Abstract
Purpose
A shift toward an evening circadian preference and the onset of mood problems often occur during adolescence. While these changes are linked to poorer outcomes, few studies have considered how positive and negative affect are related to the circadian rhythm during adolescence. This study examined the relationship between evening and morning affect ratings and dim light melatonin onset (DLMO), a measure of endogenous circadian rhythm. Age and sex were tested as moderators.
Methods
This study is based on a subset of 163 (94 female, age = 14.7) adolescents with an evening circadian preference from an NIMH-funded study. Participants provided saliva for melatonin analysis and rated evening and morning affect.
Results
Higher evening negative affect was related to a later DLMO. Evening positive affect was not significantly related to DLMO timing. Age but not sex was a significant moderator such that higher negative and lower positive affect were related to a later DLMO for 10–13 year olds whereas higher positive affect was related to a later DLMO for 17–18 year olds. DLMO was not significantly related to morning affect ratings.
Conclusions
There is evidence that higher negative and lower positive affect may be related to the shift toward an evening circadian preference observed in adolescents, particularly for younger adolescents.
Keywords: Adolescence, Circadian rhythm, Sleep, Development, Affect
Introduction
Adolescence is a developmental stage associated with change across important domains of life. Adolescence is also a period of increased risk for mental illness, behavioral problems, substance use, and relationship difficulties. Given the potential for long-term consequences, there is a need to identify mechanisms contributing to vulnerability among adolescents. One potential contributor is the shift toward an evening circadian preference that occurs during adolescence and may be triggered by the onset of puberty [1–3]. An evening circadian preference is characterized by physical and mental activity in the evening compared to the morning. An individual’s circadian preference is influenced by genetic variation in circadian polymorphisms (e.g., PER3, CLOCK, or BMAL1) and environmental factors (e.g., light exposure, exercise, or socializing), which are orchestrated by the circadian rhythm oscillator in the suprachiasmatic nucleus (SCN) [4,5]. Approximately 40% of adolescents have an evening circadian preference [6,7]. The onset of puberty in combination with social changes including reduced parental involvement with sleep and evening electronic use may contribute to the shift toward an evening circadian preference in adolescence [8,9]. Sleep behaviors characteristic of an evening circadian preference (e.g., late bedtimes or a large discrepancy between weeknight and weekend sleep patterns) may combine with early morning school start times to contribute to other sleep-related changes observed during adolescence such as inadequate sleep duration and daytime sleepiness [10–12].
An evening circadian preference may be connected to the increase in mood difficulties observed during adolescence [13,14]. Adolescents with an evening circadian preference experience increased depression symptoms compared to adolescents with a morning circadian preference [15,16]. In a large sample of adolescents ages 12–16, an evening circadian preference was also linked to high anxiety symptoms [17]. Adolescents with an evening circadian preference also differ in terms of self-reported mood and affect. Ratings of momentary mood at three time points during the school day indicated that adolescents with an evening preference experience lower mood compared to adolescents with a morning or no circadian preference [18]. Additionally, an experimental sleep deprivation study with adolescents ages 10–16 reported that those with an evening circadian preference experienced less positive affect compared to adolescents with a morning circadian preference [19]. Later bedtime but not short sleep duration has also been prospectively linked to increased emotional distress 6–8 years later [20]. While previous research provides encouraging evidence that mood and affect are related to an evening circadian preference, it has been noted that studies have typically relied upon measures that may reflect mood or affect over the past week or month rather than current affective state [19]. This is potentially problematic given evidence that adolescent mood increases throughout the school day independent of circadian preference as well as evidence that women with high depression symptoms experience increased positive affect in the evening compared to the morning [18,21]. Measuring affect in the evening and morning may help to further elucidate the relationship between circadian preference and positive or negative affect.
An objective measure of the circadian rhythm may also help to further clarify the relationship between affect and circadian changes in adolescents. Melatonin is a hormone secreted by the pineal gland that has a diurnal pattern whereby circulating levels of melatonin remain low during the day, quickly increase in the evening, peak at night, and decrease in the morning. Dim light melatonin onset (DLMO) is an accurate, non-invasive, and reliable measure of the endogenous circadian rhythm [22], and has been validated in adolescents [23,24]. Circulating melatonin levels are a preferred circadian marker because it is comparatively robust and less prone to masking from other external influences compared to measures such as core body temperature, cortisol, and heart rate [24,25]. However, bright light in the evening can suppress, or “mask”, nighttime melatonin production [22], which necessitates its measurement in dim light conditions. DLMO is associated with self-reported sleep and wake parameters for adolescents during both the school year and the summer [23], and a later DLMO is indicative of a delayed circadian rhythm. DLMO may also be connected with affect given that mood ratings are lowest during the morning on school days and early risetimes and shorter time in bed on weekdays are related to higher anxiety [17,18]. Further, reduced melatonin secretion has been observed among individuals ages 12–30 with an affective disorder [26,27]. Despite promising evidence that DLMO and affect may be connected, it remains unclear whether a biological index of the circadian rhythm may also be related to affect among adolescents.
