Abstract
Introduction
Colorectal cancer remains the second leading cause of cancer death in the United States, and new strategies to prevent, detect, and treat the disease are needed. The receptor, guanylate cyclase C (GUCY2C), a tumor suppressor expressed by the intestinal epithelium, has emerged as a promising target.
Areas Covered
This review outlines the role of GUCY2C in tumorigenesis, and steps to translate GUCY2C-targeting schemes to the clinic. Endogenous GUCY2C-activating ligands disappear early in tumorigenesis, silencing its signaling axis and enabling transformation. Pre-clinical models support GUCY2C ligand supplementation as a novel disease prevention paradigm. With the recent FDA approval of the GUCY2C ligand, linaclotide, and two more synthetic ligands in the pipeline, this strategy can be tested in human trials. In addition to primary tumor prevention, we also review immunotherapies targeting GUCY2C expressed by metastatic lesions, and platforms using GUCY2C as a biomarker for detection and patient staging.
Expert Commentary
Results of the first GUCY2C targeting schemes in patients will become available in the coming years. The identification of GUCY2C ligand loss as a requirement for colorectal tumorigenesis has the potential to change the treatment paradigm from an irreversible disease of genetic mutation, to a treatable disease of ligand insufficiency.
Keywords: Colorectal cancer, GUCY2C, hormone replacement, immunotherapy, linaclotide
2.0 Current Challenges in Colorectal Cancer
Colorectal cancer (CRC) remains the fourth most diagnosed cancer, and the second leading cause of cancer death in the United States [1]. Worldwide, it accounts for as many as 1.2 million new cases and 600,000 deaths per year [2]. CRC incidence and mortality has declined since the 1980s, paralleling adoption of screening; however, available screening methods (e.g. fecal occult blood, flexible sigmoidoscopy, colonoscopy) vary in terms of sensitivity and specificity, risks, and evidence supporting their implementation. Unfortunately, no screening method has proven to reduce all-cause mortality [3]. Colonoscopy has become the gold standard, enabling removal of dysplastic lesions before progression to cancer. Yet, sensitivity decreases for lesions <1 cm, and the adenoma detection rate and completeness of polyp removal varies between providers [4, 5]. Indeed, the superiority of colonoscopy over other screening approaches recently was questioned [6].
Complicating limitations of current screening tools, widespread and ill-defined risk factors for CRC make it difficult to develop screening guidelines. A fraction of new tumors arise in patients with known genetic syndromes (e.g. Lynch syndrome, familial adenomatous polyposis); however >90% of cases are thought to be sporadic [7]. Age and family history play a role, hence colonoscopy is indicated at age 50 for patients with average risk, and at age 40 for patients with a first degree relative diagnosed at a young age [2]. But other risk factors of unclear significance include high-fat diets, tobacco smoking, alcohol consumption, and body mass index [8, 9]. Patients with inflammatory conditions of the bowel, such as ulcerative colitis, are particularly predisposed to CRC [10], and several studies have suggested benefits from low dose non-steroidal anti-inflammatory drugs [11, 12, 13]. However, a pathophysiological link between these risk factors and tumorigenesis remains unclear, delaying the development of disease prevention schemes.
Despite progress in early detection and treatment, ~25% of patients present with late stage disease [14]. Many promising agents for metastatic disease have become clinically available (e.g. tyrosine kinase inhibitors, epidermal growth factor inhibitors, anti-angiogenesis agents, etc.), but the five-year survival rate for this population remains only 11.7% [2, 14]. Hence, strategies for prevention, detection, and treatment of primary and metastatic CRC are needed.
3.0 Genetic Basis of Colorectal Cancer
CRCs develop slowly, often requiring over a decade to accumulate mutations required for epithelial transformation (providing a long window for detection). The average tumor contains 90 different mutations [15], but despite this genetic heterogeneity, 70–80% of sporadic (non-hereditary) colorectal tumors arise by a series of mutations typically described as the adenoma-carcinoma sequence [7, 16]. Canonically, this sequence begins with an inactivating mutation of the adenomatous polyposis coli (APC) tumor suppressor gene. The APC protein normally inhibits the accumulation of β-catenin, the downstream mediator of the Wnt signaling pathway. APC loss enables phosphorylation and aberrant translocation of β-catenin to the nucleus in the absence of the Wnt ligand, where it participates in oncogenic transcription (activation of c-MYC and cyclin D1) [17]. Subsequent activating mutations of oncogenes (e.g. KRAS), and deactivating mutations of tumor suppressors (e.g. TP53) characterize the progression from normal epithelium, to adenoma, to carcinoma [7, 16]. About 15% of sporadic tumors arise by a different mechanism, characterized by dysfunction of DNA mismatch repair genes, such as MLH1 and MSH2. Here, defective DNA repair permits accumulation of mutations in short repeated genetic sequences (microsatellite sequences), resulting in a microsatellite instability phenotype. Finally, a third tumorigenic pathway is characterized by aberrant gene silencing via CpG island methylation, an epigenetic phenomenon. These adenomas often harbor mutational activation of the BRAF oncogene, and exhibit a characteristic sessile serrated architecture [7, 16]. Many tumors contain elements of more than one pathway; for example, hypermethylation of the MLH1 mismatch repair gene contributes to a large subset of microsatellite unstable tumors.
By comparison, tumors arising from hereditary CRC syndromes occur less frequently (3-5% of cases), and arise from germline, rather than somatic, mutations in the aforementioned pathways [7, 16]. Hereditary non-polyposis CRC (HNPCC, or Lynch syndrome) occurs most commonly, and arises from mismatch repair gene mutation. Familial adenomatous polyposis (FAP) arises from germline mutations of APC, resulting in thousands of colonic polyps early in life and 100% risk of cancer by age 40.
4.0 GUCY2C as a Target in Colorectal Cancer
Although a wealth of genetic and epigenetic changes have been associated with intestinal transformation, a common causative agent has yet to be found. Recent studies have defined a role for the intestinal surface receptor, guanylate cyclase C (GUCY2C), as a tumor suppressor involved in the earliest stages of transformation.
GUCY2C belongs to the particulate guanylate cyclase class of receptors, and appears on the apical brush border of the intestinal epithelium [18]. Early studies defined its role in regulating luminal secretion, specifically as the receptor for the bacterial heat-stable enterotoxin, ST, the causative agent of traveler’s diarrhea [19]. ST binding to the extracellular domain of GUCY2C activates the intracellular catalytic domain, converting GTP to cyclic GMP [18]. This second messenger activates cGMP-dependent protein kinase II (PKGII), leading to downstream phosphorylation and activation events, including water and electrolyte secretion via the cystic fibrosis transmembrane conductance regulator (CFTR) [18]. Predictably, persons with activating or deactivating mutations of GUCY2C exhibit intestinal hyper- or hypo- secretory syndromes, respectively [20, 21]. Our understanding of GUCY2C-induced signaling has since expanded to include regulatory roles in epithelial renewal along the crypt-villus axis [22, 23], GI barrier integrity [24, 25], injury response [26, 27], and the gut-brain satiety axis [28, 29]. Importantly, GUCY2C is densely expressed throughout the intestine, and overexpressed by tumor tissue, features that can be exploited for diagnostic and therapeutic goals [30, 31, 32].
