Abstract
Anomalous brain structure and function are implicated in children with attention-deficit/hyperactivity disorder (ADHD). Most neuroimaging research, however, has examined school-aged children, despite the typical onset of symptoms in early childhood. This study compared the volumes of subcortical structures (caudate nucleus, putamen, globus pallidus, and thalamus) among preschoolers with ADHD and typically developing (TD) children. High resolution T1-weighted 3D MPRAGE images covering the whole brain were acquired on a 3T scanner and subcortical volumes were automatically extracted. Analyses were conducted in a total of 87 medicationnaïve preschoolers, ages 4–5 years (47 with ADHD, 40 controls; 63% boys). ADHD was diagnosed using modified DSM-IV criteria based on review of developmental history, structured psychiatric interview and caregiver ratings. Compared to typically developing children, subcortical volumes were reduced among preschoolers with ADHD, with largest reductions in the caudate, globus pallidus, and thalamus. Among girls (but not boys) with ADHD, putamen and thalamus volumes were associated with ADHD symptom severity. The observed patterns of subcortical differences in preschoolers with ADHD (larger reductions in girls), contrasted with differences observed among school-aged children, (larger reductions in boys) suggests that children with ADHD show sexual dimorphism in neuroanatomical development that parallels early trajectory of symptom onset and attenuation.
Keywords: MRI, Basal ganglia, Sex differences, Neuroanatomy, Thalamus, Development
1. Introduction
Attention-deficit hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder characterized by developmentally inappropriate symptoms of inattention and/or hyperactivity/impulsivity that emerge in childhood. Neurobiological models of ADHD have centered on the frontal lobe and its interconnections with sub-cortical structures as contributing to executive dysfunction, motor deficits, and difficulties regulating attention, motivation, and affect, which in turn produce the behavioral symptoms of ADHD. An alternative neurodevelopmental model of ADHD (Halperin and Schulz, 2006) posits that anomalous development of the earlier maturing (relative to the frontal lobe) subcortical brain regions may contribute to the etiology of ADHD given the typical onset of ADHD symptoms during the preschool years. Nearly all neuroimaging studies of children with ADHD have included only children of school-age (i.e., age 6 years and older). Thus, there is a need for neuroimaging studies of preschool-age children to determine whether anomalous basal ganglia morphology is evident as ADHD symptoms begin to emerge earlier in development.
By school-age, ADHD is associated with widespread structural brain abnormalities including smaller total cerebral volumes (Friedman and Rapoport, 2015), reductions in total gray matter volumes (Batty et al., 2010), and more localized anomalies in prefrontal and premotor areas (Dirlikov et al., 2015; Mahone et al., 2011b; Shaw et al., 2006), and subcortical structures (Ellison-Wright et al., 2008; Frodl and Skokauskas, 2012; Hoogman et al., 2017; Nakao et al., 2011; Norman et al., 2016). Despite the fairly consistent evidence of frontal lobe anomalies in individuals with ADHD, it is not clear whether these volumetric reductions are secondary to developmental deviations in subcortical regions. Although some studies of the basal ganglia in ADHD have included children as young as 4–5 years (Castellanos et al., 2002; Shaw et al., 2014a), the analyses have spanned a large age range often from childhood through adolescence and even later adulthood. Only one previous neuroimaging study examined a small sample (n=26) of preschoolers with and without ADHD (Mahone et al., 2011a), reporting reduced caudate volumes whereas globus pallidus, putamen, and cortical volumes did not differ. Furthermore, the majority of studies examining structural brain differences in ADHD have included individuals treated with stimulant medication, which may affect the trajectory of brain development in ADHD (Shaw et al., 2009). In addition, little attention has been paid to the thalamus in the ADHD literature (c.f., Batty et al., 2015; Greven et al., 2015; Ivanov et al., 2010) despite its central role in the cortico-striato-thalamo-cortical loops that govern motor, cognitive, and socio-emotional functions that tend to be affected in ADHD.
Whether these abnormalities in subcortical structures are similar for girls and boys with ADHD relative to TD same-sex peers has also not been comprehensively investigated, due in large part to the examination of primarily male samples. Structural neuroimaging studies have reported greater motor region abnormalities among boys with ADHD and greater prefrontal region abnormalities among girls with ADHD (Dirlikov et al., 2015; Jacobson et al., 2015). Furthermore, the sexually dimorphic developmental course of the basal ganglia (Raznahan et al., 2014) suggests that whether girls and boys with ADHD display basal ganglia anomalies may depend on their age. Specifically, Raznahan et al. (2014) suggest that the estimated peak volume of the globus pallidus is earlier in males (age 7.7) than females (age 9.5) whereas the estimated peak volume of the striatum and thalamus is earlier in girls (ages 12 and 13.8, respectively) than boys (ages 14.7 and 17.4, respectively). Of the neuroimaging studies that included a greater proportion of females with ADHD allowing for examination of sex differences, some have reported that diagnosis-by-sex interactions in basal ganglia structures did not emerge (Castellanos et al., 2002; Hoogman et al., 2017; Villemonteix et al., 2015; Yang et al., 2008), although other studies suggested that diagnostic differences in basal ganglia morphology are specific to boys (Qiu et al., 2009; Seymour et al., 2017) or (for studies including girls only) not observed in girls (Castellanos et al., 2001). These inconsistent findings may be partially due to the age range of samples in these studies and or methodological differences, such as diagnostic methods, screening for comorbidities, image acquisition, processing, and statistical modeling, and, importantly, differences in the proportion and length of medication treatment for ADHD that have been shown to affect subcortical morphology (Sobel et al., 2010).
The current study extends previous investigations of basal ganglia morphology in ADHD through examination of stimulant-naïve pre-school-age children (ages 4–5) oversampled for girls to test for diagnostic group and sex differences without the confound of medication usage. In addition, we have used an automated subcortical segmentation procedure that has shown to be superior to other segmentation algorithms, such as those implemented in Freesurfer (Tang et al., 2015). Given previous findings of sex differences in anomalous basal ganglia morphology among children with ADHD (Qiu et al., 2009; Seymour et al., 2017), we specifically compared subcortical volumes among girls and boys with ADHD to same-sex TD children. We hypothesized that preschool children with ADHD would show reduced subcortical volumes compared to age- and sex-matched TD preschoolers, but (in contrast to findings in school-aged children) the patterns of greater relative anomaly among boys with ADHD would be attenuated in pre-schoolers, given the earlier patterns of brain development in girls (Giedd and Rapoport, 2010; Giedd et al., 2015; Raznahan et al., 2014; Shaw et al., 2008). We also hypothesized that reduced subcortical volumes in ADHD would be associated with severity of symptoms as shown in previous studies (Dirlikov et al., 2015; Mahone et al., 2011a), perhaps more so among girls with ADHD who may be undergoing greater developmental changes at this age.
