Abstract
Advance care planning (ACP) is a central tenet of dialysis care, but the vast majority of dialysis patients report never engaging in ACP discussions with their care providers. Over the last decade, we have developed and iteratively tested SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), a theory-based, patient- and family-centered advance care planning intervention. SPIRIT is a six-step, two-session, face-to-face intervention to promote cognitive and emotional preparation for end-of-life decision making for patients with ESRD and their surrogates. In these explanatory trials, SPIRIT was delivered by trained research nurses. Findings consistently revealed that patients and surrogates in SPIRIT showed significant improvement in preparedness for end-of-life decision making, and surrogates in SPIRIT reported significantly improved post-bereavement psychological outcomes after the patient’s death compared to a no treatment comparison condition. As a critical next step, we are conducting an effectiveness-implementation study. This study is a multicenter, clinic- level cluster randomized pragmatic trial to evaluate the effectiveness of SPIRIT delivered by dialysis care providers as part of routine care in free-standing outpatient dialysis clinics, compared to usual care plus delayed SPIRIT implementation. Simultaneously, we will evaluate the implementation of SPIRIT, including sustainability. We will recruit 400 dyads of patients at high risk of death in the next year and their surrogates from 30 dialysis clinics in four states. This trial of SPIRIT will generate novel, meaningful insights about improving ACP in dialysis care.
Keywords: End-stage renal disease, dialysis, advance care planning, pragmatic trial, cluster randomized trial
INTRODUCTION
End-stage renal disease (ESRD) currently affects nearly 660,000 people in the United States [1]. While over 70% of patients with ESRD are treated with dialysis, the likelihood that dialysis can restore health or prolong life is limited; only 50% of dialysis patients are alive 3 years after the onset of ESRD [1]. Thus, many dialysis patients and their family members or surrogate decision-makers have to face difficult end-of-life decisions. Although advance care planning (ACP), in which patients and surrogate decision-makers discuss future health states and treatment options, is a central tenet of dialysis care [2–5], the vast majority of dialysis patients (>90%) report never engaging in ACP discussions with their care providers [6, 7]. The lack of effective ACP to prepare patients and their surrogates for making end-of-life decisions with sufficient time before death has deleterious consequences at all levels of society. Patients may experience physical and emotional suffering from the prolonged use of futile treatment at the end of life, surrogates may have high levels of stress from making decisions about care both during the loved one’s hospitalizations and after their death, and the costs of care that had little or no impact on survival burden hospitals and society as a whole [8–14].
SPIRIT (Sharing Patient’s Illness Representation to Increase Trust), a patient- and family-centered ACP intervention based on the Representational Approach to Patient Education [15, 16], is a testable model of how end-of-life care discussions can occur between a dialysis patient and his/her chosen surrogate (usually a spouse or adult child). The discussions, which are facilitated by a trained care provider, are framed around addressing each individual’s representations of (beliefs about) the illness and views of life-sustaining measures at the end of life. SPIRIT follows a six-step process over two sessions, which together take about 60 minutes. The care provider guides the patient in examining his/her values related to end-of-life care, helps the surrogate understand the patient’s illness progression, and prepares the surrogate for his/her role as a surrogate in a highly emotionally charged medical setting.
Over the last decade, we have iteratively tested SPIRIT to establish feasibility, patient-surrogate acceptability, and efficacy [17–20]. In these explanatory trials carried out in dialysis clinics, SPIRIT was delivered by trained research nurses. Patients and surrogates in SPIRIT showed significant improvement in preparedness for end-of-life decision making, including the extent to which: a) the patient and surrogate agreed on end-of-life care goals, b) the patient had reduced conflict about the benefits and burdens of life-sustaining treatments, and c) the surrogate had increased confidence about the role of surrogate. In addition, surrogates who received SPIRIT reported significantly improved post-bereavement psychological outcomes after the patient’s death compared to those who did not. The purpose of this paper is to describe the rationale, design and methods of the next logical step in this program of research, a pragmatic trial of SPIRIT.
METHODS
Study design overview
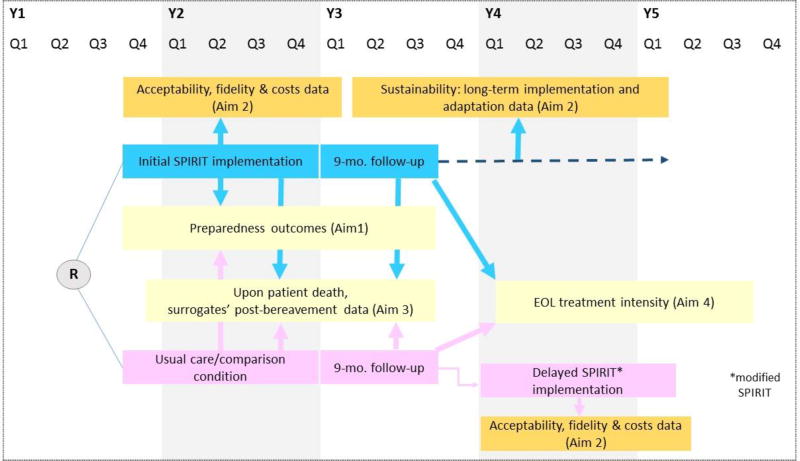
The study is a phase III, dialysis clinic- level cluster randomized trial with two groups, SPIRIT (initial implementation) and usual care followed by delayed SPIRIT (delayed implementation). See Figure 1. We will recruit 400 dyads of patients on chronic (“prevalent”) dialysis who are at high risk of death in the next year and their surrogate decision-makers (total 800 individuals) from 30 free-standing dialysis clinics in four states (GA, NM, NC, and PA). The primary outcomes are patient and surrogate self-reported preparedness for end-of-life decision making (Aim 1). Implementation evaluation data will be obtained throughout the study course (Aim 2). Upon patient death, we will assess surrogates’ post-bereavement distress (Aim 3). Medicare claims data will be obtained to assess end-of-life treatment intensity (Aim 4).
