Abstract
The intestinal epithelial barrier plays an essential role in maintaining host homeostasis. The barrier regulates nutrient absorption as well as prevents the invasion of pathogenic bacteria in the host. It is composed of epithelial cells, tight junctions, and a mucus layer. Several factors, such as cytokines, diet, and diseases can affect this barrier. These factors have been shown to increase intestinal permeability, inflammation, and translocation of pathogenic bacteria. In addition, dysregulation of the epithelial barrier can result in inflammatory diseases such as inflammatory bowel disease. Our lab and others have also shown that barrier disruption can have systemic effects including bone loss. In this chapter, we will discuss the current literature to understand the link between intestinal barrier and bone. We will discuss how inflammation, aging, dysbiosis and metabolic diseases can affect intestinal barrier-bone link. In addition, we will highlight the current suggested mechanism between intestinal barrier and bone.
1. Introduction
The gastrointestinal (GI) epithelium plays an essential role in maintaining host health through its ability to digest and absorb nutrients. At the same time, it is essential for providing a selective barrier that prevents translocation of harmful substances as well as pathogens and their products from the external environment to the blood stream. The intestinal epithelium is composed of a continuous single layer of intestinal epithelial cells (IECs) that are sealed together by tight junctions (TJ) proteins. This epithelial layer allows the movement of materials from the mucosal side of the epithelium to the serosal side via transcellular and paracellular pathways. A mucus layer, secreted by specialized epithelial cells (goblet cells), is located on the surface of the epithelium and is important for limiting the ability of gut bacteria and pathogens to access host cells. The lumen of the GI tract also harbors a variety of commensal microorganisms referred to as the gut microbiota which accounts for 90% of the cells in the human body, approximately 1014 bacteria total. The intestine also secretes immunoglobulins, defensins, and other antimicrobial products that contribute to maintaining a healthy environment. Beneath the epithelial layer is the lamina propria which contains immune cells, fibroblasts and plasma cells.
Disruption of the epithelial barrier can 1) affect efficient nutrient absorption, 2) facilitate pathogen translocation into the bloodstream and cause systemic inflammation, and 3) alter gut microbiota composition [1]. As a consequence, barrier disruption can trigger the development of GI diseases such as inflammatory bowel disease (IBD), celiac disease, and colon cancer [2–5]. Other systemic and metabolic diseases such as type I diabetes can also be influenced by barrier changes [6, 7]. However, whether barrier dysfunction is causal or consequence of these systemic and metabolic diseases is controversial. Recent studies from our lab and others demonstrate that GI barrier dysregulation can critically affect bone health [8, 9]. In this chapter, we will review several important aspects of intestinal epithelial barrier function including: tight junction protein composition, the mucus layer, epithelial barrier integrity measurements, barrier alterations associated with disease processes, and barrier dysregulation-induced bone loss during aging, dysbiosis, and metabolic diseases.
1.1 Pathophysiology of tight junction proteins
Tight junction (TJ) proteins connect adjacent epithelial cells on their apical side and therefore are critical for controlling paracellular permeability by selectively regulating the flow of ions, solutes, and small molecules across the epithelium. TJ proteins respond to a variety of stimuli including changes in diet, dysbiosis, viruses, inflammation, antibiotic treatment, and/or humoral or neuronal signals [10][1][4]. Stimuli can have positive or adverse effects on paracellular permeability depending on the physiological status of the host [1, 11–13].
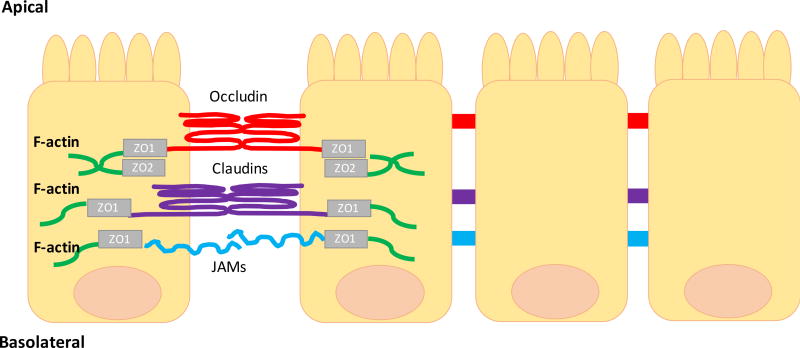
TJ protein complexes are composed of junctional adhesion molecules (JAM), occludins, desmosomes, claudins, and cytoskeletal linker proteins such as zonula occludens (ZO) (1–3) (Figure 1). The ZO is a family of proteins (ZO-1, ZO-2, ZO-3) that link the TJ proteins to the actin cytoskeleton. This interaction, between the TJ and the actin cytoskeleton, is essential to maintain TJ structure and cytoskeletal regulation of the epithelial barrier. Desmosomes do not directly connect adjacent epithelial cells. Instead, they provide the adhesive force to ensure the integrity of the epithelial layer [14][1]. Alterations of the TJ complexes can increase paracellular permeability and pathogen translocation that can induce sustained activation of the mucosal immune system and tissue damage.
Figure 1. Schematic representation of the intestinal tight junctions proteins and their location.
Tight junctions are protein complexes that span between epithelial cells to form a tight barrier. They are comprised of transmembrane proteins, such as occludin (red) and claudins (purple) and they are connected to the actin cytoskeleton via a zona occludens (ZO-1 and ZO-2 (grey)). The transmembrane receptor JAM (junctional adhesion molecule (blue)) is also found at tight junctions complexes. Abbreviations: JAM, junctional adhesion molecule; ZO, zona occludens.
Several cytokines can modulate TJ complexes and affect intestinal permeability. For example, the proinflammatory cytokine tumor necrosis factor alpha (TNFα) can directly increase intestinal permeability in cultured intestinal epithelial cells and mouse epithelium by dysregulating TJ proteins. In vitro, in a colon epithelial cell line (Caco-2), TNFα-induced increases in TJ permeability were associated with increased nuclear factor kappa beta (NF-κB) activation and nuclear translocation of NF-κB p65 [13]. TNFα has also been shown to increase the expression of the myosin light-chain kinase (MLCK), and inhibition of MLCK can prevent TNFα induced increases in permeability in intestinal epithelial cells [15, 16]. Similarly, interferon-γ (IFN-γ) increases paracellular permeability in T84 colonic epithelial cells. IFN-γ decreases ZO-1 protein synthesis, increases internalization of the TJ proteins and rearranges the actin cytoskeleton [17]. Interleukin-1β (IL-1β) can also regulate transepithelial permeability in vitro. IL-1β caused a progressive time-dependent increase in transepithelial permeability in Caco-2 cells. This increase in permeability was attributed to the rapid activation of the NF-κB by IL-1β [18]. On the other hand, the role of the anti-inflammatory cytokine interleukin 10 (IL-10) in TJ regulation has been demonstrated in IL-10 knockout mice. IL-10 knockout mice present with an increase in ileal and colonic permeability at 2 weeks of age. However, this effect was ablated in IL-10 gene-deficient mice raised under germ-free conditions, suggesting a role of the microbiota in intestinal permeability in IL-10 knockout mice [19].
Studies in IBD patients indicate that TNFα levels are increased in the serum, stool, and intestinal mucosa [20] and, correspondingly, patients display increased intestinal paracellular permeability that is characterized by suppression and re-distribution of occludins, and claudins 5,8, whereas claudin-2 expression was upregulated [21]. Interestingly, people at high risk of developing Crohn’s disease exhibit increases in small intestinal permeability [22]. Together, these studies demonstrate that TJ proteins are regulated by cytokines, modify intestinal permeability and are linked to disease pathogenesis.
1.2 GI Mucus Layer
The GI epithelium is covered by a layer of mucus that creates a physical barrier. This barrier prevents the interaction of luminal microorganism with the surface of the epithelium. It also serves as the first line of host defense and allows the exchange of water, nutrients and gases with the underlying epithelium. This layer is formed by high molecular weight glycoproteins called mucins (MUC) that are synthesized and secreted by goblet cells. Mucins are produced and stored in granules in the goblet cell and then are transported and secreted into the lumen. They can be secreted by continuous fusion (constitutive/basal secretion) or by exocytosis (exocytosis/regulated secretion). There are two groups of mucins: secreted mucins and transmembrane mucins. MUC2, MUC5AC, MUC5B and MUC6 constitute the secreted mucins and are responsible for the formation of the mucus layer. The transmembrane mucins (MUC1, MUC4, MUC13 and MUC16) don’t play a role in mucus production. Under normal physiological conditions, goblet cells continually produce mucins, however, factors such as cytokines, toxins, microbes and microbial product can negative or positively regulate this process [23]. Disruption of this process has been associated with GI diseases.
Inflammatory cytokines such as TNFα, IL-1β, and IL-6 are known to be major regulators of mucin synthesis and exocytosis. In vitro, in colon cells, TNFα and IL-6 increased the expression of the secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) [24]. TNF-α enhanced MUC2 transcription through the activation of the NF-κB pathway in colon cells [25]. Similarly, the anti-inflammatory cytokine IL-10 enhances MUC2 expression in goblet cells [26].
The gut microbiota depends on the mucus layer which serves as an energy source for bacteria. The mucus layer also serves as a matrix for commensal bacteria attachment and colonization that ultimately prevents opportunistic pathogenic bacteria from binding/growing within the mucus. Cross talk between the intestinal microbiota and mucus layer contributes to the regulated production of mucin by goblet cells [27–29]. Studies in germ-free mice demonstrate that microbiota-deficiency leads to fewer goblet cells and thinner mucus layer in comparison with conventionally-raised mice; supporting the role of the microbiota and/or microbial products in modulation of mucin synthesis and secretion [27]. Correspondingly, treatment of intestinal epithelial cells (HT-29) with the probiotic Lactobacillus planetarium enhanced MUC2 and MUC3 mRNA expression levels [30]. Similarly, commensal microbiota break down non-digestible carbohydrates into short-chain fatty acids (SCFAs) such as acetate, propionate, and especially butyrate, which at low concentration can increase mucus production and secretion [28, 29, 31]. Other microbial products, such as lipopolysaccharides and flagellin, also increase mucin synthesis [32]. If mucin production is chronically stimulated, goblet cells become depleted of mucin and the lack of mucus secretion can lead to increased permeability and disease [33]. Taken together, mucus secretion is highly regulated by a variety of conditions/factors and, along with tight junctions, contributes to intestinal barrier integrity.
