Catheters
Background
Catheter-related bloodstream infections (CRBSI) are serious nosocomial infections associated with high mortality, prolonged intensive care unit (ICU) stay, and increased healthcare costs [1–3]. The incidence of CRBSI reportedly decreased to 0.7 cases per 1000 catheter-days [4], probably due to more widespread use of a prevention bundle, but further reduction is a meaningful and achievable goal [1].
The current Centers for Disease Control and Prevention (CDC) guidelines for preventing intravascular catheter-related infections published in 2011 [5] recommends skin preparation using >0.5% chlorhexidine solution with alcohol before central venous catheter (CVC) or arterial catheter (AC) placement and during dressing changes. Although several studies have demonstrated that chlorhexidine gluconate (CHG) solution decreases the incidence of CRBSI associated with peripheral venous catheters, CVCs, and ACs to a greater extent than 10% aqueous povidone-iodine (PVI) [6–10], few high-quality studies have compared the efficacy of 0.5% and 1% CHG in preventing catheter colonization with that of 10% PVI [11–15]. Furthermore, 2% CHG solutions are not available in several countries, including Japan. Therefore, a study to compare 0.5% and 1% CHG with 10% PVI is essential.
To identify the ideal topical antiseptic solution for clinical use, however, CRBSI itself may not be feasible as an outcome measure due to the low incidence of CRBSI. Instead, catheter colonization is a reasonable and durable surrogate endpoint [16] when performing a meaningful study with a minimum number of participants. For this reason, this study was undertaken to compare the effectiveness of three topical antiseptic solutions—0.5% CHG, 1% CHG, and 10% PVI—for the prevention of CVC and AC colonization. We hypothesize that catheter colonization incidence is lower after skin preparation using 0.5% or 1% CHG compared with the incidence after using 10% PVI.
Methods
Trial design
This multicenter, open-label, parallel randomized controlled study was conducted in 16 ICUs (1 December 2012 to 31 March 2014) including four university hospitals and 12 general hospitals in Japan. Nine intensive care units were medical ICUs only and seven were combined medical-surgical ICUs. The review boards of all participating institutions approved the study protocol, and patients/close relatives provided written informed consent. This study was registered with the Japanese Clinical Research Registration System of the University Hospital Medical Information Network (UMIN) (registration number UMIN000008725).
Patients
All consecutive CVCs and ACs placed in patients ≥18 years old after admission to the ICU for the intravenous administration of medications, hemodynamic monitoring and frequent blood sampling were eligible for inclusion. Exclusion criteria included: catheters inserted before ICU admission, catheters inserted for long-term total parenteral nutrition or chemotherapy for seven or more days, patients with a history of drug allergy to the study antiseptic solutions, patients with systemic adverse events or skin reactions at the catheter insertion site, and catheters changed using a guidewire.
Intervention/randomization
The enrolled catheters were randomly allocated to the following three groups according to the antiseptic solution used for initial and subsequent cutaneous antisepsis: 0.5% alcohol/CHG solution with 79% ethanol (Maskin Wethanol Solution 0.5% w/v; Maruishi Pharmaceutical Company, Osaka, Japan), 1.0% alcohol/CHG solution with 79% ethanol (Hexizac AL Solution 1%; Yoshida Pharmaceutical Company, Tokyo, Japan), and 10% aqueous PVI solution (10% PVI; Isodine Solution 10%; Meiji Seika Pharma Co., Tokyo, Japan). Until the randomized catheter was removed, a subsequently placed catheter in the same patient was not included the study. The same antiseptic solution was used during dressing changes and before catheter removal. Randomization was stratified by the hospital and catheter type (CVCs or ACs) using the sealed opaque envelope method with computer-generated randomized blocks of nine which was generated at the central institution and not revealed to the participating patients/physicians/nurses. Although patients/physicians/nurses were not blinded to the antiseptic solution group, the microbiologists performing the catheter-tip cultures and data analyst were blinded to this information.
Clinical assessment/procedure and bacteriological methods
The type of catheters and insertion sites in this study were selected at the discretion of individual physicians. At the time of conducting the study, silver antiseptic or antimicrobial-impregnated catheters were unavailable in Japan.
Before catheter insertion, the site was prepared using the allocated antiseptic solution for 30 s and allowed to dry according to standardized protocols (i.e., CHG 30s; PVI 2 min). Physicians used maximal barrier precautions (i.e., sterile gloves, gowns, masks, and large drapes covering the chest), as per CDC guidelines [5]. After catheter insertion, sterile polyurethane or gauze dressings (if an exudate was observed) were placed at the insertion site. The dressings were changed every 7 days for polyurethane dressings, every 72 h for gauze dressings, or sooner if soiled or wet. No antiseptic-containing dressings were used. Physicians/nurses inspected the insertion site at ≤24-h intervals for any evidence of infection (e.g., erythema, pain, swelling, or purulent discharge).
Catheters were removed if their use was not required or if a CRBSI was suspected [e.g., fever (temperature of ≥38.5 °C); leukocytosis (leukocyte count ≥14,000 cells/mm3) without any apparent cause; and/or pus, extensive erythema, or tenderness at the insertion site]. Before catheter removal, the same antiseptic solution was used at the site in an identical manner.
