Abstract
Background
Frailty is an important prognostic factor for adverse outcomes and increased resource use in the growing population of older surgical patients. We identified and appraised studies that tested interventions in populations of frail surgical patients to improve perioperative outcomes.
Methods
We systematically searched Cochrane, CINAHL, EMBASE and Medline to identify studies that tested interventions in populations of frail patients having surgery. All phases of study selection, data extraction, and risk of bias assessment were done in duplicate. Results were synthesized qualitatively per a prespecified protocol (CRD42016039909).
Results
We identified 2 593 titles; 11 were included for final analysis, representing 1 668 participants in orthopedic, general, cardiac, and mixed surgical populations. Only one study was multicenter and risk of bias was moderate to high in all studies. Interventions were applied pre- and postoperatively, and included exercise therapy (n = 4), multicomponent geriatric care protocols (n = 5), and blood transfusion triggers (n = 1); no specific surgical techniques were compared. Exercise therapy, applied pre-, or post-operatively, was associated with significant improvements in functional outcomes and improved quality of life. Multicomponent protocols suffered from poor compliance and difficulties in implementation. Transfusion triggers had no significant impact on mortality or other outcomes.
Conclusions
Despite a growing literature that demonstrates strong independent associations between frailty and adverse outcomes, few interventions have been tested to improve the outcomes of frail surgical patients, and most available studies are at substantial risk of bias. Multicenter, low risk of bias, studies of perioperative exercise are needed, while substantial efforts are required to develop and test other interventions to improve the outcomes of frail people having surgery.
Introduction
Western populations are aging rapidly.[1,2] Older people have surgery at over two times the rate of younger individuals,[3] and advanced age is a well-established risk factor for adverse postoperative outcomes.[4,5] However, amongst the older surgical population, outcomes vary substantially.[6] Frailty, a state of increased vulnerability to stressors due to age-, and disease-related deficits that accumulate across multiple domains, is a key factor in explaining the increased rates of complications, healthcare resource use, loss of independence, and mortality experienced by older surgical patients[7–11]
The prevalence of frailty increases exponentially with age.[12] Therefore, as our population ages, an increasing number of frail patients are expected to present for surgery. In fact, contemporary studies estimate that 25–40% of older patients having major surgery are frail or pre-frail.[13–15] Based on a conservative estimate that frailty is associated with a 2- to 3-fold increase in the relative risk of adverse postoperative events,[8] we estimate that the proportion of adverse events attributable[16] to frailty is 25–50%. However, despite the strong and increasingly well-recognized association of frailty with adverse postoperative events and increased resource use across surgical specialties,[8,9,17] and the multitude of instruments that have been used to diagnose frailty,[18] interventions specifically tailored to frail surgical patients are not commonly described in the literature, and have not been systematically reviewed. Knowledge generated from such a synthesis is needed to inform current care and future research. Therefore, we undertook a systematic review to identify interventions that have been tested in populations of frail surgical populations to improve health outcomes, patient experience or costs of care.[19]
Materials and methods
This systematic review was performed in accordance with guidelines from the Cochrane Collaboration,[20] and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (see checklist in S1 File).[21] The study protocol was registered with the International Prospective Register of Systematic Reviews (2016:CRD42016039909).
Search strategy
A systematic search strategy was designed in consultation with an information specialist, and then reviewed and finalized using the peer review of electronic search strategy checklist.[22] The search strategy is provided in Table A in S2 File. We employed a broad strategy using keywords and controlled vocabulary to identify frailty and surgical procedures. The search did not place limitations on outcomes or study designs. No language restrictions were applied, and all databases (Cochrane, Medline, Cumulative Index of Nursing and Allied Health Literature, and the Excerpta Medica Database) were searched from inception to February 14, 2016. Grey literature was searched and considered, including conference proceedings (2010–2016) from the American College of Surgeons, American Geriatrics Society, American Society of Anesthesiology, British Geriatrics Society, and the European Geriatrics Society, as well as conference abstracts identified through our database searches. We also searched ClinicalTrials.gov to identify planned, in-progress or completed studies that had not yet been reported.
Inclusion and exclusion criteria
Randomized and non-randomized (e.g., cohort, controlled before after, interrupted time series, other quasi-randomized designs) studies were eligible for inclusion, however, non-experimental studies (such as case reports or case series) were excluded. To be included, studies had to evaluate a population of frail individuals having surgery (endovascular cardiac valve procedures, endoscopic procedures, and cataract surgery were not included as perioperative processes and trajectories were felt to differ substantially from prototypical surgical procedures), or have a specific subgroup of frail patients where frailty-specific intervention and outcome data could be extracted. In the case of a mixed population (i.e., surgical and medical), surgical patients had to represent the majority of included participants. Included studies had to state the specific method used to define individuals as frail, however, we placed no limitations on what frailty definitions were acceptable. Studies could test any intervention, so long as it was applied in the perioperative period and was related to the fact that patients were having, or had surgery. We did not limit inclusion to specific outcome types, however, we did categorize outcomes in one of the three domains of the IHI Triple Aim outcome framework (health, cost, experience).[19]
Selection of included studies and data extraction
All identified titles and abstracts, and conference proceedings were screened in duplicate by two independent reviewers. When adherence to inclusion/exclusion criteria was unclear, studies were moved forward for full text review. Full text review was also performed in duplicate, and disagreement at any stage was resolved in discussion with the primary investigator (DM). The reference lists of all included articles were searched to identify any other studies that may have been missed by our search strategy.
