Abstract
Introduction:
Evidence from animal models suggests that prenatal exposure to bisphenol A (BPA), a ubiquitous endocrine-disrupting chemical, is associated with adverse reproductive outcomes in females. Exposure during early gestation, a critical period for reproductive development, is of particular concern. Anogenital distance (AGD) is a sensitive biomarker of the fetal hormonal milieu and a measure of reproductive toxicity in animal models. In some studies, the daughters of BPA-exposed dams have shorter AGD than controls. Here, we investigate this relationship in humans.
Methods:
BPA was assayed in first-trimester urine samples from 385 participants who delivered infant girls in a multicenter pregnancy cohort study. After birth, daughters underwent exams that included two measures of AGD (AGD-AC: distance from center of anus to clitoris; AGD-AF: distance from center of anus to fourchette). We fit linear regression models to examine the association between specific gravity–adjusted (SPG-adj) maternal BPA concentrations and infant AGD, adjusting for covariates.
Results:
BPA was detectable in 94% of women. In covariate-adjusted models fit on 381 eligible subjects, the natural logarithm of SpG-adj maternal BPA concentration was inversely associated with infant AGD-AC [, 95% confidence interval (CI): , ]. We observed no association between maternal BPA and infant AGD-AF.
Conclusion:
BPA may have toxic effects on the female reproductive system in humans, as it does in animal models. Higher first-trimester BPA exposure was associated with significantly shorter AGD in daughters, suggesting that BPA may alter the hormonal environment of the female fetus. https://doi.org/10.1289/EHP875
Introduction
Bisphenol A (BPA) is a synthetic chemical widely used in consumer products, including food and drink containers, thermal receipts, medical equipment, and other plastic products (CDC 2013). BPA is detectable in over 90% of the population in the United States (Calafat et al. 2008), and may act on the endocrine system in numerous ways, including binding to and activating numerous nuclear and membrane endocrine receptors, and stimulating changes in estrogen, androgen, progesterone, and thyroid hormone activity (Gentilcore et al. 2013; Jones et al. 2016; Rehan et al. 2015; Sohoni and Sumpter 1998; Teng et al. 2013; Vandenberg et al. 2009). Dozens of studies in humans have examined BPA exposure in relation to a wide range of health end points, including reproductive, perinatal, and pediatric outcomes. That epidemiological research is complemented by findings from animal models and in vitro studies indicating that many tissues and organ systems (including the mammary gland, prostate gland, adipose tissue, reproductive system, and brain) are sensitive to BPA (Ariemma et al. 2016; Berger et al. 2016; de Lima et al. 2015; Vandenberg et al. 2007; Wolstenholme et al. 2011).
In animal models and humans, BPA can cross the placenta to enter fetal circulation (Balakrishnan et al. 2010; Gerona et al. 2013; Ikezuki et al. 2002; Nishikawa et al. 2010; Takahashi and Oishi 2000). Because fetal development is a period of rapid cell proliferation and differentiation, tissue development, and organ growth, prenatal exposure to environmental chemicals such as BPA may be of particular concern. In humans, linking prenatal BPA exposure to postnatal outcomes can be challenging for many reasons, including the long lag between exposure and the outcomes of interest. This is particularly true of reproductive end points because decades may lapse between prenatal exposure and outcomes such as pubertal development, sex steroid production, and, ultimately, fertility.
However, even in the case of these reproductive end points, there are early indicators that may reflect signal altered development. For example, anogenital distance (AGD), the distance from the anus to the genitals, can be measured from birth and is important because a) it is a well-documented index of prenatal androgen exposure in animal models; and b) in humans, it has been linked to clinically relevant measures of adult reproductive health in both sexes (Castaño-Vinyals et al. 2012; Eisenberg et al. 2012; Mendiola et al. 2011; Mendiola et al. 2016). Across numerous mammalian species (including humans), AGD is 50–100% longer in males than in females, and in animal models, it is responsive to experimental manipulation of the prenatal endocrine environment (Dean and Sharpe 2013). Here, we focus on females, acknowledging there is an even larger literature on AGD in males. When androgens are administered to a pregnant dam, her female offspring have longer, more masculine AGD than controls (Hotchkiss et al. 2007). In humans, an early study observed that three infant girls with congenital adrenal hyperplasia (a condition characterized by supranormal adrenal androgen exposures in utero) all had AGD ratios above the 95% confidence limit, as established based on measurements in 115 healthy infant girls (Callegari et al. 1987). We have reported, furthermore, that infant girls born to mothers with polycystic ovary syndrome (PCOS) (), another hyperandrogenic condition, have longer AGD at birth than infant girls born to a PCOS-free comparison group () (Barrett et al. 2016). In a study of 100 young adult women, longer AGD was associated with higher testosterone levels (Mira-Escolano et al. 2014), as well as multifollicular ovaries (Mendiola et al. 2012). Most recently, a case–control study demonstrated that women with endometriosis () had shorter AGD than controls () (Mendiola et al. 2016).
Thus, AGD may be an important marker of prenatal endocrine-disrupting exposures and potentially a predictor of reproductive sequelae in females. Several studies in rodents have examined the effect of low-dose maternal BPA exposure on AGD in female offspring, but the results have been inconsistent. In a rat study, decreases in female AGD at birth were observed following prenatal and lactational BPA exposure at body weight (BW) per d, an amount well below the no observed adverse effect level of (Christiansen et al. 2014), but still considerably higher than the likely human daily exposure, estimated at (EFSA 2015; Lakind and Naiman 2011; WHO 2011). In another rat study, at age 1 mo, AGD was shorter in the female offspring of dams exposed to BPA (at doses of 0.17 and BW per d) compared with controls; however, the differences in AGD were attenuated by 3 mo of age (Kobayashi et al. 2012). At the same time, a handful of other studies in mice and rats have observed no significant changes in AGD in female offspring following gestational BPA exposure at similar or lower concentrations () (Honma et al. 2002; Howdeshell et al. 2008; Ryan et al. 2010). Differences in timing of exposure, dose, strain, sample size, and age at AGD measurement all may contribute to the inconsistencies across studies.