After catheter removal, 5 cm and 1–2 cm of the distal CVC and AC tips, respectively, were immediately sent for semi-quantitative culture analysis. The catheter tip is rolled 5–7 times across a 5% sheep blood agar plate and then aerobically cultured at 35 °C–37 °C for 24 h to 7 days [17]. All isolated bacteria were identified using standard methods. When the Maki method was unavailable, the sonication technique was used. Antimicrobial susceptibility testing was performed according to the recommendations of the Clinical and Laboratory Standards Institute.
The procedures including skin site preparation and barrier precautions during catheter insertion and removal, site preparation and dressing method during the dressing change, and the catheter culture method were standardized and explained to physicians in all ICUs before the study began. Video lectures of the standardized procedures were also distributed to the study ICUs. Definitions of study parameters (e.g., acute kidney injury and diabetes) were standardized in all ICUs before the study began.
Outcomes
The primary outcome of this study is catheter colonization incidence per 1000 catheter-days at the time of catheter removal. Secondary outcomes are CRBSI incidence, ICU length of stay, hospital mortality, and antiseptic solution-related adverse events.
Definition of catheter colonization and catheter-related bloodstream infection
Catheter colonization was defined as ≥15 colony-forming units (CFUs) in a semi-quantitative catheter tip culture using the roll-plate technique (i.e., by rolling the catheter segment across 5% sheep blood agar plate) after 24 h [17]. In the sonication technique, the definition of catheter colonization was at the discretion of the microbiological laboratory at each study institution. In the absence of colonization at 24 h, the culture was allowed to grow for up to 7 days. CRBSI was defined according to the CDC [18] and IDSA [19] guidelines, and the definition included three major criteria (Additional file 1: Table S1).
Statistical analysis
In a previous study, the incidence of catheter colonization with 10% PVI and 0.5% CHG was 24.8% and 15.1%, respectively, whereas another study reported an incidence of 10.2% with 1.0% CHG [12, 13]. Based on these findings, we hypothesized that the incidence of catheter colonization will be 40% lower with CHG (0.5% or 1.0%) than with 10% PVI. We assumed that the incidence of catheter colonization in the PVI group would be 25%. The sample-size estimation showed that 245 catheters are required in each of the three arms of this study to ensure 80% power and a two-sided α error of 5%.
The data is presented as the mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables and percentages for categorical variables. One-way analysis of variance (ANOVA) and the Kruskal–Wallis test or χ2 test and Fisher’s exact test, as appropriate, were used for comparison. The incidence of catheter colonization and CRBSI were compared among the three groups using the log-rank test. Pairwise comparisons of the antiseptic solutions were conducted using a marginal Cox proportional hazards model with a closed testing procedure, and hazard ratios (HR) and 95% confidence intervals (CIs) were calculated. In the closed testing procedure, 0.5% CHG vs. 10% PVI was tested only if 1.0% CHG vs. 10% PVI was significant and 0.5% CHG vs. 1.0% CHG was tested if both were significant. For repeated catheter insertions in a single patient, the correlation between patient factors and catheter factors should be considered. Therefore, we applied a marginal Cox regression hazard model to compare the efficacy of the antiseptic solutions despite low proportion of repeated catheter insertions (13.4%) and a minimal intra-class correlation (r = 0.02) as shown in a previous study [5]. Subgroup analyses by catheter type (CVCs or ACs) and catheter duration (≥72 h or <72 h) were planned before commencement of the study and written in the protocol.
The primary population analyzed in this study is the full analysis set (FAS), while patients without catheter colonization data were excluded from the intention-to-treat (ITT) population (Fig. 1). The groups were compared for differences in catheter site and patient characteristics missing primary outcomes, to evaluate the degree of randomization. As a sensitivity analysis to evaluate the impact of the exclusion of patients with missing data in the primary FAS analysis, multiple imputation (MI) was performed for the imputed missing data [20]. Multiple imputation (MI) is a principled method if the mechanism of missing data is missing at random. The MI by chained equations, applicable for imputations of continuous and categorical variables with a non-monotone missing pattern, was used as an imputation algorithm. The number of imputations in MI was 100, and the number of burn-ins between each imputation was 200. The incomplete response variables were catheter colonization (binary) and the duration of catheterization (continuous; log transformed). The observed covariates in the imputation were the antiseptic solution (categorical) and type of catheter (binary). In MI by chained equations, each incomplete variable was sequentially imputed, given all other variables. Analysis was performed using PROC MI and PROC MIANALYZE in SAS (SAS Inc, Cary, NC, USA). Planned interim analysis was conducted to recalculate the sample size after registration of 50 catheters in each group. The significance level for all tests was two-sided at 5%. All statistical analyses were performed using SAS, version 9.4 (SAS Inc, Cary, NC, USA).
Fig. 1.
