Abstract
Background
Airway mucociliary clearance (MCC) is an important defense mechanism against pulmonary infections and is compromised in cystic fibrosis (CF). Cl- and HCO3- epithelial transport are integral to MCC. During pulmonary infections prostaglandin E2 (PGE2) production is abundant.
Aim
To determine the effect of PGE2 on airway Cl- and HCO3- secretion and MCC in normal and CF airways.
Methods
We examined PGE2 stimulated MCC, Cl- and HCO3- secretion using ferret trachea, human bronchial epithelial cell cultures (CFBE41o- with wildtype CFTR (CFBE41 WT) or homozygous F508del CFTR (CFBE41 CF) and human normal bronchial submucosal gland cell line (Calu-3) in Ussing chambers with or without pH-stat.
Results
PGE2 stimulated MCC in a dose-dependent manner and was partially impaired by CFTRinh-172. PGE2-stimulated Cl- current in ferret trachea was partially inhibited by CFTRinh-172, with niflumic acid eliminating the residual current. CFBE41 WT cell monolayers produced a robust Cl- and HCO3- secretory response to PGE2, both of which were completely inhibited by CFTRinh-172. CFBE41 CF cells exhibited no response to PGE2. In Calu-3 cells, PGE2 stimulated Cl- and HCO3- secretion. Cl- secretion was partially inhibited by CFTRinh-172, with additional inhibition by niflumic acid. HCO3- secretion was completely inhibited by CFTRinh-172.
Conclusions
PGE2 stimulates bronchotracheal MCC and this response is decreased in CF. In CF airway, PGE2-stimulated Cl- and HCO3- conductance is impaired and may contribute to decreased MCC. There remains a CFTR-independent Cl- current in submucosal glands, which if exploited, could represent a means of improving airway Cl- secretion and MCC in CF.
Introduction
Cystic fibrosis, caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), is characterized by defective Cl- and HCO3- epithelial ion transport. In the airways this results in thick, sticky mucus, impairing airway surface liquid (ASL) height and mucociliary clearance (MCC). In healthy individuals, routine microbial insults of the lung are cleared through a non-pathologic inflammatory response, coupled with bronchotracheal MCC of mucus-trapped pathogens, thereby preventing obstruction and infection [1]. In cystic fibrosis (CF), defective MCC leads to bronchiectasis, chronic infections, and progressive loss of lung function. Bronchotracheal Cl- and HCO3- secretion contribute to ASL height and MCC through effects on extracellular hydration and mucin expansion [2–4]. In the model put forth by Haq et al., defective Cl- and HCO3- transport in CF leads to a dehydrated and acidic ASL. Dysregulation of the epithelial Na+ channel (ENaC) causes Na+ hyperabsorption, further dehydrating the ASL layer. Water moves out of the mucus layer and eventually out of the periciliary layer, which coupled with increases mucus viscosity due to the acidic environment, results in a thick, viscous layer that compresses the cilia and impairs MCC [5].
Airway anion secretion occurs in response to microbial infection [6] and inflammatory mediators. In infected airways, prostaglandin E2 (PGE2) is abundantly produced by epithelia and infiltrating inflammatory cells, and is found in bronchioalveolar lavage fluid, sputum, and airway epithelium [7–10]. During acute CF pulmonary exacerbations, sputum PGE2 levels can increase over four-fold [9]. In the intestines, PGE2 stimulates Cl-, HCO3-, and mucin secretion via cAMP, Ca2+, and PI3K (phosphatidylinositol 3-kinase) signaling [11, 12]. In the duodenum CFTR is an important HCO3- exit pathway for PGE2-stimulated HCO3- secretion, but unlike many other stimuli, PGE2 may also stimulate HCO3- secretion through CFTR-independent exit pathways [13, 14]. In the airways, PGE2 has been shown to increase iodide transport and short-circuit current (Isc), which has led to a presupposition that PGE2 stimulates anion transport through CFTR [15–17], however, its specific role in Cl- and HCO3- secretion in CF airways remains unclear.
We hypothesized that PGE2 signaling plays an important role in the normal response to airway insult by activating, via CFTR, Cl- and HCO3- dependent fluid secretion that optimizes mucus clearance, and that in CF, defective PGE2-stimulated anion secretion contributes to CF airway disease. In order to specifically study Cl- vs. HCO3- transport, we crafted a series of experiments that promoted preferential transport of Cl- vs. HCO3-, performed ion substitution studies, and used pH-stat titration for measurement of HCO3- secretion. We studied this process in cell culture models of bronchial surface epithelial cells, submucosal glandular cells, and intact trachea to determine the effects of PGE2 on Cl- and HCO3- secretion in distinct components of the airway, and assessed how these components may contribute to MCC.
Materials and methods
Cell culture and tissues
16HBE14o-, CFBE41o- + wildtype CFTR (CFBE41 WT), CFBE41o- + homozygous F508del CFTR (CFBE41 CF), and Calu-3 cell lines were cultured using procedures similar to previously, according to standard protocols [18–20]. Primary cultures from human bronchial epithelial cells and CF nasal polyp explant epithelial cells were obtained from Dr. Walter Finkbeiner (University of California, San Francisco) and were cultured using published protocols [21, 22]. Calu-3 cells were purchased from ATCC (Manassas, VA). All cells were grown at air-liquid interface and used when transepithelial resistance indicated intact monolayer. Calu-3 cells were used at about 300Ω.cm2 and bronchial epithelial cell lines at about 1000Ω.cm2. Mustela putorius ferrets of 6–36 months old were obtained 1–2 hours postmortem by pentobarbital sodium injection and tissues were transported in ice-cold PhysioSolTM (Hospira, IL) solution. Trachea was obtained from just below the larynx to just above the carina. Tissues were transferred to ice-cold Krebs Ringer HCO3--buffered solution and gassed with 95% O2/5% CO2 until used, usually within 6 hours of procurement [2]. All protocols for handling animal tissues at Stanford were approved by the Administrative Panel on Laboratory Animal Care (Stanford’s Institutional Animal Care and Use Committee: IACUC protocol#: 10048).
Mucociliary clearance
Experiments were performed in a manner similar to that done previously [2]. The dorsal muscular portion of the trachea was cut along its entire length and the opened trachea with cartilage intact was pinned mucosal side up in a chamber allowing the serosal side to be bathed in a 37°C Kreb’s Ringer HCO3--buffered solution with indomethacin (1 μM). The mucosal side was exposed to warm, humidified air (95% O2/5% CO2). Mounted trachea was stabilized in the chamber for 15 minutes, except when pretreated with CFTRinh-172 inhibitor, and then the bath was discarded and replaced with fresh solution. For CFTR inhibitor studies, the trachea was bathed bilaterally with CFTRinh-172 (20 μM) for 30 minutes and then CFTRinh-172 remained in the serosal bath for the entire experiment. Xerox ink particles were deposited at the proximal portion of the trachea and a video camera captured images every 20 seconds, tracking the particles as they moved towards the distal end. Measurements (mm/min) were averaged over 5 minutes and tracked for 30 minutes. Tissue viability was tested at the end of each experiment with forskolin (10 μM) and carbachol (0.3 μM).
Measurement of Isc
Snapwell inserts with confluent cell culture monolayers were mounted in an Ussing chamber (Physiologic Instruments P2300), and transepithelial voltage was clamped to zero millivolts using a voltage clamp meter (Physiologic Instruments VCC600), and Isc recorded on a computer using data acquisition software (LabChart 8, ADInstruments). To monitor changes in transepithelial resistance, a voltage pulse of 1 mV was applied every 60 seconds with measurement of resultant deflections of Isc. Ohm’s Law was used to calculate transepithelial resistance. Ussing chambers were kept at 37°C with a temperature-controlled water bath circulator and both mucosal and serosal solutions were continuously gassed with 95% O2/5% CO2. For HCO3--free experiments mucosal and serosal solutions were gassed with 100% O2. All snapwells were rinsed in unbuffered HCO3--free solution prior to placement in Ussing chambers. For tracheal tissues, the tissue was placed in ice-cold PhysioSolTM (Hospira, IL) solution until further dissection, at which time it was placed in solution containing indomethacin (10 μM) to inhibit endogenous prostaglandin release due to dissection trauma. The tracheal submucosal layer containing cartilage was left intact, however, the outer layer covering the cartilage was bluntly dissected under a dissecting microscope with transillumination to ensure no over dissection. Tissue was bathed in indomethacin-containing solution during the entire dissection. Tissues were secured in sliders with steel pins, which are located sufficient distance away from the aperture so as not to interfere with ion transport measurements. Indomethacin (10 μM, bilaterally) was present during Ussing chamber experiments to prevent de novo formation of prostaglandins. Amiloride (10 μM, mucosal), to inhibit epithelial Na+ channel (ENaC), was added at the beginning of the experiment and was present throughout the entire experiment. For CFTRinh-172 pre-treatment, CFTRinh-172 (20 μM, mucosal) was added at least 30 minutes prior to PGE2 stimulation. For HCO3--free experiments, acetazolamide (300 μM, bilateral) was used to inhibit carbonic anhydrase, in addition to O2 gassing and HCO3- removal from solutions.
