Abstract
During neonatal testis development, centrally located gonocytes migrate to basement membrane of the seminiferous cords, where physical contact with a niche established by Sertoli cells is essential for transition of gonocytes into spermatogonial stem cells (SSCs). To provide structural support and signaling stimuli for the gonocyte-to-SSC transition that occurs at a specific location during a finite phase, temporal-spatial establishment of the niche is critical. To date, the factors that guide Sertoli cells to establish the initial stem cell niche remain largely unknown. Using the Sertoli cell-specific Arid4b knockout (Arid4bSCKO) mice, we demonstrated that ablation of AT-rich interaction domain 4B (ARID4B) resulted in abnormal detachment of Sertoli cells from the basement membrane of seminiferous cords during the gonocyte-to-SSC transition phase, suggesting failure to establish a niche for the SSC formation. Without support by a niche environment, gonocytes showed disarranged cell distribution in the Arid4bSCKO testes and underwent apoptosis. The commitment of gonocytes to differentiate into the spermatogonial lineage was broken and the capability of SSCs to self-renew and differentiate was also impaired. Gene expression profiling revealed the molecular mechanisms responsible for the phenotypic changes in the Arid4bSCKO testes, by identifying genes important for stem cell niche function as downstream effectors of ARID4B, including genes that encode gap junction protein alpha-1, KIT ligand, anti-Müllerian hormone, Glial cell-line derived neurotrophic factor, inhibin alpha, inhibin beta, and cytochrome P450 family 26 subfamily b polypeptide 1. Our results identified ARID4B as a master regulator of a signaling network that governs the establishment of a niche during the critical gonocyte-to-SSC transition phase to control the fate of gonocytes and SSCs.
Keywords: AT-rich interaction domain 4B, Sertoli cell, Gonocyte, Spermatogonia, Spermatogonial stem cell niche
Introduction
Spermatogonial stem cells (SSCs) continuously produced spermatozoa through spermatogenesis [1]. Gonocytes are direct precursors of the SSCs. During neonatal development between postnatal day (P) 0–6, gonocytes enter mitosis to expand the population and differentiate into SSCs [2, 3]. These primary SSCs serve as a foundational self-renewing reservoir to ensure continuous production of spermatozoa throughout adult life. Failure to form the primary SSC pool results in loss of the germ cell lineage, which can lead to male infertility. In rodents, the SSCs are A-single (Asingle) spermatogonia and have the ability to self-renew by undergoing cell divisions to produce two new Asingle spermatogonia. In addition, SSCs can undergo differentiating divisions to form progenitor spermatogonia (Apaired and Aaligned). SSCs and progenitor spermatogonia are collectively described as the undifferentiated spermatogonial population [3, 4]. The Aaligned spermatogonia further differentiate into differentiating spermatogonia that undergo spermatogenic differentiation to produce spermatozoa. While a portion of the gonocyte population transforms into SSCs, another portion of gonocytes bypasses the undifferentiated spermatogonial phase and directly differentiates into differentiating spermatogonia [5]. These gonocyte-derived differentiating spermatogonia contribute to the first round of spermatogenesis, whereas all subsequent rounds of spermatogenesis originate from SSCs [5]. The events that gonocytes differentiate to the spermatogonial lineage occur at a defined phase of neonatal development, whereas SSCs maintain a undifferentiated state with the ability to either self-renew or differentiate throughout adulthood [6]. Although genes and pathways that regulate SSC self-renewal and differentiation have recently garnered extensive interest, the mechanisms that regulate the formation of the primary SSCs from gonocytes are seldom investigated, despite being important for male reproduction.
Sertoli cells, the somatic cells and epithelium inside the seminiferous cords, are the major component of the niche that provides critical architectural support and regulatory factors to establish a niche microenvironment for the formation and maintenance of the SSC population [7, 8]. Before birth, gonocytes are located at the center of the seminiferous cords, whereas Sertoli cells are anchored to the basement membrane. Shortly after birth (P0.5–P3 in mice), gonocytes migrate toward the basement membrane, where physical contact with Sertoli cells occurs [2]. During migration or shortly after contact with Sertoli cells, gonocytes undergo mitotic cell cycle progression and start differentiation, resulting in the formation of the primary SSC population [2, 3]. These events are tightly regulated by extrinsic factors in the niche microenvironment provided by Sertoli cells. For example, cytokines KIT ligand (KITL) and platelet-derived growth factor (PDGF) promote gonocyte migration from the center of seminiferous cords to the basement membrane during neonatal development [9, 10]. Retinoic acid (RA) stimulates differentiation of gonocytes and SSCs [11, 12]. Anti-Müllerian hormone (AMH) increases the number of gonocytes that undergo differentiation to become SSCs [13]. Notch signaling regulates the timing of gonocyte differentiation [14]. Other proteins produced by Sertoli cells, such as Glial cell-line derived neurotrophic factor (GDNF) [15], gap junction protein alpha-1 (GJA1) [16], cytochrome P450 family 26 subfamily b polypeptide 1 (CYP26B1) [17], and ets variant 5 (ETV5) [18] are indispensable for maintenance of gonocytes and/or SSCs in their niche. Collectively, the formation and maintenance of the SSC pool is controlled by a signaling network provided by the niche microenvironment. However, it remains to be determined how these different signaling pathways are regulated and coordinated to mediate the establishment of the initial SSC niche during neonatal development.
AT-rich interaction domain 4B (ARID4B) is a chromatin remodeling protein that belongs to the ARID family. ARID4B has been identified as a component of the HDAC1/SIN3A chromatin remodeling complex [19]. Although ARID4B does not have the intrinsic methyltransferase nor acetyltransferase activities, ARID4B contains a Tudor domain and a chromo domain [20]. These two domains bind to methylated histones and promote subsequent assembly of the chromatin remodeling complexes [21, 22]. Previously, we reported that mice globally deficient for Arid4b (Arid4b−/−) died during early embryogenesis between embryonic day (E) 3.5 and 7.5 [23]. To overcome embryonic lethality and investigate the function of Arid4b in specific tissue, the conditional knockout mice were generated. Given that ARID4B was mainly expressed in Sertoli cells of the testis [24], we generated the Sertoli cell-specific Arid4b knockout (Arid4bSCKO) mice and uncovered that the mutant males were infertile throughout their lifespan [25]. The Arid4bSCKO mice contain a deleted allele and a floxed allele in the Arid4b gene and a transgene of Cre recombinase driven by the anti-Müllerian hormone promoter (Arid4b−/flox Amh-cre) to obtain the most complete Arid4b deletion in Sertoli cells [25]. In this study, we used the Arid4bSCKO mice and identified Arid4b as an essential gene that governs the temporal and spatial establishment of the initial SSC niche by Sertoli cells during the neonatal testis development. Importantly, we showed that the establishment of an elaborate SSC niche is indispensable for the successful formation of the primary SSC pool from gonocytes and impacts the cell fate decisions of gonocytes and SSCs. Analysis of the mechanisms further revealed that ARID4B functions as a master regulator to control expression of factors critical for the stem cell niche function, including GJA1, KITL, CYP26B1, AMH, GDNF, inhibin alpha (INHA), and inhibin beta B (INHBB). Our study underscores an important role of ARID4B in regulation of the gonocyte-to-SSC transition.
Materials and Methods
Mouse Lines
The Arid4bSCKO (Arid4b−/flox; Amh-cre) mice and their control (Arid4b+/flox; Amh-cre) mice have been described previously [25]. These mice are maintained on a hybrid C57BL6/J and 129/SvEv genetic background. Experimental animals and studies were approved by the Institutional Animal Care and User Committee (IACUC) of The George Washington University (protocol number: A208). All of the mice were bred and maintained at the institution’s specific pathogen-free mouse facility. The facility is approved by the American Association for Accreditation of Laboratory Animal Care and operated in accordance with current regulations and standards of the US Department of Agriculture and the Department of Health and Human Services.
