Abstract
We summarize here, mainly for mammalian systems, the present knowledge of (a) the membrane CO2 permeabilities in various tissues; (b) the physiological significance of the value of the CO2 permeability; (c) the mechanisms by which membrane CO2 permeability is modulated; (d) the role of the intracellular diffusivity of CO2 for the quantitative significance of cell membrane CO2 permeability; (e) the available evidence for the existence of CO2 channels in mammalian and artificial systems, with a brief view on CO2 channels in fishes and plants; and, (f) the possible significance of CO2 channels in mammalian systems.
Keywords: CO2 permeability, membrane cholesterol, protein CO2 channels, aquaporins, Rhesus proteins, aquaporin-1-deficient mice
1. Introduction
This review intends to update the state of this field as it has been given by Endeward et al. [1] in 2014. In addition, we attempt to give a compilation of all of the lines of evidence that have so far been published demonstrating the existence of protein CO2 channels and their contributions to membrane CO2 permeability. We also give a compilation of the recently described remarkable variability of the CO2 permeability in mammalian cell membranes. Because a discussion of the true value of intracellular CO2 diffusivity has come up [2], we estimate the relative roles of the intracellular diffusion resistance vs. the diffusion resistance of the cell membrane for exit of CO2 from the cell, and discuss the crucial role of the intracellular diffusion coefficient for this relation. A novel development in the field is the prominent role, which membrane cholesterol seems to play in determining the CO2 permeability of a large number of mammalian biological membranes [3]. A further, newly evolving principle is the amazingly good positive correlation between the values of cellular oxygen consumption and CO2 permeability, suggesting that cells adapt their CO2 permeability to their level of CO2 production. Finally, we extensively discuss the role of CO2 channels for gas exchange by red cells, since this is presently the in vivo system whose CO2 transport properties have been characterized most quantitatively and in the most detail [4,5,6].
2. Measurement and Variability of CO2 Permeabilities of Biological Membranes
Table 1 compiles the CO2 permeabilities that we have measured with the 18O mass spectrometric method for a broad variety of cells or organelles. Derivation of PCO2 from mass spectrometric recordings of the time course of C18O16O in cell suspensions requires an extensive system of mathematical equations describing the complete process of 18O exchange between CO2, HCO3−, and H2O. However, it nevertheless constitutes at present the most direct method to obtain quantitative estimates of cell membrane permeabilities. The method has been introduced in a simpler form without consideration of the CO2 permeability by Itada and Forster [7]. The theory, as developed further by our group, as well as the handling of the technique, have been presented in their definitive form by Endeward and Gros [8], and several strengths and limitations of the method have been extensively discussed by Endeward et al. [1]. Very recently, another group has used this 18O technique to measure the CO2 permeability of isolated chloroplasts [9]. Unfortunately, the authors were not able to measure the internal stroma carbonic anhydrase activity of the chloroplasts, a quantity that is essential for the evaluation of the mass spectrometric records [8]. Lack of precise knowledge of this figure is expected to constitute a limitation to the reliability of the derived CO2 permeabilities.
Table 1.
CO2 permeabilities of cells and organelles of various mammalian tissues.
| Type of Cell/Organelle | PCO2 (cm/s) | |
|---|---|---|
| Apical membrane PCE a | 0.0015 | Endeward and Gros [8] |
| MDCK b | 0.017 | Itel et al. [3] |
| Basolateral membrane PCE a | 0.022 | Endeward and Gros [8] |
| Rat Cardiomyocytes | 0.1 | Arias-Hidalgo et al. [18] |
| Human Erythrocytes | 0.15 | Endeward et al. [4,6] |
| Mitochondria c | 0.33 | Arias-Hidalgo et al. [19] |
a proximal colon epithelium of guinea pig; b Madin Darby canine kidney cells; c isolated from rat liver.
Another approach that is often used to estimate membrane CO2 permeabilities, is that employed by Boron’s group [4,10,11], which records the alkaline pH shift on the cell surface that is associated with an influx of CO2 into oocytes. This allows one to indirectly quantitate that flux. It is clear that the surface pH amplitude is related to membrane CO2 permeability, but it is hardly feasible to derive an explicit value of PCO2 from these pH shifts [12,13]. So the method can be considered semi-quantitative, but has been important in providing further corroboration of the gas channel property of aquaporin 1 and has successfully been used to provide the important comparison of the CO2 permeabilities of the aquaporin and Rhesus protein isoforms [10,11,14].
A further important method to quantitate membrane CO2 permeabilities, especially of artificial phospholipid vesicles, is the stopped-flow technique. An intravesicular fluorescent pH indicator is used to follow the uptake of CO2 by the vesicles. This requires extremely rapid intravesicular conversion of CO2 into HCO3− and H+, i.e., the presence of high activities of carbonic anhydrase inside the liposomes. Tsiavaliaris et al. [15] have shown that in some earlier studies this approach has suffered from (a) an intravesicular carbonic anhydrase activity that was not sufficient to render the CO2 hydration reaction non-limiting, in combination with (b) the use of an equation describing the process of CO2 uptake that assumes instantaneous chemical equilibrium of CO2, HCO3−, and H+. This led to erroneously low PCO2 values for liposomes of around 0.001 cm/s [16,17]. When these problems are circumvented, it can be shown that cholesterol-free liposomes have a PCO2 of >0.1 cm/s, and liposomes with 50% cholesterol have a PCO2 of 0.01 cm/s [15]. As will be discussed below, these PCO2 values agree very well with those obtained with the mass spectrometric 18O technique. Thus, the stopped flow method seems very suitable in principle to quantitate CO2 permeabilities. However, because of the high pressures in the syringes and the high flow rates arising in the mixing chamber, it will be difficult to apply this technique to cell suspensions when cell lysis is to be avoided.
Table 1 shows the CO2 permeabilities determined by our lab for various cellular and organellar membranes, all by the 18O technique. The PCO2 values vary enormously, by a factor of 200, from 0.0015 to 0.3 cm/s. This seems to suggest that PCO2 is a highly regulated quantity and helps cells adapt to physiological challenges, whose nature shall be discussed below.
3. Is There a Physiological Parameter Governing the Adaptation of CO2 Permeabilities?
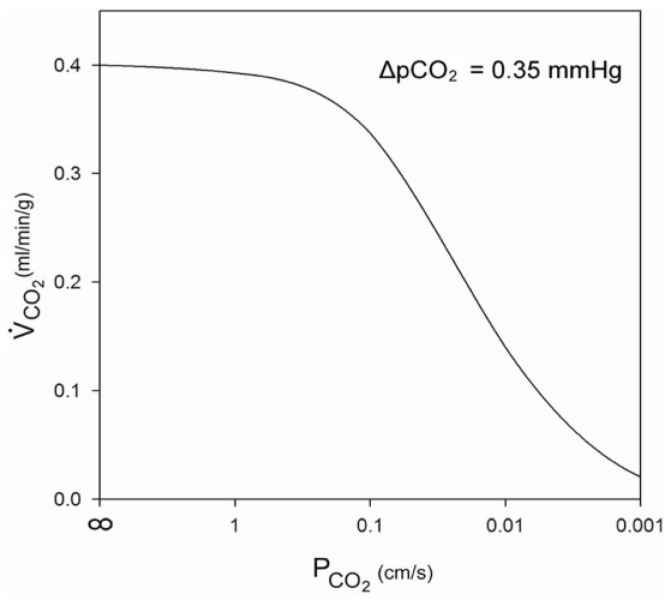
We ask here, whether the variability of the CO2 permeabilities seen in Table 1 serves a definable physiological purpose. Endeward et al. [1] have theoretically shown that the resistance of the cell membrane can become significant for cellular CO2 release—in addition to that of the cytoplasmic diffusion path—when PCO2 falls below about 0.1 cm/s. They also showed that, for a given intra- to extracellular CO2 partial pressure gradient, a membrane PCO2 of, say, 0.01 cm/s would markedly limit CO2 exchange of a cell of very high oxidative metabolic rate, such the 300 nmol O2/g tissue/s (=0.40 mL O2/g tissue/min) found in maximally working cardiomyocytes [20], and reduce CO2 efflux to about 1/4 of what is required (Figure 1). However, for a cell of a low O2 consumption, such as exhibited by cells in culture, e.g., with the number of 12 nmol O2/g cell/s (0.016 mL O2/g tissue/min) reported for MDCK cells [21], this same PCO2 would have no effect on the rate of CO2 exchange at all. This can also be appreciated from Figure 1. We should note that a limitation of CO2 efflux by a high membrane diffusion resistance would require a build-up of the intracellular CO2 partial pressure to ensure sufficient CO2 removal. This would imply an increased acid load for this cell.
