Abstract
Heart failure with preserved ejection fraction (HFpEF) now accounts for the majority of confirmed HF cases in the United States. However, there are no highly effective evidence‐based treatments currently available for these patients. Inflammation correlates positively with adverse outcomes in HF patients. Interleukin (IL)‐1, a prototypical inflammatory cytokine, has been implicated as a driver of diastolic dysfunction in preclinical animal models and a pilot clinical trial. The Diastolic Heart Failure Anakinra Response Trial 2 (D‐HART2) is a phase 2, 2:1 randomized, double‐blind, placebo‐controlled clinical trial that will test the hypothesis that IL‐1 blockade with anakinra (recombinant human IL‐1 receptor antagonist) improves (1) cardiorespiratory fitness, (2) objective evidence of diastolic dysfunction, and (3) elevated inflammation in patients with HFpEF (http://www.ClinicalTrials.gov NCT02173548). The co–primary endpoints will be placebo‐corrected interval changes in peak oxygen consumption and ventilatory efficiency at week 12. In addition, secondary and exploratory analyses will investigate the effects of IL‐1 blockade on cardiac structure and function, systemic inflammation, endothelial function, quality of life, body composition, nutritional status, and clinical outcomes. The D‐HART2 clinical trial will add to the growing body of evidence on the role of inflammation in cardiovascular disease, specifically focusing on patients with HFpEF.
Keywords: Interleukin‐1, Heart Failure, clinical trial study design
1. INTRODUCTION
Patients with heart failure (HF) and a preserved left ventricular (LV) ejection fraction (HFpEF) comprise >50% of the overall HF population.1 The management of HFpEF represents a significant challenge, as currently available therapies do not significantly improve exertional dyspnea, exercise tolerance, or survival.1
Evidence of the presence of heightened inflammation is well established in HF.2 Interleukin‐1 (IL‐1), an apical cytokine, was isolated first by Dinarello and colleagues as a leukocytic pyrogen factor.3 Subsequent investigations have shown that IL‐1 is a master regulator not only of fever, but also of a complex humoral and cellular inflammatory response.3
In addition to its role in systemic inflammation, early investigations noted that IL‐1 was a soluble cardiodepressant factor in septic shock.4 This initial clinical observation has led to a deeper understanding of IL‐1's effects on the myocardium, including impairment of cardiac diastolic function, a hallmark of HFpEF, through modulation of sarcoplasmic reticulum phospholamban and calcium‐ATPase.2, 5
In experimental models, the administration of IL‐1 to healthy mice increases isovolumetric relaxation time and LV end‐diastolic pressure, impairs LV − dP/dT, and induces chronotropic incompetence.6, 7 IL‐1 blockade prevents and restores diastolic dysfunction in a murine heart failure model.7 (For a summary of the effects of IL‐1 on myocardial structure and function, see Supporting Information, Figure 1, in the online version of this article.)
Figure 1.
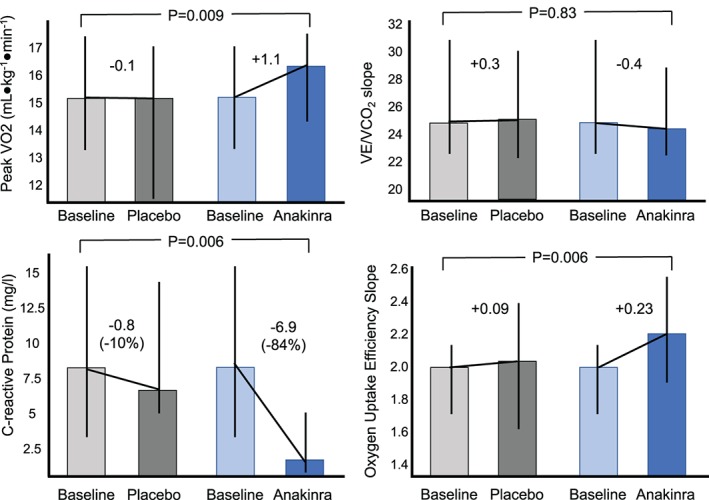
Effects of IL‐1 blockade on cardiorespiratory fitness in a pilot clinical trial. A randomized, double‐blind, crossover, pilot study evaluated the effects of IL‐1 blockade in patients with HFpEF. Abbreviations: HFpEF, heart failure with preserved ejection fraction; IL‐1, interleukin 1; peak VO2, peak oxygen consumption; VE/VCO2, minute ventilation–carbon dioxide production relationship
IL‐1 hyperactivity in patients with HF is supported by a large body of clinical evidence.8, 9, 10 Moreover, C‐reactive protein (CRP), which is considered a marker of IL‐1 activity, correlates positively with LV end‐diastolic pressure11 as well as peak oxygen consumption (VO2) and ventilatory efficiency—both prognostic indicators in HF.12