Results
Failure to Establish the Initial Stem Cell Niche in the Arid4bSCKO Testes During Neonatal Development
Previously, we reported expression of Arid4b in Sertoli cells of testes from embryonic day (E)15.5 through P42 [25]. Sertoli cells are the major component of the SSC niche. Using the Arid4bSCKO mice, we investigated whether ablation of ARID4B in Sertoli cells affects the niche establishment. Before birth, gonocytes are in a quiescent state and centrally located in the seminiferous cords, whereas Sertoli cells reside along the periphery of the cords. Histological analyses revealed similar structure and cell distribution of the seminiferous cords between the control and Arid4bSCKO testes at E18.5 (Fig. 1A–1D). To further analyze the cell distribution clearly, double immunofluorescent staining for two Sertoli cells markers, AMH (cytoplasm) and Wilms Tumor 1 (WT1, nuclear), was performed. The result showed that Sertoli cells were properly located along the periphery of the seminiferous cords in both control and Arid4bSCKO testes (Fig. 1E, 1F). To clearly define the location of gonocytes in the seminiferous cords, double immunofluorescent staining for AMH and the gonocyte/undifferentiated spermatogonia marker, promyelocytic leukemia zinc finger (PLZF), was performed. The result showed that gonocytes were located in the lumen of seminiferous cords in the control and Arid4bSCKO testes at E18.5 (Fig. 1G, 1H). These results suggest that no change in cellular distribution was observed in the E18.5 Arid4bSCKO testes. As expected, Arid4b was knocked out in Sertoli cells at this stage, because immunofluorescent analysis detected ARID4B protein in the nuclear region of Sertoli cells only in the control testes but not in the Arid4bSCKO testes (Supporting Information Fig. S1).
Figure 1.
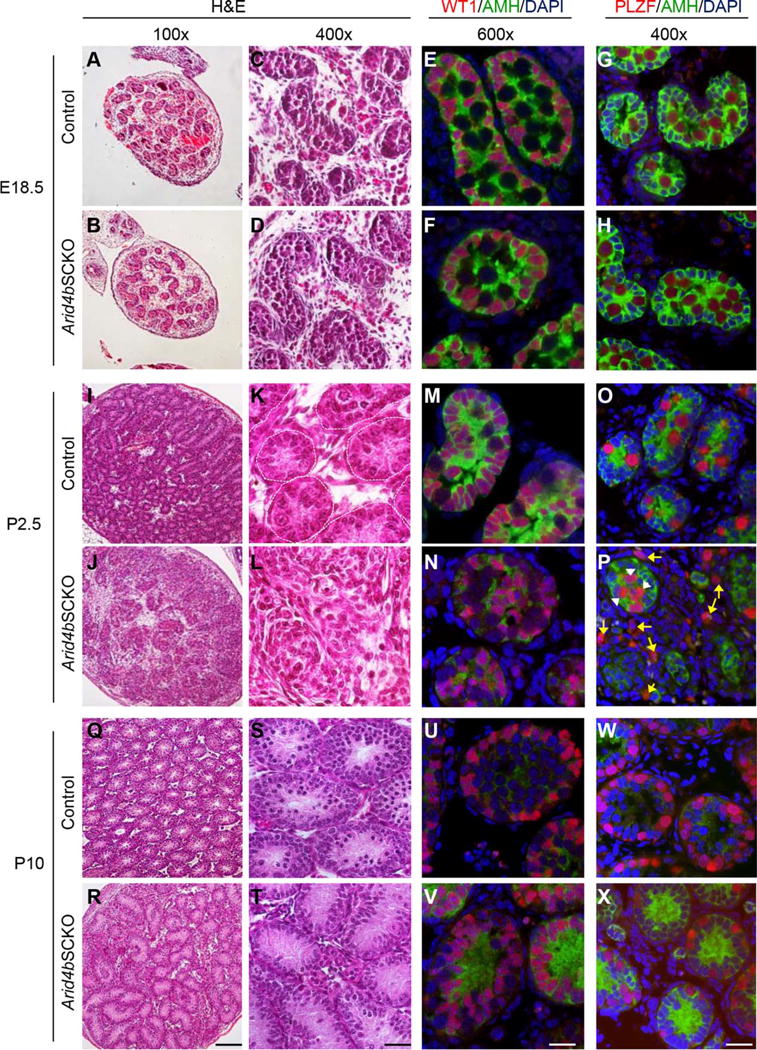
Failure to establish the spermatogonial stem cell niche in the Arid4bSCKO testes at P2.5 of age. (A–D, I–L, Q–T): Histological analyses of the control and Arid4bSCKO testes at E18.5, P2.5, and P10. Paraffin-embedded testis sections were stained with H&E. The basement membrane of the seminiferous tubules is outlined with dashed lines (K). Original magnifications of images were 100× (A, B, I, J, Q, R) and 400× (C, D, K, L, S, T). Scale bars = 100 μm (R) and 25 μm (T). (E, F, M, N, U, V): Double immunofluorescent staining of anti-Müllerian hormone (AMH) (green, cytoplasmic) and Wilms Tumor 1 (red, nuclear) to detect Sertoli cells in testis sections from the Arid4bSCKO and control mice at E18.5, P2.5, and P10. DNA was stained by DAPI (blue). Scale bar = 20 μm. (G, H, O, P, W, X): Double immunofluorescent staining of AMH (green, cytoplasmic) and promyelocytic leukemia zinc finger (red, nuclear) to detect Sertoli cells and gonocytes, respectively. Testis sections were from the Arid4bSCKO and control mice at E18.5, P2.5, and P10. Nuclear DNA was stained by DAPI (blue). White arrowheads point to gonocytes at central location within the seminiferous cords, and yellow arrows point to gonocytes scattered outside the cords in the Arid4bSCKO testes at P2.5 (P). Scale bar = 25 μm. Abbreviations: AMH, anti-Müllerian hormone; DAPI, 4′,6-diamidino-2-phenylindole; PLZF, promyelocytic leukemia zinc finger; WT1, Wilms Tumor 1.
The transformation of gonocytes into SSCs begins shortly after birth when gonocytes exit from quiescence. Between P0.5–P3, centrally located gonocytes migrate to the periphery of seminiferous cords, where gonocytes are in close contact with Sertoli cells [2]. Sertoli cells that reside along the basement membrane of the seminiferous cords are responsible for establishing a permissive niche to direct gonocyte migration and facilitate gonocyte differentiation into SSCs [2]. Histological analyses indeed showed that cells distributed along the periphery of seminiferous cords in the control testes at P2.5 (Fig. 1I, 1K). However, the P2.5 Arid4bSCKO testes showed disarranged cell distribution in the seminiferous cords, and the boundary of the seminiferous cords was not clearly recognizable by H&E staining (Fig. 1J, 1L). To detect Sertoli cells, double immunofluorescent staining for AMH and WT1 was performed. The result showed that Sertoli cells in the control testes resided along the periphery of seminiferous cords (Fig. 1M). In contrast, most of Sertoli cells in the Arid4bSCKO testes no longer anchored to the basement membrane of the seminiferous cords, but instead, translocated to the lumen of the cords (Fig. 1N). Next, double immunofluorescent staining for PLZF and AMH showed that gonocytes had migrated to the basement membrane of the seminiferous cords in the control testes (Fig. 1O). In the Arid4bSCKO testes, some gonocytes remained in the center of the seminiferous cords (Fig. 1P, white arrowheads), while some gonocytes were found scattered outside of the cord structure (Fig. 1P, yellow arrows). Furthermore, while some Sertoli cells broke away from the seminiferous cords in the Arid4bSCKO testes (Supporting Information Fig. S2, orange arrows), it is of note that the integrity of the seminiferous cords was perturbed with breaches on the basement membrane (Supporting Information Results; Supporting Information Fig. S3), which could cause gonocytes escaping from the seminiferous cords (Supporting Information Fig. S2, yellow arrows). These results suggest that ablation of ARID4B resulted in failure to retain Sertoli cells on the basement membrane of seminiferous cords during neonatal development, leading to a defect in establishment of a niche environment. Without support from the niche, the homing of gonocytes was disrupted, resulting in abnormal distribution of gonocytes in the Arid4bSCKO testes.
