Abstract
The inability of axons to regenerate over long-distances in the central nervous system (CNS) limits the recovery of sensory, motor, and cognitive functions after various CNS injuries and diseases. Although pre-clinical studies have identified a number of manipulations that stimulate some degree of axon growth after CNS damage, the extent of recovery remains quite limited, emphasizing the need for improved therapies. Here, we used traumatic injury to the mouse optic nerve as a model system to test the effects of combining several treatments that have recently been found to promote axon regeneration without the risks associated with manipulating known tumor suppressors or oncogenes. The treatments tested here include TPEN, a chelator of mobile (free) zinc (Zn2+); shRNA against the axon growth-suppressing transcription factor Klf9; and the atypical growth factor oncomodulin combined with a cAMP analog. Whereas some combinatorial treatments produced only marginally stronger effects than the individual treatments alone, co-treatment with TPEN and Klf9 knockdown had a substantially stronger effect on axon regeneration than either one alone. This combination also promoted a high level of cell survival at longer time points. Thus, Zn2+ chelation in combination with Klf9 suppression holds therapeutic potential for promoting axon regeneration after optic nerve injury, and may also be effective for treating other CNS injuries and diseases.
Keywords: Optic neuropathy, Axon regeneration, Retinal ganglion cell, Zinc chelation, Klf9, Oncomodulin, Gene therapy
Introduction
The inability of neurons in the central nervous system (CNS) to survive or to regenerate damaged axons over long distances limits recovery after multiple types of damage, including spinal cord injury (Hug and Weidner, 2012), stroke (Benowitz and Carmichael, 2010; Sozmen et al., 2012), and traumatic or ischemic optic neuropathy (Benowitz et al., 2015; Ghaffarieh and Levin, 2012; Wang et al., 2013; You et al., 2013). In the case of the optic nerve, manipulation of various factors can promote long-distance regeneration and some reinnervation of central target areas, but these treatments have generally involved intraocular inflammation and/or deletion or knock-down of genes that can act as tumor suppressors, raising questions about their clinical utility (Bei et al., 2016; Belin et al., 2015; de Lima et al., 2012a; de Lima et al., 2012b; Duan et al., 2015; Kurimoto et al., 2010; Lee et al., 2010; Lim et al., 2016; Liu et al., 2010; Park et al., 2008; Smith et al., 2009; Sun et al., 2011).
Here, we used the optic nerve crush (ONC) model in mice to evaluate combinatorial effects of three recently established treatments that stimulate retinal ganglion cells (RGCs), the projection neurons of the eye, to re-grow injured axons over long-distances. We focused on treatments that do not involve manipulation of factors such as Pten, Socs3, Sox11, Bcl2, and Myc (Belin et al., 2015; Chen et al., 1997; de Lima et al., 2012b; Jayaprakash et al., 2016; Kurimoto et al., 2010; Park et al., 2008; Smith et al., 2009; Sun et al., 2011), which are established tumor suppressors or oncogenes (Cory et al., 2003; He et al., 2003; Keniry and Parsons, 2008; Kuo et al., 2015; Nilsson and Cleveland, 2003; Rigby et al., 2007). We tested combinatorial effects of the following treatments: the Zn2+ chelator TPEN (Li et al., 2017), shRNA gene therapy-mediated knockdown (KD) of the axon-growth suppressing transcription factor Klf9 (Apara et al., 2017), and the atypical growth factor oncomodulin (Ocm) combined with a cAMP analog (CPT-cAMP) (Kurimoto et al., 2010; Yin et al., 2009; Yin et al., 2006).
We used a Zn2+ chelator based on our recent finding that injury to the optic nerve induces a rapid and profound elevation of mobile Zn2+ in the retina, and that intraocular injection of Zn2+-selective chelators such as TPEN [N,N,N′,N′-tetrakis(2- pyridyl methyl) ethylenediamine] lead to long-lasting RGC protection and considerable axon regeneration (Li et al., 2017). Other studies have shown that intraocular inflammation also induces substantial levels of optic nerve regeneration and that this effect is mediated in large part by the atypical growth factor oncomodulin (Ocm), which binds to its cognate receptor on RGCs in a cAMP-dependent manner (Kurimoto et al., 2010; Yin et al., 2009; Yin et al., 2006). The combination of Ocm and a cAMP analog (e.g., CPT-cAMP) captures most of the effects of intraocular inflammation on axon growth without the potentially harmful effects of intraocular inflammation, although Ocm/cAMP has only a small effect on cell survival (Andereggen et al., 2015; Yin et al., 2006). Our other recent work has shown that several members of the Kruppel-like transcription factor (KLF) family contribute to the developmental changes in RGCs’ intrinsic capacity to extend axons, and that knock-down of KLF-4 or KLF-9, two developmentally-upregulated suppressors of axon growth, promotes axon regeneration in mature mice (Apara et al., 2017; Moore et al., 2009).
