Abstract
Single-trial-level analyses afford the ability to link neural indices of elaborative attention (such as the late positive potential [LPP], an event-related potential) with downstream markers of attentional processing (such as reaction time [RT]). This approach can provide useful information about individual differences in information processing, such as the ability to adapt behavior based on attentional demands (“brain-behavioral adaptability”). Anxiety and depression are associated with maladaptive information processing implicating aberrant cognition-emotion interactions, but whether brain-behavioral adaptability predicts response to psychotherapy is not known. We used a novel person-centered, trial-level analysis approach to link neural indices of stimulus processing to behavioral responses and to predict treatment outcome. Thirty-nine patients with anxiety and/or depression received 12 weeks of cognitive behavioral therapy (CBT). Prior to treatment, patients performed a speeded reaction-time task involving briefly-presented pairs of aversive and neutral pictures while electroencephalography was recorded. Multilevel modeling demonstrated that larger LPPs predicted slower responses on subsequent trials, suggesting that increased attention to the task-irrelevant nature of pictures interfered with reaction time on subsequent trials. Whereas using LPP and RT averages did not distinguish CBT responders from nonresponders, in trial-level analyses individuals who demonstrated greater ability to benefit behaviorally (i.e., faster RT) from smaller LPPs on the previous trial (greater brain-behavioral adaptability) were more likely to respond to treatment and showed greater improvements in depressive symptoms. These results highlight the utility of trial-level analyses to elucidate variability in within-subjects, brain-behavioral attentional coupling in the context of emotion processing, in predicting response to CBT for emotional disorders.
Keywords: late positive potential, event-related potential, attention, anxiety, depression, CBT
Anxiety and depression are prevalent, frequently comorbid and can be highly impairing (Kessler et al., 2005, 2006; Mineka et al., 1998; Kaufman & Charney, 2000). Difficulties with flexibly adapting behavior based on attentional demands could underlie dysfunction in these disorders and play a role in treatment outcome (Johnco et al., 2014; Mennin & Fresco, 2013; Stange et al., 2017a, b, in press). Cognitive behavioral therapy (CBT) is a gold-standard learning-based psychological treatment for anxiety and depressive disorders (Beck et al., 1979; Hofmann et al., 2012a) that involves practicing adaptive coping strategies to effectively modify maladaptive responses to emotional events (Arch & Craske, 2009). Although CBT is moderately effective for these disorders, many patients remain symptomatic after an initial intervention (Hofmann & Smits, 2008; Kemp et al., 2008). Thus, identifying patient characteristics that are associated with response to CBT may lead to more personalized treatment decision-making and better outcomes (Paulus, 2015). Linking neural and behavioral indices of attentional processing could facilitate the identification of individuals who are most likely to benefit from CBT.
Given the high degree of comorbidity between anxiety and depression, it is likely that common factors, such as increased attention toward aversive information, underlie these disorders (Bar-Haim et al., 2007; Mathews & MacLeod, 2002; Peckham et al., 2010; Pessoa et al., 2002; Mineka et al., 1998). One tool that can be used to study individual differences in elaborative attention at the neural level is the late positive potential (LPP), an event-related potential (ERP) that is larger for emotional relative to neutral stimuli (e.g., Bar-Haim et al., 2005; Hajcak et al., 2012; MacNamara & Hajcak, 2009; MacNamara et al., 2011, 2013). For example, using the LPP, prior work has found that individuals who are more anxious (elevated stated anxiety and generalized anxiety disorder) are less able to modulate attention to emotional distracters during a working memory task (MacNamara et al., 2011; MacNamara & Proudfit, 2014).
In addition, the ability to adapt attention and behavior to changes in contextual demands may be important to recovering from depression and anxiety and may facilitate psychological health more broadly (e.g., Kashdan & Rottenberg, 2010; Bonanno & Burton, 2013; Aldao, Sheppes, & Gross, 2015; Cheng, Lau, & Chan, 2014; Stange et al., 2016, in press). Importantly, the use of single-trial-level analyses may permit the assessment of components of cognitive and behavioral flexibility. For example, it affords the ability to link neural indices of elaborative attention (such as the LPP) with downstream markers of attentional processing (such as reaction time [RT]) (e.g., Egner & Hirsch, 2005; Saville et al., 2012; Kerns et al., 2004; Botvinick et al., 2001), providing information about individual differences in brain-behavioral adaptability. Among healthy individuals, greater attention to task-irrelevant stimuli (indexed by the LPP) was associated with slowed responses to subsequently-presented target stimuli (geometric shapes), whereas trials with smaller LPPs were associated with faster RTs on subsequent trials (Weinberg & Hajcak, 2011). An important implication for brain-behavioral adaptability is that in the absence of attentionally-salient stimuli, healthy individuals are able to adjust their behavior by directing cognitive resources instead to the task at hand (Desimone & Duncan, 1995). Thus, the ability to adapt behavior based on attentional demands allows individuals to benefit (e.g., as reflected by improved performance) when task-irrelevant stimuli are processed as less salient.
Importantly, individual differences in this type of brain-behavioral coupling (or adaptability) could provide useful data about information processing and response to psychotherapy for emotional disorders. No studies to our knowledge have examined relationships between brain and behavior in the context of emotional disorders. Indeed, most prior studies of ERPs, including those in the context of treatment outcome (e.g., Burkhouse et al., 2016; Leutgeb et al., 2009; Stange et al., 2017a), have ignored trial-to-trial variability in ERPs and RT (i.e., collapsing across trials to create averages), even though within-subject variation may provide important information about brain-behavioral adaptability that average scores cannot (e.g., Weinberg & Hajcak, 2011; Egner & Hirsch, 2005; Saville et al., 2012; Kerns et al., 2004; Botvinick et al., 2001). In the present study we made use of these within-subject brain-behavior patterns by assessing attention allocation on a given trial (indexed by the LPP) and performance on the subsequent trial (indexed by RT), to provide a measure of brain-behavior coupling (e.g., Adamo et al., 2015; Bender et al., 2015; Saville et al., 2012). This allows for a novel approach to evaluating predictors of treatment response, one that is not possible when relying exclusively on between-subjects assessments of brain and behavior.