Given that Sertoli cells and gonocytes abnormally distributed in the P2.5 Arid4bSCKO testes, we examined whether the disarranged cellular distribution is permanent. Histological and immunostaining analyses were performed on the control and Arid4bSCKO testes at P10. The results showed that Sertoli cells were present along the periphery of seminiferous tubules in the Arid4bSCKO testes at P10 (Fig. 1R, 1T, 1V), which is similar to that found in the age-matched control testes (Fig. 1U). Concomitantly, undifferentiated spermatogonia, including SSCs and progenitor spermatogonia both stained by PLZF, were also in close association with the basement membrane of the tubules in the P10 Arid4bSCKO testes (Fig. 1X), as they were in the P10 control testes (Fig. 1W). These results suggest that Sertoli cells with ablation of ARID4B might be able to establish the niche that allowed homing and retention of the residual undifferentiated spermatogonia in the Arid4bSCKO testes at P10. However, it is clear that the establishment of initial stem cell niche in the Arid4bSCKO testes lag behind developmentally, as it occurred later than the timeframe (P2.5) during which the niche has been established in the control testes (Fig. 1M, 1N). These results indicate that ARID4B is required by Sertoli cells for timely establishment of the initial niche during the critical gonocyte-to-SSC transition phase.
Notably, the P10 Arid4bSCKO testes contained fewer undifferentiated spermatogonia than the control testes (Fig. 1W, 1X), which is possibly due to increased apoptosis and failure of SSC self-renewal in the Arid4bSCKO testes (Figs. 2 and 3, respectively). In addition, AMH was found to be decreased in the P2.5 Arid4bSCKO testes (Fig. 1N), but elevated in the P10 Arid4bSCKO testes (Fig. 1V). The differential expression of Amh was confirmed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) (Fig. 4B) and was further discussed in Supporting Information Results and Supporting Information Figure S9.
Figure 2.
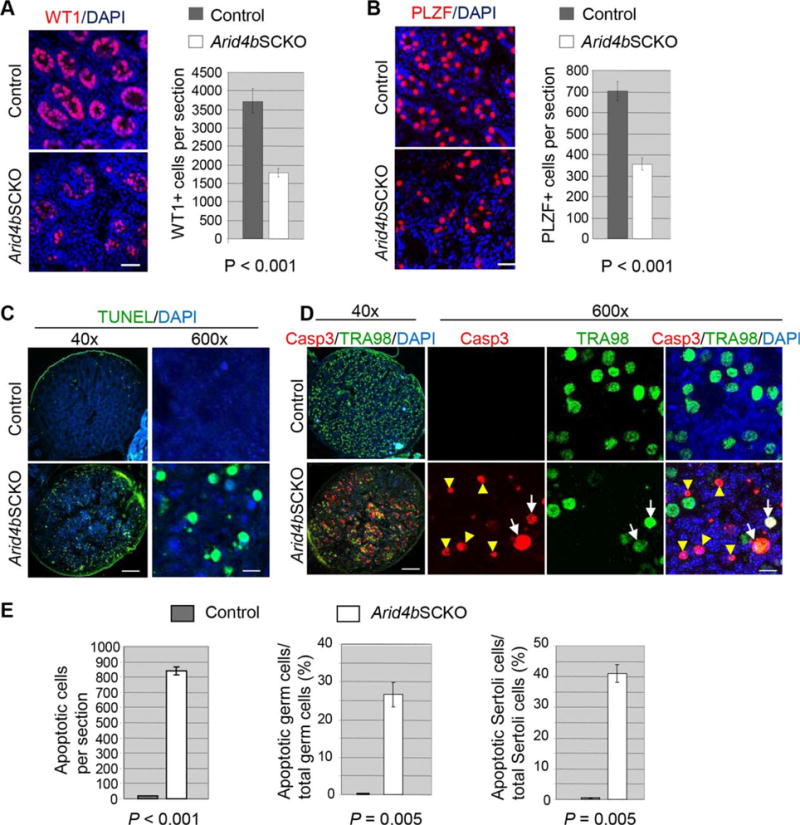
Increased apoptosis in the P2.5 Arid4bSCKO testes. (A): Reduced number of Sertoli cells in the P2.5 Arid4bSCKO testes. Immunostaining of Wilms Tumor 1 (WT1) detected Sertoli cells (red) in the control and Arid4bSCKO testes at P2.5 (left). Nuclear DNA was stained by DAPI (blue). Scale bar = 50 μm. For quantification of Sertoli cells, WT1-positive cells per testis section of the P2.5 control and Arid4bSCKO mice were counted (right). Three mice from each genotype and different litters were analyzed. Data are means ± SEM. (B): Depletion of gonocytes in the Arid4bSCKO testes at P2.5. Immunostaining of promyelocytic leukemia zinc finger (PLZF) detected gonocytes (red) in the control and Arid4bSCKO testes at P2.5 (left). Nuclear DNA was stained by DAPI (blue). Scale bar = 50 μm. For quantification, PLZF-positive cells per testis section of the P2.5 control and Arid4bSCKO mice were counted (right). Three mice from each genotype and different litters were analyzed. Data are means ± SEM. (C): Detection of apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in testis sections from the control and Arid4bSCKO littermates at P2.5. Apoptotic cells were TUNEL-positive (green). Nuclear DNA was stained by DAPI (blue). Original magnifications of images were 40× (left) and 600× (right). Scale bars = 250 μm (left) and 10 μm (right). (D): Double immunofluorescent staining using the anti-cleaved Casp3 antibody (red, nuclear) and the anti-germ cell specific antigen TRA98 antibody (green, nuclear) in testis sections from the control and Arid4bSCKO littermates at P2.5. The white arrows point to cleaved Casp3-positive apoptotic germ cells (Casp3+TRA98+). The yellow arrowheads point to cleaved Casp3-positive apoptotic Sertoli cells (Casp3+TRA98−). Original magnifications of images were 40× (left) and 600× (right). Scale bars = 250 μm (left) and 10 μm (right). (E): Quantification of apoptotic cells in the control and Arid4bSCKO testes at P2.5. Total apoptotic cells, the percentage of apoptotic germ cells, and the percentage of apoptotic Sertoli cells were compared between the control and Arid4bSCKO testes. Three mice from each genotype and different litters were analyzed. Data are means ± SEM. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PLZF, promyelocytic leukemia zinc finger; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; WT1, Wilms Tumor 1.
Figure 3.
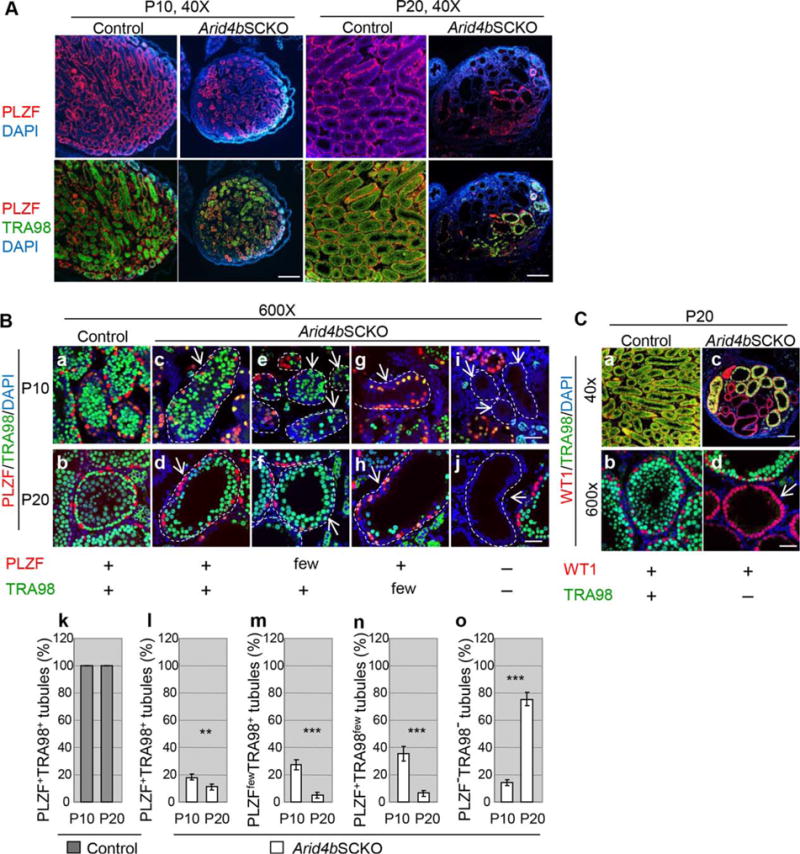
Defects in the formation, self-renewal, and differentiation of spermatogonial stem cells in the Arid4bSCKO testes. (A): Loss of undifferentiated spermatogonia and germ cells in the Arid4bSCKO testes at P10 and P20. Double immunofluorescent staining using the anti-promyelocytic leukemia zinc finger (PLZF) (red) and TRA98 (green) antibodies which detect undifferentiated spermatogonia and germ cells, respectively, was performed in testis sections from the control and Arid4bSCKO littermates at P10 and P20. Nuclear DNA was stained by DAPI (blue). Original magnifications of images were 40×. Scale bars = 300 μm. (B): High magnification of seminiferous tubules in the control and Arid4bSCKO testes at P10 and P20 with double immunofluorescent staining using the anti-PLZF (red) and TRA98 (green) antibodies. Seminiferous tubules from the control and Arid4bSCKO testes contained both undifferentiated spermatogonia (PLZF+) and germ cells (TRA98+) (a–d). In the Arid4bSCKO testes, some seminiferous tubules showed depletion of undifferentiated spermatogonia (PLZFfewTRA98+) (e, f), very few differentiating germ cells (PLZF+TRA98few) (g, h), or loss of germ cells altogether (PLZF−TRA98−) (i, j). The basement membrane of the tubule is outlined with a dashed line. Arrows indicate seminiferous tubules. Original magnification of images was 600×. Scale bar = 20 μm. Percentages of the PLZF+TRA98+ seminiferous tubules in the control and Arid4bSCKO testes (k, l), the PLZFfewTRA98+ tubules in the Arid4bSCKO testes (m), the PLZF+TRA98few tubules in the Arid4bSCKO testes (n), or the PLZF−TRA98− tubules in the Arid4bSCKO testes (o) at P10 and P20 were shown. Three mice from different litters of each genotype and each age group were analyzed. Data are means ± SEM. ***, p < 0.001; **, p < 0.01. (C): Sertoli-cell-only tubules in the Arid4bSCKO testes. Double immunofluorescent staining for Wilms Tumor 1 (red) and TRA98 (green) to detect Sertoli cells and germ cells, respectively, was performed using the control and Arid4bSCKO testes at P20. Nuclear DNA was stained by DAPI (blue). The arrow points to one of the Sertoli-cell-only tubules (d). Original magnifications of images were 40× (top) and 6003 (bottom). Scale bars = 300 μm (top) and 20 lm (bottom). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; PLZF, promyelocytic leukemia zinc finger; WT1, Wilms Tumor 1.
