Abstract
According to the latest report from the World Health Organization, an estimated 265,000 deaths still occur every year as a direct result of burn injuries. A widespread range of these deaths induced by burn wound happens in low- and middle-income countries, where survivors face a lifetime of morbidity. Most of the deaths occur due to infections when a high percentage of the external regions of the body area is affected. Microbial nutrient availability, skin barrier disruption, and vascular supply destruction in burn injuries as well as systemic immunosuppression are important parameters that cause burns to be susceptible to infections. Topical antimicrobials and dressings are generally employed to inhibit burn infections followed by a burn wound therapy, because systemic antibiotics have problems in reaching the infected site, coupled with increasing microbial drug resistance. Nanotechnology has provided a range of molecular designed nanostructures (NS) that can be used in both therapeutic and diagnostic applications in burns. These NSs can be divided into organic and non-organic (such as polymeric nanoparticles (NPs) and silver NPs, respectively), and many have been designed to display multifunctional activity. The present review covers the physiology of skin, burn classification, burn wound pathogenesis, animal models of burn wound infection, and various topical therapeutic approaches designed to combat infection and stimulate healing. These include biological based approaches (e.g. immune-based antimicrobial molecules, therapeutic microorganisms, antimicrobial agents, etc.), antimicrobial photo- and ultrasound-therapy, as well as nanotechnology-based wound healing approaches as a revolutionizing area. Thus, we focus on organic and non-organic NSs designed to deliver growth factors to burned skin, and scaffolds, dressings, etc. for exogenous stem cells to aid skin regeneration. Eventually, recent breakthroughs and technologies with substantial potentials in tissue regeneration and skin wound therapy (that are as the basis of burn wound therapies) are briefly taken into consideration including 3D-printing, cell-imprinted substrates, nano-architectured surfaces, and novel gene-editing tools such as CRISPR-Cas.
Keywords: Burn wound infection, Wound healing, Topical treatment, Nanomedicine, Nanoparticles, Stem cells, Stimulus-responsive drug delivery, Growth factors, Gene therapy, CRISPR, 3D printing, Cell-Imprinting
Graphical Abstract
1. Introduction
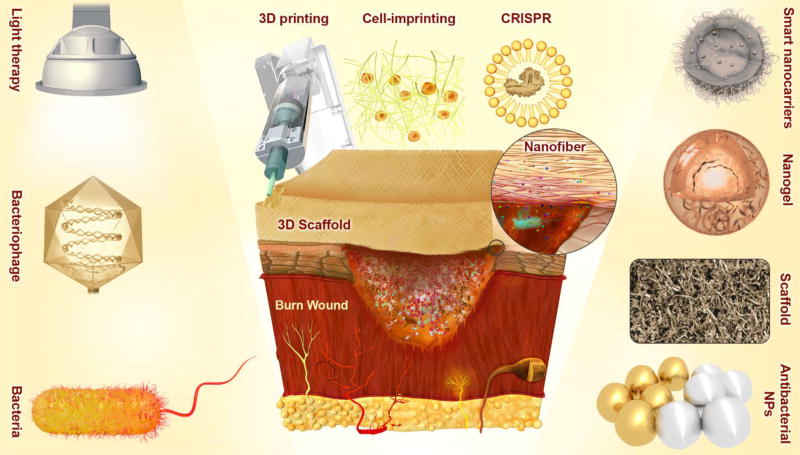
Burns are generally defined as skin wounds caused by thermal/heat exposure (e.g. fire, hot liquids, solids or gases), electricity, chemical materials (e.g. strong bases or strong acids), radiation exposure (e.g. ultraviolet light, ionizing radiations such as X-ray, microwaves, etc.), and so forth. The ultimate therapeutic goal is to prevent and treat infection, while encouraging satisfactory healing that preserves function [1]. In accordance with the latest report from the World Health Organization, about 265,000 deaths occur due to thermal burn wounds each year; these fatalities are especially common in low-to-middle-income countries, and particularly in the South-East Asia Region [2]. In India alone, over 1,000,000 people are inflicted with moderate or severe burns every year. More than 88% of these burns are caused by flames or contact with hot or boiling liquids. Patients with severe burns over a significant proportion of the body surface have received a potentially devastating injury, and need immediate skillful emergency care to minimize mortality, and will often require long-term rehabilitation and recovery to prevent the occurrence of crippling scars and other morbidity. The skin surface has a pivotal role in the maintenance of body fluid homeostasis, thermoregulation, serves as a barrier against the outside world (in particular excluding pathogenic microorganisms), and regulates many metabolic processes [3]. The skin is also the body’s largest and most active immune organ [4]. Thermal injury is a complex process that needs advanced methods to prevent burn infection, and the application of effective antimicrobial therapy if infection has taken place (as it most often does), together with strategies to stimulate wound healing and prevent scarring. Initially burned patients should be provided with fluid resuscitation, pulmonary care, nutritional support, as well as antimicrobial control as emergency measures [5]. The survival of thermally burned victims may critically depend on exactly what is done, particularly to the burn sites themselves [6]. Remarkable advances have been obtained in wound dressing platforms, advent of new antimicrobial agents, and recommendations have been made for early full thickness burn excision and grafting. Nevertheless studies still are ongoing to improve the wound healing process and its effectiveness. Most efforts have been conducted to boost the antimicrobial activity of a broad range of topically applied therapeutic agents, facilitating disinfection in wound sites, as well as improving wound healing processes and tissue regeneration mechanisms [7, 8]. Some conventional treatments are based on application of topical antimicrobial substances such as combinations of topical antibiotics, povidone-iodine, silver-sulfadiazine, chlorhexidine, mafenide acetate and such like. Newer avenues that are being explored are immune-based antimicrobial molecules (polypeptides like defensins) and therapeutic microorganisms (probiotics and bacteriophages). In addition, remarkable results have recently been reported for a range of light therapies using NIR, visible light (blue or green); ultraviolet light, and the combination of light with photosensitizing dyes [9]. Moreover, some other innovative approaches such as stem and other types of cell therapy have led to significantly improved wound healing. In recent years, despite the noticeable progress, the relatively new areas of nanomedicine and nanotechnology in general, have been strongly pressed into service against burn infections. In this regard, nanostructures (NSs) have been developed as advanced delivery vehicles for: cell therapies, growth factors, gene therapy vectors, advanced antimicrobial agents, and agents that will stimulate wound healing. Moreover, a whole new arsenal of smart stimulus-responsive delivery vehicles and nano-robots have emerged using nanostructured platforms e.g. films, 3D-scaffolds, nanoparticles (NPs) (activated by e.g. light, enzymes, pH, ultrasound, etc.), photoactivated NS-based approaches. The most-often utilized NSs platforms for wound healing and burn reconstruction can be categorized into nanofibers, NPs, films and 3D-scaffolds. Figure 1 schematically illustrates several important conventional, developing or innovative approaches for wound healing and burn infection inhibition, which have all been under investigation in recent years.
Figure. 1.
Schematic illustration of important conventional, developing or innovative approaches, (such as light therapy; therapeutic microorganisms e.g. bacteriophages and bacteria with bactericidal effects; antibacterial NPs or NPs with therapeutic effects e.g. silver NPs and gold NPs; innovative nanocarriers e.g. smart nanocarriers and nanogels; novel scaffolds for wound dressing, (stem) cell therapy and regenerative medicine, therapeutic (drug, growth factor, gene, etc.) delivery, and so forth; and innovative technologies e.g. 3D-printing, cell-imprinting, and CRISPR-Cas9 gene editing), which have been the subject of various researches, conducted with the aim of wound healing and prevention of infection in burns.
Animal models are critically important for laboratory investigations into burn wound healing, as in vitro studies can only go so far, and there is a reluctance to test nanotechnology-based products directly on human burn victims. These models will allow better understanding of wound-associated processes and mechanisms, which then can lead to substantial advancements in burn injury therapies.
2. Skin structure and the burn wound healing process
An understanding of normal skin physiology, pathogenesis of burns, as well as appreciation of infections that develop in burns (with different classes of microorgansism arriving at different stages of burn development) are all important for understanding the role of nanotechnology in burn wounds.
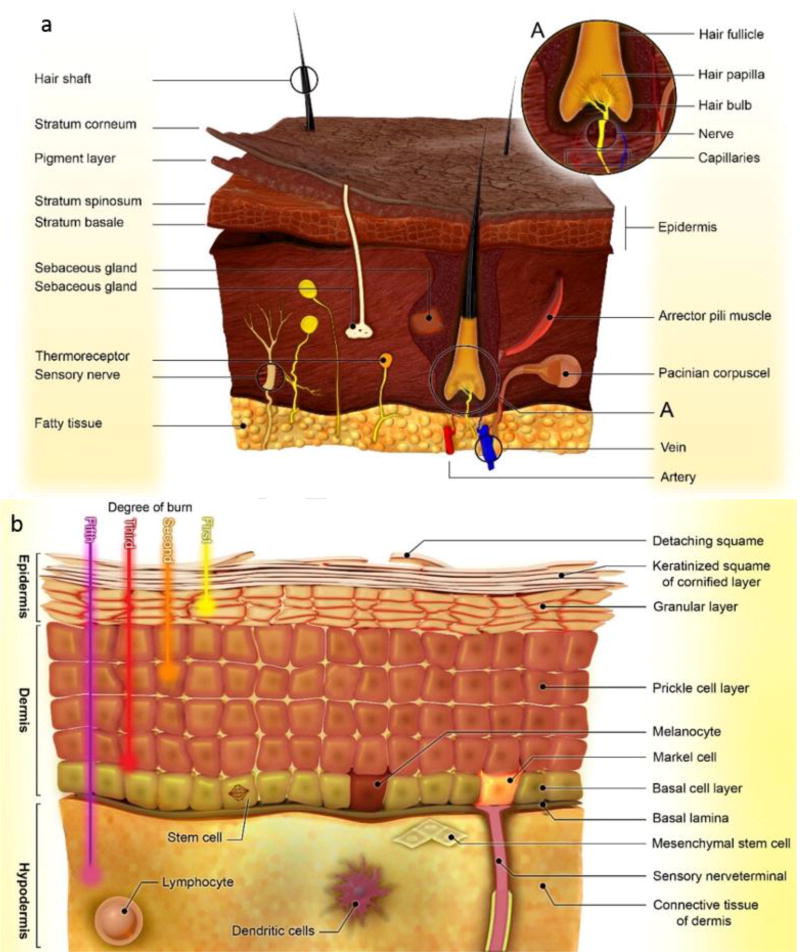
Generally, the skin is composed of three distinct layers (Figure 2-a) including the epidermis, dermis and hypodermis [10]. The epidermis prevents the entry of virulent microorganisms, as maintaining body hydration. The five layers (called stratums) consisting of: basale, spinosum, granulosum, lucidum, and corneum form the epidermis layers of skin, respectively. [11]. These layers are made from diverse immune and non-immune cell types such as keratinocytes, melanocytes, merkel cells, langerhans cells, CD8+ T cells, corneocytes and stem cells [11–13]. The basal layer is the proliferative portion of the epidermis producing keratinocytes that can go on to differentiate into the spinous layer, granular layer as well as outermost stratum corneum layers. The skin of the palms and soles has an additional stratum lucidum or clear layer. Stem cells residing in the basal epidermis are responsible for continually replacing keratinocytes that are normally lost by exfoliation, as well as for regenerating wounded skin [14]. The dermis layer is a layer between the hypodermis and epidermis, which is largely made of collagen protein, blood vessels (arterioles, venules, capillaries) sweat glands, hair roots, nerve cells, lymphatic vessels and mesenchymal stem cells (MSCs) [15]. The primary function of the dermis is to provide structural toughness to the skin. The differentiation of dermal MSCs cultured in the laboratoryinto chondrocytes,, osteocytes, adipocytes,, smooth muscle cells, hematopoietic cells, neurons and glia have been reported [16]. The hypodermis or subcutaneous fat, is comprised of adipocytes, macrophages, vasculature, nerves and fibroblasts, and its function is to support and anchor the dermal and epidermal layers [17]. Figure 2 schematically illustrates the structure of skin (Figure 2-a), and its different constituent layers and cells (Figure 2-b).
Figure 2.
Schematic illustration of: a) the structure of skin, b) layers of skin including epidermis, dermis and hypodermis, and their constituent cells and sub-layers.
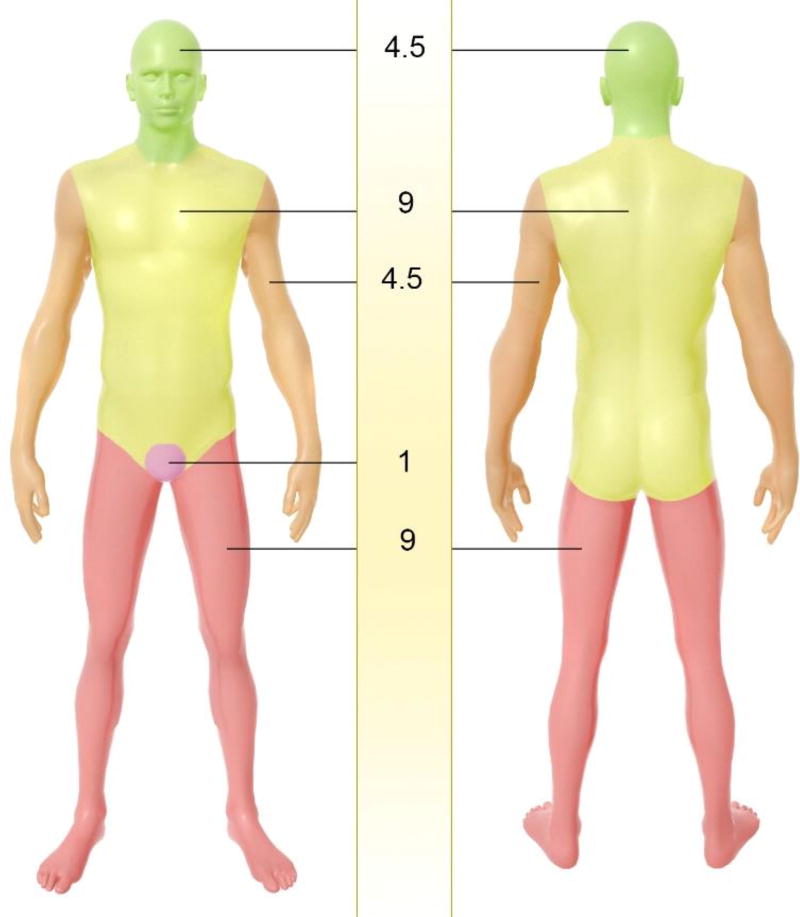
Classification of the severity of burn injuries usually is evaluated using two criteria; assessment of the depth of the thermal damage, and an estimation of percentage of total body surface area (TBSA) affected by a burn injury. In the first method burn wounds are classified into four degrees (Figure 2-a). First degree burns that involve only the epidermis display redness, pain at the site of damage, and dryness. Second degree burns can affect either papillary layer (i.e. the upper dermis) or reticular layer (i.e. the deeper dermis) with a white or yellow color, blistering of the skin, and a moist appearance. In third-degree burns, there is full thickness destruction of both epidermis and dermis, and a stiff or leathery consistency. Scarring and contractures will develop after healing. Fourth-degree burns cause catastrophic damage in underlying tissues, muscle, tendons, ligaments and even bone [18]. As mentioned, burns are also assessed in terms of percentage of TBSA. The rule of nines in the affected area is a quick way to estimate the percent TBSA affected (Figure 3) [3, 5, 19]. More precise assessment can be evaluated by Lund & Browder charts where diverse proportions of body parts in children and adults are accounted for; e.g. burn wounds greater than 10% in children, have the same severity as 15% for adults. This is important because hypovolemic shock can be potentially life threatening. When about 25% of the body area is affected by burns, loss of fluid from the damaged microvessels produces edema and hypoproteinemia. The volume of fluid replacement with isotonic crystalloid solution (Ringer’s lactate) can be calculated by the Parkland formula: V(mL) = 4 × body weight(kg) × %TBSA [7, 20].
Figure 3.
Assessment of skin burn in terms of total body surface area (TBSA) percent, using the rule of nines in applied area, thus to quickly estimate the affected TBSA percent.
The final goal in burn injury healing is the rapid replacement of normal skin with minimal scarring. The healing of burn damage is a biological process including four overlapping phases (Table 1), homeostasis phase, inflammatory phase, proliferation phase and remodeling phase [21].
Table 1.
Cellular and molecular events in normal wound healing
| Phases | |
|---|---|
| Homeostasis | Platelet aggregation, immunity activation, blood clotting and complement system induction |
| Inflammination | Recruitment of neutrophils and macrophages as well as ECM molecule production by resident cells in the skin |
| Proliferation | Re-epithelialization; expansion of keratinocytes, epithelial cells, stem cells and fibroblasts |
| Angiogenesis; activation of endothelial cells and creation of a site in which these cells can proliferate in the wound | |
| Granulation; the promotion of tissue granulation by fibroblasts, granulocytes and macrophages | |
| Remodeling | Production of collagen and elastin as well as fibroblasts maturing to myofibroblasts in the presence of T cells and macrophages |
2.1 Homeostasis phase
About 10-min after the thermal insult occurs, the initial phase commences as an autonomic response to attempt to minimize the damage. In this phase, several phenomena occur including platelet aggregation, immune activation, blood clotting and complement system activation. Here, the blood clot encompasses vitronectin, fibronectin, fibrin and thrombospondins, which supply a scaffold-like matrix for the migration of fibroblasts leukocytes, keratinocytes and endothelial cells as well as causing the accumulation of growth factors [15].
2.2 Inflammatory phase
This occurs 1–3 days after the infliction of the burn wound. This phase can be divided into the early phase with neutrophils arriving in the first days and a late phase; 3 days after the initiation of damage, when monocytes transform into macrophages. Neutrophils produce tumor necrosis factor (TNF-α), interleukin-1 (IL-1) and (IL-6) that activate the inflammatory response and stimulate vascular endothelial growth factor (VEGF) and IL-8 secretion to repair blood vessels, and macrophages produce transforming growth factors (TGF-α, and TGF-β), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF) and VEGF, to stimulate cell expansion and migration and the extracellular matrix (ECM) generation molecules by resident cells of the skin. Adaptive immunity takes at least 4 days after the damage initiation involving lymphocytes (T-cells and B-cells) [22, 23].
2.3 Proliferation phase
Following the inflammatory response, a coordinated proliferation phase occurs, which includes three steps; re-epithelialization, angiogenesis and formation of granulation tissue. The proliferation phase occurs 3–10 days after the initiation of injury. Re-epithelialization is induced by activation of cytokines and growth factors including insulin-like growth factors (IGF-1), nerve growth factor (NGF), epidermal growth factor (EGF), and keratinocyte growth factor (KGF), which cause expansion of keratinocytes, epithelial cells, stem cells and fibroblasts. Keratinocytes, fibroblasts and fibroblast-derived macrophages immigrate into the wound and expand during the regeneration of the burn-damaged tissue. Some fibroblasts differentiate into myofibroblasts; then the fibroblasts and myofibroblasts manufacture ECM, particularly collagen that produces the mature scar [15, 24]. In the proliferation phase, new blood vessels are created (angiogenesis). In this phase, several growth factors are involved such as VEGF, PDGF, FGF-β, granulocyte-macrophage colony-stimulating factor (GM-CSF) and thrombin, which are the foremost activating molecules for endothelial cell growth. The proteolytic enzymes secreted by the activated endothelial cells secrete dissolve the basal lamina and create an environment in which they can proliferate and immigrate into the wound [15, 25]. The final step of the proliferation phase is the production of granulation tissue, which can be specified by the presence of many fibroblasts, granulocytes and macrophages. The main cells in the granulation phase are fibroblasts that produce collagen and other ECM molecules. The ECM supplies an appropriate scaffold for cell adhesion, and organizes the growth and differentiation of the cells including fibroblasts. At the end of this phase, the differentiation of the fibroblasts into myofibroblasts occurs (forming a scar) or else undergo apoptosis [26, 27].
2.4 Remodeling phase
This final step of wound healing is called the remodeling phase. During this step, wound remodeling initiates 2–3 weeks after the burn damage, and continues for as long as a year or more; here the wound scar produces more collagen and elastin, and also fibroblasts mature into myofibroblasts. Beside fibroblast transformation, death of keratinocytes and inflammatory cells (such as T cells and macrophages) play a vital role in the ending of the response to the injury [15, 28].
Topical methods have been conventionally used as treatments for burns. Many of these are traditional disinfectants and antiseptics such as povidone-iodine, chlorhexidine, combinations of topical antibiotics, silver-sulfadiazine, and mafenide acetate. In the following sections, we will cover more innovative approaches such as immune-based antimicrobial molecules, nanotechnology based antimicrobial agents, therapeutic microorganisms, and antimicrobial phototherapy and ultrasound therapy, and stem cell-based treatments for therapy of burn infection and burn wound healing. Since many of these agents are intrinsically cytotoxic towards bacteria, fungi and other microorganisms, a clear concern that must be addressed is whether they will damage host cells in the burn site, or maybe adversely affect wound healing Table 2.
Table2.
Topical methods for prevention and treatment of burn infection and wound healing
| Biological-based approaches for burn wounds healing: |
|
|
| - Immune-based antimicrobial molecules |
|
|
| Antimicrobial peptides (AMPs) |
| Passive immunotherapy |
|
|
| - Reactive oxygen species and nitric oxide generators |
|
|
| - |
|
|
| - Antimicrobial agents |
|
|
| Metallic elements |
| Biopolymeric agents |
|
|
| - Therapeutic microorganisms |
|
|
| Antimicrobial light and ultrasound-based wound therapy: |
|
|
| Phototherapy |
| Shockwave and ultrasound based-therapy |
|
|
| Stem cell based-burn therapy |
|
|
| Burn wound infection therapy using nanostructures (NSs) and NPs |
3. Biological-based approaches for burn wound healing
Some conventional treatments are based on application of topical antimicrobial substances such as combinations of topical antibiotics, povidone-iodine, silver-sulfadiazine, chlorhexidine, mafenide acetate and such like. Newer avenues that are being explored are immune-based antimicrobial molecules (polypeptides like defensins) and therapeutic microorganisms (probiotics and bacteriophages).
3.1 Immune-based antimicrobial molecules
3.1.1 Antimicrobial peptides (AMPs)
AMPs are naturally or synthetically generated cationic polypeptide molecules, which include some specific amino acids that produce an overall amphipathic conformation [29, 30]. These molecules can be naturally produced by many different organisms including bacteria, amphibians, insects and mammals and an active research effort in screening and discovery is still underway. AMPs are also produced by a diverse range of cell types well found in the skin, mucosal surfaces and immune cells. Synthetic approaches are also available for generation of AMPs. AMPs as antimicrobial agents are able to destroy an wide range of microorganisms. Their antimicrobial mechanism of action operates via attaching to and disrupting the negatively charged microbial cell membrane as well as suppressing the generation of important molecules of microorganisms consisting of proteins and nucleic acids, thus inhibiting the microorganism growth. Hence, they can be applied for the treatment of burn infections [29, 31–33].
Various natural AMPs such as indolicidin and ranalexin have been introduced as potential antimicrobial agents to treat infectious diseases [34, 35]. Cathelicidins (a member of the AMP family) are found in lysosomes of macrophages, polymorphonuclear leukocytes (PMNs), and keratinocytes. They are pivotal molecules for immune responses to microbial infections. They have broad-spectrum antimicrobial effects against fungi, viruses, and Gram-positive and Gram-negative bacteria. These small peptides kill virulent pathogens by destroying their cell membrane, and can neutralize the biological activity of their endotoxins [36]. Moreover, they can adhere to membrane receptors of immune cells such as toll-like receptors (TLRs) on antigen-presenting cell including monocytes, macrophages, and dendritic cells, as well as non-immune cells such as epithelial cells and keratinocytes. Cathelicidins and their derivative peptides such as LL-37 can be used topically to treat burn wound infections [37, 38]. Lactoferrin is an AMP, and binds to iron in a similar manner to transferrin (serum carrier of iron). This peptide exists in neutrophil granules, milk, tears, saliva, sweat, nasal secretions, and the genital tract. It exerts antibacterial and antifungal activity through microbial membrane disruption as well as iron sequestration, thus repressing microorganism growth [39–41].
Synthetically-obtained AMPs have been also investigated for their enhanced antimicrobial activity. In an investigation Zhong et al. developed β-sheet SAMPs including IK8D, IK8L, and IK8-2D to inhibit multidrug-resistant P. aeruginosa, the most common Gram negative bacteria isolated from burn wound infections of humans. These SAMPs were applied for treatment of P. aeruginosa-infected burn wounds in mice. Findings revealed that among the SAMPs, IK8L could significantly inhibit P. aeruginosa, and its successive administration did not cause drug resistance [31]. Recently, Mohamed et al. evaluated the antibacterial activity of SAMPs for treatment of MRSA-induced infection, one of the most virulent microorganisms in human wounds. Herein, the antibacterial function of two topically applied SAMPs including WR12 (consisting of a 12 residue peptide of arginine and tryptophan) and D-IK8 (made of an 8 β-sheet peptide structure) against MRSA was evaluated. These SAMPs were found to inhibit MRSA in both stationary and intracellular bacterial growth phases compared to the control groups (i.e. linezolid and vancomycin) in vivo, as well eradication of S. aureus and S. epidermidis in vitro. The in-vivo evaluation indicated both the SAMPs regulated the immune system and decreased generation of pro-inflammatory cytokines e.g. TNF-α and IL-6 [42]. In another study, charge- and structure-manipulated SAMPs showed enhanced antimicrobial activity and immunomodulatory ability. Herein, a pendant aromatic group was added to the SAMP sequence to achieve an enhanced antibacterial activity against Gram negative bacteria e.g. E. coli. The SAMP with the addition of 6 charges and a naphthalene ring showed increased selectivity against both Gram negative- and Gram positive bacteria e.g. E. coli and S. aureus. The regulation of macrophage immunomodulatory function with and without a TLR agonist (i.e. lipopolysaccharide) as well as the generation of neutrophil chemoattractant activity were also demonstrated [43].
3.1.2 Passive immunotherapy
This approach is based on humanized and chimerized forms of specific monoclonal antibodies (mAb) generated by engineering transgenic mice and cultured hybridoma cells. For example, antibodies against the Pseudomonas aeruginosa lipopolysaccharide O-side chain structure can be produced in transgenic mice. Other examples of passive immunotherapeutics are flagellin-binding antibodies that recognize; bacterial flagellins. These structures are essential virulence factors responsible for the rapid movement of bacteria and facilitate bacterial invasion into tissue. High titer of mAbs such as anti-flagellin mAb could be used as an effective molecular approach to diminish mortality and morbidity induced through burn wounds with P. aeruginosa infection [44–46]. Exopolysaccharide (EPS) of P. aeruginosa is biosynthesized by the polysaccharide synthesis locus (Psl) and includes galactose- and mannose-rich molecules. The psl gene cluster consists of 15 genes encoding proteins capable for EPS synthesis, which is considered to be an essential factor for bacterial biofilm formation [47]. Another approach uses antibodies that bind to P. aeruginosa V-antigen (PcrV). PcrV is involved in the type III toxins secretion system (TTSS) that allows toxins to be released from the cells. PcrV is a structural protein and hydrophilic translocator of TTSS, which has a pivotal role in bacterial injection into host cells in order to initiate an infection [48, 49]. PcrG is also a cytoplasmic regulator that can interact with the intramolecular coiled-coil region of PcrV protein and regulates TTSS [50]. Passive mAb treatment against PcrV, so-called PcrV immunization, enhances survival rate in murine burn models infected with P. aeruginosa [51, 52].
3.2 Reactive oxygen species and nitric oxide generators
Reactive oxygen species (ROS) as potent antimicrobial agents are defined as very reactive molecules containing oxygen including hydroxyl radicals(•OH), hydrogen peroxide (H2O2), superoxide (O2․−), and singlet oxygen(1O2), produced by oxygen reduction in inflammatory leukocytes particularly neutrophils at infectious damaged sites [53]. ROS produced by defending host cells are able to inhibit a diverse range of microorganisms. The activity of superoxide and hydrogen peroxide is less effective than both hydroxyl radical and single oxygen, because of their detoxification due to presence of endogenous anti-oxidants related to enzymatic and non-enzymatic mechanisms; while no enzyme can detoxify hydroxyl radical or single oxygen (although organic antioxidant molecules can quench them), so they are extra lethal for pathogenic microorganisms [54]. Unfortunately, ROS are able to interact with host biomolecules and cause cellular and finally tissue damage. Pathogens can inhibit ROS by their own enzymes with anti-oxidant activity such as catalase and superoxide dismutase.. ROS have bactericidal and virucidal effects that are utilized by human cells against microorganisms in burn wound infections [55, 56]. Nitric oxide (NO) is a reactive free radical with a short half-life. NO is made from L-arginine by the enzyme NO synthase both constitutively and induced pathways of human immune cells [57]. Its antimicrobial activity is caused by damage to microbial DNA, DNA repair enzymes and lipid damage to microorganisms. NO generation has been reported as one of the main processes occurring in wounds and during skin reconstruction. Many recently produced topical gels can deliver NO content for treatment of skin wounds. NO recruits hair follicle stem cells for the re-epithelialization of skin wounds. It also improves angiogenesis and accelerates collagen production in the damaged site. Therefore NO has been utilized for burn wound healing through several mechanisms [58, 59].