Here, we found that out of these approaches, a co-treatment with TPEN and Klf9 KD had a substantially stronger effect than either one alone, while the other combinatorial treatments produced only marginally stronger effects than the individual treatments. Thus, Zn2+ chelation in combination with Klf9 suppression holds therapeutic potential for promoting RGC survival and axon regeneration after optic nerve injury and potentially in other parts of the CNS as well.
Results
We tested three pro-regenerative approaches individually and then in 2-way combinations for possible additive or synergistic effects in promoting RGC survival and axon regeneration after optic nerve crush (ONC) injury in mice, a widely used model of CNS injury, and more specifically, traumatic optic neuropathy. Where information was available, we used the doses and timing of treatments as previously described (Kurimoto et al., 2010; Li et al., 2017; Yin et al., 2009; Yin et al., 2006) (experimental time-line is shown in Fig. 1): For localized gene therapy, we injected intravitreally 3 μl of a high titer (1 × 1012) adeno-associated virus serotype 2 (AAV2) expressing anti-KLF9 shRNAs with a GFP marker, or GFP control, two weeks prior to ONC injury. For targeted drug delivery, we injected intravitreally immediately after ONC 3 μl of either Ocm (15 ng/μl) combined with CPT-cAMP (50 μM; as described previously for this treatment, Yin et al., 2006), or TPEN (100 μM; also injected 4 days later as described previously for this treatment, Li et al., 2017), or phosphate-buffered saline (PBS) as a control. Across studies, the investigator performing the surgeries and quantification was masked to the treatment (i.e., the tube with virus or drug was prepared and coded by another researcher) until the end of the experiment. Animals that received viral injections were randomly selected for subsequent injections of different drugs or PBS alone. The animals were euthanized two weeks after injury and analyzed for axon regeneration in the optic nerve and RGC survival in the retina as described in Methods (Fig. 1). A few animals that developed cataract in the injured eye were excluded.
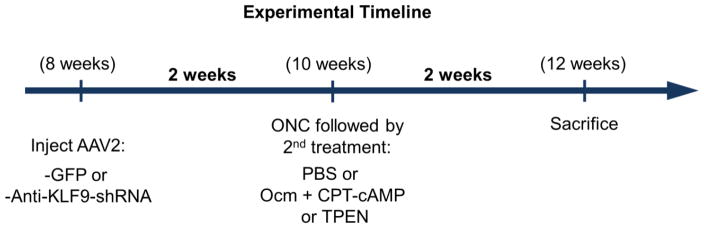
Figure 1. Experimental timeline.
Adult mice were pre-treated with anti-Klf9 shRNA gene therapy or GFP control virus 2 weeks prior to optic nerve crush (ONC) injury. The indicated treatments were injected intravitreally immediately after the injury and animals were sacrificed for histological analysis 2 weeks later. For the long-term experiment shown in Fig. 6, mice were sacrificed 6 weeks after injury.
As expected, the negative control group (AAV2-GFP injected prior to nerve injury, PBS injected shortly after injury) showed very few axons growing beyond the injury site, whereas each of the individual experimental treatments promoted considerable regeneration (Figs. 2–3; Table 1). Of these, pre-injury KLF9 KD resulted in greater regeneration than post-injury administration of Ocm + CPT-cAMP or TPEN (Fig. 2–3; Table 1). The extent of axon regeneration induced by Ocm + CPT-cAMP or TPEN was comparable to previous reports from our lab (Li et al., 2017; Yin et al., 2009; Yin et al., 2006).
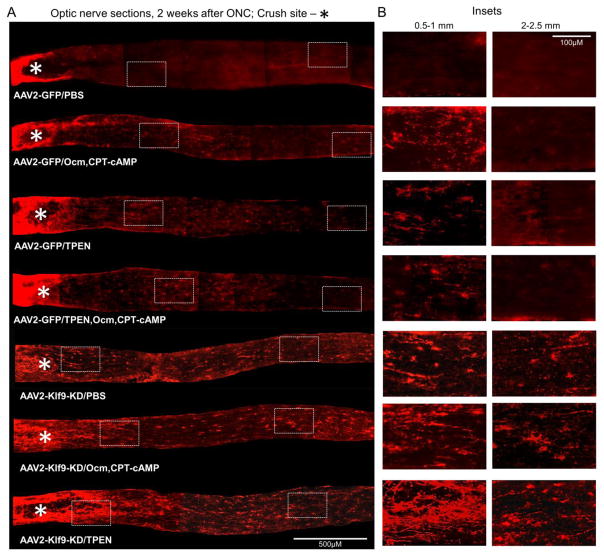
Figure 2. Axon regeneration 2 weeks after various treatments.
