Abstract
Objectives
To test multiple adiposity measures and prostate cancer (PC) risk in men undergoing prostate biopsy. We hypothesized that BMI, body fat and waist circumference would be highly correlated and all would be associated with aggressive PC, but not overall risk.
Subjects and methods
A case (483) –control (496) study among men undergoing prostate biopsy from 2007–2016 was conducted at the Durham Veterans Affairs Medical Center. Anthropometric measurements and self-reported were taken. Percent body fat was measured. Associations between adiposity measures and PC risk and high-grade PC (Gleason ≥7) were examined using logistic regression.
Results
BMI, percent body fat, and waist circumference were highly correlated (ρ ≥0.79) (p<0.001). On multivariable analysis, BMI (p=0.011) was associated with overall PC risk, but percent body fat (p=0.16) and waist circumference (p=0.19) were not. However, all adiposity measurements were associated with high-grade disease (p<0.001). We found a strong relationship between self-reported and measured weight (ρ=0.97) and height (ρ=0.92).
Conclusions
BMI, body fat and waist circumference were all highly correlated and associated with aggressive PC. This study supports the idea that higher adiposity is selectively associated with high-grade PC and reinforces the continued use of self-reported BMI as a measure of obesity in epidemiological studies of PC.
Keywords: Adiposity measures, Prostate Cancer Risk, Obesity
INTRODUCTION
Prostate cancer (PC) and obesity are major public health problems. The association between these common entities has been subject to increased investigation in the last decade and multiple epidemiologic studies have suggested that obesity is associated with increased risk and death from multiple types of cancer such as breast and colon cancer. (1, 2, 3) However, the relationship between obesity and PC risk is less clear (4, 5). Current evidence suggests that obesity, typically assessed by BMI, is a risk factor for aggressive PC but is unrelated or even protective for overall PC. (6,7,8) BMI is widely used as a surrogate marker for obesity, as it is easy to measure, inexpensive, routinely collected in clinic settings and is available in most patient medical records or can be calculated using self-reported weight and height. (9)
However, it may not be an ideal surrogate, as BMI is influenced by both adipose and non-adipose tissue (i.e. bone, muscle mass, etc.) (10,11). Moreover, BMI does not take into consideration adipose distribution (central vs. peripheral adiposity). (11) Finally, the degree to which self-reported body weight correlates with actual weight is of concern and likely dependent on the lag time that a participant is required to recall. Collectively, these limitations might explain some of the inconsistent findings and obscure the association between obesity and PC. The use of bioelectric impedance analysis to estimate the percent of body fat and waist circumference to estimate central adiposity might provide a clearer picture of the association between adiposity and PC. Waist circumference is a measure of abdominal fat and is a simple technique that can be used to screen for obesity in men. (12,13) However, few PC research studies have evaluated the associations between BMI, waist circumference, body fat and PC risk simultaneously in the same population to determine which is the best predictor. (14)
To evaluate the associations between multiple measures of adiposity and PC risk, we examined measured and self-reported data and took clinical measurements from men undergoing prostate biopsies at the Durham Veterans Affairs Medical Center in North Carolina. We hypothesized that BMI, body fat and waist circumference would be highly correlated with each other, and that all of them would be associated with aggressive PC, but not overall PC risk. In addition, we studied if self-reported height and weight strongly correlate with measured values, which would obviate the need to measure these in future epidemiological studies of PC risk. If true, despite the limitations of BMI at an individual level, these results would support the use of self-reported BMI in epidemiological studies of PC.
Subjects and Methods
Study Design
Data were obtained from an ongoing case-control study of veterans undergoing prostate biopsy for concerns about PC at the Durham Veterans Affair Medical Center (DVAMC) in Durham, North Carolina. The study was approved by the institutional review board at the DVAMC and written informed consent was obtained from all subjects before enrollment. Subjects were recruited between January 2007 and July 2016 from the urology clinic at the DVAMC. Eligible subjects were men with no prior history of PC who were undergoing a prostate needle biopsy because of abnormal PSA and/or suspicious digital rectal exam (DRE) as clinically indicated. Biopsy was typically done for elevated PSA or rectal examination though there was no set threshold to define elevated and was at the discretion of the treating physician. Of the 1,714 eligible cases, 1116 consented to participate (65% response rate). We excluded 131 patients due to missing age, race, PSA, DRE, any previous biopsy, prostate volume, percent body fat, waist circumference, or BMI. Of the 979 men who underwent a biopsy and were included in the analysis, 483 (49%) were biopsy-positive (cases) and 496 (51%) were biopsy negative (controls).