3.3 Antimicrobial agents
3.3.1 Metallic elements
Several metallic elements have been tested as effective materials to treat microbial infections [60]. Silver compounds have been utilized in burn infection treatment. Ionized silver (Ag+) compounds can react with thiol moieties of enzymes and proteins in the microbial cells, thus inhibiting growth and metabolism of a wide range of microorganisms. Moreover, silver ions are able to bind to cell walls and intracellular vacuoles of bacteria, which inhibit microbial division. Silver also causes membrane damage and disrupts electrical conductivity and membrane potential [61]. Silver nitrate was the original agent used for burns, which is utilized to inhibit the growth of microorganisms. It is still utilized as a treatment for burns, but concerns about host toxicity have led to the development of other silver derivatives [62]. Silver sulfadiazine (SSD) is another derivative that can be applied to prevent against microbial wound dressing and cell proliferating uses on the skin damaged surface [63].
Bismuth-thiols are other metal compounds that have antibacterial activity against staphylococci causing wound damage [64]. Copper (Cu) as a metal element and its derivatives such as Cu-nicotinate complex have natural antimicrobial activity [65]. Cu ions bind to some vital groups of microorganisms and suppress their enzymes. It has been reported that Cu salts can be used on infected skin wounds such as burns [66]. Cu compounds have also been indicated to have antibacterial activity against Vibrio cholerae, Shigella flexneri, Escherichia coli, Salmonella typhi and Salmonella paratyphi [67]. Gallium (Ga) is a metallic element that is not found in its pure form in nature, while its ionic Ga3+ form has been found in many minerals such as those containing aluminum and zinc. Ga3+ compounds have been shown to have antimicrobial activity. Another metal element is ferric iron (Fe3+), which is a vital element that has a critical role in bacterial growth and metabolic pathways [68]. The antimicrobial activity of Ga3+ maltolate salts against P. aeruginosa infections in murine burn wounds, was shown to induce inactivation of Fe3+-dependent redox enzymes, which had important roles in cell proliferation of bacteria. The results showed substantial decreases in the colony forming units of Acinetobacter baumannii and Staphylococcus aureus in the wound site [69].
Halogen based compounds including: fluorine, chlorine, bromine and iodine, have been used as antimicrobials. Hypochlorous acid is a reactive chlorine compound that can damage DNA and inactivate oxidative phosphorylation enzymes in microorganisms. HClO has a potential virucidal effect and disturbs the nucleic acids and capsids of viruses, and can be also used for disinfection of contaminated burn wounds [70]. Iodine molecules suppress essential enzymes of microorganisms by attaching to the sulfur groups of their proteins [71]. Iodine has effective antimicrobial activity against fungi and spores. Iodophors are preparations that contain iodine in combination with a solubilizing agent that can release free iodine molecules in situ. Iodine compounds have been applied as antiseptics and surface disinfectants for treatment of burn wound infections [72, 73].
3.3.2 Biopolymeric agents
Other naturally occurring antimicrobial compounds can also interact with microbial cells and induce membrane disruption. Chitin is the second most commonly-found biopolymer (behind cellulose) and is a linear polysaccharide isolated from crustacean shells, fungal cells and yeast. A common chitin derivative, chitosan, can be obtained by chemical extraction and hydrolysis of acetyl groups. The positively-charged structure of chitosan (CS) is the base for its therapeutic properties such as antimicrobial activity, fast blood coagulation and electrostatic immobilization of wound microorganisms [74, 75]. XF porphyrins such as XF-73 and XF-70 are dicationic porphyrin compounds, originally developed for photodynamic therapy [76]. However these agents were also found to disrupt the bacterial membrane in many bacterial species. XF-70 and XF-73 have been reported as potential suppressors against non-dividing or slow-growing forms of bacteria such as S. aureus. Additionally, these compounds have shown an effective inhibition activity against bacterial biofilms [77, 78]. Polyphenolic compounds such as tannins can be purified from several plant species such as tea, vegetables, cereals, beverages, grapes, apples, pears and cherries. Plants containing tannins have been used in medicine for their antimicrobial properties [79]. Essential oils such as Valencia orange oil and tea tree oil are substances extracted from various plants and used in cosmetics, cuisine and medicine [80]. Many researchers have reported specific oils display antimicrobial activity, applicable as a topical therapy against infections induced in burn wound [81]. Alginate is a derivative of Laminaria digitata brown seaweed [82]. Alginate-silver compounds and alginate oligomer-loaded with antibiotics have been utilized as antimicrobial agents against various virulence microorganisms such as Enterococcus faecalis, Enterococcus faecium, P. aeruginosa, E. coli, and so forth [83, 84]. Polyamines inhibit efflux pumps situated on microbial membranes that function to pump out antibiotics, and which are a major cause of microbial multi-drug resistance. Infections in burns induced by drug-resistant bacteria e.g. S. aureus have been reported to be treated using preparations containing polyamine compounds [85]. Aerobic (and anaerobic microorganisms) quickly colonize skin wounds after burn damage. Partly owing to the widespread application of antimicrobial compounds e.g. antibiotics, fungi and yeasts are observed to colonize burns particularly at later time points [86]. Mafenide acetate is a dose-depended bactericidal and bacteriostatic agent that can inhibit Gram-negative and Gram-positive bacteria. Mafenide acetate is widely applied in burn injury treatment [87, 88]. Firmocidin is a compound derived from the normal commensal bacterial species that lives on the skin, Staphylococcus epidermidis, and which can inhibit other pathogenic bacteria such as Staphylococci and Streptococci spp. This bacterial agent has been found to cause antimicrobial and immunomodulatory (i.e. anti-inflammatory) activity in burns. Firmocidin can be utilized against many burn pathogens such as S. aureus, group A and group B streptococcus Streptococci [8]. Fusidic acid is a bacteriostatic agent derived from Fusidium coccineum fungi. It is capable of suppressing protein production in Gram-positive bacteria such as Staphylococcus spp. and Streptococcus spp. in burn wound infections [89]. Usnic acid is an antibiotic and anti-inflammatory molecule containing a dibenzofuran structure. Thentimicrobial activity of this compound against Gram-positive bacteria has been reported. Hence, it has been used in treatment of burn wounds [8, 90].
Nubiotics are novel antibiotics derived from proprietary protonated nucleic acid and applied against some of infectious pathogens. They were tested in a mouse model of burn wounds infected with a lethal dose of P. aeruginosa and gave a significant survival advantage [91]. Biofilms are formed through complex multilayer communities comprised of bacterial cells embedded within a matrix with self-secreting capability, containing extracellular polymeric substance (EPS), proteins and nucleic acids. They exist in an extensive span of natural and artificial environments, which can supply a protective microenvironment for microbial cells. Biofilm-growing cells have been shown to express various set of genes, thus are highly resistant to most antimicrobial treatments. A multilayer biofilm model was described that could be used for testing antimicrobial approaches (such as antibiotic ointments) against burn wound bacterial isolates [92]. The compound 2-aminobenzimidazole (2-ABI) can be found in sea sponges. It can protect the sessile sea sponges against the growth of marine biofilms. 2-ABI analogs exert anti-biofilm activity against biofilms of Gram-negative bacteria such as P. aeruginosa [93]. Polyanionic polyphosphate materials can chelate metallic elements including calcium, magnesium, manganese and iron. As a result, they possess antimicrobial activity and are used to treat burn infections [8]. A biofilm destruction device was designed to generate ozone and oxygen at a certain ratio. Ozone has a potent antimicrobial activity and can be used in a controlled concentration to deactivate bacteria and fungi in treatment of burns e.g. S. aureus and P. aeruginosa [94, 95]. D-amino acids also show a dispersal activity against biofilms, and can prevent important microorganisms such as S. aureus and P. aeruginosa from forming biofilms. These D-amino acids are also generated by the bacteria themselves [96, 97]. Dispersin B via an enzymatic anti-biofilm activity cleaves the poly-N-acetylglucosamine molecules, one of the polysaccharide constituents of the biofilm produced by S. aureus and E. coli, Therefore, this enzyme can be used to treat burn wound biofilms [98, 99].
Various mechanisms for development of antimicrobial resistance have been detected in microorganisms [100]. These resistance mechanisms consist of chemical modification of the antimicrobial agent, pumping out the drug from the cells, and modification of the microbial target. Antimicrobial resistance may be an inherent property of the organism, e.g. related to type of the cell wall structure, rendering it resistant, or it may be achieved via mutation in its microbial genome and by acquiring mobile DNA elements such as plasmids from other species [101, 102].
3.4 Therapeutic microorganisms
Therapeutic microorganisms exert antimicrobial activities against the growth, adhesion of various pathogens to host cells, as well as biofilm generation, and can be used for burn infection therapy [103]. Probiotic bacteria include a group of Lactobacillus strains. L. acidophilus is commercially available in fermented milk, yogurt and other foods. These bacteria can significantly inhibit Gram-positive and Gram-negative bacterial growth in burns [104]. Bdellovibrio bacteriovorus and Bdellovibrio like organisms (BALO) are naturally small and motive predatory bacteria belonging to the α- and δ-proteobacteria phylum, which can only replicate inside Gram-negative bacteria, thereby inducing their death [105–107]. Recently, researchers have tested the activity of BALOs against E. coli as well as in burn wound infections caused by P. aeruginosa [108].
Bacteriophages as viruses can enter their host bacterium, within which they can replicate, effectively infecting the bacterial cell. Bacteriophage amplification occurs into the host bacterial cells, which enable bacteriophages to be widely used as gene carrier platforms. Different types of phages have specific shape, a diverse loading capacity, a particular mechanism and rate of molecular assembly [109]. Antibacterial phages have been developed due to their efficacy against antibiotic resistant bacteria [110], however the high specificity of phages for their individual bacterial host, means that a cocktail of phages must be used to obtaining any broad-spectrum activity. In a recent study, bacteriophages were utilized for wound infection treatment in mice induced by Klebsiella pneumonia suggesting that bacteriophage therapy could be applied for K. pneumoniae induced wound infections [111]. In one study, phage therapy was tested in a preliminary clinical trial. Here, a bacteriophage cocktail (BFC-1) was employed to act against P. aeruginosa and S. aureus in colonized burn wounds. No adverse effects or abnormality was detected due to the phage, opening the way for further investigation [112]. In another clinical trial, phage therapy gave fewer complications, temperature normalization, as well maintained the level of phagocytosis. Streptococcus and Staphylococcus strains were cultured less often in wound secretions [113]. Elsewhere, a phage cocktail was used against K. pneumonia-mediated burn wound infection, and its effectiveness for arresting infection processes without any likelihood of generating resistant strain mutants [114]. Multidrug-resistant P. aeruginosa infection has been also illustrated to be controlled in mice through exploiting a lytic phage [115]. In Holguin’s work, phage Φ Pan70 showed a significant effect against P. aeruginosa infections. Herein, all mice were rescued in the burned model, although the skin lesions remained present in several experimental groups. Thus, bacterial invasion into the blood was hindered by the phages. However, some side-effects such as an intensified immunological response and mastocytosis in the skin was reported at the inoculation site [116].
4. Antimicrobial light and ultrasound-based wound therapy
4.1 Phototherapy
Antimicrobial phototherapy (PT) can utilize both visible and non-visible light (ultraviolet and near-infrared), to treat infectious diseases in a minimally invasive manner. Light irradiation has been indicated to cause improvement in the healing process of burn wounds [117–123]. PT consists of several therapeutic methods such as UV irradiation (200–280 nm), blue light therapy (BLT) (400–470 nm) and low-level laser therapy (LLLT) (390–1100 nm). Also, NP-based photodynamic therapy (PDT) and photothermal therapy (PTT) are promising varieties of phototherapy, which will be discussed in the following sections [124–128].
Although UV light is supposed to have adverse effects on mammalian cells (e.g. by damaging their DNA) particularly upon prolonged or repeated exposure, several investigations have been conducted that showed that microorganisms are overall much more susceptible to UV inactivation than mammalian cells. Therefore, it has been suggested that UV light could be used as a viable approach for infection therapy [129, 130]. UV irradiation damages microbial nucleic acids, and induces repression of the microorganism replication [131]. Investigations have also reported UV light for sterilization and wound care [132]. The chief disadvantage of UV light is its poor penetration into tissue so it can only be used for fairly superficial infections.
LLLT is a non-thermal and non-antimicrobial phototherapy that utilizes laser or light irradiation [133]. LLLT has been tested in studies to treat several disorders, and was shown to increase cellular proliferation, modulate inflammation, and accelerate re-epithelization and repair of the injured skin [125, 134]. In one study by Yadav et al. [117] a super-pulsed 904 nm laser (200 ns pulse width, 100 Hz, and mean output power equal to 0.7 mW) was used to increase ATP levels in burn wounds. The results indicated improved aerobic metabolism (by increasing the activity of enzymes e.g. PFK, CS, G6PD, and CCO and higher ATP levels, and lower NADP/NADPH ratio), decreased expression of GS1 and up-regulation of GLUT1 and pAMPKα. These changes led to activation of cellular bioenergetics, regulation of the redox homeostasis and reduced inflammatory response, thus explaining the improved wound healing.
Blue light therapy (BLT) has shown impressive antimicrobial effects, especially for treatment of infected skin wounds including burns. In an investigation, Dai et al. [118] interrogated the mechanism of action of 415 nm blue light in P. aeruginosa infected wounds. P. aeruginosa cells displayed 35-fold higher levels of killing by blue light, compared to the killing seen with keratinocytes. Results showed intracellular damage to P. aeruginosa cells. Using an animal model where the infection could be monitored using bioluminescence imaging, there was a reduced area-under-the-bioluminescence-time-curve after blue light exposure, improved survival rate of infected mice, and negligible damage to the skin and keratinocytes of the mice caused by blue light. Elsewhere, the antimicrobial activity of 415nm blue light was exploited against multidrug-resistant A. baumannii infection in mice with third-degree burn wounds. The multidrug-resistant bacteria showed higher blue light-susceptibility than human keratinocytes [123]. Also, other results were reported including blue light-triggered ultrastructural bacterial cell damage, negligible damage to DNA of mouse skin, no resistance to blue light inactivation, and highly attenuated bacterial burden in mouse tissue samples.
Furthermore, green light has also been the subject of some studies. Catão used green LED light to tackle skin burns in rats by exerting anti-inflammatory effects. The results showed a substantial decrease in inflammatory cells [119].
Far-infrared (FIR, 3um-100um) light has also shown beneficial effects on wound healing. In a research by Chiu et al., the exploitation of FIR reduced NLRP3 inflammasome activity and IL-1β generation caused ASC ubiquitination (a regulatory role of inflammasomes), followed by enhanced transport of inflammasomes to autophagosomes. FIR irradiation also lessened the infiltration of inflammatory cells, prevented loss of specific collagen fibers, and burn-triggered epidermal thickening, as well enhanced autophagy. Hence, these results suggested FIR could promote burn wound healing [120]. In another study, a single exposure of a low power laser (830nm) equal to3 J/cm2 was applied to thermally-injured Swiss albino mice. Histological and immunohistochemical analyses indicated improved tissue healing in the NIR-illuminated mice, and regulation of several cellular markers [122].
Recently, low power laser (LPL) and LED have been employed to realize the molecular mechanisms related to epidermal healing in burn wounds. The results signified efficacy of 660nm LPL and 850 nm LED to decrease oxidative stress levels and lower expression of IL-6 and pERK1/2, leading to downregulated inflammatory responses, less dermal necrosis, and enhanced granulation tissue generation, subsequently all causing betterment of burn wound healing process [121].
4.2 Shockwave and ultrasound based-therapy
Therapeutic shockwaves and ultrasound have been indicated to have advantageous effects on wound treatment, thus to accelerate the wound healing process in burns. recently, Rünzler et al. indicated the efficacy of shockwave treatment for wound healing by activating purinergic ERK1/2 signaling pathways by inducing ATP release, and then enhancing cell proliferation [135]. In another study, Fantinati et al. investigated chronic third-degree burn wounds and reported that low-intensity ultrasound could control the extent of tissue necrosis, enhance granulation tissue formation, as well as accelerate wound closure (via contraction). They suggested that ultrasound would have useful effects on the inflammatory and proliferative phases in burn treatment process; but could induce undesirable angiogenesis as well as inflammation in the remodeling phase [136].
5. Stem cell based-burn therapy
Considering their regenerative ability, stem cells have been regarded as a versatile and efficacious therapy in biomedicine [137], and could provide new solutions for many diseases including, osteoporosis, osteoarthritis, cartilage regeneration, and cardiovascular. In the case of wound treatment, the combination of stem cells with nanostructures has opened up new horizons. Stem cells can be distinguished from other kinds of cells by three main attributes: 1) renewability and ability to divide themselves for prolonged periods of time; 2) they are non-specialized cells; and 3) they potentially can differentiate into miscellaneous specified cell types. Diverse stem cells derived from humans and animals have been reported including induced pluripotent stem cells (iPSCs), non-embryonic "somatic" or "adult" stem cells, and embryonic stem cells. In this regard, stem cells have shown promising potential to treat burn wounds.
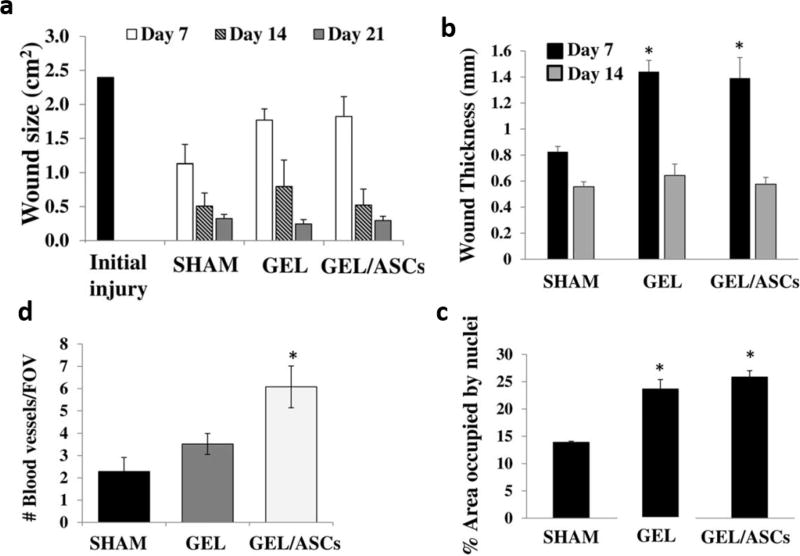
Stem cells can improve wound healing through differentiation into skin cells, by a pronounced anti-inflammatory effect and by stimulating angiogenesis. Foubert et al. [138], for instance, found acceleration of re-epithelialization in treated wounds by using adipose-derived stem cells (ASC). Subsequently, an increase in blood vessel density and deposition of ECM (collagen) was indicated. Directly injecting stem cells into the wound, or their intravenously administrating both led to comparable beneficial outcomes.
Yang et al. [139], showed epidermal stem cells that overexpressed the EGF-like domain 7 (EGFL7-ESCs) could stimulate fibroblasts to proliferate and migrate, and this effect could be used for enhanced healing of wounds. Bliley et al. [140] employed adipose derived stem cells (ASCs), and demonstrated an enhancements in the mRNA expression of collagen (type I and III), collagen deposition vascularity, which eventually followed by adipogenesis. They suggested ASCs could have a vital role as an adjunctive therapy for full-thickness burn wounds.
Recently, in a research [141],bone marrow–derived mesenchymal stem cells (BM-MSCs) migration into the burn wound margins enhanced the re-epithelialization, as well, the mobilization of BM-MSCs was regulated via CXCL12/CXCR4 signaling.
In another study [142] allogeneic MSCs were shown to improve burn wound healing via reducing unhealed areas, and enhancement of the dermal thickness, epidermal area and collagen content. In a study by Chen et al. [143], adipogenic differentiated ASCs (using +3-isobutyl-1-methylxanthine (IBMX) [D1–5]+INSULIN) enhanced early wound better compared to ASCs, as well as decreasing fibrosis and inflammatory infiltration in the superficial dermis.
6. Nanotechnology for treatment of burn infections and wound healing
Treatment of burn wound infections and skin regeneration are complex processes, and have been researched for decades. Recently the rise of antibiotic-resistant pathogenic microorganisms has correlated to greater resistance to topical antimicrobial agents in burn injuries, which is regarded as an important threat to public health. Hence, developing new even more potential antimicrobial agents is a necessity. Nanotechnology has introduced particles made of a wide variety of materials, but what they have in common is a size range of 10–1000 nm [144, 145]. Nanotechnology has been called “a new paradigm” in medicine, and new therapeutic methods based on NSs have been developed to tackle many diseases [146, 147], particularly burn wounds and burn infections. Nanomedicine can lead to effective improvements in therapy for infected burns in the post-antibiotic era and better skin regeneration [148]. In this section, we review new nanotechnology-based approaches for of burn infection treatment and healing of wounds. NSs have shown impressive antimicrobial activities against resistant strains of microorganisms, and are also capable of accelerating regeneration of burn-injured skin [149, 150]. NSs utilized in burn therapy can be classified as two main groups: organic NPs and inorganic NPs; each of which contains diverse types of NPs utilized for burn infections infection and wound healing [151, 152]. Organic NSs include polymeric NPs, nanoemulsions, nanogels, liposomes, micelles and solid lipid-based NPs (SLBNs), while inorganic NSs contain nanocarbons, AuNPs, CuNPs, AgNPs, TiO2 NPs, magnetic NPs (MNPs) and quantum dots (QDs). Nanocarbons comprise fullerenes, carbon nanotubes (CNTs), graphene, nanodiamonds, etc. [150, 153]. Furthermore, in recent years, NS-based structures such as nanofibers (NFs), 3D-scaffolds and films have been developed for wound therapy, which also will be discussed in the following sections.
6.1 Therapy of burn wound infections using NSs with antimicrobial effects
6.1.1 Organic NPs
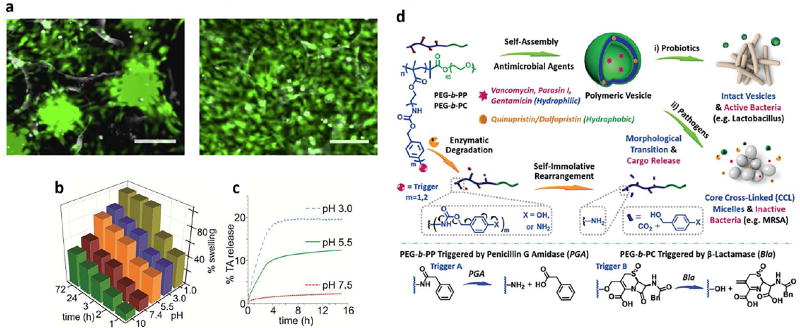
Polymeric NPs can be fabricated through implementing various materials, and methods. They are conventionally synthesized via self-assembly of ingredients such as chitosan (CS), curcumin, poly lactic-co-glycolic acid (PLGA), as well as having different shapes including dendrimers, hydrogels and Nanomicelles (NMs) [154]. Polymeric NPs have been utilized in different biomedical applications [155, 156]. They are usually synthesized by self-assembly of charged polymers mediated by interactions between chains with cationic and anionic groups. This is a relatively mild process and will not damage cargo components such as proteins that would be deactivated by high temperatures and organic solvents. These methods use an emulsification process to synthesize polymeric NPs. Such methods can be used as a strategy for production of others polymeric NPs incorporating therapeutic nucleic acids, peptides or proteins inside the NPs [157]. KGF is a potent growth factor involved in re-epithelization of skin wounds [158]. In two recent investigations it was shown that that KGF contained in self-assembled NPs could stimulate healing of skin wounds, giving increased re-epithelization, better tissue remodeling and skin regeneration [159, 160]. PLGA is a FDA approved material used in the production of polymeric NPs (100–200 nm) due to its advantages including biodegradability, biocompatibility and non-toxicity [161]. PLGA NPs are normally fabricated by emulsification of hydrophobic components, using an organic solvent and various surfactants [162]. In one study, PLGA was used to manufacture EGF-loaded NPs for wound healing. Full-thickness wounds treated with EGF-loaded PLGA-NPs gave the highest level of fibroblast proliferation and the fastest healing [163]. Researchers have reported that PLGA could provide a biocompatible system for delivery of growth factors. In one study, the delivery of the host defense peptide called LL37, incorporated into PLGA NPs reduced the release of lactate and accelerated wound healing [164]. Studies have clearly indicated that PLGA-NPs loaded with LL-37 antimicrobial peptide could be utilized as an effective agent against (polymicrobial) infected wounds [164, 165]. CS NPs (150–300 nm) usually are made of pristine soluble CS by using ionic gelation methods. CS can be employed as a natural and safe biodegradable substance in the design of drug/gene delivery systems [166]. Recently, CS due to considerable advantages has been utilized in burn wounds, and fabrication of haemostatic dressings, bandages, etc. In addition, CS can also be used as a prophylactic agent that prevents the progression of infections, and has also been described to produce significant acceleration of wound-healing [167–169]. Because CS is a cationic polymer, CS NPs have been used as intrinsic antimicrobial agents for prevention of microbial infections [170, 171]. Many studies have reported that CS NPs and analogs could facilitate wound treatment process by increasing the function of inflammatory cells in the wounds, and stimulating fibroblasts and osteolasts [172]. In two independent studies, it was shown that CS NPs could facilitate coagulation by attaching to red blood cells, and improve inflammatory cell functions. In addition, CS NPs are used as components of dressings and bandages designed for skin wounds to support the mechanical strength of the wound, and increase the wound healing rate both in humans and animals [169, 173]. In another research, CS NPs were employed for the preparation of films in combination with alginate, and loaded with SSD cream to treat infected open wounds [174]. Curcumin NPs, are formed from curcumin (40–200 nm), which is a natural yellow pigment with a diarylheptanoid structure, an example of curcuminoid-related molecules extracted from turmeric, a spice prepared from the root of Curcuma longa, which has been exploited in traditional medicine for centuries [175]. Encapsulation of curcumin into NPs is an advantageous delivery method for this water-insoluble substance. In one study, curcumin NPs were found to have strong antibacterial activity to suppress the growth of P. aeruginosa and S. aureus, and treat skin infections in mouse wounds [176]. In other studies, it was found that curcumin NPs could reduce inflammation and induce cell expansion that was useful in reconstruction of damaged tissue [177–179].
• Dendrimers
Dendrimers are a type of nano-scale (1–10 nm) polymeric macromolecule with a homogeneous and monodisperse structure, which have applications in both diagnosis and therapy. Dendrimers possess a symmetric core, an inner shell and an outer shell. Dendrimers can be prepared from phenyl acetylene subunits [180, 181]. Moreover, the surface functional groups of dendrimers can act as antibacterial agents themselves (especially if cationic). In one study, the interaction of the positively charged groups of a dendrimer (quaternary ammonium groups) and the negatively charged groups of the bacterial cell wall could lead disruption of the bacterial structure. Moreover water-soluble dendrimers such as polyamidoamine -based dendrimers can be loaded with water-insoluble antibiotics (e.g. nadifloxacin and prolifloxacin), which increases their bioavailability and enhances their antibacterial properties [181]. In another approach, silver-dendrimeric NSs were found to have synergistic effects in terms of anti-inflammatory and antimicrobial activity; dendrimers were shown to be an ideal carrier to diminish inflammation, while disinfecting the wound and stimulate healing [182].
• Hydrogels
Hydrogels as polymeric networks are regarded as hydrophilic three-dimensional structures, which potentiate absorption of large amounts of aqueous fluid. Because they have high porosity and a soft consistency, they can be used for dressings and to help tissue regeneration [183]. These materials are ideal for wound dressings, because they can protect the injured tissues make the patients more comfortable as they exert a cooling function, and their non-adhesive nature preserves the wound tissue [184]. Recent advances have shown that hydrogel NPs combined with other components including CS, keratin, PVA, PEG, AMPS, PEGDA, self-assembled peptides, dextran, hyaluronan, collagen, fibrin and antibiotics can induce skin regeneration in burns. [185]. In one study, the antimicrobial activity and scar preventive (antifibrotic) properties of hydrogels were optimized for wound dressings using a coating comprised of a combination of PVP, PEG and CS coated onto a cotton fabric. A model drug, tetracycline hydrochloride was loaded in the hydrogel structure. The Cumulative drug release was indicated to be 80% over 48 hours, followed by improved antimicrobial effect of the dressings against both Gram-positive and Gram-negative bacteria, and minimal scarring when the dressing was applied to wounds [186]. Hydrogels also possess other features making them substantially invaluable for tissue regeneration and engineering. A 3D network-like matrix composed of hydrogel behaves more like natural biological ECM to allow cell growth, compared to cells seeded on two-dimensional substrates [187]. The nanoplatforms can be modulated to release various bioactive molecules such as antibacterial agents, growth factors, and cytokinesin tissue damage site, as well to assist beneficial implementation of exogenous epithelial cells, fibroblasts or stem cells [188, 189]. Recently, basic fibroblast growth factor (bFGF) transport by gelatin-based hydrogels, and their subsequent release were reported to help the healing of acute vocal fold damage without scarring. Using this bFGF hydrogel-based treatment enhanced tissue regeneration [190]. In another study, ionic micellar NPs were designed using chitosan with hydrophobic modifications, combined with fatty acids, which undergo self-assembly in aqueous medium. Results indicated biocompatibility of the NPs, successful encapsulation of a poorly-soluble drug (clarithromycin), and the ability to be administered both parenterally and topically. Thus, this nanoplatform was suggested to be beneficial for skin regeneration and wound healing [191].