(A) Representative images of GAP-43-immunostained longitudinal sections through the optic nerve 2 weeks after ONC from the various conditions, as marked. The edges of the tissue were optically trimmed (i.e., cropped-out) due to artefactual autofluorescence that is common at tissue edges. (B) Representative images of the optic nerve regions proximal and distal to the injury site are magnified for better visualization of the axons or their absence.
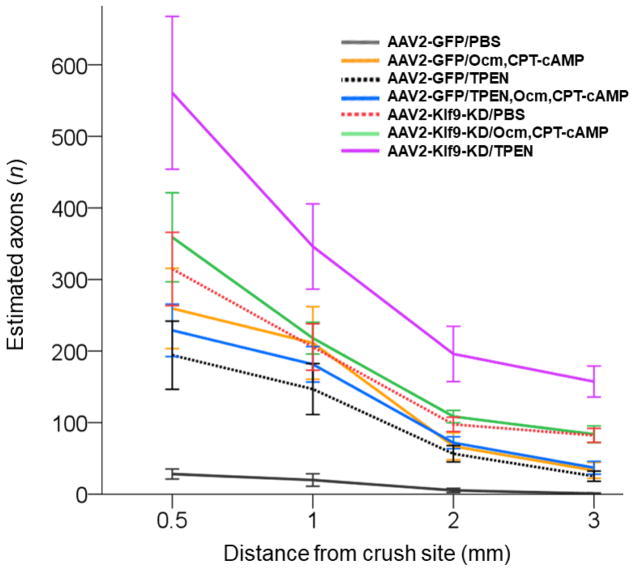
Figure 3. Quantitation of axon regeneration 2 weeks after individual and combinatorial treatments.
Regenerating axons visualized by GAP-43 immunostaining quantified 2 weeks after ONC at increasing distances from the injury site across various conditions, as marked (Mean ± S.E.M; n = 5–6 cases for each treatment). ANOVA with repeated measures, sphericity assumed, overall F = 13.7, p < 0.0001; p-values of pairwise comparisons by posthoc LSD are shown in Table 1.
Table 1. Pairwise comparisons p-values for Figure 3.
Pairwise comparisons between the treatments in Figure 3 were performed by ANOVA with repeated measures and posthoc LSD. The p-values for each individual treatment compared to control treatment are shown in the first column. The p-values in rest of the columns are for comparisons between each combination treatment and the individual treatments, as well as between the combinatorial treatments themselves, as marked. Significant differences indicated by a star and bold p-value.
| AAV2-GFP/PBS | AAV2-GFP/TPEN, Ocm, CPT-cAMP | AAV2-Klf9-KD/Ocm, CPT-cAMP | AAV2-Klf9-KD/TPEN | |
|---|---|---|---|---|
| AAV2-GFP/Ocm, CPT-cAMP | 0.009* | 0.050* | 0.050* | 0.012* |
| AAV2-GFP/TPEN | 0.020* | 0.011* | 0.011* | 0.020* |
| AAV2-GFP/TPEN, Ocm, CPT-cAMP | 0.001** | – | 0.033* | 0.019* |
| AAV2-Klf9-KD/PBS | 0.001** | 0.180 | 0.494 | 0.075 |
| AAV2-Klf9-KD/Ocm, CPT-cAMP | 0.001** | 0.033* | – | 0.115 |
| AAV2-Klf9-KD/TPEN | 0.003* | 0.019* | 0.115 | – |
Combining KLF9 KD with TPEN resulted in substantially stronger regeneration than either one alone, enabling ~200 axons to regenerate more than half-way down the optic nerve in just two weeks (total length of optic nerve ~5 mm) (Fig. 2–3; Table 1). A few axons even entered the optic chiasm in these mice (Fig. 4). In contrast, the effect of combining Ocm + CPT-cAMP with either TPEN or KLF9 KD was only marginally stronger than the individual treatments (Fig. 2–3; Table 1). Some of the treatments improved RGC survival compared to mice receiving the control virus + PBS injections, although the treated groups did not differ significantly from one another even though the averages differed slightly (Fig. 5).