2. Methods
2.1. Participants
Participants included in the current analyses were 47 medicationnaïve children with ADHD (30 boys) and 40 typically developing (TD) children (25 boys) between the ages of 4 years, 0 months and 5 years, 11 months. Participants were recruited from the community, local daycare centers, community publications, pediatricians' offices, and word-of-mouth. All procedures were approved by the hospital Institutional Review Board. Interested parents were provided with a description of the study, after which they signed informed consent and children provided verbal assent. Participants were initially screened via parent telephone interview to determine eligibility. After enrollment, participants attended two laboratory sessions during which they completed a neuropsychological assessment battery, including measures of cognitive ability and language functioning. Parents completed behavior rating scales at the time of the assessment visit. All participants underwent mock MRI scan training (detailed below) prior to the MRI scan to acclimate children to the scanner environment and train children to lie still in the scanner.
2.1.1. Exclusion and diagnostic procedures
Participants were excluded for any of the following, established via review of medical/developmental history, and/or by study assessment: 1) diagnosis of Intellectual Disability or Autism Spectrum Disorder; 2) known visual impairment; 3) treatment for psychiatric disorder with psychotropic medications; 4) history of DSM-IV Axis I diagnosis, other than Oppositional Defiant Disorder or Adjustment Disorder; 5) neurological disorder (e.g., epilepsy, cerebral palsy, traumatic brain injury, Tourette syndrome); 6) documented hearing loss ≥ 25 dB; 7) history of physical, sexual, or emotional abuse; 8) medical contraindication to MRI; or 9) Full Scale IQ < 80 assessed using the Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III; Wechsler, 2002). In addition, children were excluded if Developmental Language Disorder (DLD) was determined either during the initial phone screen, based on prior assessment (completed within one year of the current assessment), or during screening visit assessed using the Clinical Evaluation of Language Functions-Preschool-2 (CELF-P-2) (Semel et al., 2004). Children scoring below −1.5 SD on either the Receptive Language or Expressive Language Index of the CELF-P-2, or below −1.0 SD on both indices, were excluded (n=1).
Diagnostic methods for group assignment were adapted from the NIH Preschoolers with Attention-deficit/Hyperactivity Disorder Treatment (PATS) Study (Kollins et al., 2006; Posner et al., 2007). Diagnosis of ADHD was made using modified DSM-IV-TR criteria, based on parent report on the Diagnostic Interview Schedule for Children-Young Child (YC-DISC) (Lucas et al., 1998, 2008) or Diagnostic Interview for Children and Adolescents, Fourth Edition—DICA-IV (Reich et al., 1997), depending upon child age, and the DSM-IV ADHD Scales (Scales L and M) of the Conners' Parent Rating Scales-Revised (CPRS) (Conners, 1997; Conners et al., 1998). The YC-DISC is a highly structured, computer-assisted diagnostic instrument that assesses common psychiatric disorders, as defined by DSM-IV, presenting in young children. The DICA-IV is the parallel version of the computer-assisted, structured interview for older children and adolescents. In the present study, the DISC-YC was developed for 3–4 year olds and was therefore administered to 4-year olds in the current study. The DICA-IV, designed for children ages 6 and older, was used for 5-year olds in the current study because these children are of school-age and their environment may be more similar to a 6-year old than a 4-year old. To be included in the ADHD group, children in the ADHD group were required to have T-scores ≥ 65 on one or both of the DSM-IV ADHD Scales of the CPRS. Additionally, symptoms must have been present for at least 6 months as documented via structured interview, with evidence of cross-situational impairment (defined as parent report of problems at home and with peers, as not all children were enrolled in school).
Children were included in the typically developing group only if they did not meet categorical diagnostic criteria for any disorder on the YC-DISC or DICA-IV. Additionally, children in the control group were required to have T-scores < 65 on the CPRS DSM-IV ADHD Scales and they could not have a biological sibling diagnosed with ADHD (n=1). Five participants were excluded because they did not meet criteria for either diagnostic group.
2.2. MRI methods
2.2.1. Preparation of preschoolers for scans
The present study used a brief (15–30 min) behavioral protocol involving practice with a mock MRI scanner, designed for young children and those with developmental disabilities (Slifer et al., 1993, 2002). Procedures employed during the mock scan targeted getting children to enter the MRI environment willingly and lying still. A full description of behavioral procedures employed can be found in Mahone et al. (2011a). In the current study, 10 participants (4 ADHD boys, 3 ADHD girls, 2 TD girls, 1 TD boy) were excluded as they were unwilling to complete the mock scan. An additional six participants (4 TD girls, 1 TD boy, 1 ADHD boy) successfully completed the mock scan, but did not complete the actual scan and were excluded from these analyses.
2.2.2. Acquisition methods
All scanning was completed on a Philips 3T scanner (Achieva; Phillips Healthcare, Best, The Netherlands) with an 8-channel coil. Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE) images were used for volumetric assessment. Slice thickness = 1.0 mm; FOV = 26 cm; Matrix size: 256 × 256. A Sensitivity Encoding (SENSE) coil was used to address geometric distortion artifacts due to macroscopic magnetic susceptibility effects that can cause signal dropout at the air-tissue interface.
In order to ensure good data quality, excessive motion is qualified in 3 stages. First, the MPRAGE is visually inspected for gross motion artifacts (e.g., ringing, blurred gray-white matter boundaries, frame shifts, pixelation) at the scanner and a new MPRAGE would be collected if excessive motion is detected. In cases where a second MPRAGE is attempted, corrective feedback is provided to the child. Additionally, in more challenging children, a trained research team member enters the MRI room and sits with the child to help minimize movement. Second, the MPRAGE scan(s) quality is rated (Good, Borderline +, Borderline, Borderline -, and unusable) by a trained research team member who is blind to the participant's clinical diagnosis. The ratings reflect the amount of motion artifact (e.g., ringing, blurred gray-white matter boundaries, frame shifts, and pixelation) in the MPRAGE. Third, the parcellation maps are visually inspected for errors. Scans receiving a quality rating below Borderline (i.e., Borderline- and unusable) were excluded from these analyses (n = 17).
