Abstract
Over the past few years, our understanding of estrogen signaling in the brain has expanded rapidly. Estrogens are synthesized in the periphery and in the brain, acting on multiple receptors to regulate gene transcription, neural function, and behavior. Various estrogen-sensitive signaling pathways often work in concert within the same cell, increasing the complexity of the system. In females, estrogen concentrations fluctuate over the estrous/menstrual cycle, dynamically modulating estrogen receptor expression, activity and trafficking. These dynamic changes influence multiple behaviors, but are particularly important for reproduction. Using the female rodent model, we review our current understanding of estradiol signaling in the regulation of sexual receptivity.
Keywords: ERα, GPER, caveolin, lordosis behavior, estrogen feedback, mGluR
Estradiol actions in the brain: historical context
The actions of estradiol on brain function have been studied for decades. Principally synthesized in the gonads, estradiol was initially characterized as binding to a single intracellular estrogen receptor (ER), now termed ERα, and regulating gene expression through binding to estrogen response elements (EREs) located in the promoter regions of specific genes [1]. There is excellent accord regarding the distribution of ERα to specific subpopulations of neurons known to play critical roles in sexual maturation and sexual receptivity. For example, estradiol activation of ERα in regions such as the rodent ventromedial hypothalamus (VMH), medial preoptic area and central gray region of the midbrain are critical for the display of lordosis (see Glossary) [2]. Hence, it was once believed that the story of estrogen action in brain was both simple and straightforward. We now know this is not the case.
Approximately ten years after the identification of ERα, a second estrogen receptor, ERβ was cloned. Through direct transcriptional regulation, ERα and ERβ can have either complementary or opposing actions, and can influence gene expression independent of EREs or estradiol [1, 3, 4]. Even with all this complexity, activation of intracellular ERs is only one of the major mechanisms of estradiol action. Estradiol mediates a variety of other responses, many of which are initiated at the membrane surface, across neuronal and non-neuronal tissue [5]. Within the nervous system, rapid estradiol action was first demonstrated in preoptic/septal neurons, where changes in electrophysiological properties were observed within seconds of estradiol exposure [6]. For years, the identity of membrane-localized ERs was unclear, but these actions of estradiol appeared to require the activation of G protein-coupled receptors (GPCRs) [7]. Due to the multiple amplification steps associated with activation GPCRs, the relative expression of membrane-localized estrogen receptors required for physiological impact is low, which made their identification difficult.
The first indication that classical ERs mediate membrane-initiated estrogen signaling was an experiment that found overexpressed ERα and ERβ trafficked to the membrane and activated cell signaling [8]. This was followed by ER knockout experiments indicating that rapid estrogen signaling was dependent on ERα and/or ERβ [9]. Within the nervous system, membrane-localized ERα and ERβ were then found to functionally couple to group I and II metabotropic glutamate receptors (mGluRs), initiating mGluR signaling upon estradiol stimulation, independent of glutamate [10]. This provided an explanation as to how estradiol was able to affect a wide array of signaling pathways, although the mechanism underlying the functional pairing of ERs with mGluRs remained a mystery. This review will outline the mechanisms by which membrane ERs (mERs) are able to signal at the neuronal and glial membrane surface through mGluRs, and how this and other estrogen-sensitive signaling pathways coordinate female receptivity.
Estrogen Receptor Signaling through metabotropic glutamate receptors (mGluRs)
Caveolin proteins mediate ER and mGluRs interactions
The initial finding that ERs can be separately coupled to different types of mGluRs [10] led to additional studies to determine the mechanisms by which discrete estrogen-responsive signaling pathways were coupled. Given the degree of fine spatial tuning, caveolin proteins (Cav1-3) were candidates for an intermediary protein that allowed ERs and mGluRs to interact functionally in a spatially localized manner. Caveolins (Cavs) are small integral membrane proteins that organize signaling molecules into functional microdomains [11–13]. In non-neural tissue, they form oligomers (i.e., caveolae) that produce invaginations in the plasma membrane. Caveolae had not been observed in the brain, which led to initial reports that Cav expression in the nervous system was limited to endothelial and glial cells [14].
At the time when ERs were found to couple to mGluRs, a single report indicated that Cav1 was, in fact, expressed in neurons [15]. This led to the discovery that all three Cavs are expressed in neurons [16]. Furthermore, it was determined that Cav1 and Cav3 were responsible for generating distinct signaling complexes within individual neurons, thereby isolating estrogen activation of group I from group II mGluR signaling [16] (Fig 1). In addition, Cavs facilitate trafficking of ERs to the plasma membrane, as has been shown for a number of surface signaling proteins [17–19]. The disruption of Cav1 expression decreases membrane-localized ERα [20].
Figure 1. Schema of classical nuclear receptor signaling from the plasma membrane.
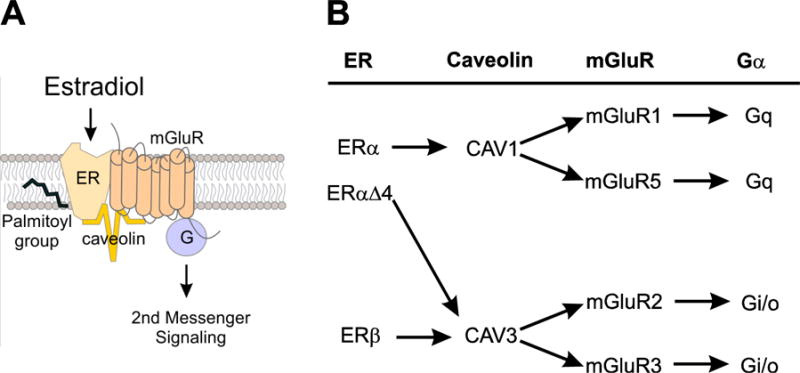
(A). Across the nervous system, ERα and ERβ have been found to functionally couple to group I and II mGluRs. Surface trafficking requires palmitoylation of the estrogen receptor. Activation of mGluR signaling by the ER also requires interaction with palmitoylated caveolin proteins. (B) Caveolins determine the association of ERs with mGluRs, which allow estradiol to be either excitatory, by interacting with mGluR1a/5, or inhibitory through mGluR2/3. The ERαΔ4 splice variant is associated with mGluR2 through their interaction with Cav3 produces inhibition. Figure modified from [16]; data from [114].
Palmitoylation regulates ER trafficking to the plasma membrane and ER signaling
ERα and ERβ can mediate both direct nuclear- and membrane-initiated estradiol signaling. Post-translational modifications appear to determine whether ERα and ERβ are targeted to the plasma membrane or the nucleus. There are several forms of palmitoylation. ERs are regulated by S-Palmitoylation, which is a reversible lipid modification, shown to control transient membrane tethering of otherwise cytosolic proteins [21–23].
