Abstract
Subsocial wood feeding cockroaches in the genus Cryptocercus, the sister group of termites, retain their symbiotic gut flagellates during the host molting cycle, but in lower termites, closely related flagellates die prior to host ecdysis. Although the prevalent view is that termite flagellates die because of conditions of starvation and desiccation in the gut during the host molting cycle, the work of L.R. Cleveland in the 1930s through the 1960s provides a strong alternate hypothesis: it was the changed hormonal environment associated with the origin of eusociality and its concomitant shift in termite developmental ontogeny that instigates the death of the flagellates in termites. Although the research on termite gut microbial communities has exploded since the advent of modern molecular techniques, the role of the host hormonal environment on the life cycle of its gut flagellates has been neglected. Here Cleveland’s studies are revisited to provide a basis for re-examination of the problem, and the results framed in the context of two alternate hypotheses: the flagellate symbionts are victims of the change in host social status, or the flagellates have become incorporated into the life cycle of the eusocial termite colony. Recent work on parasitic protists suggests clear paths for exploring these hypotheses and for resolving long standing issues regarding sexual-encystment cycles in flagellates of the Cryptocercus-termite lineage using molecular methodologies, bringing the problem into the modern era.
Keywords: symbiosis, microbiome, caste determination, polyphenism, molt, eusociality, interdependency, cyst, hormones
‘One in general does not kill the goose that laid the golden egg’
[1]
1. Introduction
Overwhelming evidence is now available from morphological characters and genomic analyses of both hosts and symbionts that termites are a clade nested within the cockroaches, with the wood-feeding, subsocial cockroach Cryptocercus as their undisputed sister group [2,3,4,5]. A prominent feature uniting Cryptocercus with the termites is a symbiotic relationship with single-celled anaerobic eukaryotes living in the enlarged hindgut paunch of the insects. These gut protists fall into two main groups traditionally known as flagellates—the Phylum Parabasalia and the Order Oxymonadida (Phylum Preaxostyla) [6], with the former historically divided into the trichomonads and hypermastigotes. Nearly 450 distinct flagellate species have been described in the termite and Cryptocercus hosts that have been investigated (see [7,8] for a catalogue of those reported). The morphological characters historically used to classify species, however, are proving in many cases to be inadequate, and new species and relationships are continually being described based on molecular sequencing techniques, e.g., [9,10,11,12,13].
The flagellates of the two host insect groups have similar taxonomic affiliations, and their distributions are either limited, or they cannot be found in other hosts or in the free-living state [2,8,14]. It has been long thought [15,16] and is now strongly established that divergence of the flagellates took place in a common ancestor of Cryptocercus and termites, currently estimated as occurring ~170 mya [17]. Intergenerational transmission is vertical [18,19]: they are transferred from parents to offspring via proctodeal (anal) trophallaxis (the hatchlings feed on parental hindgut fluids) in both Cryptocercus and termites; in the latter during the incipient, subsocial stage. In both taxa, the parents are the source of all flagellates for a given social group. In termites, however, the flagellates are re-circulated among colony members via trophallaxis once the colony becomes established. As expected in vertically transmitted symbionts, flagellate communities of different host lineages indicate co-speciation, with possible horizontal shifts due to stochastic, dietary or ecological effects [20,21,22,23].
Functionally, the gut microbiota partner with the host and each other to interactively degrade plant polymers, fix atmospheric nitrogen, synthesize amino acids, and recycle nitrogenous waste products [24,25,26]. Despite the dominance of the single celled eukaryotes, the Cryptocercus/termite hindgut system is clearly a multiple symbiosis that involves flagellates, bacteria, and archaea [27]; all organisms involved are symbionts as well as mutualists [8]. The vast majority of prokaryotes in the gut (up to 85%) are associated with the protists [28,29] and exhibit a high level of integration with their flagellate hosts: bacteria or spirochaetes are attached to the cell surface, or located in the cytoplasm or nucleoplasm. Further prokaryotes live free in the gut fluid or are attached to the gut wall of the insect host [27,30,31,32,33,34,35,36,37,38,39]. Clearly the Cryptocercus/lower termite gut microbiome is an interdependent, multifactorial, synergistic system whose subtle dynamics cannot be analyzed as simple binary interactions. That being said, the focus here is on the flagellates, which not only drive cellulose degradation [40,41], but also are more exacting in their requirements than prokaryotes [42].
Recently, significant advances have been made in the understanding of these host-symbiont relationships, prompted by the application of new, sophisticated technologies that are independent of microbial culturing. Well over 3000 primary articles and 150 reviews that address termite symbioses were published in the period 2000–2014, inclusive [8]. Emphasis has been primarily on identification of the components of the microbiota in a phylogenetic context, and of individual biochemical functionality and interdependence. Our increased understanding of the sophistication of the microbiome, however, is not matched by an understanding of how it is integrated into the biology, behavior and life history of the insect host.