2.2.3. Subcortical segmentation
From each T1-weighted image, subcortical structures (specifically the caudate, putamen, globus pallidus, and thalamus, which are included in the automated protocol described below) were delineated in both hemispheres using MRICloud, a high-throughput neuroinformatics platform for automated brain MRI segmentation. This platform uses the multi-atlas likelihood-fusion (MALF) algorithm (Tang et al., 2013), which better accounts for anatomic variability by relying on information from multiple pediatric atlases. The MALF pipeline leverages the sophisticated brain warping technique, large deformation diffeomorphic metric mapping (LDDMM), to identify the optimal deformation between the atlases and each subject image. More specifically, for any subject image, we assume that every atlas image is a possible generator of it. Thus in the estimation of the segmentation label, the choice of the deformable atlas has become a random variable. Integral to this estimation is identification of the optimal diffeomorphism acting on the background space of coordinates which affects the evolution with least energy from the randomly selected atlas image to the subject image. The atlas used in this study for subcortical structure segmentation is based on a highly reliable manual parcellation schema and has been demonstrated to produce subcortical segmentations with a high degree of correspondence to the manual ‘gold-standard’ labels (Tang et al., 2015) In addition, scans were visually inspected to ensure that the automated parcellations were properly delineated. The MALF pipeline has been validated in pediatric and elderly clinical populations and has been shown to be superior to other automated algorithms, such as Freesurfer, for performing subcortical segmentation (Tang et al., 2015). Additional details on the algorithm and validation analyses can be found in previous studies (Tang et al., 2015, 2013).
Subcortical volumes were normalized in all analyses to correct for differences in total cerebral volume between diagnostic groups and sexes, using the procedure recommended by Kramer et al. (2007). Specifically, we calculated the average total cerebral volume (TCV) for each diagnostic group separately for girls and boys and multiplied the absolute ROI volume for each individual by the average TCV of the diagnostic group and divided by the individual's TCV (Mahone et al., 2011b; Ranta et al., 2009). For each participant, TCV estimation was generated using the Freesurfer pial surface and includes total cerebral gray and white matter and excludes CSF (Fischl et al., 2004). This process was unsuccessful for seven participants due to registration errors, resulting in their exclusion from these analyses.
2.3. Data analysis
Diagnostic group and sex differences in TCV were examined using factorial analysis of variance (ANOVA) with the between-subjects factors of diagnostic group (ADHD vs. TD) and sex (girls vs. boys). Differences in subcortical volumes were examined using mixed-factor ANOVAs with the between-subjects factors of diagnostic group (ADHD vs. TD) and sex (girls vs. boys), and the within-subjects factors of ROI (caudate, putamen, globus pallidus, and thalamus) and hemisphere (left vs. right). Interpretation of results focused on effects involving diagnostic group as this is our primary interest. We also explored ADHD-related sex differences given our previous findings of reduced basal ganglia volumes in boys, but not girls, with ADHD compared to same-sex TD peers (Qiu et al., 2009; Seymour et al., 2017). Cohen's d is reported as a measure of effect size for diagnostic group differences (absolute value) in volume such that d = 0.2, 0.5, and 0.8 are considered small, medium, and large effects, respectively (Cohen, 1988). In addition, correlations between parent-reported ADHD symptoms on the CPRS and raw subcortical volumes were also examined among girls and boys with ADHD.
3. Results
3.1. Demographics
Diagnostic group differences in demographic and clinical measures are reported in Table 1. There were no significant differences between groups in sex (χ2(1) =0.016, p = 0.898; 63% male), or racial distribution (χ2(3) =4.9, p = 0.177; overall sample: 85.7% Caucasian, 9.9% African-American, 3.3% Asian, 1.1% not reported) or handedness (χ2(2) = 0.449, p = 0.799; 84% right-handed). The ADHD and TD groups also did not differ significantly in age, socioeconomic status, FSIQ, or CELF-Core Language scores (see Table 1). However, girls with ADHD were significantly older than TD girls, F(1, 30) = 5.1, p = 0.032.1 Girls and boys with ADHD did not differ in age, socioeconomic status, FSIQ, or CELF-Core Language scores (see Table 1) nor do they differ in raw scores on the CPRS. However, ADHD girls have higher T-scores on the CPRS inattention scale (p = 0.015), but not on the CPRS hyperactive/impulsive scale (p = 0.138). All participants were naïve to stimulant medication at the time of participation.
Table 1. Demographic and clinical characteristics of ADHD and TD groups overall and within sex.
| Control | ADHD | Group Comparisons | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||
| Girls (n = 15) | Boys (n = 25) | All (n = 40) | Girls (n = 17) | Boys (n = 30) | All (n = 47) | Girls TD vs. ADHD | Boys TD vs. ADHD | All TD vs. ADHD | ADHD Boys vs. Girls | |||||||
|
|
||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-values | ||||
| Age (years) | 4.8 | 0.5 | 5.0 | 0.6 | 5.0 | 0.6 | 5.3 | 0.6 | 4.9 | 0.6 | 5.1 | 0.6 | 0.032 | 0.488 | 0.432 | 0.082 |
| % Male | n/a | n/a | 62.5% | n/a | n/a | 63.8% | n/a | n/a | 0.898 | n/a | ||||||
| SESa | 58.9 | 7.5 | 55.5 | 12.8 | 56.8 | 11.1 | 55.8 | 13.0 | 57.4 | 9.7 | 56.8 | 10.9 | 0.421 | 0.541 | 0.993 | 0.639 |
