Abstract
Background
The neuropeptide oxytocin (OT) is a key regulator of social and emotional behaviors. The effects of OT are context-dependent, and it has been proposed that OT increases the salience of both positive and negative social cues. Here we tested whether the bed nucleus of the stria terminalis (BNST) mediates anxiogenic effects of OT.
Methods
First, we studied the effects of systemic administration of an OT receptor (OTR) antagonist L-368,899 on social behavior in male and female California mice exposed to social defeat. We examined the effect of L-368,899 on G protein activation and used EGR1 immunohistochemistry to identify potential sites of OTR action. Finally, we examined the effects of L-368,899 infused in the BNST on behavior.
Results
A single dose of systemic L-368,899 increased social approach in stressed females and decreased social approach in males naïve to defeat. L-368,899 prevented OT activation of G proteins, and did not activate G-proteins in the absence of OT. Intranasal OT, which reduces social approach in females but not males, increased EGR1 immunoreactivity in the nucleus accumbens (NAc) core and anteromedial BNST in females but not males. Stressed females that received an infusion of L-368,899 in to anteromedial BNST but not the NAc core increased social approach and decreased social vigilance responses.
Conclusions
Our results suggest that OTR activation in anteromedial BNST induces a vigilance response in which individuals avoid, yet attend to unfamiliar social contexts. Our results suggest that OTR antagonists may have unappreciated therapeutic potential for stress-induced psychiatric disorders.
Keywords: oxytocin, social anxiety, bed nucleus of stria terminalis, stress, social defeat, sex differences
INTRODUCTION
Oxytocin (OT) is an evolutionary conserved neuropeptide that is a key regulator of social and emotional behaviors (1–4). Reports in humans that OT has anxiolytic effects (5–8) have sparked interest in the use of OT as a therapeutic. Nonetheless, it is clear in some cases that OT is anxiogenic (9). Intriguingly, post-mortem analyses showed that humans suffering from depression had more OT immunoreactive neurons in the hypothalamus (10). These results might reflect OT production as a homeostatic response to stress, but could also indicate that OT contributes to behavioral pathology. When assessing these hypotheses, sex specific effects of OT must be considered. Intranasal OT reduces amygdala reactivity in men (5, 7), but increases it in women (11, 12). Sex specific effects of OT in humans are consistent with findings in animal research (1, 13, 14), indicating the importance including both males and females in studies of OT function.
We previously observed that social defeat increases the activity of OT neurons in the medioventral bed nucleus of the stria terminalis (BNSTmv) and paraventricular nucleus (PVN) in female but not male California mice (15). We also found that intranasal OT reduced social interaction in unstressed females, mirroring the effect of social defeat stress. We hypothesized that stress-induced increases in the activity of OT neurons contribute to social avoidance in females by activating OT receptors (OTR). We studied the effects of systemic administration of the OTR antagonist L-368,899 (OTA) on social and non-social behavior in control and stressed males and females. To identify potential sites of action of OTR, we used receptor autoradiography to examine effects of defeat on OTR binding as well as immunohistochemistry to measure effects of intranasal OT or systemic administration of OTA on immediate early gene activation. Based on these results, we then performed site-specific injections of OTA in either the dorsolateral nucleus accumbens core (NAcdl) or anteromedial bed nucleus of the stria terminalis (BNSTam) of stressed females. Our main finding is that a single dose of OTA, either systemically or within the BNSTam, rapidly reverses stress-induced social avoidance in females, without affecting behavior in non-social contexts. Our results support the hypothesis that stress-induced increases in the activation OTR induce social withdrawal and imply that OTR antagonists may have unappreciated therapeutic potential for stress-induced psychiatric disorders.
MATERIALS AND METHODS
Full details of experimental procedures are provided in the Supplementary Methods and Materials.
Animals
All studies on male and female California mice were approved by the Institutional Animal Care and Use Committee (IACUC) and conformed to NIH guidelines.
Social defeat
Male and female mice were randomly assigned to control handling or social defeat for 3 consecutive days as described (16). Behavioral and receptor binding analyses were conducted two weeks after social defeat (17).
Effects of systemic OTA treatment
To inhibit OTR, we used the high affinity non-peptide OTA L-368,899 (L2540, Sigma-Aldrich, St. Louis, MO), which readily passes through the brain-blood barrier (18, 19). Males and females exposed to social defeat or control conditions were given intraperitoneal injections of either saline (sterile phosphate-buffered saline) or one of two doses of L-368,899 (1 or 5 mg/kg). These doses were based on previous behavioral studies of L-368,899 (20–22). Injections were administered 30 min before testing based on pharmacokinetic data (23).
Social interaction test (SI)
Social interaction testing was performed as previously described (16, 31). The interaction phase videos were later reanalyzed by an observer blind to treatment assignment to manually score active investigation, time spent inactive in interaction zone, auto-grooming, and time spent in sides (for details see Supplementary Table S1). We also manually recorded head orientation toward the target mouse when the focal mouse was outside of the interaction zone. This behavior is referred to as “risk assessment behavior” or vigilance (Supplementary Video1, (24)).
Odor preference test (OP)
One day after SI, OP was assessed using the same arena used for SI. During odor preference phase, diluted urine of a known conspecific (cagemate odor) and an unknown conspecific (unfamiliar odor) were added to pre-defined interaction areas (Figure 1E). Odor preference ratio was defined as time spent withthe head in unfamiliar odor divided by total time spent with the head in both cage-mate and unfamiliar odors.