Subjects were asked to self-report weight, height, and race. Age and history of prior biopsy were abstracted from the medical records. Measurements of weight and height were taken by trained personnel and used to calculate body mass index (BMI; kg/m2). Percent body fat was measured using bioelectrical impedance (Omron HBF-306 Fat Loss Monitor, Omron Healthcare, Inc.). Prostate volume (in cubic centimeters) was estimated using trans-rectal ultrasound (TRUS) at the time of biopsy. Gleason score, obtained from the pathological report of the biopsy, was categorized as low-grade disease (Gleason sum 2–6) or high-grade (Gleason sum 7–10).
Statistical Analysis
We tested the association between biopsy outcome (cancer vs. no cancer) and clinical variables using chi-square (χ2) for categorical variables, t-tests for normally distributed continuous variables, and rank-sum for non-normally distributed continuous variables. Similarly, we examined the association between cancer grade (low-grade: Gleason 2–6 vs. high-grade: Gleason 7–10) and clinical variables. Variables included age (continuous), race (black vs. non-black), PSA (logarithmically transformed, continuous), DRE findings (abnormal vs. normal), TRUS prostate volume (logarithmically transformed, continuous), BMI (continuous), family history of PC (yes vs. no vs. unknown), percent body fat (continuous), waist circumference (continuous), and Gleason score (2–6, 7, and 8–10). The correlations between adiposity measures and both self-reported and measured body height and weight were tested using the Spearman correlation test. Similarly, Spearman correlation was used to test the correlation between adiposity measures and clinical characteristics.
We used logistic regression to assess risk of cancer versus no cancer. Because BMI, percent body fat, and waist circumference were highly correlated, we fit them in separate models to avoid collinearity. A Bland-Altman plot for self –reported vs. actual weight was used to compare both measurement techniques. We also used multinomial logistic regression models to examine the relationships between the adiposity measures and risk of low-grade cancer (vs. no cancer) and high-grade cancer (vs. no cancer). For all logistic and multinomial logistic regression models, we fit both unadjusted and adjusted models to account for confounders. In the multivariable model, we adjusted for age(continuous variable), race (black, white, other), previous biopsy(yes, no), family history of PC(yes, no) and factors that could predict the detectability of an existent tumor including PSA (continuous logarithmically transformed), DRE findings (suspicious for cancer vs. not), and TRUS prostate volume(continuous logarithmically transformed). A sensitivity analysis was performed to assess risk of high-grade PC with high-grade defined as Gleason ≥4+3. An α-level of 0.05 was set as the threshold for statistical significance for all analyses. All statistical analyses were performed using Stata 13.0 (Stata, Corp., College Station, TX).
Results
Of the 979 men in our study cohort, 483 (49%) were diagnosed with PC from the biopsy. Patients who were diagnosed with PC were more likely to be black (64% vs. 51%; p=0.001), had higher median PSA (6.4 vs. 5.5 ng/ml; p<0.001), were more likely to have an abnormal DRE (28% vs. 20%; p=0.007), and had smaller median prostate volumes (34 vs. 49 cc; p<0.001), relative to biopsy-negative men (table 1). There were no statistically significant differences between biopsy status and age, BMI, percent body fat, or waist circumference (all p>0.3).
Table 1.