| WPPSI-3b FSIQ | 110.5 | 13.4 | 110.4 | 16.7 | 110.5 | 15.4 | 110.0 | 12.0 | 110.0 | 14.6 | 110.0 | 13.5 | 0.906 | 0.912 | 0.874 | 0.993 |
| CELF-P2c Core Lang | 110.3 | 10.4 | 104.2 | 11.7 | 106.5 | 11.5 | 109.3 | 7.9 | 105.9 | 13.0 | 107.2 | 11.4 | 0.766 | 0.619 | 0.785 | 0.334 |
| CPRSd IA raw | 0.8 | 1.3 | 2.7 | 3.4 | 2.0 | 2.9 | 15.4 | 4.5 | 16.0 | 6.3 | 15.8 | 5.6 | < 0.001 | < 0.001 | < 0.001 | 0.740 |
| CPRS IA T | 43.1 | 3.5 | 45.3 | 6.6 | 44.5 | 5.7 | 79.4 | 8.9 | 70.8 | 11.8 | 73.9 | 11.5 | < 0.001 | < 0.001 | < 0.001 | 0.015 |
| CPRS HI raw | 3.2 | 2.8 | 3.4 | 3.6 | 3.3 | 3.3 | 15.6 | 5.6 | 18.3 | 5.5 | 17.3 | 5.6 | < 0.001 | < 0.001 | < 0.001 | 0.124 |
| CPRS HI T | 47.5 | 6.2 | 45.0 | 6.1 | 45.9 | 6.2 | 75.3 | 12.6 | 70.3 | 9.3 | 72.1 | 10.8 | < 0.001 | < 0.001 | < 0.001 | 0.138 |
| CPRS Tot raw | 4.0 | 3.1 | 6.1 | 6.0 | 5.3 | 5.2 | 31.0 | 8.7 | 34.3 | 10.0 | 33.1 | 9.6 | < 0.001 | < 0.001 | < 0.001 | 0.274 |
| CPRS Tot T | 45.3 | 4.0 | 44.8 | 5.6 | 45.0 | 5.0 | 79.6 | 10.1 | 72.0 | 9.6 | 74.8 | 10.4 | < 0.001 | < 0.001 | < 0.001 | 0.018 |
| ADHD subtype (count) CO:IA:HI | n/a | n/a | n/a | 13:2:2 | 17:6:7 | 30:8:9 | n/a | n/a | n/a | 0.395 | ||||||
Note. ADHD = Attention-deficit/Hyperactivity Disorder; SES=Hollingshead Four-Factor Index of Socioeconomic Status; WPPSI-3 = Wechsler Preschool and Primary Scale of Intelligence, 3rd Ed.; FSIQ = Full Scale IQ score; CELF-P2 = Clinical Evaluation of Language Fundamentals, Preschool, 2nd Ed.; Core Lang = Core Language score; CPRS IA = Conners' Parent Rating Scale-Revised Scale L (DSM-IV Inattentive raw/T-score); CPRS HI = Conners' Parent Rating Scale-Revised Scale M (DSM-IV Hyperactive/Impulsive raw/T-score); CPRS Tot = Conners' Parent Rating Scale-Revised Scale N (DSM-IV ADHD Total raw/T-score); CO= Combined subtype; IA= Inattentive subtype; HI = Hyperactive/Impulsive subtype.
SES missing for 3 children (3 ADHD boys);
WPPSI-3 missing for 1 child (1 ADHD boy);
CELF-P2 missing for 1 child (1 ADHD boy);
CPRS missing for 3 children (1 ADHD girl, 2 ADHD boys).
3.2. Total cerebral volume
For TCV, there were significant main effects of diagnostic group (ADHD < TD; p = 0.010) and sex (girls < boys, p = 0.002), but no group-by-sex interaction (p = 0.590). Within the narrow age range of the sample, age was not correlated with TCV (r = 0.118, p = 0.278). Given the differences in TCV between diagnostic group and sexes, subcortical volumes were normalized in all analyses testing for diagnostic group differences using the approach described above. Results are also reported for the raw volumes in Supplement 1.
3.3. Volume analysis
Results of the ANOVA indicate significant main effects of diagnostic group, F(1,83) = 10.0, p = 0.002, d = 0.69, and sex, F(1, 83) = 30.5, p < 0.001, d = 1.24. The main effect of diagnostic group was qualified by a diagnostic group × ROI interaction, F(3, 81) = 4.2, p = 0.008, d = 0.45, such that reduced volumes were observed among children with ADHD compared to controls in the caudate (p = 0.008, d = 0.52), globus pallidus (p = 0.031, d = 0.44), and thalamus, (p = 0.001, d = 0.62), but not the putamen (p = 0.270, d = 0.25; see Table 2).
Table 2. Subcortical volumes (raw) for girls and boys with ADHD and typically developing (TD) controls.
| All | TD n = 40 | ADHD n = 47 | p | d [CI] | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | SD | Mean | SD | |||
| Caudate | 8066 | 1103 | 7680 | 672 | 0.008 | 0.52 [0.09,0.94] |
| Putamen | 9058 | 935 | 8892 | 753 | 0.270 | 0.25 [−0.18, 0.67] |
| Glob Pal | 3184 | 317 | 3081 | 232 | 0.031 | 0.44 [0.01, 0.87] |
| Thalamus | 13,529 | 1030 | 13,028 | 972 | 0.001 | 0.62 [0.18, 1.04] |
| Girls | n = 15 | n = 17 | ||||
| Caudate | 7818 | 948 | 7297 | 614 | 0.034 | 0.81 [0.07, 1.51] |
| Putamen | 8516 | 565 | 8495 | 582 | 0.937 | 0.03 [−0.66, 0.72] |
| Glob Pal | 3048 | 212 | 2950 | 195 | 0.203 | 0.44 [−0.28, 1.13] |
| Thalamus | 13,222 | 641 | 12,490 | 922 | 0.007 | 1.20 [0.42, 1.92] |
| Boys | n = 25 | n = 30 | ||||
| Caudate | 8214 | 1180 | 7896 | 613 | 0.104 | 0.43 [−0.11, 0.96] |
| Putamen | 9384 | 969 | 9117 | 754 | 0.088 | 0.45 [−0.09, 0.98] |
| Glob Pal | 3266 | 345 | 3155 | 220 | 0.057 | 0.53 [−0.01, 1.07] |
| Thalamus | 13,714 | 1180 | 13,333 | 873 | 0.063 | 0.47 [−0.08, 1.00] |
Notes. Glob Pal = globus pallidus. p = p-value for the post-hoc comparisons from the mixed factors ANOVA with the normalized volumes. d = Cohen's d effect size estimate for the normalized volumes; [CI] = 95% confidence interval for Cohen's d effect size.
3.4. ADHD-related sex differences
Based on previous findings of ADHD-related sex differences in basal ganglia volumes (Qiu et al., 2009; Seymour et al., 2017), we compared the magnitude of diagnostic group differences separately among girls and boys for each of the subcortical structures despite the absence of a diagnostic group × sex × ROI interaction, F(3, 81) = 1.4, p = 0.242, d = 0.26. Among boys, although volume was generally reduced across all subcortical structures, there were no significant diagnostic group differences (ps > 0.05), with medium effect sizes observed across all subcortical structures, (ds ranging from 0.43 − 0.53), suggesting insufficient power to detect these effects. In contrast, among girls, diagnostic group differences (with moderate to large effect size) were observed in the caudate (p = 0.034, d = 0.81) and thalamus (p = 0.007, d = 1.20). In addition, a non-significant volume difference of medium effect size was observed in the globus pallidus (p = 0.203, d = 0.44) whereas putamen volumes were highly similar among girls with and without ADHD (p = 0.937, d = 0.03; see Table 2).