Importantly, IL‐1 blockade is distinct from tumor necrosis factor‐α (TNF‐α) inhibition.2 IL‐1 and TNF‐α are distinct cytokines, regulated by different families of genes, different families of receptors, and different signaling cascades. IL‐1, in its 2 isoforms (IL‐1α and IL‐1β), binds a single signaling membrane receptor (IL‐1R) that is part of the Toll‐IL‐1 family of receptors.3 In contrast, TNF‐α binds one of 2 membrane receptors, type I and type II, that are part of a large family of receptors called the TNF‐α superfamily of receptors, which includes ≥27 subtypes that share the ability to bind the ligand via an extracellular cysteine‐rich domain and include many death‐ligand receptors.13 The 2 TNF‐α receptors have different, and often opposing, downstream signaling.14 Moreover, TNF‐α inhibition causes immunosuppression and has a nonlinear dose‐response relationship; IL‐1 blockade does not appear to have these limitations.2
Based on these data, the Diastolic Heart Failure–Anakinra Response Trial (D‐HART, http://www.ClinicalTrials.gov NCT01542502), a randomized, double‐blind, placebo‐controlled crossover study of 12 patients with HFpEF and objective diastolic dysfunction, was conducted.15 Patients with stable HFpEF and heightened systemic inflammation (high‐sensitivity CRP [hsCRP] >2 mg/L) were eligible. Patients were treated with anakinra 100 mg daily for 14 days or placebo, followed by crossover to the other treatment arm.
Treatment with anakinra (Kineret; Swedish Orphan Biovitrum, Stockholm, Sweden), a recombinant nonglycosylated human IL‐1 receptor antagonist, for 14 days provided a placebo‐corrected interval change in peak VO2 of +1.1 mL•kg−1•min−1 (Figure 1).15 CRP was reduced by 84% during the 14 days of anakinra treatment, and changes in CRP correlated significantly with changes in peak VO2. Oxygen uptake efficiency slope, a measure of ventilatory efficiency, significantly improved during anakinra treatment. The minute ventilation–carbon dioxide production slope, another measure of ventilatory efficiency, was abnormal in 5 of the 12 patients at baseline and significantly improved with anakinra treatment.
We designed the D‐HART2 trial to confirm the effects of anakinra on cardiorespiratory fitness (CRF) and inform the design of a larger outcomes trial.
2. METHODS
2.1. Study design and oversight
The D‐HART2 trial (http://www.ClinicalTrials.gov NCT02173548) is a single‐center, randomized, double‐blind, phase 2 clinical trial with 2:1 allocation to anakinra or placebo, designed to investigate the effects of IL‐1 blockade on CRF and cardiac structure and function in patients with symptomatic HFpEF (New York Heart Association class II–III), confirmed diastolic dysfunction, and evidence of systemic inflammation (Figure 2). Exploratory analyses of body composition, nutritional status, circulating biomarkers (except hsCRP, which is a secondary endpoint), and vascular function will also be conducted.
Figure 2.
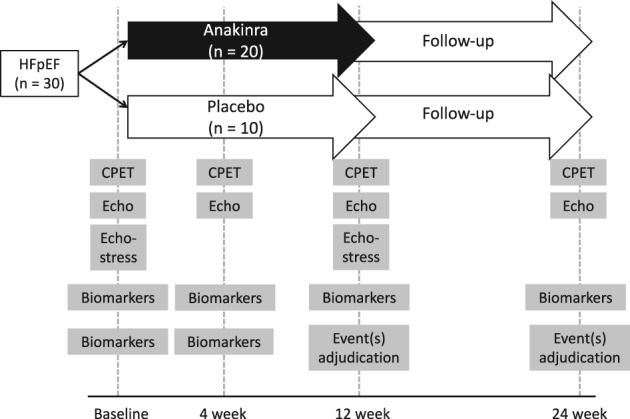
Design of the Diastolic Heart Failure–Anakinra Response Trial 2 (D‐HART2). Abbreviations: CPET, cardiopulmonary exercise testing; echo, echocardiography; HFpEF, heart failure with preserved ejection fraction
The Clinical Event Committee is composed of Michael Kontos, MD (chair), Keyur Shah, MD, and Jaideep Patel, MD. The Data and Safety Monitoring Board includes Dominick Angiolillo, MD (chair), Richard Cooke, MD, Gonzalo Bearman, MD, Ion Jovin, MD, Jeffrey Kushinka, MD, and Christine DeWilde, RN (coordinator). For a complete list of study personnel, see Supporting Information, Appendix, in the online version of this article.