Baseline patient characteristics by prostate cancer status from biopsy result
| Prostate Cancer | Prostate Cancer Grade | |||||
|---|---|---|---|---|---|---|
| No PC | PC | p | Low-grade PC (Gleason 6) |
High-grade PC (Gleason 7–10) |
p | |
| No. Patients | 496 (51%) | 483 (49%) | – | 246 (51%) | 237 (49%) | – |
| Age, mean ± SD | 62.3 ± 6.1 | 62.5 ± 6.4 | 0.52* | 62.1 ± 6.3 | 63.0 ± 6.5 | 0.13* |
| Race | 0.001† | 0.07† | ||||
| Non-black | 244 (49%) | 172 (36%) | 78 (32%) | 94 (40%) | ||
| Black | 252 (51%) | 311 (64%) | 168 (68%) | 143 (60%) | ||
| PSA (ng/ml), median (IQR) | 5.5 (4.3, 7.2) | 6.4 (4.9, 9.4) | <0.001 ‡ | 5.8 (4.6, 7.6) | 7.4 (5.3, 12.9) | <0.001‡ |
| DRE | 0.007† | <0.001† | ||||
| Normal | 396 (80%) | 351 (72%) | 205 (83%) | 145 (61%) | ||
| Abnormal | 100 (20%) | 133 (28%) | 41 (17%) | 92 (39%) | ||
| TRUS volume (cc), median (IQR) | 49 (35, 68) | 34 (26, 50) | <0.001 ‡ | 37 (26, 54) | 31 (25, 44) | 0.002‡ |
| Gleason Score | – | – | ||||
| 6 | 0 | 246 51%) | 246 (100%) | 0 | ||
| 3+4 | 0 | 130 (27%) | 0 | 130 (55%) | ||
| 4+3 | 0 | 56 (12%) | 0 | 56 (24%) | ||
| 8−10 | 0 | 51 (10%) | 0 | 51 (22%) | ||
| Family history of PC | 0.64† | 0.45† | ||||
| No | 260 (52%) | 248 (51%) | 124 (50%) | 124 (52%) | ||
| Yes | 96 (19%) | 105 (22%) | 50 (20%) | 55 (23%) | ||
| Unknown | 140 (28%) | 130 (27%) | 72 (29%) | 58 (25%) | ||
| BMI (kg/m2), mean ± SD | 29.7 ± 5.1 | 29.8 ± 5.8 | 0.81* | 29.4 ± 5.8 | 30.3 ± 5.7 | 0.09* |
| % Body Fat, mean ± SD | 28.6 ± 6.0 | 28.3 ± 6.7 | 0.49* | 27.4 ± 6.9 | 29.3 ± 6.4 | 0.002* |
| Waist Circumference (cm), mean ± SD | 105. 9 ± 14.1 | 104.7 ± 14.7 | 0.18* | 103.0 ± 14.2 | 106.5 ± 15.0 | 0.010* |
Table cells are n (%) unless otherwise specified
PC = prostate cancer; SD = standard deviation, IQR = interquartile range, BMI = body mass index, DRE = previous digital rectal exam, PSA = prostate-specific antigen, TRUS = transrectal prostate volume
p-value calculated using *t-test, †chi-squared test, or ‡rank sum test
There were 237 (49%) patients diagnosed with high-grade PC and 237 (51%) with low-grade PC. Patients with high-grade PC had higher median PSA (7.4 vs. 5.8 ng/ml; p<0.001), relative to patients with low-grade PC, more often had abnormal DREs (39% vs. 17%; p<0.001), and had smaller median prostate volume (31 vs. 37; p=0.002) (table 2). The association between PC grade and age or race was not significant. High-grade cancer was also associated with higher percent body fat (29% vs. 27%; p=0.002) and higher waist circumference (103.0 vs. 106.5 cm; p=0.010), but the trend between high-grade PC and higher BMI did not reach the level of statistical significance (30.3 vs. 29.4 kg/m2; p=0.09).
Table 2.