3.5. Correlations with symptom severity
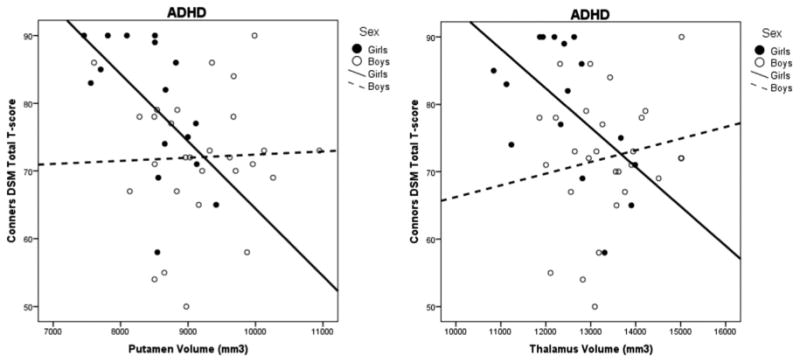
Associations between subcortical volumes and parent-reported ADHD symptom severity were examined within the ADHD group separately for girls and boys (see Table 3). Among girls with ADHD (n = 16), reduced putamen and thalamus volumes were associated with greater symptom severity (see Fig. 1). Specifically, putamen volume was significantly correlated with total symptom severity (r = −0.582, p = 0.018) and inattention symptom severity (r = −0.553, p = 0.026), whereas thalamus volume was significantly correlated with total symptom severity (r = −0.548, p = 0.028) and hyperactivity/impulsivity symptom severity (r = −0.517, p = 0.040). Among boys with ADHD, there were no significant associations between subcortical volumes and ADHD symptom ratings (ps > 0.29).
Table 3. Correlations between subcortical volumes (raw) symptom severity for girls and boys with ADHD.
| CPRS Total | CPRS Inattention | CPRS Hyp/Imp | |
|---|---|---|---|
| ADHD Girls (n = 16) | |||
| Caudate | −0.134 | −0.063 | −0.086 |
| Putamen | −0.582* | −0.553* | −0.406 |
| Globus Pallidus | −0.426 | −0.342 | −0.360 |
| Thalamus | −0.548* | −0.372 | −0.517* |
| ADHD Boys (n = 28) | |||
| Caudate | 0.113 | 0.082 | 0.100 |
| Putamen | 0.036 | −0.025 | 0.084 |
| Globus Pallidus | 0.143 | 0.179 | 0.053 |
| Thalamus | 0.162 | 0.204 | 0.062 |
p < 0.05; CPRS = Conners' Parent Rating Scale-Revised, DSM-IV Total (Scale N), Inattention (Scale L) and Hyperactivity/Impulsivity (Scale M) T scores.
Fig. 1. Relationship between volume of the putamen and thalamus with ADHD symptom severity for girls and boys with ADHD.
4. Discussion
The current study contributes valuable knowledge to the extant literature on anomalous subcortical morphology in ADHD by comparing basal ganglia and thalamus volumes among a carefully characterized sample of medication naïve preschool-age children with ADHD relative to age- and sex-matched typically developing children. Despite the vast literature on anatomic studies of individuals with ADHD and a recent mega-analysis of subcortical volumes (e.g., Hoogman et al., 2017), this study is unique in its focus on 4–5 year olds, all of whom are naïve to stimulant medication, and the use of validated protocols for optimizing subcortical segmentation (Tang et al., 2015). Furthermore, this is the first study to show greater relative subcortical reductions among girls with ADHD (compared to TD girls), with medium to large effects — ds ranging from 0.44 to 1.20 — contrasted with boys with ADHD (vs. TD boys) who showed smaller, non-significant reductions — ds ranging from 0.43 to 0.53. However, it is important to note that these findings are preliminary given our relatively small sample of girls and require replication in a larger, separate sample. Further, effect size estimates for boys are in the moderate range, suggesting our study was underpowered to detect these effects, all of which just escaped traditional levels of statistical significance (e.g., p-values range from 0.057 to 0.104). In addition, putamen and thalamus volumes were significantly associated with symptom severity only among girls with ADHD. Taken together, these findings are in contrast to the limited literature examining sexually dimorphic, ADHD-related basal ganglia morphology anomalies among school-age children, in which subcortical differences were primarily observed in boys with ADHD. In addition, the diagnostic group effects are much larger than those reported in a recent mega-analysis of approximately 1500 children younger than age 15 (Hoogman et al., 2017), although it is unclear whether this is a function of the younger age of our sample, methodological differences, or a combination of both factors. A developmental perspective is critical in understanding these patterns, both in terms of the course of ADHD symptoms (Willcutt et al., 2012) and the sexually dimorphic growth trajectory of subcortical structures (Raznahan et al., 2014).
In a preliminary study examining structural brain anomalies among a small sample (n=26) of preschool-age children with and without ADHD, there was evidence of significantly reduced caudate (but not putamen or globus pallidus) volumes in preschoolers with ADHD (Mahone et al., 2011a). In this larger sample, we have been able to expand these preliminary findings using an updated (automated) parcellation protocol, and extend analyses to better characterize the (potentially moderating) effect of sex. In doing so, we found stronger evidence of widespread, ADHD-related subcortical reductions in the caudate, globus pallidus, and thalamus, particularly among girls with ADHD. The current findings, along with emerging evidence of reduced cortical (prefrontal, premotor) volumes among preschoolers with ADHD (Jacobson et al., under review) suggest that brain differences in ADHD are evident at least as early as symptom onset. Together, these observations inform our understanding of the neurobiology of ADHD in preschool-age children, and lay the foundation for future longitudinal investigations. Specifically, it remains unclear whether (or when) these observed subcortical anomalies among girls with ADHD diminish with age, and whether the degree to which changes in ADHD symptom presentation parallel sex-specific “normalization” of subcortical volumes in girls. Although the current study does not address the etiology of these brain differences, a recent review of brain imaging genetic studies in ADHD provides some evidence of an association between the dopamine transporter gene and reduced striatal volume in children and adolescents with ADHD (Klein et al., 2017). To better understand the etiology of atypical brain structure in ADHD, longitudinal research conducted at multiple levels of analysis (e.g., cellular, multi-modal neuroimaging, neuropsychological, behavioral, and presentation of symptoms) is recommended.