2.2. Screening and enrollment
Patients are eligible for inclusion (Table) if they have signs and symptoms of HF and objective confirmation of LV ejection fraction >50%, diastolic dysfunction or elevated LV filling pressure,16 and hsCRP ≥2 mg/L.
The initial inclusion criteria required a previous hospitalization for HF. In light of the fact that such criterion is no longer considered to be a reliable marker of HF severity, this criterion was subsequently removed to facilitate patient enrollment.17 Twenty patients had been enrolled prior to the removal of the previous‐hospitalization criterion.
Major exclusion criteria (Table) include (1) an inability to complete or a contraindication to maximal cardiopulmonary exercise testing (CPET); (2) any auto‐inflammatory or autoimmune disease; and (3) chronic, long‐term treatment with an anti‐inflammatory or immunosuppressant agent.
2.3. Randomization and allocation concealment
The randomization log was prepared by an outside consultant and sent electronically to the director of the investigational pharmacy at Virginia Commonwealth University. Investigators were blinded to all CRP levels during the study. Identical anakinra or placebo syringes were dispensed by the investigational pharmacy.
2.4. Study treatment
Patients were randomized 2:1 to either anakinra (Kineret) 100 mg or placebo, administered once daily for 12 weeks. The primary side effects of anakinra are injection‐site reactions, such as erythema or pain. By blocking IL‐1, anakinra prevents fever and other systemic manifestation of disease, and as such it may delay the diagnosis of infection.
Patients in the study will receive standard‐of‐care medical treatments, such as diuretics, β‐adrenergic receptor blockers, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, aldosterone blockers, isosorbide dinitrate, hydralazine, digoxin, aspirin, and statins.
2.5. Endpoints
Placebo‐controlled interval changes in peak VO2 and the minute ventilation (VE)/VCO2 slope after 12 weeks of treatment with anakinra or placebo are the co–primary endpoints (see Supporting Information, Table 1, in the online version of this article).
Table 1.
| Inclusion Criteria (all must be present) | Exclusion Criteria |
|---|---|
|
|
Secondary CPET endpoints include interval changes in peak VO2 and VE/VCO2 slope at 4 and 24 weeks, as well as the oxygen uptake efficiency slope, anaerobic threshold, and chronotropic response (see Supporting Information, Table 1, in the online version of this article).
Structural and functional myocardial parameters captured with transthoracic echocardiography according to current guidelines include left and right ventricular and atrial dimensions, left and right ventricular systolic function, transmitral flow Doppler spectra, mitral and tricuspid valve annulus tissue Doppler spectra, ejection time, stroke volume, inferior vena cava, and aorta and pulmonary artery diameters and Doppler spectra.18 Patients also undergo a limited stress echocardiography and Doppler study immediately after peak exercise at baseline and 12 weeks to measure placebo‐controlled interval changes in diastolic reserve measured as changes in E/e´ ratio with exercise.
Blood will be analyzed for complete blood count, metabolic markers (including lipids and lipoproteins, glycemic control and insulin resistance, hepatic and renal panels, sterols), inflammation (hsCRP, galectin‐3), and cardiac biomarkers (N‐terminal pro‐brain natriuretic peptide and troponin I). Galectin‐3, an established prognostic marker in HF and a surrogate for fibrosis, was included given the importance of fibrotic adverse LV remodeling and to investigate its potential relationship to inflammation.19 With the exception of hsCRP, biomarker assessments are considered exploratory.
Clinical endpoints will include death (cardiac and noncardiac), hospitalizations (cardiac, including HF and non‐HF and noncardiac), and serious and nonserious adverse events through study conclusion or 108 weeks, whichever occurs last. Detailed definitions of each endpoint are provided in Supporting Information, Table 2, in the online version of this article.
Dietary assessment and body‐composition analysis will be conducted by trained dietitians using a standardized 24‐hour dietary recall method and bioelectrical impedance analysis, respectively.
