Abstract
Mucus is a selective barrier to particles and molecules, preventing penetration to the epithelial surface of mucosal tissues. Significant advances in transmucosal drug delivery have recently been made and have emphasized that an understanding of the basic structure, viscoelastic properties, and interactions of mucus is of great value in the design of efficient drug delivery systems. Mucins, the primary non-aqueous component of mucus, are polymers carrying a complex and heterogeneous structure with domains that undergo a variety of molecular interactions, such as hydrophilic/hydrophobic, hydrogen bonds and electrostatic interactions. These properties are directly relevant to the numerous mucin-associated diseases, as well as delivering drugs across the mucus barrier. Therefore, in this review we discuss regional differences in mucus composition, mucus physicochemical properties, such as pore size, viscoelasticity, pH, and ionic strength. These factors are also discussed with respect to changes in mucus properties as a function of disease state. Collectively, the review seeks to provide a state of the art roadmap for researchers who must contend with this critical barrier to drug delivery.
Keywords: Mucus barrier, mucins, viscoelasticity, mucus filtering mechanisms, drug delivery
Graphical Abstract
1. Introduction
One of the major challenges to transmucosal drug delivery is the presence of a complex mucus barrier lining the mucosal epithelium of tissues. Mucus is a complex hydrogel biopolymer barrier located in the airways, gastrointestinal tract, reproductive tract, and the eyes (Bansil and Turner, 2006; Lieleg and Ribbeck, 2011). It is continuously produced, secreted, and finally digested, recycled, or discarded (Lai et al., 2009d) and its main functions include lubrication of the epithelia, maintenance of a hydrated layer, exchange of gases and nutrients with the underlying epithelium, as well as acting as a barrier to pathogens and foreign substances (Bansil and Turner, 2006; Lai et al., 2009d). Moreover, mucus is involved in various disease states like asthma, bronchitis, cystic fibrosis, and cancer (Bansil and Turner, 2006; Hollingsworth and Swanson, 2004; Khanvilkar et al., 2001; Rose and Voynow, 2006; Thornton et al., 2008; Williams et al., 2006). Mucus is a barrier which impedes transport of drugs and other molecules, and its physicochemical properties such as pore size, viscoelasticity, pH, ionic strength, and charge can impact the eventual fate and delivery of drug delivery systems in mucosal tissues (Bansil and Turner, 2006; Cone, 2009).
During homeostasis, the airway epithelium contains a protective continuous mucus layer, with a thickness of 5–15 μm in the nasal cavity (Beule, 2010; Ugwoke et al., 2005), 10–30 μm in the trachea and 2–5 μm in the bronchi (Patton, 1996; Sanders et al., 2009; Wine, 1999). The gastrointestinal tract not only allows the digestion and absorption of nutrients, electrolytes, and fluids but also acts as a barrier against environmental threats. The gastrointestinal epithelium has a protective continuous mucus layer preventing direct access by microorganisms to the intestinal mucosa (Ensign et al., 2012a; Goldberg and Gomez-Orellana, 2003; Macpherson and Harris, 2004), with a mean thickness of 100–300 μm in the stomachs of rats and 100–900 μm in the intestine (Atuma et al., 2001; Jordan et al., 1998). The stomach and colon are comprised of two mucus layers, a loosely adherent mucus layer and an underlying firmly adherent mucus layer attached to the mucosa; the small intestine contains a single mucus layer. The gastrointestinal mucus hinders the diffusion of bacteria (Macpherson and Harris, 2004) and macromolecules (Schenk and Mueller, 2008), decreasing permeation across this barrier. In the vagina, its surface is lined and protected by cervical mucus (thickness ~ 50 μm (McKinley et al., 2014)), which changes in rate of production and viscoelastic properties throughout the menstrual cycle. During ovulation, cervical mucus is less viscoelastic, resulting in higher permeability of molecules (Wolf et al., 1978). In the eye, the conjunctiva is a mucosal epithelium that has a mucus layer secreted by goblet cells lined with the cornea to form a precorneal mucin gel with a reported thickness of 3 μm to more than 30 μm depending on the measurement method used. The conjunctiva acts as a lubricant and a stabilizer of the tear film (Ellingham et al., 1999; Greaves and Wilson, 1993; Ludwig, 2005; Prydal and Campbell, 1992).
Mucus is composed of water, mucins, globular proteins, salts, DNA, lipids, cells and cellular debris (Bansil and Turner, 2006; Button et al., 2013; Carlstedt and Sheehan, 1989; Cone, 2005; Thornton and Sheehan, 2004), wherein the homeostasis of these several components is complex and highly interdependent. Minor changes within mucus constituents can significantly alter the physicochemical properties and affect disease states. For instance, the mucus layer hydration state is directly related to ions, salts and water concentrations in the lungs (Button and Button, 2013) and is critical for the mucociliary clearance process, which rapidly removes particles that remain deposited on the ciliated cells (Boucher, 2007b; Knowles and Boucher, 2002). Indeed, there is a negative correlation between airway surface mucus concentration (i.e. percentage solids and total mucins concentration) and mucociliary clearance in patients with chronic bronchitis (Anderson et al., 2015). Additionally, there is evidence that mucociliary clearance in the airways can be regulated by luminal ATP concentrations as a feedback system in response to changes in the hydration status of the mucus layer to maintain rheological properties that ensure efficient mucus clearance (Button et al., 2013). Mucus concentration is also a dominant variable affecting mucus viscosity (Button et al., 2016). Recently, a sialic acid/urea ratio methodology has been developed to measure mucus hydration as an alternative to the conventional percent solids method. This method can be useful experimentally, as airway samples are often diluted during collection, for example in bronchoalveolar lavage (BAL) samples (Esther et al., 2017). It has also been proposed that ionic strength and pH also modulate the mucus hydration and viscoelasticity (Celli et al., 2005; Georgiades et al., 2014; Tam and Verdugo, 1981). Specifically, there is evidence suggesting that not only chloride ions, but calcium and bicarbonate ions play an important role in the expansion of polymeric mucins after their secretion, a fundamental process in mucus formation and transport (Cooper et al., 2013; Thornton et al., 2008). Moreover, understanding mucus viscoelasticity is critical to elucidate mucus physiological processes and disease states, relevant for the design of mucosal drug delivery systems (Khanvilkar et al., 2001; Lai et al., 2009d).
In this review, we discuss physicochemical properties of mucus in different tissues and how the mucus barrier impacts on drug delivery systems to mucosal surfaces. The gastrointestinal tract, nose, lungs, vagina, and eyes are sites with mucosal epithelia that are accessible for mucosal drug delivery systems.
2. Mucus composition
In general, mucus is mainly composed of water (~95% w/w), mucins (~0.2 to 5.0% w/v), globular proteins (~0.5% w/v), salts (~0.5 to 1.0% w/w), lipids (1–2% w/w), DNA, cells, and cellular debris (Allen et al., 1993; Bansil and Turner, 2006; Boegh et al., 2013; Button et al., 2013; Carlstedt and Sheehan, 1989; Cone, 2005; Fahy et al., 1993; Ghani and Soothill, 1997; Khanvilkar et al., 2001; Kilbourn, 1978; Lai et al., 2009d; Lopata et al., 1974; Matthews et al., 1963; Thornton and Sheehan, 2004) and forms a dense, viscoelastic layer over epithelial cells to serve as a selective barrier to drugs and other molecules. The mucus layer has a high number of physical entanglements stabilized by covalent and noncovalent interactions, including hydrophobic, electrostatic, hydrogen bonds, or other specific binding interactions that contribute to the mucus viscoelasticity, creating a mesh network filter that decreases penetration of molecules and particles and their diffusion rates (Lieleg and Ribbeck, 2011; Sanders et al., 2009).
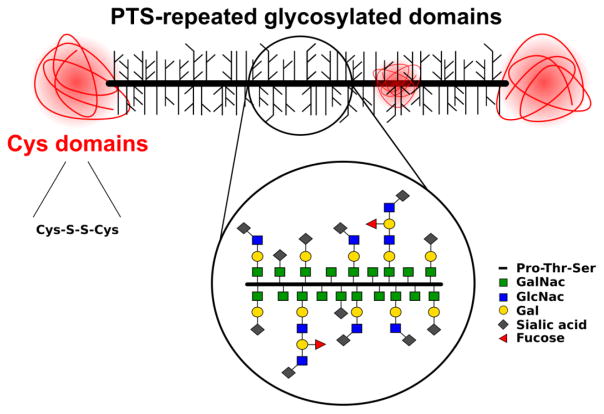
Mucins are very high molecular weight (10–40 MDa) polymeric gel-forming glycoproteins (Sheehan et al., 1986) secreted by epithelial goblet cells and submucosal glands (Carlstedt and Sheehan, 1989; Thornton and Sheehan, 2004). Mucin fibers are filamentous O-linked glycoproteins with ‘PTS’ (proline, threonine, and serine) repeated domains, which are highly glycosylated with a carbohydrate density of more than 70% (Figure 1) (Lamblin et al., 1991; Thornton et al., 2008). Glycosylation involves primarily N-acetylgalactosamine (GalNac), N-acetylglucosamine (GlcNac), fucose, galactose (Gal), and sialic acid and low amounts of mannose and sulfate (Bansil and Turner, 2006). Due to their dense glycosylation, mucins are arranged in a brush-like structure (Lieleg and Ribbeck, 2011). Inside the secretory glands, high concentrations of calcium ions aid mucin condensation by shielding negatively charged sulfate and sialic acid groups. After secretion, mucins undergo dramatic swelling, with over 500-fold expansion in volume (Cone, 2009; Verdugo, 1990). Additionally, steric interactions of O-linked GalNac residues with the protein core contribute to the expanded mucin structure (Shogren et al., 1989). Furthermore, the PTS-domains are interspersed with hydrophobic globular regions with a high proportion of cysteine, which form intradisulfide bonds, and the subsequent polymerization forms long linear oligomers that provide the adhesive and swellable properties of the mucus layer (Bansil and Turner, 2006; Sheehan et al., 1986). High sialic acid and sulfate content in most mucin glycoproteins confer a strongly net-negative surface charge which increase the stiffness via charge repulsion (Shogren et al., 1989). At acidic pH, mucins change conformation from random coil to extended conformation and form a gel phase in mucus (Cao et al., 1999). These conformational changes were proposed to facilitate cross-links among mucin macromolecules through hydrophobic interactions at a low pH, leading to a sol-gel transition state (Cao et al., 1999). Additionally, changes in ionic strength may play a role in the formation of a gel phase in mucus, demonstrated by a study that calcium ions might promote assembly of mucins into large linear or branched aggregates (Raynal et al., 2003). Thus, physicochemical characteristics like composition, pH, ionic strength, conformation are important in the formation, function and rheological properties of mucus.
Figure 1.
Mucin O-linked glycoproteins. A mucin protein backbone typically consists of ‘PTS’ (proline, threonine, and serine) repeated domains, and interspersed with cysteine domains stabilized by internal disulfide bonds. Various O-glycans are linked to threonine or serine residues in the ‘PTS’ repeated domains. N-terminus to C-terminus from left to right, respectively. GalNac - N-acetylgalactosamine, GlcNac - N-acetylglucosamine, and Gal - galactose, not drawn to scale
Currently, there are 21 mucin genes (MUC) identified in humans according to the HUGO gene nomenclature committee (http://www.genenames.org/, accessed 05.04.17). There are two types of mucins: membrane-bound mucins and secreted mucins. Membrane-bound mucins are related to cellular adhesion, pathogen binding, and signal transduction functions, while secreted mucins are highly related to the viscoelastic properties of mucus (Hollingsworth and Swanson, 2004; Williams et al., 2006).
Although the PTS-repeated domains are common to all mucins, their glycosylation, their specific sequence and number of tandem repeats are variable, and different collections of mucin genes are expressed in different tissues, suggesting that individual mucins have evolved to perform specific roles where they are expressed. Specific organs and correspondent secreted mucins are described in Table 1.
Table 1.
Mucins and expression in different organs.
2.1 Mucus in airways
In the airways, MUC5AC and MUC5B are the major polymeric mucins present (Davies et al., 1999; Hovenberg et al., 1996b; Kirkham et al., 2002), secreted from goblet cells in the surface epithelium and mucous cells located in submucosal glands, respectively (Groneberg et al., 2002; Hovenberg et al., 1996a; Wickström et al., 1998). MUC5B has two different glycoforms, occurring in low- and high-charged variants, being the low-charge variant enriched in mucin derived from human respiratory tract submucosal tissue (Thornton et al., 1997). MUC2 is present in the respiratory tract, albeit in lower concentrations (Cone, 2009; Davies et al., 1999; Hovenberg et al., 1996b).
Mucins exert a key function in airway defense by protecting the epithelium against foreign pathogens. Despite their important role in innate defense of the airways, hypersecretion and upregulation of mucin expression are associated with chronic airway diseases (Groneberg et al., 2003). While MUC5AC is the main mucin present in healthy airway secretions, MUC5B has been predominant in more chronic conditions, as in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) (Kirkham et al., 2002), although both have been shown to increase in concentration in CF airway secretions during pulmonary exacerbation (Henke et al., 2007). It was demonstrated that MUC5B, but not MUC5AC, is required for airway defense, playing an important role in controlling infections in the airways, as well as maintaining lungs immune response and mucociliary clearance in mice (Roy et al., 2014). Moreover, these findings were confirmed recently, and it has been suggested that mucus hyperconcentration controls mucociliary clearance deficit in the production of bronchitic airway pathology (Livraghi-Butrico et al., 2016). Thus, although therapies that reduce mucus production and secretion might decrease obstruction, careful design of therapeutic strategies to promote adequate mucociliary clearance should be taken, considering a complete MUC5B removal from the airways may be undesirable and result in adverse events, such as chronic bacterial infections, macrophages with impaired phagocytic functions, and increased airway inflammation (Livraghi-Butrico et al., 2016; Roy et al., 2014). Investigating the role of mucins both in health and disease has been of great interest, and from substantial progress in the past two decades. Specific mucins decreased production, secretion, and expression are potential novel strategies being evaluated for the treatment of pathologic mucus in airway inflammatory diseases (Bae et al., 2014; Dickinson et al., 2016; Fahy and Dickey, 2010; Ha and Rogers, 2016; Lee et al., 2016).
2.2 Gastrointestinal mucus
The gastrointestinal (GI) tract exerts a complex dual function as a selective barrier for transport of molecules. While the GI tract allows the absorption and passage of nutrients and other molecules to the systemic circulation, it also serves as the first line of defense against pathogens. The selective filtering properties of mucus is critically responsible to modulate gastrointestinal permeability. Along the gastrointestinal tract, mucus disposition varies significantly in the stomach, small intestine, and colon. The major secreted mucin present in the GI tract is MUC2 (Strugala et al., 2003), followed by MUC5AC, and lower concentrations of MUC5B, MUC6, and MUC7 (Thornton et al., 2008). In the salivary glands, MUC5B and MUC7 are produced and serve as lubricants for passage of food throughout the esophagus (Bobek et al., 1993; Wickström et al., 1998). The gastrointestinal mucus barrier decreases permeation and hinders the diffusion of bacteria (Brieland et al., 2001; Linden et al., 2002), drugs, and macromolecules (Boegh et al., 2015; Larhed et al., 1998; Larhed et al., 1997; Schenk and Mueller, 2008). In the stomach, epithelial cells secrete bicarbonate, and the inner mucus layer containing mainly MUC5AC is a diffusion barrier for hydrochloric acid (Schade et al.), which creates a pH gradient from the acidic lumen to the neutral epithelium. MUC5AC and MUC6 mucins protect the gastric epithelium from Helicobacter pylori bacteria, where the former contains specific glycan structures and acts as ligands to bind bacteria (Linden et al., 2002) whereas the latter performs a natural antibiotic function (Kawakubo et al., 2004). Unlike the airways where mucus is motile to maintain homeostasis for mucociliary clearance, gastric mucus is an adherent unstirred layer to act as a barrier against invasive pathogens.
