Abstract
BACKGROUND AND PURPOSE
The purpose of this case-cohort study was to examine urinary arsenic levels in relation to incident ischemic stroke in the United States.
METHODS
We performed a case-cohort study nested within the REasons for Geographic and Racial Differences in Stroke(REGARDS) cohort. A subcohort(n=2,486) of controls was randomly sampled within region-race-sex strata, while all incident ischemic stroke cases from the full REGARDS cohort(n=671) were included. Baseline urinary arsenic was measured by inductively coupled plasma mass spectrometry. Arsenic species, including urinary inorganic arsenic(iAs) and its metabolites monomethylarsonic acid(MMA) and dimethylarsinic acid(DMA), were measured in a random subset(n=199). Weighted Cox’s proportional hazards models were used to calculate hazard ratios(HRs) and 95% confidence intervals(CIs) of ischemic stroke by arsenic and its species.
RESULTS
The average follow-up was 6.7 years. While incident ischemic stroke showed no association with total arsenic or total iAs, for each unit higher level of urinary MMA on a log-scale, after adjustment for potential confounders, ischemic stroke risk increased nearly 2-fold(HR=1.98; 95%CI: 1.12–3.50). Effect modification by age, race, sex, or geographic region was not evident.
CONCLUSIONS
A metabolite of arsenic was positively associated with incident ischemic stroke in this case-cohort study of the U.S. general population, a low-to-moderate exposure area. Overall, these findings suggest a potential role for arsenic methylation in the etiology of stroke, having important implications for future cerebrovascular research.
Keywords: Ischemic stroke, minerals, arsenic, stroke belt, stroke disparities
Introduction
Stroke is the fifth leading cause of death among adults in the United States and a significant source of disability1. The mortality rate in stroke varies geographically in the United States with a “Stroke Belt” traversing the Southeastern region(Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Virginia)2, where individuals have an elevated risk of dying from stroke compared to other states3. Within the Stroke Belt states, there is an area with an even higher risk of stroke mortality known as the “Stroke Buckle.” This includes North Carolina, South Carolina, and Georgia4. While some factors have been found to explain this geographic disparity in part5, a conclusive answer has yet to be found.
Because of its role in inflammation6 and atherosclerosis7, inorganic arsenic(iAs) may be part of the reason for the Stroke Belt. Organic arsenic compounds, such as arsenobetaine(AB), occur naturally in seafood and are considered non-toxic8. Inorganic arsenic, alternatively, is considered highly toxic, and occurs in the environment as arsenite(iAsIII) and arsenate(iAsV). Both forms can be found in contaminated food sources, drinking water, occupational exposure, and industrial sources9. In the liver, iAsIII is metabolized into a trivalent form(MMAIII) and then a pentavalent form of monomethylarsonic acid(MMAV), followed by trivalent and pentavalent forms of dimethylarsinic acid(DMAIII and DMAV)10. After ingestion, iAs is excreted in the urine with concentrations in the range of 10–20% iAs, 10–15% MMA, and 60–75% DMA11. Together, exposure to the trivalent and pentavalent forms of iAs, MMA, and DMA have the potential to inflict widespread cardiovascular damage. Underreporting iAs and its metabolites is a serious limitation of research on the health effects of arsenic.
Much of the evidence for iAs toxicity and vascular disease risk comes from regions of high groundwater exposure, such as Taiwan12, Bangladesh13, Chile14, and China Inner Mongolia15. Although iAs concentrations as high as 100 ppb have been reported in some areas16, exposure in the U.S. overall is considered as low-to-moderate. For this reason, few studies have investigated the potential adverse effects of iAs in the U.S.17–20, and none have represented the Stroke Belt region.
While increasing evidence suggests that long-term exposure to iAs is associated with risk of a variety of vascular diseases21, evidence that iAs is an independent predictor of ischemic stroke remains inconclusive. A greater risk of stroke mortality or hospitalizations in households, villages or counties with high-total-arsenic drinking water was found in some12, 13, 18, 19, 22, but not all studies14, 15, 23–26. Three studies have assessed the relation between urinary arsenic and stroke incidence or mortality20, 24, 26; none demonstrated a significant association. A recent meta-analysis across 31 studies(8 reporting on stroke prevalence/mortality) found a positive association between arsenic exposure and cardiovascular disease, coronary heart disease, and peripheral arterial disease, but not stroke27.
This study aimed to explore the association between urinary arsenic(organic and inorganic) and incident ischemic stroke using data from the U.S. national REasons for Geographic and Racial Differences in Stroke(REGARDS) Study.