Figure 1.
Study design and overview
Clinics will be randomized to either SPIRIT or usual care in Study Year 1. Clinics randomized to usual care will implement SPIRIT in Year 4, after they serve as control for effectiveness evaluation. The process outcomes from the initial implementation of SPIRIT will be used to determine if any modifications to SPIRIT are necessary. Clinics in the delayed implementation phase will be evaluated on the process outcomes, which will provide data on the iterated version of SPIRIT without having to conduct another trial.
Study participants
The study requires both the patient and his/her chosen surrogate decision maker to participate as a dyad (pair). Patients meeting the following criteria will be deemed eligible for the study: 1) 18 years or older; 2) on either hemodialysis or peritoneal dialysis; 3) seriously ill based on clinician’s heuristic prediction of survival using the validated [21–23] Surprise Question (SQ): “Would I be surprised if this patient died in the next year?” (patients for whom the answer is “No, I wouldn’t be surprised” are 4 times more likely to die in the next year); and 4) able to understand and speak English. Patients who lack an available surrogate, are too ill or cognitively impaired to participate based on clinicians’ judgment, or are already enrolled in hospice will be excluded. We will use a short investigator-developed set of questions [24] to help patients identify a surrogate decision-maker if they have not already done so. We will not exclude patients who already have an advance directive or a medical order; previous completion of those documents was not associated with the study outcomes in previous trials [18–20] and a study of physician orders for life-sustaining treatment (POLST) suggested that these medical order forms are often completed without an in-depth ACP discussion [25].
Surrogate eligibility criteria are 1) 18 years or older (to serve as a surrogate decision-maker, the individual must be an adult); and 2) being chosen by the patient. Those who cannot complete questionnaires due to physical or cognitive limitations will be excluded. Roughly 76 providers at the 30 clinics, including all medical directors, nurse managers, social workers, and those who are selected to conduct SPIRIT sessions will participate in the implementation evaluation.
Quarterly, in both groups, a dialysis care provider at each clinic will generate a list of patients each quarter for whom the SQ answer is “No”. The care provider will then assess whether the patient meets the other criteria and whether the patient is willing to meet with a recruiter from the research team. The recruiter will approach eligible and willing patients during their scheduled dialysis clinic appointment to explain the study purposes and procedures. The recruiter will then provide the patient with a study brochure, obtain a written consent, and encourage him/her to talk to the surrogate regarding the study within the next 2–3 days. Several days later, the recruiter will telephone the surrogate to assess his/her willingness to participate. Upon the surrogate’s verbal consent, the recruiter will conduct a baseline survey by phone. For surrogates in the SPIRIT implementation clinics, the recruiter will schedule the first SPIRIT session to take place at the clinic 2 weeks hence. A printed consent form with a stamped return envelope will be mailed to the surrogate in the meantime. For surrogates in the usual care clinics, a verbal consent will be obtained over the telephone.
Randomization
Clinics covered by the same care provider who will deliver SPIRIT sessions will be combined into one cluster to avoid risk of contamination; this results in 19 clusters (3 in GA, 3 in NC, 8 in NM, and 5 in PA). Because the numbers of available patients substantially vary across the clusters, to minimize group imbalance, clusters will be stratified to three cluster sizes: small (patient census ≤ 52), medium (53–105), and large (≥ 106). We will randomize clusters to either SPIRIT or usual care plus delayed SPIRIT, with randomly permuted blocks (sizes of 2 and 4) within cluster size stratum nested in each state, using a pseudo-random-number generator, by the Study Coordination Center. Before the study starts, the Study Coordination Center will inform the group assignment for each cluster to the site PIs, with a unique ID consisting of abbreviations for state and cluster size stratum.
While the heterogeneity in minority race/ethnicity across clusters improves the study’s generalizability, stratification or pairing clusters by race/ethnicity is not feasible because some clinics serve only one race with no comparable clinics within the state. Group imbalances on race/ethnicity and other potential confounding factors will be examined and adjusted for in the analyses. The cluster randomized design prevents potential spillover effects between the treatment conditions. Research staff assessing effectiveness outcomes will be blind to group assignment.
Treatment conditions
The comparison condition is usual care related to ACP at the participating clinics. As required by CMS [26], written information on advance directives is provided to a patient on the first day of dialysis, and a social worker reviews this information with patients and encourages them to complete an advance directives. This typically takes about 10 minutes. If completed, the presence of an advance directives is documented on the Plan of Care form. If a patient expresses a desire not to be resuscitated in the dialysis unit, a Do-Not-Resuscitate (DNR) order is written by a nephrologist and placed in the clinic record. If there is no DNR order in the record, a full code is presumed. A social worker or charge nurse reviews code status and updates it annually. Currently, all four states endorse the POLST paradigm; NM and NC use medical orders for scope of treatment (MOST), while PA and GA use POLST; these forms may be completed to complement advance directives. None of the clinics employ routine identification of patients at high risk for death or structured ACP sessions targeting those patients. As in our previous studies, we will review the patient’s electronic medical records (EMR) at the clinic at baseline and quarterly to track usual care. To capture any changes in usual care, policy and procedures related to ACP at the clinic will be reviewed every 6 months by each site coordinator.
