Summary
Post-ingestive signals related to nutrient metabolism are thought to be the primary drivers of reinforcement potency of energy sources. Here, in a series of neuroimaging and indirect calorimetry human studies, we examine the relative roles of caloric load and perceived sweetness in driving metabolic, perceptual and brain responses to sugared beverages. Whereas caloric load was manipulated using the tasteless carbohydrate maltodextrin, sweetness levels were manipulated using the non-nutritive sweetener sucralose. By formulating beverages that contain different amounts of maltodextrin+sucralose, we demonstrate a non-linear association between caloric load, metabolic response and reinforcement potency, which is driven in part by the extent to which sweetness is proportional to caloric load. In particular, we show that (1) lower calorie beverages can produce greater metabolic response and condition greater brain response and liking than higher calorie beverages and (2) when sweetness is proportional to caloric load greater metabolic responses are observed. These results demonstrate a non-linear association between caloric load and reward and describe an unanticipated role for sweet taste in regulating carbohydrate metabolism, revealing a novel mechanism by which sugar-sweetened beverages influence physiological responses to carbohydrate ingestion.
Keywords: gut-brain axis, dopamine, sugar, nucleus accumbens, obesity, gustation, fMRI, metabolism
Introduction
Traditionally, sweet taste has been considered an unconditioned stimulus that promotes ingestive behavior without requiring learning. Supporting this contention, newborns of a variety of species show appetitive responses to the taste of sugars [1]. However, recent work calls this view into question. Mice genetically engineered to lack the ability to sense sweet taste nevertheless form powerful preferences for nutritive, but not for non-nutritive sugars [2] and isocaloric sucrose and glucose are preferred over fructose [3]. In rodents, preferences are formed for non-caloric flavored liquids sensed during intra-gastric infusion of glucose but not saline [4], and in humans preferences are formed for flavors paired with the tasteless and odorless carbohydrate maltodextrin, but not for the sweet, but non-nutritive sweetener aspartame [5]. Furthermore, non-caloric flavored beverages previously consumed with 112.5 kcals of maltodextrin (i.e. conditioned stimulus “CS+” flavors) preferentially recruit striatal and hypothalamic circuits over non-caloric flavored beverages previously consumed without calories (i.e. CS- flavors) [6]. Importantly, the magnitude of this effect is proportional to the change in plasma glucose that occurred in the days preceding the fMRI study when participants consumed the caloric version of the beverage. Since glucose must be available in order to be metabolized, this finding provides indirect support that metabolic signals regulate brain response to calorie-predictive cues. Additional indirect evidence comes from reports that nucleus accumbens (NAcc) response to a variety of food cues is sensitive to peripheral metabolic signals associated with glucose metabolism [7–14].
Collectively, these studies from humans and rodents suggest that the reinforcing effects of sugar derive not from the perceived pleasantness of sweet taste but rather from a post-ingestive signal that regulates neural circuits that control feeding. However, controversy surrounds the nature of this signal, with some studies suggesting that the mechanism depends upon the interaction of nutrients with sensors in the gut lumen [15] and others indicating that metabolic response is critical. For example, preferences may be formed for non-metabolizable sugars [16] and at the same time blocking the metabolism of intra-gastrically administered glucose with intra-venous injection of 2-deoxy-glucose disrupts appetitive responding for glucose and blunts striatal dopamine release [17]. Thus, despite the importance of increased sugar consumption, especially in beverage form, in promoting diabetes and obesity [18–22], the mechanism behind sugar reward is incompletely understood.
Also controversial is the extent to which post-ingestive effects are relevant for understanding feeding in humans. For example, in two bottle preference tests thirsty rodents will overwhelming consume liquids from a bottle containing a flavor previously paired with intra-gastric glucose administration compared to one previously paired with saline infusion [23]. In contrast, in humans, although this “flavor-nutrient learning” has been demonstrated, effects are frequently weak or absent [5, 24–28], leading to the description of flavor nutrient learning in humans as an elusive phenomenon [28] that may be of minimal relevance for ingestive behavior. However, the vast majority of these studies rely on measuring liking and intake in a laboratory setting to assess learning. In contrast, when brain response is used as the outcome measure robust responses are observed in the NAcc and hypothalamus to flavors paired with, compared to without calories [6]. This suggests that flavor nutrient learning does occur in humans, but that it is unrelated to liking and unreliably translates into food intake in laboratory settings. However, since higher NAcc and hypothalamic responses to food cues are associated with obesity [29–32], genetic risk for obesity [33–35], eating in the absence of hunger [36], food choice [37, 38] future weight gain [6, 11, 39, 40], poorer performance on weight-loss trials [41] and overfeeding [42] these responses are clearly associated with behaviors related to overeating and obesity, underscoring the importance of better understanding of the role of the gut-brain axis in ingestive behavior and obesity. We therefore performed a series of fMRI, metabolic and behavioral studies to examine the relative roles of caloric load and perceived sweetness in driving metabolic and affective responses to sugared beverages.
We reasoned that if metabolic signals drive reinforcement then the caloric content of novel flavored beverages should predict conditioned flavor liking and brain response, reflecting the ability of higher caloric loads to generate greater metabolic responses. We further predicted that reinforcement potency should be linearly related to caloric load even if sweetness is held constant, reflecting the importance of post-ingestive signals in determining reward. To test these hypotheses we used the carbohydrate maltodextrin, which we verified was not detectable in our beverages (Figure 2 and Stars Methods), to provide calories, and the non-nutritive sweetener sucralose, which has no calories, to provide sweetness. This enabled us to generate beverages in which calories and sweetness were independently manipulated and where caloric and non-caloric versions of the beverages were indistinguishable. We then assessed the reinforcement value of five caloric loads of maltodextrin delivered in equally sweet and novel flavored beverages using an fMRI flavor-nutrient learning paradigm [6]. Contrary to our initial prediction we found that NAcc response depended upon the “match” between sweetness and caloric load rather than the absolute amount of calories ingested (i.e. the ratio between sucralose and maltodextrin). Subsequent fMRI flavor-nutrient learning and indirect calorimetry studies then confirmed that lower calorie beverages in which sweetness is matched to caloric load produce greater metabolic response and are more reinforcing than higher-calorie mismatched beverages. These surprising findings indicate that post-ingestive metabolic signals and not caloric load drive sugar reward and that sweet taste regulates the generation of these signals.
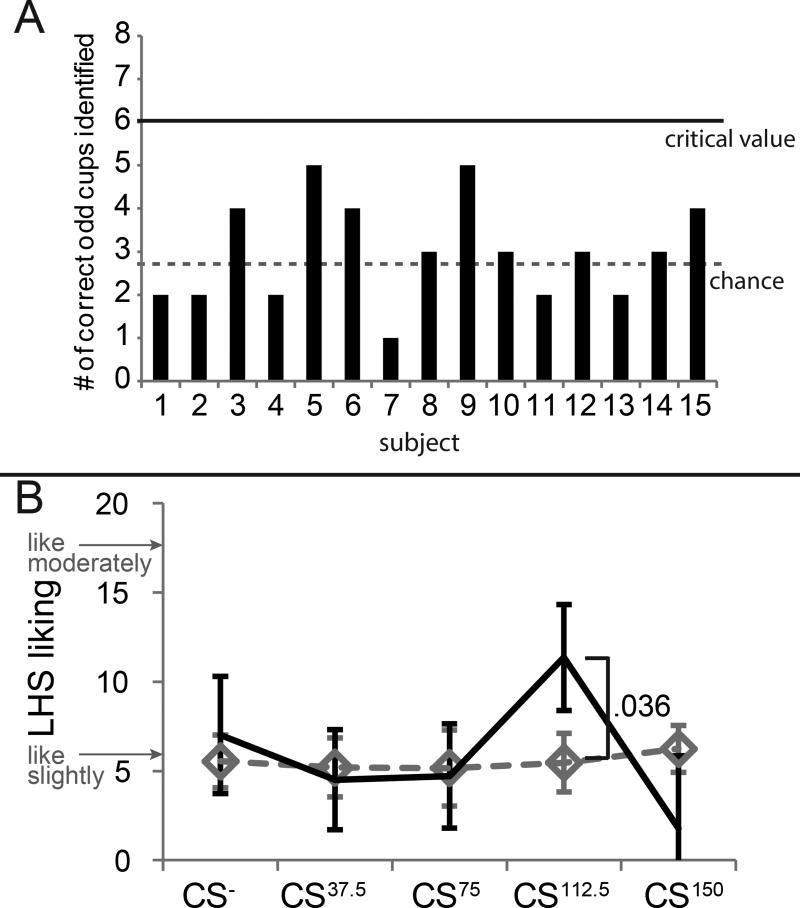
Figure 2. Experiment 1 perceptual results.
(A) Triangle Test. Y-axis = number of correct responses. X-axis = subjects. Six correct responses were required for reliable detection (critical value). All qualifying subjects performed at chance. (B) Liking ratings: Y-axis = liking rating on the labeled hedonic scale. X-axis = conditioned stimuli (CS), corresponding to the 5 beverages before (dotted) and after (solid) pairing with the caloric load indicated in superscript. Error bars (+/− SEM). See also Table S1.