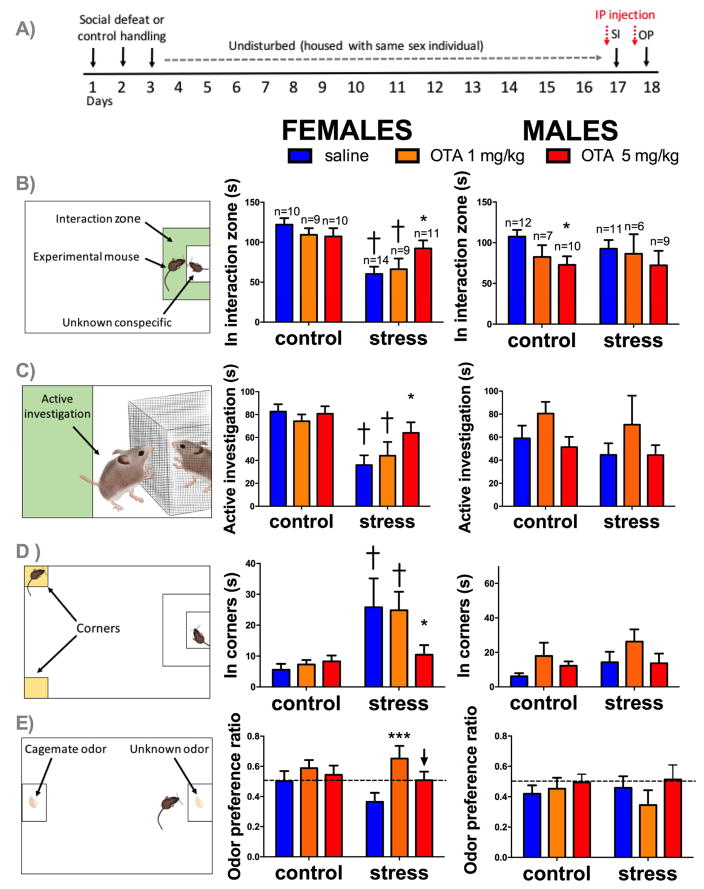
Figure 1. Effects of systemic administration of OTA and social defeat stress on behavior.
Timeline of experiment (A). Mean and SEM time spent in the interaction zone (B), in active investigation (C), and in corner zones (D) during the social interaction test. Mean and SEM of odor preference ratio during OP test (E). Left column shows diagrams representing behavior measured, middle column shows female data, right column shows male data, *=p < 0.05 effect of 5mg/Kg OTA vs. saline, †=p<0.01 effect of stress in saline treated animals, ***=p<0.01 effect of 1mg/Kg OTA vs. saline in stressed animals, ↓ p=0.08 effects of 5mg/Kg OTA vs. saline in stressed animals.
OTR autoradiography
Males and females were exposed to control or defeat conditions and then euthanized two weeks later (25, 26). Brains were snap frozen, sliced, and processed for OTR autoradiography as previously described (27).
Effects of intranasal OT and systemic OTA on EGR1 immunoreactivity
Immunohistochemistry for early growth response factor 1 (EGR1) in mice treated intranasally with 0.8 IU of OT or saline (15) was used to identify possible sites of OT action. EGR1 has been shown to be useful as an indirect marker of neuronal activation in a variety of species and contexts (28–30). We used animals naïve to defeat since intranasal administration of OT did not affect social behavior in stressed females while it reduced SI in naïve females, which mimics the effects that stress has on this behavior (full details of in Supplementary methods). Based on the results obtained in this study, we analyzed EGR1 expression in NAcdl and BNSTam of control and stressed animals receiving systemic injections of saline or OTA.
Site-specific injection of OTA
Seven days after social defeat, females were implanted with bilateral guide cannula (Plastics One, Roanoke, VA) aimed at either the NAcdl (AP: + 0.51, ML: +/−1.5, DV: + 6.0) or BNSTam (AP: + 0.45, ML: +/−1.0, DV: + 5.6)(31). After recovery, females were randomly assigned to receive bilateral 200 nl infusions of either aCSF or L-368,899 (1 ug per side) into NAcdl or BNSTam (Plastics One, Roanoke, VA) that projected 1 mm past the guides. The dose used is within the range of previous studies using intracerebral injections (32–35). Thirty minutes later each mouse was run in the SI test. Histology was used to determine injection sites (see Supplementary methods).
Effects of OTA on OT activation of G-proteins and β-arrestins recruitment
OT facilitates OTR coupling to several different G-proteins and β-arrestins, allowing for multiple degrees of freedom for effects on neural activity (36, 37). While L-368–899 is known to be a selective OTR antagonist, its effects on OTR signaling is unknown. This is an important question because some OTR antagonists, such as atosiban, can activate selective Gi pathways (36, 38). We performed a complete bioluminescene resonance energy transfer (BRET) characterization of the coupling properties of L-368,899 (39) to test if this compound is a complete antagonist or shows biased agonism (See Supplementary Methods).
Statistical analysis
We used two-way ANOVA to analyze receptor autoradiography (sex*stress), behavior from systemic OTA administration (stress*drug), behavior from site-specific injections (drug*injection site), odor preference ratio, and EGR1 immunoreactivity (sex*drug). For significant two-way ANOVA analyses, planned comparisons were used to detect differences between groups (package “lsmeans” in R, Bonferroni, 0.95 confidence interval). Effect size is reported as Cohen’s d. Spearman correlations were used to correlate autoradiography data with behavior. Finally, we performed 3-way ANOVA to assess effects of estrous cycle (stress*drug*estrous) in the SI and OP tests. Estrous cycle was assessed post mortem to avoid disrupting behavior (40). There were no main effects of estrous cycle or interaction with stress or treatment (Supplementary Table S2).
RESULTS
Effects of systemic administration of OTA on social interaction behavior
In females, there was evidence suggesting that the effects of systemic OTA treatment on social interaction behavior were different in control vs. stressed females (Figure 1B; stress*drug interaction, p=0.06). Stressed females spent less time in the interaction zone than controls if they were treated with saline (p<0.001, d=2.04) or 1 mg/Kg of OTA (p<0.01, d=1.29). In contrast, stressed females treated with 5mg/Kg of OTA spent significantly more time in the interaction zone than stressed females treated with saline (p<0.05, d=1.03) and were no different from control females (all treatments, d=0.45). Effects of OTA were stronger on the amount of time females actively interacted (by rearing or sniffing) with the cage containing the target mouse (Figure 1C; stress*drug interaction; p<0.001). Stressed females showed reduced interaction with the target mouse if treated with saline (p<0.001, d=1.6) or 1mg/Kg dose of OTA (p=0.02, d=1.1) but not with 5mg/Kg dose of OTA (d=0.6). In females, stress also significantly increased time spent in corners opposite the interaction zone (Figure 1D; main effect of stress, p<0.001), but only in females receiving saline (p=0.01, d=0.8) or 1 mg/Kg of OTA (p= 0.047, d=1.3), but not 5 mg/Kg of OTA (d=0.25). There were no significant differences in any of these variables during acclimation phase when the target mouse was absent (Supplementary Figure S1A, S1B) or other variables quantified during manual scoring (Supplementary Figure S2). During the open field phase (when the empty cage was absent), there were no effects of treatment on time spent in center, but there was a main effect of stress reducing time spent in center (Supplementary Figure S1C, p<0.001).