2.6. Cardiopulmonary exercise testing
A physician‐supervised maximal CPET will be administered using a metabolic cart interfaced with a treadmill. A standard conservative ramping treadmill protocol is used as previously reported and according to American Heart Association guidelines.20, 21, 22 All patients must achieve a respiratory exchange ratio >1.00, and preferably >1.10. Tests were repeated if a patient did not achieve a maximal effort.
The highest 10‐second average value within the 30 seconds prior to study completion for O2 uptake defines peak VO2 in mL•kg−1•min−1. The dual‐methods criteria wherein the V‐slope method and the ventilatory equivalents method will be employed to determine VO2 at ventilatory threshold (VAT). Ten‐second averaged VE and VCO2 data, from the initiation of exercise to peak, will be utilized to calculate the VE/VCO2 slope via least squares linear regression (y = mx + b, m = slope). Chronotropic response will be determined from the chronotropic index, which is the difference between the peak heart rate (HR) and the resting HR relative to the metabolic requirement of exercise (peak VO2 − resting VO2).
2.7. Symptom assessment
Patient‐reported symptoms will be assessed at each study visit. The Minnesota Living with Heart Failure Questionnaire (MLHFQ) is a 21‐item graded questionnaire that has been extensively used to measure impairment in quality of life in patients with HF.23 The Duke Activity Status Index (DASI) is a 12‐item instrument that allows for the calculation of perceived functional capacity, in which each question describes a different physical activity and the questions are weighted according to their degree of physical exertion.24
2.8. Bioelectrical impedance analysis
Body‐composition assessment will be conducted at each visit with bioelectrical impedance analysis, a noninvasive, quick, and safe technique that allows for an accurate estimation of fluid status and body composition. Two electrodes on the foot and 2 on the homolateral hand (superior limbs abducted at 30° and inferior limbs at 45°) measure electrical signals with a Quantum IV Body Composition Analyzer (RJL Systems, Clinton Township, MI). Body composition (total body water, extracellular water, intracellular water, lean fat‐free mass, and fat mass) will be calculated using dedicated software.
2.9. Vascular assessment
As an exploratory analysis, vascular assessment was conducted at each study visit. A small plethysmography cuff (ccNexfin; Edwards Lifesciences, Irvine, CA) will be placed on the subject's finger to measure blood flow. After capture of baseline variables, a standard blood pressure (BP) cuff will then be placed on the arm and inflated for 3 minutes. The finger cuff will measure baseline and change in BP, HR, estimated cardiac output, and systemic vascular resistance. Reactive hyperemia will be calculated as the highest 10‐second average cardiac output within 3 minutes post‐deflation divided by baseline cardiac output. Flow‐mediated dilatation will be estimated from reactive hyperemia using Poiseuille's law. Effective arterial elastance and end‐systolic elastance will be calculated as previously reported.25
2.10. Safety assessment
Safety parameters will include data deriving from history and physical examination performed at each visit, laboratory data, and results of functional and imaging tests. To enhance detection of adverse events between visits, all patients will be encouraged to contact the research team at any time with concerns or any perceived changes in their clinical status. Particular attention will be given toward symptoms and signs of infection.
2.11. Statistical analysis
The CPET data will be collected and electronically transferred to the Core Laboratory at the University of Illinois at Chicago for analysis, under the guidance of Ross Arena, PhD. After locking the database, Dr. Arena will be provided a group allocation according to treatment A or B (blinded to real treatment). Data will then be analyzed according to blinded group allocation and the description of the true group allocation will be disclosed only after completion of the analysis. All other data will be analyzed at VCU in an identical fashion. For all endpoints, an unadjusted P value of <0.05 will be considered statistically significant.
2.11.1. Sample size calculation
The sample size for this pilot study was calculated according to the primary endpoint of difference in interval change in peak VO2 at 12 weeks between anakinra and placebo. The initial calculation based on an expected average peak VO2 of 15 ± 3.5 mL•kg−1•min−1, and randomization of 40 subjects to anakinra and 20 subjects to placebo indicated >99%, >99%, 99%, and 86% power to detect a difference in peak VO2 of 3.5, 3.0, 2.5, and 2.0 mL•kg−1•min−1 in peak VO2, respectively.