Proteins belonging to the palmitoyl acyltransferase (PAT) family of enzymes are responsible palmitoylation of target proteins, usually via a thiol-ester bond at cysteine residues [24]. Palmitate attachment increases the lipophilicity/hydrophobicity of the protein, facilitating association with lipid membranes and lipophilic proteins (Fig 1). In addition to serving as a lipophilic anchor, palmitate may also signal to cellular trafficking mechanisms [25]. To date, there are 23 PAT members of the DHHC family of enzymes [26].
Two DHHC enzymes, DHHC7 and DHHC21, palmitoylate and promote surface trafficking of ERα [27]. Interestingly, both ERα and ERβ (as well as other steroid hormone receptors) contain conserved palmitoylation sequences that appear regulated by the same two DHHC enzymes [28]. More recent studies verified, within neurons, that membrane-initiated signaling by ERα and ERβ are dependent on DHHC7 and DHHC21 [29]. Furthermore, mutation of the ER palmitoylation site eliminates membrane, but not nuclear function of the receptor [29–31].
With only one palmitoylation site on each ER, it is somewhat surprising that disruption of either DHHC7 or DHHC21 (as opposed to simultaneous knockdown) results in a loss of mER signaling. The cause for this result is currently unclear, but there are several possible explanations. First, DHHC7 and DHHC21 may palmitoylate steroid hormone receptors as a heterodimer [26]. Second, DHHC7 and DHHC21 may act independently, but sequentially. A third possibility is that either DHHC7 or DHHC21 directly palmitoylates the steroid hormone receptor, while the other DHHC enzyme palmitoylates a required accessory protein. Notably, Cav proteins are regulated through palmitoylation [9, 32, 33].
Membrane estrogen receptor signaling dynamics
Interestingly, trafficking of ERα to the surface membrane is itself highly regulated by estradiol (Fig 2). Estradiol first promotes ERα trafficking to the membrane, then reduces the levels through receptor internalization [34]. Mechanistically, activated ERα is removed from the cell membrane through a mechanism involving phosphorylation by G protein-coupled receptor kinase 2 (GRK2) and recruitment of β-arrestin-1 (Arrb1), leading to internalization of the receptor complex [35]. Arrb1 links ERα to the AP-2 adaptor complex assisting clathrin-mediated endocytosis [36, 37]. Internalization is important for the immediate reduction of receptors on the cell surface that curtails signaling [34]. Internalized receptors release their ligands in early lysosomes, and the ligand-free ERα can be recycled back to the cell membrane and restimulated. In this manner, recycling restores cellular responsiveness to estradiol. Upon prolonged stimulation, internalized receptors can be sorted to lysosomes where they are proteolytically degraded leading to a down-regulation of receptors and an extended attenuation of cellular responsiveness to estradiol. In in vitro preparations, this decrease in membrane ERα signaling occurs within two hours of estradiol stimulation [34, 38].
Figure 2. Proposed mechanism of β-arrestin1 (Arrb1) in internalization and signaling of membrane ERα (mERα).
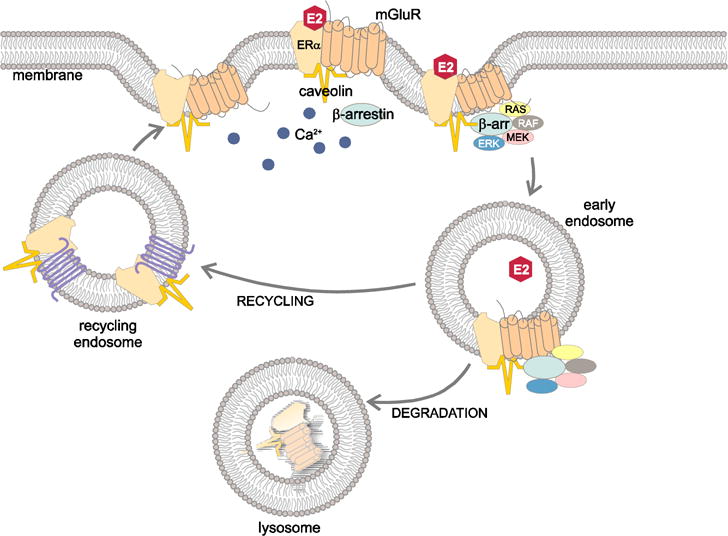
Membrane ERα is part of a G-protein coupled receptor complex, which includes mGluR1a and caveolin (Fig 1). Following estradiol (E2) activation of mERα, Arrb1 is recruited to this receptor complex where it organizes Raf/MEK/ERK signaling and the endocytic machinery needed to internalize mERα into endosomes. In the absence of Arrb1, mERα internalization and ERK1/2 (MAPK) signaling are blocked. Eventually, the internalized mERα-mGluR1a loses Arrb1 and signaling ceases. The receptor complex is either recycled and trafficked to the cell surface, or sorted to lysosomes for degradation. Modified from [39] and [115].
Experiments in immortalized hypothalamic neurons suggest that Arrb1 is also directly involved in membrane-initiated estradiol signaling [39], possibly as a scaffold protein to recruit and organize downstream signaling molecules (e.g., Ras/Raf/MEK) at the cell membrane [40, 41]. Moreover, Arrb1 has been implicated as a key player through which endosomal signaling extends cellular responsiveness (reviewed in [42, 43]). Our results suggest Arrb1 functions in this way mediating estradiol signaling in hypothalamic cells. Specifically, estradiol-induced ERK1/2 phosphorylation and internalization both depend on Arrb1, and membrane-initiated estradiol signaling persists as long as Arrb1 remains associated with the receptor including after sequestration into endosomes [39, 44].
In addition to estradiol and Cav1, PKC regulate ERα trafficking to the membrane, which is necessary for ERα-dependent lordosis behavior [45, 46]. Additionally, disruption of Arrb1 expression in the arcuate nucleus of the hypothalamus (ARH) eliminates lordosis behavior. Furthermore, these results provide a mechanism that underlies activation of female sexual receptivity by estradiol-only treatment. A large dose of estradiol benzoate (EB) alone induces lordosis within 48 hours, while more physiological doses do not (e.g., [47]). This effect appears to be due to low doses of estradiol prolonging Arrb1-mediated activation of membrane-initiated estradiol signaling, which extends inhibition of lordosis. Progesterone relieves the opioid inhibition thereby facilitating lordosis [48].