Figure 4. The combination of TPEN and Klf9-KD enables some axons to regenerate into the optic chiasm by 2 weeks after injury.
(A) Micrograph of a horizontal section through the optic chiasm. Outlined regions indicate where axons were detected. (B) GAP-43-immunostained axons detected in the optic chiasm regions outlined in A.
Figure 5. Improved regeneration with TPEN/Klf9 KD is not due to increased RGC survival.
(A) RGC survival quantified in retinal flat-mounts immunostained for the RGC marker βIII-Tubulin (Tuj1 antibody) 2 weeks after ONC with treatment conditions as marked (mean ± S.E.M; n = 5–6; overall F = 13.9, p < 0.0001 by ANOVA, comparisons * p < 0.05 with posthoc LSD); AAV2-GFP and PBS were used as controls. (B) Representative images of Tuj1-labled RGCs show a control that received PBS + GFP and experimentally treated with TPEN + GFP cases, 2 weeks after ONC.
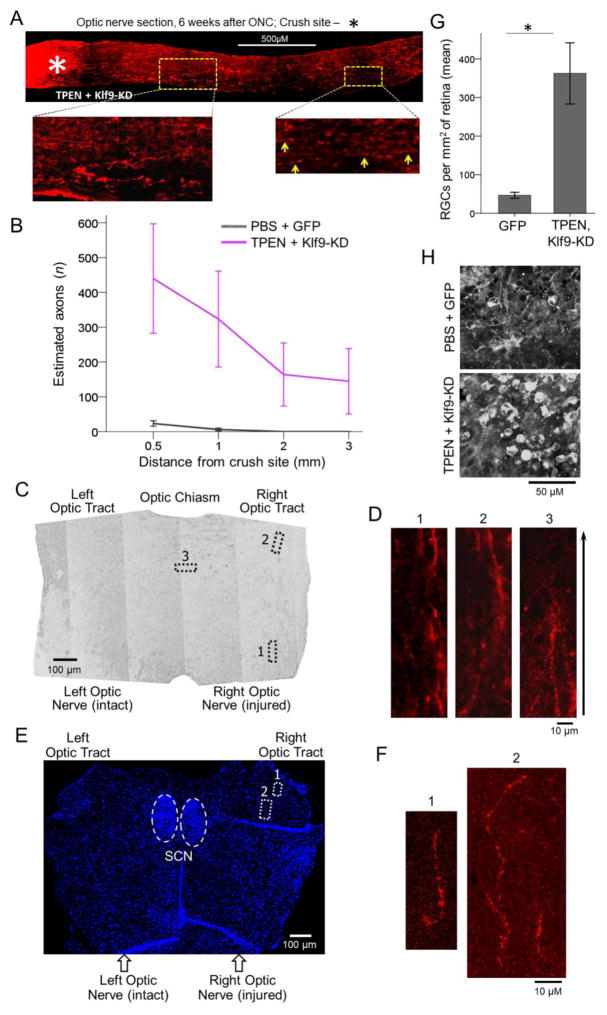
Next, we investigated the long-term effect of the lead combinatorial treatment of TPEN and KLF9 KD. The experiment was performed as above except that animals were sacrificed at 6 weeks after injury. Regenerating axons in these studies were labeled with intravitreal injection of the anterograde tracer cholera toxin subunit B (CTB), as our prior work has shown that levels of GAP-43 immunostaining begin to decline several weeks after the initiation of axon regeneration (Kurimoto et al., 2010). Mice were also tested at 4 weeks after injury and the day before sacrifice for possible recovery of simple visual functions using the optomotor response (OMR) (de Lima et al., 2012b). The number of regenerated axons at 6 weeks after injury was unchanged from what we observed at 2 weeks (Fig. 6A–B), although a few axons regenerated through the optic chiasm and into the ipsilateral optic tract, with some stalling at the midline (Fig. 6C–F). Mice receiving the combinatorial treatment showed ~7-fold higher levels of RGC survival than controls (Fig. 6G–H). These studies did not continue past 6 weeks after injury, and no evidence of visual recovery was found on the OMR tests by the latest time-point we tested, although the experiments were focused primarily on anatomic and not functional endpoints.
Figure 6. The combination of TPEN and Klf9-KD promotes lengthy axon regeneration and persistent RGC survival 6 weeks after injury.