Odds ratios and 95% confidence intervals for risk of PC, low-grade PC, and high-grade PC versus no PC based on patients who had a prostate cancer biopsy
| All PC* | Low-Grade PC* | High-Grade PC* | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Unadjusted | ||||||
| BMI | 1.00 (0.98, 1.03) | 0.81 | 0.98 (0.96, 1.02) | 0.40 | 1.02 (0.99, 1.05) | 0.22 |
| Body Fat % | 0.99 (0.97, 1.01) | 0.49 | 0.97 (0.95, 0.99) | 0.017 | 1.02 (0.99, 1.04) | 0.17 |
| Waist Circumference | 0.99 (0.99, 1.00) | 0.18 | 0.99 (0.97, 0.996) | 0.009 | 1.00 (0.99, 1.01) | 0.64 |
| Adjusted** | ||||||
| BMI | 1.04 (1.01, 1.06) | 0.011 | 1.01 (0.98, 1.04) | 0.53 | 1.08 (1.04, 1.11) | <0.001 |
| Body Fat % | 1.02 (0.99, 1.04) | 0.16 | 0.99 (0.97, 1.02) | 0.47 | 1.06 (1.03, 1.09) | <0.001 |
| Waist Circumference | 1.01 (0.997, 1.02) | 0.19 | 1.00 (0.98, 1.01) | 0.41 | 1.02 (1.01, 1.04) | <0.001 |
Odds ratios are for risk of disease grade relative to the risk of not having cancer among patients who had a biopsy
Adjusted for age, race, PSA, DRE, previous biopsy, TRUS prostate volume, and family history of PC
PC=prostate cancer; BMI=body mass index (kg/m2); OR=odds ratio; CI=confidence interval
PSA and the adiposity measures were very weakly correlated (ρ −0.06 to −0.02), as were the correlations between prostate volume and the adiposity measures (ρ 0.17–0.19). (Supplementary Table 1) BMI, percent body fat, and waist circumference were strongly correlated (ρ 0.79–0.85, p<0.001, figure 1). Although BMI was calculated using height and weight measured by trained personnel, figure 2 shows a very strong relationship between self-reported weight and measured weight (Spearman, r=0.96). Similarly, self-reported and measured height were strongly related (Spearman, r=0.92, figure 2). On the contrary, the Bland-Altman plot for self-reported vs. actual weight showed only 7% of the observations were outside the limits of agreement, indicating that self -reported weight is a good proxy for measured weight (Supplementary figure 1). Nevertheless, taking in account the fanning pattern of the plot, the agreement between the two measures are accurate for average values rather than extremes. When we repeated the analyses using self-reported height and weight to calculate BMI, results were nearly identical.
Figure 1.
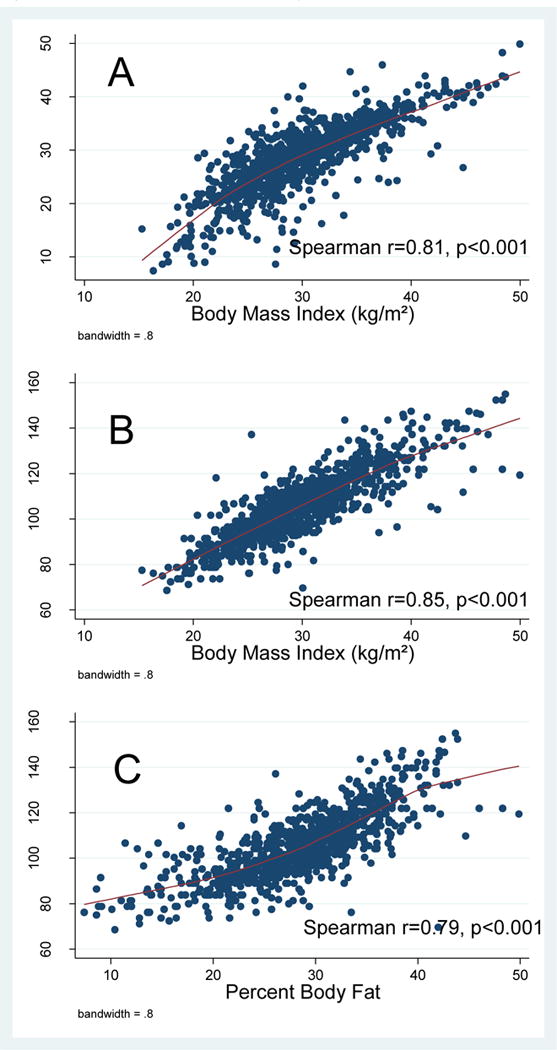
Scatterplots of adiposity measures
Figure 2.