Figure 4.
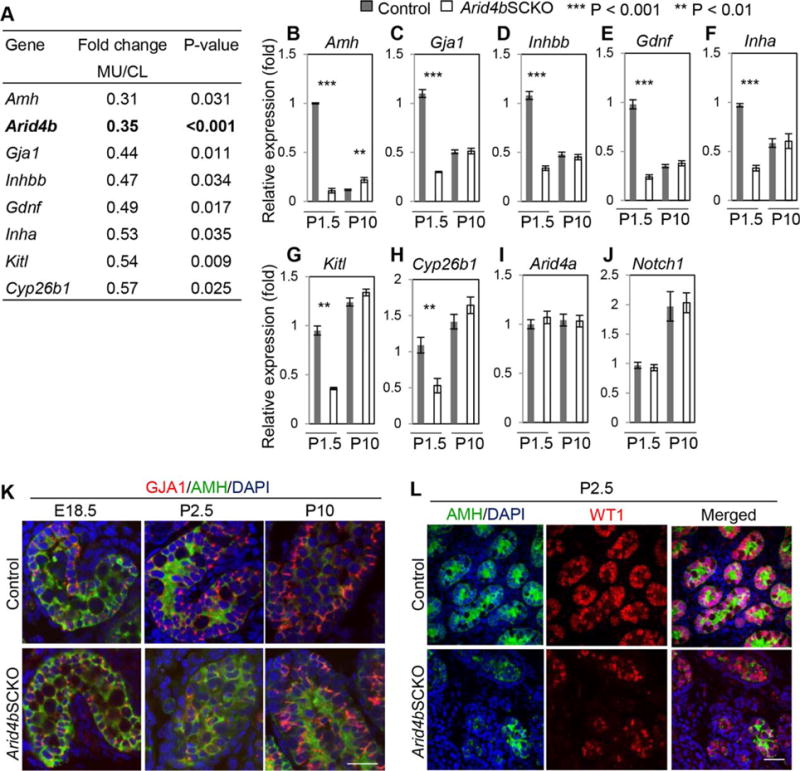
Downregulation of Sertoli cell-expressed genes important for the function of stem cell niche in the Arid4bSCKO testes at P1.5. (A): RNA-Seq analysis showed decreased expression of Amh, Arid4b, Gja1, Inhbb, Gdnf, Inha, Kitl, and Cyp26b1 in the Arid4bSCKO (MU) testes at P1.5 compared with the age-matched control (CL) testes. (B–J): Quantitative reverse transcriptase polymerase chain reaction analyses measured the mRNA levels of Amh, Gja1, Inhbb, Gdnf, Inha, Kitl, Cyp26b1, Arid4a, and Notch1 in testes from the control and Arid4bSCKO mice at P1.5 and P10. Three mice from different litters of each genotype and each age group were analyzed. The level of gene expression from one of the control testis at P1.5 was set as 1. Data are means ± SEM. (K): Gap junction protein alpha-1 (GJA1) protein was decreased in the Arid4bSCKO testes at P2.5. Double immunofluorescent staining of GJA1 (red) and anti-Müllerian hormone (AMH) (green) was performed on testis sections from the control and Arid4bSCKO mice at E18.5, P2.5, and P10. Scale bar = 20 μm. (L): AMH protein was decreased in the Arid4bSCKO testes at P2.5. Double immunofluorescent staining of AMH (green, cytoplasmic) and Wilms Tumor 1 (red, nuclear) was performed in testis sections from the control and Arid4bSCKO mice at P2.5. Scale bar = 50 μm. Abbreviations: AMH, anti-Müllerian hormone; DAPI, 4′,6-diamidino-2-phenylindole; GJA1, gap junction protein alpha-1; WT1, Wilms Tumor 1.
Increased Apoptosis in Sertoli Cells and Gonocytes in the Arid4bSCKO Testes
We investigated the consequence of abnormal distribution of Sertoli cells and gonocytes in the P2.5 Arid4bSCKO testes. As evidenced by immunofluorescent staining of WT1, the mutant testes contained fewer Sertoli cells than the control testes (Fig. 2A, left). Concomitant with the reduced number of Sertoli cells, immunofluorescent staining of PLZF showed a striking decrease in gonocytes in the mutant testes (Fig. 2B, left). Quantification results showed approximately 53% reduction in Sertoli cells and approximately 50% reduction in gonocytes in the Arid4bSCKO testes when compared with the control testes (Fig. 2A, 2B, right).
Given the significant reduction in the number of Sertoli cells and gonocytes in the P2.5 Arid4bSCKO testes (Fig. 2A, 2B), we next examined whether cell death was responsible. Results from terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay which detects apoptotic DNA fragmentation clearly showed an increase in the TUNEL-positive cells in the Arid4bSCKO testes compared with controls (Fig. 2C), suggesting increased apoptosis in the Arid4bSCKO testes.
The ability of gonocytes to migrate from the center of seminiferous cords toward the basement membrane to occupy the appropriate niche is essential for gonocyte survival [26]. Given that failure of the niche establishment affected the homing of gonocytes in the Arid4bSCKO testes at P2.5 (Fig. 1P), we examined whether gonocytes undergo apoptosis in the mutant testes. Co-immunostaining using the antibody against the apoptosis marker cleaved caspase 3 (Casp3) and the anti-germ cell specific antigen TRA98 antibody showed a marked increase in the population of the Casp3+ TRA98+ cells in the P2.5 Arid4bSCKO testes, indicating presence of apoptotic gonocytes (Fig. 2D, white arrows). In addition, the Arid4bSCKO testes contained an increased number of Casp3+ TRA98− cells inside the seminiferous cords (Fig. 2D, yellow arrowheads), indicating that Sertoli cells also underwent apoptosis. Quantification results confirmed that total apoptotic cells increased dramatically in the P2.5 Arid4bSCKO testes (Fig. 2E, left panel), with approximately 30% of gonocytes and approximately 40% of Sertoli cells undergoing apoptosis (Fig. 2E, middle and right panels, respectively). These results suggest that expression of Arid4b in Sertoli cells is required for the survival of Sertoli cells and gonocytes during neonatal development.
