Abstract
Introduction
Cultured primary human trophoblasts (PHT), derived from term placentas, are relatively resistant to infection by diverse viruses. The resistance can be conferred to non-trophoblastic cells by pre-exposing them to medium that was conditioned by PHT cells. This antiviral effect is mediated, at least in part, by microRNAs (miRNA) expressed from the chromosome 19 microRNA cluster (C19MC). Recently we showed that PHT cells and cells pre-exposed to PHT medium are also resistant to infection by Zika virus (ZIKV), an effect mediated by the constitutive release of the type III interferons (IFN) IFN lambda-1 and IFN lambda-2 in trophoblastic medium. We hypothesized that trophoblastic C19MC miRNA are active against ZIKV, and assessed the interaction of this pathway with IFN lambda-1 - mediated resistance.
Methods
Term PHT cells were cultured using standard techniques. An osteosarcoma cell line (U2OS) was used as non-trophoblastic cells, which were infected with either ZIKV or vesicular stomatitis virus (VSV). Trophoblastic extracellular vesicles (EVs) were produced by gradient ultracentrifugation. RT-qPCR was used to determine viral infection, cellular or medium miRNA levels and the expression of interferon-stimulated genes.
Results
We showed that C19MC miRNA attenuate infection of U2OS cells by ZIKV, and that C19MC miRNA or exosomes that contain C19MC miRNA did not influence the type III IFN pathway. Similarly, cell exposure to recombinant IFN lambda-1 had no effect on miRNA expression, and these pathways did not exhibit synergistic interaction.
Discussion
PHT cells exert antiviral activity by at least two independent mechanisms, mediated by C19MC miRNA and by type III IFNs.
Keywords: Placenta, Trophoblast C19MC, IFN lambda-1, ZIKV
INTRODUCTION
Infection of pregnant women by certain viruses, such as CMV, Rubella, and parvovirus, have been associated with congenital anomalies and other devastating pregnancy outcomes [1, 2]. In humans and non-human primates, Zika virus (ZIKV), an arthropod-borne flavivirus, has recently been linked to severe birth defects, including brain parenchymal lesions, microcephaly, ocular lesions and other developmental abnormalities [3–6]. The barrier that protects a fetus from vertical transmission of pathogens is the placenta, which forms the main interface of the fetus with the maternal host. In mammalian species, such as primates and rodents that possess a hemochorial placenta [7], placental trophoblasts are directly bathed in maternal blood and forms the forefront of fetal exposure to maternal blood-borne pathogens.
Among the more than 400 species of microRNAs (miRNA) expressed by human trophoblasts, the chromosome 19 microRNA cluster (C19MC) constitutes the largest human miRNA cluster [8]. C19MC miRNA are expressed from chromosome 19q13.41. This cluster includes 58 mature miRNA species that are encoded by 46 miRNA genes, spanning over 100 kb [8]. Intriguingly, this cluster is expressed exclusively in primates, and expressed almost exclusively from the placenta [8–10].
The full landscape of trophoblast-dependent mechanical and immunological defense pathways against viral infection remains under-explored. We recently found that cultured primary human trophoblasts (PHT), derived from term placentas, are relatively resistant to infection by diverse DNA and RNA viruses [11]. We also reported that cultured medium that was conditioned by PHT cells can attenuate subsequent viral replication in non-trophoblastic cells. PHT conditioned medium contains C19MC miRNA in PHT-derived extracellular vesicles (EVs) or in a protein-bound form. Indeed, C19MC miRNA or exosomes containing these miRNAs also reduce viral replication in non-placental cells, an effect mediated by induction of autophagy [11]. We recently also showed that primary syncytiotrophoblasts, through the constitutive production of the type III interferon (IFNs), including IFN lambda-1, are resistant to ZIKV and can confer that resistance to recipient non-placental cells by pre-exposing these cells to conditioned medium, including that depleted of exosomes [12]. By contrast, a primitive type of trophoblast that can be generated from pluripotent stem cells by a simple differentiation protocol involving BMP4 and inferred to be homologous to the very early placenta that surrounds the embryo proper during very early pregnancy [13], is highly susceptible to ZIKV and appears not to express type III IFNs [14]. Whether or not C19MC miRNA can attenuate ZIKV infection in any of these model systems remains unknown. Therefore, we tested the hypothesis that trophoblastic C19MC can reduce ZIKV infection in non-trophoblastic cells. Here we provide evidence that the C19MC miRNAs stimulate resistance to ZIKV through a type III IFN-independent pathway. Moreover, the antiviral pathways by C19MC miRNA and IFN lambda-1 are autonomous, and do not exhibit synergistic interactions.