Measurement of HCO3- secretion by pH-stat
The pH-stat method, which measures the amount of HCl needed to keep the luminal bath at a constant pH using a pH electrode, was used to measure HCO3- secretion. Automatic titrators (Metrohm Titrando 902) were used to titrate 0.2 μL aliquots of 5 mM HCl into the mucosal bath at a steady rate in order to keep from under- or overshooting the set pH. The pH was set to 6.9 in order to prevent activation of apical HCVN1 proton channels which are activated at pH >7.0 [23]. Tiamo software (Metrohm) was used to control the rate of titration and continuously measure the amount titrated and pH. Bicarbonate secretory rates (μmol.cm2.h-1) were calculated in 5 minute intervals by noting the amount titrated, the concentration of titrant, and the surface area of the slider aperture. Short-circuit measurements were simultaneously performed during pH-stat measurements in a similar manner as Cl- secretion measurements, with a few exceptions. First, cell monolayers were not voltage pulsed. To monitor transepithelial resistance, the voltage clamp was released and the open circuit voltage was recorded every 10 minutes. During this time the auto-titrator was briefly paused to ensure no interference. Second, the serosal solution was bathed with 95% O2/5% CO2 (similar to Cl- experiments), but the mucosal solution was bathed with 100% O2 to prevent base formation from carbonhic anhydrase conversion of CO2.
Solutions
The Krebs Ringer HCO3--buffered solution for MCC consisted of (in mM): NaCl 115, K2HPO4 2.4, KH2PO4 0.4, NaHCO3 25, MgCl2 1.2, CaCl2 1.2, Glucose 10. Solutions for tracheal Ussing chamber experiments consisted of the following in mM. Mucosal: NaGluconate 115, K2HPO4 2.4, KH2PO4 0.4, NaHCO3 25, Mg(Gluconate)2 1.2, Ca(Gluconate)2 4, Mannitol 10; Serosal: NaCl 115, K2HPO4 2.4, KH2PO4 0.4, NaHCO3 25, MgCl2 1.2, CaCl2 1.2, Glucose 10. For Cl- secretion experiments with human bronchial epithelial cells and Calu-3 cells, solutions were as following in mM: Mucosal: NaGluconate 120, NaHCO3 25, KH2PO4 3.3, K2HPO4 0.8, Ca(Gluconate)2 4, Mg(Gluconate)2 1.2, Mannitol 10; Serosal: NaCl 120, NaHCO3 25, KH2PO4 3.3, K2HPO4 0.8, CaCl2 1.2, MgCl2 1.2, Glucose 10. For HCO3- secretion measurements by Isc only, solutions were similar to the above, except (in mM): Mucosal: NaCl 120, NaHEPES 25. For pH-stat experiments, solutions were as follows (in mM): Mucosal: NaCl 115, NaGluconate 25, KCl 5, CaCl2 1.2, MgCl2 1.2, Mannitol 10; Serosal: NaCl 120, NaHCO3 25, KH2PO4 3.3, K2HPO4 0.8, CaCl2 1.2, MgCl2 1.2, Glucose 10. All solutions had an osmolarity of approximately 290 mOsm, as determined by a vapor pressure osmometer (Wescor, 5500).
Inhibitors
As stated above, CFTRinh-172 (20 μM, mucosal) was used to inhibit CFTR [24], amiloride (10 μM, mucosal) to inhibit ENaC, acetazolamide (300 μM, bilaterally) to inhibit carbonic anhydrase, and indomethacin (10 μM, bilaterally) to inhibit prostaglandin formation via cycloxygenase. Additionally, bumetanide (10 μM, serosal) was used to inhibit the basolateral Na+:K+/2Cl- (NKCC) channel and niflumic acid (100 μM, mucosal) to inhibit Ca2+-activated Cl- channels. Oubain (10 μM, mucosal) was used to inhibit apical non-gastric H+/K+ ATPase. All drugs (inhibitors plus PGE2, forskolin, carbachol, adenosine-triphosphate (ATP)) were obtained from Sigma-Aldrich.
Statistical analysis
Mean ± standard error of the mean (SEM) were calculated for all experiments with at least three replicates. Statistical significance between groups was determined using paired and unpaired Student’s t-test, as appropriate. Time course comparisons were performed using one-way analysis of variance (ANOVA). Significance was determined at P values < 0.05.
Results
Mucociliary clearance
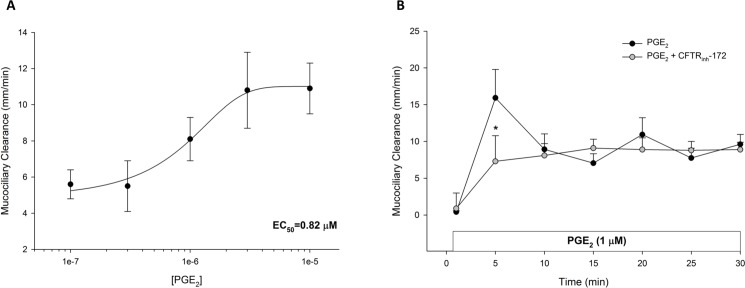
We first examined the effect of PGE2 on MCC, with a validated model of MCC using ferret trachea ex vivo [2]. Serosal exposure of PGE2 in concentrations ranging from 10−7 M to 10−5 M (n = 3 each dose), produced a dose-dependent increase in MCC with an EC50 of 0.82 μM (Fig 1A). In examining the timecourse of stimulation, PGE2 (1 μM, serosal) increased MCC with an initial peak at 5 minutes, followed by a lower sustained response (n ≥ 6 each). Pre-treatment with CFTRinh-172 (20 μM; n ≥ 6 each) attenuated the initial peak (P < 0.05), but did not affect the sustained plateau (Fig 1B). These data suggest that PGE2-stimulated MCC is partially CFTR-dependent, and may contain a CFTR-independent mechanism for clearance.
Fig 1. PGE2-stimulated mucociliary transport in ferret trachea.
A. PGE2 stimulates a dose-dependent increase in MCC in ferret trachea. Each tissue was exposed to 2–3 doses of PGE2 for 30 minutes each (n = 3 each dose). Data are shown as the mean PGE2-stimulated increase in MCC over baseline ± SEM. The half-maximal effective concentration (EC50) is noted in lower right corner. B. Timecourse of PGE2-stimulated MCC with and without CFTR inhibition (n ≥ 6 each). For CFTR inhibition, tissues were bathed in apical and serosal solution for 30 minutes with CFTRinh-172 (20 μM) prior to the 15-minute period and kept in the serosal bath for the length of the experiment. PGE2 (1 μM) was added to the serosal bath. Circles represent means with bars indicating SEM. Asterisks represent P < 0.05 by ANOVA.