In males, social defeat stress did not reduce social interaction behavior, consistent with previous studies (15, 41, 42). There was no significant effect of OTA on time spent in the interaction zone in the presence of a target mouse (Figure 1B; main effect of drug; p=0.1). However, a planned comparison showed that 5 mg/Kg of OTA reduced time spent in the interaction zone of control males (p=0.02, d=1.25). No significant differences were observed in stressed males. There were no significant differences in time spent in active investigation (Figure 1C) or in corner zones (Figure 1D). There were no effects of stress, treatment, or their interaction on time spent in interaction zone or corners during acclimation phase and time spent in the center of the open field during open field phase (Supplementary Figures S1).
Effects of systemic administration of OTA on odor preference behavior
For females there was a main effect of treatment on odor preference during interaction phase (Figure 1E, main effect of drug; p<0.001). In stressed females 1 mg/Kg of OTA significantly increased preference for an unfamiliar odor vs cage-mate compared to saline treated animals (p<0.001, d=0.94). Intriguingly, the effect of 5 mg/Kg of OTA was less pronounced (p=0.08, d=0.78). In control females, OTA had no significant effect on preference ratios. Surprisingly, neither control nor stressed males showed a preference for any odor, and this was not affected by OTA treatment.
Effects of OTA on OT activation of G-proteins and β-arrestins recruitment
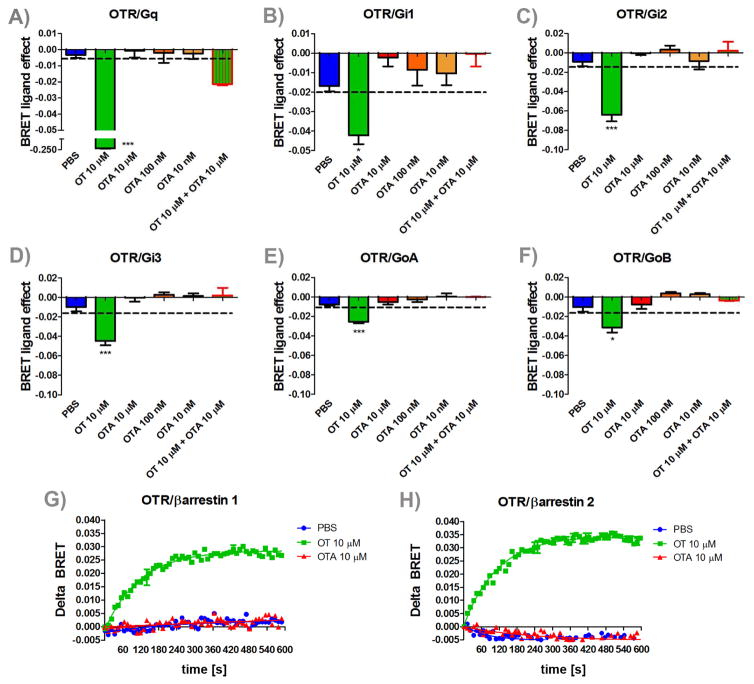
Incubation with OT significantly reduced energy transfer (BRET) between all 6 G-proteins analyzed (Figure 2, all p’s < 0.05), consistent with previous observations that OT is capable of activating Gq and Gi/o pathways. None of the three concentrations of OTA alone led to activation of G-proteins. However, OTA treatment fully prevented G-protein activation of OT. Similarly, in cells co-expressing OTR-Rluc and β-arrestin1-YFP, OT increased the BRET ratio, indicating agonist-induced association between the OTR and β-arrestin 1. Similar results were observed using the β-arrestin2 YFP construct. OTA treatment alone didn’t induce changes in BRET ratio. Thus, L368, 899 is a full antagonist at all known OTR dependent second messenger systems.
Figure 2. Effects of OTA on OTR activation of G-proteins and β-arrestins recruitment.
Mean and SEM of BRET ligand effect of OT, PBS, OT+ OTA and three different concentrations of OTA for (A) Gq, (B) Gi1, (C) Gi2, (D) Gi3, (E) GoA, and (F) GoB activation and (G) β-arrestin 1 and (H) β-arrestin 2 recruitment *=p < 0.05 effect of drug treatment vs. PBS, ***=p < 0.001 effect of drug treatment vs. PBS.
Effects of stress on OTR expression in males and females
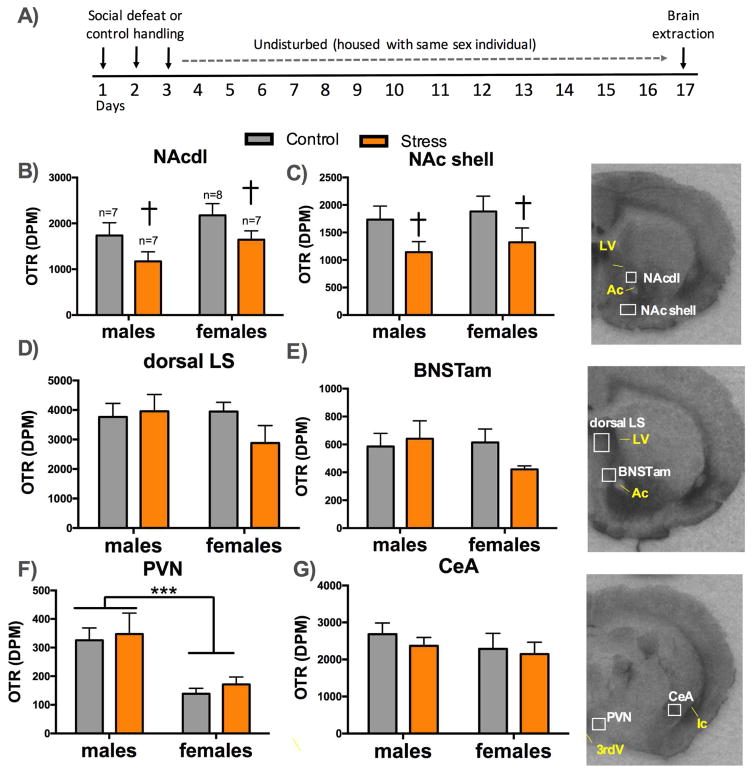
In the NAcdl (Figure 3B; main effect of stress; p=0.03), and NAc shell (Figure 3C; main effect of stress; p=0.03) stressed males and females had significantly less OTR binding than controls. In the PVN, females had significantly less OTR binding than males (Figure 3F; main effect of sex; p<0.001) and there was no effect of stress. There were no effects of sex or stress in dorsal LS (Figure 3D), BNSTam (Figure 3E), or central nucleus of the amygdala (CeA, Figure 3G) any other areas investigated (Supplementary Figure S3). In males, OTR in the NAc shell and CeA were positively correlated with time spent in the interaction zone (Supplementary Table S3). Time spent in the interaction zone was not correlated with OTR in females.