(A) Representative image of the optic nerve longitudinal section with CTB-labeled axons, from a TPEN + Klf9-KD treated case at 6 weeks after ONC. The edges of the tissue were trimmed (i.e., cropped-out) due to artefactual autofluorescence that is common at tissue edges. Inset images of the optic nerve regions proximal and distal to the injury site are magnified for better visualization of the axons, indicated by yellow arrows in the distal region. (B) Regenerated axons visualized by CTB labeling quantified 6 weeks after ONC at increasing distances from the injury site (mean ± S.E.M; n = 4; ANOVA with repeated measures, posthoc LSD). (C) RGC survival quantified in retinal flat-mounts immunostained for an RGC marker βIII-Tubulin (Tuj1 antibody) at 6 weeks after ONC in the treatment conditions as marked (mean ± S.E.M; n = 4; t-test, * p < 0.05). (D) Representative images of Tuj1-labled RGCs show a control that received PBS + GFP and experimentally treated with TPEN + Klf9 KD cases, 6 weeks after ONC. (E) Micrograph of the optic chiasm (horizontal section) with outlined regions where the regenerated axons were detected. (F) Insets of CTB-labeled axons detected in the optic chiasm regions outlined in E. (G) Nuclei marker (DAPI) stained horizontal section through the suprachiasmatic nucleus (SCN) above the optic chiasm along with the initial segments of the optic tracts. The SCN is identified by its typical olive-shaped pattern formed by densely concentrated DAPI-labeled nuclei (outlined by dashed white lines ovals). Regions of the optic tract where CTB-labeled regenerated axons were detected are outlined by doted white lines rectangulars. No axons at, or growing towards, the SCN were found. (H) Insets of the CTB-labeled axons detected in the optic tact regions outlined in G.
Discussion
The failure of CNS projection neurons to regenerate axons damaged by injury or disease remains a major unmet problem in neurology and ophthalmology. No therapeutics exist yet to help patients regenerate damaged axons over long distances, and pre-clinical studies have shown only modest improvements, with some of the most successful approaches involving in-traocular inflammation or manipulation of tumor suppressors and oncogenes such as Pten, Socs3, Sox11, Bcl2, and Myc (Belin et al., 2015; Chen et al., 1997; Jayaprakash et al., 2016; Kurimoto et al., 2010; Park et al., 2008; Smith et al., 2009; Sun et al., 2011), which may be too risky for clinical use in humans (Cory et al., 2003; He et al., 2003; Keniry and Parsons, 2008; Kuo et al., 2015; Nilsson and Cleveland, 2003; Rigby et al., 2007). Here, we carried out a systematic investigation of 2-way combinatorial effects of recently identified axon-regenerating treatments that do not involve direct manipulation of tumor suppressors or oncogenes. Our main result is that localized co-treatment involving attenuation of injury-induced elevation of mobile Zn2+ using TPEN (Li et al., 2017) and shRNA gene therapy-mediated KD of the axon-growth suppressing transcription factor Klf9 (Moore et al., 2009) resulted in a substantially stronger effect on axon regeneration than either treatment alone (although still only ~0.5% of normal at the furthest distance from the injury site we quantified at). Whereas the chelator TPEN in principle could also neutralize other divalent cations such as calcium, iron, manganese, cobalt, and copper (Cho et al., 2007; Ollig et al., 2016), we previously showed (using various Zn2+ sensors, chelators, equimolar pre-mixing with cations, Zn2+ transporter ZnT-3 knockout mice, and other approaches) that in our retinal model system the neuroprotective effect of TPEN was due to chelation of mobile Zn2+.
We further discovered that the effect on axon regeneration was sustained for at least 6 weeks, with some axons growing through the optic chiasm and into the optic tract, albeit only ipsilaterally and not very far. The treatment-induced gains in RGC survival (at 6 weeks after injury) were substantial (~10% of normal), consistent with the effects of TPEN reported recently (Li et al., 2017) and reflecting sustained long-term neuroprotection of RGCs that would otherwise die in the control condition (~ 98.5%). The absence of visual recovery as measured by the OMR test is consistent with the failure of axons to extend far into the optic tract, although the shorter, 6-week time course of the experiments and lack of additional visual assays (e.g., circadian rhythm or visual cliff) suggest that further experimentation will be critical to addressing functional improvement.
A possible mechanistic explanation for the additive effect on axon regeneration might be that TPEN substantially improved long-term RGC health and survival, enhancing the effects of Klf9 KD on long-distance axon regeneration. Mathematically, the effect of the combined treatment appears to be super-additive at the most distal points in the axon, in that the amount of long-distance axon growth seen with the combined treatment (at the furthest distances from the injury site that we quantified at, see Fig. 3) is greater than the sum of the effects of the individual treatments (TPEN or Klf9 KD) in isolation. At this stage, we do not yet know whether the two approaches act on complementary axon-growth pathways or whether they increase the activity of the same molecular pathway. The fact that only a few axons regenerated beyond the optic chiasm despite many more surviving the injury suggests other hypotheses, such as that extrinsic glia-associated inhibitors (Dickendesher et al., 2012; Yiu and He, 2006) may prevent more axons from growing over long distances, or that only a specific subtype of RGCs responds to the treatment robustly (Duan et al., 2015). The precise nature of cooperativity between mobile Zn2+ chelation and Klf9 KD, and the molecular mechanisms that underlie this effect, will be important to investigate in future studies.