SPIRIT is a patient and family-centered ACP and has beneficial effects on a range of psychosocial outcomes for dialysis patients and their surrogates. The goals of SPIRIT are to help patients clarify their end-of-life preferences and help surrogates understand the patient’s wishes and prepare for the surrogate role. All care providers responsible for SPIRIT delivery will follow six steps using the structured SPIRIT Interview Guide: 1) assessing illness presentation, 2) identifying gaps and concerns, 3) creating conditions for conceptual change, 4) introducing replacement information, 5) summarizing, and 6) setting goals and planning. SPIRIT first establishes an understanding of the cognitive, emotional and spiritual aspects of the patient’s representation of his/her illness. This understanding enables the care provider to provide individualized medical information and to assist the patient in examining his/her own values related to life-sustaining treatment at the end of life. In this way, the patient can more readily express his/her treatment preferences to the surrogate. SPIRIT also enables the surrogate to understand the patient’s illness experiences and values and to be prepared for the responsibility and emotional turmoil that can arise during decision making at the end of life. Each element of SPIRIT is designed to enhance the quality and authenticity of exchanges between patient and surrogate about experiences surrounding illness and values. During the process, the patient discovers his/her own representations about illness and dialysis and examines thresholds and/or conditions for (dis)continuing life support measures. The surrogate gains an understanding of the patient’s illness experience and begins to see his/her limited life expectancy. The surrogate also validates similarities or differences with the patient in regard to life support measures and examines his/her own ability to follow the patient’s wishes. This process is critical to preparation for end-of-life decision making.
All sessions will be conducted in a private room in the clinic. SPIRIT has two face-to-face sessions with patient and surrogate together. During the first session (~45 minutes), the care provider will assess the patient’s and surrogate’s cognitive, emotional, and spiritual/religious representations of the patient’s illness, prognosis, and end-of-life care. This will allow the care provider to provide individualized information about topics, such as the effectiveness of life-sustaining treatment for people with end-organ failure, and assist the patient to examine his/her values about life-sustaining treatment at the end of life. The care provider will help the surrogate prepare for end-of-life decision-making and for the emotional burden of decision-making by actively involving the surrogate in the discussion. If the surrogate is someone out of the order of the hierarchical compensatory model [27] (e.g., a sibling is chosen instead of a spouse), the care provider will explore potential family conflicts and encourage the dyad to talk with other family members and complete a healthcare power of attorney. A Goals-of-Care document will be completed at the end of the session to indicate the patient’s preferences. A brief second session (~15 minutes) will be delivered about 2 weeks later. This session is a follow-up to address remaining or new concerns and questions raised after the first session. The patient’s Goals-of-Care document will be reviewed and revised if clarification or correction is needed. The provider will document the patient’s end-of-life preferences and the surrogate’s name and relationship to the patient in the medical record. If the patient desires a DNR order, POLST, or MOST, the care provider will inform with the patient’s nephrologist and arrange a meeting to complete a treatment order form. We will track completion of these forms.
Care provider training for SPIRIT
Each dialysis clinic has identified two care providers (primary and secondary) designated to conduct SPIRIT sessions (e.g., advanced nurse practitioner, registered nurse, social worker). The reason for selecting and training two care providers is to help address staff turnover. SPIRIT training will occur shortly after randomization for the initial implementation clinics and in Year 4 for the delayed implementation clinics. The SPIRIT trainer, trained and certified by the PI at the Study Coordination Center, will conduct in-person SPIRIT training for all four sites based on the manualized curriculum. Care provider training will consist of a 1½-day, competency-based program that has been used in our previous trials. Module 1 (1/2 day) focuses on understanding of end-of-life care issues and communication as key to improving end-of-life care and the Representational Approach (theoretical underpinnings of SPIRIT). Module 2 (1/2 day) is a skill-base session to foster understanding of the SPIRIT intervention and delivery, including role plays. A 2-week practice period will be scheduled to integrate skills and explore additional learning needs. Finally, Module 3 (1/2 day) involves skill-demonstration and certification.
Intervention fidelity and monitoring
To maintain internal validity, certain components of the intervention will be standardized. As described above, care provider training is standardized using the training modules. Session 1 will be conducted face-to-face using the SPIRIT Interview Guide to promote consistency and quality of intervention delivery. We have developed a template of policies and procedures related to SPIRIT implementation that each clinic will customize and use as a resource.
To assess fidelity, we will use two independent data sources: 1) the SPIRIT Interview Guide will direct the care provider to document performance data after each patient-surrogate dyad encounter. The Guide has a checklist of SPIRIT components, including start and finis h times and a brief self-evaluation section; and 2) at the 2-week post-intervention follow-up, a research assistant will query patients and surrogates about the SPIRIT sessions using the checklist of SPIRIT components. After the first 50 dyads (~first 4 months) have participated, clinics with < 80% adherence on either data source will receive corrective feedback and by the Site PI and the SPIRIT trainer.