Figure 3. OTR binding in naïve and stressed males and females.
Timeline of experiment (A). Mean and SEM of OTR binding in (B) the dorsolateral nucleus accumbens core (NAcdl) core, (C) NAc shell, (D) dorsal lateral septum, (E) anteromedial bed nucleus of the stria terminalis (BNSTam), (F) paraventricular nucleus (PVN), and (G) central nucleus of the amygdala (CeA). † =p < 0.05 main effect of stress ***=p < 0.01 main effect of sex. Ac=anterior commissure, LV= lateral ventricle,3rdV= third ventricle, Ic= internal capsule.
Effects of intranasal administration of OT and systemic administration of OTA on EGR1 immunoreactivity
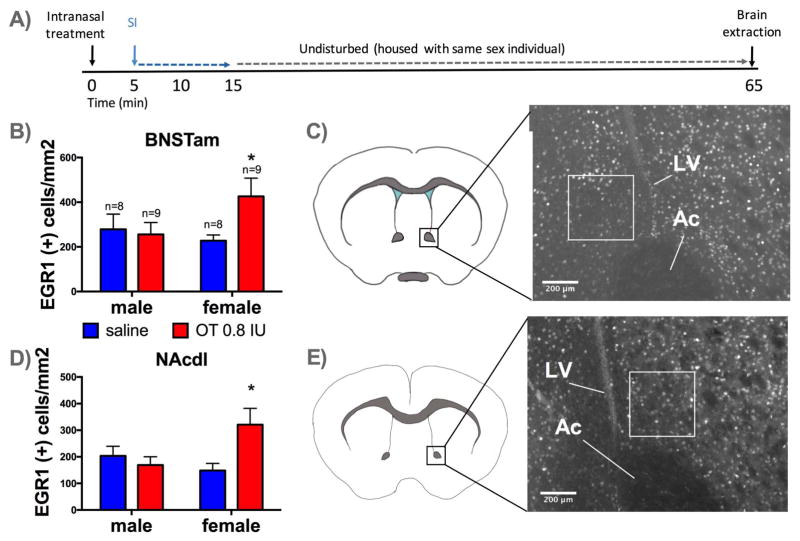
In the BNSTam there was evidence for sex-specific effects of intranasal OT on EGR1 immunoreactivity (Figure 4B; sex*drug interaction, p=0.08). Planned comparisons showed that intranasal OT increased the number of EGR1 positive cells in females (p= 0.03, d=1.09) but not males (d=0.32). A similar pattern was observed in the NAcdl (Figure 4D; sex*drug interaction, p=0.06). Planned comparisons showed that intranasal OT significantly increased EGR1 positive cells in females (p= 0.03, d=0.93) but not males (p= 0.58, d=0.31). In the NAc shell, females had more EGR1 positive cells than males (Supplementary Figure S4, main effect of sex, p=0.03). There were no effects of stress, treatment, or their interaction on EGR1 positive cells detected in NAc core ventromedial, LS or PVN (Supplementary Figure S4). There were no effects of stress or 5 mg/kg OTA treatment on EGR1 expression in NAcdl or BNSTam following the odor preference test (Supplementary Figure S5). However, females had more EGR1 positive cells in both NAcdl (F1,59=47.58, p<0.01) and BNSTam (F1,56=26.47, p<0.01) than males.
Figure 4. Effects of intranasal administration of OT on EGR1 immunoreactivity in naïve males and females.