Methods
Study population
Methodological details of REGARDS can be found elsewhere28. In summary, REGARDS is a population-based study of adults aged ≥45 years28. The cohort includes 30,239 individuals enrolled between January 2003 and October 2007, with follow-up every six months. The cohort is 55% female, 41% black, and 56% from Stroke Belt region.
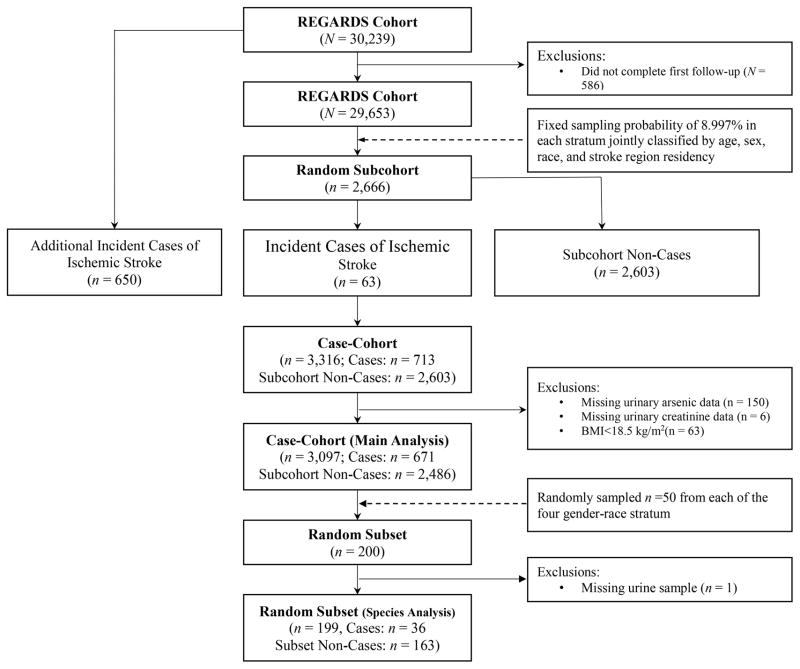
This study uses a case-cohort design. The subcohort(n=2,666) was selected from the entire cohort of REGARDS participants having at least one follow-up(N=29,653) with a fixed sampling probability of 8.997% in each stratum jointly classified by age(<55, 55–64, 65–74, 75–84, and ≥85 years), sex, race(black and white), and stroke region residency(non-Stroke Belt, Stroke Belt, and Stroke Buckle)29. A flow chart of sample exclusion process is listed in Figure 1.
Figure 1.
Flow chart featuring the case-cohort sampling scheme of this study.
REGARDS was approved by the Institutional Review Boards of each participating institution, and all participants provided written informed consent. Because of the sensitive nature of data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be send to the REGARDS administrative staff at regardsadmin@uab.edu.
Measures
Primary Exposure
Urine samples were obtained during a home visit at baseline. All urine samples for this study were analyzed by the University of Missouri-Columbia Research Reactor Center(MURR). Specific laboratory methods are described in more detail elsewhere30. In brief, total urinary arsenic was measured using inductively coupled plasma-mass spectrometry(ICP-MS). Urinary creatinine was measured using standard methods31. We report urinary arsenic and arsenic species as a normalized ratio of arsenic/creatinine to control for variation in urine flow rate.
Because of budget constraints, arsenic species and metabolites were measured in a random subset of 200 urine samples from the case-cohort sample(n=3,097), with 50 samples from each of the four gender-race stratum. Species were separated via liquid chromatography and measured using ICP-MS. Arsenic species have been shown to have long-term stability in urine excretions among those exposed32.
Outcome
The outcome was incident ischemic stroke. Methods of determination of incident stroke have previously been reported33. Suspected cases of incident stroke were obtained every six months via telephone and verified using medical record review. The final adjudication was based on the WHO definition of stroke and/or imaging results consistent with stroke. All ischemic stroke cases adjudicated by September 30, 2012, were included.
Covariates
Baseline demographic and health behavior measures were collected via telephone interview. Covariates considered were age, sex, race(black or white), region(non-Stroke Belt, Stroke Belt, and Stroke Buckle residence), education(<high school, high school graduate, some college, or ≥college graduate), smoking status(never, past or current smoker), alcohol consumption(never, past or current user), physical activity(none, 1–3/week, or ≥4/week), body mass index(BMI), systolic and diastolic blood pressure(BP), total cholesterol, low-density lipoprotein-cholesterol, and glucose. History of type 2 diabetes, myocardial infarction, hypertension, and albuminuria were defined using self-report and measured data. Myocardial infarction was defined by self-report or centrally-read ECG evidence of MI. Hypertension was defined as a systolic BP≥140 mmHg, a diastolic BP ≥90 mmHg, or the self-reported hypertension medication use. Albuminuria was defined as a urinary albumin level of ≥30 mg/g creatinine. BMI was based on measured height and weight and categorized into underweight/normal(<25 kg/m2), overweight(25–<30 kg/m2), and obese(≥30 kg/m2).