Endogenous GUCY2C ligands, the peptides guanylin and uroguanylin, are among the most commonly lost gene products in mouse models and human CRC [33, 34, 35]. In a study of 300 patients, >85% of colorectal tumors exhibited disappearance of guanylin mRNA and protein compared to normal adjacent tissue [33]. This loss occurs early in intestinal transformation, suggesting that an intact GUCY2C signaling axis opposes tumorigenesis [23, 36, 37]. Indeed, mice in which GUCY2C expression is eliminated (Gucy2c−/−) exhibit a tumorigenic phenotype, including epithelial dysfunction, DNA mutation, cellular proliferation and migration, and metabolic reprogramming (Figure 1) [24, 37]. Interestingly, diet-induced obesity in mice also leads to guanylin loss and tumor formation, suggesting a mechanistic link between CRC and a well-described risk factor, obesity [28]. Conversely, GUCY2C activating ligands and downstream mediators suppress oncogenic drivers (e.g. pRb, cyclin D1, B-catenin, pAKT) and increase tumor suppressors (e.g. p21, p27) [23, 25, 38, 39]. These findings underlie the paracrine hormone hypothesis, whereby CRC arises from an environment of ligand loss and functional GUCY2C inactivation. This pathophysiological paradigm could transform colon cancer from an irreversible disease of genetic origin, to a treatable disorder of ligand insufficiency. Recent FDA-approval of the GUCY2C ligand, linaclotide, and the entrance of two others in the clinical pipeline, makes it feasible to test ligand supplementation for chemoprevention of CRC in humans.
Figure 1.
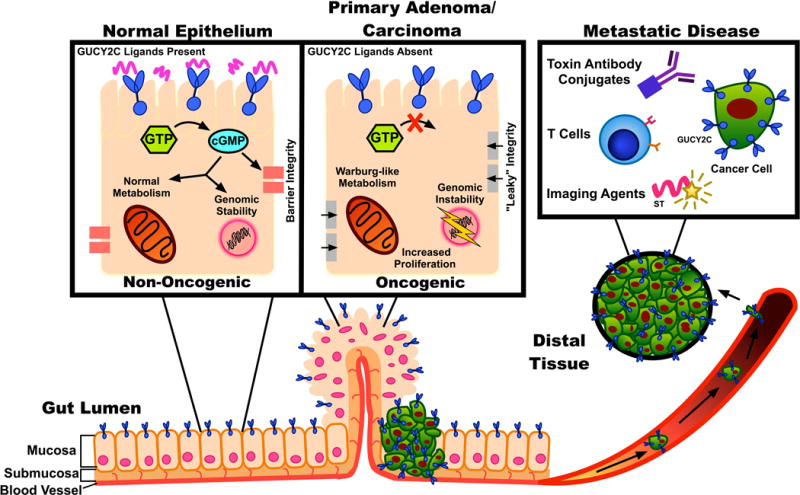
GUCY2C-targeting approaches. GUCY2C is an intestinal mucosal receptor involved in epithelial homeostasis and tumor suppression. Loss of GUCY2C ligands, silencing GUCY2C signaling, is an early step in tumorigenesis, leading to oncogenic signaling, metabolic reprogramming, loss of epithelial barrier integrity, and cell proliferation. Oral replacement of lost ligands is a strategy to prevent colorectal cancer. Furthermore, colorectal cancer cells overexpress GUCY2C, providing a target for immunotoxin therapy or T cell-mediated immunotherapies to eliminate metastases. Imaging agents also can target GUCY2C, improving disease detection and the monitoring of treatment progress.
5.0 GUCY2C Agonists for Colorectal Cancer Prevention
GUCY2C peptide agonists available for chemoprevention of primary colorectal tumors include the endogenous peptides guanylin and uroguanylin, bacterial diarrheagenic heat-stable enterotoxins (STs), and the synthetic peptides linaclotide, plecanatide, and dolcanatide. These ligands share structural homologies and conserved mechanisms of action through GUCY2C activation and downstream cGMP production.
5.1 Endogenous Ligands
Guanylin and uroguanylin, the endogenous ligands for GUCY2C in the intestine, were first described in the early 1990s [40, 41]. They are produced and stored as propeptides, and undergo processing to their mature 15-mer (guanylin) or 16-mer (uroguanylin) forms. The mature peptides are thought to act on GUCY2C in a paracrine fashion to maintain epithelial homeostasis and fluid secretion [18]. Uroguanylin also serves an endocrine role in gut-brain satiety signaling [28]. The peptides exhibit complimentary roles along the axis of the intestine, with maximal uroguanylin expression in the small intestine, and guanylin in the large intestine [42]. However, even after two decades of study, the cells of origin remain controversial, and may continue to evolve as we elucidate the multiple roles of the GUCY2C signaling axis [43, 44, 45, 46]. Interestingly, despite >50% sequence homology, uroguanylin is principally active in acidic pH and guanylin in basic pH, further reflecting regional specificity [47].
The disappearance of guanylin/uroguanylin early in colorectal tumorigenesis reflects a tumor suppressive function. Preclinical studies in mice have demonstrated the potential of therapeutic ligand replacement. For example, in Apcmin/+ mice (a CRC model) oral uroguanylin supplementation inhibited tumorigenesis [48]. In another model, mice genetically modified to overexpress guanylin were resistant to DSS-induced colitis [25]. Further, it was recently shown that diet-induced obesity suppressed guanylin expression in mice, leading to tumorigenesis, and specific enforcement of guanylin expression prevented obesity-related tumors [49]. In all of these studies, no adverse effects were observed over the lifetime of the mice.
5.2 Enterotoxins
Heat-stable enterotoxins (STs) are produced by several diarrheagenic bacteria, including enterotoxigenic E. coli, K. pneumonia, V. cholera, and Y. enterocolitica [18]. First described as GUCY2C agonists in 1990, STs include a family of peptides with a conserved C-terminal region [19]. Structurally similar to guanylin and uroguanylin, STs contain an additional disulfide bond, contributing to their canonical heat stability and increased receptor binding affinity [18]. Ligand-receptor binding activates GUCY2C, leading to CFTR-driven fluid and electrolyte transport into the intestinal lumen, manifesting as secretory diarrhea. Enterotoxigenic E. coli is endemic in developing countries with poor sanitation infrastructure. Interestingly, these regions have a lower incidence of CRC, which may reflect life-long exposure to STs, increased GUCY2C activation, and suppression of epithelial dysplasia [39, 50].