In October 2016, given a slower than expected enrollment rate, the investigators reviewed the baseline data and original power calculations. Data from prior HF clinical trials of CPET26, 27, 28, 29 and our own preliminary data15 suggested that a more accurate expected SD for within‐subject changes is 1.9 mL•kg−1•min−1, as opposed to the original 3.5 mL•kg−1•min−1 (see Supporting Information, Figure 2, in the online version of this article). Based on this new estimate of variance, enrollment of 30 patients in the same 2:1 ratio would retain >99%, 90%, and 78% power to detect a minimum difference in peak VO2 of 3.5, 3.0, and 2.0 mL•kg−1•min−1. The sample size was adjusted to 30 patients.
2.11.2. Demographics and baseline characteristics
Baseline measurements and demographic characteristics will be summarized for the patients in each of the treatment arms. Descriptive summaries of continuous measurements will consist of medians and interquartile ranges due to potential deviation from Gaussian distribution. Descriptive summaries of categorical measurements will consist of frequencies, proportions, and 95% confidence intervals on those proportions. Baseline characteristics for treatment and control groups will be compared with the χ2 test for categorical variables or the Mann‐Whitney U test for continuous variables.
2.11.3. Endpoint Analysis
The analysis will be performed at the conclusion of the study as described above. The interval change between treatment groups will be compared using random effects analysis of variance (ANOVA) for repeated measures assessing for the interaction between “time” and “group allocation.” For clinical events, Kaplan‐Meier curves will be constructed and compared using log‐rank testing. The primary analysis for all endpoints will be conducted in a modified intention‐to‐treat population, which includes all patients who were randomized and have complete data for the primary endpoints at baseline and 12 weeks or have complete data at baseline and at 4 weeks, in which case the 4‐weeks value will be used according to the last‐observation‐carried‐forward principle.
A landmark analysis will be performed as an on‐treatment and off‐treatment analysis in which the Kaplan‐Meier curves created for patients treated with placebo and anakinra are compared for the first 12 weeks (on‐treatment) and separately for the following 12 weeks (off‐treatment) using the log‐rank test for event rates to assess for the potential loss of benefit with cessation of treatment.
In addition, a sensitivity analysis will be conducted after stratification of patients according to presence or absence of prior hospitalization.
3. RESULTS
Preliminary results: The first patient was enrolled on September 3, 2014. DHART2 has completed enrollment of the 30 patients, and the last patient's last visit is on June 20, 2017.
4. DISCUSSION
A significant link between increased IL‐1 activity and outcomes in patients with HF has been established. The D‐HART2 study is testing whether IL‐1 blockade improves the co–primary endpoints of peak VO2 and ventilatory efficiency in patients with symptomatic HFpEF, evidence of impaired diastolic function, and heightened systemic inflammation. Because effective treatment options for HFpEF are limited, IL‐1 blockade may fill an important unmet medical need.
Prior HFpEF clinical trials have failed to demonstrate significant improvements in clinical outcomes. One explanation for these disappointing results is that HFpEF itself is a heterogeneous syndrome with multiple subphenotypes of varying etiology and pathophysiology. Enrollment of a broad cohort, as conducted in prior studies, may have diluted any potential signal of benefit. Similarly, HFpEF symptoms can be mimicked by other conditions, suggesting the need for inclusion criteria that identify structural and/or functional myocardial impairment.
Thus, D‐HART2 was designed with strict inclusion criteria to ensure the enrollment of a homogenous cohort of patients who had both impaired cardiac diastolic function and reduced exercise capacity. Supporting Information, Table 3, in the online version of this article compares eligibility criteria across HFpEF clinical trials that utilized CPET as an endpoint. Slow enrollment in DHART2 can likely be attributed to these strict criteria. The best approach for the design of future HFpEF clinical trials likely represents a hybrid of the 2 approaches discussed here.
The co–primary endpoints were selected on the basis that they each correlate significantly with mortality in HF and provide a functional assessment of exercise capacity—sufficient justification for subsequent clinical trials.30, 31, 32 Moreover, these outcomes are ideal surrogates for a phase 2 trial, as they are immune to bias from overexertion, show minimal training effects over time, and exhibit high intraclass correlation coefficients (>0.90).26, 33, 34, 35, 36, 37 Assessment of clinical outcomes will further provide possible justification for future clinical trials.