Based on animal models of ADHD, which highlight early striatal delays that stabilize by age 6 weeks (human equivalent of 7–9 years) (Hsu et al., 2010), and cross-sectional studies of subcortical volume reductions in ADHD suggesting reduced subcortical differences over development (e.g., Hoogman et al., 2017), one might hypothesize that these subcortical anomalies observed in preschoolers will diminish with age. However, a longitudinal neuroimaging study of school-aged children and adolescents with and without ADHD (Shaw et al., 2014a) suggests similar trajectories of striatal and globus pallidus volumes among individuals with and without ADHD. Thus, the developmental course of subcortical anomalies associated with ADHD remains unclear and is likely complicated by treatment with medication and sex differences. These findings raise another important question about the “distal” impact of early subcortical abnormalities in ADHD, and their possible contribution to more widespread cortical (predominantly frontal) and cerebellar dysfunction later in development (i.e., the “crossed trophic effect”). Specifically, it is conceivable that these brain regions share a pattern of reciprocal influence, such that early anomalies in subcortical structures (basal ganglia, thalamus) impairs cortical and cerebellar development, and vice versa (Inder et al., 1999; Limperopoulos et al., 2005)—a hypothesis that can be optimally tested in future longitudinal studies.
The current findings add to a growing literature demonstrating the importance of examining ADHD-related sex differences in brain structure and function given evidence of sexually dimorphic development of the basal ganglia (Raznahan et al., 2014). In light of evidence that ADHD is associated with a delay in cortical maturation (Shaw et al., 2012), diagnostic group differences observed in any cross-sectional study may vary, based on the age range of the sample examined. Complicating the matter further, subcortical structures appear to follow a curvilinear developmental trajectory (Raznahan et al., 2014), such that comparisons made across wide age-ranges may be also misleading. The current findings provide a foundation for future longitudinal studies including younger children either with ADHD (or at-risk for ADHD) and highlight the importance of explicitly examining sex differences.
In the absence of longitudinal studies examining sex differences among large samples of well-characterized girls and boys with and without ADHD, valuable insight may still be gained from cross-sectional comparisons. In particular, a recently published study from our research group identified sex-differences in subcortical morphology (volume and shape) among school-age children (8–12 years) with and without ADHD (Seymour et al., 2017). In this older sample, boys with ADHD showed reduced volumes and localized compression in the globus pallidus and putamen, but not the caudate or thalamus, compared to TD boys, whereas no diagnostic group differences were observed in girls with ADHD compared to TD girls. Due to the use of identical image acquisition and image processing methods as used in the current study, comparison of subcortical volumes in our sample of 4–5 year-olds with the earlier published study of 8–12 year-olds may be particularly informative. As shown in Fig. 2, there are strong differences in caudate and thalamus volumes among 4–5 year-olds (effect sizes 2–3 times larger among girls) that are greatly reduced among children ages 8–12 years. In contrast, globus pallidus differences emerge early and are of similar magnitude in 4–5 year-old girls and boys, but seem to persist and perhaps increase in 8–12 year-old boys and diminish in girls. Finally, putamen differences were not observed among 4–5 year-olds, but emerge among 8–12 year-old boys only. This pattern of findings comparing preschool and school age samples might suggest that the pattern of subcortical differences among children with ADHD may be sex- and age-dependent. Longitudinal research may reveal that atypical behavior (e.g., symptoms) and anatomical differences in frontostriatal circuitry will be observed earlier in girls than in boys, relative to typically developing same-sex peers, and some of these differences will dissipate by school-age among girls, but not in boys, in conjunction with girls' earlier maturation.
Fig. 2.
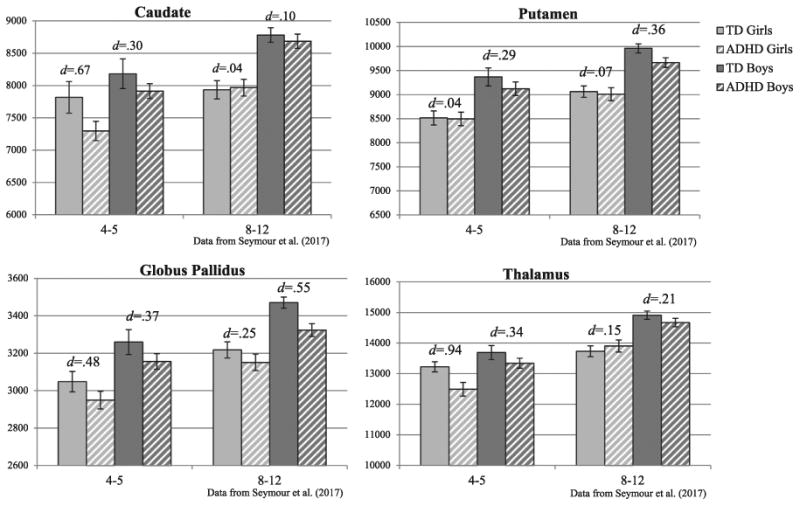
Cross-sectional comparison of subcortical volumes (raw) for different cohorts of girls and boys with ADHD and typically developing (TD) controls at ages 4–5(n = 87, 32 girls) and 8–12 (n = 217, 76 girls; Seymour et al., 2017) years.
However, another important difference between the preschool and school-age samples is that the majority (75% of girls and boys) of the 8–12 year-olds underwent treatment with stimulant medication whereas the 4–5 year-old sample is entirely stimulant naïve. Studies have now shown that stimulant medication may normalize brain structure and function in children with ADHD (see review by Spencer et al., 2013), including subcortical (Sobel et al., 2010) and cortical morphology (Shaw et al., 2009) and cerebellar anomalies (Bledsoe et al., 2009; Ivanov et al., 2014). Although highly speculative at this point, it may be that stimulant medication facilitates development of specific subcortical structures (e.g., caudate and thalamus) among individuals with ADHD. This hypothesis highlights the need for long-itudinal research with careful attention to stimulant medication treatment.