When studying the dynamics of ERα in vitro using both primary cultures of neurons and astrocytes, we and others identified a splice variant of the ESR1 gene encoding ERα that is missing exon 4 (ERαΔ4) [34, 38, 46, 49]. Interestingly, ERαΔ4 is the more prevalent ERα variant in membranes obtained from cultured or immortalized neurons and astrocytes. In contrast, in membranes obtained directly from the brain, levels of full length ERα are greater than ERαΔ4 levels [20]. At this point, we do not understand the conditions that shift the ERαΔ4:ERα ratio in vitro compared with in vivo tissue. ERαΔ4 is missing exon 4, resulting in an in-frame deletion, giving rise to a truncated ERα protein [50–52]. This alternatively spliced ERα lacks the nuclear translocalization sequence, and after translation ERαΔ4 is not transported to the nucleus and builds-up in the cytoplasm, increasing trafficking to the cell membrane ([53]; but see [54]). Functionally, ERαΔ4 does not stimulate transcription ([55], but see [54]); it does inhibit though ERα-mediated transcription [56]. Some researchers posit that ERαΔ4 does not bind estradiol or interact with the ERE [57]. However, estradiol treatment induces internalization of membrane ERαΔ4 in cultured astrocytes and neurons – actions associated with ligand bound receptors. In vivo knockdown of Cav1 did not prevent ERαΔ4 trafficking to the membrane [20], but recent experiments indicate that membrane levels of ERαΔ4 require Cav3, the Cav isoform implicated in functional coupling of ERα and ERβ with group II mGluRs [16]. Consistent with this hypothesis, ERαΔ4 co-immunoprecipitates with mGluR2, and thus, may mediate inhibitory estradiol actions.
Membrane estrogen receptors regulating sexual receptivity
Estrogen actions on the neural circuitry controlling sexual receptivity
A classic example of estrogen action in the female brain is the induction of lordosis. Sexually receptive female rodents, when mounted by a male, respond with the stereotypic arching of the back that allows copulation to occur. Over the years, studies have clarified much of the neurocircuitry required for this behavior [2]. Display of lordosis requires the precise timing of ERα activation within the circuit. We now know that both nuclear and membrane-initiated mechanisms are needed to induce sexual receptivity. For example, nuclear ER signaling that induces protein synthesis is necessary [58], as are rapid membrane signaling pathways [45, 59, 60]. In terms of nuclear receptor signaling, ERα, but not ERβ, is essential for facilitation of lordosis [61, 62]. Under certain conditions, the G protein-coupled estrogen receptor (GPER) also has an essential role in facilitation of lordosis by estradiol [60, 63]. To further complicate matters, there is an overlap in the actions of ERα and another ER, the Gq-mER, facilitating lordosis [64].
Models for understanding steroid activation of sexual receptivity
Studies of steroid treatments in ovariectomized (ovx) rodents demonstrate several principles required for the induction of lordosis: 1) if used alone, more estradiol is needed than if estradiol priming is followed by progesterone (reviewed in [47, 65]); 2) a single dose of estradiol produces a delayed onset of sexual receptivity, which lasts longer compared with lordosis induced by estradiol + progesterone (reviewed in [65, 66]); 3) maximal sexual receptivity can eventually be achieved by repeated lower doses of estradiol whereas, estradiol + progesterone treatments produce consistently high levels of sexual receptivity (reviewed in [67]); 4) treating an ovx rat with a priming dose of EB followed by a dose of a nonesterified estradiol facilitates lordosis without progesterone [60, 63, 68].
A commonality for all these paradigms is that for lordosis to occur, requires an extended estradiol exposure is needed (20 to 48 hours). During this time, estradiol activates inhibitory neuropathways to prevent copulation from occurring before ovulation. This interval allows for protein expression in the lordosis circuit neurons mediated by a combination of nuclear and extranuclear estrogen signaling pathways; and the functional coupling of receptors to intracellular signaling cascades. [69–71]. In ovx rats, estradiol exposure of about 20-24 hours is needed for progesterone or other agents to induce moderate to high levels of sexual receptivity[48, 72, 73]. The quintessential protein upregulated by estradiol is the classical progesterone receptor (PGR) in the VMH, and medial preoptic nucleus (MPN) [74–79].
Although estradiol priming through ERα is sufficient for upregulation of PGR and facilitation of lordosis [62], there is evidence that ERα and ERβ both have a role PGR induction [80]. However, simultaneous ERα and ERβ activation does not replicate estradiol treatment, pointing to the involvement of an additional ER, such as GPER, which induces enough PRG for progesterone for a moderate level lordosis [81]. Thus, multiple ERs appear to underlie progesterone’s facilitation of lordosis.
An interesting aspect of inducing lordosis in ovx rodents is that PGR is not needed for inducing sexual receptivity. Estradiol-only facilitation of lordosis is neither blocked by PGR antagonists nor dependent on classical PGR activity [82, 83]. Significantly, both estradiol-only and estradiol + progesterone facilitation of lordosis modulate ARH β-endorphin (β-end) neurons that project to the MPN [48, 84], but via different pathways [47].
In this light, attention has shifted from slow actions of estradiol (> 24 h) to actions occurring within minutes of estradiol administration [85]. Estradiol signaling rapidly induces neurotransmitter release [45, 86–88] and regulates neurotransmission [89]. Indeed, estradiol (and steroid hormones in general) signaling through membrane receptors resembles GPCR neurotransmitter signaling [90]. Such analyses revealed that the initial action of estradiol, in terms of lordosis, is to engage an inhibitory circuit that involved MPN-projecting β-end neurons [84, 86]. A transient opioid inhibition action is needed for maximal sexual receptivity, but sustained activation of MOR in the MPN inhibits sexual receptivity [59, 84, 91, 92]. Subsequent steroid treatments and pharmacological treatments that reduce estradiol-induced MOR activation facilitate lordosis [9, 47, 48, 60, 63, 93]. These lordosis-facilitating steroid priming paradigms converge on ARH β-end neurons that project to the MPN [84, 94].
The lordosis lordosis-regulating ARH-MPN circuitry
Over the years, examination of the ARH-MPN circuit has provided an excellent opportunity to study steroid mechanisms regulating sexual receptivity (Fig 3; reviewed in [67, 85]). Initially demonstrated in maximally receptive rats, the MPN is an inhibitory node for female sexual receptivity, which is mediated by MOR activation. Stimulation of the MPN inhibits lordosis, whereas MPN lesions facilitate lordosis in rats treated with subthreshold doses of estradiol [95–99]. The MPN acts on downstream lordosis regulatory nodes, including the VMH and ventral tegmental area [99]. Thus, the ARH-MPN MOR system regulates the onset of sexual receptivity by preventing copulation from occurring until all reproductive organs are exposed to the necessary levels and duration of steroid hormones so that sexual activity is coordinated with ovulation, maximizing the chances of fertilization and zygote implantation.