2. Epithelial barrier integrity measurements
The intestinal epithelial barrier plays an essential role in host health as well as in GI diseases. Several tests have been developed to improve the diagnosis of GI diseases such as IBD. These tests (discussed below and in Table 1) include measures of serum, fecal, and urine biomarkers that are altered as a consequence of intestinal barrier dysfunction (i.e., inflammation) or that directly assess barrier permeability (i.e., endotoxin).
Table 1.
Methods for the assessment of intestinal epithelial barrier integrity.
| Test | Measured in | Advantages | Disadvantages |
|---|---|---|---|
|
| |||
| Ex vivo | |||
|
| |||
| Ussing chamber | Small and large intestine | Site specific | Invasive |
|
| |||
| In vivo | |||
|
| |||
| C-reactive protein (CRP) | Serum | Non-invasive | Low sensitivity |
|
| |||
| Inflammatory markers (e.g. cytokines) | Serum | Non-invasive | Non- specific |
|
| |||
| Bacteria metabolic products (e.g. LPS) | Serum | Non-invasive | High sensitivity |
|
| |||
| Fatty acid binding proteins (FABP) | Seum /urine | Non-invasive | |
|
| |||
| Calprotectin and lactoferrin | Feces | Non-invasive | Non-specific |
|
| |||
| Dual sugar test (e.g. mannitol, lactulose) | Urine | Non-invasive | Time consuming |
|
| |||
| Polyethylene glycols (PEG) | Urine | Non-invasive | Time consuming |
| Small and large intestine | |||
2.1. Serum
Serologic markers for epithelial barrier integrity in IBD patients includes C-reactive protein (CRP) measurements [34, 35]. CRP levels are elevated in the serum of patients with acute IBD, with almost 100% of patients with CD showing an increase of this protein in the serum [34][36]. Serum measurements of inflammatory markers such as cytokines and neutrophils can also be performed. However, plasma/serum levels of inflammatory markers, including CRP, are not specific for gut inflammation since they can also be increased in other inflammatory conditions.
Breakdown of the epithelial barrier can lead to the translocation of the microbiota or their toxic products. Markers of bacterial antibodies for Escherichia coli and Pseudomonas fluorescens have been used to identify children with IBD with 67% sensitivity and 76% specificity [37]. Bacterial metabolic products, such as D-lactate or cell wall components such as LPS/endotoxin, are also commonly measured. Baseline levels of these markers are low in healthy individuals, whereas increased circulating LPS/endotoxin levels are related to an impaired mucosal barrier and increased levels of D-lactate are correlated with intestinal injury [38].
To estimate enterocyte damage, measurement of the fatty acid binding proteins (FABP) can be performed in the urine or plasma. FABP is located on top of the villi and an increase in FABP in the blood or urine can be used as a marker of early stage intestinal diseases [39–41]. Levels of FABP rise rapidly after GI inflammation and have been correlated with the histological status of the epithelium after GI inflammation.
2.2 Feces
Invasive methods to test gut epithelial barrier inflammation in humans are not feasible. However, fecal proteins such as calprotectin and lactoferrin are specific markers for mucosal inflammation in intestinal diseases [42] and can identify patients with IBD, assess disease activity, and predict relapse [43, 44]. Calprotectin is a 36 kDa calcium- and zinc-binding protein. Approximately 60% of the cytosolic protein content in the neutrophils is made up of calprotectin. During intestinal inflammation, neutrophils migrate to the mucosa and any break in the mucosal barrier results in the leakage of neutrophils into the lumen. Hence the presence of calprotectin in the feces indicates the migration of neutrophils to the intestinal mucosa and potential leakage of these cells into the lumen. Calprotecin is stable in feces, and its concentration represents an indirect measure of neutrophil infiltration and barrier breaks. Lactoferrin is an iron-binding protein that is also found in neutrophils, specifically neutrophil granules. Lactoferrin is secreted during inflammation; when the intestine is inflamed and neutrophils are present, lactoferrin levels increase in the lumen and stool [42][45].
2.3 Urine
Intestinal permeability can also be assessed by using small to large-sized probe molecules [46, 47]. This approach involves oral ingestion of sugars, such as mannitol and lactulose, and measuring their subsequent concentration in the urine over a period of time (usually 5 hours). Mannitol is a monosaccharide with a molecular weight (MW) of 182 Da and a molecular radius of ≤ 0.4 nm. Lactulose is a disaccharide with an MW of 342 Da and a molecular radius of 0.42 nm. The different sizes of the molecules allows for the measurement of transcellular and paracellular routes of permeability across the epithelia [45]. Large molecules, such as lactulose, are thought to traverse the epithelium by paracellular pathways. Small molecules, such as mannitol, cross the epithelium predominantly by the transcellular pathways. Neither sugar should be fermented by bacteria or metabolized in the body. Thus, the ratio of urinary excretion of the relatively large molecule is compared with that of the relatively small molecule and permeability is expressed as the ratio [46].
One concern with this approach is that many factors can influence the uptake of these sugars by epithelial cells, including (1) GI motility, (2) the use of medications such as nonsteroidal anti-inflammatory drugs, (3) intestinal transit time and surface area, (4) mucosal blood flow and (5) renal clearance; these effects can potentially yield false-positive results. However, when both the large and small molecules are combined in the test solution at a fixed concentration ratio, the effects of variables, such as gastric emptying, intestinal transit time, and renal clearance will apply equally to both. Thus, the urinary excretion ratio will be influenced only by the difference in gut permeability for each molecule.
Polyethylene glycols (PEG) have also been used to test intestinal barrier function. PEGs that have a molecular weight of 400–4000 Da can only cross the intestinal mucosa under conditions of barrier integrity loss. PEG can be used to measure both small and large intestinal permeability and are not degraded by bacteria. PEG have been used to test changes in permeability in IBD and Crohn’s patients [48, 49].
3. Barrier pathophysiology in development
3.1 Pediatrics
The intestine at birth is not fully developed and many factors, such as diet, stress and microbiota have been implicated in influencing its permeability during development [50][51][52]. Increased intestinal permeability in infancy may lead to diseases that persist throughout childhood as well as those that appear later in life, such as IBD. Immune system involvement, which is developed alongside microbiota and diet changes, is also a significant indicator of the health of the intestinal barrier. Because of the fundamental differences in development between children and adults, it is important to consider pediatric intestinal barrier physiology separately.
The intestinal mucosal barrier significantly matures after birth, coinciding with changes in microbial composition and diet changes. At birth, the child is introduced to microbes, traditionally through contact with the birth canal, that colonize the intestine. It has been shown that vaginally born infants have higher numbers of Bifidobacteria and Bacteroides when compared with infants born through cesarean section [53]. In addition, the presence of Bifidobacteria in breast-fed infants, corresponds with breast-fed infants having lower intestinal permeability than cow’s milk formula-fed infants [54]. Other studies have shown that B. infantis promotes intestinal barrier function by regulating tight junctions. Infant mice treated with B. infantis exhibited decreased internalization of claudin 4 and occludin, which effectively decreased the incidence of necrotizing enterocolitis [55]. Mucin production also contributes to barrier integrity due to its importance in building the mucosal layer. In mice, the maturation and production of these glycoproteins occurs after weaning, signifying the role of diet as well as hormonal and other age-associated factors in barrier development [56].
Increased intestinal permeability is associated with a variety of intestinal as well as extra-intestinal diseases, many of which persist or manifest in adulthood. Since the intestinal microbiota takes approximately 2.5 years to become functionally mature, the clinical impact of any large shifts in microbial composition during this developmental period can significantly impact intestinal permeability [57]. It has been suggested that traumatic GI events in early infancy, during the period of barrier maturation, are more powerful indicators of eventual disease than events occurring outside this “critical window”. As evidence for the possibility of an early life disturbance creating lasting effects on barrier function, the trauma of maternal separation during weaning has been shown to predispose adult rats to enhanced intestinal permeability in response to stress [51].
The intestinal immune system in infants develops upon antigen exposure, directing attention to the importance of gut microbial colonization in relation to successful barrier function. Immunoglobulin A (IgA) is a class of antibody first received in breast milk and is then produced by the gut mucosa. Interestingly, by 24 months, both mono- and dizygotic twins had IgA responses comparable to unrelated children although significant differences were observed at older ages, suggesting a level of maturation acquired by age two [58]. This roughly coincides with the stabilization of the makeup of the microbiome at 2.5 years. The interplay among microbial colonization, diet, immune system and the intestinal barrier is fundamental to the health of the gastrointestinal tract. Insults to the intestinal barrier at a young age can induce diseases such as IBD that appear in childhood and possibly persist through adulthood.
Early life disturbances such as premature birth, which can increase intestinal permeability, can also affect bone density [59]. In fact, 16–40 % of very low birth weight (VLBW, <1.500 kg) and extremely low birth weight (ELBW, <1.000 kg) infants are estimated to develop bone metabolic diseases [60]. Examination of growth and bone mineralization among children born prematurely (birth weight less than 1.5 kg) indicates reduced lumbar bone mineral density and content compared to full-term children [60]. During this early period of life, the intestinal barrier is particulary permeable to allow antibodies in the mother’s colostrum to cross into the infant’s blood. This increased permeability in neonates can cause intestinal inflammation and can lead to necrotizing enterocolitis (NEC) [61]. While a direct link between early changes in bone density with reduced barrier function in neonates and children has yet to be proven, studies in inflammatory bowel patients and animal models support this link (see section on IBD below). For example, chemically increasing barrier permeability in young (5 week old) growing mice causes reduced bone density and stunted growth compared to control mice [62]. It is noteworthy, that when the inflammatory insult is removed the young mice are able to fully regain bone density and length 5 weeks later [62]. Taken together, the data support the need for more studies to understand the role of the gut epithelial barrier in early life disturbances in bone physiology.
3.2 Aging
Several studies have shown that aging can have profound effects on the GI tract. Approximately 35–40% of elderly patients report having at least one GI tract complication during a routine medical exam [63]. Effects of aging in the GI tract includes changes in permeability, motility, inflammation, and disruption of the gut microbiota. However, the mechanisms by which aging contribute to shifts in any of these effects and their influence on epithelial barrier and bone health are poorly understood. This is in part because it is difficult to discern if changes are due to normal aging, common age-related disorders, or result from disease treatments. This section will discuss the effects of aging on intestinal permeability, inflammation and microbiome and while no papers directly link barrier changes with age related bone loss, we will discuss potential connections.