The environment in which the flagellates live is unusually homeostatic and closely comparable from host to host [43]. The protists are housed in the gut of an individual insect, living within a family (Cryptocercus) or colony (termites), which is lodged within the buffered environment of a nest. They are supplied with a steady stream of food, in a liquid, temperature-controlled, safe haven. Their world, then, is fairly constant, with one notable exception: the molting cycle of their insect host.
If the relationship of these two insect taxa to their gut fauna during developmental ontogeny is compared, it is obvious that there has been an evolutionary change during the host molting cycle. In both Cryptocercus and lower termites, neonates acquire the flagellates from a parent or a sibling (the latter in established colonies of termites); the symbiosis is established at about the third instar in both taxa. The big difference lies in what subsequently occurs during the molting cycles of the developing juvenile host. After the symbiosis is established in Cryptocercus, it is retained through all subsequent ecdyses; it is never lost under natural conditions [15,44,45]. Developing nymphs of this cockroach are thereafter nutritionally autonomous and capable of a solitary lifestyle. In termites, however, the large flagellates die prior to subsequent ecdyses and must be re-acquired by feeding on the hindgut fluids of a nest mate. Unlike most vertically transmitted symbioses [46] then, developing termites have an aposymbiotic phase with respect to the larger gut flagellates. If cockroaches in the genus Cryptocercus are used as a model of the termite ancestral state, that shift in the host-symbiont relationship can be pinned to a specific node of the Dictyopteran phylogenetic tree. This paper is an attempt to explore the foundations of that evolutionary transition.
2. Why Is Death of Protists in Termites of Significance?
Among eusocial insects, the symbiosis with these gut flagellates is a feature unique to the Cryptocercus-termite lineage. Historically, the death of these flagellates at molt was thought to be a key factor associated with termite eusocial origins, e.g., [47,48] but more recent work habitually excludes its influence on social structure. Any hypothesis that ignores this termite specific life history characteristic, however, is unsatisfactory and serves to emphasize the current deep-seated bias in analyzing Isopteran eusocial origins in terms of hymenopteran attributes [49,50]. A re-examination of this symbiotic relationship during host molt is overdue for two compelling reasons: first, because the need to re-acquire the symbiosis after molt precludes independent living by termite individuals, and second, because current literature rarely describes the symbiotic relationship during the host molting cycle in either insect taxon accurately.
2.1. Interdependence
In both Cryptocercus and lower termites there are two levels of dependence. First, individuals rely on gut flagellates to metabolize and supplement their wood diet; and second, neonates in both taxa rely on their parents (in incipient colonies of termites) for vertical transfer of the symbionts. In termites alone, there is a third level of dependence: because the flagellates die at molt, developing termite individuals do not survive unless they have access to the hindgut fluids of a relative [51]. The dependence on gut flagellates to help metabolize their wood diet, combined with the periodic death of these symbionts precludes independent living in termites. If the host-symbiont relationship in Cryptocercus is used as a model of the termite ancestral state, previously independent insects evolved interdependency. Up to the threshold when the protistans began dying during the termite molting cycle, eusociality in termites may have been reversible, but in interdependent organisms the fitness of one depends on the fitness of another, making cooperation the best strategy [52]. The death of protists at molt was a ‘tipping point’ [51], in that beyond that threshold, intrinsic processes in the system drove accelerating change [53].
2.2. Misconceptions
How the gut symbiosis is established in neonates of Cryptocercus and lower termites, and how the symbiotic bond between the host and its gut flagellates is either maintained, or broken and subsequently re-established during the host molting period are consistently misunderstood in current literature [51]. Prior to the 1980s, the confusion was understandable because Cleveland et al. (Figure 1) [15], the early authorities on these flagellates, did not understand the social structure and behavior of Cryptocercus; rather, these authors assumed two things about the natural history of the cockroach based on their observations of gut flagellates during Cryptocercus molting cycles. The first assumption was that neonates of Cryptocercus acquired their gut symbionts by feeding on the fecal pellets of recently molted older siblings, because these pellets contained resistant stages of the gut protists. The second assumption was that, because adults do not molt (and therefore their feces would not contain resistant stages of the symbionts), an adult pair of these cockroaches could not found a colony independently. These authors speculated that new colonies were formed by a ‘combined creeping migration’ of adults and older nymphs to a new log. Cleveland et al. ([15] p. 209) did not rule out that trophallaxis may play a role in establishing the gut microbiota in neonate Cryptocercus, but they did not observe the behavior during their studies.
Figure 1.