To date, very few studies have examined the relationship between prenatal BPA exposure and AGD in humans, and thus far, all of them have focused on male offspring. In a Chinese study, the sons of workers with occupational BPA exposure during pregnancy () had shorter AGD than the sons of controls who worked in related industries () (Miao et al. 2011). However, maternal urinary BPA (a more precise exposure metric) was not measured, and AGD was measured in the sons at a wide range of ages (0–17 yr). By contrast, in a second study, maternal urinary BPA concentration in late pregnancy was not associated with AGD in male infants at birth (), but it was inversely associated with testosterone concentrations and the testosterone-to-estradiol ratio in cord blood (Liu et al. 2016). In this case, the timing of urine and blood collection was outside of the period of greatest relevance, the reproductive programming window (estimated to be approximately 8–14 weeks gestation), during which AGD appears to be most responsive to exposures (Welsh et al. 2008). The objective of the current study was to expand upon this small literature by using data from a large pregnancy cohort study to examine the relationship between maternal BPA concentrations in the early pregnancy reproductive programming window and AGD in the resulting daughters at birth.
Methods
Study Population and Overview
The Infant Development and the Environment Study (TIDES) was a pregnancy cohort study designed to examine exposure to endocrine disrupting chemicals in relation to infant reproductive development. Women in their first trimester of pregnancy were recruited in 2010–2012 at four academic medical centers: University of California, San Francisco (UCSF), University of Minnesota (UMN), University of Rochester Medical Center (New York) (URMC), and University of Washington (UW). Eligibility criteria included: age 18 or older, able to read and write English, no major medical complications, weeks pregnant, and planning to deliver in a participating study hospital. In each trimester, subjects gave urine samples and completed questionnaires, which included items on demographics, health, lifestyle, and reproductive history. All study activities were approved by the relevant institutional review boards prior to study implementation, and all subjects signed informed consent. The current analysis includes TIDES subjects who gave a first-trimester urine sample and went on to deliver a daughter who underwent a TIDES physical examination shortly after birth. Gestational age at birth was determined based on the first ultrasound in the medical record. When that was not available, the physician’s estimate of gestational age at birth was used instead.
Bisphenol A Measurement and Analysis
Urine samples were collected in BPA-free containers and frozen at until they were shipped on dry ice to the Division of Laboratory Sciences, National Center for Environmental Health at the Centers for Disease Control and Prevention (CDC). Due to funding constraints, BPA was only analyzed in first trimester urine samples from mothers who gave birth to girls. At the CDC, total urinary BPA (free plus conjugated species) was measured using online solid phase extraction–high-performance liquid chromatography–isotope dilution mass spectrometry (Ye et al. 2005). For quality control, each batch also included field blanks, reagent blanks, analytical standards, and matrix-based quality control materials.
Samples with BPA concentrations below the limit of detection () were assigned a value of LOD divided by the square root of 2, following convention (Hornung and Reed 1990). We adjusted for urine dilution using a standard formula: , where is the specific gravity–adjusted (SpG-adj) BPA concentration, BPA is the ssconcentration measured in the individual sample, 1.014 is the mean SpG for all TIDES samples, and SpG is the SpG of the individual urine sample (Boeniger et al. 1993). SpG-adj BPA concentrations were then natural log–transformed.
Infant Physical Examinations and Anogenital Distance
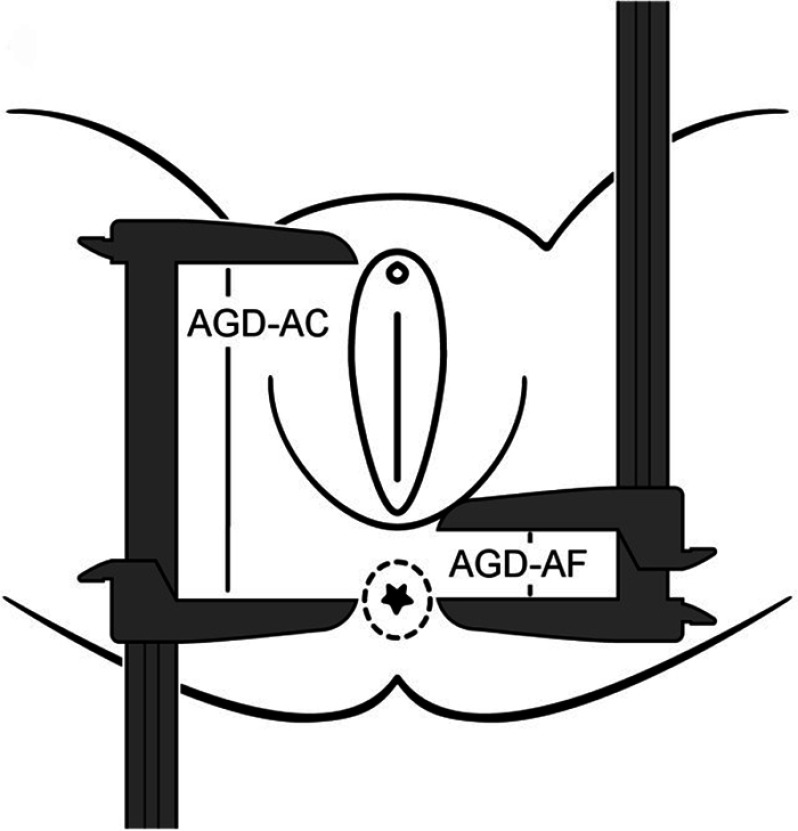
Prior to hospital discharge (typically at 1–2d of age), the study team visited the TIDES mother and child to conduct the infant physical examination. For infants born preterm or fragile, exams were delayed until the clinical team felt the infant was ready. TIDES exams consisted of weight and length measurements (following standardized protocols), as well as comprehensive genital exams conducted by experienced examiners who had undergone 2-d, face-to-face, multicenter intensive standardized trainings at the beginning and middle of the study (Sathyanarayana et al. 2015). AGD was measured following protocols developed based on our previous work (Swan et al. 2005; Swan 2008). The infant was placed flat on her back with her legs held in a “frog-leg” position. Trained study coordinators obtained two measurements of AGD (in mm) using dial calipers, shown in Figure 1. The shorter measurement, the anus–fourchette distance (AGD-AF), is from the posterior end of the fourchette to the center of the anus (1). The longer measurement, the anus–clitoris distance (AGD-AC), is from the anterior surface of the clitoral hood to the center of the anus (Figure 1). Each measurement was repeated three times, and the mean of the three measurements was used in data analysis. At each study center, measurements were independently repeated by a different examiner in at least 10% of infants to assess quality control. The repeated measurements allowed us to quantify inter- and intraobserver (intraclass correlations) variation (as further described by Sathyanarayana et al. 2015). On a monthly basis, the TIDES coordinating center assessed interexaminer and intercenter variation, and any issues were immediately addressed to ensure data quality.