Because evidence suggests that combined exposure of arsenic and cadmium, as well as arsenic and mercury, induces greater toxicity than either alone34, cadmium and mercury were included as covariates. Cadmium was measured in urine using ICP-MS. Mercury was measured in serum using a direct mercury analyzer(Nippon MA-3000).
Statistical Analysis
Distributions of baseline characteristics in the random subcohort were presented based on quintiles of total urinary arsenic(creatinine adjusted). The differences in baseline characteristics across urinary arsenic subgroups were compared using analysis of variance, Kruskal-Wallis test, or chi-squared test, as appropriate.
Participants contributed to person-time from baseline to the time when a case was identified, a participant was censored, or the end of the study(September 30, 2012). To determine the association[hazard ratios(HRs) and 95% confidence intervals(CIs)] between urinary arsenic levels and incident ischemic stroke, a weighted Cox model was used. The variance of the estimator was estimated using the robust variance35. We sequentially adjusted for potential confounders in three models.
We assessed effect modification by race, age, sex, and geographic variables(e.g., urban vs. rural, and Stroke Belt vs. non-Stroke Belt residence) with likelihood ratio tests.
We examined the associations of urinary iAs(iAsIII+iAsV), iAs+MMA, iAs+MMA+DMA, MMA, and DMA with incident ischemic stroke in the random subsample. The second-stage sampling weight was also considered.
All analyses were conducted using SAS 9.4(SAS Institute, Inc., Cary, NC, USA). P≤0.05 was considered statistically significant.
Results
There were 63 and 713 incident ischemic stroke cases in the subcohort(n=2,666) and the entire case-cohort sample(n=3,316), respectively. Among the total 3,316 participants, urinary arsenic data were available in 3,166 individuals. We further excluded participants with missing data on urine creatinine(n=6) and those who had BMI<18.5 kg/m2(n=63). Therefore, 3,097 participants remained in the main analysis(subcohort: n=2,486; incident cases: n=671).
Baseline characteristics of the subcohort across quintiles of creatinine-adjusted total urinary arsenic exposure are represented in Table 1. Those with total urinary arsenic levels in the highest quintile tended to be older, female, past or never smokers, current alcohol users, college graduates, overweight or obese, and Stroke Buckle residents compared with those in the lowest quintile. Total urinary arsenic exhibited an inverse correlation with urinary cadmium, as well as a positive trend with serum mercury. Baseline characteristics of ischemic stroke cases across quintiles of creatinine-adjusted total urinary arsenic exposure are represented in Supplement Table 1. In summary, ischemic stroke cases with total urinary arsenic in the highest quintile tended to be older, current smokers, college graduates, have lower diastolic BP, lower LDL-C, and higher serum mercury.
Table 1.