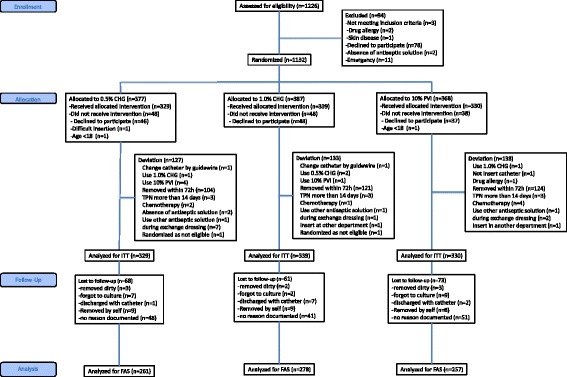
Flow chart showing randomization of skin preparation for catheter insertion. CHG chlorhexidine gluconate, FAS full analysis set, ITT Intention to treat, PVI povidone-iodine
Results
A total of 1226 catheter placements were reviewed for eligibility, and the remaining 1132 catheters, after obtaining informed consent, were randomized to one of three solutions. Several catheters were excluded because of withdrawal of informed consent, leaving 998 (88%) catheters eligible for analysis. However, substantial numbers of catheters were not cultured for various reasons (Fig. 1). Therefore, the FAS analysis is limited to 796 (70%) catheters.
There are no significant differences in the baseline characteristics among the groups (Table 1). The characteristics of the 202 patients excluded because of the lack of catheter colonization data are similar among the three groups and between the FAS population and patients excluded from the FAS (Fig. 1, Additional file 1: Table S2 and S3). No significant differences in the insertion sites selected were observed (data not shown). The characteristics of the catheters are similar among the groups (Table 2).
Table 1.
Characteristics of study participants
| Characteristic | All N = 796 |
0.5% CHG N = 261 |
1.0% CHG N = 278 |
10% PVI N = 257 |
|---|---|---|---|---|
| Age, mean (SD), years | 66.2 (16.3) | 65.7 (16.3) | 65.9 (16.3) | 67.1 (16.4) |
| Gender, male (n, %) | 504 (63.3%) | 162 (62.1%) | 187 (67.3%) | 155 (60.3%) |
| APACHE 2, mean (SD) | 21.0 (8.5) | 21.0 (8.6) | 21.5 (8.6) | 20.5 (8.4) |
| SAPS 2, mean (SD) | 49.4 (18.6) | 48.5 (19.2) | 50.9 (18.2) | 48.8 (18.4) |
| SOFA, mean (SD) | 7.2 (4.0) | 7.1 (3.9) | 7.5 (4.3) | 7.0 (3.9) |
| Comorbidities (n, %) | ||||
| Immunodeficiency | 39 (4.9%) | 10 (3.8%) | 17 (6.1%) | 12 (4.7%) |
| Steroid administration | 90 (11.3%) | 32 (12.3%) | 35 (12.6%) | 23 (9.0%) |
| Trauma | 64 (8.0%) | 27 (10.3%) | 16 (5.8%) | 21 (8.2%) |
| Cancer | 84 (10.6%) | 29 (11.1%) | 31 (11.2%) | 24 (9.3%) |
| Diabetes | 153 (19.2%) | 57 (21.8%) | 51 (18.4%) | 45 (17.5%) |
| HIV | 1 (0.1%) | 0 (0%) | 1 (0.4%) | 0 (0%) |
| Cirrhosis | 40 (5.0%) | 13 (5.0%) | 14 (5.0%) | 13 (5.1%) |
| Acute kidney injury | 245 (30.8%) | 85 (32.6%) | 81 (29.1%) | 79 (30.7%) |
| Chronic kidney disease | 137 (17.2%) | 51 (19.5%) | 47 (16.9%) | 39 (15.2%) |
| Admission category (n, %) | ||||
| Medical | 645 (81.0%) | 203 (77.8%) | 231 (83.1%) | 211 (82.1%) |
| Scheduled surgery | 23 (2.9%) | 9 (3.5%) | 5 (1.8%) | 9 (3.5%) |
| Emergency surgery | 128 (16.1%) | 49 (18.8%) | 42 (15.1%) | 37 (14.4%) |
| Infections before catheter insertion (n, %) | 448 (56.3%) | 150 (57.5%) | 162 (58.3%) | 136 (52.9%) |
| Origin of infection (n, %) | ||||
| Central nervous system | 4 (0.5%) | 1 (0.4%) | 2 (0.7%) | 1 (0.4%) |
| Pulmonary | 195 (24.5%) | 69 (26.5%) | 63 (22.7%) | 63 (24.5%) |
| Cardiovascular | 5 (0.6%) | 2 (0.8%) | 2 (0.7%) | 1 (0.4%) |
| Abdominal | 92 (11.6%) | 23 (8.9%) | 44 (15.8%) | 25 (9.7%) |
| Urinary tract | 44 (5.5%) | 19 (7.3%) | 10 (3.6%) | 15 (5.8%) |
| Skin | 14 (1.8%) | 7 (2.7%) | 3 (1.1%) | 4 (1.6%) |
| Catheter | 8 (1.0%) | 2 (0.8%) | 5 (1.8%) | 1 (0.4%) |
| Others | 82 (10.3%) | 25 (9.6%) | 31 (11.2%) | 26 (10.1%) |
| None | 351 (44.2%) | 112 (43.1%) | 118 (42.5%) | 121 (47.1%) |
| Antibiotics before catheter insertion (n, %) | 445 (55.9%) | 153 (58.6%) | 155 (55.8%) | 137 (53.3%) |
CHG chlorhexidine gluconate, PVI povidone-iodine, SD standard deviation, APACHE acute physiology and chronic health evaluation, SAPS simplified acute physiology score, SOFA sequential organ failure assessment, HIV human immunodeficiency virus
Table 2.