The number of axons that were initiated to regenerate by combining TPEN and KLF9 KD did not increase from 2 to 6 weeks after injury, suggesting that this combination does not also have a delayed onset effect on stimulating axon regeneration and/or that only some RGC subtypes are responsive to this treatment. Future studies are needed to test whether regeneration could be further enhanced by additional factors (e.g., attenuating myelin-associated inhibitors), towards achieving functional recovery of at least simple visual parameters.
As this approach does not involve manipulation of tumor suppressors or oncogenes, it may hold therapeutic potential for promoting axon regeneration after CNS injury. Gene therapies are becoming increasingly more accepted for clinical trials and zinc chelation therapy has been tested for safely in other conditions. Thus, localized intraocular injection of Klf9 shRNA clinical grade viruses and TPEN or other chelators has the potential to be translated to the clinic for safety and efficacy profiles, at least providing strong neuroprotection while continuing to develop means to enhance axon regeneration further.
Methods
Animal Use, Surgeries, and Intraocular Injections
Animal studies were performed at Boston Children’s Hospital with approval of the Institutional Animal Care and Use Committee. Mice were housed in the animal facility with a 12-h light/12-h dark cycle (lights on from 7:00 AM to 7:00 PM) and a maximum of five adult mice per cage. The study used wild-type C57BL/6J mice (Jackson Laboratory). Optic nerve surgeries and intravitreal injections were carried out on male mice 8–10 weeks of age (average body weight, 20–26 g) under general anesthesia, as described previously (de Lima et al., 2012b; Li et al., 2017; Yin et al., 2006)
Viruses (3 μl per eye) were injected intravitreally, avoiding injury to the lens, in 8 week-old mice, 2 weeks prior to optic nerve surgery. This lead time allowed for sufficient transduction and expression of the shRNA to knock down expression of Klf9 in RGCs at the time of ONC. The viruses included AAV2 expressing anti-KLF9 shRNAs (target sequences are as follows: 5′-GGAGGCGCTGCCGGTTACGTA-3′, 5′-TGGCTGCCCAGTGTCTGGTTT-3′, 5′-CGGGGGACACCTGGAAGGATT-3′, and 5′-GCAAATAAATGCTTTTGGTAC-3′) and a GFP reporter (1 × 1012 GC/mL; the Miami Project Viral Vector Core, University of Miami Miller School of Medicine, Miami, FL) to knockdown the Klf9 gene expression in RGCs; and as a control, AAV2 expressing a GFP reporter (1 × 1012 GC/mL; Viral Vector Core, Boston Children’s Hospital, Bos-ton, MA). Agents (3 μl per eye) were injected intravitreally immediately after ONC, including recombinant Oncomodulin (15 ng/μl; as described previously (Yin et al., 2006); 8-(4-chlorophenylthio) (CPT)-cAMP (50 μM; Sigma), a membrane permeable, nonhydrolyzable cAMP analog; the specific Zn2+ chelator TPEN (100 μM, Calbiochem), also injected 4 days later, as described (Li et al., 2017); and phosphate-buffered saline (PBS, 1X) as a vehicle control.
Investigators performing the surgeries, injections, and quantifications were masked to the treatment identity (i.e., tube with virus or drug was prepared and coded by another researcher) until the end of the experiment, and the animals that received viral injections were randomly pulled for subsequent injections of different drugs or PBS control. A few animals that developed cataract in the injured eye were excluded.