We used a task previously shown to elicit the LPP in healthy and anxious samples (MacNamara & Hajcak, 2009, 2010; Stange et al., 2017a), but never before analyzed in a trial-to-trial manner that links brain to behavior. First, we hypothesized that aversive targets would lead to larger LPPs than neutral targets, and that these larger LPPs would lead to slower RTs on the next trial. Second, we hypothesized that greater coupling (i.e., greater slowing following trials with larger LPPs, and faster responding following trials with smaller LPPs) would be positively associated with treatment outcome. This hypothesis was based on the premise that (1) healthy individuals show positive associations between the LPP and RT (i.e., brain-behavior coupling; Weinberg & Hajcak, 2011), (2) brain-behavioral coupling might represent a form of flexibility in that individuals are able to adapt attention based on the demands of the situation, and (3) evidence that cognitive flexibility is associated with better treatment outcome (e.g., Johnco et al., 2014), while noting that this is the first study to evaluate brain-behavioral coupling as a predictor of treatment outcome. However, given evidence that anxiety and depression may be characterized by persistent attention toward aversive stimuli (Gibb, McGeary, & Beevers, 2015; Mathews & MacLeod, 2005; Peckham et al., 2010; Hofmann et al., 2012b; Dai et al., 2016), we also considered the possibility that greater RT slowing following trials with larger LPPs might predict poorer treatment outcome. To evaluate these questions in line with contemporary perspectives that emphasize substantial overlap in the mechanisms and treatments for emotional disorders (e.g., Cuthbert, 2014; Sanislow et al., 2010), we examined data from a transdiagnostic sample of adults who received CBT for an anxiety and/or depressive disorder.
Method
Participants
All participants met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for a current anxiety or depressive diagnosis (i.e., social anxiety disorder, panic disorder, generalized anxiety disorder or major depressive disorder diagnosis; see Table 1). All participants were free of psychotropic medication for > 8 weeks prior to and throughout the study. Exclusionary criteria were a) substance abuse or dependence in the prior six months, b) history of bipolar disorder or schizophrenia, or the presence of an organic mental syndrome, intellectual disability, or pervasive developmental disorder, c) ongoing psychotherapy and/or current treatment with any psychotropic medication, and d) clinically significant medical or neurologic condition. Participants were between 18 and 55 years of age. The study protocol was approved by the Institutional Review Boards of the University of Michigan Medical School and the University of Illinois at Chicago. All participants provided written informed consent.
Table 1.
Sample characteristics and diagnoses.
| Mean | SD | |
|---|---|---|
| Age (years) | 25.36 | 6.77 |
| Education (years) | 15.31 | 2.03 |
| CGI Severity (pre-treatment) | 4.22 | 0.47 |
| CGI Severity (post-treatment) | 2.95 | 1.22 |
| HAM-A (pre-treatment) | 15.49 | 7.98 |
| HAM-A (post-treatment) | 6.56 | 5.04 |
| HAM-D (pre-treatment) | 9.59 | 6.31 |
| HAM-D (post-treatment) | 3.95 | 4.08 |
| n | % | |
| Female | 33 | 84.6 |
| Race | ||
| Caucasian | 25 | 64.1 |
| African American | 1 | 2.6 |
| Asian | 8 | 20.5 |
| More than one race | 5 | 12.8 |
| Hispanic or Latino/a | 9 | 23.1 |
| Principal Diagnosis | ||
| Social Anxiety Disorder | 20 | 51.3 |
| Major Depressive Disorder | 12 | 30.8 |
| Generalized Anxiety Disorder | 5 | 12.8 |
| Panic Disorder | 2 | 5.1 |
| Any Current Diagnosis | ||
| Social Anxiety Disorder | 28 | 71.8 |
| Major Depressive Disorder | 13 | 33.3 |
| Generalized Anxiety Disorder | 14 | 35.9 |
| Panic Disorder | 11 | 28.2 |
| Specific Phobia | 3 | 7.7 |
| Post-Traumatic Stress Disorder | 4 | 10.3 |
Note. N = 39. CGI = Clinical Global Impression scale.
Materials and Measures
Diagnostic Interview
Participants were interviewed by Master’s- or Doctoral-level clinicians using the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 1996) to assess Axis I disorders (see Table 1).
Treatment Outcome Measures
To assess illness baseline severity and response to CBT, clinicians completed the Clinical Global Impression (CGI) Severity and Improvement scales (Busner & Targum, 2007). Both measures use 7-point scales, ranging from 1 (normal, not at all ill) to 7 (extremely ill) for CGI Severity (CGI-S) and from 1 (very much improved) to 7 (very much worse) for CGI Improvement (CGI-I). CGI-Improvement was considered the primary index of treatment response. Participants were determined to have achieved clinically-significant treatment response and were classified as “Responders” if they were rated to be “very much improved” or “much improved” (CGI-I score of 1 or 2). Participants with CGI-I scores > 3 were classified as “Non-Responders.”
The 17-item Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960), a widely-used interview-based measure of depression symptom severity and the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), a 14-item clinician-administered measure of severity of anxious symptomatology, were administered by trained, independent evaluators at pre- and post-treatment to assess changes in symptoms of depression and anxiety, respectively.
Affective pictures
Forty-eight aversive (e.g., mutilated bodies, attack scenes) and 48 neutral pictures (e.g., neutral faces, household objects) were selected from the International Affective Picture System (Lang et al., 2005).1 Aversive pictures were selected because they had been rated as less pleasant and higher in arousal than neutral pictures (see MacNamara & Hajcak, 2009, for details). Stimuli were presented on a Dell Optiplex 750 computer, using Presentation software (Neurobehavioral Systems, Inc.). Participants were seated approximately 60 cm from the screen.
Procedures
CBT
Patients received 12 weeks of individual manualized CBT conducted by doctoral-level clinical psychologists (Beck et al., 1979; Craske et al., 1992; Hope et al., 2006; Martell et al., 2010). A licensed clinical psychologist with expertise in CBT provided supervision to ensure adherence to treatment. CBT included psychoeducation, cognitive restructuring, in vivo exposures, behavioral activation, and relapse prevention, targeted toward each patient’s principal diagnosis.
Affective picture task
At pre-treatment, participants completed a computerized task while EEG was recorded (MacNamara & Hajcak, 2009). Briefly, four pictures – two to the left and right, and two above and below the center of the screen – were presented simultaneously on each trial. Participants were asked to indicate whether two of the pictures (either the vertical or horizontal picture pairs) were pictorially the same or different. Picture valence (aversive or neutral) was always the same in both the horizontal and vertical pairs. From here on, stimuli presented in task-relevant spatial locations will be referred to as “targets,” and stimuli presented in task-irrelevant locations will be referred to as “distracters.”