Cl- secretion
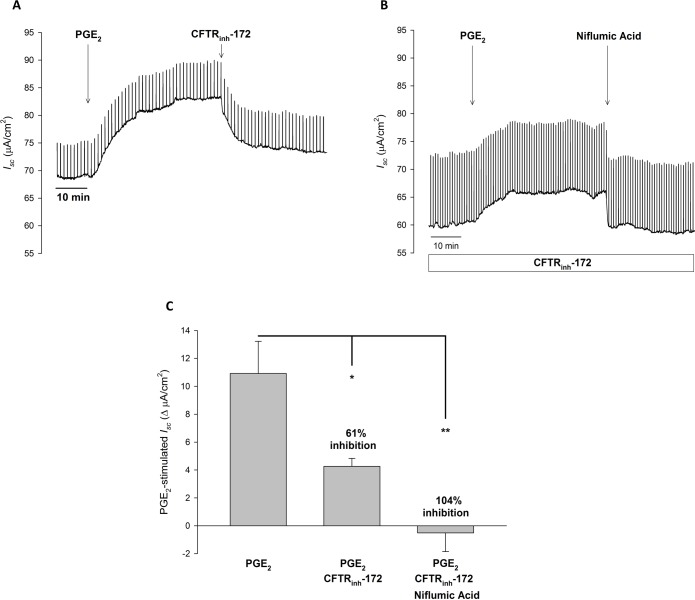
Ferret tracheal MCC has been shown to be highly dependent on transepithelial Cl- transport [2]. Thus, to correlate PGE2-stimulated MCC rate to Cl- transport, we examined PGE2-stimulated Isc with ferret trachea mounted in Ussing chambers with a serosal to mucosal Cl- gradient. As seen in Fig 2A, in the presence of amiloride (10 μM, mucosal), PGE2 (1 μM, serosal) stimulated a significant increase in Isc over baseline (65.83 ± 12.01 vs. 78.61 ± 14.43 μA/cm2, P < 0.01, n = 7). Subsequent addition of CFTRinh-172 (20 μM, mucosal) caused a significant, but not complete, inhibition of PGE2-stimulated Isc (PGE2: 76.75 ± 14.24 vs. CFTRinh-172: 69.90 ± 13.82 μA/cm2, P < 0.01, n = 7) (Fig 2A and 2C). Further addition of bumetanide (10 μM, serosal) to block basolateral Cl- uptake via NKCC, completely abolished the remaining PGE2-stimulated Isc (-10.23 ± 4.33 ΔμA/cm2 from baseline, n = 7). These results show that: 1) PGE2-stimulated Isc is reflective of transepithelial Cl- secretion, and 2) CFTR is responsible for the majority, but not all, of PGE2-stimulated Cl- secretion. With the ability of PGE2 to stimulate cAMP and Ca2+ intracellular signaling pathways, we next examined if the remaining bumetanide-sensitive Isc was from activation of Ca2+-activated Cl- channels. In similar experiments, we examined the ability of niflumic acid (NFA: 100 μM, mucosal), a Ca2+-activated Cl- channel inhibitor, to inhibit CFTR-independent Isc. In these experiments tissues were pre-treated with amiloride (10 μM, mucosal) and CFTRinh-172 (20 μM, mucosal) for at least 30 minutes prior to PGE2 stimulation. Fig 2B and 2C show that NFA eliminates PGE2-stimulated Isc in the presence of CFTRinh-172 (Baseline: 62.93 ± 8.58 vs. NFA: 60.22 ± 8.11 μA/cm2, n = 5), suggesting that Ca2+-activated Cl- channels may be responsible for CFTR-independent PGE2-stimulated Cl- secretion in ferret trachea.
Fig 2. In ferret trachea, PGE2 stimulated Isc is mediated by CFTR and Ca2+-activated Cl- channels.
A. Representative Isc trace with vertical deflections indicating the change in Isc after a 1 mV pulse was applied (every 1 minute). Ferret trachea was exposed to serosal to mucosal Cl- gradient with equivalent bilateral HCO3-. PGE2 (1 μM, serosal) was added to ferret trachea after a baseline period of ≥ 10 minutes, with CFTRinh-172 (20 μM, mucosal) added after 30 minutes. B. Representative Isc trace of ferret trachea incubated in CFTRinh-172 (20 μM, mucosal) for at least 30 minutes prior to PGE2 (1 μM, serosal) stimulation. After 30 minutes, niflumic acid (100 μM, mucosal) was added. C. Change in PGE2-stimulated Isc (mean ± SEM, n ≥ 5) in ferret trachea, with comparisons between no inhibition, CFTR inhibition, or CFTR and Ca2+-activated Cl- inhibition. Asterisks denote significance by Student’s t-test (*, P < 0.05, **, P < 0.01). Mean percent inhibition compared to PGE2 stimulation alone noted.
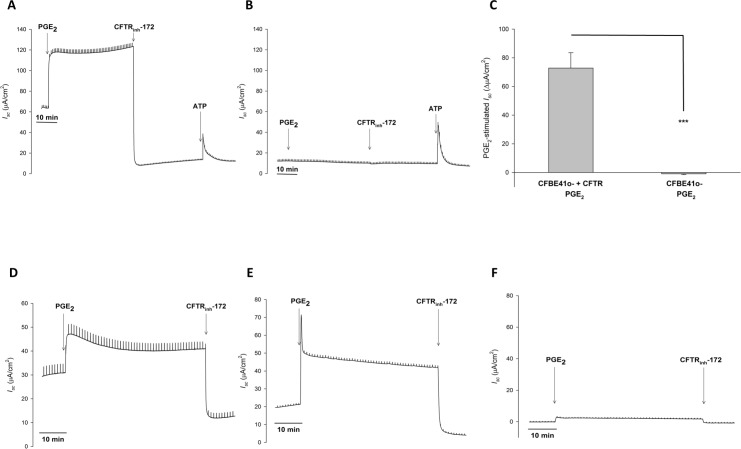
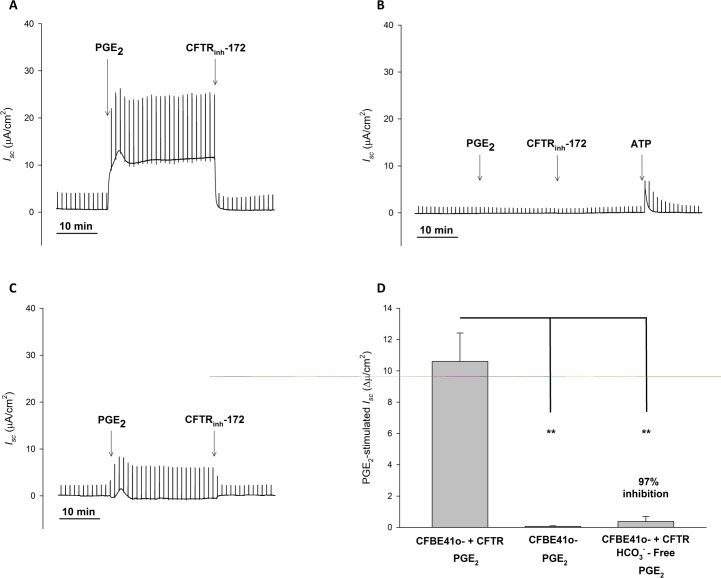
Since airway fluid is composed of secretions from both surface epithelial cells and submucosal glands, we next examined the individual contributions from cell culture models of bronchial epithelial cells and serous gland cells. We first examined PGE2-stimulated Isc in CFBE41o- with transfected wildtype CFTR (CFBE41 WT) and with transfected F508del CFTR (CFBE41 CF) as models of surface epithelial cells. In the presence of amiloride (10 μM, mucosal), PGE2 (1 μM, serosal) stimulated a rapid and significant increase in Isc over baseline in CFBE41 WT cells (47.84 ± 12.02 vs. 120.67 ± 4.75 μA/cm2, P < 0.01, n = 4). This response was completely abolished with CFTRinh-172 (20 μM, mucosal) (-41.47 ± 11.98 ΔμA/cm2 from baseline, n = 4). Given the magnitude of this inhibition, to ensure cells were still viable, ATP (500 μM, mucosal) was added after CFTRinh-172. ATP produced a rapid and transient increase in Isc (Fig 3A). Similar experiments were performed with CFBE41 CF cells, which have little to no CFTR activity. In these cells PGE2 (1 μM, serosal) failed to stimulate Isc (6.79 ± 1.69 vs. 5.84 ± 1.20 μA/cm2, P > 0.05, n = 5). CFTRinh-172 (20 μM, mucosal) had no effect (PGE2: 5.84 ± 1.20 vs. CFTRinh-172: 5.47 ± 1.39 μA/cm2, P > 0.05, n = 5), but ATP (500 μM, mucosal) did stimulate an increase in Isc (Fig 3B). Thus, in bronchial epithelial cells, PGE2-stimulates transepithelial Cl- secretion that is entirely CFTR-dependent (Fig 3C). Similar experiments were performed in the normal bronchial epithelial cell line 16HBE14o-, primary human bronchial epithelial cultures, and nasal cultures from CF patients, with similar responses to that in CFBE41 WT and CF cells (Fig 3D–3F), confirming that this was not a cell line-specific phenomenon.
Fig 3. In human bronchial epithelial cells, PGE2 stimulated Cl- secretion is completely CFTR dependent.
A. Representative Isc trace with vertical deflections indicating the change in Isc after a 1 mV pulse was applied (every 1 minute). Bronchial epithelial cells were exposed to serosal to mucosal Cl- gradient with equivalent bilateral HCO3-. PGE2 (1 μM, serosal) was added to HBE41 WT cells after a baseline period of ≥ 10 minutes, with CFTRinh-172 (20 μM, mucosal) added afterwards. To verify cell viability, ATP (500 μM, mucosal) was added. B. Representative Isc trace from a similar experiment with CFBE41 CF cells. C. Change in PGE2-stimulated Isc (mean ± SEM, n ≥ 4) in CFBE41 WT and CF cells. Asterisks denote significance by Student’s t-test (***, P < 0.001). Mean percent inhibition compared to CFBE41 WT noted. D-F. PGE2 stimulated Cl- secretion in 16HBE14o- cells (D), primary cultures of human bronchial epithelial cells (E), and primary cultures from CF nasal polyp extract (F). Experiments were performed in the same manner as Fig 3A and representative Isc traces are shown. N ≥ 3 experiments were performed for each set of cells with similar responses.