Results
Four experiments were performed; using a combination of flavor nutrient learning paradigms coupled with fMRI to assess the reinforcing effects of the carbohydrate maltodextrin, and indirect calorimetry to assess metabolic response to different combinations of maltodextrin and sucralose. Detailed methods are provided in the STAR Methods.
Experiment 1: Lower calorie beverages are more reinforcing than equally sweet higher calorie beverages
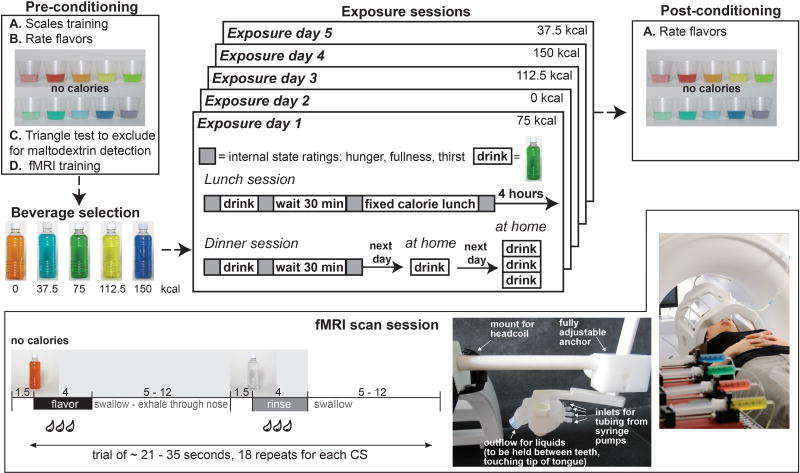
Our first experiment employed a flavor-nutrient conditioning paradigm in conjunction with fMRI to test if brain and behavioral measures of conditioning depend on caloric load independently of sweetness (Figure 1). Fifteen healthy weight participants (7 women, mean age 23.9 ± 3.5 years, mean body mass index (BMI) 22.4 ± 1.4 kg/m2 with average BMI always reflecting the full sample, i.e. both genders) rated their liking for 10 non-caloric beverages before and after 5 of the beverages were repeatedly consumed with one of 5 caloric loads of maltodextrin (0, 37.5; 75; 112.5 and 150 kcal). Post-conditioning fMRI was used to measure brain response to the non-caloric versions of the exposed beverages (CS- CS37.5, CS75, CS112.5, CS150). Importantly, triangle tests confirmed that participants could not differentiate caloric from non-caloric versions of the beverages (Figure 2A). All beverages were sweetened with sucralose to be approximately equivalent to the middle load (75 kcal) if the calories had come from sucrose. Sucrose was selected as our standard because it is used as table sugar and most people are familiar with adding sucrose to foods and beverages to adjust sweetness.
Figure 1. Design.
Pre-conditioning session: Subjects rated ten non-caloric versions of the flavored beverages. If five were rated similarly and slightly liked subjects performed a triangle test to rule out oral detection of maltodextrin (Figure 2A). Exposure sessions: Qualifying subjects were scheduled for 5 exposure days (1 for each of 5 beverages differing in maltodextrin load as shown). At each session two bottles of the same beverage were consumed in the lab, one in the evening and three the next day at home for a total of 6 exposures per beverage. Post-conditioning session: Subjects again rated the 10 non-caloric versions of the flavored beverages. fMRI scan session: Brain response to the non-caloric versions of the five beverages was assessed using previously validated fMRI protocols and flavor delivery methods. For further details please refer to the STARS methods.
Beverages were exposed over 5 sessions. During each session participants consumed one of the beverages 6 times: twice in the lab and four times at home. This provided 6 opportunities to associate the flavor of the beverage with its caloric load. Our primary prediction was that post-conditioning liking and mesolimbic response to the calorie predictive flavors would be proportional to caloric load, reflecting a linear association between calories and the generation of the metabolic signals that drive mesolimbic circuits.
Perceptual Ratings
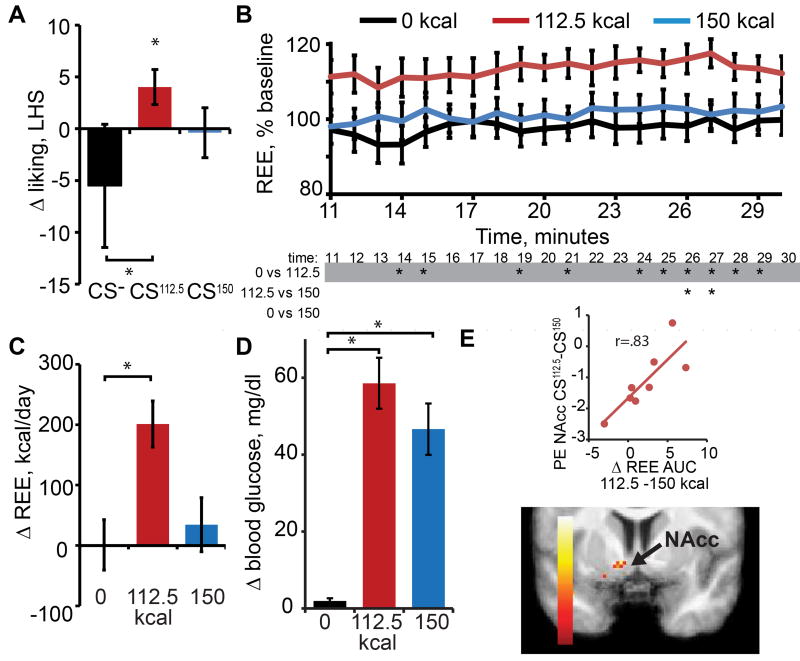
Repeated measures analyses of variance (ANOVA) of the perceptual ratings with time (pre/post) and beverage (CS- CS37.5, CS75, CS112.5, CS150) as a within-subject variables yielded no significant main effects or interactions for any of the ratings assessed (liking, wanting, intensity or sweetness) (Supplemental Table 1). However, based on our prior study showing increased liking for a flavor previously paired with 112.5 Kcal (CS112.5) [6], we performed a planned t-test to compare liking of the CS112.5 flavor before vs. after conditioning. Replicating that study, we found that liking increased from “like slightly” to just below “like moderately” at post-conditioning (p = .036) (Figure 2B). Interestingly, the liking ratings of CS150 showed that not only did liking not increase, but trended towards becoming less liked than the CS112.5 (p = .042 uncorrected or p = .168 with Bonferroni correction).
Repeated measures ANOVAs of hunger, fullness, and thirst ratings revealed a significant effect of time on hunger, fullness and thirst ratings. Post-hoc t-tests using Bonferroni correction showed that after consuming the beverage hunger and thirst decreases (p = .022 and p < .001 respectively) and fullness increases (p = .007). Results not shown.
Brain response
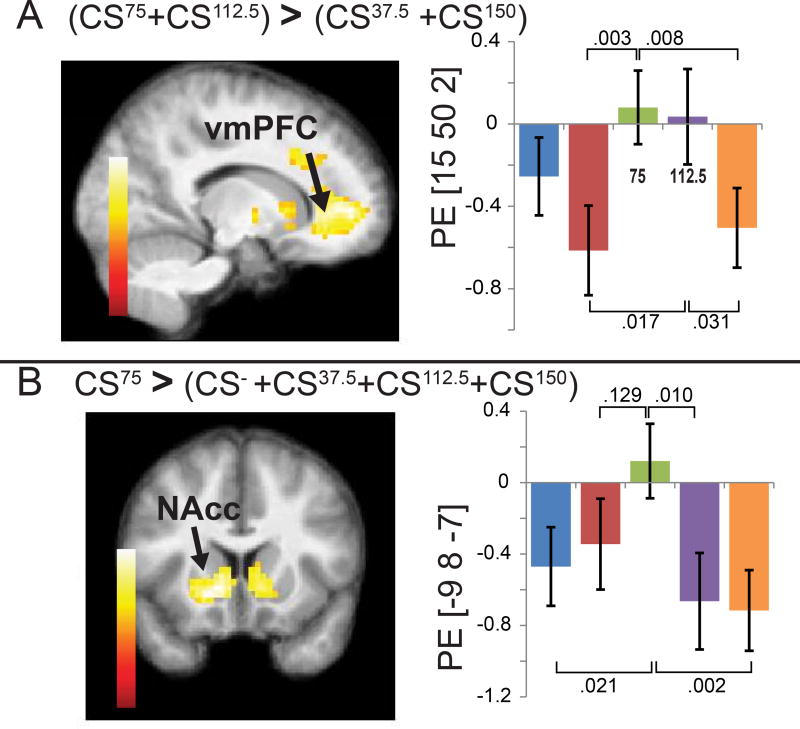
Data were pre and post-processed using Statistical Parametric Mapping (SPM) 12 according to our standard and previously published procedures [6] (and STAR Methods). We first tested for linear effects of caloric load on brain response by entering the SPM for each CS into an ANOVA and specifying a custom contrast of a monotonically increasing function of weights (CS−; −2, CS37.5; −1, CS75; 0, CS112.5; 1, CS150; 2) but, mirroring the perceptual data, none were observed. Therefore, we next examined the SPM of the F-contrast of caloric load to determine if there were nonlinear effects of load. This revealed significant differential response in the NAcc and medial prefrontal cortex (mPFC). Surprisingly, follow-up t-contrasts with Bonferroni correction showed that the NAcc response was driven by significantly greater response to the CS75 compared to all other stimuli, whereas the mPFC effect was driven by greater response to the CS75 and CS112.5 vs. the CS37.5 and CS150 (Figure 3 and Supplemental Table 1).The results from experiment 1 lead us to conclude that contrary to our prediction, the relationship between the caloric load and reinforcement is not linear, with lower calorie beverages conditioning greater liking or mesolimbic response than a higher calorie beverage. We therefore next set out to determine if a lower calorie beverage could induce greater metabolic response than a higher calorie beverage.