There were four trial types: neutral targets paired with neutral distracters, neutral targets paired with aversive distracters, aversive targets paired with neutral distracters, and aversive targets paired with aversive distracters. Participants used the left and right mouse buttons (counterbalanced across participants) to indicate if targets were identical (“same”) or different (“different”); participants were encouraged to respond as quickly and as accurately as possible. Before each trial, two white rectangles appeared on a black background for 1,000 ms to indicate which picture pair (horizontal or vertical) would be the targets for the same/different decision in the upcoming trial. Pictures were displayed in color for 250 ms. Participants completed 10 practice trials and 320 experimental trials. Pictures presented during practice trials were not repeated during experimental trials. Trial order and pictures were presented pseudo-randomly, with each picture repeated 10 times across the task (for more details see MacNamara & Hajcak, 2009).
Electroencephalographic Recording and Behavioral Responses
An elastic cap and the ActiveTwo BioSemi system (Amsterdam, Netherlands) were used to continuously record the EEG. Thirty-four electrode sites (standard 32 channel setup plus Iz and FCz) based on the 10/20 system were used, with one additional electrode on each of the left and right mastoids. Four facial electrodes recorded the electrooculogram generated from eye blinks and eye movements: horizontal eye movements were measured with two electrodes placed approximately 1 cm beyond the outer edge of each eye; vertical eye movements and blinks were measured with two electrodes placed approximately 1 cm above and below the right eye. Online data were referenced according to BioSemi’s design using two separate electrodes for grounding (the Common Mode Sense active electrode and the Driven Right Leg passive electrode) and data were digitized at 1024 Hz.
Off-line analyses were performed using Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were re-referenced to the average of the two mastoids and were band-pass filtered with low and high cutoffs of 0.01 and 30Hz, respectively. ERPs were segmented for each trial beginning 200ms before picture and continuing for 1200ms (1000ms beyond picture onset). Eye blink and ocular corrections were made using the algorithm developed by Miller et al. (1988). Artifact analysis identified a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100 ms intervals. Trials were also inspected visually for any remaining artifacts; intervals containing artifacts were rejected from individual channels in each trial. Baseline correction was performed using the 200ms prior to picture onset on each trial. Amplitudes (and RTs) for each correct trial were exported on a trial-by-trial basis to allow for within-subject trial-level analysis. As in prior work, to assess between-subject differences in average LPP amplitude, the LPP was scored by averaging activity from 400 to 1000ms at four centro-parietal sites where the LPP was maximal: CP1, CP2, Cz, and Pz (e.g., Weinberg & Hajcak, 2010; Hajcak et al., 2007). Only trials associated with a correct response made within 1,800 ms following picture offset were included in the ERP analyses. Reaction time (RT) was determined as the time taken to respond following picture onset on correct trials and accuracy (across the task) was assessed as the percentage of correct responses.
Participants generally performed well on the task (M = 89.35% correct, SD = 8.72%). One (female) participant was removed from analyses because of excessive EEG artifacts (> 50% of trials excluded); two participants (one male, one female) were excluded because of poor task performance (less than 50% accuracy), yielding a final sample of n = 39 for analyses (Table 1).
Statistical Analyses
Given the nested structure of the data (multiple trials, including RT and LPPs, nested within each person), multilevel modeling (MLM) was used (Raudenbush & Bryk, 2002) with Mplus 7.4 software (Muthen & Muthen, 1998–2015). This approach allowed for a person-centered (within-subjects) approach to measuring the relationship between LPPs and RTs on subsequent trials. To facilitate a person-centered approach, variables that were nested within participants (“Level 1” variables) were centered around each person’s own mean, so that fluctuations represented deviations from each person’s average level (e.g., deviations from each person’s average LPP or RT across trials). Numerous studies have supported the utility of trial-level analyses of ERPs and RTs (Adamo et al., 2015; Bender et al., 2015; Berchicci et al., 2016; Gaspar et al., 2011; Leue et al., 2013; Ramchurn et al., 2014; Saville et al., 2011, 2012).
To examine the primary hypotheses, we selected pairs of consecutive trials in which both trials in the pair were accurate. Out of 319 possible accurate trial pairings for each participant, a mean of 257 pairings (SD = 45.56, range = 134–309) met this criterion. The relationship between LPP on trial N and RT on trial N+1 was modeled with a random intercept (allowing individuals to vary in their mean RT) and a random slope (allowing the strength of the relationship between fluctuations in LPPs and RTs on subsequent trials, i.e., brain-behavioral adaptability, to vary between individuals). In these models, at Level 1, we included as covariates in the analyses the following variables, which were person-centered, as predictors of RT: target type (aversive or neutral), distracter type (aversive or neutral), and the interaction between target and distracter type on trial N (to reduce variance in RT on trial N+1 not associated with the relationship with LPP on trial N). In addition, as RTs on proximal trials are likely to be more similar than are RTs on more distal trials (i.e., an autoregressive effect), we also controlled for RT on trial N to account for changes in RT that occurred across the task, which were not the primary question of interest here.
Furthermore, in the event that LPPs were larger when target images were aversive than when they were neutral, and that larger LPPs led to slower RTs on subsequent trials, we planned to conduct multilevel mediation analyses to evaluate the presence of an indirect within-subjects mediation effect. This model would evaluate whether target valence (aversive vs. neutral) on trial N would predict LPP on trial N, which subsequently would lead to RT on trial N+1.
In addition to examining the strength of brain-behavioral adaptability across individuals, we then examined whether individual differences in brain-behavioral adaptability would predict treatment Responder status. We extracted each person’s adaptability score (factor scores of the slope of LPPs on trial N on RTs on trial N+1) from the models described above, and used these adaptability scores as predictors of (1) CGI-I Responder status using logistic regression, controlling for pre-treatment CGI-S, (2) post-treatment HAM-D scores using linear regression, controlling for pre-treatment HAM-D scores, and (3) post-treatment HAM-A scores using linear regression, controlling for pre-treatment HAM-A scores. As a conservative approach, these analyses also controlled for mean RT (to account for between-subjects differences in average RT), mean LPP (to account for between-subjects differences in LPP amplitude), and the number of consecutive correct trials in the analysis (to account for between-subjects differences in the tendency to respond accurately to consecutive trials). Brain-behavioral adaptability slopes were standardized prior to entering into regressions predicting treatment outcome, for ease of interpretation.