The D‐HART2 trial will also provide insight into possible mechanisms through which IL‐1 affects cardiac function. Doppler echocardiography will aid in determining the effects of IL‐1 blockade on cardiac structure and function. An important assessment will be the effects of IL‐1 blockade on early diastolic E wave deceleration time and E´ mitral annular velocity, measures of active relaxation, both at rest and at peak exercise.38 Estimates of flow‐mediated dilatation will evaluate whether IL‐1 blockade affects endothelial function. Recent studies have suggested that ventricular‐arterial uncoupling plays an important role in the syndrome of HFpEF.39 Measurement of CRP will be utilized to confirm that adequate inflammation suppression was achieved and to determine if improvements in CRF correlate with changes in inflammation.
There is growing appreciation for the interaction between total body weight, total fat mass, and inflammation in HFpEF.40 In fact, one study demonstrated improvements in peak VO2 after caloric restriction with or without a structured exercise program in obese HFpEF patients despite no change in measures of diastolic function.29 The dietary recall and body composition assessments will further support novel hypotheses on the relationship between specific dietary components (ie, mono‐ or polyunsaturated fatty acids) and HFpEF.
4.1. Study limitations
There are potential limitations to the design of D‐HART2, many of which are inherent to pilot clinical trials. Secondary outcomes will be considered hypothesis‐generating, and the study is not intended to evaluate clinical outcomes such as hospitalizations or mortality. Results may not be applicable to other centers with different HF management practices due to the single‐center design. It will remain unclear whether patients with hsCRP < 2 mg/L would also benefit from anakinra. At present, in the D‐HART pilot trial, 14 of 23 patients (60%) who were screened for eligibility had an hsCRP >2 mg/dL.
5. CONCLUSION
The D‐HART2 pilot trial will test the novel hypothesis that IL‐1 impairs CRF in patients with HFpEF. The data collected with CPET, echocardiography, and clinical assessment will inform the design of potential larger studies aimed at assessing the effects of IL‐1 blockade on clinical outcomes. This pilot trial adds to the current portfolio of ongoing clinical trials that are currently assessing the role of IL‐1 in acute myocardial infarction, prior myocardial infarction, systolic HF, systolic HF in end‐stage renal disease, and pericarditis.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1. Supporting Appendix
Figure S1. Role of Inflammation in Heart Failure with Preserved Ejection Fraction.
Figure S2. Comparison of Standard Deviation Relative to Effect Size across Select Studies of Heart Failure with Preserved Ejection Fraction
Acknowledgments
D‐HART2 is supported by a grant from the National Heart, Lung, and Blood Institute (R34HL118348). Swedish Orphan Biovitrum LLC (Stockholm, Sweden) has provided study medication (anakinra) at a discounted price and matching placebo, but had no role in the study design, conduct, analysis or reporting.
Van Tassell BW, Buckley LF, Carbone S, Trankle CR, Canada JM, Dixon DL, Abouzaki N, Oddi‐Erdle C, Biondi‐Zoccai G, Arena R and Abbate A. Interleukin‐1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D‐HART2). Clin Cardiol. 2017;40:626–632. 10.1002/clc.22719
Clinicaltrials.gov Identification Number: NCT02173548
Funding Information D‐HART2 is supported by a grant from the National Heart, Lung, and Blood Institute, Grant/Award number: R34HL118348; Swedish Orphan Biovitrum LLC (Stockholm, Sweden) has provided study medication (anakinra) at a discounted price and matching placebo, but had no role in the study design, conduct, analysis or reporting.