Figure 1.
Influence of membrane CO2 permeability on CO2 release from a maximally metabolizing cardiomyocyte at a given partial pressure difference of 0.35 mmHg over the half-thickness of the cell. From: Endeward et al. [1].
In view of these considerations, it appears conceivable that the value of membrane CO2 permeability is adapted to the level of aerobic metabolism of the cell, i.e., to the level of the rate of CO2 production.
To test this hypothesis, we can take a look at the specific rates of oxygen consumption of some of the CO2 permeabilities of Table 1. As just mentioned, MDCK cells with a PCO2 of 0.017 cm/s have an oxygen consumption of 12 nmol O2/g cell/s, rat cardiomyocytes exhibiting a PCO2 of 0.1 cm/s possess an O2 consumption of 300 nmol O2/g tissue/s, and rat liver mitochondria with a PCO2 of 0.33 cm/s have a maximal respiratory rate of 1000 nmol O2/mL/s [19]. Thus, there is a clear increase of PCO2 with an increasing rate of O2 consumption or CO2 production, respectively. This principle will have to be further substantiated by obtaining more data pairs of PCO2 and O2 consumption for various tissue cells. However, we can already conclude from these values that PCO2 of cell membranes appears to be adapted to—and possibly regulated by—the level of oxygen consumption.
Other physiological processes to which membrane CO2 permeabilities seem to adapt in a major way are (a) the especially high rate of gas exchange across red blood cell membranes, and possibly (b) the high rate of CO2 diffusion across the apical membrane of the proximal tubule required for tubular bicarbonate reabsorption. Both these adaptations will be discussed below.
4. Cholesterol—One of Two Mechanisms Regulating CO2 Permeability
Two factors that affect the CO2 permeability of a membrane have been identified so far: the presence or absence of protein gas channels, and the content of cholesterol in the membrane. Gas channels have been expressed in oocytes to demonstrate this, and 90% of the CO2 permeability of the human red cell membrane has been shown be due to the channels aquaporin-1 and Rhesus-associated glycoprotein. This evidence for gas channels will be discussed in detail below.
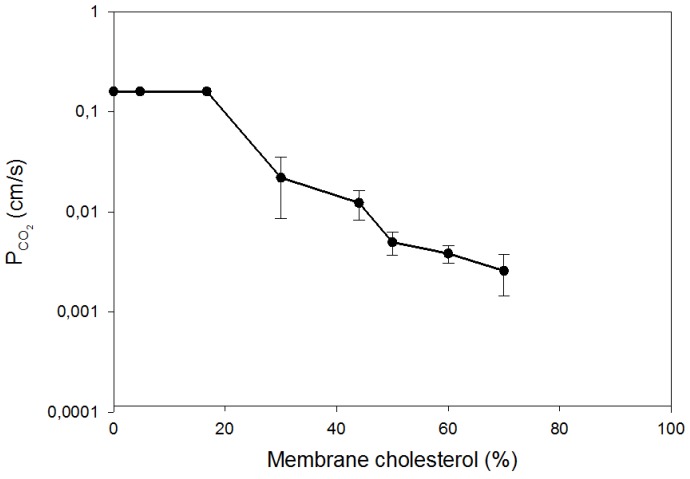
The second important determinant of PCO2 is the content of cholesterol in the membrane. As shown in Figure 2, this has been studied systematically by Itel et al. [3], who used artificial phospholipid vesicles with cholesterol contents of between 0% and 70%, and measured their CO2 permeability by the 18O mass spectrometric method. Between 20% and 70% cholesterol PCO2 of these vesicles falls by two orders of magnitude, from ≥0.16 cm/s to about 0.002 cm/s. Thus, between these concentrations, PCO2 depends drastically on cholesterol. It is in agreement with other observations on cholesterol in membranes that there is no effect of cholesterol on PCO2 between 0% and 20% (c.f. [3]).
Figure 2.
CO2 permeability (PCO2) of phospholipid vesicles with variable concentration of cholesterol as a function of cholesterol content in mol % cholesterol per mol total membrane lipids. PCO2 determination by the mass spectrometric 18O exchange method. From: Itel et al. [3].
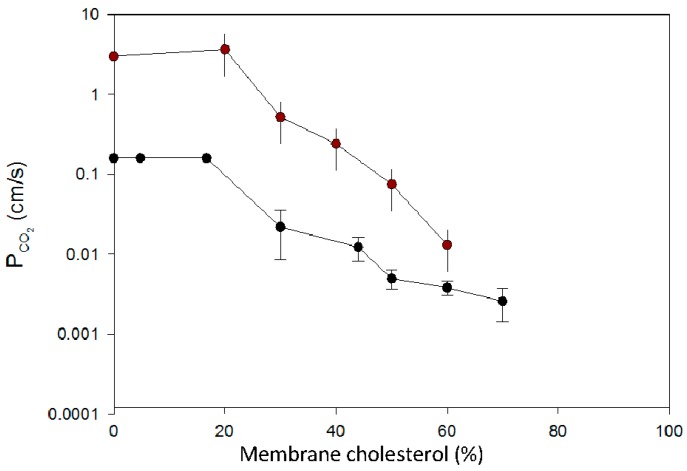
Figure 3 shows a dependency of PCO2 on membrane cholesterol as it can be derived from the molecular dynamics simulation of Hub et al. [22] (upper curve) in comparison to the experimental curve by Itel et al. [3] (lower curve). The starting points of PCO2 at 0% cholesterol are about an order of magnitude apart, possibly because Hub et al. based their calculation on a mixture of phospholipids quite different from the mixture used by Itel et al. However, in all other respects, there is an astonishing agreement between theory and experiment: (a) Hub’s curve as well as Itel’s curve fall by about two orders of magnitude between 20% and 60 to 70% cholesterol content in the membrane, and (b) Hub et al. [22] conform with the observation of Itel et al. [3] that cholesterol does not affect PCO2 between concentrations of 0 and 20%. Thus, molecular dynamics calculations and 18O measurements of liposomes both show the drastic reduction of PCO2 by increasing concentrations of cholesterol.
Figure 3.
Cholesterol dependency of the CO2 permeability of phospholipid vesicles. Upper curve: calculated from the molecular dynamics simulation data of Hub et al. [22], lower curve: experimental curve of Itel et al. [3] as shown in Figure 2.
Recently, for the case of CO2, this effect has been confirmed by an entirely different experimental technique, i.e., stopped flow observations of the uptake kinetics of CO2 by the type of vesicles studied by Itel et al. [3]. With this technique, Tsiavaliaris et al. [15] have observed a reduction of PCO2 of liposomes from >0.1 cm/s to 0.01 cm/s, when membrane cholesterol was increased from 0 to 50%. This effect agrees well with what one predicts from the curve of Itel et al. [3], as shown in Figure 2. Thus, three entirely different approaches lead to the same result: a most drastic effect of cholesterol on membrane CO2 permeability.