The overall aim of this study was to examine the relationship between affect and DLMO in adolescents with an evening circadian preference, and determine if this relationship is moderated by age and sex. The first aim was to test the hypothesis that higher negative and lower positive affect the night of melatonin collection will be associated with a later DLMO. The second aim was to test the hypothesis that a later DLMO will be related to higher negative and lower positive affect measured the morning following melatonin collection. Given that adolescence is a period of rapid developmental change that occurs at differing rates for males and females, this study will also test if the preceding hypotheses are moderated by age and sex.
Methods
Participants
The 163 participants (94 female and 69 male) for the current study were drawn from a subset of those enrolled in an NIMH randomized controlled trial. A total of 396 participants were assessed for eligibility, and 220 (55.6%) were excluded for not meeting inclusion criteria (n = 154) or refusing to participate (n = 66). 176 participants were enrolled and all provided saliva samples for melatonin assay. Thirteen (7.4%) participants were not included because a DLMO was not observed during the sampling period. Participants were eligible if they scored within the lowest quartile of the Children’s Morningness-Eveningness Preferences Scale (27 or lower); had a 7-day sleep diary showing a sleep onset time of 10:40 pm or later for 10–13 year olds, 11:00 pm or later for 14–16 year olds, and 11:20 pm or later for 17–18 year olds at least 3 nights per week; and had a current pattern of late bedtimes for the last 3 months. These age-group cutoffs reflect developmental changes in sleep [28,29]. Pre–treatment demographic, DLMO, sleep, and affect characteristics are displayed in Table 1. All study procedures were approved by the University of California, Berkeley Institutional Review Board. Informed assent and/or consent was obtained for all participants.
Table 1.
Means, standard deviations, and/or percentages for demographic variables, dim light melatonin onset, affect, and sleep.
| Characteristic | M or N | % or SD |
|---|---|---|
| Age (Years) | 14.7 | 1.8 |
| Age group | ||
| 10–13 years old | 42 | 25.8% |
| 14–16 years old | 92 | 56.4% |
| 17–18 years old | 29 | 17.8% |
| Female | 94 | 57.7% |
| Dim light melatonin onset* | 21.31 | 1.08 |
| Positive and negative affect | ||
| Negative affect (PM) | 2.96 | 1.10 |
| Positive affect (PM) | 2.83 | 1.11 |
| Negative affect (AM) | 1.39 | 0.70 |
| Positive affect (AM) | 2.85 | 1.12 |
| Sleep | ||
| Bedtime | 23.10 | 1.04 |
| Waketime | 7.56 | 0.76 |
| Time in Bed | 524.37 | 67.15 |
| Total Sleep Time | 462.22 | 67.45 |
Decimal hours
Materials and Procedure
Dim light melatonin onset (DLMO)
Melatonin was collected by serial saliva sampling on one night pretreatment in a lab overnight stay. Dim light (<50 lux) was initiated 1 hour before the earliest melatonin onset calculated from the previous week sleep diary [30]. Saliva (1 ml) was collected in 30-minute intervals in dim light using untreated Sarsedt Salivettes (Starstedt, Germany). Light levels were determined using Fisher Scientific Extech Light Meter 407026 (Pittsburgh, PA). Thirteen saliva samples were collected for each participant 5.5 hours before average bedtime and continued to 30 minutes following average bedtime from the previous week determined by sleep diary. The sleep diary was based on the Expanded Consensus Sleep Diary for Morning [31,32]. If participants ingested anything except water before the saliva sample, they rinsed their mouths and brushed their teeth with water. Ingestion of caffeine, fruits, chocolate, non-steroidal anti-inflammatory drugs, and alcohol were prohibited. Saliva samples were centrifuged at 3300 g for 5 minutes. If <1ml saliva yielded after centrifuging, samples were centrifuged for an additional 5 minutes at 3500 g. Samples were frozen and stored at −80 °C and later assayed for melatonin by SolidPhase (Portland, Maine) using radioimmunoassay test kits (APLCO Diagnostics, Windham, NH). Assay sensitivities were 0.3 pg/ml and the minimum detectable dose was 0.05 pg/ml. Mean intra- and inter-assay coefficients of variation (CV) were 7.9 and 9.4%, respectively. DLMO was defined as the interpolated time at which melatonin exceeded 3.0 pg/ml. The selection of this threshold was based upon prior experience with melatonin and the visual inspection of each participant’s DLMO record [33].