Sertoli Cell-Specific Ablation of ARID4B Impairs Gonocyte and SSC Fate
Although the sizes of the control and Arid4bSCKO testes were similar at P2.5 of age, testis sizes of the Arid4bSCKO mice barely increased from P2.5 to P20, whereas the sizes of the control testes increased with maturity as expected (Supporting Information Fig. S4) [25]. Therefore, the Arid4bSCKO testes at P10 and P20 were markedly smaller. At P3–P6 after gonocyte homing, contributions from the niche microenvironment provided by Sertoli cells facilitate gonocytes to either differentiate into SSCs or directly into differentiating spermatogonia that enter the first wave of spermatogenesis [2]. The primary SSCs first appear at P5 and become enriched at P7–P10 [27, 28]. An elaborate niche microenvironment established by Sertoli cells is required to support the gonocyte-to-spermatogonia transition. The niche also supports SSCs to self-renew and to undergo differentiation for all subsequent rounds of spermatogenesis. Given the fact that ablation of ARID4B in Sertoli cells impaired the timely establishment of the niche at P2.5 (Fig. 1), we examined whether the delay in establishing the initial niche has lasting effects on the cell fate decisions of gonocytes and SSCs. To this end, the Arid4bSCKO and control testes at P10 and P20 were analyzed by double immunofluorescent staining using PLZF and TRA98 antibodies. While the TRA98 antibody recognizes the germ cell specific antigen expressed in both undifferentiated and differentiating germ cells, expression of PLZF is restricted to the undifferentiated spermatogonia population that includes SSCs. Strikingly, our results showed a reduced number of undifferentiated spermatogonia (PLZF+) and total germ cells (TRA98+) in the Arid4bSCKO testes at P10 and P20 compared with that in the age-matched controls (Fig. 3A). Depending on the presence of undifferentiated spermatogonia and differentiating germ cells, the following four different types of seminiferous tubule in the Arid4bSCKO testes were identified. First, when compared with the seminiferous tubules in the control testes (Fig. 3Ba, 3Bb), some of the Arid4bSCKO tubules containing both undifferentiated spermatogonia and differentiating germ cells (PLZF+TRA98+) exhibited a relatively normal appearance (Fig. 3Bc, 3Bd). Second, some Arid4bSCKO tubules contained differentiating germ cells but showed depletion of undifferentiated spermatogonia (PLZFfewTRA98+) (Fig. 3Be, 3Bf), suggesting failure to establish a self-renewing pool of SSCs. Third, many Arid4bSCKO tubules contained mainly undifferentiated spermatogonia, but few or no differentiating germ cells (PLZF+TRA98few) (Fig. 3Bg, 3Bh), suggesting a defect in the ability of gonocytes and/or SSCs to differentiate into differentiating spermatogonia. Finally, numerous Arid4bSCKO tubules were devoid of any germ cell (PLZF−TRA98−) (Fig. 3BI, 3Bj). These tubules contained only Sertoli cells as identified by presence of Sertoli cell marker WT1 and absence of TRA98+ germ cells in seminiferous tubules of the Arid4bSCKO testes (Fig. 3Cc, 3Cd), while all of the control tubules contain Sertoli cells and germ cells (Fig. 3Ca, 3Cb). Together, ablation of ARID4B in Sertoli cells resulted in loss of either undifferentiated spermatogonia or differentiating germ cells, or lack of germ cells altogether in the seminiferous tubules.
Quantification results clearly showed that undifferentiated spermatogonia and differentiating germ cells were present in all seminiferous tubules (PLZF+TRA98+) in the control testes at P10 and P20 as expected (Fig. 3Bk). The percentages of the PLZF+TRA98+ tubules were severely decreased in the Arid4bSCKO testes at P10 and P20 (Fig. 3Bl). In the P10 Arid4bSCKO testes, the presence of the PLZFfewTRA98+ and PLZF+TRA98few tubules suggests that differentiation of gonocytes into SSCs or differentiating spermatogonia, respectively, was impaired (Fig. 3Bm, 3Bn). In addition, the Sertolicell-only tubules (PLZF−TRA98−) were found in the Arid4bSCKO testes at P10, suggesting that the commitment of gonocytes to differentiate into a spermatogonial lineage was completely disrupted (Fig. 3Bo). In the P20 Arid4bSCKO testes, the decreased percentage of the PLZF+TRA98+ tubules and the increased percentage of the PLZF−TRA98− tubules were even more pronounced (Fig. 3Bl, 3Bo), suggesting that the ability of SSCs to self-renew and differentiate was compromised. These data demonstrated a progressive loss of undifferentiated spermatogonia and differentiating germ cells in the Arid4bSCKO testes, leading to increased number of Sertoli-cell-only tubules. Collectively, ablation of ARID4B in Sertoli cells that resulted in failure to establish a stem cell niche during neonatal development (Fig. 1N) affected gonocyte and SSC fate.
Ablation of ARID4B Impaired Expression of Genes Critical for Function of the Stem Cell Niche
To gain insight into the molecular mechanisms responsible for the phenotypic changes caused by ablation of ARID4B in Sertoli cells, gene expression profiling by whole transcriptome sequencing (RNA-Seq) analysis was performed using the control and Arid4bSCKO testes collected at P1.5. The P1.5 testes were used because Sertoli cells were dislocated in the Arid4bSCKO testes at P2.5 (Fig. 1N), but remained at the periphery of seminiferous cords in the Arid4bSCKO testes at P1.5 (Supporting Information Results; Supporting Information Fig. S5E–S5H). Compared with the control, ablation of ARID4B resulted in significant upregulation or downregulation (p < 0.05, |Log2FC| ≥ 0.59) of 608 and 555 genes, respectively (Supporting Information Table S1). It is of note that Arid4b was identified as one of the genes whose expression was significantly reduced in the Arid4bSCKO testes (Fig. 4A), which validated our RNA-Seq analysis. Functional classification of gene annotations revealed that the Arid4b regulated genes are involved in extracellular stimuli, cell–cell adhesion, and cell–cell signaling (Supporting Information Fig. S6; Supporting Information Table S2).
Consistent with the observed phenotypic changes, transcripts of numerous genes expressed by Sertoli cells and known to be important for development of gonocytes and SSCs, including Amh, Gja1, Inhbb, Gdnf, Inha, Kitl, and Cyp26b1, were decreased in the Arid4bSCKO testes at P1.5 (Fig. 4A). Using newly collected RNA different from that used for RNA-seq, qRT-PCR analysis confirmed decreased expression of these genes in the P1.5 Arid4bSCKO testes (Fig. 4B–4H). In contrast, Arid4a, an Arid4b related gene, and Notch1, a gene known to regulate maintenance and differentiation of gonocytes [14], were not downregulated (Fig. 4I, 4J), which were used as controls for qRT-PCR analysis to suggest specific downregulation of Amh, Gja1, Inhbb, Gdnf, Inha, Kitl, and Cyp26b1. Collectively, ARID4B is required for the optimal expression of Amh, Gja1, Inhbb, Gdnf, Inha, Kitl, and Cyp26b1 at the time when gonocytes migrate to the basement membrane of the seminiferous cords to contact with Sertoli cells and differentiate into spermatogonia (P0–P6). Interestingly, expression of Gja1, Inhbb, Gdnf, Inha, Kitl, and Cyp26b1 was comparable between the control and Arid4bSCKO testes at P10 (Fig. 4C–4H). These results are in line with the phenotypes that the niche in the Arid4bSCKO testes was established at P10 (Fig. 1V), but not at P2.5 (Fig. 1N).