3.2.1 Changes in permeability
Several studies have shown that aging can have detrimental effects on intestinal barrier permeability. A study looking at 34 vs. 133-week-old rats demonstrated that the younger rats excreted 34.3% of the administrated PEG 400 in the urine, while 43.6% was excreted by the older rats [64]. Similarly, another study showed that as rats age (12–112 weeks), intestinal permeability to PEG 400 and mannitol increased [65]. Colonic mucosal biopsies from young and old non-human primates (baboons) demonstrate significant differences in permeability and TJ proteins. The older baboons displayed a significant decrease in ZO-1, occludin, and JAM-A proteins, and an increase in claudin-2 expression, all of which correlated with increased permeability in the old aged group [66]. Another study in monkeys found that old monkeys have an increase in FITC dextran flux compared to young monkeys [67].
A cross-sectional study of non-smoking healthy adults, between 60 and 85 years old, showed no difference in the permeability index (PI= lactulose/mannitol) between young and older humans. However, the study had some limitations such as that the older age group consisted of predominantly males and sex difference may play a role in intestinal permeability [68]. In an ex vivo assay by Man et.al [69], the authors demonstrated that the transepithelial electric resistance (TEER) is affected in the aged humans. In their study, the effects of age on TEER was tested using ileal biopsies from healthy humans, young (7–12 years), adult (20–40 years), and aging (67–77 years). The TEER was significantly reduced in the aging biopsies whereas no difference was observed between the two younger groups. The increase in permeability in the aging group appeared to be restricted to solutes since the permeability to macromolecules was not affected by aging [69]. There were no changes in mRNA expression of ZO-1, occludin, and JAMA-1 in the aging group compared with adults and young individuals. On the other hand, they observed that levels of claudin-2 were significantly increased in the aging group and not in the adult group; suggesting that claudin-2 play an important role in intestinal permeability [69]. It has also been proposed that the changes seen in intestinal permeability in aging people can be due to an increase in inflammation and/or disruption of the gut microbiota.
3.2.2 Changes in mucosal immune system
Aging is associated with a decline in the immune response [70, 71]. About 50% of the older age group are affected by low-grade chronic inflammation known as “inflammageing” [72]. In a steady-state situation, the IECs communicate with the intestinal immune system to regulate intestinal homeostasis. IECs regulate the intestinal immune homeostasis through the secretion of cytokines that control dendritic cells and T-regulatory cells. This interaction helps to discriminate between invasive pathogenic organisms and harmless antigens. Several studies have reported a dysregulation in the intestinal immune homeostasis in different models of aging. Specifically, it has been shown that the function of the gut-associated immune system is impaired in elderly humans [67, 69, 73, 74].
Using monkeys as a model of human aging, researchers found that old monkeys have greater systemic inflammation as compared to young monkeys. This increase in inflammation was attributed to an increase in serum CRP [67]. In a similar study using baboons, it was found that aged baboons have a significant increase in IFN-γ, IL-6, and IL-β in colonic biopsies. In the same study, the old animals presented an increase in colonic permeability [75]. These results suggest that dysregulation of the immune system can alter intestinal permeability.
Several studies in humans have also confirmed the effects of aging on the intestinal immune response. Ileal biopsies from young (7–12 years), adult (20–40 years) and aging (67–77 years) individuals were assessed for inflammatory cytokines levels. They noticed an increase in the expression of IL-6, but not IFNγ, TNF-α, and IL-1β in the aging group. The increase in IL-6 was attributed to an increase in dendritic cells. They also demonstrated a correlation between IL-6, claudin 2 and permeability [69]. Many studies indicate that IL-6 expression is induced with aging. Animal studies showed that the decline in the production of IL-1 β, TNFα, and IL-12, in response to LPS in aging is restored in aging IL-6-deficient knock-out mice, suggesting that IL-6 is responsible for the changes in the mucosal immune system during aging [76]. In conclusion, the pro-inflammatory state observed in aging populations may be related to dysfunction of the intestinal barrier.
3.2.3 Changes in intestinal microbiota
In a healthy intestinal tract, the microbiota and the gut immune system interact to maintain a homeostatic equilibrium. Perturbation of this homeostatic equilibrium has been strongly associated with many human diseases such as obesity and IBD [77, 78]. The intestinal microbiota supplies nutrients as well as protects the intestinal barrier against pathogens [79]. A variety of factors including the host, microbiological, dietary and environmental factors can disrupt the gut microbiota. Because of the crucial role of the intestinal microbiota in host homeostasis, it is important to study the age-related differences in microbiota and how it influences intestinal function.
It has been demonstrated that the human intestinal microbiota undergoes maturation from birth to adulthood and is further altered with aging. A study looking at age-related differences in the gut microbiota composition among young (average 31-years old), elderly (average 72-years old), and centenarians humans (average 100- years old) demonstrated that the composition and diversity of the gut microbiota do not differ between young adults and elderly groups. However, there was a significant difference between the elderly and centenaries [80]. These differences were attributed to an increase in facultative anaerobes, mostly belonging to Proteobacteria and Bacilli in the centenarians group. The Firmicutes/Bacteroidetes ratios did not differ between the young and centenarian groups. In the same study, measurements of inflammatory cytokines were performed. An increase in IL-6 and IL-8 was observed, but not TNFα. They also found a positive correlation between bacteria belonging to the phylum Proteobacteria with IL-6 and IL-8 [80]. Species diversity was found to change with age in bacteria isolated from fecal samples from healthy young and elderly adults. On the other hand, the overall numbers of organisms were similar at the genus level [81]. In a different study, the results showed a change in bacterial genera with age and a reduction in the numbers Bacteroides and Bifidobacteria in the elderly group. These reductions were accompanied by reduced species diversity [82]. The Firmicutes/Bacteroidetes ratio of the human microbiota increased with age [83]. This shift in microbiota composition might result in a greater susceptibility to diseases by altering intestinal permeability among other consequences.
3.2.4 Intestinal changes and bone health
While we do not know of any study directly linking the effect of aging on gut barrier-to-bone signaling, the intestinal changes that occur with aging are associated with bone loss in other conditions. Specifically, the strongest link between gut permeability and bone loss comes from colitis studies where barrier disruption in adult animal models leads to bone loss, even without weight loss [84][85][62]. Thus, as animals and humans age, intestinal permeability increases [64][65][66][67][62][69] and could contributes to age-related bone loss. In addition, increased permeability likely promotes the low-grade chronic inflammation, termed “inflammageing” [72][67][75], and many studies link low grade inflammation with bone loss [84][85][62]. In the future, studies are need to test if a direct link exists between barrier function and bone health in the elderly, since this could be a promising target for new therapeutics.
3.3 Menopause
Menopause is the natural cessation of menstruation and decline in reproductive hormones. One of the most significant reproductive hormones in females is estrogen, produced primarily in the ovaries. It is also produced at extra-gonadal sites including adipose tissue, skin, osteoblasts, osteoclasts, aorta, and the brain. After menopause, adipose tissue is the main source of estrogen. There are several forms of estrogen, 17β estradiol being the most prevalent circulating estrogen. Only a small amount in the plasma is free and active, most is bound to globulin or albumin. The two primary receptors for estrogen are estrogen receptor α (ERα) and estrogen receptor β (ERβ), both of which are nuclear receptors. ERα is typically associated with secondary sex characteristics and regulation of the menstrual cycle in females and sperm maturation in males [86]. ERβ has less of a role in the classical estrogen target tissues and has been found to be more dominant in the brain, cardiovascular system and the colon [87, 88]. A decrease in estrogen levels during menopause has been attributed to osteoporosis that occurs in post-menopausal women but the role of declining estrogen in intestinal permeability is only beginning to be understood. In ovariectomized Wistar rats, colonic paracellular permeability was increased significantly and this was reversed by estrogen treatment (oestradiol benzoate) [89]. Consistent with this, colonic paracellular permeability decreases during the oestrus phase (high levels of estrogen) of the rat when compared to the dioestrus phase (low levels of estrogen) [89]. Although estrogen treatment has been shown to predispose ovariectomized rats to development of ulcerative colitis-induced tumor development [90], most studies to-date have shown that estrogen treatment decreases colonic paracellular permeability and reduces IBD symptom severity [89, 91, 92]. ERβ is the predominant estrogen receptor in the intestinal tract. Whole body ERβ knockout mice display altered intestinal cell proliferation, decreased apoptosis and abnormal villus/crypt architecture throughout the intestine [93]. One potential mechanism that could account for estrogen effects on the intestine is through its alterations in TJ and adhesion molecules which would alter intestinal permeability [94]. In models of IBD (IL-10 deficient mice and HLA-B27 rats), ERβ mRNA levels were decreased and colonic permeability increased [92]. Similarly, treatment of cell culture models of intestinal epithelial layers (HT-29, T84, Caco-2) with estrogen receptor antagonists increases permeability while estrogen treatment prevents this outcome [89, 92]. Our lab demonstrated a significant increase in intestinal permeability 1 week post-surgery in the absence of estrogen (OVX model) in mice. Section specific changes in permeability were also measured ex vivo by Ussing chambers which demonstrated that the ileum had the most dynamic changes. This study indicates that estrogen deficiency induces region-specific effects on intestinal permeability [94]. Thus, estrogen appears to predominantly inhibit increases in intestinal permeability and therefore an increase in permeability during menopause could lead to increase in systemic and bone inflammation that could contribute to bone loss.
In addition to altering epithelial barrier function, estrogen has also been shown to impact calcium absorption in the intestine, which is important for bone maintenance. Several studies identified decreases in intestinal and renal calcium absorption following estrogen deficiency [95–100]. Though the exact mechanism is not well understood, it is thought that estrogen deficiency leads to down regulation of the expression of transcellular calcium transport proteins plasma membrane calcium pump 1b (PMCA1b), transient receptor potential cation channel subfamily V member 5 (TRPV5) and calbindin-D 28K (CaBP28k) [95]. Furthermore, estrogen has been found to increase vitamin D receptor (VDR) gene and protein expression as well as 1,25(OH)2D3 activity in the colon, which leads to increased intestinal calcium absorption [101, 102]. Taken together, these studies suggest that estrogen could modulate bone heath via multiple mechanisms that depend on intestinal barrier function (permeability and calcium/vitamin-D metabolism).