In the intestine, MUC2 mucin is the major component of the intestinal mucus (Pelaseyed et al., 2014). An outer loosely adherent mucus layer and an underlying firmly adherent mucus layer line the stomach and colon, whereas a single mucus layer protects the small intestine (Figure 2) (Johansson et al., 2013; Lundquist and Artursson, 2016). The small intestine mucus layer is not attached to the epithelium under normal conditions, however it was found to be firmly adherent in the cystic fibrosis disease due to dysfunctional CFTR-secreted bicarbonate (Pelaseyed et al., 2014). The thicker double layer of mucus in the stomach and colon functions as a protecting mechanism to the lining epithelium against the stomach acidic pH and pathogens. As an additional defense against foreign pathogens in the colon, the inner mucus layer is constantly renewed by secreting surface goblet cells, with a 1–2 h turnover estimated from murine distal colonic tissue (Johansson, 2012). The thinner and loosely adherent mucus in the small intestine contributes to absorption of nutrients and other molecules, as more than 90% of nutrients (carbohydrates, proteins, lipids, water, vitamins, and minerals) are absorbed by the small intestine, while the rest is absorbed in the stomach and large intestine (Renukuntla et al., 2013).
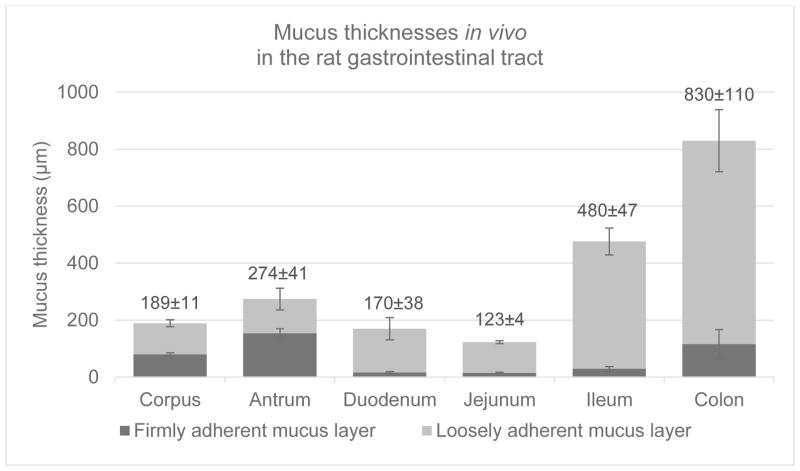
Figure 2.
Graph showing the thicknesses of the mucus gel layers in vivo in the rat gastrointestinal tract. The values for total mucus thickness in μm are reported on top as means ± SE for each group. Adapted from (Atuma et al., 2001)
2.3 Cervical mucus
Mucus in the endocervical epithelium concomitantly acts as a barrier against pathogens and helps regulate the reproductive function of the female reproductive tract by modulating sperm entry into the uterus. The primary mucins in the cervix are MUC4 and MUC5B, along with smaller amounts of MUC5AC and MUC6. During ovulation there is a peak in mucus production and MUC5B secretion, which correlate with high estrogen levels (Curlin and Bursac, 2013), higher pH, and decreased viscoelasticity of mucus (Svensson and Arnebrant, 2010), and subsequently, these factors combine to facilitate sperm mucus permeation. Also during ovulation, there are changes to the structure and glycosylation of mucus; specifically, there is a decrease in the number of sugar residues containing sulfate groups and sialic acid residues, and there is a resulting increase in pH (Curlin and Bursac, 2013). The cervical mucus plug comprises additionally antimicrobial activity from components such as secretory leukoprotease inhibitor, lysozyme, lactoferrin, and neutrophil defensins (Bernkop-Schnürch and Hornof, 2003; Hein et al., 2002)
2.4 Ocular mucus
On the surface of the eye, mucus lining the conjunctival epithelium is secreted by goblet cells and functions as a lubricant and a stabilizer of the tear film. The precorneal tear film is composed of a superficial lipid layer, a central aqueous layer, and an inner mucus layer (Figure 3) (Ludwig, 2005).
Figure 3.
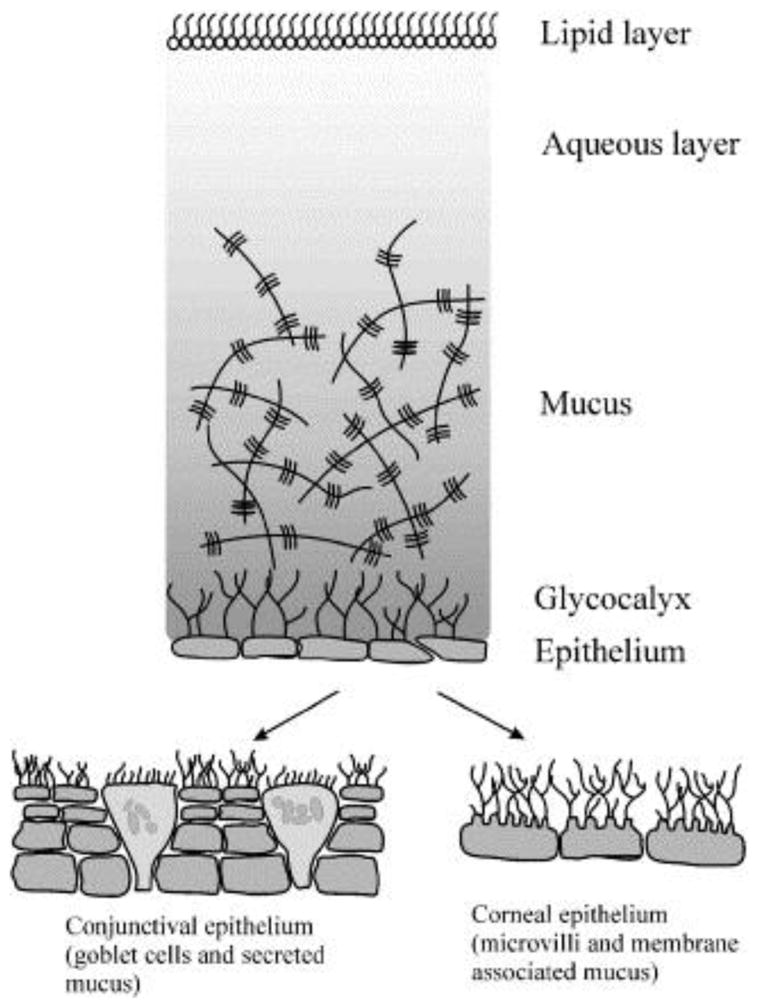
Schematic of the precorneal tear film, composed of three layers lining the conjunctival epithelium and the corneal epithelium. Reprinted from reference (Ludwig, 2005), with permission from Elsevier.
The major gel-forming mucin in human tears is MUC5AC (Inatomi et al., 1996; Jumblatt et al., 1999). Conjunctival goblet cells produce MUC5AC, along with TFF peptides contributing to the viscoelasticity of the tear film (Langer et al., 1999; Ludwig, 2005). MUC1, MUC2, MUC4, and MUC5AC are expressed in the conjunctiva, being MUC5AC synthetized in the goblet cells (Jumblatt et al., 2003). MUC2, also a gel-forming mucin, has been found expressed in normal human conjunctiva, although at levels 5900-fold lower than MUC5AC (McKenzie et al., 2000). MUC4 is also present in the tear fluid and conjunctival epithelium, and a deficiency of MUC4 along with MUC5AC has been found in dry eye disorders (Yeo et al., 2003). MUC1 is a transmembrane mucin produced by the corneal and conjunctival epithelium and may play a role in tear film spread, as well as preventing adhesion of pathogens, debris, and cells to the ocular surface (Inatomi et al., 1995). Goblet cells-associated mucins are produced in the conjunctiva, and nongoblet epithelium of the cornea and conjunctiva are also likely sources of tear film mucins. Indeed, MUC1, MUC4, MUC5B, and MUC7 were found to be present in normal human lacrimal gland, indicating that the lacrimal gland, together with the conjunctiva, may contribute to mucin composition on the ocular surface (Jumblatt et al., 2003). More recently, it has been demonstrated a correlation between MUC16 and MUC5AC amounts in human tears, but not with the other membrane associated mucin MUC1, suggesting that the MUC16 in tears is associated with goblet cell secretion (Gipson et al., 2016). Other mucins found to be present in the ocular surface are MUC2, MUC6, MUC13, MUC15, and MUC17, which may possibly contribute to the protective ocular mucus barrier, although their specific role in the tear film remains to be elucidated (Argüeso and Gipson, 2001; Argüeso et al., 2003; Corraleset al., 2003a; Corrales et al., 2003b; Ellingham et al., 1997; Gipson and Inatomi, 1998; Ohashi et al., 2006; Paulsen et al., 2004).
3. Mucus physicochemical properties and filtering mechanisms
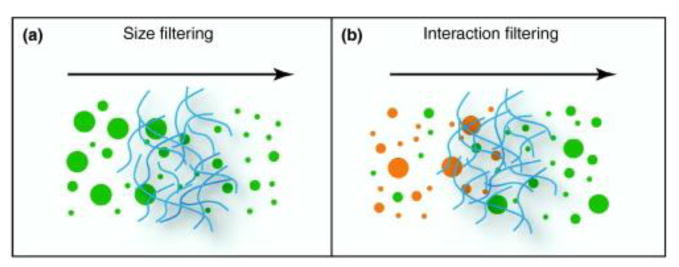
Mucus regulates permeability to molecules and particles through various suggested mechanisms including size exclusion, hydrogen bonding, electrostatic and hydrophobic interactions, and other specific binding interactions (Figure 4).
Figure 4.
Filtering mechanisms regulating mucus permeability: (a) size exclusion mechanism. Size filtering allows molecules and particles that are smaller than the mesh spacing between the mucin fibers to cross, whereas larger molecules are rejected. (b) interaction filtering including electrostatic and hydrophobic interactions, hydrogen bonds and other specific binding interactions allow particles to be selected according to their surface properties: a subset of particles (orange) interact strongly with the mucus and are trapped, whereas other particles (green) exhibit only weak interactions and thus are able to cross. Reprinted from reference (Lieleg and Ribbeck, 2011), with permission from Elsevier.
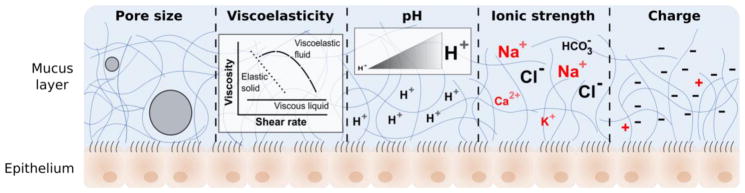
These mechanisms are mainly governed by mucus physicochemical properties such as pore size, viscoelasticity, pH, ionic strength, and charge, illustrated in figure 5.
Figure 5.
Pore size, viscoelasticity, pH, ionic strength, and charge are the main physicochemical properties governing translocation of molecules and drug delivery systems across the mucus barrier. The impact of these properties on healthy and disease states is discussed in the next sections.
Moreover, mucus permeation may vary according to different organs, pathological conditions, and even patients. Table 2 compiles measurements of mucus physicochemical properties in different organs and diseases reported in the literature, which will be discussed further in this review.
Table 2.
Mucus physicochemical properties in different organs. Abbreviations and symbols: NF, not found. IQR, interquartile range
| Organs Properties |
Nose | Lungs | Cervix | Stomach | Intestine | Eyes20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Cystic Fibrosis |
Asthma | COPD | Proliferative phase |
Ovulation | Secretory phase |
Other | Small intestine |
Colon | |||||
| Ionic strength | HCO3− | NF | 28.1 ± 4.2 mM (Pezzulo et al., 2012) | 13.1 ± 2.4 mM (Pezzulo et al., 2012) | NF | NF | 35 mM9 (Maas et al., 1977) | NF | 90 mM9 (Maas et al., 1977) | - | 8 mM (Allen and Garner, 1980) | 10–15 μEq.cm−1.h−1
15 (Flemström et al., 2003) 5.85 ± 0.82 μEq.cm−1.h−1 15 (Sjoblom and Flemstrom, 2003) 7.20 ± 1.35 μEq.cm−1.h−1 15 (Sjoblom and Flemstrom, 2003) |
87 mEq/L (Crane, 1965) | 26 mEq/L (Botelho, 1964) |
| Viscosity | 1.6 ± 1.5 Pa-s (Majima et al., 1999)3 1.8 ± 1.7 Pa-s (Viswanathan et al., 2006) |
10 Pa-s (Schuster et al., 2013) 0.04–0.49 Pa-s (Serisier et al., 2009) 150–28,000 poises (Luk and Dulfano, 1983) |
322 ± 199 Pa-s (Vasconcellos et al., 1994) 0.04–0.38 Pa-s (Serisier et al., 2009) 0.04–1.60 poises10 (Lopez-Vidriero and Reid, 1978) |
0.09–3.1 poises10 (Lopez-Vidriero and Reid, 1978) | 0.16–1.8 Pa-s (Serisier et al., 2009) 0.08–1.63 poises10 (Lopez-Vidriero and Reid, 1978) |
NF | NF | NF | 1,000–100,000 mPa-s8,12 (Lai et al., 2009b) 10–100 mPa-s 12,17 (Lai et al., 2009b) |
2.9–3.1 mPa-s (Larhed et al., 1997) | 1.1–25.816 mPa-s (Larhed et al., 1997) | NF | 4.4–8.3 mPa-s (Greaves and Wilson, 1993) 0.97–2.33 mPa.s (Gouveia and Tiffany, 2005) 2–9 mPa-s (Pandit et al., 1999) 6.6±1.6 mPa-s (Tiffany, 1991) |
|
human nasal pH measured in situ
human chronic rhinosinusitis mucus
complex viscosity at frequency of 1 Hz in patients with chronic sinusitis
poor asthma control
stable asthma
mucoid sputum samples
purulent sputum samples
frequency from 0.1–100 rad/s
oviductal fluid of rhesus monkey
range between three different macroscopic types of sputum: mucoid, mucopurulent and purulent
samples collected during active labor
menstrual cycle phase not indicated
samples collected at random times throughout the menstrual cycle, excluding the mid-cycle ovulatory interval
suggested size range cutoff for polystyrene microspheres translocation through gastrointestinal mucin
HCO3− secretion by rats duodenal mucosa
pig intestinal mucus with concentrations ranging from 1.5–8%, and shear rates ranging from 11.6–1162 mPa-s
microrheology of 100 – 1000 nm probe diameter
mouse colon mucus
human tears, except when indicated differently
bovine vitreous
3.1 Pore size
From diffusivity studies primarily in intestinal mucus, small molecules were shown to diffuse freely across the mucus barrier, while larger macromolecules like globular proteins did not penetrate mucus (Cone, 2009). The mucus mesh network encloses a heterogeneous mesh spacing, ranging from 20 to 1800 nm across different organs and diseases (Table 2), wherein particles and small molecules are transported across lower viscosity pores within an elastic matrix (Lai et al., 2009d). From this perspective, it is reasonable to postulate that mucus permeability might be limited by its pore size. Indeed, it has been reported in different studies a decrease in particle mobility in mucus with increasing particle size (Figure 6) (Dawson et al., 2003; Murgia et al., 2016; Saltzman et al., 1994). However, mucus permeability is affected not only by size, as other studies have demonstrated that larger, virus-like particles (~38–55 nm) had unhindered diffusion in human cervical mucus compared to smaller particles (Olmsted et al., 2001). Furthermore, diffusion of IgM (11–16 nm) was three-fold less in human cervical mucus than saline which suggests that immunoglobulins form low-affinity bonds with the mucins, thereby decreasing their diffusivity in mucus (Olmsted et al., 2001). In addition, studies have shown an increased diffusivity of more neutral polyethylene glycol (PEG)-coated particles in human cervical mucus and sputum from cystic fibrosis patients compared to uncoated particles within average pore size (Lai et al., 2007; Suk et al., 2009). Increased diffusivities have also been correlated ex vivo and in vivo with higher PEG-densities coatings in poly(lactic-co-glycolic acid) (PLGA) nanoparticles in human cervicovaginal mucus and mouse cervicovaginal tract (Xu et al., 2015), suggesting that other mechanisms of filtration in addition to size exclusion seem to govern mucus permeation, such as electrostatic or hydrophobic interactions mainly due to the negatively charged and hydrophobic regions in mucin fibers.