Characteristics of the catheters
| Characteristic | All N = 796 |
0.5% CHG N = 261 |
1.0% CHG N = 278 |
10% PVI N = 257 |
P value |
|---|---|---|---|---|---|
| Type of catheter (n, %) | 0.69 | ||||
| Central venous catheter | 285 (35.8%) | 93 (35.6%) | 95 (34.2%) | 97 (37.7%) | |
| Arterial catheter | 511 (64.2%) | 168 (64.4%) | 183 (65.8%) | 160 (62.3%) | |
| Insertion site (n, %) | 0.10 | ||||
| Central venous catheter | |||||
| Internal jugular vein | 265 (93.0%) | 86 (92.5%) | 93 (97.9%) | 86 (88.7%) | |
| Subclavian vein | 11 (3.9%) | 5 (5.4%) | 1 (1.1%) | 5 (5.2%) | |
| Femoral vein | 9 (3.2%) | 2 (2.2%) | 1 (1.1%) | 6 (6.2%) | |
| Arterial catheter | 0.20 | ||||
| Radial artery | 481 (96.0%) | 160 (97.0%) | 173 (96.1%) | 148 (94.9%) | |
| Femoral artery | 15 (3.0%) | 5 (3.0%) | 6 (3.3%) | 4 (2.6%) | |
| Dorsalis artery | 5 (1.0%) | 0 | 1 (0.6%) | 4 (2.6%) | |
| Duration of catheterization, median (IQR), days | 3.8 (2.0–6.7) | 3.8 (2.1–6.7) | 4.0 (2.0–7.0) | 3.7 (2.0–6.0) | 0.43 |
| Methods for catheter culture (n, %) | 0.49 | ||||
| Maki methods | 663 (83.3%) | 223 (85.4%) | 227 (81.7%) | 213 (82.9%) | |
| Sonication methods | 133 (16.7%) | 38 (14.6%) | 51 (18.4%) | 44 (17.1%) | |
| Dressing (n, %) | 0.41 | ||||
| Film | 668 (83.9%) | 225 (86.2%) | 228 (82.0%) | 215 (83.7%) | |
| Gauze | 128 (16.1%) | 36 (13.8%) | 50 (18.0%) | 42 (16.3%) | |
CHG chlorhexidine gluconate, PVI povidone-iodine, IQR interquartile range
The incidence of catheter-tip colonization (per 1000 catheter-days) is 3.7, 3.9, and 10.5 events in the 0.5% CHG, 1% CHG and 10% PVI groups, respectively (Table 3 and Fig. 2 (Kaplan–Meier curve) (p = 0.03)). Pairwise comparison of catheter colonization between groups shows that the risk is significantly higher in the 10% PVI group (0.5% CHG vs. 10% PVI: HR 0.33, 95% CI 0.12–0.95, p = 0.04; 1.0% CHG vs. 10% PVI: HR0.35, 95% CI 0.13–0.93, p = 0.04). However, there are no differences in colonization risk between the 0.5% and 1.0% CHG groups (HR 0.95, 95% CI 0.29–3.13, p = 0.94). Of all analyzed catheters, 134 (16.8%) were further randomized to the three solutions for second or later insertions, although the initially placed catheters were already included in the analysis. A sensitivity analysis for initially inserted catheters, excluding these 134 catheters, showed no differences in patient characteristics between the three groups, and the same conclusion was obtained, showing superiority of 0.5%/1.0% CHG over 10% PVI, as confirmed in the FAS analysis (Additional file 1: Table S4).
Table 3.
Catheter outcomes: colonization and CRBSI
| (a) No. of catheters and incidence per 1000 catheter-days | |||||
| All N = 796 |
0.5% CHG N = 261 |
1.0% CHG N = 278 |
10% PVI N = 257 |
P value | |
| Colonization | |||||
| Number of catheters (%) | 24 (3.0%) | 5 (1.9%) | 6 (2.2%) | 13 (5.1%) | 0.07 |
| Incidence per 1000 catheter-days, n (95% CI) | 5.8 (3.5–8.2) | 3.7 (0.5–6.9) | 3.9 (0.8–7.0) | 10.5 (4.8–16.3) | 0.03 |
| CRBSI | |||||
| Number of catheters (%) | 13 (1.6%) | 4 (1.5%) | 3 (1.1%) | 6 (2.3%) | 0.51 |
| Incidence, per 1000 catheter-days, n (95% CI) | 3.2 (1.4–4.9) | 3.0 (0.1–5.8) | 2.0 (0–4.2) | 4.9 (1.0–8.8) | 0.41 |
| (b) Hazard ratio for each combination of antiseptic solution | |||||
| Colonization | CRBSI | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| 0.5% CHG vs. 10% PVI | 0.33 (0.12–0.95) | 0.04 | 0.63 (0.18–2.22) | 0.47 | |
| 1.0% CHG vs. 10% PVI | 0.35 (0.13–0.93) | 0.04 | 0.41 (0.10–1.63) | 0.20 | |
| 0.5% CHG vs. 1.0% CHG | 0.95 (0.29–3.13) | 0.94 | 1.54 (0.35–6.90) | 0.57 | |
CHG chlorhexidine gluconate, PVI povidone-iodine, HR hazard ratio, CI confidence interval, CRBSI catheter-related bloodstream infection
Fig. 2.