Given that one out of two postmenopausal women will fracture a bone [103], the potential for using the gut as a therapeutic target to treat osteoporosis has increased research in this area. Recent studies support a role for intestinal health in the prevention of bone loss in ovariectomy (Ovx) mice [104][8]. Decreasing intestinal inflammation or altering the gut microbiome leads to the prevention of bone loss [104][8]. Our lab has shown that treatment with the probiotic Lactobacillus reuteri significantly protected Ovx mice from bone loss. This prevention of bone loss by Lactobacillus reuteri was attributed to a decrease in osteoclastogenesis and an increase in bone marrow CD4+ T-lymphocytes. Lactobacillus reuteri also modifies microbial communities in the Ovx mouse gut [104]. In a different study, researchers found an increase in gut permeability and cytokines (TNFα and IL-17) in the small intestine of Ovx mice. Surprisingly, in the germ-free mice the effect of estrogen deficiency in gut permeability and cytokines dysregulation was ablated; suggesting a role of the gut microbiota in Ovx induce bone loss. Treatment with the probiotic Lactobacillus rhamnosus GG (LGG) or the probiotic supplement VSL#3 reduces gut permeability, intestinal inflammation, and completely protects against bone loss induced by estrogen deficiency [8]. Together, these data highlight the role that of the gut epithelial barrier and microbiota in bone loss induced by estrogen-deficiency.
4. Barrier pathophysiology in disease
4.1 Dysbiosis
The intestinal microbiota has been described as a virtual organ that exhibits a complex bidirectional crosstalk with the environment and other systems throughout the body [105, 106]. The intestinal barrier acts as a wall between the intestinal microbiota, and the host’s immune system. Under normal conditions the intestinal epithelium has numerous adaptations such as anti-microbial peptides and mucins that keep the intestinal microbiota away from the gut epithelial layer [107–110]. The TJ also impede microbial invasion into the host tissue [111]. This intestinal epithelial barrier and its adaptations are not static, but can be regulated by a variety of external factors such as alteration to the gut microbiota (i.e. dysbiosis).
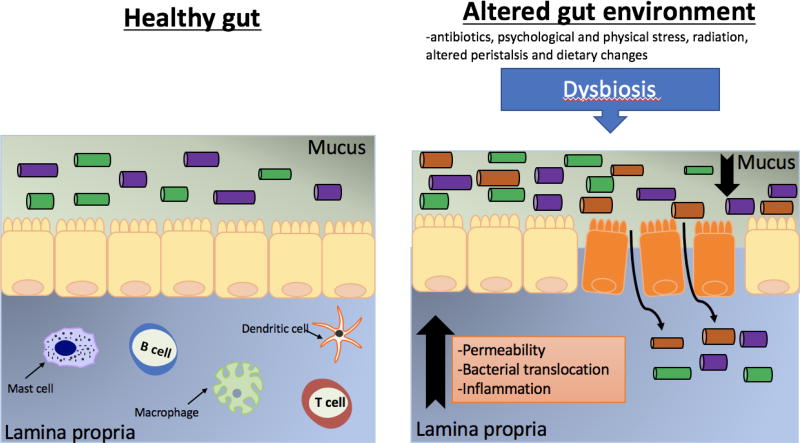
A number of factors can alter intestinal microbial composition. These include medications such as antibiotics, psychological and physical stress, radiation, altered peristalsis and dietary changes [112–116]. This can lead to alterations in bacterial metabolism as well as overgrowth of potential pathogenic bacteria [117]. Changes to the gut microbiota during dysbiosis have now been linked to a myriad of diseases such as IBD, irritable bowel syndrome (IBS), obesity and rheumatoid arthritis [118–121]. Importantly, dysbiosis can also lead to disruption of epithelial barrier leading to unwanted consequences [50] (Figure 2).
Figure 2. Schematic representation of the gut epithelial layer in healthy gut vs dysbiosis.
In the normal state, the mucus layer prevents the interaction between the gut microbiota and the intestinal epithelial barrier. Underneath the epithelial layer is the lamina propria. The lamina propria is composed of connective tissue and cells of the innate and adaptive immune system: mast cells, macrophages, dendritic cells, and lymphocytes (T and B). The composition of the intestinal epithelial layer can be influenced by many factors including antibiotic treatment, psychological and physical stress, radiation, age, and diet. This can lead to alterations in bacterial metabolism as well as overgrowth of potential pathogenic bacteria. This dysbiosis is associated with increased levels of permeability, bacterial translocation and inflammation.
Altered gut microbiota can signal through pattern recognition receptors on gut epithelial cells, activating the NF-κB pathway and leading to changes in gut homeostasis [122]. Epithelial cell NF-κB activation increases pro-inflammatory cytokines such as TNFα, IL-1, and INFγ [123]. An increase in gut INFγ and TNFα protein levels have been shown to increase intestinal permeability [124, 125]. This altered protein composition decreases barrier properties and leads to leaky gut properties. The exact mechanism behind these effects is not well characterized (20). Dysbiosis has also been linked to increase in IL-1β, which has also been shown to increase permeability by decreasing TJ protein occludin expression [18].
Additionally, dysbiosis of the gut microbiota also influences other adaptations such as mucin production which in turn influences gut barrier function. Intestinal mucins can inhibit bacterial adhesion to the intestinal epithelial cells, limiting immune responses and maintaining barrier function. The commensal microbiota has been shown to regulate production of intestinal mucins [126]. The abundance of mucolytic bacterium, which has the ability to degrade mucins, has been shown to increase 100 fold during dysbiosis observed in IBD [127]. In addition, under dysbiosis pathogens can secrete proteases that have been shown to cleave MUC2, the main mucin-component, thereby decreasing mucin levels [128]. Decreases in the mucin layers have been shown to compromise the intestinal barrier function leading to increases in intestinal permeability and microbe penetration [129]. All of the effects of dysbiosis on the gut barrier function can also lead to systemic changes in the body including bone loss [130–132].
Intestinal dybiosis has also been shown to affect bone density [8, 104, 133–135]. The role of the microbiome in regulating bone remodeling was shown in germ free mice (in C57BL/6 background) which have increased femoral bone density (both trabecular and cortical) when compared to conventionally raised mice [132]. This increase in bone density was attributed to a decrease in osteoclast, as well as, inflammatory cytokines in the bone and bone marrow in the germ free mice vs conventionally raised mice [132]. However, the effects of microbiome on bone density, as determined by studies using germ free mice, are not consistent across mouse strains and/or sex [136][8][130][132]. In addition, the impact of the microbiota changes on the epithelial barrier were not been fully examined. It is possible that changes in the microbiome that promote greater barrier function could benefit bone density while a more pro-inflammatory microbiome could cause bone loss, thereby explaining the inconsistencies between studies.
Previous work has demonstrated that gut dysbiosis promotes inflammation in the bone marrow that correlates with bone loss [137]. It has been hypothesized, that dysbiosis disrupts barrier function leading to increases in inflammation and activates T-cells leading to enhanced expression of TNFα in bone marrow [138, 139]. The increase in TNFα stimulates osteoclastogenesis and/or enhances osteoblast apoptosis, thus disrupting normal bone homeostasis leading to bone loss [138, 139]. The mechanism by which activated T-cells are increased in the bone marrow in response to changes in the gut microbiota are not completely understood; T-cell activation could be due to gut antigens crossing the intestinal barrier consequent to dysbiosis [140].
The finding that dysbiosis can alter bone density led to studies investigating the role of both pre- and probiotics in bone health. Prebiotics are non-digestible fermentable nutrients which promote the growth of beneficial microorganisms [141]. In vitro studies indicate that prebiotics can enhance intestinal epithelial barrier function and increase tight junction protein expression [142]. Under healthy and estrogen-deficient conditions, prebiotics (such as fructo-oligosaccharides (FOS) and inulin) also increase bone health parameters [143, 144][145, 146]. In addition, probiotics (live microorganisms which have a beneficial effect on the host) have been shown to increase barrier function and bone health. The probiotic Lactobacillus reuteri has anti-TNFα properties, reduces gut inflammation, and strengthens gut barrier function in vitro [104][134][147]. When given to mice, L. reuteri treatment was found to increase bone density in healthy male mice in addition to preventing bone loss in both female ovariectomized mice and type 1 diabetic male mice[134][104, 148][149]. Taken together, these data demonstrate the role of the microbiome and intestine in maintaining bone density.
4.2 Colitis/IBD
Inflammatory bowel disease is characterized by damage to the intestinal epithelial barrier resulting in increased permeability and the resultant dissemination of the commensal microbiota. This translocation of the luminal contents into the lamina propria persistently stimulates the immune system leading to its hyper-activation and eventual damage to the intestine. IBD can occur in two different forms, through either ulcerative colitis, which affects only the large intestine, or Crohn’s Disease, which can occur anywhere in the gastrointestinal tract. This idea that IBD is caused by the improper localization of the microbiota and other luminal contents is largely supported through animal models of intestinal inflammation in that it is difficult to elicit these diseases in germ-free conditions [150]. In animal models, decreased epithelial resistance has been shown to precede microscopic inflammation [151]. This highlights the importance of maintaining a healthy epithelial barrier to protect and regulate the permeability and translocation of the microbiota.
An important element in maintaining this healthy barrier is the constant maintenance and restoration of the epithelial cells comprising this barrier as these cells age and eventually undergo apoptosis (approximately every week). To maintain a healthy barrier the epithelial cells are constantly in a balance of proliferation, migration, and differentiation, migrating from the base of the crypts to the crypt surface or villous tip. Once their journey is complete, these epithelial cells are removed through shedding/apoptosis that does not result in inflammation, normally associated with mass apoptosis. In a disease state, such as IBD, this apoptosis is greatly upregulated resulting in damage and increased permeability in the epithelial barrier and impairment of its basic functions.
As mentioned before, one of the key factors maintaining the integrity and permeability of the epithelial barrier are the TJ. Additionally, inflammatory conditions can influence this regulation resulting in alterations in the mucosal barrier. Increases in proinflammatory cytokine such as TNFα, IL-4, IL-6 and IL-13 have all been shown to increase epithelial permeability and have been tied to increased expression of claudin-2 in animal and human models as well as decreased expression of JAM-A and occludin [152, 153]. For example, TNFα is responsible for the removal of claudin-1 from tight junctions. TNFα also induces occludin degradation while promoting MLCK phosphorylation thus resulting in augmented paracellular permeability [154, 155]. Not only can the expression of these TJ proteins be influenced but also their localization within the cell can be dysregulated. Claudins 3, 5 and 8 as well as occludin and JAM-A have all been observed to be internalized rather than expressed on the membrane in biopsies from patients with colitis [156]. These effects on the TJ proteins by inflammatory cytokines is in part mediated by myosin light-chain phosphorylation through myosin light-chain kinase (MLCK). This phosphorylation induces actomyosin contraction that can lead to openings in the junctional gap. In fact, mice continuously expressing MLCK are more susceptible to experimental colitis [157]. Furthermore, improper activation of protein kinase C and Rho can modulate the actin cytoskeleton and influence tight junction regulation and function [158].