Figure 3. Experiment 1 fMRI results.
Neural response in the amygdala (Amy, A) we observed decreased response to CS37.5 compared to all other stimuli. Neural response in Nacc (B), was greater to the CS75 compared to all other stimuli. Brain sections show significant clusters (corrected for multiple comparisons) of voxels, with the bargraphs showing average parameter estimate (PE) in arbitrary units (+/− SEM) of the peak voxel in the cluster. For illustrative purposes we performed post-hoc t-test using Bonferonni correction to determine which CSs specifically were significantly different from CS75 or CS 37.5. Color bar indicates T-values of voxels. See also Figure S1 and Table S2.
Experiment 2: Lower calorie beverages generate greater metabolic response than equally sweet higher calorie beverages
We reasoned that if the critical signal for sugar reward derived from a metabolic response then ingestion of the 75 and/or 112.5 kcal-flavored beverage should result in a greater metabolic response compared to the 150 kcal beverage. To test this prediction, we performed a second experiment where we employed indirect calorimetry to measure diet-induced thermogenesis, which refers to the increase in energy expenditure above the resting metabolic rate that is due to the cost of processing food for use and storage. Resting energy expenditure (REE) was measured before and after 15 healthy weight participants (7 women, mean age 25.5 ± 3.6 years; mean BMI 22.2 ± 2.1) consumed equi-sweet (sweetened with sucralose to be equivalent to 75 Kcal of sucrose as in experiment 1) flavored beverages with 0-, 112.5-, and a 150 kcal (supplementary materials). The 112.5 kcal dose was selected over the 75kcal dose because we have previously demonstrated that this dose is an effective reinforcer, conditioning BOLD NAcc response to flavor as a function of a metabolic response (change in plasma glucose) [6].
Subjects first participated in an intake session, to which they arrived fasted (4 hours). Non-caloric versions of the beverages were sampled and rated as in study 1 and a triangle test was performed to exclude subjects who could detect maltodextrin in the flavored beverages. Subjects then returned to the lab for 3 subsequent sessions for calorimetry measurements (one beverage per day with order counterbalanced).
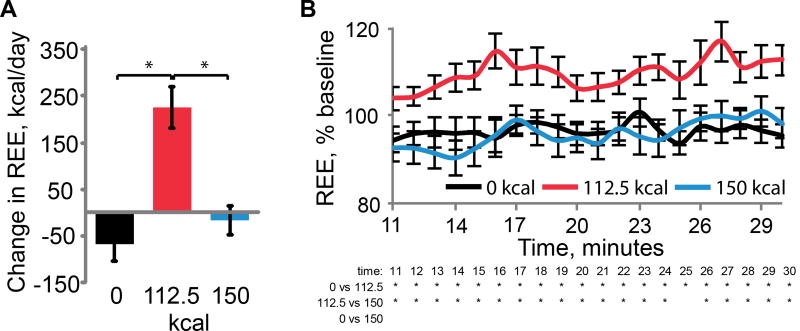
As predicted, but still remarkably, a repeated measures ANOVA looking at the average REE for 5 minutes preceding and 5 minutes post consumption (26–30 min post consumption) revealed a significant time by beverage interaction (F = 16.7; p <.001, with greater change in REE following consumption of the 112.5 Kcal compared to the 0 Kcal and 150 Kcal beverages (p-values .001 and .002 respectively, following Bonferroni correction) (Figure 4). Fourteen of 15 subjects showed this effect. We performed the analyses on the 26–30 min time point because this corresponds to the time when glucose should peak. The results of study 2 are therefore consistent with the findings from experiment 1 and further support the hypothesis that metabolic signals associated with changes in REE in response to nutrient consumption provide a critical signal for sugar reward, which is surprisingly independent of caloric load.
Figure 4. Experiment 2.
(A) Change in the average REE for 5 minutes preceding, and for 5 minutes post beverage consumption (25–30 min post consumption; y-axis) for each beverage (x-axis). (B) Line graphs present the data from 10 – 30 minutes post beverage consumption expressed as a percent of the mean REE for all beverages 10 minutes prior to consumption. A repeated-measures ANOVA revealed a main effect of beverage (F = 19.653, p<.001), with post-hoc t-test showing change in REE greater following consumption of the 112.5kcal beverage relative to both the 0kcal and 150kcal beverages at each time point. In this and following graphs asterisk stands for significant post-hoc t-test at α = .05 corrected with Bonferonni adjustment for multiple comparisons.
Experiment 3. Metabolic signals drive NAcc response independently of plasma glucose
We designed a third experiment seeking to replicate findings from experiments 1 and 2 and to determine if change in REE following consumption of caloric beverages correlates with NAcc response and change in plasma glucose. Nine healthy weight subjects (7 women; mean age 23.5 ± 1.9; mean BMI 21.2 ± 0.5;) performed the flavor-nutrient learning experiment described in study 1 concomitant to performing calorimetric and plasma measurements before and after consumption of the beverages on the exposure days. In addition to glucose, we measured insulin, ghrelin, leptin, hematocrit, hemoglobin, triglycerides and lactate. Our primary prediction was that change in REE following consumption of the 112.5 kcal beverage compared to the 150 kcal beverage would be correlated with NAcc response to the CS112.5 vs. CS150.
Perceptual Ratings
Replicating our previous report [6] and the results from study 1, planned comparisons (with Bonferroni corrections) showed that liking increased slightly for CS112.5 (p = .045), but not for CS150 (p = .877), and that there was a trend for liking at CS112.5 at post-test to be greater than for CS150 (trend at p=.056) (Figure 5A). No other effects were observed.
Figure 5. Experiment 3.
A) Change in liking (y – axis) pre vs. post-conditioning for each beverage (x-axis). Replicating study 1, planned comparisons reveal an increase in liking for CS112.5, but not CS150 (p = .03 and p = .83). (B) Change in REE across time (as in Figure 4B) depicting the main effect of beverage (F=5.994, p=0.011), with post-hoc t-tests showing change in REE greater following consumption of the 112.5kcal beverage relative to the 0kcal beverage at each time point and relative to the 150kcal at four timepoints. (C) The average change in REE as described in Figure 4A. Replicating study 2, there is greater change following the 112.5-kcal compared to the 0-kcal and 150-kcal beverages. (D) Change in blood glucose (y-axis) after consumption of each beverage (x-axis) depicting the effect of beverage, with greater change after 150- and 112.5-kcal beverages versus the non-caloric beverage. (E) Brain section shows significant clusters (corrected for multiple comparisons) of voxels. See also Table S3.
Metabolic measures
Replicating study 2, REE increased after consuming the 112.5 Kcal vs. 0 but not the 150 Kcal vs. 0 flavored beverages (time by beverage interaction, F=5.368, p = .016; with 112.5 vs. 0 p = 0.008, 150 vs. 0 p = 1.00) (Figure 5B). Repeated measures ANOVAs also showed that plasma glucose and insulin levels increased significantly following consumption of both caloric beverages compared to the non-caloric beverage (Table 1 and Figure 5D). However, changes were similar following consumption of the 112.5 and 150 Kcal beverages (Table 1). We also identified a significant time * beverage interaction for ghrelin, with significant decrease observed for the 112.5 vs. 0 but not he 150 vs 0. However, again the magnitude of change did not differ significantly for the 112.5 vs. the 150 Kcal beverage (Table 1). No other significant effects were observed. Therefore, the only metabolic measure distinguishing 112.5 and 150 Kcal beverages was change in REE. Finally, correlations were run to determine if change in liking or blood measures correlated with change in REE. No significant effects were observed after correcting for multiple comparisons.
Table 1.
