Abstract
Background & Aims
There are few effective treatments for nausea and other symptoms in patients with gastroparesis and related syndromes. We performed a randomized trial of the ability of the neurokinin-1 receptor antagonist aprepitant to reduce symptoms in patients with chronic nausea and vomiting due to gastroparesis or gastroparesis-like syndrome.
Methods
We conducted a 4-week multicenter, double-masked trial of 126 patients with at leastmoderate symptoms of chronic nausea and vomiting of presumed gastric origin for at least 6 months. Patients were randomly assigned to groups given oral aprepitant (125 mg/day, n=63) or placebo (n=63). The primary outcome from the intention-to-treat analysis was reduction in nausea, defined as a decrease of 25 mm or more, or absolute level below 25 mm, on a daily patient-reported 0–100 visual analog scale (VAS) of nausea severity. We calculated relative risks of nausea improvement using stratified Cochran-Mental-Haenszel analysis.
Results
Aprepitant did not reduce symptoms of nausea, based on the primary outcome measure (46% reduction in the VAS score in the aprepitant group vs 40% reduction in the placebo group; relative risk, 1.2; 95% CI, 0.8–1.7) (P=.43). However, patients in the aprepitant group had significant changes in secondary outcomes such as reduction in symptom severity (measured by the 0–5 Gastroparesis Clinical Symptom Index) for nausea (1.8 vs 1.0; P=.005), vomiting (1.6 vs 0.5; P=.001), and overall symptoms (1.3 vs 0.7; P=.001). Adverse events, predominantly mild or moderate in severity grade, were more common in aprepitant (22/63 patients, 35% vs 11/63, 17% in the placebo group) (P=.04).
Conclusions
In a randomized trial of patients with chronic nausea and vomiting due to gastroparesis or gastroparesis-like syndrome, aprepitant did not reduce the severity of nausea, when reduction in VAS score was used at the primary outcome. However, aprepitant had varying effects on secondary outcomes of symptom improvement. These findings support the need to identify appropriate patient outcomes for trials of therapies for gastroparesis, including potential additional trials for aprepitant. ClinicalTrials.gov no: NCT01149369.
Keywords: Gastroparesis, chronic nausea and vomiting, aprepitant treatment, RCT
Introduction
Chronic nausea is a debilitating symptom characteristic of gastric neuromuscular disorders such as gastroparesis and the closely related syndrome of chronic unexplained nausea and vomiting (CUNV), also known as gastroparesis-like syndrome.1–4 These syndromes are difficult to treat, with less than a third of patients with gastroparesis showing significant clinical improvement after a year or more of treatment.5 A variety of agents have been used for the treatment of gastroparesis including classic agents such as the prokinetic metoclopramide and domperidone (not approved in the USA). The utility of these drugs, however, is limited by concerns about serious neurological and cardiovascular adverse effects.6 Many of these patients with refractory nausea are treated with older anti-emetics, neuromodulators or invasive therapies such as gastric electrical stimulation, with little evidence for their effectiveness.6–10 There is therefore a need for new and innovative therapies for these syndromes.
Afferent and efferent signals involved in nausea and vomiting are conveyed by the vagus nerve and NK1R activity is prominent in both sensory and motor vagal nuclei in the brainstem; therefore, NK1 receptor antagonism may be an effective anti-emetic strategy regardless of the nature of the inciting stimulus.11 Aprepitant is a neurokinin-1 receptor (NK1R) antagonist, currently approved as a three-day regimen for chemotherapy-related nausea due to agents such as cis-platinum and for the prevention of post-operative nausea and vomiting. Here we report the results of a 4-week multicenter, double-masked, randomized clinical trial comparing aprepitant (125 mg orally daily) with placebo for symptomatic relief of patients with chronic nausea and vomiting due to suspected gastric origin (gastroparesis and gastroparesis-like syndrome, also known as CUNV- chronic unexplained nausea and vomiting)4.
Methods
STUDY DESIGN
The Aprepitant for the Relief of Nausea in patients with gastroparesis or chronic nausea and vomiting of presumed gastric origin (APRON) trial was conducted by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Gastroparesis Clinical Research Consortium (GpCRC)(ClinicalTrials.gov number, NCT01149369). This was a multicenter, randomized, double-masked, placebo-controlled trial of 4 weeks of aprepitant versus placebo in patients with at least moderate symptoms of chronic nausea and vomiting of presumed gastric origin.
Adult patients, aged 18 years or older, with nausea along with other symptoms suggestive of a gastric origin (early satiety, fullness, bloating and epigastric pain) for at least 6 months, who had a 4-hour gastric emptying scintigraphy test and a normal upper endoscopy within 2 years of registration were considered for enrollment, Patients needed a total score ≥21 on the 0–45 point 9-symptom Gastroparesis Cardinal Symptom Index (GCSI)12 over a two-week period and a visual analog scale (VAS) mean score of nausea after 7 days of >25 mm on a 0 to 100 mm scale. Patients were excluded if using narcotics for more than three days per week, using warfarin, pimozide, terfenadine, astemizole, or cisapride, had elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) at twice the upper limit of normal or a Child-Pugh score≥10, or were allergic to aprepitant. However, patients on stable doses of other gastrointestinal drugs including metoclopramide or erythromycin were not excluded. The gastric emptying study (GES) was performed using a low-fat, egg white meal with imaging at 0, 1, 2, 4 hours,13 with delayed gastric emptying present if gastric retention >60% at 2 hours and/or >10% at 4 hours. However, having delayed gastric emptying was not an inclusion criteria.
Patients meeting eligibility criteria were randomly assigned (1:1) to either once-daily aprepitant (125 mg) or a matching placebo using a computer-generated, centrally-administered procedure developed and managed by the Data Coordinating Center (DCC). The randomization scheme assigned patients in randomly permuted blocks stratified by clinical center, which ensured that the two groups were balanced by calendar time of enrollment and by clinic. Patients, investigators, and clinical site staff were masked to treatment assignment. After randomization, patients were seen at 2-week intervals for a total of 4 weeks treatment to assess nausea symptom improvement and safety of the drug. Patients returned 2 weeks post-treatment to assess further safety of the drug.