Data collection and measures
Data collection procedures
Collection of effectiveness outcome data will be centralized; research staff at the Study Coordinating Center will collect the data from patients and surrogates by phone. Centralized data collection maintains blinding of data collectors to group assignment. The preparedness outcomes will be assessed 2 weeks post intervention, as in our previous trial [18]. This low level of followup intensity is consistent with the focus in pragmatic trials of keeping the study condition as close to the real-world setting as possible [28, 29]. We expect that roughly 40% of study patients (~160) will die by the 9-month follow-up. Deaths are readily identifiable through dialysis clinics and will trigger post-bereavement surveys with surrogates at 3 months.. Post-bereavement assessment at 3 months is based on our efficacy data showing that distress symptoms sharply rose in both groups 2-weeks post-bereavement and then stabilized at 3 months [20].
Effectiveness Outcomes
Preparedness for end-of-life decision making (primary outcomes) will be measured at baseline and 2 weeks post-intervention. Dyad congruence will be assessed using the Goals-of-Care Tool [18, 20], which includes two scenarios describing medical conditions commonly occurring in patients with ESRD. In the first, the patient develops a severe complication and cannot speak for himself/herself; the medical team believes recovery is unlikely and continuing life-sustaining treatment, including dialysis, will no longer be beneficial. In the second scenario, the patient develops advanced dementia. Each scenario has three response options: “The goals of care should focus on delaying my death, and thus I want to continue life-sustaining treatment”, “The goals of care should focus on my comfort and peace, and thus I do not want life-sustaining treatment, including dialysis”, and “I am not sure”. Patients and surrogates complete this tool independently and their responses are then compared to determine dyad congruence -- either congruent in both scenarios or incongruent. If both members of the dyad endorse “I am not sure”, they are considered incongruent.
Patient decisional conflict will be measured using the 13-item Decisional Conflict Scale (DCS), a validated measure in the context of end-of-life decision making [17]; higher scores indicate greater difficulty in weighing benefits and burdens of life-sustaining treatments and decision making (range 1–5; Cronbach’s α = 0.8 – 0.93[17, 18, 20, 30]).
Surrogate decision-making confidence will be measured using the 5-item Decision Making Confidence (DMC) scale (Cronbach’s α = 0.81–0.90 [18, 31]) on which higher scores reflect greater comfort in performing as a surrogate (range 0, not confident at all-4, very confident). DMC items ask the surrogate’s confidence in: knowledge of the patient’s wishes, ability to make treatment decisions even in a highly stressful situation, ability to seek information about risks and benefits of medical choices, ability to handle unwanted pressure from others, and ability to communicate with health care providers about the patient’s wishes.
We will also create a composite outcome combining dyad congruence and surrogate DMC to differentiate surrogates who both understand the patient’s wishes and feel confident in their role from those who don’t (understand the wishes but lack confidence, misunderstand the wishes but feel confident, neither understand nor feel confident) [18, 20, 31].
Surrogates’ post-bereavement psychological distress will be measured at baseline and 3 months after the patient’s death. Symptoms of anxiety, depression, and post-traumatic distress [32, 33] will be measured using the Hospital Anxiety and Depression Scale (HADS) [34]; subscale scores range from 0 to 21 with higher scores indicating greater symptom severity. Internal consistencies and test-retest reliabilities are 0.88–0.90 and 0.84–0.94, respectively [35]. Intensity of post-traumatic distress symptoms will be assessed using the Post-Traumatic Symptoms Scale-10 (PTSS-10) [36]. Higher scores indicate more intense symptoms (range 10–70) [37]. The PTSS-10 has been shown to have high sensitivity and specificity [36, 38, 39].
End-of-life treatment intensity will be assessed upon patient death. We will link study data to publicly available United States Renal Data System (USRDS) data. From the inpatient and outpatient claims data, we will obtain dates and attributed causes of death; dates of hospital, skilled nursing facility, and hospice admissions and discharges; dates of outpatient encounters (including dialysis sessions and ED visits); and diagnostic and procedure codes for all inpatient and outpatient encounters. Thus, we will be able to determine hospitalization, ICU days, days hospitalized, use of intensive procedure, (dis)continuation of dialysis (also from CMS Form 2746), and hospice use during the final month of life.
We will consider the following to be intensive procedures: mechanical ventilation, feeding tube placement, dialysis, and cardiopulmonary resuscitation [40]. These intensive procedures will be identified using Healthcare Common Procedure Coding System and International Classification of Disease-9-CM codes (e.g., ICD-9 codes for intubation and mechanical ventilation: 96.04, 96.05, 96.7X) [41].
Implementation process outcomes
For the initial SPIRIT intervention, after all dyads have completed the SPIRIT sessions (~Year 3, Q1), care providers will complete a survey and an interview. Care provider acceptability will be evaluated using the 7-item Care Providers’ Perceived Acceptability Survey to assess their perceptions about SPIRIT implementation, including the time required, impact on interactions with patients, and whether they would recommend SPIRIT to other clinics [42]. Response options range from 1=strongly disagree to 4=strongly agree; higher scores indicate greater acceptability.
A semi-structured interview (face-to-face or by telephone; audio-recorded) will obtain providers’ perspectives on whether the implementation of SPIRIT is compatible with their setting and the workflow, its perceived utility, their willingness to continue using it after the study, factors influencing implementation (barriers, logistical constraints, and facilitators), and suggestions for improvement.