Figure 3. Estradiol induction of sexual receptivity (lordosis behavior) in the female rat.
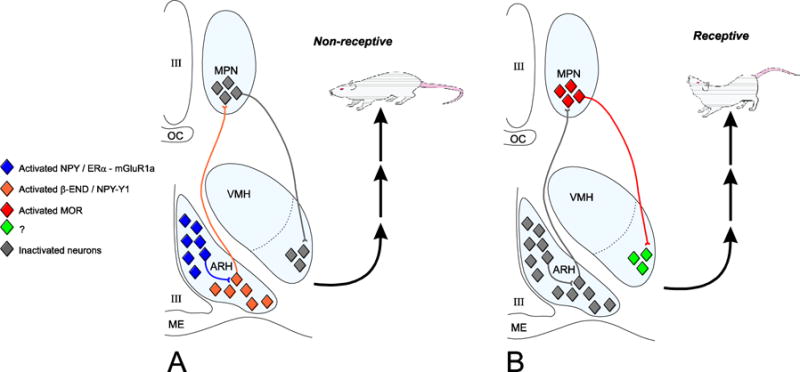
A widespread circuit that extends from the limbic system to the spinal cord underlies the CNS regulation of this global response to hormonal and sensory input. Within this lordosis regulating circuit, E2 acts rapidly through estradiol membrane signaling to release neuropeptide Y (NPY) in the arcuate nucleus of the hypothalamus (ARH) activating β-endorphin (β-END) projection neurons that terminate in the medial preoptic nucleus (MPN). The MPN is an important integrative node receiving accessory olfactory and limbic input. β-END activates MOR, producing a transient inhibition and a “non-receptive” female. This opioid inhibition is overcome by progesterone in the cycling female leading to disinhibition of medial preoptic nucleus (MPN) MOR neurons projecting to the ventromedial nucleus of the hypothalamus (VMH) and activation of hypothalamic outflow producing a sexually “receptive” female. The estradiol membrane signaling requires ERα transactivation of mGluR1a and subsequent phosphorylation of the protein kinase C, PKCθ. Both the transient inhibition and activation of VMH are necessary for the full expression of lordosis behavior in the rodent. Modified from [115].
In the forebrain, proopiomelanocortin (POMC) neurons are located in the ARH. Of the several post-translational products of POMC, the most important for reproduction is β-end, an endogenous MOR ligand. While β-end neurons project to a number of hypothalamic regions, a population of β-end neurons projects to the MPN activating MORs. In the MPN, MOR activation increases in estradiol-primed rodents, inhibiting sexual receptivity, whereas reducing estradiol-induced MPN MOR activation facilitates lordosis [48, 59, 60, 63, 84, 86, 100, 101]. MPN MOR neurons mediate hormonal regulation of sexual receptivity, through a population of ERα or ORL-1 neurons projecting to the VMH.
Thus far, all steroid paradigms studied regulate the output of ARH β-end neurons in a manner that is congruent with the rat’s sexual behavioral state [9, 47, 48, 59, 60, 84, 90, 93, 101–105]. Importantly, the association of behavior and MPN MOR activity is observed in intact cycling rats [95].
Estradiol rapidly activates the ARH-MPN circuit through of mERα-mGluR1a [38]. Activation of mERα-mGluR1a induces of neuropeptide Y (NPY) release that activates ARH β-end neurons projecting to the MPN [59, 84, 94]. In ARH plasma membrane fractions, ERα co-immunoprecipitates with mGluR1a. This signaling complex is essential for activation of β-end and subsequent facilitation of lordosis [45, 59, 104]. Observations that support this idea include the rapidity of the estradiol-induced activation of MOR in the MPN [86, 106], and that MPN MOR activation by estradiol infusion into the ARH is blocked by pretreatment with an ER antagonist, fulvestrant (ICI 182,780) or an mGluR1a antagonist indicating that membrane-initiated estradiol signaling involving mERα and mGluR1a mediated the rapid actions [59]. Blocking ERα trafficking to the membrane by disrupting Cav-1 expression prevents lordosis [20]. Finally, preventing mERα-mGluR1a signaling in the ARH with a PKC antagonist also prevents MOR internalization and lordosis behavior [45]. Concurrent ARH infusion of mGluR1a agonists with estradiol priming that does not produce receptivity on its own (2 µg EB), facilitates lordosis and reduces MPN MOR activation in a manner similar to a single, high dose of estradiol [59]. In summary, in this behavioral circuit estradiol activates both mERα signaling and direct ERα transcriptional events that are important for facilitating lordosis. However, at this point, the proportion of membrane to nuclear signaling vs. direct (nuclear) signaling is not known.
Interestingly, various doses of estradiol regulate mERα signaling by regulating its levels on the membrane [107]. Rats given a low dose of EB continue to have mERα-mGluR1a in the ARH. In contrast, a high dose of EB, reduces mERα-mGluR1a levels. Thus, a priming dose, which does not induce lordosis, maintains the mERα-mGluR1a signaling complex that maintains opioid inhibition. Significantly, 48 hours after a 50 μg EB dose, when the female is sexually receptive, mERα-mGluR1a levels are reduced, and so is β-end neurotransmission. As in vitro, estradiol modulates membrane-initiated signaling by regulating levels of mERα [38].
In addition to modulation of ER signaling, waning estradiol levels induce OFQ/N release (Fig 4) that further reduces β-end neurotransmission, and MPN MOR activation, allowing lordosis behavior [47]. Deactivation of the ARH-MPN pathway and facilitation of lordosis by estradiol-only treatments require activation of ORL-1 on β-end neurons. The mechanism for this appears to be through GPER. Thus, multiple ERs appear to regulate lordosis-facilitating pathways.
Figure 4. Model of early and late actions of estradiol (E2) regulating arcuate (ARH) to medial preoptic nucleus (MPN) lordosis circuit.