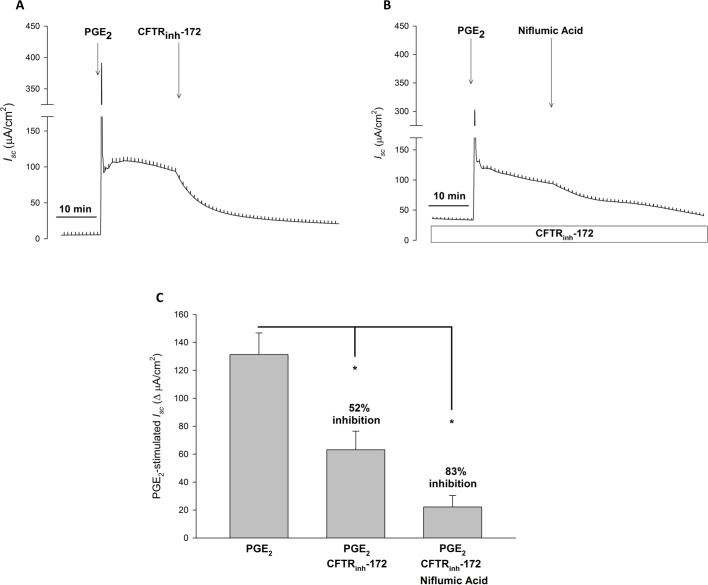
To examine PGE2-stimulated Cl- secretion in serous gland cells, we used the Calu-3 cell line as a model. Experiments were performed in a similar manner as those done with bronchial epithelial cells. In the presence of amiloride (10 μM, mucosal), PGE2 (1 μM, serosal) stimulated a rapid and large transient increase in Isc, followed by a sustained significant increase in Isc over baseline (17.61 ± 4.67 vs. 148.95 ± 18.51 μA/cm2, P < 0.001, n = 8). Subsequent addition of CFTRinh-172 (20 μM, mucosal) caused a robust, but incomplete, inhibition of PGE2-stimulated Isc (PGE2: 148.95 ± 18.51 vs. CFTRinh-172: 53.90 ± 11.70 μA/cm2, P < 0.001, n = 8) (Fig 4A and 4C). Subsequent addition of bumetanide (10 μM, serosal), nearly eliminated the remaining PGE2-stimulated Isc (8.15 ± 3.31 ΔμA/cm2 from baseline, n = 8), inhibiting PGE2-stimulated current by 94 ± 2%. Given the residual Cl- current not inhibited by CFTRinh-172, we performed similar experiments to that done in ferret trachea and examined if NFA could inhibit this bumetanide-sensitive current. In the presence of CFTRinh-172 (20 μM, mucosal), NFA significantly inhibited PGE2-stimulated Isc (PGE2+CFTRinh-172: 79.37 ± 16.57 vs. NFA: 38.40 ± 9.68 μA/cm2, P < 0.05, n = 4) (Fig 4B). Thus, similar to bronchial epithelial cells, PGE2 stimulates Cl- secretion in Calu-3 cells, however, in contrast to bronchial epithelial cells, this current is not completely CFTR-dependent. Similar to what is seen in ferret trachea, CFTR-independent PGE2-stimulated Cl- secretion is predominantly NFA-sensitive (Fig 4C).
Fig 4. In Calu-3 cells, PGE2 stimulated Cl- secretion is mediated by CFTR and Ca2+-activated Cl- channels.
A. Representative Isc trace with vertical deflections indicating the change in Isc after a 1 mV pulse was applied (every 1 minute). Calu-3 cells were exposed to serosal to mucosal Cl- gradient with equivalent bilateral HCO3-. PGE2 (1 μM, serosal) was added to Calu-3 cells after a baseline period of ≥ 10 minutes, with CFTRinh-172 (20 μM, mucosal) added after 30 minutes. B. Representative Isc trace of Calu-3 cells incubated in CFTRinh-172 (20 μM, mucosal) for at least 30 minutes prior to PGE2 (1 μM, serosal) stimulation. After 30 minutes, niflumic acid (100 μM, mucosal) was added. C. Change in PGE2-stimulated Isc (mean ± SEM, n ≥ 4) in Calu-3 cells, with comparisons between no inhibition, CFTR inhibition, or CFTR and Ca2+-activated Cl- inhibition. Asterisks denote significance by Student’s t-test (*, P < 0.05). Mean percent inhibition compared to PGE2 stimulation alone noted.
HCO3- secretion
Having evaluated the effect of PGE2 on airway Cl- secretion, we next sought to determine if PGE2 also stimulates airway HCO3- secretion. To do so, we used the same human bronchial epithelial (CFBE41 WT and CF) and serous gland (Calu-3) cell models, and measured PGE2-stimulated Isc with a serosal to mucosal HCO3- gradient and symmetrical Cl-, in the presence of amiloride (10 μM, mucosal). In this configuration, PGE2 (1 μM, serosal) stimulated a significant increase in Isc over baseline in CFBE41 WT cells (0.53 ± 0.05 vs. 11.13 ± 1.87 μA/cm2, P < 0.05, n = 3). Addition of CFTRinh-172 (20 μM, mucosal) abolished this response with Isc returning to baseline levels (8.79 ± 2.003 vs. 0.57 ± 0.08 μA/cm2, P < 0.05, n = 3) (Fig 5A). PGE2 (1 μM, serosal) failed to stimulate Isc in CFBE41 CF cells (Fig 5B), further supporting that the PGE2-stimulated HCO3- conductance in bronchial epithelial cells relies on CFTR. Since this set-up contains both Cl- and HCO3- anions that can contribute to Isc, we next examined if PGE2-stimulated increases in Isc were from HCO3- or Cl-. To do so we performed identical experiments in HCO3- free conditions with acetazolamide (300 μM, serosal) and 100% O2 mucosal gassing. In HCO3- free conditions, PGE2 failed to increase Isc above baseline (0.26 ± 0.15 vs. 0.65 ± 0.27 μA/cm2, P > 0.05, n = 3) (Fig 5C). These studies indicate that in CFBE41 WT cells, PGE2 stimulates HCO3- transport that, similar to Cl- transport in these cells, is entirely CFTR-dependent (Fig 5D).
Fig 5. In CFBE41 cells, PGE2 stimulated HCO3- secretion is completely CFTR dependent.
A. Representative Isc trace with vertical deflections indicating the change in Isc after a 1 mV pulse was applied (every 1 minute). CFBE41 WT cells were exposed to serosal to mucosal HCO3- gradient with equivalent bilateral Cl-. PGE2 (1 μM, serosal) was added to CFBE41 WT cells after a baseline period of ≥ 10 minutes, with CFTRinh-172 (20 μM, mucosal) added afterwards. B. Representative Isc trace from a similar experiment with CFBE41 CF cells. To verify cell viability, ATP (500 μM, mucosal) was added. C. Representative Isc trace from a similar experiment as Fig 5A with CFBE41 WT cells, except experiments were performed in HCO3--free conditions. D. Change in PGE2-stimulated Isc (mean ± SEM, n = 3) in CFBE41 WT and CF cells in HCO3- containing and HCO3--free conditions. Asterisks denote significance by Student’s t-test (**, P < 0.01). Mean percent inhibition compared to CFBE41 WT noted.
