Summary
Eukaryotic 60S ribosomal subunits are comprised of three rRNAs and ∼50 ribosomal proteins. The initial steps of their formation take place in the nucleolus, but, owing to a lack of structural information, this process is poorly understood. Using cryo-EM, we solved structures of early 60S biogenesis intermediates at 3.3 Å to 4.5 Å resolution, thereby providing insights into their sequential folding and assembly pathway. Besides revealing distinct immature rRNA conformations, we map 25 assembly factors in six different assembly states. Notably, the Nsa1-Rrp1-Rpf1-Mak16 module stabilizes the solvent side of the 60S subunit, and the Erb1-Ytm1-Nop7 complex organizes and connects through Erb1’s meandering N-terminal extension, eight assembly factors, three ribosomal proteins, and three 25S rRNA domains. Our structural snapshots reveal the order of integration and compaction of the six major 60S domains within early nucleolar 60S particles developing stepwise from the solvent side around the exit tunnel to the central protuberance.
Keywords: ribosome, assembly, ribosome biogenesis, nucleolus, cryo-electron microscopy, Nsa1, Erb1, pre-60S, large subunit assembly
Graphical Abstract
Highlights
-
•
Structures of five nucleolar pre-60S intermediates by cryo-EM
-
•
Assembly factors Nsa1, Mak16, Rpf1, and Rrp1 form a module at the solvent side
-
•
Erb1 acts as a central coordinator at the intersubunit side
-
•
A sequential assembly pathway follows after the 5′ to 3′ circular formation of pre-rRNA
Cryo-EM analysis of the architecture of pre-60S ribosomes provides insights into the sequential events and intermediate states critical for ribosome assembly, as well as the functions of many associated factors.
Introduction
Eukaryotic ribosomes, the protein-synthesizing molecular machines, consist of a small subunit (SSU) (40S) and a large subunit (LSU) (60S), which comprise four ribosomal RNAs (18S, 5S, 5.8S, and 25S/28S) and around 80 ribosomal proteins (RPs). The assembly of ribosomal subunits starts in the nucleolus, where RNA polymerase I transcribes the rRNA precursor (35S pre-RNA in yeast), from which, after processing and removal of the external and internal transcribed spacers (ETS and ITS), the mature 18S, 5.8S, and 25S rRNAs are generated (Woolford and Baserga, 2013). The pre-RNA is modified during transcription by small nucleolar ribonucleoproteins (snoRNPs), processed by RNA nucleases, and assembled with numerous RPs (Turowski and Tollervey, 2015). After endonucleolytic cleavage of the rRNA precursor at site A2 in yeast, the pre-40S and pre-60S subunits follow separate biogenesis routes.
Co-transcriptional folding and assembly intermediates of pre-ribosomes were first visualized as compact knobs on rDNA-chromatin spreads, representing the 90S pre-ribosome or SSU processome (Grandi et al., 2002, Miller and Beatty, 1969). Here, the modular assembly of the 90S particle provides a scaffold for the domain-wise folding of the 18S precursor rRNA (Chaker-Margot et al., 2017, Kornprobst et al., 2016, Sun et al., 2017). In contrast to the 40S subunit, the architecture of the 60S subunit is more complex with its six highly intertwined domains of the 25S rRNA (Ben-Shem et al., 2011). Around 90 assembly factors (AFs) have been associated with 60S biogenesis (Woolford and Baserga, 2013). Proteomic analysis has revealed that early nucleolar intermediates show a high degree of complexity with respect to AF association and that the number of AFs generally decreases throughout the maturation pathway (Nissan et al., 2002, Wu et al., 2016). During this development, it is thought that the incorporation of RPs occurs hierarchically via the sequential integration of early-, middle-, and late-acting groups of RPs (Gamalinda et al., 2014).
Complex early pre-60S intermediates containing the 27SB pre-rRNA and the 5S rRNA could be isolated by affinity purification methods based on tagged assembly factors, among which Nsa1 yielded a nucleolar pre-60S particle of distinct protein and rRNA composition (Kressler et al., 2008). Notably, these particles contained numerous early-acting AFs, including the conserved Erb1-Ytm1 complex (Kressler et al., 2008). Release of Nsa1 and the Erb1-Ytm1 complex from these particles is mediated through the action of the remodeling AAA-ATPases Rix7 and Rea1, respectively (Bassler et al., 2010, Kressler et al., 2008). To date, however, nothing is known about the structure of such early biogenesis intermediates, and, accordingly, it remains to be determined how pre-60S subunits form and evolve during the first steps of their nucleolar assembly. Major structural transitions are expected to occur during this early phase, as indicated by the finding that the release of the Erb1-Ytm1 complex is linked to the exit of the particle from the nucleolus to the nucleoplasm (Bassler et al., 2010). Here, another set of AFs associates with these intermediate pre-60S particles, including the Arx1-Alb1 dimer that binds at the already formed ribosomal tunnel exit site (Bradatsch et al., 2012, Leidig et al., 2014, Wu et al., 2016). As there is no structural characterization of any nucleolar intermediate, this nucleoplasmic Arx1-containing particle (also referred to as Arx1 or Nog2 particle) represents the earliest observed pre-60S structure at present. In addition to Arx1, this pre-60S particle is associated with further AFs, including Rsa4, Nog2, and the Rpf2-Rrs1 complex, in the area of the unrotated 5S RNP (Bradatsch et al., 2012, Leidig et al., 2014, Nissan et al., 2002, Ulbrich et al., 2009, Wu et al., 2016) and typically exhibits a landmark structure, called “foot,” consisting of five biogenesis factors (Nop15, Cic1, Nop7, Rlp7, and Nop53) and partially processed ITS2 (Wu et al., 2016). Surprisingly, in this intermediate, the central protuberance (CP) together with the bound 5S RNP (5S rRNA and RPs uL18 and uL5) is stabilized in an immature, 180°-rotated state (Leidig et al., 2014, Wu et al., 2016). However, apart from the CP, several helices on the inter-subunit side and the ITS2, the rRNA within the Arx1 particle has acquired a close-to-mature globular conformation. Prior to nuclear export, the Rix1-Rea1 complex exerts a further remodeling step, likely releasing the Rpf2-Rrs1 complex and resulting in the rotation of the 5S RNP, as well as the release of Rsa4 (Barrio-Garcia et al., 2016, Ulbrich et al., 2009, Wu et al., 2016). Export competence of the particle is achieved in two steps, release of the guanosine triphosphase (GTPase) Nog2 allows recruitment of the export adaptor Nmd3, whereas Yvh1-binding-mediated release of Mrt4 allows binding of the export receptor Mex67-Mtr2 (Matsuo et al., 2014, Sarkar et al., 2016). Upon exit into the cytoplasm, the pre-60S particle undergoes final maturation, which is mostly dedicated to AF removal and incorporation of the last remaining RPs (Lo et al., 2010). Here, Drg1, a further AAA-type ATPase, is required for the recycling of the assembly factors Rlp24 and Nog1, resulting in an ordered cascade of assembly factor release events (Kappel et al., 2012, Lo et al., 2010, Pertschy et al., 2007). The removal of the export factor Nmd3 is enabled through incorporation of the RP uL16 and the action of the GTPase Lsg1 (Ma et al., 2017, Malyutin et al., 2017). It is thought that in a final step, removal of the anti-association factor Tif6 by the combined action of the Shwachman-Bodian-Diamond syndrome homolog Sdo1 and the elongation factor G homolog Efl1 leads to translationally competent LSU (Menne et al., 2007, Weis et al., 2015).
The known pre-60S structures depict the rRNA to a large extent already in a mature state. As even the nucleoplasmic Arx1 or Nog2 particle only show rRNA at the intersubunit side and the central protuberance in a pre-mature state, the question arises of how the majority of the rRNA folds in preceding nucleolar pre-60S intermediates to adopt a monolithic and intertwined conformation. To elucidate the order of these folding events and the underlying mechanisms, we have performed a cryoelectron microscopy (cryo-EM) analysis of nucleolar pre-60S particles isolated from yeast. This analysis provided insight into the nucleolar pre-60S assembly steps, which allowed us to assign roles to the multiple biogenesis factors and deduce the principles of rRNA folding during 60S biogenesis.
Results
Cryo-EM Analysis of Stage-Ordered Nucleolar Pre-60S Particles
We selected several biogenesis factors that allow isolation of nucleolar pre-60S particles upon affinity purification following two different strategies. First, we applied the split-tag affinity purification method (based on two different bait proteins, each labeled with a different affinity tag followed by two subsequent affinity purifications) to restrict the pool of assembly intermediates. Second, we aimed to isolate early pre-60S particles via a single bait protein from yeast mutants arresting at, and thus accumulating, a distinct stage during early pre-60S biogenesis.
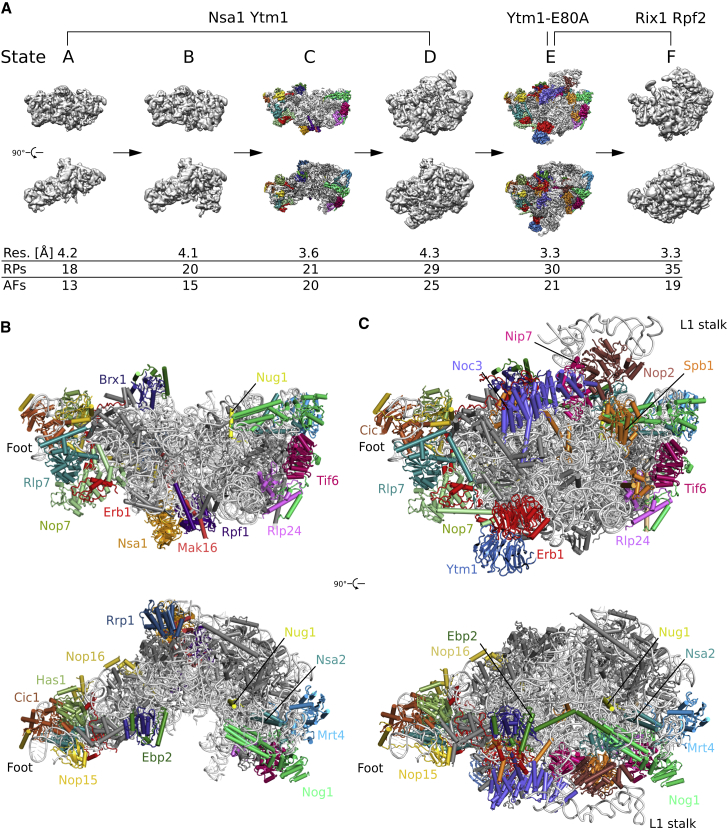
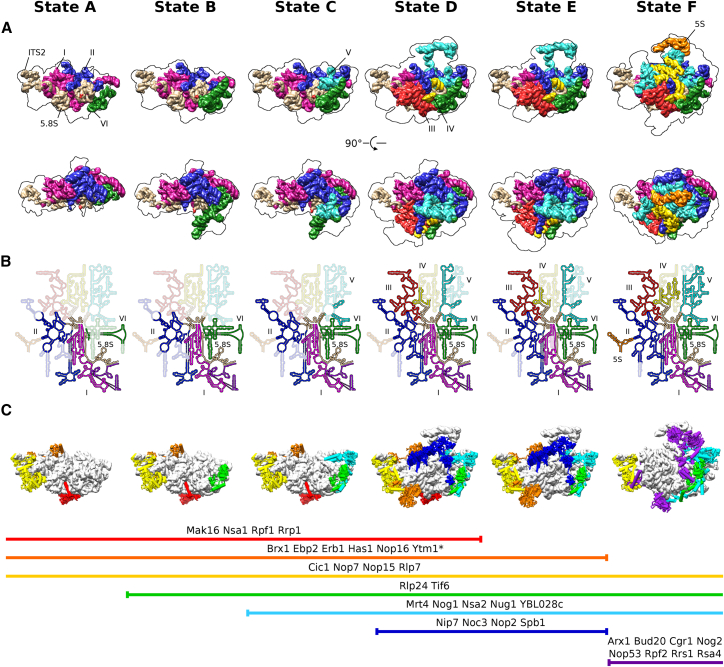
Following the first strategy, we affinity purified bona fide pre-60S particles from wild-type yeast cells using combinations of tagged early pre-60S assembly factors, the first pair consisting of Nsa1 and Ytm1 (Nsa1-TAP, Flag-Ytm1) and expected to enrich nucleolar pre-60S assembly intermediates, and the second pair of Rix1 and Rpf2 (Rix1-TAP, Rpf2-Flag), which could enrich further progressed pre-60S particles (Figures S1A and S1B). Regarding the mutant approach, we selected the dominant-lethal TAP-tagged Ytm1 E80A allele (Figures S1C and S1D), which allows affinity purification of a distinct pre-60S particle. It harbors a single amino-acid substitution in the N-terminal ubiquitin-like (UBL) domain of Ytm1, and, similar to the complete deletion of the UBL domain, this mutant is impaired in the interaction with the AAA-type ATPase Rea1. Consequently, Rea1 cannot remove the Erb1-Ytm1 complex, thereby blocking further maturation (Bassler et al., 2010). When all these different affinity purified pre-60S particles were analyzed by single-particle cryo-EM and 3D classification, six distinct and stable pre-60S maturation states could be obtained, which in the following will be referred to as states A to F (Figures 1A, S2, and S3). These intermediates were put into a consecutive order (from states A to F), based on the increase of structured rRNA and incorporation of RPs, which are thought to follow a hierarchical assembly pathway (Gamalinda et al., 2014) (Figures 1A and S4). Consistent with our experimental design, the four earliest intermediates (A to D) were isolated via the Nsa1-Ytm1 baits, whereas the Rix1-Rpf2 pair yielded the intermediates of the two later states (E and F). Importantly, only state F pre-60S particles displayed a clearly visible and stably incorporated 5S RNP module. This state represents the previously observed nucleoplasmic pre-60S particle, which can be affinity purified with either Arx1 or Nog2 as bait (Leidig et al., 2014, Wu et al., 2016). Therefore, we used the Arx1/Nog2 particle model as basis for the interpretation of the State F map (Wu et al., 2016). Using the dominant-lethal Ytm1 E80A mutant to affinity purify pre-60S subunits, we indeed observed an enrichment of particles, which are structurally indistinguishable from type E particles derived from wild-type cells (Rix1-TAP/Rpf2-Flag), with only a minor portion of particles resembling earlier intermediates similar to states A–C. Importantly, the state E pre-60S population yielded the highest resolved structure at 3.3 Å average resolution (Figures 1A, S2, and S3).