Figure 6.
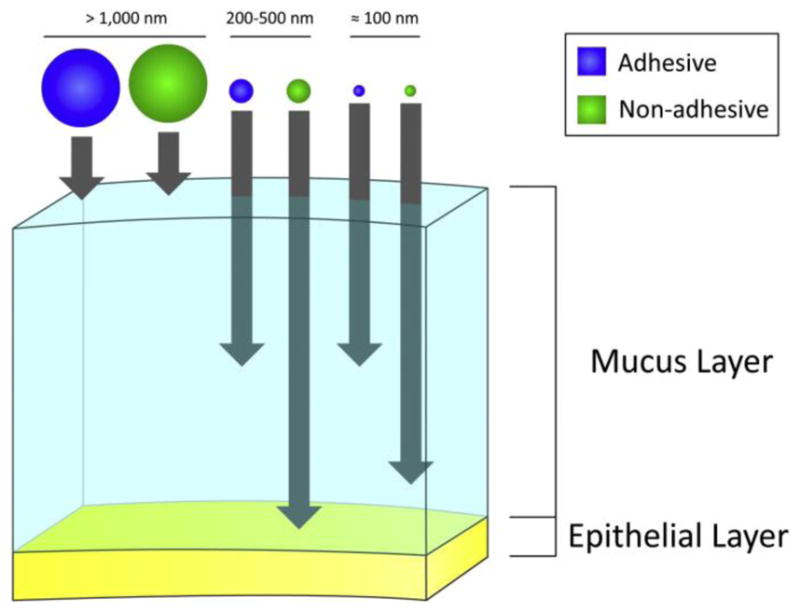
A schematic representation of the relative transport of particles across mucus, considering their size and surface properties (i.e. mucus adhesive or non-adhesive particles). Reprinted from (Neves et al., 2011), Copyright 2011, with permission from John Wiley & Sons, Inc.
3.2 Viscoelasticity
Mucins are the main component responsible for the viscoelastic properties of mucus (Bansil and Turner, 2006), although other mucus constituents such as DNA (Lethem et al., 1990; Mrsny et al., 1996; Potter et al., 1967), lipids (Galabert et al., 1987; Murty et al., 1984), salts (Crowther et al., 1984; Raynal et al., 2003) and proteins (Harbitz et al., 1984; Olmsted et al., 2001; Saltzman et al., 1994) also have been shown to contribute to mucus viscoelasticity. Furthermore, interactions including physically entangled, low-affinity non-covalent bonds (Carlstedt and Sheehan, 1984) and stronger covalent disulfide bonds (Bansil and Turner, 2006; Meyer and Silberberg, 1980) between mucin fibers and other mucus components affect viscoelasticity.
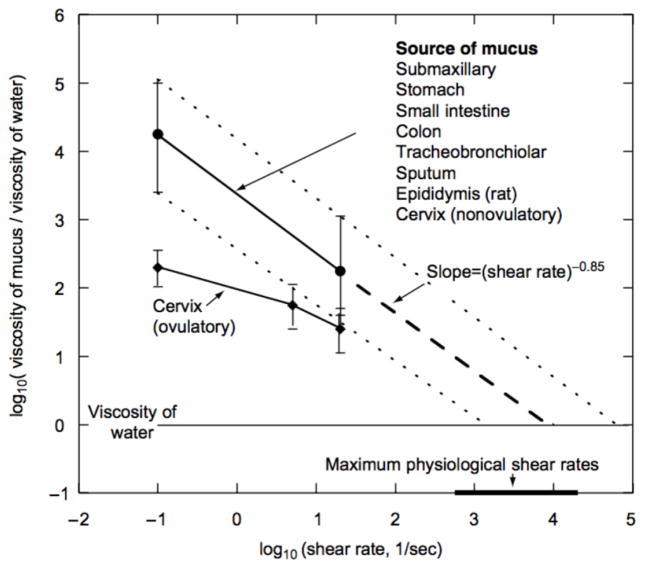
At the macroscale, mucus behaves as a complex non-Newtonian viscoelastic material, and it contains both viscous (flow) and elastic (resistance to deformation) components (Lai et al., 2009d). At low shear rates, the viscosity of mucus is 100–10,000 times higher compared to water, while at higher shear rates approaching the maximum physiological rates, mucus behaves as a shear thinning material with viscosity comparable to water (Figure 7) (Cone, 2005; Cone, 2009). This shear-thinning viscoelasticity contributes to a dual biological function; mucus acts as both a low viscosity fluid lubricating the mucosa epithelia in the eyes, endocervical epithelium and in the gastrointestinal tract and an elastic gel for mucociliary clearance in the lungs (Lai et al., 2009d) and for sperm permeation in the cervix (Svensson and Arnebrant, 2010). Rheology measurements indicate that elastic properties dominate in mucus. In a frequency sweep test, the elastic storage modulus showed to be higher than the loss modulus in cystic fibrosis sputum samples (Nielsen et al., 2004). Furthermore, following the removal of a constant shear stress, more than 75% of the strain is rapidly recovered in cystic fibrosis sputum and mucus secretions from different sources, fully recovering over minutes to hours (Cone, 2005; Nielsen et al., 2004). Rapid recovery is critical in mucociliary transport to prevent mucus removed by coughing from flowing backward to the alveoli.
Figure 7.
A summary of mucus viscosity measurements as a function of shear rate performed on a variety of mucus secretions from different species, using various methodologies (source references cited in reference (Cone, 2005)). Viscosity decreases as shear rate increases, characteristic of a shear thinning behavior. At maximum physiological rates, mucus approaches the viscosity of water. Recovery after a removal of the shear rate is not depicted in the figure. Reprinted from reference (Cone, 2005), with permission from Elsevier.
Macrorheological bulk measurements are useful for predicting the viscoelastic behavior of mucus on the macroscale, and these measurements are largely reported in the literature for the viscoelastic properties of mucus (Bell et al., 1985; Cone, 2005; Nielsen et al., 2004; Sanders et al., 2000; Taylor et al., 2003). Macrorheological bulk characteristics are averaged measurements of physical properties in mucus and are important to understand clearance and lubrication functions. However, macrorheological measurements cannot provide direct information about the local environment of the biopolymer mesh network in mucus, comprised of entangled mucin polymers with microscopic domains in water, a low viscosity fluid. At the microscale, entangled mucin polymers with microscopic domains filled with a low viscosity liquid create a three-dimensional crosslinked mucus network (Lai et al., 2009d).
Microrheology accounts for physical properties at a higher spatial resolution, providing a detailed characterization of the viscosity and elasticity of the local biopolymer network, along with the network mesh itself. Hence, microrheology of mucus determines the effective local viscoelastic properties at the microscale, which is relevant to the transport scale of pathogens and small drug molecules through the barrier (Lai et al., 2009d). Mucus microrheology has been studied with particle tracking microscopy (Lai et al., 2007; Lai et al., 2009c; Lieleg et al., 2010; Schuster et al., 2013; Suk et al., 2009; Tang et al., 2009a), a non-destructive, sensitive method to quantify the heterogeneity of the mucus pore network and to determine the effective viscoelastic properties of mucus at the micron scale.
Macro- and microrheology comparative studies of respiratory mucus from healthy humans demonstrated a mucus bulk viscosity more than 13,000 times compared to water. Using particle tracking with PEG-coated polystyrene particles, 100, 200 and 500 nm PEG-coated polystyrene nanoparticles presented viscosities 250-, 100-, and 4-fold lower compared to the former bulk viscosity of mucus, respectively (Schuster et al., 2013), indicating differences between macro and micro scale environments in mucus.
Other physicochemical properties of mucus may contribute to changes in the viscoelasticity. In gastric mucus, a decrease in pH (i.e. more acidic) can increase the mucus viscoelasticity, due to a reduction of the negative charges of the carboxyl groups on sialic acids in the mucin glycosylated regions (Lamont, 1992). Furthermore, the abnormal viscoelasticity of mucus has been proposed to contribute to the clinical manifestations of respiratory diseases like cystic fibrosis, asthma and bronchitis, as discussed in later in this review (Nielsen et al., 2004; Sanders et al., 2000).
3.3 pH
Mucus pH varies across different organs in the body. In the airways, the mucus layer is approximately neutral (Karnad et al., 1990; Pezzulo et al., 2012; Soyenkoff and Hinck, 1935; Washington et al., 2000). In addition, the pH varies throughout the gastrointestinal tract and between species (subsequent values reported are from a range of studies in mucus derived from humans and pigs species). In oral delivery, drugs will initially encounter the harsh acidic environment of the stomach. In the stomach, pH varies from 1 to 2 on the luminal surface to almost neutral pH (~6.0) at the epithelial surface (Schreiber and Scheid, 1997). The pH in the duodenum is 6.1 and steadily increases through the rest of the GI tract, reaching a pH of 7–8 in the colon and rectum (Dressman et al., 1990; Langguth et al., 1997). In the endocervical mucus, pH conditions significantly change during the menstrual cycle, ranging from acidic to basic conditions (pH 5.4 to 8.2) (Eggert-Kruse et al., 1993).
A determinant of the pH of mucus is the ionic concentration, specifically HCO3− and H+ ions. Airway primary cultures and cell lines actively secrete HCO3−, which is regulated by apical CF transmembrane conductance regulator (CFTR) Cl− channels, and mediated by mucosal histamine or ATP (Fischer et al., 2002; Lee et al., 1998; Smith and Welsh, 1992). Changes in pH can alter the conformation of mucus by promoting the exposure of hydrophobic domains of the mucins, changing electrostatic charges of their glycosylated regions, influencing non-covalent mucin-mucin interactions thereby increasing the viscoelasticity (Bhaskar et al., 1991; Lieleg et al., 2010). Additionally, H+ concentration determines the net charge of sulfated and sialylated glycosylated domains of mucins, and its hydration state (Tam and Verdugo, 1981). While the pH-mediated conformational changes in mucin and subsequent increase in viscoelasticity can serve a protective effect in the gastrointestinal tract, these same pH changes can be deleterious in certain diseases such as cystic fibrosis, whereby the acidic pH decreases repulsion between mucins and the enhanced viscosity decreases mucociliary clearance of pathogens (Coakley and Boucher, 2001).[LJ1] This underscores that the effects of pH on mucus and its function are tissue- and context-dependent. More basic pH in healthy airways results in decreased mucus viscosity, thereby facilitating transport of bacteria and viruses across the mucosal barrier. Similarly, Helicobacter pylori increases the pH of their surroundings to reduce the mucus viscoelasticity and increase their motility across the gastrointestinal mucus barrier (Celli et al., 2009).
Several studies have shown that pH can regulate mucus filtration via electrostatic interactions between the mucin polymers. For example, in an experiment with porcine gastric mucins (Lieleg et al., 2010), positively and negatively charged particles were less mobile and their transport was more hindered at acidic pH 3 compared with neutral PEGylated particles. In comparison, at neutral pH 7 both charged and neutral PEGylated particles diffused almost freely in mucus. An additional study showed that the human immunodeficiency virus type 1, which has a net-negative surface charge, was trapped in acidic human cervicovaginal mucus, but not at neutral pH (Lai et al., 2009a). Collectively, these studies suggest that non-covalent mucin-mucin interactions are regulated either by the mucins or charge interactions of the particles and are pH sensitive, depending on the protonation levels of the glycosylated domains and amino acids.[LJ2]
3.4 Ionic strength
The ionic strength of mucus is regulated by epithelial channels and other cellular ions transport mechanisms. Normal mucus formation relies on cAMP-dependent HCO3− secretion and exocytosis mediated by Ca2+ (Yang et al., 2013). Before being secreted, negatively charged mucin polymers are packed within granules at a low pH and high Ca2+ concentrations, neutralizing the negative charges in mucins (Chen et al., 2010). To be secreted, mucins undergo swelling and hydration processes, mediated by an expansion mechanism via electrostatic forces, essentially exchanging H+ and divalent Ca2+ in the matrices for monovalent cations (Na+ and K+) in the medium, unshielding mucin anions and removing inter- and intramolecular cross-links to facilitate mucin secretion. Additionally, HCO3− is also involved in mucin swelling and hydration mechanisms by reducing Ca2+ cross-linking in mucins, thereby decreasing its viscosity (Chen et al., 2010). CFTR channels directly mediate Cl− and HCO3− transport across the apical membrane of several epithelial cells from different tissues (Yang et al., 2013) and indirectly downregulates the activity of the epithelial sodium channel (ENaC) (Boucher, 2007a). At the macroscale, increase in ion concentration generally correlate with a decrease of mucus viscosity (Lai et al., 2009d), although mucus elasticity increases with greater ion valency (Crowther et al., 1984; Steiner et al., 1984).
Mucus hydration state is closely related to ionic strength, and its osmolarity is isotonic compared to plasma (Tarran et al., 2001a). A reduced secretion of electrolytes correlates with mucus dehydration and subsequent increased viscoelasticity, impacting diseases such as cystic fibrosis, where CFTR channel function is impaired leading to a severe imbalance in ion and water transport (Boucher, 2007a, b; Henderson et al., 2014). Without a functional CFTR, water is hyperabsorbed, driven by enhanced absorption of Na+ via the ENaC, resulting in mucus concentration, and the formation of a stationary mucus layer. Moreover, with a CFTR ion transport dysfunction, there is an acidification of the mucus layer (pH <6.5) due to defective HCO3– ion transport (Yoon et al., 2006). In the eye, electrolytes present in the tear film regulate the osmolarity of tears, acting as a buffer to maintain pH at a relatively constant level and maintaining epithelial integrity (Johnson and Murphy, 2004). Increased electrolyte concentration in the tear film and altered electrolyte composition have been associated with dry eye syndrome, predominantly due to decreased tear turnover leading to increased tear evaporation and consequently, ocular surface damage (Gilbard, 1994; Johnson and Murphy, 2004; Lemp, 1995).