Change in blood assay values after beverage consumption in Study 3.
| 0 kcal | 112.5 kcal | 150 kcal | F time * beverage |
P 0 vs 112.5 |
P 0 vs 150 |
P 112.5 vs 150 |
|
|---|---|---|---|---|---|---|---|
| Δ Glucose(xxx) | 1.95 ± 0.69 | 58.55 ± 6.63 | 46.62 ± 6.68 | 40.013* | <.001* | .001* | .283 |
| Δ Insulin (mIU/ml) | −0.45 ± 0.24a | 37.30 ± 4.99 | 33.23 ± 5.41 | 37.180* | .001* | .002* | 1.00 |
| Δ Ghrelin (pg/ml) | 45.56 ± 36.24 | −140.79 ± 31.60b | −82.88 ± 49.08 | 4.202* | .011* | .467 | 1.00 |
| Δ Leptin (µg/L) | −0.50 ± 0.20 | −0.028 ± 0.12 | 0.012 ± 0.13 | 3.397 | .378 | .087 | 1.00 |
| Δ Hematocrit (%) | 0.21 ± 0.15 | 0.04 ± 0.21 | −0.39 ± 0.25 | 2.326 | 1.00 | .367 | .399 |
| Δ Hemoglobin (g/dl) | −0.14 ± 0.19 | −0.26 ± 0.13 | −0.19 ± 0.08 | .157 | 1.00 | 1.00 | 1.00 |
| Δ Triglycerides (mg/dl) | 3.98 ± 4.79 | −0.20 ± 3.78 | 1.73 ± 1.53 | .503 | .309 | 1.00 | 1.00 |
| Δ Lactate (mmol/L) | −0.15 ± 0.09 | −0.14 ± 0.04 | 0.01 ± 0.04 | 2.358 | 1.00 | .289 | .187 |
All data represent mean ± SEM.
significant at α = .05, post-hoc t-tests Bonferroni corrected for multiple comparisons
Brain response
Pre and post-processing proceeded as in study 1. To test our primary prediction that differential response in the NAcc to the CS112.5 vs. the CS150 would be correlated with differential metabolic response following the ingestion of the caloric versions of the beverages, we regressed the difference in change in REE for 112.5 vs 150 against the differential brain response to the CS112.5 vs. the CS150. As predicted we identified a significant positive association between NAcc response to the CS112.5 vs. the CS150 and change in REE (Figure 5E, Supplemental Table 2). Thus we are able to replicate, in the same participants, the paradoxical effects observed in study 1 and 2 and show that change in REE is related to NAcc response. In addition, consistent with a prior report [6], NAcc response was unrelated to change in liking.
Experiment 4: Sweet taste regulates metabolic response
Collectively, the findings from experiments 1–3 reveal that a lower calorie beverage becomes more liked, conditions greater brain response, and elicits greater metabolic response than a higher calorie, but equally sweet beverage. This suggests that metabolic response, rather than caloric load determines the reinforcement potency of the carbohydrate maltodextrin. Still unexplained, however, is why the lower calorie beverage produces greater metabolic response than the higher calorie beverage. Since sweet taste perception can produce cephalic phase responses such as insulin secretion [43], we reasoned that one possibility might be that holding sweetness constant resulted in an inappropriate cephalic response for the amount of ingested nutrient. If so, then altering the sweetness of a fixed calorie beverage should influence change in REE. To test this possibility we next examined change in REE following consumption of novel beverages with caloric load from maltodextrin held constant (either 150 kcal or 75 kcal) and sweetness varied, such that (1) sucralose was added to “match” sweetness to 150 kcal or 75 kcal (i.e. 150/150 and 75/75, where kcal/sweetness) or (2) sucralose was added to produce a “mismatch” to create a beverage that was not sweet enough 150/75 or too sweet 75/150. Ten participants (7 female; age 22.7 ± 4.74; BMI 22.62 ± 1.225) completed the experiment, which was conducted over 4 days using similar procedures as above.
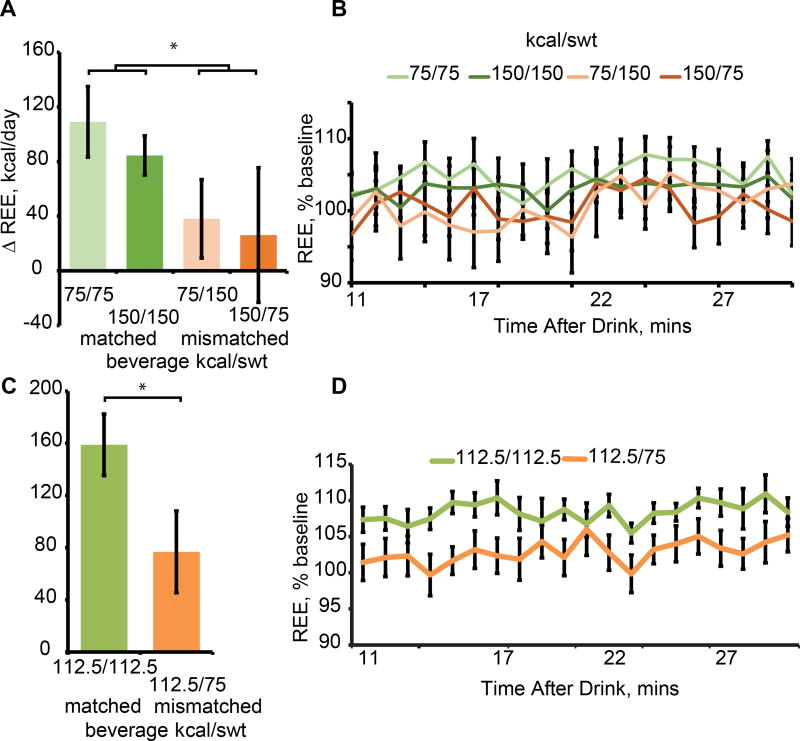
As predicted, but nevertheless remarkably, a repeated measures ANOVA yielded a significant time * stimulus interaction (F =3.056, p = .018) such that REE changed significantly for the two matched compared to the two mismatched beverages (p = .003) (Figure 6A and B). However, comparison of the overall magnitude of change shows that the matched 150 Kcal beverage produces a similar change as the matched 75 Kcal beverage, with both changes considerably smaller (approximately half the magnitude) compared to the changes observed in response to the 112.5 Kcal beverage in experiments 2 and 3. To determine if sweetness also influences metabolic response to 112.5 we measured REE in a second group of 18 participants (12 female; age 24.1 ± 3.6; BMI 21.9 ± 2.1) before and after drinking 112.5 kcal beverages sweetened appropriately or to 75 kcal. A paired t-test again indicated that REE changed significantly more for the matched beverage (t(17) = −2.208, p =.041) (Figure 6C and D). These data demonstrate that sweetness and caloric load influence change in REE, producing a highly non-linear association between caloric load and metabolic response.
Figure 6. Experiment 4.
(A) The average change in REE as described in Figure 4A for the 75 Kcal and 150 Kcal beverages with matched and mismatched calories and sweetness. (B) Change in REE across time. (C) The average change in REE for the matched and mismatched 112.5 Kcal beverage. (D) Change in REE across time.
Post-hoc Analysis
Finally, we reasoned that if sweet taste influences the metabolic signal underlying reinforcement then response in the NAcc, observed in experiment 1, should be proportional to the extent of mismatch between sweet taste and calories. We therefore returned to the data collected in experiment 1 and tested whether response in the mesolimbic reward system is influenced by the degree of mismatch between sweet taste and caloric load. Using a factorial ANOVA with contrasts weighted by degree of mismatch, we verified this prediction. Specifically, the degree of mismatch for each of the flavors was associated with NAcc response (Supplemental Information Figure 1). No other significant associations were observed. We therefore conclude that the nonlinear effect identified in the NAcc in experiment 1 reflects the ratio between sweet taste (sucralose) and carbohydrate load (maltodextrin).
Discussion
Our experiments reveal three novel findings, two of which were very unsuspected. First, as predicted, we have found that metabolic response, independent of caloric load and plasma nutrient level provides a critical signal by which flavor cues are associated with nutritional value in the mesolimbic reward system. Second, we show that this metabolic response is regulated by sweet taste perception, establishing a novel and unanticipated role for sweet taste perception in carbohydrate metabolism. Third, we observed an independent influence of caloric load on metabolic response resulting in lower calorie beverages being more reinforcing than higher calorie beverages. Collectively, these findings reveal a non-linear relationship between caloric load and metabolic response and suggest a novel mechanism by which artificial sweeteners and sugar-sweetened beverages might disrupt normal physiological responses to carbohydrate ingestion.
Metabolic signals drive nutrient reinforcement in mesolimbic circuits
Our results provide strong evidence that in humans, as in animals [2, 17] it is a metabolic signal that determines the reinforcing value of carbohydrates. More specifically, we demonstrate that an initially similarly liked lower calorie beverage produces greater change in REE, stronger conditioned responses in the mesolimbic system, and greater increases in flavor liking compared to a higher calorie beverage, suggesting that it is the metabolic fate of the ingested nutrient that drives reinforcement rather than its caloric load. This is further supported by the observation that both the 150 kcal and the 112.5 kcal beverages increase blood glucose and insulin compared to the non-caloric drink, whereas only the 112.5 kcal beverage increases REE, and conditions liking and NAcc response (Figure 5). In other words, ingestion of the nutrient and its transport from the gut to the blood stream is not sufficient to condition reinforcement. Rather, a metabolic response must be generated. One caveat, is that compared to the 0 Kcal beverage, ghrelin levels changed significantly following consumption of the 112.5 Kcal but not the 150 Kcal beverage. This is consistent with more efficient absorption of 112.5 Kcal and raises the possibility that ghrelin signaling may be involved in the observed effects.
The current findings also highlight the implicit nature of this signal. Although we replicate prior work in showing weak increases in liking following the pairing of a flavor with 112.5 Kcal [5, 6], this change in liking is consistently not related to NAcc or metabolic response. First, in our prior report, neither NAcc response nor change in plasma glucose correlated with change in liking [6]. Second, in experiment 1 of the current work liking increased for CS112.5 flavor but NAcc response was driven by the CS75 flavor. Third, in experiment 3 change in REE, but not change in liking, was correlated with NAc response, and change in liking was unrelated to change in REE. Collectively, these findings underscore the existence of separate mechanisms supporting the effects of conditioning on liking versus the neural representation of calorie predictive cues in the striatum, which is consistent with the distinctions draw between wanting and liking [44], and may explain the failure to observe robust flavor-nutrient learning in humans when explicit ratings of liking are measured [26, 28].