METHODS
Culture of PHTs and purification of extracellular vesicles
PHT cells were isolated from healthy singleton term pregnancies, based on previously published protocols, by using trypsin-DNase-dispase/Percoll method [15, 16]. Placenta collection and isolation of cells for culture were in accordance with reviewed and approved protocols from the Institutional Review Board at the University of Pittsburgh. PHT cells were cultured in 7 × 15 cm plates at 37 °C for 72 h in 5% CO2 air atmosphere, grown in DMEM supplemented with 1% antibiotics and 10% fetal bovine serum (FBS) that had been depleted of bovine exosomes (A27208-01, Thermo Fisher Scientific). Cell quality was monitored by microscopy and production of human chorionic gonadotropin (hCG), quantified by ELISA (EIA-1911R, DRG Instrument GmbH, Marburg, Germany). Medium conditioned by PHT cells was collected at 72 h, when the majority of the cells had differentiated to syncytialized cells [15]. The conditioned medium was collected and vesicles were isolated by using differential ultracentrifugation and OptiPrep continuous gradient ultracentrifugation, according to a previously described protocol [17].
Trophoblasts from induced human embryonic stem cells
H1 human embryonic stem cells were differentiated to trophoblasts by use of BMP4 and inhibitors of FGF2 and ACTIVIN signaling through use of previously published protocols [13]. Cell lysates for C19MC expression analysis were prepared at days 0, 2, 4, 6, and 8 of differentiation.
Cells and viruses
Human osteosarcoma U2OS cells were cultured in DMEM supplemented with 10% bovine growth serum (BGS) and 1% antibiotics. BeWo cells were maintained in F12K Kaighn’s modified medium supplemented with 10% BGS and antibiotics. ZIKV Paraiba 2015 was propagated in Vero cells. Viral titers were determined by fluorescent focus assay, with anti-double stranded RNA monoclonal antibody (a gift from Abraham Brass, University of Massachusetts). Experiments measuring infection of ZIKV were performed by using 1 focus-forming unit/cell for 24 h. Viral titers were determined by fluorescent focus assay, as previously described [18]. Infection of ZIKV or VSV was determined by RT-qPCR, measuring the respective viral transcripts. Experiments measuring vesicular stomatitis virus (VSV) infections were performed using a multiplicity of infection (MOI) of 1 for approximately 5 h or until cytopathic effects were evident.
RNA Extraction, cDNA synthesis, and RT-qPCR
For cellular mRNA analysis and miRNA analysis, total RNA was extracted with TRI reagent (Cat# TB 126, MRC, Cincinnati, OH) as we previously described [19]. RNA samples were treated with RNAse-free DNase (Cat# 79254, Qiagen, Valencia, CA). For mRNA analysis, total RNA was reverse transcribed by using High-Capacity cDNA Reverse Transcription kit (Cat# 4368814, Thermo Fisher Scientific) according to the manufacturers protocol. RT-qPCR was performed by means of SYBR Select (Cat# 4472908, Thermo Fisher Scientific) in a ViiA 7 system (Life Technologies). Gene expression was calculated by the 2−CTΔΔ CT method normalized to GAPDH [20].
RT-qPCR Primers
The following RT-qPCR primers were used: GAPDH (5′-GAAGGTCGGAGTCAACGGATTT -3′ and 5′-GAATTTGCCATGGGTGGAAT -3′); ZIKV (5′-AGATGACTGCGTTGTGAAGC-3′ and 5′-GAGCAGAACGGGACTTCTTC-3′); OAS1 (5′-ATAAAAGCAAACAGGTCTGG-3′ and 5′-TCTGGCAAGAGATAGTCTTC-3′); IFI44L (5′-TGCAGAGAGGATGAGAATATC-3′ and 5′-ACTAAAGTGGATGATTGCAG-3′); VSV (5′-TGCAAGGAAAGCATTGAACAA-3′ and 5′-GAGGAGTCACCTGGACAATCACT-3′); IFIT1 (5′-CAACCAAGCAAATGTGAGGA-3′ and 5′-GGAGACTTGCCTGGTGAAAA-3′); IFIT2 (5′-ACCATGAGTGAGAACAATAAG-3′ and 5′-TTAGATAGGCCAGTAGGTTG-3′).