5.3 Synthetic Peptides
Synthetic peptides sharing homology with natural GUCY2C ligands target the secretory function of GUCY2C for therapeutic purposes. The first agent developed, linaclotide (Ironwood Pharmaceuticals, Inc., Cambridge, MA), is an ST analog approved by the FDA for the treatment of chronic idiopathic constipation (CIC) and constipation-predominant irritable bowel syndrome (IBS-C). Linaclotide binds GUCY2C, inducing cGMP accumulation and fluid secretion. Double-blind, placebo-controlled, phase III clinical trials were completed for patients with IBS-C (MCP-103-302 and LIN-MD-31) and CIC (MCP-103-303 and LIN-MD-01) [51, 52, 53]. Linaclotide met all primary endpoints, significantly reducing abdominal symptoms and severity of constipation. No differences in serious adverse events were observed between linaclotide and placebo. The most commonly reported side effect was diarrhea, an effect predicted by its mechanism of action. New agents, plecanatide and dolcanatide (Synergy Pharmaceuticals Inc., New York, NY), are uroguanylin analogs with increased potency [54]. Like linaclotide, these agents agonize GUCY2C and stimulate cGMP production. They reduced disease severity (e.g. weight loss, inflammatory infiltrate, destruction of crypt architecture) in pharmacologic and genetic murine models of colitis [54]. In a phase I trial of 72 healthy volunteers, up to 48.6 mg of plecanatide was safe and well-tolerated [55]. Currently, plecanatide is in phase III clinical trials for CIC and IBS-C [56].
Given their safety in human trials, these compounds could be used as oral-chemopreventive agents for CRC. In principle, exogenous GUCY2C ligand administration would reconstitute the tumor-suppressing GUCY2C signaling axis, preventing colorectal tumorigenesis. A phase I trial is underway to identify oral linaclotide dosing regimens that stimulate GUCY2C in the rectum. Study participants receive a single oral dose of linaclotide daily for 7 days, and then are assessed for increases in cGMP levels in rectal biopsy, as well as safety and tolerability (Linaclotide Acetate in Preventing Colorectal Cancer in Healthy Volunteers, clinicaltrials.gov NCT01950403).
6.0 GUCY2C-Targeted Immunotherapies for Metastatic Colorectal Cancer
While prevention of CRC remains the clinical ideal, therapeutic strategies for advanced disease are also needed. A growing body of literature endorses immunotherapy for cancer treatment. The immune system has a remarkable ability to suppress neoplastic proliferation, as demonstrated by heightened cancer risk in immunocompromised patients [57, 58]. In part, this risk reflects diminished immune control of oncogenic viruses (e.g. human herpes virus 8 and Kaposi sarcoma, hepatitis B and C viruses and liver cancer, or Epstein-Barr virus and Hodgkin’s lymphoma) [58]; however, these patients also are predisposed to cancers without known infectious etiologies (e.g. melanoma, thyroid, and colorectal cancers) [58]. Instead, these are thought to arise from poor immune surveillance against cancer cells in tumors and the circulation. For example, the presence of lymphocytes in CRC tumors is associated with delayed metastasis and prolonged survival [59]. Tumor cells have a propensity to bypass or overcome these natural defense mechanisms, creating an unmet need for therapies that improve the immune response to cancer antigens (e.g. vaccines, adoptive T cell therapy) or target cancer cells directly (e.g. immunotoxins) [60, 61].
Effective CRC immunotherapies require antigenic targets that maximize immunogenicity and minimize autoimmunity. The most explored target, the glycoprotein carcinoembryonic antigen (CEA), is upregulated in CRC, but also appears in organs outside the GI tract, leading to potential autoimmunity and immunological tolerance [62, 63]. In contrast, GUCY2C has unique anatomic and biological characteristics that appear to circumvent these issues. GUCY2C is expressed by intestinal mucosa from the small bowel to the rectum, and is overexpressed in primary and metastatic colorectal neoplasms [30, 31, 32]. Further, expression is largely restricted to the luminal aspect of the GI mucosa, and its extracellular domain is antigenically distinct from other members of the guanylate cyclase family found in other tissues [64, 65, 66]. Importantly, GUCY2C resides in an immune privileged compartment, with minimal exposure to the systemic immune response [64, 65, 66]. Limited cross-talk between systemic and mucosal immune elements protects normal mucosa expressing GUCY2C from autoimmune toxicity, while also limiting systemic tolerance to the antigen [64, 65, 66]. These advantages have led to the exploration of several GUCY2C-targeted immunotherapeutic strategies (Figure 1).
6.1 Vaccines
Similar to the yearly-recommended flu vaccine, cancer vaccines stimulate the immune system to destroy cancer cells by targeting tumor-specific antigens, while also generating long-lasting immunity [60]. Viral vector vaccines, engineered to contain the genes for cancer antigens, enhance antitumor immunity by stimulating the expansion of adaptive immune system elements, namely Type 1 CD4+ T-helper cells, cytotoxic CD8+ T cells and antibodies [61]. This paradigm forms the basis for a GUCY2C-targeted vaccine, designed to elicit immune responses to metastatic CRC.
The first GUCY2C-specific vaccine incorporated replication-deficient type 5 recombinant adenovirus (Ad5) encoding the extracellular domain of GUCY2C (Ad5-GUCY2C) [64, 65, 66]. In a murine pre-clinical proof-of-concept study, the vaccine stimulated a GUCY2C-specific CD8+ cytotoxic T-cell response, which killed GUCY2C-expressing colon cancer cells. Remarkably, survival in mice with lung and liver metastases improved, without signs of inflammatory bowel disease, organ or metabolic dysfunction, or autoimmune tissue damage [64]. Interestingly, the vaccine produced strong CD4+ T-cell, CD8+ T-cell, and B-cell responses in Gucy2c−/− mice, but produced only a modest CD8+ T-cell response in Gucy2c+/+ mice, which was attributed to GUCY2C-specific CD4+ T-cell tolerance [66]. To overcome this, the vaccine was modified to include an immunogenic T-helper epitope from foreign protein [66, 67]. This new vector reconstituted CD4+ T-cell, CD8+ T-cell, and memory responses [66]. This was the first demonstration that selective CD4+ T-cell tolerance blocks GUCY2C-specific immunity and memory responses. Importantly, this paradigm may extend to other antigens, including those in melanoma and breast cancer, suggesting that overcoming CD4+ T-cell tolerance may be a requirement in many cancer vaccine approaches [66, 68, 69].
Preliminary results were recently reported for a phase I clinical trial exploring the safety and immunogenicity of this vaccination scheme in stage I/II colon cancer patients (clincialtrials.gov NCT01972737)[70]. The vaccine is analogous to the murine vaccine, but encodes the human GUCY2C extracellular domain fused to the T-helper epitope PAn DR Epitope (Ad5-GUCY2C-PADRE). Preliminary findings are consistent with the pre-clinical studies, with patients responding to the vaccine by producing GUCY2C-specific CD8+ T-cell and B-cell responses, but not a CD4+ T-cell response, suggesting that selective CD4+ T-cell tolerance governs GUCY2C-specific immune responses in humans, as well as mice [70]. Moreover, like preclinical studies, the vaccine did not induce GUCY2C-targeted toxicity in any GUCY2C-expressing tissue. Importantly, these first findings in humans support Ad5-GUCY2C-PADRE as a promising therapeutic approach for patients with GUCY2C-expressing malignancies.