Histological Procedures
Standard histological procedures were used, as described previously (de Lima et al., 2012b; Kurimoto et al., 2010; Li et al., 2017; Yin et al., 2006). Briefly, anesthetized mice were transcardially perfused with isotonic saline followed by 4% paraformal-dehyde (PFA) at 2 or 6 weeks after optic nerve injury, the eyes, optic nerves, and the optic chi-asms were dissected, postfixed 2 hours, the retinas were dissected-out, and optic nerves and the optic chiasms were transferred to 30% sucrose overnight at 4 °C. Free-floating retinas were immunostained in 24-well plate wells and, after making 4 symmetrical slits, flat-mounted on coated glass slides for imaging. The optic nerves and the optic chiasms were embedded in OCT Tissue Tek Medium (Sakura Finetek), frozen, cryostat-sectioned at 14 μm (longitudinally for the optic nerves and horizontally for the optic chiasms), immunostained on coated glass slides, and then mounted for imaging. For immunostaining, the retinas and tissue sections were blocked with the appropriate sera, incubated overnight at 4 °C with primary antibodies described in the main text, then washed three times, incubated with appropriate fluorescent secondary antibodies (1:500; Alexa Fluor, Life Technologies) 3 hours at room temperature, washed three times again, and mounted. Images were acquired using fluorescent microscope (E800, Nikon).
Quantitation of Axon Regeneration and RGC Survival
Regenerating axons were visualized at 2 weeks after optic nerve injury by immunostaining with a GAP-43 antibody [1:2,000; custom-made in sheep (Benowitz et al., 1988)] and at 6 weeks after optic nerve injury by immunostaining for the cholera toxin B fragment (CTB, 1% in 3 μl; List Biological), which was injected intravitreally 2 days prior to sacrifice (anti-CTB 1:500, rabbit polyclonal; GenWay, #GWB-7B96E4). Sections were examined for possible axon sparing; no spared axons were found in controls and no evidence of axon sparing was found in experimental conditions (i.e., at 2 weeks after injury no axons were found beyond the initial segment of the optic chiasm and at 6 weeks no axons were found beyond the initial segment of the optic tract). Axons (defined as fibers continuous for > 100 μm, which are absent in controls and are discernible from background puncta and artefactual structures) were counted manually using fluorescent microscope (E800, Nikon) in at least 4 longitudinal sections per optic nerve at 0.5 mm, 1 mm, 2 mm, and 3 mm distances from the injury site (identified by the abrupt disruption of the densely packed axons near the optic nerve head, as marked by an asterisk in Figs. 2 and 6A), and these values were used to estimate the total number of regenerating axons per nerve, as described (de Lima et al., 2012b; Yin et al., 2006).
RGC survival was quantified as in earlier studies from our laboratory and others (de Lima et al., 2012b; Guo et al., 2016; Kurimoto et al., 2010; Leon et al., 2000; Yin et al., 2009; Yin et al., 2006), by immunostaining with an antibody to βIII-tubulin (1:500, rabbit polyclonal; Abcam, #Ab18207), taking advantage of the selective expression of βIII-tubulin in RGCs. ImageJ software was used to count βIII-tubulin+ cells from 6–8 images per retina (200×, E800; Nikon) taken at prespecified areas, at 1 mm and four at 2 mm from the optic nerve head in four directions, then averaged to estimate overall RGC survival per mm2.
Statistical Analyses
All tissue processing, quantification, and data analysis were done masked throughout the study. Sample sizes were based on accepted standards in the literature and prior experiences from our laboratory. Sample size (n) represents total number of biological replicates in each condition. All experiments included appropriate controls. No cases were excluded in our data analysis, although a few animals that developed cataract in the injured eye were excluded from the study and their tissues were not processed. The data was analyzed by ANOVA, with Repeated Measures where appropriate, and a posthoc LSD test, or by unpaired Student’s t-test, two-tailed (SPSS). Data are presented as Means ± SEM. All differences were considered significant at p < 0.05.
Highlights.
The effects of combining treatments that promote axon regeneration were tested.
Traumatic injury to the mouse optic nerve was used as a model system.
Zinc chelator TPEN and knockdown of Klf9 had the strongest effect on axon regeneration.
TPEN and knockdown of Klf9 increased retinal ganglion cell survival by 7-fold.