Patient and surrogate acceptability will be assessed using the 10-item ACP Acceptability Questionnaire. Participants are asked how strongly they agree or disagree (4 to 1) with statements about their experience with SPIRIT sessions, including duration, interactions with the care provider, level of comfort and satisfaction. Higher scores indicate greater acceptability. Each patient and surrogate will complete this survey at the 2-week post-intervention follow-up. Fidelity/adherence will be assessed using two independent data sources, (a) the SPIRIT Interview Guide, Checklist, and self-evaluation completed by the care provider after each SPIRIT session and (b) patient and surrogate responses to the SPIRIT components coverage during the 2-week post-intervention follow-up as described above. The number of SPIRIT components covered, the minutes required for the care provider to complete the sessions, the number of dyads who complete SPIRIT sessions, and the number of incomplete or interrupted sessions will be aggregated.
Intervention costs will be estimated based on the actual time the care provider spent in carrying out SPIRIT, multiplied by hourly wage (+benefits), plus costs of materials. Overhead (e.g., facility) costs and research staff’s time will not be included.
We define sustainability as the extent to which a newly implemented intervention is maintained within a service setting’s ongoing, stable operations [43]. In Y4, Q4, we will conduct a brief interview with care providers to ask about sustainability: (a) Is SPIRIT on-going (and at what frequency)? (b) What components of the SPIRIT protocol have been retained? and (c) Has SPIRIT implementation been evaluated at the clinic level? If so, how? [44]
For the delayed SPIRIT implementation, the process evaluation will involve care providers only since patient and surrogate study participation will have ended at the end of 9-month follow-up or patient death. Care provider acceptability, fidelity, and intervention costs will be determined as described above.
Descriptors and Potential Covariates
Patients and surrogates will complete a Sociodemographic Profile which includes age, gender, race and ethnicity, type of relationship between patient and surrogate, marital status, religious affiliation, education, household income, previous end-of-life decision-making experience, and previous participation in an ACP discussion or AD completion. To describe the sample, the patient’s clinical characteristics, including dialysis modality, years on dialysis, and comorbid conditions will be abstracted from the patient’s EMR. Care providers’ sociodemographic data will include age, gender, race and ethnicity, education, and years of practice. At baseline, we will collect clinic- level contextual data [45] that could facilitate understanding study results, including staffing, patient census, rural-urban status [46–50], palliative care and hospice availability, and proximity of hospitals.
SPIRIT Modification
The analysis of the qualitative implementation process data from the initial implementation group may offer an opportunity to modify SPIRIT to enhance its acceptability for care providers. We will begin analyzing the implementation data as they are collected. Themes identified from the acceptability interview data will be organized to facilitate identifying SPIRIT’s aspects that contributed to a perception of SPIRIT as less than positive. Using these data we will make changes to facets of SPIRIT that are modifiable, e.g., delivery mode of the training modules, workflow issues, timing of delivery, or moving some facets of Session 1 into Session 2.
Data analysis plan
Patient-surrogate dyads will be the primary unit of analysis; all analyses will be intent-to-treat with all available data from all participants. The preliminary analysis will include summarizing variables with standard descriptive statistics and graphical displays or frequency tables. Distributional assumptions will be assessed and the data will be transformed as necessary. Clinic characteristics (e.g., rural-urban status) will be compared using χ2 tests for categorical variables and t-tests for continuous variables. We will compare SPIRIT and usual care participants on baseline characteristics (e.g., age, race/ethnicity) to explore possible between-group differences using generalized estimating equation (GEE) methods, accounting for the observed correlation within the same cluster [51, 52]. In our previous work, SPIRIT had no effect on patient mortality [20]; however, we will compare survival time between SPIRIT and usual care using Cox proportional hazards models with the log-rank test, adjusted for cluster effects. If imbalanced, we will consider adjustment for the group difference.
Although we expect minimal missing data given the short-term follow-up, we will investigate missing data with pattern analysis for data missing completely at random, missing at random or missing not at random, and use maximum likelihood or multiple imputation appropriate for each type to impute missing values. We will also conduct sensitivity analyses to encompass different scenarios of assumptions and evaluate consistency or discrepancy among them.
SPIRIT effectiveness on the preparedness outcomes (Aim 1)
Dyad congruence and the composite outcome (binary variables) will be analyzed by fitting a generalized mixed effects model for each, where the binary outcome is modeled in terms of a logit link [51] with both a random intercept and random slope to control for variation within and between subjects and clusters. For patient DCS and surrogate DMC scores, we will replace the logit link by the identity link with an additional error term. These models will allow us to examine whether SPIRIT was superior to usual care in the primary outcomes at 2 weeks and whether the effect of SPIRIT varies by cluster size. The analysis will be adjusted for potential covariates, such as race/ethnicity, and rural-urban status, in the model, including interaction between treatment and race/ethnicity.
Implementation process outcomes (Aim 2)
Quantitative data on acceptability, fidelity, and costs of SPIRIT will be summarized using descriptive statistics. SPIRIT will be determined to be acceptable to care providers and patients and surrogates if over 75% of responses exceed an average summative score ≥3 (of 4) on the acceptability measures. Cost estimates will be used to ascertain resources needed to implement or replicate SPIRIT in the future [53]. We will also explore the relationships of these quantitative data with characteristics of settings and stakeholders. Transcripts of acceptability interviews will be transferred to ATLAS.ti for analysis. Content analysis techniques [54] will be used without preconceived categories [55]. Open coding will be applied [56] and disagreements on coding will be resolved by consensus. We will examine the data for patterns or differences in themes between those with acceptability scores ≥3 and scores <3 and setting characteristics. This analysis will be facilitated by creating matrixes that organize textual and numeric data for comparing and contrasting [57] so that what contributed to acceptability scores that are positive and less than positive may be identified. We will use a similar approach, content analysis, to evaluate the sustainability data.