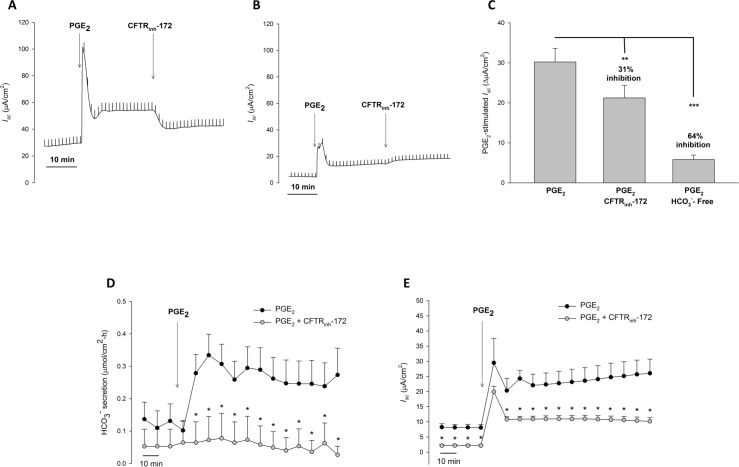
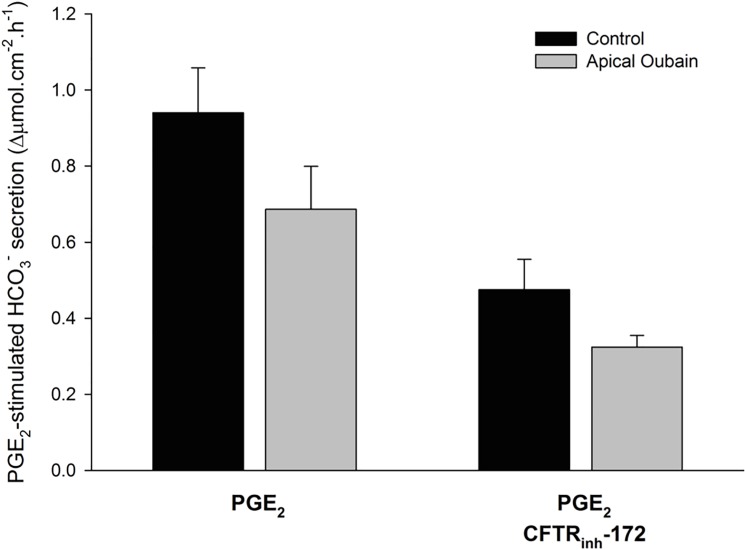
We next examined PGE2-stimulated HCO3- secretion in Calu-3 cells. We first performed Isc measurements in Ussing chambers, similar to that done with CFBE41 cells. Under these circumstances PGE2 caused a large, transient increase in Isc, followed by a sustained significant increase in Isc (21.00 ± 2.30 vs. 51.22 ± 2.43 μA/cm2, P < 0.001, n = 7), which was markedly decreased (31%), but not completely eliminated by CFTR inhibition with CFTRinh-172 (51.22 ± 2.43 vs. 42.24 ± 1.53 μA/cm2, P < 0.01, n = 7) (Fig 6A and 6C). When repeating these experiments in HCO3-- free conditions, there remained a residual anion current stimulated by PGE2 (51.22 ± 2.43 vs. 42.24 ± 1.53 μA/cm2, P < 0.01, n = 7) that was resistant to CFTRinh-172 (Fig 6B and 6C). Given our prior findings suggesting that PGE2 can stimulate a Ca2+-activated Cl- channel current in Calu-3 cells, we employed the pH-stat technique to measure HCO3- secretion in a more direct manner. With this method, voltage clamp and pH-stat were simultaneously measured with Calu-3 cells exposed to symmetrical Cl- and a serosal to mucosal HCO3- gradient with mucosal O2 gassing to prevent the generation of apical base from gassed CO2. To mitigate any potential influences of drugs on apical pH, these experiments were performed with DMSO (5 μL; 1:1000 with bath) or CFTRinh-172 (20 μM, mucosal) added prior to PGE2 stimulation. With this method, PGE2-stimulated a significant increase in HCO3- secretion in control conditions (n = 10, P > 0.05). In contrast, CFTR inhibition ameliorated this response (n = 6, P < 0.05) (Fig 6D). Similar to prior experiments, PGE2-stimulated a significant increase in Isc, that was partially inhibited with CFTRinh-172 (P < 0.05) (Fig 6E). To ensure that activation of apical non-gastric H+/K+ ATPase did not cause falsely low HCO3- secretory rates, we performed similar experiments with or without apical ouabain (10 μM). Oubain did not significantly alter PGE2-stimulated HCO3- secretion, with or without CFTRinh-172 (n ≥ 5, P > 0.05) (Fig 7).
Fig 6. In Calu-3 cells, PGE2 stimulated HCO3- secretion is completely CFTR dependent.
A. Representative Isc trace with vertical deflections indicating the change in Isc after a 1 mV pulse was applied (every 1 minute). Calu-3 cells were exposed to serosal to mucosal HCO3- gradient with equivalent bilateral Cl-. PGE2 (1 μM, serosal) was added to Calu-3 cells after a baseline period of ≥ 10 minutes, with CFTRinh-172 (20 μM, mucosal) added 30 minutes after. B. Representative Isc trace from a similar experiment with Calu-3 cells in HCO3--free conditions. C. Change in PGE2-stimulated Isc (mean ± SEM, n = 3) in Calu-3 cells, with comparisons between no inhibition, CFTR inhibition, and HCO3--free conditions. Asterisks denote significance by Student’s t-test (**, P < 0.01, ***, P < 0.001). Mean percent inhibition compared to Calu-3 cells under control conditions. D. Timecourse of HCO3- secretion measured by pH-stat. The serosal solution was bathed with 95% O2/5% CO2 (similar to experiments in A-C), but the mucosal solution was bathed with 100% O2 to prevent base formation from carbonhic anhydrase conversion of CO2. Calu-3 cells were incubated in DMSO (5 μL; 1:1000 with bath; n = 10) or CFTRinh-172 (20 μM, mucosal; n = 6) for 30–60 minutes prior to PGE2 stimulation (1 μM, serosal). Circles represent means with bars indicating SEM. Asterisks represent P < 0.05 by ANOVA. E. Timecourse of Isc measured by pH-stat measured simultaneously as pH-stat. Circles represent means with bars indicating SEM. Asterisks represent P < 0.05 by ANOVA.
Fig 7. In Calu-3 cells, PGE2 stimulated HCO3- secretion is not affected by apical oubain, an inhibitor of the non-gastric H+/K+ ATPase.
Experiments were performed to determine the potential role of ATP12A in measured PGE2-stimulated HCO3- secretion in normal and CF conditions. Calu-3 experiments were performed similar to that in Fig 6, with the exception that an additional set of experiments were done with oubain (10 μM, mucosal) pre-treatment for ≥ 40 minutes prior to PGE2 stimulation. Bars represent change in PGE2-stimulated Isc (mean ± SEM, n ≥ 5) in Calu-3 cells. Statistical comparisons were done between PGE2 with and without oubain and PGE2 with CFTR inhibition with and without oubain. No statistical difference was noted in either case (P > 0.05 by Student’s t-test).
Discussion
PGE2 and CF airway disease
Cystic fibrosis affects approximately 30,000 people in the U.S., with an estimated annual mean healthcare cost of approximately 1.5 Billion U.S. dollars [25]. The majority of healthcare costs, morbidity, and mortality associated with CF are attributed to pulmonary infections and their associated complications. Amongst the inflammatory milieu of the infected airways, PGE2 is abundantly produced by epithelia and infiltrating inflammatory cells, and is found in bronchioalveolar lavage fluid, sputum, and airway epithelium [7–10]. Lack of functional CFTR may tilt the balance into excessive PGE2 production, leading to a positive proinflammatory loop of NF-kβ (nuclear factor-kappa beta) and CREB (cAMP response element binding protein) activation, causing an upregulation of cyclooxygenase-2 (COX-2) and increased PGE2 production [26]. The overall result being an exaggerated inflammatory condition. Ibuprofen, which can be a useful therapeutic agent in CF [27], may help tip the balance of PGE2 back to appropriate levels. In addition to promoting inflammation, PGE2 also helps resolve inflammation by stimulating Cl-, HCO3-, and mucin secretion [11]. Bronchotracheal MCC is integral to the innate mucosal defense against microbial insults and is regulated by coordinated efforts between transepithelial Cl- secretion and submucosal gland mucus secretion. In this study, we have shown for the first time that PGE2 stimulates MCC in ferret trachea. We also showed that CFTR inhibition causes a significant decrease (~50%) in the initial phase of MCC, indicating that PGE2-stimulated MCC in CF patients may be impaired. The inability of CFTRinh-172 to have a more substantial impact on MCC may be related to the activation of non-CFTR Cl- channels, supported by the ability of niflumic acid to further inhibit CFTRinh-172-independent Isc, or relative insensitivity of ferret CFTR channels to CFTRinh-172 [28]. The former hypothesis is supported by our Calu-3 data, which also showed sensitivity to both CFTRinh-172 and niflumic acid. Activation of TMEM16A channels increases ciliary beat frequency and ASL height, both of which would increase MCC [29]. Likewise, Joo et al. found that in the ferret trachea forskolin-, but not carbachol-stimulated MCC was inhibited by CFTRinh-172 [30]. Thus, we speculate that the residual PGE2-induced MCC during CFTR inhibition may be due to Ca2+-activated Cl- channel activity.