In addition to the diagnostic group differences, the magnitude of subcortical volume reduction predicted ADHD symptom severity among girls, but not boys, with ADHD. The general pattern of associations suggests that reduced putamen, globus pallidus, and thalamus volumes are associated (rs ranging from −0.46 to −0.58) with increased ADHD symptom severity (Conners T-scores) among girls with ADHD. This observation might imply that basal ganglia and thalamus abnormalities contribute to difficulties characteristic of ADHD to a greater extent among 4–5 year-old girls than boys of the same age. Given the relatively small sample (n = 17) of girls with ADHD, however, only the strongest correlations between ADHD symptoms with thalamus and putamen volumes were statistically significant. Thus, it will be important to replicate these findings in a larger sample of young girls with ADHD. Interestingly, caudate volumes were unrelated to ADHD symptom severity in preschool girls with ADHD and none of the brain-behavior correlations were significant among boys with ADHD (rs all < 0.17). This brain-behavior relationship may also reflect a sensitive period in development among 4–5 year-old girls in which changes in subcortical volume are very relevant for symptom onset. Given evidence suggesting that the striatum and thalamus mature earlier in girls (Raznahan et al., 2014), the sensitive period in which volumetric changes translate to behavioral symptoms may occur later in boys.
Although this study has several important strengths, it is also not without its limitations. First, the use of a validated automated protocol for subcortical segmentation is a strength of the current study, but it also did not allow for examination of other subcortical structures that may be relevant for ADHD such as the amygdala and hippocampus. It will be important for future research to examine these additional sub-cortical structures, particularly given evidence of emotion dysregulation in ADHD (Shaw et al., 2014b) and associations between subcortical morphology and emotion dysregulation (Seymour et al., 2017). Second, the inclusion of a relatively large number of girls is an advancement over previous studies, but the study may have been underpowered to detect the diagnosis-by-sex interaction and significant differences in boys, which were less pronounced in 4–5 year olds.
In summary, the results of this study contribute to the extant literature establishing the neurobiological basis of ADHD. These findings expand upon this literature by showing evidence of atypical basal ganglia and thalamus volumes at least as early as symptom onset in a well-characterized sample of preschool-age girls and boys with ADHD compared to typically developing children. In future research, it will be important to replicate these findings and to conduct shape-based analyses to identify whether specific subregions of the basal ganglia and thalamus are affected in children with ADHD to inform our understanding of dysfunctional cortico-striato-thalamo-cortical loops in ADHD. Furthermore, consideration of the current findings relative to a previous study examining ADHD-related sex differences in subcortical morphology among school-age children suggests a sexually dimorphic developmental trajectory of subcortical abnormalities associated with ADHD. However, longitudinal research is necessary to determine the developmental course of these differences. Through improved characterization of ADHD-related sex differences in brain development, we may be better able to understand sex differences in the clinical presentation and course of ADHD as well as neurobiological markers of risk for the emergence of additional comorbidities in adolescence.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 HD068425, U54 HD079123, UL1 RR025005, R01 MH078160, R01 MH085328, K23 MH101322, UL1 TR 000424 and the Johns Hopkins Brain Sciences Institute UL1 RR025005.
Footnotes
In order to determine whether the older age of girls with ADHD influenced the observed diagnostic group differences, analysis of covariance (ANCOVA) was employed for the comparison of subcortical volumes with age as a covariate. All significant effects from the ANOVA remained significant and all non-significant effects remained non-significant, suggesting that age did not impact the observed results.
Conflict of Interest: None to declare.
Contributors: KSR and EMM developed the plan for the statistical analysis, interpretation of results, and wrote the majority of the manuscript. KSR conducted the statistical analyses. DC and KH collected and processed the neuroimaging data, assisted with writing the neuroimaging methods, and provided feedback on other sections of the manuscript. EMM and SHM contributed to the conceptualization, implementation, and design of the study. SHM assisted with interpretation of results and provided critical feedback on the manuscript.
Appendix A. Supporting information: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.pscychresns.2017.10.013.
References
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, et al. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. http://dx.doi.org/10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty MJ, Palaniyappan L, Scerif G, Groom MJ, Liddle EB, Liddle PF, et al. Morphological abnormalities in prefrontal surface area and thalamic volume in attention deficit/hyperactivity disorder. Psychiatry Res. 2015;233(2):225–232. doi: 10.1016/j.pscychresns.2015.07.004. http://dx.doi.org/10.1016/j.pscychresns.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65(7):620–624. doi: 10.1016/j.biopsych.2008.11.030. http://dx.doi.org/10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58(3):289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Cohen D. Statistical Power Analyses for the Behavioral Sciences. 2nd. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Conners CK. Conners Parent Rating Scale - Revised (CPRS-R): Technical Manual. Multi-Health Systems, Inc; North Tonawanda, NY: 1997. [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Rosch KS, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage: Clin. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. http://dx.doi.org/10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E. Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. http://dx.doi.org/10.1186/1471-244X-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friedman LA, Rapoport JL. Brain development in ADHD. Curr Opin Neurobiol. 2015;30:106–111. doi: 10.1016/j.conb.2014.11.007. http://dx.doi.org/10.1016/j.conb.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125(2):114–126. doi: 10.1111/j.1600-0447.2011.01786.x. http://dx.doi.org/10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. http://dx.doi.org/10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2015;40(1):43–49. doi: 10.1038/npp.2014.236. http://dx.doi.org/10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven CU, Bralten J, Mennes M, O'Dwyer L, van Hulzen KJE, Rommelse N, et al. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry. 2015;72(5):490–499. doi: 10.1001/jamapsychiatry.2014.3162. http://dx.doi.org/10.1001/jamapsychiatry.2014.3162. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. http://dx.doi.org/10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, et al. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 2017 doi: 10.1016/S2215-0366(17)30049-4. http://dx.doi.org/10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed]