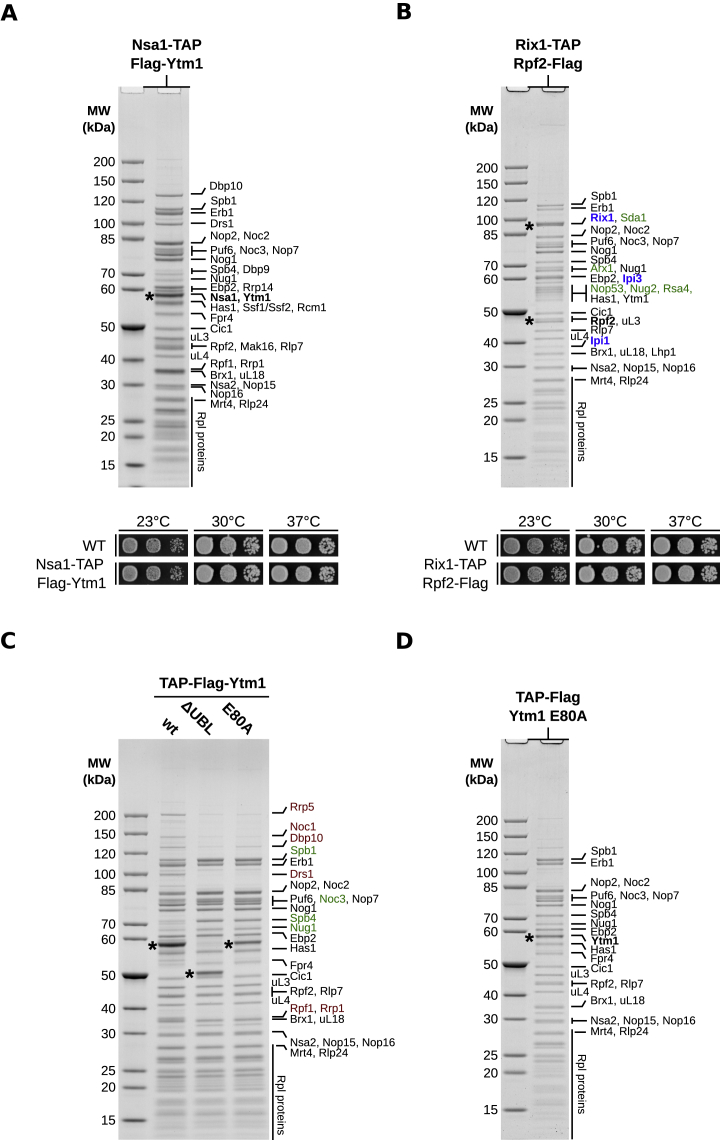
Figure S1.
Affinity Purification of Nucleolar Pre-60S Particles for Cryo-EM Analysis, Related to Figure 1
(A and B) Split affinity purifications of pre-60S intermediates (upper panels) purified through Nsa1-TAP Flag-Ytm1 (A) and Rix1-TAP Rpf2-Flag (B). Final eluates were used for cryo-EM and analyzed by SDS-PAGE and Coomassie staining. Major protein bands were identified by mass-spectrometry and are labeled on the right side of the gels. The bait proteins are shown in bold and indicated by asterisks. The Rix1-TAP Rpf2-Flag purification purifies nucleolar (state E) and nucleoplasmic (state F) pre-60S particles (B). Nucleoplasmic assembly factors are indicated in green and the Rix1 subcomplex members (Rix1, Ipi3, Ipi1) in blue. Growth analysis (lower panels) of the Nsa1-TAP Flag-Ytm1 (A) and the Rix1-TAP Rpf2-Flag (B) strains in comparison to the untagged wild-type strain (DS1-2b). Cells were spotted in 10-fold serial dilution on YPD medium and cell growth at the indicated temperatures was monitored after 2 days.
(C) Affinity purifications of the indicated plasmid-encoded Ytm1 variants fused to an N-terminal TAP-Flag tag and expressed from the endogenous promoter. The plasmids were transformed into a wild-type strain (W303). Final eluates of the purifications were analyzed by SDS-PAGE and Coomassie staining and co-purifying proteins are indicated on the right side of the gel and bands corresponding to the Ytm1 bait proteins are marked with an asterisk. Proteins decreased in the Ytm1 ΔUBL/E80A purifications compared to the Ytm1 wild-type particle are shown in red and factors increased on the mutant particles are indicated in green.
(D) Affinity purification of the Ytm1 E80A particle used for cryo-EM analyzed by SDS-PAGE. Proteins bands were identified by mass-spectrometry and are labeled on the right side of the Coomassie stained gel.
Figure 1.
Cryo-EM Structures of Nucleolar Pre-60S Assembly Intermediates
(A) Cryo-EM densities filtered to 10 Å in the case of states A, B, D, and F. Maps of states C and E are filtered to 3.6 Å with the identified biogenesis factors highlighted in color. Below each map, the overall resolution (res.), the number ribosomal proteins (RPs), and assembly factors (AFs), which are stably associated with the core particle and thus are resolved in the cryo-EM structures, are shown. The resolution of state E corresponds to the Ytm1-E80A particle-derived map.
(B and C) Front and top views of the model of state C (B) and state E (C). rRNA and RPs are colored in light and dark gray, respectively. Biogenesis factors are highlighted in color. Helices 76–79 of the L1 stalk (C) are shown as backbone only and not included in the deposited model.
See also Figures S2 and S4.
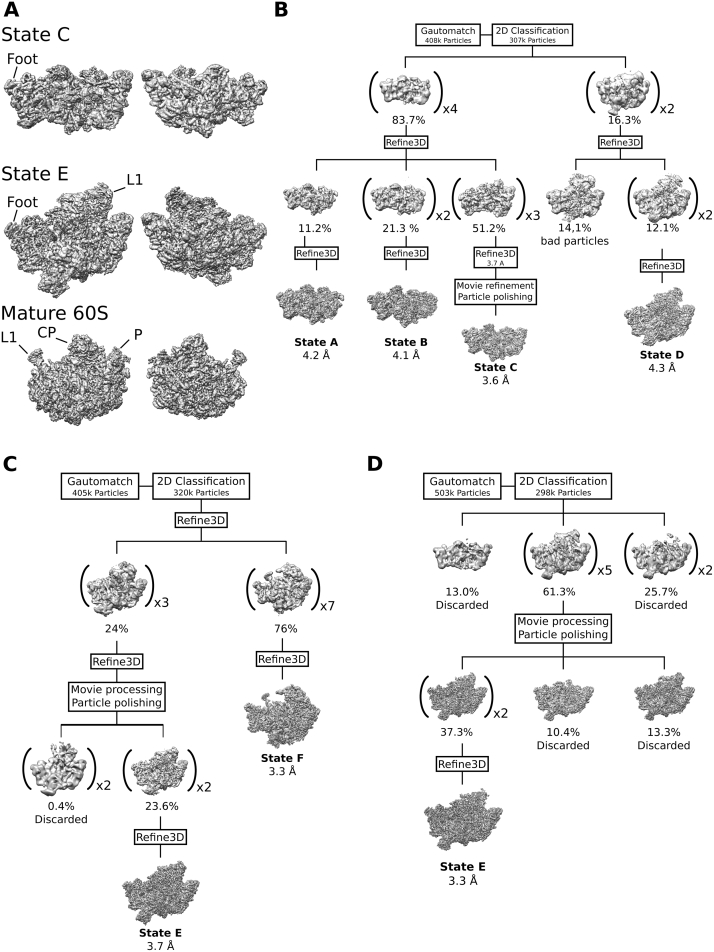
Figure S2.
Comparison of States C and E to the Mature 60S and Cryo-EM Processing Schemes, Related to Figure 1
(A) Pre-60S States C and D and the mature 60S (EMDB: 6478) (Passos and Lyumkis, 2015) showing the intersubunit side (left) and the solvent exposed side (right).
(B–D) Cryo-EM processing schemes for the Nsa1-TAP Flag-Ytm1 (B), Rix1-TAP Rpf2-Flag (C), and the TAP-Flag-Ytm1 E80A (D) sample. Percentages indicate the fraction of total particles after 2D classification in a class or set of classes. Brackets indicate the joining of several highly similar classes.
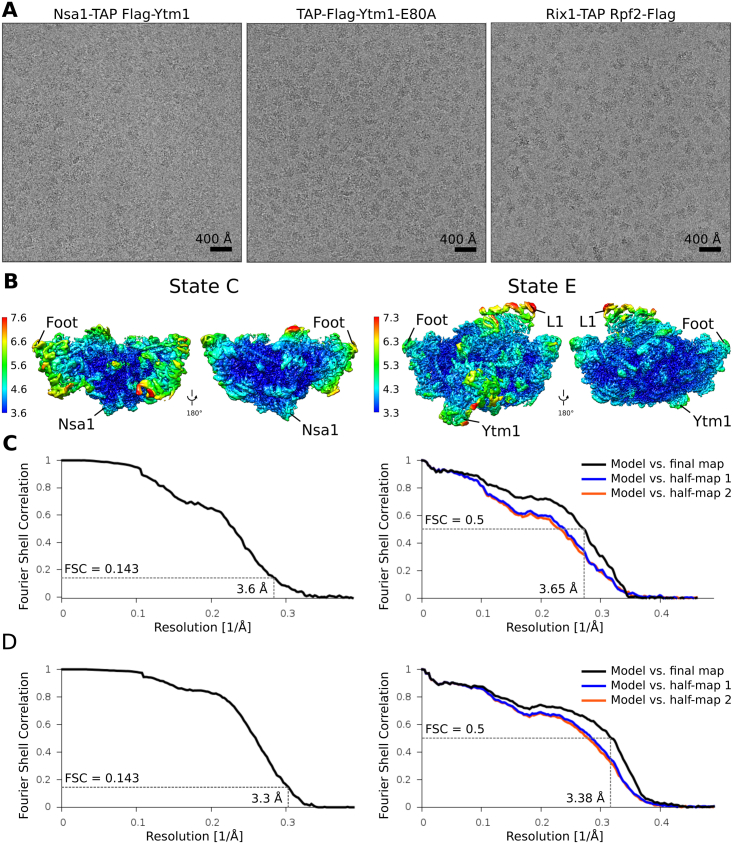
Figure S3.
Resolution Estimation and Model Validation, Related to Figure 1
(A) Exemplary micrographs of the three different biochemical samples.
(B–D) Two views rotated by 180° of volumes of state C and E filtered and colored according to local resolution as provided by Relion-2.0 (Kimanius et al., 2016). Gold standard FSC-curves and FSC curves calculated between the cryo-EM maps and the models for the State C particle (C), and for the State E particle (D).
Figure S4.