For data collection, a form designed specifically for this review was first piloted on six studies, and then applied to all studies. Data was extracted in duplicate, and reviewed in a triad that included both reviewers and the primary investigator. Publication characteristics, patient and surgical factors, details of the intervention, and study outcomes were extracted for all included studies. All citation screening, full text review, and data collection was performed using DistillerSR® (Evidence Partners, Ottawa, Canada).
Risk of bias assessment
Risk of bias assessments were conducted for all studies. Non-randomized studies were assessed using the Risk of Bias in Non-randomized studies of Interventions (ROBINS-I);[23] randomized controlled trials (RCTs) were assessed with the Cochrane Risk of Bias Tool for randomized trials.[20] The scales for each risk of bias tool were modified to provide consistent scoring across study designs. All risk of bias assessments were done in duplicate by the primary investigator and a second team member; disagreements were resolved by consensus.
Analysis and data synthesis
We summarized the study designs, frailty instruments, surgeries, patient characteristics, intervention characteristics and outcomes reported. We did not anticipate identifying adequately homogenous data to support formal meta-analysis, and we therefore pre-specified a qualitative approach to data synthesis. We organized our qualitative synthesis first around the type of intervention, then by surgical population, and finally be phase of the perioperative period where intervention was employed. We also synthesized the types of outcomes that were studied within these groupings.
Results
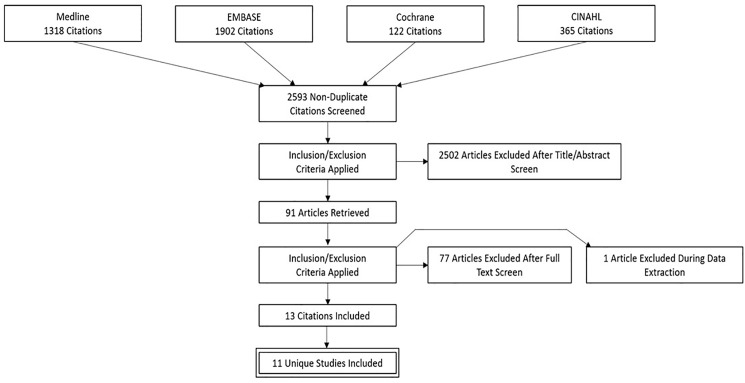
Following removal of duplicate records, we identified 2 593 unique title and abstracts to review, and as described in Fig 1, included 11 studies for final analysis (1 study generated 3 unique citations the result of which were considered together as a single study). The one conference abstract identified was not included in our formal synthesis, as frailty definitions used were not described, and because inadequate information was available to assess risk of bias. Seven trials were identified through ClinicalTrials.gov (November 23rd, 2016); one had completed recruitment (an email to the investigators requesting data was not returned), four were currently recruiting, and two were not yet open for recruitment. The conference abstract and summaries of ClincalTrials.gov protocols are provided in Table B in S2 File.
Fig 1. Flow diagram outlining selection of studies.
Study and population characteristics
Six of the included studies were RCTs, and five were observational (four controlled before after, and one whose design was unclear but which appeared to be most consistent with a prospective non-randomized trial;[24] Table 1). Sample sizes ranged from 21 to 386 participants (1 668 total). Mean participant age was older than 70 years in all studies. Surgery types included general surgery (three studies), cardiac (two studies), orthopedic (four studies), solid tumor (one study) and mixed (one study). Surgical urgency included elective (six studies), emergency (two studies), mixed (one study), and not reported (two studies). Other details are provided in Table 1. Frailty was defined by geriatric assessment in three studies, the Identification of Seniors at Risk questionnaire in two studies, Fried’s Frailty Phenotype in one study, Groningen Frailty Indicator (GFI) for one study, Clinical Frailty Scale for one study, and physical performance measures in three studies.
Table 1. Characteristics of included studies.
| Source | Study Type | Surgery | Frailty Instrument | Control (n) | Intervention (n) | Mean age | Intervention |
|---|---|---|---|---|---|---|---|
| Bakker et al, 201427 | CBA | Mixed | Geriatric examination | 191 | 195 | 77 | Enhanced care protocol |
| Binder et al, 200433 | RCT | Hip Fracture | mPPT score and ADLs | 44 | 46 | 80 | Post-operative exercise |
| Chen et al, 201426 | CBA | General | Fried’s frailty phenotype | 52 | 52 | 73 | Enhanced care protocol |
| Gorelik et al, 201524 | Unclear | General | Geriatric examination | 35 | 36 | 82 | Enhanced care protocol |
| Gregersen et al, 201535−37 | RCT | Hip Fracture | Comprehensive Geriatric Assessment | 140 | 144 | 86 | Blood transfusion trigger |
| Hempenius et al, 201329 | RCT | Solid tumor | Groningen Frailty Indicator | 149 | 148 | 77 | Enhanced care protocol |
| Hoogeboom et al, 201030 | RCT | Hip replacement | Clinical Frailty Scale | 11 | 10 | 77 | Pre-operative exercise |
| Indrakusuma et al, 201425 | CBA | General | ISAR | 50 | 50 | 81 | Enhanced care protocol |
| Molino-Lova et al, 201134 | RCT | Cardiac | SPPB score | 48 | 51 | 75 | Post-operative exercise |
| Oosting et al, 201231 | RCT | Hip replacement | ISAR | 15 | 15 | 77 | Pre-operative exercise |
| Opasich et al, 201032 | CBA | Cardiac | BPOMA | 74 | 150 | 75 | Post-operative exercise |
BPOMA: Balance Performance Oriented Mobility Assessment; CBA: controlled before after; ISAR: Identification of Seniors At Risk; mPPT: modified version of the Physical Performance Test; RCT: randomized controlled study; SPPB: Short Physical Performance Battery score
Intervention characteristics
Interventions were applied in the pre- and postoperative period; however, no specific intraoperative interventions were identified. Three categories of interventions were identified: multicomponent geriatric care protocols (n = 5), exercise interventions (n = 5), and transfusion triggers (n = 1). Specific details for each intervention are provided in Table 2, while trends in outcome effects across intervention types and surgical populations are described in Fig 2.