Figure 1.
Measurement of anogenital distance in female newborns [adapted from Sathyanarayana et al. (2010) and reprinted with permission from John Wiley and Sons].
Statistical Methods
We first calculated univariate statistics for all variables of interest, including counts and percentages for categorical variables, means, and summary statistics (minimum, 25th percentile, median, 75th percentile, maximum) for continuous variables (Table 1). Variables of interest included our main exposure (log–transformed, SpG-adj BPA) and outcome variables (AGD-AF and AGD-AC), all of which were continuous. We selected a set of covariates a priori based on our prior analyses and the previous literature (Swan et al. 2015). Those covariates were postconception age at the time of exam (calculated as gestational age at birth plus age at exam, continuous), infant size at exam [weight-for-length z-score (ZWL), continuous], mother’s age (continuous), child’s race (non-Hispanic white vs. other; categorical indicator) time of urine collection (time since midnight in hours), and study center (categorical indicator). We elected to use ZWL based on World Health Organization (WHO) standard curves (WHO Multicentre Growth Reference Study Group 2009) to adjust for body size in our models because our previous work in this cohort suggests that among possible infant size metrics to consider (including weight, weight for age, and length-for-age z-scores), ZWL is the strongest predictor of genital measurements (Swan et al. 2015).
Table 1.
Characteristics of the study population ().
| Continuous variables | Min | Percentiles | Max | Association with log(SpG-adj BPA) (r)c | |||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| Maternal age (years) | 18.3 | 27.4 | 31.8 | 35.3 | 45.2 | ||
| Gestational age at birth (weeks) | 32.9 | 38.9 | 39.6 | 40.6 | 42.3 | 0.03 | |
| Age at exam (days) | 0 | 1.0 | 1.0 | 2.0 | 65.0 | 0.11 | |
| z-score weight for age | 0.4 | 8.6 | 0.06 | ||||
| Postconception age at exam (weeks) | 35.0 | 39.1 | 40.0 | 41.0 | 49.0 | 0.11 | |
| AGD-AC (mm)a | 16.5 | 34.1 | 36.8 | 39.1 | 53.3 | ||
| AGD-AF (mm) | 8.0 | 13.9 | 15.7 | 18.2 | 28.5 | 0.03 | |
| Time of urine collection (hours since midnight) | 7.5 | 10.5 | 12.5 | 14.8 | 19.8 | 0.16 | |
| Gestational age at urine collection (weeks) | 5.1 | 9.4 | 11.0 | 12.3 | 15.7 | 0.01 | |
| SpG-adj | 0.04 | 0.6 | 1.0 | 1.9 | 27.1 | — | |
| Categorical variables | (%)b | ||||||
| Center | |||||||
| UCSF | 98 (25.7) | ||||||
| UMN | 94 (24.7) | ||||||
| URMC | 109 (28.6) | ||||||
| UW | 80 (21.0) | ||||||
| Race/ethnicity | |||||||
| White/non-Hispanic | 225 (60.5) | ||||||
| Other | 147 (39.5) | ||||||
| Education | |||||||
| High school or less | 55 (14.7) | ||||||
| Some college or more | 320 (85.3) | ||||||
Note: AGD-AC, anogenital distance from the anus to the clitoris; AGD-AF, anogenital distance from the anus to the fourchette; BPA, Bisphenol A; Max, maximum; Min, minimum; SD, standard deviation; SpG-adj, specific gravity–adjusted; UCSF, University of California, San Francisco; UMN, University of Minnesota; URMC, University of Rochester Medical Center; UW, University of Washington.
One infant did not have an AGD-AC measurement, so .
Percentages may not total exactly 100% due to rounding.
Log-transformed specific gravity–adjusted concentrations. Pearson’s correlation was used to examine associations with continuous variables.
We used scatterplots to examine the relationships between our outcome variables (AGD-AF and AGD-AC) and all continuous covariates. Four unusual observations were detected in bivariate analyses. Three babies were measured at ages much older than the median of 1 d (110, 132, and 153 d). An additional mother had a urinary SpG value that was biologically implausible (1.062) (Boeniger et al. 1993). Those four mother–infant dyads were removed from all subsequent analyses.