6.2 Adoptive T Cell Therapies
The past decade has witnessed remarkable progress in an immunotherapy approach known as adoptive cell therapy (ACT). Rather than employing a vaccine or other drug to induce an immune response within a patient, this strategy employs ex vivo tissue culture to expand naturally-occurring immune effectors or create them de novo for administration to the patient [71]. One approach involves boosting the activity of naturally occurring immune responses present in tumors, called tumor-infiltrating lymphocytes (TILs), which are suppressed by the tumor microenvironment [61]. TILs can be isolated from patient tumors, activated and expanded ex vivo, and reintroduced to the patient, bypassing immunosuppressive elements. Another approach involves ex vivo genetic manipulation of peripheral blood lymphocytes to retarget them to tumors by expressing cancer-specific T-cell receptors (TCRs). Both TIL and TCR-gene transfer approaches have been efficacious in mouse models and humans with metastatic melanoma [72, 73, 74, 75, 76]. An ACT alternative approach employs chimeric antigen receptors (CARs). Here, T lymphocytes are modified to express an engineered receptor comprised of intracellular T-cell signaling motifs and an extracellular antibody domain that recognizes antigens in an MHC/HLA-independent fashion [77, 78]. CD19-targeted CAR-T cells have shown remarkable promise in the treatment of refractory leukemia in humans [79, 80, 81]. Because CARs can theoretically employ antibodies targeting any cell surface antigen, ACT approaches may be vastly expanded and personalized for other malignancies, including solid tumors.
While efficacious for certain cancers, ACT has had mixed results in CRC patients. A recent report demonstrated regression of lung metastases in a patient with colorectal cancer injected with TILs targeting mutant KRAS [82]. However, prior trials of ACT targeting CEA and Her-2 resulted in adverse autoimmune effects, including death [83, 84]. In contrast, GUC2YC-targeted CAR-T cells may target metastatic CRC cells without destroying healthy tissue, given the anatomical compartmentalization of GUCY2C on the luminal aspect of the intestine, beyond access by CAR-T cells, which recognize native GUCY2C. As a proof-of-concept, CD8+ T cells bearing CARs targeted to mouse GUCY2C lysed murine colon cancer cells, eliminated colorectal cancer metastases, and prolonged survival in a mouse model of metastatic CRC, without toxicity [85].
6.3 GUCY2C-targeted Immunotoxins
Antibodies offer several advantages as an immunotherapeutic tool, including immunomodulatory capacity, interference in ligand-receptor interactions, and relative ease of mass-production. Indeed, antibody-therapies are well-established in the clinic, with over 50 FDA-approved therapeutics [86]. For example, the monoclonal antibody bevacizumab (Avastin), which targets the vascular endothelial growth factor pathway, is FDA-approved as first line treatment for metastatic CRC. Others include cetuximab (Erbitux) and panitumumab (Vectibix), antibodies which bind to the extracellular domain of the epidermal growth factor receptor, blocking ligand binding and tumorigenic signaling [87, 88]. Still, these agents offer limited improvements in survival: bevacizumab was approved as a first line agent for metastatic CRC in 2004, but only increased median survival from 15 to 20 months [87].
The next generation of antibody therapies, antibody-drug conjugates (ADCs) enable targeted delivery of cytotoxic agents to specific tissues [89, 90]. ADCs are engineered by linking a cytotoxin to a monoclonal antibody, facilitating targeting to cells expressing cancer antigens, endocytic uptake, and intracellular delivery of the toxic payload. Conceptually, the targeted nature of ADCs reduces systemic exposure, and endocytic uptake reduces drug resistance by P-glycoprotein efflux pump, two of the pitfalls of existing chemotherapeutics [89]. However, as a relatively new drug class, ADCs historically have proven difficult to optimize, and have been associated with significant side effects due to non-specific targeting [90]. For this reason, only two have achieved FDA approval, adotrastuzumab emtasine and brentuximab vedotin, although several others have entered clinical trials.
Recently, a model GUCY2C-targeted ADC was devised, consisting of a GUCY2C antibody, ricin toxin payload, and cleavable disulfide linker (4-succinimidyloxycarbonyl-α-methyl-α-[2-pyridyldithio]- toluene; SMPT) [91]. The ADC specifically targeted GUCY2C, underwent endocytosis, trafficked to lysosomes, and delivered a toxic payload to colon cancer cells [91]. In mice with CRC lung metastases, the ADC prolonged survival without compromising normal tissue [91]. A subsequent phase I clinical trial was recently completed, examining a human IgG1 monoclonal antibody to GUCY2C conjugated via a protease-cleavable linker to monomethyl auristatin E, an anti-microtubule agent. The ADC (TAK-264) was tested for safety and tolerability in 41 patients with GUCY2C-expressing metastatic gastrointestinal disease. Four patients in the highest dose group experienced dose-limiting toxicity (neutropenia), but the safety profile was deemed manageable, and preliminary data suggest antitumor activity [92].
7.0 GUCY2C as a Biomarker in Colorectal Cancer Detection
Features that elevate GUCY2C as a target for immunotherapy (overexpression by tumors, limited expression outside the gastrointestinal tract [30, 31, 32]) also have value for cancer detection and staging. Disease stage remains a key prognostic and therapeutic factor in the management of patients with CRC [93]. Whereas the resection of tumors restricted to the bowel wall (stage II) is often curative, patients with metastasis of tumor cells to lymph nodes (stage III) experience recurrence rates of up to 50% with surgery alone [2]. Although adjuvant chemotherapy remains controversial at stage II, progression to stage III is an indication for chemotherapy, increasing survival as much as 15% [2, 94]. Unfortunately, traditional staging by histopathological examination of lymph node tissue remains insensitive, leading to missed metastases, patient under-staging, and inappropriate patient management. For example, less than 0.01% of available tissue is typically reviewed, and as many as 25% of supposedly lymph node-negative (pN0) patients die of disease recurrence [93], suggesting undetected metastatic cells.
7.1 GUCY2C mRNA as a biomarker
The expression profile of GUCY2C makes it uniquely suited for the staging of primary colorectal tumors and occult metastases [32, 95]. In a blinded multicenter prospective trial, 2570 lymph nodes from 257 pN0 colorectal cancer patients were examined for GUCY2C mRNA by quantitative real-time PCR [96]. Patients were followed for 24 months, and the primary outcome measure was time to recurrence. Remarkably, 87% of patients considered stage II by traditional histopathological techniques were found to harbor occult metastases by GUCY2C molecular staging, correlating with earlier time to recurrence. Furthermore, qRT-PCR was used to stratify patients by tumor burden, based on the number of positive nodes and relative GUCY2C expression across nodes [97]. For the first time, it was shown that patients with greater occult tumor burden had a greater risk of recurrence, and this method could be used to stratify patients based on prognostic risk. Importantly, molecular staging by GUCY2C RT-PCR has been validated across multiple users and laboratories and may replace conventional histopathologic evaluation for staging and therapeutic decision making in colorectal cancer [98, 99, 100].