Charged particles or mucin polymers undergo electrostatic interactions in solution, wherein attractive or repulsive forces between diffusing particles and the mucus depend on the ionic strength. This has been shown experimentally, where positively charged particles presented up to a 10-fold increase in diffusivity in porcine gastric mucin solutions with high concentrations of either NaCl or CaCl2, while neutral PEG particles diffusivity remained unaffected by changes in the salt concentration (Lieleg et al., 2010). Therefore, high salt content seems to increase the mobility of charged particles by a charge shielding effect, which weakens their interactions with mucins.
4. Mucus in disease states
The physical state of mucus, changes in concentration of mucus and secreted mucins, and the strong dependence of its physicochemical properties on environmental factors such as pH, ionic strength, and shear play an important role in many diseases. Mucus overproduction has been correlated with chronic airway diseases like cystic fibrosis (CF) (Boucher, 2004), chronic obstructive pulmonary disease (COPD), and asthma (Rose, 1992) and is related to an upregulation of MUC genes, as well as goblet cell hyperplasia (Rose and Voynow, 2006). Mucus underproduction is associated with dry eye syndromes (Argüeso and Gipson, 2001). Moreover, it is known that mucus expression and composition is also altered in different types of cancers (Hollingsworth and Swanson, 2004; Khanvilkar et al., 2001). Therefore, an understanding of the nature of mucus in relevant disease conditions is critical to design efficient drug delivery systems for mucosal applications.
4.1 Cystic fibrosis
Cystic fibrosis disease (CF) is an autosomal recessive disorder that affects multiple organs, characterized by defective chloride and bicarbonate ion transport due to defective cystic fibrosis transmembrane conductance regulator (CFTR) expression primarily in epithelial cells (Bobadilla et al., 2002; Tang et al., 2009b). Additionally, there is an increased activity of the ENaC in CF airways that has also been linked to CF pathogenesis (Boucher, 2007a; Mall and Galietta, 2015). Lack of CFTR function also leads to chronic respiratory infections, pancreatic dysfunction, intestinal obstruction disorders, liver dysfunction, and infertility. In CF, mucus has an increased viscoelasticity and a higher concentration of physical entanglements, which results in a decreased permeability to drugs. Impaired chloride ion channels in CF patients promotes a failure to secrete chloride ions, and there is increased water absorption and dehydration of the epithelia, resulting in thickened mucus. Furthermore, defective bicarbonate secretion impairs the release and expansion of mucins from goblet cells and submucosal glands (Chen et al., 2010; Gustafsson et al., 2012). Analysis of the mucin content of expectorated and induced sputum from CF, COPD, and asthmatic patients has shown an increase in mucin concentrations compared with normal induced sputum (Henke et al., 2007; Kirkham et al., 2002). MUC5AC and MUC5B are both present in the sputum of patients with CF, and studies have shown that the levels of MUC5B are increased compared with MUC5AC (Henke et al., 2007). Ultimately, CF patients develop intractable chronical infections due to a dehydrated, concentrated mucus and an impaired mucociliary clearance that traps pathogens organisms and hinders effective diffusion of drugs.
Alterations in CF mucins glycosylation patterns have been reported in different studies. In CF mucus from the duodenum, there was an increase in fucose and sulfate levels, while a decrease in sialic acid content in CF samples, compared to non-CF samples (Dische et al., 1959). Several O-glycan structures in mucins from CF and bronchitis patients have been determined, with an increased sulfation been reported (Cheng et al., 1989; Holmén et al., 2004; Klein et al., 1992; Lo-Guidice et al., 1994). Although the significance of altered glycosylation in the CF pathogenesis has not been elucidated, it is believed that they might contribute to changes in viscoelastic properties of mucus, since sialic acid and sulfates contribute to overall anionic charge of the mucins, while fucosylation can impact their hydrophobic properties (Rose and Voynow, 2006). In contrast, a recent study showed no differences in glycosylation between CF and non-CF native ASL mucus from piglets, suggesting that glycosylation changes in CF mucus might occur due to secondary factors, such as infections and inflammation. The same study demonstrated a 2-fold increase of sulfate levels in CF ASL samples (Tang et al., 2016).
The use of inhaled therapies, including the mechanisms of action of various drugs in CF has been reviewed elsewhere (Doring et al., 2012; Heijerman et al., 2009; Máiz et al., 2013). Since CF mucus impairs the activity of inhaled drugs (Heijerman et al., 2009), strategies to improve the efficacy of aerosolized antibiotics in CF have been exploited, including coadministration of bronchodilators or mucolytic agents such as recombinant human deoxyribonuclease (e.g. rhDNase) (Frederiksen et al., 2006; Fuchs et al., 1994), as well as physiotherapy. In addition, hypertonic saline may promote beneficial clinical effects in patients with cystic fibrosis by improving mucociliary clearance (Donaldson et al., 2006; Elkins et al., 2006; Robinson et al., 1997). Indeed, in vitro studies with CF human airway epithelia demonstrated that hypertonic saline increases the volume of airway surface liquid, suggesting that therapies can be potentially designed to restore normal ASL volume in CF and improve mucus transport, although effects were typically short acting (Tarran et al., 2001b). Since there is no curative treatment for correction for the genetic disorder, current treatments for CF mainly focus on symptomatic management, antibiotic treatment, and mucociliary improvement.
4.2 Chronic obstructive pulmonary disease
COPD is a complex of inflammatory diseases characterized by airflow obstruction due to chronic bronchitis or emphysema and is the fourth leading cause of patient deaths in adults in the United States (Rose and Voynow, 2006). Mucus production and secretion are exacerbated in COPD due to gland hypertrophy in the trachea and bronchi, and goblet cell metaplasia of the surface epithelium in the bronchi and bronchioles, leading to impaired mucociliary clearance in the airways and chronical infections (Rose and Voynow, 2006). Patients with COPD have shown a 3- to 6-fold increase in goblet cells in the airways and increased concentration of MUC5AC and MUC5B mucins in the lumen of small airways compared to healthy individuals (Caramori et al., 2004; Hogg et al., 2004; Lumsden et al., 1984), leading to a mechanical obstruction of the small airways, and significantly impacting the disease pathogenesis. While current studies suggest that mucus hypersecretion plays a critical role in airflow obstruction in COPD, further studies are still needed to elucidate mucin expression and secretion mechanisms and how they correlate with the clinical manifestations of the disease, in order to improve patient outcomes.
4.3 Asthma
Asthma is a common chronic respiratory disease characterized by mucus hypersecretion, inflammation and occlusion of small and medium-sized airways by mucus and cellular debris (Williams et al., 2006), mainly due to gland hypertrophy in the trachea and bronchi, and goblet cell metaplasia of the surface epithelium in the bronchi and bronchioles. Patients with asthma presented a 2-fold increased number of goblet cells in the airway surface epithelium (Fahy, 2002; Ordoñez et al., 2001). The major secreted mucins in the airways of individuals with asthma are MUC5AC and MUC5B, and their levels have been shown 2-fold and approximately 7-fold higher in secretions from asthmatic airways compared to nonasthmatic, respectively, albeit MUC5AC concentration is more prominent (Kirkham et al., 2002). Additionally, the low charge form of MUC5B is predominant in individuals with asthma, but not in non-asthmatic individuals (Kirkham et al., 2002), although it is still unclear the clinical significance of these findings. Thus, additional studies are needed to systematically evaluate the mucus of asthmatic patients and understand how mucus alterations may affect patients with asthma and contribute to progression of the disease.
4.4 Cancer
Mucins overproduction has also been correlated to cancer pathogenesis, in particular, adenocarcinomas (Hukill and Vidone, 1965). It has been suggested that tumors might utilize mucins as a mechanism for configuration of the microenvironment during invasion, metastasis, and growth in hostile conditions (Hollingsworth and Swanson, 2004). An increase of heterogeneity of mucin glycoproteins in serum and gastric adenocarcinomas has also been correlated with advanced staging and poor prognosis (Ho et al., 1995; Hollingsworth and Swanson, 2004). It was found a 60% coexpression of multiple mucins in advanced cancers (stages III and IV) compared to only 10% in early cancers (stages I and II), demonstrating an altered mucin gene expression pattern in gastric neoplastic specimens (Ho et al., 1995). The glycosylation density in mucins by different tumors is also highly variable, which might contribute significantly to the abnormal functions associated with mucins during the pathogenesis of cancer and other diseases (Hanisch and Muller, 2000). Moreover, mucin overexpression in pancreatic cancer cells has been correlated with decreased intracellular uptake of antineoplastic agents fluorouracil (5-FU), bortezomib, and gemcitabine thereby decreasing its therapeutic efficacy (Kalra and Campbell, 2007; Kalra and Campbell, 2009; Skrypek et al., 2013; Tréhoux et al., 2015; Wissniowski et al., 2012).
4.5 Dry eye syndrome
Dry eye syndrome (DES) is characterized by ocular surface disorders with diverse and multiple etiology. Abnormal tear film is a common manifestation of DES, along with tear hyperosmolarity, inflammation, and symptoms of ocular irritation (Lemp, 1995). Alteration in mucin distribution and post-translational modifications at the ocular surface have been correlated with dry eye syndrome (Danjo et al., 1998; Gipson and Inatomi, 1997). Patients with Sjögren syndrome had less MUC5AC mucin expression and secretion in the tear fluid compared to normal individuals, with negligible differences in MUC1 and MUC4 expression; this finding suggests that deficiency of MUC5AC mucin in tears constitutes one of the mechanisms responsible for tear film instability in this disease (Argüeso et al., 2002). Additional studies conducted on MUC1 deficient mice showed a correlation between lack of MUC1 expression and secretion and development of eye inflammation, suggesting a critical protective role of MUC1 on the ocular surface (Kardon et al., 1999). Indeed, patients with Sjögren syndrome presented decreased expression of MUC1 gene and an increase in inflammatory mediators in the tear film (Jones et al., 1998). Current treatment strategies focus on the stabilization of the tear film, decreasing evaporative tear loss by the introduction of lipids, protection of the corneal and conjunctival cells, enhanced lubrication and healing of the ocular surface (Lemp, 2008). However, treatment options have been limited largely to over-the-counter tear substitutes and the approved therapeutic drug cyclosporine A to modulate immune activity and to suppress inflammation in DES. Although additional therapies directed to specific disease mechanisms have been investigated, such as doxycycline (De Paiva et al., 2006) and topical corticosteroids (Hyon et al., 2007), their focus have been predominantly on anti-inflammatory properties.
5. Transmucosal drug delivery
Considerable advances in drug formulations and innovative routes of administration have been made to increase drug transport across mucosal barriers. Indeed, extensive knowledge has been gained in the past few decades about drug absorption mechanisms and delivery strategies to overcome these selective barriers.
Development of drugs and drug delivery systems able to successfully traverse the mucosa requires an in-depth knowledge of their physicochemical properties, such as molecular weight, size, hydrophobicity, and stability, as well as the biological barriers that limit their permeation. Frequently, there is an inverse correlation between drug molecular weight and diffusion coefficient in mucus (Bolister et al., 1991; Khanvilkar et al., 2001). A drug diffusion study in native pig intestinal mucus showed that size affected diffusion of both larger hydrophobic and hydrophilic drugs, demonstrating that molecular size limits the diffusion of larger molecules (Larhed et al., 1997). Drug binding to mucins due to intermolecular interactions also limits their diffusion in mucus. For instance, it has been demonstrated that positively-charged, low molecular weight drugs such as tobramycin, gentamicin, amikacin, and some β-lactam antibiotics bind to negatively charged mucin fibers in rat intestinal mucus and sputum from cystic fibrosis, chronic bronchitis or bronchiectasis patients (Bataillon et al., 1992; Bolister et al., 1991; Niibuchi et al., 1986). However, in another study drugs with large differences in charge did not exert significant differences in the diffusion coefficients in native pig intestinal mucus, suggesting that charge has a small effect on diffusion in mucus (Larhed et al., 1997). To determine the effect of mucin on drug transport, five drugs diverse in structure and physicochemical properties (isoniazid, pentamidine, rifampicin, p-aminosalicylic acid, and pyrazinamide) were tested in vitro with purified porcine gastric mucus and exhibited 2.3- to 11-fold decrease in permeability compared to buffer solution (Bhat et al., 1995). Lipophilic molecules had much more decreased diffusion than those seen with charged molecules, which suggests that lipophilicity has a greater influence on mucus diffusion compared to charge. Additional permeability studies with three of the aforementioned drugs in human bronchial secretions from cystic fibrosis patients demonstrated 28% to 75% decreased permeability coefficients (Bhat et al., 1996), which suggests that disease states may specifically affect drug delivery across mucus barriers.
Hydrophobic molecules typically exhibit low permeability across the mucus barrier due to interactions with the glycoproteins and lipids in the mucus, resulting in low bioavailability (Sigurdsson et al., 2013). A diffusion study of nine different drugs displaying large differences in charge and octanol/water distribution ratios (K) in native pig intestinal mucus revealed a negative correlation between the diffusion coefficient and log K (Larhed et al., 1997). Moreover, a diffusion study of a range of polar and nonpolar drugs with pig intestinal mucus demonstrated that more non polar drugs displayed higher affinity and slower diffusion through mucus, compared to the more hydrophilic drugs (Matthes et al., 1992).
Different models of mucus can impact drug transport and their diffusivity through the mucus barrier. For example, the permeability of testosterone in three different mucus systems was found to be much greater in reconstituted porcine gastric mucin than in both native porcine intestinal mucus and a synthetic mucus model containing mucin, lipids, albumin, immunoglobulin, and DNA. This difference in was mainly attributed to the presence of lipids in the mucus reducing the permeation of testosterone in the experiments (Larhed et al., 1998; Larhed et al., 1997). The diffusion of other drugs across mucus models has also been reviewed elsewhere (Cu and Saltzman, 2009; Khanvilkar et al., 2001).