To examine the relationship between maternal BPA concentrations and infant AGD, we fit unadjusted and adjusted linear regression models. In the unadjusted models, only SpG-adj BPA was used as a covariate, while in the adjusted models, all covariates specified above were included. We conducted several sets of sensitivity analyses. First, we refit models stratifying based on whether the infants were examined by the study team within 2 d after birth. Second, we refit models replacing the composite variable “postconception age at exam” with its constituent variables, gestational age at birth and age at exam. Model diagnostics were conducted to investigate any possible violations of the linear model assumptions of normality (Q-Q plots), homogeneity of variance (residuals as a function of predicted values), independence of the observations (Durbin-Watson test), and linearity of the relationship between the outcome variables and the covariates (plots of residual vs. predicted values as well as component plus residual plots). Outliers were identified using the studentized residuals ( or ), influential observations were identified using Cook’s distance (), and leverage points were identified using the diagonal elements of the hat matrix (for our sample, ). Adjusted models were also checked for collinearity using the variance inflation factors (VIFs) of each variable ( indicating collinearity issues). In adjusted models, several violations were noted, including violation of the constant variance assumption (AGD-AC models only), and nonlinear relationships between some covariates (postconception age, ZWL, and BPA) and outcome variables in some models. Although several potential outliers were noted across the various models and some observations had slightly high leverage (but were not influential), lacking further justification for excluding them, they were retained. Given the model violations noted above, we log–transformed the outcome variables and refit models. This did not lead to significant improvement in satisfying the linear assumptions; therefore, we also explored generalized additive models (GAMs). Ultimately, because the intent of this analysis is inference, not prediction, we present the linear models as primary, with the nonlinear models presented secondarily for reference. All analyses were done using R (version 3.23; R Development Core Team), and were considered significant.
Results
Demographic characteristics of TIDES participants have been previously described (Barrett et al. 2014; Swan et al. 2015), and are summarized here briefly. A total of 385 mothers (and their infant daughters) had data on first-trimester maternal BPA concentrations, infant AGD, and relevant covariates. After removing from the analysis the four excluded mother–child pairs, 381 dyads were included in the current analyses (Table 1). On average, mothers in the study were yr of age. Participants were predominantly white (65.3%). The remaining women self-identified as black (14.8%), Asian (6.7%), and other/unknown (13.2%). Most women were non-Hispanic (87.5%), and 85.3% had at least a high school education. There was roughly equal representation across the four study centers (California: 25.7%; Minnesota: 24.7%; New York: 28.6%; Washington: 21.0%) (see Table S1). Eight women (2.1%) gave a urine sample outside of the first trimester (range: 14–15 weeks ); however, they were retained in analyses given that the reproductive programming window is believed to extend into that approximate gestational age range.
The infants in the current analyses were mostly born at term (91.9% at weeks gestation). Median infant weight for length z-score at the time of examination was slightly negative (), presumably due to the water weight loss that typically occurs in the days immediately following birth (Mulder and Gardner 2015). Of the 381 babies included in this analysis, exams were conducted on 68 babies (17.8%) at greater than 2 d old. Of these, sixteen exams were delayed due to neonatal intensive care unit (NICU) admission (following preterm birth), while the remaining 52 exams were delayed due to logistical issues, where mothers were discharged from the hospital before they could be reached by the study team.
Across all girls, mean AGD-AC was , and mean AGD-AF was . The intraexaminer intraclass correlations (ICCs) (looking at consistency of measurements within a single examiner) were 0.92 for both AGD measures. Fifty-four infants in this analysis underwent repeated measurements by two examiners, and the interexaminer ICCs were 0.73 and 0.79 for AGD-AF and AGD-AC, respectively. BPA concentration was below the limit of detection in 6.3% of samples, and the median BPA concentration was ( after SpG adjustment). A check of model assumptions showed evidence of nonhomogeneous residual variance with untransformed AGD, so models were also fit using the natural logarithm of AGD. Conclusions were similar and results from the transformed models are presented in Table S2. Scatterplots of AGD in relation to covariates are shown in Figures S1 and S2.
In unadjusted models, log(SpG-adj BPA) showed a nonsignificant, inverse association with AGD-AC [, 95% confidence interval (CI): , 0.06] and a nonsignificant, weakly positive relationship with AGD-AF (, 95% CI: , 0.52) (Table 2). In multivariable models adjusting for mother’s age, infant’s postconception age, weight-for-length z-score, time of urine collection, infant’s race, and study center, log(SpG-adj BPA) was significantly associated with AGD-AC (, 95% CI: , ), but not with AGD-AF (, 95% CI: , 0.37). Since the third quartile of SpG-adj BPA is 317% that of the first quartile, multiplying the slopes for log(SpG-adj BPA) by estimates the change in AGD for a change in BPA from the first to the third quartile. The transformed slopes in the adjusted model are for AGD-AC and 0.03 mm for AGD-AF. The infant’s postconception age and ZWL were both strongly and positively associated with AGD-AC (Table 2). AGD-AF varied by study center and was inversely associated with maternal age, but positively associated with infant’s postconception age (Table 2).
Table 2.
Linear regression models examining the relationship between log(SpG-adj BPA) and covariates and anogenital distance measures in newborn daughters ().
| Characteristic | AGD-AC | AGD-AF | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Log(SpG-adj BPA) | ||||
| Maternal age | ||||
| Infant’s postconception age | 0.54 (0.35, 0.73) | 0.17 (0.02, 0.33) | ||
| Weight-for-length z-score | 0.66 (0.35, 0.96) | |||
| Race | ||||
| Urine collection time | ||||
| UCSF center | ||||
| URMC center | ||||
| UW center | 1.99 (1.07, 2.90) | |||
Note: AGD-AC, anogenital distance from the anus to the clitoris; AGD-AF, anogenital distance from the anus to the fourchette; BPA, Bisphenol A; mm, millimeters; CI, confidence interval; SpG-adj, specific gravity–adjusted; UCSF, University of California, San Francisco; URMC, University of Rochester Medical Center; UW, University of Washington. For race, the referent is non-Hispanic, white. For center, the referent is UMN. All other variables are continuous.
In secondary GAM models that allowed for nonlinearity between covariates and AGD, AGD-AC showed nonlinear associations with both log(SpG-adj BPA) and ZWL, while AGD-AF showed nonlinear associations with log(SpG-adj BPA) and postconception age. The association between log(SpG-adj BPA) on AGD-AC was relatively constant for small values of BPA, but AGD-AC decreased more rapidly in association with higher BPA concentrations (Figure S3). The nature of this relationship was similar when stratified by postconception age greater or less than 2 d (not shown). GAM models for AGD-AF showed nonmonotonic relationships with log(SpG-adj BPA) (Figure S4).