Lemuel Roscoe Cleveland (1892–1969). Smithsonian Institution Archives, Image #SIA2008-0153. The Smithsonian has granted permission for use.
Nutting [44] was the first to question the described mode of symbiont transfer in Cryptocercus. A subsequent study was done on adults collected in the field as mated pairs [54], suggesting that adults may be able to found colonies independently. In 1983, field work by Seelinger and Seelinger [55] demonstrated that molting older siblings were not present in the nest when Cryptocercus egg cases hatch; the typical family consists of a pair of adults with a single cohort of offspring. This social structure, together with the observation of parent to offspring vertical transmission of gut symbionts via proctodeal trophallaxis, was subsequently supported by Nalepa [56] and Park et al. [57]. The subsocial, semelparous life history of Cryptocercus, including parent to offspring vertical transmission of gut symbionts, is now well established, and it is generally accepted that extended parental care in an ancestral cockroach modeled after Cryptocercus was the key basal condition in the pre-eusocial termite ancestor [8]. Misconceptions regarding the host-symbiont relationship in this lineage nevertheless continue to cloud interpretations of its evolutionary trajectory, e.g., [58,59,60].
3. Molting in Cryptocercus
The gut microbiota of insects have a unique challenge not faced by those in vertebrate taxa. Because the insect hindgut lining is ectoderm, it is shed along with the rest of the exoskeleton during the molting period throughout host development. Consequently, insect gut microbiota may require measures to cope with the conditions of the digestive tract during this time. As Cryptocercus enters its molting period, rising titers of the molting hormone ecdysone trigger the sexual/encystment (SE) cycles (addressed below) of the flagellates in the gut. During the SE cycle, the protistans become more resistant by producing cysts, pseudocysts, or by employing other mechanisms. The lining of the cockroach hindgut is then shed whole, but retained within the host body. The resistant flagellates are then held within the old gut lining (intima) until formation of the new lining is completed. The old intima ruptures, and the protists escape into and repopulate the gut lumen. The old hindgut lining subsequently breaks into small pieces, and is voided in fecal pellets along with some resistant protists [15,61]. Although these protists may pass with host feces shortly before or after molting (in some hosts half or more, in others just a few), they play no role in intergenerational transmission under natural conditions.
Prior to the work on social structure of Cryptocercus in the 1980s, it was thought that SE cycles in flagellates of Cryptocercus were rooted in a fecal-oral mechanism of transmission between generations. The cysts were considered mandatory to withstand the stress of being subjected to the environment outside the insect host, i.e., in fecal pellets lying on the floor of the nest. We now know that flagellates are transferred to neonates via ingestion of the trophozoite stage in the hindgut fluids of parents. After their hindgut microbiota have become established at about the third instar, trophallactic behavior in young nymphs of Cryptocercus ceases. The fact that some cysts may be found in the fecal pellets of post-molt juveniles of Cryptocercus, however, suggests that contemporaneous members of the family may ingest some of these; trophozoites that subsequently emerge from these cysts are probably re-integrated into the existing gut microbiota of the coprophage. Feces are a part of the diet in most cockroaches, including Cryptocercus [18,62]. However, because all flagellates in a given family originate with the parents, no new flagellate taxa would enter the social group via coprophagy.
4. Molting in Termites
The most common error regarding the disappearance of the protistans in molting termites is that the hindgut lining is excreted whole with the protists still in it; prevalent fallacies regarding how the symbiosis is re-established after molt is that it is restored by coprophagy or by feeding on an intact, shed hindgut lining. With a few possible exceptions (discussed below), however, the flagellates are dead and gone from the hindgut prior to the molt of their termite host [44,63,64,65,66,67]. The symbionts are not ‘shed’, ‘discarded’ or ‘cast’ with the hindgut lining. The gut is devoid of flagellates seven days before molt in Kalotermes flavicollis [68] and six days before molt in Coptotermes formosanus [69]. The physical process of molt in termites is the same as in Cryptocercus. The lining of the hindgut is shed but retained within the body for two or three days; it later breaks up and is voided. Consequently, the gut protists cannot be re-acquired by a post-molt termite via feeding on its own shed exuvium, that of a nestmate, or by feeding on feces. The hindgut lining is not attached to the remainder of the shed exuvia [70], and termite feces never contain active flagellates, cysts, or other resistant stages [15,43,47,61,64,71]. The sole mechanism of refaunation in a newly molted termite is via interaction with a conspecific: repeated proctodeal trophallaxis with intermolt donor nestmates [51]. If Cryptocercus is used as a model of the termite ancestor, the host-symbiont relationship during molt changed from retention by the individual host via the behavioral and physiological responses of the flagellates (SE cycles), to retention by the colony via host behavioral interaction with other colony members [18].