Table 2. Description of interventions and outcomes.
| Source | Intervention Timing | Intervention | Control Group Intervention | Outcome(s) | Outcome Window | Result |
|---|---|---|---|---|---|---|
| Bakker, 201427 | Pre & Post | Care Protocol: | Standard care | Hospital-acquired delirium | In-hospital | No difference |
| Orientation | Cognitive decline | In-hospital | No difference | |||
| Mobilization | Physical decline | At discharge | Worse with intervention group | |||
| Day program activities | ADL | At discharge | Worse with intervention | |||
| Physiotherapy consult | ADL | At discharge | No difference | |||
| Dietitian consult | ADL | 3 months post-discharge | Better with intervention | |||
| Discharge planning | Readmission | 30 days post-discharge | No difference | |||
| Medication review | Unplanned readmission | 30 days post-discharge | No difference | |||
| CGA by geriatrician | Caregiver burden | 3 months post-discharge | No difference | |||
| Binder, 200433 | Post | Exercise | Non-personalized exercise without weight training | Modified Physical Performance Test | 6 months after surgery | Better with intervention |
| Functional Status Questionnaire | 6 months after surgery | Better with intervention | ||||
| Basic ADL | 6 months after surgery | No difference | ||||
| Instrumental ADL | 6 months after surgery | No difference | ||||
| Assistive devices use | 6 months after surgery | Less use with intervention | ||||
| Knee extension strength | 6 months after surgery | Better with intervention | ||||
| Walking speed | 6 months after surgery | Better with intervention | ||||
| Single limb stance time | 6 months after surgery | Better with intervention | ||||
| Berg balance score | 6 months after surgery | Better with intervention | ||||
| Total fat-free mass | 6 months after surgery | No difference | ||||
| Bone mineral density | 6 months after surgery | No difference | ||||
| SF-36 score | 6 months after surgery | Better health, physicial and social function with intervention | ||||
| Hip Rating Questionnaire | 6 months after surgery | Better with intervention | ||||
| Chen, 201426 | Post | Care Protocol: | Standard care | *Frailty rate | At discharge | Better with intervention |
| 3 months post-discharge | No difference | |||||
| Early mobilization | Transitions between frailty states | From admission to discharge | Better with intervention | |||
| Oral and nutritional assistance | ||||||
| Orientating communication | From admission to 3-months post-discharge | No difference | ||||
| Gorelik, 201524 | Post | Care Protocol: | Standard care | *Stability | 6 months after surgery | Better with intervention |
| Walking | 6 months after surgery | Better with intervention | ||||
| Rehabilitation | Malnutrition | 6 months after surgery | Better with intervention | |||
| Nutrition support | Cognitive disorders | 6 months after surgery | Better with intervention | |||
| Psychotherapy | Moral status | 6 months after surgery | Better with intervention | |||
| Home care for some | Independence | 6 months after surgery | Better with intervention | |||
| Gregersen, 201535−37 | Post | Restrictive blood transfusion | Liberal blood transfusion | Modified Barthel Index | 10 days after surgery | No difference |
| New Mobility Score | 10 days after surgery | No difference | ||||
| Ambulation score | 10 days after surgery | No difference | ||||
| Transfer independence | 10 days after surgery | No difference | ||||
| Walking independence | 10 days after surgery | No difference | ||||
| Mortality, per protocol | 30-day | Worse with restrictive | ||||
| Mortality | 90-day | No difference | ||||
| Leukocyte counts | 30 days post-operatively | No difference | ||||
| CRP concentration | 30 days post-operatively | No difference | ||||
| Infection | 10 days post-operatively | No difference | ||||
| Complications | 10 days post-operatively | No difference | ||||
| Modified Barthel Index | Day 30 to 1 year post-operatively | Better with liberal | ||||
| Depression | Day 30 post-operatively | No difference | ||||
| 1 year post-operatively | No difference | |||||
| Hempenius, 201329 | Pre & Post | Standard care | Postoperative delirium | 10 days after surgery | No difference | |
| Care Protocol: | Severity of delirium | 10 days after surgery | No difference | |||
| Complications, >1 | 10 days after surgery | No difference | ||||
| Individualized geriatric care plan | Mortality | In-hospital | No difference | |||
| SF-36 score | Discharge | No difference | ||||
| Care dependency | Assessed at discharge | No difference | ||||
| Return to an independent living situation | Assessed at discharge | Worse with intervention | ||||
| Additional care at home | Assessed at discharge | No difference | ||||
| Length of stay | In-hospital | No difference | ||||
| Hoogeboom, 201030 | Pre | Exercise | Standard care | Osteoarthritis Outcome Score | Week before surgery | No difference |
| Longitudinal Aging Study Amsterdam Physical Activity Questionnaire | Week before surgery | No difference | ||||
| Physical Working Capacity | Week before surgery | No difference | ||||
| 6-MWT | Week before surgery | No difference | ||||
| Timed Up & Go Test | Week before surgery | No difference | ||||
| Chair Rise Time | Week before surgery | No difference | ||||
| Grip Strength | Week before surgery | No difference | ||||
| Time needed to functional independence | In-hospital | No difference | ||||
| Patient-Specific Complaints Questionnaire | Week before surgery | No difference | ||||
| Length of stay | In-hospital | No difference | ||||
| Indrakusuma, 201425 | Pre | Care Protocol: | Standard care | Mortality | 30 days post-operatively | No difference |