Positive and negative affect
Participants were asked “How negative do you feel right now?” and “How positive do you feel right now?” at each saliva collection including an additional affect assessment after the final saliva collection. Responses were provided on a Likert-type scale and included “Very slightly or not at all,” “A little,” “Moderately,” “Quite a bit,” and “Extremely.” These questions were derived from the Positive and Negative Affect Schedule [34], which has high reliability and convergent validity with measures of depression and anxiety [35]. Affect was assessed 14 times in the evening and three times in the morning. Test-retest reliability of these measures was excellent for positive (ICC = 0.96) and negative affect (ICC = 0.94). Internal consistency was good for positive and negative affect (Cronbach’s α = 0.82).
Statistical analysis
Hierarchical linear models (HLM) with restricted maximum likelihood estimation were used to address the aims of this study. This statistical method can appropriately account for the relationships between repeated measurements and does not have the same missing data restrictions of traditional regression or ANOVA analyses. Following the convention described by Aiken and West [36], simple slopes were used to probe significant interactions. Simple slopes for age were examined for ages 10–13, 14–16, and 17–18 to reflect developmental changes in sleep [28,29]. The 14 repeated evening affect measurements and the three repeated morning affect measurements were processed by linear detrend. HLMs included a random intercept for collection time and all analyses controlled for age, sex, and total sleep time (TST).
Results
Evening affect and DLMO timing
The association between affect measured the night of melatonin collection and DLMO was examined first (Table 2). Negative affect was significantly related to DLMO such that higher negative affect was associated with a later DLMO. Positive affect was not significantly related to DLMO.
Table 2.
Coefficient estimates from hierarchical linear models of the relationship between DLMO and affect.
| β | se | z | p | 95% CI | |
|---|---|---|---|---|---|
|
|
|||||
| Predictor: Evening negative affect Outcome: DLMO | 0.008 | 0.002 | 4.91 | <.01 | [0.005, 0.011] |
| Predictor: Evening positive affect Outcome: DLMO | 0.001 | 0.001 | −1.20 | .23 | [−0.004, 0.000] |
| Predictor: DLMO Outcome: Morning negative affect | 0.021 | 0.961 | 0.02 | .98 | [−2.565, 1.140] |
| Predictor: DLMO Outcome: Morning positive affect | −1.365 | 1.536 | −0.89 | .37 | [−4.375, 1.646] |
Note. The z statistic and p values were derived from hierarchical linear models. All analyses also included age, sex, and total sleep time (TST) as covariates.
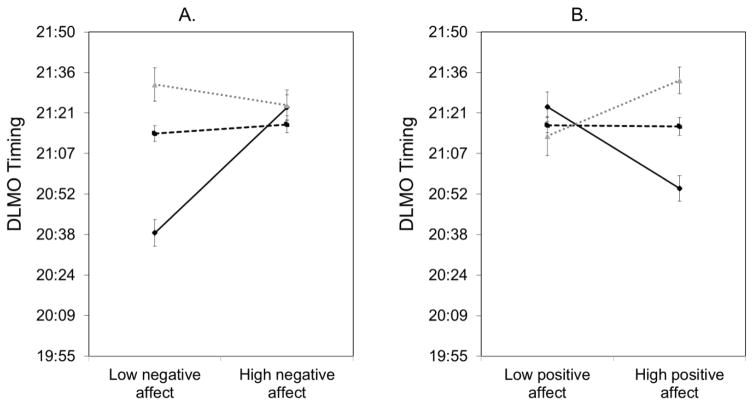
Age and sex were examined as moderators of the relationship between affect measured the night of melatonin collection and DLMO. The three-way interaction between age, sex, and negative affect was significantly related to DLMO (Table 3). Post-estimation parameter tests indicated that the two-way interaction between age and negative affect was significant whereas the interaction between sex and negative affect was non-significant (Table 3). Hence, simple slopes were examined for age group but not stratified by gender. For adolescents 10–13 years old, higher negative affect was related to a later DLMO (Figure 1A, Table 3). The slope for negative affect and DLMO for older adolescents (i.e., 14–16 years old or 17–18 years old) was not significant (Figure 1A, Table 3). The slope for 10–13 year olds was significantly different compared to 14–16 year olds, z = 5.39, p < .01, and 17–18 year olds, z = 4.98, p < .01.