When we refit models stratifying by age at examination, in the subset of infants measured within 2 d of birth (), the adjusted slope for BPA was (95% CI: , 0.01), whereas the relationship was stronger among infants measured after 2 d of age (; ; 95% CI: , 0.59). In sensitivity models replacing postconception age at exam with gestational age at birth and age at exam, results were virtually identical to our primary models (AGD-AC: , 95% CI: , ; AGD-AF: , 95%CI: , 0.37).
Discussion
In this pregnancy cohort study, first-trimester maternal urinary BPA concentration was inversely associated with one of two measures of daughters’ AGD at birth. To our knowledge, this is the first study to examine BPA exposure during early pregnancy in relation to AGD. It is also the first study to consider prenatal BPA exposure and AGD in females. Our findings are consistent with several (but not all) rodent studies that found BPA administration during gestation to be associated with shortened AGD in newborn female offspring (Christiansen et al. 2014; Kobayashi et al. 2012), and it has been hypothesized that shorter female AGD may represent hyperfeminization, possibly resulting from estrogen receptor agonism (Christiansen et al. 2014). Given the body of evidence that AGD is, in fact, a sensitive measure of endocrine activity during early fetal development, our results provide further support for the hypothesis that BPA exposure during pregnancy alters typical gestational endocrine signaling pathways. The extent to which early endocrine changes may program long-term reproductive development and trajectories is uncertain, but merits additional research.
Research across a number of model species has linked prenatal BPA exposure to outcomes including changes in oogenesis and ovarian steroidogenesis (Fernández et al. 2010; Hunt et al. 2012; Susiarjo et al. 2007; Xi et al. 2011), a polycystic ovarian syndrome-like phenotype (Adewale et al. 2009; Fernández et al. 2010), uterine and endometrial defects (Newbold et al. 2009; Signorile et al. 2010), and morphological changes in the mammary gland (Paulose et al. 2015). Although there has been considerable epidemiological research on adolescent and adult BPA exposure in relation to reproductive outcomes (reviewed by Peretz et al. 2014), to our knowledge, there is currently only one study that measured prenatal BPA levels and followed the resulting children to reproductive maturity, when reproductive outcomes are more easily measured and clinically relevant. In that Mexican cohort, BPA concentration in third-trimester urine was not associated with steroid hormone levels or sexual maturation in girls at age 8–13 y () (Watkins et al. 2014), but was inversely associated with odds of having reached adrenarche or pubarche in boys () (Ferguson et al. 2014). Additional longitudinal research is needed to replicate these findings in other populations to examine exposures earlier in pregnancy and to investigate reproductive outcomes beyond the peripubertal period in humans.
Based on the body of evidence from numerous in vitro, animal model, and human studies, AGD is usually considered an androgen-sensitive measure (reviewed in Dean and Sharpe 2013; Thankamony et al. 2016). By contrast, BPA is best known for its estrogenic activity (Alonso-Magdalena et al. 2012; Vom Saal et al. 2012), and the mechanisms underlying the relationship between prenatal BPA exposure and AGD are uncertain. In animal models, there is evidence that BPA may act as an androgen receptor antagonist (vom Saal and Hughes 2005; Wetherill et al. 2007), a mechanism that is consistent with findings from a Chinese study demonstrating that maternal occupational exposure to BPA (as estimated by personal air sampling during pregnancy) was associated with shorter age- and weight-adjusted AGD in sons during childhood () (Miao et al. 2011). In that study, daughters were not examined. However previous work in animal models and humans suggests that prenatal exposure to antiandrogens [such as di(2-ethylhexyl) phthalate (DEHP)] is not associated with alterations in AGD in females (Christiansen et al. 2009; Hass et al. 2007; Swan et al. 2015). Thus, if BPA exposure in early gestation affects AGD in females, it may be through an alternative mechanism. For instance, BPA may increase activity of the enzyme aromatase, which converts testosterone to estradiol (Castro et al. 2013; Séralini and Moslemi 2001). This possibility is supported by a cross-sectional study that found that BPA concentration in third-trimester maternal urine was inversely associated with the testosterone to estradiol ratio in cord blood of 137 newborn boys (Liu et al. 2016). It is also possible that BPA exposure may affect AGD through estrogenic pathways. Some studies have found that gestational exposure to known estrogenic compounds, like diethylstilbestrol (DES), ethinyl estradiol (), or genistein, decreases AGD in female rodents (Delclos et al. 2009; Levy et al. 1995), whereas other studies have found increases in AGD, depending on the compound used, dose, species, and the study design (Casanova et al. 1999; Mandrup et al. 2013). Finally, it is worth considering other potential mechanisms; in large-scale toxicity testing (ToxCast program), BPA had effects in 101 of 467 in vitro screening assays. Notably, of 309 environmental chemicals studied, BPA had the second-highest toxic potential score, suggesting its ability to act through numerous endocrine pathways (Reif et al. 2010).
A strength of our study is the longitudinal cohort design with maternal samples collected from early pregnancy, arguably the period of most relevance for fetal reproductive system development. In addition, we recruited healthy pregnant women broadly from four U.S. cities, giving us a relatively diverse sample (in terms of age, race, education, and socioeconomic status) compared to some other notable pregnancy cohorts, which have focused on a very specific subject population, such as migrant farm workers (Harley et al. 2013b) or low-income inner-city families (Braun et al. 2011; Harley et al. 2013a; Wolff et al. 2008). Notably, BPA concentrations varied quite considerably across study centers. For instance, women at the UCSF study center, who tended to be very well educated and had high income, had much lower BPA levels () than women at the other TIDES study centers (URMC: ; UMN: , UW: ). Their BPA levels were also lower than those reported in a number of other recent studies of pregnant women in Boston (geometric mean: ), Denmark (), and at National Children’s Study Vanguard sites (geometric mean: ) (Cantonwine et al. 2016; Frederiksen et al. 2014; Mortensen et al. 2014). Given the widespread concern about BPA exposure, better understanding the specific factors (including consumer choices) linked to low BPA levels (such as those seen in our UCSF population) may shed light on potential ways to reduce exposure.