Another paper, by Kai and Kaldenhoff [23], uses pH measurements on the surface of planar lipid bilayers to measure lipid bilayer PCO2, a method introduced by Missner et al. [24]. They find a six times lower PCO2 in phosphatidylcholine bilayers with 67% as compared to 0% cholesterol. Thus, their measurements confirm a strong effect of cholesterol, but not as strong as that observed by Itel et al. [3], Hub et al. [22] and Tsiavaliaris et al. [15], who would predict an effect of around two orders of magnitude by 67% cholesterol. It should be noted that in an early study, Missner et al. [24] report no effect of cholesterol at all on PCO2 of phospholipid bilayers. We add that these latter discrepancies may be related to the difficulty of achieving the same ratio of cholesterol/total lipids in the membranes of the liposomes as established in the solution from which the liposomes are generated. In our experience, the final liposomes sometimes take up substantially less cholesterol than is present in the solution, although they never seem to take up more cholesterol/total lipids than present in that solution.
The strong cholesterol effects on PCO2 observed by stopped flow, 18O exchange and molecular dynamics simulation, are paralleled by findings of Hill and Zeidel [25] of a 45-fold decrease in water permeability, when 52% cholesterol was added liposomes containing 9% phosphatidycholine, 18% sphingomyelin, and 21% glycosphingolipid. Similarly, the same maneuver reduced in their work the permeabilities for formamide by 54-fold, for acetamide by 130-fold, for urea by 20-fold, and for NH3 by 90-fold. Since CO2 is quite hydrophilic [26], it is especially interesting that both water and the hydrophilic NH3 exhibit a similar or greater reduction of their permeability by cholesterol when compared to CO2. We may add that Zocher et al. [27] have observed by molecular dynamics simulation a reduction of NH3 permeability in the presence of 50% cholesterol to 3% of its value in the absence of cholesterol, a reduction approaching that observed experimentally for NH3 by Hill and Zeidel [25], and similar to the reduction of PCO2 measured by Itel et al. [3] (Figure 2).
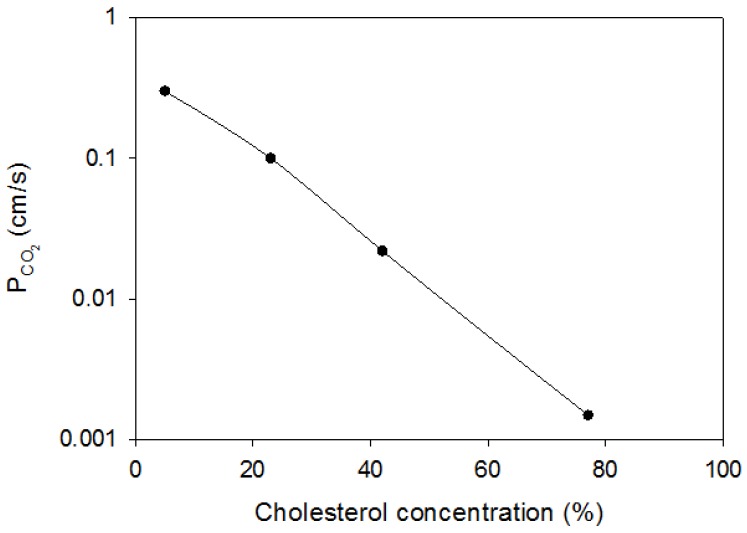
We conclude from this discussion that the available evidence predominantly suggests that cholesterol can reduce the CO2 permeability of membranes by about two orders of magnitude like it does with other hydrophilic (and non-hydrophilic) substrates. A further important support for this view comes from the measurements of the CO2 permeability of various biological membranes, which exhibit different concentrations of cholesterol but lack protein gas channels. Some such measurements have been reported by Itel et al. [3], and others are given above in Table 1. In Figure 4, we have plotted the PCO2 values of these biological membranes versus their cholesterol content as taken from the literature. The permeabilities in the figure are those for mitochondria [19], cardiomyocytes [18], the basolateral membrane of proximal colon epithelium [8], and the apical membrane of proximal colonic epithelium [8]. None of these membranes has a significant amount of protein CO2 channels. It is apparent in a semilogarithmic plot, like the one used in Figure 2 for liposomes, that there is an almost linear relation between PCO2 and cholesterol. Quite similar to what is shown in Figure 2 for liposomes, PCO2 falls by a little more than two orders of magnitude, when membrane cholesterol is raised from 5% to 77%. This shows that cholesterol is as powerful in lowering PCO2 in biological membranes as it is in artificial liposomes.
Figure 4.
CO2 permeability of biological membranes as a function of their cholesterol content (% mol cholesterol/mol total membrane lipids). From left to right: rat liver mitochondria [17], cardiomyocytes [18], basolateral membrane of proximal guinea pig colon [8], and apical membrane of proximal guinea pig colon [8].
5. Significance of Membrane vs. Cytoplasmic Diffusion Resistance
Figure 1 suggests that membrane CO2 permeability can affect cellular CO2 release when it falls below a value of 0.1 cm/s. This calculation is based on a certain intracellular cytoplasmic diffusion resistance Rcyto, which is given by an assumed intracellular diffusion path length d from mitochondria to plasma membrane of 7 μm, and an intracellular CO2 diffusion coefficient DCO2 of 1.3 × 10−5 cm/s, using the Equation (1):
| Rcyto = d/DCO2. | (1) |
The total cellular CO2 diffusion resistance is then given by the sum of cytoplasmic and membrane diffusion resistances, i.e.,
| Rtot = Rcyto + Rmembr = d/DCO2 + 1/PCO2. | (2) |
This equation illustrates that the membrane resistance Rmembr becomes important when it contributes significantly to the total resistance. With the numbers given above, Rcyto is 54 s/cm, and with P = 0.1 cm/s, Rmembr is 10 s/cm, i.e., the membrane contributes 15% of the total diffusion resistance.
It is clear that the relative contribution of the membrane resistance depends on the value of the intracellular CO2 diffusion coefficient. Recently, it has been suggested that the intracellular CO2 diffusion—at least in the case of red blood cells—is much lower than thought heretofore [2]. For this reason, we will discuss the available evidence for the value of the intracellular diffusion coefficient of CO2.
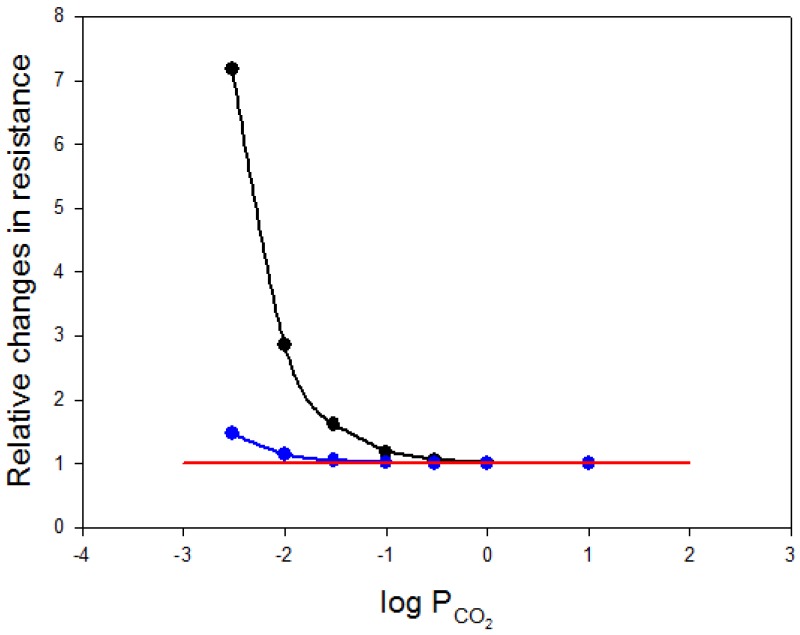
Richardson and Swietach [2] uncaged protons locally within red cells from 6-nitroveratraldehyde and observed the dissipation of these protons by imaging the intracellular fluorescence of a pH indicator. The kinetics of dissipation essentially reflects the facilitated transport of H+ by translational hemoglobin diffusion [28,29] and by the combined reaction and diffusion processes of H+, HCO3−, and CO2. The latter also involves, among other steps, the intraerythrocytic diffusion of CO2. If the complete process of facilitated H+ transport is modelled correctly, in principle, information on CO2 diffusivity can be derived from the fluorescence measurements. It is clear that the entire process is highly complex and further complicated by the red cell geometry, and that this approach to determining intraerythrocytic CO2 diffusion is quite indirect. In their article, Richardson and Swietach [2] come to the conclusion that the CO2 diffusivity inside red cells is 23 times lower than in water. With a DCO2 in water of 2.4 × 10−5 cm2/s (37 °C; [30]) Rcyto becomes 671 s/cm. This value of course renders a contribution of the membrane to Rtot of 10 s/cm entirely insignificant. This is illustrated in Figure 5, which shows the relative change in total resistance as a function of PCO2. The lower curve (blue) has been calculated for an intraerythrocytic CO2 diffusion coefficient of 2.4 × 10−5/23 = 0.10 × 10−5 cm2/s, as reported by Richardson and Swietach [2], the upper curve (black) represents the same calculation for the classically used DCO2 of the order of 1.3 × 10−5 cm2/s [31]. Whereas, the black curve shows a 7-fold increase in total resistance when PCO2 is lowered to an extreme 0.003 cm/s, total resistance increases by only 30% in the case of the blue curve for the low DCO2. Thus, whether low PCO2 values are relevant for cellular CO2 release, depends strongly on the true intracellular CO2 diffusion coefficient.