Table 3.
Coefficient estimates from hierarchical linear models of the moderating effect of age, sex, and evening affect on DLMO or age, sex, and DLMO on morning affect.
| β | se | z | p | 95% CI | χ 2 | p | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Evening negative affect x age x sex (outcome: DLMO) | 13.39 | <.01 | |||||
| Evening negative affect x age | 31.58 | <.01 | |||||
| 10–13 years old | 0.022 | 0.003 | 6.88 | <.01 | [0.016, 0.028] | ||
| 14–16 years old | 0.002 | 0.002 | 0.77 | .44 | [−0.002, 0.006] | ||
| 17–18 years old | −0.004 | 0.004 | −0.89 | .37 | [−0.012, 0.004] | ||
| Evening negative affect x sex | 0.31 | .57 | |||||
| Evening positive affect x age x sex (outcome: DLMO) | 8.21 | .02 | |||||
| Evening positive affect x age | 18.79 | <.01 | |||||
| 10–13 years old | −0.009 | 0.002 | −4.25 | <.01 | [−0.013, −0.005] | ||
| 14–16 years old | 0.000 | 0.001 | −0.11 | .91 | [−0.003, 0.002] | ||
| 17–18 years old | 0.006 | 0.003 | 2.46 | .01 | [0.001, 0.011] | ||
| Evening positive affect x sex | 0.01 | .92 | |||||
| DLMO x age x sex (outcome: morning negative affect) | 0.96 | .62 | |||||
| DLMO x age x sex (outcome: morning positive affect) | 0.72 | .70 | |||||
Note. The χ2 statistic and corresponding p value were derived from the post-estimation parameter test of two- and three-way interactions. The z statistic and p values were derived from simple slopes. All analyses also included age, sex, and total sleep time (TST) as covariates.
Figure 1.
Simple slopes of the moderating effect of age on the relationship between (A) evening negative affect and DLMO timing and (B) evening positive affect and DLMO timing.
The interaction between age, sex, and positive affect was also significantly related to DLMO (Table 3). Similarly, post-estimation parameter tests only indicated that the two-way interaction between age and positive affect was significant, but the interaction between sex and positive affect was non-significant (Table 3). For adolescents 10–13 years old, lower positive affect was associated with a later DLMO (Figure 1B). However for 17–18 years old adolescents, higher positive affect was associated with a later DLMO (Figure 1B). The slope for 14–16 year olds was not significant. The slope for 10–13 year olds was significantly different compared to 14–16 year olds, z = –3.57, p < .01, and 17–18 year olds, z = −4.62, p < .01. The slope for 17–18 year olds was also significantly different compared to 14–16 year olds, z = −2.23, p = .03.
DLMO timing and morning affect
The relationship between DLMO and affect measured the morning following melatonin collection was also examined (Table 2). The main effect of DLMO on negative or positive affect the morning following melatonin collection was not significant. The interaction between age, sex, and DLMO was not significantly related to negative or positive affect the morning following melatonin collection (Table 3).
Discussion
The present study was designed to examine the relationship between affect and DLMO among adolescents with a self-reported evening circadian preference, and also evaluate if this relationship is moderated by age and/or sex. We examined the association between evening affect and DLMO and found partial support for our first hypothesis. Higher reported negative affect but not positive affect was associated with a later DLMO timing. These findings are consistent with other research that indicates that adolescents with an evening circadian preference experience increased symptoms of depression and less positive mood and affect compared to adolescents with a morning circadian preference [15,18,19]. We did not find support for our second hypothesis. The relationship between DLMO timing and affect ratings the following morning was not significant. The protocol for this study involved sleeping in a controlled laboratory environment, which may have disrupted participants’ typical routine and negatively influenced morning affect. While it is possible that DLMO timing may be less related to morning affect, it may also be the case that the influence of the study protocol on morning affect made it difficult to observe this relationship. Future studies should examine DLMO timing and morning affect in a free-living environment.