Several strategies have been exploited to improve mucosal permeability of drugs, including nanoparticle-based formulations, permeation enhancers, nanoemulsions, mucoadhesive and mucopenetrating systems, polymers, enzymes, liposomes, and peptides. In order to reach the underlying epithelium and avoid rapid clearance and degradation, drug molecules must be able to rapidly traverse the mucus barrier. As a result, it is challenging to develop effective drug delivery systems to mucosal tissues. Nanoparticle-based systems offer the advantages of size and protection of drug from degradation to enhance delivery to target mucosal tissues (Alonso, 2004). Additionally, the transport of nanoparticles with different surface chemistries across the mucus barriers has been extensively studied, and also reviewed elsewhere (Bernkop-Schnürch, 2013; Dünnhaupt et al., 2015b; Ensign et al., 2012a; Ensign et al., 2014; Lai et al., 2009c; Netsomboon and Bernkop-Schnürch, 2016). For a nanoparticle to traverse mucus, it must possess desired surface characteristics to avoid adhesion and steric inhibition by the mucin fiber mesh. It has been demonstrated that poly(lactic-co-glycolic acid) (PLGA) and polystyrene particles functionalized with mucus inert polymers such as poly(ethylene glycol) (PEG) enhanced mucus transport in pig gastric mucus (Dawson et al., 2004; Griffiths et al., 2015), pig intestinal mucus (Abdulkarim et al., 2015; Groo et al., 2014), mouse vaginal mucus (Ensign et al., 2012b), human cervicovaginal mucus (Lai et al., 2007; Mert et al., 2012; Tang et al., 2009a; Xu et al., 2013a; Xu et al., 2015), human respiratory mucus (Schuster et al., 2013), cystic fibrosis (CF) sputum (Suk et al., 2011; Suk et al., 2009; Tang et al., 2009a), and bovine vitreous ex vivo (Xu et al., 2013b). Moreover, our group has previously demonstrated that carboxyl and amine-functionalized nanoparticles disrupt the mucus barrier and improve drug permeation up to 4.9-fold with 200 nm carboxyl-nanoparticles in porcine gastric mucus compared controls without particles (McGill and Smyth, 2010). Additional strategies to improve mucus permeation include nanoparticles functionalized with mucolytic enzymes such as papain and bromelain (de Sousa et al., 2015a; Köllner et al., 2015; Müller et al., 2013), which degrade mucus locally and improved transport from 2-fold to up to ~5-fold in intestinal porcine mucus when compared with non-functionalized particles. However, this approach may cause dose-dependent toxicity and local damage to the epithelial tissues, as well as increase incidence of infections due to lack of mucosa protection. Thus, the use of mucolytic agents as adjuvants to improve particle transport is likely limited to diseases where mucus represents a dense barrier, such as cystic fibrosis (CF) and chronic obstruction pulmonary disease (COPD). Regardless, collectively these findings demonstrate that nanoparticles can be engineered with surface chemistries to overcome the mucus barrier in different organs in the body.
Self-nanoemulsifying drug delivery systems (SNEDDS) have also been exploited as a strategy to improve mucus permeation. SNEDDS are based on an isotropic mixture of oil, surfactants and co-surfactants that are able to form a fine oil-in-water nanoemulsion, followed by administration into aqueous media (Wang et al., 2009). These systems usually produce droplet sizes between 20 and 200 nm (Balakumar et al., 2013), and their subsequent small size facilitates diffusion across mucus layers. Here is hypothesized that their hydrophobic surface allows lower interactions with the hydrophilic regions of mucin fibers (Dünnhaupt et al., 2015b). In a diffusion study with pig intestinal mucus, SNEDDS with mean particle sizes of 12.0 nm showed an 8.5-fold increased diffusion through the mucus layer compared to particle sizes of 455.5 nm (Friedl et al., 2013). Similarly, insulin loaded SNEDDS coupled with the anionic phospholipid dimyristoyl phosphatidylglycerol with an average droplet size of 30–45 nm demonstrated up to 40% diffusion across purified porcine intestinal mucus, protection from intestinal enzymes, and a sustained release profile (Karamanidou et al., 2015).
An interesting approach to achieve greater amount of drug permeation is to maximize residence time in the mucus barrier through the use of mucoadhesive drug delivery systems. The cationic polymer chitosan has been used for a variety of oral and nasal drug delivery applications (Bernkop-Schnürch, 2005; Davis and Illum, 2003; Prego et al., 2005). The nasal cavity has a large surface area and a highly vascularized mucosa, allowing drugs to be readily absorbed and pass directly into the systemic circulation or to the brain via a nose-to-brain pathway, thereby avoiding first-pass metabolism. However, nasal mucociliary transport has been found to clear drug formulations with a half-life of clearance of about 15 min, making it challenging for hydrophilic drugs to be efficiently absorbed and transported across the nasal membrane (Illum, 2002). Therefore, the use of mucoadhesive systems can be effective to improve the nasal absorption of hydrophilic drugs and increase residence time for drugs to penetrate. For example, the addition of chitosan to a nasal formulation of insulin resulted in a 5.6-fold increase in the peak plasma insulin levels in sheep nasal mucosa, and a 7-fold increase in the area under the curve (AUC) (Illum et al., 1994). Additionally, thiolated polymers are interesting strategies to achieve mucoadhesion and mucus penetrating delivery due to their pH dependency. Specifically in the gastrointestinal or cervical mucus, where a pH gradient is present, thiomer-based nanoparticles might be able to permeate the acidic luminal mucus layer without forming disulfide bonds. However, as functionalized particles approach the epithelium with a higher pH, the thiol groups are more reactive and form disulfide bonds with mucus glycoproteins rich in cysteine domains, as previously discussed, thereby increasing their mucoadhesion (Dünnhaupt et al., 2015b). The use of thiolated chitosan functionalized particles has also been exploited for enhanced particle permeation in pig intestinal mucus (de Sousa et al., 2015b), and the oral delivery of insulin in Caco-2/HT29-MTX co-cultures (Jin et al., 2012), human cervical mucus (Liu et al., 2016), and a combined in vitro system containing both porcine gastric mucin and a Caco-2 monolayer (Dünnhaupt et al., 2015a). In addition, thiolated chitosan nanoparticles enhanced leuprolide delivery, with up to 5.2-fold improved transport in porcine respiratory mucosa compared to leuprolide drug solution, as well as a 6.9-fold increased AUC and higher bioavailability compared to unmodified chitosan NPs intranasally delivered in rats (Shahnaz et al., 2012). Additionally in this study, these thiolated NPs did not alter human nasal ciliary beat frequency (CBF) in vitro, which suggests that these NPs are not toxic and did not adversely impact the function of mucociliary clearance. Thiol-conjugates were also incorporated into SNEDDS, leading to an average droplet size of 50 nm and neutral charges (Rohrer et al., 2016). In this study, those systems were used in vitro for permeation studies in pig intestinal mucus using multiple particle tracking (MPT) technique, demonstrating a 66-fold increase in diffusion coefficient when compared to unthiolated control.
Mucoadhesive, thiolated polymers have also been investigated in ocular delivery. For instance, thiolated poly(acrylic acid) ocular inserts were tested in vitro and in vivo in human cornea/tear film using fluorescein and diclofenac salts as model drugs. In vitro release studies demonstrated controlled release for the incorporated model drugs on the thiolated poly(acrylic acid) inserts for up to 8 h. Similarly, in vivo studies showed a fluorescein concentration on the eye surface for more than 8 h, whereas the fluorescein concentration rapidly decreased after application of aqueous eye drops or inserts based on unmodified poly(acrylic acid) (Hornof et al., 2003).
Although mucoadhesive systems represent a promising strategy to increase drugs bioavailability to mucosal tissues, there are potential challenges to their successful translation. Mucoadhesive systems bind to the mucus layer via interactions with mucin fibers and their residence time is dependent on physiological mucus turnover. Moreover, most canonical mucoadhesive systems are unable to traverse the mucus layer and enter the underlying epithelium, making them unsuitable for gene and systemic delivery. Therefore, to achieve efficient transmucosal delivery with desired bioavailability, it is necessary to design systems with both mucus adhesive and permeating properties, with improved residence time and transport across the mucosal surface.
Surface charge-switching (i.e. zwitterionic) carriers have been studied as promising delivery systems to traverse the mucus layer and reach the underlying epithelium. Particles are engineered to initially display an overall negative charge and cross the net-negatively charged mucus layer (Shogren et al., 1989). Once reaching the epithelia, particles undergo an overall change to positive charges, getting immobilized and facilitating cellular uptake (Dünnhaupt et al., 2015b). This strategy can avoid back-diffusion and increase transfection efficiencies of gene delivery systems (Schmitz et al., 2007). A recent study demonstrated the ability of polyethylene imine-6-phosphogluconic acid nanoparticles (PEI-6-PGA) to shift their zeta potential from 6.4 mV to +2.8 mV by enzymatic cleavage of their phosphate ester moiety by alkaline phosphatase secreted by Caco-2 cells monolayer (Bonengel et al., 2015). Similarly, carboxymethylcellulose and chitosan nanoparticles surface-functionalized with phosphotyrosine demonstrated a change in their zeta potential (−5 mV to up to +8 mV) after incubation with intestinal alkaline phosphatase (Perera et al., 2015). In another study, PEI-6-PGA nanoparticles (NPs) were evaluated for their ability to delivery plasmid DNA encoding green fluorescent protein (GFP) in cell lines. GFP expression was observed in both Caco-2 and HEK-293 cells and their transfection efficiencies were decreased in the presence of a phosphatase inhibitor, which suggests the role of charge-switching in improving cell binding (Bonengel et al., 2016). However, mucus permeation of the tested nanocomplexes remains to be elucidated. Recently, a combined strategy of zeta potential changing and self-emulsifying drug delivery systems was tested in vitro for enhanced mucus permeation in porcine intestinal mucus. A small zeta potential change within the range of 1–3 mV was observed after phosphate release studies for the formulations tested. Negatively charged SEDDS diffused more across the porcine intestinal mucus compared to positively charged ones in the first two segments of the mucus layer (Suchaoin et al., 2016).
Various strategies have been exploited for enhanced drug delivery across transmucosal barriers. Advances in knowledge of mucus interactions mechanisms are critical to overcome this barrier and efficiently deliver therapeutics through mucosal membranes.
6. Conclusions
The complex structure and functionality of mucus hinders the transport of particles and molecules across mucosae and remain as a critical bottleneck in the therapeutic delivery of drugs across the mucosal epithelia. Alterations in physiological conditions imposed by different disease states can affect the efficiency of drug delivery systems to mucosal barriers. Mucus pore size, thickness, composition, and viscoelasticity may vary depending on the pathological condition, as well as intersubjects, suggesting a strong variability of mucus molecular structures to different environments. Moreover, the strong dependence of mucus physicochemical properties on environmental factors such as ionic strength and pH might impose additional complications to drug delivery systems.
Currently, there is a tremendous effort in designing effective drug delivery systems to overcome the mucus barrier and deliver therapeutics through the mucosal membrane. The fundamental principles governing mucus permeation are still an object of research, considering its relevance to physiological conditions and diseases. The mucus barrier represents a rate limiting step to drug transport. A better understanding of the nature of mucus and mechanisms associated with different disease states is crucial to design efficient drug delivery systems for mucosal applications.
Promising strategies in transmucosal drug delivery rely on the development of technologies to enhance mucus permeation as well as targeting efficacy. Extension of the current knowledge may provide new avenues for drug development of mucus-penetrating drug delivery systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulkarim M, Agulló N, Cattoz B, Griffiths P, Bernkop-Schnürch A, Borros SG, Gumbleton M. Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:230–238. doi: 10.1016/j.ejpb.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Adler K, Wooten O, Philippoff W, Lerner E, Dulfano MJ. Physical Properties of Sputum. American Review of Respiratory Disease. 1972;106:86–96. doi: 10.1164/arrd.1972.106.1.86. [DOI] [PubMed] [Google Scholar]
- Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiological reviews. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- Allen A, Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980;21:249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MJ. Nanomedicines for overcoming biological barriers. Biomedicine & Pharmacotherapy. 2004;58:168–172. doi: 10.1016/j.biopha.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden DB, Lazarowski ER, Davis CW, Bailey S, Fuller F, Almond M, Qaqish B, Bordonali E, Rubinstein M, Bennett WD, Kesimer M, Boucher RC. The Relationship of Mucus Concentration (Hydration) to Mucus Osmotic Pressure and Transport in Chronic Bronchitis. Am J Respir Crit Care Med. 2015;192:182–190. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Investigative ophthalmology & visual science. 2002;43:1004–1011. [PubMed] [Google Scholar]
- Argüeso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Experimental eye research. 2001;73:281–289. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- Argüeso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investigative ophthalmology & visual science. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. American journal of physiology. Gastrointestinal and liver physiology. 2001;280:G922–929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- Avetisov S, Safonova T, Novikov I, Pateiuk L, Griboedova I. Ocular surface acidity and buffering system (by studying the conjunctival sac) Vestnik oftalmologii. 2014;130:5–10. [PubMed] [Google Scholar]
- Avisar R, Savir H, Sidi Y, Pinkhas J. Tear calcium and magnesium levels of normal subjects and patients with hypocalcemia or hypercalcemia. Investigative ophthalmology & visual science. 1977;16:1150–1151. [PubMed] [Google Scholar]
- Bae CH, Jeon BS, Choi YS, Song SY, Kim YD. Delphinidin inhibits LPS-induced MUC8 and MUC5B expression through toll-like receptor 4-mediated ERK1/2 and p38 MAPK in human airway epithelial cells. Clinical and experimental otorhinolaryngology. 2014;7:198. doi: 10.3342/ceo.2014.7.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar K, Raghavan CV, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids and Surfaces B: Biointerfaces. 2013;112:337–343. doi: 10.1016/j.colsurfb.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Bansil R, Turner BS. Mucin structure, aggregation, physiological functions and biomedical applications. Current Opinion in Colloid & Interface Science. 2006;11:164–170. [Google Scholar]
- Bartman AE. The MUC6 secretory mucin genets expressed in a wide variety of epithelial tissues. J Pathol. 1998;186:398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bataillon V, Lhermitte M, Lafitte JJ, Pommery J, Roussel P. The binding of amikacin to macromolecules from the sputum of patients suffering from respiratory diseases. Journal of Antimicrobial Chemotherapy. 1992;29:499–508. doi: 10.1093/jac/29.5.499. [DOI] [PubMed] [Google Scholar]
- Bell AE, Sellers LA, Allen A, Cunliffe WJ, Morris ER, Ross-Murphy SB. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985;88:269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A. Thiomers: a new generation of mucoadhesive polymers. Advanced drug delivery reviews. 2005;57:1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A. Reprint of: Nanocarrier systems for oral drug delivery: Do we really need them? European Journal of Pharmaceutical Sciences. 2013;50:2–7. doi: 10.1016/j.ejps.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Bernkop-Schnürch A, Hornof M. Intravaginal drug delivery systems. American Journal of Drug Delivery. 2003;1:241–254. [Google Scholar]
- Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS current topics in otorhinolaryngology, head and neck surgery. 2010:9. doi: 10.3205/cto000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar KR, Gong DH, Bansil R, Pajevic S, Hamilton JA, Turner BS, LaMont JT. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. The American journal of physiology. 1991;261:G827–832. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- Bhat PG, Flanagan DR, Donovan MD. The limiting role of mucus in drug absorption: Drug permeation through mucus solution. International journal of pharmaceutics. 1995;126:179–187. [Google Scholar]
- Bhat PG, Flanagan DR, Donovan MD. Drug diffusion through cystic fibrotic mucus: Steady-state permeation, rheologic properties, and glycoprotein morphology. Journal of pharmaceutical sciences. 1996;85:624–630. doi: 10.1021/js950381s. [DOI] [PubMed] [Google Scholar]
- Biesbrock AR, Bobek LA, Levine MJ. MUC7 gene expression and genetic polymorphism. Glycoconjugate journal. 1997;14:415–422. doi: 10.1023/a:1018587031814. [DOI] [PubMed] [Google Scholar]
- Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J Biol Chem. 1993;268:20563–20569. [PubMed] [Google Scholar]
- Boegh M, Foged C, Müllertz A, Nielsen HM. Mucosal drug delivery: barriers, in vitro models and formulation strategies. Journal of Drug Delivery Science and Technology. 2013;23:383–391. [Google Scholar]
- Boegh M, Garcia-Diaz M, Mullertz A, Nielsen HM. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur J Pharm Biopharm. 2015;95:136–143. doi: 10.1016/j.ejpb.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Bolister N, Basker M, Hodges N, Marriott C. The diffusion of β-lactam antibiotics through mixed gels of cystic fibrosis-derived mucin and Pseudomonas aeruginosa alginate. Journal of antimicrobial chemotherapy. 1991;27:285–293. doi: 10.1093/jac/27.3.285. [DOI] [PubMed] [Google Scholar]
- Bonengel S, Prüfert F, Jelkmann M, Bernkop-Schnürch A. Zeta potential changing phosphorylated nanocomplexes for pDNA delivery. International Journal of Pharmaceutics. 2016;504:117–124. doi: 10.1016/j.ijpharm.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Bonengel S, Prüfert F, Perera G, Schauer J, Bernkop-Schnürch A. Polyethylene imine-6-phosphogluconic acid nanoparticles a novel zeta potential changing system. International Journal of Pharmaceutics. 2015;483:19–25. doi: 10.1016/j.ijpharm.2015.01.041. [DOI] [PubMed] [Google Scholar]
- Botelho SY. Tears and the lacrimal gland. Scientific American. 1964;211:78–87. doi: 10.1038/scientificamerican1064-78. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007a;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007b;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- Brieland JK, Jackson C, Menzel F, Loebenberg D, Cacciapuoti A, Halpern J, Hurst S, Muchamuel T, Debets R, Kastelein R, Churakova T, Abrams J, Hare R, O’Garra A. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect Immun. 2001;69:1554–1560. doi: 10.1128/IAI.69.3.1554-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Anderson WH, Boucher RC. Mucus Hyperconcentration as a Unifying Aspect of the Chronic Bronchitic Phenotype. Annals of the American Thoracic Society. 2016;13:S156–S162. doi: 10.1513/AnnalsATS.201507-455KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP Release Maintains Proper Mucus Hydration of Airways. Science signaling. 2013;6:ra46–ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button BM, Button B. Structure and function of the mucus clearance system of the lung. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Bansil R, Bhaskar KR, Turner BS, LaMont JT, Niu N, Afdhal NH. pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys J. 1999;76:1250–1258. doi: 10.1016/S0006-3495(99)77288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, Papi A. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004;45:477–484. doi: 10.1111/j.1365-2559.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- Carlstedt I, Sheehan JK. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Foundation symposium. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Carlstedt I, Sheehan JK. Structure and macromolecular properties of cervical mucus glycoproteins. Symposia of the Society for Experimental Biology. 1989;43:289–316. [PubMed] [Google Scholar]
- Celli J, Gregor B, Turner B, Afdhal NH, Bansil R, Erramilli S. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules. 2005;6:1329–1333. doi: 10.1021/bm0493990. [DOI] [PubMed] [Google Scholar]
- Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S, Bansil R. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proceedings of the National Academy of Sciences. 2009;106:14321–14326. doi: 10.1073/pnas.0903438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, Ann DK, Wu R. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Boat TF, Cranfill K, Yankaskas JR, Boucher RC. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Invest. 1989;84:68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley RD, Boucher RC. Regulation and functional significance of airway surface liquid pH. JOP : Journal of the pancreas. 2001;2:294–300. [PubMed] [Google Scholar]
- Cone RA. Chapter 4 - Mucus A2 - Mestecky, Jiri. In: Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W, editors. Mucosal Immunology. 3. Academic Press; Burlington: 2005. pp. 49–72. [Google Scholar]
- Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Cooper JL, Quinton PM, Ballard ST. Mucociliary transport in porcine trachea: differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol. 2013;304:L184–190. doi: 10.1152/ajplung.00143.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales R, Calonge M, Herreras J, Saez V, Mayo A, Chaves F. Levels of mucin gene expression in normal human conjunctival epithelium in vivo. Current eye research. 2003a;27:323–328. doi: 10.1076/ceyr.27.5.323.17221. [DOI] [PubMed] [Google Scholar]
- Corrales R, Galarreta D, Herreras J, Calonge M, Chaves F. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Archivos de la Sociedad Espanola de Oftalmologia. 2003b;78:375–381. [PubMed] [Google Scholar]
- Crane CW. Observations on the sodium and potassium content of mucus from the large intestine. Gut. 1965;6:439–443. doi: 10.1136/gut.6.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RS, Marriott C, James SL. Cation induced changes in the rheological properties of purified mucus glycoprotein gels. Biorheology. 1984;21:253–263. doi: 10.3233/bir-1984-211-227. [DOI] [PubMed] [Google Scholar]
- Cu Y, Saltzman WM. Mathematical modeling of molecular diffusion through mucus. Advanced drug delivery reviews. 2009;61:101–114. doi: 10.1016/j.addr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlin M, Bursac D. Cervical mucus: from biochemical structure to clinical implications. Frontiers in bioscience (Scholar edition) 2013;5:507–515. doi: 10.2741/s386. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK. Alteration of mucin in human conjunctival epithelia in dry eye. Investigative ophthalmology & visual science. 1998;39:2602–2609. [PubMed] [Google Scholar]
- Davies JR, Svitacheva N, Lannefors L, Kornfält R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochemical Journal. 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- Davis SS, Illum L. Absorption enhancers for nasal drug delivery. Clinical pharmacokinetics. 2003;42:1107–1128. doi: 10.2165/00003088-200342130-00003. [DOI] [PubMed] [Google Scholar]
- Dawson M, Krauland E, Wirtz D, Hanes J. Transport of polymeric nanoparticle gene carriers in gastric mucus. Biotechnology progress. 2004;20:851–857. doi: 10.1021/bp0342553. [DOI] [PubMed] [Google Scholar]
- Dawson M, Wirtz D, Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J Biol Chem. 2003;278:50393–50401. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Experimental eye research. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- de Sousa IP, Cattoz B, Wilcox MD, Griffiths PC, Dalgliesh R, Rogers S, Bernkop-Schnürch A. Nanoparticles decorated with proteolytic enzymes, a promising strategy to overcome the mucus barrier. European Journal of Pharmaceutics and Biopharmaceutics. 2015a;97:257–264. doi: 10.1016/j.ejpb.2015.01.008. [DOI] [PubMed] [Google Scholar]
- de Sousa IP, Steiner C, Schmutzler M, Wilcox MD, Veldhuis GJ, Pearson JP, Huck CW, Salvenmoser W, Bernkop-Schnürch A. Mucus permeating carriers: formulation and characterization of highly densely charged nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2015b;97:273–279. doi: 10.1016/j.ejpb.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Debailleul V. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. J Biol Chem. 1998;273:881–890. doi: 10.1074/jbc.273.2.881. [DOI] [PubMed] [Google Scholar]
- Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ, Stappenbeck TS, Brody SL. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy. 2016;12:397–409. doi: 10.1080/15548627.2015.1056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische Z, Di Sant’Agnese P, Pallavicini C, Youlos J. Composition of mucoprotein fractions from duodenal fluid of patients with cystic fibrosis of the pancreas and from controls. Pediatrics. 1959;24:74–91. [PubMed] [Google Scholar]
- Dohrman A, Tsuda T, Escudier E, Cardone M, Jany B, Gum J, Kim Y, Basbaum C. Distribution of Lysozyme and Mucin (MUC2 and MUC3) mRNA in Human Bronchus. Experimental Lung Research. 1994;20:367–380. doi: 10.3109/01902149409064393. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl j med. 2006;2006:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- Doring G, Flume P, Heijerman H, Elborn JS Consensus Study G. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, Jarvenpaa KM. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7:756–761. doi: 10.1023/a:1015827908309. [DOI] [PubMed] [Google Scholar]
- Dünnhaupt S, Barthelmes J, Köllner S, Sakloetsakun D, Shahnaz G, Düregger A, Bernkop-Schnürch A. Thiolated nanocarriers for oral delivery of hydrophilic macromolecular drugs. Carbohydrate polymers. 2015a;117:577–584. doi: 10.1016/j.carbpol.2014.09.078. [DOI] [PubMed] [Google Scholar]
- Dünnhaupt S, Kammona O, Waldner C, Kiparissides C, Bernkop-Schnürch A. Nano-carrier systems: strategies to overcome the mucus gel barrier. European Journal of Pharmaceutics and Biopharmaceutics. 2015b;96:447–453. doi: 10.1016/j.ejpb.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Eggert-Kruse W, Kohler A, Rohr G, Runnebaum B. The pH as an important determinant of sperm-mucus interaction. Fertility and sterility. 1993;59:617–628. [PubMed] [Google Scholar]
- Eichner H, Behbehani AA, Hochstrasser K. Diagnostic value of nasal secretions, current state: normal values. 1. Laryngol Rhinol Otol (Stuttg) 1983;62:561–565. [PubMed] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. New England Journal of Medicine. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Ellingham RB, Berry M, Stevenson D, Corfield AP. Secreted human conjunctival mucus contains MUC5AC glycoforms. Glycobiology. 1999;9:1181–1189. doi: 10.1093/glycob/9.11.1181. [DOI] [PubMed] [Google Scholar]
- Ellingham RB, Myerscough N, Gout II, Berry M, Corfield AP. Soluble mucins in human aqueous tears. Biochemical Society Transactions. 1997;25:12S–12S. doi: 10.1042/bst025012s. [DOI] [PubMed] [Google Scholar]
- Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012a;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign LM, Cone R, Hanes J. Nanoparticle-based drug delivery to the vagina: a review. Journal of Controlled Release. 2014;190:500–514. doi: 10.1016/j.jconrel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign LM, Henning A, Schneider CS, Maisel K, Wang YY, Porosoff MD, Cone R, Hanes J. Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Mol Pharm. 2013;10:2176–2182. doi: 10.1021/mp400087y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign LM, Tang BC, Wang YY, Terence AT, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Science translational medicine. 2012b;4:138ra179–138ra179. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande F. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur J Biochem. 2002;269:3637–3644. doi: 10.1046/j.1432-1033.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- Esther CR, Hill DB, Button B, Shi S, Jania CM, Duncan EA, Doerschuk CM, Chen G, Ranganathan S, Stick SM, Boucher RC. The sialic acid to urea ratio as a measure of airway surface hydration. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2017 doi: 10.1152/ajplung.00398.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV. Goblet Cell and Mucin Gene Abnormalities in Asthma*. Chest. 2002;122:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. New England Journal of Medicine. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV, Steiger DJ, UUJ, Basbaum CB, Finkbeiner WE, Boushey HA. Marken of Mucus Secretion and DNA Levels in Induced Sputum from Asthmatic and from Healthy Subjects. The American review of respiratory disease. 1993;147:1132–1137. doi: 10.1164/ajrccm/147.5.1132. [DOI] [PubMed] [Google Scholar]
- Fischer H, Widdicombe JH, Illek B. Acid secretion and proton conductance in human airway epithelium. American journal of physiology Cell physiology. 2002;282:C736–743. doi: 10.1152/ajpcell.00369.2001. [DOI] [PubMed] [Google Scholar]
- Flemström G, Sjöblom M, Jedstedt G, Åkerman KEO. Short fasting dramatically decreases rat duodenal secretory responsiveness to orexin A but not to VIP or melatonin. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2003;285:G1091–G1096. doi: 10.1152/ajpgi.00193.2003. [DOI] [PubMed] [Google Scholar]
- Frederiksen B, Pressler T, Hansen A, Koch C, Høiby N. Effect of aerosolized rhDNase (Pulmozyme®) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica. 2006;95:1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- Friedl H, Dünnhaupt S, Hintzen F, Waldner C, Parikh S, Pearson JP, Wilcox MD, Bernkop-Schnürch A. Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems. Journal of pharmaceutical sciences. 2013;102:4406–4413. doi: 10.1002/jps.23757. [DOI] [PubMed] [Google Scholar]
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- Galabert C, Jacquot J, Zahm JM, Puchelle E. Relationships between the lipid content and the rheological properties of airway secretions in cystic fibrosis. Clinica chimica acta; international journal of clinical chemistry. 1987;164:139–149. doi: 10.1016/0009-8981(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Pudney PD, Thornton DJ, Waigh TA. Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers. 2014;101:366–377. doi: 10.1002/bip.22372. [DOI] [PubMed] [Google Scholar]
- Ghani M, Soothill JS. Ceftazidime, gentamicin, and rifampicin, in combination, kill biofilms of mucoid Pseudomonas aeruginosa. Canadian journal of microbiology. 1997;43:999–1004. doi: 10.1139/m97-144. [DOI] [PubMed] [Google Scholar]
- Gilbard JP. Human tear film electrolyte concentrations in health and dry-eye disease. International ophthalmology clinics. 1994;34:27–36. doi: 10.1097/00004397-199403410-00005. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Inatomi T. Mucin genes expressed by the ocular surface epithelium. Progress in Retinal and eye research. 1997;16:81–98. [Google Scholar]
- Gipson IK, Inatomi T. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2. Springer; 1998. Cellular origin of mucins of the ocular surface tear film; pp. 221–227. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Moccia R, Zhan Q, Toribara N, Ho SB, Gargiulo AR, Hill JA., 3rd MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biology of reproduction. 1999;60:58–64. doi: 10.1095/biolreprod60.1.58. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Experimental Eye Research. 2016;145:230–234. doi: 10.1016/j.exer.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godl K. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- Gouveia SM, Tiffany JM. Human tear viscosity: An interactive role for proteins and lipids. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2005;1753:155–163. doi: 10.1016/j.bbapap.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Grant R. Calcium in gastric mucus and regulation of gastric acidity. American Journal of Physiology--Legacy Content. 1941;135:496–503. [Google Scholar]
- Greaves JL, Wilson CG. Treatment of diseases of the eye with mucoadhesive delivery systems. Advanced Drug Delivery Reviews. 1993;11:349–383. [Google Scholar]
- Griffiths PC, Cattoz B, Ibrahim MS, Anuonye JC. Probing the interaction of nanoparticles with mucin for drug delivery applications using dynamic light scattering. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:218–222. doi: 10.1016/j.ejpb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respiratory Medicine. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Peiser C, Dinh QT, Matthias J, Eynott PR, Heppt W, Carlstedt I, Witt C, Fischer A, Chung KF. Distribution of Respiratory Mucin Proteins in Human Nasal Mucosa. The Laryngoscope. 2003;113:520–524. doi: 10.1097/00005537-200303000-00023. [DOI] [PubMed] [Google Scholar]