Characteristics of the random subcohort by quintiles of urinary total arsenic(n=2,486)*
| Random subcohort | Quintiles of urinary total arsenic(μg/g creatinine)† | P-value‡ | |||||
|---|---|---|---|---|---|---|---|
| <4.22 | 4.23–6.55 | 6.56–10.48 | 10.49–20.54 | ≥20.55 | |||
| n | 2,486 | 498 | 497 | 497 | 497 | 497 | -- |
| Age(year) | 64.9±9.3 | 64.1±9.6 | 64.8±9.5 | 65.1±8.9 | 65.8±9.3 | 64.7±9.1 | 0.09 |
| Female(%) | 54.4 | 48.8 | 53.9 | 57.6 | 58.2 | 53.7 | 0.02 |
| Black(%) | 40.8 | 40.2 | 42.7 | 40.9 | 39.2 | 41.1 | 0.86 |
| Smoke(%) | |||||||
| Never | 46.4 | 43.6 | 46.9 | 47.9 | 48.1 | 45.5 | <0.01 |
| Past | 39.0 | 35.5 | 36.4 | 39.4 | 40.2 | 43.5 | |
| Current | 14.6 | 20.9 | 16.7 | 12.7 | 11.7 | 11.1 | |
| Region(%) | |||||||
| Non-Stroke Belt | 43.9 | 36.7 | 44.7 | 42.9 | 47.5 | 47.7 | <0.01 |
| Stroke Belt | 34.9 | 43.2 | 38.6 | 34.4 | 30.0 | 28.2 | |
| Stroke Buckle | 21.2 | 20.1 | 16.7 | 22.7 | 22.5 | 24.1 | |
| Alcohol(%) | |||||||
| Never | 30.0 | 35.5 | 29.4 | 33.0 | 29.2 | 23.1 | <0.01 |
| Past | 18.3 | 21.1 | 21.3 | 16.1 | 16.1 | 16.9 | |
| Current | 51.6 | 43.4 | 49.3 | 50.9 | 54.7 | 60.0 | |
| Education(%) | |||||||
| <High school | 12.3 | 12.7 | 14.9 | 10.3 | 12.1 | 11.7 | <0.01 |
| High school graduate | 24.4 | 27.9 | 23.6 | 23.1 | 22.2 | 25.0 | |
| Some college | 27.4 | 29.9 | 29.4 | 27.0 | 28.6 | 22.1 | |
| ≥College graduate | 35.9 | 29.5 | 32.1 | 39.6 | 37.1 | 41.3 | |
| Physical activity(%) | |||||||
| None | 33.2 | 36.9 | 34.9 | 29.7 | 32.2 | 32.1 | 0.09 |
| 1~3/week | 35.4 | 33.9 | 36.1 | 35.6 | 36.3 | 35.4 | |
| ≥4/week | 31.4 | 29.2 | 29.0 | 34.8 | 31.4 | 32.5 | |
| Body mass index(%) | |||||||
| <25.0kg/m2 | 23.4 | 19.9 | 23.5 | 24.1 | 25.0 | 24.6 | 0.10 |
| 25~29.9kg/m2 | 38.0 | 37.4 | 36.4 | 38.0 | 38.8 | 39.4 | |
| ≥30.0kg/m2 | 38.6 | 42.8 | 40.0 | 37.8 | 36.2 | 36.0 | |
| SBP, mmHg | 127.7±16.6 | 128.5±17.9 | 126.9±16.1 | 127.8±16.7 | 127.7±16.3 | 127.4±16.0 | 0.67 |
| DBP, mmHg | 76.6±9.5 | 76.8±10.2 | 76.0±9.6 | 76.4±9.4 | 76.6±8.9 | 77.2±9.3 | 0.31 |
| TC, mg/dL | 191.9±40.0 | 191.9±39.1 | 190.9±37.9 | 191.1±40.3 | 192.3±42.2 | 193.3±40.7 | 0.89 |
| LDL-C, mg/dL | 114.1±34.5 | 114.8±34.8 | 112.7±33.3 | 114.3±35.5 | 113.5±33.9 | 115.0±35.0 | 0.84 |
| Glucose, mg/dL | 104.2±35.4 | 105.0±32.9 | 106.6±37.7 | 101.3±29.6 | 102.9±31.8 | 104.9±43.5 | 0.17 |
| Hypertension(%) | 59.1 | 59.2 | 58.0 | 60.4 | 57.6 | 60.6 | 0.82 |
| Myocardial infaction(%) | 11.8 | 12.9 | 11.9 | 11.3 | 10.9 | 12.1 | 0.89 |
| Type 2 diabetes(%) | 21.4 | 21.9 | 23.1 | 19.7 | 21.1 | 21.1 | 0.76 |
| Albuminuria(%) | 14.0 | 13.7 | 11.3 | 15.1 | 15.9 | 14.1 | 0.28 |
| Urine cadmium(ng/g) | 0.48(0.28–0.85) | 0.59(0.31–1.05) | 0.48(0.27–0.83) | 0.48(0.28–0.80) | 0.46(0.27–0.81) | 0.45(0.26–0.79) | <0.01 |
| Serum mercury(ng/g) | 0.33(0.18–0.58) | 0.20(0.12–0.38) | 0.29(0.17–0.50) | 0.32(0.18–0.56) | 0.37(0.22–0.59) | 0.49(0.29–0.84) | <0.01 |
DBP=diastolic blood pressure; LDL-C=low-density lipoprotion-cholesterol; SBP=systolic blood pressure; TC=total cholesterol.
Data are means±standard deviations, medians(inter-quartile ranges), or proportions.
Quintiles of urinary total arsenic were calculated based on the random sub-cohort.
P values are for any difference across subgroups by using analysis of variance, Kruskal-Wallis test, or chi-squared test as appropriate.