Results
Sample demographics and clinical characteristics are presented in Table 1.
Task Effects
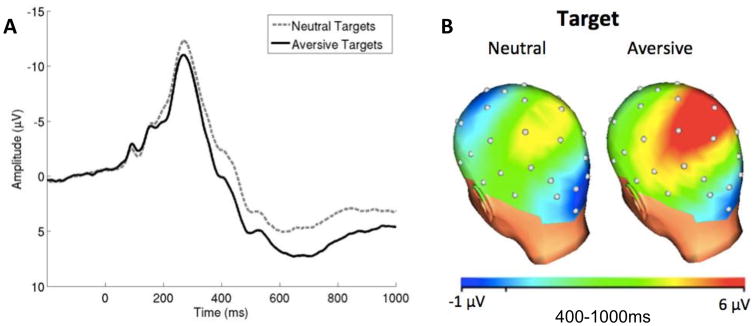
Figure 1 displays grand-average (between-subject) waveforms and scalp distributions to illustrate the overall pattern of effects and to provide context for the trial-level effects we report below. Findings are in line with previous studies using this task (MacNamara & Hajcak, 2009, 2010; Stange et al., 2017a) verifying the paradigm probed LPP as expected. Among the 39 participants, a total of 9,688 consecutive correct trial observations were available that also contained usable LPP data. The intra-class correlation (ICC) for RT in an empty model was .222, indicating that 22.2% of the variance in RT occurred at the between-subjects level (Level 2), whereas 77.8% of the variance in RT occurred at the within-subjects level (Level 1), suggesting adequate variability to evaluate intra-individual (within-subjects) processes. Comparable models also indicated that there was adequate variability in the LPP (ICC = .104, suggesting 89.6% of the variance in LPP occurred within-subjects).
Figure 1.
Grand average amplitudes at pooling of CP1, CP2, Cz, and Pz (panel A) and scalp distributions of amplitudes from 400–1000 ms after picture onset (panel B) for neutral and aversive trials.
To determine whether behavioral or neural responses differed based on the type of stimuli at the trial level, MLMs were conducted with target type (aversive or neutral), distracter type (aversive or neutral), and their interaction predicting LPP, RT, and accuracy, with all variables person-centered and at the within-subjects level.
As expected based on prior work (MacNamara & Hajcak, 2009, 2010), there was a significant effect of target type on the LPP (B = 2.07, t = 6.42, p < .001), such that larger LPPs were elicited for aversive (M = 5.43 μV, SD = 4.91 μV) relative to neutral targets (M = 3.18 μV, SD = 4.53 μV). However, there was no main effect of distracter type (B = −0.06, t = −0.23, p = .82), nor was there an interaction between target and distracter type (B = 0.26, t = 0.61, p = .67).
In terms of RT, there was a significant main effect of target type (B = 8.71, t = 2.33, p = .02) such that aversive targets (M = 703.89 ms, SD = 116.24 ms) were associated with longer RTs than neutral targets (M = 693.66 ms, SD = 112.95 ms). Neither the main effect of distracter type (B = −0.03, t = −0.01, p = .99), nor the interaction between target and distracter type (B = 11.22, t = 1.55, p = .12) were significant. When examining accuracy on all trials containing a valid response (non-omission; n = 12,361 trials; ICC = .068), no significant effects of target (B < 0.001, t = 0.09, p = .93), distracter (B = 0.002, t = 0.666, p = .51), or their interaction (B = −0.01, t = −1.90, p = .06) were observed. Given the lack of differences (in LPP, RT, and accuracy) in terms of distracter valence, we collapsed across distracter valence for the primary analyses of interest below.
Within-Subjects Relationships between Neural and Behavioral Responses to Stimuli
Consistent with hypotheses, larger LPPs (relative to participant’s own average LPP) on trial N were associated with slower RTs on trials N+1 (B = 3.60, t = 1.98, p < .05),2 even after controlling for RT on trial N, the patient’s mean (Level 2) RT, mean LPP, and the number of accurate trial pairs.3,4 This suggests that on trials when LPPs were smaller than average for a given individual, performance efficiency improved on the next trial. The strength of this relationship did not differ (was not predicted) by principal diagnosis of MDD (B = −3.31, t = −0.83, p = .41) or SAD5 (B = −0.02, t = −0.01, p = .99), nor did it differ by pre-treatment HAM-D (B = 0.07, t = 0.29, p = .77) or HAM-A (B = 0.14, t = 0.73, p = .47), suggesting that the ability to benefit behaviorally from a smaller LPP on the prior trial was consistent across diagnoses and pre-treatment symptom severity.
Associations between Aversive Stimuli and Impaired Subsequent Behavioral Performance via Neural Indices of Elaborated Processing
Given that aversive targets elicited larger LPPs, and larger LPPs predicted slower behavioral performance on subsequent trials, we conducted multilevel mediation analyses to evaluate the presence of an indirect within-subjects mediation effect. This model evaluated whether target valence (aversive vs. neutral) on trial N would predict LPP on trial N, which subsequently would lead to RT on trial N+1. Although there was not a significant direct effect of target valence on RT on subsequent trials (B = 2.69, t = 0.81, p = .42),6 there was a significant indirect effect of target valence on subsequent RTs via LPPs (B = 0.43, 95% CI = 0.06–0.80, t = 2.28, p = .02). This result is consistent with the possibility that exposure to aversive stimuli may interfere with the efficient processing of new information due to elaborated and continued processing of salient stimuli. Stated differently, overall, individuals were able to process new information more efficiently when previous stimuli were neutral and therefore less salient, representing an ability to benefit behaviorally from less-salient stimuli.
Response to CBT
Based on the CGI-I, 64% of the sample (25 of 39) were considered to be “Responders” as they were rated to be “very much improved” or “much improved” (CGI-I score of 1 or 2), whereas 36% of the sample (14 of 39) had a CGI-I score of three or higher at post-treatment and thus were considered to be “Non-Responders.” Principal diagnosis was not significantly associated with treatment responder status (SAD principal: 14 of 20 were responders (70%), χ2(1) = 0. 62, p = .51; MDD principal: 8 of 12 were responders (67%); χ2(1) = 0.05, p > .99). At baseline, Responders and Non-Responders did not differ in CGI-Severity (t(37) = −0.32, p = .76).