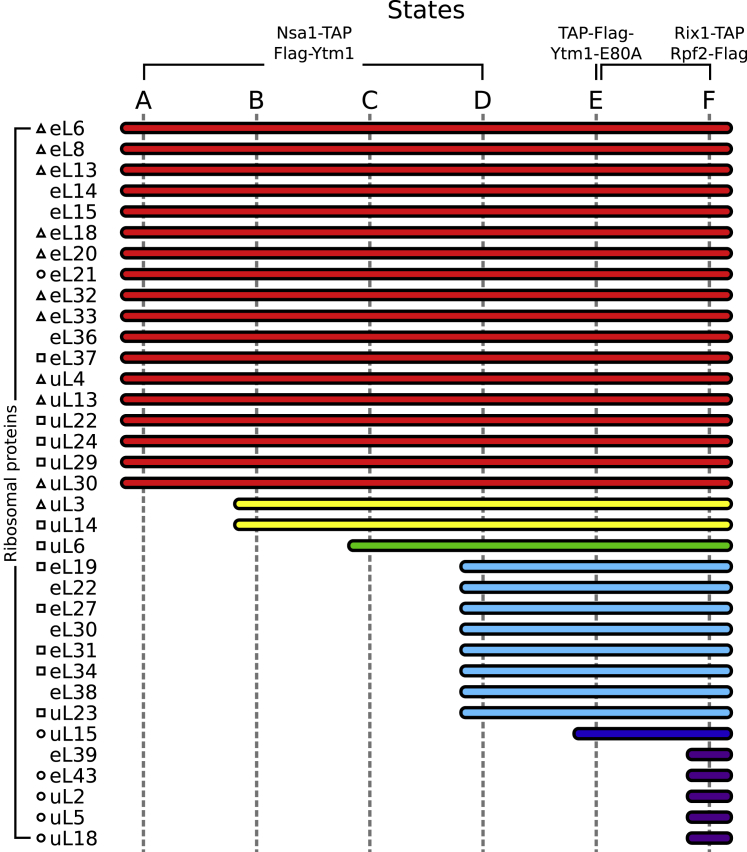
RP Composition of the Six Identified States, Related to Figure 1
RPs clustered and colored according to time of stable association with the core particle. RPs are marked with a triangle, square or circle according to depletion phenotypes leading to maturation arrests at early, middle, and late assembly stages, respectively (Gamalinda et al., 2014).
Atomic Structure of Early States C and E Pre-60S Particles
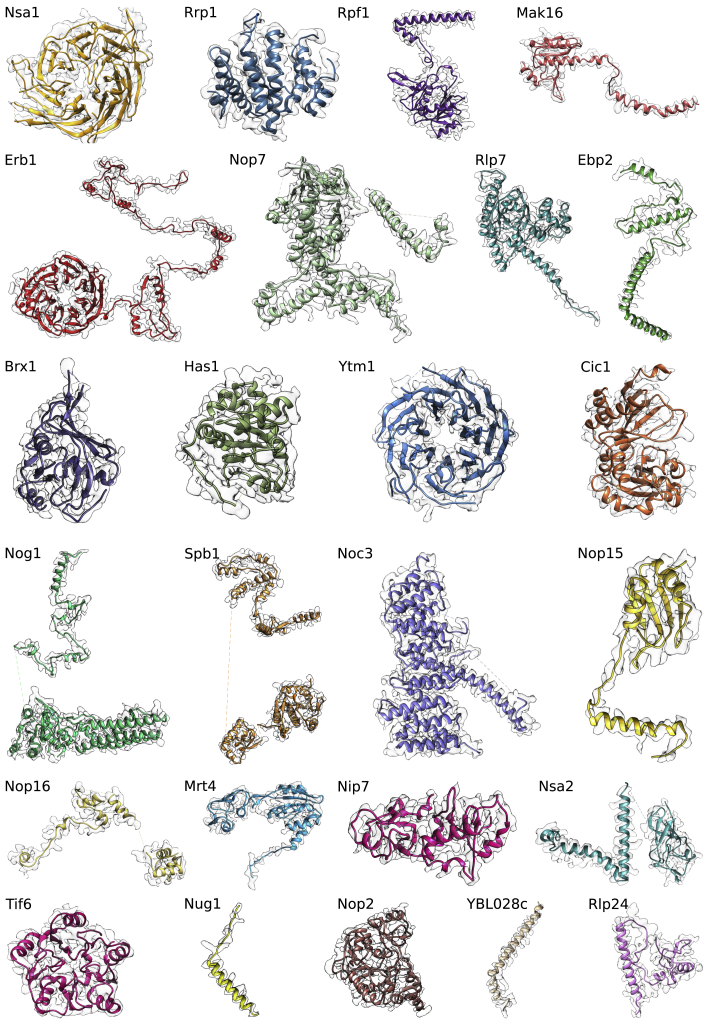
We built atomic structures for the two highest resolved pre-60S particles, states C and E, with average resolutions of 3.6 Å and 3.3 Å, respectively. These models comprise a total of 25 localized ribosome assembly factors, of which 13 were built de novo, including Nsa1, Rrp1, Rpf1, Mak16, Erb1, Has1, Nop16, Ebp2, Brx1, Noc3, Nop2, Nip7, and Spb1 (Figures 1B and S5). The models for Nsa1 and Rrp1 agree nicely with the respective crystal structures, indicating that both behave as rigid bodies in the pre-60S particles (Figures S6B and S6C).
Figure S5.
Gallery of All Modeled AFs, Related to Figures 1, 2, 3, and 4
AF models overlaid with the corresponding segmented map volumes. All AFs are taken from state E, except for Rrp1, Rpf1, Nsa1, and Mak16, which are taken from state C. Map densities are filtered to 3.3 Å and 3.6 Å for State E and C respectively.
Figure S6.
CRAC Crosslinks and Crystal Structures, Related to Figure 2
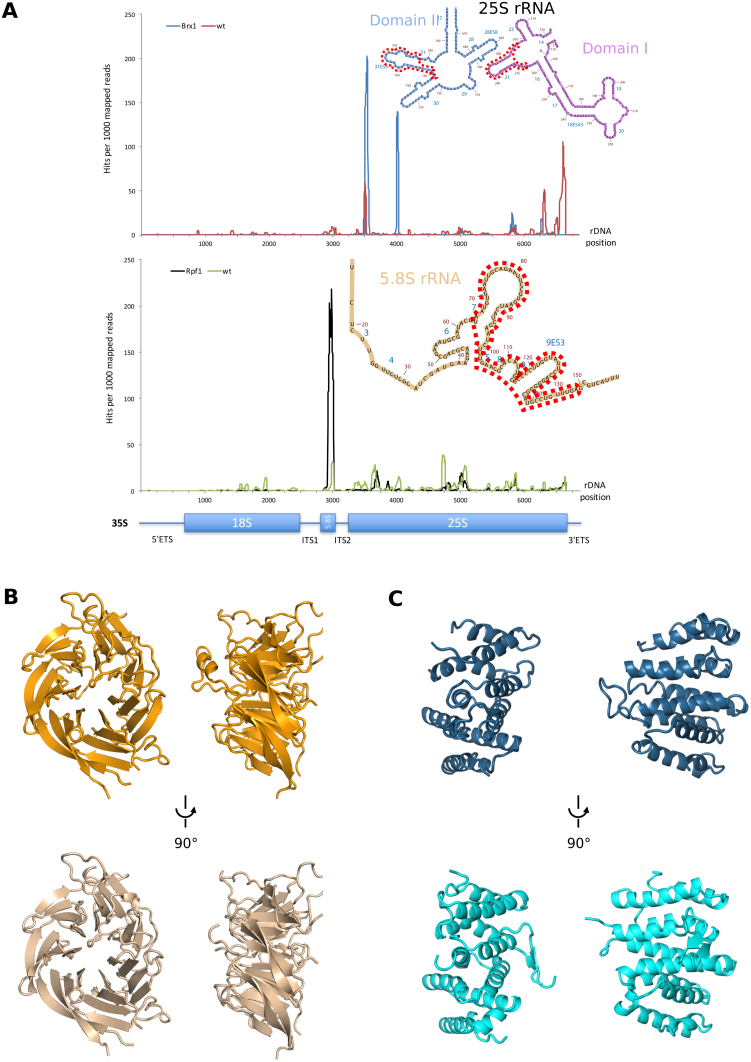
(A) CRAC analysis hits of yeast Brx1 (blue, top plot) and untagged wild-type strain (red, top plot) as well as Rpf1 (black, bottom plot) and untagged wild-type strain (green, bottom plot). A schematic representation of the 35S pre-rRNA is drawn below the x axis. The number of hits per 1000 total mapped reads is plotted against the nucleotide position on the rDNA.
(B) Comparison of the cryo-EM model (top) of Nsa1 with its crystal structure (bottom).
(C) Comparison of the cryo-EM model (top) of Rrp1 with the crystal structure of Chaetomium thermophilum Rrp1 (bottom).
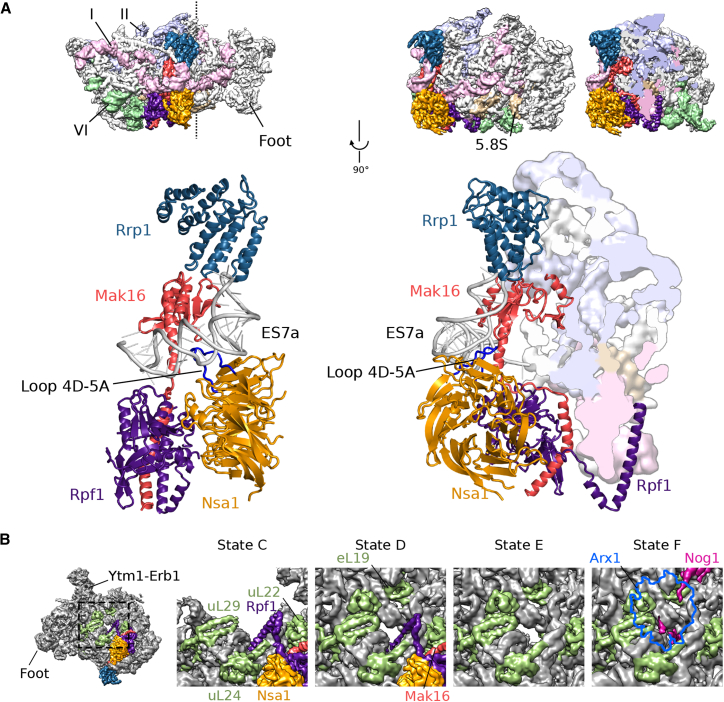
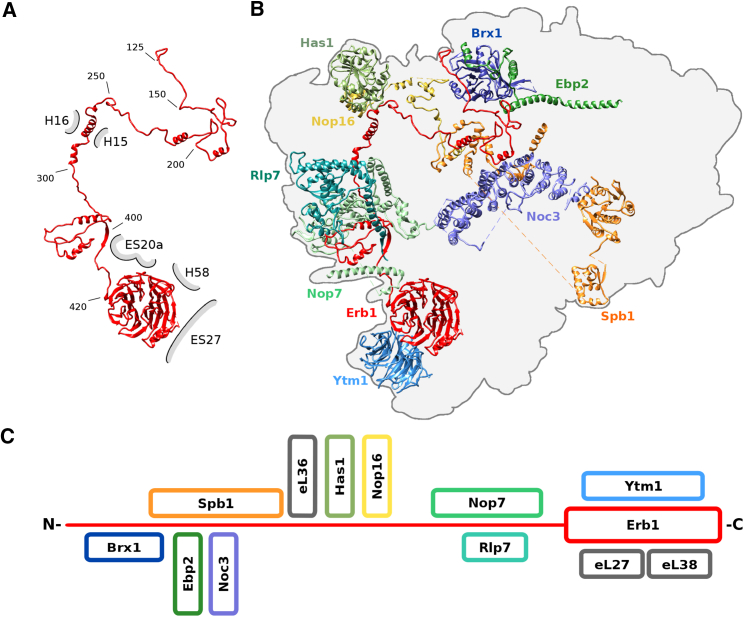
The nucleolar pre-60S particles in states A–C show a characteristic arch-like morphology. This strongly deviates from the later D, E, and F state particles, which already display a globular shape similar to the mature 60S (Figure S2A). However, states A–C already contain a hallmark structure typically seen on Arx1 or Nog2 particles, termed the “foot,” which is formed by ITS2 rRNA and its associated “A3” cluster factors Cic1/Nsa3, Nop7, Nop15, and Rlp7 (Bradatsch et al., 2012, Leidig et al., 2014, Wu et al., 2016). In contrast to the “foot” in the Arx1 particle, the A–C state particles contain a part of the Erb1 N terminus (amino acid residues 328–400) instead of Nop53 (Figure 1B). More importantly, however, adjacent to the foot, the 5.8S portion of the pre-rRNA and the 25S rRNA domains I and II and the increasing parts of domain VI complete, together with eventually 21 RPs (in state C), the solvent-exposed back side of these pre-60S particles. Strikingly, in state C the polypeptide exit tunnel (PET) and the entire inter-subunit side, including the CP, the peptidyl transferase center, and the L1 stalk, are not yet formed, since domains III and IV and most of domain V of the 25S rRNA are not stably incorporated into the core particle (Figures 1A–1C). Notably, these domains are inevitably tethered to the particle (linked between domains II and VI); however, they are not yet rigidly oriented with respect to the developing core particle. Therefore, we cannot draw any conclusion regarding their intra-domain folding state and association mode of ribosomal and non-ribosomal proteins. However, we can clearly observe how the core particle develops, which apparently starts with the formation of the solvent side of the 60S subunit. Here, a cluster of four AFs—the Nsa1 module—is positioned; it consists of the β-propeller protein Nsa1 (used as a bait), the α-helical protein Rrp1, and the Brix-fold protein Rpf1 with its known interaction partner Mak16 (Baßler et al., 2017, McCann et al., 2015). The Nsa1 module binds the rRNA expansion segment (ES) 7a, which adopts a distinct L-shaped conformation, and thereby interacts with and clamps together rRNA domains I and II (Figure 2). This solvent-side location of Nsa1 explains why failure of its removal during 60S biogenesis, due to an N-terminal deletion in the Rix7 AAA ATPase, still allows the formation of functional 80S ribosomes and polysomes (Kressler et al., 2008). The loop between the fourth and fifth β-blade of Nsa1 (Loop 4D-5A) (Figure 2A) was described to mediate the interaction between the human homologs of Nsa1 (WDR74) and Rix7 (NVL2) (Lo et al., 2017). Yet in yeast, we found that the Nsa1 loop 4D-5A is involved in interactions with ES7a and Mak16; it is therefore not readily accessible for interaction with Rix7 (Figure 2A). Thus, a conformational rearrangement may be required for Rix7-mediated dissociation of Nsa1 from the pre-60S particle.