Figure 5.
Relative changes in the combined diffusion resistances of 7 μm of cytoplasm and the cell membrane (Y-axis), versus varying values of CO2 permeability (X-axis). Relative changes in total resistance are defined as total resistance per resistance of the cytoplasm alone. Note that PCO2 on the X-axis is plotted logarithmically and varies between 10 and 0.003 cm/s. The upper curve (black) is calculated for an intracellular CO2 diffusion coefficient of 1.3 × 10−5 cm2/s, the lower curve (blue) for a more than 10 times lower DCO2 of 0.104 × 10−5 cm2/s as it has been postulated by Richardson and Swietach [2]. The horizontal red line represents total diffusion resistance for infinitely high PCO2, i.e., the normalized cytoplasmic resistance alone. It is apparent that, for “normal” PCO2 values between 0.02 and 0.3 cm/s (Table 1), the relative contribution of membrane CO2 permeability to the total cellular diffusion resistance becomes almost negligible for this very low cytoplasmic CO2 diffusivity (blue curve), whereas for the “classical” diffusion coefficient of 1.3 × 10−5 cm2/s (black curve) this contribution rises enormously with lower PCO2 values.
What is the evidence for a much higher intraerythrocytic CO2 diffusion coefficient than that postulated by Richardson and Swietach [2]? Gros and Moll [31] have reported CO2 diffusion measurements across 1 mm thick layers of packed intact mammalian red cells, hemolysed red cells, and of pure hemoglobin solutions of identical hemoglobin concentration. In all three cases, they obtained practically identical DCO2 values of 1.14 × 10−5 cm2/s at 37 °C. Very similar results have been obtained for the diffusion of O2 across layers of red cells and hemoglobin solutions of identical hemoglobin concentration by Kreuzer and Yahr [32] and by Kutchai and Staub [33]. Although these results immediately seem incompatible with the idea of a 10 times lower “true” intracellular CO2 (or O2) diffusivity, one might ask whether in layers of packed red cells the extracellular, intercellular, fluid might constitute a shunt pathway of hemoglobin-free fluid, and consequently of low diffusion resistance. The volume fraction of extracellular fluid in centrifuged red cells has been estimated to be ~2% [34]. Gros and Moll [31] report a hemoglobin concentration of packed beef red cells of 33 g/100 mL, while the intraerythrocytic hemoglobin concentration is ~34 g/100 mL (Documenta Geigy Wissenschaftliche Tabellen [35]). This would imply an extracellular space of 3% in their packed red cells. A “shunt” pathway for CO2 through this extracellular space would be most effective under the unrealistic condition that the entire extracellular space constitutes a coherent parallel diffusion resistance of a path length identical to the thickness of the layer. The diffusion resistance of this composite layer can then be obtained as the sum of reciprocals of parallel resistances of (i) packed red cells, comprising 97–98% of the diffusion area, and of (ii) a water column comprising 2–3% of the diffusion area. Thus, with 1/Rtot = 1/Rery + 1/Rwater, we can write (3)
| 1/Rtot = (0.98 × 1.14 × 10−5 cm2/s) + (0.02 × 2.4 × 10−5 cm2/s) = 1.12 × 10−5 cm2/s + 4.8 × 10−7 cm2/s = 1.17 × 10−5 cm2/s, |
(3) |
where the diffusion areas have been normalized and the thickness of the diffusion layer has been omitted, because it is identical for both components. Thus, for an extracellular space of 2%, the intracellular diffusivity of CO2 is overestimated by 2%, and for an extracellular space of 3%, it is overestimated by 3%. Conversely, with the above-mentioned low intracellular CO2 diffusion coefficient of 0.104 × 10−5 cm2/s inserted into equation (3), we predict an apparent DCO2 of 0.15 × 10−5 cm2/s for this experiment, almost 10 times lower than actually measured. In reality, the extracellular shunt diffusion paths will run around red cells and thus be much longer than the thickness of the layer, and this will even further reduce their contribution to 1/Rtot of the layer. Therefore, the measurements of gas diffusion through layers of packed cells will, for CO2 as well as for O2, lead to diffusion coefficients hardly different from the true intracellular gas diffusion coefficients. We conclude that a 10 times lower DCO2 inside red cells is clearly incompatible with these diffusion measurements. Thus, the role of membrane PCO2 will be as shown by the upper black curve of Figure 5, rather than as that illustrated by the lower blue curve. It is expected that the same conclusion holds for other tissues such as muscle [31]. By the same token, PCO2 will affect CO2 release by tissues in about the way illustrated in Figure 1. We may finally consider how the intraerythrocytic CO2 diffusion coefficient influences the time required by the blood in the lung capillaries to release its CO2 (see Table 2 below). As seen in the first line of Table 2, the process of RBC CO2 release in the absence of an effect of the cell membrane is estimated to be completed by 95% after 50 ms, when the classical intracellular CO2 diffusivity is used. With the 10 times lower CO2 diffusivity of Richardson and Swietach [2], this time is calculated to be prolonged to 480 ms, almost 10-fold. Such a time interval may exceed the capillary transit time available in the lung under conditions of heavy exercise.
Table 2.
Time required by human red cells (RBCs) to complete CO2 release during passage through the lung capillary by 95% (t95%).
| PCO2 (cm/s) | t95% (ms) | |
|---|---|---|
| No membrane resistance | ∞ | 50 |
| Normal membrane permeability | 0.15 | 110 |
| AQP-1null or Rhnull RBCs | 0.07 | 180 |
| Basal permeability (AQP-1null + DIDS) | 0.01 | 1000 |
Values calculated for different CO2 permeabilities of the cell membrane. Intraerythrocytic carbonic anhydrase activity is assumed to be nonlimiting (from: Endeward et al. [6]). On the basis of the in vivo shape of red cells in the lung capillary [57], RBCs were approximated in the model by a sphere of radius 2.5 μm.
6. Evidence for Protein CO2 Channels
The earliest evidence for the existence of membrane proteins that conduct CO2 came from (A) analyses of the CO2 permeability of red cells and (B) the observation of CO2 fluxes into aquaporin 1-expressing Xenopus laevis oocytes.