- Hsu JW, Lee LC, Chen RF, Yen CT, Chen YS, Tsai ML. Striatal volume changes in a rat model of childhood attention-deficit/hyperactivity disorder. Psychiatry Res. 2010;179(3):338–341. doi: 10.1016/j.psychres.2009.08.008. http://dx.doi.org/10.1016/j.psychres.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(4):397–408. doi: 10.1176/appi.ajp.2009.09030398. http://dx.doi.org/10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Murrough JW, Bansal R, Hao X, Peterson BS. Cerebellar morphology and the effects of stimulant medications in youths with attention deficithyperactivity disorder. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2014;39(3):718–726. doi: 10.1038/npp.2013.257. http://dx.doi.org/10.1038/npp.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LA, Peterson DJ, Rosch KS, Crocetti D, Mori S, Mostofsky S. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(11):938–946. doi: 10.1016/j.jaac.2015.08.014. http://dx.doi.org/10.1016/j.jaac.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Onnink M, van Donkelaar M, Wolfers T, Harich B, Shi Y, et al. Brain imaging genetics in ADHD and beyond - mapping pathways from gene to disorder at different levels of complexity. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.01.013. http://dx.doi.org/10.1016/j.neubiorev.2017.01.013. [DOI] [PMC free article] [PubMed]
- Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, et al. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS) J Am Acad Child Adolesc Psychiatry. 2006;45(11):1275–1283. doi: 10.1097/01.chi.0000235074.86919.dc. http://dx.doi.org/10.1097/01.chi.0000235074.86919.dc. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Quitania L, Dean D, Neuhaus J, Rosen HJ, Halabi C, et al. Magnetic resonance imaging correlates of set shifting. J Int Neuropsychol Soc: JINS. 2007;13(3):386–392. doi: 10.1017/S1355617707070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116(4):844–850. doi: 10.1542/peds.2004-2282. http://dx.doi.org/10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Fisher P, Luby J. Young Child DISC-IV Research Draft: Diagnostic Interview Schedule for Children Division of Child Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders. Columbia University; New York, NY: 1998. [Google Scholar]
- Lucas CP, Fisher P, Luby J. Young Child DISC-IV Research Draft: Diagnostic Interview Schedule for Children Division of Child Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders. Columbia University; New York, NY: 2008. [Google Scholar]
- Mahone EM, Crocetti D, Ranta ME, Gaddis A, Cataldo M, Slifer KJ, et al. A preliminary neuroimaging study of preschool children with ADHD. Clin Neuropsychol. 2011a;25(6):1009–1028. doi: 10.1080/13854046.2011.580784. http://dx.doi.org/10.1080/13854046.2011.580784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O'Brien J, Kaufmann WE, Denckla MB, et al. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc: JINS. 2011b;17(6):1047–1057. doi: 10.1017/S1355617711001056. http://dx.doi.org/10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168(11):1154–1163. doi: 10.1176/appi.ajp.2011.11020281. http://dx.doi.org/10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73(8):815–825. doi: 10.1001/jamapsychiatry.2016.0700. http://dx.doi.org/10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- Posner K, Melvin GA, Murray DW, Gugga SS, Fisher P, Skrobala A, et al. Clinical presentation of attention-deficit/hyperactivity disorder in preschool children: the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) J Child Adolesc Psychopharmacol. 2007;17(5):547–562. doi: 10.1089/cap.2007.0075. http://dx.doi.org/10.1089/cap.2007.0075. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. http://dx.doi.org/10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta ME, Crocetti D, Clauss JA, Kraut MA, Mostofsky SH, Kaufmann WE. Manual MRI parcellation of the frontal lobe. Psychiatry Res. 2009;172(2):147–154. doi: 10.1016/j.pscychresns.2009.01.006. http://dx.doi.org/10.1016/j.pscychresns.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci USA. 2014;111(4):1592–1597. doi: 10.1073/pnas.1316911111. http://dx.doi.org/10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. Diagnostic Interview for Children and Adolescents-IV (DICA-IV) Multi-Health Systems, Inc; North Tonawanda, NY: 1997. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals Preschool-2 (CELF-Preschool-2) Psychological Corporation; San Antontio, TX: 2004. [Google Scholar]
- Seymour KE, Tang X, Crocetti D, Mostofsky SH, Miller MI, Rosch KS. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Res: Neuroimaging. 2017;261:20–28. doi: 10.1016/j.pscychresns.2017.01.002. http://dx.doi.org/10.1016/j.pscychresns.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, De Rossi P, Watson B, Wharton A, Greenstein D, Raznahan A, et al. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder (e711) J Am Acad Child Adolesc Psychiatry. 2014a;53(7):780–789. doi: 10.1016/j.jaac.2014.05.003. http://dx.doi.org/10.1016/j.jaac.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci: Off J Soc Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. http://dx.doi.org/10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. http://dx.doi.org/10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191–197. doi: 10.1016/j.biopsych.2012.01.031. http://dx.doi.org/10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166(1):58–63. doi: 10.1176/appi.ajp.2008.08050781. http://dx.doi.org/10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014b;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. http://dx.doi.org/10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer KJ, Cataldo MF, Cataldo MD, Llorente AM, Gerson AC. Behavior analysis of motion control for pediatric neuroimaging. J Appl Behav Anal. 1993;26(4):469–470. doi: 10.1901/jaba.1993.26-469. http://dx.doi.org/10.1901/jaba.1993.26-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer KJ, Koontz KL, Cataldo MF. Operant-contingency-based preparation of children for functional magnetic resonance imaging. J Appl Behav Anal. 2002;35(2):191–194. doi: 10.1901/jaba.2002.35-191. http://dx.doi.org/10.1901/jaba.2002.35-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K, et al. Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry. 2010;167(8):977–986. doi: 10.1176/appi.ajp.2010.09091259. http://dx.doi.org/10.1176/appi.ajp.2010.09091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J Clin Psychiatry. 2013;74(9):902–917. doi: 10.4088/JCP.12r08287. http://dx.doi.org/10.4088/JCP.12r08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Crocetti D, Kutten K, Ceritoglu C, Albert MS, Mori S, et al. Segmentation of brain magnetic resonance images based on multi-atlas likelihood fusion: testing using data with a broad range of anatomical and photometric profiles. Front Neurosci. 2015;9:61. doi: 10.3389/fnins.2015.00061. http://dx.doi.org/10.3389/fnins.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Oishi K, Faria AV, Hillis AE, Albert MS, Mori S, et al. Bayesian parameter estimation and segmentation in the multi-atlas random orbit model. PloS One. 2013;8(6):e65591. doi: 10.1371/journal.pone.0065591. http://dx.doi.org/10.1371/journal.pone.0065591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemonteix T, De Brito SA, Slama H, Kavec M, Balériaux D, Metens T. Grey matter volume differences associated with gender in children with attention-deficit/hyper-activity disorder: A voxel-based morphometry study. doi: 10.1016/j.dcn.2015.06.001. < http://dx.doi.org/142015323710.1016/j.dcn.2015.06.001. [DOI] [PMC free article] [PubMed]
- Wechsler DL. Wechsler Preschool and Primary Scale of Intelligence. Third. Psychological Corporation; San Antonio, TX: 2002. (WPPSI-III). [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012 doi: 10.1037/a0027347. http://dx.doi.org/10.1037/a0027347. [DOI] [PMC free article] [PubMed]
- Yang PC, Wang PN, Chuang KH, Jong YJ, Chao TC, Wu MT. Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Res-Neuroimaging. 2008;164(3):245–253. doi: 10.1016/j.pscychresns.2007.12.013. http://dx.doi.org/10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