RT-qPCR for miRNA
cDNA synthesis and qPCR were performed with miRScript PCR system (Cat# 218161, Qiagen) and ViiA 7 Real Time PCR system according to the manufacturer’s protocols. RNU6B was used for normalization, and relative fold change was determined by the 2−CTΔΔ CT method, as previously reported [20]. The catalog number for the miRScript primer pairs were: miR-517a-3p (MS00004459), miR-525-5p (MS00004557), miR-1323 (MS00014658), RNU6B (MS00033740), miR-512-3p (MS00007000), and miR-516b (MS00007749).
miRNA Mimics and Transfections
Mimics for C19MC miRNAs (miRIDIAN) as well as non-targeting control miRNA mimic were obtained from Thermo Fisher Scientific (Dharmacon) as described [21]. U2OS cells were reverse transfected with one or multiple miRNA mimics or miRNA mimic control (final concentration 40 nM for individual mimics, or an equal concentration for combined mimics) by using HiPerFect Transfection Reagent (Cat# 301704, Qiagen) according to the manufacturers protocol. Cells were assayed 48 h post-transfection. The total concentration of the non-targeting control miRNA mimic was adjusted to that of all active miRNA mimics.
C19MC BAC preparation and Recombinant IFN
U2OS cells stably expressing a bacterial artificial chromosome (BAC) containing 160 kb of genomic DNA from the q13-42 region of chromosome 19, which contains the entire C19MC miRNA cluster, was generated as previously reported [11]. Recombinant IFN beta (100 U/mL; PBL Source), or recombinant IFN lambda-1 (10 ng/mL; R&D Systems) was added to cells. After 24 h exposure, the RNA was harvested and analyzed for miRNA levels.
Statistics
Experiments were performed at least in triplicate as detailed in the figure legends. Data are presented as the mean ±SD. Except where specified, a Student’s t test was used to determine statistical significance for virus infection assays when two sets were compared, and one-way ANOVA with a Bonferroni correction for multiple comparisons applied post hoc to determine statistical significance. Specific p values are provided in each figure legend.
RESULTS
C19MC miRNAs attenuate infection by ZIKV in non-trophoblastic cells
To assess the ability of C19MC miRNAs to confer resistance to ZIKV, we transfected four of the most highly expressed members of the cluster that we previously showed to have anti-viral activity (miR-512-3p, miR-516b, miR-517a, and miR-525-5p) [11] into U2OS cells. 48 h following introduction of miRNAs, we infected the cells with ZIKV or with VSV, as control [11, 12]. We found that the C19MC miRNA mimic-mix, but not non-C19MC mix or scramble RNA control, reduced ZIKV and VSV infection, as evident by a ~50% reduction in the production of viral RNA (vRNA) (Fig. 1A–B).
Figure 1. C19MC miRNAs confer resistance to ZIKV.
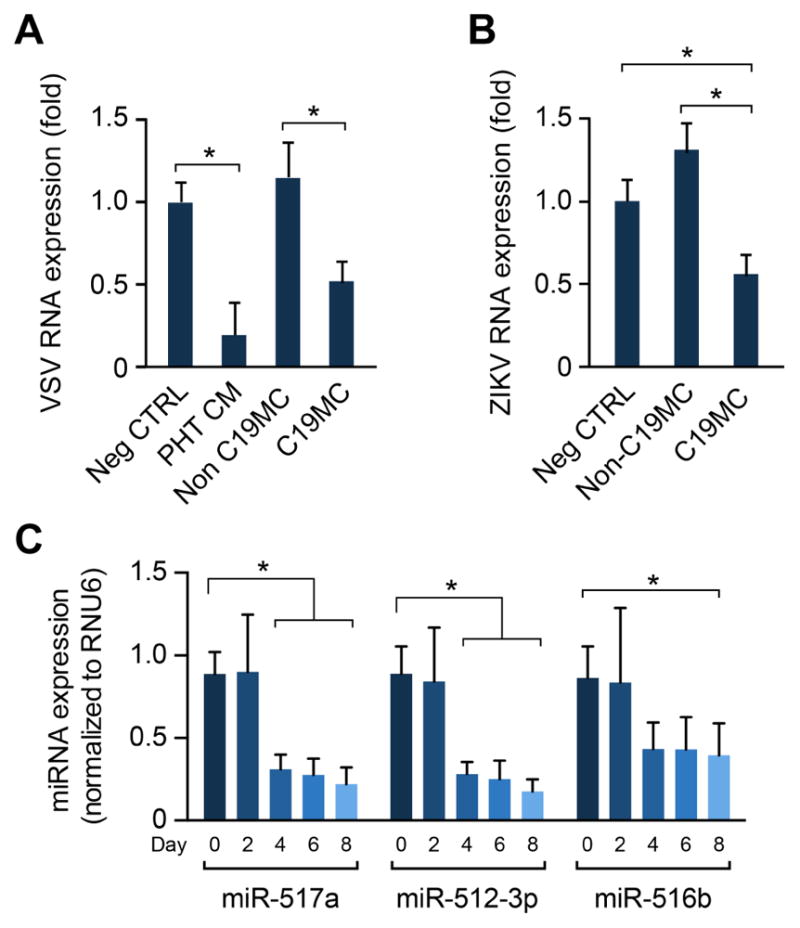
(A) U2OS osteosarcoma cells were transfected with a non-targeting control miRNA mimic, a pool of 4 miRNAs from the C19MC (miR-512-3p, miR-525-5p, miR-516b-5p, and miR-517a) to a final concentration of 40 nM, or a corresponding concentration of non-C19MC miRNA mix (miR-141, miR-29b, miR-320). PHT conditioned medium (CM), prepared as described in Methods, was used as a positive control. At 48 h after transfection, cells were infected with VSV for 5 h, and viral RNA levels were determined by RT-qPCR. (B) the same experiment was performed using ZIKV, infected for 24 h. (C) Cellular miRNA levels for miR-512-3p, miR-517a, and miR-516b-5p were measured by RT-qPCR from ESC differentiated into an early trophoblast lineage at day 0, 2, 4, 6, and 8. Differences were determined using ANOVA, with post hoc Bonferroni correction. Experiments were performed three independent times. Data are shown as mean ±SD. (*p<0.05).