During the initial visit and at weeks 2 and 4, surveys were administered to assess upper gastrointestinal and gastroparetic symptoms (Patient Assessment of Upper GI Symptoms (PAGI-SYM, which includes the GCSI)12, 14 and Gastrointestinal Symptom Rating Scale, GSRS)15. Symptom assessment surveys, including the daily visual analog scale (VAS) on a 0–100 mm scale, and the daily diary version of the GCSI (GCSI-DD)16 completed by the patient each night, psychometric measures (Beck Depression Inventory, and State and Trait Anxiety Inventories) and quality of life measures such as Patient Health Questionnaire 15 (PHQ15) and Short Form 36 version (SF36 v2)) were collected. At the initial and 4 week visits, anthropometric measures and fasting blood samples were collected for routine biochemical tests (hematology and complete metabolic panel), and a satiety test with electrogastrography (EGG) was performed, as previously described.10 All protocol deviations were recorded.
Symptom severity during the previous two weeks using the PAGI-SYM questionnaire were graded from 0 (no symptoms) to 5 (very severe). GCSI was computed as the average of the following subscores: nausea/vomiting (3 items), fullness/early satiety (4 items), bloating (2 items). The GCSI-DD rated symptoms during the past 24-hours on a 0 (no symptoms) to 4 (very severe) scale. The GSRS measures gastrointestinal discomfort on a 0 to 7 scale (0=no to 7=very severe discomfort).15
Improvement in nausea by each treatment (aprepitant and placebo) was assessed by several means. The primary outcome, chosen to mirror prior studies with aprepitant in chemotherapy, was a composite measure defined as either a mean 28-day treatment VAS of <25 mm or a decline of >25 mm in the 28-day treatment period mean as compared with the 7-day baseline VAS mean. Secondary outcomes included different combinations of the above, daily hours of nausea, the percent of nausea-free days during treatment, and a reduction in the nausea symptom severity score on the PAGI-SYM. Improvement in other symptoms was assessed by the difference from baseline at the 4-week visit, the difference between the average 28-day value from the mean 7-day baseline value (for daily measures), and overall substantial symptom improvement defined as a ≥1 point decrease in GCSI at 4 weeks from baseline5.
STATISTICAL ANALYSIS
Primary analysis was made on “intention-to-treat” basis and patients without any nausea VAS measures during the follow-up were imputed as not improved. The primary outcome and binary secondary and sensitivity outcomes were analyzed using the Cochran-Mantel-Haenszel chi-square test, stratified by clinic; continuous secondary outcomes were analyzed using analysis of covariance models (ANCOVA), regressing the change from baseline to follow-up on treatment group and baseline value of the outcome. A P-value (two-sided) was considered significant if ≤0.05.
Post-hoc analysis of the subgroup variation in the odds of nausea improvement between treatment groups using baseline and post-randomization subgroups was done using logistic regression of the odds of the primary outcome by treatment group within each stratum of the subgroup. The treatment by subgroup P-value was derived from a Wald’s test of one or more indicator varibles of the interaction of the treatment group and subgroup within each stratum of the subgroup, with a two-sided P-value <0.01 defined as significant.
The planned sample size was 120 patients with equal assignment to two groups (60 per group). Given this sample size, the study had 90% power to detect 30 percent increase in the symptomatic improvement rate assuming 10% loss to follow-up, 25% symptomatic improvement rate in placebo group and a two-sided Type I error of 5%. Statistical analyses for this study were generated using SAS (SAS/STAT version 9.3, SAS Institute Inc. Cary, NC, USA) and Stata (StataCorp. 2011. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
All authors had access to the study data and reviewed and approved the final manuscript.
STUDY OVERSIGHT AND SAFETY
The trial was designed by the first author, other GpCRC investigators, and the DCC and approved by the GpCRC Steering Committee, local institutional review boards (IRB) and a central data safety and monitoring board (DSMB) appointed by NIDDK. Performance and safety data were monitored biannually by the DSMB and members of the NIDDK. All patients provided written consent prior to study enrollment. Adverse events from treatment were monitored and recorded throughout the trial and those with severity grade of 3 (severe) or higher were reviewed by the medical safety officer. The drug (IND#108939) and placebo used in the study were donated by a pharmaceutical company (listed in Funding Support statement), which had no role in the study design, data accrual, data analysis, data interpretation or manuscript preparation.
Results
PATIENTS
Between April 2013 and July 2015, 126 patients were enrolled at eight participating medical centers, of whom 63 were randomized to aprepitant and 63 to placebo (Figure S1, Appendix 2). 72 (57%) patients had delayed gastric emptying (gastroparesis) and the rest had normal or rapid emptying (40 and 3% respectively), a group that we have previously described by the term chronic unexplained nausea and vomiting (CUNV). 4 Baseline characteristics were balanced between the treatment groups, except for higher proportion of diabetes and mean vomiting severity in the aprepitant group, and a higher proportion with delayed gastric emptying and anxiolytic use in the placebo group (Table 1, Table S1).
Table 1.