7.2 GUCY2C as a target for diagnostic imaging agents
Positron emission tomography has become a mainstay for staging CRC and monitoring treatment response [101]. This method capitalizes on the increased metabolic demand, and therefore increased glycolysis, by cancer cells. Cancer cells take up the glucose analog, 2-[18F]fluoro-2-deoxy-D-glucose (FDG) to a greater extent than surrounding normal tissue, allowing visualization by PET. However, glucose requirements by other tissues decreases specificity; false positives (due to inflammation, surgery, diverticulitis, etc.) lead to unnecessary follow-up colonoscopy or inappropriate staging [101]. Alternative imaging modalities using molecular targets, rather than metabolic patterns, may address these issues [102]. Targeting imaging probes to GUCY2C offers a sensitive means of detecting tumors derived from intestinal epithelium. Conjugates of radionuclides and GUCY2C ligands (e.g. ST, uroguanylin analogs) specifically target GUCY2C-expressing xenografts [103, 104]. These agents can be visualized with gamma camera scintigraphy, and accurately differentiate tumors of gastrointestinal origin from surrounding tissue [103, 104]. Further, GUCY2C-directed antibodies accumulate in cells via clathrin-mediated endocytosis of the antibody-receptor complex, with the potential to amplify delivery of imaging agents or therapeutic cargo [91].
8.0 Conclusion
Despite improvements in CRC screening, incidence and mortality are among the highest of all cancers, and while the genetic basis has been well described, therapeutic targets remain elusive. The intestinal receptor GUCY2C has emerged as a target uniquely suited for prevention, therapy, and diagnostics. Its role as a tumor suppressor, inactivated by ligand loss early in tumorigenesis, suggests a novel disease prevention paradigm focused on GUCY2C ligand replacement. A clinical program is underway ultimately to test this strategy with the FDA-approved agent, linaclotide, and other promising agents are emerging. Further, its expression profile in the intestinal lumen and metastatic CRC tumors offers an ideal target for a rapidly expanding array of cancer immunotherapies, including vaccines, T-cell therapies, and antibody-drug conjugates. Finally, GUCY2C can be exploited as a sensitive biomarker for the detection and staging of CRC. Translation to the clinics is underway on multiple fronts. Novel approaches targeting GUCY2C could revolutionize the treatment of CRC.
9.0 Expert Commentary
Cancer research remains an ever-changing field, with exciting advances in the past few decades that have shifted traditional treatment approaches. Preventative strategies are the clinical ideal and successes have been achieved for several neoplasms, such as the decreased incidence of gastric cancer following the identification and reduction of H. pylori infections [1]. Likewise, colonoscopy has reduced the incidence of colorectal cancers by eliminating lesions before they become invasive and metastatic.
While screening has reduced the incidence of colorectal cancer, it remains the fourth most diagnosed cancer, and the second leading cause of cancer death, with a 5-year survival <15% in metastatic disease. GUCY2C appears to play a pivotal role in epithelial homeostasis, including intestinal barrier integrity and obesity, known risk factors for colon cancer, suggesting novel molecular pathways that may be pharmacologically targetable. The revelation of GUCY2C ligand loss and receptor silencing early in tumorigenesis may have a transformative impact, supported by the exploration of multiple translational avenues. With regards to cancer prevention, reactivation of the GUCY2C tumor suppressor pathway with exogenous peptides has shown promise in pre-clinical models. Though initially formulated for the treatment of irritable bowel disease and chronic constipation, the translation of the FDA-approved GUCY2C ligand, linaclotide, to CRC is feasible, as safety and efficacy are already established. However, long-term effects of linaclotide and other synthetic GUCY2C ligands have not yet been defined and longitudinal chemoprevention trials are required.
In the context of CRC treatment, the identification of GUCY2C as a biomarker and cell-surface target of metastatic CRC cells may usher in new biologics and immunotherapies. GUCY2C-targeted vaccines and antibody-drug conjugates have advanced into clinical testing. Further, detection of GUCY2C mRNA in lymph nodes offers a sensitive means of staging the disease, enabling more accurate identification of patients at risk for disease recurrence. Appropriate intervention in patients with previously unrecognized occult metastases may improve survival, especially as targeted therapeutics enter the clinic.
Another area of interest and debate is the nature of cancer inception, and implications for targeting strategies. Traditionally, disease recurrence and treatment failure are thought to result from the inevitable acquisition of mutations and epigenetic changes that allow cancer cells to evade destruction [105]. However, evidence increasingly indicates the presence of “cancer stem cells”, a subpopulation of cancer cells with stem-like characteristics (e.g., tumorigenesis, self-renewal, and differentiation) that underlie metastasis, recurrence, and chemoresistance [106]. Identification and targeting of cancer stem cell markers could enhance CRC therapies. For example, a recent study demonstrated co-expression of CD133 and the breast cancer resistance protein (BCRP)/ATP-binding cassette subfamily G member 2 (ABCG2) by human colorectal tumors [107]. Downregulation of ABCG2 inhibited self-renewal capabilities and enhanced chemotherapeutic effects in double-positive colon adenocarcinoma cells. Dual-therapies, potentially targeting a universal CRC marker like GUCY2C as well as a marker of the stem cell subpopulation may be a new translational avenue. As we better-characterize these neoplastic markers, therapeutic strategies will continue to evolve.
10.0 Five Year View
A large body of work across multiple laboratories supports the hypothesis that GUCY2C ligand loss is a necessary step in tumorigenesis. In the next five years, the molecular steps in this process likely will be defined, potentially leading to new clinical targets. Furthermore, results of the first trials translating GUCY2C-targeting schemes to the clinic will become available, including the effectiveness of GUCY2C ligand supplementation with linaclotide, a GUCY2C-targeted vaccine, a GUCY2C-targeted antibody-drug conjugate, and GUCY2C-targeted CAR-T cells. Additional GUCY2C ligands (dolcanatide and plecanatide) entering the pipeline will likely be explored for similar use as chemoprevention agents. Ultimately, the next five years should provide the first insights into the potential for GUCY2C-targeting to influence human colorectal cancer outcomes.
Key Issues.
The gastrointestinal epithelial receptor, guanylate cyclase C (GUCY2C) has been described as a novel tumor suppressor and reliable biomarker of colorectal cancer.
Endogenous GUCY2C ligand loss has been widely-described as an early step in colorectal tumorigenesis, suggesting a therapeutic strategy of ligand replacement for chemoprevention. The GUCY2C agonist linaclotide is FDA approved for other indications and a phase I clinical trial examining its use for colorectal cancer prevention is underway.