Yabe et al recently showed that ESCs that were exposed to bone morphogenic protein 4 (BMP-4), activin A inhibitor and FGF2 signaling inhibitor differentiate into trophoblasts resembling trophoblasts of the primitive placenta during implantation [13]. Interestingly, these cells express ZIKV attachment factors and can be readily infected by ZIKV [14]. Based on our results, we surmised that these cells might have a lower expression level of C19MC miRNAs. Using RT-qPCR, we found that the level of three highly expressed C19MC miRNA, miR-512-3p, miR-516b, and miR-517a), declined significantly immediately after initiating differentiation to trophoblasts ([13], and Fig. 1C), which is consistent with their enhanced susceptibility to infection by ZIKV relative to ESC [14].
Trophoblastic exosomes do not induce IFN-stimulated genes
We recently reported that PHT cells constitutively release type III IFNs, including IFN lambda-1, which can reduce infection by ZIKV in non-trophoblastic cells through paracrine effects [12]. We also showed that trophoblastic exosomes, which contain C19MC, exhibit antiviral activity [11, 17, 21]. To assess the impact of trophoblastic EVs on the expression of interferon stimulated genes (ISGs), we added EVs that we isolated from PHT cells to U2OS cells, and assessed the induction of IFN-induced protein 44-like (IFI44L) [22], as well as IFN-induced protein with tetratricopeptide repreats (IFIT)-1 and -2, and ISG 2′-5′ oligoadenylate synthase 1 (OAS1) (see also Fig. 4). Whereas PHT conditioned medium stimulated the induction of all ISGs tested, none of the trophoblastic EVs recapitulated this effect (Fig. 2A). We further confirmed this effect using individual C19MC miRNA, and found that unlike the robust induction of the ISGs by recombinant IFN lambda-1, transfection of the highly expressed miR-512-3p, miR-516b, or miR-517a had no effect on ISG expression (Fig. 2B). Together, these results suggest that the antiviral effects observed using trophoblast exosomes or C19MC miRNAs are independent of the ISG induction by type III IFNs.
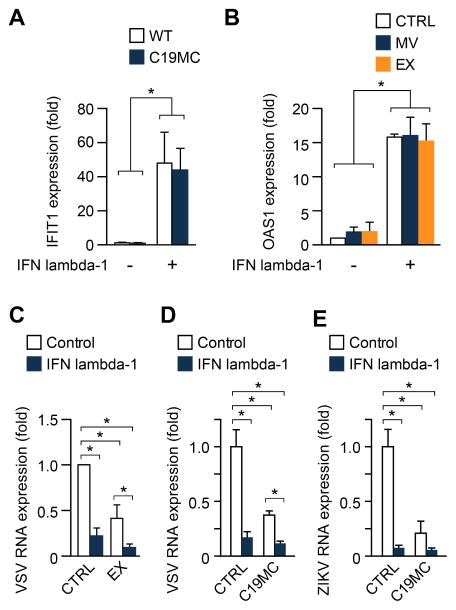
Figure 4. IFN lambda-1 and C19MC miRNAs function independently to confer viral resistance.