Baseline characteristics of the study population
| Characteristic | Aprepitant (N=63) | Mean (SD)* Placebo (N=63) | Total (N=126) |
|---|---|---|---|
| Demographic/anthropometric | |||
| Age (years) | 42.9 (14.8) | 46.8 (13.5) | 44.8 (14.3) |
| Women, No. (%) | 49 (78%) | 52 (83%) | 101 (80%) |
| Hispanic, No. (%) | 14 (11%) | 14 (11%) | 28 (22%) |
| Race, No. (%) | |||
| White | 53 (84%) | 59 (94%) | 112 (89%) |
| Black | 8 (13%) | 2 (3%) | 10 (3%) |
| Other† | 2 (3%) | 2 (3%) | 4 (3%) |
| Diabetes type 1 or type 2, No. (%) | 24 (38%) | 13 (21%) | 37 (29%)* |
| Body mass index (BMI) (kg/m2) | 27.8 (8.3) | 28.0 (7.5) | 27.9 (7.9) |
| Weight (kg) | 75.4 (22.8) | 75.1 (20.6) | 75.2 (21.7) |
| Waist circumference (cm) | 90.9 (17.2) | 91.3 (18.0) | 91.1 (17.5) |
| Medications taken in past month, No. (%) | |||
| Proton pump inhibitors | 42 (67%) | 51 (81%) | 93 (74%) |
| Benzodiazepine or anxiolytic | 13 (21%) | 27 (43%) | 40 (32%)* |
| Prokinetic | 25 (40%) | 18 (29%) | 43 (34%) |
| Antiemetic | 44 (70%) | 49 (78%) | 93 (74%) |
| Narcotic | 6 (10%) | 4 (6%) | 10 (8%) |
| Neuropathic or pain modulator, anti-seizure, or other psychiatric medication | 27 (43%) | 29 (46%) | 56 (44%) |
| Daily diary symptoms evaluation | |||
| 7-day Nausea visual analog scale (VAS) score, mm | 63.0 (21.5) | 64.1 (20.2) | 63.6 (20.8) |
| Gastroparesis Cardinal Symptom Index Daily Diary (GCSI-DD) (all items scored 0 to 4, none to very severe) | |||
| Nausea (hours) | 9.0 (7.0) | 9.3 (7.1) | 9.2 (7.1) |
| GCSI total score | 2.2 (0.9) | 2.3 (0.7) | 2.7 (0.9) |
| Nausea severity | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) |
| Vomiting severity | 1.0 (1.0) | 0.9 (1.0) | 1.0 (1.0) |
| Early satiety severity | 2.6 (1.1) | 2.7 (0.9) | 2.6 (1.0) |
| Excessive fullness severity | 2.7 (1.1) | 2.8 (0.9) | 2.7 (1.0) |
| Bloating severity | 2.2 (1.4) | 2.6 (1.1) | 2.4 (1.2) |
| Upper abdominal pain severity | 2.3 (1.2) | 2.3 (1.0) | 2.3 (1.1) |
| Vomiting (No. episodes) | 1.3 (1.6) | 1.1 (1.5) | 1.2 (1.5) |
| Retching (No. episodes) | 2.0 (2.5) | 2.3 (2.8) | 2.1 (2.6) |
| Overall symptom severity | 2.5 (0.8) | 2.6 (0.7) | 2.6 (0.8) |
| Gastroparesis symptoms inventories | |||
| PAGI-SYM Severity index (symptoms each scored 0 to 5, none to very severe) | |||
| Gastroparesis Cardinal Symptom Index (GCSI), total score | 3.4 (0.9) | 3.3 (0.7) | 3.4 (0.8) |
| Nausea/vomiting severity subscore | 3.3 (1.1) | 2.8 (0.9) | 3.0 (1.1)* |
| Nausea severity | 4.2 (0.8) | 4.0 (0.9) | 4.1 (0.9) |
| Retching severity | 3.0 (1.6) | 2.6 (1.3 ) | 2.8 (1.5) |
| Vomiting severity | 2.6 (1.8) | 1.9 (1.6) | 2.2 (1.7)* |
| Fullness/early satiety subscore | 3.7 (1.0) | 3.7 (0.8) | 3.7 (0.9) |
| Bloating subscore | 3.3 (1.4) | 3.4 (1.4) | 3.4 (1.4) |
| Upper abdominal pain/discomfort subscore | 3.4 (1.3) | 3.3 (1.3) | 3.4 (1.3) |
| Lower abdominal pain | 2.4 (1.6) | 2.5 (1.4) | 2.5 (1.5) |
| Lower abdominal discomfort | 2.5 (1.6) | 2.6 (1.4) | 2.6 (1.5) |
| Gastroesophageal Reflux (GERD) subscore | 2.4 (1.4) | 2.3 (1.4) | 2.3 (1.4) |
| Constipation severity | 2.9 (1.7) | 2.6 (1.8) | 2.8 (1.8) |
| Diarrhea severity | 1.6 (1.7) | 1.8 (1.7) | 1.7 (1.7) |
| Nausea/vomiting predominant symptom, No. (%) | 38 (61%) | 39 (64%) | 77 (63%) |
| Gastrointestinal Symptom Rating Scale (GSRS) (items coded 0 to 7, no to very severe discomfort) | |||
| Total score | 3.6 (1.1) | 3.7 (1.0) | 3.7 (1.1) |
| Scintigraphic gastric emptying (GES)† | |||
| Percent gastric retention at: | |||
| 1 hour (%) | 69.7 (20.0) | 73.0 (19.3) | 71.3 (19.6) |
| 2 hours (%) | 45.6 (24.9) | 53.1 (23.4) | 49.4 (24.4) |
| 4 hours (%) | 17.8 (21.8) | 20.4 (17.5) | 19.1 (19.7) |
| Delayed gastric emptying‡, No. (%) | 29 (46%) | 43 (68%) | 72 (57%)* |
| Rapid gastric emptying‡, No. (%) | 2 (3%) | 2 (3%) | 4 (3%) |
Data are mean (SD), unless otherwise noted.
There were 4 significant differences by treatment group due to chance of the 47 baseline characteristics analyzed (denoted by an asterisk (*)). These were: diabetes status (P=0.05), anxiolytic use (P=0.01), PAGI-SYM vomiting symptom severity and nausea subscore (both P=0.02), delayed gastric emptying at either 2 or 4 hours (P=0.02). P value determined using Fisher’s exact test for categorical variables and a t-test for continuous variables.
Other race: 1 aprepitant, 2 placebo subjects reported Asian, 1 aprepitant subject reported mixed race; PAGI-SYM predominant symptom: 2 aprepitant, 1 placebo subjects did not report a predominant symptom; for gastric emptying scintigraphy (GES): 2 aprepitant, 3 placebo subjects did not have GES recorded at 1 hour or 4 hours, 1 aprepitant subject did not have GES recorded at 2 hours
Delayed gastric emptying defined as gastric emptying scintigraphy of > 60% retention at 2 hours OR > 10% retention at 4 hours; rapid gastric emptying defined as gastric emptying scintigraphy of < 30% retention at 1 hour.
Patients completed 97% of the study visits; 94% in the aprepitant group and 100% in the placebo group (P=0.12). There were no differences by treatment group in adherence to treatment defined as taking the drug on at least 80% of the days in treatment: 56/59 (95%) aprepitant vs 60/63 (95%) of placebo patients (P=1.00). There were no differences in treatment group by use of a rescue medication during the trial compared to post-treatment phase (P=1.00) (Table S2).