Figure 2.
Nsa1 Module and Formation of the PET
(A) Binding site of the Nsa1 module consisting of Mak16 (red), Nsa1 (orange), Rpf1 (purple), and Rrp1 (dark blue) bound to ES7a (light gray). A back and side view of the particle is shown. Densities for rRNA domains I, II, and VI as well as the 5.8S rRNA are, respectively, colored in light pink, purple, green, and beige. The vertical dashed line through the particle in the upper left view indicates the clipping plane for the cropped density. Loop 4D-5A, suggested to interact with the AAA-ATPase Rix7, is indicated in blue.
(B) Maturation of the PET from states C–F. The N terminus of Rpf1 occupies the tunnel in states A–D; state E shows a free tunnel; and state F displays the C terminus of Nog1 residing in the tunnel.
Another assembly factor of the Nsa1 module, prominently seen in our pre-60S particle, is the Brix protein Rpf1 (Wehner and Baserga, 2002). Notably, its N terminus extends from the solvent side of the particle to a location where later the PET will form and, moreover, binds close to helix H24 of the 25S rRNA and the RP uL24 (Figure 2B). This N-terminal helix remains in place during the further construction of the tunnel until state D, thus it may act as a “place marker” for where the PET will be constructed. After dissociation of the Nsa1 module from the pre-60S particle, likely triggered by Rix7, the tunnel is seen unoccupied in state E, but becomes filled with the C-terminal extension of Nog1 in state F, where typically Arx1-Alb1 decorate the exit site (Figure 2B) (Bradatsch et al., 2012, Wu et al., 2016). In addition to the aforementioned assembly factors, state C particles already contain a subset of the so-called B-factors: Nog1, Mrt4, Tif6, and Rlp24, which are essential for the later separation of the 27SB precursor into 26S and 7S pre-rRNA (Talkish et al., 2012). These B-factors, together with the N-terminal anchor helices of Nsa2 and Nug1, bind to the side of the pre-60S particle opposing the “foot” (Figure 1B). Notably, although present from state C onward, Nog1’s C-terminal extension, which can reach toward and into the peptide exit tunnel, is not localized at states C–E.
Erb1 Functions as a Multivalent Interaction Hub
In the further matured states D and E, the rRNA domain III with parts of domain IV and additional stretches of domain V of the 25S rRNA become visible after incorporation into the core particle, resulting in the lateral closure and, thus, completion of exit tunnel formation. This correlates with the stable incorporation of nine additional RPs (Figure S4) and five AFs (Nip7, Noc3, Nop2, Spb1, and Ytm1) at the maturing inter-subunit interface of state E, which are not seen in state C (Figure 1). Notably, state E pre-60S particles are very similar to state D particles, except that in state E the Nsa1 module is absent and RP uL15 has been acquired. This suggests that E-type pre-60S particles have undergone the AAA-type ATPase Rix7-mediated dissociation of Nsa1.
A central assembly factor already present on the earliest pre-60S particles is Erb1, for which a large number of biochemical, structural, and genetic data have been obtained in previous studies (Konikkat et al., 2017, Miles et al., 2005, Thoms et al., 2016, Wegrecki et al., 2015). It is composed of an N-terminal region containing the nuclear localization signal, a long N-terminal stretch, and a C-terminal β-propeller domain (Konikkat et al., 2017, Pestov et al., 2001, Thoms et al., 2016, Wegrecki et al., 2015). Whereas part of its N terminus is already visible in states A–C, most of Erb1 (amino acids 125 to 807 of 807) can only be seen in the states D and E particles (Figure 3A). Here, the Erb1 β-propeller binds at the edge of the maturing inter-subunit side, in close proximity to the RPs eL27 and eL38, rRNA helix H58, and ES20a and ES27 (Figure 3). Notably, Erb1 contacts its nearby interaction partner Ytm1, as previously observed in the crystal structure of the Chaetomium thermophilum Erb1-Ytm1 complex (Thoms et al., 2016, Wegrecki et al., 2015). Ytm1 contains an N-terminal domain, called MIDO (MIDAS-interacting domain) or UBL domain, which binds the MIDAS (metal ion-dependent adhesion site) domain of Rea1 during the first Rea1 remodeling step (Bassler et al., 2010). However, due to flexibility the MIDO is not well resolved in state D and E. Based on the crystal structure of Ytm1 (Thoms et al., 2016, Wegrecki et al., 2015), the Ytm1-UBL domain is strategically located at the distal edge of the pre-60S particle, thus allowing access for the Rea1 MIDAS-domain and enabling the Rea1-mediated removal of the Ytm1-Erb1 heterodimer from the pre-ribosomal particle.
Figure 3.
Erb1 Functions as a Multivalent Interaction Hub
(A) Overview of the Erb1 domain architecture showing the extended N terminus and the doughnut-shaped WD40 repeat domain. Interactions with rRNA are indicated in gray.
(B) Localization of Erb1 in the state E particle (gray silhouette) with interacting AFs.
(C) Schematic overview of protein contacts between Erb1 and other AFs and RPs.
Following the Erb1 N-terminal tail meandering over the pre-60S particle reveals a number of different AFs contacts on the early pre-60S particles (states A–E). In state D and E, it extends toward the base of the foot, where it interacts with Nop7 and forms a short two-stranded intermolecular β sheet with the N terminus of Rlp7. The Erb1 tail then wraps along the back side of the “foot” to the top of the pre-60S particle, where it comes in contact with Nop16, the helicase Has1, and RP eL36. From there, it proceeds to the rather immature inter-subunit side, where it interacts with further four AFs: the C terminus of the Spb1 methyltransferase (Kressler et al., 1999, Lapeyre and Purushothaman, 2004), Noc3, Ebp2 and its binding partner Brx1. In this way, the Erb1 N-terminal extension coordinates three 25S rRNA domains (I, III, and IV), three ribosomal L-proteins, and nine different biogenesis factors, in total reaching around half of the pre-60S particle (Figures 3A–3C). Thus, our cryo-EM structures explain at molecular level many of the described in vivo effects of the A3-factor Erb1 and its partner Ytm1, and clarify its key role as coordination hub in the early nucleolar pre-60S particles of states D and E.
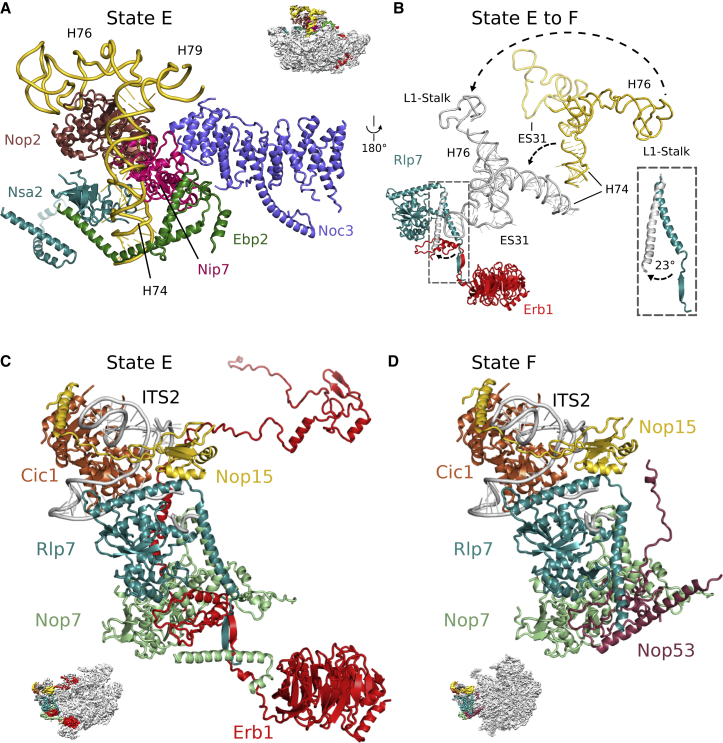
Notably, although the state E pre-60S particle is well on its way toward maturation by compaction of domains III, IV, and V, it is striking to see that the CP (i.e., the area around 5S RNP in the mature 60S) has not developed at all, and that the L1-stalk (rRNA H74-H79) exhibits a strong displacement from its mature position in the 60S subunit to a stable immature conformation in the pre-60S particle. Specifically, a rotation of the L1 stalk at the base of helix H74 is required to bring this hallmark structure of the large subunit from its outward conformation to the mature position. Interestingly, the immature position of rRNA helices H76 to H79 is similar to the immature non-rotated location of the 5S rRNP in the later Arx1 particle (Leidig et al., 2014) (Figures 1 and 4). The L1 stalk RNA is chaperoned and kept in this unusual conformation by a set of five AFs, consisting of the C terminus of Ebp2, Noc3, the methyltransferase domain of Nop2 (Sharma et al., 2013) its binding partner Nip7 (Talkish et al., 2012), and the C-terminal β-barrel domain of Nsa2. As a consequence, accommodation of the entire rRNA segment, later forming the L1 stalk, into the conformation observed in the further progressed F state particles (also Arx1 particles) is prevented (Bradatsch et al., 2012, Leidig et al., 2014, Wu et al., 2016). Thus, a set of factors, including Nip7, Nop2, Noc3, and Ebp2, has to dissociate from the developing pre-60S particle before the L1 stalk domain can adopt its mature position. Importantly, the E state structure reveals how the rearrangement of the L1-stalk toward a more mature conformation as seen in state F particles is linked to distinct changes in the “foot” structure (Figures 4C and 4D). Removal of Erb1-Ytm1 from the pre-60S particle by Rea1 will also disrupt a small antiparallel β sheet between Erb1 and Rlp7, which in turn allows the adjoining C-terminal α helix of Rlp7 to rotate toward the “foot.” One long distance effect of this β sheet splitting is that the rotation of the entire L1 stalk becomes possible, which otherwise would clash with Rlp7 via its rRNA ES31. Another consequence is that the exosome recruiting factor Nop53 (Thoms et al., 2015, Wu et al., 2016) can be recruited to the position previously occupied by Erb1 (Figures 4C and 4D), explaining why 7S pre-rRNA processing by the nuclear exosome can only occur after the first Rea1 remodeling step.
Figure 4.
Maturation of the L1-Stalk Segment Is Linked to “Foot” Remodeling
(A) The L1 segment (rRNA helices H74–H79, gold) is stabilized in a pre-mature conformation by a set of AFs, including Ebp2 (green), Nip7 (magenta), Noc3 (purple), Nop2 (brown), and Nsa2 (turquoise).
(B) The L1 segment undergoes large-scale conformational changes during maturation, which requires an outward rotation of the Rlp7 N-terminal α helix. The conformations as observed in states D and E of the L1 segment and Rlp7 are colored in gold and turquoise. Gray denotes their conformation as observed in state F.
(A and B) Helices H76–H79 are displayed as backbone only and are not included in the provided model. In states D and E, the position of ES31 (gold, semi-transparent, backbone only) is indicated based on connectivity to helix H79, but not observed in the respective maps because of flexibility.
(C and D) Structure of the “foot” in states E (C) and F (D). In state F, Nop53 takes the place of Erb1, resulting in a conformational change in the N terminus of Rlp7.
The molecular interpretation of state F is based on the Arx1/Nog2 particle model (PDB: 3JCT) (Wu et al., 2016).