Given that Arid4b is required for Sertoli cells to remain attached along the basement membrane of seminiferous cords and for gonocytes to contact with Sertoli cells during neonatal development (Fig. 1N, 1P), it is plausible to suggest that ARID4B regulates genes involved in cell–cell adhesion. One such gene whose transcripts were significantly reduced in the Arid4bSCKO testes at P1.5 (Fig. 4A, 4C) is Gja1. GJA1, also known as Connexin-43, is a predominant gap junction protein expressed by Sertoli cells, and is located between neighboring Sertoli cells and between Sertoli cells and germ cells [29]. Although GJA1 is known to be critical for maintaining the cell composition of the bone marrow niche and controlling migration and homing of hematopoietic stem cells [30], the role of GJA1 in the establishment of the SSC niche in testes is not clear. By immunofluorescent staining, we found that the abundance of GJA1 protein was low in both control and Arid4bSCKO testes at E18.5, and no clear difference was observed between the E18.5 control and Arid4bSCKO testes (Fig. 4K). The abundance of GJA1 was elevated in seminiferous tubules of the control testes from P2.5 onward (Fig. 4K). In contrast, GJA1 was reduced in the P2.5 Arid4bSCKO tubules (Fig. 4K). However, GJA1 in the P10 Arid4bSCKO tubules was elevated to the abundance similar to that in the P10 control tubules (Fig. 4K). These results are consistent with the mRNA level of Gja1 which was decreased in the P1.5 Arid4bSCKO testes, but was increased to the level comparable with that in the P10 control testes (Fig. 4C). It should be emphasized that the period (P2.5) during which GJA1 in the Arid4bSCKO testes was strikingly less abundant than that in the age-matched control testes coincides with the timing when Sertoli cells failed to establish the initial niche (Fig. 1N) and gonocytes failed to reside in an appropriate niche in the P2.5 Arid4bSCKO testes (Fig. 1P). It is also interesting to note that at P10 when GJA1 in the mutant testes increased to the similar abundance as the control testes (Fig. 4K), Sertoli cells were found located at the basement membrane of the tubules (Fig. 1V) and homing of undifferentiated spermatogonia were also observed in the Arid4bSCKO testes (Fig. 1X). Together, these results suggest that Gja1 is regulated by ARID4B and may be involved in the timely establishment of the initial niche required for the homing of gonocytes.
To establish the niche, Sertoli cells not only provide architectural support for gonocyte and SSC homing but also secrete factors in the niche microenvironment that impinge on the signaling pathways in gonocytes and SSCs [4, 7, 8]. RNA-Seq and qRT-PCR analyses showed decreased expression of Amh, Gdnf, Kitl, Cyp26b1, Inha, and Inhbb in the Arid4bSCKO testes at P1.5 (Fig. 4A, 4B, 4D–4H). These Sertoli cell-expressed genes encode factors that have been shown to be involved in the cell fate decision of gonocytes and SSCs. Regulation of these genes by ARID4B is consistent with the results suggesting that ARID4B in Sertoli cells is required for gonocytes to differentiate into spermatogonia and for SSCs to self-renew and differentiate (Fig. 3). Double immunofluorescent staining for AMH and WT1 confirmed a striking decrease in the protein level of AMH in the P2.5 Arid4bSCKO Sertoli cells, where expression of WT1 was relatively abundant, suggesting selective downregulation of AMH in Sertoli cells (Fig. 4L). This result is consistent with the decrease at the mRNA level of Amh in the P1.5 Arid4bSCKO testes (Fig. 4A, 4C). During neonatal development, AMH induces gonocytes to differentiate into SSCs [13]. Decreased expression of AMH in the Arid4bSCKO testes during the gonocyte-to-SSC transition phase (P0–P6) may be responsible for the defect of gonocyte differentiation into SSCs (Fig. 3Ce, 3Cf). Decreased levels of other Sertoli cell-produced factors GDNF, INHA, CYP26B1, and KITL in the P2.5 Arid4bSCKO testes were also verified by immunofluorescent staining (Supporting Information Fig. S7). Together, the immunofluorescent staining results along with RNA-Seq and qRT-PCR analyses identified ARID4B-regulated genes which express key factors in Sertoli cells to allow the appropriate establishment of a niche environment supporting the gonocyte-to-SSC transition.
Gdnf Is a Direct Target of ARID4B
To globally identify ARID4B direct targets, chromatin immunoprecipitation (ChIP) using the antibody against ARID4B followed by DNA sequencing analysis (ChIP-Seq) was performed on wild type testes collected at P1.5. In all, 6,503 genes were identified to contain the ARID4B binding sites within 10 Kb of their gene margins (Supporting Information Table. S3). Among these genes, 6,231 genes (94%) have the ARID4B binding sites within the CpG islands. In addition, massive enrichment of ARID4B binding was found to center around the transcriptional start sites of genes (Fig. 5A), with 4,529 genes having the ARID4B binding sites within ± 500 bp of the transcriptional start sites (Fig. 5B). While RNA-Seq analysis identified both directly and indirectly regulated genes of ARID4B, ChIP-Seq analysis revealed the ARID4B direct binding targets. To look for the ARID4B directly regulated targets, 426 genes were identified by both RNA-Seq and ChIP-Seq analyses (Supporting Information Table. S4). Among these 426 potential ARID4B direct target genes, 211 of them (approximately 50%) contain the ARID4B binding sites within ± 500 bp of the transcriptional start sites (Fig. 5B). Notably, Gdnf is one of these potential ARID4B direct targets (Fig. 5B), with ARID4B binding to the Gdnf promoter from 275 bp upstream (−275) of the transcriptional start site (Fig. 5C, red line). To confirm the recruitment of ARID4B to the Gdnf promoter, we performed ChIP-qPCR analysis using the antibody against endogenous ARID4B in the control and Arid4bSCKO testes collected at P1.5. Our results showed that ARID4B was recruited to the Gdnf promoter in the control testes (Fig. 5D). The recruitment of ARID4B to the Gdnf promoter was specific, as only little ARID4B recruitment to this promoter was detected in the Arid4bSCKO testes (Fig. 5D). Small nuclear ribonucleoprotein N (Snrpn), a known ARID4B direct target gene [23], was used as a control for ChIP-qPCR analysis, showing that ARID4B was recruited to the promoter of Snrpn (exon 1) but not exon 7 (Fig. 5D). Together with the fact that ARID4B regulated expression of Gdnf (Fig. 4E), our results suggest that Gdnf is a direct target gene of ARID4B.
Figure 5.
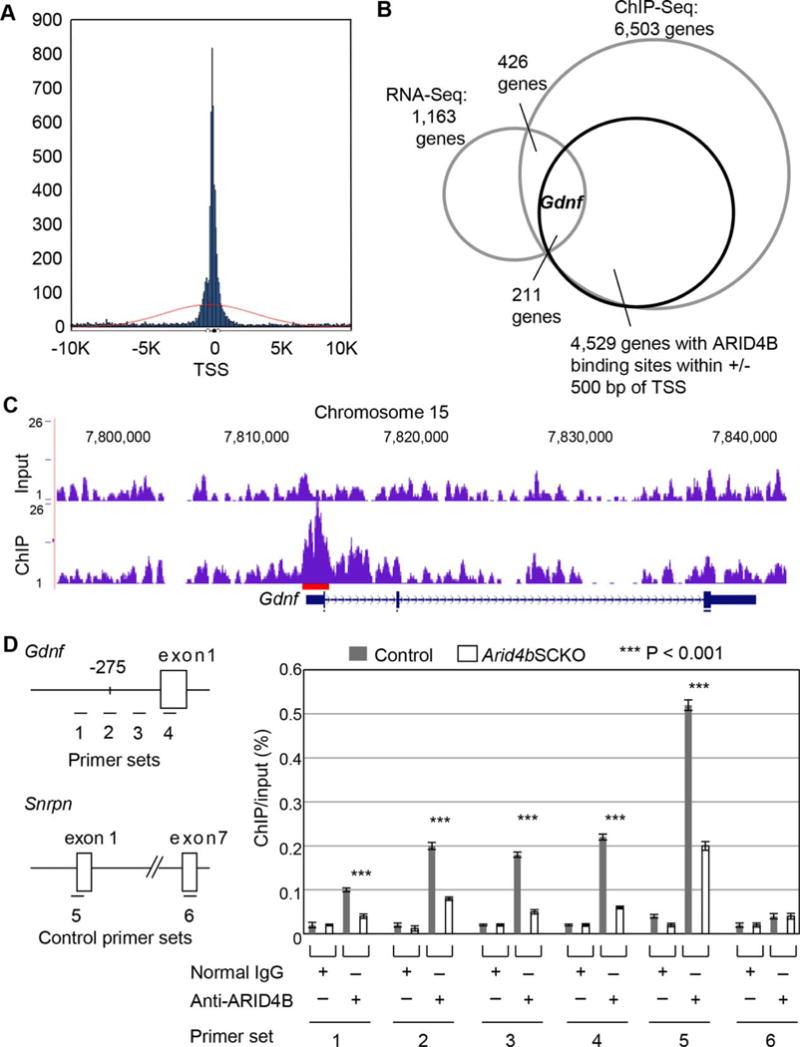
Identification of Gdnf as an ARID4B direct target in the neonatal testes. (A): Histogram of ARID4B binding sites within ± 10 Kb ranges of transcriptional start sites (TSS) of target genes identified by chromatin immunoprecipitation (ChIP)-Seq analysis. Results of ChIP-Seq analysis using anti-ARID4B antibody (blue peaks) and input (red line) showed enrichment of ARID4B binding close to the TSS of genes. (B): RNA-Seq and ChIP-Seq analyses identified Gdnf as one of the potential ARID4B direct targets with ARID4B binding sites within ± 500 bp of the transcriptional start sites. (C): ChIP-Seq analysis showed the enrichment of ARID4B binding to the promoter region of Gdnf. Results were analyzed using the mouse mm10 assembly on UCSC genome browser. Red line indicates the ARID4B binding region (D): ChIP-qPCR analysis showed that ARID4B was recruited to the Gdnf promoter. Cross-linked chromatin from testes of the control and Arid4bSCKO mice at P1.5 was immunoprecipitated with normal rabbit IgG or anti-ARID4B antibody. Immunoprecipitated DNA was subject to qPCR analysis using the primer sets 1–6. The promoter region of Gdnf is shown with four primer sets 1–4, which indicate the region of each qPCR product in ChIP-qPCR analysis. The distance (bp) upstream of −275 that indicates from the transcriptional start site of Gdnf is the starting point of the ARID4B binding region identified by ChIP-Seq analysis. The primer set 5 that overlaps the Snrpn exon 1 was used as a positive control, and the primer set 6 that overlaps the Snrpn exon 7 was used as a negative control. Data are means ± SEM. Abbreviations: ChIP, chromatin immunoprecipitation; TSS, transcriptional start site.