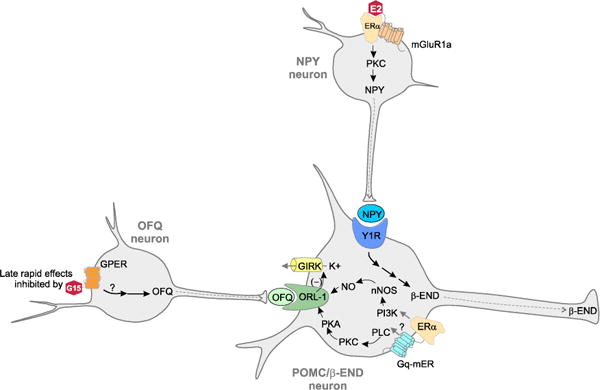
E2 rapidly initiates signaling through membrane associated ERα that complexes with and signals through metabotropic glutamate receptor 1a (mGluR1a). This activates a PKC pathway that stimulates the release of neuropeptide Y (NPY), which binds to the NPY-Y1 receptor (Y1R) on β-END neurons that project to the medial preoptic nucleus (MPN). NPY increases β-END transmission to inhibit lordosis through MOR activation. Simultaneously, E2 acts on membrane associated ERα and STX responsive Gq-mER signaling through PLC/PKC/PKA and PI3K/nNOS/NO pathways to decouple the opioid receptor-like receptor-1 (ORL-1). This reduces inhibitory K+ currents and increase β-END transmission to the MPN MOR to inhibiting lordosis. Later actions of E2 that rapidly facilitate lordosis activate of G protein-coupled ER (GPER) located in orphanin FQ (OFQ/N) neurons. OFQ/N binds to its receptor, ORL-1, on MPN projecting β-END neurons. Activation of ORL-1 increases potassium (K+) currents through G protein-coupled inwardly-rectifying potassium (GIRK) channels that inhibit β-END transmission, allowing lordosis to proceed. Modified from [67, 89, 94].
ER signaling-regulated synaptic responses
Steroid priming regulates the coupling of ORL-1 to the GIRK-1 channel in β-end neurons in a manner that is associated with the animal’s behavioral state (Fig 4). In ovx rats, OFQ/N-induced robust GIRK-1 currents [102], but, a dose of estradiol that does not induce receptivity reduces OFQ/N-induced GIRK-1 currents effectively increasing β-end neuron excitation. On the other hand, steroid treatments that induce sexual receptivity (estradiol, STX and PPT, an ERα agonist) have robust GIRK-1 currents, inhibiting β-end neurotransmission [108]. The decoupling of ORL-1 from GIRK-1 is mediated by activation of PLC/PKC/PKA and the phosphatidylinositol-3-kinase (PI3K)/neuronal nitric oxide synthase (nNOS) pathways [89; Fig 4].
Morphological plasticity and sexual receptivity
There is an interesting discrepancy between mice that are missing MOR (MOR-KO) and the blockade of mGluR1a in the ARH. MOR-KO mice have a ~20% decrease in lordosis quotient compared with wild type controls. In contrast, pharmacological blockade of mGluR1a virtually abolishes sexually receptive behavior [59]. Thus, membrane-initiated estradiol signaling affects something in the ARH besides the β-end neuron. Estradiol-induced morphological plasticity was an obvious choice based on demonstrated changes in the ARH, VMH and hippocampus [109–111]. Indeed, estradiol rapidly induces spinogenesis that is dependent on mERα-mGluR1a signaling [104]. Fulvestrant, or the mGluR1a antagonist LY 367,385 prevents spinogenesis. This estradiol membrane-initiated signaling rapidly induces phosphorylation of the actin severing protein, cofilin. Phosphorylated cofilin is inactive, which allows for the formation of new dendritic spines. Significantly, blocking spinogenesis in the ARH blocks estradiol induced lordosis [104]. The formation of new spines is rapid and spine numbers remain stable for 48 hours. During this time, there is a shift in spine morphology with numbers of mushroom-shaped spines significantly increasing. This suggests that a portion of the newly formed spines mature over these days and take on a morphology indicative of functional synapses. The time course of the increase in the number of mushroom-shaped spines coincides with the appearance of lordosis behavior.
While spines are rapidly formed, they are labile. An additional stimulus appears to be necessary to stabilize them. In the cortex, estradiol paired with a long-term potentiation (LTP) protocol results in a sustained increase in connectivity ([112]). Formation of a functional synapse would accomplish the same thing in vivo. Estradiol increased the expression of postsynaptic density protein-95 (PSD-95) in the ARH, but pretreatment with fulvestrant prevented the increase. Similarly, the axonal growth associated protein, GAP43, was upregulated by estradiol treatment and blocked by antagonism of ERα [90]. The estradiol induction of PSD-95 and GAP43 were blockade by antagonism of mGluR1a. Thus, acting in the ARH, estradiol increases behaviorally relevant synapses.
Concluding Remarks
Reproductive neuroendocrinologists have known for many years that in females, steroid actions exist in the context of a complex dance with time and concentration. Unexpectedly, it turned out that the same receptors that mediated direct nuclear action also mediated membrane-initiated cell signaling. ERα and ERβ are chaperoned to the membrane by Cav proteins that mediate the association of the ERs with specific mGluRs, whose transduction is the mechanism through which these nuclear receptors signal at the membrane. This places estradiol membrane-initiated signaling in the realm of GPCRs. The mERs behave like typical membrane receptors: cycled into and out of the membrane in response to the presence of estradiol, their native ligand. The discovery of membrane estrogen receptors, forced a reevaluation of the traditional understanding of steroid actions both at the cellular and the circuit level. If membrane-initiated estradiol signaling occurs on a time scale of seconds to minutes rather than hours to days, it was necessary to examine activation of lordosis-regulating circuits in that same time frame. It was discovered that the initial action of estradiol induces an inhibition of lordosis that is centered in the MPN. While the source of that inhibition was not known, electrophysiological recordings revealed an estradiol-induced inhibition of lordosis behavior [113]. Later, the inhibition was understood to be through the activation of β-end neurons acting on MPN MOR. Examination of the other time points between estradiol administration and lordosis demonstrated that other ERs also contributed to the regulation of behavior. The GPER activates lordosis, hours after the initial estradiol treatment [60]. This indicates that several sequential membrane-initiated actions underlie the estradiol-induction of lordosis behavior, in addition to direct nuclear actions. At this point, we do not know the proportion of estradiol membrane-initiated signaling compared with direct nuclear action that underlies circuit activation (see Outstanding Questions). It is likely that what has been assumed to be direct nuclear action may turn out to be the result of membrane to nucleus signaling and the activation of CREB. Indeed, there are hints that this may be the case. Lordosis behavior is dependent on the formation of new spines in the ARH [104] induced by initial estradiol actions through ERα-mGluR1a signaling. The later stabilization and spine maturation is associated with the expression of PSD-95 and GAP43, which themselves are regulated by the same mERα action [90].