In addition to causing a leaky barrier, IBD and ulcerative colitis negatively impact bone [62, 137, 159, 160]. Patients with IBD have a 40% higher risk of developing osteoporosis than the general population [161]. Inflammatory cytokines also increase in IBD and it is know that they can have negative effects in the bone [162]. Although it is not well known whether loss of intestinal barrier per se in IBD patients is causal to bone loss in these patients (see chapter on IBD and bone for further details), our lab has shown that intestinal inflammation without weight loss in an IBD model can lead to significant bone loss suggesting a link [85]. Despite these results, more studies need to be perform to further understand the role of intestinal disruption in IBD effects on bone.
4.3 Type 1 Diabetes
Type 1 diabetes (T1D) is characterized by hyperglycemia and hypoinsulinemia and requires treatment with exogenous insulin therapy. Intestinal health has been shown to play a key role in the development of T1D [163–165]. Additionally, T1D induced changes in intestinal health and function have been suggested to contribute to further T1D complications, such as osteoporosis [166]. Intestinal changes that have been reported to precede or be caused by T1D which can influence bone health include intestinal barrier function or permeability and the intestinal microbiota [167–174].
Intestinal barrier function has been implicated in the development of T1D [167, 171, 174–177]. In rodent models of T1D, intestinal permeability or the “leakiness” of the gut has been studied by measuring the amount of disaccharides and monosaccharides in the urine following their oral administration. The spontaneously diabetic biobreeding (BB) rat model of T1D shows an increased amount of permeability in the stomach, small intestine and the colon [169]. The increased permeability in both the stomach and small intestine appear prior to the development of overt diabetic symptoms [169]. During the pre-diabetes stage, BB rats that are diabetes prone have increased intestinal permeability, altered tight junction proteins (specifically claudin 7), increased gut infiltration by neutrophils and decreased numbers of gut natural killer cells in comparison to BB rats which were diabetes resistant [168, 178].
Examination of intestinal permeability in human patients with T1D has been limited and has shown diverse outcomes. An initial study examining the permeability of the monosaccharide mannitol in T1D patients showed an increase in intestinal permeability [172, 179]. However, a subsequent study using pediatric T1D patients showed no difference in the permeability to lactulose or mannitol except in patients with a high-risk allele for celiac disease [172]. As of now, the role of gut permeability in T1D is not well understood and further research is needed to understand how hypoinsulinemia affects barrier function.
The gastrointestinal system has the largest immune population in the body and creates an interface between the external environment and the host and has been linked with numerous other autoimmune diseases [180] in addition to T1D. T1D is an autoimmune condition resulting from T-cell mediated destruction of insulin-secreting pancreatic β cells. As the gut houses the largest population of immune cells, it is not surprising that alterations in the intestine can predispose patients to the development of T1D. Furthermore, microbial communities within the intestine have also been shown to alter immune cell number and differentiation [181–186].
Development of T1D has been shown to be preceded by changes in the microbiome in both human and rodent studies. Studies examining the microbiome in T1D patients and control subjects found that T1D patients had less microbial diversity, less microbial population similarities between individual patients, as well as an increase in non-butyrate producing bacteria when compared with non-diabetic subjects [187]. In a study examining the microbiota in children prior to the development to T1D (children were negative for anti-islet antibodies, however, they possessed the predisposing HLA genotypes), several bacterial taxa correlated with the development of anti-islet antibodies found in T1D children; indicating that alterations in the microbiome may precede the development of T1D [188]. In rodent models of T1D (non-obese diabetic (NOD) mouse and biobreeding rat (BBR)), exposure to specific bacterial strains or metabolic products as well as vivarium hygiene can modulate T1D incidence [189–199]. Furthermore, comparison of the microbiome between NOD and the genetically related, but T1D-resistant mouse (NOR) demonstrated an increase in beneficial microbe populations in NOR mice [200]. Fecal transplant of NOD stool into NOR mice increased insulin resistance, however NOR stool transplant into NOD mice did not prevent the development of T1D [200]. As the microbiome is highly influential in the development and activity of the immune system within the gut, researchers have sought to determine the role of intestinal immune function in the development of T1D. In humans with T1D, duodenal samples had increased expression of pro-inflammatory cytokines, increased leukocytic infiltration, as well as alterations in microbial populations within the microbiome (increase in Firmicutes), which all contributed to a pro-inflammatory environment as compared to healthy controls [201].
As the microbiome was found to be altered in both human and rodents with T1D, studies have gone on to show that T1D prevention can be achieved by supplementation with both prebiotics and probiotics. Recently, in the non-obese diabetic (NOD) mouse model, T1D severity was shown to be inversely correlated with levels of acetate and butyrate, microbial metabolites. When these metabolites were replaced in the diet, NOD mice were protected from the development of T1D [202]. Furthermore, the authors found that acetate supplementation decreased the activation of autoreactive T cells while butyrate supplementation increased the number and function of regulatory T cells, thereby preventing autoimmune development of T1D [202]. Several studies examining different probiotics have shown that altering the microbiome can influence both the inflammatory state of the intestine as well as prevent the progression of T1D [203].
In addition to intestinal changes, T1D is associated with many complications including bone loss [204][205][206] (Figure 3). Our lab demonstrated that treatment with the probiotic Lactobacillus reuteri 6475 prevents T1D-induced bone loss in mice; suggesting a role of the gut microbiota in T1D-induce bone loss. Interestingly, the probiotic treatment prevented several of the bone pathologies of T1D including marrow adiposity, suprpressed Wnt10b expression and suppressed osteoblast activity [149]. The prevention of bone loss occurred despite metabolic dysregulation as indicated by high blood glucose levels. It remains to be seen whether T1D-induced changes in gut permeability can directly influence T1D bone loss. Even if not causal, it would be of interest to examine if reversing increases in T1D-induced intestinal permeability can also reverse T1D bone loss.
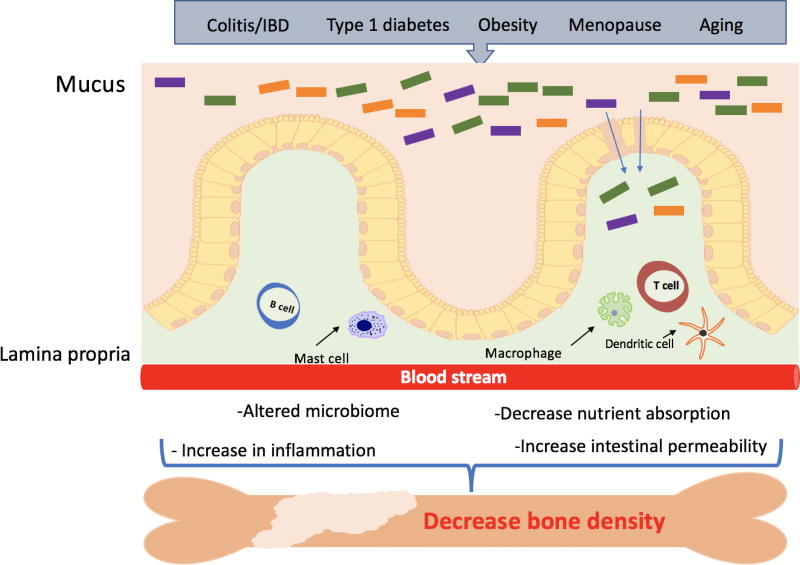
Figure 3. Model of intestinal epithelial disruption signals that can regulate bone density.
Many factors such as aging, menopause and metabolic diseases are known to disrupt the intestinal epithelial layer. They can modulate gut microbiota composition and activity, increase intestinal permeability, inflammation and decrease nutrient absorption. These changes can result in local and systemic responses that can affect bone density.
4.4 Obesity
Obesity is typically associated with several metabolic disorders and is characterized by low-grade inflammation; the molecular origin of which remains unclear [207]. Several studies have reported that serum levels of bacterial LPS are modestly increased in a high-fat diet and that LPS is capable of inducing metabolic disease onset [208–211]. This increase in serum LPS suggests that in obesity the intestinal barrier is compromised. In vivo, animal models of obesity have demonstrated increased whole intestinal permeability via measurement of 4kD FITC-dextran transport to the serum [209, 210] (Figure 3). Ex vivo, studies utilizing the Ussing chamber have reported increased permeability in the small intestine [211]. Interestingly, while the large intestine has the highest bacterial density and highest levels of LPS, experimental models do not support a definitive causative role for colonic gut barrier dysfunction in obesity. However, a role for increased colonic permeability in obesity cannot be conclusively ruled out as further specific studies are required [212].
The effect of obesity on intestinal permeability in humans is inconclusive. Studies using lactulose (L) and mannitol (M), two sugar probes commonly used to evaluate small intestinal permeability in humans, have shown either no change or modestly increased permeability [212]. In a study investigating obese patients with nonalcoholic steatohepatitis (NASH) the ratio of L/M excreted was similar to healthy controls suggesting no change in intestinal permeability [213]. This is supported in a study by Brignardello et al [214] that looked at gut permeability in asymptomatic, non-smoking obese volunteers and observed no differences compared to healthy controls. In contrast, a study by Teixeira et al [215] reported obese females exhibited higher levels of lactulose excretion but not mannitol than the lean controls; suggesting that small intestinal paracellular permeability may be altered in obese individuals.
Investigations into the mechanisms behind the increased intestinal permeability in animal models have focused on expression of the TJ proteins. In a study by Brun et al [211] using ob/ob and db/db mice, distribution of occludin and zonula occludens-1 (ZO-1) were reported to be profoundly modified in the small intestine; suggesting disruption of TJ links with the cytoskeleton, a condition known to compromise the sealing properties of TJs [211]. Obesity-induced changes to TJ expression are further supported in studies by Cani et al [209, 210]. In these studies, small intestine gene expression of ZO-1 and occludin were reduced in mice fed a high-fat diet and distribution altered in ob/ob mice. Expression of these genes had a significant negative correlation with intestinal permeability [209, 210].