A total of 671 participants developed ischemic stroke over an average follow-up of 6.7 years. Table 2 shows the multivariable-adjusted HR of ischemic stroke by quintiles of creatinine-corrected total urinary arsenic levels. After adjustment for confounders, there were no associations between total urinary arsenic levels and ischemic stroke incidence. Neither a linear nor non-linear association was detected by a restricted cubic spline analysis. Effect modification by race, sex, age, or geographic region was not observed(data not shown).
Table 2.
Hazard ratio(95% confidence intervals) of incident ischemic stroke by quintiles of urinary total arsenic levels)(n=3,097)*
| Quintiles of urinary total arsenic(μg/g creatinine)† | Ptrend‡ | ||||||
|---|---|---|---|---|---|---|---|
| <4.22 | 4.23–6.55 | 6.56–10.48 | 10.49–20.54 | ≥20.55 | Linear | Non-linear | |
| Median(inter-quartile range) | 3.29(2.72–3.72) | 5.26(4.75–5.88) | 8.07(78.26–9.18) | 13.88(11.99–16.72) | 34.06(26.11–54.81) | ||
| No. of events/participants | 150/637 | 138/622 | 139/624 | 119/606 | 125/608 | ||
| Model 1§ | 1.00 | 0.92(0.70–1.22) | 0.90(0.69–1.19) | 0.74(0.56–0.99) | 0.84(0.63–1.11) | 0.69 | 0.51 |
| Model 2| | | 1.00 | 0.93(0.70–1.23) | 0.95(0.72–1.27) | 0.79(0.59–1.06) | 0.89(0.67–1.19) | 0.66 | 0.83 |
| Model 3# | 1.00 | 0.97(0.73–1.30) | 1.03(0.77–1.38) | 0.87(0.64–1.18) | 1.01(0.74–1.36) | 0.91 | 0.998 |
All models were constructed using weighted Cox regression for case-cohort study.
Quintiles were calculated based on the random subcohort(n=2,486).
P values for linear/non-linear trend were tested using restricted cubic spline method.
Model 1: adjusted for age at baseline, sex, race, age*race, and stroke region.
Model 2: additionally adjusted for body mass index, education, smoking status, alcohol consumption, and physical activity.
Model 3: additionally adjusted for quintiles of urine cadmium and serum mercury.
The multivariable-adjusted HRs of ischemic stroke by iAs and metabolite species are presented in Table 3. Among the 200 participants in random subsample, 1 sample was contaminated. Therefore, there were 199 participants in the species analysis(random subset: n=199; incident cases: n=41). There was a significant linear association of iAs(iAsIII+iAsV)+MMA on a log-scale with stroke risk(HR=1.69; 95%CI: 1.06–2.72), but the statistical significance of this association was attenuated after further adjustment. The levels of MMA ranged from 0.01 to 0.77 μg/g creatinine, and for each unit higher level of urinary MMA on a log-scale, after adjustment for potential confounders, ischemic stroke risk increased about 2-fold(HR=1.98; 95%CI: 1.12–3.50).
Table 3.
Hazard ratio(95% confidence intervals) of incident ischemic stroke by urinary arsenic species and its metabolites(μg/g creatinine) based on the random subset(n=199)*
| <Median † | ≥Median† | Linear association‡ | |
|---|---|---|---|
| Total arsenic | |||
| Range | 2.11–7.48 | 7.49–289.91 | |
| No. of events/participants | 20/100 | 21/99 | |
| Model 1§ | 1.00 | 0.93(0.44–1.98) | 0.95(0.62–1.47) |
| Model 2| | | 1.00 | 1.34(0.47–3.88) | 1.06(0.62–1.83) |
| Model 3# | 1.00 | 1.66(0.48–5.72) | 1.11(0.63–1.97) |
| Total iAs(iAsIII+iAsV) | |||
| Range | 0.01–0.15 | 0.16–2.57 | |
| No. of events/participants | 22/101 | 19/98 | |
| Model 1§ | 1.00 | 1.30(0.54 3.13) | 1.01(0.78–1.33) |
| Model 2| | | 1.00 | 0.89(0.27–2.90) | 0.91(0.64–1.30) |
| Model 3# | 1.00 | 0.91(0.26–3.18) | 0.91(0.64–.30) |
| Total iAs+MMA | |||
| Range | 0.01–0.54 | 0.55–5.75 | |
| No. of events/participants | 19/98 | 22/101 | |
| Model 1§ | 1.00 | 2.03(0.77–5.41) | 1.69(1.06–2.72) |
| Model 2| | | 1.00 | 1.98(0.55–7.15) | 1.69(0.92–3.10) |
| Model 3# | 1.00 | 2.21(0.53–9.15) | 1.79(0.91–3.53) |
| Total iAs+MMA+DMA | |||
| Range | 0.10–3.52 | 3.53–66.82 | |
| No. of events/participants | 17/97 | 24/102 | |
| Model 1§ | 1.00 | 1.55(0.71–3.41) | 1.22(0.86–1.72) |