Timeline of experiment (A). Mean and SEM of EGR1 positive cells per mm2 detected in BNSTam (B), and NAcdl (D), *=p < 0.05 effect of intranasal OT vs. saline. Diagrams of BNSTam (C) and NAc (E) quantification regions with representative photomicrographs and box placement. Ac=anterior commissure, LV=lateral ventricle
Effects of OTA infusion into BNSTam or NAcdl in stressed females on SI
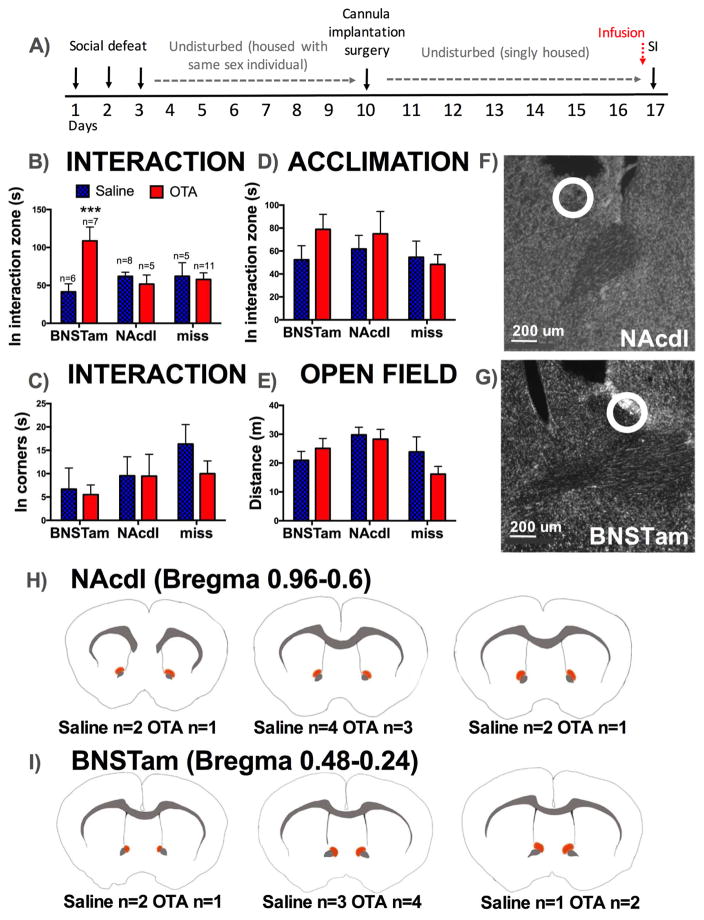
The effects of OTA on social interaction behavior were dependent on the site of injection (Figure 5B, injection site*drug, p<0.001). Stressed females that received infusions of OTA into the BNSTam (Figure 5B, 5G, 5I) spent significantly more time in the interaction zone with the target mouse than females that received saline (p<0.001, d=1.27). In contrast, OTA injections in to the NAcdl (Figure 5B, 5F, 5H d=0.07) or misses (Figure 5B, Supplementary Table S4, d=0.12) had no effect on time spent in the interaction zone with the target mouse. No effects of treatment or injection site were detected in time spent in corners during interaction (Figure 5C), time in the interaction zone during acclimation (Figure 5D) or distance traveled during open field (Figure 5E).
Figure 5. Effects of site-specific administration of OTA on behavior in stressed females.
Timeline of experiment (A). Mean and SEM time spent in the interaction zone (B) and in corners (C) during social interaction. There were no differences in time spent in the interaction zone during acclimation (D) or in locomotor behavior during open field phase (E). Example of hits in Nissl stained slices in NAcdl (F) and BNSTam (G) with injections sites indicated by circles. Diagrams showing location of injections considered as hits for NAcdl (H) and BNSTam (I). Under each diagram, the number of animals receiving injection in each site is indicated. ***=p < 0.01 effect of OTA vs. saline in animals receiving injections in BNSTam.
Effects of OTA on risk assessment behavior
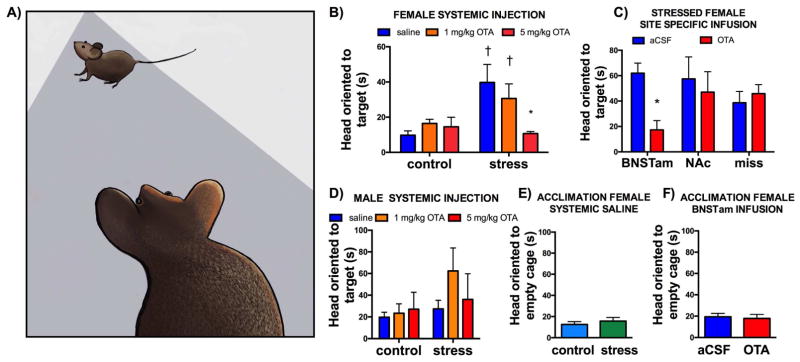
While performing experiments we noticed that stressed females would orient towards the target mouse when outside of the interaction zone (Supplementary Video 1, Figure 6A). Head orientation was recorded by an observer blind to treatment groups. In the systemic OTA experiment, the effect of OTA on time spent oriented towards the target mouse was different in control and stressed females (Figure 6B, stress*drug interaction, P= 0.02). Specifically, for saline treated females, stressed mice spent more time oriented towards the target mouse than control females (Fig. 6B, P<0.01). Treatment with 5 mg/kg of OTA but not 1 mg/kg OTA eliminated the stress-induced increase in time spent oriented toward the target mouse (Figure 6B, P<0.01). No differences in orientation responses were observed in control vs. stressed males (Figure 6D) or during the acclimation phase when there was no target mouse present in control vs. stressed females (Figure 6E). In site-specific studies on stressed females, only OTA infusion in the BNSTam reduced the amount of time the focal mouse was oriented toward the target (Figure 6C, P<0.05). As in the systemic OTA experiment, OTA infusions in the BNSTam had no effect on orientation to an empty cage (Figure 6F). These results suggest that OTR activation in the BNST induces a response in which an individual avoids yet attends an unfamiliar social context.
Figure 6. Effects of OTA on risk assessment behavior.
Drawing of focal mouse oriented towards target mouse (A). Mean and SEM time spent with head oriented towards the target mouse in control and stressed females receiving systemic injections of saline, 1 mg/kg OTA or 5 mg/Kg OTA (B). Stressed females receiving site specific injections of aCSF or OTA (C). Control and stressed males receiving systemic injections of saline, 1 or 5 mg/Kg OTA (D). There was no difference between control and stressed females during acclimation phase (E, when the cage was empty). Similarly, OTA infusion in to the BNSTam had no effect during the acclimation phase (F). *=p<0.05 main effect of OTA vs saline or aCSF, †=p=0.01 effect of stress vs. same drug treatment (aCSF or 1 mg/kg OTA).
DISCUSSION
Our results show that a single administration of systemic OTA is sufficient to reverse stress-induced deficits in social interaction behavior. To achieve similar effects with a selective serotonin reuptake inhibitor, 4 weeks of daily treatment was required (31). Local infusion of OTA in to the BNSTam, but not NAcdl, mimics the effects of systemic administration, indicating that the BNSTam is a critical site of action for OTR-dependent social withdrawal in females. Importantly, our data suggest that the effects of OTA are limited to social contexts. Results from the odor preference test suggest that defeat induces a preference for familiar social contexts and that OTR facilitates this preference. Furthermore, analyses of orientation responses suggest that OTR in the BNSTam promotes a vigilance response to unfamiliar social contexts that inhibits social approach. The rapid action of OTA on social behavior suggests that further dissection of OTR-dependent behavioral phenotypes could lead to important insights for novel uses for OTR ligands.