SPIRIT effectiveness on surrogates’ post-bereavement psychological distress (Aim 3)
We will use the same approach as in Aim 1 to compare anxiety, depression, and post-traumatic distress symptoms in SPIRIT vs. usual care among surrogates of patients who die during the 9-month follow-up of the initial implementation.
SPIRIT effectiveness on end-of-life treatment intensity (Aim 4)
Among patients who die during the 9-month follow-up of the initial SPIRIT implementation, percentages of patients hospitalized, having ICU admission, having intensive procedures and length of hospital stay in the final month of life will be summarized using descriptive statistics, 95% CIs, and graphical displays. The exploratory examination of SPIRIT’s effectiveness on improving these outcomes, we will use the same analytic approach as in Aims 1 and 3.
Power Calculation
We have an adequate sample size (19 clusters) to detect clinically meaningful differences between SPIRIT and usual care for our primary outcomes. Statistical power is based on a random effects models: a generalized linear mixed model for binary outcomes (e.g., dyad congruence) and a linear mixed model for continuous outcomes (e.g., patient decisional conflict). We conducted a simulation study to estimate power with 2-sided significance level alpha = 0.05, corrected for anticipated dropout and potential intraclass correlations (ICCs) ranging 0.01– 0.04, based on ICCs observed in our prior work. Since the anticipated cluster sizes are known we simulated data from a generalized model with the adjustments made for the stratification variable, varying cluster size. To estimate the power more conservatively, we used the effect sizes observed at 2 months [20] rather than those at 2 weeks [18], which are larger (e.g., for the composite outcome, OR=1.8 at 2 months, OR=4.4 at 2 weeks). The estimated power to detect clinically meaningful differences is at least 80% for all of the primary outcomes and the post-death outcomes.
Discussion
This paper describes the study design and methods of a pragmatic trial to evaluate the effectiveness of SPIRIT delivered by dialysis care providers as part of routine care in free-standing outpatient clinics compared to usual care plus delayed SPIRIT implementation. Simultaneously, we will evaluate the implementation of SPIRIT, including its sustainability. This is a Type I effectiveness-implementation hybrid approach [58, 59] that combines testing intervention effectiveness and gathering information about implementation of an efficacious intervention in a real world setting. If study outcomes are congruent with the positive outcomes seen in our previous trials, there will be sufficient evidence to accelerate the integration of SPIRIT into dialysis practice and policy.
Despite the urgent need to promote ACP to improve end-of-life care for patients with serious chronic illnesses and their families [5], rarely have trials demonstrated successful translation of an effective ACP intervention into practice. SPIRIT is the only ACP intervention that has demonstrated efficacy in improving outcomes for ESRD patients and their surrogates. An evidence-based, potentially billable ACP intervention could help policy makers expand metrics for patient-centered dialysis care to go beyond current services driven by disease-specific quality indicators such as rate of infections and anemia management.
Dialysis care providers currently practice within the guidelines of the Medicare End-Stage Renal Disease Quality Incentive Program, which includes metrics related to infections, anemia, and urea clearance. Although clinical practice guidelines and the National Quality Foundation strongly endorse ACP as a quality indicator, ACP is not a clinical benchmark for care of ESRD patients [2, 60]. In fact, very little is known about implementing an efficacious ACP intervention in dialysis clinical practice [61]. To advance to widespread implementation of SPIRIT, it is essential to generate data to inform future implementation processes [62]. This study will systematically evaluate implementation process outcomes (i.e., ones that are distinct from effectiveness outcomes) based on the implementation research framework delineated by Proctor et al. [63], including acceptability, fidelity and costs. We will also gather long-term implementation and adaptation data. These data will enable us to answer process evaluation questions [58], such as “What are the potential barriers and facilitators to real-world implementation of SPIRIT?” “What problems were associated with delivering SPIRIT in the real-word?” “What modifications to SPIRIT could be made to maximize implementation based on clinic-specific feedback?” “Will care providers continue to use SPIRIT after the initial implementation ends?”
Hybrid designs are increasingly used to expedite the sequential process, “efficacy to effectiveness to preliminary implementation,” [59] and are recommended when there is strong evidence of the intervention effects and the intervention is low risk for participants [58]. To maximize data on the implementation process and sustainability, we chose a delayed intervention design [64] in which clinics are randomized either to implement SPIRIT immediately after randomization (i.e., initial implementation) or to maintain usual care for a comparison condition and then implement the intervention in Year 4 (i.e., delayed implementation). The delayed implementation group will effectively serve as control for effectiveness evaluation.
Virtually no trials are purely pragmatic or purely explanatory. Our study is more pragmatic than explanatory in the pragmatic-explanatory continuum because it will include diverse patient populations, multiple heterogeneous settings, few inclusion and exclusion criteria, and the comparison condition is a real-world alternative (i.e., usual care), not a placebo [28, 29]. Further, the study is built around normal dialysis care operations as much as possible with flexible study protocols that minimize intrusion in daily work flow at the dialysis facilities. Of direct relevance to dialysis care, the intervention will be implemented by dialysis care providers, such as nurses and social workers. This real-world trial of SPIRIT will generate meaningful insights in improving ACP in dialysis care to prevent prolonged use of futile treatment at the end of life, prevent high levels of distress during decision making, maximize opportunities to benefit from palliative care services or hospice, and minimize post-bereavement anxiety and depression frequently observed in surrogates.