(A) The level of the ISG IFIT1 was determined by RT-qPCR in U2OS cells stably expressing the C19MC BAC compared to wild-type, non-transfected cells. Cells were exposed to 10 ng of IFN lambda-1. (B) The level of the ISG OAS1 was determined by RT-qPCR in U2OS cells exposed to microvesicles (MV), or exosomes (EX) with or without the addition of IFN lambda-1 (10 ng). (C) U2OS cells exposed to PHT-derived EX with or without the addition of IFN lambda-1 (10 ng) were infected with VSV at an MOI of 1 for 5 h, and infection was determined by RT-qPCR. Infection level was expressed relative to negative control non-conditioned medium. (D–E) represent the same experiments as in (C), but with C19MC mix (Fig. 1) replacing the EX in cells infected by VSV (D) or ZIKV (E). Experiments were performed three independent times. Differences were determined using ANOVA, with post hoc Bonferroni test. Data are shown as mean ±SD. (*p<0.05).
Figure 2. Trophoblastic extracellular vesicles or C19MC miRNAs do not enhance the expression of ISGs.
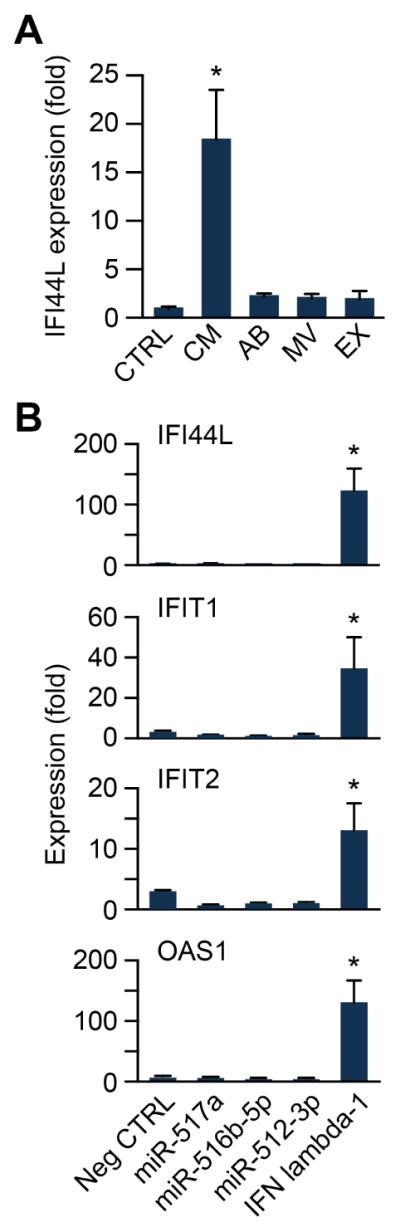
(A) RT- qPCR analysis of IFI44L expression in U2OS cells exposed to the PHT-derived extracellular vesicles apoptotic bodies (AB), microvesicles (MV), or exosomes (EX). PHT conditioned medium (CM) was used as a positive control. Results are expressed relative to non-conditioned medium (CTRL). (B) RT-qPCR analysis of the expression of the ISGs IFI44L, IFIT1, IFIT2 and OAS1 in U2OS cells transfected with either 40 nM of miR-517a, miR-516b-5p, miR-512-3p, or an equal concentration of control non- targeting miRNA (Neg CTRL). 10 ng IFN lambda-1 was used as a positive control. Experiments were performed three independent times. Data are presented as fold-change relative to control non-targeting miRNA, and shown as mean ±SD (* p<0.05). Differences were determined using ANOVA, with post hoc Bonferroni correction.
Type III IFNs do not affect the expression levels of miRNAs
To determine whether or not exposure to type III IFNs influences C19MC miRNA levels, we exposed the trophoblast line BeWo, which endogenously expresses C19MC, to recombinant IFN lambda-1. Notably, these cells do not express IFN lambda-1. We found that while IFN lambda-1 had a negligible, yet statistically significant effect on miR-1323 (<20%), with no change in any of the other miRNA tested (Fig. 3A). Similarly, exposure of cells to recombinant IFN beta also had no effect on C19MC mirna expression (not shown). Using U2OS cells that were stably transfected with a bacterial artificial chromosome that contained the entire C19MC gene [11], we also found no effect of recombinant IFN lambda-1 on the expression levels of representative C19MC miRNA, including miR-517a, -512-3p, -1323, and -525-5p (Fig. 3B).
Figure 3. C19MC miRNA levels are unaffected by exposure of cells to IFN lambda-1.