Sex-specific effects of systemic inhibition of OTR
Our results show that OTR inhibition has opposing effects on social approach in males versus females in a novel environment. This is consistent with our previous data showing that intranasal OT induces social withdrawal in females but not males, and that stress induces hyperactivation of OT neurons in females but not males (15). Studies in rodents and humans have consistently shown robust sex-specific effects of OT on social behavior. For example, in rats intracerebroventricular (ICV) administration of OT increases social investigation of a novel conspecifics in stressed males (32) but not females (43). Injections of OT into the lateral septum reduce social play in juvenile female rats but not male rats (44). Similarly, in prairie voles OT administration during development facilitates partner preference behavior in males (45) but not females (46). In humans, intranasal OT increases anxiety in women but is anxiolytic in men following a social stress test (47). There are several possible explanations for sex-specific actions of OT.
An intuitive explanation for sex-specific effects of OT could involve sex differences in expression of OT or its cognate receptors including OTR and V1aR, as OT can activate multiple receptor types (48–50). Although sex differences in vasopressin immunoreactivity are well documented, there is little evidence for sex differences in OT immunoreactivity (51). In California mice, few sex differences in OT immunoreactivity are observed, and only in mice exposed to defeat stress (15). For receptors, males rats have significantly more V1aR binding than females in 8 brain regions (52). However, other species report few or no sex differences in V1aR binding (53, 54). In a previous study we considered the hypothesis that sex differences in V1aR expression or function contributed to stress-induced social withdrawal (26). There were few sex differences in V1aR binding in California mice and V1aR antagonist infusions in to the BNST reduced social interaction in both males and females. Also, V1aR antagonist infusions in to the NAc had no effects on social interaction in females. In the present study, we investigated whether sex differences or stress-induced changes in OTR expression could account for sex differences in behavioral responses to defeat. There were no sex differences in OTR expression, consistent with previous reports in other species (53, 54). When effects of stress were observed, OTR expression was decreased, possibly as a negative feedback response to elevated OT release (55). On balance, it does not appear that sex differences in OT or OTR expression can account for the sex-specific effects of OTR we report.
An alternative possibility is that there are sex differences in OTR activation of G proteins. OT facilitates OTR coupling to several different G-proteins and β-arrestins, which provides multiple degrees of freedom for effects on neural activity (36, 37). For example, OT activation of OTR can inhibit inward rectifier (IR) currents of immortalized gonadotropin releasing hormone cells (GN11) through activation of Gq/11 whereas the same IR current can be activated by OT via OTR coupling with Gi/o protein (56). Interestingly, the OTR ligand atosiban, considered to be an OTR antagonist, actually activates Gi-mediated pathways in vitro (36, 38) and in vivo (57). Atosiban mimicked the inhibitory effect of OT on sensory neurons of the spinal cord, providing clear evidence of its unique agonist activity on restricted OTR signaling pathways (57). Using BRET assays we showed that unlike atosiban, L368, 899 blocks OTR activation of Gq, Gi, and β-arrestin by OT. To our knowledge, no study has considered whether sex differences in G-protein activation by a single receptor contributes to sex differences in behavioral effects. This would appear to be possible, as sex differences in the cellular trafficking of corticotropin releasing factor receptors was linked to sex differences in the activity of norepinephrine neurons in the locus coeruleus (58). While biased agonists such as carbetocin (which selectively activates Gq) have been reported to have different behavioral effects than OT (59, 60), no study has systematically compared these ligands in males and females. Our finding that a broad-spectrum OTR antagonist has opposite effects on social behavior in males and females suggest that this is a promising direction for further study.
Specific-site effects of OTR on social avoidance
We used several approaches to identify a site of action of OTR. The EGR1 immunoreactivity was effective at identifying nuclei that responded more strongly to intranasal OT in females compared to males. Intranasal OT reduced social interaction behavior in female but not male California mice (15), so we hypothesized that NAcdl and/or BNSTam could be sites of OTR action on behavior. A single injection of OTA in to BNSTam but not the NAcdl, increased social approach in stressed females. This is interesting because there has been increased focus on the BNST as a key locus contributing to stress-induced psychiatric disorders (61, 62).
This sexually dimorphic forebrain structure is involved in the regulation of anxiety (63) and social behaviors including aggression (64, 65) and attachment (66). The BNST is a heterogeneous structure containing both excitatory and inhibitory neurons, and has strong connections with stress response circuits (67), motivational systems (68), and social behavior circuits (69). The BNST is thought to be a key center for integrating information from social and physical environments to generate avoidance/approach responses (61). Anteromedial portions of BNST receive inputs from the medial amygdala and posterior BNST, and send direct projections to PVN and CeA (70, 71). Further study is needed to identify the downstream effects of OTR activation in the BNSTam. Interestingly, social defeat increases levels of brain-derived neurotrophic factor (BNDF) in BNSTam of female but not male California mice (31). Furthermore, infusion of a selective TrkB inhibitior in to BNSTam had an identical effect as OTA, increasing social approach in stressed females. Infusion of TrkB inhibitor in unstressed females had no effect on behavior, suggesting that the role of the BNSTam in modulating social behavior is more important following a stressful experience. It has been reported that OT can increase BDNF expression in hippocampus (72) and neuroblastoma cells (73), but otherwise little is known about OT-BDNF interactions.
Stress induces vigilance and avoidance of unfamiliar social contexts
An intriguing finding was that stressed females spent more time oriented towards the target mouse, but not an empty cage, when outside of the interaction zone. This response was inhibited by systemic or intra-BNST OTA treatment. Male rats confronted with threatening stimuli such as a predator or predator odor exhibit similar orienting responses (74), which have been described as risk assessment or vigilance behavior (24). It has been proposed that the BNST plays a critical role in ‘valence surveillance’, in which an individual assesses social contexts and assigns a positive or negative valence (61). Similarly, the social salience hypothesis posits that OT enhances the salience of both positive and negative social experiences (75). Our results suggest that these hypotheses converge at the BNSTam, where OTR activation appears to inhibit social approach not by reducing social motivation, but by increasing vigilance towards unfamiliar and possibly dangerous social contexts. It has been previously reported that OTR play a critical role in learning during aversive social contexts (76). Our results expand on this finding by showing that OTR mediate behavioral responses that occur weeks after an aversive social experience.