Acknowledgments
This work was supported in part by American Heart Association Postdoctoral Fellowship 15POST25080290 (E.F.T.); National Eye Institute Grant R01-EY024481 (P.A.R. and L.B.); National Eye Institute Grant R01-EY020913 (J.L.G.); US Department of Defense Grant CDMRP W81XWH-14-1-0488 (L.B. and J.L.G.); the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (L.B.), and Research to Prevent Blindness, Inc (J.L.G.). We thank Hui-Ya Gilbert and Yuqin Yin (Boston Children’s Hospital, Harvard Medical School) for technical assistance and advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andereggen L, Trakhtenberg EF, Yin Y, Benowitz LI. Inflammation and Optic Nerve Regeneration, Neuroinflammation: New Insights into Beneficial and Detrimental Functions. Wiley; Hoboken, NJ: 2015. pp. 189–204. [Google Scholar]
- Apara A, Galvao J, Wang Y, Blackmore M, Trillo A, Iwao K, Brown DP, Fernandes KA, Huang A, Nguyen T, Ashouri M, Zhang X, Shaw PX, Kunzevitzky NJ, Moore DL, Libby RT, Goldberg JL. KLF9 and JNK3 Interact to Suppress Axon Regeneration in the Adult CNS. J Neurosci. 2017;37:9632–9644. doi: 10.1523/JNEUROSCI.0643-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei F, Lee HH, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E, Sanes JR, Chen C, Fagiolini M, He Z. Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell. 2016;164:219–232. doi: 10.1016/j.cell.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, Steen JA. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis. 2010;37:259–266. doi: 10.1016/j.nbd.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, He Z, Goldberg JL. Reaching the brain: Advances in optic nerve regeneration. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- Cho YE, Lomeda RA, Ryu SH, Lee JH, Beattie JH, Kwun IS. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2007;1:29–35. doi: 10.4162/nrp.2007.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- de Lima S, Habboub G, Benowitz LI. Combinatorial therapy stimulates long-distance regeneration, target reinnervation, and partial recovery of vision after optic nerve injury in mice. Int Rev Neurobiol. 2012a;106:153–172. doi: 10.1016/B978-0-12-407178-0.00007-7. [DOI] [PubMed] [Google Scholar]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012b;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR. Subtype-Specific Regeneration of Retinal Ganglion Cells following Axotomy: Effects of Osteopontin and mTOR Signaling. Neuron. 2015;85:1244–1256. doi: 10.1016/j.neuron.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarieh A, Levin LA. Optic nerve disease and axon pathophysiology. Int Rev Neurobiol. 2012;105:1–17. doi: 10.1016/B978-0-12-398309-1.00002-0. [DOI] [PubMed] [Google Scholar]
- Guo X, Snider WD, Chen B. GSK3β regulates AKT-induced central nervous system axon regeneration via an eIF2Bε-dependent, mTORC1-independent pathway. Elife. 2016;5:e11903. doi: 10.7554/eLife.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci U S A. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug A, Weidner N. From bench to beside to cure spinal cord injury: lost in translation? Int Rev Neurobiol. 2012;106:173–196. doi: 10.1016/B978-0-12-407178-0.00008-9. [DOI] [PubMed] [Google Scholar]
- Jayaprakash N, Wang Z, Hoeynck B, Krueger N, Kramer A, Balle E, Wheeler DS, Wheeler RA, Blackmore MG. Optogenetic Interrogation of Functional Synapse Formation by Corticospinal Tract Axons in the Injured Spinal Cord. J Neurosci. 2016;36:5877–5890. doi: 10.1523/JNEUROSCI.4203-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- Kuo PY, Leshchenko VV, Fazzari MJ, Perumal D, Gellen T, He T, Iqbal J, Baumgartner-Wennerholm S, Nygren L, Zhang F, Zhang W, Suh KS, Goy A, Yang DT, Chan WC, Kahl BS, Verma AK, Gascoyne RD, Kimby E, Sander B, Ye BH, Melnick AM, Parekh S. High-resolution chromatin immunoprecipitation (ChIP) sequencing reveals novel binding targets and prognostic role for SOX11 in mantle cell lymphoma. Oncogene. 2015;34:1231–1240. doi: 10.1038/onc.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kügler S, Benowitz LI. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010;30:15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Andereggen L, Yuki K, Omura K, Yin Y, Gilbert HY, Erdogan B, Asdourian MS, Shrock C, de Lima S, Apfel UP, Zhuo Y, Hershfinkel M, Lippard SJ, Rosenberg PA, Benowitz L. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc Natl Acad Sci U S A. 2017;114:E209–E218. doi: 10.1073/pnas.1616811114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat Neurosci. 2016;19:1073–1084. doi: 10.1038/nn.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- Ollig J, Kloubert V, Weßels I, Haase H, Rink L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals. 2016;6:1–16. [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozmen EG, Hinman JD, Carmichael ST. Models that matter: white matter stroke models. Neurotherapeutics. 2012;9:349–358. doi: 10.1007/s13311-012-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brown DP, Duan Y, Kong W, Watson BD, Goldberg JL. A novel rodent model of posterior ischemic optic neuropathy. JAMA Ophthalmol. 2013;131:194–204. doi: 10.1001/2013.jamaophthalmol.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y, Gupta VK, Li JC, Klistorner A, Graham SL. Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci. 2013;24:301–321. doi: 10.1515/revneuro-2013-0003. [DOI] [PubMed] [Google Scholar]