Moderator analyses highlighted the importance of age in relation to affect and DLMO. Age but not sex was a significant moderator of the relationship between evening affect and DLMO timing. Analyses indicated that these effects may be specific to younger adolescents (ages 10–13). Compared to older adolescents, younger adolescents experienced a later DLMO in the context of higher negative and lower positive affect. Previous research has indicated that the tendency towards an evening circadian preference and the development of mood difficulties increases during the years of adolescence [1–3,13,14]. The present study may provide preliminary evidence that greater negative and lower positive affect are related to a biological index of the circadian rhythm. Given evidence that the circadian rhythm of younger adolescents may be more malleable than older adolescents [37], future experimental studies are needed to test whether the circadian rhythm is responsive to affective state, above and beyond more powerful environmental circadian cues such as light exposure.
Although these findings provide evidence that affect and a measure of the endogenous circadian rhythm are related, there are limitations to this study. First, this study only included adolescents with a self-reported evening circadian preference, which may provide a restricted range for DLMO and limit the generalizability of these findings. The average DLMO for this study was 21:19 (SD = 68.4 minutes), which is comparable to other studies that have examined DLMO in adolescents. These studies have reported DLMOs of 21:17 for adolescents ages 14.8–17.8 [24], 20:42 for adolescents ages 9–12 [23], 21:09 for adolescents ages 13–16 [23], and a range of 20:32–21:53 for adolescents ages 9–18 [38]. Despite similar DLMO timings, future studies may benefit from recruiting adolescents across a broader range of self-reported circadian preference. Second, affect was measured with two questions that targeted valence, which may have missed other important features of affect that could influence sleep such as arousal. Indeed, we also observed that higher evening positive affect was related to a later DLMO for 17–18 year old adolescents, which may reflect high arousal positive affect. Future studies should consider how affect valence and arousal are related to DLMO among adolescents. Third, although a strength of this study was using repeated ratings of current affect, the present study was not designed for causal inference. Experimental studies that utilize an affect induction or manipulate evening melatonin levels may help to specify the directionality of these effects. Finally, puberty is a biological event that occurs during adolescence, and is associated with a shift toward an evening circadian preference as well as the onset of mood difficulties [1–3,13,14]. Although analyses did include correlates of puberty such as age and sex, future research should examine the role of pubertal status in the relation between affect and DLMO timing.
In sum, the current study provides support for the hypothesis that greater negative and lower positive affect are related to a later DLMO in adolescents with a self-reported evening circadian preference. This relationship was moderated by age, and suggested that these relationships are stronger for younger compared to older adolescents. Results did not support the hypothesis that DLMO timing is related to morning affect. Adolescence is a developmental stage associated with increased risk for mood difficulties and sleep problems, which is compounded by competing social and environmental demands such as early morning school start times, extracurricular activities, and peer relationships [9,12]. Future studies should examine how the association between affect and DLMO timing is influenced by the adolescent social environment.
Implications and Contribution.
Adolescence is associated with sleep changes and the onset of mood difficulties. This study examined if ratings of affect were associated with a measure of the endogenous circadian rhythm. Findings suggested that lower evening affect may be related to the shift toward an evening circadian preference, particularly for younger adolescents.
Acknowledgments
The authors would like to thank James Wyatt for his guidance regarding DLMO analysis. The authors are also grateful to the following team members for their assistance with project set-up and project co-ordination: Kerrie Hein, Lulu Dong, Sophia Rabe-Hesketh, Nicole B. Gumport, Jennifer Kanady, Stephen P. Hinshaw, Jennifer S. Silk, Rita L. Smith, Monique A. Thompson, Nancee Zannone, Daniel Jin Blum, Emily M. Clark, Brenden Mei, Xin Zhao, Leah M. Miller, Lauren Asarnow, O’Min Kwon, Shay K. O'Brien, Aaron T. Daley, Armando Martinez, Eve Fine, Elizabeth McCoy, Davin Duval, Chia Okwu , Annie Liang, Caitlin Eggleston, Deidre Abrons, Cynthia Oei, Ania Foster, Elizabeth Mason, Adriane Soehner, Emily Pfannenstiel, and Jane Chen. This work was supported by the National Institutes of Health [grant numbers R01HD071065 (AGH) and T32MH020006 (MRD)].