EFFICACY
The major target symptom in this trial was nausea. We used a variety of metrics to measure this and in the absence of any scientific precedence in patients with symptoms of gastroparesis, the VAS was selected to define the primary outcome as used in trials of aprepitant in chemotherapy-induced and post-operative nausea and vomiting.17, 18 The primary outcome showed no difference between the treatment groups: 29 (46%) in aprepitant group versus 25 (40%) in placebo group (relative risk, 1.2 [95% CI: 0.8–1.7]); P=0.43 (Table 2). However, a sensitivity analysis showed that nausea improvement, if defined as meeting both conditions of the primary outcome (that is, both mean 28-day VAS<25 mm and a decrease of the mean 28-day VAS from baseline of ≤25 mm), was seen in a significantly greater proportion of patients in the aprepitant group, 22/59 (37%) versus 11/63 (17%) placebo (relative risk 2.1 [95% CI: 1.1–4.1]); P=0.01. Outcomes of improvement in other secondary outcomes of nausea were also significantly greater in patients in the aprepitant group. Nausea, vomiting and retching severity improved significantly as measured on a 0–5 GCSI scale. The number of daily hours of nausea declined significantly more in the aprepitant group (adjusted mean change, −1.5; P=0.03) and the proportion of nausea-free days over the 28-day period was significantly higher (adjusted mean change, 10.5%; P=0.03) (Table 2, Figure 1). With respect to vomiting, although the frequency of daily episodes did not change, overall vomiting severity as measured by the GCSI (both nausea/vomiting cluster as a whole as well as individual vomiting severity) was significantly improved after aprepitant treatment.
Table 2.
Improvement in nausea and vomiting: primary and secondary outcomes and sensitivity analysis
| Outcomes | Aprepitant | Placebo | Relative risk ratio or adjusted mean changes from baseline (95% CI) Aprepitant vs. placebo† | P† |
|---|---|---|---|---|
| Primary analysis | ||||
| Improvement in nausea (ITT)*‡ | ||||
| No. of patients randomized | 63 | 63 | ||
| Improved nausea, No. (%) | 29 (46%) | 25 (40%) | 1.2 (0.8, 1.7) | 0.43 |
| Sensitivity analysis | ||||
| No. evaluable patients | 59 | 63 | ||
| Improvement in components of composite primary outcome, No. (%) | ||||
| 1) Mean change of 28-day VAS versus baseline VAS ≤ −25 mm | 25 (42%) | 20 (32%) | 1.3 (0.8, 2.1) | 0.24 |
| 2) Mean 28-day VAS < 25 mm | 26 (44%) | 16 (25%) | 1.7 (1.1, 2.8) | 0.02 |
| 3) Both mean change in VAS ≤ −25 mm and mean 28-day VAS < 25 mm | 22 (37%) | 11 (17%) | 2.1 (1.1, 4.1) | 0.01 |
| Secondary outcomes of nausea/vomiting | ||||
| Measured change (mm) 28-day VAS versus baseline VAS, mean change (95% CI)§ | −21.4 (−27.0, −15.8) | −14.9 (−20.3, −9.5) | −6.5 (−14.3, 1.3) | 0.10 |
| Repeated measures of daily VAS¶ | ||||
| No. of measures of VAS change | 1701 | 1833 | ||
| Change (mm) 28-day VAS versus baseline VAS, adjusted mean change (95% CI) | −21.8 (−28.2, −15.1) | −14.9 (−19.3, −15.3) | −6.9 (−14.7, 0.95) | 0.09 |
| PAGI-SYM Severity index (0=none to 5=very severe) | ||||
| Nausea severity | −1.8 (1.5) | −1.0 (1.4) | −0.7 (−1.3, −0.2) | 0.005 |
| Vomiting severity | −1.6 (1.7) | −0.5 (1.4) | −0.7 (−1.1, −0.3) | 0.001 |
| Retching severity | −1.7 (1.6) | −0.8 (1.4) | −0.8 (−2.3, −0.3) | 0.003 |
| Daily Diary (GCSI-DD) symptom severities (0=none to 4=very severe) | ||||
| Nausea severity | −0.8 (1.0) | −0.5 (0.7) | −0.3 (−0.6, 0.0) | 0.06 |
| Vomiting severity | −0.4 (0.8) | −0.2 (0.5) | −0.1 (−0.3, 0.1) | 0.24 |
| Daily Nausea (hours) | −2.5 (3.2) | −1.2 (4.3) | −1.5 (−2.8, −0.1) | 0.03 |
| Percent of nausea-free days during 28-day follow-up (mean, 95% CI) | 21.8% (14.0, 29.6%) | 11.3% (5.5, 17.1%) | 10.5 (0.9, 20.2) | 0.03 |
| Imputation analyses of primary outcome|| | ||||
| Best case: missing outcome=improve | 33 (52%) | 25 (40%) | ||
| Improved nausea, No. (%) | 33 (52%) | 25 (40%) | 1.3 (0.9, 1.9) | 0.13 |
| Worst case: missing outcome=not improve | ||||
| Improved nausea, No. (%) | 29 (46%) | 25 (40%) | 1.2 (0.8, 1.7) | 0.43 |
| Multiple imputation of primary outcome | ||||
| Improved nausea, % (range) | 50% (46% – 52%) | 40% (40% – 40%) | 1.2 (0.8, 1.9) | 0.28 |
The primary outcome of Improvement in nausea is a binary composite outcome defined as either 1) an improvement in the mean of available nausea visual analog scale (VAS) scores over the 28-day treatment period compared to the means of VAS during the 7-day baseline (BL) period being ≤ −25 mm, or 2) the mean VAS after 28-days of treatment was < 25 mm. The number of 28-day treatment VAS available per subject ranged from 14 to 41 (median=28.5, IQR:27,33; PAprepitant Vs Placebo=0.76).
Baseline VAS denotes the mean of VAS scores over the 7-day pre-randomization period.
28-day VAS denotes the mean of all available VAS scores over the 28-day treatment period.
P-values, relative risk ratios, and 95% confidence limits (CI) for the primary ITT were calculated using the Cochran-Mantel-Haenszel chi-square test, stratified by clinic. Mean adjusted change from baseline, 95% confidence limits (CI), and P-values were calculated using ANCOVA, regressing change from baseline to 28 days on treatment group and baseline value of the secondary outcome.
There were 4 subjects missing all 28-day VAS scores; the primary outcome for these subjects was imputed to no improvement for the intention-to-treat (ITT) analyses.
Improvement indicated by a decrease in the change from the average 7-day baseline VAS of the average available VAS at 28-days of treatment as a percent of the baseline VAS.
Adjusted mean changes from baseline for each treatment group, and adjusted mean change from baseline between treatment groups, P-values, and 95% CI were determined from multiple linear regression of daily change of the VAS during follow-up in relation to the mean 7-day baseline VAS, with an indicator for treatment group. A GEE random effects model with a robust variance estimate was used to account for repeated subject measures.
No. patients in imputation sensitivity analyses: 63 (aprepitant), 63 (placebo).