| Model 2| | | 1.00 | 1.77(0.62–5.02) | 1.22(0.78–1.90) |
| Model 3# | 1.00 | 2.18(0.64–7.43) | 1.27(0.81–2.02) |
| MMA | |||
| Range | 0.01–0.40 | 0.40–0.77 | |
| No. of events/participants | 15/93 | 26/106 | |
| Model 1§ | 1.00 | 2.89(1.23–6.76) | 1.65(1.14–2.39) |
| Model 2| | | 1.00 | 2.61(0.89–7.68) | 1.68(1.09–2.60) |
| Model 3# | 1.00 | 2.94(0.87–10.01) | 1.98(1.12–3.50) |
| DMA | |||
| Range | 0.01–0.40 | 0.40–10.40 | |
| No. of events/participants | 19/98 | 22/101 | |
| Model 1§ | 1.00 | 1.27(0.62–2.63) | 1.14(0.83–1.57) |
| Model 2| | | 1.00 | 1.31(0.53–3.25) | 1.20(0.74–1.95) |
| Model 3# | 1.00 | 1.45(0.54–3.93) | 1.31(0.81–2.13) |
AsIII=arsenite; AsV=arsenate; DMA=dimethylarsinic acid; MMA=monomethylarsonic acid.
All models were constructed using weighted Cox regression for case-cohort study.
Medians were calculated based on the subset(n=163) of the random subcohort.
Linear associations were examined with 1 unit increment in Ln(exposure) with extreme values(>99th percentile) deleted.
Model 1: adjusted for age at baseline, sex, race, age*race, and stroke region.
Model 2: additionally adjusted for body mass index, education, smoking status, alcohol consumption, and physical activity.
Model 3: additionally adjusted for urine cadmium(≥or<median) and serum mercury(≥or<median).
Discussion
In this population-based case-cohort study, we found that MMA, a metabolite of iAs, but not total arsenic, was positively associated with incident ischemic stroke. While the findings are generally consistent with results from ecological studies, this study provides important additional information on urinary arsenic species and stroke incidence from an area with low-to-moderate arsenic exposure.
To date, few studies have investigated urinary arsenic(total and/or speciated) exposure and ischemic stroke incidence, although the possible adverse effects of chronic exposure to high arsenic levels in drinking water in relation to stroke have been documented many times in ecological studies13, 19. Ecological data can help generate hypotheses, but it is prone to bias, so must be interpreted with caution. Our study found no association between total arsenic and incident stroke. These findings are in agreement with a study between total urinary arsenic exposure and stroke mortality in a sample of 11,746 Bangladeshi men and women24 that found no association. A prospective study in 3,575 American Indians showed similar findings20.
Our study is the first we are aware of to show a significant relation between exposure to inorganic arsenic and ischemic stroke incidence. We are aware of only one other study investigating iAs metabolites and stroke risk. A case-cohort study of the Health Effects of Arsenic Longitudinal Study26 cohort found no association between MMA% methylation and stroke risk, although associations were evident with cardiovascular disease. The investigators note, however, that they were unable to distinguish between stroke subtypes in this analysis, and so the inclusion of hemorrhagic stroke may have undermined any possible association. The metabolism of iAs varies across individuals and is considered reflective of an individual’s ability to methylate to MMA and then DMA36. This is particularly important, since MMAIII is considered more toxic than either iAs or DMA37. After ingestion, typical iAs methylation results in 10–15% and 60–75% concentrations of MMA and DMA, respectively11. Those with a higher proportion of MMA(and thus a lower proportions of iAs and DMA) are considered “poor methylators”(>15% MMA), and are at a higher risk for atherosclerosis, peripheral vascular disease, heart disease, hypertension and various types of cancer38. The possibility cannot be excluded that the observed association between MMA and ischemic stroke may only be a proxy of individual methylation poor capacity to all external substances.