Sequential Incorporation of the Six 25S rRNA Domains into the Developing Pre-60S Core Particle
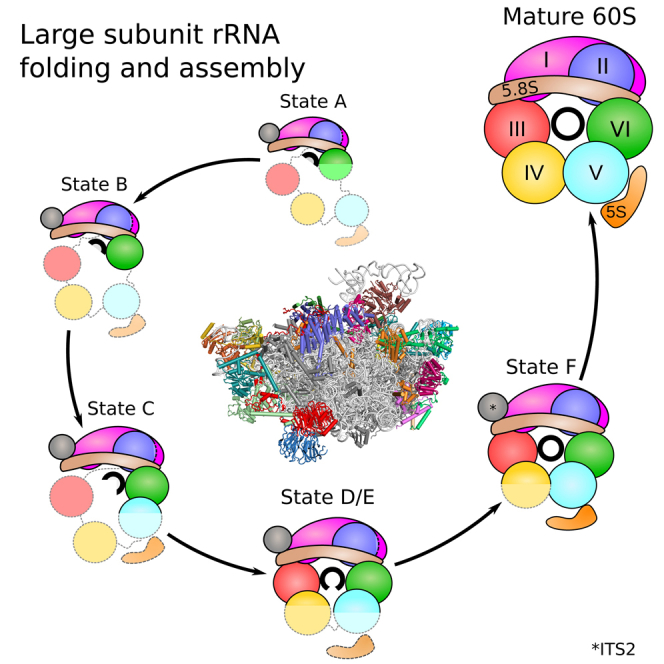
The 25S rRNA can be divided into six domains (I–VI) that assemble into a monolithic compact mature 60S subunit (Ben-Shem et al., 2011). We analyzed the immature pre-60S states A–F to derive the most plausible order of 25S rRNA domain folding and compaction, when transiting from state A to F (Figure 5).
Figure 5.
Sequential Incorporation of the rRNA Domains into a Developing Pre-60S Core Particle
(A) Front and top views of structural rRNA representations based on Chimera molmaps for states A–F. The molmaps are color-coded by the rRNA domains: domain 0, ITS2, and the 5.8S rRNA portions are displayed in light brown; the 5S rRNA is in orange; domains I–VI are in magenta, blue, red, yellow, cyan, and green, respectively.
(B) Secondary structure plots indicating folded rRNA for each state following the same color-code used in (A).
(C) Schematic representation of AFs associated with all states. Clustering and coloration is based on the time point of stable association and dissociation from the maturing particle as indicated by the horizontal lines. (∗) Ytm1 was clustered with Erb1, as the two proteins form a tight complex (Thoms et al., 2016, Wegrecki et al., 2015), and it was used as a bait protein to purify states A–D. It is therefore clear that Ytm1 is present in these states (A–D), but not yet stably localized in the core particle.
The molecular interpretation of state F is based on the Arx1/Nog2 particle model (PDB: 3JCT) (Wu et al., 2016).
The very early state A pre-60S particle shows density only for 5.8S rRNA, ITS2, and 25S rRNA domain I, whereas 25S rRNA domains II and VI are only partly visible and domains III to V are not at all visible. Structure-wise, this corresponds to the “foot” and the adjacent portion of the solvent-exposed (back) side of the pre-60S particle. Upon progression to state B, the solvent side becomes enlarged with the full incorporation of domain VI. Upon further progression to state C, the additional folding of helices H42–H44 of domain II and of helices H89–H91 of domain V can be seen. As maturation progresses further to state D, the PET and the tunnel exit site are formed, with the stable folding of domain III and stabilization of the L1 stalk in its premature state. States D and E show very similar rRNA conformation, except for the 50-nt-long ES7a, which is only rigidly incorporated up to state D particles, stabilized by the Nsa1 module. As the L1 stalk moves into a mature-like position in state F, domains IV and V and the rotated 5S RNP form additional domains at the inter-subunit face and the immature CP (Leidig et al., 2014, Wu et al., 2016). Notably, the 5S RNP, comprising 5S rRNA, uL5, and uL18, and its assembly factors Rpf2 and Rrs1 are all present in nucleolar pre-60S particles affinity purified via the Nsa1 bait (Kressler et al., 2008) or in our early pre-60S particles (corresponding to states A–D), obtained via the Nsa1-Ytm1 split-tag approach (see above) (Figure S1). This suggests that the 5S RNP associates from very early on with the nucleolar pre-60S particles, before it becomes stably incorporated as domain V into the developing pre-60S core particle, which is prior to formation of the CP.
Finally, our structures agree with the postulated hierarchical incorporation of ribosomal L-proteins into the newly forming 60S subunit (Gamalinda et al., 2014). We observe that many new RPs become stably incorporated into the core particle upon rRNA compaction, from 18 in the earliest particle (state A) to the 35 in the latest particle (state F) (Figure S4). Along the maturation trajectory, the AF composition varies in complexity, with distinct sets of AFs, such as the Nsa1 module, being present only for a certain assembly interval in a subset of states (Figure 5C). Altogether, these data indicate that folding and stable integration of rRNA domains into the developing 60S core particle proceeds in a non-transcriptional yet linear series of steps by first forming the solvent-exposed side like an exoskeleton, followed by formation of the exit tunnel and the inter-subunit interface at last.
Discussion
Function of Assembly Factors Nsa1, Erb1, and Rea1
The Nsa1 module was identified on the early pre-60S particles (states A–D) at a position where it clamps the early-forming rRNA domains I and II and marks the future PET construction site, explaining why the Nsa1 module has a key role in the early steps of 60S assembly. Many interactions involving the Nsa1 module are clustered at the rRNA expansion segment ES7a. Besides ES7a, ES27 also has been observed to interact with AFs, namely with the Erb1-Ytm1 complex in the nucleolus and Arx1 during later stages of 60S assembly (Bradatsch et al., 2012, Greber et al., 2012, Leidig et al., 2014, Wu et al., 2016). Therefore, our findings support the importance of ES in eukaryotic rRNA for ribosomal assembly (Ramesh and Woolford, 2016), likely by acting as a binding hub for assembly factors and eukaryote-specific extensions of RPs (Bradatsch et al., 2012, Granneman et al., 2011, Greber et al., 2012, Leidig et al., 2014, Wu et al., 2016).
In addition to the Nsa1 module, we identified Erb1 as a key connector, which wraps around the maturing 60S subunit with its long N-terminal extension, thereby establishing a multitude of interactions with different rRNA domains, AFs, and RPs (Figure 3). Our structural data explain why deletions in the Erb1 N-terminal extension caused many different in vivo defects in 60S biogenesis (Konikkat et al., 2017).
The C-terminal β-propeller domain of Erb1 forms a tight complex with Ytm1 and removal of the Erb1-Ytm1 heterodimer from the early pre-60S particles by Rea1 is an essential remodeling step to induce the transition from the nucleolus to the nucleoplasm (Bassler et al., 2010). While investigating the structure of the pre-60S particle isolated from the dominant-lethal Ytm1 E80A mutant accumulating in state E, we found that this intermediate is identical to wild-type pre-60S particles split-tag affinity purified via Rix1-TAP/Rpf2-Flag (state E), indicating that the impaired release of the Erb1-Ytm1 complex blocks further maturation, with the L1-stalk domain stabilized by several AFs in its pre-translocation conformation. To overcome this immature state and allow rRNA helices H74–H79 to properly rearrange, dissociation of multiple factors (Ebp2, Noc3, the Nop2-Nip7 sub-complex, and the C terminus of Spb1) and remodeling of Rlp7 are required (Figure 4). As many of these factors directly interact with Erb1, we suggest that the timely release of Erb1-Ytm1 is the trigger for the observed large-scale rearrangement at the inter-subunit face that eventually leads to the stabilization of rRNA domains IV and V (as observed in state F). Notably, a related restructuring step from a stabilized immature rRNA conformation occurs during the 180° rotation of the 5S rRNA on a later pre-60S particle, which was suggested to be part of a checkpoint control (Barrio-Garcia et al., 2016, Wu et al., 2016). Thus, it is intriguing that these two large-scale rRNA reorganization steps during 60S biogenesis rely on Rea1 AAA-type ATPase, which apparently facilitates the transition from one state characterized by a stabilized immature rRNA conformation (of the L1 domain and 5S RNP, respectively) to the next, more mature state. For the remodeling step allowing 5S RNP rearrangement, the Rix1-Rea1 complex was found to bind at the inter-subunit surface and the CP (Barrio-Garcia et al., 2016). Interestingly, the structural landscape of this area is completely different in the nucleolar pre-60S particles. Nevertheless, Rix1 as a bait can be used to purify nucleolar state E particles (Figures 1 and S1). This suggests that Rix1 might also be involved in the nucleolar Rea1 remodeling step. Taken together and considering the distinct positions of the Rea1 target proteins Ytm1 and Rsa4, Rea1 appears to employ two distinct interaction modes with pre-60S particles.
Early 60S Assembly Employs a Sequential rRNA Folding Pathway
The diverse structural states observed in the nucleolar pre-60S particles are likely to be of physiological relevance since they were affinity purified via functional bait proteins from wild-type-like yeast cells (Figures S1A and S1B). Analyzing these states provided mechanistic insight into how the six rRNA domains (I to VI) are compacted and subsequently decorated with a number of ribosomal L-proteins, finally leading to monolithic 60S core particles. Our structural data do not point to parallel 60S assembly pathways, as suggested for bacterial 50S subunits (Davis et al., 2016). However, this previous study was based on 50S assembly intermediates obtained from bacterial cells, in which the early RP bL17 was depleted, causing a severe growth defect and a 20-fold reduced ribosome assembly rate. Hence, the isolated 50S assembly intermediates may have used alternative assembly pathways for compensation. Importantly, however, there are major kingdom-specific differences between eukaryotic and bacterial ribosome assembly, which is illustrated by the almost complete lack of ribosomal AFs in the observed bacterial pre-50S intermediates (Davis et al., 2016).
During the earliest steps of 60S assembly, the different rRNA domains may already have folded into their secondary structures and recruited the earliest ribosomal L-proteins to these sites, which in consequence may stabilize first tertiary folds. For example, nucleolar pre-60S particles purified via Nsa1 contain already the 5S RNP, but it can only be seen as a structurally distinct entity in the 60S core when reaching state F (Arx1/Nog2 particle). Therefore, when analyzing and interpreting ribosomal assembly intermediates, one has to distinguish between initial rRNA secondary structure formation, partial tertiary structure construction, ordered incorporation into the core particle, and, eventually, condensation into a fully mature conformation. Similarly, RPs may already bind early during the assembly process, but become part of the core particle only later, and when absent could prevent further maturation. In the past, different approaches have been used to follow the consecutive steps in 60S assembly, e.g., by using truncated rDNA transcripts (Chen et al., 2017) or by depletion of specific RPs (Davis et al., 2016, Gamalinda et al., 2014). However, blocking formation of full-length rRNA particles, as in the first case, may lead to kinetic off-path intermediates, and the absence of a given RP, as in the second case, can identify its rate limiting role during 60S assembly, but without a clear connection to its initial or later interaction characteristics in the evolving particle.
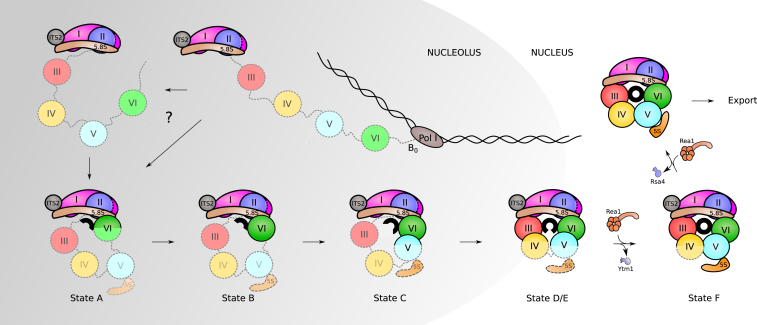
On the basis of these considerations, we propose a model for the early structural assembly of the 60S subunit. A hallmark of this model is that in the nucleolus, after circularization of rRNA domains, early 60S assembly into a growing core particle is carried out in a sequential fashion (Figure 6): as RNA-polymerase-I-driven transcription of the 35S pre-rRNA proceeds, fast secondary structure formation and assembly of first AFs and RPs is thought to begin co-transcriptionally. However, instead of following the transcriptional sequence, circularization of the pre-rRNA is one of the first steps in the formation of a rigid core structure, as only the 5.8S portion, ITS2, and domains I and II and partially domain VI are folded in the earliest observed intermediate (state A). Thus, initially the solvent-exposed back side of the LSU forms like an exoskeleton and construction proceeds by formation of the exit tunnel, which is in agreement with a previously suggested model of hierarchical folding based on RP depletion phenotypes (Gamalinda et al., 2014). Although the very late-folding peptidyl transferase center is the evolutionary oldest part of the ribosome, it is likely that folding the solvent side first brings the advantage of providing a stable scaffold for the developing 60S subunit.
Figure 6.