Taste regulates metabolic response
Our data also show that sweet taste regulates the metabolic fate of carbohydrates. More specifically, we demonstrate that metabolic response to carbohydrate ingestion depends, in part, upon the match between the sweet taste and the caloric load. This is demonstrated in experiment 4 where change in REE is greater for 75, 112.5 and 150 kcal beverages when sweetness is matched to the load compared to when it is mismatched (either too high (75/150) or too low (112.5/75; (150/75)). Of note, however, is the fact that change in REE for the matched beverages is greatest for the 112.5 Kcal beverage (Figures 4–6). This has two implications. First, it suggests that the match between sweet and caloric load and the absolute caloric load independently influence change in REE. Second, it suggests that the influence of taste on metabolic response will vary as a function of caloric load. Testing this will require a comprehensive analysis of taste – calorie associations.
Error signaling
Another important avenue for future work will be to disentangle the role of metabolic response versus error signaling in driving NAcc response. More specifically, when the match between sweet taste and caloric load was used to model BOLD response to 0, 37.5, 75, 112.5 and 150 kcal predictive flavors robust, bilateral, whole brain corrected responses are observed in NAcc (Supplemental Figure 1 and Figure 3). This suggests that NAcc response best reflects the match between sweetness and caloric load. However, here match is directly proportional to the generation of prediction errors, which are known drivers of striatal dopamine [45] and BOLD response [46, 47]. In other words, the extent to which the metabolic response following calorie ingestion deviates from that predicted by the sweet taste cue may drive NAcc response, rather than, or in addition to, a metabolic signal. As such, response to the CS75 is greater than the CS112.5 (Figure 3B). Nevertheless, it is clear that change in REE following consumption of the 112.5 Kcal beverage is greater than change in REE following consumption of the 75 kcal beverage and that change in REE is a strong predictor of BOLD response to the CS112.5 (Figure 5). Also of note, is the relatively weaker response to the matched 150 kcal beverage compared to the matched 75 kcal beverage. This is noteworthy because it suggests that gastric emptying, which is slower for higher caloric loads [48], plays a role in the underlying mechanism.
Another unanswered question is the precise role of change in REE in post-ingestive reward. Change in REE reflects diet induced thermogenesis (also known as the thermic effect of food), which reflects the amount of every expenditure required to digest, store, use and dispose of nutrients. Although it is possible that this dose-independent effect reflects a signaling mechanism it is equally possible that it is epiphenomenal and simply correlated with the as-of-yet unidentified critical mechanism. Further, as our data are correlational, it is not possible to know whether metabolic signals drive brain response or brain response drives metabolic signals.
Regardless of the mechanistic underpinnings, the regulation of carbohydrate by sweet taste, and the non-linear effect of caloric load on the metabolic signal that drives reward have important implications. The modern food environment offers many energy sources where sweet taste ligands and carbohydrate co-occur but in “artificial” combinations. Sugar-sweetened beverages now constitute the largest source of calories and added sugars for both children and adults in the United States [19]. Accordingly, epidemiological studies report strong associations between sugar-sweetened beverage intake and weight gain, obesity, type2 diabetes, and coronary heart disease [18, 21, 22, 49]. Notably, although some sugar-sweetened beverages are sweetened with only a single sugar, these beverages are in the minority. For example, Powerade contains glucose, fructose, sucralose and Acesulfame K while Coca cola ™, Sprite ™ and Pepsi ™ all contain fructose and glucose [50]. Yogurts also frequently contain multiple varieties of sweeteners. The popular American yogurt Chobani Simply 100 ™ contains 14 grams of carbohydrate, 6 of which are from sugars but also locust bean gum and stevia leaf extract, which are sweet. Our findings raise the possibility that nutrient partitioning is altered under these mismatched conditions, which suggests a novel mechanism by which sugar-sweetened beverages might negatively influence physiological responses to carbohydrate ingestion. Accordingly, a critical next step will be to determine the fate of the glucose that is not metabolized following consumption of mismatched beverages. Presumably, metabolism is either delayed or the glucose is stored. Determining whether, and if so, how this impacts metabolic health is therefore an important avenue for future research. It will also be important to investigate if the observations we report with liquids generalize to solid food, and/or if consumption of diet or mismatched beverages with energy dense foods impacts the metabolic fate of nutrients. This is an important question because energy dense meals are frequently consumed with diet drinks.
One important consideration is that there is no a priori reason why our biology would not be geared to learn that certain foods contain more energy than others even though this is not reflected in their sweetness. There are many natural foods where sweet taste and caloric load are mismatched, and many carbohydrates that are not sweet (e.g. potato). We have previously argued that flavor perception evolved to facilitate this learning, while providing a mechanism for maintaining the integrity of sweet taste as an indicator of sugar availability [51]. Sweetness reflects the quantity of sugar present across many foods. Flavor, on the other hand, which occurs when taste and smell are integrated, is unique to particular energy sources (in nature), with preferences learned. As such, “strawberry” can be associated with the overall nutritional value while sweetness remains an important indicator of sugar availability (which, for example, will vary as a function ripeness). We therefore suggest that the metabolism of sugars in novel foods and beverages, such as the ones used here, will be initially determined by sweetness. However, we predict that overtime, learning will occur as the flavor becomes a better predictor of the overall nutritional properties. Future work is required to test this hypothesis.
Conclusion
In summary, we have found that metabolic response, independent of caloric load and plasma nutrient level provides a critical signal by which food cues are associated with nutritional value in the mesolimbic reward system. We also show that metabolic response is influenced by sweetness, establishing a novel and unanticipated role for sweet taste perception in carbohydrate metabolism and reward.
STARS METHODS
CONTACT FOR REAGENT AND RESOURCES SHARING
“Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dana Small (dana.small@yale.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Participants in all studies provided informed consent on protocols approved by the Yale University School of Medicine Human Investigation Committee. All subjects reported having no known taste, smell, metabolic, neurologic or psychiatric disorder. Subjects were recruited from the New Haven area by means of flyers, Facebook and radio advertisements.
Experiment 1
Fifteen healthy weight participants (7 women, mean age 23.9 ± 3.5 years, mean body mass index (BMI) 22.4 ± 1.4 kg/m2 with average BMI always reflecting the full sample, i.e. both genders).
Experiment 2
Fifteen healthy weight participants (7 women, mean age 25.5 ± 3.6 years; mean BMI 22.2 ± 2.1).
Experiment 3
Nine healthy weight subjects (7 women; mean age 23.5 ± 1.9; mean BMI 21.2 ± 0.5).
Experiment 4
Sample A) Ten participants (7 women; mean age 22.7 ± 4.74; mean BMI 22.62 ± 1.225) completed the experiment. Sample B) Eighteen; mean age 24.1 ± 3.6.
METHODS DETAILS
Experiment 1
The full design of study 1 is depicted in Figure 1. Subjects participated in a preconditioning session, 5 exposure days, a post-conditioning session and an fMRI scan session.
Pre-Conditioning Session
Stimuli
Stimuli were ten differently flavored non-caloric beverages, contained 0.1% (w/v) citric and 0.0078% sucralose (Sigma-Aldrich Inc. MO, USA) dissolved in demineralized water. The beverages also each had a flavor and a color that were assigned in a counterbalanced manner prior to the pretest, such that there was no consistent flavor-color pairing across subjects (flavor-color pairing was kept constant for each subject across sessions). The flavors used were 0.002% acerola, 0.5% bilberry, 0.1% horchata, 0.1% lulo, 0.2% yuzu. 0.1% papaya, 0.1% chamomile, 0.1% aloe vera, 0.1% mamey, and 0.2% maqui berry (Bell Labs Flavors and Fragrances Inc., IL, USA, product numbers: 33.81940, 15.80182, 132.81478, 141.14606. 101.29478, 102.82506, 141.31243, 141.31480, 46.29969 and 13.32059). The flavors are relatively novel and absent of gustatory or trigeminal components. Food coloring (McCormick & Co, Inc., MD, USA) was used to make the beverages pink, yellow, green, blue, purple, red, orange, teal, yellow-green or indigo. Pilot testing with the in-lab formulated beverages showed that they were near neutral and similarly liked (data not shown). For the maltodextrin triangle test we randomly selected a SoBe lifewater (“mango melon”, “black and blueberry”, “yumberry pomegranate”, “acai fruit punch” or “Fuji apple pear” from PepsiCo, NY, USA) that contained <5 calories. The lifewater was mixed with maltodextrin (Spectrum, CA, USA) at 34% (w/v), equivalent to 150 calories in 355 ml. Since water activates taste cortex [52, 53] and has a taste [54], we used “artificial saliva” as a flavorless control stimulus and rinse for the fMRI scan [55]. Stimuli in the pretest phase were all presented as 10 ml aliquots in 30 ml medicine cups. Demineralized water was available for rinsing between stimuli.