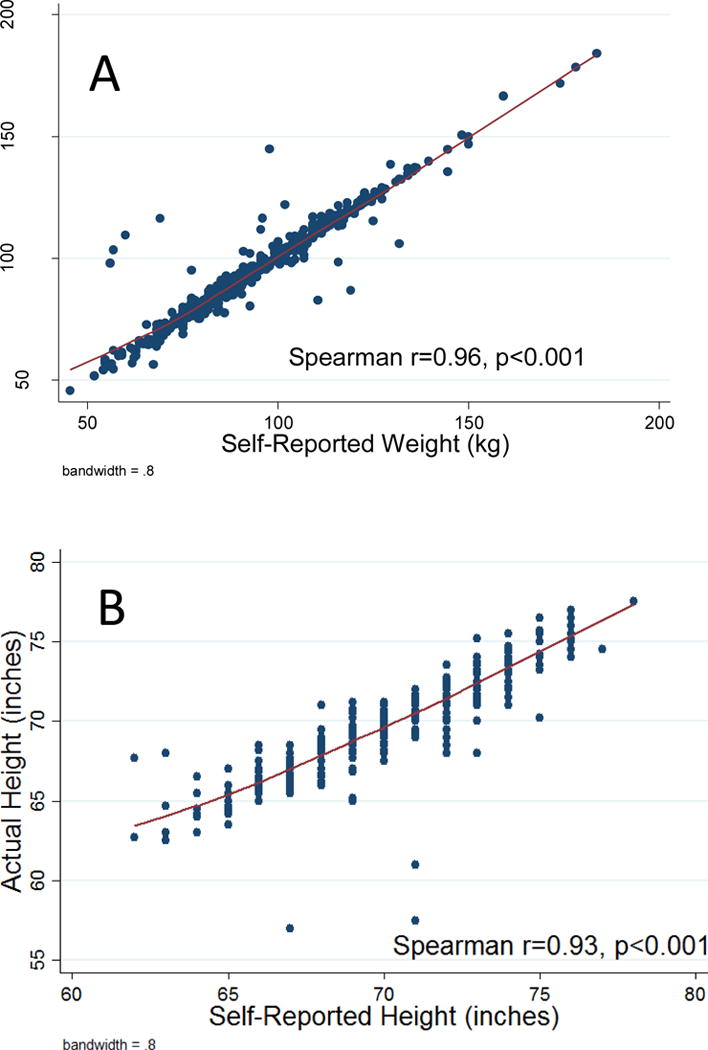
Plot of self-reported versus measured (A) weight and (B) height
In unadjusted logistic regression models, none of the adiposity measures were statistically significant in predicting overall risk of PC (table 2). On multivariable analysis, higher BMI was associated with increased risk of PC (OR 1.04, 95% CI 1.01–1.06, p=0.011), but there was no association with percent body fat or waist circumference. When stratified by low-grade vs. high-grade disease, an interesting pattern appeared. Specifically, the unadjusted analysis revealed that all adiposity measures were inversely associated with low-grade disease and positively associated with high-grade disease. However, only percent body fat (p=0.017) and waist circumference (p=0.009) reached statistical significance in predicting a lower risk of low-grade disease. After adjusting for multiple clinical characteristics, there was no association between adiposity measures and low-grade disease, but all three adiposity measures were associated with a significantly increased risk of high-grade disease (OR 1.02–1.08, p<0.001). In a sensitivity analysis defining high-grade PC as Gleason ≥4+3, results were similar in that positive associations were seen for all three adiposity measures and high-grade disease, though due to small numbers of men with high-grade disease this only reached significance for BMI. Likewise, all three adiposity measures were unrelated to low-grade with the exception that BMI was positively associated with increased risk (OR 1.03, p=0.019, results not shown).
Given differences in result between the null univariable analyses and the positive multivariable analyses for predicting high-grade disease, we explored which clinical characteristic had the greatest impact on changing both the estimated odds ratios and p-values of the adiposity measures. When adding each potential confounder one at a time, we noted that adjusting for TRUS prostate volume had the greatest effect on the adiposity measures in predicting risk of high-grade disease.