The true mechanism of iAs metabolism is still little understood. One proposed model suggests that iAsIII is methylated to MMAV and DMAV, forming a transient MMAIII species in the process10. The urinary MMA level reported in this study is the sum of MMAIII and MMAV. For this reason, the MMA metabolite measured in this study was most likely the pentavalent form and any MMAIII that may have been present likely would have been methylated to the pentavalent form after the addition of hydrogen peroxide during the laboratory analysis stage39.
Finally, it is important to note that the methylation of iAs is not the only possible source of MMA. MMA can also be found in monosodium methyl arsenate(MSMA), a commonly used organo-arsenate-based herbicide. MSMA seems to persist in foliage and soil for long periods of time and may pose a threat to those exposed to these treated areas40. MMA can be measured in rice and foods derived from rice long after the herbicidal treatments have ceased41. In sum, we must consider two possible sources of exposure to MMA–the methylation of iAs to MMA and exposure to MSMA. The observed association between MMA, but not iAs, and incident ischemic stroke suggests the latter requires serious consideration. A deeper understanding of MSMA exposure in this cohort is required.
Inflammation may mediate the association of MMA with ischemic stroke. Human models indicate an increase in the expression of proinflammatory cytokines in circulating lymphocytes after arsenic exposure42. For example, individuals with prolonged exposure to arsenic exhibited an upregulation of a variety of growth factors and cytokines related to inflammation in their circulating lymphocytes42. Inflammation plays a significant role in atherosclerotic plaque formation and vascular damage43. Taken together, arsenic-induced inflammation is a plausible mechanism of atherosclerosis and, by extension, ischemic stroke.
Limitations of this study require consideration. The small sample size of the subcohort in which arsenic species were measured limited our capability to detect heterogeneity, so further study is needed. Another limitation was that the exposure was only measured at baseline, although several studies have demonstrated the temporal stability of arsenic in private and public drinking water sources, as well as levels in urine32, 44. Furthermore, arsenic levels in drinking water from individual households were unavailable, limiting our ability to speculate on the source of iAs exposure.
Our study has a number of strengths. The greatest strength is the measurement of arsenic species, a rarity in stroke research involving arsenic. Another strength is the use of a large, national sample that over-represents blacks and southerners. Also, because REGARDS was specifically designed to explore risk factors for stroke, its characterization of vascular risk factors is excellent. Furthermore, urinary arsenic measurement that incorporates all sources of exposure, including both water, food, and environment, is a great objective measure of arsenic exposure45. The case-cohort study design also represents a strength, as it is both efficient and flexible.
Supplementary Material
Acknowledgments
Funding: NIH(R01ES021735, U01NS041588); NSF instrument grant(BCS-0922374)
We thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Sources of Funding
This work was supported by a grant from NIH(R01ES021735). The REGARDS research project is supported by a cooperative agreement(U01NS041588) from the NIH. This research was performed using instrumentation purchased by NSF(BCS-0922374).
Footnotes
Conflicts of Interest
None to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:447. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke. 1995;26:1145–1149. doi: 10.1161/01.str.26.7.1145. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Howard VJ, Katholi C, Oli MK, Huston S. Decline in US stroke mortality an analysis of temporal patterns by sex, race, and geographic region. Stroke. 2001;32:2213–2220. doi: 10.1161/hs1001.096047. [DOI] [PubMed] [Google Scholar]
- 4.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 28:936–940. doi: 10.1161/01.str.28.5.936. 997. [DOI] [PubMed] [Google Scholar]
- 5.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, et al. Life’s Simple 7 and risk of incident stroke the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dutta K, Prasad P, Sinha D. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. International Journal of Hygiene and Environmental Health. 2015;218:564–574. doi: 10.1016/j.ijheh.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Aalbers TG, Houtman J. Relationships between trace elements and atherosclerosis. Science of the Total Environment. 1985;43:255–283. doi: 10.1016/0048-9697(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 8.Benramdane L, Bressolle F, Vallon J. Arsenic speciation in humans and food products: a review. Journal of Chromatographic Science. 1999;37:330–344. doi: 10.1093/chromsci/37.9.330. [DOI] [PubMed] [Google Scholar]
- 9.Gorby M. Arsenic in human medicine. Arsenic n the Environment, Part II. In: Nriagu JO, editor. Human Health and Ecosystem Effects. New York: Wiley Interscience; 1994. [Google Scholar]
- 10.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 11.Hopenhaynrich C, Smith AH, Goeden HM. Human studies do not support the methylation threshold hypothesis for the toxicity of inorganic arsenic. Environmental Research. 1993;60:161–177. doi: 10.1006/enrs.1993.1024. [DOI] [PubMed] [Google Scholar]