Discussion
In this study, we demonstrated that ARID4B is essential for the temporal–spatial establishment of the initial SSC niche during neonatal development. Study into the molecular mechanism identified several new downstream effectors of ARID4B and provides evidence that ARID4B is a master regulator of a gene network governing the establishment of SSC niche. These ARID4B downstream effectors include GJA1, KITL, CYP26B1, AMH, GDNF, INHA, and INHBB.
GJA1, also known as connexin-43, is a predominant gap junction protein produced by Sertoli cells and located between neighboring Sertoli cells and between Sertoli cells and germ cells. In addition, GJA1 is shown to maintain the cell composition in the bone marrow niche and is involved in migration and homing of hematopoietic stem cells [31]. It has been reported that the Sertoli cell-specific Gja1 knockout male mice are infertile and display Sertoli-cell-only syndrome or an arrest of spermatogenesis at the level of spermatogonia [16, 32]. However, whether GJA1 is involved in the establishment of the initial SSC niche in testes during neonatal development remains unclear. In the Arid4bSCKO testes, disassociation of Sertoli cells from the basement membrane of the seminiferous cords was detected, concomitant with downregulation of Gja1 at the critical gonocyte-to-SSC transition period. It is plausible that GJA1 is the key structural protein maintaining Sertoli cells in proper organization for the establishment of the initial niche during neonatal development.
Decreased abundance of GJA1 in the Arid4bSCKO testes could also impair the interaction between Sertoli cells and gonocytes, leading to a defect in gonocyte homing to the niche. Consistent with this hypothesis, our results showed that some gonocytes were scattered outside of the seminiferous cord structure, and some remained in the center of the seminiferous cords in the neonatal Arid4bSCKO testes, as opposed to gonocytes in the neonatal control testes that reside in the niche located at the basement membrane of the seminiferous cords. In addition to the defects in gonocyte homing, the fact that some gonocytes remained in the center of the seminiferous cords in mutant testes suggests a potential defect in gonocyte migration. KITL is secreted by Sertoli cells and signals via binding to its cognate receptor, the transmembrane tyrosine kinase receptor c-KIT, on gonocytes. KITL/c-KIT signaling promotes migration of gonocytes from the center of seminiferous cords to the periphery during neonatal development [10]. In the Arid4bSCKO testes, decreased expression of KITL is consistent with the defect that gonocytes fail to migrate to the basement membrane of seminiferous cords.
CYP26B1 is another downstream effector of ARID4B. CYP26B1 is expressed in Sertoli cells, and is able to metabolize and inactivate RA. RA signaling promotes differentiation of gonocytes and SSCs. Knockout of Cyp26b1 in mice increases RA levels in embryonic testes, resulting in abnormal development, increased apoptosis, and complete absence of male germ cells in mutant male neonates [33]. Therefore, CYP26B1 acts as a survival factor to prevent apoptosis and maintains gonocytes and undifferentiated spermatogonia by metabolizing RA [17]. Because ablation of ARID4B in Sertoli cells resulted in decreased expression of Cyp26b1, we measured the levels of RA in the P1.5 control and Arid4bSCKO testes. The result showed upregulation of RA in the Arid4bSCKO testes (Supporting Information Fig. S8), which could medicate aberrant entry of gonocytes and SSCs into differentiation. Therefore, ablation of ARID4B in Sertoli cells that caused a decrease in CYP26B1 during early postnatal development may not be beneficial for the formation and maintenance of a self-renewing SSC population.
In the Arid4bSCKO testes, failure to establish the niche impairs the transition of gonocytes into the SSCs and differentiating spermatogonia. SSCs in the Arid4bSCKO testes also lost their ability to self-renew and differentiate. We demonstrated that expression of Amh is downregulated in the Arid4bSCKO mice. AMH produced by Sertoli cells has been shown to induce gonocytes to differentiate into SSCs [13]. In this regard, decreased expression of AMH is consistent with the failure of gonocytes to transition into the SSCs in the Arid4bSCKO mice. In addition, activin, a homodimers of two inhibin β-subunits, βa (INHBA) or a βb (INHBB), is able to induce differentiation of gonocytes and SSCs. Inhibin, a heterodimer consisting of an inhibin α-subunit (INHA) and an INHBA or an INHBB subunit, exerts an opposite biological effect on activin. In the testes from the Arid4bSCKO mice, reduced expression of Inha and Inhbb could result in the deficiency of both activin and inhibin proteins, which may be responsible for the defect in differentiation of gonocytes and SSCs.
Finally, we demonstrated that Gdnf is a direct target gene of ARID4B. As a secreted factor produced by Sertoli cells, GDNF acts as a chemoattractant for SSCs and promotes self-renewal of SSCs [15, 34–36]. Consequently, disruption of GDNF signaling resulted in loss of SSCs and led to the appearance of Sertoli-cell-only seminiferous tubules [37, 38]. In the Arid4bSCKO testes, ablation of ARID4B in Sertoli cells resulted in downregulation of Gdnf, which may be one of the factors responsible for the defect in SSC self-renewal, leading to depletion of SSCs and Sertoli-cell-only tubules.
Defects in spermatogenesis decrease production of spermatozoa, which could result in male infertility. Spermatogenesis includes two phases. The first phase consists of gonocytes and subsequent formation of SSCs from gonocytes. The second phase starts from differentiation of undifferentiated spermatogonia, progressing through spermatogenic differentiation, and end with the formation of spermatozoa. Spermatogenic progression is highly dependent on a microenvironment provided by Sertoli cells. Here, we showed that ARID4B regulated the establishment of SSC niche by Sertoli cells to control gonocyte differentiation into SSCs and SSC self-renewal and differentiation. In our previous reports [24, 25], we demonstrated that ARID4B, as an AR coactivator, is involved in regulation of the second phase spermatogenesis, from undifferentiated spermatogonia through spermatogenic differentiation (Supporting Information Fig. S10). Using the Arid4b knockout mouse model, these studies advance our understanding into the cause of spermatogenesis defects, which could improve the evaluation and therapeutic intervention for patients with idiopathic infertility.