| Nutrition supplements | Postoperative delirium | Not reported | No difference | |||
| Cardiology consult | Postoperative complications | Not reported | No difference | |||
| Blood transfusion | Length of stay | In-hospital | No difference | |||
| Haloperidol prophylaxis | ||||||
| Molino-Lova, 201134 | Post | Exercise | Usual aerobic exercise | Short Physical Performance Battery | 1 year | Better with intervention |
| Oosting et al, 201231 | Pre | Exercise | Standard care | Timed Up & Go Test | 6 weeks post-discharge | No difference |
| 6-MWT | 6 weeks post-discharge | Better with intervention | ||||
| Chair Rise Time | 6 weeks post-discharge | Better with intervetion | ||||
| Hip disability and Osteoarthritis Outcome Score | 6 weeks post-discharge | No difference | ||||
| Longitudinal Aging Study Amsterdam Physical Activity Questionnaire | 6 weeks post-discharge | No difference | ||||
| Pain | 6 weeks post-discharge | No difference | ||||
| Patient Specific Complaints Questionnaire | 6 weeks post-discharge | No difference | ||||
| Opasich et al, 201032 | Post | Exercise | Traditional physiotherapy program | *Nursing needs | At discharge | Better with intervention |
| Balance Performance Oriented Mobility Assessment | At discharge | Better with intervention | ||||
| Timed Up & Go Test | At discharge | Better with intervention | ||||
| Arm Curl | At discharge | Better with intervention | ||||
| Chair Stand | At discharge | Better with intervention | ||||
| 6-MWT | At discharge | No difference | ||||
| Health related quality of life | At discharge | No difference | ||||
| Length of Stay | In-hospital | Shorter with intervention |
* Primary outcome not specified in study. 6-MWT: 6 minute walk test; ADL: activities of daily living; CRP: c-reactive protein; SF: short form
Bolded and underlined text = Primary outcomes
Bolded outcomes reached statistical significance
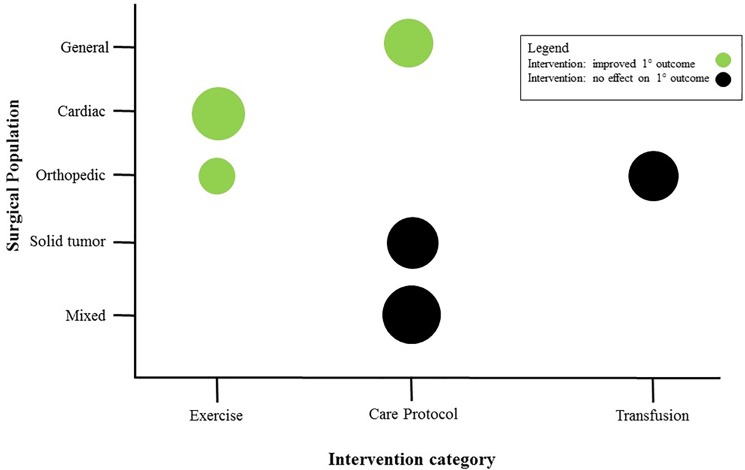
Fig 2. Summary of study outcomes by intervention type and surgical population.
The size of each circle is proportional to the number of participants in each grouping.
Exercise interventions
Two studies evaluated the impact of preoperative exercise programs for elective total hip arthroplasty patients.[25,26] Participants in both trials were satisfied with the interventions, and both studies found positive impacts of exercise on functional outcomes. No improvements in postoperative function were noted.[25] Three studies evaluated postoperative exercise interventions, two in cardiac surgery and one after hip fracture surgery.[27–29] All three studies found positive impacts of the exercise intervention on functional outcomes, while in the lowest risk of bias study, the exercise intervention significantly improved quality of life outcomes.(28) Detailed description of the exercise interventions is provided in the Table C in S2 File, while a summary of evidence using the GRADE Framework[30] is provided in Table 3.
Table 3. GRADE summary of evidence.
| Population-People with frailty having surgery | ||||||
|---|---|---|---|---|---|---|
| Intervention-Exercise therapy | ||||||
| Control-No or non-standardized exercise therapy | ||||||
| Quality assessment | ||||||
| Participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Overall quality | Comment |
| Postoperative function | ||||||
| 503 (4) | Moderate | Low | No serious indirectness | No serious imprecision | Moderate1 | Significant improvement in most physical performance measures in 3/4 studies |
| Postoperative health related quality of life | ||||||
| 314 (2) | Serious | Moderate | No serious indirectness | No serious imprecision | Low2 | Significant improvement in physical and mental health in a randomized trial |
| Postoperative length of stay | ||||||
| 245 (2) | Moderate | Moderate | No serious indirectness | Moderate imprecision | Very low3 | Decreased length of stay in larger observational study; none in small pilot randomized trial |
1. Downgraded as not all studies showed improvement, and 1 was non-randomized
2. Downgraded due to unclear allocation concealment and blinding in RCT, no effect in observational study
3. Downgraded due to inconsistency, positive effect was from a high risk of bias observational study
Multicomponent geriatric care protocols
Prior to elective colorectal surgery, geriatric assessment to guide perioperative care planning was associated with decreased length of hospital stay, however no differences in primary or other outcomes were identified.[31]
Geriatric-specific multicomponent interventions were tested in three observational studies, two of which included general surgery patients,[24,32] and the third which included a mix of surgical specialties.[33] Following elective general surgery, institution of a modified hospital elder life program (a formal evidence-based program to optimize care of older patients in hospital[34]) was associated with a lower rate of frailty at hospital discharge.[32] Following institution of a team-based complex geriatric intervention for a mixed surgical population, there was no significant difference in primary or most secondary outcomes.[33] A structured geriatric rehabilitation program after laparoscopic cholecystectomy was associated with improvements in functional, nutritional and cognitive outcomes.[24]
A single RCT evaluated a geriatric care protocol with pre- and postoperative components in elective cancer surgery.[35] The authors found that the individuals in the intervention group, who underwent preoperative geriatric assessment, individualized delirium prevention plans, daily geriatric nurse liaison while in hospital and consultative treatment advice experienced similar rates of delirium and other outcomes compared to those who received standard care.