Acknowledgments
Funding source: This work is supported by NIH/NINR 1R01NR017018-01 (PI, Song)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial registration: ClinicalTrials.gov NCT03138564, registered 05/01/2017
Disclosure The authors do not have any potential conflicts of interest to disclose.
References
- 1.United States Renal Data System. 2015 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. [Google Scholar]
- 2.Renal Physicians Association. Shared decision-making in the appropriate initiation of and withdrawal from dialysis: Clinical practice guideline. 2. Rockville, MD: Renal Physicians Association; 2010. [Google Scholar]
- 3.Kolarik RC, et al. Objectives for advance care planning. J Palliat Med. 2002;5(5):697–704. doi: 10.1089/109662102320880516. [DOI] [PubMed] [Google Scholar]
- 4.Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. Jama. 2005;294(3):359–65. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine. Dying in America: Improving quality and honoring individual preferences near the end of life. The National Academy of Sciences; Washington, D.C: 2014. [PubMed] [Google Scholar]
- 6.Davison SN. Facilitating advance care planning for patients with end-stage renal disease: The patient perspectives. Clinical Journal of the Amedican Society of Nephrology. 2006;1:1023–1028. doi: 10.2215/CJN.01050306. [DOI] [PubMed] [Google Scholar]
- 7.Goff SL, et al. Advance Care Planning: A Qualitative Study of Dialysis Patients and Families. Clin J Am Soc Nephrol. 2015;10(3):390–400. doi: 10.2215/CJN.07490714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun UK, et al. Voices of African American, Caucasian, and Hispanic surrogates on the burdens of end-of-life decision making. J Gen Intern Med. 2008;23(3):267–74. doi: 10.1007/s11606-007-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherlin E, et al. Communication between physicians and family caregivers about care at the end of life: when do discussions occur and what is said? J Palliat Med. 2005;8(6):1176–85. doi: 10.1089/jpm.2005.8.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LM, et al. The family perspective of ESRD deaths. Am J Kidney Dis. 2005;45(1):154–61. doi: 10.1053/j.ajkd.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Cohen LM, et al. A very good death: measuring quality of dying in end-stage renal disease. J Palliat Med. 2001;4(2):167–72. doi: 10.1089/109662101750290209. [DOI] [PubMed] [Google Scholar]
- 12.Hebert RS, Dang Q, Schulz R. Preparedness for the death of a loved one and mental health in bereaved caregivers of patients with dementia: findings from the REACH study. J Palliat Med. 2006;9(3):683–93. doi: 10.1089/jpm.2006.9.683. [DOI] [PubMed] [Google Scholar]
- 13.Hebert RS, et al. Preparing Family Caregivers for Death and Bereavement. Insights from Caregivers of Terminally Ill Patients. J Pain Symptom Manage. 2009;37(1):3–12. doi: 10.1016/j.jpainsymman.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–46. doi: 10.7326/0003-4819-154-5-201103010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Donovan HS, Ward S. A representational approach to patient education. J Nurs Scholarsh. 2001;33(3):211–6. doi: 10.1111/j.1547-5069.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 16.Donovan HS, et al. An update on the representational approach to patient education. J Nurs Scholarsh. 2007;39(3):259–65. doi: 10.1111/j.1547-5069.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song MK, et al. A randomized, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Medical Care. 2005;43(10):1049–1053. doi: 10.1097/01.mlr.0000178192.10283.b4. [DOI] [PubMed] [Google Scholar]
- 18.Song MK, et al. Randomized controlled trial of SPIRIT: An effective approach to preparing African American dialysis patients and families for end-of-life. Research in Nursing & Health. 2009;32:260–273. doi: 10.1002/nur.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song MK, et al. Effects of an intervention to improve communication about end-of-life care among African Americans with chronic kidney disease. Applied Nursing Research. 2010;23:65–72. doi: 10.1016/j.apnr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Song MK, et al. Advance care planning and end-of-life decision making in dialysis: A randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis. 2015;66(5):813–22. doi: 10.1053/j.ajkd.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynn J, Schuster JL, Kabcenell A. Improving care for the end of life: A sourncebook for health care managers and clinicians. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 22.Moss AH, et al. Utility of the "surprise" question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379–84. doi: 10.2215/CJN.00940208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen LM, et al. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5(1):72–9. doi: 10.2215/CJN.03860609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song MK, Ward SE. Disconnect between emergency contacts and surrogate decision-makers in the absence of advance directives. Palliat Med. 2013;27(8):789–92. doi: 10.1177/0269216312474486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickman SE, Keevern E, Hammes BJ. Use of the physician orders for life-sustaining treatment program in the clinical setting: a systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–50. doi: 10.1111/jgs.13248. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. Final Rule. D.o.H.a.H. Services; 2008. Medicare and Medicaid Programs; Conditions for Coverage for End-Stage Renal Disease Facilities. [PubMed] [Google Scholar]
- 27.Carr D, Khodyakov D. Health care proxies: whom do young old adults choose and why? J Health Soc Behav. 2007;48(2):180–94. doi: 10.1177/002214650704800206. [DOI] [PubMed] [Google Scholar]