Abbreviations
- DLMO
dim light melatonin onset
- SCN
suprachiasmatic nucleus
- TST
total sleep time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 2.Roenneberg T, Kuehnle T, Pramstaller PP, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Borbély AA, Daan S, Wirz-Justice A, et al. The two-process model of sleep regulation: A reappraisal. J Sleep Res. 2016;25:131–43. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 5.Borbély AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1:205–10. [PubMed] [Google Scholar]
- 6.Tonetti L, Fabbri M, Natale V. Sex difference in sleep-time preference and sleep need: A cross-sectional survey among italian pre-adolescents, adolescents, and adults. Chronobiol Int. 2008;25:745–59. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- 7.Carskadon MA, Acebo C, Richardson GS, et al. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 8.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11:735–42. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Dahl RE, Lewin DS. Pathways to adolescent health: Sleep regulation and behavior. J Adolesc Heal. 2002;31:175–84. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- 10.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Hansen M, Janssen I, Schiff A, et al. The Impact of School Daily Schedule on Adolescent Sleep. Pediatrics. 2005:115. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- 12.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–47. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankin BL, Abramson LY, Moffitt TE, et al. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107:128–40. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 14.Newman DL, Moffitt TE, Caspi A, et al. Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol. 1996;64:552–62. [PubMed] [Google Scholar]
- 15.Gau SS-F, Soong W-T, Merikangas KR. Correlates of sleep-wake patterns among children and young adolescents in Taiwan. Sleep. 2004;27:512–9. [PubMed] [Google Scholar]
- 16.Fares S, Hermens DF, Naismith SL, et al. Clinical correlates of chronotypes in young persons with mental disorders. Chronobiol Int. 2015;32:1183–91. doi: 10.3109/07420528.2015.1078346. [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Morales JF. Anxiety during adolescence: Considering morningness-eveningness as a risk factor. Sleep Biol Rhythms. 2015;14:141–7. [Google Scholar]
- 18.Díaz-Morales JF, Escribano C, Jankowski KS. Chronotype and time-of-day effects on mood during school day. Chronobiol Int. 2015;32:37–42. doi: 10.3109/07420528.2014.949736. [DOI] [PubMed] [Google Scholar]
- 19.Dagys N, McGlinchey EL, Talbot LS, et al. Double trouble? The effects of sleep deprivation and chronotype on adolescent affect. J Child Psychol Psychiatry. 2012;53:660–7. doi: 10.1111/j.1469-7610.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asarnow LD, McGlinchey E, Harvey AG. The Effects of Bedtime and Sleep Duration on Academic and Emotional Outcomes in a Nationally Representative Sample of Adolescents. J Adolesc Heal. 2014;54:350–6. doi: 10.1016/j.jadohealth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray G. Diurnal mood variation in depression: A signal of disturbed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Lewy AJ. Circadian rhythms and mood disorders: a guide for the perplexed. J Clin Psychiatry. 2015;76:e662–4. doi: 10.4088/JCP.14com09716. [DOI] [PubMed] [Google Scholar]
- 23.Crowley SJ, Acebo C, Fallone G, et al. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep. 2006;29:1632–41. doi: 10.1093/sleep/29.12.1632. [DOI] [PubMed] [Google Scholar]
- 24.Crowley SJ, Suh C, Molina TA, et al. Estimating the dim light melatonin onset of adolescents within a 6-h sampling window: the impact of sampling rate and threshold method. Sleep Med. 2016;20:59–66. doi: 10.1016/j.sleep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Robillard R, Naismith SL, Rogers NL, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: Comparison of unipolar and bipolar phenotypes. 2013;28 doi: 10.1016/j.eurpsy.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Naismith SL, Hermens DF, Ip TKC, et al. Circadian profiles in young people during the early stages of affective disorder. Transl Psychiatry. 2012;2:e123. doi: 10.1038/tp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national United States sample. J Adolesc Heal. 2014;54:691–7. doi: 10.1016/j.jadohealth.2013.10.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carskadon MA. Adolescent Sleep Patterns. Biological, Social, and Psychological Influences. 2004;37 [Google Scholar]
- 30.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 31.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29:1075–80. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Positive and negative affect schedule (PANAS) J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 35.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–65. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 36.Aiken LS, West SG. Multiple regression. Thousand Oaks; 1991. [Google Scholar]
- 37.Crowley SJ, Cain SW, Burns AC, et al. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J Clin Endocrinol Metab. 2015;100:4067–73. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley SJ, Van Reen E, LeBourgeois MK, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]