Discussion
There is consistent evidence that greater body fat is related to advanced prostate cancer,(15) but which measure of adiposity is most strongly linked with aggressive PC is unclear. We hypothesized that BMI, which is easy to measure, would correlate with more “precise” measures of adiposity such as waist circumference and body fat and that all three would correlate with aggressive PC risk. To test this hypothesis, we analyzed data from men undergoing prostate biopsy. Indeed, we found that only BMI was associated with overall risk of PC, but all were significantly associated with increased risk of high-grade disease on multivariable analysis, consistent with several prior studies. (8, 16) In sharp contrast, neither percent of body fat nor waist circumference were associated with PC risk. Nevertheless, all three adiposity measures were highly correlated among them. Our findings, may suggest that anthropometric indices may measure different features of body fat distribution but still retain their relationship between them. A recent Canadian case-control study evaluating PC risk among anthropometric measures, showed that abdominal fat more than overall BMI was associated with PC risk suggesting that population diversity may have a role in adiposity measurements and body fat distribution. (17) This study adds to the current knowledge that despite limitations of BMI on an individual level, at a population level, BMI is just as strongly associated with aggressive PC as other adiposity measures. This coupled with the near identical findings between measured and self-reported height and weight, support continued use of self-reported BMI for epidemiological studies of PC.
Multiple prior studies have examined the association between obesity, typically measured by BMI, and PC risk. (5,6,7) There is a growing consensus that while obesity may be associated with a lower risk of localized or low grade PC disease, (18) it is also associated with an increased risk of aggressive or high risk disease, PC mortality and biochemical progression after surgery (19, 20, 21). Indeed, a meta-analysis found that obesity was inversely associated with risk of low-grade PC but positively associated with risk of advanced PC (8). Based upon this, we expected to find similar observations in our data. Consistent with these prior studies, using BMI as a measure of obesity, we found that obesity was associated with increased risk of overall PC risk, as well associated with aggressive PC providing further confirmation of the link between obesity and aggressive PC. The biological reasons for these findings may be related to the fact that carcinogenesis is a distinct process from cancer progression. Once cancer cells are formed, the rate they progress to clinical diseases, end-stage disease and death is influenced by many factors including their genetic makeup, the stromal micro-environment, and the systemic metabolic state of the body. Obesity is a systemic inflammatory state with a cross communication between insulin-like growth factor (IGF)-1 axis, sex hormones, and adipokine signaling among other cytokines. (22) If we add the plausible effect of obesity in decreasing androgen levels (23) due to increased peripheral conversion of androgens to estrogens in adipose tissue by aromatase activity affecting the estrogens to testosterones ratios, obese men with an aggressive PC phenotype will have low testosterone in addition to a chronic subclinical inflammation mediated by cytokines and leptin (24) favoring the progression of their PC disease state.
The majority of the world’s literature on obesity and PC is based on BMI. While BMI is often used as a surrogate of adiposity, in the individual subject, BMI may not always reflect the level of body fat. For example, men who are extremely muscular can have a high BMI and yet have low body fat. Alternatively, frail men can have a “normal” BMI but have much body fat. To overcome this, other measures such as percent body fat and waist circumference have been proposed to better reflect adiposity. However, whether these more “precise” measures of adiposity yield similar associations is unclear. In one prior study, Giovannucci et al. examined over 47,000 men from the Health Professionals Follow-up Study and concluded that as it relates to obesity and PC risk, “Patterns for BMI and waist circumference were similar.” (25) Based upon this, we hypothesized that for a group of men, BMI, body fat and waist circumference would be highly correlated with each other and each would show similar results: associated with aggressive PC, but not overall PC risk. We examined these alternative measures and found that on the whole they were highly correlated with BMI, as we hypothesized. Substituting one measure for another in our models – only BMI was associated with PC risk but all had an increased risk of high-grade PC. Our study supports that using BMI, which is inexpensive and easy to measure, will give similar conclusions as more difficult to obtain obesity measures such as waist circumference and percent body fat.
Of note, on univariable analyses we found that all obesity measures especially body fat and waist circumference were inversely associated with PC risk. This mirrors the epidemiological literature regarding obesity and PC risk, which shows that in locations with high PSA screening prevalence (i.e. the US), obesity is inversely associated with PC risk (6, 7). However, these associations were attenuated on multivariable analysis. We have seen similar findings before: controlling for factors associated with PC detection (i.e. lower PSA levels in obese men and larger prostate volumes), the “inverse” association between obesity and PC risk is negated. (26) These findings lend further support to the notion that PC detection is more difficult in obese men making obesity appear protective for low-grade PC. (6,7) We found that both PSA levels and prostate volume were weakly correlated with the adiposity measures, this may be explained by the dilutional effect of PSA in obese men (27) and although usually obese men have larger prostates volumes, in our study, there may be other factors that must be considered when interpreting the results, as inter-observer variations and controversies in prostate volume estimation by ultrasonography (28) and possible confounders not measured.