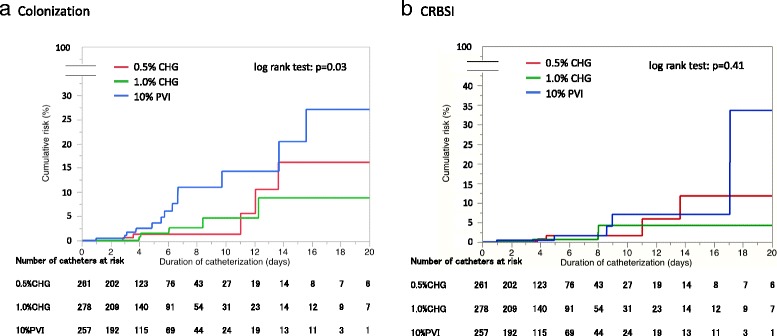
Cumulative catheter colonization and catheter-related bloodstream infection risk (Kaplan–Meier curves). a catheter colonization, b catheter-related bloodstream infection. CHG chlorhexidine gluconate, PVI povidone-iodine
In another sensitivity analysis conducted with MIs for evaluation of the impact of missing data, the marginal Cox proportional hazards model yielded results similar to those from the FAS analysis (Additional file 1: Table S5).
Subgroup analyses by catheter type (CVCs or ACs) and duration of catheter placement (≥72 h or <72 h) show that catheter colonization incidence does not significantly differ among the groups, regardless of catheter type. However, catheter duration ≥72 h poses a greater colonization risk in the 10% PVI group than that in the 0.5% CHG group (Additional file 1: Table S6 and Additional files 2, 3, 4, 5, 6 and 7: Figure S1A-F).
Since the rates of catheter insertion in the femoral vein differed among the three antiseptic solutions used, an additional analysis was conducted excluding catheters placed in the femoral vein. The results were similar to those of the primary outcome in this study, which showed that the rate of catheter colonization increased in the 10% PVI group compared to the 0.5% and 1% CHG groups (0.5% CHG vs. 10% PVI: HR 0.35, 95% CI 0.11–0.95, p = 0.04; 1.0% CHG vs. 10% PVI: HR0.37, 95% CI 0.13–0.97, p = 0.04) (Additional file 1: Table S7).
There are no significant differences in the probability of developing CRBSI among the groups (3.0 vs. 2.0 vs. 4.9 per 1000 catheter-days in 0.5% CHG, 1.0% CHG, and 10% PVI, p = 0.41) (Table 3). Figure 2 shows the related Kaplan–Meier plots. Pairwise between-group comparisons of CRBSI showed no significant differences, but the risk exhibits a decreasing trend in the 0.5% and 1.0% CHG groups (0.5% CHG vs. 10% PVI: HR 0.63, 95% CI 0.18–2.22, p = 0.47; 1.0% CHG vs. 10% PVI: HR 0.41, 95% CI 0.10–1.63, p = 0.20). Similar results were obtained in other subgroup analyses (Additional file 1: Table S6). A summary of patient characteristics and outcomes in patients with/without colonization or CRBSI and antibiotics before catheter insertion are shown in Additional file 1: Table S8 and S9.
Microorganisms isolated from each colonized catheter and blood in patients diagnosed with CRBSI are shown in Additional file 1: Table S10 and S11. There are no significant differences in microorganism type that caused catheter colonization and CRBSI between the groups. Systemic and local unknown serious adverse events were not observed in any of the three groups (Additional file 1: Table S12).
Discussion
The results of this open-label, randomized controlled study show that alcohol/0.5% CHG and 1.0% CHG were superior to aqueous 10% PVI for the prevention of CVC and AC colonization, but the incidence of CRBSI is similar among the groups. The use of 10% PVI with catheters in situ for ≥72 h increases the risk of catheter colonization, whereas there was no difference in catheter colonization rates among the three solutions tested for catheters in place <72 h.
The superiority of alcohol/1% CHG over 10% aqueous PVI or alcohol/0.5% CHG for the prevention of CRBSI has not been fully examined [11–14, 21, 22]. Also, no comparative studies have examined the effect of differences in CHG concentration. Two studies [13, 14] showed that alcohol/1.0% CHG is superior to 10% aqueous PVI, but the studies had significant methodological limitations. One [13] study was prepared as an abstract for a meeting in 2001 with no subsequent publication as a full paper. The other [14] study included patients with hematological disorders alone. The only study comparing CHG at different concentrations was conducted by Valles et al [12], reporting that both alcohol with 0.5% and aqueous 2% CHG were associated with a significantly lower incidence of catheter colonization than 10% PVI. A meta-analysis published in 2016 [23] evaluated the effects of skin antisepsis as part of CVC care for reducing CRBSIs, catheter colonization, and patient mortality and morbidity. The results of subgroup comparisons based on the solution used, concluded that chlorhexidine solution may reduce rates of CRBSI and catheter colonization compared with povidone iodine. However, this study included several types of CHG solutions, including various concentrations (0.25–2.0%) of chlorhexidine and diluents (alcohol or aqueous). To the best of our knowledge, this is the first randomized control trial to demonstrate the efficacy of alcohol/1.0% CHG and 0.5% CHG over 10% aqueous PVI in preventing catheter colonization. The results also suggest non-inferiority of 0.5% CHG to 1.0% CHG.