Poor protocol adherence was noted in two of five multicomponent studies, [31,33] while another multicomponent study reported difficulties with the complexity of applying and measuring adherence to the study’s specific protocol components.[35] Details of each multicomponent intervention and control group care are provided in theTable D in S2 File.
Transfusion trigger
Following hip fracture surgery, one study of a restrictive vs. liberal red blood cell transfusion strategy found no differences in mortality, quality of life, functional outcomes, or infectious complications between arms. The authors did report an increase in 30-day mortality in the restrictive arm per their secondary per protocol analysis, however, there were an equal number of protocol violations in both study arms, and at 90 days there was no difference in mortality, even when analyzed per protocol.[36–38]
Risk of bias
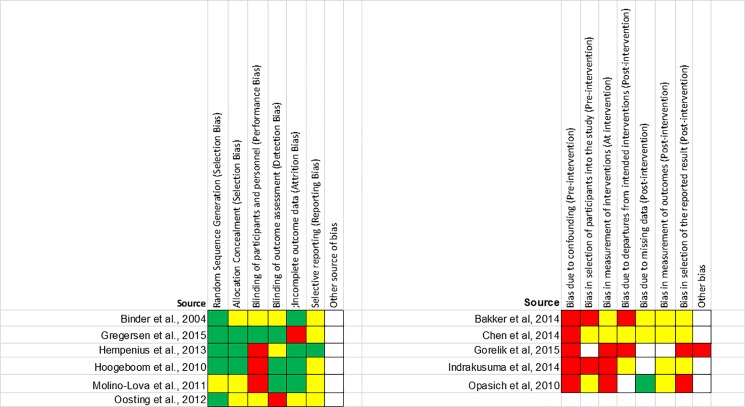
Two RCTs were assessed as moderate risk of bias; all others were at high risk of bias. Performance bias related to blinding of participants, and selective outcome reporting were the domains most often rated as moderate to high risk of bias. All observational studies were at high risk of bias, and in particular suffered from confounding bias (Fig 3).
Fig 3. Risk of bias assessment.
Green represents low risk of bias, yellow moderate risk of bias and red high risk of bias. For domains with white squares, risk of bias was unclear.
Outcomes
Based on the Triple Aim Framework, all studies reported at least one health outcome, eight studies reported a patient experience outcome, and cost outcomes were reported in four studies. Seven studies specified a primary outcome, while four studies reported on multiple outcomes without specifying a primary outcome of interest. A formal meta-analysis was not possible due to the heterogeneity of study designs, interventions and outcomes.
Discussion
A substantial proportion of postoperative adverse events in older surgical patients are attributable to the presence of frailty. However, despite a marked increase in the epidemiological literature describing associations between frailty and adverse postoperative outcomes, we identified only eleven studies that tested interventions in populations of frail patients having surgery. Although six out of eleven studies identified were RCTs, only two of eleven studies were not at high risk of bias. The small number of studies identified, and the high risk of bias present in most studies, highlights a substantial knowledge gap in surgery and perioperative medicine. There is an urgent need for the development and testing of new interventions to improve the outcomes of frail people having surgery, as well as large, multicenter RCTs at low risk of bias to evaluate promising interventions, such as perioperative exercise therapy in the frail elderly.
Even with a broad search strategy and no specific limitations on the frailty definitions eligible for inclusion, or the intervention types considered, our systematic review identified only eleven studies that tested perioperative interventions in frail patients. In part, this does reflect our protocol’s requirement that a frailty definition be used. This lead to exclusion of studies of hip fracture patients which did not include specified frailty definitions. While some consider a hip fracture to be frailty-defining, not all older hip fracture patients are found to be frail when frailty criteria are applied.[39,40] Furthermore, geriatric-specific interventions, such as the Proactive care of Older People having Surgery,[41] have been tested in higher-risk older surgical patients, and show promising impacts on outcomes. While some included patients in this study were likely frail, the frailty definition requirement of our protocol excluded this study as our aim was to identify evidence that could be generalized specifically to frail older people, who are a unique stratum of the population of older people having surgery.
Only one conference abstract and seven study protocols were identified, suggesting that the small pool of published studies identified is not about to increase substantially. Given our study’s strengths, including pre-registration of our study protocol, and adherence to best practice methodologies (such as duplicate handling of all stages of the review, grey literature searches, and hand searching of study reference lists) the paucity of identified studies underlies an urgent call for a transition from the current focus of describing the epidemiology of perioperative frailty to efforts to prospectively address the risk of frailty in patients having surgery. These efforts should also include younger people with frailty, who were not represented in any of our included studies. Moving forward, investigators will need to study interventions which address the factors that we currently understand to contribute to the adverse outcome burden experienced by frail people having surgery. Although not yet comprehensively understood, these factors include vulnerability to intrinsic and extrinsic stressors, decreased cognitive reserve, and dysregulation of immune and inflammatory mechanisms.[8,42] To support this move, investigators must commit to performing low risk of bias randomized trials (with a particular focus on improved blinding, allocation concealment, and outcome pre-specification). Where randomized trials aren’t indicated or feasible, improved observational study methodologies, such as interrupted time series analyses or other quasi-randomized designs should be considered in place of controlled before after studies. Furthermore, no intraoperative interventions, such as comparison of specific surgical techniques for frail patients, have been reported.