• Nanoemulsions
Nanoemulsions (NEs) are made of oil-based and aqueous solutions forming nano-sized particles in the range of 20–200 nm [192]. NMs are generally made of amphiphilic molecules that adopt a spherical form in aqueous solutions [193]; they have two parts including a hydrophobic core as well as a hydrophilic coating. The hydrophobic core can be efficiently loaded with insoluble agents, and the hydrophilic coating allows the NPs to be soluble in aqueous media [194]. The aqueous phase of NE contains water and co-surfactants e.g. glycerin and ethyleneglycol. NEs can be produced via mixing the constituents using ultrasonication and stirring. In one study, chlorhexidine acetate formulated in a nanoemulsion was used against a methicillin-resistant S. aurerus (MRSA) infection in a skin burn wound model. Here, the nanoemulsion showed effective and rapid activity against MRSA in-vitro and in-vivo and hindered formation of biofilm, disrupted MRSA cell walls, led to increased leakage of DNA, protein, Mg2+, K+ and alkaline phosphates out of the cells [195].
In another study, a CS film was impregnated with NEs encapsulating eucalyptus oil, produced by an emulsification method, which showed a good antibacterial effect against S. aureus. The mechanism of action of this eucalyptus oil-NE-CS film was attributed to cell membrane damage, and wound healing efficacy to a non-irritant activity in the wounds [196]. NMs made via self-assembly have been recently used to encapsulate hydrophobic drugs to improve their solubility [197]. Recently, ionic micelles have been fabricated using ionically-modified CS, and loaded with SSD as an antimicrobial. These micelles were also loaded with platelet lysate and used for treatment of chronic burn wounds. Unloaded CSs demonstrated good biocompatibility against fibroblasts. High concentrations of SSD could be obtained using the micelles as well as reduction in the cytotoxicity and bio-incompatibility of SSD towards fibroblasts, and improvement in the the activity PDGF-AB (platelet derived growth factor (PDGF-AB) [198].
• Liposomal NPs
Liposomal NPs consist of spherical vesicles made of one or more lamellar lipid-bilayers that spontaneously self-assemble when amphipathic precursors are mixed. Unilamellar liposomes have a single shell, while multilamellar liposomes have several concentraic shells. These bilayers can be produced from cholesterol and naturally occurring phospholipids including phosphatidylcholine [199]. One study reported that dihydroquercetin formulated as a liposomal nanocomplex could improve endogenous antioxidant activity, reduce the necrotic area in burned skin, and finally induce better healing [200]. Elsewhere, the use of other liposomal components e.g. DOTAP/cholesterol for encapsulating DNA ending the IGF-I and KGF genes into dermis and epidermis was reported to be an effective method to improve epithelial cell differentiation and expansion [201, 202].
SLBNs have been tested as potential drug delivery systems (DDSs) that could be topically administered to the skin and eyes, or else delivered by parenteral, oral, and inhalational routes. SLBNs can be classified into two main groups including solid lipid NPs (SLNs) and nanostructured lipid carriers (NLCs). Various methods have been developed for synthesis of these NPs including microemulsion, solvent emulsification, evaporation, and high pressure homogenization [203].
SLNs are solid colloidal drug delivery vehicles at room temperature, made of solid lipids, instead of the liquid lipids that are used in NEs. SLNs made from spherical solid lipids can also include hydrophilic molecules such as PEG derivatives, and can be stabilized using surfactants. These vehicles have been suggested to be as an alternative to, microparticles, liposomes, and emulsions. It has been also shown that coating SLNs with hydrophilic agents enhances their plasma stability and improves the bioactivity of drug-loaded SLNs [204]. Several types of drugs can be delivered to chosen organs with high specificity using ligand-targeted or stimulus-responsive SLNs such as antibody-SLN conjugates, magnetic-SLN, pH sensitive SLN and cationic-SLN [205]. In one study, SSD-loaded-SLNs were tested for prolonged release of the cargo, and to reduce the pain in severe skin wounds. In addition, the use of SLNs increased cell migration and improved wound closure ratres [206]. Another study used a novel cell-based 3D-wound treatment approach, showing that empty SLNs facilitated re-epithelialization with low cytotoxicity and no irritation. Moreover these NPs could also encapsulate and allow release of morphine in a wound dressing [207]. In another investigation, SLNs loaded with nitrofurazone acted as a delivery vehicle to get topical drug into the burn eschar, to improve the antimicrobial activity of nitrofurazone for treatment of infection in burn patients [208].
The second generation of SLNs, called nanostructured lipid carriers (NLCs) are comprised of both solid and liquid components, and have a higher drug-loading capacity and better stability [209, 210]. NLCs are comprised of physiologically biocompatible, and biodegradable and lipids and surfactants [211]. NLCs can be used to deliver highly lipophilic drugs into targeted organs, and damaged tissues such as burn wounds [212]. Miconazole-encapsulated NLCs were recently used to enhance miconazole activity (a poorly-water soluble antifungal agent). It was reported that the miconazole-encapsulated NLCs displayed enhanced antifungal activity against Candida albicans yeast cells [213]. In recent studies, emulsified recombinant human (rh)EGF-loaded into NLCs by ultrasonication was indicated to improve wound healing, as well to stimulate fibroblast expansion and collagen production [214, 215]. These rhEGF-loaded NLCs are potential candidates for healing of chronic wounds and burns. In a study by Gainza et al., dressings were fabricated in the form of fibrin-based solid scaffolds or semi-solid hydrogels, and loaded with lipid NP-encapsulated rhEGF. The growth factor underwent sustained release into wounded skin. The fibrin-based scaffolds were suggested as the best approach for rhEGF delivery due to their superior prolonged stability [216].
6.1.2 Inorganic NPs
• Metallic NPs
Metallic NPs mostly consist of AuNPs, CuNPs, and AgNPs have been employed in wound healing researches. Recently, the therapeutic activity of phytochemical-decorated AuNPs (Phyto-AuNPs) was studied for treatment of surgical wounds and burn wounds in a rat model. The Phyto-AuNPs were shown to act as an efficient antioxidant that could stimulate high expression of the pro-angiogenic agents (VEGF and Ang-2) thus improving skin regeneration. These Phyto-AuNPs were also suggested to be useful for healing of burn wounds [217]. In a similar study, cryopreserved human fibroblast cells (CrHFC) cultured with AuNPs were used to treat third-degree skin burns in white male rats. CrHFC-AuNPs facilitated skin repair and improved collagen production by the third week after treatment [218]. Interestingly, a novel modifiable collagen-gelatin fleece also improved burn wound healing without any added AuNPs [219]. In another study, AuNPs-incorporated collagen scaffolds (AuNP-SCs) were designed and cross-liked with glutaraldehyde. Use of AuNPs, allowed the scaffolds to have good biocompatibility, high mechanical strength, enhanced stability against enzymatic degradation, and reduced hydrolytic activity, compared to non-AuNP-incorporated scaffolds. Cross-linked AuNP-SCs inhibited inflammation and greatly improved the granulation tissue generation, and produced more neovascularization.
Wound closure was improved for both cross-linked AuNP-SCs and non-Au containing scaffolds and the AuNP-SCs were suggested as a potent skin substitute for coverage of wounds [220]. Cu is considered to possess a broad-spectrum antimicrobial activity against bacteria, yeasts as well as viruses [221]. Despite its beneficial properties, Cu can damage proteins (especially lipoiproteins), can cause rapid oxidation reactions in unsaturated lipids, therefore there is a possibility of Cu causing cytotoxicity and tissue damage. Thus the use of innovative nanotechnology-based methods are essential to diminish the side effects of Cu, while still allowing it to act as a powerful antimicrobial agent. Recently, one study showed that CuNPs were effective antimicrobial agents against E. coli, S. aureus, Micrococcus luteus, K. pneumoniae, P. aeruginosa as well as fungi such as Aspergillus flavus, Aspergillus niger and Candida albicans: all of these species are commonly found found in burn wounds [222]. A similar study examined Cu-encapsulated-CS-NPs for antimicrobial applications. As mentioned previously, CS is a natural antimicrobial polymer, that could exert synergistic activity between Cu and CS NPs [223].
For decades silver nitrate and SSD have been utilized as topical antimicrobial agents in burn wound treatment. Recently nano-scale silver-containing platforms such as AgNPs, and Ag-containing hydrogels have been applied in burn wound therapy. Two recent studies found that hydrogels containing AgNPs were a potent alternative to SSD for healing of burn wound infections, without causing cytotoxicity [224, 225]. In another study, AgNPs were used as an antimicrobial material for healing of skin wounds, where also showed a good antimicrobial effect and the capability to be used in wound dressings [226]. In a similar study, AgNPs loaded with additional components such as enoxaparin (a low molecular weight heparin used as an anticoagulant) were used to induce anti-inflammatory and angiogenic activity for wound treatment. The enoxaparin-loaded AgNPs could shorten the healing time of the wounds [227]. In a similar study, cotton fabrics containing stable 22 nm diameter AgNP suspensions were used as wound dressings and with effective antimicrobial and anti-inflammatory functions. Here, 250 ppm AgNP-containing cotton fabric demonstrated good anti-microbial activity compared to fabric containing less (60 and 125 ppm) AgNPs. Furthermore, using AgNPs led to same healing consequences in comparison with Dermazin® cream (SSD), and an anti-inflammatory effect, the same as indomethacin drug with dose of 20 ml [228]. In another study, an AgNP-based dressing with 3D-cultured fibroblast cells was used for full-thickness skin damage in burned patients. The results indicated that AgNPs reduced mitochondrial activity without activating cell death mechanisms, as well In vivo results showed AgNPs were localized in the cytoplasm of fibroblasts with no cytotoxicity evident [229]. The presence of normal human epidermal keratinocytes (NHEKs) and normal human dermal fibroblasts (NHDFs) is essential for good wound healing, but because they synthesize interleukin 12 (IL-12), TNF-α, and VEGF, they can cause excessive inflammation in the first two days. AgNPs could decrease the secretion of VEGF and pro-inflammatory cytokines. The results indicated NHEKs were more susceptible to AgNPs than NHDFs [230].
• Carbon-based NPs
Carbon-based NPs include CNTs, fullerenes, graphene, nano-diamonds and so forth. CNTs have recently been studied as novel drug and gene delivery platforms [231]. The molecular structure of CNTs is extremely hydrophobic, and CNTs are highly prone to aggregation. Nevertheless they can be attached to biomolecules by covalent and non-covalent bonds and used as delivery vehicles. CNTs have also been explored as potential antimicrobial agents with an intrinsic antibiotic function. [232]. In one research, Cu/Zn bimetallic NPs were combined with carbon nanofibers (CNFs) as a novel antimicrobial agent for wound dressing and burn healing. This antibacterial platform suppressed the growth of Gram-negative bacteria and Gram-positive bacteria e.g. E. coli and methicillin-resistant MRSA, respectively [233]. In one study, SWCNTs showed effective bactericidal activity against bacteria e.g. Salmonella enterica and E. coli. Here, SWCNTs caused the aggregation of bacterial cells and led to death of the microorganisms. Fullerenes have also shown an intrinsic antimicrobial function against different bacteria such as E. coli, Salmonella and Streptococcus spp. This antibacterial activity may be due to inhibition of energy metabolism and impairment of respiration by entry of the NPs into the cytoplasm [234].
Graphene is defined as a single monolayer of carbon atoms forming a two-dimensional crystal, with derivatives such as graphite oxide (GO). GO is hydrophilic (while graphene itself is highly hydrophobic) and can be produced by chemical modification of graphene. Graphene and GO NSs have been reported as appropriate carriers of drugs while showing antimicrobial activity against microbes such as E. coli., S. aureus, P. aeruginosa and C. albicans, besides can be exploited for fabrication of wound dressings and also for water disinfection [235]. In one study, it was reported that GO NPs could interact with bacteria, causing RNA efflux, and could disrupt cell membranes of Gram-negative bacteria and Gram-positive bacteria [236]. The povidone-iodine complex (PVPI) has a potent antiseptic activity against several microorganisms e.g. E. coli. In one study, SWCNTs were coated with PVPI to produce water-soluble and stable structures with monolayer polymeric helical coil morphology. Eventually, a composite film composed of these NSs served as a conductive bandage for skin wounds with improved flexibility, besides demonstrating the slow-release of antiseptic iodine [237].
• QDs
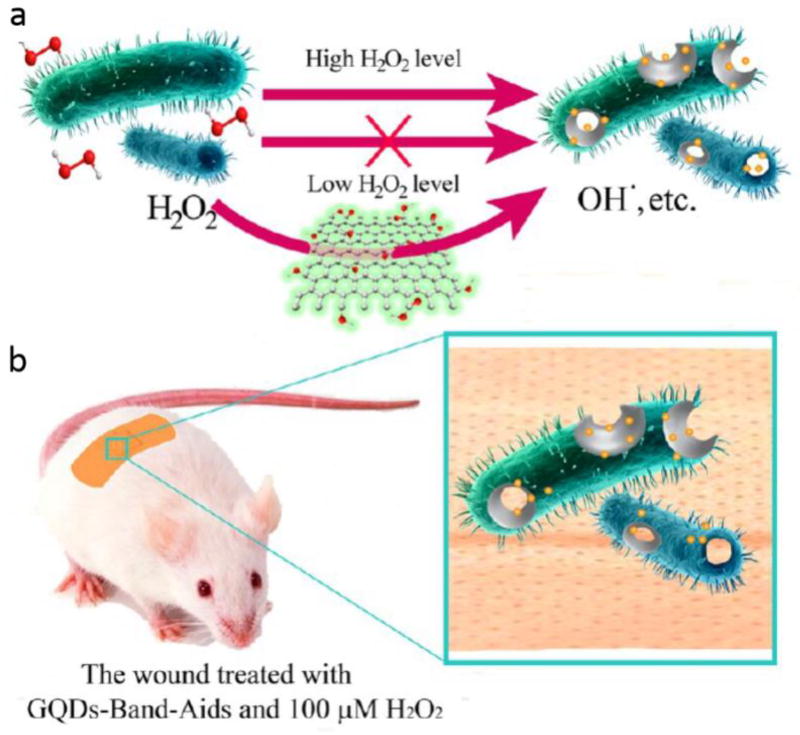
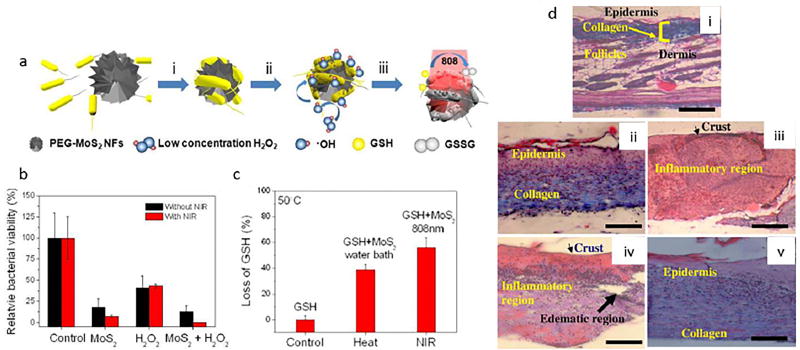
QDs are semiconductor nanocrystals with a three-dimensional structure and a tunable luminescence emission in any color, that are widely as fluorescent dyes and reporters in biomedicine [238]. Graphene QDs (GQDs) were shown to have an intrinsic peroxidase-like activity, whereby they functioned as a catalyst similar to an enzyme. The GQDs were able to generate hydroxyl radicals from hydrogen peroxide. Therefore, since •OH has a potent antibacterial activity, the reaction of H2O2 to produce •OH dramatically increased the antibacterial function of H2O2 meaning that high concentrations H2O2 in the wound were not needed and host toxicity was reduced (Figure 4) [239]. In another research, antibiotic NSs were fabricated comprised of AgNPs combined with GQDs coated with PEGylation and produced by a laser synthesis process. The antibacterial activity of Ag-GQD complexes was evaluated against bacteria including S. aureus and P. aeruginosa. Ag-GQDs acted as an antimicrobial agent with synergistic activity of its two components [240].
Figure 4.
a) The platform based on GQDs/low level H2O2 used as antibacterial agent, b) The GQD equipped bandage for disinfection of the wound site in vivo Reprinted with permission from ref. [239], copyright 2014, American Chemical Society.
• Ceramic NPs
Ceramic NPs include oxides, carbides, phosphates, carbonates, calcium, and generally have porous structures such as silicon oxide (silica) and aluminum oxide (alumina) [241, 242]. They have a wide range of industrial, diagnostic, and therapeutic applications because of their high heat resistance and chemical inertness. Targeted ceramic NPs have been explored as potential carriers for drugs, genes, proteins, imaging agents and photosensitizers in a broad span range of biomedical applications. Ceramic NPs have also been utilized as drug delivery platforms to enhance antimicrobial activity against pathogens [243, 244]. In one study, the antibiotics gentamicin and rifamycin were loaded into silica NPs immobilized inside collagen-hydrogels, and were used to treat wound infections and to evaluate their antibacterial activity against P. aeruginosa and S. aureus wound pathogens. Results indicated the gentamicin-loaded silica NPs embedded in collagen hydrogels could significantly improve wound healing and demonstrated a long-lasting antibacterial activity, with low cytotoxicity toward fibroblast cells. On the other hand, the rifamycin-loaded silica NPs in collagen hydrogels showed no bactericidal activity, because of intense absorption of rifamycin onto the collagen fibers and no subsequent release [245]. As mentioned above NO is an antimicrobial agent that can prevent infections developing in burn wound sites [246]. Recent studies have assessed the potential of NO-loaded silica NPs to act as an antimicrobial agents against various microorganisms in burn infections. Delivery of high doses of NO-loaded silica NPs has been shown to inhibit fibroblast proliferation compared to clinically relevant doses of NO. Furthermore, these NO-NPs have been shown to have antimicrobial activity against biofilms [246, 247]. TiO2 NPs show antibacterial activity upon UV irradiation due to ROS generation by the process known as photocatalysis. The aggregates/agglomerates of TiO2 NPs greater than 100 nm in diameter could not photo-inactivate E. coli and Saccharomyces cerevisiae, but conversely were able to act as a “sunscreen” and protect the microbial cells from inactivation by UV light alone [248, 249]. In one study, ZnO and TiO2 NPs demonstrated a strong inhibition against some species of bacteria including MRSA biofilms [250]. In another study, TiO2 NPs were applied as an antibacterial agent against Gram-negative and Gram-positive bacteria to improve wound treatment. Furthermore, the “green-synthesized” TiO2 NPs extracted from Moringa oleifera in a pH and temperature dependent process, stimulated wound healing in rats [251]. In a similar research, films equipped with green TiO2 NPs, isolated from the plant Psidium guajava, exhibited an antibacterial and antioxidant mechanism against E. coli and S. aureus [252]. In another investigation, the antimicrobial activity of TiO2 NPs and the related mechanism of action against P. aeruginosa was studied [253].
6.2 Nanofibers
Nanofibers (NFs) are fabricated from natural and synthetic continuous polymer chains. Nanoscale biodegradable NFs can enhance cellular and drug interactions with scaffolds used in tissue regenerating and engineering as well anti-microbial drug delivery applications. NFs with a diameter near 5 nm, can emulate collagen in size and the fibril structure that exists in the ECM. Hence, these NFs have been applied as beneficial nano-textured materials and scaffolds in burn wound care. NFs can be fabricated with diverse pore sizes, diameters and density [254, 255].
Electrospinning is a well-known technique to produce electrospun biodegradable NFs [256]. In this technique, a charged liquid jet of substrate is injected into a quickly rotating collector. Subsequently, the NFs is fabricated taking advantage of an electrostatic charge imbalance that overcomes the surface tension of the solution and the solvent is evaporated [257]. Regarding the high area to volume ratio of electrospun NFs, they can increase transfer of various molecules e.g. antibacterial agents, anticancer drugs, nucleic acids, and diverse growth factors, as well improve cellular attachment to tissues. Thus, electrospun NFs have been extensively employed in the form of NFs scaffolds used in tissue regeneration and tissue engineering [258]. In a study, Jayesh et al. loaded the iron chelator 2,3-dihydroxybenzoic acid (DHBA) within poly D, L-lactide (PDLLA)-poly ethylene oxide (PEO) NFs (called DF) created by electrospinning. They used DF for therapy of chronic infections induced by P. aeruginosa biofilms in wounds. Thus it was found that DF could decrease biofilm creation by nearly 75% in 8 h and could be incorporated into a wound dressing to cure P. aeruginosa chronic infections. Here, DHBA was released from DF for 4 h, comparable to the time needed for bacterial growth inhibition [259].
Copper can be incorporated in the regeneration and healing of damaged tissue. Cu can increase integrin expression (as an adhesive molecule), collagen fabrication and fibrinogen stabilization [260]. More recently, a copper containing NF structure (326 ± 149 nm in diameter) fabricated via electrospinning of PDLLA and poly-ethylene oxide PEO was shown to inhibit S. aureus and P. aeruginosa biofilm formation, by 41% and 50%, respectively [261].
NFs can be also fabricated by thermally induced phase separation (TIPS). In a study Li et al. generated a silk fibroin-PLGA NF scaffold (to mimic the ECM) by the TIPS method for a wound dressing. This structure showed the highest equilibrium water uptake rate of 72.2% and a water vapor transmission rate of 5591.88 g/m2 per day. Furthermore, the optimum properties of silk fibroin-PLGA NF scaffolds including water vapor permeability, water uptake rate/loss rate (to absorb wound skin humidity for suppression of wound infection and protection of damaged skin humidity) were achieved at the weight ratio of 8/8. The scaffolds were also reported to show sustained release of curcumin (as a model drug). [262].
Shape memory polymer–based NFs can be designed to control drug release, and as smart wound healing dressings are able to be applied for chronic wound healing and regeneration of damaged skin [263, 264]. In a study, Tan et al. generated a multifunctional and smart electrospun-based NF for wound repair. They fabricated the NF from chitosan, gelatin and shape-memory polyurethane, loaded with silver nitrate (AgNO3). The antibacterial activity of the NF was evaluated against bacteria including E. coli, P. aeruginosa and S. aureus. The results demonstrated higher antibacterial activity against E. coli and P. aeruginosa compared to S. aureus [265].
In a recent research, dendrimers (as branched monodisperse nanoscale molecules) were incorporated in a NF structure due to properties such as biocompatibility, flexibility, solubility, chemical stability, etc. Here, Dongargaonkar et al. designed electrospun blends, comprised of gelatin and highly branched star-shaped polyamidoamine dendrimer covalently conjugated to gelatin. Silver acetate (0.83, 1.65, and 3.30% w/w) as an antibacterial agent was loaded into the constructed NFs. Silver was shown to be significantly released at 48 h. antibacterial activity of the NF was evaluated on S. aureus and P. aeruginosa at 37 °C for 4, 24, and 48 h. In control groups including a blend of gelatin and silver acetate, S. aureus and P. aeruginosa showed rapid growth for 4 and 24 h while in active groups, S. aureus and P. aeruginosa growth was completely inhibited by silver acetate released from the constructed NF in 4 h. Thus, dendrimers covalently associated to gelatin both increased loading capacity as well bestowed immense flexibility to NFs as a candidate for dressing materials [266].
6.3 3D-scaffolds, films and wound dressings
New platforms including 3D-scaffolds, engineered films and hi-tech wound dressings are regarded as promising materials for tissue engineering, and improved results have been reported in wound healing studies. For example, ASC-containing PEGylated fibrin-based gel scaffolds used as a construct for wound coverage were shown to improve burn wound healing. Using this scaffold, ASCs were still present at the wound site 14 days after wound initiation. The scaffold enhanced the vascularization and attracted mannose receptor-expressing cells into the wound, improved the generation of granulation tissue as well as early mononuclear cell recruitment, but did not show any negative effects on wound closure. Figure 5 shows the measurements of wound size 1, 2, and 3 weeks after initiation of burn injury (Figure 5-a), the wound thickness 1 and 2 weeks after initiation of burn injury (Figure 5-b), the area occupied by nuclei 1 week after the injury initiation (Figure 5-c) and of blood vessel quantification in regenerating areas of the wound site 1 week after the initiation of the burn injury (Figure 5-d) [267].
Figure 5.
Curves showing morphological measurements evaluations from mice treated with SHAM group, GEL (hydrogel), GEL/ASCs at the burn wound site: a) wound size measurement; 1, 2, and 3 weeks after burn injury initiation compared to initial injury. Here, no effect of the gel wound dressing and wound closure was reported, b) and c) wound thickness (mm) after 1 and 2 week, and area occupied by nuclei, respectively, indicative of granulation tissue, which was facilitated by ASC-containing gels, c) Quantitative measurement of blood vessels in regenerating regions 1 week after the burn initiation. SHAM group (untreated), GEL group (treated only with P-fibrin gel), and GEL/ASCs group (GEL group integrated with adipose-derived stem cells) Reprinted with permission from ref. [267], copyright 2016, John Wiley & Sons, Inc.
Bacterial cellulose (BC) is defined as a natural polysaccharide with well-known beneficial properties such as biocompatibility, biodegradability, mechanical strength, nanofibrillar structure, moldability, water absorbency, porosity, and noticeable biological affinity in general. The ability of BCs to play a role in the fabrication and construction of 3D-scaffolds applied for tissue regeneration and healing has been reported [268, 269].
In the literature, various reports of a diverse assortment of films/membranes acting as wound healing dressings have been published. For example Cu2+-loaded bioactive glass/eggshell films [270], NO-releasing CS films [271], and Ag/ZnO-microporous CS composite dressings [272]. All these have resulted in substantial improvements in antibacterial activity, angiogenesis, re-epithelialization, skin reconstruction, blood clotting ability, and so forth leading to improved wound healing. Particularly concentrating on burn wounds, miscellaneous dressings have been reported such as Ag+ sulfadiazine-containing nanosheet-based ultra-thin films [273], NO-releasing polymeric films [274], tyrothricin (cyclic polypeptide antibiotic)-containing CS-based film-forming gel layers [275] with particular advantages including insignificant cytotoxicity, high activity against burn-wound bacterial pathogens, blocking bacterial invasion, inhibition of inflammation, reduced scarring, enhanced epithelialization and formation of granulation tissues, and so forth. Namazi et al. fabricated a carboxymethylcellulose hydrogel that incorporated MCM-41 mesoporous silica nanoparticles (MSN) nanocarriers, loaded with tetracycline and methylene blue. The MCNs helped control the hydrogel swelling and provided controlled drug release, potentiating the antimicrobial effects against S. aureus. These benefits suggested that the time intervals between changing the antimicrobial bandage could be elongated [276]. Zhao et al. also reported an electroactive injectable hydrogel wound dressing for cutaneous wounds with properties such as antibacterial activity, antioxidant, self-healing, improved hemostasis and adhesiveness. Figure 6 illustrates the synthesis process of these hydrogels [277].
Figure 6.
Schematic of the synthesis procedure of QCSP/PEGS-FA hydrogels. Reprinted with permission from ref. [277], copyright 2017, Elsevier.
Recently, a wound-dressing platform was developed based upon oxidized pectin-gelatin nano-hydrocolloids (OP-Gel). These were prepared by dip coating technique, consisting the entrapment of silver NPs (NS-OP-Gel) or ciprofloxacin (Cipro-OP-Gel) inside the hydrogel (Figure 7-a). The results (compared to commercially available Bactigras® chlorhexidine dressing) indicated high antimicrobial activity, enhanced neovascularization, stimulated cell migration, better-organized deposition of collagen, and good hydrophilicity for both NS-OP-Gel and Cipro-OP-Gel dressings. The NS-OP-Gel showed enhanced antibacterial function (at very low loading of 3.75mg per cm2) and Cipro-Op-Gel (at 1% drug loading) against E. coli and S. aureus. NIH3T3 fibroblast proliferation was inhibited most by Bactigras® slightly by NS-OP-Gel andf not all by Cipro-OP-Gel. Figure 7-b shows the microscopic images of full thickness wounds of C57BL/6J mice on day 21 after the burn injury. The histological analysis of the excised tissue at wound site is also shown in figure 7-c. Healing rate was enhanced by NS-OP-Gel, while there were similar rates for Cipro-OP-Gel and Bactrigas® [278].
Figure 7.
a) Schematic illustrating the fabrication process of the biocomposite wound dressing, b) microscopic images of 8mm full thickness wounds of C57BL/6J mice on day 21 after the burn injury initiation: (i) Spontaneous healing, (ii) OP-Gel, (iii) OP-Gel-NS, (iv) OP-Gel-Cipro, and (v) Bactigras, c) Histological analysis of the excised tissue at wound site: (i) intact tissue at first day, treated tissue after 3 weeks under different conditions of: (ii) Spontaneous healing, (iii) using OP-Gel, (iv) using OP-Gel- NS, (v) using OP-Gel-Cipro and (vi) using Bactigras (commercially available dressing). Reprinted with permission from ref. [278], copyright 2016, Elsevier.
Microspheres can be also employed to prepare wound dressings. Romic et al. showed the ability of CS/Pluronic® F127 microspheres loaded with melatonin to serve as wound healing dressings A good sustained release of melatonin, showed an antibacterial effect against S. aureus and clinical isolates of MRSA, along with high biocompatibility with keratinocytes (e.g. HaCaT) and fibroblasts (e.g. BJ) [279].