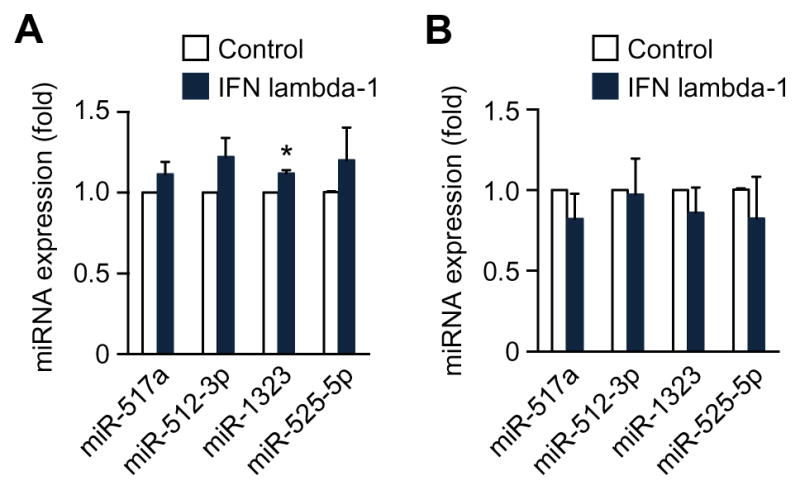
The levels of C19MC miRNA were determined by RT-qPCR in BeWo cells (A) or U2OS cells stably expressing a BAC containing the C19MC miRNA cluster (B). The cells were exposed to IFN lambda-1 (10 ng), and the expression levels of miR-512-3p, miR-517a, miR-525-5p, and miR-1323 were quantified, normalized to RNU6B. Experiments were performed three independent times. Data are shown as mean ±SD (* p<0.05).
Type III IFNs and C19MC miRNAs function independently to attenuate viral infection
To assess whether or not C19MC miRNAs and type III IFNs were synergistic in their antiviral effects, we first compared the level of ISG induction in U2OS cells that stably express the C19MC BAC to that in wild type control U2OS cells, and found that the addition of recombinant IFN lambda-1 had no effect on the induction of the ISG IFN-induced protein with tetratricopeptide repeats 1 (IFIT1, Fig. 4A). We next added isolated EVs, derived from PHT cells, to wild type U2OS cells, in the presence or absence of recombinant IFN lambda-1. Comparing the effects of microvesicles or exosomes derived from PHTs in the absence or presence of IFN lambda-1, we observed no significant difference in the expression of the ISG 2′-5′ oligoadenylate synthetase 1 (OAS1, Fig. 4B). To assess functional synergy in the antiviral activity of IFN lambda-1 and trophoblastic exosomes, we exposed U2OS cells to IFN lambda-1, in the presence or absence of PHT exosomes, and then infected them with VSV. As expected, both IFN lambda-1 and PHT exosomes, when used alone, significantly reduced the levels of VSV in U2OS cells. Exposure of cells to IFN lambda-1 and PHT exosomes together led to a significant additive effect on VSV levels. Furthermore, we exposed U2OS cells to IFN lambda-1, in the presence or absence of C19MC miRNAs, and found a similar effect on infection by VSV or ZIKV, even though the reduction in ZIKV infection when cells were exposed to IFN lambda-1 and C19MC miRNAs was not significantly different from C19MC alone. Together, these results support the conclusion that PHT cells employ at least two distinct and non-interacting antiviral mechanisms.
DISCUSSION
We have previously shown that the resistance of PHT cells to viral infection, and the ability of these cells to attenuate viral replication in non-trophoblastic cells, is mediated by at least two pathways: (a) the expression of human trophoblast-specific C19MC miRNA, and (b) the production of the type III IFNs, including IFN lambda-1 [11, 23, 24]. Both C19MC miRNA and IFN lambda-1 are released from PHT cells, and may therefore influence other, non-trophoblastic cells in a paracrine manner. Building on our previous observations, we have now expanded our studies, and showed that C19MC miRNAs limit ZIKV infection. We observed a reduction in ZIKV infection in cells that were transiently transfected with miRNA mimics from the C19MC. Additionally, the resistance to ZIKV seen in ESC is lost in embryonic stem cells when they are differentiated into an early trophoblast lineage. This increased vulnerability correlates with a decline in miRNA expression ([14, 25] and Fig. 1B). These data suggest that term trophoblasts utilize a diverse array of antiviral pathways to protect the placenta, and consequently, the fetus, against ZIKV infection, but that very early in pregnancy, when embryonic lineages are being specified, there is likely a period of trophoblast vulnerability which ends when the subsequent villous placenta emerges and begins to mature.