Despite the limitations present in our included studies, including the heterogeneity of frailty definitions, intervention types, surgical populations and outcome measures that precluded meta-analysis and formal assessment of publication bias, and the substantial risk of bias across studies, our findings do provide important insights to guide the improvement of outcomes for frail surgical patients. Perioperative exercise therapy appears to be a promising intervention to improve function and quality of life, and we identified consistent barriers in studies which attempted to implement and test multicomponent geriatric-specific care protocols. These insights are discussed in the following paragraphs.
Exercise therapy
In all five studies that evaluated perioperative exercise therapy, the intervention was positively associated with improved function, quality of life, or both. Findings from two RCTs[28,29] and one before after study[27] found that postoperative exercise therapy in cardiac and orthopedic surgery populations improved outcomes. Therefore, while confirmation of these findings in a high quality multicenter RCT would be preferable, we suggest that the consistent directional association that was generalized across surgical populations supports inclusion of postoperative exercise therapy in the perioperative care of frail surgical patients. Preoperative exercise therapy requires a more thorough evaluation in future studies, as the two small RCTs that we identified primarily evaluated changes in preoperative function. Furthermore, neither was designed or powered to adequately evaluate the impact of preoperative exercise on postoperative functional recovery or other outcomes. Therefore, a high quality RCT of preoperative exercise in frail older patients that is properly powered and designed to evaluate meaningful differences in long-term postoperative outcomes is needed. Studies that include pre- and postoperative exercise interventions should also be considered.
Multicomponent geriatric interventions
Despite the positive impact on outcomes of multicomponent interventions such as orthogeriatric care in older hip fracture surgery patients (who are often frail),[43,44] the five studies of multicomponent geriatric-focused care protocols included in our study did not demonstrate consistent improvements in outcomes. In fact, only one study clearly found a positive association between protocol implementation and the primary study outcome. Chen et. al.,[32] who implemented a modified version of a pre-existing evidence-based intervention found that protocol implementation was associated with improved frailty status at hospital discharge. Interestingly, the authors describe use of standardized training materials and a specially trained nurse-educator to implement and support compliance with the protocol. In contrast, the three studies that clearly failed to demonstrate an improvement in their primary outcome all reported issues with protocol implementation and non-compliance[31,33,35] (methodological and reporting limitations from the fifth care protocol study precluded clear interpretation of its findings[24]). Therefore, in addition to ensuring that interventions included in geriatric-focused multicomponent interventions for frail surgical patients are evidence based, there is also a need to consider the feasibility of each intervention, as well as the clinical context, to support success.
Choice of frailty instrument
Although the adverse outcome effect of frailty appears to generalize across different frailty instruments, the generalizability of current and future interventional study findings will be limited in the absence of efforts to standardize, or at least limit, the number of different frailty instruments used in perioperative research. Consistent with previous reports from other areas of frailty research (such as non-surgical frailty,[45] or non-interventional studies of perioperative frailty[8,17]), we identified substantial heterogeneity in the instruments used to define frailty. In the eleven included studies, eight different frailty definitions were used. Although the modified Fried Index,[46] a phenotypic approach to frailty diagnosis, is recommended by practice guidelines,[47] only one recent publication has compared the predictive performance of different frailty instruments to inform the choice of an appropriate perioperative tool.[48] Further comparative research and consensus building is needed. Without consensus, clinicians will be limited in their ability to apply study findings to people with frailty having surgery, and future efforts in knowledge synthesis will be significantly hindered by heterogeneity in frailty definitions.
Outcomes reported
The variety of outcomes evaluated in identified studies is both promising, and a cause for concern. Encouragingly, studies did not focus only on traditional outcomes such as morbidity, mortality and length of stay, but also evaluated patient experience, function, and quality of life. In fact, all three domains of the IHI Triple Aim were well-represented. However, the heterogeneity in outcome measures also draws attention to the lack of agreed upon core outcomes for the frail elderly in general, or frail people having surgery more specifically. Engagement of processes such as the Core Outcome Measures in Effectiveness Trials (COMET) initiative[49] to define a minimum set of key outcomes for frail surgical patients is needed.
Conclusions
Only a small number of studies exist which investigate the impact of perioperative interventions on outcomes in frail surgical patients. Although exercise interventions appear to show promise in improving functional and quality of life outcomes, further studies are needed to address methodological limitations identified in the existing literature. Development of multicomponent geriatric care protocols require consideration of anticipated efficacy as well as feasibility to support effective implementation. Significant efforts are needed to develop evidence-informed interventions to improve the outcomes of our growing frail surgical population, and to evaluate these interventions in low risk of bias studies.
Supporting information
(DOC)
Table A- Search strategies for included databases; Table B—Conference abstracts and study protocols identified; Table C—Description of exercise interventions and control conditions; Table D- Description of multicomponent geriatric care protocols and control conditions.