Nanofibers have also been investigated for wound therapy as dressings or membranes, and so forth. In a recent study, N,N,N-trimethyl CS fibers were used as wound dressings and showed good antibacterial activity against S. aureus (>99%) and E. coli (>63%), while showing a lack of toxicity to mouse embryonic fibroblast cells at low concentrations. In-vivo tests revealed improved re-epithelialization and better wound contraction [280]. Elsewhere, electrospun SF nanofiber membranes were designed and loaded with an antimicrobial peptide immobilized via EDC/NHS and thiol-maleimide click chemistry conjugation. This platform illustrated a long-lasting antibacterial activity. Beneficial effects on monocytes, keratinocytes and fibroblasts were reported, such as enhanced proliferation and/or differentiation of various cells (HaCaT cells and NHDF cells) and improved cell-cell attachment, together with inhibition of the LPS-triggered expression of TNF-α from monocytes e.g. Raw264.7 cells [281]. Multifunctional core-shell NFs can be utilized for wound dressings [282]. Recently, Wei et al. used such structures to accelerate wound healing. They applied polycaprolactone (PCL) as a mechanically strong core and used collagen as a biocompatible shell. In this NF scaffold, vitamin A palmitate was encapsulated in the PCL core as a pharmaceutical to accelerate wound healing, and Ag-NPs as an antibacterial material was integrated in the collagen shell. The encapsulated Ag-NPs demonstrated antibacterial activity against S. aureus, and nearly 70% of loaded vitamin A palmitate was released after 6 days. In addition, L929 cells (mouse fibroblasts) attached well on the PCL-collagen core-shell NFs after 5 days [283].
Three-dimensional (3D) NFs scaffolds are considered to be a promising approach in tissue engineering to emulate ECM [284, 285]. In one study, Park et al. designed a 3D electrospun-based silk-fibroin NF scaffold using freeze drying, and salt-leaching followed by electrospinning with NaCl. Moreover, human skin keratinocytes (HaCAT) and fibroblasts (NIH 3T3) were co-cultured in 3D silk-fibroin NF scaffolds to mimic a bilayered skin structure in vitro. Their finding showed improved fibroblast proliferation in the internal layer of electrospun/NaCl based-scaffold compared to freeze dried and salt-leached 3D scaffolds. Better keratinocyte differentiation was demonstrated in the external layer of salt-leached 3D scaffolds than that prepared by freeze dried and electrospun with NaCl methods [286].
Self-assembled NFs produced from self-assembled peptides (SAP) can be employed to fabricate self-assembled hydrogels as scaffolds, applied for the proliferation and regeneration of skin cells in wound healing [287]. In one report, Bradshaw et al. generated hydrogel NF scaffolds from RADA16 (1% w/v) self-assembled peptide. They studied the effect of three peptides including RADA16, RADA16 containing a fibronectin motif (RADA16-GG-RGDS) and RADA16 containing a collagen type I motif (RADA16-GG-FPGERGVEGPGP) on the migration and expansion of immortalized human keratinocytes as well as mouse embryonic fibroblasts. Two glycine residues (GG) were also added to ensure the motif flexibility. RADA16-FPG effectively inhibited fibroblast proliferation and mildly suppressed keratinocyte expansion (Figure 8), compared with RADA16 and RADA16-RGD (Arg-Gly-Asp) scaffolds, at 72 hours [288].
Figure 8.
a) Scanning electron microscopy of RADA16, (b) RADA16-FPG and (c) RADA16-RGD. (d) Cell viability result for the implemented keratinocyte (i.e. HaCaT) and fibroblast (i.e. NIH/3T3) using the three 1% w/v RADA16 NF scaffolds and cell control after 0 hour and 3 days of incubation. Reprinted from ref. [288], copyright 2014, Nature (licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License).
6.4 Smart stimulus-responsive NSs for burn wounds
Stimuli-responsive smart materials are starting to open new horizons in nanomedicine [289–295], and their abilities are expected to potentiate development of effective therapies for burn wound injuries and infections. This aim is possible due to the ability of so-called “smart nanomaterials” to respond to cues in their microenvironment, so they can provide “on-demand” interaction with cells (e.g. gene expression only in specific cells and only when desired). Smart nanomaterials can control their own behavior and fate, resulting in improved signaling and treatment mechanisms in burn wounds and infections, and minimize side-effects. In addition, stimulus-responsive smart nanomaterials can provide on-demand and appropriately-controlled liberation of miscellaneous therapeutic agents [296–298].
Stimulus-responsive NSs in the form of 3D-scaffolds are able to undergo on-demand morphological deformation such as volumetric swelling in response to environmental changes e.g. changes in the cellular environment and in the surrounding medium. This could lead to improved healing of burns and chronic wounds. For example, You et al. hypothesized that local pH changes (e.g. when hypoxia occurs) could change the ability of a pH-dependent scaffold to transport vital molecules (e. g. oxygen, glucose and other nutrients), as well as affecting the local cell density growing on the scaffold. Here, acidic pH-induced swelling of dimethylaminoethyl methacrylate (DMAEMA)/ 2-hydroxyethyl methacrylate (HEMA) scaffolds enhanced the penetration of oxygen and increased cell infiltration, compared to non-pH sensitive scaffolds. Furthermore, other results showed improved formation of granulation tissue, increased expression of tissue-regenerating factors and pro-healing genes, more angiogenesis and better tissue remodeling were reported. Mechanical stretching of the scaffold was also suggested as a probable factor that could induce tissue regeneration (Figure 9-a) [299]. In another study, a tannic acid (TA) loaded hydrogel designed by Voelcker et al. was employed in which carboxylated agarose was cross-linked with zinc ions to allow pH-dependent liberation of the cargo [300]. The results showed sustained TA release at acidic pH values along with significant swelling (with a highly enhanced compressive modulus of 0.947 MPa), and wioth insignificant release at alkaline and neutral pH values (Figure 9-b and -c). No cytotoxicity was seen towards 3T3 fibroblasts, while good anti-bacterial and anti-inflammatory effects were observed (the latter due to TA-induced inhibition of NO generation in activated human macrophages). Grützner et al. [301] studied the effect of enzyme-sensitive antimicrobial nanoplatforms loaded with polyhexanide biguanide (cationic polymer) and octenidine (cationic surfactant) for prophylaxis against infection in cutaneous burns. The results showed time-dependent cellular uptake, along with no major effect on expression of cytokines and pro-inflammatory molecules by endothelial cells, insignificant cytotoxicity compared to solutions of antiseptic agents, and no inhibition of angiogenesis. Elsewhere, an antimicrobial enzyme-responsive antibiotic-loaded nanocarrier was reported that could respond to microbial drug-resistance enzymes such as penicillin G amidase and β-lactamase. (Figure 9-d) shows enhanced wound healing, bacterial-selective delivery, high structural stability with modular design, and decreased side-effects using this system [302].
Figure 9.
a) Fluorescence micrographs of cells showing clustered growth after days 3 on HEMA (left; scaffolds coated with fibronectin were seeded with NIH/3T3 mouse fibroblasts); with homogenous growth of the cells cultured on pH-sensitive DMAEMA/HEMA scaffolds (right) [299], b) percentage swelling of zinc ion containing hydrogels; c) TA release from the hydrogels, at various pH values Reprinted with permission from ref. [300], copyright 2016, American Chemical Society; d) Scheme illustrating the synthesis of enzyme-sensitive self-assembled polymeric vehicles (from PEG-b-PP and PEG-b-PC) for selective delivery of antibiotics. They were designed to be responsive to penicillin G amidase (side chain cleavage) and to β-lactamase (microstructural transformation). This stimulus-responsive nanoplatform resulted in the constant cargo liberation and bioactivity of the encapsulated antimicrobial agent Reprinted with permission from ref. [302], copyright 2015, John Wiley & Sons, Inc.
Temperature-responsive NSs have also been investigated for disinfection purposes. A smart-stimulus-responsive nanocarrier equipped with the thermo-sensitive moiety, poly-N-isopropylacrylamide, showed triggered release of bacteriophages targeted against specific bacterial infections. This innovative concept could be used for therapy of infected burns [303].
Ultrasound irradiation has been used to activate NPs in infected wounds. Osumi et al used ultrasound (1.0 MHz, intensity of 0.4 W cm−2) to activate TiO2 NPs, which produced ROS. They claimed that ROS at physiological level could improve cell response and angiesis, whereas higher levels could carry out disinfection; therefore enhancing healing of the infected sites. Ultrasound therapy could enhance the penetration of NP into the tissues to treat deeper infected sites. Figure 10 shows (a) images of the infected wounds treated with or without using ultrasound-activated TiO2 NPs, (b) curves illustrating relative wound size percentage calculated compared to the initial wound size [304].
Figure 10.
a) Images of the infected wounds with different treatments: (i, ii) Untreated wounds on control at postoperative day 0 and 7. (iii, iv) Infected untreated wounds on postoperative day 0 and 7. (v, vi) Infected wounds treated ultrasound-activated TiO2 (UIT) on postoperative day 0 and 7, b) Effect of ultrasound-activated TiO2 on the treatment of the infected wound in regard to wound size percentage according to the initial wound size (day 0) Reprinted with permission from ref. [304] copyright 2016, John Wiley & Sons, Inc., c) Scheme of the smart antimicrobial system. Here, the exposure to lysozyme led to hydrolysis of NAc-CTS to give COS. The generated COS served as a substrate for CDH that produces the antimicrobial H2O2. Reprinted with permission from ref. [305], copyright 2017, John Wiley & Sons, Inc.
The family of matrix metallo-proteinase (MMP) enzymes, which mediate the balance between the generation and degradation of ECM, has an inevitable function in the healing of wounds, as well as in the remodeling of epidermal layers. In one study Kim et al. [306] showed the MMP2-responsiveness of a 4-arm PEG-conjugated siRNA molecule that underwent clustering leading to formation of nanoscale particles containing MMP-cleavable linkers. These NPs were resistant to RNase hydrolysis (compared with free siRNA) and could mediate gene transfection, leading to enhanced wound healing. There was low cellular uptake of the clusters in the absence of MMP, while in cells that expressed MMP, the clusters dissociated and endocytosis occurred. Wound closure was enhanced, while decreased MMP2 expression was caused by gene silencing together with higher cytokeratin expression in the healed wound.
Elsewhere, the use of a nanofibrous matrix containing a MMP-cleavable linker and decorated with siRNA encoding the silencing of the MMP2-gene was tested in a wound healing model [307]. In another study, MMP secretion was shown to have a key role in re-epithelialization and acceleration of healing as well as extracellular-induced regulation of kinase signaling in the wound healing process [308]. In a recent study elevated lysozyme activity was exploited as a biomarker of infection in order to activate a stimulus-responsive antimicrobial platform. N-acetyl chitosan (degree of N-acetylation: 40%) was hydrolyzed by lysozyme in artificial wound fluid to give N-acetylated chito oligosaccharides (COS). These COS were shown to serve as substrates for added cellobiose dehydrogenase (CDH) leading to the production of 1 mM hydrogen peroxide (H2O2) after 24 h incubation. This was a sufficient concentration to induce an antimicrobial effect against S. aureus and E. coli (Figure 10-c) [305].
6.5 Photothermal and photodynamic therapy for antimicrobial activity and wound healing
NPs can be activated by incident light taking advantage of either photothermal or photodynamic therapy-based mechanisms, and can kill microbial cells in wound sites and improve wound healing. The principle of photothermal therapy (PTT) is conversion of light to a localized heating effect due to photo-excitation of metallic NPs (AuNPs) or inorganic NPs (graphene, magnetic NPs. MoS2) [309–313]. Various materials can be utilized to obtain the PTT-activated antibacterial effect such as photothermal NPs or agents such as molybdenum disulfide (MoS2), GO, and polypyrrole NPs (PPy) [127, 314, 315].
In one study, a 1064 nm NIR laser was used for surface photothermal activation of GO, which led to highly efficacious antibacterial activity in a non-invasive and inexpensive approach. This nanoplatform was applied against pathogens including P. aeruginosa and S. aureus [127]. Elsewhere, an NIR-activated nanoplatform was designed for combination therapy, comprised of a photothermal agent (PPy) and an antibiotic (Van), both incorporated into hollow microspheres made of PLGA. When administered into the wound site, NIR light photothermally triggered both localized hyperthermia and antibiotic drug release, resulting in activation of a synergistic antibacterial activity [314].
Recently, PEG-functionalized MoS2 nanoparticles were synthesized, and exploited for simultaneous NIR-induced photothermal and peroxidase-like catalytic activity. Figure 11-a shows the mechanism of action of this multifunctional nanosystem. Peroxidase activity allows conversion of H2O2 to hydroxyl radical (•OH) that have strong antibacterial activity. The synergistic effect of the catalysis and 808nm-irradiation-triggered PTT of PEG-MoS2 NPs led to effective eradication of Gram-negative ampicillin resistant E. coli and Gram-positive endospore-forming B. subtilis (Figure 11-b) in wounds. The photothermal effect was also reported to cause glutathione (GSH) oxidation, inducing even more effective bacterial death (Figure 11-c). [315].
Figure 11.
a) Scheme of a PEG-MoS2 nanoplatform to destroy bacteria via a synergistic combination of peroxidase catalysis and photothermal activation: (i) Capture of the nanoplatform by bacteria; (ii) catalytic activity of the nanoplatform decomposes low-concentration H2O2, leading to generation of ·OH that damages cell wall integrity; 808 nm laser treatment induces hyperthermia, causing accelerated GSH oxidation, b) Relative bacterial viability for B. subtilis, obtained after incubation with PBS, MoS2 (100 µg mL−1), H2O2 (100 µM), or MoS2+H2O2 for 20 min, with or without the laser treatment, c) GSH loss after heating by water bath or 808 nm NIR-irradiation compared to control (non-treated GSH) for 20 min at 50 °C. Reprinted with permission from ref. [315], copyright 2016, American Chemical Society, d) Photomicrographs indicating PDT-triggered healing: (i) normal skin, (ii) wound without infection, (iii) infected wounds, (iv) infected wounds with 200 µM pl–cp6 treatment, and (v) infected wounds with 200 µM pl–cp6 treatment plus with exposure to the light dose of 60 J/cm2. Reprinted with permission from ref. [316], copyright 2013, Springer.
The concept of PDT is that photon absorption can excite the ground state of a photosensitizing agent (incorporated for instance in NPs) to a long-lived triplet state, leading to an energy transfer reaction with molecular oxygen, thus generating singlet oxygen as reactive species. Electron transfer reactions can also take place producing other ROS e.g. superoxide radicals, free hydroxyl radicals and hydrogen peroxide [317, 318]. PDT can be used to kill various microorganisms (bacteria, viruses, yeasts, fungi, parasites and cancer cells) [319, 320]. PDT has been reported to treat dermatological diseases and infections [321]. Two recent studies suggested that PDT could be used as an effective antimicrobial method for burn infections [322, 323].
Nafee et al. reported a polymeric nanoplatform encapsulating the naturally-occurring photosensitizer, hypericin, as an antibiotic-free agent for PDT-based antimicrobial therapy. They tested clinical isolates of MRSA biofilms and planktonic cells. Excitation of the photoactivatable 45 nm-diameter NPs generatied ROS and gave a higher antibacterial effect against biofilms than planktonic cells, and in-vivo tests showed accelerated healing, and improved epithelialization, keratinization and generation of collagen fibers [128]. In a recent study, poly-cationic poly-lysine was conjugated to chlorin p6 (photosensitizer) was reported for topical PDT of mice with wounds infected with P. aeruginosa. To this end, hyper-inflammatory response of bacteria was investigated. Figure 11 shows histological analysis including unwounded tissue (Figure 11-d (i)) and wounded tissue with different treatments (Figure 11-d (ii–v)). Complete re-epithelialization, and no necrotic or inflammatory areas were reported in the uninfected wounds, while the non-treated infected wound and infected wound treated only with drug-conjugate (no light) and slow healing, and disorganized collagen (due to excessive production of TNF-α). Light irradiation with doses of 60 and 120 J/cm2 onto the drug-treated infected wound area decreased the bacterial load, led to 5-days earlier healing, and the wounds showed parallel collagen fibers, no edema, shortened inflammatory phase, fewer inflammatory cells, and faster re-epithelialization. Significant reductions in TNF-α and cytokines IL-6 were reported at 96h post-treatment. Culture supernatants showed decreased bacterial protease activity (~ 50%) indicative of inactivation of extracellular virulence factors [316].
6.6 NS-based cell therapy for wound healing
Stem cell therapy is an example of advanced tissue-engineering techniques, which can be potentiated by different nanotechnology-based platforms, such as 3D cell-scaffold biostructures and hydrogels to improve wound healing. Kamp et al. [324] interrogated the capability of MSCs for therapy of chronic wounds or burns, that were combined with an MSC chemoattractant, hepatocyte growth factor (HGF) loaded in collagen and silk fibroin scaffolds. Here, the release of active HGF from the scaffolds was indicated to induce direct cell migration, leading to endogenous recruitment of MSCs from the local environment in the scaffolds
In one study Chung et al. [325] developed an engineered 3D-scaffold made of PEGylated fibrin gel that was assessed for delivery of ASCs into the burn injury site. ASC delivery led to enhanced vascularization and more cells expressing the mannose receptor, as well as early recruitment of mononuclear cells with improved granulation tissue generation. No negative influence on connective tissue extension or wound closure kinetics was reported. Elsewhere, Burmeister et al. [326] reported a platform comprised of ASCs and PEG-fibrin hydrogels as a scaffold, which was tested in a porcine model of debrided burn wounds. The delivery of ASCs into the wound site using the scaffold produced improved skin angiogenesis (in a dose-related manner) and better wound healing. Furthermore, the PEG-fibrin scaffolds could inhibit skin contraction after the meshing of autografts. In another study, Bio-NSs based on BC and MNPs were investigated an as effective tool for healing of chronic wounds. The morphology, viability and expansion of human ASCs, and the low cell toxicity of the scaffolds indicated BC-MNPs as a smart NS for chronic wound healing [269].
6.7 NSs for growth factor delivery
Growth factors can be exogenously delivered into wounds so as to activate healing process. Basic fibroblast growth factors (bFGFs) can induce fibroblasts to proliferate and migrate, endothelial cells to proliferate, as well to carry out angiogenesis. In research conducted by Butko et al. [327] bFGF was loaded into NPs made from succinylated-CS. The results showed a burst release profile, while co-encapsulation of bFGF together with heparin led to the maximum release (18%).
In another research [328], a hydrogel-sheet based on thiolated heparin attached to PEG and loaded with human EGF was used for wound healing (Figure 12-a). The profile showed a sustained release pattern. Applying the hydrogel sheet onto deep wounds on the back of mice improved the wound closure rate (Figure 12-b and -c), while stimulating granulation tissue formation and better epithelialization and capillary formation.
Figure 12.
a) Scheme illustrating the preparation of hydrogel sheet loaded with hEGF, used for healing of full thickness wounds, b) Wound closure-time curve in mice; and c) Macroscopic images of wound sites, obtained for different samples compared to non-treated controls. Reprinted with permission from ref. [328], copyright 2016, Elsevier.
6.8 NSs for gene delivery
Gene therapy is regarded as a novel and powerful technique for treatment of wounds and skin damages. In one study, Du et al. reported the rapid closure of burn wounds and improved healing stimulated by the gene transfection of DNA coding for hypoxia inducible factor-1 (HIF-1α) to induce angiogenesis, integrated with intravenous infusion of HIF-1-activated angiogenic cells cultured with the hydroxylase inhibitor, dimethyloxalylglycine [329]. Moreover, because highly efficient delivery of genes into cells and tissues is regarded as the path to success for gene therapy, NSs can function as a non-viral platform for efficacious delivery of genes. For example in one study, gene-silencing using Flightless I (Flii) siRNA loaded into CS-coated porous silicon NPs was used in a cutaneous wound site for down-regulation of Flii (a cytoskeletal actin remodelling protein, which negatively affects wound healing). The addition of this nanoplatform to keratinocytes caused a reduction in Flii gene-expression and lower Flii protein production. Cell migration and proliferation were enhanced, and were followed by better healing in acute excisional wounds [330]. Peng et al. prepared pDNA-VEGF165/β-cyclodextrin- linked polyethylenimine (CYD-PEI) polyplex NPs embedded in a gelatin/β -TCP scaffold. This 3D transfection platform was utilized for manipulation of ESCs in wound site. The results indicated prolonged VEGF expression in the ESCs and topical administration led to angiogenesis, accelerated re-epithelialization, and increased dermal collagen and hair follicles [331].
6.9 Combination therapies for wound healing
Various approaches have been developed for wound treatment, and recently it has been realized that these approaches will probably need to be used in combination if a substantial advance in potentiating the healing process is to be achieved. In recent investigations, different combination wound therapies have been reported including combinations of already approved clinical modalities, negative-pressure wound therapy combined with pulsed radiofrequency energy [332]; and a combination of photobiomodulation with antidiabetic medication [333]. The combination of topical negative pressure and silver-impregnated foam has also shown a synergistic inactivation against bacterial biofilms in the wound site [334].
Integration of stem cells with other potent therapeutics have demonstrated significant effects such as improved wound closure and angiogenesis, enhanced cell migration and proliferation, as well as negligible toxicity or inflammation in wounds [335–340]. Such investigations have designed stem cell-integrated combination therapies, which can also possibly be considered for burn wounds.
External stimuli have been also utilized in combination wound treatments. Some examples are the combined use of pulsed wave low-level laser therapy and stem cells [340]; the combined administration of LLLT/light-emitting diode (LED) [341]; and the concurrent exploitation of therapeutic high-frequency ultrasound and microcurrent electrical stimulation [342].
The introduction of nanotechnology to wound therapy has been investigated via diverse routes. Some recent examples are: a complex of different GF combinations and hyaluronic acid (HA) loaded into liposomes [343]; and the synchronous use of an ointment containing therapeutic NP and wound healing medicines, which have been reported to have beneficial results for skin wound healing [344]. In another investigation, the targeting ability of magnetic NPs was combined with an angiogenic gene therapy using a gene transfer technique to improve angiogenesis and wound healing [345]. In another study on wound treatment, a “lipoproteoplex” (i.e. liposome and protein hybrid) NP preparation was developed for efficient siRNA delivery as a gene therapy approach for wound therapy [346]. Hydrogels have also been attractive components in combination therapies; some recent examples are as follows: combination of LLLT and policaju (derived from Anacardium occidentale L gum)/chitosan hybrid hydrogels [347]; the integration of hydrogels and stem cells [337], the combination of hydrogels and platelet-rich plasma [348, 349], and so forth.
Several combination therapies aimed at wound healing have been studied in different phases of clinical trials, such as a clinical trial recently conducted to investigate the effect of simultaneously using gelatin sheet and platelet-rich plasma release [350].
6.10 New technologies in tissue regeneration and wound healing
In the era of translational medicine with a range of bench-to-bedside efforts being conducted to transfer preclinical studies to clinical products, well-designed efforts are absolutely required in order to eliminate previous deficiencies and shortcomings, especially in the fabrication methods of therapeutic products, such as complicated design methods and the high-cost of processes and materials, but also to produce user-friendly and low cost technologies. In this regard, some innovative approaches have recently been introduced in tissue engineering. Among such advances, nano-scale architectured substrates, cell-imprinted substrates and 3D-printed scaffolds are of particular interest. Furthermore, from a biotechnology aspect, some revolutionary techniques have emerged, including CRISPR-Cas9 gene editing, which will change the future of genetic engineering, tissue regeneration and may lead to new therapies for serious diseases such as cancer, HIV, and genetic defects. Hence, these newly emerged technologies are briefly discussed below:
6.10.1 Nanoscale architectured and textured substrates
Cells live in an environment with features on both the micrometer and nanometer scales where they govern various functional behaviors. Nanoscale-architectured structures employed in implantable biomaterials can be used to mimic bio-physicochemical environments and cellular niches to control cellular behavior [351]. More recently, advances in nanotechnology have provided new methods to create substrates equipped with surface nanotopography, emulating ECM architecture or providing suitable cell microenvironments. Nanotopography or nano-architectures have been shown to have a vital function in inducing or modulating cellular activity, motility, viability, morphology, adhesion, differentiation, expansion and migration [352, 353]. In the body environment, stem cells of various lineages e.g. human embryonic stem cells (hESCs) and hMSCs have the potential to differentiate into diverse cell types and can be applied in regeneration and tissue engineering. Stem cells can be influenced by employing tailored biochemical micro- and nano-architectures consisting of both solid materials and soluble molecules. The use of nano-topographies and nano-architectures as advanced implantable platforms that mimic the biophysical properties of the cellular microenvironment can accurately guide stem cells in their self-renewal, proliferation and differentiation processes [354–356]. In the recent literature, a wide range of nano-topographic geometries and arrays including nanopits, nanoposts, nanogratings, pillars, grooves, ridges, nanotubes, nanopores, nanowires, and so forth, have all been reported to have tissue engineering applications [357–362].
The use of nano-architectured substrates that incorporate stem cells in cell-based therapy approaches is of high interest. Recently, the effect of controllable biophysical signals on the mechanosensitivity of cells has gained substantial attention. In one study, Chen et al. designed patterns with controllable nano-roughness on glass surfaces as a culture substrate for hESCs and NIH/3T3 fibroblasts. Their finding revealed that the nano-topography created a potent regulatory environment for hESC responses related to cell signaling and functions such as cell morphology, adhesion, self-renewal and proliferation, which were cell-type specific. Here, the topological sensing ability of hESCs resulted in feedback regulation, associated with the mechanosensory integrin-mediated adhesion to the cell-matrix, E-cadherin, and myosin II [363]. In a similar study, Yim et al. observed that the nano-topography and stiffness of substrates could modulate the behavior of hMSCs by altering the formation of activated integrin clusters and assembly of focal adhesion complexes. This, consequently, regulated the organization of the cell cytoskeleton of hMSCs cultured on a substrate with 350 nm grating nano-topographies on the surface of tissue-culture grade polystyrene and polydimethylsiloxane (PDMS) substrates. Here, by employing the nano-topographies, the expression of various integrin molecules including alpha, alpha 2, alpha V, beta2, beta 3 and beta 4 was decreased in comparison with the control group (i.e. non-patterned substrates). Moreover, the mechanical properties (e.g. Young's modulus and apparent viscosity) and expression of cytoskeleton and focal adhesion components were also changed on the patterned substrates. The grating nano-topography induced alignment of the actin cytoskeleton of the elongated hMSCs whereas the non-patterned substrate led to a dense and random spreading of the actin cytoskeleton network [364].
The differentiation of fibroblasts into myofibroblasts is an important stage in wound healing because of the important role of myofibroblasts in restoring skin homeostasis and modulating tissue physiology in the wound healing process [365, 366]. Keratinocyte behaviors can be influenced by modification of nanoscale topographies, which may cause improvements in wound healing [367, 368]. Parkinson et al. in a study, evaluated the effect of nano-topography on keratinocytes including their morphology, proliferation and migration for treatment of burn wounds. Herein, nanoporous anodic aluminum oxide (AAO) membranes were employed with a pore size of 300 nm, which increased keratinocyte expansion and migration and could cover a partial thickness burn wound. A burn injury dressing incorporating a nano-topographical AAO membrane enhanced skin healing with a significantly reduction in tissue granulation and maturation of epidermal layers, compared with a control group [369]. In a similar study, Pot et al. reported nano-topography could regulate keratocytes, fibroblasts and myofibroblasts, which have a major role in corneal wound healing. These cells were seeded onto planar polyurethane surfaces. After 24 h, the keratocytes, fibroblasts and myofibroblasts were stained and imaged for cell shape analysis as well as for studying migration rates. Here, the elongation of keratocytes, fibroblasts and myofibroblasts were reported to be more than 1000 nm on a pitch (i.e. groove + ridge) patterned surfaces where cells responded to topographic features that had a lower limit of size with a transition zone of 800 to 1200-nm pitch size. Moreover, using this nano-topography, while keratocytes remained motionless, the migration of fibroblasts as well as myofibroblasts toward surface ridges larger than 1 µm was also reported; however, directional guidance on submicron/nano-topographic or planar surfaces was lacking [370].
6.10.2 Cell-imprinted substrates
Cell-imprinted biomaterials are able to mimic the natural environment of stem cells, and induce stem cell differentiation and expansion for wound healing and artificial tissue applications [371, 372]. In this regard, various cell types and morphologies can be imprinted to design a proper cell niche micro-/nano-environment. The cost-effectiveness and high efficiency of this approach is likely to have many advantages in tissue engineering and regeneration as well as wound therapies, using various cell casting processes on surfaces [373]. In one study, cell-imprinted substrates were reported to function as an artificial NS for skin repair. Herein, PDMS casting based-cell-imprinted micro-/nano-environments were created using human keratinocyte patterned templates. The keratinocyte-cell-imprinted biomimetic substrates could mimic the surface morphology of the cell membrane, which created a suitable micro-/nano-environment for topographical-induced cell differentiation. Moreover, seeded ASCs on the cell-imprinted surfaces were stimulated to adopt keratinocyte morphology and properties. Molecular dynamics simulation of the stem cell nucleus interestingly revealed that changing the shape of stem cells into that resembling mature cells could affect the deformation of the nucleus and subsequent gene expression. The results demonstrated the cell-imprinted substrate could be utilized as an effective strategy to differentiate stem cells to skin cells for wound healing [374]. More recently, a similar research reported cell-imprinted surfaces as a smart nano-environment, constructed by utilizing templates so that different cell types, including chondrocytes, tenocytes and semifibroblasts had potentials to differentiate, re-differentiate and trans-differentiate from the seeded ASCs. Moreover, immunofluorescent staining of proteins such as collagen type II in chondrocytes revealed that ASCs, semifibroblasts and tenocytes were able to attain the chondrocyte phenotype after 2 weeks of being implanted on chondrocyte-imprinted substrates. These results indicated that cell-Imprinted substrates could be applied in skin regeneration and cell-based therapy [375].