Conclusion
Although GJA1, GDNF, AMH, CYP26B1, INHA, and INHBB expressed by Sertoli cells are critical for gonocyte homing and cell fate decision of gonocytes and/or SSCs, how these genes are regulated and coordinated remains poorly understood. Our data showed that ARID4B is required for the optimal expression of these genes. Using the Arid4bSCKO mice, we demonstrated that ARID4B is essential for the temporal–spatial establishment of the initial SSC niche during neonatal development. More importantly, downregulation of Gja1, Gdnf, Amh, Cyp26b1, Inha, and Inhbb in the Arid4bSCKO testes is consistent with the failure in the commitment of gonocyte to differentiate into a spermatogonial lineage and the impairment in the capabilities of SSCs to self-renew and differentiate. Taken together, our study identified ARID4B as the master coordinator of a gene network that regulates the establishment of the initial SSC niche to dictate the fate of gonocytes and SSCs.
Supplementary Material
Significance Statement.
The primary spermatogonial stem cells (SSCs), which arise from gonocytes during neonatal development, serve as a foundational self-renewing reservoir to ensure continuous production of spermatozoa throughout adulthood. The transformation of gonocytes into SSCs takes place in a niche established by Sertoli cells. We provide the first evidence that the AT-rich interaction domain 4B regulatory network determines the timing and location for the establishment of initial stem cell niche during the crucial gonocyte-to-SSC transition stage. The establishment of an elaborate niche impacts the cell fate decision of gonocytes and SSCs. These findings uncover the mechanisms that control the formation of the primary SSC population.
Acknowledgments
We thank Dr. Sophia Y. Tsai for her insightful comments and critical reading of the manuscript. This work was supported in part by Grants CA188471 and CA187857 from the NIH and McCormick Genomic and Proteomic Center, George Washington University (to R.-C.W. and M.-Y.W.).
Footnotes
AUTHOR CONTRIBUTIONS
R.-C.W. and M.-Y.W: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.Z. and Y.-F.C.: collection and assembly of data; R.B.L.: data analysis and interpretation.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
References
- 1.Jan SZ, Hamer G, Repping S, et al. Molecular control of rodent spermatogenesis. Biochim Biophys Acta. 2012;1822:1838–1850. doi: 10.1016/j.bbadis.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Yang QE, Oatley JM. Early postnatal interactions between Sertoli and germ cells. In: Griswold MD, editor. Sertoli Cell Biology. 2nd. Waltham, MA: Elsevier Inc.; 2015. pp. 81–98. [Google Scholar]
- 3.Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction. 2015;149:R139–R157. doi: 10.1530/REP-14-0431. [DOI] [PubMed] [Google Scholar]
- 4.Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149:R159–1R167. doi: 10.1530/REP-14-0481. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida S, Sukeno M, Nakagawa T, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 6.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne CJ. Cycling to and from a stem cell niche: the temporal and spatial odyssey of mitotic male germ cells. Int J Dev Biol. 2013;57:169–177. doi: 10.1387/ijdb.130006cp. [DOI] [PubMed] [Google Scholar]
- 9.Basciani S, De Luca G, Dolci S, et al. Platelet-derived growth factor receptor beta-subtype regulates proliferation and migration of gonocytes. Endocrinology. 2008;149:6226–6235. doi: 10.1210/en.2008-0349. [DOI] [PubMed] [Google Scholar]
- 10.Marziali G, Lazzaro D, Sorrentino V. Binding of germ cells to mutant Sld Sertoli cells is defective and is rescued by expression of the transmembrane form of the c-kit ligand. Dev Biol. 1993;157:182–190. doi: 10.1006/dbio.1993.1122. [DOI] [PubMed] [Google Scholar]
- 11.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Culty M. Identification and distribution of a novel platelet-derived growth factor receptor beta variant: effect of retinoic acid and involvement in cell differentiation. Endocrinology. 2007;148:2233–2250. doi: 10.1210/en.2006-1206. [DOI] [PubMed] [Google Scholar]
- 13.Zhou B, Hutson JM. Human chorionic gonadotropin (hCG) fails to stimulate gonocyte differentiation in newborn mouse testes in organ culture. J Urol. 1995;153:501–505. doi: 10.1097/00005392-199502000-00071. [DOI] [PubMed] [Google Scholar]
- 14.Garcia TX, DeFalco T, Capel B, et al. Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev Biol. 2013;377:188–201. doi: 10.1016/j.ydbio.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 16.Brehm R, Zeiler M, Ruttinger C, et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, MacLean G, Cameron D, et al. Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One. 2009;4:e7501. doi: 10.1371/journal.pone.0007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Ouyang W, Grigura V, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleischer TC, Yun UJ, Ayer DE. Identification and characterization of three new components of the mSin3A corepressor complex. Mol Cell Biol. 2003;23:3456–3467. doi: 10.1128/MCB.23.10.3456-3467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai A, Lee JM, Yang WM, et al. RBP1 recruits both histone deacetylase-dependent and -independent repression activities to retinoblastoma family proteins. Mol Cell Biol. 1999;19:6632–6641. doi: 10.1128/mcb.19.10.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu R, Wang GG. Tudor: a versatile family of histone methylation ’readers’. Trends Biochem Sci. 2013;38:546–555. doi: 10.1016/j.tibs.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishibuchi G, Nakayama J. Biochemical and structural properties of heterochromatin protein 1: understanding its role in chromatin assembly. J Biochem. 2014;156:11–20. doi: 10.1093/jb/mvu032. [DOI] [PubMed] [Google Scholar]
- 23.Wu MY, Tsai TF, Beaudet AL. Deficiency of Rbbp1/Arid4a and Rbbp1l1/Arid4b alters epigenetic modifications and suppresses an imprinting defect in the PWS/AS domain. Genes Dev. 2006;20:2859–2870. doi: 10.1101/gad.1452206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu RC, Jiang M, Beaudet AL, et al. ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc Natl Acad Sci USA. 2013;110:4616–4621. doi: 10.1073/pnas.1218318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu RC, Zeng Y, Pan IW, et al. Androgen receptor coactivator ARID4B is required for the function of sertoli cells in spermatogenesis. Mol Endocrinol. 2015;29:1334–1346. doi: 10.1210/me.2015-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tres LL, Kierszenbaum AL. The ADAM-integrin-tetraspanin complex in fetal and postnatal testicular cords. Birth Defects Res C Embryo Today. 2005;75:130–141. doi: 10.1002/bdrc.20041. [DOI] [PubMed] [Google Scholar]
- 27.Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26. doi: 10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- 28.Culty M. Gonocytes, from the fifties to the present: is there a reason to change the name? Biol Reprod. 2013;89:46. doi: 10.1095/biolreprod.113.110544. [DOI] [PubMed] [Google Scholar]
- 29.Sridharan S, Brehm R, Bergmann M, et al. Role of connexin 43 in Sertoli cells of testis. Ann NY Acad Sci. 2007;1120:131–143. doi: 10.1196/annals.1411.004. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Nieto D, Li L, Kohler A, et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood. 2012;119:5144–5154. doi: 10.1182/blood-2011-07-368506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heazlewood SY, Oteiza A, Cao H, et al. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche. Ann NY Acad Sci. 2014;1310:119–128. doi: 10.1111/nyas.12329. [DOI] [PubMed] [Google Scholar]
- 32.Sridharan S, Simon L, Meling DD, et al. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76:804–812. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- 33.MacLean G, Li H, Metzger D, et al. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology. 2007;148:4560–4567. doi: 10.1210/en.2007-0492. [DOI] [PubMed] [Google Scholar]
- 34.Dovere L, Fera S, Grasso M, et al. The niche-derived glial cell line-derived neurotrophic factor (GDNF) induces migration of mouse spermatogonial stem/progenitor cells. PloS One. 2013;8:e59431. doi: 10.1371/journal.pone.0059431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huleihel M, Fadlon E, Abuelhija A, et al. Glial cell line-derived neurotrophic factor (GDNF) induced migration of spermatogonial cells in vitro via MEK and NF-kB pathways. Differentiation. 2013;86:38–47. doi: 10.1016/j.diff.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Kanatsu-Shinohara M, Inoue K, Takashima S, et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell. 2012;11:567–578. doi: 10.1016/j.stem.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Jijiwa M, Kawai K, Fukihara J, et al. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells. 2008;13:365–374. doi: 10.1111/j.1365-2443.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 38.Savitt J, Singh D, Zhang C, et al. The in vivo response of stem and other undifferentiated spermatogonia to the reversible inhibition of glial cell line-derived neurotrophic factor signaling in the adult. STEM CELLS. 2012;30:732–740. doi: 10.1002/stem.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