(A) Forster et al. [36] observed in human red cells using the 18O-exchange technique that 4,4′-diisothiocyanato-stilbene-2′2′-disulfonate (DIDS) not only inhibits the permeation of HCO3− by inhibiting the Cl−–HCO3− exchanger, but in addition, affects the mass spectrometric signal in an additional way. By applying the extended theory of 18O-exchange developed in our lab [37], they were able to show that this additional effect constituted an inhibitory effect of DIDS on the membrane permeability for CO2. They concluded that the membrane must possess a protein that acts as a CO2 channel, and that this channel is inhibited by DIDS. These studies were followed by studies of human red cells lacking potential candidates for the role of a CO2 channel. Firstly, aquaporin-1-deficient human red cells (RBC) were shown by Endeward et al. [4] to exhibit (1) only about 50% of the CO2 permeability of normal RBCs, (2) a reduced but still present inhibitability by DIDS, together with (3), a largely lost inhibitability by pCMBS. The presently likely but not fully proven explanation for the behavior of these inhibitors is that one part of the aquaporin-1 (AQP1)-mediated CO2 flux uses the AQP1 water channel of the AQP1 monomer and is therefore inhibited by the inhibitor of this channel pCMBS. DIDS, which is not an inhibitor of the AQP1 water channel, is thought to inhibit the flux of CO2 that occurs through the central pore of the AQP1 tetramer [38]. Therefore, the effect of pCMBS is lost, when AQP1 is lacking.
Secondly, Endeward et al. [5,6] studied human RBCs that were devoid of the Rhesus protein complex, including the Rhesus-associated glycoprotein RhAG, which had then already been known to provide a channel for NH3 [39]. Endeward et al. [5,6] found that RhAG-deficient RBC (1) possess only 50% of the CO2 permeability of normal RBC; (2) exhibit a reduced but still present inhibition by DIDS; and, (3) show a competition between the transport of CO2 and NH3, suggesting that CO2 and NH3 use the same channel in RhAG; (4) exhibit no effect of the more specific inhibitor of the Cl−–HCO3− exchanger DiBAC on CO2 permeability. Since both AQP1 and Rh protein complex are blood groups in human RBC, the authors also studied several other blood group deficiencies, finding that none of the other blood group proteins acts as a CO2 channel. From all of these studies, it can be concluded that at least 90% of the CO2 permeability of the human red cell membrane is due to the two CO2 channels AQP1 and RhAG. Both of these channels are inhibited by DIDS, which makes DIDS a quite efficient gas channel inhibitor, in line with the original findings by Forster et al. [36].
While normal human RBC exhibit a CO2 permeability of 0.15 cm/s, AQP1-deficient RBC in the presence of 100 μM DIDS have a PCO2 of 0.015 cm/s only. Human red cells possess a cholesterol content in their membrane of 45% [40,41]. Inspection of Figure 2 as well as Figure 4 shows that about a value of 0.015 cm/s for PCO2 can be expected for a membrane cholesterol of 45%. This low PCO2 can be considered the “intrinsic” PCO2 of this membrane. The actual value is ten times higher due to the presence of two protein CO2 channels that occur in this membrane in large numbers [42]. The CO2 permeability of several other cells, such as those shown in Figure 4, is exclusively defined by their membrane’s cholesterol content. The red cell, by contrast, has a comparatively high cholesterol content, but, presumably on account of its central role in gas transport, compensates the effect of this on PCO2 by the incorporation of two abundant gas channels. It should be noted that apparently a majority of cells regulate their CO2 permeability by their membrane’s cholesterol content, only a lesser number of cells use gas channels in addition.
(B) Further important evidence for the existence of gas channels comes from work on AQP1-expressing oocytes. Nakhoul et al. [43] and Cooper and Boron [44] showed by intracellular pH recordings that the influx of CO2 into oocytes is accelerated by about a factor of two upon expression of AQP1. Later on, this group used the more sensitive surface pH measurements to follow the influx of CO2 into oocytes. Endeward et al. [4] showed that after the expression of AQP1 in oocytes the alkaline surface pH transient associated with CO2 influx was drastically enhanced and was inhibitable by DIDS. Musa-Aziz et al. [10] also used surface pH measurements on oocytes expressing the particular proteins, to confirm the previous findings by Endeward et al. [4,5,6] that AQP1 as well as the erythrocytic RhAG represent channels for CO2. In addition, they showed that the other aquaporin isoforms AQP4 and AQP5 and another member of the Rh family, the bacterial AmtB, conduct CO2. The same group then extended the range of aquaporin isoforms to all of the AQP isoforms 0–9 [11], and observed that CO2 is conducted by the AQP isoforms 0, 1, 4-M23, 5, 6, and 9, but hardly by 2, 3, 4-M1, 7 and 8. AQP0 is present in the lens fibers of the eye, and may facilitate CO2 release by the superficial lens-fiber cells. AQP1 is present in the RBC membrane, as discussed above, in proximal kidney tubules, where it may mediate the large amounts of CO2 reabsorbed in the proximal tubule [45], and almost universally in blood capillaries, where it may contribute to CO2 in addition to water transfer. AQP5 is strongly expressed in type I alveolar epithelial cells [46], where its CO2 conductivity may contribute to pulmonary CO2 exchange. AQP6 is present in H+-ATPase-carrying intracellular vesicles in renal collecting duct epithelia [47], where its CO2 pathway may serve the exit from the vesicle of CO2 produced by the intravesicular reaction H+ + HCO3− → CO2 + H2O. AQP9 is present in the plasma membrane of hepatocytes [48], where its CO2 conductivity may be involved in providing the substrate CO2 to urea synthesis. It may be noted that the mitochondrial AQP8 is not a CO2 channel and has been shown not to contribute to the high CO2 permeability of the inner mitochondrial membrane [19].
In addition to this substantial amount of evidence derived from studies of red blood cells and gas-channel-expressing oocytes, further compelling evidence has been reported for the existence of gas channels in membranes. Some of this evidence comes from plant aquaporins, the largest group being called PIP (plasma membrane intrinsic protein). For the case of the tobacco aquaporin NtAQP1, Uehlein et al. [49] have provided the same kind of evidence that Nakhoul et al. [43] have given for human AQP1: expression in oocytes accelerated the kinetics of CO2 influx, as observed by intracellular pH measurement. Moreover, overexpression of NtAQP1 in tobacco plant was shown to raise membrane CO2 permeability and to increase plant growth. Later on, the same group confirmed this by presenting a most striking further piece of evidence for the CO2 channel properties of plant aquaporins [50]. They used virtually CO2-impermeable planar triblock-copolymer (ABA1) membranes that separated a fluid chamber with high CO2 partial pressure from one containing no CO2. The flux of CO2 across this layer was observed by surface pH measurement on the low CO2 side, using the experimental principle of Missner et al. [24]. In the absence of plant aquaporins, the membrane conducted no CO2 at all. After incorporation of one of the plant aquaporins NtAQP1 and NtPIP2;1, a most impressive flux of CO2 through the layer was observed, providing quite direct proof for the properties of these aquaporins as CO2 channels. Similarly, a little later this same group [23] reported measurements on lipid bilayers of l-a-Phosphatidylcholine (PC) using again the same surface pH technique. They first showed that incorporation of stigmasterol (Stig) into the bilayer drastically reduced its PCO2, similar to what has above been demonstrated for cholesterol. They went on to show that incorporation of NtAQP1 into the PC:Stig membrane caused an approximately 3-fold increase in PCO2. Lastly, they also illustrated the relevance of the background permeability for the visibility of the CO2 channel property of aquaporin by incorporating NtAQP1 into a pure PC membrane. The membrane without Stig had a substantially higher PCO2, rendering the effect of NtAQP1 almost invisible. This latter observation provides further reinforcement of the concept that gas channels are effective in membranes with a low intrinsic CO2 permeability, but not in membranes of high basal PCO2 [3,51].