Our data also indicate that the two antiviral pathways are distinct: we showed that C19MC miRNAs do not influence type III IFN pathways, examined by the level of diverse ISGs, including IFI44L, IFIT1, IFIT2, and OAS1 in U2OS cells. This is consistent with our previous finding that C19MC mimics do not enhance the expression of IFIT1 [11]. Likewise, recombinant IFN lambda-1 had no impact on C19MC miRNAs expression. Finally, we found no evidence for functional synergy in the antiviral activity of IFN and C19MC miRNA signals in non-trophoblastic cells. However, we did observe an additive effect, supporting the hypothesis that the two pathways operate independently of one another. While the precise mechanism by which C19MC miRNAs stimulate autophagy [11] and exert antiviral action remains to be determined, our results strongly suggest that the two antiviral pathways are independent of each other.
Our results described here and in our previous publications [11, 12, 23, 24, 26] shed light on pathways that may explain the relative resistance of villous trophoblasts to infection by ZIKV and other viruses. Recently published histopathological analyses of placentas from human pregnancies infected by ZIKV at different gestational ages showed that ZIKV was localized mainly to Hofbauer cells and other villous core cells [27–31], with some staining in the amnion [32]. A relatively higher affinity to Hofbauer cells was found by several groups using cultured placental cells [33, 34]. The susceptibility of human amniocytes to ZIKV infection was also shown by using primary cell culture [35]. Other researchers infected trophoblast cultures and placental explants and detected some level of ZIKV in trophoblasts [36, 37] yet with greater susceptibility of first trimester cytotrophoblasts to ZIKV infection [35, 38]. In a mouse model reported by Miner et al, placental infection by ZIKV was more prominent in the spongiotrophoblast than in cytotrophoblasts or syncytiotrophoblasts of the labyrinth layer, which, in the mouse, forms the main interface with the maternal bloodstream [4]. Infection in mouse trophoblasts and endothelial cells has been documented by some researchers [39], but was found to be less defined by others [6, 40, 41]. While our results, and information published by others, suggest that human trophoblasts are less permissive than other placental cell types to ZIKV infection, definitive conclusions are hampered by insufficiently large studies performed on human placentas infected in vivo, and a lack of systematic analyses of human and non-human primate placentas infected following infection with a wider range of ZIKV strains and performed at different stages of pregnancy. Importantly, the relative resistance of villous trophoblasts to ZIKV infection may not prevent feto-placental infection that occurs via the fetal membranes [35] and/or extravillous trophoblasts at the of the placental anchoring villi [14].
HIGHLIGHTS.
The expression of C19MC miRNA attenuates ZIKV infection
Trophoblastic exosomes do not induce IFN-stimulated genes
Type III IFNs and C19MC miRNA act independently to lessen viral replication
Acknowledgments
We thank Judy Ziegler and Tiffany Coon for technical assistance, Lori Rideout for assistance in manuscript preparation, and Bruce Campbell for editing. This project was supported by NIH R01HD075665 (C.B.C. and Y.S.), F32AI122456 (N.J.L.), R21HD071707 (to Y.S), the Richard King Mellon Foundation 8069 (Y.S.), the Twenty-Five Club of Magee-Womens Hospital and The Margaret Ritchie R. Battle Family Charitable Fund. In addition, C.B.C. is supported by Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Awards.
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest, and alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Racicot K, Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127(5):1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199–213. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coyne CB, Lazear HM. Zika virus - reigniting the TORCH. Nat Rev Microbiol. 2016;14(11):707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 4.Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe. 2017;21(2):134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–63. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM, Barry GL, Weisgrau KL, Vosler LJ, Mohns MS, Breitbach ME, Stewart LM, Rasheed MN, Newman CM, Graham ME, Wieben OE, Turski PA, Johnson KM, Post J, Hayes JM, Schultz-Darken N, Schotzko ML, Eudailey JA, Permar SR, Rakasz EG, Mohr EL, Capuano S, 3rd, Tarantal AF, Osorio JE, O’Connor SL, Friedrich TC, O’Connor DH, Golos TG. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog. 2017;13(5):e1006378. doi: 10.1371/journal.ppat.1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RM, Green JA, Schulz LC. The evolution of the placenta. Reproduction. 2016;152(5):R179–89. doi: 10.1530/REP-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolin-Cavaille ML, Dance M, Weber M, Cavaille J. C19MC microRNAs are processed from introns of large Pol-II, non-protein-coding transcripts. Nucleic Acids Res. 2009;37(10):3464–73. doi: 10.1093/nar/gkp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37(7):766–70. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 10.Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefevre A, Coullin P, Moore GE, Cavaille J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19(18):3566–82. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- 11.Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110(29):12048–53. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19(5):705–12. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yabe S, Alexenko AP, Amita M, Yang Y, Schust DJ, Sadovsky Y, Ezashi T, Roberts RM. Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc Natl Acad Sci USA. 2016;113(19):E2598–607. doi: 10.1073/pnas.1601630113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, Roberts RM. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci USA. 2017;114(9):E1587–E1596. doi: 10.1073/pnas.1616097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouillet JF, Chu T, Nelson DM, Mishima T, Sadovsky Y. MiR-205 silences MED1 in hypoxic primary human trophoblasts. FASEB J. 2010;24(6):2030–9. doi: 10.1096/fj.09-149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86–95. doi: 10.1016/j.placenta.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of flaviviruses by fluorescent focus assay. J Virol Methods. 2006;134(1–2):183–9. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Chang G, Mouillet JF, Mishima T, Chu T, Sadovsky E, Coyne CB, Parks WT, Surti U, Sadovsky Y. Expression and trafficking of placental microRNAs at the feto-maternal interface. FASEB J. 2017;31(7):2760–2770. doi: 10.1096/fj.201601146R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Donker RB, Mouillet JF, Chu T, Hubel CA, Stolz DB, Morelli AE, Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod. 2012;18(8):417–24. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472(7344):481–5. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayer A, Delorme-Axford E, Sleigher C, Frey TK, Trobaugh DW, Klimstra WB, Emert-Sedlak LA, Smithgall TE, Kinchington PR, Vadia S, Seveau S, Boyle JP, Coyne CB, Sadovsky Y. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212(1):71 e1–8. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadovsky Y, Mouillet JF, Ouyang Y, Bayer A, Coyne CB. The function of trophomiRs and other micrornas in the human placenta. Cold Spring Harb Perspect Med. 2015;5(8):a023036. doi: 10.1101/cshperspect.a023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CQ, Gardner L, Turco M, Zhao N, Murray MJ, Coleman N, Rossant J, Hemberger M, Moffett A. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Reports. 2016;6(2):257–72. doi: 10.1016/j.stemcr.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol. 2014;1(1):133–46. doi: 10.1146/annurev-virology-031413-085524. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental pathology of Zika virus: Viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med. 2017;141(1):43–48. doi: 10.5858/arpa.2016-0401-OA. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz DA. Viral infection, proliferation, and hyperplasia of Hofbauer cells and absence of inflammation characterize the placental pathology of fetuses with congenital Zika virus infection. Arch Gynecol Obstet. 2017;295(6):1361–1368. doi: 10.1007/s00404-017-4361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritter JM, Martines RB, Zaki SR. Zika virus: Pathology from the pandemic. Arch Pathol Lab Med. 2017;141(1):49–59. doi: 10.5858/arpa.2016-0397-SA. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, Gary J, Muehlenbachs A, Lambert A, Lanciotti R, Oduyebo T, Meaney-Delman D, Bolanos F, Saad EA, Shieh WJ, Zaki SR. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis. 2017;23(3):405–414. doi: 10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, Ritter J, Gary J, Rollin D, Goldsmith CS, Reagan-Steiner S, Ermias Y, Suzuki T, Luz KG, de Oliveira WK, Lanciotti R, Lambert A, Shieh WJ, Zaki SR. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388(10047):898–904. doi: 10.1016/S0140-6736(16)30883-2. [DOI] [PubMed] [Google Scholar]
- 32.van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mogling R, van Kampen JJ, Widagdo W, Aron GI, GeurtsvanKessel CH, Pas SD, Raj VS, Haagmans BL, Koopmans MP. Miscarriage associated with Zika virus infection. N Engl J Med. 2016;375(10):1002–4. doi: 10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- 33.Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, Fikrig E. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight. 2016;1(13) doi: 10.1172/jci.insight.88461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20(1):83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe. 2016;20(2):155–66. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep. 2016;6:35296. doi: 10.1038/srep35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB, Hu M, Stossi F, Mancini MA, Harris RA, Kahr M, Eppes C, Rac M, Belfort MA, Park CS, Lacorazza D, Rico-Hesse R. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci Rep. 2017;7:41389. doi: 10.1038/srep41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, Bronstein M, Stockheim D, From I, Eisenberg I, Lewkowicz AA, Yagel S, Panet A, Wolf DG. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J Virol. 2017;91(4) doi: 10.1128/JVI.01905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vermillion MS, Lei J, Shabi Y, Baxter VK, Crilly NP, McLane M, Griffin DE, Pekosz A, Klein SL, Burd I. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun. 2017;8:14575. doi: 10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siddharthan V, Van Wettere AJ, Li R, Miao J, Wang Z, Morrey JD, Julander JG. Zika virus infection of adult and fetal STAT2 knock-out hamsters. Virology. 2017;507:89–95. doi: 10.1016/j.virol.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534(7606):267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]