Procedure
Subjects were instructed not to eat or drink anything except water for four hours prior to each session since conditioning has been shown to be stronger when hungry [56]. Upon arrival we used a Q-tip to swab the subject’s cheek and told them that this would allow us to detect whether she/he had fasted as instructed. These samples were not actually assayed, but were intended to encourage compliance. Each subject was asked to indicate her/his internal state by rating how hungry, full, and thirsty she/he was, on VAS scales that recorded responses from 0 (e.g. “Not Hungry At all”) to 100 (e.g. “Very Hungry”), a recurring set of ratings that we will refer to as “internal state ratings” from here on. The control solution was then selected by presenting the dilution series of artificial saliva pairwise in a two alternative-forced-choice procedure in which the subject indicated “which of these two solutions tastes most like nothing?” The subject was next trained to make ratings using the general labeled magnitude scale (gLMS) for intensity ratings [57, 58] and the labeled hedonic scale (LHS) for liking ratings [59].
In order to familiarize the subject with the stimuli, the ten different beverages were first presented (once each) without requiring the subject to make any ratings. The subject was instructed to take the solution entirely into her/his mouth, swish it around, and expectorate into a sink. The subject then rinsed and paused for 30 seconds before continuing to the next sample. Next, utilizing the same sip-and-spit procedure the ten different beverages were each presented three times (counterbalanced pseudo-random orders), but before rinsing the subject was instructed to rate the stimulus for overall intensity, sweetness intensity, liking and wanting. Wanting was measured with a VAS with the labels “I would never want to drink this” at the left anchor, “neutral” in the middle and “I would want to drink this more than anything” on the right anchor). The presentation order of the scales was counterbalanced across subjects. Averages were obtained for each flavor rating. The averaged liking ratings were evaluated to determine if a subset of five flavors was rated above “neutral” (0) but below “like moderately” (17.82) on the LHS. The five flavors were also required to be similarly liked, for which there needed to be overlap between standard deviations of the averaged liking ratings. If such a subset of flavors could be determined, the subject continued in the preconditioning session (and the subset of five eligible flavors became that subject’s “conditioning” flavors for the exposure phase). If not, the subject was excluded from further participation. Approximately 40% of subjects were excluded for this reason, but we felt that it was justified so that we could rule out baseline liking ratings as contributing to the predicted increases in liking resulting from conditioning.
To verify that study participants could not detect the presence of maltodextrin (purportedly a tasteless and odorless carbohydrate) in the exposure phase of the experiment, they participated in a triangle test in which they indicated which of three cups was different. All cups contained the same flavor, but for each trial, either one or two cups also contained maltodextrin. Eight trials were conducted. We used the binomial distribution to set our criteria for maltodextrin detection; specifically, the minimum number of correct judgments to establish significance for the triangle test (one-tailed, α = 0.05, z = 1.64, probability of guessing p = 1/3) was calculated according to the formula
where n = number of trials, X = minimum number of correct judgments, and z = 1.64 if α is set to 5% [60]. Therefore, for n = 8 tests, the minimum number of correct judgments X=6.
Following this the subject participated in a training session in the fMRI simulator, in order to familiarize the individual with the task and to confirm they were comfortable with all aspects of the fMRI experimental procedure (no subject reported experiencing discomfort with the procedure). For the training, the subject underwent one full 13-minute run in the fMRI simulator. Briefly, while lying supine in an fMRI simulator, we presented 1ml aliquots Sobe Lifewaters (mango melon and black and blueberry), and a photo of a PET-bottle containing the Sobe Lifewater.
Exposure phase
Stimuli
Based on the subject’s liking of the ten flavors, a subset of five were selected to be paired with the five caloric doses, and consumed by the subjects during the exposure sessions. Each of these flavors was paired with a specific nutrient dose by adding maltodextrin at five different increments (0, 37.5, 75, 112.5, or 150 calories), with the highest dose equivalent to a standard 12 fl oz (355 ml) can of soda. We created a total of six 355 ml beverages for each flavor-nutrient load combination, in PET-bottles. Beverage flavor, color, caloric load, and presentation order were all counterbalanced and mixed by one of the authors (MGV) who was not in contact with the subject during the exposure sessions. The beverages were not labeled for their caloric content at any time. Thus the conditioning phase of this study was double-blind.
Procedure
There were a total of five exposure days (1 per beverage) conducted within a two-week period. In each session one of the five selected beverages was consumed on six different occasions. The first two occasions were in-lab and the remaining four were at home. Subjects were requested to consume the same breakfast at the same time of morning for each exposure day. The in-lab session then began 30 minutes before the subject’s normal lunch time and at least four hours after their normal breakfast time. The session began with a cheek swab, completion of a food diary on the content and time of their breakfast that morning, and rating their internal state as outlined above. Following their ratings, the subject consumed the beverage at their own pace but within five minutes. Immediately after the drink the subject made another set of internal state ratings, relaxed for 30 minutes, and then again rated their internal state. Subsequently, they were provided with a lunch (of their choice, but fixed across lunch sessions) that they consumed in the lab before rating their internal state one final time. The subject returned to the lab four hours later for their dinner session having not consumed anything (but water) between sessions. The dinner session followed the same procedure as the lunch session, except that the dinner session ended with the ratings made after the 30-minute wait, at which time the subject was instructed to eat dinner autonomously outside the lab. In order to maximize the number of exposures subjects were given another four bottles to consume at home: 1) one hour after dinner, 2) one hour before lunch the next day, 3) one hour before dinner the next day, and 4) one hour after dinner the next day. Prior work suggests has reported success with at-home conditioning sessions [61]. To encourage compliance subjects signed a sheet stating that they adhered to the instructions to the best of their ability. They were also asked to return the empty bottles to the lab. All subjects complied with these instructions.
Post-conditioning session
The same stimuli and procedures from pretest were used to collect ratings for the ten beverages (without calories added) at a post-conditioning session that occurred before the fMRI scan.
fMRI scan
Stimuli and stimulus delivery
The subjects’ five exposure beverages (without calories added) were used during the fMRI session, and the flavorless solution was used as both the rinse and a control stimulus. The subject’s exposure flavors and selected tasteless solution were delivered as 1mL of solution over 4 s from syringe pumps with a gustometer system that has previously been described in detail [55]. In brief, this system consists of computer-controlled syringe pumps which infuse liquids from syringes filled with flavor solutions into an fMRI-compatible custom designed gustatory manifold via 25-foot lengths of Tygon beverage tubing (Saint-Gobain Performance Plastics, Akron, OH, USA). The gustatory manifold is mounted on the MRI headcoil and the tubes anchor into separate channels that converge over a stylus, which rests just inside the subject’s mouth. When a pump is triggered liquid drops from the channel onto the stylus and comes in contact with the tongue.
Experimental Design
During the fMRI scanning session, the subject performed one anatomical run and a total of three functional runs, each 13 minutes long. A long event-related design was used (for details of events within each trial see Figure 1). During each of the runs, each flavor that was selected for the exposure sessions as well as the flavorless control was presented six times in a pseudo-random order, resulting in a total of eighteen repeats for each stimulus.
fMRI Data Acquisition
We measure the blood oxygenation-level dependent (BOLD) signal as an indication of cerebral brain activation with echo planar imaging, acquired on a Siemens 3T TIM Trio scanner. Echo planar imaging was used to measure the blood oxygenation-level dependent (BOLD) signal as an indication of cerebral brain activation. A susceptibility-weighted single-shot echo planar method was used to image the regional distribution of the BOLD signal with parameters of: TR: 2000ms; TE: 20 ms; flip angle: 90°; FOV: 220 mm; matrix: 64 × 64; slice thickness: 3 mm, and number of slices: 40. Slices were acquired in an interleaved mode to reduce the crosstalk of the slice selection pulse. At the beginning of each functional run, the MR signal was allowed to equilibrate over six scans for a total of 12 s, which were then excluded from analysis. The anatomical scan used a T1-weighted 3D FLASH sequence (TR/TE: 2530/3.66 ms; flip angle: 20°; FOV: 256; matrix: 256 × 256; slice thickness: 1 mm; number of slices: 176).
Debriefing
Immediately after the fMRI scan the subject was debriefed about the goal of the study, the manipulation with the caloric dose of the beverages, and saliva collection. None of the subjects professed any awareness of the manipulation.
Experiment 2
In experiment 2 we used indirect calorimetry to test the hypothesis that the 112.5 Kcal beverage would produce greater change in resting energy expenditure (REE) compared to a non-caloric and a 150 Kcal beverage, reflecting a larger metabolic response (i.e. thermic effect of food). Subjects participated in three calorimetry sessions (one for each beverage). Beverages were prepared and selected as in experiment 1, with the exception that only three were used. Triangle tests as described above were used to exclude individuals that could detect the maltodextrin.
Participants arrived in the lab having consumed only water for at least four hours prior to arrival. Resting energy expenditure (REE) was measured with a TrueOne 2400 Metabolic Measurement System (ParvoMedics, Inc., Sandy, Utah) for at least 15 minutes before and for at least 30 minutes immediately after consumption of one of the 355 ml-beverages. The participant, lying supine on a reclined dentist chair, with a clear metabolic canopy placed over head and neck, remained motionless without falling asleep. The system measures the ratio of carbon dioxide production over oxygen consumption from the expired air and calculates REE for every minute of the testing session [62]. Beverages contained 0, 112.5, or 150 kilocalories provided by maltodextrin, and were presented in a randomized crossover design on separate days. Beverage color and flavor were randomly selected from those described in study 1 and remained constant for each participant across caloric dose.