Observations with missing 28-day mean VAS score were imputed using 500 datasets. Imputation model included the following baseline variables: treatment group indicator, baseline VAS, clinic, delayed gastric retention, diabetes status, age and sex.
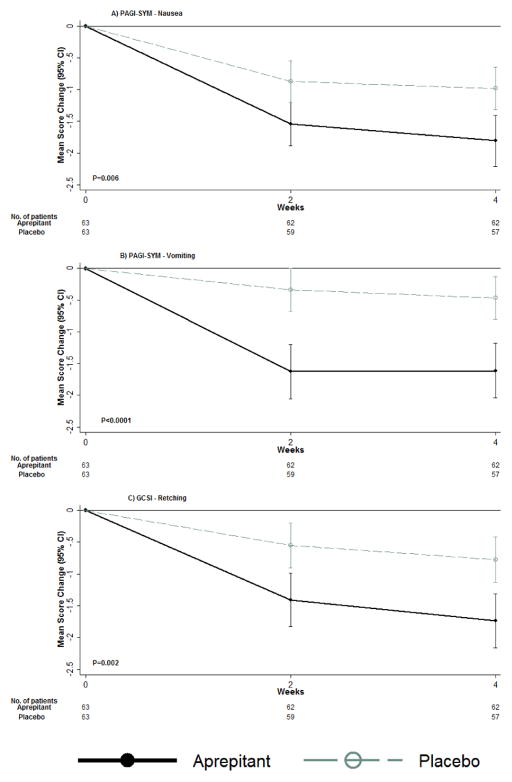
Figure 1.
Changes from baseline in nausea, vomiting and retching severity scores, measured by PAGI-SYM, and overall symptom severity using GCSI, by treatment group Mean values of changes from baseline during treatment with aprepitant (63 patients) or placebo (63 patients) for up to 4-weeks are shown. P-values for overall treatment effect of change over time were derived from GEE linear regression, modeling change as a function of treatment group, visit code indicator, baseline value of the outcome, and a treatment group by visit code interaction term. Nausea (A), vomiting (B), and retching (C) severity and overall symptoms (GCSI) decreased in both treatment groups over follow-up, but patients assigned to aprepitant had significantly greater decreases over time compared to patients assigned to placebo, P=0.006, P<0.0001, P=0.002, and P=0.001, respectively.
Aprepitant treatment was also accompanied by a decrease in the severity of several other symptoms. Overall symptom severity as measured by PAGI-SYM and GCSI-DD, showed an adjusted mean change from baseline of GCSI as −0.6 [95% CI: −0.9,−0.3]; P=0.001] and of daily overall symptom relief as −0.3 [95% CI: −0.6,−0.1]; P=0.02], respectively. Further, the percentage of patients with substantial symptomatic improvement of ≥1 on GCSI5 was 34/57 (60%) in aprepitant and 20/62 (32%) in placebo (P=0.002).
Significant improvement was also seen in several other individual symptoms of the GCSI, including fullness and bloating as well as in other PAGI-SYM measures, particularly symptoms of gastroesophageal reflux disease (GERD), including both heartburn and regurgitation (Table 2, Table S3, Figure S2). Notable changes were also seen in measures of abdominal pain, using both the Daily Diary and PAGI-SYM. In addition to these improvements in PAGI-SYM measures, GSRS total scores showed a significant improvement with an adjusted mean change from baseline of −0.4 [95% CI: −0.7,−0.1]; P=0.007] at 28-days. Satiety test results showed a significant improvement in post-meal tachygastria in aprepitant (adjusted mean change, −5.5; P=0.004), but maximum tolerated Ensure volumes remained the same between groups. Finally, though not significant, aprepitant treatment was associated with improvement in the Beck Depression Inventory score (adjusted mean change=−2.2; P=0.09), but there was no change in state or trait anxiety scores or in the physical or mental component of the SF-36v2 Quality of Life scores.
Post-hoc analysis of subgroup variation in the odds of nausea improvement (as measured by the primary outcome) did not differ between treatment groups using baseline and post-randomization subgroups with respect to a variety of attributes including etiology (diabetes versus idiopathic), the presence or absence of delayed emptying, baseline anti-emetic or prokinetic use, baseline symptom severity subscores, narcotic use during treatment and adherence (Table S4).
SAFETY
There was one (1%) serious adverse event reported during the trial, which occurred in the aprepitant group (1/63 (2%) vs 0/63 (0%); P=0.50). This adverse event (severity grade 2) occurred to a diabetic patient hospitalized overnight due to nausea and judged by the investigator to be probably unrelated to treatment and to be expected from their underlying disorder. Overall adverse event (AE) rates were low and did not differ statistically by group. There were more patients in the aprepitant group with at least 1 AE compared to placebo (22/63 (35%) vs 11/63 (17%); P=0.04); however, the number of AEs reported did not differ between treatment groups by maximum severity grade or classification by body category (Table 4, Table S5).
Table 4.
Frequency and severity of reported adverse events by treatment
| Frequency (No. of events (%))* | Aprepitant (N=63) | Placebo (N=63) | P-value† | Total (N=126) |
|---|---|---|---|---|
| Total Adverse Event rates | 0.12 | |||
| Total events | 26 | 15 | 41 | |
| Patient-months (pt-mo) of follow-up | 46.1 | 62.4 | 108.4 | |
| Rate (/patient-months) | 0.56 | 0.24 | ||
| Rate by severity grade, Rate (No./pt-mo) | ||||
| Mild (grade 1) | 0.28 (13/46.1) | 0.08 (5/62.4) | 0.10 | |
| Moderate (grade 2) | 0.26 (12/46.1) | 0.11 (7/62.4) | 0.007 | |
| Severe (grade 3) | 0.02 (1/46.1) | 0.05 (3/62.4) | 0.63 | |
| Frequency of events by patient | 0.02 | |||
| 0 | 41 (65%) | 52 (83%) | 93 (74%) | |
| 1 | 20 (32%) | 8 (13%) | 28 (22%) | |
| 2 | 1 (2%) | 2 (3%) | 3 (2%) | |
| 3 to 4 | 1 (2%) | 1 (2%) | 2 (2%) | |
| Maximum severity grade per patient‡ | 0.08 | |||
| No AE | 41 (65%) | 52 (83%) | 93 (74%) | |
| 1-mild | 9 (14%) | 4 (6%) | 13 (10%) | |
| 2-moderate | 12 (19%) | 5 (8%) | 17 (13%) | |
| 3-severe | 1 (2%) | 2 (3%) | 3 (2%) | |
| 4-life threatening | 0 (0%) | 0 (0%) | 0 (0%) | |
| 5-death | 0 (0%) | 0 (0%) | 0 (0%) | |
| Serious adverse event (SAE)‡ | ||||
| Yes | 1 (2%) | 0 (0%) | 1.00 | 1 (1%) |
As reported on Adverse Event Report (AE) form by clinical center study physician.