Only one of the two infant female AGD measurements, AGD-AC, was significantly associated with maternal BPA concentration. Overall, the high intra- and interobserver ICCs indicate that our infant AGD measurements were consistent and reproducible. All examiners were highly trained, and our protocols were carefully designed to be quick, minimally invasive, and acceptable to families. Nevertheless, as previously reported, the AGF-AF measurement tends to be relatively difficult to replicate because the fourchette landmark can be difficult to visualize (Sathyanarayana et al. 2015), and there was significant intercenter variation in that measurement. Based on these data, we believe that AGD-AC is the more reliable measurement in the current study; however, it is also worth considering the possibility that AGD-AF was not associated with first-trimester maternal BPA exposure because that distance reflects different developmental processes that may be affected by different exposures and/or during a different critical period. It is worth noting, however, that even for AGD-AC, the magnitude of the observed differences was small. Holding all other covariates constant, the daughter of a mother in the 75th percentile of BPA concentration would be expected to have 0.63 mm shorter AGD than the daughter of a mother in the 25th percentile. This difference corresponds to only a 2% shorter AGD for the average girl. While this difference is small, it is on par with the magnitude of observed associations between prenatal phthalate exposure and AGD in male infants. Our previous work suggests that depending on the AGD measurement and the metabolite, a maternal increase from the 10th to the 90th percentile of first-trimester DEHP exposure corresponds to a 2–5% decrease in boys’ AGD at birth (Swan et al. 2015). The clinical relevance of these subtle variations in AGD is an important question that requires continued longitudinal follow-up.
Our study has several limitations of note. First, women who agree to participate in intensive, longitudinal research studies may not be representative of pregnant women as a whole; however, we cannot directly address this issue, as we do not have data on women who declined to participate or could not be approached. Another concern is the use of single urine samples to assess maternal BPA exposure. BPA levels can vary considerably over time; two studies have estimated the interclass correlation for serial BPA samples collected across pregnancy to be roughly 0.2 to 0.3 (Meeker et al. 2013; Teitelbaum et al. 2008). Moreover, we did not measure BPA in maternal samples collected later in pregnancy, nor in mothers of boys, which would be informative as far as further understanding critical windows and potential sex differences. Finally, although AGD was measured by highly trained examiners and quality control measures were implemented throughout the study, there was significant intercenter variation in AGD-AF, making that measurement less informative. As with any multicenter study, there is the possibility of uncontrolled confounding by unidentified factors that may vary across the study sites. Finally, we do not have serial measures of AGD at multiple ages in girls, so we are unable to address whether associations observed between prenatal BPA concentrations and AGD are stable across childhood and into adulthood. Regardless of whether the relationship between early BPA exposure and AGD persists throughout life, our findings provide further evidence suggesting that BPA may disrupt the early endocrine environment.
Conclusion
Our study provides further evidence that prenatal exposure to BPA may impact reproductive development in females. More research is needed to confirm the current findings and investigate additional reproductive end points in human females, including reproductive hormones and ovarian reserve in infancy, as well as reproductive outcomes later in life. Whether the changes we observed persist across the lifespan and contribute to clinically relevant outcomes should be investigated further. These results may further inform the ongoing controversy over the widespread use of BPA in consumer products and provide evidence regarding policy to limit its use in manufactured goods in the United States as well as abroad (Heindel et al. 2015; Metz 2016).
Supplemental Material
Acknowledgments
We thank the TIDES Study Team for their contributions. Coordinating Center: F. Liu, E. Scher, S. Evans; UCSF: M. Stasenko, E. Ayash, M. Schirmer, J. Farrell, M.-P. Thiet, L. Baskin; UMN: H. L. Gray, C. Georgesen, B. J. Rody, C. A. Terrell, K. Kaur; URMC: E. Brantley, H. Fiore, L. Kochman, J. Marino, W. Hulbert, R. Mevorach, E. Pressman; UW/SCH: R. Grady, K. Ivicek, B. Salveson, G. Alcedo; and the families who participated in the study. In addition, we thank A. Calafat (Centers for Disease Control and Prevention) for BPA analysis, the TIDES families for their participation, and the residents at URMC and UCSF who assisted in birth exams. Funding for TIDES was provided by the following grants from the National Institute of Environmental Health Sciences: R01ES016863-04 and R01ES016863-02S4. Support for the current analysis was provided by T32ES007271, P30ES001247, and P30ES005022.