The results of the present study have several important implications for clinical practice. This result may be beneficial in several countries, including Japan, where 2% CHG is not approved for cutaneous application for the prevention of CRBSI. In addition, it is beneficial for patients in countries that currently use 2% CHG because the risk of adverse events (e.g., anaphylaxis) may increase with CHG concentration [24].
This study has several limitations. First, the primary outcome evaluated is catheter colonization, which is only a surrogate outcome for CRBSI. The incidence of CRBSI has decreased with time [4]. Based on this, the sample size required to demonstrate differences in CRBSI incidence is enormous, making it difficult to conduct the study in a manner that will permit verification of the effectiveness of various antiseptic solutions. According to a sample size calculation based on the incidence of CRBSI in the current study, 8460 catheters would be required (80% power and a two-sided α error of 5%). However, it has been reported that there is a strong correlation between CRBSI and catheter colonization (r = 0.8) [16]. In fact, many previous studies have not defined CRBSI as the primary outcome [11, 12, 21]. To our knowledge, the only study designating CRBSI as a primary outcome is the CLEAN study [25] published in 2016, in which a total of 5159 catheters were included. Taking feasibility into consideration, we used catheter colonization incidence as the primary outcome.
Second, this study was unable to completely fulfill the ITT analysis because some of the catheter colonization data was missing after randomization due to self-removal or contamination of the catheters (Fig. 1). This may have led to confounding by unknown factors and need of the competing risk analysis. However, efforts were made to minimize this by confirming that the demographics of the excluded patients did not differ between the three groups and between the FAS and the excluded patients (Additional file 1: Table S2 and S3). We also conducted between-group comparison, with multiple imputation of missing data to perform the ITT analysis. The results using a marginal Cox proportional hazards model were similar to those obtained using FAS analysis. In the current study, the multiple imputation analysis is considered equivalent to the competing risk analysis to avoid overestimation or underestimation of the true effects. All these analyses support the robustness of our results.
Third, patient and physician blinding was not possible because the antiseptic solutions have different colors. However, a detailed study protocol, including handling of antiseptic solutions, skin preparation, and catheter insertion technique was pre-defined, and distributed to each institution. The data manager at the central hospital site was in regular communication with each hospital investigator to ensure compliance with the study protocol. In addition, the microbiologists involved in the study were blinded to the solution used. Therefore, we believe that the lack of blinding did not significantly affect the study results.
Fourth, in this study, randomization was performed at the catheter level and not at the patient level, and this may have influenced the analysis of repeated measurements. Since 16.8% of the catheters in this study represent repeat insertions in several patients, the primary outcome may have been affected because of intra-class correlations. However, taking a previous large randomized controlled study [25] (in which repeated measurements were conducted and the intra-class correlation was 0.02) into consideration, we believe that the repeated measurements in this study had little impact on the results because we were analyzing using a marginal Cox regression model. We conducted a sensitivity analysis including only the first catheter inserted in each patient, and no differences in patient characteristics were observed among the three groups. The results are similar to that those obtained using the FAS analysis.
Finally, the effectiveness of alcohol/CHG and PVI-alcohol were not compared because PVI-alcohol has not been approved for use in skin disinfection of the catheter insertion site in Japan. It has previously been reported that alcohol/2% CHG provides greater protection against short-term catheter-related infections than PVI-alcohol [25]. Further studies are necessary to verify differences in the efficacy of alcohol/0.5% or 1.0% CHG and 5% PVI-alcohol for the prevention of CRBSI.
Conclusions
The results of this trial demonstrate that both alcohol/0.5% and 1.0% CHG are superior to 10% aqueous PVI for the prevention of catheter colonization. Hospitals where higher chlorhexidine concentrations are not permitted can use alcohol/0.5% CHG as an alternative to alcohol/1% CHG for the prevention of CRBSI. However, the optimal chlorhexidine concentration for the prevention of CRBSI has not yet been elucidated.
Additional files
Definition of catheter-related bloodstream infection. Table S2. Summary of patient characteristics and outcomes in patients excluded from the FAS in the three study groups. Table S3. Comparison of patient characteristics of the FAS population and patients excluded from the FAS. Table S4. Catheter outcomes for the first catheter inserted in each patient (repeat insertions in 16.8% of patients). Table S5. Hazard ratio for each combination of antiseptic solution after multiple imputations. Table S6. Subgroup analysis for colonization and catheter-related blood stream infections. Table S7. Catheter outcomes for central venous catheters excluding those inserted in the femoral vein (sensitivity analysis). Table S8. Summary of patient characteristics and outcomes in patients with/without colonization and antibiotics before catheter insertion. Table S9. Summary of patient characteristics and outcomes in patients with/without catheter-related blood stream infection and antibiotics before catheter insertion. Table S10. Microorganisms isolated from catheter-tips in the three study groups. Table S11. Microorganisms isolated in catheter-related bloodstream infections in the three study groups. Table S12. Adverse events. Table S13. Patient outcomes. Table S14. Proportion of missing data for each variable in the three study groups. Table S15. The specific names of all ethical bodies that approved this study in various participating sites. (DOCX 77 kb)
(A) Colonization of central venous catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 108 kb)
(B) Colonization of arterial catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 102 kb)
(C) Catheter-related bloodstream infection of central venous catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 116 kb)
(D) Catheter-related bloodstream infection of arterial catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine (JPG 108 kb)
(E) Catheter colonization after 72 h. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 107 kb)
(F) Catheter-related bloodstream infections after 72 h. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 115 kb)
Acknowledgements
We particularly thank the core members of JSEPTIC-CTG: Dr. Shigeki Fujitani, Dr. Tetsuhiro Takei, Dr. Shigehiko Uchino, and Dr. Yoshiro Hayashi, for suggesting the study concept and design.