126 patients randomized; 33 patients reported a total of 41 AE’s occurring during the treatment period; adverse events occurring during the 6 week study visit (post-treatment phase) were excluded.
P-values for comparisons by treatment group were determined from an exact binomial proportion test for event rates and a Fisher’s Exact test differences by frequency, severity grade and SAEs reported by patient.
Based on NCI’s Common Terminology Criteria for Adverse Events (v3.0 CTCAE). Patients with multiple adverse events were included at the maximum severity grade.
Serious Adverse Event (SAE) defined by FDA as an event meeting one or more of the following criteria: inpatient hospitalization or prolonged existing hospitalization; persistent or significant incapacity or substantial disruption of ability to conduct normal life functions; jeopardized patient and required medical or surgical intervention to prevent a serious event; or congenital anomaly or birth defect.
The patient reported with a SAE as determined by the investigator was: diabetic and had delayed gastric retention. The event reported was graded as severity grade=2, the event was hospitalization due to nausea/vomiting and rated as “probably not related’ to the treatment.
Discussion
In this randomized controlled study, aprepitant, a first generation NK1R antagonist, failed to significantly improve the pre-specified primary outcome for nausea in patients with gastroparesis and gastroparesis-like syndrome (also known as chronic unexplained nausea and vomiting). However, aprepitant treatment resulted in significant improvement of several secondary outcome measures for nausea and other symptoms. These results, in our opinion, suggest aprepitant may be a treatment for nausea in gastroparesis and related disorders and support the need for additional trials with aprepitant for this group of patients, possibly with the use of a different primary outcome.
Our designated primary outcome for this trial was a composite outcome consisting of either an absolute or a relative reduction in a quantitative nausea score based on VAS measuring daily nausea severity. Although this trial is negative based on the protocol based, pre-specified primary outcome, aprepitant did improve other measures of nausea as well as the decreasing the severity of several other symptoms. Had we used the VAS measures in a more rigorous combination (i.e., requiring both components rather than either one), there are significant differences in improvement between aprepitant and placebo. Further, examining other, more commonly used and valideated measures for gastroparesis symptom severity in this patient group, such as the 2 week GCSI, robust improvement was seen in nausea, vomiting and retching scores. Though not significant, there was also a improvement in nausea severity by the GCSI daily diary (adjusted mean change= −0.3; P=0.06) that was reinforced by changes in other daily measures including a nearly two-fold reduction in the daily duration of nausea and the percent of nausea-free days in patients receiving aprepitant. While no change was seen in GCSI-daily diary scores for vomiting severity, it should be noted that vomiting and retching are the least responsive to change by this measure.19 Aprepitant treatment had no effect on the use of anti-emetics, which could reflect lack of efficacy, but may also be due to habituation and in some cases with dependence on these drugs. It can therefore be argued that use of the VAS for nausea may not be the best measure for assessing improvement when it comes to chronic symptoms observed in patients with gastroparesis and related disorders. Further trials will be needed to assess which, if any, of the multiple secondary outcomes is the best one to assess the efficacy of this agent for chronic nausea.
It can also be argued that this trial was negative because of other reasons such as heterogeneity in the patient population, consisting of patients with both normal and delayed emptying. However, sensitivity analysis showed no effects of gastric emptying on the outcome. Further, in the setting of chronic nausea of suspected gastric origin, we have previously shown that these two groups are clinically indistinguishable and the burden of illness is equally severe, with little or no correlation between symptom severity (particularly nausea) and the degree of delay in gastric emptying.4 Given that the primary target of aprepitant is nausea and the large unmet need for symptomatic relief in these patients, we believe we are justified in our study design and that the trial was adequately powered.
Aprepitant also resulted in significant improvements in global measures such as total GCSI and GSRS scores, along with robust improvements in difficult-to-treat symptoms such as fullness, bloating and distention. The mean decline in overall GCSI was 1.3 which is clinically meaningful as the minimal important difference (MID) value for this measure is most likely 1.5, 19 The MID for nausea using the GCSI-DD is reported as 0.55, whereas that for the overall (composite score) is 0.73.20 In this study, aprepitant resulted in a 0.8 change in nausea and 0.6 change in overall score using the PAGI-SYM, which would be considered clinically meaningful. It is possible that improvement in these measures was mediated by acceleration in gastric emptying (which was not measured in this trial). In experimental models, activation of NK1 receptors delays gastric emptying, in part by inducing pyloric spasm as well as by central mechansims.21–24 On the other hand, as previously noted, gastric emptying correlates poorly with symptom severity. Further, these changes were not associated with any improvement in liquid satiety testing that if seen, would have indicated improved gastric fundic accommodation. Finally, although aprepitant was associated with statistically significant improvement in post-prandial tachygastria on electrogastrography, the clinical significance of this is uncertain. Thus, the underlying mechanism for such widespread relief, if true, remains unclear.
Aprepitant also resulted in significant improvement in symptoms less specific for gastroparesis, notably gastro-esophageal reflux (heartburn and reflux). Around 75% of these patients were on acid suppressant medications so it is unlikely that they began with uncontrolled acid reflux. Alternatively, the mechanism could plausibly involve the modulation of neural reflexes and/or sensation 25 Also of interest in this regard is the improvement noted in abdominal pain/discomfort indices. There is a large body of evidence implicating substance P/NK1R signaling in nociception, but trials of NK1 receptor antagonists in somatic pain conditions have failed to show efficacy.26 However, it is possible that visceral pain may be more responsive to this pharmacological strategy based on the much higher expression of substance P in visceral compared with cutaneous afferents.27–29 Our study should stimulate further research on the role of the NK1R in gastroesophageal reflux or reflux-like symptoms and chronic abdominal pain. Finally, aprepitant has also been shown to have efficacy in preclinical models of depression, with mixed results in patients.30 Consistent with this, there was improvement of depression scores after 4 weeks of treatment with aprepitant, though not significant.