To determine whether Responders and Non-Responders differed in RT, accuracy, and LPPs and whether this differed by target and distracter valence, multilevel models were conducted. Predictors included main effects of target and distracter valence and their interaction; interactions between each of these three terms and Responder status; and the main effect of Responder status. Results indicated that Responders and Non-Responders did not differ in RT (ps > .49 for all predictors), accuracy (ps > .46), or LPP amplitude (ps > .10).
Associations between Brain-Behavioral Coupling and Response to CBT
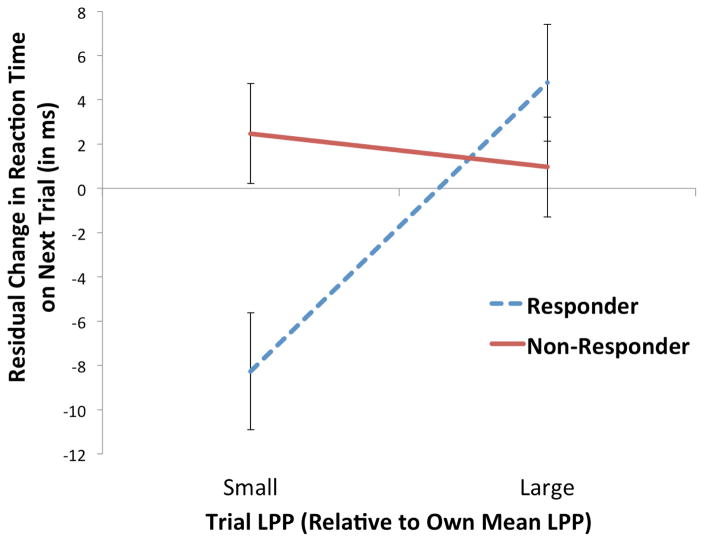
Next, we examined our primary question about how brain-behavioral coupling, i.e., improvements in performance (in terms of shorter RTs) following trials with smaller LPPs, would be associated with response to CBT. Brain-behavioral coupling was a significant predictor of treatment Responder status (B = 1.27, OR = 3.56, 95% CI = 1.17–10.82, Wald = 5.01, p = .03, ΔR2 = .23; Figure 2). Individuals who showed improved performance following trials with smaller LPPs were more likely to respond to treatment than were individuals who showed less improved performance following trials with smaller LPPs. Indeed, a one standard deviation increase in brain-behavioral coupling was associated with a 3.56 times greater likelihood of response to CBT. Greater brain-behavioral coupling was similarly associated with significantly fewer symptoms of depression (HAM-D) at post-treatment (B = −1.35, t = −2.06, p < .05, ΔR2 = .09); the association with changes in anxiety symptoms (HAM-A) across treatment was in a similar direction but was not significant (B = −0.89, t = −1.03, p = .31, ΔR2 = .02).7
Figure 2.
Relationship between pre-treatment person-centered trial-level LPP and residual change in person-centered reaction time (in milliseconds) on subsequent trial, i.e., brain-behavioral adaptability, as a function of Responder status on the CGI-Improvement scale following 12 weeks of CBT. Error bars represent standard errors of the simple slopes of LPP on residual change in reaction time.
Importantly, these analyses demonstrated that greater brain-behavioral adaptability predicted response to CBT even after accounting for differences in between-subjects predictors of treatment outcome, including pre-treatment symptom severity, mean RT, mean LPP, and the number of accurate trial pairs.8,9,10 Thus, these results suggest that the use of within-subject trial-level analysis allowed for the detection of a set of relationships that were not detectable at the between-subjects level, and that predicted treatment response.
Discussion
This is the first ERP study to integrate measures of brain and behavior with trial-level analysis in the context of treatment outcome. To this end, the present study had two aims. First, we sought to replicate and extend the results of prior work by examining whether elaborative processing (indexed by the LPP) could account for the relationship between aversive stimuli and interference with the processing of subsequent stimuli, using a treatment-seeking sample of individuals with anxiety and/or depression. Using a person-centered approach, with multiple trials nested within each person, we evaluated within-individual variability in LPPs and RTs. As hypothesized, our results indicate that overall, individuals exhibited larger LPPs on trials with aversive targets than trials with neutral targets; consistent with the presence of aversive-related “interference,” larger LPPs then led to slower responses on subsequent trials, with LPPs mediating the relationship between aversive targets and slower responses on subsequent trials. Second, we extended the literature by evaluating how response to CBT is related to between-individual differences in brain-behavioral adaptability. We found that individuals who responded to CBT (in terms of CGI improvement and depressive symptom reduction) benefited more (behaviorally) from smaller LPPs (i.e., demonstrating greater adaptability), relative to individuals who did not respond to CBT, whose performance (RT) did not improve following smaller LPPs. Findings support a new approach to evaluating individual differences by linking brain-behavioral adaptability to treatment response.
Consistent with prior work in a healthy sample (Weinberg & Hajcak, 2011), our results suggest that in general, elaborative attention (indexed by the LPP) interferes with the processing of subsequent stimuli; in contrast, patients were able to benefit when task-irrelevant stimuli were processed to a lesser extent, adapting their behavior to focus on the task at hand. This is consistent with the notion that having a well-coordinated brain-behavior response represents an adaptive process that facilitates the ability to attend to potential threats (e.g., aversive stimuli) or rewards (e.g., pleasant stimuli) when necessary (i.e., bottom-up processing), which could confer evolutionary advantages (LeDoux, 1998). As a different example of how coordinated brain-behavior responses facilitate adaptability, Thayer and Lane’s (2000, 2009) theory of neurovisceral integration suggests that behavioral adaptation to changes in contextual demands is facilitated by a core set of neural structures (e.g., the medial prefrontal cortex) that allow the body to integrate internal and external signals and adaptively regulate cognition, behavior, and physiology (Thayer et al., 2012). Consistent with this theory, recent evidence has suggested that flexible autonomic responses to emotional stimuli are associated with adaptive emotion regulation (Stange et al., 2017c; Yaroslavsky et al., 2016) and with psychological health, including lower levels of depression and anxiety (Kashdan & Rottenberg, 2010; Stange et al., 2017b, in press). Although we used a different set of methods and constructs, our results are in line with such data suggesting that the ability to adjust behavior based on neural or physiological input may facilitate adaptive outcomes.