6.10.3 3D bio-printed scaffolds
3D printing is an advanced technology that has been progressing rapidly, and demonstrating revolutionary effects in biomedical research and therapies. In 3D printing, complex biomolecules and even living components such as (stem) cells, proteins (e.g. fibrin, collagen, growth factors, etc.), and therapeutic molecules can be precisely patterned and assembled through layer-by-layer deposition on scaffolds e.g. hydrogels, with the aid of computer-aided designs and manufacturing (e.g. CAD and CAM) [373]. This technology can be used in several health care applications, including tissue regeneration; prosthetics fabrication, implant creation, anatomical model fabrication and drug delivery investigations [376, 377]. The potential of 3D printing and its ability to construct complex and elaborate patterns and architectures can be utilized in the study of wound healing therapies to manufacture 3D-scaffolds, dressings, etc. or to create powerful platforms as skin models to investigate and find treatments for skin diseases such as burn wounds (Figure 13). Bio-printing based formation of cell-matrix components has been reported for the regeneration of skin tissue and formation of stem cell-scaffold complexes to thoroughly cover the full-thickness skin damage in burn wounds [373]. As an example of recent pioneering studies, Lee et al. produced eight collagen layers as human-like skin using a 3D printing platform. The structure of the 3D printed platform consisted of implanted cells within a multi-layered collagen construction on poly-d-lysine-coated glass petri dishes previously nebulized by sodium bicarbonate steam. The eight layers of collagen consisted of six collagen layers sandwiching three layers of fibroblasts and two additional collagen layers that included keratinocytes [378].
Figure 13.
Schematic of 3D-printing technology developed for the fabrication of 3D-scaffolds and dressings, within which cell-matrix components can be implanted, with applications in burn wound healing and tissue regeneration.
As 3D-printable platforms have been dramatically progressing in recent years, unprecedented concepts have been also introduced to this area such as "4D-printing". In this regard, the introduction of smart materials into 3D-printable platforms has been called as "4D-printing", which means the shape, performance, functionality and properties of printed smart materials can be altered over time (as the 4th dimension) and in response to different stimuli. 4D-printed structures can be designed to be self-assembled, multi-functional, self-repairable, etc. In this regard, 4D-bioprintable platforms will likely become an important area of research in tissue regeneration and engineering, particularly for printable smart scaffolds, skin regeneration and artificial skin, with applications in (burn) wound healing and regeneration [379, 380]. From the bio-inspired aspect, 4D-bio-pintable platforms are printed objects that can show smart stimuli-responsive behavior when for instance, external stimuli are applied, or when cell-fusion or post-printing self-assembly are employed [381].
6.10.4 CRISPR-Cas9: new gene editing tool for reprogramming of stem cells
Gene editing through the clustered regularly interspaced palindromic repeats (CRISPR)-Cas system has been introduced for the modification of the genome in many microorganisms and eukaryotic cells [382]. Originally discovered as a form of bacterial immunity to viruses, type 2 known as CRISPR-Cas9 system is a simple and efficient tool employed for manipulation of heterogenous and endogenous gene expression. This breakthrough approach can be applied for somatic and embryonic gene therapy, genomic screening and the generation of animal models that have been exploited in all biomedical-related researches, ranges from basic research to gene therapy [383, 384]. CRISPR/Cas9 system can be applied to reprogram primary fibroblasts so as to produce iPSCs. iPSCs themselves have the potential to differentiate into other cell types such as keratinocytes and MSCs, which are the most important cells for wound regeneration [385, 386]. More recently, a CRISPR-Cas system was reported, which could be applied in gene therapy for skin diseases and wound healing. Herein, the designed gene therapy system enhanced skin growth factors expression such as EGF, TGF-β, PDGF and FGF [387]. Hence, CRISPR-Cas9 gene editing technology can be a highly potent tool in cell and tissue engineering as well as regenerative medicine. In addition, it suggests new horizons in wound healing and infection prevention, which requires more research to be conducted to fully realize the potential. Figure 14 illustrates the concept of exploiting the gene editing tool, CRISPR-Cas, for reprogramming of stem cells (e.g. iPSCs), where reprogrammed (i.e. differentiated) cells can be integrated in fabricated 3D-scaffolds, for wound healing applications.
Figure 14.
Schematic representing the concept of using gene editing tools such as CRISPR-Cas for reprogramming of stem cells (e.g. iPSCs) in wound healing applications
7. Animal models for burn wounds
Animal models of burn wounds are widely used in the laboratory to evaluate different drugs and treatments that are being developed for treatment of human burn victims [388].
In wound therapy research, regarding various wound models, different aspects should be considered including incision or excision models, acute, chronic or diabetic wounds, administration methods for therapies (e.g. topically, intravenously, intradermally, and spray), in-vitro models (e.g. EpiDermFT as an epidermal full-thickness model, and human/mouse fibroblast and keratinocyte models as popular models), in-vivo models (e.g. rat and mouse that are cost-effective and user-friendly, as well as pig and rabbit), and so forth [154].
Burn wounds are usually created on the shaved backs of animal models such as mice, rats, rabbits and pigs, with a range from 5% to 30% TBSA for 3 to 30 seconds to produce partial to full thickness thermal damage. As an advantage, anatomically and physiologically the skin structure of the guinea pig is more analogous to human skin compared with mouse and rat. The epidermal thickness of pig is 30 to 140 µm similar to the human epidermal thickness which is 50 to 120 µm [389, 390]. Thus guinea pig burn models are better able to emulate human thermal wounds. However, mice and rats are less expensive than guinea pigs. Studies have revealed that inoculating infectious microorganisms topically is more clinically relevant than injecting them under the burn wound [391]. Investigations have also shown that acute infections can be induced in animal models e.g. mouse, rat and pig by invasive microorganisms such as P. aeruginosa, whereas chronic infections can be created using less virulent microorganisms such as S. aureus [7, 392]. Here we briefly discuss important animal models for burn wounds, which are classified as: gas flame burn model, burning ethanol bath burn model, pre-heated single metal plate/bar boiling or hot water burn models, and pre-heated double brass block burn model [391].
7.1 Gas flame burn model
Katakura burn model
In this model, a 2.5 × 3.5 cm window equipped template is placed on the shaved back of mice, and subsequently, the exposed site on the skin is heated for 9 second using gas flame. This flame-induced burn model can create a third degree burn with a nearly 15% TBSA on 26 g SCID-bg mice. Subsequently, gas flame burned immune-deficient mice are inculcated with a 2 × 103CFU MRSA and various numbers of CFU E. faecalis to test the influence of an antimicrobial agent on antibiotic-resistant burn infections [393].
7.2 Burning ethanol bath burn model
Stieritz-Holder burn model
These authors applied an ethanol bath to make a burn wound animal model. In this model, anesthetized and back-shaved female BALB/c mice weighing 22–24 g are used. To generate a nearly 30% of TBSA, back-shaved mice are exposed to an asbestos sheet containing a window, firmly pressed on the back. Subsequently, ethanol is evenly sprayed on the region specified by the window, and then ignited and allowed to burn for 10 sec. The bacterial infection models are created via inoculating 100 CFU of P. aeruginosa and K. pneumoniae into the burned subcutaneous area. Here, the infections can rapidly lead to death depending on the virulence of the bacterial strain chosen. This model has been employed to investigate the antibacterial function of polyclonal immunoglobulin antibodies for burn wound healing [391, 394].
7.3 Pre-heated single metal plate/bar burn models
Tavares burn model
In this model, a pre-heated aluminum rod (51 g in weight and 10 mm in diameter) is utilized. To make a second degree burn wound, it is heated to about 98 to 100°C for 10 min. Subsequently, the heated aluminum rod is pressed on the shaved back of male rats for 15 sec. Every seven-days for 4 weeks, the anesthetized burned animal is evaluated for bleeding and infection. After 4 weeks, re-epithelialization cis usually completed through fibroblast expansion and new collagen synthesis [395].
Orenstein burn model
In this model, to create a third degree burn, a pre-heated 1 × 1 × 3 cm plate is used, with a 20 cm handle. The temperature of the copper plate is increased to 150°C where the plate is pressed on the back-shaved guinea pigs for 10 second. The burned area is inoculated after 15 min by 1mL suspension containing 108 S. aureus. This model can be employed for the investigation of prevention and treatment of infected burns [396].
7.4 Boiling or hot water burn models
Suzuki burn models
In this model, male Wistar rats weighing 450–550 g are used and their skin is brought into contact with a glass chamber device through which water circulates for 1 min at 35–60°C over a seven-day period. The important variable is how firmly the device is pressed onto the skin (a common applied pressure is 10 g/cm2). This model benefits from the ability to vary exposure time and temperature [391, 397].
Bahar burn model
This burn model involves the application of a 2.5 cm2 thick piece of absorbent lint cloth submerged in boiling water. This cloth is then applied to the back of male Wistar rats weighing 250–300 g for varying times (2 or 10 seconds) [398].
Bjornson burn model
Here, female or male guinea pigs are utilized weighing 300–350 g. as a burn model. In this model, 60 cm2 area is submerged in water at 99°C for 13 second to make an about 15% TBSA. To create second thermal wound, after 1 h, the guinea pigs are again exposed in the template. Subsequently, the animals are inoculated with 5 × 105CFU of P. aeruginosa, S. aureus and C. albicans under the burn area [399].
Mason and Walker burn model
These workers developed the first animal burn model in rats using boiling water, which is a standard boiling water burn model used in diverse investigations. In this model, a template is employed in the form of a thin metal half-cylinder that fits over the back of the rat. It has a hole of calculated dimensions in the central region of the half-cylinder. The size of the hole is determined to correspond with the size of the burn in the rat. The animal is anesthetized, then shaved on the back, followed by placing supine in the template. The exposed region is submerged into boiling water. To create a partial thickness (as 2nd degree) and full-thickness (as 3rd degree) burn model, back-shaved rats are exposed 3 and 10 seconds to boiling water, respectively. The upper restriction on wound burn size, permitting by Mason and Walker model is about 30% of TBSA. This model has been broadly employed in studies conducted on the suppression and therapy of burn wound infections, for example: bacterial translocation, testing various antimicrobial agents, gene therapy, wound dressing for inhibiting burn infections such as fungal (e.g. candidiasis) infections [391, 400].
7.5 Pre-heated double brass blocks burned models
Kaufman burn model
In this model, a cylindrical aluminum rod is utilized weighing at least 500 g, with diameter and length of the handle of 3.78 cm and 24 cm, respectively. The heating of the equipment is done while submerged in water for 120 minute, and then to create a deep partial skin thickness thermal wound at 75°C on shaved back of an anesthetized guinea-pig. The model can be applied to investigate epithelialization, contraction and scar formation of burn wounds [401]
Stevens burn model
This model uses two pre-heated brass blocks applied to the back of shaved mice for between 3 and 10 seconds depending on the depth of burn required. In this model, pre-heated double brass blocks (heated to 92–95°C) are placed onto either side of a raised skin fold on the back of the shaved mice for between 5 seconds depending on the depth of burn required, resulting in a 1.8 cm2 burned area being created with nearly 5% TBSA (as an example see Figure 15). Subsequently, to generate a bacterial infection model, various numbers of P. aeruginosa CFU are inoculated onto the burned region or else are intradermally injected into the eschars. Bacteria can be recovered from the unburned muscle by 1 day for some bacteria. In this burn model, survival at 10 d has been reported to be 60% with P. aeruginosa infection [391, 402].
Figure 15.
I–II) In Stevens burn model, a deep burn wound is generated using two blocks of brass with pre-heating of 92–95°C on a shaved mouse back. III) Square show burned region. IV) Bacterial suspension in PBS was used on the surface of burn. Reprinted from ref. [391], copyright 2011, Taylor and Francis Group (open access, licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.)
8. Recent clinical trial evaluations for wound healing
An essential step in using the various wound healing approaches discussed in the above-mentioned sections, is their translation into clinical applications and bench-to-bedside evaluation. In this regard, in this section, some of recent clinical trials in different stages of completion are summarized.
Essentially, clinical trials to understand the efficacy of (stem) cell therapy on the healing process of burn wounds are still needed so as to confirm their efficacy and wound-healing properties. In April 2017, Cytori Therapeutics Inc. obtained FDA approval for a pilot clinical trial of their product "Cytori Cell TherapyTM" using for thermal-burn injured patients. Here, the safety and feasibility of the product along with standard care for patients with 20% to 50% TBSA injuries was assessed [403]. In March 2017, a Phase I clinical trial in 2nd degree burn wound patients with less than 20% TBSA was verified to be conducted by University of Miami. The purpose of this investigation was to evaluate the safety of allogeneic MSC based treatment, and then to find the maximum safe dose to be used in the Phase II of this study [404].
Among currently used approaches for wound therapy, skin allografts have been suggested for dermo-protection and enhancing re-epithelialization in burn injuries [405].
The wound closure effect of “nitrosating substances” e.g. nitroso-thiols or RSNOs have been suggested due to their inflammation modulation, cytokine generation and vascular improvement functions. Employing nanotechnology can enhance the effect of such products. For example, recently an S-nitroso-N-acetyl cysteine containing hydrogel-based NP platform (NAC-SNO-np) was developed, with encouraging results in translational and bench-to-bedside studies. This nanoplatform could release NO in a timely manner, besides facilitating nitrosation. NAC-SNO-np also has been licensed by the company Nano Biomed Inc.[406, 407]. In another approach in 2007, Phase I and II clinical trials were designed and funded by National Institute of Health (NIH) to support filing with the FDA and marketing potential of a NO-generating topical gel for burn wound healing [408].
Ag NPs provide great promise in clinical applications of wound healing therapies. This is due to their intrinsic antimicrobial activity along with anti-inflammatory and wound healing effects resulting in scar reduction, suppression of local and systemic inflammation, modulation of cytokine levels, increasing wound closure via proliferation and migration of keratinocytes, and enhancing wound contraction via myoblast generation by fibroblast differentiation. Furthermore, the effect of Ag NPs as a UV and radio-therapy sensitizing agent and their effect on the immune system can be also considered [409].
Growth factors can influence wound healing, and clinical evaluation of their exogeneous application is required. PDGF, a growth factor and cytokine, can facilitate the transition from a chronic wound to short-term healing by stimulation of granulation tissue formation, and has been tested in clinical trials [405]. Delivery of PDGF as a topical agent to burn wounds may be achieved by nano-platform approaches. In 2010, a clinical study was reported to evaluate healing of second degree burn wounds using the growth factor, rhEGF. In this study, 80 elderly patients were studied from 2003 to 2008, and the results indicated that rhEGF enhanced burn wound healing [410]. In a recent first-in-man clinical trial, a collagen/gelatin scaffold was developed capable of sustained release of FGFs. The results of this clinical trial indicated the efficacy and safety of the scaffold applied on patients [411].
A phase I clinical trial carried out by Stanford University Medical Center (CA, USA) used a skin-based gene therapy for wound healing, and was hailed as the first safe and effective skin-based gene therapy. This study aimed at grafting genetically altered skin onto chronic wounds of epidermolysis bullosa patients [412].
In 2012, a randomized controlled trial was carried out where the analgesia effect of a combined donor compound that could release nitrous oxide (N2O)/oxygen was investigated to reduce pain associated with changing burn dressings [413].
In an recently conducted phase 1 clinical trial, the combination of high-voltage, pulsed-current electric therapy and silver-collagen dressing were shown to be efficacious for chronic full-thickness wounds [414].
In addition to the aforementioned case studies, the literature also contains examples, where the effects of keratinocytes and stem cells, pirfenidone NPs, hyperbaric oxygen, opioids, and so forth, on burn wound healing have been explored [415].
9. Concluding remarks and perspectives
Burns pose a highly complex problem in medical care. The high susceptibility to microbial infection coupled with the need to achieve rapid and satisfactory wound healing, while at the same time avoiding undesirable scarring taxes the resources of even specialized burns units. While topical application of antimicrobial agents has formed the bedrock of burns care, alternative approaches including immune-based antimicrobial molecules, therapeutic microorganisms, antimicrobial phototherapy (PTT, PDT, blue and green light or NIR based therapies), and different kinds of cell therapy have been explored. Nonetheless, antimicrobial resistance developing in a wide range of microorganisms has created vast problems for topical treatment of burned infections in recent years. Since antibiotic-resistant microorganisms are now the main threat to public health, new advanced approaches such as nanotechnology are being investigated that may be able to offer novel therapeutic strategies in the post-antibiotic era.
Up until the present the most important problem in burn wounds has been chronic infections caused by some microorganisms such as P. aeruginosa, that are especially serious in deep burns with large areas involved. This is due to the intrinsic and acquired resistance of bacteria to antibiotics resulting in high mortality in burn wound patients.
A wide range of sophisticated nanostructures are now being explored in burn wounds, which may be able to overcome the problem of infection, while at the same time stimulating skin regeneration. Hence, in this review we have focused on both organic and non-organic NSs including polymeric NPs, NEs, SLNs, hydrogels, ceramic NPs, metal NPs, TiO2 NPs, MNPs, carbon-based NPs, QDs and NFs all of which could be possibly utilized as potential antimicrobial agents in burns. In addition, other NS-based platforms may be more directed towards improving burn wound healing such as 3D-scaffolds, wound dressings and films, strategies to deliver growth factors, and stimuli-responsive NSs. These various platforms can be effectively utilized for wound therapy applications such as PDT, PTT, stimulus-sensitive drug delivery, cell therapy, growth factor delivery, and gene therapy.
NSs can be incorporated into dressings that are applied to the burned area, especially for inhibition of microbial proliferation, modulation of immune response, and acceleration of wound healing. Studies have shown that NSs either used alone or as multifunctional targeted and smart agents can be effective strategies to inhibit microbial growth in the burn wound microenvironment. NSs can also be implemented to design stem cell–based scaffolds for skin regeneration and remodeling. The unique properties of stem cells combined with nano-structured scaffolds may give rise to insights that may lead to substantial advancements in burn therapy. The advanced properties of NSs suggest they can be a technological step forward to prevent infections in thermal wounds and improved healing of damaged tissues compared with conventional topical treatments.
Burn wound healing can also be improved by using scaffolds possessing innovative features that have been fabricated via novel and user-friendly techniques. Materials such as electrospun NFs, smart stimuli-responsive and multifunctional 3D-scaffolds with favorable properties including the ability to control proliferation, migration and differentiation of various cell types, loading and on-demand release of antimicrobial therapeutics, and so forth, will lead to more effective wound therapies and suppression of burn infection. More importantly, recently developed technologies including 3D/4D-printing, use of cell-imprinted substrates, and nano-scale architectured surfaces have emerged with beneficial properties and features. In addition, some biotechnology-related advances such as powerful gene-editing techniques including CRISPR-Cas-based systems will create a milestone in the therapy of various diseases, particularly with major effects on treatment of (burn) wound healing and suppression of pathogens and infections, which are expected to become reality in the coming years.
Considering the immense research effort that is currently being invested in nanomedcine applications, future progress in burn treatment is expected to result from these above-mentioned innovative approaches.
Supplementary Material
Acknowledgments
Michael R. Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700
Abbreviations
- 2ABI
2-Aminobenzimidazole
- Ang-2
Angiopoietin=2
- ASC
Adipose-derived stem cell
- ATP
Adenosine triphosphate
- AuNP
Gold nanoparticles
- BALO
Bdellovibrio and like organisms
- BC
Bacterial cellulose
- BLT
Blue light therapy
- CNF
Carbon nanofibers
- CNP
Carbon NP
- CNT
Carbon nanotubes
- CrHFC
Crypreserved human cultured fibroblasts
- CRISPR
clustered regularly interspaced palindromic repeats
- CS
Chitosan
- DC
Dendritic cell
- DOTAP
N-(2,3-Dioleoyloxy-1-propyl) trimethylammonium methyl sulfate
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EPS
Exopolysaccharide
- ERK
Extracellular regulated kinase
- FDA
US Food and drug administration
- FGF
Fibroblast growth factor
- FIR
Far infrared
- GMCSF
Granulocyte macrophage colony stimulating factor
- GO
Graphene oxide
- GQD
Graphene quantum dots
- IGF
Insulin-C12like growth factor
- iPSC
Induced pluripotent stem cell
- KGF
Keratinocye growth factor
- IL
Interleukin
- LED
Light emitting diode
- LLLT
Low-level laser (light) therapy
- LPL
Low power laser
- mAb
Monoclonal antibody
- MNP
Magnetic NP
- MRSA
Methicillin resistant Staphylococcus aureus
- MSC
Mesenchymal stem cells
- MSN
Mesoporous silica nanoparticle
- NE
Nanoemulsion
- NGF
Nerve growth factor
- NHDF
Normal human dermal fibroblasts
- NHEK
Normal human epidermal keratinocytes
- NIR
Near infrared
- NO
Nitric oxide
- NP
Nanoparticle
- NS
Nanostructure
- OP-GEL
Oxidized pectin gelatin
- PcrV(A)
P. aeruginosa V(A) antigen
- PDGF
Platelet-derived growth factor
- PDMS
polydimethylsiloxane
- PDT
Photodynamic therapy
- PEG
Polyethylene glycol
- PGLA
Poly-lactic-co-glycolic acid
- Phyto-AuNP
Phytochemical decorated AuNP
- PMN
Polymorphonuclear neutrophil
- PS
Photosensitizer
- Psl
Polysaccharide synthesis locus
- PTT
Photothermal therapy
- PVPI
Povidone iodine complex
- QD
Quantum dot
- rhEGF
Recombinant human EGF
- ROS
Reactive oxygen species
- siRNA
Small interfering RNA
- SLBN
Solid lipid-based NP
- SSD
Silver sulfadiazine
- SWCNT
Single wall CNT
- TGF
Transforming growth factor
- TiO2 NP
Titanium dioxide NP
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- TTSS
Type III secretion system
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peck M, Molnar J, Swart D. A global plan for burn prevention and care. Bulletin of the World Health Organization. 2009;87:802–803. doi: 10.2471/BLT.08.059733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.http://www.who.int.