One explanation offered for the death of termite flagellates during host molt was that because termites exhibit colony-wide proctodeal trophallaxis, the SE cycle in their flagellates was superfluous and expendable, as the flagellates were never exposed to the outside environment [16]; this explanation is still popular [72,73]. It is the premise of this paper that the directionality of cause-and-effect of this common assumption should be reversed. Instead of colony-wide trophallaxis leading to the death of flagellates at host molt, the early stages of eusociality led to dysfunctional SE cycles in the flagellates and required a behavioral solution on the part of the host: colony-wide trophallaxis [18,51].
5. Why Is the Partnership Dissolved at Every Molt in Termites?
It is commonly assumed that abiotic factors in the gut environment are responsible for killing termite protistans during molt, and that encystment by the flagellates in Cryptocercus protect them during this period. The flagellates are known to be extremely sensitive to changing conditions of temperature, food, and water [74]; indeed, subjecting Cryptocercus and termites to heat, starvation or oxygen is a standard technique for rendering them aposymbiotic for experimental purposes, e.g., [75,76]. The assumption that such conditions kill termite flagellates during molt is therefore understandable, since these symbionts pass through a gauntlet of harsh conditions each time the insect undergoes a molting cycle. The host ceases to feed, and the gut fluid increases in viscosity to a heavy paste [61,77]. Large bubbles also appear in the hindgut ([15] Plate 7), suggesting potential vulnerability to oxygen poisoning. In free living and parasitic protists, stimuli such as these are often the first triggers of a cell differentiation process that leads to encystment, thus enabling resistance to a deteriorating environment [78,79,80]. Starvation, desiccation or oxygen, however, play no role in initiating the SE cycles of the flagellates in Cryptocercus [44,81]. The cycle is triggered by rising titers of host ecdysone long before there is a change in the feeding habits of the host or a change in viscosity of the gut fluid [81,82]. Furthermore, SE cycles can be induced with ecdysone in the flagellates of intermolt Cryptocercus nymphs as well as in adults, both of which have normal, wood-and fluid-filled guts [45]. The relevant question, then, is why does a rising titer of the molting hormone ecdysone trigger a differentiation process in the flagellates of Cryptocercus, but lead to disintegration of the flagellates in termites? To address this question, the host-symbiont relationship at molt displayed by Cryptocercus will be examined in detail.
6. Flagellate Differentiation during the Cryptocercus Molting Cycle
Protists in Cryptocercus exhibit two morphologically distinct stages in the life cycle. Intermolt Cryptocercus typically harbor the trophozoite stage, which reproduces by mitosis. Flagellates change from asexual to sexual methods of reproduction (i.e., initiate an SE cycle) at the time their host enters its molting period. The molting period of Cryptocercus begins with the first appearance of ecdysone in the insect and ends with the disappearance of this hormone. Ecdysone can begin rising as much as 50 days prior to ecdysis, and persists until three days after [81,83,84]. Ecdysis is defined as the behavioral act of shedding the exoskeleton. Note that for simplicity, I am here calling the differentiation events in flagellates during host molt a sexual-encystment (SE) cycle, regardless of the genetic consequences of the cycle and the mechanism of resistance. There is extraordinary variation in the sexual cycle of the flagellates during the Cryptocercus molting cycle; likewise, the morphological indications of flagellate resistance vary, both in degree and in the timing of appearance.
6.1. Cryptocercus: Variation in Flagellate Sexual Cycles
Cleveland [61] thought that the flagellates in Cryptocercus exhibited a variety of sexual forms as extensive as all other protistan groups known at his time. In general, his accounts of the SE cycles in the flagellates indicate little opportunity for genetic recombination. There are reports of remarkable abbreviations of, and variations on, standard sexual processes that are difficult to interpret (summarized in [85,86,87]). In Pyrsonympha, for example, meiosis-like reductional divisions can be considered either degenerated meiosis or as precursors of conventional meiosis [88]. Haploid lineages undergo postzygotic meiosis, some with one, others with two divisions [89]. Diploid flagellates show pre-gametic meiosis requiring two divisions in some species and just one in others. Autogamy occurs in a few species. One-divisional meiosis probably exists only in flagellates from Cryptocercus (reviewed by Raikov [90]). Even within a given flagellate species, SE cycles do not always follow a uniform pattern. Leptospironympha eupora, for example, may encyst either before or after gametogenesis; the usual pattern being encystment following fertilization [91].
Although meiosis is highly conserved in eukaryotes, deviations from the norm are ubiquitous and the evolutionary significance of these deviations is largely unknown. In many protists meiosis is not tightly associated with reproduction [88,92,93], and may have a functional basis in DNA restoration rather than in recombination, i.e., elimination of deleterious mutations, direct repair of DNA breaks, or removal of oxidative DNA damage [94]. Other apparent advantages include recombination fidelity, ploidy reduction and epigenetic resetting (refs. in [95]).