References
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. 2009. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 81(4):690–699, PMID: 19535786, 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, Fuentes E, et al. . 2012. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol 355(2):201–207, PMID: 22227557, 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Ariemma F, D'Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, et al. . 2016. Low-dose Bisphenol-A impairs adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PLoS One 11(3):e0150762, PMID: 26942597, 10.1371/journal.pone.0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. 2010. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol 202(4):393.e1–7, PMID: 20350650, 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Barrett E, Hoeger K, Sathyanarayana S, Redmon JB, Nguyen RH, Swan SH. 2016. Anogenital distance, a biomarker of prenatal androgen exposure, is longer among newborn daughters of women with polycystic ovary syndrome (PCOS). In: Endocrine Society's 98th Annual Meeting and Expo, April 1–4, 2016 – Boston. Boston, MA:Endocrine Society (ENDO). [Google Scholar]
- Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RH, Kobrosly R, et al. . 2014. Environmental health attitudes and behaviors: Findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol 176:119–125, PMID: 24647207, 10.1016/j.ejogrb.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. 2016. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol 60:39–52, PMID: 26746108, 10.1016/j.reprotox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am Ind Hyg Assoc J 54(10):615–627, PMID: 8237794, 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. . 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128(5):873–882, PMID: 22025598, 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Exposure of the U.S. Population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 116(1):39–44, PMID: 18197297, 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegari C, Everett S, Ross M, Brasel JA. 1987. Anogenital ratio: Measure of fetal virilization in premature and full-term newborn infants. J Pediatr 111(2):240–243, PMID: 3612396, 10.1016/S0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. 2016. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect 124(10):1651–1655, PMID: 27177253, 10.1289/EHP188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HA. 1999. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci 51(2):236–244, PMID: 10543025, 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- Castaño-Vinyals G, Carrasco E, Lorente JA, Sabaté Y, Cirac-Claveras J, Pollán M, et al. . 2012. Anogenital distance and the risk of prostate cancer. BJU Int 110(11 Pt B):E707–E710, PMID: 22984847, 10.1111/j.1464-410X.2012.11516.x. [DOI] [PubMed] [Google Scholar]
- Castro B, Sanchez P, Torres JM, Preda O, del Moral RG, Ortega E. 2013. Bisphenol a exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS One 8(2):e55905, PMID: 23405234, 10.1371/journal.pone.0055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2013. Bisphenol A (BPA) Factsheet Atlanta, GA:Centers for Disease Control National Biomonitoring Program. [Google Scholar]
- Christiansen S, Axelstad M, Boberg J, Vinggaard AM, Pedersen GA, Hass U. 2014. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 147(4):477–487, PMID: 24298045, 10.1530/REP-13-0377. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, et al. . 2009. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ Health Perspect 117(12):1839–1846, PMID: 20049201, 10.1289/ehp.0900689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima RF, Rodriguez DA, Campos MS, Biancardi MF, dos Santos IF, de Oliveira WD, et al. . 2015. Bisphenol-A promotes antiproliferative effects during neonatal prostate development in male and female gerbils. Reprod Toxicol 58:238–245, PMID: 26529182, 10.1016/j.reprotox.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Dean A, Sharpe RM. 2013. Anogenital distance or digit length ratio as measures of fetal androgen exposure: Relationship to male reproductive development and its disorders. J Clin Endocrinol Metab 98(6):2230–2238, PMID: 23569219, 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, et al. . 2009. Overlapping but distinct effects of genistein and ethinyl estradiol (EE2) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol 27(2):117–132, PMID: 19159674, 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA. 2015. Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 13(1):3978, 10.2903/j.efsa.2015.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Shy M, Walters RC, Lipshultz LI. 2012. The relationship between anogenital distance and azoospermia in adult men. Int J Androl 35(5):726–730, PMID: 22519659, 10.1111/j.1365-2605.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Peterson KE, Lee JM, Mercado-García A, Blank-Goldenberg C, Téllez-Rojo MM, et al. . 2014. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol 47:70–76, PMID: 24945889, 10.1016/j.reprotox.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. 2010. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 118(9):1217–1222, PMID: 20413367, 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Jensen TK, Jørgensen N, Kyhl HB, Husby S, Skakkebæk NE, et al. . 2014. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 147(4):555–565, PMID: 24395915, 10.1530/REP-13-0522. [DOI] [PubMed] [Google Scholar]
- Gentilcore D, Porreca I, Rizzo F, Ganbaatar E, Carchia E, Mallardo M, et al. . 2013. Bisphenol A interferes with thyroid specific gene expression. Toxicology 304:21–31, PMID: 23238275, 10.1016/j.tox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, et al. . 2013. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol 47(21):12477–12485, PMID: 23941471, 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, et al. . 2013a. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect 121(4):514–520, PMID: 23416456, 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, et al. . 2013b. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res 126:43–50, PMID: 23870093, 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, et al. . 2007. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect 115 (Suppl 1):122–128, PMID: 18174960, 10.1289/ehp.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, et al. . 2015. NIEHS/FDA CLARITY-BPA research program update. Reprod Toxicol 58:33–44, PMID: 26232693, 10.1016/j.reprotox.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. 2002. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol 16(2):117–122, PMID: 11955942, 10.1016/S0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE Jr. 2007. Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci 96(2):335–345, PMID: 17218470, 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- Howdeshell HL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE Jr. 2008. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci 102(2):371–382, PMID: 18096570, 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, et al. . 2012. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci USA 109(43):17525–17530, PMID: 23012422, 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. 2002. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 17(11):2839–2841, PMID: 12407035, 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Jones BA, Wagner LS, Watson NV. 2016. The effects of bisphenol A exposure at different developmental time points in an androgen-sensitive neuromuscular system in male rats. Endocrinology 157(8):2972–2977, PMID: 27022676, 10.1210/en.2015-1574. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kubota H, Ohtani K, Hojo R, Miyagawa M. 2012. Lack of effects for dietary exposure of bisphenol A during in utero and lactational periods on reproductive development in rat offspring. J Toxicol Sci 37(3):565–573, PMID: 22687996, 10.2131/jts.37.565. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. 2011. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 national health and nutrition examination survey. J Expos Sci Environ Epidemiol 21(3):272–279, PMID: 20237498, 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JR, Faber KA, Ayyash L, Hughes CL Jr. 1995. The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proc Soc Exp Biol Med 208(1):60–66, PMID: 7892297, 10.3181/00379727-208-43832. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu X, Zhang Y, Li W, Huo X. 2016. Associations between maternal phenolic exposure and cord sex hormones in male newborns. Hum Reprod 31(3):648–656, PMID: 26724800, 10.1093/humrep/dev327. [DOI] [PubMed] [Google Scholar]