Importantly, however, in our data not all individuals showed the same ability to benefit behaviorally when stimuli were processed to a lesser degree. Individual differences in brain-behavioral coupling predicted treatment response: patients who responded to CBT showed greater adaptability prior to treatment than did patients who did not respond to CBT. This suggests that evaluating brain-behavioral adaptability prior to treatment may be useful in determining which patients are most likely to benefit from CBT. It is possible that patients who are able to implicitly adapt their attention to external information when previous stimuli are perceived as less relevant to the task at hand are more able to benefit from learning skills in the course of therapy than are individuals who are less able to adapt. Potentially, individuals with more brain-behavioral adaptability might be more able to benefit behaviorally from reductions in elaborative processing that might occur when practicing cognitive and behavioral techniques in the face of salient information (e.g., feared stimuli, negative thoughts) resulting in more efficient or effective learning of adaptive coping strategies.
It is not immediately apparent why brain-behavioral adaptability predicted improvements in symptoms of depression, but not anxiety, although these effects were in the same direction. Although speculative, one possibility is that depression often is characterized by context insensitivity, or difficulty adapting to meet the situational demands (Bonanno & Burton, 2013; Rottenberg et al., 2005; Stange et al., in press). Although brain-behavioral adaptability was not associated with pre-treatment depressive symptoms, it did predict fewer symptoms of depression at post-treatment, consistent with a prior study of cardiovascular context sensitivity (Somers et al., 2015). This lack of appropriate adaptability could present an obstacle to overcome when learning new skills for managing environmental demands that are taught during the course of CBT. Nevertheless, as symptoms of depression and anxiety and global treatment response (CGI) were moderately correlated, these results require replication before concluding that they would not extend to symptoms of anxiety.
Despite the strengths of this study, several limitations should be noted. The sample size was relatively small, which prevented us from determining if results differed by principal diagnosis and by subtypes of anxiety disorders. We evaluated brain-behavioral coupling on pairs of trials in which responses were accurate, but not on pairs of trials in which one trial was inaccurate, as few trials of this type were available due to high overall accuracy on the task. Future studies could consider evaluating similar relationships in tasks designed to elicit greater variability in trial-to-trial accuracy, which could provide additional information about links between brain and behavioral performance. In addition, not including a wait-list control group means that our findings could be indicative of symptom-based change more broadly (e.g., reductions in severity due to the passage of time), rather than CBT-based change specifically (though note that this would not negate the finding that brain-behavior relationships predicted symptom reduction). Future studies might benefit from employing multiple treatments (e.g., other types of psychotherapies or pharmacotherapy) to determine whether brain-behavior links can be used to identify which patients are most likely to benefit from one treatment versus another, with the goal of personalized medicine (Tracy, Klonsky, & Proudfit, 2014). Although predictors of treatment response are not necessarily the same as those that are changed by treatment (e.g., Doehrmann et al., 2013; Klumpp et al., 2013; MacNamara et al., 2015; Phan et al., 2013), examining the degree to which brain-behavioral coupling improves following treatment might also help to clarify whether it could serve as a mechanism, in addition to being a predictor, of treatment response. Next, the CGI was administered by the treating clinician, which could have biased rates of treatment response; nevertheless, that brain-behavioral adaptability also predicted improvement in symptoms of depression (which were rated by an independent interviewer) suggests that this concern is unlikely to fully explain the effects reported here. Finally, in the present study we report analyses of pre-post treatment changes in symptoms; future studies should consider evaluating trajectories of symptoms across multiple points of follow-up.
In conclusion, the current results extend prior work by demonstrating the utility of trial-level analysis in elucidating within-subjects processes such as brain-behavioral adaptability, which may be helpful in predicting treatment response in transdiagnostic clinical samples with anxiety and/or depression. This research represents a promising avenue for future studies, with the long-term goal of directing individuals to treatments that are most likely to benefit them and in identifying brain-behavioral mechanisms underlying treatment response.
Highlights.
We used a novel person-centered, trial-level analysis approach among adults receiving CBT for anxiety or depression.
Attention to the task-irrelevant nature of pictures was indexed by the late positive potential (LPP).
We linked neural indices of stimulus processing (the LPP) to behavioral responses (reaction time).
Individuals with greater ability to benefit behaviorally from smaller LPPs on the previous trial (greater brain-behavioral adaptability) were more likely to respond to CBT and showed greater improvements in depressive symptoms.
Trial-level analyses can elucidate variability in within-subjects, brain-behavioral attentional coupling in the context of emotion processing.
Footnotes
This work was supported by National Institute of Mental Health (NIMH) K23MH093679 and Brain and Behavior Research Foundation (formerly NARSAD) Award to HK and in part by NIMH R01MH101497 (to KLP) and the Center for Clinical and Translational Research (CCTS) grant, UL1RR029879. JS is supported by NIMH 5T32MH067631-12. AM is supported by NIMH K23MH105553.
As reported in MacNamara and Hajcak (2009), the IAPS pictures used were aversive (1050, 1090, 1120, 1205, 1220, 1240, 1270, 1280, 1300, 1930, 1120, 1205, 1220, 1240, 1270, 1280, 1300, 1932, 2120, 2800, 2811, 3030, 3051, 3060, 3068, 3069, 3080, 3100, 3102, 3120, 3140, 3170, 3230, 3250, 3261, 3350, 3530, 6260, 6313, 6315, 6350, 6360, 6370, 6510, 6530, 6540, 6550, 6560, 6570, 9040, 9042, 9140, 9301, 9320, 9570) and neutral (1390, 1450, 1650, 1670, 18101390, 1450, 1650, 1670, 19351390, 1450, 1650, 1670, 2038, 2102, 2190, 2200, 2210, 2214, 2357, 2383, 2393, 2397, 2446, 5500, 5510, 5530, 7000, 7002, 7030, 7034, 7036, 7037, 7040, 7041, 7054, 7057, 7060, 7110, 7130, 7175, 7234, 7491, 7493, 7496, 7500, 7501, 7546, 7547, 7550, 7560, 7595, 7620, 7710, 7920).