In fishes, interesting evidence has been obtained on Rh and AQP gas channels. Perry et al. [52] have shown that the Rh proteins Rhcg1 and Rhbg show competition between the transport of CO2 and NH3, i.e., they facilitate transport of both gases: (a) in Zebrafish larvae knockdown of Rhcg1 and/or Rhbg decreased both CO2 and ammonia efflux across the skin of the larvae, (b) in adult zebrafish the increased efflux of CO2 across the gills after exposure of the animals to hypercapnia was accompanied by a decrease in ammonia efflux. The conclusion was that the Rh proteins are channels for both CO2 and NH3, which agrees with the competition between CO2 and NH3, as demonstrated by Endeward et al. [6] for RhAG of the human red cell membrane. Later, the same group [53] showed that the zebrafish aquaporin-1a1 exhibits an analogous dual role as gas channel for both CO2 and NH3. Morpholino knockdown of AQP-1a1 protein expression in zebrafish larvae, while not affecting oxygen uptake of the larvae, reduced their CO2 release as well as their ammonia efflux, in addition to reducing their exchange of water. Talbot et al. [53] suggest that the localization of AQP-1a1 in the yolk sac epithelium rather than its localization in RBCs is the crucial one for the transport of the two gases in the early stages of zebrafish development.
The evidence presented in this section collectively indicates compellingly that protein CO2 channels exist and that they are functionally important in some cells, although not in others.
7. Physiological Role of CO2 Channels in Mammals
As just discussed, CO2 channels in plants appear to have an important functional role. Uptake of CO2 by plants occurs, due to the low atmospheric CO2 partial pressure (pCO2) of 0.3 mmHg, along an extremely small pCO2 gradient. This renders membrane CO2 permeability crucial, especially when compared to mammals, which have an arterial pCO2 of 40 mmHg. Plant aquaporin-mediated CO2 transport has been implicated in several plant functions and importantly in plant growth [49,54,55,56]. Similarly, Perry and Gilmour and colleagues [52,53] have shown that the central function of the gills of zebrafish, or skin in larvae, to excrete ammonia and CO2 is greatly supported by CO2/NH3 channels.
The contributions of CO2 channels of mammalian origin to CO2 transport have been well documented in artificial systems such as liposomes and oocytes. The only mammalian in vivo system, in which the presence, as well as the role in membrane CO2 permeation, have experimentally been documented thoroughly, are human red blood cells. This has already been discussed above, and will be elaborated further below. In addition, there are preliminary data showing a similar role of the AQP1 CO2 channel in the proximal tubular epithelium of the kidney, as will also be discussed below.
What is the role of the RBC CO2 channels for CO2 exchange of the body? Endeward et al. [4,6] have used a mathematical model taking into account the n vivo RBC geometry in the lung capillary [57], transmembrane CO2 diffusion, and intraerythrocytic reaction and diffusion of CO2/HCO3−, to estimate the influence of PCO2 on the kinetics of release of CO2 as it occurs in the lung. Bicarbonate movements across the RBC membrane were not taken into account in this model. From these calculations, Table 2 shows that in the case of zero diffusion resistance of the RBC membrane, 50 ms are required for the red cell to release 95% of the CO2 that is due to be exchanged in the lung. This compares with a capillary transit time in the lung of 700 ms at rest and about 350 ms under high workload. With the diffusion resistance exerted by the normal RBC CO2 permeability of 0.15 cm/s, this time rises to 110 ms, still sufficiently fast under all of the conditions. Even when only one of the CO2 channels, either AQP1 or RhAG, is lacking, the t95% of 180 ms is still short enough. However, when the RBC CO2 permeability falls to its basal level in the absence of both channels and assumes a value of ~0.01 cm/s, the t95% increases to 1000 ms, which is critical under conditions of rest but clearly too long under heavy exercise. This predicts that a complete lack of CO2 channels in the RBC membrane should become apparent under conditions of maximally increased oxygen consumption.
We have started to study whether this can be demonstrated experimentally in mice. When oxygen consumption of mice was increased to its maximum by means of the Helox technique, we found indeed a reduction of maximal oxygen consumption by 16% in mice lacking AQP1 [58]. This observation indicates a new phenotype of AQP1-kockout mice. We then further analysed the possible cause of this interesting finding, and obtained a surprising result [59]. In this second study, our group observed that the hearts of AQP1-deficient mice possess a 12% thinner wall of the left ventricle than WT mice, a 10% reduced capillary density, and a left ventricular weight normalized to tibia length reduced by 20% for males and by 8% for females. The latter finding has also been reported previously by Montiel et al. [60]. The conclusion from these results must be that, with less ventricle mass und thinner ventricle walls, the AQP1-deficient hearts are expected to exhibit a smaller maximal cardiac output and thus a lower maximal oxygen consumption. A possible cause for the reduced muscle mass and capillarisation of the AQP1-deficient hearts has been proposed by Al-Samir et al. [59] to be the known important role of AQP1 in capillary growth. Thus, the reduction of maximal oxygen consumption in AQP1-knockout mice cannot safely be attributed to the gas channel function of AQP1 in red cells or other tissues. This is not unexpected in view of the calculations of Endeward et al. [4,6] as described in Table 2 above, whose result is that in human RBCs lack of only one type of gas channel will not noticeably affect the efficiency of CO2 exchange in the body. This in line with the other findings of Al-Samir et al. [58] that neither Rhag-KO nor AQP9-KO (AQP9 being another CO2-conducting AQP of the mouse red cell membrane) show a significant reduction of maximal oxygen consumption when compared to controls. However, their finding that also Rhag-AQP1-double KO mice show no reduction of maximal O2 consumption beyond that already seen in AQP1-single KO mice, is difficult to reconcile with the results of Table 2. An explanation may come from unpublished work of our lab (Endeward and Gros), which shows that the CO2 permeability of the red cell membranes of all these gas channel-knockout mice, single as well as double, is affected to a much lesser extent by the knockouts than that of human red cell membranes by the lack of one, either AQP1 or RhAG. Our conclusion at present is therefore that mouse red cells deficient in these gas channels are not a suitable model to study the significance of RBC gas channels for the human organism; their contribution to the CO2 permeability of the red cell membrane of mice appears to be too small. New approaches are required to assess the role of AQP1 in gas exchange in humans. Likewise, it will be important to study the contribution to CO2 exchange in the body of other CO2-conducting AQP isoforms such as the AQP5 of the lung.
Another physiological process that may strongly depend on protein CO2 channels, is the bicarbonate reabsorption of the proximal kidney tubule. It is quite conceivable that the epithelial cell membrane adapts to this situation by establishing a high membrane CO2 permeability, which has, however, not been quantitated so far. The apical membrane of kidney proximal tubules has a fraction of cholesterol per total membrane lipids of about 50% [60], which would suggest a rather low intrinsic CO2 permeability for this membrane (see the above discussion). This permeability may be increased substantially by the high level of aquaporin 1 expressed in this membrane [61]. Indeed, Boron [45] has reported preliminary evidence showing that about 60% of proximal HCO3− reabsorption is lost, when AQP1 is missing in the kidney, indicating that transepithelial CO2 flux is reduced by 60% in the absence of AQP1.
8. Conclusions
We believe there is now ample and largely undisputed evidence that protein CO2 channels exist in several mammalian and non-mammalian membranes. We have presented here, in addition, more recent evidence indicating that membrane CO2 permeabilities can be modulated over a wide range by CO2 channels, and probably even more often by the cholesterol content of the membrane. One physiological purpose of this modulation seems to be to adapt the ease of CO2 permeation across the membrane to the amount of CO2 produced per time by the specific cell. This becomes apparent from an excellent linear relation between cellular rates of oxygen consumption and membrane CO2 permeabilities. In special cases, membrane CO2 permeabilities are increased above the level resulting from membrane cholesterol by the incorporation of CO2 channels, such as aquaporin or Rhesus protein. Examples are the red blood cells, whose CO2 permeability is 10x the value expected from their membrane cholesterol content. Their high CO2 permeability most certainly is related to the specialized role of red cells in CO2 exchange in the body. Another example may be the apical membrane of proximal tubules, where AQP1 may serve to accomplish the high transmembrane CO2 fluxes necessary for proximal bicarbonate reabsorption. Because of species-specific properties of mice that differ from those of humans, it has so far not been possible to use this animal model to investigate the role of protein gas channels in systemic CO2 (or O2) exchange. Further efforts are necessary to evaluate this role.