- Mandrup KR, Jacobsen PR, Isling LK, Axelstad M, Dreisig K, Hadrup N, et al. . 2013. Effects of perinatal ethinyl estradiol exposure in male and female Wistar rats. Reprod Toxicol 42:180–191, PMID: 24036065, 10.1016/j.reprotox.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. . 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol 47(7):3439–3447, PMID: 23469879, 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Roca M, Mínguez-Alarcón L, Mira-Escolano MP, López-Espín JJ, Barrett ES, et al. . 2012. Anogenital distance is related to ovarian follicular number in young Spanish women: A cross-sectional study. Environ Health 11:90, PMID: 23217457, 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Sánchez-Ferrer ML, Jiménez-Velázquez R, Cánovas-López L, Hernández-Peñalver AI, Corbalán-Biyang S, et al. . 2016. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum Reprod 31(10):2377–2383, PMID: 27357299, 10.1093/humrep/dew163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. 2011. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect 119(7):958–963, PMID: 21377950, 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz CM. 2016. Bisphenol A: Understanding the controversy. Workplace Health Saf 64(1):28–36; quiz 37, PMID: 26800896, 10.1177/2165079915623790. [DOI] [PubMed] [Google Scholar]
- Miao M, Yuan W, He Y, Zhou Z, Wang J, Gao E, et al. . 2011. In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res Part A Clin Mol Teratol 91(10):867–872, PMID: 21987463, 10.1002/bdra.22845. [DOI] [PubMed] [Google Scholar]
- Mira-Escolano MP, Mendiola J, Mínguez-Alarcón L, Melgarejo M, Cutillas-Tolín A, Roca M, et al. . 2014. Longer anogenital distance is associated with higher testosterone levels in women: A cross-sectional study. BJOG 121(11):1359–1364, 10.1111/1471-0528.12627. [DOI] [PubMed] [Google Scholar]
- Mortensen ME, Calafat AM, Ye X, Wong LY, Wright DJ, Pirkle JL, et al. . 2014. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ Res 129:32–38, PMID: 24529000, 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder PJ, Gardner SE. 2015. The healthy newborn hydration model: A new model for understanding newborn hydration immediately after birth. Biol Res Nurs 17(1):94–99, PMID: 25504955, 10.1177/1099800414529362. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. 2009. Prenatal exposure to bisphenol A at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 117(6):879–885, PMID: 19590677, 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. 2010. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect 118(9):1196–1203, PMID: 20382578, 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose T, Speroni L, Sonnenschein C, Soto AM. 2015. Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer. Reprod Toxicol 54:58–65, PMID: 25277313, 10.1016/j.reprotox.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. . 2014. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007-2013. Environ Health Perspect 122(8):775–786, PMID: 24896072, 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan M, Ahmad E, Sheikh IA, Abuzenadah AM, Damanhouri GA, Bajouh OS, et al. . 2015. Androgen and progesterone receptors are targets for bisphenol A (BPA), 4-methyl-2,4-bis-(p-hydroxyphenyl)pent-1-ene–a potent metabolite of BPA, and 4-tert-octylphenol: A computational insight. PLoS One 10(9):e0138438, PMID: 26379041, 10.1371/journal.pone.0138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, et al. . 2010. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 118(12):1714–1720, PMID: 20826373, 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE Jr. 2010. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci 114(1):133–148, PMID: 19864446, 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Beard L, Zhou C, Grady R. 2010. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int J Androl 33(2):317–323, PMID: 20132349, 10.1111/j.1365-2605.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Grady R, Redmon JB, Ivicek K, Barrett E, Janssen S, et al. . 2015. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): Methods and predictors. J Pediatr Urol 11(2):76.e1–e6, PMID: 25824881, 10.1016/j.jpurol.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séralini G, Moslemi S. 2001. Aromatase inhibitors: Past, present and future. Mol Cell Endocrinol 178(1-2):117–131, PMID: 11403901, 10.1016/S0303-7207(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Signorile PG, Spugnini EP, Mita L, Mellone P, D'Avino A, Bianco M, et al. . 2010. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol 168(3):318–325, PMID: 20350546, 10.1016/j.ygcen.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. 1998. Several environmental oestrogens are also anti-androgens. J Endocrinol 158(3):327–339, PMID: 9846162, 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. 2007. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet 3(1):e5, PMID: 17222059, 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. 2008. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 108(2):177–184, PMID: 18949837, 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. . 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113(8):1056–1061, PMID: 16079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, Redmon JB, et al. . 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod 30(4):963–972, PMID: 25697839, 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. 2000. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect 108(10):931–935, PMID: 11049811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. . 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106(2):257–269, PMID: 17976571, 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, et al. . 2013. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact 203(3):556–564, PMID: 23562765, 10.1016/j.cbi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Pasterski V, Ong KK, Acerini CL, Hughes IA. 2016. Anogenital distance as a marker of androgen exposure in humans. Andrology 4(4):616–625, PMID: 26846869, 10.1111/andr.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev 30(1):75–95, PMID: 19074586, 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. 2007. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology 148(1):116–127, PMID: 17023525, 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. 2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113(8):926–933, PMID: 16079060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. 2012. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 354(1-2):74–84, PMID: 22249005, 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Téllez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, et al. . 2014. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res 134:233–241, PMID: 25173057, 10.1016/j.envres.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, et al. . 2008. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118(4):1479–1490, PMID: 18340380, 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, et al. . 2007. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 24(2):178–198, PMID: 17628395, 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2011. Toxicological and health aspects of bisphenol A. In: Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A: Final Report, Including Report of Stakeholder Meeting on Bisphenol A, 1-5 November 2010, Ottawa, Canada. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. 2009. WHO Child Growth Standards: Growth Velocity Based on Weight, Length, and Head Circumference. Methods and Development. Geneva, Switzerland:World Health Organization. [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. . 2008. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect 116(8):1092–1097, PMID: 18709157, 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. 2011. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav 59(3):296–305, PMID: 21029734, 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Lee CK, Yeung WS, Giesy JP, Wong MH, Zhang X, et al. . 2011. Effect of perinatal and postnatal bisphenol a exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol 31(4):409–417, PMID: 21182934, 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 383(4):638–644, PMID: 16132150, 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.