- 28.Loudon K, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe KE, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62(5):464–75. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Song MK, Sereika SM. An evaluation of the Decisional Conflict Scale for measuring the quality of end-of-life decision making. Patient Educ Couns. 2006;61(3):397–404. doi: 10.1016/j.pec.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Song MK, Ward SE, Lin FC. End-of-life decision-making confidence in surrogates of African-American dialysis patients is overly optimistic. J Palliat Med. 2012;15(4):412–7. doi: 10.1089/jpm.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lautrette A, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–78. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 33.Siegel MD, et al. Psychiatric illness in the next of kin of patients who die in the intensive care unit. Crit Care Med. 2008;36(6):1722–8. doi: 10.1097/CCM.0b013e318174da72. [DOI] [PubMed] [Google Scholar]
- 34.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Lowe B, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J Affect Disord. 2004;78(2):131–40. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 36.Eid J, Thayer JF, Johnsen BH. Measuring post-traumatic stress: a psychometric evaluation of symptom--and coping questionnaires based on a Norwegian sample. Scand J Psychol. 1999;40(2):101–8. doi: 10.1111/1467-9450.00105. [DOI] [PubMed] [Google Scholar]
- 37.Weisaeth L. Torture of a Norwegian ship's crew. In: Wilson JP, Raphael B, editors. Stress reactions, coping, and psychiatric aftereffects, in International Handbook of Traumatic Stress Syndromes. Plenum Press; London: 1993. [Google Scholar]
- 38.Schelling G, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26(4):651–9. doi: 10.1097/00003246-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Johansen VA, et al. Prevalence and predictors of post-traumatic stress disorder (PTSD) in physically injured victims of non-domestic violence. A longitudinal study. Soc Psychiatry Psychiatr Epidemiol. 2007;42(7):583–93. doi: 10.1007/s00127-007-0205-0. [DOI] [PubMed] [Google Scholar]
- 40.Wong SP, Kreuter W, O'Hare AM. Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med. 2012;172(8):661–3. doi: 10.1001/archinternmed.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnato AE, et al. Development and validation of hospital "end-of-life" treatment intensity measures. Med Care. 2009;47(10):1098–105. doi: 10.1097/MLR.0b013e3181993191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean KA. Department of Psychology. University of Miami; 2013. Healthcare provider acceptability of a behavioral intervention to promote adherence; p. 434. [Google Scholar]
- 43.Glasgow RE, et al. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns. 2001;44(2):119–27. doi: 10.1016/s0738-3991(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 44.Wiltsey Stirman S, et al. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17. doi: 10.1186/1748-5908-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stange KC, Glasgow RE. In: Considering and Reporting Important Contextual Factors in Research on the Patient-Centered Medical Home. A.f.H.R.a. Quality, editor. Agency for Healthcare Research and Quality; Rockville, MD: 2013. [Google Scholar]
- 46.United States Department of Agriculture. Urban influence code. 2013 [Google Scholar]
- 47.Gessert CE, Haller IV, Johnson BP. Regional variation in care at the end of life: discontinuation of dialysis. BMC Geriatr. 2013;13:39. doi: 10.1186/1471-2318-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe-Galloway S, et al. Quality of end-of-life care among rural Medicare beneficiaries with colorectal cancer. J Rural Health. 2014;30(4):397–405. doi: 10.1111/jrh.12074. [DOI] [PubMed] [Google Scholar]
- 49.Lavergne MR, et al. Examining palliative care program use and place of death in rural and urban contexts: a Canadian population-based study using linked data. Rural Remote Health. 2015;15(2):3134. [PubMed] [Google Scholar]
- 50.Wang H, et al. Rural-Urban Differences in Costs of End-of-Life Care for Elderly Cancer Patients in the United States. J Rural Health. 2016;32(4):353–62. doi: 10.1111/jrh.12160. [DOI] [PubMed] [Google Scholar]
- 51.Verbeke G, Molenberghs G. Linear mized models for longitudinal data. New York: Springer; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molenberghs G, Verbeke G. Models for discrete longitudinal data. New York: Springer; 2005. [Google Scholar]
- 53.Ritzwoller DP, et al. Costing behavioral interventions: a practical guide to enhance translation. Ann Behav Med. 2009;37(2):218–27. doi: 10.1007/s12160-009-9088-5. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 55.Kondracki NL, Wellman NS, Amundson DR. Content analysis: review of methods and their applications in nutrition education. J Nutr Educ Behav. 2002;34(4):224–30. doi: 10.1016/s1499-4046(06)60097-3. [DOI] [PubMed] [Google Scholar]
- 56.Miles MB, Huberman AM. Qualitative Data Analysis: An expanded sourcebook. 2. Thousand Oaks, CA: Sage Publishing; 1994. [Google Scholar]
- 57.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. [Google Scholar]
- 58.Curran GM, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NIH Health Care Systems Research Collaboratory. Pragmatic Trials eBook. [cited 2016 March 18th];In rethinking Clinical Trials: A Living Texbook of Pragmatic Clinical Trials. Available from: http://www.crispebooks.org/PragmaticTrialsEbook_Preview/Section-V-11U7-1159S.html.
- 60.Tamura MK, Meier DE. Five policies to promote palliative care for patients with ESRD. Clin J Am Soc Nephrol. 2013;8(10):1783–90. doi: 10.2215/CJN.02180213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holley JL, Davison SN. Advance Care Planning for Patients with Advanced CKD: A Need to Move Forward. Clin J Am Soc Nephrol. 2015;10(3):344–6. doi: 10.2215/CJN.00290115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proctor E, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Proctor EK, et al. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36(1):24–34. doi: 10.1007/s10488-008-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krist AH, et al. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implement Sci. 2013;8:73. doi: 10.1186/1748-5908-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]