An increase in body weight due to obesity has been commonly considered to have a positive effect on bone. However, recent studies demonstrated that bone quality can be compromised in obesity [216, 217]. As mentioned before, obesity can have several effects on gut epithelium, but their effects on bone density are not well known. It has been suggested that a high fat diet may affect intestinal calcium absorption and therefore decrease bone formation. Free fatty acids can form unabsorbable insoluble calcium soaps and therefore decrease calcium absorption [218]. Several gut peptides whose levels are altered in obesity, such as ghrelin and incretins, may be involved in bone metabolism [219][220][221]. Future studies need to be perform to further understand the role of obesity and complex effects on the intestine and their subsequent impact on bone health.
Conclusions
In this chapter, we discussed the importance of the intestinal barrier in maintaining host homeostasis. We discussed different non-invasive methods such as serum, fecal, and urine biomarkers, to further understand intestinal health. We also present information in how disruption of this barrier can have detrimental effects in the host including effects on bone. Factors such as inflammation, changes in microbiota, aging, menopause and disease have been shown to dysregulate this barrier. In addition, there is now emerging evidence that dysregulation of the intestinal barrier can affect distant organs such as the bone.
References
- 1.Ma TY, Anderson JM, Turner JR. Tight Junctions and the Intestinal Barrier. Physiol Gastrointest Tract. 2012 doi: 10.1016/B978-0-12-382026-6.00038-5. [DOI] [Google Scholar]
- 2.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016;14:127–38. doi: 10.5217/ir.2016.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH. Intestinal Permeability Regulation by Tight Junction: Implication on Inflammatory Bowel Diseases. Intest Res. 2015;13:11–8. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 6.Gong J, Hu M, Huang Z, Fang K, Wang D, Chen Q, Li J, Yang D, Zou X, Xu L, Wang K, Dong H, Lu F. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front Pharmacol. 2017;8:42. doi: 10.3389/fphar.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox AJ, Zhang P, Bowden DW, Devereaux B, Davoren PM, Cripps AW, West NP. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2016;43:2–5. doi: 10.1016/j.diabet.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz AT, Jones RM, Pacifici R. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol. 2010;6:506–517. [PMC free article] [PubMed] [Google Scholar]
- 10.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 11.Tulstrup MVL, Christensen EG, Carvalho V, Linninge C, Ahrné S, Højberg O, Licht TR, Bahl MI. Antibiotic Treatment Affects Intestinal Permeability and Gut Microbial Composition in Wistar Rats Dependent on Antibiotic Class. PLoS One. 2015 doi: 10.1371/journal.pone.0144854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. doi: 10.4049/jimmunol.178.7.4641. doi: 178/7/4641 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol - Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 14.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124 doi: 10.1016/j.jaci.2009.05.038. 3-20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183:1871–1884. doi: 10.1016/j.ajpath.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2363. [PubMed] [Google Scholar]
- 18.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke J-D. Changes in expression and distribution of claudin 2,5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratù R. Intestinal permeability in Crohn’s disease patients and their first degree relatives. Dig Liver Dis. 2001;33:680–685. doi: 10.1016/s1590-8658(01)80045-1. [DOI] [PubMed] [Google Scholar]
- 23.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enss ML, Cornberg M, Wagner S, Gebert A, Henrichs M, Eisenblätter R, Beil W, Kownatzki R, Hedrich HJ. Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res. 2000;49:162–169. doi: 10.1007/s000110050576. [DOI] [PubMed] [Google Scholar]
- 25.Biochemistr. Ahn D-H, Crawley SC, Hokari R, Kato S, Yang SC, Li J-D, Kim Young S, Kim YS. TNF-alpha Activates MUC2 Transcription via NF-kappaB but Inhibits via JNK Activation. Cell Physiol Biochem. 2005;15:29–40. doi: 10.1159/000083636. [DOI] [PubMed] [Google Scholar]
- 26.Hasnain SZ, Tauro S, Das I, Tong H, Chen AH, Jeffery PL, McDonald V, Florin TH, McGuckin MA. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology. 2013;144:357–368.e9. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 27.Deplancke B, Gaskins HR. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001;73 doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 28.Finnie IA, Dwarakanath AD, Taylor BA, Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut. 1995;36:93–9. doi: 10.1136/gut.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 30.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:941–950. doi: 10.1136/gut.35.4.483. [DOI] [PubMed] [Google Scholar]
- 31.Barcelo A, Claustre J, Moro F, Chayvialle Ja, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JD, Feng W, Gallup M, Kim JH, Gum J, Kim Y, Basbaum C. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc Natl Acad Sci U S A. 1998;95:5718–23. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogler G, Biedermann L. Clinical Utility of Biomarkers in IBD. Curr Gastroenterol Rep. 2015 doi: 10.1007/s11894-015-0449-x. [DOI] [PubMed] [Google Scholar]
- 35.Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, Moum B. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–23. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 36.Shine B, Berghouse L, Jones JEL, Landon J. C-Reactive protein as an aid in the differentiation of functional and inflammatory bowel disorders. Clin Chim Acta. 1985;148:105–109. doi: 10.1016/0009-8981(85)90219-0. [DOI] [PubMed] [Google Scholar]
- 37.Benor S, Russell GH, Silver M, Israel EJ, Yuan Q, Winter HS. Shortcomings of the inflammatory bowel disease Serology 7 panel. Pediatrics. 2010;125:1230–6. doi: 10.1542/peds.2009-1936. [DOI] [PubMed] [Google Scholar]
- 38.Sun XQ, Fu XB, Zhang R-, Lü Y, Deng Q, Jiang XG, Sheng ZY. Relationship between plasma D(−)-lactate and intestinal damage after severe injuries in rats. World J Gastroenterol. 2001;7:555–558. doi: 10.3748/wjg.v7.i4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vreugdenhil AC, Wolters VM, Adriaanse MP, Van den Neucker AM, van Bijnen AA, Houwen R, Buurman WA. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol. 2011;46:1435–1441. doi: 10.3109/00365521.2011.627447. [DOI] [PubMed] [Google Scholar]
- 40.Adriaanse MPM, Tack GJ, Passos VL, Damoiseaux JGMC, Schreurs MWJ, Van Wijck K, Riedl RG, Masclee AAM, Buurman WA, Mulder CJJ, Vreugdenhil ACE. Serum I-FABP as marker for enterocyte damage in coeliac disease and its relation to villous atrophy and circulating autoantibodies. Aliment Pharmacol Ther. 2013;37:482–490. doi: 10.1111/apt.12194. [DOI] [PubMed] [Google Scholar]
- 41.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339–343. doi: 10.1053/gast.1996.v110.pm8566578. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundberg J, Hellström P. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract. 2005;2:96–102. doi: 10.1038/ncpgasthep0094. [DOI] [PubMed] [Google Scholar]
- 44.Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14–22. doi: 10.1097/00005176-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Derikx JP, Luyer MD, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol. 2010;16:5272–5279. doi: 10.3748/wjg.v16.i42.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med. 2013;19:12–24. [PubMed] [Google Scholar]
- 47.Fink MP. Interpreting dual-sugar absorption studies in critically ill patients: What are the implications of apparent increases in intestinal permeability to hydrophilic solutes? Intensive Care Med. 1997;23:489–492. doi: 10.1007/s001340050363. [DOI] [PubMed] [Google Scholar]
- 48.Kerckhoffs APM, Akkermans LMA, De Smet MBM, Besselink MGH, Hietbrink F, Bartelink IH, Busschers WB, Samsom M, Renooij W. Intestinal permeability in irritable bowel syndrome patients: Effects of NSAIDs. Dig Dis Sci. 2010;55:716–723. doi: 10.1007/s10620-009-0765-9. [DOI] [PubMed] [Google Scholar]
- 49.Olaison G, Leandersson P, Sjödahl R, Tagesson C. Intestinal permeability to polyethyleneglycol 600 in Crohn’s disease. Peroperative determination in a defined segment of the small intestine. Gut. 1988;29:196–199. doi: 10.1136/gut.29.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Söderholm JD, Yates DA, Gareau MG, Yang P, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- 52.Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18:923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 54.Weaver LT, Laker MF, Nelson R, Lucas A. Milk feeding and changes in intestinal permeability and morphology in the newborn. J Pediatr Gastroenterol Nutr. 1987;6:351–358. doi: 10.1097/00005176-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Bergmann KR, Liu SXL, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1596–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biol-N’garagba MC, Louisot P. Regulation of the intestinal glycoprotein glycosylation during postnatal development: Role of hormonal and nutritional factors. Biochimie. 2003;85:331–352. doi: 10.1016/S0300-9084(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 57.Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI, Warner BB, Gordon JI. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534:263–6. doi: 10.1038/nature17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurl S, Heinonen K, Lansimies E, Launiala K. Determinants of bone mineral density in prematurely born children aged 6–7 years. Acta Paediatr. 1998;87:650–653. doi: 10.1111/j.1651-2227.1998.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 60.Takada M, Shimada M, Hosono S, Tauchi M, Minato S, Takahashi M, Okuni S, Takeuchi S. Trace elements and mineral requirements for very low birth weight infants in rickets of prematurity. Early Hum Dev. 1992;29:333–338. doi: 10.1016/0378-3782(92)90188-M. [DOI] [PubMed] [Google Scholar]
- 61.Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3:e1000707. doi: 10.1080/21688370.2014.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris L, Senagore P, Young VB, McCabe LR. Inflammatory bowel disease causes reversible suppression of osteoblast and chondrocyte function in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1020–9. doi: 10.1152/ajpgi.90696.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall KE, Proctor DD, Fisher L, Rose S. American Gastroenterological Association future trends committee report: Effects of aging of the population on gastroenterology practice, education, and research. Gastroenterology. 2005;129:1305–1338. doi: 10.1053/j.gastro.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Hollander D, Tarnawski H. Aging-associated increase in intestinal absorption of macromolecules. Gerontology. 1985;31:133–137. doi: 10.1159/000212694. [DOI] [PubMed] [Google Scholar]
- 65.Ma TY, Hollander D, Dadufalza V, Krugliak P. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol. 1992;27:321–333. doi: 10.1016/0531-5565(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal Physiology and Function. Handb Exp Pharmacol. 2017 doi: 10.1007/164_2016_118. [DOI] [PubMed] [Google Scholar]
- 67.Mitchell EL, Davis AT, Brass K, Dendinger M, Barner R, Gharaibeh R, Fodor AA, Kavanagh K. Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J Nutr Heal Aging. 2017;21:354–361. doi: 10.1007/s12603-016-0725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valentini L, Ramminger S, Haas V, Postrach E, Werich M, Fischer A, Koller M, Swidsinski A, Bereswill S, Lochs H, Schulzke J-D. Small intestinal permeability in older adults. Physiol Rep. 2014;2:e00281–e00281. doi: 10.14814/phy2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, Watson AJM, Nicoletti C. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci. 2015;129:515–527. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]