Chloride secretion
We are not the first group to examine PGE2-stimulated anion transport in the airway, however, we have undertaken the most comprehensive examination of PGE2-stimulated Cl- and HCO3- secretion to date. Cullen and Widdicombe et al. showed that PGE2 increases Isc in canine and human trachea [15, 31], while Cowley showed the same in Calu-3 cells [32]. In the latter study, PGE2-stimulated Isc was inhibited 87% by pre-incubation with DPC (diphenylamine-2-carboxylate), suggesting significant CFTR-dependence [32]. Before newer generation CFTR inhibitors, DPC was commonly used to inhibit CFTR. However, DPC is not specific for CFTR and can inhibit other Cl- channels [33]. In our bronchial epithelial experiments, we found that CFTRinh-172 was a potent and complete inhibitor of PGE2-stimulated Cl- secretion. As such, we speculate that the CFTRinh-172-independent Isc observed in ferret trachea and Calu-3 cells is due to non-CFTR Cl- channels. Widdicombe et al. observed small increases in PGE2-stimulated Isc in CF human trachea that was unresponsive to isoproterenol [31]. With the ability of niflumic acid to inhibit our observed residual current, we hypothesize that Ca2+-activated Cl- channels account for the CFTRinh-172-independent Isc in ferret trachea and Calu-3 cells. Shamsuddin et al found that complete inhibition of PGE2-stimulated Isc in small porcine airways required both CFTR and Ca2+-activated channel inhibition (GlyH-101 and niflumic acid, respectively) [16].
We found differential responses to PGE2-stimulated Cl- secretion between bronchial epithelial cell lines and submucosal gland cell lines. In WT CFBE41 and other bronchial epithelial cell lines (including primary culture), PGE2-stimulated Cl- secretion required CFTR. However, Calu-3 cells appear to utilize both CFTR and Ca2+-activated Cl- channels. Ferret trachea showed similar responses to Calu-3 cells, likely due to the presence of submucosal glands. The difference in responses is unlikely to be due to a lack of Ca2+-activated Cl- channels in our bronchial epithelial cell cultures since apical ATP stimulated Cl- current in both WT and CFBE41 cells. It is possible that there is differential PGE2 receptor expression between the two cell types. Four different receptors for PGE2 have been described (EP1-EP4), with all four being expressed in Calu-3 cells. EP1 and EP2 receptors are located at the apical membrane, while EP3 and EP4 receptors are located at both the apical and basolateral membranes [17]. In normal human tracheobronchial epithelial (NHTBE) cells, EP1-EP4 mRNA are present [34], however, to our knowledge, there are no published reports examining EP receptor membrane expression in CFBE41o- cells or other surface airway epithelial cells. EP1 and EP3 signaling increases intracellular Ca2+, while EP3 can also stimulate inositol triphosphate (IP3). EP2 and EP4 increase cAMP, while EP4 also stimulates PI3K [35]. In the duodenum, PGE2 stimulates HCO3- secretion via cAMP, Ca2+, and PI3K through EP3 and EP4 receptors [12]. In Calu-3 cells, Joy et al. found that CFTR-dependent PGE2-stimulated iodide efflux was mediated by EP4 [17]. This leads one to hypothesize that CFTR-dependent Cl- secretion in bronchial epithelial cells and Calu-3 cells may be mediated by EP4, whereas Ca2+-activated Cl- secretion in Calu-3 cells may occur through EP3 activation. Ongoing studies examining the EP receptor membrane distribution in bronchial epithelial cells may shed light on this hypothesis (Fig 8). It may also be possible that there is different intracellular signaling machinery in bronchial epithelial cells and Calu-3 cells, leading to cAMP and Ca2+ crosstalk in Calu-3, but not, bronchial epithelial cells. In mouse inner medullary collecting duct cells, PGE2 stimulated CFTRinh-172- and flufenamic acid-sensitive Isc exclusively through EP4 receptors. Inhibition of IP3 receptors with 2-APB (aminoethoxyldiphenyl borate) blocked PGE2-stimulated Isc by nearly 80%, with complete inhibition of the Ca2+-activated Cl- current. [36]. Lee et al. have described cAMP-dependent activation of IP3-dependent Ca2+ release in submucosal glands and Joo et al. have recently shown that low dose forskolin and carbachol can generate a synergistic Isc and MCC response in ferret trachea [30, 37]. Intracellular increases in cAMP may bind to Epac (exchange protein directly activated by cAMP), catalyzing the generation of IP3 by phospholipase C, resulting in release of intracellular Ca2+ stores and eventual Ca2+-activated Cl- channel activation [38, 39]. Namkung et al. also showed that elevations in intracellular Ca2+ can also lead to activation of Ca2+/calmodulin-sensitive adenylyl cyclase 1, further illustrating the possible bidirectional activation of cAMP and Ca2+ signaling pathways [40]. In the intestine, lubiprostone, a prostaglandin derivative, increases the trafficking of EP4 and CFTR to the membrane, which would be anticipated to increase ion transport [41]. It is unclear if a similar mechanism occurs in bronchial epithelial cells or submucosal glands when exposed to PGE2. Ongoing research into the receptor dependence of PGE2 stimulation in bronchial epithelial cells and submucosal glands, and the intracellular signaling and trafficking involved in Ca2+-activated Cl- channel activation may lead to new ideas on how to coopt this mechanism as a therapeutic target in CF.
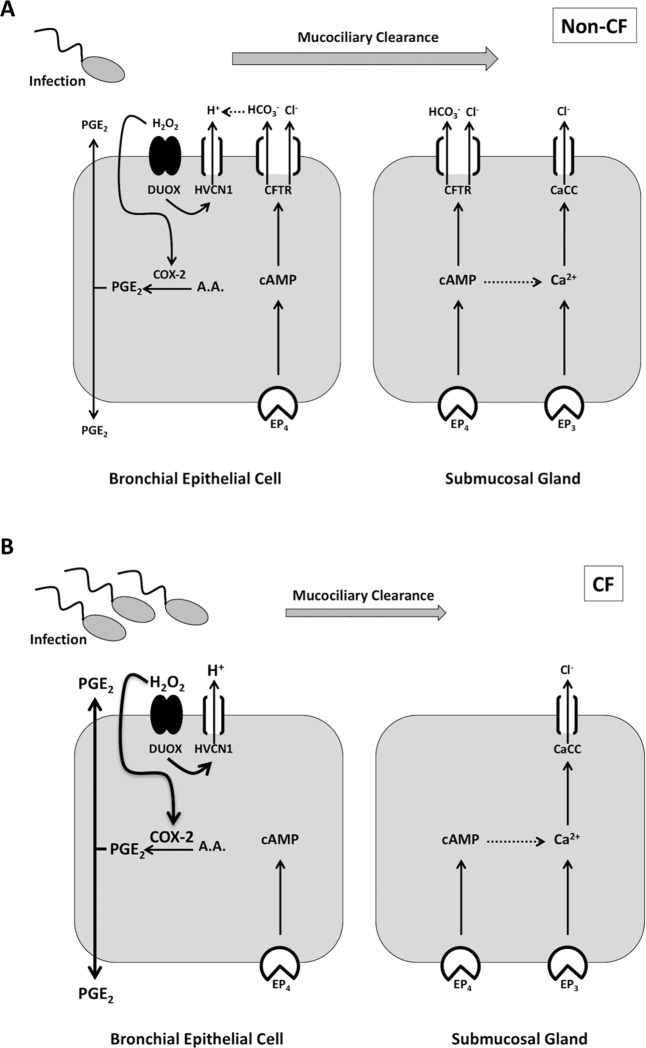
Fig 8. Simplified working model of PGE2-stimulated Cl- and HCO3- secretion and mucociliary clearance in non-CF and CF airway.
A. In the airway, microbial infections cause an increase in PGE2 through release from infiltrating inflammatory cells (not pictured) and production by airway epithelia via COX-2 activation. H2O2 produced by DUOX activates COX-2 and HVCN1 channels provide the H+ shunt from H2O2 production. PGE2 exits the cell and activates PGE2 (EP) receptors. In the current study we did not examine specific EP receptor involvement, however, we propose that EP4 is the predominant mediator of serosal PGE2 stimulation in bronchial epithelial cells. Submucosal gland cells may also utilize the EP3 receptor, or Ca2+-activated Cl- channels (CaCC) may get activated via EP4-mediated cAMP-Ca2+ crosstalk. In bronchial epithelial cells, PGE2 stimulates Cl- and HCO3- secretion via CFTR, whereas in submucosal glands, both CFTR and CaCC are activated. Cl- and HCO3- secretion will then influence airway pH, mucus properties, hydration, and ultimately, mucociliary clearance. B. In CF airway, lack of CFTR-dependent Cl- and HCO3- secretion in bronchial epithelial cells, coupled with no HCO3- secretion and decreased Cl- secretion from submucosal glands, leads to an acidic airway pH, thick-adherent mucus, and decreased mucociliary clearance. This results in increased microbial infection and rampant inflammation, in part by increased PGE2 production.