- 3.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clinical microbiology reviews. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salmon JK, Armstrong CA, Ansel JC. The skin as an immune organ. West J Med. 1994;160:146–152. [PMC free article] [PubMed] [Google Scholar]
- 5.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: review and advancements. Critical care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kallinen O, Koljonen V, Tukiainen E, Randell T, Kirves H. Prehospital Care of Burn Patients and Trajectories on Survival. Prehospital emergency care : official journal of the National Association of EMS Physicians and the National Association of State EMS Directors. 2016;20:97–105. doi: 10.3109/10903127.2015.1056895. [DOI] [PubMed] [Google Scholar]
- 7.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent patents on anti-infective drug discovery. 2010;5:124–151. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sevgi M, Toklu A, Vecchio D, Hamblin MR. Topical antimicrobials for burn infections - an update. Recent patents on anti-infective drug discovery. 2013;8:161–197. doi: 10.2174/1574891x08666131112143447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin R, Dai T, Avci P, Jorge AE, de Melo WC, Vecchio D, Huang YY, Gupta A, Hamblin MR. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol. 2013 doi: 10.1016/j.coph.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marquart-Elbaz C, Lipsker D, Sick H, Grosshans E, Cribier B. Does subcutaneous cellular tissue exist? Annales de dermatologie et de venereologie. 2001:1201–1205. [PubMed] [Google Scholar]
- 11.Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 13.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 16.Sellheyer K, Krahl D. Skin mesenchymal stem cells: prospects for clinical dermatology. J Am Acad Dermatol. 2010;63:859–865. doi: 10.1016/j.jaad.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Bellas E, Seiberg M, Garlick J, Kaplan DL. In vitro 3D Full-Thickness Skin-Equivalent Tissue Model Using Silk and Collagen Biomaterials. Macromolecular bioscience. 2012;12:1627–1636. doi: 10.1002/mabi.201200262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo S, Dipietro LA. Factors affecting wound healing. Journal of dental research. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novelli S, Garcia-Muret P, Mozos A, Sierra J, Briones J. Total body-surface area as a new prognostic variable in mycosis fungoides and Sezary syndrome. Leukemia & lymphoma. 2016;57:1060–1066. doi: 10.3109/10428194.2015.1057894. [DOI] [PubMed] [Google Scholar]
- 20.Staley M, Richard R. Management of the acute burn wound: an overview. Advances in wound care : the journal for prevention and healing. 1997;10:39–44. [PubMed] [Google Scholar]
- 21.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Strbo N, Yin N, Stojadinovic O. Innate and Adaptive Immune Responses in Wound Epithelialization. Advances in wound care. 2014;3:492–501. doi: 10.1089/wound.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clinics in dermatology. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Advances in wound care. 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 26.Reinke JM, Sorg H. Wound repair and regeneration. European surgical research. Europaische chirurgische Forschung. Recherches chirurgicales europeennes. 2012;49:35–43. doi: 10.1159/000339613. [DOI] [PubMed] [Google Scholar]
- 27.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Advances in skin & wound care. 2012;25:349–370. doi: 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinz B. Formation and function of the myofibroblast during tissue repair. The Journal of investigative dermatology. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 29.Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals. 2013;6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong M, Chen M, Zhang J. Rational Evolution of Antimicrobial Peptides Containing Unnatural Amino Acids to Combat Burn Wound Infections. Chemical biology & drug design. 2016 doi: 10.1111/cbdd.12768. [DOI] [PubMed] [Google Scholar]
- 31.Zhong G, Cheng J, Liang ZC, Xu L, Lou W, Bao C, Ong ZY, Dong H, Yang YY, Fan W. Short Synthetic beta-Sheet Antimicrobial Peptides for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Burn Wound Infections. Advanced healthcare materials. 2017;6 doi: 10.1002/adhm.201601134. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida S, Lee JO, Nakamura K, Suzuki S, Hendon DN, Kobayashi M, Suzuki F. Lineage - CD34+CD31+ cells that appear in association with severe burn injury are inhibitory on the production of antimicrobial peptides by epidermal keratinocytes. PloS one. 2014;9:e82926. doi: 10.1371/journal.pone.0082926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lahoda LU, Wang SC, Vogt PM. A mixture of antimicrobial peptides and fibrin glue in treatment of partial-thickness burn wounds. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2006;77:251–256. doi: 10.1007/s00104-005-1089-8. [DOI] [PubMed] [Google Scholar]
- 34.Jaskiewicz M, Orlowska M, Olizarowicz G, Migon D, Grzywacz D, Kamysz W. Rapid Screening of Antimicrobial Synthetic Peptides. International journal of peptide research and therapeutics. 2016;22:155–161. doi: 10.1007/s10989-015-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jindal HM, Le CF, Mohd Yusof MY, Velayuthan RD, Lee VS, Zain SM, Isa DM, Sekaran SD. Antimicrobial Activity of Novel Synthetic Peptides Derived from Indolicidin and Ranalexin against Streptococcus pneumoniae. PLoS One. 2015;10:e0128532. doi: 10.1371/journal.pone.0128532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agier J, Brzezinska-Blaszczyk E. Cathelicidins and defensins regulate mast cell antimicrobial activity. Postepy higieny i medycyny doswiadczalnej. 2016;70:618–636. doi: 10.5604/17322693.1205357. [DOI] [PubMed] [Google Scholar]
- 37.Fabisiak A, Murawska N, Fichna J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacological reports : PR. 2016 doi: 10.1016/j.pharep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Agier J, Efenberger M, Brzezinska-Blaszczyk E. Cathelicidin impact on inflammatory cells. Central-European journal of immunology / Polish Society for Immunology and eleven other Central-European immunological societies. 2015;40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Actor JK. Lactoferrin: A Modulator for Immunity against Tuberculosis Related Granulomatous Pathology. Mediators of inflammation. 2015;2015:409596. doi: 10.1155/2015/409596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. The Journal of allergy and clinical immunology. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annual review of nutrition. 1995;15:93–110. doi: 10.1146/annurev.nu.15.070195.000521. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed MF, Abdelkhalek A, Seleem MN. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Scientific reports. 2016;6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thaker HD, Som A, Ayaz F, Lui D, Pan W, Scott RW, Anguita J, Tew GN. Synthetic mimics of antimicrobial peptides with immunomodulatory responses. J Am Chem Soc. 2012;134:11088–11091. doi: 10.1021/ja303304j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemachandra S, Kamboj K, Copfer J, Pier G, Green LL, Schreiber JR. Human Monoclonal Antibodies against Pseudomonas aeruginosa Lipopolysaccharide Derived from Transgenic Mice Containing Megabase Human Immunoglobulin Loci Are Opsonic and Protective against Fatal Pseudomonas Sepsis. Infection and immunity. 2001;69:2223–2229. doi: 10.1128/IAI.69.4.2223-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Z, Kimmel R, Petersen S, Thomas S, Pier G, Bezabeh B, Luo R, Schreiber JR. Multi-valent human monoclonal antibody preparation against Pseudomonas aeruginosa derived from transgenic mice containing human immunoglobulin loci is protective against fatal pseudomonas sepsis caused by multiple serotypes. Vaccine. 2005;23:3264–3271. doi: 10.1016/j.vaccine.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 46.Barnea Y, Carmeli Y, Neville LF, Kahel-Reifer H, Eren R, Dagan S, Navon-Venezia S. Therapy with anti-flagellin A monoclonal antibody limits Pseudomonas aeruginosa invasiveness in a mouse burn wound sepsis model. Burns : journal of the International Society for Burn Injuries. 2009;35:390–396. doi: 10.1016/j.burns.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, Lu H, Sprinkle A, Parsek MR, Wozniak DJ. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. Journal of bacteriology. 2007;189:8353–8356. doi: 10.1128/JB.00620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawa T, Ito E, Nguyen VH, Haight M. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Human vaccines & immunotherapeutics. 2014;10:2843–2852. doi: 10.4161/21645515.2014.971641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warrener P, Varkey R, Bonnell JC, DiGiandomenico A, Camara M, Cook K, Peng L, Zha J, Chowdury P, Sellman B, Stover CK. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrobial agents and chemotherapy. 2014;58:4384–4391. doi: 10.1128/AAC.02643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu A, Das U, Dey S, Datta S. PcrG protects the two long helical oligomerization domains of PcrV, by an interaction mediated by the intramolecular coiled-coil region of PcrG. BMC structural biology. 2014;14:5. doi: 10.1186/1472-6807-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holder IA, Neely AN, Frank DW. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect Immun. 2001;69:5908–5910. doi: 10.1128/IAI.69.9.5908-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neely AN, Holder IA, Wiener-Kronish JP, Sawa T. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns : journal of the International Society for Burn Injuries. 2005;31:153–158. doi: 10.1016/j.burns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxidants & redox signaling. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR. Antimicrobial strategies centered around reactive oxygen species--bactericidal antibiotics, photodynamic therapy, and beyond. FEMS microbiology reviews. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terashvili M, Pratt PF, Gebremedhin D, Narayanan J, Harder DR. Reactive oxygen species cerebral autoregulation in health and disease. Pediatric clinics of North America. 2006;53:1029–1037. xi. doi: 10.1016/j.pcl.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. BioMed Research International. 2012;2012 doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3:271–279. doi: 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu H, Ka B, Murad F. Nitric oxide accelerates the recovery from burn wounds. World journal of surgery. 2007;31:624–631. doi: 10.1007/s00268-007-0727-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhu H, Wei X, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. Journal of burn care & research : official publication of the American Burn Association. 2008;29:804–814. doi: 10.1097/BCR.0b013e3181848119. [DOI] [PubMed] [Google Scholar]
- 60.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nature reviews. Microbiology. 2013;11:371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 61.Heyneman A, Hoeksema H, Vandekerckhove D, Pirayesh A, Monstrey S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns : journal of the International Society for Burn Injuries. 2016 doi: 10.1016/j.burns.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 62.Ganatra S, Cohen D. Silver nitrate burn of the lower lip: a case report. General dentistry. 2016;64:75–77. [PubMed] [Google Scholar]
- 63.Mohseni M, Shamloo A, Aghababaei Z, Vossoughi M, Moravvej H. Antimicrobial Wound Dressing Containing Silver Sulfadiazine With High Biocompatibility: In Vitro Study. Artificial organs. 2016 doi: 10.1111/aor.12682. [DOI] [PubMed] [Google Scholar]
- 64.Domenico P, Baldassarri L, Schoch PE, Kaehler K, Sasatsu M, Cunha BA. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrobial agents and chemotherapy. 2001;45:1417–1421. doi: 10.1128/AAC.45.5.1417-1421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nassar MA, Eldien HM, Tawab HS, Saleem TH, Omar HM, Nassar AY, Hussein MR. Time-dependent morphological and biochemical changes following cutaneous thermal burn injury and their modulation by copper nicotinate complex: an animal model. Ultrastructural pathology. 2012;36:343–355. doi: 10.3109/01913123.2012.685687. [DOI] [PubMed] [Google Scholar]
- 66.Champagne VK, Helfritch DJ. A demonstration of the antimicrobial effectiveness of various copper surfaces. Journal of biological engineering. 2013;7:1. doi: 10.1186/1754-1611-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sudha VB, Ganesan S, Pazhani GP, Ramamurthy T, Nair GB, Venkatasubramanian P. Storing drinking-water in copper pots kills contaminating diarrhoeagenic bacteria. Journal of health, population, and nutrition. 2012;30:17–21. doi: 10.3329/jhpn.v30i1.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dzyuba MV, Mardanov AV, Beletskii AV, Kolganova TV, Sukhacheva MV, Shelenkov AA, Gorlenko VM, Kuznetsov BB, Skryabin KG. Reconstruction of iron metabolism pathways of bacteria Magnetospirillum aberrantis SpK spp. based on sequenced genome analysis. Doklady biological sciences : proceedings of the Academy of Sciences of the USSR, Biological sciences sections / translated from Russian. 2012;444:202–205. doi: 10.1134/S001249661203009X. [DOI] [PubMed] [Google Scholar]
- 69.DeLeon K, Balldin F, Watters C, Hamood A, Griswold J, Sreedharan S, Rumbaugh KP. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrobial agents and chemotherapy. 2009;53:1331–1337. doi: 10.1128/AAC.01330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mangat HS, Stewart TL, Dibden L, Tredget EE. Complications of chlorine inhalation in a pediatric chemical burn patient: a case report. Journal of burn care & research : official publication of the American Burn Association. 2012;33:e216–221. doi: 10.1097/BCR.0b013e318254d1c8. [DOI] [PubMed] [Google Scholar]
- 71.Yuksel EB, Yildirim AM, Bal A, Kuloglu T. The effect of different topical agents (silver sulfadiazine, povidone-iodine, and sodium chloride 0.9%) on burn injuries in rats. Plastic surgery international. 2014;2014:907082. doi: 10.1155/2014/907082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lau YS, Brooks P. Innovative use of povidone-iodine to guide burn wound debridement and predict the success of biobrane as a definitive treatment for burns. Advances in skin & wound care. 2014;27:111–113. doi: 10.1097/01.ASW.0000442875.94332.fd. [DOI] [PubMed] [Google Scholar]
- 73.Rodeheaver GT, Wheeler CB, Vensko JA, Edgerton MT, Edlich RF. Extinguishing the flaming burn victim. Jacep. 1979;8:307–311. doi: 10.1016/s0361-1124(79)80370-6. [DOI] [PubMed] [Google Scholar]
- 74.Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Marine drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moghaddam AS, Raji A, Movaffagh J, Yazdi AT, Mahmoudi M. Effects of autologous keratinocyte cell spray with and without chitosan on third degree burn healing: an animal experiment. Wounds : a compendium of clinical research and practice. 2014;26:109–117. [PubMed] [Google Scholar]
- 76.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrobial agents and chemotherapy. 2005;49:1542–1552. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. Journal of antimicrobial chemotherapy. 2009:dkp409. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]
- 78.Ooi N, Miller K, Randall C, Rhys-Williams W, Love W, Chopra I. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. The Journal of antimicrobial chemotherapy. 2010;65:72–78. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]
- 79.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative medicine and cellular longevity. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The Home Reference to Holistic Health & Healing: Easy-To-Use Natural Remedies, Herbs, Flower Essences, Essential Oils, Supplements, and Therapeutic Practices for Health, Happiness, and Well-Being. Library Journal. 2015;140:106–107. [Google Scholar]
- 81.Sienkiewicz M, Łysakowska M, Pastuszka M, Bienias W, Kowalczyk E. The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules. 2013;18:9334–9351. doi: 10.3390/molecules18089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fertah M, Belfkira A, Dahmane Em, Taourirte M, Brouillette F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arabian Journal of Chemistry [Google Scholar]
- 83.Thomas JG, Slone W, Linton S, Okel T, Corum L, Percival SL. In vitro antimicrobial efficacy of a silver alginate dressing on burn wound isolates. Journal of wound care. 2011;20:124, 126–128. doi: 10.12968/jowc.2011.20.3.124. [DOI] [PubMed] [Google Scholar]
- 84.Brachkova MI, Marques P, Rocha J, Sepodes B, Duarte MA, Pinto JF. Alginate films containing Lactobacillus plantarum as wound dressing for prevention of burn infection. The Journal of hospital infection. 2011;79:375–377. doi: 10.1016/j.jhin.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Truong-Bolduc QC, Villet RA, Estabrooks ZA, Hooper DC. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. The Journal of infectious diseases. 2014;209:1485–1493. doi: 10.1093/infdis/jit660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clinical microbiology reviews. 2001;14:244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falcone PA, Harrison HN, Sowemimo GO, Reading GP. Mafenide acetate concentrations and bacteriostasis in experimental burn wounds treated with a three-layered laminated mafenide-saline dressing. Annals of plastic surgery. 1980;5:266–269. doi: 10.1097/00000637-198010000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Zahmatkesh M, Manesh MJ, Babashahabi R. Effect of Olea ointment and Acetate Mafenide on burn wounds - A randomized clinical trial. Iranian journal of nursing and midwifery research. 2015;20:599–603. doi: 10.4103/1735-9066.164507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ulkur E, Oncul O, Karagoz H, Yeniz E, Celikoz B. Comparison of silver-coated dressing (Acticoat), chlorhexidine acetate 0.5% (Bactigrass), and fusidic acid 2% (Fucidin) for topical antibacterial effect in methicillin-resistant Staphylococci-contaminated, full-skin thickness rat burn wounds. Burns : journal of the International Society for Burn Injuries. 2005;31:874–877. doi: 10.1016/j.burns.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Nunes PS, Albuquerque RL, Jr, Cavalcante DR, Dantas MD, Cardoso JC, Bezerra MS, Souza JC, Serafini MR, Quitans LJ, Jr, Bonjardim LR, Araujo AA. Collagen-based films containing liposome-loaded usnic acid as dressing for dermal burn healing. Journal of biomedicine & biotechnology. 2011;2011:761593. doi: 10.1155/2011/761593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dale RM, Schnell G, Wong JP. Therapeutic efficacy of "nubiotics" against burn wound infection by Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy. 2004;48:2918–2923. doi: 10.1128/AAC.48.8.2918-2923.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hammond AA, Miller KG, Kruczek CJ, Dertien J, Colmer-Hamood JA, Griswold JA, Horswill AR, Hamood AN. An in vitro biofilm model to examine the effect of antibiotic ointments on biofilms produced by burn wound bacterial isolates. Burns : journal of the International Society for Burn Injuries. 2011;37:312–321. doi: 10.1016/j.burns.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frei R, Breitbach AS, Blackwell HE. 2-Aminobenzimidazole derivatives strongly inhibit and disperse Pseudomonas aeruginosa biofilms. Angewandte Chemie. 2012;51:5226–5229. doi: 10.1002/anie.201109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bialoszewski D, Pietruczuk-Padzik A, Kalicinska A, Bocian E, Czajkowska M, Bukowska B, Tyski S. Activity of ozonated water and ozone against Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Medical science monitor : international medical journal of experimental and clinical research. 2011;17:BR339–344. doi: 10.12659/MSM.882044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sunnen GV. Method and apparatus for the deactivation of bacterial and fungal toxins in wounds, and for the disruption of wound biofilms. Google Patents. 2010 [Google Scholar]
- 96.Leiman SA, May JM, Lebar MD, Kahne D, Kolter R, Losick R. D-amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. Journal of bacteriology. 2013;195:5391–5395. doi: 10.1128/JB.00975-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joo HS, Otto M. Molecular basis of in vivo biofilm formation by bacterial pathogens. Chemistry & biology. 2012;19:1503–1513. doi: 10.1016/j.chembiol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manuel SG, Ragunath C, Sait HB, Izano EA, Kaplan JB, Ramasubbu N. Role of active-site residues of dispersin B, a biofilm-releasing beta-hexosaminidase from a periodontal pathogen, in substrate hydrolysis. The FEBS journal. 2007;274:5987–5999. doi: 10.1111/j.1742-4658.2007.06121.x. [DOI] [PubMed] [Google Scholar]
- 99.Donelli G, Francolini I, Romoli D, Guaglianone E, Piozzi A, Ragunath C, Kaplan JB. Synergistic activity of dispersin B and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrobial agents and chemotherapy. 2007;51:2733–2740. doi: 10.1128/AAC.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soares GM, Figueiredo LC, Faveri M, Cortelli SC, Duarte PM, Feres M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. Journal of applied oral science : revista FOB. 2012;20:295–309. doi: 10.1590/S1678-77572012000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heidari H, Emaneini M, Dabiri H, Jabalameli F. Virulence factors, antimicrobial resistance pattern and molecular analysis of Enterococcal strains isolated from burn patients. Microbial pathogenesis. 2016;90:93–97. doi: 10.1016/j.micpath.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Ohadian Moghadam S, Pourmand MR, Aminharati F. Biofilm formation and antimicrobial resistance in methicillin-resistant Staphylococcus aureus isolated from burn patients, Iran. Journal of infection in developing countries. 2014;8:1511–1517. doi: 10.3855/jidc.5514. [DOI] [PubMed] [Google Scholar]
- 103.Gudina EJ, Teixeira JA, Rodrigues LR. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Marine drugs. 2016;14 doi: 10.3390/md14020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jebur M. Therapeutic efficacy of Lactobacillus acidophilus against bacterial isolates from burn wounds. North American journal of medical sciences. 2010;2:586–591. doi: 10.4297/najms.2010.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sockett RE. Predatory lifestyle of Bdellovibrio bacteriovorus. Annual review of microbiology. 2009;63:523–539. doi: 10.1146/annurev.micro.091208.073346. [DOI] [PubMed] [Google Scholar]
- 106.Pasternak Z, Njagi M, Shani Y, Chanyi R, Rotem O, Lurie-Weinberger MN, Koval S, Pietrokovski S, Gophna U, Jurkevitch E. In and out: an analysis of epibiotic vs periplasmic bacterial predators. The ISME journal. 2014;8:625–635. doi: 10.1038/ismej.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iebba V, Totino V, Santangelo F, Gagliardi A, Ciotoli L, Virga A, Ambrosi C, Pompili M, De Biase RV, Selan L, Artini M, Pantanella F, Mura F, Passariello C, Nicoletti M, Nencioni L, Trancassini M, Quattrucci S, Schippa S. Bdellovibrio bacteriovorus directly attacks Pseudomonas aeruginosa and Staphylococcus aureus Cystic fibrosis isolates. Frontiers in microbiology. 2014;5:280. doi: 10.3389/fmicb.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harini K, Ajila V, Hegde S. Bdellovibrio bacteriovorus : A future antimicrobial agent? Journal of Indian Society of Periodontology. 2013;17:823–825. doi: 10.4103/0972-124X.124534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karimi M, Mirshekari H, Moosavi Basri SM, Bahrami S, Moghoofei M, Hamblin MR. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoff-Lenczewska D, Kawecki M, Glik J, Klama-Baryla A, Nowak M. The potential of bacteriophages in the treatment of burn wounds. Polski przeglad chirurgiczny. 2013;85:615–618. doi: 10.2478/pjs-2013-0092. [DOI] [PubMed] [Google Scholar]
- 111.Kumari S, Harjai K, Chhibber S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. Journal of medical microbiology. 2011;60:205–210. doi: 10.1099/jmm.0.018580-0. [DOI] [PubMed] [Google Scholar]
- 112.Rose T, Verbeken G, De Vos D, Merabishvili M, Vaneechoutte M, Lavigne R, Jennes S, Zizi M, Pirnay JP. Experimental phage therapy of burn wound infection: difficult first steps. International journal of burns and trauma. 2014;4:66–73. [PMC free article] [PubMed] [Google Scholar]
- 113.Lazareva E, Smirnov S, Khvatov V, Spiridonova T, Bitkova E, Darbeeva O, Maĭskaia L, Parfeniuk R, Men'shikov D. Efficacy of bacteriophages in complex treatment of patients with burn wounds. Antibiotiki i khimioterapiia= Antibiotics and chemoterapy[sic]/Ministerstvo meditsinskoi i mikrobiologicheskoi promyshlennosti SSSR. 2000;46:10–14. [PubMed] [Google Scholar]
- 114.Chadha P, Katare OP, Chhibber S. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microbial Pathogenesis. 2016;99:68–77. doi: 10.1016/j.micpath.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 115.Golkar Z, Bagasra O, Jamil N. Experimental phage therapy on multiple drug resistant Pseudomonas aeruginosa infection in mice. Journal of Antivirals & Antiretrovirals. 2013;2013 [Google Scholar]
- 116.Holguín AV, Rangel G, Clavijo V, Prada C, Mantilla M, Gomez MC, Kutter E, Taylor C, Fineran PC, Barrios AFG. Phage ΦPan70, a Putative Temperate Phage, Controls Pseudomonas aeruginosa in Planktonic. Biofilm and Burn Mouse Model Assays, Viruses. 2015;7:4602–4623. doi: 10.3390/v7082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yadav A, Gupta A, Keshri GK, Verma S, Sharma SK, Singh SB. Photobiomodulatory effects of superpulsed 904 nm laser therapy on bioenergetics status in burn wound healing. Journal of Photochemistry and Photobiology B: Biology. 2016;162:77–85. doi: 10.1016/j.jphotobiol.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 118.Dai T, Gupta A, Huang Y-Y, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrobial agents and chemotherapy. 2013;57:1238–1245. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Catão MHCV, Costa RO, Nonaka CFW, Junior RLCA, Costa IRRS. Green LED light has anti-inflammatory effects on burns in rats. Burns. 2016;42:392–396. doi: 10.1016/j.burns.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 120.Chiu H-W, Chen C-H, Chang J-N, Chen C-H, Hsu Y-H. Far-infrared promotes burn wound healing by suppressing NLRP3 inflammasome caused by enhanced autophagy. Journal of Molecular Medicine. 2016;94:809–819. doi: 10.1007/s00109-016-1389-0. [DOI] [PubMed] [Google Scholar]
- 121.Silveira PCL, Ferreira KB, da Rocha FR, Pieri BLS, Pedroso GS, De Souza CT, Nesi RT, Pinho RA. Effect of Low-Power Laser (LPL) and Light-Emitting Diode (LED) on Inflammatory Response in Burn Wound Healing. Inflammation. 2016;39:1395–1404. doi: 10.1007/s10753-016-0371-x. [DOI] [PubMed] [Google Scholar]
- 122.Rathnakar B, Prabhu V, Rao BSS, Chandra S, Rai S, Mahato KK. Regulation of cellular marker modulated upon irradiation of low power laser light in burn injured mice, SPIE BioPhotonics Australasia. International Society for Optics and Photonics. 2016 100132Z-100132Z-100136. [Google Scholar]
- 123.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. Antimicrobial Blue Light Therapy for Multidrug-Resistant Acinetobacter baumannii Infection in a Mouse Burn Model: Implications for Prophylaxis and Treatment of Combat-related Wound Infections. The Journal of Infectious Diseases. 2013;209:1963–1971. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bay C, Togsverd-Bo K, Lerche CM, Haedersdal M. Skin tumor development after UV irradiation and photodynamic therapy is unaffected by short-term pretreatment with 5-fluorouracil, imiquimod and calcipotriol. An experimental hairless mouse study. Journal of photochemistry and photobiology. B, Biology. 2016;154:34–39. doi: 10.1016/j.jphotobiol.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 125.Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in cutaneous medicine and surgery. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 126.Gold MH, Andriessen A, Biron J, Andriessen H. Clinical Efficacy of Self-applied Blue Light Therapy for Mild-to-Moderate Facial Acne. The Journal of clinical and aesthetic dermatology. 2009;2:44–50. [PMC free article] [PubMed] [Google Scholar]
- 127.Khan MS, Abdelhamid HN, Wu H-F. Near infrared (NIR) laser mediated surface activation of graphene oxide nanoflakes for efficient antibacterial, antifungal and wound healing treatment. Colloids and Surfaces B: Biointerfaces. 2015;127:281–291. doi: 10.1016/j.colsurfb.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 128.Nafee N, Youssef A, El-Gowelli H, Asem H, Kandil S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. International journal of pharmaceutics. 2013;454:249–258. doi: 10.1016/j.ijpharm.2013.06.067. [DOI] [PubMed] [Google Scholar]
- 129.Garcia VG, de Lima MA, Okamoto T, Milanezi LA, Júnior ECG, Fernandes LA, de Almeida JM, Theodoro LH. Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: histological study in rats. Lasers in medical science. 2010;25:221–228. doi: 10.1007/s10103-009-0694-z. [DOI] [PubMed] [Google Scholar]
- 130.Aleem NA, Aslam M, Zahid MF, Rahman AJ, Rehman FU. Treatment of burn wound infection using ultraviolet light: a case report. Journal of the American College of Clinical Wound Specialists. 2013;5:19–22. doi: 10.1016/j.jccw.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bohrerova Z, Linden KG. Assessment of DNA damage and repair in Mycobacterium terrae after exposure to UV irradiation. J Appl Microbiol. 2006;101:995–1001. doi: 10.1111/j.1365-2672.2006.03023.x. [DOI] [PubMed] [Google Scholar]
- 132.Gupta A, Avci P, Dai T, Huang YY, Hamblin MR. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Advances in wound care. 2013;2:422–437. doi: 10.1089/wound.2012.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics and Lasers in Medicine. 2012;1:267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta biomaterialia. 2014;10:4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rünzler D, Weihs AM, Redl H, Teuschl AH, Fuchs C. Shockwave treatment enhances proliferation and improves wound healing via purinergic signaling induced ERK1/2 pathway. 2016 [Google Scholar]
- 136.Fantinati MS, Mendonça DEO, Fantinati AMM, Santos BFd, Reis JCO, Afonso CL, Vinaud MC, Júnior L, de Souza R. Low intensity ultrasound therapy induces angiogenesis and persistent inflammation in the chronic phase of the healing process of third degree burn wounds experimentally induced in diabetic and non-diabetic rats. Acta Cirurgica Brasileira. 2016;31:463–471. doi: 10.1590/S0102-865020160070000006. [DOI] [PubMed] [Google Scholar]
- 137.Karimi M, Bahrami S, Mirshekari H, Basri SMM, Nik AB, Aref AR, Akbari M, Hamblin MR. Microfluidic systems for stem cell-based neural tissue engineering. Lab on a Chip. 2016;16:2551–2571. doi: 10.1039/c6lc00489j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Foubert P, Doyle-Eisele M, Gonzalez A, Berard F, Weber W, Zafra D, Alfonso Z, Zhao S, Tenenhaus M, Fraser JK. Development of a combined radiation and full thickness burn injury minipig model to study the effects of uncultured adipose-derived regenerative cell therapy in wound healing. International Journal of Radiation Biology. 2016:1–11. doi: 10.1080/09553002.2017.1242814. [DOI] [PubMed] [Google Scholar]
- 139.Yang R-H, Qi S-H, Ruan S-B, Lin Z-P, Lin Y, Zhang F-G, Chen X-D, Xie J-L. EGFL7-overexpressing epidermal stem cells promotes fibroblast proliferation and migration via mediating cell adhesion and strengthening cytoskeleton. Molecular and Cellular Biochemistry. 2016;423:1–8. doi: 10.1007/s11010-016-2812-0. [DOI] [PubMed] [Google Scholar]
- 140.Bliley JM, Argenta A, Satish L, McLaughlin MM, Dees A, Tompkins-Rhoades C, Marra KG, Rubin JP. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns. 2016;42:1212–1222. doi: 10.1016/j.burns.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 141.Hu C, Yong X, Li C, Lü M, Liu D, Chen L, Hu J, Teng M, Zhang D, Fan Y, Liang G. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. Journal of Surgical Research. 2013;183:427–434. doi: 10.1016/j.jss.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 142.Clover AJP, Kumar AHS, Isakson M, Whelan D, Stocca A, Gleeson BM, Caplice NM. Allogeneic mesenchymal stem cells, but not culture modified monocytes, improve burn wound healing. Burns. 2015;41:548–557. doi: 10.1016/j.burns.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y-W, Scutaru TT, Ghetu N, Carasevici E, Lupascu CD, Ferariu D, Pieptu D, Coman C-G, Danciu M. The Effects of Adipose-Derived Stem Cell–Differentiated Adipocytes on Skin Burn Wound Healing in Rats. Journal of Burn Care & Research. 2017;38:1–10. doi: 10.1097/BCR.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 144.Kranz I, Gonzalez JB, Dorfel I, Gemeinert M, Griepentrog M, Klaffke D, Knabe C, Osterle W, Gross U. Biological response to micron- and nanometer-sized particles known as potential wear products from artificial hip joints: Part II: Reaction of murine macrophages to corundum particles of different size distributions. J Biomed Mater Res A. 2009;89:390–401. doi: 10.1002/jbm.a.32121. [DOI] [PubMed] [Google Scholar]