(DOCX)
Acknowledgments
We would like to acknowledge Ms. Sascha Davis for her expertise in developing our search strategy. Use of DistillerSR® is provided by The Ottawa Hospital Department of Anesthesiology.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sixty-five plus in the United States [Internet]. U.S. Census Bureau Statistical Brief. 1995. Available from: http://www.census.gov/population/socdemo/statbriefs/agebrief.html
- 2.Statistics Canada. The Canadian Population in 2011: Age and Sex [Internet]. Ottawa, ON; 2011. Available from: http://www12.statcan.gc.ca/census-recensement/2011/as-sa/98-311-x/98-311-x2011001-eng.cfm#a2
- 3.Etzioni DA, Liu JH, Maggard MA, Ko CY. The aging population and its impact on the surgery workforce. Ann Surg [Internet]. 2003. August [cited 2012 Nov 16];238(2):170–7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1422682&tool=pmcentrez&rendertype=abstract doi: 10.1097/01.SLA.0000081085.98792.3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg [Internet]. 2006. December [cited 2012 Nov 11];203(6):865–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17116555 doi: 10.1016/j.jamcollsurg.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 5.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc [Internet]. 2005. March [cited 2013 Jan 17];53(3):424–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15743284 doi: 10.1111/j.1532-5415.2005.53159.x [DOI] [PubMed] [Google Scholar]
- 6.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA [Internet]. 2014. May [cited 2014 Aug 25];311(20):2110–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24867014 doi: 10.1001/jama.2014.4573 [DOI] [PubMed] [Google Scholar]
- 7.Berian J, Mohanty S, Ko CY, Rosenthal RA, Robinson TN. Association of Loss of Independence With Readmission and Death After Discharge in Older Patients After Surgical Procedures. JAMA Surg. 2016;1–7. [DOI] [PubMed] [Google Scholar]
- 8.Beggs T, Sepehri A, Szwajcer A, Tangri N, Arora RC. Frailty and perioperative outcomes: a narrative review. Can J Anesth Can d’anesthésie [Internet]. 2015. February 25;62(2):143–57. Available from: http://link.springer.com/10.1007/S22630-014-0273-z [DOI] [PubMed] [Google Scholar]
- 9.McIsaac DI, Bryson GL, van Walraven C. Association of Frailty and 1-Year Postoperative Mortality Following Major Elective Noncardiac Surgery. JAMA Surg [Internet]. 2016. January 20;online ahe. Available from: http://archsurg.jamanetwork.com/article.aspx?doi=10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 10.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-roche K, Patel P, et al. Frailty as a Predictor of Surgical Outcomes in Older Patients. ACS [Internet]. 2010;210(6):901–8. Available from: http://dx.doi.org/10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 11.McIsaac DI, Beaule PE, Bryson GL, van Walraven C. The impact of frailty on outcomes and healthcare resource utilization after total joint arthroplasty: a population-based cohort study. Bone Jt J. 2016;98:799–805. [DOI] [PubMed] [Google Scholar]
- 12.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing. 2014;0:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenig J, Zychiewicz B, Olszewska U, Richter P. Screening for frailty among older patients with cancer that qualify for abdominal surgery. J Geriatr Oncol [Internet]. 2015;6(1):52–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2879406814003294 doi: 10.1016/j.jgo.2014.09.179 [DOI] [PubMed] [Google Scholar]
- 14.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg [Internet]. 2013;206(4):544–50. Available from: http://dx.doi.org/10.1016/j.amjsurg.2013.03.012 doi: 10.1016/j.amjsurg.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg [Internet]. 2010. June;210(6):901–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20510798 doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 16.Metrics: Population Attributable Fracion [Internet]. World Health Organization: Health Statistics and Information Systems. [cited 2016 Dec 13]. Available from: http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/
- 17.Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative Frailty Assessment and Outcomes at 6 Months or Later in Older Adults Undergoing Cardiac Surgical Procedures. Ann Intern Med [Internet]. 2016; Available from: http://annals.org/article.aspx?doi=10.7326/M16-0652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johanning JM, Hall D, Arya S. Frailty and Mortality After Noncardiac Surgery in Elderly Individuals. JAMA Surg [Internet]. 2016. June 1;151(6):545 Available from: http://archsurg.jamanetwork.com/article.aspx?doi=10.1001/jamasurg.2015.5235 doi: 10.1001/jamasurg.2015.5235 [DOI] [PubMed] [Google Scholar]
- 19.Berwick DM, Nolan TW, Whittington J. The Triple Aim: Care, Health, And Cost. Health Aff. 2008;3(3):759–69. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. 5.1.0. The Cochrane Collaboration; 2011. Available from: www.handbook.cochrane.org [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med [Internet]. 2009. July 21 [cited 2012 Nov 2];6(7):e1000097 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2707599&tool=pmcentrez&rendertype=abstract doi: 10.1371/journal.pmed.1000097 [PMC free article] [PubMed] [Google Scholar]
- 22.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol [Internet]. 2016. July;75:40–6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0895435616000585 doi: 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ [Internet]. 2016. October 12;i4919 Available from: http://www.bmj.com/lookup/doi/10.1136/bmj.i4919 doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelik S, Lutsenko V, Prashchayeu K, Tatyaneko T. The Effectof a Frailty Management Program on the Rehabilitation of Elderly Patients after Surgical Treatment. Res J Pharm Biol Chem Sci. 2015;6(4):183–7. [Google Scholar]
- 25.Hoogeboom TJ, Dronkers JJ, van den Ende CH, Oosting E, van Meeteren NL. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil [Internet]. 2010. October 1;24(10):901–10. Available from: http://cre.sagepub.com/cgi/doi/10.1177/0269215510371427 doi: 10.1177/0269215510371427 [DOI] [PubMed] [Google Scholar]
- 26.Oosting E, Jans MP, Dronkers JJ, Naber RH, Dronkers-Landman CM, Appelman-de Vries SM, et al. Preoperative Home-Based Physical Therapy Versus Usual Care to Improve Functional Health of Frail Older Adults Scheduled for Elective Total Hip Arthroplasty: A Pilot Randomized Controlled Trial. Arch Phys Med Rehabil [Internet]. 2012. April;93(4):610–6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0003999311009701 doi: 10.1016/j.apmr.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Opasich C, Patrignani A, Mazza A, Gualco A, Cobelli F, Pinna GD. An elderly-centered, personalized, physiotherapy program early after cardiac surgery. Eur J Cardiovasc Prev Rehabil [Internet]. 2010. October;17(5):582–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20941843 [DOI] [PubMed] [Google Scholar]