Funding
This study was supported by Maruishi Pharmaceutical and Yoshida Pharmaceutical Company.
The sponsor and funder had no input in the development of the research or the manuscript, including the data collection, the analysis, the interpretation of data and the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AC
Arterial catheter
- ANOVA
Analysis of variance
- CDC
Centers for Disease Control and Prevention
- CFU
Colony-forming unit
- CHG
Chlorhexidine gluconate
- CI
Confidence interval
- CRBSI
Catheter-related bloodstream infection
- CVC
Central venous catheter
- FAS
Full analysis set
- HR
Hazard ratio
- ICU
Intensive care unit
- IQR
Interquartile range
- ITT
Intention-to-treat
- MI
Multiple imputation
- PVI
Povidone-iodine
- SD
Standard deviation
- UMIN
University Hospital Medical Information Network
Authors’ contributions
HY is the guarantor of the content of the manuscript, including the data and analysis. He had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially and adverse effects. MS contributed substantially to the study concept and design, data analysis and interpretation, and critical revision of the manuscript for important intellectual content. TA contributed to the accuracy of the data analysis. NS and AK contributed to the study concept and design, the acquisition and the interpretation of data, and drafting of the manuscript. TK, JH, SM, SK, HY, KA, RS, KI, EN, NS, SN, KO, RF and YG contributed to the study design, the acquisition and the interpretation of data, and drafting of the manuscript. TT contributed to the study design and drafting of the manuscript. AKL contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The review boards of all participating institutions approved the study protocol, and patients/close relatives provided written informed consent. The specific names of all ethical bodies that approved this study in various participating sites involved in Additional file 1: Table S15.
Consent for publication
Not applicable
Competing interests
HY received lecture fees from Maruishi Pharmaceutical Company. MS received lecture fees from Maruishi Pharmaceutical Company. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1890-z) contains supplementary material, which is available to authorized users.
Contributor Information
Hideto Yasuda, Email: yasudahideto@me.com.
Masamitsu Sanui, Email: msanui@mac.com.
Takayuki Abe, Email: abe.t@keio.jp.
Nobuaki Shime, Email: shime@koto.kpu-m.ac.jp.
Tetsuya Komuro, Email: tk.fake1019@gmail.com.
Junji Hatakeyama, Email: s-hatakeyama.icu@yokohama.jrc.or.jp.
Shohei Matsukubo, Email: s.makkubo@gmail.com.
Shinji Kawano, Email: kwnsnj@jikei.ac.jp.
Hiroshi Yamamoto, Email: hiroyamaoosaka@gmail.com.
Kohkichi Andoh, Email: ko.andoh@gmail.com.
Ryutaro Seo, Email: ryutaro_seo@hotmail.com.
Kyo Inoue, Email: warabimochi22@yahoo.co.jp.
Eiichiro Noda, Email: eichannoda@mac.com.
Nobuyuki Saito, Email: nobu99@nms.ac.jp.
Satoshi Nogami, Email: noga.satoshi@gmail.com.
Kentaro Okamoto, Email: beetle5522@yahoo.co.jp.
Ryota Fuke, Email: drmagicianearl@yahoo.co.jp.
Yasuhiro Gushima, Email: yasuhiro-gushima@saiseikaikumamoto.jp.
Atsuko Kobayashi, Email: oba_93happy@hotmail.com.
Toru Takebayashi, Email: ttakebayashi@a3.keio.jp.
Alan Kawarai Lefor, Email: alefor@jichi.ac.jp.