6.2. Cryptocercus: Variation in Flagellate Mechanism of Resistance
The flagellates in Cryptocercus exhibit the entire spectrum of the morphological indications of resistance during host molt, ranging from those that produce a thick, double-walled cyst to those that undergo a differentiation cycle during host molt but display no physical evidence of a resistant outer wall. Individuals of more than one species can skip an SE cycle altogether but are nonetheless retained during host molt.
True cysts are defined as having a thick hyaline wall formed from the outer layer of the cytoplasm, with an empty space between the inner margin of the cyst wall and the living cytoplasm [96]. Examples of flagellates that clearly form cysts include Trichonympha and Macrospironympha; Cleveland [82] considered the latter as the best flagellate for studying encystment. The definition of a pseudocyst is more nebulous. A general definition is that it is a compact, resistant, resting form, where the cell rounds up without forming a cyst wall [97]. Cleveland, however, struggled with defining this mechanism of resistance. In Barbulanympha, for example, he described the organism as losing water, taking on a hyaline appearance, discarding its flagella, becoming circular and immobile, and becoming capable of withstanding conditions outside the host body. He considered it a ‘clear cut case of protozoa becoming resistant without formation of an external, protective cyst membrane or wall’ but refrained from naming the condition [15]. He later, however, indicated that Barbulanympha forms a pseudocyst [98]. Rhynchonympha was described as undergoing ‘partial or pseudoencystation’, but it was also noted as ‘sometimes’ forming a cyst [96,99]. Variation within a genus was noted: Oxymonas nana forms a cyst, but O. dorsalis forms a pseudocyst [89]. Leptospironympha typically forms a cyst, but there is no encystment or pseudoencystment in L. wachula [91,96]. As in L. wachula, Eucomonympha, Notila and Saccinobaculus apparently undergo sexual cycles with neither encystment nor pseudoencystment [96,100,101].
Finally, Cleveland described instances of flagellates that do not undergo an SE cycle but are nonetheless retained by Cryptocercus during molt. Although in most flagellates all individuals undergo an SE cycle when their host molts [61], a small percentage of those in the genera Trichonympha, Leptospironympha and possibly others do not. These individuals are actively motile, free of wood particles, and location specific: they are always found in the very anterior part of the hindgut [15,61,91]. The number of these individuals that do not undergo an SE cycle is always sufficient to repopulate the gut after molt. Consequently, Cleveland et al. ([15] p. 262) concluded that the function of flagellate encystment was to produce resistant organisms capable of withstanding conditions external to the host, so that they may be transmitted in feces to neonates. Since we now know that these flagellates are vertically transmitted between generations via proctodeal trophallaxis, another possibility suggests itself: cysts in flagellates of Cryptocercus are currently of no functional consequence, but are vestiges from a distant gregarious ancestor when externally excreted cysts functioned in horizontal transmission [85]. Fewer than half of the flagellates studied by Cleveland exhibit the thick outer wall associated with a true cyst ([83] Table 2), and the morphological variation in resistance exhibited by the flagellates in this host may represent stages in dispensing with the costly encystment process. Encystment in the infectious vertebrate parasite Giardia lamblia, for example, involves pulsed production, processing and secretion of cyst wall material, and neogenesis of encystment specific vesicles [80,102], indicating substantial metabolic and material investment.
7. Link between Social Behavior and Symbiont Transmission
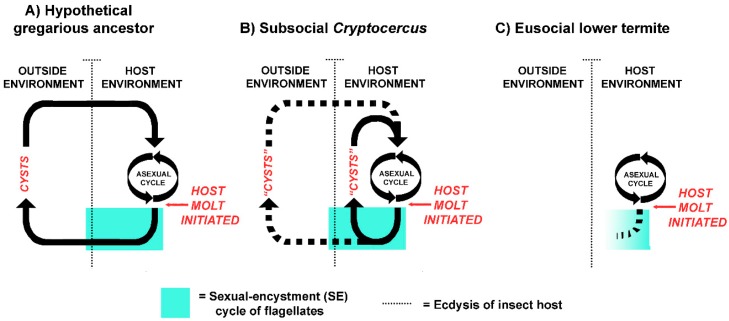
The host-symbiont patterns currently seen in Cryptocercus and extant lower termites suggests a logical evolutionary sequence (Figure 2). In a distant gregarious cockroach ancestor, the host-symbiont relationship was not obligate, and the flagellates likely had two distinct differentiated states: the vegetative state inside of the insect host, and the encysted state in fecal pellets in the outside environment. Encystment was a crucial part of the life cycle, as it was the mechanism of horizontal transmission via the fecal-oral route (coprophagy) [18]. As in well-studied parasitic protists from diverse lineages, meiosis and resistance to the outside environment were linked to dispersal from the host [103]; it allowed the flagellates to withstand external conditions if new hosts were not immediately available. Cysts within fecal pellets served as an ‘infection bank’, much like the gregarines that parasitize extant gregarious cockroaches [104,105]. Aggregation behavior of the distant cockroach ancestor served as a mechanism that concentrated both the cysts and the hosts in a limited space.