- Groo AC, Mircheva K, Bejaud J, Ailhas C, Panaiotov I, Saulnier P, Ivanova T, Lagarce F. Development of 2D and 3D mucus models and their interactions with mucus-penetrating paclitaxel-loaded lipid nanocapsules. Pharmaceutical research. 2014;31:1753–1765. doi: 10.1007/s11095-013-1280-4. [DOI] [PubMed] [Google Scholar]
- Gum JR, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EV, Rogers DF. Novel therapies to inhibit mucus synthesis and secretion in airway hypersecretory diseases. Pharmacology. 2016;97:84–100. doi: 10.1159/000442794. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt K. Intrauterine contraception with the copper-T device. Contraception. 1972;6:37–54. doi: 10.1016/s0010-7824(72)80004-0. [DOI] [PubMed] [Google Scholar]
- Hanisch FG, Muller S. MUC1: the polymorphic appearance of a human mucin. Glycobiology. 2000;10:439–449. doi: 10.1093/glycob/10.5.439. [DOI] [PubMed] [Google Scholar]
- Harbitz O, Jenssen AO, Smidsrod O. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. European journal of respiratory diseases. 1984;65:512–520. [PubMed] [Google Scholar]
- Heijerman H, Westerman E, Conway S, Touw D. Inhaled medication and inhalation devices for lung disease in patients with cystic fibrosis: A European consensus. Journal of Cystic Fibrosis. 2009;8:295–315. doi: 10.1016/j.jcf.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. American Journal of Obstetrics and Gynecology. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, Sheehan JK, Boucher RC, Kesimer M. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175:816–821. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun HW, Falk RJ, Jennette JC, Alcorta DA, Li H, Yamamoto T, Saito Y, Nakamura M. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- Ho SB. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer research. 1995;55:2681–2690. [PubMed] [Google Scholar]
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Holmén JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, Davis CW. Mucins and their O-Glycans from human bronchial epithelial cell cultures. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- Hornof M, Weyenberg W, Ludwig A, Bernkop-Schnürch A. Mucoadhesive ocular insert based on thiolated poly (acrylic acid): development and in vivo evaluation in humans. Journal of Controlled Release. 2003;89:419–428. doi: 10.1016/s0168-3659(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. The Biochemical journal. 1996a;318( Pt 1):319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovenberg HW, Davies JR, Herrmann A, Linden CJ, Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconjugate journal. 1996b;13:839–847. doi: 10.1007/BF00702348. [DOI] [PubMed] [Google Scholar]
- Hukill PB, Vidone RA. Histochemistry of mucus and other polysaccharides in tumors. I. Carcinoma of the bladder. Laboratory investigation; a journal of technical methods and pathology. 1965;14:1624–1635. [PubMed] [Google Scholar]
- Hyon JY, Lee YJ, Yun PY. Management of ocular surface inflammation in Sjögren syndrome. Cornea. 2007;26:S13–S15. doi: 10.1097/ICO.0b013e31812f6782. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery: new developments and strategies. Drug discovery today. 2002;7:1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- Illum L, Farraj NF, Davis SS. Chitosan as a novel nasal delivery system for peptide drugs. Pharmaceutical research. 1994;11:1186–1189. doi: 10.1023/a:1018901302450. [DOI] [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Investigative Ophthalmology and Visual Science. 1995;36:1818–1827. [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Investigative ophthalmology & visual science. 1996;37:1684–1692. [PubMed] [Google Scholar]
- Jany BH, Gallup MW, Yan PS, Gum JR, Kim YS, Basbaum CB. Human bronchus and intestine express the same mucin gene. Journal of Clinical Investigation. 1991;87:77–82. doi: 10.1172/JCI115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Song Y, Zhu X, Zhou D, Chen C, Zhang Z, Huang Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- Johansson ME. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS One. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ. Changes in the tear film and ocular surface from dry eye syndrome. Progress in Retinal and Eye Research. 2004;23:449–474. doi: 10.1016/j.preteyeres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Jones DT, Monroy D, Ji Z, Pflugfelder SC. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2. Springer; 1998. Alterations of ocular surface gene expression in Sjögren’s syndrome; pp. 533–536. [DOI] [PubMed] [Google Scholar]
- Jordan N, Newton J, Pearson J, Allen A. A novel method for the visualization of the in situ mucus layer in rat and man. Clinical science (London, England : 1979) 1998;95:97–106. [PubMed] [Google Scholar]
- Joris L, Dab I, Quinton PM. Elemental Composition of Human Airway Surface Fluid in Healthy and Diseased Airways. American Review of Respiratory Disease. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Jumblatt JE. MUC5AC mucin is a component of the human precorneal tear film. Investigative ophthalmology & visual science. 1999;40:43–49. [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–45. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Kalra A, Campbell R. Mucin impedes cytotoxic effect of 5-FU against growth of human pancreatic cancer cells: overcoming cellular barriers for therapeutic gain. British journal of cancer. 2007;97:910. doi: 10.1038/sj.bjc.6603972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. European Journal of Cancer. 2009;45:164–173. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Karamanidou T, Karidi K, Bourganis V, Kontonikola K, Kammona O, Kiparissides C. Effective incorporation of insulin in mucus permeating self-nanoemulsifying drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:223–229. doi: 10.1016/j.ejpb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Kardon R, Price RE, Julian J, Lagow E, Tseng S, Gendler SJ, Carson DD. Bacterial conjunctivitis in Muc1 null mice. Investigative ophthalmology & visual science. 1999;40:1328–1335. [PubMed] [Google Scholar]
- Karnad DR, Mhaisekar DG, Moralwar KV. Respiratory mucus pH in tracheostomized intensive care unit patients: Effects of colonization and pneumonia. Critical care medicine. 1990;18:699–701. doi: 10.1097/00003246-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- Khanvilkar K, Donovan MD, Flanagan DR. Drug transfer through mucus. Advanced Drug Delivery Reviews. 2001;48:173–193. doi: 10.1016/s0169-409x(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Kilbourn JP. Bacterial content and ionic composition of sputum in cystic fibrosis. Lancet. 1978;1:334. doi: 10.1016/s0140-6736(78)90111-3. [DOI] [PubMed] [Google Scholar]
- Kirch J, Schneider A, Abou B, Hopf A, Schaefer UF, Schneider M, Schall C, Wagner C, Lehr CM. Optical tweezers reveal relationship between microstructure and nanoparticle penetration of pulmonary mucus. Proceedings of the National Academy of Sciences. 2012;109:18355–18360. doi: 10.1073/pnas.1214066109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. The Biochemical journal. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Carnoy C, Lo-Guidice JM, Lamblin G, Roussel P. Separation of mucin oligosaccharide-alditols by high performance liquid chromatography on alkylamine-bonded silica columns. Effects of structural parameters. Carbohydrate research. 1992;236:9–16. doi: 10.1016/0008-6215(92)85003-i. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodric M, Shah AN, Fabbri LM, Confalonieri M. An Investigation of Airway Acidification in Asthma Using Induced Sputum. American Journal of Respiratory and Critical Care Medicine. 2007;175:905–910. doi: 10.1164/rccm.200607-940OC. [DOI] [PubMed] [Google Scholar]
- Köllner S, Dünnhaupt S, Waldner C, Hauptstein S, de Sousa IP, Bernkop-Schnürch A. Mucus permeating thiomer nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:265–272. doi: 10.1016/j.ejpb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009a;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Suk JS, Pace A, Wang YY, Yang M, Mert O, Chen J, Kim J, Hanes J. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials. 2011;32:6285–6290. doi: 10.1016/j.biomaterials.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One. 2009b;4:e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009c;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009d;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamblin G, Lhermitte M, Klein A, Houdret N, Scharfman A, Ramphal R, Roussel P. The carbohydrate diversity of human respiratory mucins: a protection of the underlying mucosa? The American review of respiratory disease. 1991;144:S19–24. doi: 10.1164/ajrccm/144.3_pt_2.S19. [DOI] [PubMed] [Google Scholar]
- Lamont JT. Mucus: the front line of intestinal mucosal defense. Annals of the New York Academy of Sciences. 1992;664:190–201. doi: 10.1111/j.1749-6632.1992.tb39760.x. [DOI] [PubMed] [Google Scholar]
- Langer G, Jagla W, Behrens–Baumann W, Walter S, Hoffmann W. Secretory peptides TFF1 and TFF3 synthesized in human conjunctival goblet cells. Investigative ophthalmology & visual science. 1999;40:2220–2224. [PubMed] [Google Scholar]
- Langguth P, Bohner V, Heizmann J, Merkle HP, Wolffram S, Amidon GL, Yamashita S. The challenge of proteolytic enzymes in intestinal peptide delivery. Journal of Controlled Release. 1997;46:39–57. [Google Scholar]
- Larhed AW, Artursson P, Bjork E. The influence of intestinal mucus components on the diffusion of drugs. Pharm Res. 1998;15:66–71. doi: 10.1023/a:1011948703571. [DOI] [PubMed] [Google Scholar]
- Larhed AW, Artursson P, Grasjo J, Bjork E. Diffusion of drugs in native and purified gastrointestinal mucus. J Pharm Sci. 1997;86:660–665. doi: 10.1021/js960503w. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Song KJ, Hwang HJ, Kim KS. International forum of allergy & rhinology. Wiley Online Library; 2016. Effectiveness of atorvastatin in suppressing MUC5AC gene expression in human airway epithelial cells; pp. 1159–1166. [DOI] [PubMed] [Google Scholar]
- Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. The American journal of physiology. 1998;274:L450–453. doi: 10.1152/ajplung.1998.274.3.L450. [DOI] [PubMed] [Google Scholar]
- Lemp A. Report of the National Eye Institute/Industry Workshop on clinical trials in dry eyes. Eye & Contact Lens. 1995;21:221–232. [PubMed] [Google Scholar]
- Lemp MA. Advances in Understanding and Managing Dry Eye Disease. American Journal of Ophthalmology. 2008;146:350–356. e351. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Lethem MI, James SL, Marriott C. The role of mucous glycoproteins in the rheologic properties of cystic fibrosis sputum. The American review of respiratory disease. 1990;142:1053–1058. doi: 10.1164/ajrccm/142.5.1053. [DOI] [PubMed] [Google Scholar]
- Lieleg O, Ribbeck K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011;21:543–551. doi: 10.1016/j.tcb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieleg O, Vladescu I, Ribbeck K. Characterization of particle translocation through mucin hydrogels. Biophys J. 2010;98:1782–1789. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden S, Nordman H, Hedenbro J, Hurtig M, Boren T, Carlstedt I. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology. 2002;123:1923–1930. doi: 10.1053/gast.2002.37076. [DOI] [PubMed] [Google Scholar]
- Linden SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS ONE. 2008;3:e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang J, Zhu X, Shan W, Li L, Zhong J, Zhang Z, Huang Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. Journal of Controlled Release. 2016;222:67–77. doi: 10.1016/j.jconrel.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, O’Neal WK, Boucher RC. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2016 doi: 10.1038/mi.2017.29. [DOI] [PubMed] [Google Scholar]
- Lo-Guidice JM, Wieruszeski JM, Lemoine J, Verbert A, Roussel P, Lamblin G. Sialylation and sulfation of the carbohydrate chains in respiratory mucins from a patient with cystic fibrosis. Journal of Biological Chemistry. 1994;269:18794–18813. [PubMed] [Google Scholar]
- Lopata M, Barton AD, Lourenço RV. Biochemical Characteristics of Bronchial Secretions in Chronic Obstructive Pulmonary Disease 1–3. American Review of Respiratory Disease. 1974;110:730–739. doi: 10.1164/arrd.1974.110.6P1.730. [DOI] [PubMed] [Google Scholar]
- Lopez-Vidriero MT, Reid L. Chemical markers of mucous and serum glycoproteins and their relation to viscosity in mucoid and purulent sputum from various hypersecretory diseases. The American review of respiratory disease. 1978;117:465–477. doi: 10.1164/arrd.1978.117.3.465. [DOI] [PubMed] [Google Scholar]
- Lowther GE, Miller RB, Hill RM. TEAR CONCENTRATIONS OF SODIUM AND POTASSIUM DURING ADAPTATION TO CONTACT LENSES: 1. SODIUM OBSERVATIONS*. Optometry & Vision Science. 1970;47:266–275. doi: 10.1097/00006324-197004000-00002. [DOI] [PubMed] [Google Scholar]
- Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Advanced Drug Delivery Reviews. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clinical science (London, England : 1979) 1983;64:449–451. doi: 10.1042/cs0640449. [DOI] [PubMed] [Google Scholar]
- Lumsden AB, McLean A, Lamb D. Goblet and Clara cells of human distal airways: evidence for smoking induced changes in their numbers. Thorax. 1984;39:844–849. doi: 10.1136/thx.39.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Maas DHA, Storey BT, Mastroianni L. Hydrogen Ion and Carbon Dioxide Content of the Oviductal Fluid of the Rhesus Monkey (Macaca Mulatta)**Supported by United States Public Health Service Grant HD-06274 and by a grant from the National Foundation-March of Dimes.††. Fertility and sterility; Presented at the Ninth World Congress on Fertility and Sterility and the Thirty-Third Annual Meeting of The American Fertility Society; April 12 to 16, 1977; Miami Beach, Fla. 1977. pp. 981–985. [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Máiz L, Girón RM, Olveira C, Quintana E, Lamas A, Pastor D, Cantón R, Mensa J. Inhaled antibiotics for the treatment of chronic bronchopulmonary Pseudomonas aeruginosa infection in cystic fibrosis: systematic review of randomised controlled trials. Expert Opinion on Pharmacotherapy. 2013;14:1135–1149. doi: 10.1517/14656566.2013.790366. [DOI] [PubMed] [Google Scholar]
- Majima Y, Harada T, Shimizu T, Takeuchi K, Sakakura Y, Yasuoka S, Yoshinaga S. Effect of Biochemical Components on Rheologic Properties of Nasal Mucus in Chronic Sinusitis. American Journal of Respiratory and Critical Care Medicine. 1999;160:421–426. doi: 10.1164/ajrccm.160.2.9805117. [DOI] [PubMed] [Google Scholar]
- Mall MA, Galietta LJV. Targeting ion channels in cystic fibrosis. Journal of Cystic Fibrosis. 2015;14:561–570. doi: 10.1016/j.jcf.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Matthes I, Nimmerfall F, Sucker H. Mucus models for investigation of intestinal absorption mechanisms. 2. Mechanisms of drug interactions with intestinal mucus. Die Pharmazie. 1992;47:609–613. [PubMed] [Google Scholar]
- Matthews LW, Spector S, Lemm J, Potter JL. Studies on Pulmonary Secretions 1, 2, 3: I. The Over-all Chemical Composition of Pulmonary Secretions from Patients with Cystic Fibrosis, Bronchiectasis, and Laryngectomy. American Review of Respiratory Disease. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- McGill SL, Smyth HD. Disruption of the mucus barrier by topically applied exogenous particles. Molecular pharmaceutics. 2010;7:2280–2288. doi: 10.1021/mp100242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin MA, Thornton DJ, Whitsett JA. Chapter 14 - Mucins and Mucus A2 - Mestecky, Jiri. In: Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. 4. Academic Press; Boston: 2015. pp. 231–250. [Google Scholar]
- McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Investigative ophthalmology & visual science. 2000;41:703–708. [PubMed] [Google Scholar]
- McKinley SA, Chen A, Shi F, Wang S, Mucha PJ, Forest MG, Lai SK. Modeling neutralization kinetics of HIV by broadly neutralizing monoclonal antibodies in genital secretions coating the cervicovaginal mucosa. PloS one. 2014;9:e100598. doi: 10.1371/journal.pone.0100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mert O, Lai SK, Ensign L, Yang M, Wang YY, Wood J, Hanes J. A poly (ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. Journal of controlled release. 2012;157:455–460. doi: 10.1016/j.jconrel.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer FA, Silberberg A. The rheology and molecular organization of epithelial mucus. Biorheology. 1980;17:163–168. doi: 10.3233/bir-1980-171-217. [DOI] [PubMed] [Google Scholar]
- Miller RB. TEAR CONCENTRATIONS OF SODIUM AND POTASSIUM DURING ADAPTATION TO CONTACT LENSES-II. POTASSIUM OBSERVATIONS*. Optometry & Vision Science. 1970;47:773–779. [PubMed] [Google Scholar]
- Mrsny RJ, Daugherty AL, Short SM, Widmer R, Siegel MW, Keller GA. Distribution of DNA and alginate in purulent cystic fibrosis sputum: implications to pulmonary targeting strategies. J Drug Target. 1996;4:233–243. doi: 10.3109/10611869608995625. [DOI] [PubMed] [Google Scholar]