During the 4-week duration of this study, aprepitant appeared to be safe. Aprepitant has been tested in clinical trials with over 2000 patients in doses that ranged from 40 to 240 mg per day and for durations up to 10 months for a variety of indications including acute chemotherapy-induced nausea and vomiting, motion sickness, postoperative nausea and vomiting, and urge urinary incontinence of postmenopausal women with overactive bladder.31–34 In these trials, aprepitant was well tolerated, with generally mild adverse experiences.
In conclusion, the APRON trial was a negative trial using the pre-specified VAS-based primary outcome of nausea improvement. However, aprepitant was effective in relieving nausea as assessed by a variety of secondary outcome measures and improving several markers of global severity as well as symptoms of reflux and pain in patients with gastroparesis and chronic nausea and vomiting of suspected gastric origin. Given the paucity of effective treatments for these conditions, our results warrant further trials of this agent with different primary outcomes.
Supplementary Material
Table 3.
Changes in other secondary outcomes by treatment
| Outcomes | Aprepitant | Placebo | Relative risk ratio or adjusted mean changes from baseline (95% CI) Aprepitant vs. placebo* | P* |
|---|---|---|---|---|
| No. evaluable patients† | 59 | 63 | ||
| Gastroparesis symptoms inventories PAGI-SYM Severity index (0=none to 5=very severe) | ||||
| Gastroparesis Cardinal Symptom Index (GCSI) score | −1.3 (1.0) | −0.7 (0.9) | −0.6 (−0.9, −0.3) | 0.001 |
| Substantial symptomatic improvement‡, No. (%) | 34 (60%) | 20 (32%) | 1.8 (1.2, 2.8) | 0.002 |
| Nausea/vomiting severity subscore | −1.7 (1.3) | −0.7 (1.1) | −0.8 (−1.2, −0.4) | <0.001 |
| Fullness/early satiety subscore | −1.0 (1.3) | −0.7 (1.0) | −0.3 (−0.7, 0.1) | 0.13 |
| Bloating subscore | −1.2 (1.2) | −0.6 (1.2) | −0.6 (−1.2, −0.2) | 0.004 |
| Upper abdominal pain subscore | −1.1 (1.5) | −0.6 (1.2) | −0.4 (−0.9, 0.1) | 0.08 |
| GERD subscore | −1.1 (1.3) | −0.6 (0.9) | −0.5 (−0.8, −0.1) | 0.007 |
| Daily Diary Gastroparesis Cardinal Symptom Index (GCSI-DD) (0=none to 4=very severe) | ||||
| GCSI total score | −0.5 (0.9) | −0.4 (0.5) | −0.2 (−0.4, 0.1) | 0.22 |
| Upper abdominal pain severity | −0.7 (0.9) | −0.3 (0.7) | −0.4 (−0.7, −0.1) | 0.01 |
| Vomiting (No. episodes) | −0.5 (1.0) | −0.4 (1.1) | −0.1 (−0.3, 0.3) | 0.94 |
| Retching (No. episodes) | −0.5 (1.5) | −0.7 (2.0) | 0.1 (−0.4, 0.6) | 0.73 |
| Overall symptom severity | −0.7 (0.8) | −0.4 (0.6) | −0.3 (−0.6, −0.1) | 0.02 |
| Gastrointestinal Symptom Rating Scale (GSRS) (0=no to 7=very severe discomfort) | ||||
| Total score | −0.8 (0.9) | −0.5 (0.9) | −0.4 (−0.7, 0.1) | 0.007 |
NOTE: The minimal important differences (MIDs) for: GCSI range from 0.75 to 1; GCSI-DD is 0.55.20
Mean adjusted change from baseline, 95% confidence limits (CI), and P-values were calculated using ANCOVA, regressing change from baseline to 28 days on treatment group and baseline value of the secondary outcome. P-value, relative risk ratio and 95% confidence limits (CI) for substantial symptomatic improvement‡ were calculated using the Cochran-Mantel-Haenszel chi-square test, stratified by clinic.
There were 4 subjects missing all 28-day VAS scores or other symptom data.
In addition, 2 aprepitant subjects did not provide 28 day data (GSRS).
Substantial symptomatic improvement defined as a decrease of at least 1 point from baseline in GCSI score at 28-days. No. patients in analyses: 57 (aprepitant), 62 (placebo)
Acknowledgments
Grant support statement:
This project is supported through federal funding from the National Institutes of Diabetes, Digestive and Kidney Diseases (grants: U01DK073983, U01DK073985, U01DK073975, U01DK074035, U01DK074007, U01DK073974, U01DK074008,) and the following Clinical and Translational Science Award Grants (UL1TR000424 (Johns Hopkins University), UL1TR000093 (Stanford University), UL1TR001105 (Texas Tech Health Sciences Center), UL1TR000433 (University of Michigan), UL1TR000135 (Mayo Clinic College of Medicine). This study also was funded in part by Merck & Co., Inc. via a Collaborative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- AE
Adverse event
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CUNV
Chronic unexplained nausea and vomiting
- DSMB
Data safety and monitoring board
- EGG
Electrogastrography
- GES
Gastric emtyping scintigraphy study
- GCSI
Gastroparesis Cardinal Symptom Index
- GCSI-DD
Gastroparesis Cardinal Symptom Index-Daily Diary
- GpCRC
Gastroparesis Clinical Research Consortium
- GSRS
Gastrointestinal Symptom Rating Scale
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NK1R
Neurokinin-1 receptor
- PAGI-SYM
Patient Assessment of Upper GI Symptoms
- VAS
Visual analog scale
Footnotes
This paper is subject to the NIH Public Access policy (http://publicaccess.nih.gov/).
The APRON trial is registered on clinicaltrials.gov (NCT01149369; https://clinicaltrials.gov/ct2/show/NCT01149369).