Bicarbonate secretion
In recent years there has been increased focus on airway HCO3- transport, as it has become apparent that defective ASL formation cannot be accounted for by altered Cl- and Na+ alone [42–44]. The role of HCO3- secretion in MCC is less clear than that of Na+ or Cl-. Jeong et al. found that HCO3- removal did not decrease MCC in a statistically significant manner [2]. However, HCO3- removal impairs submucosal gland secretion and mucus detachment, both of which would negatively affect MCC [45, 46]. Airway HCO3- secretion has been confirmed previously in both surface epithelial cells and submucosal glands [44, 47]. Shamsuddin et al. showed that PGE2 stimulates HCO3- transport in porcine small airways [16], but we are the first to examine PGE2-stimulated HCO3- secretion in both bronchial epithelial and Calu-3 cells. In addition to CFTR, HCO3- conductance can be regulated through increased HCO3- uptake by the Na:HCO3- cotransporter, basolateral Cl-/HCO3- exchange and/or intracellular HCO3- generation by carbonic anhydrase. In the current study, we did not examine the individual roles of these processes in generating HCO3- substrate, however, we did show that PGE2-stimulated HCO3- secretion in the airway requires CFTR. This may occur through direct HCO3- transport through CFTR and/or apical recycling of CFTR-mediated Cl- secretion through apical Cl-/HCO3- anion exchangers [19, 48]. In the duodenum, apical HCO3- conductance through Cl-/HCO3- exchangers can occur in an electroneutral manner, independent of CFTR [13]. Our pH-stat data did not show any electroneutral HCO3- secretion, indicating that, in contrast to the duodenum [13], CFTR-independent Cl-/HCO3- exchange is likely not involved in PGE2-stimulated HCO3- secretion in Calu-3 cells.
It remains unclear whether the effects of HCO3- transport loss in CF are due to acidic pH or HCO3- itself. To address this question, Tang et al. examined CF porcine ASL viscosity at variable HCO3- concentrations and pH values. In their experiments, ASL viscosity was primarily affected by pH, not HCO3- concentration itself [3]. In addition to HCO3- transport, H+ secretion also helps to regulate ASL pH. Lung epithelium contains DUOX NADPH oxidase, which produces H2O2 for release during pulmonary microbial infections. Schwarzer et al. showed that Zn2+-sensitive HVCN1 channels shunt H+ generated during DUOX NADPH oxidase reactions out of the cell [49]. This would serve to lower ASL pH. Interestingly, H2O2 causes autocrine release of PGE2 and stimulates CFTR-dependent increases in Isc [50]. Thus, one might hypothesize that H2O2 release during acute pulmonary infections may increase PGE2-stimulated Cl- secretion to increase MCC and increase HCO3- secretion to limit the negative effect of H2O2-induced H+ secretion on ASL pH (Fig 8). Iovannisci et al. also showed that HVCN1 H+ channels are activated by ASL pH, being closed at resting ASL pH of 6.85 and become active as the pH alkalinizes above that [23]. In our Isc experiments we did not examine H+ secretion specifically. These experiments were done at pH 7.4 so in theory the HVCN1 H+ channel could be active. The lack of Isc response to PGE2 in bronchial epithelial cells without CFTR or with HCO3- removal suggests that HVCN1 H+ channels were either not activated by PGE2 or they play an insignificant role. Likewise, our pH-stat experiments were performed at a set point of pH 6.9 to ensure that HVCN1 activation did not mask HCO3- secretion. Another potential contributor to apical H+ transport and ASL pH in porcine and human airway epithelium is ATP12A (the α subunit of non-gastric H+/K+ ATPase). Shah et al. showed that at pH 7.0, in CF human and pig airway epithelia, cAMP stimulated a decrease in ASL pH, which was inhibited by apical oubain or siRNA against ATP12A [51]. Thus, it has been proposed that in the absence of CFTR, increases in cAMP may lead to ATP12A activation and acidify the ASL. In our bronchial epithelial studies, similar to the reasons stated above, we did not examine ATP12A channel activation. However, we did perform a set of experiments in Calu-3 cells with pH-stat where we applied apical oubain to determine if this unmasked a change in PGE2-stimulated apical pH. We found no change with or without ouabain, leading us to conclude that in Calu-3 cells, under our experimental conditions, PGE2 does not activate ATP12A channels. Altogether, we do not have any evidence that PGE2 activates HVCN1 or ATP12A channels, although specific studies examining PGE2-stimulated H+ secretion in different pH environments may be warranted. What we can say is that PGE2 does stimulate HCO3- secretion in bronchial epithelial cells and submucosal glands and we propose that together with Cl- secretion, this contributes to increasing MCC and microbial removal during infection.
Conclusions
In summary, we have shown that PGE2, an inflammatory mediator produced during CF pulmonary exacerbations, is involved in bronchotracheal MCC and the stimulation of Cl- and HCO3- secretion from bronchial epithelial cells and submucosal glands. Absence of CFTR activity in bronchial epithelial cells leads to a total loss of both Cl- and HCO3- secretion. In submucosal glands, HCO3- secretion is absent, yet some niflumic acid-sensitive Cl- secretion remains, suggesting involvement of Ca2+-activated Cl- secretion. This residual anion current may mitigate some of the deleterious effects of CFTR loss on MCC. These studies provide further information on the role of CFTR in maintaining airway health and provide additional insight into CF airway pathology. Further work to understand the mechanism whereby PGE2 may stimulate Ca2+-activated Cl- channels and MCC in CF, may help identify new therapeutic targets that may assist in the normalization of airway ion transport and clearance of pulmonary microbial insults.
Acknowledgments
This research was supported by the Cystic Fibrosis Foundation (SELLER15B0, SELLER16LO to Z.M.S.; ILLEK15P0, ILLEK16G0 to B.I.; WINE17G0, WINE07XXO to JJW), Elizabeth Nash Foundation (BI), and CAPES (10054-14-6 to MFF). The authors would like to thank Dr. Walter Finkbeiner and Lorna Zlock (Department of Pathology, University of California, San Francisco) for providing primary airway cultures.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by the Cystic Fibrosis Foundation (www.cff.org; SELLER15B0, SELLER16LO to Z.M.S.; ILLEK15P0, ILLEK16G0 to B.I.; WINE17G0, WINE07XXO to JJW), Elizabeth Nash Foundation (http://www.elizabethnashfoundation.org; BI), and CAPES (http://www.capes.gov.br; 10054-14-6 to MFF).