REFERENCES
- 1. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 2. Van Tassell BW, Toldo S, Mezzaroma E, et al. Targeting interleukin‐1 in heart disease. Circulation. 2013;128:1910–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinarello CA. Interleukin‐1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar A, Thota V, Dee L, et al. Tumor necrosis factor α and interleukin 1‐β are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Tassell BW, Raleigh JMV, Abbate A. Targeting interleukin‐1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep. 2015;12:33–41. [DOI] [PubMed] [Google Scholar]
- 6. Van Tassell BW, Seropian IM, Toldo S, et al. Interleukin‐1β induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–640. [DOI] [PubMed] [Google Scholar]
- 7. Toldo S, Mezzaroma E, Bressi E, et al. Interleukin‐1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J Cardiovasc Pharmacol. 2014;64:1–6. [DOI] [PubMed] [Google Scholar]
- 8. Chow SL, O'Barr SA, Peng J, et al. Renal function and neurohormonal changes following intravenous infusions of nitroglycerin versus nesiritide in patients with acute decompensated heart failure. J Card Fail. 2011;17:181–187. [DOI] [PubMed] [Google Scholar]
- 9. Deswal A, Petersen NJ, Feldman AM, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 10. Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. [DOI] [PubMed] [Google Scholar]
- 11. Shah SJ, Marcus GM, Gerber IL, et al. High‐sensitivity C‐reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12:61–65. [DOI] [PubMed] [Google Scholar]
- 12. Canada JM, Fronk DT, Cei LF, et al. Usefulness of C‐reactive protein plasma levels to predict exercise intolerance in patients with chronic systolic heart failure. Am J Cardiol. 2015;117:116–120. [DOI] [PubMed] [Google Scholar]
- 13. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mann DL. Targeted anticytokine therapy and the failing heart. Am J Cardiol. 2005;95:9C–16C. [DOI] [PubMed] [Google Scholar]
- 15. Van Tassell BW, Arena R, Biondi‐Zoccai G, et al. Effects of interleukin‐1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D‐HART Pilot Study). Am J Cardiol. 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1892. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. AbouEzzeddine OF, Haines P, Stevens S, et al. Galectin‐3 in heart failure with preserved ejection fraction A RELAX trial substudy. JACC Heart Fail. 2015;3:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol . 2002;40:1531–1540. [DOI] [PubMed] [Google Scholar]
- 21. Balady GJ, Arena R, Sietsema K, et al. Clinician's guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 22. Arena R, Humphrey R, Peberdy MA, et al. Predicting peak oxygen consumption during a conservative ramping protocol: implications for the heart failure population. J Cardiopulm Rehabil. 2003;23:183–189. [DOI] [PubMed] [Google Scholar]
- 23. Rector T. Minnesota Living with Heart Failure Questionnaire. http://license.umn.edu/technologies/94019_minnesota‐living‐with‐heart‐failure‐questionnaire. Accessed December 11, 2015.
- 24. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self‐administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 25. Schwartzenberg S, Redfield MM, From AM, et al. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. [DOI] [PubMed] [Google Scholar]
- 26. Bensimhon DR, Leifer ES, Ellis SJ, et al; HF‐ACTION Trial Investigators. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training). Am J Cardiol . 2008;102:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redfield MM, Chen HH, Borlaug BA, et al; RELAX Trial. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA . 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Edelmann F, Wachter R, Schmidt AG, et al; Aldo‐DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 29. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cahalin LP, Chase P, Arena R, et al. A meta‐analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart Fail Rev. 2013;18:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arena R, Myers J, Aslam SS, et al. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. 2004;147:354–360. [DOI] [PubMed] [Google Scholar]
- 32. Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 33. Barron A, Dhutia N, Mayet J, et al. Test‐retest repeatability of cardiopulmonary exercise test variables in patients with cardiac or respiratory disease. Eur J Prev Cardiol. 2014;21:445–453. [DOI] [PubMed] [Google Scholar]
- 34. Scott JM, Haykowsky MJ, Eggebeen J, et al. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:1809–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Skinner JS, Wilmore KM, Jaskolska A, et al. Reproducibility of maximal exercise test data in the HERITAGE family study. Med Sci Sports Exerc. 1999;31:1623–1628. [DOI] [PubMed] [Google Scholar]
- 36. Russell SD, McNeer FR, Beere PA, et al. Improvement in the mechanical efficiency of walking: an explanation for the “placebo effect” seen during repeated exercise testing of patients with heart failure. Duke University Clinical Cardiology Studies (DUCCS) Exercise Group. Am Heart J . 1998;135:107–114. [DOI] [PubMed] [Google Scholar]
- 37. Van Laethem C, De Sutter J, Peersman W, et al. Intratest reliability and test‐retest reproducibility of the oxygen uptake efficiency slope in healthy participants. Eur J Cardiovasc Prev Rehabil. 2009;16:493–498. [DOI] [PubMed] [Google Scholar]
- 38. Wang M, Yip GW, Wang AY, et al. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–826. [DOI] [PubMed] [Google Scholar]
- 39. Borlaug BA, Kass DA. Ventricular‐vascular interaction in heart failure. Heart Fail Clin. 2008;4:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carbone S, Lavie CJ, Arena RA. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Appendix
Figure S1. Role of Inflammation in Heart Failure with Preserved Ejection Fraction.
Figure S2. Comparison of Standard Deviation Relative to Effect Size across Select Studies of Heart Failure with Preserved Ejection Fraction