Acknowledgments
This work was supported by the Deutsche Forschnungsgemeinschaft grant No. EN 908/1-2. No funds were received to cover the cost of open access publishing.
Author Contributions
V.E. and G.G. conceived the concept of this review article. V.E., M.A.-H., S.A.-S. and G.G. contributed parts of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Endeward V., Al-Samir S., Itel F., Gros G. How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Front. Physiol. 2014;4 doi: 10.3389/fphys.2013.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S.L., Swietach P. Red blood cell thickness is evolutionarily constrained by slow, hemoglobin-restricted diffusion in cytoplasm. Sci. Rep. 2016;6 doi: 10.1038/srep36018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itel F., Al-Samir S., Öberg F., Chami M., Kumar M., Supuran C.T., Deen P.M.T., Meier W., Hedfalk K., Gros G., et al. CO2 permeability of cell membranes is regulated by membrane cholesterol and protein gas channels. FASEB J. 2012;26:5182–5191. doi: 10.1096/fj.12-209916. [DOI] [PubMed] [Google Scholar]
- 4.Endeward V., Cooper G.J., Chen L.-M., Pelletier M.F., Virkki L.V., Supuran C.T., King L.S., Boron W.F., Gros G., Musa-Aziz R., et al. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J. 2006;20:1974–1981. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- 5.Endeward V., Cartron J.P., Ripoche P., Gros G. Red cell membrane CO2 permeability in normal human blood and in blood deficient in various blood groups, and effect of DIDS. Transfus. Clin. Biol. 2006;13:123–127. doi: 10.1016/j.tracli.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Endeward V., Cartron J.-P., Ripoche P., Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008;22:64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- 7.Itada N., Forster R.E. Carbonic anhydrase activity in intact red blood cells measured with 18 O exchange. J. Biol. Chem. 1977;252:3881–3890. [PubMed] [Google Scholar]
- 8.Endeward V., Gros G. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. J. Physiol. 2005;567:253–265. doi: 10.1113/jphysiol.2005.085761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolleter D., Chochois V., Poiré R., Price G.D., Badger M.R. Measuring CO2 and HCO3− permeabilities of isolated chloroplasts using a MIMS-18O approach. J. Exp. Bot. 2017;34:303–310. doi: 10.1093/jxb/erx188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musa-Aziz R., Chen L.-M., Pelletier M.F., Boron W.F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl. Acad. Sci. USA. 2009;106:5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer R.R., Musa-Aziz R., Qin X., Boron W.F. Relative CO2/NH3 selectivities of mammalian aquaporins 0–9. AJP Cell Physiol. 2013;304:C985–C994. doi: 10.1152/ajpcell.00033.2013. [DOI] [PubMed] [Google Scholar]
- 12.Somersalo E., Occhipinti R., Boron W., Calvetti D. A reaction-diffusion model of CO2 influx into an oocyte. J. Theor. Biol. 2012;309:185–203. doi: 10.1016/j.jtbi.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Occhipinti R., Musa-Aziz R., Boron W.F. Evidence from mathematical modeling that carbonic anhydrase II and IV enhance CO2 fluxes across Xenopus oocyte plasma membranes. Am. J. Physiol. Cell Physiol. 2014;307:C841–C858. doi: 10.1152/ajpcell.00049.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geyer R.R., Parker M.D., Toye A.M., Boron W.F., Musa-Aziz R. Relative CO2/NH3 permeabilities of human RhAG, RhBG and RhCG. J. Membr. Biol. 2013;246:915–926. doi: 10.1007/s00232-013-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsiavaliaris G., Itel F., Hedfalk K., Al-Samir S., Meier W., Gros G., Endeward V. Low CO2 permeability of cholesterol-containing liposomes detected by stopped-flow fluorescence spectroscopy. FASEB J. 2015;29:1780–1793. doi: 10.1096/fj.14-263988. [DOI] [PubMed] [Google Scholar]
- 16.Prasad G.V., Coury L.A., Finn F., Zeidel M.L. Reconstituted aquaporin 1 water channels transport CO2 across membranes. J. Biol. Chem. 1998;273:33123–33126. doi: 10.1074/jbc.273.50.33123. [DOI] [PubMed] [Google Scholar]
- 17.Yang B., Fukuda N., Van Hoek A., Matthay M.A., Ma T., Verkman A.S. Carbon dioxide permeability of aquaporin-1 measured in erythrocytes and lung of aquaporin-1 null mice and in reconstituted proteoliposomes. J. Biol. Chem. 2000;275:2686–2692. doi: 10.1074/jbc.275.4.2686. [DOI] [PubMed] [Google Scholar]
- 18.Arias-Hidalgo M., Al-Samir S., Weber N., Geers-Knörr C., Gros G., Endeward V. CO2 permeability and carbonic anhydrase activity of rat cardiomyocytes. Acta Physiol. (Oxf.) 2017;38:42–49. doi: 10.1111/apha.12887. [DOI] [PubMed] [Google Scholar]
- 19.Arias-Hidalgo M., Hegermann J., Tsiavaliaris G., Carta F., Supuran C.T., Gros G., Endeward V. CO2 and HCO3− Permeability of the Rat Liver Mitochondrial Membrane. Cell. Physiol. Biochem. 2016;39:2014–2024. doi: 10.1159/000447897. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt R., Thews G. Physiologie des Menschen 23. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 1987. [Google Scholar]
- 21.Guarino R.D., Dike L.E., Haq T.A., Rowley J.A., Pitner J.B., Timmins M. Method for determining oxygen consumption rates of static cultures from microplate measurements of pericellular dissolved oxygen concentration. Biotechnol. Bioeng. 2004;86:775–787. doi: 10.1002/bit.20072. [DOI] [PubMed] [Google Scholar]
- 22.Hub J.S., Winkler F.K., Merrick M., de Groot B.L. Potentials of mean force and permeabilities for carbon dioxide, ammonia, and water flux across a Rhesus protein channel and lipid membranes. J. Am. Chem. Soc. 2010;132:13251–13263. doi: 10.1021/ja102133x. [DOI] [PubMed] [Google Scholar]
- 23.Kai L., Kaldenhoff R. A refined model of water and CO2 membrane diffusion: Effects and contribution of sterols and proteins. Sci. Rep. 2014;4:6665. doi: 10.1038/srep06665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Missner A., Kügler P., Saparov S.M., Sommer K., Mathai J.C., Zeidel M.L., Pohl P. Carbon dioxide transport through membranes. J. Biol. Chem. 2008;283:25340–25347. doi: 10.1074/jbc.M800096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill W.G., Zeidel M.L. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J. Biol. Chem. 2000;275:30176–30185. doi: 10.1074/jbc.M003494200. [DOI] [PubMed] [Google Scholar]
- 26.Edsall J.T. Carbon dioxide, carbonic acid, and bicarbonate ion: Physical properties and kinetics of interconversion. In: Forster R.E., Edsall J.T., Otis A.B., Roughton F.J.W., editors. CO2: Chemical, Biochemical, and Physiological Aspects. National Aeronautics and Space Administration; Washington, DC, USA: 1969. pp. 15–27. [Google Scholar]
- 27.Zocher F., Van Der Spoel D., Pohl P., Hub J.S. Local partition coefficients govern solute permeability of cholesterol-containing membranes. Biophys. J. 2013;105:2760–2770. doi: 10.1016/j.bpj.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gros G., Moll W. Facilitated diffusion of CO2 across albumin solutions. J. Gen. Physiol. 1974;64:356–371. doi: 10.1085/jgp.64.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gros G., Gros H., Lavalette D. Facilitated proton transfer in protein solutions by rotational and translational diffusion. In: Franks F., Mathias S.F., editors. Biophysics of Water. John Wiley Sons, Ltd.; Chichester, UK: New York, NY, USA: Brisbane, Australian: Toronto, ON, Canada: Singapore: 1982. pp. 225–230. [Google Scholar]