GUCY2C is overexpressed in colorectal cancer metastases and several immunotherapies targeting GUCY2C are being explored, including adoptive T-cell therapy with GUCY2C-targeted CAR-T cells, a viral vector vaccine, and a GUCY2C-targeted antibody-drug conjugate. The latter two are currently in early human trials.
Cancer staging and imaging strategies targeting GUCY2C also are being explored. GUCY2C mRNA is a sensitive biomarker of occult lymph node metastases, improving cancer detection and staging.
References
Papers of interest (*)
Papers of considerable interest (**)
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Lin JS, Piper MA, Perdue LA, Rutter CM, Webber EM, O’Connor E, Smith N, Whitlock EP. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576–94. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 4.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg DS, Barkun A, Turner BJ. Colorectal Cancer Screening in the United States: What Is the Best FIT? Ann Intern Med. 2016 doi: 10.7326/M16-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogaert J, Prenen H. Molecular genetics of colorectal cancer. Ann Gastroenterol. 2014;27:9–14. [PMC free article] [PubMed] [Google Scholar]
- 8.Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7:105–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 10.Kinugasa T, Akagi Y. Status of colitis-associated cancer in ulcerative colitis. World J Gastrointest Oncol. 2016;8:351–7. doi: 10.4251/wjgo.v8.i4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106:1340–50. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friis S, Riis AH, Erichsen R, Baron JA, Sorensen HT. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk: A Population-Based, Case-Control Study. Ann Intern Med. 2015;163:347–55. doi: 10.7326/M15-0039. [DOI] [PubMed] [Google Scholar]
- 13.Hamoya T, Fujii G, Miyamoto S, Takahashi M, Totsuka Y, Wakabayashi K, Toshima J, Mutoh M. Effects of NSAIDs on the risk factors of colorectal cancer: a mini review. Genes Environ. 2016;38:6. doi: 10.1186/s41021-016-0033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai SG, Fuloria J. Novel therapeutic agents in the treatment of metastatic colorectal cancer. World J Gastrointest Oncol. 2016;8:99–104. doi: 10.4251/wjgo.v8.i1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 16.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 17.Gerlach JP, Emmink BL, Nojima H, Kranenburg O, Maurice MM. Wnt signaling induces accumulation of phosphorylated beta-catenin in two distinct cytosolic complexes. Open Biol. 2014;4:140120. doi: 10.1098/rsob.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev. 2016;96:751–804. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 19.Schulz S, Green CK, Yuen PS, Garbers DL. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990;63:941–8. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 20.Fiskerstrand T, Arshad N, Haukanes BI, Tronstad RR, Pham KD, Johansson S, Havik B, Tonder SL, Levy SE, Brackman D, Boman H, Biswas KH, Apold J, Hovdenak N, Visweswariah SS, Knappskog PM. Familial diarrhea syndrome caused by an activating GUCY2C mutation. N Engl J Med. 2012;366:1586–95. doi: 10.1056/NEJMoa1110132. [DOI] [PubMed] [Google Scholar]
- 21.Romi H, Cohen I, Landau D, Alkrinawi S, Yerushalmi B, Hershkovitz R, Newman-Heiman N, Cutting GR, Ofir R, Sivan S, Birk OS. Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C. Am J Hum Genet. 2012;90:893–9. doi: 10.1016/j.ajhg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu N, Saha S, Khan I, Ramachandra SG, Visweswariah SS. Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem. 2014;289:581–93. doi: 10.1074/jbc.M113.511311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase C restricts the proliferating compartment in intestine. Am J Pathol. 2007;171:1847–58. doi: 10.2353/ajpath.2007.070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One. 2011;6:e16139. doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S, Waldman SA. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One. 2012;7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenna O, Bruland T, Furnes MW, Granlund A, Drozdov I, Emgard J, Bronstad G, Kidd M, Sandvik AK, Gustafsson BI. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol. 2015;50:1241–52. doi: 10.3109/00365521.2015.1038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garin-Laflam MP, Steinbrecher KA, Rudolph JA, Mao J, Cohen MB. Activation of guanylate cyclase C signaling pathway protects intestinal epithelial cells from acute radiation-induced apoptosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G740–9. doi: 10.1152/ajpgi.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121:3578–88. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim GW, Lin JE, Snook AE, Aing AS, Merlino DJ, Li P, Waldman SA. Calorie-induced ER stress suppresses uroguanylin satiety signaling in diet-induced obesity. Nutr Diabetes. 2016;6:e211. doi: 10.1038/nutd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005;36:170–9. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Carrithers SL, Barber MT, Biswas S, Parkinson SJ, Park PK, Goldstein SD, Waldman SA. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996;93:14827–32. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cagir B, Gelmann A, Park J, Fava T, Tankelevitch A, Bittner EW, Weaver EJ, Palazzo JP, Weinberg D, Fry RD, Waldman SA. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999;131:805–12. doi: 10.7326/0003-4819-131-11-199912070-00002. [DOI] [PubMed] [Google Scholar]
- 33**.Wilson C, Lin JE, Li P, Snook AE, Gong J, Sato T, Liu C, Girondo MA, Rui H, Hyslop T, Waldman SA. The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:2328–37. doi: 10.1158/1055-9965.EPI-14-0440. Demonstrates near universal loss of the GUCY2C ligand, guanylin, in primary colorectal tumors compared to matched normal adjacent tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun. 2000;273:225–30. doi: 10.1006/bbrc.2000.2917. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 36.Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol. 2002;161:2169–78. doi: 10.1016/S0002-9440(10)64494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Baran AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. Describes the mechanism of GUCY2C as a colorectal tumor suppressor. [DOI] [PubMed] [Google Scholar]
- 38*.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, Gibbons AV, Marszlowicz G, Pitari GM, Waldman SA. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–54. doi: 10.1053/j.gastro.2009.08.064. Describes the mechanism of GUCY2C as a colorectal tumor suppressor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–51. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992;89:947–51. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, et al. Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1993;90:10464–8. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology. 2000;141:3210–24. doi: 10.1210/endo.141.9.7644. [DOI] [PubMed] [Google Scholar]