Disclosures
None of the authors report any conflict of interest with respect to this manuscript or the data in it
Author Contributions:
Pankaj J. Pasricha: study concept and design, acquisition of data, writing of manuscript
Katherine P. Yates: statistical analysis and presentation of data, writing and revision of manuscript
Irene Sarosiek: acquisition of data, critical revision of the manuscript for important intellectual content
Richard W McCallum: acquisition of data, critical revision of the manuscript for important intellectual content
Thomas L. Abell: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content
Kenneth L. Koch: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content
Linda Nguyen: acquisition of data; critical revision of the manuscript for important intellectual content
William J. Snape: acquisition of data, critical revision of the manuscript for important intellectual content
William L. Hasler: acquisition of data, critical revision of the manuscript for important intellectual content
John Clarke: acquisition of data
Sameer Dhalla: acquisition of data
Ellen Stein: acquisition of data
Linda Lee: review of safety data, technical support
Laura Miriel: technical support
Mark Van Natta: statistical analysis
Madhusudan Grover: critical revision of the manuscript for important intellectual content
Gianrico Farrugia: critical revision of the manuscript for important intellectual content
James Tonascia: study concept and design, statistical analysis, critical revision of the manuscript for important intellectual content
Frank Hamilton: study supervision, critical revision of the manuscript for important intellectual content
Henry P. Parkman: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–15. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkman HP, Yates K, Hasler WL, et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin Gastroenterol Hepatol. 2011;9:1056–64. doi: 10.1016/j.cgh.2011.08.013. quiz e133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harer KN, Pasricha PJ. Chronic Unexplained Nausea and Vomiting or Gastric Neuromuscular Dysfunction (GND)? An Update on Nomenclature, Pathophysiology and Treatment, and Relationship to Gastroparesis. Curr Treat Options Gastroenterol. 2016;14:410–419. doi: 10.1007/s11938-016-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasricha PJ, Colvin R, Yates K, et al. Characteristics of patients with chronic unexplained nausea and vomiting and normal gastric emptying. Clin Gastroenterol Hepatol. 2011;9:567–76. e1–4. doi: 10.1016/j.cgh.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasricha PJ, Yates KP, Nguyen L, et al. Outcomes and Factors Associated With Reduced Symptoms in Patients With Gastroparesis. Gastroenterology. 2015;149:1762–1774. e4. doi: 10.1053/j.gastro.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanger GJ, Pasricha PJ. Investigational drug therapies for the treatment of gastroparesis. Expert Opin Investig Drugs. 2017 doi: 10.1080/13543784.2017.1288214. [DOI] [PubMed] [Google Scholar]
- 7.Hasler WL. Symptomatic management for gastroparesis: antiemetics, analgesics, and symptom modulators. Gastroenterol Clin North Am. 2015;44:113–26. doi: 10.1016/j.gtc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 8.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815–e636. doi: 10.1111/nmo.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277–81. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 10.Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640–9. doi: 10.1001/jama.2013.282833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanger GJ. Neurokinin NK1 and NK3 receptors as targets for drugs to treat gastrointestinal motility disorders and pain. Br J Pharmacol. 2004;141:1303–12. doi: 10.1038/sj.bjp.0705742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 13.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 14.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–49. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 15.Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 16.Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–80. doi: 10.1111/j.1365-2036.2009.04078.x. [DOI] [PubMed] [Google Scholar]
- 17.Jordan K, Gralla R, Rizzi G, et al. Efficacy benefit of an NK1 receptor antagonist (NK1RA) in patients receiving carboplatin: supportive evidence with NEPA (a fixed combination of the NK1 RA, netupitant, and palonosetron) and aprepitant regimens. Support Care Cancer. 2016;24:4617–25. doi: 10.1007/s00520-016-3304-1. [DOI] [PubMed] [Google Scholar]
- 18.Moon HY, Baek CW, Choi GJ, et al. Palonosetron and aprepitant for the prevention of postoperative nausea and vomiting in patients indicated for laparoscopic gynaecologic surgery: a double-blind randomised trial. BMC Anesthesiol. 2014;14:68. doi: 10.1186/1471-2253-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasricha PJ, Camilleri M, Hasler WL, et al. Gastroparesis: Clinical and Regulatory Insights for Clinical Trials. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD) Neurogastroenterol Motil. 2012 May;24(5):456–63. e215–6. doi: 10.1111/j.1365-2982.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang FY, Lee SD, Yeh GH, et al. Rat gastrointestinal motor responses mediated via activation of neurokinin receptors. J Gastroenterol Hepatol. 1999;14:39–45. doi: 10.1046/j.1440-1746.1999.01808.x. [DOI] [PubMed] [Google Scholar]
- 22.Lidberg P. On the role of substance P and serotonin in the pyloric motor control. An experimental study in cat and rat. Acta Physiol Scand Suppl. 1985;538:1–69. [PubMed] [Google Scholar]
- 23.Krowicki ZK, Hornby PJ. The inhibitory effect of substance P on gastric motor function in the nucleus raphe obscurus is mediated via nitric oxide in the dorsal vagal complex. J Auton Nerv Syst. 1996;58:177–80. doi: 10.1016/0165-1838(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 24.Improta G, Broccardo M. Tachykinins: effects on gastric secretion and emptying in rats. Pharmacol Res. 1990;22:605–10. doi: 10.1016/s1043-6618(05)80052-0. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds JC, Ouyang A, Cohen S. A lower esophageal sphincter reflex involving substance P. Am J Physiol. 1984;246:G346–54. doi: 10.1152/ajpgi.1984.246.4.G346. [DOI] [PubMed] [Google Scholar]
- 26.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–6. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 27.Cervero F, Laird JM. Understanding the signaling and transmission of visceral nociceptive events. J Neurobiol. 2004;61:45–54. doi: 10.1002/neu.20084. [DOI] [PubMed] [Google Scholar]
- 28.Brown JL, Liu H, Maggio JE, et al. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–44. doi: 10.1002/cne.903560302. [DOI] [PubMed] [Google Scholar]
- 29.Todd AJ, Spike RC, Polgar E. A quantitative study of neurons which express neurokinin-1 or somatostatin sst2a receptor in rat spinal dorsal horn. Neuroscience. 1998;85:459–73. doi: 10.1016/s0306-4522(97)00669-6. [DOI] [PubMed] [Google Scholar]
- 30.Hafizi S, Chandra P, Cowen J. Neurokinin-1 receptor antagonists as novel antidepressants: trials and tribulations. Br J Psychiatry. 2007;191:282–4. doi: 10.1192/bjp.bp.107.037879. [DOI] [PubMed] [Google Scholar]
- 31.Warr D. The neurokinin1 receptor antagonist aprepitant as an antiemetic for moderately emetogenic chemotherapy. Expert Opin Pharmacother. 2006;7:1653–8. doi: 10.1517/14656566.7.12.1653. [DOI] [PubMed] [Google Scholar]
- 32.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005;41:1278–85. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–30. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 34.Gan TJ, Apfel CC, Kovac A, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9. doi: 10.1213/01.ane.0000263277.35140.a3. tables of contents. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.