Conclusions
The current findings combined with our previous work (15) suggest a model that social defeat induces hyperactivity of BNSTmv and PVN OT neurons which in turn increase the activation of OTR in the BNSTam to induce avoidance of unfamiliar social contexts in females. The empirical data support the hypothesis that elevated OT may contribute to social vigilance in novel contexts. Interestingly, intranasal OT has been reported to increase perceived social stress (9) and mistrust (77) in humans. Together, this evidence suggests that in unfamiliar contexts, OTR antagonists may have unappreciated therapeutic potential for reducing social anxiety. Further study of OT and OTR-sensitive circuits on the behavioral effects of psychosocial stressors could greatly contribute to the understanding of mechanisms underlying social deficits associated with psychiatric disorders.
Supplementary Material
Figure S1. Effects of systemic administration of OTA on behavior during acclimation and open field phase of social interaction test in males and females. Mean and SEM time spent (a) in proximity to an empty cage (b) in corners, and (c) in center of the open field. †=p<0.01 main effect of stress.
Figure S2. Effects of stress and systemic administration of OTA on behavior reanalysis during social interaction in females and males. Mean and SEM time spent (A) inactive in interaction zone (B) autogrooming, and (C) time spent in sides of arena. *=p<0.05 OTA vs. saline.
Figure S3. OTR binding in naïve and stressed males and females. Mean and SEM of OTR binding in (A) dorsal bed nucleus of stria terminalis (BNST) (B) medioventral BNST, (C) ventral lateral septum (LS), (C) Prefrontal cortex, (D) Dentate gyrus and (F) Hippocampus area CA1.
Figure S4. Effects of intranasal administration of OT on EGR1 immunoreactivity in naïve males and females. Mean and SEM of EGR1 positive cells per mm2 detected in A) Nucleus accumbens shell (NAc), B) NAc core ventromedial, C) lateral septum (LS), and D) Paraventricular nucleus of hypothalamus (PVN). †=p<0.05 main effect of sex.
Figure S5. Effects of systemic administration of 5mg/Kg OTA on EGR1 immunoreactivity in naïve and stressed males and females. Mean and SEM of Egr-1 positive cells per mm2 detected in A) Nucleus accumbens core dorsolateral (NAcdl) and B) Bed nucleus of the stria terminalis anteromedial (BNSTam). * = p<0.01 main effect of sex.
Table S1. Social interaction reanalysis
Table S2. Estrous stage analysis on behavior (3-way ANOVAS including stress, treatment, and estrous stage)
Table S3. Correlations of OTR binding and social interaction behavior in males and females.
Table S4. Location of injection misses by treatment. Numbers in parenthesis represent number of injections hitting that region in the 14 mice with missed injection sites; injection hits were assessed unilaterally.
Acknowledgments
The authors thank D. Fox, J. Geng, A. Shackman, C. A. Marler, A.V. Williams, E. Wright, and M. Wilson for helpful discussions. This work was supported by Becas Chile Comisión Nacional de Investigación Científica y Tecnológica (to ND-W), Grant No. F31 MH095253 (to Q25 Q26 MQS), a National Science Foundation Graduate Research Fellowships grant Q27 (to SAL), and National Institutes of Health Grant no. R01 MH103322 (to BCT).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 2017;76:87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2016;79:213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Domes G, Heinrichs M, Gläscher J, Büchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 7.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci Off J Soc Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R. Oxytocin facilitates the sensation of social stress. Hum Brain Mapp. 2014;35:4741–4750. doi: 10.1002/hbm.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 11.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, et al. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci. 2009;31:332–341. doi: 10.1159/000216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 15.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, et al. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry. 2016;80:406–414. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, et al. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PloS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boccia ML, Goursaud A-PS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899®, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettibone DJ, Clineschmidt BV, Guidotti MT, Lis EV, Reiss DR, Woyden CJ, et al. L-368,899, a potent orally active oxytocin antagonist for potential use in preterm labor. Drug Dev Res. 1993;30:129–142. [Google Scholar]
- 20.Lee S-Y, Park S-H, Chung C, Kim JJ, Choi S-Y, Han J-S. Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci Rep. 2015:5. doi: 10.1038/srep18540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olszewski PK, Waas JR, Brooks LL, Herisson F, Levine AS. Oxytocin receptor blockade reduces acquisition but not retrieval of taste aversion and blunts responsiveness of amygdala neurons to an aversive stimulus. Peptides. 2013;50:36–41. doi: 10.1016/j.peptides.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci. 2015;112:14084–14089. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, et al. Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab Dispos Biol Fate Chem. 1997;25:1113–1118. [PubMed] [Google Scholar]
- 24.Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Laredo SA, Steinman MQ, Robles CF, Ferrer E, Ragen BJ, Trainor BC. Effects of defeat stress on behavioral flexibility in males and females: modulation by the mu-opioid receptor. Eur J Neurosci. 2015;41:434–441. doi: 10.1111/ejn.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC. Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology. 2016;110:59–68. doi: 10.1016/j.neuropharm.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL. Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front Behav Neurosci. 2015;9:191. doi: 10.3389/fnbeh.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasen NS, Gammie SC. Maternal aggression: New insights from Egr-1. Brain Res. 2006;1108:147–156. doi: 10.1016/j.brainres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Yochiy A, Britto LRG, Hunziker MHL. Novelty, but not operant aversive learning, enhances Fos and Egr-1 expression in the medial prefrontal cortex and hippocampal areas of rats. Behav Neurosci. 2012;126:826–834. doi: 10.1037/a0030721. [DOI] [PubMed] [Google Scholar]
- 30.Loveland JL, Fernald RD. Differential activation of vasotocin neurons in contexts that elicit aggression and courtship. Behav Brain Res. 2017;317:188–203. doi: 10.1016/j.bbr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The Neuropeptide Oxytocin Facilitates Pro-Social Behavior and Prevents Social Avoidance in Rats and Mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herisson FM, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12381. [DOI] [PubMed] [Google Scholar]