References
- 1.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–7. doi: 10.1172/JCI15217 ; PubMed Central PMCID: PMC150901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong JH, Joo NS, Hwang PH, Wine JJ. Mucociliary clearance and submucosal gland secretion in the ex vivo ferret trachea. American journal of physiology Lung cellular and molecular physiology. 2014;307(1):L83–93. doi: 10.1152/ajplung.00009.2014 . [DOI] [PubMed] [Google Scholar]
- 3.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126(3):879–91. doi: 10.1172/JCI83922 ; PubMed Central PMCID: PMCPMC4767348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209(7):1263–72. Epub 2012/06/20. doi: 10.1084/jem.20120562 ; PubMed Central PMCID: PMC3405509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016;71(3):284–7. doi: 10.1136/thoraxjnl-2015-207588 . [DOI] [PubMed] [Google Scholar]
- 6.Luan X, Campanucci VA, Nair M, Yilmaz O, Belev G, Machen TE, et al. Pseudomonas aeruginosa triggers CFTR-mediated airway surface liquid secretion in swine trachea. Proc Natl Acad Sci U S A. 2014. doi: 10.1073/pnas.1406414111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakrzewski JT, Barnes NC, Piper PJ, Costello JF. Detection of sputum eicosanoids in cystic fibrosis and in normal saliva by bioassay and radioimmunoassay. Br J Clin Pharmacol. 1987;23(1):19–27. PubMed Central PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl D, Starosta V, Maier K, Beck-Speier I, Rebhan C, Becker BF, et al. Inhaled glutathione decreases PGE2 and increases lymphocytes in cystic fibrosis lungs. Free Radic Biol Med. 2005;39(4):463–72. doi: 10.1016/j.freeradbiomed.2005.03.032 . [DOI] [PubMed] [Google Scholar]
- 9.Reid DW, Misso N, Aggarwal S, Thompson PJ, Walters EH. Oxidative stress and lipid-derived inflammatory mediators during acute exacerbations of cystic fibrosis. Respirology. 2007;12(1):63–9. doi: 10.1111/j.1440-1843.2006.00962.x . [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53(1):160–71. doi: 10.1016/j.freeradbiomed.2012.05.001 ; PubMed Central PMCID: PMC3412514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang N, Garcia MA, Quinton PM. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. The Journal of physiology. 2013;591(Pt 18): 4581–93. doi: 10.1113/jphysiol.2013.257436 ; PubMed Central PMCID: PMC3784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuo BG, Wen GR, Seidler U. Phosphatidylinositol 3-kinase is involved in prostaglandin E2-mediated murine duodenal bicarbonate secretion. American journal of physiology Gastrointestinal and liver physiology. 2007;293(1):G279–87. Epub 2007/05/15. doi: 10.1152/ajpgi.00488.2006 . [DOI] [PubMed] [Google Scholar]
- 13.Tuo B, Riederer B, Wang Z, Colledge WH, Soleimani M, Seidler U. Involvement of the anion exchanger SLC26A6 in prostaglandin E2- but not forskolin-stimulated duodenal HCO3- secretion. Gastroenterology. 2006;130(2):349–58. Epub 2006/02/14. doi: 10.1053/j.gastro.2005.10.017 . [DOI] [PubMed] [Google Scholar]
- 14.Nyberg L, Pratha V, Hogan DL, Rapier RC, Koss MA, Isenberg JI. Human proximal duodenal alkaline secretion is mediated by Cl-/HCO3- exchange and HCO3- conductance. Dig Dis Sci. 1998;43(6):1205–10. Epub 1998/07/04. . [DOI] [PubMed] [Google Scholar]
- 15.Cullen JJ, Welsh MJ. Regulation of sodium absorption by canine tracheal epithelium. J Clin Invest. 1987;79(1):73–9. doi: 10.1172/JCI112811 ; PubMed Central PMCID: PMC423989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamsuddin AK, Quinton PM. Native small airways secrete bicarbonate. Am J Respir Cell Mol Biol. 2014;50(4):796–804. doi: 10.1165/rcmb.2013-0418OC ; PubMed Central PMCID: PMC4068927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol. 2008;38(2):143–52. Epub 2007/08/11. doi: 10.1165/rcmb.2006-0295OC . [DOI] [PubMed] [Google Scholar]
- 18.Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem. 2008;22(1–4):57–68. doi: 10.1159/000149783 ; PubMed Central PMCID: PMC2927120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan J, Liao J, Huang J, Robert R, Palmer ML, Fahrenkrug SC, et al. Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. The Journal of physiology. 2012;590(Pt 21): 5273–97. doi: 10.1113/jphysiol.2012.236893 ; PubMed Central PMCID: PMC3515819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krouse ME, Talbott JF, Lee MM, Joo NS, Wine JJ. Acid and base secretion in the Calu-3 model of human serous cells. American journal of physiology Lung cellular and molecular physiology. 2004;287(6):L1274–83. Epub 2004/08/18. doi: 10.1152/ajplung.00036.2004 . [DOI] [PubMed] [Google Scholar]
- 21.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–21. doi: 10.1007/978-1-62703-125-7_8 . [DOI] [PubMed] [Google Scholar]
- 22.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci U S A. 2012;109(49):20035–40. doi: 10.1073/pnas.1213241109 ; PubMed Central PMCID: PMCPMC3523865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iovannisci D, Illek B, Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J Gen Physiol. 2010;136(1):35–46. doi: 10.1085/jgp.200910379 ; PubMed Central PMCID: PMC2894549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110(11):1651–8. doi: 10.1172/JCI16112 ; PubMed Central PMCID: PMCPMC151633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Sullivan AK, Sullivan J, Higuchi K, Montgomery AB. Health care utilization & costs for cystic fibrosis patients with pulmonary infections. Manag Care. 2011;20(2):37–44. . [PubMed] [Google Scholar]
- 26.Chen J, Jiang XH, Chen H, Guo JH, Tsang LL, Yu MK, et al. CFTR negatively regulates cyclooxygenase-2-PGE(2) positive feedback loop in inflammation. J Cell Physiol. 2012;227(6):2759–66. doi: 10.1002/jcp.23020 . [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–9. doi: 10.1164/rccm.200702-181OC ; PubMed Central PMCID: PMC2176097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol. 2007;36(3):313–23. doi: 10.1165/rcmb.2006-0286OC ; PubMed Central PMCID: PMCPMC1894945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Skinner D, Hicks SB, Bevensee MO, Sorscher EJ, Lazrak A, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PloS one. 2014;9(8):e104090 doi: 10.1371/journal.pone.0104090 ; PubMed Central PMCID: PMCPMC4130514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo NS, Jeong JH, Cho HJ, Wine JJ. Marked increases in mucociliary clearance produced by synergistic secretory agonists or inhibition of the epithelial sodium channel. Sci Rep. 2016;6:36806 doi: 10.1038/srep36806 ; PubMed Central PMCID: PMCPMC5103292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widdicombe JH, Welsh MJ, Finkbeiner WE. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985;82(18):6167–71. ; PubMed Central PMCID: PMCPMC391013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowley EA. Isoprostane-mediated secretion from human airway epithelial cells. Mol Pharmacol. 2003;64(2):298–307. doi: 10.1124/mol.64.2.298 . [DOI] [PubMed] [Google Scholar]
- 33.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82(2):503–68. doi: 10.1152/physrev.00029.2001 . [DOI] [PubMed] [Google Scholar]
- 34.Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, et al. Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase A signaling. Mol Pharmacol. 2004;66(2):337–46. doi: 10.1124/mol.66.2.337 . [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7. doi: 10.1074/jbc.R600038200 . [DOI] [PubMed] [Google Scholar]
- 36.Rajagopal M, Thomas SV, Kathpalia PP, Chen Y, Pao AC. Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells. Am J Physiol Cell Physiol. 2014;306(3):C263–78. doi: 10.1152/ajpcell.00381.2012 ; PubMed Central PMCID: PMCPMC3920002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee RJ, Foskett JK. cAMP-activated Ca2+ signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest. 2010;120(9):3137–48. doi: 10.1172/JCI42992 ; PubMed Central PMCID: PMCPMC2929731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, et al. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3(11):1020–4. doi: 10.1038/ncb1101-1020 . [DOI] [PubMed] [Google Scholar]
- 39.Ivonnet P, Salathe M, Conner GE. Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling. Br J Pharmacol. 2015;172(1):173–84. doi: 10.1111/bph.12934 ; PubMed Central PMCID: PMCPMC4280976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010;21(15):2639–48. doi: 10.1091/mbc.E09-12-1004 ; PubMed Central PMCID: PMCPMC2912350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci. 2012;57(11):2826–45. Epub 2012/08/28. doi: 10.1007/s10620-012-2352-8 ; PubMed Central PMCID: PMC3482986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Bazzaz FJ. Role of cyclic AMP in regulation of chloride secretion by canine tracheal mucosa. Am Rev Respir Dis. 1981;123:295–8. doi: 10.1164/arrd.1981.123.3.295 [DOI] [PubMed] [Google Scholar]
- 43.Al-Bazzaz FJ, Al Awqati Q. Interaction between sodium and chloride transport in canine tracheal mucosa. J App Physiol. 1979;46:111–9. [DOI] [PubMed] [Google Scholar]
- 44.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89(4):1148–53. Epub 1992/04/01. doi: 10.1172/JCI115696 ; PubMed Central PMCID: PMC442972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho HJ, Joo NS, Wine JJ. Mucus secretion from individual submucosal glands of the ferret trachea. American journal of physiology Lung cellular and molecular physiology. 2010;299(1):L124–36. doi: 10.1152/ajplung.00049.2010 ; PubMed Central PMCID: PMCPMC2904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345(6198):818–22. doi: 10.1126/science.1255825 ; PubMed Central PMCID: PMCPMC4346163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MC, Penland CM, Widdicombe JH, Wine JJ. Evidence that Calu-3 human airway cells secrete bicarbonate. Am J Physiol. 1998;274(3 Pt 1): L450–3. Epub 1998/04/08. . [DOI] [PubMed] [Google Scholar]
- 48.Garnett JP, Hickman E, Burrows R, Hegyi P, Tiszlavicz L, Cuthbert AW, et al. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem. 2011;286(47):41069–82. Epub 2011/09/15. doi: 10.1074/jbc.M111.266734 ; PubMed Central PMCID: PMC3220502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279(35):36454–61. doi: 10.1074/jbc.M404983200 . [DOI] [PubMed] [Google Scholar]
- 50.Conner GE, Ivonnet P, Gelin M, Whitney P, Salathe M. H2O2 stimulates cystic fibrosis transmembrane conductance regulator through an autocrine prostaglandin pathway, using multidrug-resistant protein-4. Am J Respir Cell Mol Biol. 2013;49(4):672–9. doi: 10.1165/rcmb.2013-0156OC ; PubMed Central PMCID: PMCPMC3824048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–7. doi: 10.1126/science.aad5589 ; PubMed Central PMCID: PMCPMC4852973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.