Figure 2.
Sexual-encystment cycles of the flagellates in molting individuals of: (A) a hypothetical gregarious cockroach ancestor, (B) the subsocial cockroach Cryptocercus, and (C) eusocial lower termites. The symbiont-host relationship logically evolved in sequence, along with shifts in host social behavior, from a distant gregarious cockroach ancestor, to a subsocial Cryptocercus-like ancestor, to eusocial termites [18]. In both Cryptocercus and termites, the SE cycle has degenerated from the strong encystment pattern likely shown by an ancestor, but in both taxa, protistan numbers and functions are reliably restored after molt, albeit in different ways (retention of protists in individuals of Cryptocercus, anal trophallaxis from nestmates in termites). Neither Cryptocercus nor termite protists currently have a functional relationship with the external environment. Inspired by Weedall and Hall ([103] Figure 1).
With Cryptocercus as model, proctodeal trophallaxis evolved from this pre-existing intraspecific coprophagous behavior when termite ancestors became semelparous and subsocial, because the physiology of encystment in the flagellates precludes their transfer via cysts in adult feces: adults don’t molt, and older developmental stages are not present in the social group. This was a key point in their evolutionary history, as it assured vertical intergenerational transmission of all pro- and eukaryotic members of the gut microbiome, led to growing interdependence of the host and symbionts, and established the behavioral basis of trophallactic exchanges [18,51]. Parent to offspring proctodeal trophallaxis in both Cryptocercus and termites during colony initiation is currently a mechanism of both inoculating neonates with the symbionts and of supplying them with nourishment until their microbiome becomes established. Table 1 summarizes the similarities and differences in the host-symbiont relationship in Cryptocercus and lower termites.
Table 1.
Comparison of the host-flagellate relationship in a hypothetical cockroach ancestor, the wood-feeding cockroach Cryptocercus, and the lower termites. Characteristics of Cryptocercus are assumed to be the state immediately ancestral to lower termites. Key differences between Cryptocercus and lower termites are in red.
| Hypothetical Gregarious Ancestor | Subsocial Cryptocercus | Eusocial Lower Termites | |
|---|---|---|---|
| Depends on flagellates to metabolize a cellulose-based diet | no | yes | yes |
| Parent to offspring vertical transmission of flagellates via trophallaxis | no | yes | yes a |
| Flagellates die prior to molt of juvenile host | no | no | yes |
| Juveniles capable of solitary living | yes | yes b | no |
| Post-molt transmission among family members via trophallaxis | no | no | yes |
| Transmission of flagellates via cysts in feces | yes | no c | no |
| Transmission of flagellates via shed hindgut linings d | no | no | no |
a During the subsocial stage of colony foundation. b After third instar. c Occasional horizontal transmission among siblings after initial vertical transmission of symbiosis is possible. d Either their own or that of a nest mate.
8. What Changed?
If Cryptocercus represents the immediate ancestral condition of termites, how was the transition in the host-symbiont relationship made? Based on his flagellate research, Cleveland’s [61,82] interpretation was that somewhere in the evolution of termites the molting process changed so that the flagellates were killed each time its host molted, instead of switching from asexual to sexual reproduction as they do in Cryptocercus. The protistan SE cycle is initiated and synchronized by host ecdysone; this phase of the flagellate life cycle is therefore indirectly dependent on the neurosecretory cells of the host [106]. It is reasonable to assume, then, that a change in host endocrinology at molt had consequences for its symbionts; a change in the developmental trajectory of an insect is invariably rooted in a change in its underlying hormonal physiology.
The origin of termite eusociality is primarily a developmental phenomenon [49,107,108,109]. Cryptocercus, like other cockroaches, has a single more or less fixed developmental track: a linear, progressive hemimetabolous development to the adult stage, within a specified, albeit flexible, time frame; size, maturity and chronological age are roughly correlated. Termites deviate from this developmental blueprint in two general ways. First, the vast majority of individuals in lower termite colonies are workers (also called alloparents, helpers), which are functionally sterile, developmentally plastic, immature stages stalled in a small bodied, altricial morphotype ([110] Figure 2); they can molt repeatedly without growth or differentiation [111]. Worker chronological age is disassociated from maturity via progressive, stationary, saltatory, and reversionary molts. As such, these workers exhibit a strong temporal deviation from standard hemimetabolous development at the level of the individual.