- 12.Cheng T-J, Ke D-S, Guo H-R. The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Research. 2010;44:5770–5776. doi: 10.1016/j.watres.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Sohel N, Yunus M, Chowdhury ME, Hore SK, Zaman K, et al. A prospective cohort study of stroke mortality and arsenic in drinking water in Bangladeshi adults. BMC Public Health. 2014;14:174. doi: 10.1186/1471-2458-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. American Journal of Epidemiology. 2007;166:1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- 15.Wade TJ, Xia Y, Wu K, Li Y, Ning Z, Le XC, et al. Increased mortality associated with well-water arsenic exposure in Inner Mongolia, China. International Journal of Environmental Research and Public Health. 2009;6:1107–1123. doi: 10.3390/ijerph6031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharif M, Davis R, Steele K, Kim B, Kresse T, Fazio J. Inverse geochemical modeling of groundwater evolution with emphasis on arsenic in the Mississippi River Valley alluvial aquifer, Arkansas (USA) Journal of Hydrology. 2008;350:41–55. [Google Scholar]
- 17.Gong G, O’Bryant SE. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environmental Research. 2012;113:52–57. doi: 10.1016/j.envres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Lisabeth LD, Ahn HJ, Chen JJ, Sealy-Jefferson S, Burke JF, Meliker JR. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke. 2010;41:2499–2504. doi: 10.1161/STROKEAHA.110.585281. [DOI] [PubMed] [Google Scholar]
- 19.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environmental Health. 2007;6:1–11. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Annals of Internal Medicine. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu F, Jasmine F, Kibriya MG, Liu M, Wójcik O, Parvez F, et al. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. American Journal of Epidemiology. 175:1252–1261. doi: 10.1093/aje/kwr464. 012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiou H-Y, Huang W-I, Su C-L, Chang S-F, Hsu Y-H, Chen C-J. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28:1717–1723. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- 23.Xia Y, Wade TJ, Wu K, Li Y, Ning Z, Le XC, et al. Well water arsenic exposure, arsenic induced skin-lesions and self-reported morbidity in Inner Mongolia. International Journal of Environmental Research and Public Health. 2009;6:1010–1025. doi: 10.3390/ijerph6031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medrano MJ, Boix R, Pastor-Barriuso R, Palau M, Damián J, Ramis R, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environmental Research. 2010;110:448–454. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Wu F, Liu M, Parvez F, Slavkovich V, Eunus M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environmental Health Perspectives. 2013;121:832. doi: 10.1289/ehp.1205797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Current Atherosclerosis Reports. 2012;14:542–555. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The REasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2004;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 29.Cai J, Zeng D. Sample size/power calculation for case–cohort studies. Biometrics. 2004;60:1015–1024. doi: 10.1111/j.0006-341X.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 30.Carioni V, McElroy J, Guthrie J, Ngwenyama R, Brockman J. Fast and reliable method for As speciation in urine samples containing low levels of As by LC-ICP-MS: focus on epidemiological studies. Talanta. 2017 doi: 10.1016/j.talanta.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 31.Tamura MK, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. American Journal of Kidney Diseases. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environmental Health Perspectives. 2009;117:1428. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of Neurology. 2011;69:619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Liu Y, Habeebu SM, Waalkes MP, Klaassen CD. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147:157–166. doi: 10.1016/s0300-483x(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 35.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994:1064–1072. [PubMed] [Google Scholar]
- 36.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Science Progress. 1998;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Archives of Toxicology. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 38.Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, et al. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicology and Applied Pharmacology. 2010;247:138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aposhian HV, Zakharyan RA, Avram MD, Sampayo-Reyes A, Wollenberg ML. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicology and Applied Pharmacology. 2004;198:327–335. doi: 10.1016/j.taap.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Matteson AR, Gannon TW, Jeffries MD, Haines S, Lewis DF, Polizzotto ML. Arsenic retention in foliage and soil after monosodium methyl arsenate (MSMA) application to turfgrass. Journal of Environmental Quality. 2014;43:379–388. doi: 10.2134/jeq2013.07.0268. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M-M, Chiou H-Y, Ho I-C, Chen C-J, Lee T-C. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environmental Health Perspectives. 2003;111:1429. doi: 10.1289/ehp.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epstein FH, Ross R. Atherosclerosis—an inflammatory disease. New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 44.Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environmental Research. 2005;99:164–168. doi: 10.1016/j.envres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat M-P. What is the best biomarker to assess arsenic exposure via drinking water? Environment International. 2012;39:150–171. doi: 10.1016/j.envint.2011.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.