In our study, we observed that prostate volume had the greatest association with the adiposity measures and the prediction of high-grade disease. This association remained after multivariate adjustment. This observation may be partially explained due to known correlation between obesity and larger prostate volume.(29) In larger prostates, less of the prostate is sampled. As such, in obese men with larger prostate, the prostate is less well sampled making it easier to miss a cancer. As such, obesity appears “protective” – or least less strongly linked with aggressive cancer. Once accounted for, it is clear that obesity – however, we measure obesity – is linked with increased risk of high-grade prostate cancer. This is analogous to prior studies of ours from men undergoing prostate biopsy, where the larger prostate size among obese men had the greatest effect on obscuring the relationship between obesity and high-grade disease. (26) This demonstrates the need for future interventions directed to improve the detection of PC in the obese population.
Another key finding from our study was that self-reported and measured body height and weights were nearly identical. Our results are consistent with the sub group analysis from Discacciati et. al meta-analysis where no statistically significant differences were observed between self -reported BMI and those that relied on BMI measured by trained personnel. (8) Although in a recent study, (30) evaluating agreement between self -reported, interviewer-observed and measured body size, participants, in particular females underestimated their body size in comparison with interviewers. They found positive correlation between self- reported body size and measured body size, which is consistent with our findings. Our data confirm that men enrolling in epidemiological studies of PC risk can accurately estimate their height and weight implying that future studies do not need to actually measure body height or weight, but can rely on self-report.
This study had several limitations. First, our measure of PC aggressiveness was Gleason grade on biopsy. While Gleason grade does correlate with long-term PC progression risk, future studies using alternative definitions of disease aggressiveness (i.e. metastases and PC death) are needed to confirm our findings. Second, our sample size was modest, which limited statistical power to detect modest, but potentially clinically important associations. In addition, due relatively small numbers of Gleason 8–10 prostate cancer cases, it may have not been sufficient for stratified analysis. Third, our findings were based on data from veterans receiving care in the VA system, the largest health care system in the United States with an equal –access setting, which may have implications in the applicability of our findings in the general population. Last, the population studied was referred for a biopsy. As such, our control population was men with an elevated PSA and a negative biopsy. Whether results would differ with a “healthy” control population remains to be determined. These limitations are balanced by key strengths of our study in that we used prospectively collected data with multiple adiposity measures assessed by trained personnel as well as self-reported weight.
In summary, we found that BMI, body fat and waist circumference were all highly correlated and all significantly predicted aggressive PC, but only BMI predicted overall PC risk. This study supports the idea that obesity is selectively associated with high-grade PC. Moreover, as self-reported and measured heights and weights were very highly correlated, this study supports the continued use of self-reported BMI as a measure of obesity in epidemiological studies of PC.
Supplementary Material
Acknowledgments
“The project described was supported by Grant Number R25RR017589 from the NCRR/R25MD007607 from the NIMHD/8U54MD 007587-03 from the NMHHD and K24 CA 160653 from NCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interest to disclose
None of the authors as well their institutions have at any time received payment or services from a third party for any aspect of the submitted work.
No relevant financial relationships with entities in the biomedical arena were present during the 36 months prior to publication.
No patents & copyrights are planned, pending or issued for all authors.
No other relationships/conditions/circumstances that present a potential conflict of interest are present for all authors.