- 34.Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology. 2015;90:74–81. doi: 10.1016/j.neuropharm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85–91. doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther. 2013;346:318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 38.Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, Chini B. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J Biol Chem. 2005;280:16311–16318. doi: 10.1074/jbc.M409945200. [DOI] [PubMed] [Google Scholar]
- 39.Busnelli M, Saulière A, Manning M, Bouvier M, Galés C, Chini B. Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 2012;287:3617–3629. doi: 10.1074/jbc.M111.277178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva AL, Fry WHD, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC. Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci. 2015;42:3081–3094. doi: 10.1111/ejn.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014:0. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lukas M, Neumann ID. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. J Neurosci Methods. 2014;234:101–107. doi: 10.1016/j.jneumeth.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Bredewold R, Smith CJW, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2003;117:854–859. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- 46.Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biol Psychol. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Z, Larkin TE, Malley MO, Albers HE. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus) Horm Behav. 2016;81:20–27. doi: 10.1016/j.yhbeh.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 51.DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017 doi: 10.1002/cne.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CJW, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2017;222:981–1006. doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43:623–630. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- 55.Phaneuf S, Rodríguez Liñares B, TambyRaja RL, MacKenzie IZ, López Bernal A. Loss of myometrial oxytocin receptors during oxytocin-induced and oxytocin-augmented labour. J Reprod Fertil. 2000;120:91–97. doi: 10.1530/jrf.0.1200091. [DOI] [PubMed] [Google Scholar]
- 56.Gravati M, Busnelli M, Bulgheroni E, Reversi A, Spaiardi P, Parenti M, et al. Dual modulation of inward rectifier potassium currents in olfactory neuronal cells by promiscuous G protein coupling of the oxytocin receptor. J Neurochem. 2010;114:1424–1435. doi: 10.1111/j.1471-4159.2010.06861.x. [DOI] [PubMed] [Google Scholar]
- 57.Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron. 2016;89:1291–1304. doi: 10.1016/j.neuron.2016.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bangasser DA, Reyes Ba S, Piel D, Garachh V, Zhang X-Y, Plona ZM, et al. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60:57–62. [PubMed] [Google Scholar]
- 60.Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Oxytocin and carbetocin ameliorating effects on restraint stress-induced short- and long-term behavioral changes in rats. Neuro Endocrinol Lett. 2010;31:622–630. [PubMed] [Google Scholar]
- 61.Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016;21:450–463. doi: 10.1038/mp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daniel SE, Rainnie DG. Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;41:103–125. doi: 10.1038/npp.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duque-Wilckens N, Trainor BC. Behavioral Neuroendocrinology of Female Aggression. 2017 doi: 10.1093/acrefore/9780190264086.013.11. [DOI] [Google Scholar]
- 65.Marsh AA. What can we learn about emotion by studying psychopathy? Front Hum Neurosci. 2013;7:181. doi: 10.3389/fnhum.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coria-Avila GA, Manzo J, Garcia LI, Carrillo P, Miquel M, Pfaus JG. Neurobiology of social attachments. Neurosci Biobehav Rev. 2014;43:173–182. doi: 10.1016/j.neubiorev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol. 2013;11:141–159. doi: 10.2174/1570159X11311020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 69.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 70.Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- 72.Dayi A, Cetin F, Sisman AR, Aksu I, Tas A, Gönenc S, Uysal N. The Effects of Oxytocin on Cognitive Defect Caused by Chronic Restraint Stress Applied to Adolescent Rats and on Hippocampal VEGF and BDNF Levels. Med Sci Monit. 2015;21:69–75. doi: 10.12659/MSM.893159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bakos J, Strbak V, Paulikova H, Krajnakova L, Lestanova Z, Bacova Z. Oxytocin receptor ligands induce changes in cytoskeleton in neuroblastoma cells. J Mol Neurosci MN. 2013;50:462–468. doi: 10.1007/s12031-013-9960-4. [DOI] [PubMed] [Google Scholar]
- 74.Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol Wash DC 1983. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 75.Shamay-Tsoory SG, Abu-Akel A. The Social Salience Hypothesis of Oxytocin. Biol Psychiatry. 2016;79:194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 76.Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, Choi GB. Oxytocin Mediates Entrainment of Sensory Stimuli to Social Cues of Opposing Valence. Neuron. 2015;87:152–163. doi: 10.1016/j.neuron.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011;6:556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of systemic administration of OTA on behavior during acclimation and open field phase of social interaction test in males and females. Mean and SEM time spent (a) in proximity to an empty cage (b) in corners, and (c) in center of the open field. †=p<0.01 main effect of stress.
Figure S2. Effects of stress and systemic administration of OTA on behavior reanalysis during social interaction in females and males. Mean and SEM time spent (A) inactive in interaction zone (B) autogrooming, and (C) time spent in sides of arena. *=p<0.05 OTA vs. saline.
Figure S3. OTR binding in naïve and stressed males and females. Mean and SEM of OTR binding in (A) dorsal bed nucleus of stria terminalis (BNST) (B) medioventral BNST, (C) ventral lateral septum (LS), (C) Prefrontal cortex, (D) Dentate gyrus and (F) Hippocampus area CA1.
Figure S4. Effects of intranasal administration of OT on EGR1 immunoreactivity in naïve males and females. Mean and SEM of EGR1 positive cells per mm2 detected in A) Nucleus accumbens shell (NAc), B) NAc core ventromedial, C) lateral septum (LS), and D) Paraventricular nucleus of hypothalamus (PVN). †=p<0.05 main effect of sex.
Figure S5. Effects of systemic administration of 5mg/Kg OTA on EGR1 immunoreactivity in naïve and stressed males and females. Mean and SEM of Egr-1 positive cells per mm2 detected in A) Nucleus accumbens core dorsolateral (NAcdl) and B) Bed nucleus of the stria terminalis anteromedial (BNSTam). * = p<0.01 main effect of sex.
Table S1. Social interaction reanalysis
Table S2. Estrous stage analysis on behavior (3-way ANOVAS including stress, treatment, and estrous stage)
Table S3. Correlations of OTR binding and social interaction behavior in males and females.
Table S4. Location of injection misses by treatment. Numbers in parenthesis represent number of injections hitting that region in the 14 mice with missed injection sites; injection hits were assessed unilaterally.