While this review has focused mostly on ERα, both ERβ, and GPER have roles in sexual receptivity. Research into ERβ has to a large extent stalled in recent years due to a lack of workable antibodies. While questions remain about the cell surface or smooth endoplasmic reticulum localization of GPER, experiments continue to demonstrate a role of GPER in the brain. Despite this, the contribution of these more recently discovered ERs, their defined roles in reproductive neuroendocrinology are not well established. ERα knockdown experiments have been very clear – no ERα, no reproduction. A large question is how ERβ, GPER, and the STX-stimulated Gq-mER interact with ERα signaling. Preliminary experiments suggest that these interactions may not be simple, and will require attention to estradiol’s concentration and timing. The past few decades teach us that estrogen signaling in the brain requires a number of membrane, cytoplasmic and nuclear receptors, all of which play a role in reproduction.
TRENDS.
-
*
Membrane-initiated signaling is mediated by classic ERα and ERβ trafficked to the membrane and through novel extra-nuclear receptors such as GPER and Gq-mER
-
*
ERα and ERβ trafficking to the membrane requires palmitoylation and caveolin proteins
-
*
Caveolin proteins determine the mGluR associated with ERα establishing whether estradiol action are stimulatory (mGluR1a) or inhibitory (mGlur2/3)
-
*
Estradiol control of sexual receptivity requires activation of several types of ERs, which are involved in cell signaling in transcriptional regulation
-
*
Control of sexual receptivity requires estradiol actions at the membrane, and involve several different both ERα and GPER
-
*
Spinogenesis in the ARH is critical for sexual receptivity and is mediated by ERα-mGluR1a signaling
Outstanding Questions.
-
*
What is the role of GPER and/or Gq-mERs in reproduction given that ERαKO animals are reproductively incompetent?
-
*
Does membrane initiated steroid signaling regulate sexual differentiation and development?
-
*
How do membrane ER-initiated signaling pathways and nuclear ER signaling interact to regulate cellular activity within a given cell?
-
*
How are dose and duration of estradiol exposure “measured” in the brain?
-
*
In females, estrogen and progestin signaling are dynamically related and regulated; how does each modulate the other during various reproductive states?
-
*
How do membrane-initiated estrogen, and membrane-initiated progesterone signaling interact to modify neurotransmitter actions?
-
*
What is the physiological role of the ERα splice variant, ERαΔ4?
Acknowledgments
The authors gratefully acknowledge the editorial support of Dr. Melinda Mittelman-Smith, Dr. Lauren Rudolph, and Katherine Tonn. Experiments from the authors’ laboratories were supported by grants: DA013185 & HD042635 to PEM; DA035008 & DA041808 to PGM; and HD058638 to KS.
GLOSSARY
- Caveolin proteins
Family of integral membrane proteins that act as scaffolding protein that compartmentalize and concentrate signaling proteins. There are 3 members: Cav1, Cav2 and Cav3. They appear to act as chaperons for nuclear steroid hormone receptors, especially ERα and ERβ
- Estrous cycle
In rodents, the ovarian cycle is composed of 4 stages, Diestrus I, Disestrus II, Proestrus, and Estrus. In Diestrus, developing ovarian follicles produce primarily estrogen which feeds back onto the hypothalamus to inhibit the release of GnRH, and onto the pituitary to inhibit FSH and LH release. On the afternoon of proestrus, the rising levels of estrogen stimulate synthesis of progesterone in the hypothalamus and together these steroids stimulate the surge release of GnRH leading to the release of LH and ovulation of an ovum from the ovary. On estrus, release of progesterone from the corpus luteum inhibits the hypothalamus, resulting in low levels of GnRH, as well as LH and FSH from the pituitary leading to low circulating levels of estrogen and progesterone
- Lordosis reflex
The naturally occurring body posture for sexual receptivity/copulation present in most mammals including rodents. Primary characteristics include raising of the hips, ventral arching of the back, lateral deviation of the tail, which present the vagina to the male and allows for intromission
- Palmitoylation
The covalent attachment of fatty acids to proteins, which increases their hydrophobicity. Palmitoylation contributes to the trafficking of proteins between intracellular compartments and to the cell membrane. Finally, palmitoylation modulates protein-protein interactions, such as between estrogen receptors and metabotropic glutamate receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heldring N, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 2.Pfaff DW, et al. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press, Ltd; 1994. pp. 107–220. [Google Scholar]
- 3.Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29(14):2905–19. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marino M, et al. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7(8):497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 2016;17(12):783–797. doi: 10.1038/nrm.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly MJ, et al. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114(1):152–7. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 7.Mermelstein P, et al. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. Journal of Neuroscience. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razandi M, et al. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13(2):307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 9.Abraham IM, et al. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145(7):3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 10.Boulware MI, et al. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(20):5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel HH, et al. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francesconi A, et al. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29(11):3590–602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayasu Y, et al. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc Natl Acad Sci U S A. 2010;107(50):21778–83. doi: 10.1073/pnas.1015553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikezu T, et al. Affinity-purification and characterization of caveolins from the brain: differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998;804(2):177–92. doi: 10.1016/s0006-8993(98)00498-3. [DOI] [PubMed] [Google Scholar]
- 15.Braun JE, Madison DV. A novel SNAP25-caveolin complex correlates with the onset of persistent synaptic potentiation. J Neurosci. 2000;20(16):5997–6006. doi: 10.1523/JNEUROSCI.20-16-05997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulware MI, et al. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. Journal of Neuroscience. 2007;27(37):9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong MM, et al. Regulation of D1 dopamine receptor trafficking and signaling by caveolin-1. Mol Pharmacol. 2007;72(5):1157–70. doi: 10.1124/mol.107.034769. [DOI] [PubMed] [Google Scholar]
- 18.Shmuel M, et al. Caveolin 2 regulates endocytosis and trafficking of the M1 muscarinic receptor in MDCK epithelial cells. Mol Biol Cell. 2007;18(5):1570–85. doi: 10.1091/mbc.E06-07-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stern CM, Mermelstein PG. Caveolin regulation of neuronal intracellular signaling. Cell Mol Life Sci. 2010;67(22):3785–95. doi: 10.1007/s00018-010-0447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012;153(8):3872–7. doi: 10.1210/en.2012-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wedegaertner PB, et al. Palmitoylation is required for signaling functions and membrane attachment of Gq alpha and Gs alpha. J Biol Chem. 1993;268(33):25001–8. [PubMed] [Google Scholar]