- 43.Brenna O, Furnes MW, Munkvold B, Kidd M, Sandvik AK, Gustafsson BI. Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa. Cell Tissue Res. 2016;365:331–41. doi: 10.1007/s00441-016-2393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikpa PT, Sleddens HF, Steinbrecher KA, Peppelenbosch MP, de Jonge HR, Smits R, Bijvelds MJ. Guanylin and uroguanylin are produced by mouse intestinal epithelial cells of columnar and secretory lineage. Histochem Cell Biol. 2016 doi: 10.1007/s00418-016-1453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perkins A, Goy MF, Li Z. Uroguanylin is expressed by enterochromaffin cells in the rat gastrointestinal tract. Gastroenterology. 1997;113:1007–14. doi: 10.1016/s0016-5085(97)70198-7. [DOI] [PubMed] [Google Scholar]
- 46.Cohen MB, Witte DP, Hawkins JA, Currie MG. Immunohistochemical localization of guanylin in the rat small intestine and colon. Biochem Biophys Res Commun. 1995;209:803–8. doi: 10.1006/bbrc.1995.1571. [DOI] [PubMed] [Google Scholar]
- 47.Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR. Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci U S A. 1997;94:2705–10. doi: 10.1073/pnas.94.6.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shailubhai K, Yu HH, Karunanandaa K, Wang JY, Eber SL, Wang Y, Joo NS, Kim HD, Miedema BW, Abbas SZ, Boddupalli SS, Currie MG, Forte LR. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60:5151–7. [PubMed] [Google Scholar]
- 49.Lin JE, Colon-Gonzalez F, Blomain E, Kim GW, Aing A, Stoecker B, Rock J, Snook AE, Zhan T, Hyslop TM, Tomczak M, Blumberg RS, Waldman SA. Obesity-Induced Colorectal Cancer Is Driven by Caloric Silencing of the Guanylin-GUCY2C Paracrine Signaling Axis. Cancer Res. 2016;76:339–46. doi: 10.1158/0008-5472.CAN-15-1467-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnoczky G, Terzic A, Waldman SA. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci U S A. 2003;100:2695–9. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, Shi K, MacDougall JE, Shao JZ, Eng P, Fox SM, Schneier HA, Kurtz CB, Johnston JM. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012;107:1714–24. doi: 10.1038/ajg.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, MacDougall JE, Jia XD, Shao JZ, Fitch DA, Baird MJ, Schneier HA, Johnston JM. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012;107:1702–12. doi: 10.1038/ajg.2012.254. [DOI] [PubMed] [Google Scholar]
- 53.Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, Shao JZ, Lavins BJ, Currie MG, Fitch DA, Jeglinski BI, Eng P, Fox SM, Johnston JM. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011;365:527–36. doi: 10.1056/NEJMoa1010863. [DOI] [PubMed] [Google Scholar]
- 54.Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA, Comiskey S, Jacob GS, Plevy SE. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015;6:213–22. doi: 10.4292/wjgpt.v6.i4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shailubhai K, Comiskey S, Foss JA, Feng R, Barrow L, Comer GM, Jacob GS. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58:2580–6. doi: 10.1007/s10620-013-2684-z. [DOI] [PubMed] [Google Scholar]
- 56.ClinicalTrials.gov. Registry [Published 2016 [cited October 24, 2016] Available from: http://www.clinicaltrials.gov.
- 57.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 58.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 60.Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20:3738–50. doi: 10.3748/wjg.v20.i14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snook AE, Waldman SA. Advances in cancer immunotherapy. Discov Med. 2013;15:120–5. [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Raju GS, Jogunoori W, Menon V, Majumdar A, Chen JS, Gi YJ, Jeong YS, Phan L, Belkin M, Gu S, Kundra S, Mistry NA, Zhang J, Su X, Li S, Lin SH, Javle M, McMurray JS, Rahlfs TF, Mishra B, White J, Rashid A, Beauchemin N, Weston BR, Shafi MA, Stroehlein JR, Davila M, Akbani R, Weinstein JN, Wu X, Mishra L. Mutational profiles reveal an aberrant TGF-beta-CEA regulated pathway in colon adenomas. PLoS One. 2016;11:e0153933. doi: 10.1371/journal.pone.0153933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCann KJ, Mander A, Cazaly A, Chudley L, Stasakova J, Thirdborough SM, King A, Lloyd-Evans P, Buxton E, Edwards C, Halford S, Bateman A, O’Callaghan A, Clive S, Anthoney A, Jodrell DI, Weinschenk T, Simon P, Sahin U, Thomas GJ, Stevenson FK, Ottensmeier CH. Targeting carcinoembryonic antigen with DNA vaccination: on-target adverse events link with immunologic and clinical outcomes. Clin Cancer Res. 2016;22:4827–36. doi: 10.1158/1078-0432.CCR-15-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snook AE, Stafford BJ, Li P, Tan G, Huang L, Birbe R, Schulz S, Schnell MJ, Thakur M, Rothstein JL, Eisenlohr LC, Waldman SA. Guanylyl cyclase C-induced immunotherapeutic responses opposing tumor metastases without autoimmunity. J Natl Cancer Inst. 2008;100:950–61. doi: 10.1093/jnci/djn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snook AE, Li P, Stafford BJ, Faul EJ, Huang L, Birbe RC, Bombonati A, Schulz S, Schnell MJ, Eisenlohr LC, Waldman SA. Lineage-specific T-cell responses to cancer mucosa antigen oppose systemic metastases without mucosal inflammatory disease. Cancer Res. 2009;69:3537–44. doi: 10.1158/0008-5472.CAN-08-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Snook AE, Magee MS, Schulz S, Waldman SA. Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol. 2014;44:1956–66. doi: 10.1002/eji.201444539. Describes the Ad5-GUCY2C vaccine, and offers the first demonstration of CD4+ T-cell tolerance as a mechanism of cancer vaccine resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snook AE, Baybutt TR, Hyslop T, Waldman SA. Preclinical evaluation of a replication-deficient recombinant adenovirus serotype 5 vaccine expressing guanylate cyclase C and the PADRE T-helper epitope. Human gene therapy methods. 2016;27:238–50. doi: 10.1089/hgtb.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snook AE. Could targeting T-helper cells aid the development of effective cancer vaccines? Immunotherapy. 2014;6:959–61. doi: 10.2217/imt.14.79. [DOI] [PubMed] [Google Scholar]
- 69.Anderson CC. Application of central immunologic concepts to cancer: Helping T cells and B cells become intolerant of tumors. Eur J Immunol. 2014;44:1921–4. doi: 10.1002/eji.201444826. [DOI] [PubMed] [Google Scholar]
- 70.Snook A, Baybutt T, Mastrangelo M, Lewis N, Goldstein S, Kraft W, Oppong Y, Hyslop T, Myers R, Alexeev V, Eisenlohr L, Sato T, Waldman S. A Phase I study of Ad5-GUCY2C-PADRE in stage I and II colon cancer patients. Journal for ImmunoTherapy of Cancer. 2015;3:P450. [Google Scholar]