- 145.Malekzad H, Mirshekari H, Sahandi Zangabad P, Moosavi Basri SM, Baniasadi F, Sharifi Aghdam M, Karimi M, Hamblin MR. Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems. Critical Reviews in Biotechnology. 2017:1–21. doi: 10.1080/07388551.2017.1312267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karimi M, Zare H, Bakhshian Nik A, Yazdani N, Hamrang M, Mohamed E, Sahandi Zangabad P, Moosavi Basri SM, Bakhtiari L, Hamblin MR. Nanotechnology in diagnosis and treatment of coronary artery disease. Nanomedicine. 2016;11:513–530. doi: 10.2217/nnm.16.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M, Shahreza S, Sori M, Hamblin MR. Albumin nanostructures as advanced drug delivery systems. Expert Opinion on Drug Delivery. 2016;13:1609–1623. doi: 10.1080/17425247.2016.1193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J. Nanomedicine in the Management of Microbial Infection - Overview and Perspectives. Nano today. 2014;9:478–498. doi: 10.1016/j.nantod.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jia F, Liu X, Li L, Mallapragada S, Narasimhan B, Wang Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. Journal of controlled release : official journal of the Controlled Release Society. 2013;172:1020–1034. doi: 10.1016/j.jconrel.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Tocco I, Zavan B, Bassetto F, Vindigni V. Nanotechnology-based therapies for skin wound regeneration. Journal of Nanomaterials. 2012;2012:4. [Google Scholar]
- 151.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nature Reviews Immunology. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yah CS, Simate GS. Nanoparticles as potential new generation broad spectrum antimicrobial agents. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2015;23:43. doi: 10.1186/s40199-015-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 154.Kalashnikova I, Das S, Seal S. Nanomaterials for wound healing: scope and advancement. Nanomedicine (Lond) 2015;10:2593–2612. doi: 10.2217/NNM.15.82. [DOI] [PubMed] [Google Scholar]
- 155.Khan IU, Serra CA, Anton N, Vandamme TF. Production of nanoparticle drug delivery systems with microfluidics tools. Expert opinion on drug delivery. 2015;12:547–562. doi: 10.1517/17425247.2015.974547. [DOI] [PubMed] [Google Scholar]
- 156.Fathi M, Zangabad PS, Aghanejad A, Barar J, Erfan-Niya H, Omidi Y. Folate-conjugated thermosensitive O-maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydrate Polymers. 2017;172:130–141. doi: 10.1016/j.carbpol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 157.Tokuda M, Yamane M, Thickett SC, Minami H, Zetterlund PB. Synthesis of polymeric nanoparticles containing reduced graphene oxide nanosheets stabilized by poly(ionic liquid) using miniemulsion polymerization. Soft matter. 2016;12:3955–3962. doi: 10.1039/c6sm00269b. [DOI] [PubMed] [Google Scholar]
- 158.Gardner JC, Wu H, Noel JG, Ramser BJ, Pitstick L, Saito A, Nikolaidis NM, McCormack FX. Keratinocyte growth factor supports pulmonary innate immune defense through maintenance of alveolar antimicrobial protein levels and macrophage function. American journal of physiology. Lung cellular and molecular physiology. 2016;310:L868–879. doi: 10.1152/ajplung.00363.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feng ZG, Pang SF, Guo DJ, Yang YT, Liu B, Wang JW, Zheng KQ, Lin Y. Recombinant keratinocyte growth factor 1 in tobacco potentially promotes wound healing in diabetic rats. Biomed Res Int. 2014;2014:579632. doi: 10.1155/2014/579632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, Yarmush ML. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1034–1039. doi: 10.1073/pnas.1009881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chereddy KK, Vandermeulen G, Preat V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2016;24:223–236. doi: 10.1111/wrr.12404. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, Wischke C, Mittal S, Mitra A, Schwendeman SP. Design of Controlled Release PLGA Microspheres for Hydrophobic Fenretinide. Molecular pharmaceutics. 2016 doi: 10.1021/acs.molpharmaceut.5b00961. [DOI] [PubMed] [Google Scholar]
- 163.Yuksel E, Karakecili A, Demirtas TT, Gumusderelioglu M. Preparation of bioactive and antimicrobial PLGA membranes by magainin II/EGF functionalization. International journal of biological macromolecules. 2016;86:162–168. doi: 10.1016/j.ijbiomac.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 164.Chereddy KK, Her CH, Comune M, Moia C, Lopes A, Porporato PE, Vanacker J, Lam MC, Steinstraesser L, Sonveaux P, Zhu H, Ferreira LS, Vandermeulen G, Preat V. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. Journal of controlled release : official journal of the Controlled Release Society. 2014;194:138–147. doi: 10.1016/j.jconrel.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 165.Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Frontiers in immunology. 2013;4:143. doi: 10.3389/fimmu.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mofazzal Jahromi M, Karimi M, Azadmanesh K, Naderi Manesh H, Hassan Z, Moazzeni S. The effect of chitosan-tripolyphosphate nanoparticles on maturation and function of dendritic cells. Comp Clin Pathol. 2013:1–7. [Google Scholar]
- 167.Shrestha A, Hamblin MR, Kishen A. Characterization of a conjugate between Rose Bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrobial agents and chemotherapy. 2012;56:4876–4884. doi: 10.1128/AAC.00810-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shrestha A, Hamblin MR, Kishen A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine : nanotechnology, biology, and medicine. 2014;10:491–501. doi: 10.1016/j.nano.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert review of anti-infective therapy. 2011;9:857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dai T, Tegos GP, Burkatovskaya M, Castano AP, Hamblin MR. Chitosan acetate bandage as a topical antimicrobial dressing for infected burns. Antimicrobial agents and chemotherapy. 2009;53:393–400. doi: 10.1128/AAC.00760-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Hamblin PCAMR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–4164. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baxter RM, Dai T, Kimball J, Wang E, Hamblin MR, Wiesmann WP, McCarthy SJ, Baker SM. Chitosan dressing promotes healing in third degree burns in mice: gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J Biomed Mater Res A. 2013;101:340–348. doi: 10.1002/jbm.a.34328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karimi M, Avci P, Ahi M, Gazori T, Hamblin MR, Naderi-Manesh H. Evaluation of Chitosan-Tripolyphosphate Nanoparticles as a p-shRNA Delivery Vector: Formulation, Optimization and Cellular Uptake Study. Journal of nanopharmaceutics and drug delivery. 2013;1:266–278. doi: 10.1166/jnd.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: efficacy against bacterial burn infections. Antimicrobial agents and chemotherapy. 2011;55:3432–3438. doi: 10.1128/AAC.01803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochemical pharmacology. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 176.Mofazzal-Jahromi MA, Reza Mirnejad. Curcumin-loaded Chitosan Tripolyphosphate Nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcus aureus and Pseudomonas aeruginosa in vivo. Iranian Journal of Biotechnology. 2014;12:1–8. [Google Scholar]
- 177.Xie M, Fan D, Zhao Z, Li Z, Li G, Chen Y, He X, Chen A, Li J, Lin X, Zhi M, Li Y, Lan P. Nanocurcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int J Pharm. 2015;496:732–740. doi: 10.1016/j.ijpharm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 178.Xu YX, Pindolia KR, Janakiraman N, Noth CJ, Chapman RA, Gautam SC. Curcumin, a compound with anti-inflammatory and anti-oxidant properties, down-regulates chemokine expression in bone marrow stromal cells. Experimental hematology. 1997;25:413–422. [PubMed] [Google Scholar]
- 179.Bhawana, Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. Journal of agricultural and food chemistry. 2011;59:2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- 180.Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R. Dendrimers: synthesis, applications, and properties. Nanoscale research letters. 2014;9:247. doi: 10.1186/1556-276X-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kalomiraki M, Thermos K, Chaniotakis NA. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. International journal of nanomedicine. 2016;11:1–12. doi: 10.2147/IJN.S93069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu X, Hao W, Lok CN, Wang YC, Zhang R, Wong KK. Dendrimer encapsulation enhances anti-inflammatory efficacy of silver nanoparticles. Journal of pediatric surgery. 2014;49:1846–1851. doi: 10.1016/j.jpedsurg.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 183.Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. European Polymer Journal. 2015;65:252–267. [Google Scholar]
- 184.Hajimiri M, Shahverdi S, Esfandiari MA, Larijani B, Atyabi F, Rajabiani A, Dehpour AR, Amini M, Dinarvand R. Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm. 2016;42:707–719. doi: 10.3109/03639045.2015.1075030. [DOI] [PubMed] [Google Scholar]
- 185.Madaghiele M, Demitri C, Sannino A, Ambrosio L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns & Trauma. 2015;2:153. doi: 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Anjum S, Arora A, Alam MS, Gupta B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int J Pharm. 2016;508:92–101. doi: 10.1016/j.ijpharm.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 187.Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research. 2015;6:105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Parker J, Mitrousis N, Shoichet MS. Hydrogel for Simultaneous Tunable Growth Factor Delivery and Enhanced Viability of Encapsulated Cells in Vitro. Biomacromolecules. 2016;17:476–484. doi: 10.1021/acs.biomac.5b01366. [DOI] [PubMed] [Google Scholar]
- 189.Skardal A, Murphy SV, Crowell K, Mack D, Atala A, Soker S. A tunable hydrogel system for long-term release of cell-secreted cytokines and bioprinted in situ wound cell delivery, Journal of biomedical materials research. Part B. Applied biomaterials. 2016 doi: 10.1002/jbm.b.33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kobayashi T, Mizuta M, Hiwatashi N, Kishimoto Y, Nakamura T, Kanemaru SI, Hirano S. Drug delivery system of basic fibroblast growth factor using gelatin hydrogel for restoration of acute vocal fold scar. Auris, nasus, larynx. 2016 doi: 10.1016/j.anl.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 191.Bonferoni MC, Sandri G, Dellera E, Rossi S, Ferrari F, Mori M, Caramella C. Ionic polymeric micelles based on chitosan and fatty acids and intended for wound healing. Comparison of linoleic and oleic acid. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;87:101–106. doi: 10.1016/j.ejpb.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 192.Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Experimental biology and medicine. 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jabbarzadegan M, Rajayi H, Mofazzal Jahromi MA, Yeganeh H, Yousefi M, Muhammad Hassan Z, Majidi J. Application of arteether-loaded polyurethane nanomicelles to induce immune response in breast cancer model. Artificial cells, nanomedicine, and biotechnology. 2016:1–9. doi: 10.1080/21691401.2016.1178131. [DOI] [PubMed] [Google Scholar]
- 195.Song Z, Sun H, Yang Y, Jing H, Yang L, Tong Y, Wei C, Wang Z, Zou Q, Zeng H. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomedicine : nanotechnology, biology, and medicine. 2016 doi: 10.1016/j.nano.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 196.Sugumar S, Mukherjee A, Chandrasekaran N. Eucalyptus oil nanoemulsion-impregnated chitosan film: antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. International journal of nanomedicine. 2015;10(Suppl 1):67–75. doi: 10.2147/IJN.S79982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tyrrell ZL, Shen Y, Radosz M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Progress in Polymer Science. 2010;35:1128–1143. [Google Scholar]
- 198.Dellera E, Bonferoni MC, Sandri G, Rossi S, Ferrari F, Del Fante C, Perotti C, Grisoli P, Caramella C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;88:643–650. doi: 10.1016/j.ejpb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 199.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale research letters. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Naumov AA, Shatalin YV, Potselueva MM. Effects of a nanocomplex containing antioxidant, lipid, and amino acid on thermal burn wound surface. Bulletin of experimental biology and medicine. 2010;149:62–66. doi: 10.1007/s10517-010-0876-5. [DOI] [PubMed] [Google Scholar]
- 201.Jeschke MG, Sandmann G, Finnerty CC, Herndon DN, Pereira CT, Schubert T, Klein D. The structure and composition of liposomes can affect skin regeneration, morphology and growth factor expression in acute wounds. Gene therapy. 2005;12:1718–1724. doi: 10.1038/sj.gt.3302582. [DOI] [PubMed] [Google Scholar]
- 202.Jeschke MG, Richter G, Hofstadter F, Herndon DN, Perez-Polo JR, Jauch KW. Non-viral liposomal keratinocyte growth factor (KGF) cDNA gene transfer improves dermal and epidermal regeneration through stimulation of epithelial and mesenchymal factors. Gene therapy. 2002;9:1065–1074. doi: 10.1038/sj.gt.3301732. [DOI] [PubMed] [Google Scholar]
- 203.Yoon G, Park JW, Yoon I-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. Journal of Pharmaceutical Investigation. 2013;43:353–362. [Google Scholar]
- 204.Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. International journal of nanomedicine. 2007;2:289–300. [PMC free article] [PubMed] [Google Scholar]
- 205.Rostami E, Kashanian S, Azandaryani AH, Faramarzi H, Dolatabadi JE, Omidfar K. Drug targeting using solid lipid nanoparticles. Chemistry and physics of lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 206.Sandri G, Bonferoni MC, D'Autilia F, Rossi S, Ferrari F, Grisoli P, Sorrenti M, Catenacci L, Del Fante C, Perotti C, Caramella C. Wound dressings based on silver sulfadiazine solid lipid nanoparticles for tissue repairing. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2013;84:84–90. doi: 10.1016/j.ejpb.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 207.Kuchler S, Wolf NB, Heilmann S, Weindl G, Helfmann J, Yahya MM, Stein C, Schafer-Korting M. 3D-wound healing model: influence of morphine and solid lipid nanoparticles. Journal of biotechnology. 2010;148:24–30. doi: 10.1016/j.jbiotec.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 208.Zadeh B, Barati N, Hassani M, Rahim F. Development of solid lipid nanoparticles as Eschar delivery system for Nitrofurazone using Taguchi design approach. Int J Res Pharm Sci. 2010;1:466–472. [Google Scholar]
- 209.Müller RH, Alexiev U, Sinambela P, Keck CM. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In: Dragicevic N, Maibach IH, editors. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers. Springer Berlin Heidelberg; Berlin, Heidelberg: 2016. pp. 161–185. [Google Scholar]
- 210.Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent patents on nanotechnology. 2013;7:41–55. [PubMed] [Google Scholar]
- 211.Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. Journal of drug targeting. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 212.Khan S, Baboota S, Ali J, Khan S, Narang RS, Narang JK. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. International journal of pharmaceutical investigation. 2015;5:182–191. doi: 10.4103/2230-973X.167661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mendes AI, Silva AC, Catita JA, Cerqueira F, Gabriel C, Lopes CM. Miconazole-loaded nanostructured lipid carriers (NLC) for local delivery to the oral mucosa: improving antifungal activity, Colloids and surfaces. B. Biointerfaces. 2013;111:755–763. doi: 10.1016/j.colsurfb.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 214.Gainza G, Bonafonte DC, Moreno B, Aguirre JJ, Gutierrez FB, Villullas S, Pedraz JL, Igartua M, Hernandez RM. The topical administration of rhEGF-loaded nanostructured lipid carriers (rhEGF-NLC) improves healing in a porcine full-thickness excisional wound model. Journal of controlled release : official journal of the Controlled Release Society. 2015;197:41–47. doi: 10.1016/j.jconrel.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 215.Gainza G, Pastor M, Aguirre JJ, Villullas S, Pedraz JL, Hernandez RM, Igartua M. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: In vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. Journal of controlled release : official journal of the Controlled Release Society. 2014;185:51–61. doi: 10.1016/j.jconrel.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 216.Gainza G, Chu WS, Guy RH, Pedraz JL, Hernandez RM, Delgado-Charro B, Igartua M. Development and in vitro evaluation of lipid nanoparticle-based dressings for topical treatment of chronic wounds. Int J Pharm. 2015;490:404–411. doi: 10.1016/j.ijpharm.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 217.Lee J, Kim J, Go J, Lee JH, Han DW, Hwang D, Lee J. Transdermal treatment of the surgical and burned wound skin via phytochemical-capped gold nanoparticles, Colloids and surfaces. B. Biointerfaces. 2015;135:166–174. doi: 10.1016/j.colsurfb.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 218.Volkova N, Yukhta M, Pavlovich O, Goltsev A. Application of Cryopreserved Fibroblast Culture with Au Nanoparticles to Treat Burns. Nanoscale research letters. 2016;11:22. doi: 10.1186/s11671-016-1242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Petersen W, Rahmanian-Schwarz A, Werner J-O, Schiefer J, Rothenberger J, Hübner G, Schaller H-E, Held M. The use of collagen-based matrices in the treatment of full-thickness wounds. Burns : journal of the International Society for Burn Injuries. 2016 doi: 10.1016/j.burns.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 220.Akturk O, Kismet K, Yasti AC, Kuru S, Duymus ME, Kaya F, Caydere M, Hucumenoglu S, Keskin D. Collagen/gold nanoparticle nanocomposites: A potential skin wound healing biomaterial. Journal of biomaterials applications. 2016 doi: 10.1177/0885328216644536. [DOI] [PubMed] [Google Scholar]
- 221.Gyawali R, Ibrahim SA, Abu Hasfa SH, Smqadri SQ, Haik Y. Antimicrobial Activity of Copper Alone and in Combination with Lactic Acid against Escherichia coli O157:H7 in Laboratory Medium and on the Surface of Lettuce and Tomatoes. Journal of pathogens. 2011;2011:650968. doi: 10.4061/2011/650968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA. Synthesis and antimicrobial activity of copper nanoparticles. Materials Letters. 2012;71:114–116. [Google Scholar]
- 223.Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. International journal of nanomedicine. 2013;8:4467–4479. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Adhya A, Bain J, Ray O, Hazra A, Adhikari S, Dutta G, Ray S, Majumdar BK. Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nanocrystalline silver. Journal of basic and clinical pharmacy. 2014;6:29–34. doi: 10.4103/0976-0105.145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Boonkaew B, Suwanpreuksa P, Cuttle L, Barber PM, Supaphol P. Hydrogels containing silver nanoparticles for burn wounds show antimicrobial activity without cytotoxicity. Journal of Applied Polymer Science. 2014;131 [Google Scholar]
- 226.Konop M, Damps T, Misicka A, Rudnicka L. Certain aspects of silver and silver nanoparticles in wound care: a minireview. Journal of Nanomaterials. 2016;2016:47. [Google Scholar]
- 227.Marcato P, De Paula L, Melo P, Ferreira I, Almeida A, Torsoni A, Alves O. In vivo evaluation of complex biogenic silver nanoparticle and enoxaparin in wound healing. Journal of Nanomaterials. 2015;2015:91. [Google Scholar]
- 228.Hebeish A, El-Rafie MH, El-Sheikh MA, Seleem AA, El-Naggar ME. Antimicrobial wound dressing and anti-inflammatory efficacy of silver nanoparticles. International journal of biological macromolecules. 2014;65:509–515. doi: 10.1016/j.ijbiomac.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 229.Rigo C, Ferroni L, Tocco I, Roman M, Munivrana I, Gardin C, Cairns WR, Vindigni V, Azzena B, Barbante C, Zavan B. Active silver nanoparticles for wound healing. International journal of molecular sciences. 2013;14:4817–4840. doi: 10.3390/ijms14034817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Frankova J, Pivodova V, Vagnerova H, Juranova J, Ulrichova J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. Journal of applied biomaterials & functional materials. 2016;14:e137–142. doi: 10.5301/jabfm.5000268. [DOI] [PubMed] [Google Scholar]
- 231.Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P, Mohamed E, Saeidi A, Taheri M, Avci P, Aref AR, Amiri M, Baniasadi F, Hamblin MR. Carbon nanotubes part II: a remarkable carrier for drug and gene delivery. Expert opinion on drug delivery. 2015;12:1089–1105. doi: 10.1517/17425247.2015.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Karimi M, Solati N, Amiri M, Mirshekari H, Mohamed E, Taheri M, Hashemkhani M, Saeidi A, Estiar MA, Kiani P, Ghasemi A, Basri SM, Aref AR, Hamblin MR. Carbon nanotubes part I: preparation of a novel and versatile drug-delivery vehicle. Expert opinion on drug delivery. 2015;12:1071–1087. doi: 10.1517/17425247.2015.1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ashfaq M, Verma N, Khan S. Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: A novel potential antibiotic material, Materials science & engineering. C. Materials for biological applications. 2016;59:938–947. doi: 10.1016/j.msec.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 234.Maleki Dizaj S, Mennati A, Jafari S, Khezri K, Adibkia K. Antimicrobial activity of carbon-based nanoparticles. Advanced pharmaceutical bulletin. 2015;5:19–23. doi: 10.5681/apb.2015.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ji H, Sun H, Qu X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv Drug Deliv Rev. 2016;105:176–189. doi: 10.1016/j.addr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 236.Ji H, Sun H, Qu X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv Drug Deliv Rev. 2016 doi: 10.1016/j.addr.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 237.Simmons TJ, Lee S-H, Park T-J, Hashim D, Ajayan P, Linhardt R. Antiseptic single wall carbon nanotube bandages. Carbon. 2009;47:1561–1564. [Google Scholar]
- 238.Ornes S. Core Concept: Quantum dots. Proceedings of the National Academy of Sciences. 2016;113:2796–2797. doi: 10.1073/pnas.1601852113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sun H, Gao N, Dong K, Ren J, Qu X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano. 2014;8:6202–6210. doi: 10.1021/nn501640q. [DOI] [PubMed] [Google Scholar]
- 240.Habiba K, Bracho-Rincon DP, Gonzalez-Feliciano JA, Villalobos-Santos JC, Makarov VI, Ortiz D, Avalos JA, Gonzalez CI, Weiner BR, Morell G. Synergistic antibacterial activity of PEGylated silver–graphene quantum dots nanocomposites. Applied Materials Today. 2015;1:80–87. [Google Scholar]
- 241.Park HK, Park KY. Control of Particle Morphology and Size in Vapor-Phase Synthesis of Titania, Silica and Alumina Nanoparticles. KONA Powder and Particle Journal. 2015;32:85–101. [Google Scholar]
- 242.Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine : nanotechnology, biology, and medicine. 2015;11:313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 243.Thomas SC, Harshita, Mishra PK, Talegaonkar S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Current pharmaceutical design. 2015;21:6165–6188. doi: 10.2174/1381612821666151027153246. [DOI] [PubMed] [Google Scholar]
- 244.Rosenholm JM, Sahlgren C, Linden M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Current drug targets. 2011;12:1166–1186. doi: 10.2174/138945011795906624. [DOI] [PubMed] [Google Scholar]
- 245.Alvarez GS, Hélary C, Mebert AM, Wang X, Coradin T, Desimone MF. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. Journal of Materials Chemistry B. 2014;2:4660–4670. doi: 10.1039/c4tb00327f. [DOI] [PubMed] [Google Scholar]
- 246.Dunn JL, Hunter RA, Gast K, Maile R, Cairns BA, Schoenfisch MH. Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns : journal of the International Society for Burn Injuries. 2016 doi: 10.1016/j.burns.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009;30:2782–2789. doi: 10.1016/j.biomaterials.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yamada I, Nomura K, Iwahashi H, Horie M. The effect of titanium dioxide (TiO2) nano-objects, and their aggregates and agglomerates greater than 100nm (NOAA) on microbes under UV irradiation. Chemosphere. 2016;143:123–127. doi: 10.1016/j.chemosphere.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 249.von Goetz N, Lorenz C, Windler L, Nowack B, Heuberger M, Hungerbuhler K. Migration of Ag- and TiO2-(Nano)particles from textiles into artificial sweat under physical stress: experiments and exposure modeling. Environmental science & technology. 2013;47:9979–9987. doi: 10.1021/es304329w. [DOI] [PubMed] [Google Scholar]
- 250.Jesline A, John NP, Narayanan P, Vani C, Murugan S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Applied Nanoscience. 2015;5:157–162. [Google Scholar]
- 251.Sivaranjani V, Philominathan P. Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Medicine. 2016;12:1–5. [Google Scholar]
- 252.Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, Kirthi AV, Velayutham K, Thomas J, Venkatesan J, Kim SK. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pacific journal of tropical medicine. 2014;7:968–976. doi: 10.1016/S1995-7645(14)60171-1. [DOI] [PubMed] [Google Scholar]
- 253.Kubacka A, Diez MS, Rojo D, Bargiela R, Ciordia S, Zapico I, Albar JP, Barbas C, Martins dos Santos VA, Fernandez-Garcia M, Ferrer M. Understanding the antimicrobial mechanism of TiO(2)-based nanocomposite films in a pathogenic bacterium. Scientific reports. 2014;4:4134. doi: 10.1038/srep04134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hromadka M, Collins JB, Reed C, Han L, Kolappa KK, Cairns BA, Andrady T, van Aalst JA. Nanofiber applications for burn care. J Burn Care Res. 2008;29:695–703. doi: 10.1097/BCR.0b013e31818480c9. [DOI] [PubMed] [Google Scholar]
- 255.Sett S, Lee M, Weith M, Pourdeyhimi B, Yarin A. Biodegradable and biocompatible soy protein/polymer/adhesive sticky nano-textured interfacial membranes for prevention of esca fungi invasion into pruning cuts and wounds of vines. Journal of Materials Chemistry B. 2015;3:2147–2162. doi: 10.1039/c4tb01887g. [DOI] [PubMed] [Google Scholar]
- 256.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 257.Leach MK, Feng ZQ, Tuck SJ, Corey JM. Electrospinning fundamentals: optimizing solution and apparatus parameters. J Vis Exp. 2011 doi: 10.3791/2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 259.Ahire JJ, Dicks LM. 2,3-dihydroxybenzoic acid-containing nanofiber wound dressings inhibit biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58:2098–2104. doi: 10.1128/AAC.02397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Borkow G, Gabbay J, Dardik R, Eidelman AI, Lavie Y, Grunfeld Y, Ikher S, Huszar M, Zatcoff RC, Marikovsky M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010;18:266–275. doi: 10.1111/j.1524-475X.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 261.Ahire JJ, Hattingh M, Neveling DP, Dicks LM. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS One. 2016;11:e0152755. doi: 10.1371/journal.pone.0152755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Liu Y, Sun Q, Wang S, Long R, Fan J, Chen A, Wu W. Studies of Silk Fibroin/Poly (Lactic-Co-Glycolic Acid) Scaffold, Prepared by Thermally Induced Phase Separation, as a Possible Wound Dressing. Science of Advanced Materials. 2016;8:1045–1052. [Google Scholar]
- 263.Weng L, Xie J. Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives. Curr Pharm Des. 2015;21:1944–1959. doi: 10.2174/1381612821666150302151959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dwivedi C, Pandey H, Pandey AC, Ramteke PW. Nanofibre Based Smart Pharmaceutical Scaffolds for Wound Repair and Regenerations. Curr Pharm Des. 2016;22:1460–1471. doi: 10.2174/1381612822666151215103553. [DOI] [PubMed] [Google Scholar]
- 265.Tan L, Hu J, Huang H, Han J, Hu H. Study of multi-functional electrospun composite nanofibrous mats for smart wound healing. International journal of biological macromolecules. 2015;79:469–476. doi: 10.1016/j.ijbiomac.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 266.Dongargaonkar AA, Bowlin GL, Yang H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules. 2013;14:4038–4045. doi: 10.1021/bm401143p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chung E, Rybalko VY, Hsieh PL, Leal SL, Samano MA, Willauer AN, Stowers RS, Natesan S, Zamora DO, Christy RJ. Fibrin-based stem cell containing scaffold improves the dynamics of burn wound healing. Wound Repair and Regeneration. 2016;24:810–819. doi: 10.1111/wrr.12459. [DOI] [PubMed] [Google Scholar]
- 268.Hu W, Chen S, Yang J, Li Z, Wang H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydrate polymers. 2014;101:1043–1060. doi: 10.1016/j.carbpol.2013.09.102. [DOI] [PubMed] [Google Scholar]
- 269.Galateanu B, Bunea MC, Stanescu P, Vasile E, Casarica A, Iovu H, Hermenean A, Zaharia C, Costache M. In Vitro Studies of Bacterial Cellulose and Magnetic Nanoparticles Smart Nanocomposites for Efficient Chronic Wounds Healing. Stem cells international. 2015;2015:195096. doi: 10.1155/2015/195096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li J, Zhai D, Lv F, Yu Q, Ma H, Yin J, Yi Z, Liu M, Chang J, Wu C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomaterialia. 2016;36:254–266. doi: 10.1016/j.actbio.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 271.Kim JO, Noh J-K, Thapa RK, Hasan N, Choi M, Kim JH, Lee J-H, Ku SK, Yoo J-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. International Journal of Biological Macromolecules. 2015;79:217–225. doi: 10.1016/j.ijbiomac.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 272.Lu Z, Gao J, He Q, Wu J, Liang D, Yang H, Chen R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydrate Polymers. 2017;156:460–469. doi: 10.1016/j.carbpol.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 273.Ito K, Saito A, Fujie T, Nishiwaki K, Miyazaki H, Kinoshita M, Saitoh D, Ohtsubo S, Takeoka S. Sustainable antimicrobial effect of silver sulfadiazine-loaded nanosheets on infection in a mouse model of partial-thickness burn injury. Acta Biomaterialia. 2015;24:87–95. doi: 10.1016/j.actbio.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 274.Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomaterialia. 2014;10:4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kim J-Y, Jun J-H, Kim S-J, Hwang K-M, Choi SR, Han SD, Son M-W, Park E-S. Wound healing efficacy of a chitosan-based film-forming gel containing tyrothricin in various rat wound models. Archives of Pharmacal Research. 2015;38:229–238. doi: 10.1007/s12272-014-0368-7. [DOI] [PubMed] [Google Scholar]
- 276.Namazi H, Rakhshaei R, Hamishehkar H, Kafil HS. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. International journal of biological macromolecules. 2016;85:327–334. doi: 10.1016/j.ijbiomac.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 277.Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials. 2017;122:34–47. doi: 10.1016/j.biomaterials.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 278.Tummalapalli M, Berthet M, Verrier B, Deopura B, Alam M, Gupta B. Drug loaded composite oxidized pectin and gelatin networks for accelerated wound healing. International journal of pharmaceutics. 2016;505:234–245. doi: 10.1016/j.ijpharm.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 279.Romić MD, Klarić MŠ, Lovrić J, Pepić I, Cetina-Čižmek B, Filipović-Grčić J, Hafner A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. European Journal of Pharmaceutics and Biopharmaceutics. 2016;107:67–79. doi: 10.1016/j.ejpb.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 280.Zhou Z, Yan D, Cheng X, Kong M, Liu Y, Feng C, Chen X. Biomaterials based on N, N, N-trimethyl chitosan fibers in wound dressing applications. International journal of biological macromolecules. 2016;89:471–476. doi: 10.1016/j.ijbiomac.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 281.Song DW, Kim SH, Kim HH, Lee KH, Ki CS, Park YH. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta biomaterialia. 2016;39:146–155. doi: 10.1016/j.actbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 282.Maleki M, Latifi M, Amani-Tehran M, Mathur S. Electrospun core–shell nanofibers for drug encapsulation and sustained release. Polymer Engineering & Science. 2013;53:1770–1779. [Google Scholar]