Assembly Sequence of the Pre-rRNA Domains
Assembly of RPs and AFs to the nascent 35S rRNA precursor starts co-transcriptionally. Very early, the pre-rRNA is circularized as domain VI binds to domains I and II and the 5.8S portion of the precursor rRNA. The formation of the PET (displayed here as a black circle) starts with this circularization. Its maturation progresses as rRNA domains fold following this order: VI, V, III, and IV. Full assembly of the PET is only achieved when domain V is completely folded as observed in state F. After that, only few nucleoplasmic steps need to occur before the particles are exported to the cytoplasm, where they undergo final maturation.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| E. coli BL21(DE3) | Merck | 69450 |
| Deposited Data | ||
| Cryo-EM density map: State A | This paper | EMD-3888 |
| Cryo-EM density map: State B | This paper | EMD-3889 |
| Cryo-EM density map: State C | This paper | EMD-3893 |
| Cryo-EM density map: State D | This paper | EMD-3890 |
| Cryo-EM density map: State E | This paper | EMD-3891 |
| Cryo-EM density map: State F | This paper | EMD-3892 |
| Atomic model: Rrp1 (Spacegroup C2) | This paper | PDB: 6EMF |
| Atomic model: Rrp1 (Spacegroup P6322) | This paper | PDB: 6EMG |
| Atomic model: Nsa1 | This paper | PDB: 6EN7 |
| Atomic model: State C | This paper | PDB: 6EM1 |
| Atomic model: State E | This paper | PDB: 6ELZ |
| Architectural model: State A | This paper | PDB: 6EM3 |
| Architectural model: State B | This paper | PDB: 6EM4 |
| Architectural model: State D | This paper | PDB: 6EM5 |
| Experimental Models: Organisms/Strains | ||
| Yeast: W303 | Thomas and Rothstein, 1989 | N/A |
| Yeast: DS1-2b | Nissan et al., 2002 | N/A |
| Yeast: NSA1-TAP Flag-YTM1 | This paper | N/A |
| Yeast: RIX1-TAP RPF2-Flag | This paper | N/A |
| Yeast: BRX1-HTpA | This paper | N/A |
| Yeast: pA-TEV-(HIS)6-RPF1 | This paper | N/A |
| Recombinant DNA | ||
| pFA6a-Flag-natNT2 | This paper | N/A |
| pFA6a-TAP-klURA3 | This paper | N/A |
| pnatNT2 PYTM1 Flag | This paper | N/A |
| YCplac111-TAP-Flag-YTM1 | Thoms et al., 2016 | N/A |
| YCplac111-TAP-Flag-ytm1 ΔUBL | This paper | N/A |
| YCplac111-TAP-Flag-ytm1 E80A | This paper | N/A |
| YCplac111-pA-TEV-(His)6-RPF1 | This paper | N/A |
| pET15b-CtRrp1-(His)6 | Baßler et al., 2017 | N/A |
| pET15b-Nsa1-(His)6 | This paper | N/A |
| Software and Algorithms | ||
| EM-TOOLS | TVIPS GmbH | http://www.tvips.com/imaging-software/em-tools/ |
| MotionCorr2.1 | Li et al., 2013 | http://cryoem.ucsf.edu/software/driftcorr.html |
| GCTF | Zhang, 2016 | http://www.mrc-lmb.cam.ac.uk/kzhang |
| Motioncor2 | Zheng et al., 2017 | http://msg.ucsf.edu/em/software/motioncor2.html |
| Gautomatch | Jack Kai Zhang, Division of structural studies, MRC Laboratory of Molecular Biology | http://www.mrc-lmb.cam.ac.uk/kzhang |
| Relion-2 | Kimanius et al., 2016 | http://www2.mrc-lmb.cam.ac.uk/relion/index.php/Main_Page |
| Phenix suite (phenix.real_space_refine, molprobity) | Afonine et al., 2012, Chen et al., 2010, Wang et al., 2014 | https://www.phenix-online.org/ |
| PSIPRED | UCL Department Of Computer Science | http://bioinf.cs.ucl.ac.uk/psipred/ |
| HHpred / Modeler | Meier and Söding, 2015 | https://toolkit.tuebingen.mpg.de/#/tools/hhpred |
| Phyre2 | Kelley et al., 2015 | http://www.sbg.bio.ic.ac.uk/∼phyre2 |
| CCP4 (LIBG, ProSMART, Refmac5, Buccaneer, AIMLESS, XDS, Phaser, COOT, SHELXC/D/E) | Amunts et al., 2014, Brown et al., 2015, Cowtan, 2006, Emsley et al., 2010, Evans and Murshudov, 2013, Kabsch, 2014, McCoy et al., 2007, Murshudov et al., 2011, Winn et al., 2002, Sheldrick, 2008 | http://www.ccp4.ac.uk/ |
| ClustalOMEGA | EMBL-EBI | https://www.ebi.ac.uk/Tools/msa/clustalo |
| RNAcomposer | Institute of Computing Science, Poznan University of Technology | http://rnacomposer.ibch.poznan.pl/ |
| Chimera | UCSF Resource for Biocomputing, Visualization, and Bioinformatics | http://www.cgl.ucsf.edu/chimera/ |
| Pymol | PyMOL Molecular Graphics System, Schrödinger, LLC | https://pymol.org/2/ |
| HKL2MAP | Pape and Schneider, 2004 | http://webapps.embl-hamburg.de/hkl2map/ |
| Other | ||
| Carbon coated holey grids (2nm) R 3/3 Copper | Quantifoil | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Roland Beckmann (beckmann@lmb.uni-muenchen.de).
Experimental Model and Subject Details
Plasmid constructs and yeast strains
Recombinant DNA techniques were performed using standard procedures. Cloning and plasmid propagation was carried out with E. coli DH5α. Cloned DNA fragments obtained by PCR amplification were verified through sequencing. Plasmids used in this study are listed in Table S1. S. cerevisiae strains used and generated in this study are listed in Table S2. Genomic tagging was performed as previously described according to standard procedures (Janke et al., 2004, Longtine et al., 1998).
Method Details
Affinity purifications from S. cerevisiae
The NSA1-TAP Flag-YTM1 and the RIX1-TAP RPF2-Flag strains were grown in YPD medium and harvested at an OD600 of 2.0-2.5. For the YTM1 wild-type, ytm1 ΔUBL and ytm1 E80A alleles plasmids were generated harboring the respective coding sequences fused to an N-terminal TAP-Flag tag under control of the endogenous PYTM1 promoter. The plasmids were transformed into a wild-type strain (W303) (Thomas and Rothstein, 1989) and selected on SDC-Leu. Cultures were grown over-night in SDC-Leu medium and shifted to YPD for additional 6-7 h until the cultures reached an OD600 of 2.0-2.5. Cell pellets were harvested by centrifugation, flash frozen in liquid nitrogen and stored at −20°C.
The affinity purifications were performed as described previously (Barrio-Garcia et al., 2016). Cells were disrupted by cryogenic grinding with a Retch grinding mill (MM 400) and the cell powder was incubated with lysis buffer containing 50 mM Tris pH 7.5, 100 mM NaCl, 5 mM MgCl2, 5% (v/v) glycerol, 1 mM DTT and 0.1% (w/v) NP40 supplemented with protease inhibitors (SIGMAFAST, Sigma Aldrich). The lysate was cleared by centrifugation and incubated with IgG Sepharose 6 Fast Flow (GE Healthcare) for 90 min at 4°C to enrich for the TAP tagged bait protein. Beads were collected and washed once with 25 mL lysis buffer (batch wash) and additionally with 10 mL lysis buffer by gravity flow. The IgG beads were collected and incubated with lysis buffer supplemented with TEV protease to release the sample from the beads. The eluate was incubated with Anti-Flag M2 Affinity gel (Sigma Aldrich) for additional 90 min at 4°C to enrich for the Flag tagged protein. The remaining steps were performed in buffer containing 50 mM Tris pH 7.5, 100 mM NaCl, 5 mM MgCl2 and 1 mM DTT. The Flag agarose beads were extensively washed and samples were eluted with ca. 60 μl buffer supplemented with Flag peptide.
Protein production, crystallization and structure determination
ScNsa1
For expression of Saccharomyces cerevisiae (Sc) Nsa1-(His)6, E. coli BL21(DE3) cells, grown in lysogeny broth (LB) medium, were used. Protein expression was induced with 1.8% (w/v) lactose, and cells were harvested after overnight growth at 30°C and stored at −80°C. Cells pellets were resuspended in 10 mL buffer A (20mM HEPES (pH 8.0), 250mM NaCl, 20mM KCl, 20mM MgCl2 and 40mM imidazole) per gram of cells and lysed with a M-110L Microfluidizer (Microfluidics). Lysate was cleared at 20,000 r.p.m and Nsa1 was enriched by Ni-NTA chromatography. Nsa1 was eluted from Ni-NTA by buffer B (20mM HEPES (pH 8.0), 250mM NaCl, 20mM KCl, 20mM MgCl2, 500mM imidazole) and further purified by size-exclusion chromatography (HiLoad 26/60 Superdex 200) equilibrated in buffer C (20mM HEPES (pH 8), 200mM NaCl, 20mM KCl and 20mM MgCl2). Protein was concentrated to approximately 30 mg/ml and crystallization screens were performed at 291K by the sitting-drop vapor-diffusion method upon mixing equal volumes (0.5 μl) of protein solution and crystallization buffer with a reservoir volume of 100 μl. Nsa1 crystallized in a wide range of polyethyleneglycol (PEG)-containing conditions (e.g., 0.1 M MES pH 6.5, 25% w/v PEG-1000). Prior data collection, crystals were flash-frozen in liquid nitrogen after cryo-protection by transfer into cryo-solution containing mother liquor and 20% (v/v) glycerol. Diffraction data were measured under cryogenic conditions (100 K; Oxford Cryosystems Cryostream) at the European Synchrotron Radiation Facility (ESRF; Grenoble). Data were processed with XDS (Kabsch, 2014). The Nsa1 structure was determined by molecular replacement using PHASER and PDB: 5SUM as search model. The structure was manually corrected with Coot (Emsley et al., 2010) and refined with REFMAC5 (Murshudov et al., 2011) and PHENIX (Afonine et al., 2012). Data collection and refinement statistics are summarized in Table S3.
CtRrp1
Rrp1 from Chaetomium thermophilum (Ct) was expressed in Rosetta 2 (DE3) T1R cells transformed with the pET15b-CtRrp1-(His)6 plasmid (Baßler et al., 2017). Briefly, cells were grown until an OD600 of 0.8 was reached and then transferred to 18°C. Protein expression was induced by the addition of 0.5 mM IPTG and cells were further grown overnight. Harvested cells were lysed in buffer L (30 mM HEPES pH7.5, 30 mM imidazole, 500 mM NaCl) using a Microfludizer (Microfluidics). Cell debris and insoluble proteins were removed by centrifugation for 35 minutes at 35000 × g and 4°C. The cleared lysate was filtered with 0.45 μm filter and applied to 2 mL NiNTA column. After washing with 30 column volumes with buffer L the protein was eluted using buffer H (buffer L plus 400 mM Imidazol). Further purification was done using a Superdex 75 26/60 column equilibrated with buffer S (20 mM HEPES pH 7.5, 400 mM NaCl, 5 mM MgCl2, 2 mM DTT). CtRrp1 containing fractions were pooled, concentrated to 20-40 mg/ml and used for crystallization trials. CtRrp1 crystallized in two conditions, giving rod-shaped crystals in PEG-3350 based condition (200 mM Proline, 100 mM HEPES pH 7.5, 10% PEG3350) and thick hexagonal shaped crystals in a phosphate-containing condition (0.8M K-H2PO4, 0.8M Na-H2PO4). For data-collection crystals from both conditions were harvested into reservoir solution supplemented with 20% glycerol or ethylene-glycol and flash cooled in liquid nitrogen. Data were collected at ESRF beamline ID 29 (de Sanctis et al., 2012). For phasing, a crystal from the phosphate condition was soaked in reservoir solution supplemented with 1 mM K2PtCl4 for 1 hour and cryo-protected as described above. Data were collected at ESRF beamline ID29. Data were processed with XDS (Kabsch, 2014) and AIMLESS (Evans and Murshudov, 2013) from the CCP4 package (Winn et al., 2002). The structure was solved with SHELXC/D/E (Sheldrick, 2008) navigated with HKL2MAP (Pape and Schneider, 2004). The initial map was readily interpretable and Buccaneer (Cowtan, 2006) could place the majority of the residues automatically. Remaining residues were built with Coot (Emsley et al., 2010) and the structure was refined with REFMAC5 (Murshudov et al., 2011) and PHENIX (Afonine et al., 2012). The C2-dataset was solved by molecular replacement as implemented in PHASER (McCoy et al., 2007)], using the previously determined structure as search model. The final structures contain 2 molecules per ASU in both space-groups, arranged in different way to each other. Data collection and refinement statistics are summarized in Table S4.
CRAC analysis
The CRAC experiments for Brx1 and Rpf1 were done as described in Granneman et al. (2009) and Thoms et al. (2015) with a modification in case of Rpf1. Brx1 was tagged at the C terminus with (HIS)6-TEV-protA (HTpA). Rpf1 was tagged at the N terminus on a plasmid (YCplac111- pA-TEV-(HIS)6-RPF1). Untagged W303 was used as a background control. The yeast cultures were grown to OD 0.6-0.7 and UV-irradiated (in vivo) using a Megatron chamber (1.6 J/cm2). Brx1 and Rpf1 were purified as described in Granneman et al. (2009) including high salt (1M NaCl) wash after IgG purification. For Brx1, the sample was transferred to nitrocellulose membrane and RNA was extracted from the membrane as described in Granneman et al. (2009). In case of Rpf1, RNA was extracted directly from the imidazole eluate. For both proteins, the cDNAs obtained were sequenced using the Illumina MiSeq sequencing platform. Analysis of the sequencing results was done as described in Thoms et al. (2015). The 5′ and 3′ linkers, RT and PCR oligos are listed in Table S6 (Thoms et al., 2015). The experiments were performed twice for both proteins and the results were reproducible.