- 30.Himmelblau D.M. Diffusion of dissolved gases in liquids. Chem. Rev. 1964;64:527–550. doi: 10.1021/cr60231a002. [DOI] [Google Scholar]
- 31.Gros G., Moll W. The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 1971;324:249–266. doi: 10.1007/BF00586422. [DOI] [PubMed] [Google Scholar]
- 32.Kreuzer F., Yahr W.Z. Influence of red cell membrane on diffusion of oxygen. J. Appl. Physiol. 1960;15:1117–1122. doi: 10.1152/jappl.1960.15.6.1117. [DOI] [PubMed] [Google Scholar]
- 33.Kutchai H., Staub N.C. Steady-state, hemoglobin-facilitated 02 transport in human erythrocytes. J. Gen. Physiol. 1969;53:576–589. doi: 10.1085/jgp.53.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConaghey P.D., Maizels M. The osmotic coefficients of haemoglobin in red cells under varying conditions. J. Physiol. 1961;155:28–45. doi: 10.1113/jphysiol.1961.sp006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geigy Pharmaceutical . Documenta Geigy. Wissenschaftliche Tabellen. 6th ed. Geigy Pharmaceutical; Basle, Switzerland: 1962. [Google Scholar]
- 36.Forster R.E., Gros G., Lin L., Ono Y., Wunder M. The effect of 4,4′-diisothiocyanato-stilbene-2′2′-disulfonate on CO2 permeability of the red blood cell membrane. Proc. Natl. Acad. Sci. USA. 1998;95:15815–15820. doi: 10.1073/pnas.95.26.15815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wunder M.A.A., Gros G. 18O exchange in suspensions of red blood cells: Determination of parameters of mass spectrometer inlet system. Isotopes Environ. Health Stud. 1998;34:303–310. doi: 10.1080/10256019808234064. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Cohen J., Boron W.F., Schulten K., Tajkhorshid E. Exploring gas permeability of cellular membranes and membrane channels with molecular dynamics. J. Struct. Biol. 2007;157:534–544. doi: 10.1016/j.jsb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Ripoche P., Bertrand O., Gane P., Birkenmeier C., Colin Y., Cartron J.P. Human Rhesus-associated glycoprotein mediates facilitated transport of NH3 into red blood cells. Proc. Natl. Acad. Sci. USA. 2004;101:17222–17227. doi: 10.1073/pnas.0403704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien J.S. Cell Membranes- Composition: Structure: Function. J. Theoret. Biol. 1967;15:307–324. doi: 10.1016/0022-5193(67)90140-3. [DOI] [PubMed] [Google Scholar]
- 41.Korn E.D. Cell Membranes: Structure and Synthesis. Annu. Rev. Biochem. 1969;38:263–288. doi: 10.1146/annurev.bi.38.070169.001403. [DOI] [PubMed] [Google Scholar]
- 42.Anstee D.J. The functional importance of blood group-active molecules in human red blood cells. Vox Sang. 2011;100:140–149. doi: 10.1111/j.1423-0410.2010.01388.x. [DOI] [PubMed] [Google Scholar]
- 43.Nakhoul N.L., Davis B.A., Romero M.F., Boron W.F. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am. J. Physiol. Cell Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 44.Cooper G.J., Boron W.F. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am. J. Physiol. 1998;275:C1481–C1486. doi: 10.1152/ajpcell.1998.275.6.C1481. [DOI] [PubMed] [Google Scholar]
- 45.Boron W.F. Sharpey-Schafer lecture: Gas channels. Exp. Physiol. 2010;95:1107–1130. doi: 10.1113/expphysiol.2010.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nielsen S., King L.S., Christensen B.M., Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am. J. Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 47.Yasui M., Kwon T.-H., Knepper M.A., Nielsen S., Agre P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elkjaer M., Vajda Z., Nejsum L.N., Kwon T., Jensen U.B., Amiry-Moghaddam M., Frøkiaer J., Nielsen S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000;276:1118–1128. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- 49.Uehlein N., Lovisolo C., Siefritz F., Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425:734–737. doi: 10.1038/nature02027. [DOI] [PubMed] [Google Scholar]
- 50.Uehlein N., Otto B., Eilingsfeld A., Itel F., Meier W., Kaldenhoff R. Gas-tight triblock-copolymer membranes are converted to CO2 permeable by insertion of plant aquaporins. Sci. Rep. 2012;2:538. doi: 10.1038/srep00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boron W.F.W.F., Endeward V., Gros G., Musa-Aziz R., Pohl P. Intrinsic CO2 Permeability of Cell Membranes and Potential Biological Relevance of CO2 Channels. Chemphyschem. 2011;12:1017–1019. doi: 10.1002/cphc.201100034. [DOI] [PubMed] [Google Scholar]
- 52.Perry S.F., Braun M.H., Noland M., Dawdy J., Walsh P.J. Do zebrafish Rh proteins act as dual ammonia-CO2 channels? J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010;313:618–621. doi: 10.1002/jez.631. [DOI] [PubMed] [Google Scholar]
- 53.Talbot K., Kwong R.W.M., Gilmour K.M., Perry S.F. The water channel aquaporin-1a1 facilitates movement of CO2 and ammonia in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2015;218:3931–3940. doi: 10.1242/jeb.129759. [DOI] [PubMed] [Google Scholar]
- 54.Uehlein N., Otto B., Hanson D.T., Fischer M., McDowell N., Kaldenhoff R. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell. 2008;20:648–657. doi: 10.1105/tpc.107.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boudichevskaia A., Heckwolf M., Kaldenhoff R. T-DNA insertion in aquaporin gene AtPIP1;2 generates transcription profiles reminiscent of a low CO2 response. Plant Cell Environ. 2015;38:2286–2298. doi: 10.1111/pce.12547. [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Hu H., Qin X., Zeise B., Xu D., Rappel W.-J., Boron W.F., Schroeder J.I. Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as carbonic anhydrase 4 interactor. Plant Cell. 2016;28:568–582. doi: 10.1105/tpc.15.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyamoto Y., Moll W. Measurements of Dimensions and Pathway of Red Cells in Rapidly Frozen Lungs In Situ. Resp. Physiol. 1971;12:141–156. doi: 10.1016/0034-5687(71)90047-8. [DOI] [PubMed] [Google Scholar]
- 58.Al-Samir S., Goossens D., Cartron J.-P., Nielsen S., Scherbarth F., Steinlechner S., Gros G., Endeward V. Maximal Oxygen Consumption Is Reduced in Aquaporin-1 Knockout Mice. Front. Physiol. 2016;7:1–8. doi: 10.3389/fphys.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Samir S., Wang Y., Meissner J.D., Gros G., Endeward V. Cardiac morphology and function, and blood gas transport in aquaporin-1 knockout mice. Front. Physiol. 2016;7:181. doi: 10.3389/fphys.2016.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Molitoris B.A., Alfrey A.C., Harris R.A., Simon F.R. Renal apical membrane cholesterol and fluidity in regulation of phosphate transport. Am. J. Physiol. 1985;249:F12–F19. doi: 10.1152/ajprenal.1985.249.1.F12. [DOI] [PubMed] [Google Scholar]
- 61.Nielsen S., Smith B.L., Christensen E.I., Knepper M.A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J. Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]