- 283.Wei Q, Xu F, Xu X, Geng X, Ye L, Zhang A, Feng Z. The multifunctional wound dressing with core–shell structured fibers prepared by coaxial electrospinning. Frontiers of Materials Science. 2016;10:113–121. [Google Scholar]
- 284.Lu T, Li Y, Chen T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int J Nanomedicine. 2013;8:337–350. doi: 10.2147/IJN.S38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hu J, Ma PX. Nano-fibrous tissue engineering scaffolds capable of growth factor delivery. Pharm Res. 2011;28:1273–1281. doi: 10.1007/s11095-011-0367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Park YR, Ju HW, Lee JM, Kim DK, Lee OJ, Moon BM, Park HJ, Jeong JY, Yeon YK, Park CH. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. International journal of biological macromolecules. 2016;93:1567–1574. doi: 10.1016/j.ijbiomac.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 287.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS One. 2008;3:e1410. doi: 10.1371/journal.pone.0001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bradshaw M, Ho D, Fear MW, Gelain F, Wood FM, Iyer KS. Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Scientific reports. 2014;4:6903. doi: 10.1038/srep06903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnology Reviews [Google Scholar]
- 290.Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H, Ghahramanzadeh Asl H, Mahdieh Z, Bozorgomid M, Ghasemi A, Rahmani Taji Boyuk MR, Hamblin MR. Temperatureresponsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS applied materials & interfaces. 2016 doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z, Ghosh D, Bozorgomid M, Dashkhaneh F, Hamblin MR. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016 doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Davaran S, Ghamkhari A, Alizadeh E, Massoumi B, Jaymand M. Novel dual stimuli-responsive ABC triblock copolymer: RAFT synthesis,“schizophrenic” micellization, and its performance as an anticancer drug delivery nanosystem. Journal of Colloid and Interface Science. 2017;488:282–293. doi: 10.1016/j.jcis.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 293.Massoumi B, Poorgholy N, Jaymand M. Multistimuli responsive polymeric nanosystems for theranostic applications. International Journal of Polymeric Materials and Polymeric Biomaterials. 2017;66:38–47. [Google Scholar]
- 294.Fathi M, Entezami AA, Pashaei-Asl R. Swelling/deswelling, thermal, and rheological behavior of PVA-g-NIPAAm nanohydrogels prepared by a facile free-radical polymerization method. Journal of Polymer Research. 2013;20:125. [Google Scholar]
- 295.Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. Journal of the American Chemical Society. 2017 doi: 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H, Amiri M, Shafaei Pishabad Z, Aslani A, Bozorgomid M, Ghosh D, Beyzavi A, Vaseghi A, Aref AR, Haghani L, Bahrami S, Hamblin MR. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chemical Society reviews. 2016 doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sahandi Zangabad P, Karimi M, Mehdizadeh F, Malekzad H, Ghasemi A, Bahrami S, Zare H, Moghoofei M, Hekmatmanesh A, Hamblin MR. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9:1356–1392. doi: 10.1039/c6nr07315h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazizadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: Uncaging and activating by light. Journal of the American Chemical Society. 2017 doi: 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.You J-O, Rafat M, Almeda D, Maldonado N, Guo P, Nabzdyk CS, Chun M, LoGerfo FW, Hutchinson JW, Pradhan-Nabzdyk LK. pH-responsive scaffolds generate a pro-healing response. Biomaterials. 2015;57:22–32. doi: 10.1016/j.biomaterials.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 300.Ninan N, Forget A, Shastri VP, Voelcker NH, Blencowe A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS applied materials & interfaces. 2016;8:28511–28521. doi: 10.1021/acsami.6b10491. [DOI] [PubMed] [Google Scholar]
- 301.Grützner V, Unger RE, Baier G, Choritz L, Freese C, Böse T, Landfester K, Kirkpatrick CJ. Enzyme-responsive nanocomposites for wound infection prophylaxis in burn management: in vitro evaluation of their compatibility with healing processes. International journal of nanomedicine. 2015;10:4111. doi: 10.2147/IJN.S81263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Li Y, Liu G, Wang X, Hu J, Liu S. Enzyme-Responsive Polymeric Vesicles for Bacterial-Strain-Selective Delivery of Antimicrobial Agents. Angewandte Chemie. 2016;128:1792–1796. doi: 10.1002/anie.201509401. [DOI] [PubMed] [Google Scholar]
- 303.Hathaway H, Alves DR, Bean J, Esteban PP, Ouadi K, Mark Sutton J, Jenkins ATA. Poly(Nisopropylacrylamide-co-allylamine) (PNIPAM-co-ALA) nanospheres for the thermally triggered release of Bacteriophage K. European Journal of Pharmaceutics and Biopharmaceutics. 2015;96:437–441. doi: 10.1016/j.ejpb.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 304.Osumi K, Matsuda S, Fujimura N, Matsubara K, Kitago M, Itano O, Ogino C, Shimizu N, Obara H, Kitagawa Y. Acceleration of wound healing by ultrasound activation of TiO2 in Escherichia coli-infected wounds in mice. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2016 doi: 10.1002/jbm.b.33774. [DOI] [PubMed] [Google Scholar]
- 305.Öhlknecht C, Tegl G, Beer B, Sygmund C, Ludwig R, Guebitz GM. Cellobiose dehydrogenase and chitosan-based lysozyme responsive materials for antimicrobial wound treatment. Biotechnology and Bioengineering. 2017;114:416–422. doi: 10.1002/bit.26070. [DOI] [PubMed] [Google Scholar]
- 306.Kim HS, Son YJ, Yoo HS. Clustering siRNA conjugates for MMP-responsive therapeutics in chronic wounds of diabetic animals. Nanoscale. 2016;8:13236–13244. doi: 10.1039/c6nr01551d. [DOI] [PubMed] [Google Scholar]
- 307.Kim HS, Yoo HS. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther. 2013;20:378–385. doi: 10.1038/gt.2012.49. [DOI] [PubMed] [Google Scholar]
- 308.Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Molecular Biology of the Cell. 2012;23:1068–1079. doi: 10.1091/mbc.E11-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhang J, Du P, Xu D, Li Y, Peng W, Zhang G, Zhang F, Fan X. Near-Infrared Responsive MoS2/Poly(N-isopropylacrylamide) Hydrogels for Remote Light-Controlled Microvalves. Industrial & Engineering Chemistry Research. 2016;55:4526–4531. [Google Scholar]
- 310.Zhang M, Cao Y, Wang L, Ma Y, Tu X, Zhang Z. Manganese Doped Iron Oxide Theranostic Nanoparticles for Combined T 1 Magnetic Resonance Imaging and Photothermal Therapy. ACS applied materials & interfaces. 2015;7:4650–4658. doi: 10.1021/am5080453. [DOI] [PubMed] [Google Scholar]
- 311.Kim H, Kim J, Lee M, Choi HC, Kim WJ. Photothermal Gene Delivery: Stimuli-Regulated Enzymatically Degradable Smart Graphene-Oxide-Polymer Nanocarrier Facilitating Photothermal Gene Delivery (Adv. Healthcare Mater. 15/2016) Advanced Healthcare Materials. 2016;5:1917–1917. doi: 10.1002/adhm.201600246. [DOI] [PubMed] [Google Scholar]
- 312.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers in medical science. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 313.Bao Z, Liu X, Liu Y, Liu H, Zhao K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian Journal of Pharmaceutical Sciences. 2016;11:349–364. [Google Scholar]
- 314.Chiang W-L, Lin T-T, Sureshbabu R, Chia W-T, Hsiao H-C, Liu H-Y, Yang C-M, Sung H-W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. Journal of Controlled Release. 2015;199:53–62. doi: 10.1016/j.jconrel.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 315.Yin W, Yu J, Lv F, Yan L, Zheng LR, Gu Z, Zhao Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS nano. 2016;10:11000–11011. doi: 10.1021/acsnano.6b05810. [DOI] [PubMed] [Google Scholar]
- 316.Sahu K, Sharma M, Bansal H, Dube A, Gupta PK. Topical photodynamic treatment with poly-l-lysine–chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa-infected wounds of mice. Lasers in medical science. 2013;28:465–471. doi: 10.1007/s10103-012-1083-6. [DOI] [PubMed] [Google Scholar]
- 317.Lucky SS, Muhammad Idris N, Li Z, Huang K, Soo KC, Zhang Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS nano. 2015;9:191–205. doi: 10.1021/nn503450t. [DOI] [PubMed] [Google Scholar]
- 318.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: clinical applications. Lasers in surgery and medicine. 2011;43:755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR. Photodynamic therapy for infections: Clinical applications. Lasers Surg Med. 2011;43:755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hamblin MR, Chiang LY, Lakshmanan S, Huang YY, Garcia-Diaz M, Karimi M, de Souza Rastelli AN, Chandran R. Nanotechnology for photodynamic therapy: a perspective from the Laboratory of Dr. Michael R. Hamblin in the Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School. Nanotechnol Rev. 2015;4:359–372. doi: 10.1515/ntrev-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Deyhimi P, Khademi H, Birang R, Akhoondzadeh M. Histological Evaluation of Wound Healing Process after Photodynamic Therapy of Rat Oral Mucosal Ulcer. Journal of dentistry. 2016;17:43–48. [PMC free article] [PubMed] [Google Scholar]
- 322.Sperandio FF, Huang Y-Y, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent patents on anti-infective drug discovery. 2013;8:108–120. doi: 10.2174/1574891x113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Hettiaratchy S, Clarke J, Taubel J, Besa C. Burns after photodynamic therapy. Bmj. 2000;320:1245. doi: 10.1136/bmj.320.7244.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.van de Kamp J, Paefgen V, Wöltje M, Böbel M, Jaekel J, Rath B, Labude N, Knüchel R, Jahnen-Dechent W, Neuss S. Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor-loaded biomaterials. Journal of Tissue Engineering and Regenerative Medicine. 2016 doi: 10.1002/term.2201. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 325.Chung E, Rybalko VY, Hsieh P-L, Leal SL, Samano MA, Willauer AN, Stowers RS, Natesan S, Zamora DO, Christy RJ, Suggs LJ. Fibrin-based stem cell containing scaffold improves the dynamics of burn wound healing. Wound Repair and Regeneration. 2016;24:810–819. doi: 10.1111/wrr.12459. [DOI] [PubMed] [Google Scholar]
- 326.Burmeister DM, Stone R, Wrice NL, Becerra SC, Natesan S, Christy RJ. Fibrin Hydrogels Prevent Contraction and Deliver Adipose Stem Cells to Debrided Deep Partial Thickness Burns for Accelerated Angiogenesis. The FASEB Journal. 2016;30:1300–1307. [Google Scholar]
- 327.Butko A, Bonat Celli G, Paulson A, Ghanem A. Entrapment of basic fibroblast growth factor (bFGF) in a succinylated chitosan nanoparticle delivery system and release profile. Journal of Biomaterials Science, Polymer Edition. 2016:1–13. doi: 10.1080/09205063.2016.1178519. [DOI] [PubMed] [Google Scholar]
- 328.Goh M, Hwang Y, Tae G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydrate polymers. 2016;147:251–260. doi: 10.1016/j.carbpol.2016.03.072. [DOI] [PubMed] [Google Scholar]
- 329.Du J, Liu L, Lay F, Wang Q, Dou C, Zhang X, Hosseini SM, Simon A, Rees DJ, Ahmed AK, Sebastian R, Sarkar K, Milner S, Marti GP, Semenza GL, Harmon JW. Combination of HIF-1[alpha] gene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice. Gene Ther. 2013;20:1070–1076. doi: 10.1038/gt.2013.32. [DOI] [PubMed] [Google Scholar]
- 330.Turner CT, Hasanzadeh Kafshgari M, Melville E, Delalat B, Harding FJ, Mäkilä E, Salonen JJ, Cowin AJ, Voelcker NH. Delivery of Flightless I siRNA from porous silicon nanoparticles improves wound healing in mice. ACS Biomaterials Science & Engineering. 2016 doi: 10.1021/acsbiomaterials.6b00550. [DOI] [PubMed] [Google Scholar]
- 331.Peng L-H, Wei W, Qi X-T, Shan Y-H, Zhang F-J, Chen X, Zhu Q-Y, Yu L, Liang W-Q, Gao J-Q. Epidermal Stem Cells Manipulated by pDNA-VEGF165/CYD-PEI Nanoparticles Loaded Gelatin/β-TCP Matrix as a Therapeutic Agent and Gene Delivery Vehicle for Wound Healing. Molecular Pharmaceutics. 2013;10:3090–3102. doi: 10.1021/mp400162k. [DOI] [PubMed] [Google Scholar]
- 332.Chen B, Kao H-K, Dong Z, Jiang Z, Guo L. Complementary Effects of Negative-Pressure Wound Therapy and Pulsed Radiofrequency Energy on Cutaneous Wound Healing in Diabetic Mice. Plastic and reconstructive surgery. 2017;139:105–117. doi: 10.1097/PRS.0000000000002909. [DOI] [PubMed] [Google Scholar]
- 333.Asghari M, Kanonisabet A, Safakhah M, Azimzadeh Z, Mostafavinia A, Taheri S, Amini A, Ghorishi SK, JalaliFiroozkohi R, Bayat S, Bayat M. The effect of combined photobiomodulation and metformin on open skin wound healing in a non-genetic model of type II diabetes. Journal of Photochemistry and Photobiology B: Biology. 2017;169:63–69. doi: 10.1016/j.jphotobiol.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 334.Valente PMDS, Deva A, Ngo Q, Vickery K. The increased killing of biofilms in vitro by combining topical silver dressings with topical negative pressure in chronic wounds. International Wound Journal. 2016;13:130–136. doi: 10.1111/iwj.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Larsen L, Tchanque-Fossuo CN, Gorouhi F, Bodreault D, Nguyen C, Crawford RW, Dahle SE, Whetzel T, Isseroff RR. Combination therapy of autologous adipose mesenchymal stem cell-enriched, high density lipoaspirate and topical timolol for healing chronic wounds. Journal of Tissue Engineering and Regenerative Medicine. 2016 doi: 10.1002/term.2390. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 336.Seo E, Lim JS, Jun J-B, Choi W, Hong I-S, Jun H-S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. Journal of Translational Medicine. 2017;15:35. doi: 10.1186/s12967-017-1145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sun L, Huang Y, Bian Z, Petrosino J, Fan Z, Wang Y, Park KH, Yue T, Schmidt M, Galster S, Ma J, Zhu H, Zhang M. Sundew-Inspired Adhesive Hydrogels Combined with Adipose-Derived Stem Cells for Wound Healing. ACS applied materials & interfaces. 2016;8:2423–2434. doi: 10.1021/acsami.5b11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zhang L, Xu P, Wang X, Zhang M, Yan Y, Chen Y, Zhang L, Zhang L. Activin B regulates adiposederived mesenchymal stem cells to promote skin wound healing via activation of the MAPK signaling pathway. The International Journal of Biochemistry & Cell Biology. 2017;87:69–76. doi: 10.1016/j.biocel.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 339.Yoshida S, Yoshimoto H, Hirano A, Akita S. Wound healing and angiogenesis through combined use of a vascularized tissue flap and adipose-derived stem cells in a rat hindlimb irradiated ischemia model. Plastic and reconstructive surgery. 2016;137:1486–1497. doi: 10.1097/PRS.0000000000002062. [DOI] [PubMed] [Google Scholar]
- 340.Pouriran R, Piryaei A, Mostafavinia A, Zandpazandi S, Hendudari F, Amini A, Bayat M. The Effect of Combined Pulsed Wave Low-Level Laser Therapy and Human Bone Marrow Mesenchymal Stem Cell-Conditioned Medium on Open Skin Wound Healing in Diabetic Rats. Photomed Laser Surg. 2016;34:345–354. doi: 10.1089/pho.2015.4020. [DOI] [PubMed] [Google Scholar]
- 341.Figurova M, Ledecky V, Karasova M, Hluchy M, Trbolova A, Capik I, Hornak S, Reichel P, Bjordal JM, Gal P. Histological Assessment of a Combined Low-Level Laser/Light-Emitting Diode Therapy (685 nm/470 nm) for Sutured Skin Incisions in a Porcine Model: A Short Report. Photomed Laser Surg. 2016;34:53–55. doi: 10.1089/pho.2015.4013. [DOI] [PubMed] [Google Scholar]
- 342.Korelo RI, Kryczyk M, Garcia C, Naliwaiko K, Fernandes LC. Wound healing treatment by high frequency ultrasound, microcurrent, and combined therapy modifies the immune response in rats. Brazilian journal of physical therapy. 2016;20:133–141. doi: 10.1590/bjpt-rbf.2014.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Choi JU, Lee SW, Pangeni R, Byun Y, Yoon I-S, Park JW. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomaterialia. 2017;57:197–215. doi: 10.1016/j.actbio.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 344.Nagai N, Ogata F, Deguchi S, Ueno A, Kawasaki N, Ito Y. Combination Ointment Containing Solid Tranilast Nanoparticles and Dissolved Sericin Is Efficacious for Treating Skin Wound-Healing Deficits and Redness in Diabetic Rats. Biological and Pharmaceutical Bulletin. 2017;40:444–450. doi: 10.1248/bpb.b16-00812. [DOI] [PubMed] [Google Scholar]
- 345.Heun Y, Pogoda K, Anton M, Pircher J, Pfeifer A, Woernle M, Ribeiro A, Kameritsch P, Mykhaylyk O, Plank C, Kroetz F, Pohl U, Mannell H. HIF-1α Dependent Wound Healing Angiogenesis In Vivo Can Be Controlled by Site-Specific Lentiviral Magnetic Targeting of SHP-2. Molecular Therapy. doi: 10.1016/j.ymthe.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rabbani PS, Zhou A, Borab ZM, Frezzo JA, Srivastava N, More HT, Rifkin WJ, David JA, Berens SJ, Chen R, Hameedi S, Junejo MH, Kim C, Sartor RA, Liu CF, Saadeh PB, Montclare JK, Ceradini DJ. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials. 2017;132:1–15. doi: 10.1016/j.biomaterials.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 347.Aragão-Neto AC, Soares PAG, Lima-Ribeiro MHM, Carvalho EJA, Correia MTS, Carneiroda-Cunha MG. Combined therapy using low level laser and chitosan-policaju hydrogel for wound healing. International Journal of Biological Macromolecules. 2017;95:268–272. doi: 10.1016/j.ijbiomac.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 348.Park YG, Lee IH, Park ES, Kim JY. Hydrogel and Platelet-Rich Plasma Combined Treatment to Accelerate Wound Healing in a Nude Mouse Model. Arch Plast Surg. 2017;44:194–201. doi: 10.5999/aps.2017.44.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Morimoto N, Kakudo N, Ogura T, Hara T, Matsui M, Yamamoto M, Tabata Y, Kusumoto K. Easy-to-Use Preservation and Application of Platelet-Rich Plasma in Combination Wound Therapy With a Gelatin Sheet and Freeze-Dried Platelet-Rich Plasma: A Case Report. Eplasty. 2016;16:e22. [PMC free article] [PubMed] [Google Scholar]
- 350.Morimoto N, Kakudo N, Matsui M, Ogura T, Hara T, Suzuki K, Yamamoto M, Tabata Y, Kusumoto K. Exploratory clinical trial of combination wound therapy with a gelatin sheet and platelet-rich plasma in patients with chronic skin ulcers: study protocol. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lamers E, van Horssen R, te Riet J, van Delft FC, Luttge R, Walboomers XF, Jansen JA. The influence of nanoscale topographical cues on initial osteoblast morphology and migration. European cells & materials. 2010;20:329–343. doi: 10.22203/ecm.v020a27. [DOI] [PubMed] [Google Scholar]
- 352.Kim D-H, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol. 2012;197:351–360. doi: 10.1083/jcb.201108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lopacinska JM, Gradinaru C, Wierzbicki R, Kobler C, Schmidt MS, Madsen MT, Skolimowski M, Dufva M, Flyvbjerg H, Molhave K. Cell motility, morphology, viability and proliferation in response to nanotopography on silicon black. Nanoscale. 2012;4:3739–3745. doi: 10.1039/c2nr11455k. [DOI] [PubMed] [Google Scholar]
- 354.Kim J, Kim HN, Lim KT, Kim Y, Seonwoo H, Park SH, Lim HJ, Kim DH, Suh KY, Choung PH, Choung YH, Chung JH. Designing nanotopographical density of extracellular matrix for controlled morphology and function of human mesenchymal stem cells. Scientific reports. 2013;3:3552. doi: 10.1038/srep03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Guasch J, Diemer J, Riahinezhad H, Neubauer S, Kessler H, Spatz JP. Synthesis of Binary Nanopatterns on Hydrogels for Initiating Cellular Responses. Chemistry of Materials. 2016;28:1806–1815. [Google Scholar]
- 356.Teo BK, Ankam S, Chan LY, Yim EK. Nanotopography/mechanical induction of stem-cell differentiation. Methods Cell Biol. 2010;98:241–294. doi: 10.1016/S0091-679X(10)98011-4. [DOI] [PubMed] [Google Scholar]
- 357.Mashinchian O, Turner LA, Dalby MJ, Laurent S, Shokrgozar MA, Bonakdar S, Imani M, Mahmoudi M. Regulation of stem cell fate by nanomaterial substrates. Nanomedicine (Lond) 2015;10:829–847. doi: 10.2217/nnm.14.225. [DOI] [PubMed] [Google Scholar]
- 358.Harding FJ, Surdo S, Delalat B, Cozzi C, Elnathan R, Gronthos S, Voelcker NH, Barillaro G. Ordered Silicon Pillar Arrays Prepared by Electrochemical Micromachining: Substrates for High-Efficiency Cell Transfection. ACS applied materials & interfaces. 2016;8:29197–29202. doi: 10.1021/acsami.6b07850. [DOI] [PubMed] [Google Scholar]
- 359.Brodoceanu D, Alhmoud HZ, Elnathan R, Delalat B, Voelcker NH, Kraus T. Fabrication of silicon nanowire arrays by near-field laser ablation and metal-assisted chemical etching. Nanotechnology. 2016;27:075301. doi: 10.1088/0957-4484/27/7/075301. [DOI] [PubMed] [Google Scholar]
- 360.Elnathan R, Delalat B, Brodoceanu D, Alhmoud H, Harding FJ, Buehler K, Nelson A, Isa L, Kraus T, Voelcker NH. Maximizing Transfection Efficiency of Vertically Aligned Silicon Nanowire Arrays. Advanced Functional Materials. 2015;25:7215–7225. [Google Scholar]
- 361.Brodoceanu D, Elnathan R, Prieto-Simón B, Delalat B, Guinan T, Kroner E, Voelcker NH, Kraus T. Dense Arrays of Uniform Submicron Pores in Silicon and Their Applications. ACS applied materials & interfaces. 2015;7:1160–1169. doi: 10.1021/am506891d. [DOI] [PubMed] [Google Scholar]
- 362.Elnathan R, Isa L, Brodoceanu D, Nelson A, Harding FJ, Delalat B, Kraus T, Voelcker NH. Versatile Particle-Based Route to Engineer Vertically Aligned Silicon Nanowire Arrays and Nanoscale Pores. ACS applied materials & interfaces. 2015;7:23717–23724. doi: 10.1021/acsami.5b07777. [DOI] [PubMed] [Google Scholar]
- 363.Chen W, Villa-Diaz LG, Sun Y, Weng S, Kim JK, Lam RH, Han L, Fan R, Krebsbach PH, Fu J. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6:4094–4103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Darby IA, Laverdet B, Bonte F, Desmouliere A. Fibroblasts and myofibroblasts in wound healing. Clinical, cosmetic and investigational dermatology. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta -induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Parkinson LG, Giles NL, Adcroft KF, Fear MW, Wood FM, Poinern GE. The potential of nanoporous anodic aluminium oxide membranes to influence skin wound repair. Tissue Eng Part A. 2009;15:3753–3763. doi: 10.1089/ten.TEA.2008.0594. [DOI] [PubMed] [Google Scholar]
- 368.Franco D, Milde F, Klingauf M, Orsenigo F, Dejana E, Poulikakos D, Cecchini M, Koumoutsakos P, Ferrari A, Kurtcuoglu V. Accelerated endothelial wound healing on microstructured substrates under flow. Biomaterials. 2013;34:1488–1497. doi: 10.1016/j.biomaterials.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 369.Parkinson LG, Rea SM, Stevenson AW, Wood FM, Fear MW. The effect of nano-scale topography on keratinocyte phenotype and wound healing following burn injury. Tissue Eng Part A. 2012;18:703–714. doi: 10.1089/ten.tea.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pot SA, Liliensiek SJ, Myrna KE, Bentley E, Jester JV, Nealey PF, Murphy CJ. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Invest Ophthalmol Vis Sci. 2010;51:1373–1381. doi: 10.1167/iovs.09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Mahmoudi M, Bonakdar S, Shokrgozar MA, Aghaverdi H, Hartmann R, Pick A, Witte G, Parak WJ. Cell-imprinted substrates direct the fate of stem cells. ACS Nano. 2013;7:8379–8384. doi: 10.1021/nn403844q. [DOI] [PubMed] [Google Scholar]
- 372.Murray LM, Nock V, Evans JJ, Alkaisi MM. Bioimprinted polymer platforms for cell culture using soft lithography. Journal of nanobiotechnology. 2014;12:60. doi: 10.1186/s12951-014-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Tavakol S, Jalili-Firoozinezhad S, Mashinchian O, Mahmoudi M. Chapter 26 - Bioinspired Nanotechnologies for Skin Regeneration A2 - Hamblin, Michael R. In: Avci P, Prow TW, editors. Nanoscience in Dermatology. Academic Press; Boston: 2016. pp. 337–352. [Google Scholar]
- 374.Mashinchian O, Bonakdar S, Taghinejad H, Satarifard V, Heidari M, Majidi M, Sharifi S, Peirovi A, Saffar S, Taghinejad M. Cell-imprinted substrates act as an artificial niche for skin regeneration. ACS applied materials & interfaces. 2014;6:13280–13292. doi: 10.1021/am503045b. [DOI] [PubMed] [Google Scholar]
- 375.Bonakdar S, Mahmoudi M, Montazeri L, Taghipoor M, Bertsch A, Shokrgozar MA, Sharifi S, Majidi M, Mashinchian O, Hamrang Sekachaei M, Zolfaghari P, Renaud P. Cell-Imprinted Substrates Modulate Differentiation, Redifferentiation, and Transdifferentiation. ACS applied materials & interfaces. 2016;8:13777–13784. doi: 10.1021/acsami.6b03302. [DOI] [PubMed] [Google Scholar]
- 376.Ventola CL. Medical Applications for 3D Printing: Current and Projected Uses. P & T : a peerreviewed journal for formulary management. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 377.Dodziuk H. Applications of 3D printing in healthcare. Kardiochirurgia i torakochirurgia polska = Polish journal of cardio-thoracic surgery. 2016;13:283–293. doi: 10.5114/kitp.2016.62625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P. Design and fabrication of human skin by three-dimensional bioprinting, Tissue engineering. Part C. Methods. 2014;20:473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Khoo ZX, Teoh JEM, Liu Y, Chua CK, Yang S, An J, Leong KF, Yeong WY. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual and Physical Prototyping. 2015;10:103–122. [Google Scholar]
- 380.Momeni F, Mehdi Hassani N SM, Liu X, Ni J. A review of 4D printing. Materials & Design. 2017;122:42–79. [Google Scholar]
- 381.Gao B, Yang Q, Zhao X, Jin G, Ma Y, Xu F. 4D Bioprinting for Biomedical Applications. Trends in Biotechnology. 2016;34:746–756. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 382.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yang X. Applications of CRISPR-Cas9 mediated genome engineering. Mil Med Res. 2015;2:11. doi: 10.1186/s40779-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Jiang W, Marraffini LA. CRISPR-Cas: New Tools for Genetic Manipulations from Bacterial Immunity Systems. Annu Rev Microbiol. 2015;69:209–228. doi: 10.1146/annurev-micro-091014-104441. [DOI] [PubMed] [Google Scholar]
- 385.Webber BR, Osborn MJ, McElroy AN, Twaroski K, Lonetree CL, DeFeo AP, Xia L, Eide C, Lees CJ, McElmurry RT, Riddle MJ, Kim CJ, Patel DD, Blazar BR, Tolar J. CRISPR/Cas9-based genetic correction for recessive dystrophic epidermolysis bullosa. NPJ Regenerative medicine. 2016;1 doi: 10.1038/npjregenmed.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Chen M, Przyborowski M, Berthiaume F. Stem cells for skin tissue engineering and wound healing. Crit Rev Biomed Eng. 2009;37:399–421. doi: 10.1615/critrevbiomedeng.v37.i4-5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Gorell E, Nguyen N, Lane A, Siprashvili Z. Gene therapy for skin diseases. Cold Spring Harbor perspectives in medicine. 2014;4:a015149. doi: 10.1101/cshperspect.a015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Nomellini V, Gomez CR, Gamelli RL, Kovacs EJ. Aging and animal models of systemic insult: trauma, burn, and sepsis. Shock. 2009;31:11–20. doi: 10.1097/SHK.0b013e318180f508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Domergue S, Jorgensen C, Noel D. Advances in Research in Animal Models of Burn-Related Hypertrophic Scarring. Journal of burn care & research : official publication of the American Burn Association. 2015;36:e259–266. doi: 10.1097/BCR.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 390.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound repair and regeneration. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 391.Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2:296–315. doi: 10.4161/viru.2.4.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ragas X, Sánchez-García D, Ruiz-González R, Dai T, Agut M, Hamblin MR, Nonell S. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. Journal of medicinal chemistry. 2010;53:7796–7803. doi: 10.1021/jm1009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Katakura T, Yoshida T, Kobayashi M, Herndon DN, Suzuki F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol. 2005;142:419–425. doi: 10.1111/j.1365-2249.2005.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Stieritz DD, Holder IA. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. Journal of Infectious Diseases. 1975;131:688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- 395.Tavares Pereira Ddos S, Lima-Ribeiro MH, de Pontes-Filho NT, Carneiro-Leao AM, Correia MT. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol. 2012;2012:460841. doi: 10.1155/2012/460841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, Nitzan Y. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunology & Medical Microbiology. 1997;19:307–314. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 397.Suzuki T, Hirayama T, Aihara K, Hirohata Y. Experimental studies of moderate temperature burns. Burns : journal of the International Society for Burn Injuries. 1991;17:443–451. doi: 10.1016/0305-4179(91)90069-s. [DOI] [PubMed] [Google Scholar]
- 398.Bahar T, Bilezikci B, Maral T, Borman H. A modified partial-thickness burn model in rats. Burns : journal of the International Society for Burn Injuries. 2007;33:S52–S53. [Google Scholar]
- 399.Bjornson AB, Bjornson HS, Lincoln NA, Altemeier WA. Relative roles of burn injury, wound colonization, and wound infection in induction of alterations of complement function in a guinea pig model of burn injury. J Trauma. 1984;24:106–115. doi: 10.1097/00005373-198402000-00003. [DOI] [PubMed] [Google Scholar]
- 400.Walker HL, Mason AD., Jr A standard animal burn. The Journal of trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 401.Kaufman T, Lusthaus SN, Sagher U, Wexler MR. Deep partial skin thickness burns: a reproducible animal model to study burn wound healing. Burns. 1990;16:13–16. doi: 10.1016/0305-4179(90)90199-7. [DOI] [PubMed] [Google Scholar]
- 402.Stevens EJ, Ryan CM, Friedberg JS, Barnhill RL, Yarmush ML, Tompkins RG. A quantitative model of invasive Pseudomonas infection in burn injury. The Journal of burn care & rehabilitation. 1994;15:232–235. doi: 10.1097/00004630-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 403.http://ir.cytori.com/investor-relations/news/news-details/2017/Cytori-Receives-US-FDA-Approval-for-Burn-Clinical-Trial-Related-to-BARDA-Contract/default.aspx.
- 404.https://clinicaltrials.gov/ct2/show/NCT02104713.
- 405.Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiation Oncology. 2012;7:162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Landriscina A, Musaev T, Rosen J, Ray A, Nacharaju P, Nosanchuk JD, Friedman AJ. N-acetylcysteine S-nitrosothiol Nanoparticles Prevent Wound Expansion and Accelerate Wound Closure in a Murine Burn Model. Journal of drugs in dermatology : JDD. 2015;14:726–732. [PubMed] [Google Scholar]
- 407.http://www.internano.org/node/4211.
- 408.http://grantome.com/grant/NIH/R43-GM081967-01.
- 409.Yamada M, Foote M, Prow TW. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;7:428–445. doi: 10.1002/wnan.1322. [DOI] [PubMed] [Google Scholar]
- 410.Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, Tada H, Teramukai S, Murayama T, Toyooka C, Miura K, Takemoto S, Kanda N, Kawai K, Yokode M, Shimizu A, Suzuki S. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue engineering. Part A. 2013;19:1931–1940. doi: 10.1089/ten.tea.2012.0634. [DOI] [PubMed] [Google Scholar]
- 412.https://www.regmednet.com/channels/544-spotlight-on-wound-healing/posts/13238-skin-based-gene-therapy-safe-and-effective-in-phase-1-clinical-trial-for-epidermolysis-bullosa-wounds.
- 413.Yuxiang L, Lu T, Jianqiang Y, Xiuying D, Wanfang Z, Wannian Z, Xiaoyan H, Shichu X, Wen N, Xiuqiang M. Analgesia effect of a fixed nitrous oxide/oxygen mixture on burn dressing pain: study protocol for a randomized controlled trial. Trials. 2012;13:67. doi: 10.1186/1745-6215-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Zhou K, Krug K, Stachura J, Niewczyk P, Ross M, Tutuska J, Ford G. Silver-Collagen Dressing and High-voltage, Pulsed-current Therapy for the Treatment of Chronic Full-thickness Wounds: A Case Series. Ostomy Wound Manage. 2016;62:36–44. [PubMed] [Google Scholar]
- 415.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: review and advancements. Critical care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.