Cryo-electron microscopy
Carbon coated holey grids (2nm, Quantifoil) were glow discharged at 2.2x10−1 mbar for 30 s. For each grid, 3.5μl of sample were applied to the grids at a concentration of 1.8 A260ml-1 for the Nsa1-TAP Flag-Ytm1 sample, 1.5 A260ml-1 for the TAP-Flag-Ytm1 E80A sample and 1.3 A260ml-1 for the Rix1-TAP Rpf2-Flag sample. Subsequent vitrification was performed by plunge freezing in liquid ethane using a Vitrobot mark IV (FEI Company, Netherlands) with a blotting time of 3 s at 4°C.
Cryo-EM data were collected semi-automatically using the acquisition software EM-TOOLS (TVIPS, Germany) on a Titan Krios transmission electron microscope (FEI Company) at a defocus range between 0.9 and 3.5 μm. All data for the Nsa1-TAP Flag-Ytm1 sample and the Rix1-TAP Rpf2-Flag sample were recorded on a Falcon II detector under low dose conditions with a nominal pixel size of 1.084 A/pixel on the object scale. For the Nsa1-TAP Flag-Ytm1 sample, a total of four datasets comprising 2547, 689, 1196 and 1046 micrographs were collected. All micrographs experienced a total exposure of ∼27 e−/A2 fractionated into 6 frames for the first dataset and 10 for all subsequent datasets. For the Rix1-Tap Rpf2-Flag sample 4447 micrographs dose fractionated into 10 frames were collected. Micrographs of the TAP-Flag-Ytm1 E80A sample were recorded using a Falcon II upgraded with a Falcon III detector chip also operating with a pixel size of 1.084 Å/pixel on the object scale. For this dataset 5812 micrographs were collected with an accumulated dose of 27e−/Å fractionated into 10 frames.
Image processing
Dose-fractionation movies were initially aligned and summed up using MotionCorr2.1 (Li et al., 2013). Determination of the contrast transfer function parameters was performed via GCTF (Zhang, 2016). For further processing, the original movies were re-aligned with Motioncor2 with anisotropic motion correction (Zheng et al., 2017) using 5x5 patches. Gautomatch (http://www.mrc-lmb.cam.ac.uk/kzhang) was used to pick particles. All further image processing (classifications, refinements, and particle polishing) was performed using Relion-2.0 (Kimanius et al., 2016).
Nsa1-TAP Flag-Ytm1 dataset
First, all picked particles were subjected to two-dimensional reference free classification into 150 classes. Based on the resulting class averages, 90 classes with a total of 306732 particles were selected for further processing. An initial round of 3D classification was performed using the Arx1 particle (EMDB: 2528) as a reference allowing rotational and translational alignment. Classes with similar map features were combined, subjected to 3D refinement and second rounds of 3D-classifications. After the final classification step, similar maps classes were joined and 3D-refinements using a wide soft edge mask and solvent flattened FSC calculation were performed. Particles of the highest resolved state C (3.7 Å) were then subjected to movie refinement of individual particle stacks using a running average window of 3 frames and a standard deviation of 1 pixel as a prior for the translations (Bai et al., 2013). Particle polishing (Scheres, 2014) was performed using default parameters, followed by a final round of 3D refinement. All final reconstructions were subjected to post processing using a wide soft edge mask. In this fashion, four distinct states were recovered from the dataset, State A at a resolution of 4.2 Å, State B at 4.1 Å, State C at 3.6 Å and State D at 4.3 Å. All resolutions are supplied according to the FSC0.143 criterion following the Relion gold-standard refinement (Figure S2).
Rix1-TAP Rpf2-Flag dataset
Reference free two dimensional classification into 150 classes yielded in 60 classes with 320144 good (ribosomal) particles. These were subjected to 3D refinement and classified into 10 classes. Based on visual inspection of the map features, classes were combined and further refined. Movie processing and particle polishing was performed as described above. Another round of 3D classification followed by a final 3D refinement and post-processing yields in State E with a nominal resolution of 3.7 Å. The remaining 7 classes of the initial 3D classification were all joined and refined, resulting in state F with an average resolution of 3.3 Å after post processing with a wide soft edge mask (Figure S2).
TAP-Flag-YtmE80A dataset
All particles were subjected to reference free 2D classification into 200 classes, yielding 78 classes with 297516 good ribosomal particles. After an initial round of 3D refinement, 3D classification of the particles into 8 classes was performed. The 5 classes representing the state E of the Rix1-TAP Rpf2-Flag sample were joined. Movie processing and particle polishing was performed as described above. A further round of 3D classification was performed followed by a final round of 3D refinement yielding a 3.3 Å reconstruction of state E after post processing with a wide soft edge mask (Figure S2).
Model building and refinements
Based on the proteins identified in the purification (Figure S1), a model was built for the Ytm1 E80A state E map. Therefore, the model of the Nog2 particle (PDB: 3jct) was first fitted as a rigid body into the State E density using UCSF Chimera (Pettersen et al., 2004). This served as a starting model for fitting the ribosomal RNA, the RPs as well as the biogenesis factors Cic1, Mrt4, Nog1, Nop7, Nop15, Nsa2, Nug1, Rlp24, Rlp7 and Tif6. Chains of factors not represented by density in this map were deleted, the rRNA stretches that were not represented by the density were pruned. The results were then manually inspected and adjusted in COOT (Emsley et al., 2010). Nucleotides 2404-2818 were independently fitted with COOT based on the crystal structure of the yeast 80S ribosome (PDB: 4V7R). For target proteins with existing homologies, secondary structure predictions were calculated by PSIPRED and homology models were created via HHpred/Modeler (Meier and Söding, 2015) and Phyre2 (Kelley et al., 2015). The homology models then served as a starting model for de-novo building in COOT. Assignments of the proteins were confirmed by side chain density and secondary structure patterns. Assignments of the structurally similar Brix proteins were verified using CRAC analysis (Figure S6).
All models were combined and subsequently refined using phenix.real_space_refine (Wang et al., 2014). Refmac5 reciprocal space refinement using restraints generated via LIBG and ProSMART was then performed as previously shown (Amunts et al., 2014, Brown et al., 2015).
The model for Nsa1-TAP Flag-Ytm1 state C was created as previously described, the final model of state E was used as a starting model. The crystal structure of Nsa1 (this study) (Figure S6B) was used as a starting model and modified in COOT. An S. cerevisiae homology model of Rrp1 was created using MODELER with the C. thermophilum crystal structure (also this study) (Figure S6C) and the respective sequence alignment from ClustalOMEGA as input. For modeling the RNA of ES7a, an RNA tertiary structure prediction by RNAComposer (http://rnacomposer.ibch.poznan.pl/) was used as an initial reference. Refinement of the model was performed as described for Ytm1 E80A state E, with the exception that the chains of the less well resolved “foot” region (ITS2 RNA, Cic1/Nsa3, Erb1, Has1, Nop7, Nop15 and Rlp7) were not subjected to refinement in Refmac5 but docked as a rigid body using chimera.
Architectural models of states A, B and D were created on the basis of the refined state C and E models. First a general model was created by clipping segments not represented by the respective density map using chimera, the chains of the resulting model were then rigid body fitted using phenix.real_space_refine and finally sidechains were removed using phenix.pdbtools (Pettersen et al., 2004, Wang et al., 2014).
Values of the final refinements and model validation for both models are provided in Table S5.
Molecular interpretation of State F was based on the Arx1/Nog2 particle model (PDB: 3JCT) (Wu et al., 2016). Figures were created using UCSF chimera and PyMOL Molecular Graphics System (Version 1.7.4, Schrödinger, LLC).
Quantification and Statistical Analyses
See Methods Details for details on image processing and model building and refinements.
Data and Software Availability
All maps are deposited at EMDB as noted in the Key Resources Table. Atomic models and architectural models are deposited at PDB as noted in the Key Resources Table.
Accession codes
Cryo-EM density map State A-F: EMDB: EMD-3888; EMDB: EMD-3889; EMDB: EMD-3893; EMDB: EMD-3890; EMDB: EMD-3891; EMDB: EMD-3892
Atomic model (PDB) Rrp1 (Spacegroup C2, P6322): PDB: 6EMF, PDB: 6EMG
Atomic model (PDB) Nsa1: PDB: 6EN7
Atomic model (PDB) State C, E: PDB: 6EM1, PDB: 6ELZ
Architectural model (PDB) state A, B, D: PDB: 6EM3, PDB: 6EM4, PDB: 6EM5
Acknowledgments
We thank S. Rieder for preparation of Cryo-EM grids; T. Becker for critical input on the manuscript; A. Heuer and the Leibniz Rechenzentrum for support with HPC infrastructure; and J. Kopp and C. Siegmann from the BZH/CellNetworks crystallization platform. This work was supported by grants from the German Research Council (QBM, GRK 1721, FOR 1805, and SFB646 to R.B.; HU363/10-5 and HU363/12-1 to E.H.; SFB638, the Leibniz Programme and the Cluster of Excellence/CellNetworks Ectop1 to I.S.; and GRK1188 to M.T.). This work was also supported by CellNetworks Ectop1 and the Leibnitz Programme (to I.S.). I.S. and E.H. are investigators of the Cluster of Excellence/CellNetworks. R.B. acknowledges support from the Center for Integrated Protein Science (CiPS-M) and the European Research Council (advanced grant CRYOTRANSLATION). L.K. was supported by a DFG fellowship through the Graduate School of Quantitative Biosciences Munich (QBM).
Author Contributions
R.B., E.H., M.T., and L.K. designed this study and the experiments. Preparation of cryo-EM samples was performed by M.T. Cryo-EM data were collected by O.B. and processed by L.K. Model building was performed by L.K. and J.C. and C.B.-G. assisted. Crystal structure determination of Nsa1 and Rrp1 was performed by G.B., D.K., Y.L.A., and I.S. CRAC analysis was performed by S.I., E.H., R.B., and M.T., and L.K. interpreted the results. E.H., R.B., M.T., C.B.-G., and L.K. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Declaration of Interests
All authors declare no competing interests.
Published: December 14, 2017
Footnotes
Supplemental Information includes six figures and five tables and can be found with this article online at https://doi.org/10.1016/j.cell.2017.11.039.