We also examined the specificity of this relationship to specific trial target pairings by testing moderation by trial type (e.g., selecting trials when the current trial target was negative, and evaluating whether the relationship between LPP and RT differed based on whether the subsequent trial target was negative or neutral). The within-subject relationship between larger LPPs and slower RTs was not affected by trial type pairings (all interaction ps > .35).
Sensitivity analyses suggested that this relationship was consistent when also controlling for LPP on the subsequent trial.
To examine the impact of between-subject (Level 2) variance in RT, we repeated the analysis predicting an uncentered RT outcome variable, which allowed between-subject (Level 2) variance in RT intercepts to exist that could be predicted by the Level 2 covariates. In this analysis, mean RT predicted greater RT intercepts (B = 103.92, t = 33.43, p < .001); neither mean LPP (B = 0.41, t = 0.25, p = .81) nor the number of accurate trial pairs (B = −0.01, t = −0.09, p = .93) significantly predicted RT intercepts.
There were too few participants with principal diagnoses of generalized anxiety disorder or panic disorder to permit examination of effects of these specific disorders.
Although historically the failure to find a direct association between a predictor (e.g., target valence) and an outcome (e.g., RT on subsequent trial) has been viewed as precluding the ability to test for mediation effects (Baron & Kenny, 1986), contemporary perspectives have provided evidence that indirect effects (i.e., mediation) may exist even in the absence of a direct effect (MacKinnon et al., 2002; Shrout & Bolger, 2002; Hayes, 2009; Hayes & Rockwood, in press; Zhao et al., 2010).
Responder status was significantly associated with residual change in HAM-D (r = −.50, p < .001) and in HAM-A (r = −.42, p < .001), suggesting that these indices may measure related, but distinct, constructs.
Additional sensitivity analyses demonstrated that these results were consistent when controlling for patients’ principal diagnoses: Responder status continued to predict degree of brain-behavioral adaptability (B = 1.56, OR = 4.76, 95% CI = 1.39–16.30, Wald = 6.18, p = .01, ΔR2 = .28). We also examined the specificity of this relationship to specific trial target pairings by testing moderation by trial type (e.g., selecting trials when the current trial target was negative, and evaluating whether the relationship between LPP and RT was differentially associated with responder status based on whether the subsequent trial target was negative or neutral, resulting in four possible comparisons); this association did not differ by any trial types (ps > .57), suggesting that brain-behavioral adaptability was similarly associated with responder status regardless of the types of targets presented on consecutive trials.
To demonstrate that associations with treatment response were specific to the brain-behavioral adaptability relationship rather than to degree of variability in brain or behavior separately, we also found that Responders and Non-Responders did not differ in within-subject standard deviation of RTs (t(37) = 0.18, p = .86), accuracy (t(37) = −0.65, p = .52), or LPPs (t(37) = 0.76, p = .45).
Alternative models of the reverse association in which slopes of RT predicted subsequent LPPs were not associated with indices of treatment response.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo N, Baumeister S, Hohmann S, Wolf I, Holz N, Boecker R, … Brandeis D. Frequency-specific coupling between trial-to-trial fluctuations of neural responses and response-time variability. Journal of Neural Transmission. 2015;122(8):1197–1202. doi: 10.1007/s00702-015-1382-8. [DOI] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, Gross JJ. Emotion regulation flexibility. Cognitive Therapy and Research. 2015;39(3):263–278. [Google Scholar]
- Andreescu C, Aizenstein H. Predicting treatment response with functional magnetic resonance imaging. Biological Psychiatry. 2016;79:262–263. doi: 10.1016/j.biopsych.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Arch JJ, Craske MG. First-line treatment: a critical appraisal of cognitive behavioral therapy developments and alternatives. Psychiatric Clinics of North America. 2009;32(3):525–547. doi: 10.1016/j.psc.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Glickman S. Attentional bias in anxiety: a behavioral and ERP study. Brain and Cognition. 2005;59:11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, et al. Cognitive therapy of depression. New York: Guilford; 1979. [Google Scholar]
- Bender S, Banaschewski T, Roessner V, Klein C, Rietschel M, Feige B, … Laucht M. Variability of single trial brain activation predicts fluctuations in reaction time. Biological Psychology. 2015;106:50–60. doi: 10.1016/j.biopsycho.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Berchicci M, Spinelli D, Di Russo F. New insights into old waves. Matching stimulus-and response-locked ERPs on the same time-window. Biological Psychology. 2016;117:202–215. doi: 10.1016/j.biopsycho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Burton CL. Regulatory flexibility: An individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science. 2013;8(6):591–612. doi: 10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, Klumpp H. Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depression and Anxiety. 2016;33(4):281–288. doi: 10.1002/da.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busner J, Targum SD. The clinical global impressions scale. Psychiatry. 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Lau HPB, Chan MPS. Coping flexibility and psychological adjustment to stressful life changes: A meta-analytic review. Psychological Bulletin. 2014;140(6):1582–1607. doi: 10.1037/a0037913. [DOI] [PubMed] [Google Scholar]
- Craske MG, Barlow DH, O’Leary TA. Mastery of your anxiety and worry. Albany: Graywind Publications; 1992. [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Wei J, Shu X, Feng Z. Negativity bias for sad faces in depression: An event-related potential study. Clinical Neurophysiology. 2016;127(12):3552–3560. doi: 10.1016/j.clinph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Schwartz S, Dang-Vu TT, et al. Depression alters “top-down” visual attention: a dynamic causal modeling comparison between depressed and healthy subjects. Neuroimage. 2011;54:1662–8. doi: 10.1016/j.neuroimage.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Balteau E, Sterpenich V, et al. Abnormal neural filtering of irrelevant visual information in depression. Journal of Neuroscience. 2009;29:1395–403. doi: 10.1523/JNEUROSCI.3341-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Rosso IM, Pechtel P, et al. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depression and Anxiety. 2014;31:233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, et al. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry. 2013;70:87–97. doi: 10.1001/2013.jamapsychiatry.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Association; 1996. [Google Scholar]
- Gaspar CM, Rousselet GA, Pernet CR. Reliability of ERP and single-trial analyses. Neuroimage. 2011;58(2):620–629. doi: 10.1016/j.neuroimage.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015;171:65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Neural response to emotional pictures is unaffected by concurrent task difficulty: an event-related potential study. Behavoral Neuroscience. 2007;121:1156–62. doi: 10.1037/0735-7044.121.6.1156. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. Oxford handbook of ERP components. New York: Oxford University Press; 2012. pp. 441–474. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–420. [Google Scholar]
- Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behaviour Research and Therapy. doi: 10.1016/j.brat.2016.11.001. in press. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk IJ, et al. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy and Research. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depression and Anxiety. 2012b;29(5):409–416. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. Journal of Clinical Psychiatry. 2008;69:621–32. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG, Turk CL. Managing social anxiety: a cognitive-behavioral therapy approach. New York: Oxford University Press; 2006. [Google Scholar]
- Johnco C, Wuthrich VM, Rapee RM. The influence of cognitive flexibility on treatment outcome and cognitive restructuring skill acquisition during cognitive behavioural treatment for anxiety and depression in older adults: Results of a pilot study. Behaviour Research and Therapy. 2014;57:55–64. doi: 10.1016/j.brat.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review. 2010;30(7):865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depression and Anxiety. 2000;12(s 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Gordon E, Rush AJ, Williams LM. Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, and genetic measures. CNS Spectrums. 2008;13(12):1066–1086. doi: 10.1017/s1092852900017120. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg P, ARM, … Wang PS. Prevalence and effects of mood disorders on work performance in a nationally representative sample of US workers. American journal of psychiatry. 2006;163(9):1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan KL. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2013;45:83–91. doi: 10.1016/j.pnpbp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- LeDoux J. The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Touchstone Books; 1998. [Google Scholar]
- Leue A, Klein C, Lange S, Beauducel A. Inter-individual and intra-individual variability of the N2 component: on reliability and signal-to-noise ratio. Brain and Cognition. 2013;83(1):61–71. doi: 10.1016/j.bandc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Rabinack CA, Kennedy AE, et al. Emotion Regulatory Brain Function and SSRI Treatment in PTSD: Neural Correlates and Predictors of Change. Neuropsychopharmacology. 2015;41:611–618. doi: 10.1038/npp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Ferri J, Hajcak G. Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective, and Behavioral Neuroscience. 2011;11:321–31. doi: 10.3758/s13415-011-0036-z. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47:2975–80. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety. 2010;27:234–43. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Kappenman ES, Black SR, et al. Integrating behavioral and electrocortical measures of attentional bias toward threat. In: Barrett KC, Fox NA, Morgan GA, Fidler DJ, Daunhauer LA, editors. Handbook of self-regulatory processes in development: New directions and international perspectives. New York: Psychology Press; 2013. pp. 215–246. [Google Scholar]
- MacNamara A, Proudfit GH. Cognitive load and emotional processing in generalized anxiety disorder: electrocortical evidence for increased distractibility. Journal of Abnormal Psychology. 2014;123:557–65. doi: 10.1037/a0036997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell CR, Dimidjian S, Herman-Dunn R. Behavioral activation for depression. New York: Guilford Press; 2010. [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–95. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16:331–354. [Google Scholar]
- Mennin DS, Fresco DM. What, Me Worry and Ruminate About DSM- 5 and RDoC? The Importance of Targeting Negative Self- Referential Processing. Clinical Psychology: Science and Practice. 2013;20(3):258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM. Generalized implementation of an eye movement correction procedure. Psychophysiology. 1988;25:241–243. [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annual Review of Clinical Psychology. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- Paulus MP. Pragmatism instead of mechanism: a call for impactful biological psychiatry. JAMA Psychiatry. 2015;72(7):631–632. doi: 10.1001/jamapsychiatry.2015.0497. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27:1135–42. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Phan KL, Coccaro EF, Angstadt M, et al. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biological Psychiatry. 2013;73(4):329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchurn A, de Fockert JW, Mason L, Darling S, Bunce D. Intraindividual reaction time variability affects P300 amplitude rather than latency. Frontiers in Human Neuroscience. 2014;8:557. doi: 10.3389/fnhum.2014.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114(4):627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, et al. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119:631–9. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Saville CW, Shikhare S, Iyengar S, Daley D, Intriligator J, Boehm SG, … Klein C. Is reaction time variability consistent across sensory modalities? Insights from latent variable analysis of single-trial P3b latencies. Biological Psychology. 2012;91(2):275–282. doi: 10.1016/j.biopsycho.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Saville CW, Dean RO, Daley D, Intriligator J, Boehm S, Feige B, Klein C. Electrocortical correlates of intra-subject variability in reaction times: average and single-trial analyses. Biological Psychology. 2011;87(1):74–83. doi: 10.1016/j.biopsycho.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Somers JA, Borelli JL, Smiley PA, West JL, Hilt LM. Concurrent and prospective associations between emotion reactivity and depressive symptoms in middle childhood. Journal of Psychopathology and Behavioral Assessment. 2015 doi: 10.1007/s10862-015-9491-0. [DOI] [Google Scholar]
- Stange JP, Alloy LB, Fresco DM. Inflexibility and vulnerability to depression: A systematic qualitative review. Clinical Psychology: Science and Practice. doi: 10.1111/cpsp.12201. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY, Alloy LB. Inflexible cognition predicts first onset of major depression in adolescence. Depression and Anxiety. 2016;33(11):1005–1012. doi: 10.1002/da.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Olino TM, Fresco DM, Alloy LB. Autonomic reactivity and vulnerability to depression: A multi-wave study. Emotion. 2017b;17(4):602–615. doi: 10.1037/emo0000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, MacNamara A, Barnas O, Kennedy AE, Hajcak G, Phan KL, Klumpp H. Neural markers of attention to aversive pictures predict response to cognitive behavioral therapy in anxiety and depression. Biological Psychology. 2017a;123:269–277. doi: 10.1016/j.biopsycho.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Hamilton JL, Fresco DM, Alloy LB. Flexible parasympathetic responses to sadness facilitate spontaneous affect regulation. Psychophysiology. 2017c doi: 10.1111/psyp.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Klonsky ED, Proudfit GH. How affective science can inform clinical science: An introduction to the special series on emotions and psychopathology. Clinical Psychological Science. 2014;2:371–86. [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Yaroslavsky I, Rottenberg J, Bylsma LM, Jennings JR, George C, Baji I, … Kiss E. Parasympathetic nervous system activity predicts mood repair use and its effectiveness among adolescents with and without histories of major depression. Journal of Abnormal Psychology. 2016;125(3):323–336. doi: 10.1037/abn0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research. 2010;37(2):197–206. [Google Scholar]