- 70.Weng N ping. Aging of the Immune System: How Much Can the Adaptive Immune System Adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez CR, Gomez CR, Boehmer ED, Boehmer ED, Kovacs EJ, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Ahmadi-Abhari S, Luben RN, Wareham NJ, Khaw KT. Distribution and determinants of C-reactive protein in the older adult population: European prospective investigation into cancer-norfolk study. Eur J Clin Invest. 2013;43:899–911. doi: 10.1111/eci.12116. [DOI] [PubMed] [Google Scholar]
- 73.Nicoletti C. Age-associated changes of the intestinal epithelial barrier: local and systemic implications. Expert Rev Gastroenterol Hepatol. 2015;9:1467–1469. doi: 10.1586/17474124.2015.1092872. [DOI] [PubMed] [Google Scholar]
- 74.Man AL, Gicheva N, Nicoletti C. The impact of ageing on the intestinal epithelial barrier and immune system. Cell Immunol. 2014;289:112–118. doi: 10.1016/j.cellimm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Tran L, Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. Journals Gerontol - Ser A Biol Sci Med Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kovacs EJ, Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm. 2010 doi: 10.1155/2010/475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 78.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic Approaches for Defining the Pathogenesis of Inflammatory Bowel Diseases. Cell Host Microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neish AS. Microbes in Gastrointestinal Health and Disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, de Vos W. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010 doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- 82.Woodmansey EJ, McMurdo MET, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–6122. doi: 10.1128/AEM.70.10.6113-6122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet J-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. 2013;19:1586–97. doi: 10.1097/MIB.0b013e318289e17b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Irwin R, Raehtz S, Parameswaran N, McCabe LR. Intestinal inflammation without weight loss decreases bone density and growth. Am J Physiol - Regul Integr Comp Physiol. 2016;311:R1149–R1157. doi: 10.1152/ajpregu.00051.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson J-A. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 87.Campbell-Thompson M, Lynch IJ, Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–40. [PubMed] [Google Scholar]
- 88.Konstantinopoulos PA, Kominea A, Vandoros G, Sykiotis GP, Andricopoulos P, Varakis I, Sotiropoulou-Bonikou G, Papavassiliou AG. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur J Cancer. 2003;39:1251–8. doi: 10.1016/s0959-8049(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 89.Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heijmans J, Wielenga MCB, Rosekrans SL, van Lidth de Jeude JF, Roelofs J, Groothuis P, Ederveen A, de Jonge-Muller ESM, Biemond I, Hardwick JCH, D’Haens G, Hommes DW, Muncan V, van den Brink GR. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. 2014;63:310–6. doi: 10.1136/gutjnl-2012-304216. [DOI] [PubMed] [Google Scholar]
- 91.Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, Martin PG, Theodorou V, Fioramonti J, Houdeau E. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107:448–53. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL, Langen ML, Kl M. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–6. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 93.Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson J-A. Role of estrogen receptor beta in colonic epithelium. Proc Natl Acad Sci U S A. 2006;103:1605–1608. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, McCabe LR, Parameswaran N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. 2017;5:e13263. doi: 10.14814/phy2.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dong XL, Zhang Y, Wong MS. Estrogen deficiency-induced Ca balance impairment is associated with decrease in expression of epithelial Ca transport proteins in aged female rats. Life Sci. 2014;96:26–32. doi: 10.1016/j.lfs.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 96.Hoenderop JGJ, Nilius B, Bindels RJM. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- 97.Prince RL, Smith M, Dick IM, Price RI, Webb PG, Henderson NK, Harris MM. Prevention of postmenopausal osteoporosis. A comparative study of exercise, calcium supplementation, and hormone-replacement therapy. N Engl J Med. 1991;325:1189–95. doi: 10.1056/NEJM199110243251701. [DOI] [PubMed] [Google Scholar]
- 98.Heshmati HM, Khosla S, Burritt MF, O’Fallon WM, Riggs BL. A defect in renal calcium conservation may contribute to the pathogenesis of postmenopausal osteoporosis. J Clin Endocrinol Metab. 1998;83:1916–20. doi: 10.1210/jcem.83.6.4854. [DOI] [PubMed] [Google Scholar]
- 99.O’Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511(Pt 1):313–22. doi: 10.1111/j.1469-7793.1998.313bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511:313–322. doi: 10.1111/j.1469-7793.1998.313bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwartz B, Smirnoff P, Shany S, Liel Y. Estrogen controls expression and bioresponse of 1,25-dihydroxyvitamin D receptors in the rat colon. Mol Cell Biochem. 2000;203:87–93. doi: 10.1023/A:1007015027268. [DOI] [PubMed] [Google Scholar]
- 102.Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577–92. doi: 10.1677/joe.1.05770. [DOI] [PubMed] [Google Scholar]
- 103.Gabriel SE, Tosteson aNa, Leibson CL, Crowson CS, Pond GR, Hammond CS, Melton LJ. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–30. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 104.Britton Ra, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri Treatment Prevents Bone Loss in a Menopausal Ovariectomized Mouse Model. J Cell Physiol. 2014;229:1822–30. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–93. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun J, Chang EB. Exploring gut microbes in human health and disease: Pushing the envelope. Genes Dis. 2014;1:132–139. doi: 10.1016/j.gendis.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frey A, Giannasca KT, Weltzin R, Giannasca PJ, Reggio H, Lencer WI, Neutra MR. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–59. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–24. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 110.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 111.Shen L, Turner JR. Role of Epithelial Cells in Initiation and Propagation of Intestinal Inflammation. Eliminating the static: tight junction dynamics exposed. Am. J. Physiol. - Gastrointest. Liver Physiol. 2006;290 doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 112.Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci. 2011;108:4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 115.Kang SS, Jeraldo PR, Kurti A, Miller ME, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, Fryer JD. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40 doi: 10.1111/apt.12878. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 117.Myers SP. The Causes of Intestinal Dysbiosis. Altern Med Rev xAltern Med Rev. 2004;99:180–197. [PubMed] [Google Scholar]
- 118.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, Inoue N, Ogata H, Iwao Y, Nomoto K, Tanaka R, Hibi T. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 119.Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42(Suppl 1):41–7. doi: 10.1007/s00535-006-1925-8. [DOI] [PubMed] [Google Scholar]
- 120.Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, Antonopoulos DA, Smith D, Chang EB, Ciancio MJ. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS One. 2014;9:e92193. doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:569. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 123.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788:864–71. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–7. [PubMed] [Google Scholar]
- 125.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–88. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 126.Wrzosek L, Miquel S, Noordine M-L, Bouet S, Chevalier-Curt M, Robert V, Philippe C, Bridonneau C, Cherbuy C, Robbe-Masselot C, Langella P, Thomas M. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa Augment In Vitro Utilization of Mucin by Other Bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 128.van der Post S, Subramani DB, Backstrom M, Johansson MEV, Vester-Christensen MB, Mandel U, Bennett EP, Clausen H, Dahlen G, Sroka A, Potempa J, Hansson GC. Site-specific O-Glycosylation on the MUC2 Mucin Protein Inhibits Cleavage by the Porphyromonas gingivalis Secreted Cysteine Protease (RgpB) J Biol Chem. 2013;288:14636–14646. doi: 10.1074/jbc.M113.459479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun J, Shen X, Li Y, Guo Z, Zhu W, Zuo L, Zhao J, Gu L, Gong J, Li J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients. 2016 doi: 10.3390/nu8010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci. 2016;113:E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Villa CR, Ward WE, Comelli EM. Gut Microbiota-bone Axis. Crit Rev Food Sci Nutr. 2015 doi: 10.1080/10408398.2015.1010034. [DOI] [PubMed] [Google Scholar]
- 132.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang K, Hornef MW, Dupont A. The intestinal epithelium as guardian of gut barrier integrity. Cell Microbiol. 2015 doi: 10.1111/cmi.12501. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 134.McCabe LR, Irwin R, Schaefer L, Britton Ra. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228:1793–8. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS One. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan J, Charles JF. Gut Microbiome and Bone: to Build, Destroy, or Both? 2017 doi: 10.1007/s11914-017-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis. 2013;19:1586. doi: 10.1097/mib.0b013e318289e17b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA. Proinflammatory cytokine (TNF?/IL-1?) induction of human osteoclast formation. J Pathol. 2002;198:220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 140.Weitzmann MN, Pacifici R. T Cells: Unexpected Players in the Bone Loss Induced by Estrogen Deficiency and in Basal Bone Homeostasis. Ann N Y Acad Sci. 2007;1116:360–375. doi: 10.1196/annals.1402.068. [DOI] [PubMed] [Google Scholar]
- 141.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco M-J, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 142.Wu RY, Abdullah M, Määttänen P, Pilar AVC, Scruten E, Johnson-Henry KC, Napper S, O’Brien C, Jones NL, Sherman PM. Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci Rep. 2017;7:40820. doi: 10.1038/srep40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.García-Vieyra MI, Del Real A, López MG. Agave fructans: their effect on mineral absorption and bone mineral content. J Med Food. 2014;17:1247–55. doi: 10.1089/jmf.2013.0137. [DOI] [PubMed] [Google Scholar]
- 144.Takahara S, Morohashi T, Sano T, Ohta A, Yamada S, Sasa R. Fructooligosaccharide consumption enhances femoral bone volume and mineral concentrations in rats. J Nutr. 2000;130:1792–5. doi: 10.1093/jn/130.7.1792. [DOI] [PubMed] [Google Scholar]
- 145.Chonan O, Matsumoto K, Watanuki M. Effect of galactooligosaccharides on calcium absorption and preventing bone loss in ovariectomized rats. Biosci Biotechnol Biochem. 1995;59:236–9. doi: 10.1271/bbb.59.236. [DOI] [PubMed] [Google Scholar]
- 146.Ohta A, Uehara M, Sakai K, Takasaki M, Adlercreutz H, Morohashi T, Ishimi Y. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr. 2002;132:2048–54. doi: 10.1093/jn/132.7.2048. [DOI] [PubMed] [Google Scholar]