Supplemental Information
References
- Afonine P.V., Grosse-Kunstleve R.W., Echols N., Headd J.J., Moriarty N.W., Mustyakimov M., Terwilliger T.C., Urzhumtsev A., Zwart P.H., Adams P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts A., Brown A., Bai X.C., Llácer J.L., Hussain T., Emsley P., Long F., Murshudov G., Scheres S.H.W., Ramakrishnan V. Structure of the yeast mitochondrial large ribosomal subunit. Science. 2014;343:1485–1489. doi: 10.1126/science.1249410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X.C., Fernandez I.S., McMullan G., Scheres S.H. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. eLife. 2013;2:e00461. doi: 10.7554/eLife.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio-Garcia C., Thoms M., Flemming D., Kater L., Berninghausen O., Baßler J., Beckmann R., Hurt E. Architecture of the Rix1-Rea1 checkpoint machinery during pre-60S-ribosome remodeling. Nat. Struct. Mol. Biol. 2016;23:37–44. doi: 10.1038/nsmb.3132. [DOI] [PubMed] [Google Scholar]
- Bassler J., Kallas M., Pertschy B., Ulbrich C., Thoms M., Hurt E. The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol. Cell. 2010;38:712–721. doi: 10.1016/j.molcel.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baßler J., Ahmed Y.L., Kallas M., Kornprobst M., Calviño F.R., Gnädig M., Thoms M., Stier G., Ismail S., Kharde S. Interaction network of the ribosome assembly machinery from a eukaryotic thermophile. Protein Sci. 2017;26:327–342. doi: 10.1002/pro.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A., Garreau de Loubresse N., Melnikov S., Jenner L., Yusupova G., Yusupov M. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science. 2011;334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- Bradatsch B., Leidig C., Granneman S., Gnädig M., Tollervey D., Böttcher B., Beckmann R., Hurt E. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. Nat. Struct. Mol. Biol. 2012;19:1234–1241. doi: 10.1038/nsmb.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Long F., Nicholls R.A., Toots J., Emsley P., Murshudov G. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D Biol. Crystallogr. 2015;71:136–153. doi: 10.1107/S1399004714021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaker-Margot M., Barandun J., Hunziker M., Klinge S. Architecture of the yeast small subunit processome. Science. 2017;355:355. doi: 10.1126/science.aal1880. [DOI] [PubMed] [Google Scholar]
- Chen V.B., Arendall W.B., 3rd, Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. MolProbity: all-atom structure validation for macromolecular crystallography. Acta. Crystallogr. D. Biol. Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Xie Z., Yang F., Ye K. Stepwise assembly of the earliest precursors of large ribosomal subunits in yeast. Nucleic Acids Res. 2017;45:6837–6847. doi: 10.1093/nar/gkx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- Davis J.H., Tan Y.Z., Carragher B., Potter C.S., Lyumkis D., Williamson J.R. Modular assembly of the bacterial large ribosomal subunit. Cell. 2016;167:1610–1622.e15. doi: 10.1016/j.cell.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sanctis D., Beteva A., Caserotto H., Dobias F., Gabadinho J., Giraud T., Gobbo A., Guijarro M., Lentini M., Lavault B. ID29: a high-intensity highly automated ESRF beamline for macromolecular crystallography experiments exploiting anomalous scattering. J. Synchrotron Radiat. 2012;19:455–461. doi: 10.1107/S0909049512009715. [DOI] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalinda M., Ohmayer U., Jakovljevic J., Kumcuoglu B., Woolford J., Mbom B., Lin L., Woolford J.L., Jr. A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev. 2014;28:198–210. doi: 10.1101/gad.228825.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi P., Rybin V., Bassler J., Petfalski E., Strauss D., Marzioch M., Schäfer T., Kuster B., Tschochner H., Tollervey D. 90S pre-ribosomes include the 35S pre-rRNA, the U3 snoRNP, and 40S subunit processing factors but predominantly lack 60S synthesis factors. Mol. Cell. 2002;10:105–115. doi: 10.1016/s1097-2765(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Granneman S., Kudla G., Petfalski E., Tollervey D. Identification of protein binding sites on U3 snoRNA and pre-rRNA by UV cross-linking and high-throughput analysis of cDNAs. Proc. Natl. Acad. Sci. USA. 2009;106:9613–9618. doi: 10.1073/pnas.0901997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman S., Petfalski E., Tollervey D. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J. 2011;30:4006–4019. doi: 10.1038/emboj.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber B.J., Boehringer D., Montellese C., Ban N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. Nat. Struct. Mol. Biol. 2012;19:1228–1233. doi: 10.1038/nsmb.2425. [DOI] [PubMed] [Google Scholar]
- Janke C., Magiera M.M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E., Knop M. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Processing of X-ray snapshots from crystals in random orientations. Acta Crystallogr. D Biol. Crystallogr. 2014;70:2204–2216. doi: 10.1107/S1399004714013534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel L., Loibl M., Zisser G., Klein I., Fruhmann G., Gruber C., Unterweger S., Rechberger G., Pertschy B., Bergler H. Rlp24 activates the AAA-ATPase Drg1 to initiate cytoplasmic pre-60S maturation. J. Cell Biol. 2012;199:771–782. doi: 10.1083/jcb.201205021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimanius D., Forsberg B.O., Scheres S.H.W., Lindahl E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife. 2016;5:5. doi: 10.7554/eLife.18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konikkat S., Biedka S., Woolford J.L., Jr. The assembly factor Erb1 functions in multiple remodeling events during 60S ribosomal subunit assembly in S. cerevisiae. Nucleic Acids Res. 2017;45:4853–4865. doi: 10.1093/nar/gkw1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornprobst M., Turk M., Kellner N., Cheng J., Flemming D., Koš-Braun I., Koš M., Thoms M., Berninghausen O., Beckmann R., Hurt E. Architecture of the 90S Pre-ribosome: a structural view on the birth of the eukaryotic ribosome. Cell. 2016;166:380–393. doi: 10.1016/j.cell.2016.06.014. [DOI] [PubMed] [Google Scholar]
- Kressler D., Rojo M., Linder P., Cruz J. Spb1p is a putative methyltransferase required for 60S ribosomal subunit biogenesis in Saccharomyces cerevisiae. Nucleic Acids Res. 1999;27:4598–4608. doi: 10.1093/nar/27.23.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D., Roser D., Pertschy B., Hurt E. The AAA ATPase Rix7 powers progression of ribosome biogenesis by stripping Nsa1 from pre-60S particles. J. Cell Biol. 2008;181:935–944. doi: 10.1083/jcb.200801181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B., Purushothaman S.K. Spb1p-directed formation of Gm2922 in the ribosome catalytic center occurs at a late processing stage. Mol. Cell. 2004;16:663–669. doi: 10.1016/j.molcel.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Leidig C., Thoms M., Holdermann I., Bradatsch B., Berninghausen O., Bange G., Sinning I., Hurt E., Beckmann R. 60S ribosome biogenesis requires rotation of the 5S ribonucleoprotein particle. Nat. Commun. 2014;5:3491. doi: 10.1038/ncomms4491. [DOI] [PubMed] [Google Scholar]
- Li X., Mooney P., Zheng S., Booth C.R., Braunfeld M.B., Gubbens S., Agard D.A., Cheng Y. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods. 2013;10:584–590. doi: 10.1038/nmeth.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K.Y., Li Z., Bussiere C., Bresson S., Marcotte E.M., Johnson A.W. Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol. Cell. 2010;39:196–208. doi: 10.1016/j.molcel.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y.-H., Romes E.M., Pillon M.C., Sobhany M., Stanley R.E. Structural analysis reveals features of ribosome assembly factor Nsa1/WDR74 important for localization and interaction with Rix7/NVL2. Structure. 2017;25:762–772.e4. doi: 10.1016/j.str.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie A., 3rd, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., Pringle J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Ma C., Wu S., Li N., Chen Y., Yan K., Li Z., Zheng L., Lei J., Woolford J.L., Jr., Gao N. Structural snapshot of cytoplasmic pre-60S ribosomal particles bound by Nmd3, Lsg1, Tif6 and Reh1. Nat. Struct. Mol. Biol. 2017;24:214–220. doi: 10.1038/nsmb.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyutin A.G., Musalgaonkar S., Patchett S., Frank J., Johnson A.W. Nmd3 is a structural mimic of eIF5A, and activates the cpGTPase Lsg1 during 60S ribosome biogenesis. EMBO J. 2017;36:854–868. doi: 10.15252/embj.201696012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y., Granneman S., Thoms M., Manikas R.G., Tollervey D., Hurt E. Coupled GTPase and remodelling ATPase activities form a checkpoint for ribosome export. Nature. 2014;505:112–116. doi: 10.1038/nature12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann K.L., Charette J.M., Vincent N.G., Baserga S.J. A protein interaction map of the LSU processome. Genes Dev. 2015;29:862–875. doi: 10.1101/gad.256370.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A., Söding J. Automatic prediction of protein 3D structures by probabilistic multi-template homology modeling. PLoS Comput. Biol. 2015;11:e1004343. doi: 10.1371/journal.pcbi.1004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne T.F., Goyenechea B., Sánchez-Puig N., Wong C.C., Tonkin L.M., Ancliff P.J., Brost R.L., Costanzo M., Boone C., Warren A.J. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat. Genet. 2007;39:486–495. doi: 10.1038/ng1994. [DOI] [PubMed] [Google Scholar]
- Miles T.D., Jakovljevic J., Horsey E.W., Harnpicharnchai P., Tang L., Woolford J.L., Jr. Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol. Cell. Biol. 2005;25:10419–10432. doi: 10.1128/MCB.25.23.10419-10432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O.L., Jr., Beatty B.R. Visualization of nucleolar genes. Science. 1969;164:955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Skubák P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissan T.A., Bassler J., Petfalski E., Tollervey D., Hurt E. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 2002;21:5539–5547. doi: 10.1093/emboj/cdf547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape T., Schneider T.R. HKL2MAP: a graphical user interface for macromolecular phasing with SHELX programs. J. Appl. Cryst. 2004;37:843–844. [Google Scholar]
- Passos D.O., Lyumkis D. Single-particle cryoEM analysis at near-atomic resolution from several thousand asymmetric subunits. J. Struct. Biol. 2015;192:235–244. doi: 10.1016/j.jsb.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Pertschy B., Saveanu C., Zisser G., Lebreton A., Tengg M., Jacquier A., Liebminger E., Nobis B., Kappel L., van der Klei I. Cytoplasmic recycling of 60S preribosomal factors depends on the AAA protein Drg1. Mol. Cell. Biol. 2007;27:6581–6592. doi: 10.1128/MCB.00668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestov D.G., Stockelman M.G., Strezoska Z., Lau L.F. ERB1, the yeast homolog of mammalian Bop1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Res. 2001;29:3621–3630. doi: 10.1093/nar/29.17.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Ramesh M., Woolford J.L., Jr. Eukaryote-specific rRNA expansion segments function in ribosome biogenesis. RNA. 2016;22:1153–1162. doi: 10.1261/rna.056705.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Pech M., Thoms M., Beckmann R., Hurt E. Ribosome-stalk biogenesis is coupled with recruitment of nuclear-export factor to the nascent 60S subunit. Nat. Struct. Mol. Biol. 2016;23:1074–1082. doi: 10.1038/nsmb.3312. [DOI] [PubMed] [Google Scholar]
- Scheres S.H. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife. 2014;3:e03665. doi: 10.7554/eLife.03665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Yang J., Watzinger P., Kötter P., Entian K.D. Yeast Nop2 and Rcm1 methylate C2870 and C2278 of the 25S rRNA, respectively. Nucleic Acids Res. 2013;41:9062–9076. doi: 10.1093/nar/gkt679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G.M. A short history of SHELX. Acta Crystallogr. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Sun Q., Zhu X., Qi J., An W., Lan P., Tan D., Chen R., Wang B., Zheng S., Zhang C. Molecular architecture of the 90S small subunit pre-ribosome. eLife. 2017;6 doi: 10.7554/eLife.22086. pii: e22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkish J., Zhang J., Jakovljevic J., Horsey E.W., Woolford J.L., Jr. Hierarchical recruitment into nascent ribosomes of assembly factors required for 27SB pre-rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res. 2012;40:8646–8661. doi: 10.1093/nar/gks609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Thoms M., Thomson E., Baßler J., Gnädig M., Griesel S., Hurt E. The exosome is recruited to RNA substrates through specific adaptor proteins. Cell. 2015;162:1029–1038. doi: 10.1016/j.cell.2015.07.060. [DOI] [PubMed] [Google Scholar]
- Thoms M., Ahmed Y.L., Maddi K., Hurt E., Sinning I. Concerted removal of the Erb1-Ytm1 complex in ribosome biogenesis relies on an elaborate interface. Nucleic Acids Res. 2016;44:926–939. doi: 10.1093/nar/gkv1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski T.W., Tollervey D. Cotranscriptional events in eukaryotic ribosome synthesis. Wiley Interdiscip. Rev. RNA. 2015;6:129–139. doi: 10.1002/wrna.1263. [DOI] [PubMed] [Google Scholar]
- Ulbrich C., Diepholz M., Bassler J., Kressler D., Pertschy B., Galani K., Böttcher B., Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009;138:911–922. doi: 10.1016/j.cell.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Wang Z., Hryc C.F., Bammes B., Afonine P.V., Jakana J., Chen D.H., Liu X., Baker M.L., Kao C., Ludtke S.J. An atomic model of brome mosaic virus using direct electron detection and real-space optimization. Nat. Commun. 2014;5:4808. doi: 10.1038/ncomms5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrecki M., Rodríguez-Galán O., de la Cruz J., Bravo J. The structure of Erb1-Ytm1 complex reveals the functional importance of a high-affinity binding between two β-propellers during the assembly of large ribosomal subunits in eukaryotes. Nucleic Acids Res. 2015;43:11017–11030. doi: 10.1093/nar/gkv1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner K.A., Baserga S.J. The sigma(70)-like motif: a eukaryotic RNA binding domain unique to a superfamily of proteins required for ribosome biogenesis. Mol. Cell. 2002;9:329–339. doi: 10.1016/s1097-2765(02)00438-0. [DOI] [PubMed] [Google Scholar]
- Weis F., Giudice E., Churcher M., Jin L., Hilcenko C., Wong C.C., Traynor D., Kay R.R., Warren A.J. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat. Struct. Mol. Biol. 2015;22:914–919. doi: 10.1038/nsmb.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn M.D., Ashton A.W., Briggs P.J., Ballard C.C., Patel P. Ongoing developments in CCP4 for high-throughput structure determination. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1929–1936. doi: 10.1107/s0907444902016116. [DOI] [PubMed] [Google Scholar]
- Woolford J.L., Jr., Baserga S.J. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics. 2013;195:643–681. doi: 10.1534/genetics.113.153197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Tutuncuoglu B., Yan K., Brown H., Zhang Y., Tan D., Gamalinda M., Yuan Y., Li Z., Jakovljevic J. Diverse roles of assembly factors revealed by structures of late nuclear pre-60S ribosomes. Nature. 2016;534:133–137. doi: 10.1038/nature17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.Q., Palovcak E., Armache J.P., Verba K.A., Cheng Y., Agard D.A. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.