- 22.Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20(1):125–34. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 23.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 24.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11(3):161–75. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 25.Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J Cell Biol. 2007;176(3):249–54. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukata M, et al. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44(6):987–96. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Pedram A, et al. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Molecular biology of the cell. 2012;23(1):188–99. doi: 10.1091/mbc.E11-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 29.Meitzen J, et al. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013;154(11):4293–304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedram A, et al. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Dev Cell. 2014;29(4):482–90. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adlanmerini M, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111(2):E283–90. doi: 10.1073/pnas.1322057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietzen DJ, et al. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270(12):6838–42. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- 33.Parat MO, Fox PL. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J Biol Chem. 2001;276(19):15776–82. doi: 10.1074/jbc.M006722200. [DOI] [PubMed] [Google Scholar]
- 34.Bondar G, et al. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29(48):15323–30. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez R, et al. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29(13):4228–38. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, et al. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272(43):27005–14. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 37.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–50. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. The Journal of Neuroscience. 2010;30(38):12589–96. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong AM, et al. beta-arrestin regulates estradiol membrane-initiated signaling in hypothalamic neurons. PLoS One. 2015;10(3):e0120530. doi: 10.1371/journal.pone.0120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 41.Ge L, et al. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278(36):34418–26. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 42.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3(8):600–14. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 43.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19(4):436–45. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feinstein TN, et al. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7(5):278–84. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dewing P, et al. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149(12):5934–42. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dominguez R, et al. Membrane-initiated estradiol signaling in immortalized hypothalamic N-38 neurons. Steroids. 2013;78(6):607–13. doi: 10.1016/j.steroids.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanathara NM, et al. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic nucleus mu-opioid receptors. Hormones and Behavior. 2011;60(5):540–8. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of m-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21(15):5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorosito SV, et al. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154(4):1173–7. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Pfeffer U, et al. Estrogen receptor variant messenger RNA lacking exon 4 in estrogen-responsive human breast cancer cell lines. Cancer research. 1993;53(4):741–3. [PubMed] [Google Scholar]
- 51.Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, Crews D. Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proceedings of the National Academy of Sciences, USA. 1993 Aug;90:7172–7175. doi: 10.1073/pnas.90.15.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koehorst SG, et al. Wild type and alternatively spliced estrogen receptor messenger RNA in human meningioma tissue and MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 1993;45(4):227–33. doi: 10.1016/0960-0760(93)90336-u. [DOI] [PubMed] [Google Scholar]
- 53.Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Molecular endocrinology. 2000;14(5):634–49. doi: 10.1210/mend.14.5.0460. [DOI] [PubMed] [Google Scholar]
- 54.Pasqualini C, et al. Differential subcellular distribution and transcriptional activity of sigmaE3, sigmaE4, and sigmaE3-4 isoforms of the rat estrogen receptor-alpha. Molecular endocrinology. 2001;15(6):894–908. doi: 10.1210/mend.15.6.0642. [DOI] [PubMed] [Google Scholar]
- 55.Koehorst SG, et al. Functional analysis of an alternatively spliced estrogen receptor lacking exon 4 isolated from MCF-7 breast cancer cells and meningioma tissue. Mol Cell Endocrinol. 1994;101(1–2):237–45. doi: 10.1016/0303-7207(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 56.Park W, et al. Identification of a variant estrogen receptor lacking exon 4 and its coexpression with wild-type estrogen receptor in ovarian carcinomas. Clin Cancer Res. 1996;2(12):2029–35. [PubMed] [Google Scholar]
- 57.Inoue S, et al. Identification of a novel isoform of estrogen receptor, a potential inhibitor of estrogen action, in vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;219(3):766–72. doi: 10.1006/bbrc.1996.0308. [DOI] [PubMed] [Google Scholar]
- 58.Rainbow T, et al. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 59.Dewing P, et al. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27(35):9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long N, et al. 17beta-estradiol rapidly facilitates lordosis through G protein-coupled estrogen receptor 1 (GPER) via deactivation of medial preoptic nucleus mu-opioid receptors in estradiol primed female rats. Horm Behav. 2014;66(4):663–6. doi: 10.1016/j.yhbeh.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rissman EF, et al. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138(1):507–10. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 62.Mazzucco CA, et al. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behav Brain Res. 2008;191(1):111–7. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Long N, et al. Tamoxifen and ICI 182,780 activate hypothalamic G protein-coupled estrogen receptor 1 to rapidly facilitate lordosis in female rats. Horm Behav. 2017;89:98–103. doi: 10.1016/j.yhbeh.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christensen A, Micevych P. A novel membrane estrogen receptor activated by STX induces female sexual receptivity through an interacation with mGluR1a. Neuroendocrinology. 2013;97(4):363–8. doi: 10.1159/000351077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clemens LG, Weaver DR. The role of gonadal hormone in the activation of feminine sexual behavior. In: Adler N, et al., editors. Handbook of Behavioral Neurobiology. Plenum Press; 1985. pp. 183–227. [Google Scholar]
- 66.Portillo W, et al. Participation of progesterone receptors in facilitation and sequential inhibition of lordosis response induced by ring A-reduced progesterone metabolites in female mice. Behav Neurosci. 2016;130(6):624–634. doi: 10.1037/bne0000167. [DOI] [PubMed] [Google Scholar]
- 67.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Frontiers in neuroendocrinology. 2012;33(4):342–63. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons B, et al. Progesterone-like effects of estradiol on reproductive behavior and hypothalamic progestin receptors in the female rat. Neuroendocrinology. 1984;39(1):25–30. doi: 10.1159/000123950. [DOI] [PubMed] [Google Scholar]
- 69.Kelly MJ, et al. Estrogen suppresses mu-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12(7):2745–50. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang SL, et al. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. Journal of neurophysiology. 2005;94(4):2983–6. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- 71.Quesada A, Micevych P. Estrogen and Progesterone Modulate [S]GTPgammaS Binding to Nociceptin Receptors. Neuroendocrinology Epub. 2008 doi: 10.1159/000XXXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green R, et al. Induction of receptivity in ovariectomized rats by a single intravenous injection of estradiol-17-B. Physiol Behav. 1970;5:137–141. doi: 10.1016/0031-9384(70)90056-9. [DOI] [PubMed] [Google Scholar]
- 73.Quadagno DM, et al. The effect of varying amounts of exogenous estradiol benzoate on estrous behavior in the rat. Horm Behav. 1972;3(3):175–9. doi: 10.1016/0018-506x(72)90029-3. [DOI] [PubMed] [Google Scholar]