- 147.Liu H-Y, Roos S, Jonsson H, Ahl D, Dicksved J, Lindberg JE, Lundh T. Effects of Lactobacillus johnsonii and Lactobacillus reuteri on gut barrier function and heat shock proteins in intestinal porcine epithelial cells. Physiol Rep. 2015;3:e12355–e12355. doi: 10.14814/phy2.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, McCabe LR. Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting. PLoS One. 2016;11:e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue Sa, Pizarro TT, Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–18. doi: 10.4049/jimmunol.178.3.1809. doi: 178/3/1809 [pii] [DOI] [PubMed] [Google Scholar]
- 151.Suenaert P, Maerten P, Van Assche G, Van Driessche W, Geboes K, Bulteel V, Simaels J, Augustijns P, Ceuppens JL, Rutgeerts P, Perrier C. Effects of T cell-induced colonic inflammation on epithelial barrier function. Inflamm Bowel Dis. 2010;16:1322–31. doi: 10.1002/ibd.21211. [DOI] [PubMed] [Google Scholar]
- 152.Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol. 2010;2010:484987. doi: 10.1155/2010/484987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–71. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke J-D. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–55. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 155.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–33. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vetrano S, Rescigno M, Cera MR, Correale C, Rumio C, Doni A, Fantini M, Sturm A, Borroni E, Repici A, Locati M, Malesci A, Dejana E, Danese S. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–84. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Investig. 2006;863700373:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 158.Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trülzsch K, Müller-Höcker J, Vogelmann R, Allgäuer M, Gerhard M, Steininger S, Wolf E, Kolligs FT. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. doi: 10.1371/journal.pone.0014325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2010;300:G191–201. doi: 10.1152/ajpgi.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bianchi ML. Inflammatory bowel diseases, celiac disease, and bone. Arch Biochem Biophys. 2010;503:54–65. doi: 10.1016/j.abb.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 161.Compston JE, Judd D, Crawley EO, Evans WD, Evans C, Church HA, Reid EM, Rhodes J. Osteoporosis in patients with inflammatory bowel disease. Gut. 1987;28:410–5. doi: 10.1136/gut.28.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NHH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000;95:359–367. doi: 10.1016/S0002-9270(99)00849-7. [DOI] [PubMed] [Google Scholar]
- 163.Daft JG, Lorenz RG. Role of the gastrointestinal ecosystem in the development of type 1 diabetes. Pediatr Diabetes. 2015;16:407–418. doi: 10.1111/pedi.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paun A, Yau C, Danska JS. Immune recognition and response to the intestinal microbiome in type 1 diabetes. J Autoimmun. 2016;71:10–18. doi: 10.1016/j.jaut.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 165.Gülden E, Wong FS, Wen L. The gut microbiota and Type 1 Diabetes. Clin Immunol. 2015;159:143–153. doi: 10.1016/j.clim.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vaarala O, Atkinson Ma, Neu J. The “perfect storm” for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–62. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, Li N, Caicedo RA, Schatz DA, Atkinson M. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2005;40:589–95. doi: 10.1097/01.mpg.0000159636.19346.c1. [DOI] [PubMed] [Google Scholar]
- 169.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 170.Graham S, Courtois P, Malaisse WJ, Rozing J, Scott FW, Mowat AMI. Enteropathy precedes type 1 diabetes in the BB rat. Gut. 2004;53:1437–44. doi: 10.1136/gut.2004.042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 172.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002;35:365–8. doi: 10.1080/0891693021000008526. [DOI] [PubMed] [Google Scholar]
- 173.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 174.Secondulfo M, Iafusco D, Carratù R, DeMagistris L, Sapone A, Generoso M, Mezzogiorno A, Sasso FC, Cartenì M, De Rosa R, Prisco F, Esposito V. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. 2004;36:35–45. doi: 10.1016/j.dld.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 175.Vaarala O. Is the origin of type 1 diabetes in the gut? Immunol Cell Biol. 2012;90:271–6. doi: 10.1038/icb.2011.115. [DOI] [PubMed] [Google Scholar]
- 176.Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–748. doi: 10.1007/s00125-009-1626-y. [DOI] [PubMed] [Google Scholar]
- 177.Hadjiyanni I, Li KK, Drucker DJ. Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology. 2009;150:592–9. doi: 10.1210/en.2008-1228. [DOI] [PubMed] [Google Scholar]
- 178.Todd DJ, Forsberg EM, Greiner DL, Mordes JP, Rossini AA, Bortell R. Deficiencies in gut NK cell number and function precede diabetes onset in BB rats. J Immunol. 2004;172:5356–62. doi: 10.4049/jimmunol.172.9.5356. [DOI] [PubMed] [Google Scholar]
- 179.Carratù R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Cartenì M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr. 1999;28:264–9. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 180.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–8. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 181.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 183.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 184.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science (80-.) 2011;331 doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science (80-.) 2013;341 doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, Casella G, Drew JC, Ilonen J, Knip M, Hyöty H, Veijola R, Simell T, Simell O, Neu J, Wasserfall CH, Schatz D, Atkinson MA, Triplett EW. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS One. 2011;6:e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, Vaarala O. Fecal Microbiota Composition Differs Between Children With -Cell Autoimmunity and Those Without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bach J-F. The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 190.Pozzilli P, Signore A, Williams AJK, Beales PE. NOD mouse colonies around the world- recent facts and figures. Immunol Today. 1993;14:193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 191.Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJC, Dunne Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 192.Shaper AG, Phillips AN, Pocock SJ, Walker M, Macfarlane PW. Risk factors for stroke in middle aged British men. BMJ. 1991;302 doi: 10.1136/bmj.302.6785.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, Alem F, Lagunoff D, Bleich D, Gause WC. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–58. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Raine T, Zaccone P, Mastroeni P, Cooke A. Salmonella typhimurium Infection in Nonobese Diabetic Mice Generates Immunomodulatory Dendritic Cells Able to Prevent Type 1 Diabetes. J. Immunol. 2006;177 doi: 10.4049/jimmunol.177.4.2224. [DOI] [PubMed] [Google Scholar]
- 195.King C, Sarvetnick N, Volchkov P, Stranges P, Avanesyan L. The Incidence of Type-1 Diabetes in NOD Mice Is Modulated by Restricted Flora Not Germ-Free Conditions. PLoS One. 2011;6:e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kriegel MA, Sefik E, Hill JA, Wu H-J, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–53. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rossini AA, Williams RM, Mordes JP, Appel MC, Like AA. Spontaneous Diabetes in the Gnotobiotic BB/W Rat. Diabetes. 1979;28 doi: 10.2337/diab.28.11.1031. [DOI] [PubMed] [Google Scholar]
- 198.Brugman S, Klatter FA, Visser JTJ, Wildeboer-Veloo ACM, Harmsen HJM, Rozing J, Bos NA. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 199.Roesch LF, Lorca GL, Casella G, Giongo A, Naranjo A, Pionzio AM, Li N, Mai V, Wasserfall CH, Schatz D, Atkinson MA, Neu J, Triplett EW. Culture-independent identification of gut bacteria correlated with the onset of diabetes in a rat model. ISME J. 2009;3:536–548. doi: 10.1038/ismej.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gorzelak MA, Chan Y, Chan JM, Lochner A, Dutz JP, Vallance BA, Gibson DL. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10:321–332. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pellegrini S, Sordi V, Bolla AM, Saita D, Ferrarese R, Canducci F, Clementi M, Invernizzi F, Mariani A, Bonfanti R, Barera G, Testoni PA, Doglioni C, Bosi E, Piemonti L. Duodenal Mucosa of Patients With Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J Clin Endocrinol Metab. 2017;102:1468–1477. doi: 10.1210/jc.2016-3222. [DOI] [PubMed] [Google Scholar]
- 202.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, Mackay CR. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol. 2017;18:552–562. doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 203.Dolpady J, Sorini C, Di Pietro C, Cosorich I, Ferrarese R, Saita D, Clementi M, Canducci F, Falcone M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J Diabetes Res. 2016;2016:1–12. doi: 10.1155/2016/7569431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Motyl K, McCabe LR. Streptozotocin, type 1 diabetes severity and bone. Biol Proced Online. 2009;11:296–315. doi: 10.1007/s12575-009-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, Schwartz RC, McCabe LR. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009;218:575–83. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- 206.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 207.Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome - A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 208.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 209.Cani PD, Bibiloni R, Knauf C, Neyrinck AM, Delzenne NM. Changes in gut microbiota control metabolic diet–induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403.Additional. [DOI] [PubMed] [Google Scholar]
- 210.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brun P, Castagliuolo I, Leo V Di, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:518–525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 212.Teixeira TFS, Collado MC, Ferreira CLLF, Bressan J, Peluzio M do CG. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–647. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 213.Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: A possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brignardello J, Morales P, Diaz E, Romero J, Brunser O, Gotteland M. Pilot study: Alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–1314. doi: 10.1111/j.1365-2036.2010.04475.x. [DOI] [PubMed] [Google Scholar]
- 215.Teixeira TFS, Souza NCS, Chiarello PG, Franceschini SCC, Bressan J, Ferreira CLLF, Peluzio M do CG. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–740. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 216.Shapses SA, Sukumar D. Bone Metabolism in Obesity and Weight Loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Carnielli VP, Luijendijk IH, Van Goudoever JB, Sulkers EJ, Boerlage AA, Degenhart HJ, Sauer PJ. Structural position and amount of palmitic acid in infant formulas: effects on fat, fatty acid, and mineral balance. J Pediatr Gastroenterol Nutr. 1996;23:553–560. doi: 10.1097/00005176-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 219.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 220.Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, Takeda S, Takeuchi Y, Fukumoto S, Kangawa K, Nagata K, Kojima M. Ghrelin Directly Regulates Bone Formation. J Bone Miner Res. 2004;20:790–798. doi: 10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- 221.Dicembrini I, Mannucci E, Rotella CM. Bone: Incretin hormones perceiver or receiver? Exp Diabetes Res. 2012;2012:519784. doi: 10.1155/2012/519784. [DOI] [PMC free article] [PubMed] [Google Scholar]