Second, workers in lower termites retain the capacity to differentiate into either soldier or reproductive caste phenotypes, in essence shifting from a hierarchical model of ontogeny (egg—embryo–juvenile–adult) to one with alternate pathways. These alternate developmental tracks are rooted in the independent ontogeny of different organs relative to each other [112]. Termite developmental evolution therefore entailed not only a drastic temporal change in the ontogeny of the whole insect, but also changes in the relative developmental timing of different organs within an individual. For example, soldier-specific cuticle formation and the exaggerated growth and cell death associated with forming the morphologically specialized head and mouthparts of this caste occurs during the molting period and inevitably involves ecdysone [113,114,115]. The timing, amplitude and duration of ecdysone pulses are key in initiating and controlling such developmental transitions by activating signaling cascades in target tissues [116]. Most work on the endocrinology of caste determination in termites to date has focused on the role of juvenile hormone (JH), e.g., [111,117,118,119,120,121], but ecdysone and JH act in concert as key endocrine timers in precisely controlling the onset of developmental transitions and in harmonizing gene expression profiles [116,122,123]. The recognition of cross-talk between these two classes of hormones may be key in determining the endocrine basis of termite caste determination [115,124,125] particularly as these hormones are thought to be overlooked players in the epigenetic control of gene expression [126].
In sum, there was extensive remodeling of the nature and timing of endocrine signaling pathways associated with host molt when the subsocial cockroach ancestor became eusocial. Because the ultrastructure, metabolism and gene expression of the flagellates was deeply integrated into the ontogenetic rhythm of their subsocial cockroach host, it is reasonable to assume that, as suggested by Cleveland, a drastic change in host developmental ontogeny had fatal consequences for the flagellates. Support for this suggestion comes from Cleveland’s work on the flagellates over the entirety of his career.
9. Titer of Ecdysone and Timing of Events during the SE Cycle
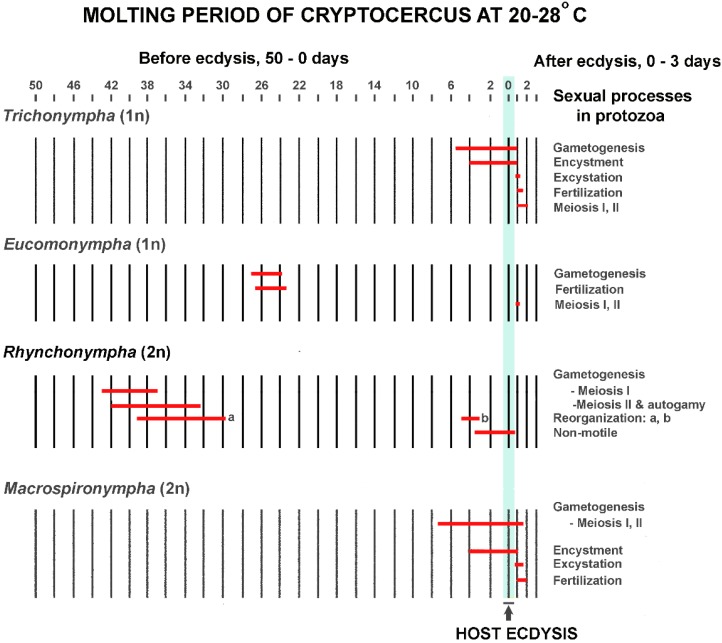
In Cryptocercus, the timing of host hormonal ebb and flow for the flagellate SE cycle is critical to the outcome, just as it is for termite caste determination. From the beginning of the molting period until several hours after ecdysis, the host is constantly changing the hormonal environment in which the hindgut protists live [81]. Each flagellate species has its own time schedule in the host molting process for initiating its SE cycle; this reflects the different thresholds of susceptibility to host ecdysone levels. For example, under natural conditions Barbulonympha, Saccinobaculus and Oxymonas begin gametogenesis 40–45 days before ecdysis of their host, while Trichonympha begins this phase of its life cycle 5–6 days before ecdysis [127]. Timing is so precise that the stage of the sexual cycle in well-studied flagellates can be used as an indicator of the host molting cycle [81]; it is rare for a given stage of the cycle in one flagellate genus to overlap in time with that of another [82]. Although the timing and details of the flagellate cycles differ (Figure 3) [83,84], the common thread is that in each studied flagellate they can be initiated with an injection of ecdysone. This occurs if an adult Cryptocercus or an intermolt nymph is injected in doses too low to trigger ecdysis in the host [84]. The protists do not have intrinsic factors that initiate the SE cycle [81], and molting cycles of the host and SE cycles in the protistans never occur separately [61].