- 74.Shughrue PJ, et al. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology. 1997;138(12):5476–84. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz SM, Blaustein JD, Wade GN. Inhibition of estrogen behavior by progesterone in rats: Role of neural estrogen and progestin receptors. Endocrinology. 1979;105(5):1078–1082. doi: 10.1210/endo-105-5-1078. [DOI] [PubMed] [Google Scholar]
- 76.MacLusky NJ, McEwen BS. Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature. 1978;274(5668):276–8. doi: 10.1038/274276a0. [DOI] [PubMed] [Google Scholar]
- 77.Moguilewsky M, Raynaud JP. The relevance of hypothalamic and hypophyseal progestin receptor regulation in the induction and inhibtion of sexual behavior in the female rat. Endocrinol. 1979;105:516–522. doi: 10.1210/endo-105-2-516. [DOI] [PubMed] [Google Scholar]
- 78.Moffatt CA, et al. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. Journal of Neuroscience. 1998;18(22):9556–63. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kraus WL, et al. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994;8(8):952–69. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- 80.Sa SI, et al. Estrogen receptors alpha and beta have different roles in the induction and trafficking of progesterone receptors in hypothalamic ventromedial neurons. FEBS J. 2015;282(6):1126–36. doi: 10.1111/febs.13207. [DOI] [PubMed] [Google Scholar]
- 81.Anchan D, et al. Activation of the GPR30 receptor promotes lordosis in female mice. Neuroendocrinology. 2014;100(1):71–80. doi: 10.1159/000365574. [DOI] [PubMed] [Google Scholar]
- 82.Mani SK, et al. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10(12):1728–1737. doi: 10.1210/mend.10.12.8961281. [published erratum appears in Mol Endocrinol 1997 Apr;11(4):423] [DOI] [PubMed] [Google Scholar]
- 83.Blaustein JD, et al. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45(2):152–9. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- 84.Mills RH, et al. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24(4):947–55. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Micevych P, Sinchak K. Temporal And Concentration Dependent Estradiol Effects On Neural Pathways Mediating Sexual Receptivity. J Neuroendocrinol. 2013;25(11):1012–23. doi: 10.1111/jne.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eckersell CB, et al. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chaban VV, et al. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145(8):3788–95. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 88.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81(1):31–7. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 89.Conde K, et al. Estradiol Rapidly Attenuates ORL-1 Receptor-Mediated Inhibition of Proopiomelanocortin Neurons via Gq-Coupled, Membrane-Initiated Signaling. Neuroendocrinology. 2016;103(6):787–805. doi: 10.1159/000443765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rudolph LM, et al. Actions of Steroids: New Neurotransmitters. J Neurosci. 2016;36(45):11449–11458. doi: 10.1523/JNEUROSCI.2473-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torii M, Kubo K. The effects of intraventricular injection of beta-endorphin on initial estrogen action to induce lordosis behavior. Physiol Behav. 1994;55(1):157–62. doi: 10.1016/0031-9384(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 92.Torii M, et al. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212(1):68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- 93.Sinchak K, et al. Modulation of the arcuate nucleus-medial preoptic nucleus lordosis regulating circuit: A role for GABAB receptors. Hormones and Behavior. 2013;64(1):136–43. doi: 10.1016/j.yhbeh.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanathara NM, et al. Estradiol Upregulates Progesterone Receptor and Orphanin FQ Colocalization in Arcuate Nucleus Neurons and Opioid Receptor-Like Receptor-1 Expression in Proopiomelanocortin Neurons that Project to the Medial Preoptic Nucleus in the Female Rat. Neuroendocrinology. 2014;100:103–118. doi: 10.1159/000363324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Molecular Neurobiology. 2003;27(2):197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 96.Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175(4025):1003–5. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- 97.Moss RL, et al. Electrical stimulation of forebrain structures and its effect on copulatory as well as stimulus-bound behavior in ovariectomized hormone-primed rats. Physiol Behav. 1974;12(6):997–1004. doi: 10.1016/0031-9384(74)90147-4. [DOI] [PubMed] [Google Scholar]
- 98.Nance DM, et al. Modifications in gonadotropin control and reproductive behavior in the female rat by hypothalamic and preoptic lesions. Brain Res Bull. 1977;2(4):307–12. doi: 10.1016/0361-9230(77)90087-9. [DOI] [PubMed] [Google Scholar]
- 99.Takeo T, C Y, Sakuma Y. Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiology and Behavior. 1993;53:831–838. doi: 10.1016/0031-9384(93)90258-h. [DOI] [PubMed] [Google Scholar]
- 100.Micevych PE, et al. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71(6):802–10. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 101.Sinchak K, et al. Sexual receptivity is reduced in the female mu-opioid receptor knockout mouse. Neuroreport. 2005;16(15):1697–700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 102.Borgquist A, et al. Estradiol Negatively Modulates the Pleiotropic Actions of Orphanin FQ/Nociceptin at Proopiomelanocortin Synapses. Neuroendocrinology. 2013;98(1):60–72. doi: 10.1159/000351868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung S, et al. Gonadal steroid hormone-dependence of b-endorphin-like immunoreactivity in the medial preoptic area of the rat. Brain Research. 1995;675:83–88. doi: 10.1016/0006-8993(95)00042-o. [DOI] [PubMed] [Google Scholar]
- 104.Christensen A, et al. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(48):17583–9. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sinchak K, et al. Orphanin FQ-ORL-1 regulation of reproduction and reproductive behavior in the female. Vitam Horm. 2015;97:187–221. doi: 10.1016/bs.vh.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Sirinathsinghji DJ, et al. Regulation of mating behaviour in the female rat by gonadotropin-releasing hormone in the ventral tegmental area: effects of selective destruction of the A10 dopamine neurones. Brain Res. 1986;374(1):167–73. doi: 10.1016/0006-8993(86)90406-3. [DOI] [PubMed] [Google Scholar]
- 107.Mahavongtrakul M, et al. Estradiol dose-dependent regulation of membrane estrogen receptor-alpha, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinology. 2013;154(9):3251–60. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borgquist A, et al. Gonadal steroids differentially modulate the actions of orphanin FQ/nociceptin at a physiologically relevant circuit controlling female sexual receptivity. J Neuroendocrinol. 2014;26(5):329–40. doi: 10.1111/jne.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsumoto A, Arai Y. Neuronal plasticity in the deafferented hypothalamic arcuate nucleus of adult female rats and its enhancement by treatment with estrogen. J Comp Neurol. 1981;197(2):197–205. doi: 10.1002/cne.901970203. [DOI] [PubMed] [Google Scholar]
- 110.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14(12):7680–7. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flanagan-Cato LM. Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol. 2011;32(2):124–36. doi: 10.1016/j.yfrne.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Srivastava DP, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(38):14650–5. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sakuma Y, Pfaff D. Mesencephalic mechanisms of integration of female reproductive behavior in the rat. American Journal of Physiology. 1979;237:R285–290. doi: 10.1152/ajpregu.1979.237.5.R285. [DOI] [PubMed] [Google Scholar]
- 114.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Molecular neurobiology. 2008;38(1):66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Micevych P, Christensen A. Membrane-initiated estradiol actions mediate structural plasticity and reproduction. Front Neuroendocrinol. 2012;33(4):331–41. doi: 10.1016/j.yfrne.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