References
- 1.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 2.Heiselman D. Nosocomial bloodstream infections in the critically ill. JAMA. 1994;272:1819–20. doi: 10.1001/jama.1994.03520230029022. [DOI] [PubMed] [Google Scholar]
- 3.Trick WE, Vernon MO, Welbel SF, Wisniewski MF, Jernigan JA, Weinstein RA. Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25:266–8. doi: 10.1086/502390. [DOI] [PubMed] [Google Scholar]
- 4.JANIS surveillance data of the incidence of CRBSI from January 2015 to June 2015. JANIS website. http://www.nih-janis.jp/report/open_report/2015/2/3/ICU_Open_Report_201501.pdf. Accessed 20 Nov 2016
- 5.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder AM, Chandar J, Billings A, Diaz R, Francoeur D, Abitbol C, Zilleruelo G. Chlorhexidine-based antiseptic solutions effectively reduce catheter-related bacteremia. Pediatr Nephrol. 2009;24:1741–7. doi: 10.1007/s00467-009-1154-5. [DOI] [PubMed] [Google Scholar]
- 7.Small H, Adams D, Casey AL, Crosby CT, Lambert PA, Elliott T. Efficacy of adding 2% (w/v) chlorhexidine gluconate to 70% (v/v) isopropyl alcohol for skin disinfection prior to peripheral venous cannulation. Infect Control Hosp Epidemiol. 2008;29:963–5. doi: 10.1086/590664. [DOI] [PubMed] [Google Scholar]
- 8.Balamongkhon B, Thamlikitkul V. Implementation of chlorhexidine gluconate for central venous catheter site care at Siriraj Hospital, Bangkok, Thailand. Am J Infect Control. 2007;35:585–8. doi: 10.1016/j.ajic.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Adams D, Quayum M, Worthington T, Lambert P, Elliott T. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect. 2005;61:287–90. doi: 10.1016/j.jhin.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127:257–66. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Humar A, Ostromecki A, Direnfeld J, et al. Prospective randomized trial of 10% povidone-iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clin Infect Dis. 2000;31:1001–7. doi: 10.1086/318145. [DOI] [PubMed] [Google Scholar]
- 12.Valles J, Fernandez I, Alcaraz D, et al. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infect Control Hosp Epidemiol. 2008;29:847–53. doi: 10.1086/590259. [DOI] [PubMed] [Google Scholar]
- 13.Maki DG KV, Narans LL et al. A randomized trial of a novel 1% chlorhexidine-75% alcohol tincture vs 10% povidone-iodine for cutaneous disinfection with vascular catheters. 11th Annual Society for Healthcare Epidemiology of America Meeting 2001:Abstract 142,2001.
- 14.Yamamoto N, Kimura H, Misao H, et al. Efficacy of 1.0% chlorhexidine-gluconate ethanol compared with 10% povidone-iodine for long-term central venous catheter care in hematology departments: a prospective study. Am J Infect Control. 2014;42:574–6. doi: 10.1016/j.ajic.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Garland JS, Buck RK, Maloney P, et al. Comparison of 10% povidone-iodine and 0.5% chlorhexidine gluconate for the prevention of peripheral intravenous catheter colonization in neonates: a prospective trial. Pediatr Infect Dis J. 1995;14:510–6. doi: 10.1097/00006454-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Rijnders BJ, Van Wijngaerden E, Peetermans WE. Catheter-tip colonization as a surrogate end point in clinical studies on catheter-related bloodstream infection: how strong is the evidence? Clin Infect Dis. 2002;35:1053–8. doi: 10.1086/342905. [DOI] [PubMed] [Google Scholar]
- 17.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296:1305–9. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley Online Library; 1987. [Google Scholar]
- 21.Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338:339–43. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 22.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792–801. doi: 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lai NM, Lai NA, O’Riordan E, Chaiyakunapruk N, Taylor JE, Tan K. Skin antisepsis for reducing central venous catheter-related infections. Cochrane Database Syst Rev. 2016;7:CD010140. doi: 10.1002/14651858.CD010140.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osakabe A, Okubo T. Survey of the Japanese literature on anaphylactic shock caused by chlorhexidine gluconate. Japanese J Infec Prev Control. 2015;30:127–34. doi: 10.4058/jsei.30.127. [DOI] [Google Scholar]
- 25.Mimoz O, Lucet JC, Kerforne T, et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386:2069–77. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of catheter-related bloodstream infection. Table S2. Summary of patient characteristics and outcomes in patients excluded from the FAS in the three study groups. Table S3. Comparison of patient characteristics of the FAS population and patients excluded from the FAS. Table S4. Catheter outcomes for the first catheter inserted in each patient (repeat insertions in 16.8% of patients). Table S5. Hazard ratio for each combination of antiseptic solution after multiple imputations. Table S6. Subgroup analysis for colonization and catheter-related blood stream infections. Table S7. Catheter outcomes for central venous catheters excluding those inserted in the femoral vein (sensitivity analysis). Table S8. Summary of patient characteristics and outcomes in patients with/without colonization and antibiotics before catheter insertion. Table S9. Summary of patient characteristics and outcomes in patients with/without catheter-related blood stream infection and antibiotics before catheter insertion. Table S10. Microorganisms isolated from catheter-tips in the three study groups. Table S11. Microorganisms isolated in catheter-related bloodstream infections in the three study groups. Table S12. Adverse events. Table S13. Patient outcomes. Table S14. Proportion of missing data for each variable in the three study groups. Table S15. The specific names of all ethical bodies that approved this study in various participating sites. (DOCX 77 kb)
(A) Colonization of central venous catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 108 kb)
(B) Colonization of arterial catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 102 kb)
(C) Catheter-related bloodstream infection of central venous catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 116 kb)
(D) Catheter-related bloodstream infection of arterial catheters. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine (JPG 108 kb)
(E) Catheter colonization after 72 h. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 107 kb)
(F) Catheter-related bloodstream infections after 72 h. Cumulative catheter colonization and catheter-related bloodstream infection risk in each subgroup (Kaplan–Meier curves). CHG chlorhexidine gluconate, PVI povidone-iodine. (JPG 115 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