*Odds ratios are for risk of disease grade relative to the risk of not having cancer among patients who had a biopsy
**Adjusted for age, race, PSA, DRE, previous biopsy, TRUS prostate volume, and family history of PC
PC=prostate cancer; BMI=body mass index (kg/m2); OR=odds ratio; CI=confidence interval
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, Million Women Study Collaboration Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007 Dec 1;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England) 2008 Feb 16;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Giles G, Ireland P. Diet, nutrition and prostate cancer. Int J cancer. 1997;(Suppl 10):13–7. doi: 10.1002/(sici)1097-0215(1997)10+<13::aid-ijc5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006 Oct;17(8):989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006 Oct;15(10):1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16(1):63–9. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 8.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012 Jul;23(7):1665–71. doi: 10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 9.Kyle UG, Genton L, Pichard C. Body composition: what’s new? Curr Opin Clin Nutr Metab Care. 2002 Jul;5(4):427–33. doi: 10.1097/00075197-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Garn SM, Leonard WR, Hawthorne VM. Three limitations of the body mass index. Am J Clin Nutr. 1986 Dec;44(6):996–7. doi: 10.1093/ajcn/44.6.996. [DOI] [PubMed] [Google Scholar]
- 11.Nomura AM. Body size and prostate cancer. Epidemiol Rev. 2001;23(1):126–31. doi: 10.1093/oxfordjournals.epirev.a000777. [DOI] [PubMed] [Google Scholar]
- 12.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001 Nov;25(11):1730–5. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 13.Koster A, Leitzmann MF, Schatzkin A, et al. Waist circumference and mortality. Am J Epidemiol. 2008 Jun 15;167(12):1465–75. doi: 10.1093/aje/kwn079. [DOI] [PubMed] [Google Scholar]
- 14.Fowke JH, Motley SS, Concepcion RS, Penson DF, Barocas DA. Obesity, body composition, and prostate cancer. BMC Cancer. 2012;12:23. doi: 10.1186/1471-2407-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014 Available at: www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf.
- 16.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013 May;63(5):800–9. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehm K, Sun M, Larcher A, et al. Waist circumference, waist-hip ratio, body mass index, and prostate cancer risk: results from the North-American case-control study Prostate Cancer & Environment Study. Urol Oncol. 2015 Nov;33(11):494.e1–7. doi: 10.1016/j.urolonc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Wallström P, Bjartell A, Gullberg B, Olsson H, Wirfält E. A prospective Swedish study on body size, body composition, diabetes, and prostate cancer risk. Br J Cancer. 2009 Jun 2;100(11):1799–805. doi: 10.1038/sj.bjc.6605077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalfin HJ, Lee SB, Jeong BC, et al. Obesity and long-term survival after radical prostatectomy. J Urol. 2014 Oct;192(4):1100–4. doi: 10.1016/j.juro.2014.04.086. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2011 Apr;4(4):486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004 Feb 1;22(3):446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 22.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 23.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-α and GPER signalling. Mol Cell Endocrinol. 2012 Apr 4;351(2):269–78. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Huang C-Y, Yu H-S, Lai T-Y, et al. Leptin increases motility and integrin up-regulation in human prostate cancer cells. J Cell Physiol. 2011 May;226(5):1274–82. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Rimm EB, Liu Y, et al. Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst. 2003 Aug 20;95(16):1240–4. doi: 10.1093/jnci/djg009. [DOI] [PubMed] [Google Scholar]
- 26.Freedland SJ, Terris MK, Platz EA, Presti JC. Body mass index as a predictor of prostate cancer: development versus detection on biopsy. Urology. 2005 Jul;66(1):108–13. doi: 10.1016/j.urology.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 27.Banez LL, Hamilton RJ, Partin AW, et al. Obesity –related plasma hemodilution and PSA concentration among men with prostate cancer. J Am Med Assoc. 2007;298:2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 28.Choi YJ, Kim JK, Kim HJ, Cho KS. Interobserver variability of transrectal ultrasound for prostate volume measurement according to volume and observer experience. AJR Am J Roentgenol. 2009;192(2):444–9. doi: 10.2214/AJR.07.3617. [DOI] [PubMed] [Google Scholar]
- 29.Wallner LP, Morgenstern H, McGree ME, et al. The effects of body mass index on changes in prostate-specific antigen levels and prostate volume over 15 years of followup: implications for prostate cancer detection. Cancer Epidemiol Biomarkers Prev. 2011;20:501–508. doi: 10.1158/1055-9965.EPI-10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutcliffe CG, Schultz K, Brannock JM, Giardiello FM, Platz EA. Do people know whether they are overweight? Concordance of self-reported, interviewer-observed, and measured body size. Cancer Causes Control. 2015 Jan;26(1):91–8. doi: 10.1007/s10552-014-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