Experiment 3
Study 3 combined the designs of the first and second experiments and in addition included collection of blood samples to assess the influence of caloric beverage consumption on plasma glucose, insulin, ghrelin, leptin, triglycerides, hematocrit and hemoglobin. Participants completed a preconditioning session using the same stimuli and procedure described in study 1, except that only 4 flavors were required to be similarly liked.
Exposure Phase
Exposure phase stimuli were prepared as described in study 1, except that we used 3 caloric loads rather than 5 (0, 112.5, and 150 kcal). Participants were exposed to 3 beverages (each containing a different load) a total of 6 times, as described above. Indirect calorimetry was performed on one afternoon session and blood draws were performed on the other.
Blood Draw Session
In order to determine the influence of beverage consumption on plasma glucose blood samples were collected at one of the lunch exposure sessions for each beverage. The IV was inserted after the cheek swab sample was collected. After IV insertion, participants waited 30 minutes to stabilize before the blood was drawn prior to drink consumption. A second blood sample was taken 30 minutes after the beverage consumption and the IV was removed (before lunch). The blood samples were immediately spun down and glucose, insulin, ghrelin, triglycerides, hematocrit and hemoglobin assayed. A small aliquot of whole blood (∼0.3ml) was used for the immediate analyses of hematocrit and hemoglobin. Another aliquot was transferred into a tube with potassium EDTA anticoagulant for the determination of ghrelin. The remaining sample was transferred into a tube with no anticoagulant for the determination of blood insulin, glucose and triglycerides. The samples were centrifuged, frozen immediately and stored at −80°C until analysis. Ser um concentration of insulin and plasma concentration of ghrelin was measured using the competitive binding radioimmunoassay method. Intra and inter assay coefficient of variation for the mid-range standard for insulin [45 (4.5) uIU/ml] was 2.3% and 3.7% (Siemens Healthcare Diagnostics, Los Angeles, CA). Intra and inter assay coefficients of variation for ghrelin (standards low range 418–868 pg/mL) were 1.6% and 2.9% (Millipore Corp., Billerica, MA). Plasma glucose and triglycerides were measured using the modified Trinder method through a colorimetric endpoint (Eagle Diagnostics, DeSoto, TX). Whole blood hemoglobin is also a colorimetric assay based on the determination of cyanmethemoglobin (Eagle Diagnostics, DeSoto, TX). Hematocrit is measured by drawing up whole blood into heparinized hematocrit tubes and centrifuged for 3 minutes. The percentage of red blood cells vs. plasma is read on a micro-capillary plate.
fMRI Scan
Stimuli, Design, and Data Acquisition
Stimulus delivery, study design, and data acquisition were the same as in study 1 with the following exceptions: Participants only received 4 non-caloric beverages during scanning (the three exposed flavors 0, 112.5, and 150 kcal and the unexposed control). Each stimulus was delivered 21 times over three 12-min 38-sec functional runs.
Experiment 4
Experiment 4 was performed to test the hypothesis that sweet taste regulates metabolic response to liquid carbohydrate ingestion. Indirect calorimetry was used as described in experiment 2. Two sub-experiments were performed with independent samples (see above).
As in the previous experiments, calories were manipulated with maltodextrin and sweetness with sucralose. We created four beverages; two with 75 Kcal and two with 150 Kcal. One of the 75 Kcal beverages was “matched” to be equivalent to the sweetness of a 75 kcal beverage and the other “mismatched” and sweetened to the equivalent of a 150 kcal beverage. Likewise, one of the 150Kcal beverages was matched and sweetened appropriately with sucralose while the other was mismatched and sweetened to the equivalent of a 75 Kcal beverage.
as if the source of calories was sucrose. These factorial combinations then led to 2 beverages that have are “matched” in terms of calories and sweetness, and two beverages that are “mismatched” in calories and sweetness, one “not sweet enough” (sweetness equivalent to 75 kcal, caloric content of 150 kcal, labelled 150/75) and one “too sweet” (sweetness equivalent 150 kcal, caloric content of 75 kcal, labelled 75/150). As in study 1, the drinks also contained citric acid and were colored with food coloring. For group 2 the same procedure was used to create the drinks; however, both beverages were of the same flavor and caloric load (112.5 Kcal). One was sweetened appropriately (112.5/112.5 and one was not sweet enough 112.5/75).
QUANTIFICATION AND STATISTICAL ANALYSIS
Experiment 1
Analysis of Behavioral data
In order to account for across-subject differences in using the various rating scales, we converted each observation to a standard score by subtracting the subject’s mean of all observations on that scale and then dividing the difference by the standard deviation of all observations made on that scale.
The five flavors that were presented during the exposure phase were sorted according to the caloric load that the flavor had been paired for that particular subject. We refer to the flavor that was paired with 0 calories (which across subjects has a different flavor and color) as CS−. For the flavors paired with calories (CS+s) we used the following notations: CS37.5, CS75, CS112.5, CS150.
We used within-subjects ANOVA analyses in PWAS for Windows (release 19.0.0, Chicago SPSS Inc.) to evaluate the effect of manipulation of the independent variable caloric load on internal state ratings and to evaluate the effect of association with caloric load on liking, intensity, sweetness and wanting (see Supplementary Results section for details). If sphericity of the data was violated (as determined by Mauchly’s test), we used adjusted df values (Greenhouse-Geisser correction). Post-hoc planned comparison t-tests were employed to examine differences between the various caloric loads. We used an alpha of 0.05 to determine significance.
fMRI analysis
Data were analyzed on Linux workstations using Matlab (MathWorks, Inc., Sherborn, MA) and SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Functional images were slice-time acquisition corrected using sinc interpolation to the slice obtained at 50% of the TR. All functional images were then realigned to the scan immediately preceding the anatomical T1 image. The images (anatomical and functional) were then normalized to the Montreal Neurological Institute template of grey matter. Images were then detrended, using a method for removing at each voxel any linear component matching the global signal [63]. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel. For the time-series analysis on all subjects, a high pass filter of 300 s was included in the filtering matrix (adapted to the period of presentation in this long event-related design) in order to remove low-frequency noise and slow drifts in the signal. Condition-specific effects at each voxel were estimated using the general linear model.
In our design there first is the presence of visual cue throughout the entire trial, the taste and oral somatosensory component while the solution drips into the mouth (although this presumably is constant across all CSs), and the retro-nasal olfactory component that peaks after swallowing the solution (see Figure 1). Therefore, we specified the onset of each event of interest as occurring 6.5 seconds post taste onset. The events of interest were the six different stimuli (taste-/odorless control, CS−, CS37.5, CS75, CS112.5, CS150), estimated from onset of the taste. Rinses were modeled as nuisance effects. No head movement regressors were included, as all subjects included had head movements within 1 mm. Parameter estimate images from each subject, for each of the six event types, were entered into a second level analysis using full factorial ANOVA with caloric load as a factor with 5 different levels. We first tested for linear effects of caloric load on brain response by specifying a custom T-contrast of a monotonically increasing function of weights (CS−; −2, CS37.5; −1, CS75; 0, CS112.5; 1, CS150; 2). We also specified an F-contrast of caloric load to determine if there were nonlinear effects of load. To follow-up on any observations in this F-contrast (which is non-directional), we specified T-contrasts to compare each of the CSs to all others. Regions of interest F and T-maps of contrasts were thresholded for display at Puncorrected = 0.005 at the voxel level, with an extent threshold of 5 contiguous voxels. Clusters were considered significant at p < 0.05 family wise error (FDR) corrected for multiple comparisons at the cluster level across the whole brain.
Experiment 2
Analysis
Changes in REE were analyzed in two ways. First to determine if caloric load differentially influences REE we calculated change in REE for each beverage by subtracting the average REE 5-minutes (1-minute time bins) before consumption (allowing subjects at least 10 minutes to “cool down”) from average REE 26–30 min after consumption. This time window was selected because this is when glucose levels should peak. A one-way ANOVA was then performed on the change scores to determine if change differed depending upon the beverage. Second, we calculated a global average baseline REE by averaging the observed REE at each 1-minute time bin for the 10 minutes preceding consumption, collapsing across beverage and participant. Change in REE at each 1-minute time bin from 11 – 30 minutes post consumption was then expressed as a percentage of the mean baseline. Percent change in REE was then contrasted in a 2-way repeated-measures ANOVA, with time and beverage (i.e. 0, 112.5, 150 kcal drinks) using SPSS. This analysis allowed us to determine the stability of change in REE.
Experiment 3
Data Analysis
Preprocessing was performed as described in study 1. The events of interest included the five different stimuli (tasteless/odorless control, CS−, CS112.5, CS150, and unexposed control), estimated from 6.5 s post onset of the taste. To determine the influence of beverage on NAcc response, parameter estimates (PE) were extracted from a 6mm sphere around the centre coordinate of (−6,8,−5) from our previous paper showing robust responses are observed in the NAcc flavor nutrient conditioning [6]. PEs were obtained for each of the three CSs by themselves and compared to each other and correlated in SPSS with the corresponding change in REE that occurred during the calorimetry exposure sessions, change in blood glucose during the blood draw exposure sessions and change in liking from pre to post conditioning sessions.