- Müller C, Leithner K, Hauptstein S, Hintzen F, Salvenmoser W, Bernkop-Schnürch A. Preparation and characterization of mucus-penetrating papain/poly (acrylic acid) nanoparticles for oral drug delivery applications. Journal of nanoparticle research. 2013;15:1353. [Google Scholar]
- Murgia X, Pawelzyk P, Schaefer UF, Wagner C, Willenbacher N, Lehr CM. Size-Limited Penetration of Nanoparticles into Porcine Respiratory Mucus after Aerosol Deposition. Biomacromolecules. 2016;17:1536–1542. doi: 10.1021/acs.biomac.6b00164. [DOI] [PubMed] [Google Scholar]
- Murty VL, Sarosiek J, Slomiany A, Slomiany BL. Effect of lipids and proteins on the viscosity of gastric mucus glycoprotein. Biochem Biophys Res Commun. 1984;121:521–529. doi: 10.1016/0006-291x(84)90213-4. [DOI] [PubMed] [Google Scholar]
- Netsomboon K, Bernkop-Schnürch A. Mucoadhesive vs. mucopenetrating particulate drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2016;98:76–89. doi: 10.1016/j.ejpb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Neves J, Amiji M, Sarmento B. Mucoadhesive nanosystems for vaginal microbicide development: Friend or foe? 2011 doi: 10.1002/wnan.144. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Hvidt S, Sheils CA, Janmey PA. Elastic contributions dominate the viscoelastic properties of sputum from cystic fibrosis patients. Biophys Chem. 2004;112:193–200. doi: 10.1016/j.bpc.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Nielsen PA, Mandel U, Therkildsen MH, Clausen H. Differential expression of human high-molecular-weight salivary mucin (MG1) and low-molecular-weight salivary mucin (MG2) J Dental Res. 1996;75:1820–1826. doi: 10.1177/00220345960750110201. [DOI] [PubMed] [Google Scholar]
- Niibuchi JJ, Aramaki Y, Tsuchiya S. Binding of antibiotics to rat intestinal mucin. International journal of pharmaceutics. 1986;30:181–187. [Google Scholar]
- Nordman H. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J. 2002;364:191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DA, Sinko PJ. Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin. Journal of Applied Polymer Science. 1997;63:1481–1492. [Google Scholar]
- Nugent S, Kumar D, Rampton D, Evans D. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52:5971–5978. [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clinica Chimica Acta. 2006;369:17–28. doi: 10.1016/j.cca.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV. Mild and Moderate Asthma Is Associated with Airway Goblet Cell Hyperplasia and Abnormalities in Mucin Gene Expression. American Journal of Respiratory and Critical Care Medicine. 2001;163:517–523. doi: 10.1164/ajrccm.163.2.2004039. [DOI] [PubMed] [Google Scholar]
- Packer LM, Williams SJ, Callaghan S, Gotley DC, McGuckin MA. Expression of the cell surface mucin gene family in adenocarcinomas. International journal of oncology. 2004;25:1119–1126. [PubMed] [Google Scholar]
- Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. European journal of biochemistry. 2002;269:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- Pandit JC, NagyovÁ B, Bron AJ, Tiffany JM. Physical Properties of Stimulated and Unstimulated Tears. Experimental Eye Research. 1999;68:247–253. doi: 10.1006/exer.1998.0600. [DOI] [PubMed] [Google Scholar]
- Patton JS. Mechanisms of macromolecule absorption by the lungs. Advanced Drug Delivery Reviews. 1996;19:3–36. [Google Scholar]
- Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell and Tissue Research. 2004;316:167–177. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EEL, Wising C, Johansson MEV, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological Reviews. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera G, Zipser M, Bonengel S, Salvenmoser W, Bernkop-Schnürch A. Development of phosphorylated nanoparticles as zeta potential inverting systems. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:250–256. doi: 10.1016/j.ejpb.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB, Jr, Welsh MJ, Zabner J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JL, Matthews LW, Spector S, Lemm J. Studies on pulmonary secretions. II. Osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. The American review of respiratory disease. 1967;96:83–87. doi: 10.1164/arrd.1967.96.1.83. [DOI] [PubMed] [Google Scholar]
- Prego C, Torres D, Alonso MJ. The potential of chitosan for the oral administration of peptides. Expert opinion on drug delivery. 2005;2:843–854. doi: 10.1517/17425247.2.5.843. [DOI] [PubMed] [Google Scholar]
- Prydal JI, Campbell FW. Study of precorneal tear film thickness and structure by interferometry and confocal microscopy. Invest Ophthalmol Vis Sci. 1992;33:1996–2005. [PubMed] [Google Scholar]
- Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem. 2003;278:28703–28710. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Hemming AL, Regnis JA, Wong AG, Bailey DL, Bautovich GJ, King M, Bye P. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax. 1997;52:900–903. doi: 10.1136/thx.52.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Partenhauser A, Hauptstein S, Gallati CM, Matuszczak B, Abdulkarim M, Gumbleton M, Bernkop-Schnürch A. Mucus permeating thiolated self-emulsifying drug delivery systems. European Journal of Pharmaceutics and Biopharmaceutics. 2016;98:90–97. doi: 10.1016/j.ejpb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Rong M. Expression and localization of Muc4/sialomucin complex (SMC) in the adult and developing rat intestine: implications for Muc4/SMC function. J Cell Physiol. 2005;202:275–284. doi: 10.1002/jcp.20121. [DOI] [PubMed] [Google Scholar]
- Rose MC. Mucins: structure, function, and role in pulmonary diseases. The American journal of physiology. 1992;263:L413–429. doi: 10.1152/ajplung.1992.263.4.L413. [DOI] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva A, Sachdeva O, Gulati S, Kakkar V. Nasal mucociliary clearance & mucus pH in patients with diabetes mellitus. The Indian journal of medical research. 1993;98:265–268. [PubMed] [Google Scholar]
- Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophysical Journal. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J. Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev. 2009;61:115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162:1905–1911. doi: 10.1164/ajrccm.162.5.9909009. [DOI] [PubMed] [Google Scholar]
- Schade C, Flemström G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22:391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Schoessler J, Hill R. EFFECTS OF HARD CONTACT LENSES ON THE CHLORIDE ION OF THE TEARS*. Optometry and Vision Science. 1974;51:84–87. [Google Scholar]
- Schmitz T, Bravo-Osuna I, Vauthier C, Ponchel G, Loretz B, Bernkop-Schnürch A. Development and in vitro evaluation of a thiomer-based nanoparticulate gene delivery system. Biomaterials. 2007;28:524–531. doi: 10.1016/j.biomaterials.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Scheid P. Gastric mucus of the guinea pig: proton carrier and diffusion barrier. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1997;272:G63–G70. doi: 10.1152/ajpgi.1997.272.1.G63. [DOI] [PubMed] [Google Scholar]
- Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34:3439–3446. doi: 10.1016/j.biomaterials.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serisier DJ, Carroll MP, Shute JK, Young SA. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respiratory Research. 2009;10:63. doi: 10.1186/1465-9921-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnaz G, Vetter A, Barthelmes J, Rahmat D, Laffleur F, Iqbal J, Perera G, Schlocker W, Dünnhaput S, Augustijns P. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: bioavailability and pharmacokinetic characterization. International journal of pharmaceutics. 2012;428:164–170. doi: 10.1016/j.ijpharm.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Shea-Donohue T, Danquechin-Dorval E, Montcalm E, El-Bayar H, Durakovic A, Conklin J, Dubois A. Alterations in gastric mucus secretion in rhesus monkeys after exposure to ionizing radiation. Gastroenterology. 1985;88:685–690. doi: 10.1016/0016-5085(85)90138-6. [DOI] [PubMed] [Google Scholar]
- Sheehan JK, Oates K, Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochemical Journal. 1986;239:147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng YH. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut. 2011;60:1661–1670. doi: 10.1136/gut.2011.239194. [DOI] [PubMed] [Google Scholar]
- Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28:5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. International Journal of Pharmaceutics. 2013;453:56–64. doi: 10.1016/j.ijpharm.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Sjoblom M, Flemstrom G. Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion. Journal of pineal research. 2003;34:288–293. doi: 10.1034/j.1600-079x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Skrypek N, Duchêne B, Hebbar M, Leteurtre E, Van Seuningen I, Jonckheere N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene. 2013;32:1714. doi: 10.1038/onc.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyenkoff BC, Hinck CF. THE MEASUREMENT OF pH AND ACID-NEUTRALIZING POWER OF SALIVA. Journal of Biological Chemistry. 1935;109:467–476. [Google Scholar]
- Steiner CA, Litt M, Nossal R. Effect of Ca++ on the structure and rheology of canine tracheal mucin. Biorheology. 1984;21:235–252. doi: 10.3233/bir-1984-211-226. [DOI] [PubMed] [Google Scholar]
- Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. The Proceedings of the Nutrition Society. 2003;62:237–243. doi: 10.1079/pns2002205. [DOI] [PubMed] [Google Scholar]
- Suchaoin W, Pereira de Sousa I, Netsomboon K, Lam HT, Laffleur F, Bernkop-Schnürch A. Development and in vitro evaluation of zeta potential changing self-emulsifying drug delivery systems for enhanced mucus permeation. International Journal of Pharmaceutics. 2016;510:255–262. doi: 10.1016/j.ijpharm.2016.06.045. [DOI] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedicine. 2011;6:365–375. doi: 10.2217/nnm.10.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk JS, Lai SK, Wang YY, Ensign LM, Zeitlin PL, Boyle MP, Hanes J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials. 2009;30:2591–2597. doi: 10.1016/j.biomaterials.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson O, Arnebrant T. Mucin layers and multilayers — Physicochemical properties and applications. Current Opinion in Colloid & Interface Science. 2010;15:395–405. [Google Scholar]
- Tam PY, Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature. 1981;292:340–342. doi: 10.1038/292340a0. [DOI] [PubMed] [Google Scholar]
- Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences. 2009a;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Fatehi M, Linsdell P. Mechanism of direct bicarbonate transport by the CFTR anion channel. Journal of Cystic Fibrosis. 2009b;8:115–121. doi: 10.1016/j.jcf.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, Choudhury B, Varki A, Stoltz DA, Welsh MJ. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. Journal of Clinical Investigation. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The Relative Roles of Passive Surface Forces and Active Ion Transport in the Modulation of Airway Surface Liquid Volume and Composition. The Journal of General Physiology. 2001a;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, Boucher RC. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001b:8. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Taylor C, Allen A, Dettmar PW, Pearson JP. The gel matrix of gastric mucus is maintained by a complex interplay of transient and nontransient associations. Biomacromolecules. 2003;4:922–927. doi: 10.1021/bm025767t. [DOI] [PubMed] [Google Scholar]
- Thaysen JH, Thorn NA. Excretion of urea, sodium, potassium and chloride in human tears. American Journal of Physiology--Legacy Content. 1954;178:160–164. doi: 10.1152/ajplegacy.1954.178.1.160. [DOI] [PubMed] [Google Scholar]
- Thornton DJ. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology. 1999;9:293–302. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Howard M, Khan N, Sheehan JK. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J Biol Chem. 1997;272:9561–9566. doi: 10.1074/jbc.272.14.9561. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Tiffany JM. The viscosity of human tears. International Ophthalmology. 1991;15:371–376. doi: 10.1007/BF00137947. [DOI] [PubMed] [Google Scholar]
- Tréhoux S, Duchêne B, Jonckheere N, Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42–44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochemical and biophysical research communications. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Advanced Drug Delivery Reviews. 2005;57:1640–1665. doi: 10.1016/j.addr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Van Haeringen NJ. Clinical biochemistry of tears. Survey of ophthalmology. 1981;26:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- Vasconcellos CA, Allen PG, Wohl ME, Drazen JM, Janmey PA, Stossel TP. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science-AAAS-Weekly Paper Edition-including Guide to Scientific Information. 1994;263:969–970. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Goblet cells secretion and mucogenesis. Annual review of physiology. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- Viswanathan H, Brownlee IA, Pearson JP, Carrie S. MUC5B secretion is up-regulated in sinusitis compared with controls. American journal of rhinology. 2006;20:554–557. doi: 10.2500/ajr.2006.20.2935. [DOI] [PubMed] [Google Scholar]
- Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. Journal of colloid and interface science. 2009;330:443–448. doi: 10.1016/j.jcis.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Washington N, Steele RJ, Jackson SJ, Bush D, Mason J, Gill DA, Pitt K, Rawlins DA. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int J Pharm. 2000;198:139–146. doi: 10.1016/s0378-5173(99)00442-1. [DOI] [PubMed] [Google Scholar]
- Wickström C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochemical Journal. 1998;334:685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway Mucus: From Production to Secretion. American Journal of Respiratory Cell and Molecular Biology. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- Winder A, Ruben M, Sheraidah G. Tear calcium levels and contact lens wear. British Journal of Ophthalmology. 1977;61:539–543. doi: 10.1136/bjo.61.8.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissniowski TT, Meister S, Hahn EG, Kalden JR, Voll R, Ocker M. Mucin production determines sensitivity to bortezomib and gemcitabine in pancreatic cancer cells. International journal of oncology. 2012;40:1581–1589. doi: 10.3892/ijo.2012.1337. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Blasco L, Khan MA, Litt M. Human cervical mucus. IV. Viscoelasticity and sperm penetrability during the ovulatory menstrual cycle. Fertility and sterility. 1978;30:163–169. doi: 10.1016/s0015-0282(16)43454-0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Boylan NJ, Cai S, Miao B, Patel H, Hanes J. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J Control Release. 2013a;170:279–286. doi: 10.1016/j.jconrel.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Boylan NJ, Suk JS, Wang YY, Nance EA, Yang JC, McDonnell PJ, Cone RA, Duh EJ, Hanes J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. Journal of controlled release. 2013b;167:76–84. doi: 10.1016/j.jconrel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Ensign LM, Boylan NJ, Schon A, Gong X, Yang JC, Lamb NW, Cai S, Yu T, Freire E, Hanes J. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS nano. 2015;9:9217–9227. doi: 10.1021/acsnano.5b03876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Garcia MAS, Quinton PM. Normal mucus formation requires cAMP-dependent HCO3– secretion and Ca2+-mediated mucin exocytosis. The Journal of Physiology. 2013;591:4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo ACH, Carkeet A, Carney LG, Yap MKH. Relationship between goblet cell density and tear function tests. Ophthalmic and Physiological Optics. 2003;23:87–94. doi: 10.1046/j.1475-1313.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- Yoon SS, Coakley R, Lau GW, Lymar SV, Gaston B, Karabulut AC, Hennigan RF, Hwang SH, Buettner G, Schurr MJ, Mortensen JE, Burns JL, Speert D, Boucher RC, Hassett DJ. Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest. 2006;116:436–446. doi: 10.1172/JCI24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yasin M, Carraway CA, Carraway KL. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell. 2006;38:271–275. doi: 10.1016/j.tice.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Jumblatt JE, Wood TO, Jumblatt MM. Quantification of MUC5AC protein in human tears. Cornea. 2001;20:873–877. doi: 10.1097/00003226-200111000-00019. [DOI] [PubMed] [Google Scholar]