- 28.Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA [Internet]. 2004. August 18;292(7):837–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15315998 doi: 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 29.Molino-Lova R, Pasquini G, Vannetti F, Paperini A, Forconi T, Polcaro P, et al. Effects of a structured physical activity intervention on measures of physical performance in frail elderly patients after cardiac rehabilitation: a pilot study with 1-year follow-up. Intern Emerg Med [Internet]. 2013. October;8(7):581–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21744061 doi: 10.1007/s11739-011-0654-z [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ [Internet]. 2008. April 26;336(7650):924–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18436948 doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Indrakusuma R, Dunker MS, Peetoom JJ, Schreurs WH. Evaluation of preoperative geriatric assessment of elderly patients with colorectal carcinoma. A retrospective study. Eur J Surg Oncol [Internet]. 2015. January;41(1):21–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25267000 doi: 10.1016/j.ejso.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 32.Chen CC-H, Chen C-N, Lai I-R, Huang G-H, Saczynski JS, Inouye SK. Effects of a modified Hospital Elder Life Program on frailty in individuals undergoing major elective abdominal surgery. J Am Geriatr Soc [Internet]. 2014. February;62(2):261–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24437990 doi: 10.1111/jgs.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker FC, Persoon A, Bredie SJH, van Haren-Willems J, Leferink VJ, Noyez L, et al. The CareWell in Hospital program to improve the quality of care for frail elderly inpatients: results of a before-after study with focus on surgical patients. Am J Surg [Internet]. 2014. November;208(5):735–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25085385 doi: 10.1016/j.amjsurg.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 34.Hospital Elder Life Program [Internet]. [cited 2016 Dec 13]. Available from: www.hospitalelderlifeprogram,org
- 35.Hempenius L, Slaets JPJ, Van Asselt D, De Bock TH, Wiggers T, Van Leeuwen BL. Long term outcomes of a geriatric liaison intervention in frail elderly cancer patients. PLoS One. 2016;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregersen M, Borris LC, Damsgaard EM. Blood transfusion and overall quality of life after hip fracture in frail elderly patients—the transfusion requirements in frail elderly randomized controlled trial. J Am Med Dir Assoc [Internet]. 2015. September 1;16(9):762–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25933728 doi: 10.1016/j.jamda.2015.03.022 [DOI] [PubMed] [Google Scholar]
- 37.Gregersen M, Damsgaard EM, Borris LC. Blood transfusion and risk of infection in frail elderly after hip fracture surgery: the TRIFE randomized controlled trial. Eur J Orthop Surg Traumatol [Internet]. 2015. August;25(6):1031–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25690514 doi: 10.1007/s00590-015-1609-2 [DOI] [PubMed] [Google Scholar]
- 38.Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthop [Internet]. 2015. June;86(3):363–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25586270 doi: 10.3109/17453674.2015.1006980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel K V., Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res. 2014;472:1010–7. doi: 10.1007/s11999-013-3334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kistler EA, Nicholas JA, Kates SL, Friedman SM. Frailty and Short-Term Outcomes in Patients With Hip Fracture. Geriatr Orthop Surg Rehabil [Internet]. 2015. September;6(3):209–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26328238 doi: 10.1177/2151458515591170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age Ageing [Internet]. 2007. March [cited 2014 Nov 17];36(2):190–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17259638 doi: 10.1093/ageing/afl163 [DOI] [PubMed] [Google Scholar]
- 42.Fried LP, Ferruci L, Darer J, Williamson J, Anderson G. Untangling the concepts of disability, frailty and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):M255–63. [DOI] [PubMed] [Google Scholar]
- 43.Prestmo A, Hagen G, Sletvold O, Helbostad JL, Thingstad P, Taraldsen K, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet [Internet]. 2015;385(9978):1623–1633. Available from: http://www.sciencedirect.com/science/article/pii/S0140673614624090 doi: 10.1016/S0140-6736(14)62409-0 [DOI] [PubMed] [Google Scholar]
- 44.Grigoryan K V, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma [Internet]. 2014. March [cited 2014 Dec 2];28(3):e49–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23912859 doi: 10.1097/BOT.0b013e3182a5a045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rockwood K, Theou O, Mitnitski A. What are frailty instruments for? Age Ageing. 2015;1–3. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol Med Sci. 2001;56(3):146–57. [DOI] [PubMed] [Google Scholar]
- 47.Chow WB, Rosenthal R a, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg [Internet]. 2012. October [cited 2013 Mar 12];215(4):453–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22917646 doi: 10.1016/j.jamcollsurg.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 48.Cooper Z, Rogers SO, Ngo L, Guess J, Schmitt E, Jones RN, et al. Comparison of Frailty Measures as Predictors of Outcomes After Orthopedic Surgery. J Am Geriatr Soc [Internet]. 2016; Available from: http://doi.wiley.com/10.1111/jgs.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prinsen CAC, Vohra S, Rose MR, King-Jones S, Ishaque S, Bhaloo Z, et al. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a “core outcome set”. Trials [Internet]. 2014;15:247 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4082295&tool=pmcentrez&rendertype=abstract doi: 10.1186/1745-6215-15-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Table A- Search strategies for included databases; Table B—Conference abstracts and study protocols identified; Table C—Description of exercise interventions and control conditions; Table D- Description of multicomponent geriatric care protocols and control conditions.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.