Experiment 4
Change in REE was calculated as described in Study 2. We performed a 2 × 4 repeated measures ANOVA with time (pre, post beverage consumption) and stimulus (75/75, 150/150, 75/150 and 150/75) as within-subjects factors and gender and age as covariates. We created custom post-hoc t-tests to contrast the matched and the mismatched beverages.
Data and software availability
Raw MRI data: OpenfMRI repository, pending
Statistical maps of the human brain: Neurovault repository, http://neurovault.org/collections/2570/ (Study 1), http://neurovault.org/collections/2588/ (Study 3).
All other data: Mendeley Data, http://dx.doi.org/10.17632/94tc9t3txs.1
Supplementary Material
Acknowledgments
Study 1 and part of study 3 was funded by PepsiCo. Studies 2–4 were funded by NIDCD (R01 DC006706) and NIDDK & NIDA (R01 R01DK085579). The authors would like to thank Nina Stachenfeld and Cheryl Leone for instruction and advice with the indirect calorimetry experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
DMS conceived and designed all studies, oversaw all analyses and wrote the paper. MGV collected data in study 1, performed all analyses and co-wrote the manuscript. NK helped with post-hoc data analyses from study 1. RKB and BP collected data and performed analyses for studies 2 and 3, and contributed to manuscript preparation. WF, collected and analyzed the data in study 1. EG collected the data in study 4. MY advised on experimental design for study 1 and contributed to manuscript preparation.
References Cited
- 1.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neuroscience and biobehavioral reviews. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 2.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sclafani A, Zukerman S, Ackroff K. Postoral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chemical senses. 2015;40:245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sclafani A. Oral and postoral determinants of food reward. Physiology & behavior. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiology & behavior. 2008;93:798–806. doi: 10.1016/j.physbeh.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 6.de Araujo IE, Lin T, Veldhuizen MG, Small DM. Metabolic regulation of brain response to food cues. Current biology : CB. 2013;23:878–883. doi: 10.1016/j.cub.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstone AP, Miras AD, Scholtz S, Jackson S, Neff KJ, Penicaud L, Geoghegan J, Chhina N, Durighel G, Bell JD, et al. Link Between Increased Satiety Gut Hormones and Reduced Food Reward After Gastric Bypass Surgery for Obesity. The Journal of clinical endocrinology and metabolism. 2016;101:599–609. doi: 10.1210/jc.2015-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, et al. Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. The American journal of clinical nutrition. 2014;99:1319–1330. doi: 10.3945/ajcn.113.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes care. 2013;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstadt-Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN. (Still) longing for food: insulin reactivity modulates response to food pictures. Hum Brain Mapp. 2013;34:2367–2380. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroemer NB, Sun X, Veldhuizen MG, Babbs AE, de Araujo IE, Small DM. Weighing the evidence: Variance in brain responses to milkshake receipt is predictive of eating behavior. NeuroImage. 2016;128:273–283. doi: 10.1016/j.neuroimage.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. 2015;6:8543. doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Vugt DA, Krzemien A, Alsaadi H, Palerme S, Reid RL. Effect of insulin sensitivity on corticolimbic responses to food picture in women with polycystic ovary syndrome. Obesity. 2013;21:1215–1222. doi: 10.1002/oby.20148. [DOI] [PubMed] [Google Scholar]
- 14.Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, Cabeza de Vaca S, Sclafani A, Carr KD. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiology & behavior. 2016;159:52–63. doi: 10.1016/j.physbeh.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drucker DB, Sclafani A. The role of gastric and postgastric sites in glucose-conditioned flavor preferences in rats. Physiology & behavior. 1997;61:351–358. doi: 10.1016/s0031-9384(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 16.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and nonmetabolizable sugar analogs. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tellez LA, Ren X, Han W, Medina S, Ferreira JG, Yeckel CW, de Araujo IE. Glucose utilization rates regulate intake levels of artificial sweeteners. The Journal of physiology. 2013;591:5727–5744. doi: 10.1113/jphysiol.2013.263103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiology & behavior. 2010;100:47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malik VS, Hu FB. Sweeteners and Risk of Obesity and Type 2 Diabetes: The Role of Sugar-Sweetened Beverages. Current diabetes reports. 2012 doi: 10.1007/s11892-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 20.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. The American journal of clinical nutrition. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference, and indifference produced by intragastric infusions of galactose, glucose, and fructose in rats. Physiology & behavior. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 24.Brunstrom JM. Dietary learning in humans: directions for future research. Physiology & behavior. 2005;85:57–65. doi: 10.1016/j.physbeh.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Brunstrom JM, Mitchell GL. Flavor-nutrient learning in restrained and unrestrained eaters. Physiology & behavior. 2007;90:133–141. doi: 10.1016/j.physbeh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Brunstrom JM, Rogers PJ, Myers KP, Holtzman JD. In search of flavour-nutrient learning. A study of the Samburu pastoralists of North-Central Kenya. Appetite. 2015;91:415–425. doi: 10.1016/j.appet.2015.04.079. [DOI] [PubMed] [Google Scholar]
- 27.Hogenkamp PS, Stafleu A, Mars M, de Graaf C. Learning about the energy density of liquid and semi-solid foods. Int J Obesity. 2012;36:1229–1235. doi: 10.1038/ijo.2011.231. [DOI] [PubMed] [Google Scholar]
- 28.Yeomans MR. Flavour-nutrient learning in humans: an elusive phenomenon? Physiology & behavior. 2012;106:345–355. doi: 10.1016/j.physbeh.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond) 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein Ewing SW, Claus ED, Hudson KA, Filbey FM, Yakes Jimenez E, Lisdahl KM, Kong AS. Overweight adolescents’ brain response to sweetened beverages mirrors addiction pathways. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Val-Laillet D, Layec S, Guerin S, Meurice P, Malbert CH. Changes in brain activity after a diet-induced obesity. Obesity. 2011;19:749–756. doi: 10.1038/oby.2010.292. [DOI] [PubMed] [Google Scholar]
- 33.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. Int J Obes (Lond) 2012;36:638–647. doi: 10.1038/ijo.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:160–165. doi: 10.1073/pnas.1605548113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. 2012;63:415–422. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- 37.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, Schur EA. Regional brain response to visual food cues is a marker of satiety that predicts food choice. The American journal of clinical nutrition. 2012;96:989–999. doi: 10.3945/ajcn.112.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. The American journal of clinical nutrition. 2013;97:15–22. doi: 10.3945/ajcn.112.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. The American journal of clinical nutrition. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 43.Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite. 2008;51:622–627. doi: 10.1016/j.appet.2008.04.271. [DOI] [PubMed] [Google Scholar]
- 44.Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience and biobehavioral reviews. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 45.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 46.Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiology of learning and memory. 2014;114:90–100. doi: 10.1016/j.nlm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14735–14744. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacGregor IL, Wiley ZD, Lavigne ME, Way LW. Slowed rate of gastric emptying of solid food in man by high caloric parenteral nutrition. American journal of surgery. 1979;138:652–654. doi: 10.1016/0002-9610(79)90338-6. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J, American Heart Association Nutrition Committee of the Council on Nutrition, P.A. et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 50.Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity. 2011;19:868–874. doi: 10.1038/oby.2010.255. [DOI] [PubMed] [Google Scholar]
- 51.Small DM. Flavor is in the brain. Physiology & behavior. 2012;107:540–552. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Frey S, Petrides M. Re-examination of the human taste region: a positron emission tomography study. European Journal of Neuroscience. 1999;11:2985–2988. doi: 10.1046/j.1460-9568.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 53.Zald DH, Pardo JV. Cortical activation induced by intraoral stimulation with water in humans. Chemical Senses. 2000;25:267–275. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- 54.Bartoshuk LM, McBurney DH, Pfaffmann C. Taste of Sodium Chloride Solutions after Adaptation to Sodium Chloride: Implications for the “Water Taste”. Science. 1964;143:967–968. doi: 10.1126/science.143.3609.967. [DOI] [PubMed] [Google Scholar]
- 55.Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to Detect Taste in a Tasteless Solution: Modulation of Early Gustatory Cortex by Attention to Taste. Chemical Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- 56.Mobini S, Chambers LC, Yeomans MR. Effects of hunger state on flavour pleasantness conditioning at home: flavour-nutrient learning vs. flavour-flavour learning. Appetite. 2007;48:20–28. doi: 10.1016/j.appet.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘labeled magnitude scale’ for measuring sensations of taste and smell. Chemical Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 58.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiology & Behavior. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Lim J, Wood A, Green BG. Derivation and Evaluation of a Labeled Hedonic Scale. Chem. Senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawless HT, Heymann H. Principles and practices. Gaithersburg: Aspen Publisher, Inc.; 1999. Sensory Evaluation of Food. [Google Scholar]
- 61.Appleton KM, Gentry RC, Shepherd R. Evidence of a role for conditioning in the development of liking for flavours in humans in everyday life. Physiology & Behavior. 2006;87:478–486. doi: 10.1016/j.physbeh.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 62.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.