- 71.Magee MS, Snook AE, Waldman SA. Adoptive cell therapy: at the forefront of cancer immunotherapy. In: Watanabe HS, editor. Horizons in Cancer Research. Hauppauge, NY: Nova Science Publishers Inc.; 2013. p. 51. [Google Scholar]
- 72.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phan GQ, Rosenberg SA. Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control. 2013;20:289–97. doi: 10.1177/107327481302000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77*.Gross G, Eshhar Z. Therapeutic potential of T cell chimeric antigen receptors (CARs) in cancer treatment: counteracting off-tumor toxicities for safe CAR T cell therapy. Annu Rev Pharmacol Toxicol. 2016;56:59–83. doi: 10.1146/annurev-pharmtox-010814-124844. Review of CAR-T-cell therapy and strategies to avoid off-target toxicity. [DOI] [PubMed] [Google Scholar]
- 78.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255–62. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85**.Magee MS, Kraft CL, Abraham TS, Baybutt TR, Marszalowicz GP, Li P, Waldman SA, Snook AE. GUCY2C-directed CAR-T cells oppose colorectal cancer metastases without autoimmunity. OncoImmunology. 2016;5:e1227897. doi: 10.1080/2162402X.2016.1227897. First pre-clinical study of a GUCY2C-directed CAR-T cell therapy for metastatic colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayyar BV, Arora S, O’Kennedy R. Coming-of-age of antibodies in cancer therapeutics. Trends Pharmacol Sci. 2016 doi: 10.1016/j.tips.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Chee CE, Sinicrope FA. Targeted therapeutic agents for colorectal cancer. Gastroenterol Clin North Am. 2010;39:601–13. doi: 10.1016/j.gtc.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35 doi: 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papachristos A, Pippa N, Demetzos C, Sivolapenko G. Antibody-drug conjugates: a mini-review. The synopsis of two approved medicines. Drug Deliv. 2016;23:1662–6. doi: 10.3109/10717544.2014.998323. [DOI] [PubMed] [Google Scholar]
- 91.Marszalowicz GP, Snook AE, Magee MS, Merlino D, Berman-Booty LD, Waldman SA. GUCY2C lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget. 2014;5:9460–71. doi: 10.18632/oncotarget.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92*.Almhanna K, Kalebic T, Cruz C, Faris JE, Ryan DP, Jung J, Wyant T, Fasanmade AA, Messersmith W, Rodon J. Phase I study of the investigational anti-guanylyl cyclase antibody-drug conjugate TAK-264 (MLN0264) in adult patients with advanced gastrointestinal malignancies. Clin Cancer Res. 2016;22:5049–57. doi: 10.1158/1078-0432.CCR-15-2474. First clinical trial results of TAK-264, a GUCY2C targeted antibody-drug conjugate. [DOI] [PubMed] [Google Scholar]
- 93.Rahbari NN, Bork U, Motschall E, Thorlund K, Buchler MW, Koch M, Weitz J. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:60–70. doi: 10.1200/JCO.2011.36.9504. [DOI] [PubMed] [Google Scholar]
- 94.Varghese A. Chemotherapy for stage II colon cancer. Clin Colon Rectal Surg. 2015;28:256–61. doi: 10.1055/s-0035-1564430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waldman SA, Cagir B, Rakinic J, Fry RD, Goldstein SD, Isenberg G, Barber M, Biswas S, Minimo C, Palazzo J, Park PK, Weinberg D. Use of guanylyl cyclase C for detecting micrometastases in lymph nodes of patients with colon cancer. Dis Colon Rectum. 1998;41:310–5. doi: 10.1007/BF02237484. [DOI] [PubMed] [Google Scholar]
- 96*.Waldman SA, Hyslop T, Schulz S, Barkun A, Nielsen K, Haaf J, Bonaccorso C, Li Y, Weinberg DS. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 2009;301:745–52. doi: 10.1001/jama.2009.141. First clinical trial defining the utility of GUCY2C qRT-PCR for molecular staging of colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hyslop T, Weinberg DS, Schulz S, Barkun A, Waldman SA. Occult tumor burden predicts disease recurrence in lymph node-negative colorectal cancer. Clin Cancer Res. 2011;17:3293–303. doi: 10.1158/1078-0432.CCR-10-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beaulieu M, Desaulniers M, Bertrand N, Deschesnes RG, Beaudry G, Garon G, Haince JF, Houde M, Holzer TJ. Analytical performance of a qRT-PCR assay to detect guanylyl cyclase C in FFPE lymph nodes of patients with colon cancer. Diagn Mol Pathol. 2010;19:20–7. doi: 10.1097/PDM.0b013e3181ad5ac3. [DOI] [PubMed] [Google Scholar]
- 99.Sargent DJ, Resnick MB, Meyers MO, Goldar-Najafi A, Clancy T, Gill S, Siemons GO, Shi Q, Bot BM, Wu TT, Beaudry G, Haince JF, Fradet Y. Evaluation of guanylyl cyclase C lymph node status for colon cancer staging and prognosis. Ann Surg Oncol. 2011;18:3261–70. doi: 10.1245/s10434-011-1731-2. [DOI] [PubMed] [Google Scholar]
- 100.Haince JF, Houde M, Beaudry G, L’Esperance S, Garon G, Desaulniers M, Hafer LJ, Heald JI, Lyle S, Grossman SR, Tetu B, Sargent DJ, Fradet Y. Comparison of histopathology and RT-qPCR amplification of guanylyl cyclase C for detection of colon cancer metastases in lymph nodes. J Clin Pathol. 2010;63:530–7. doi: 10.1136/jcp.2009.072983. [DOI] [PubMed] [Google Scholar]
- 101.Van Cutsem E, Verheul HMW, Flamen P, Rougier P, Beets-Tan R, Glynne-Jones R, Seufferlein T. Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers (Basel) 2016;8 doi: 10.3390/cancers8090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weissleder R, Schwaiger MC, Gambhir SS, Hricak H. Imaging approaches to optimize molecular therapies. Sci Transl Med. 2016;8:355ps16. doi: 10.1126/scitranslmed.aaf3936. [DOI] [PubMed] [Google Scholar]
- 103.Wolfe HR, Mendizabal M, Lleong E, Cuthbertson A, Desai V, Pullan S, Fujii DK, Morrison M, Pither R, Waldman SA. In vivo imaging of human colon cancer xenografts in immunodeficient mice using a guanylyl cyclase C–specific ligand. J Nucl Med. 2002;43:392–9. [PubMed] [Google Scholar]
- 104.Liu D, Overbey D, Watkinson LD, Daibes-Figueroa S, Hoffman TJ, Forte LR, Volkert WA, Giblin MF. In vivo imaging of human colorectal cancer using radiolabeled analogs of the uroguanylin peptide hormone. Anticancer Res. 2009;29:3777–83. [PubMed] [Google Scholar]
- 105.Maugeri-Sacca M, Vici P, Di Lauro L, Barba M, Amoreo CA, Gallo E, Mottolese M, De Maria R. Cancer stem cells: are they responsible for treatment failure? Future Oncol. 2014;10:2033–44. doi: 10.2217/fon.14.126. [DOI] [PubMed] [Google Scholar]
- 106.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2006;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 107.Ma L, Liu T, Jin Y, Wei J, Yang Y, Zhang H. ABCG2 is required for self-renewal and chemoresistance of CD133-positive human colorectal cancer cells. Tumour Biol. 2016;37:12889–96. doi: 10.1007/s13277-016-5209-5. [DOI] [PubMed] [Google Scholar]