Figure 3.
Examples of timing of different phases of the sexual/encystment (SE) cycle in relation to the molting period of the host in four genera of flagellates in the gut of Cryptocercus punctulatus. For each genus, the data are summed from several hosts, thus giving the time span during which the processes may occur. In Rhynchonympha, ‘reorganization a’ refers to unsynchronized partial re-organization of the centrioles; ‘reorganization b’ refers to synchronized loss and renewal of all the other extranuclear organelles. After Cleveland ([83] Table 2, with permission from John Wiley and Sons).
Ecdysone acts differently on each flagellate genus not only in terms of triggering the SE cycle, but also in orchestrating its details [96]. In some genera of protists, for example, the ecdysone titer must drop before meiosis can begin [66]. Timing of the various events in relation to each other is crucial; they happen in rapid succession, and a slight variation in timing with respect to other events can be lethal [96]. The discarding and renewal of extranuclear organelles during division is one example of the perfectly coordinated and delicately balanced cell mechanics [100,101]. In those flagellates that form a resistant wall or membrane, encystment is physiologically integrated with the sexual cycle. For instance, in Macrospironympha, all divisions, haploid gametogenesis as well as meiosis, are synchronized during encystation. If these same divisions occur when the cell is not encysted, synchronization is lacking and the division process speeds up [82].
Transfaunation experiments indicate that once the SE cycle has been initiated by host ecdysone, there is no turning back; the flagellates reach a ‘point of no return’ early in the process. Any hormonal change in titer or timing that interferes with the early stages of the SE cycle blocks or alters all downstream events. If there are more than a few hours difference in the molt cycles of a donor and recipient, a transfer of gut contents results in flagellate degeneration and death [81].
Period of Vulnerability
Only those flagellates that have initiated the SE cycle are susceptible to hormonal disruption; the beginning and end of the host molting cycle bracket the period of vulnerability [77,81]. Key to the arguments here is that the SE cycle is initiated before there are any visible morphological changes in the flagellates; transfaunation experiments indicate early metabolic modifications that could not be detected using microscopy techniques available at the time [81]. Cleveland and Nutting [81] concluded that the SE cycle includes a 2–3 day ‘conditioning’ period prior to the first visible indications of the cycle when ecdysone is slowly preparing the flagellates for carrying out the numerous unusual and striking differentiations of chromosomes, nuclei, and cytoplasm that subsequently occur [81,96]. If a flagellate is transferred to a recipient host three or more days before the visible SE cycle is scheduled to begin, it will proceed normally; if transferred later it dies [81]. Flagellate death in these cases is not a gradual process like it is when flagellates starve; they were completely broken down in six hours. The protists were observed in various stages of disintegration, beginning with loss of flagellar motility, then progressing with disintegration of the flagella, parabasals, chromosomes and cytoplasm.
As in other one-celled eukaryotes that encyst, morphological differentiation during SE cycles of Cryptocercus flagellates is likely coordinated at multiple levels, and involves cascades of up- and down-regulated genes associated with metabolic adjustment, nuclear division, and protein synthesis and transport [102,128,129,130,131]. Combining encystment with co-occurring sexual processes compounds the complexity, as meiosis is controlled by hundreds of genes, genes shared with mitosis as well as those that are specifically meiotic [132]. These processes are likely activated during the early ‘conditioning’ period of the flagellates in Cryptocercus, before the SE cycle is visible but when it is already susceptible to host hormonal changes. If so, there is a strong possibility that SE cycles in flagellates may be initiated during juvenile molts of lower termites, but the cycle is aborted with consequent disintegration of the flagellates before there is visible evidence that the cycles were occurring. Cleveland provided evidence that such was the case.
10. The ‘Smoking Gun’
Although as a rule, all the large protists die prior to ecdysis in termites, Cleveland documented that some flagellates undergo sexual or sex-like differentiation prior to their death. The phenomenon is especially well studied in the basal termite Mastotermes darwiniensis, where the cycles were found in the flagellates Deltotrichonympha, Koruga, and Mixotricha (Table 2). The cycles were rare (1–2% in Trichonympha), and always correlated with the termite molting cycle. Although these flagellates were sometimes successful in producing a zygote, it was nonetheless short-lived; the process was always abortive prior to host molt [133]. There is no mention of encystation in Cleveland’s descriptions of these SE cycles, and sexual phenomena could not be documented in the protists of some other termites that were studied during molt, e.g., Zootermopsis [44]. Messer and Lee [134], however, fed ecdysone treated paper to workers of Zootermopsis and observed both sexual stages and cysts in Trichonympha. The evidence suggests, then, that there was not a complete loss of the SE cycle during molt in juvenile stages of termites; there was loss of a functional SE cycle. The underlying machinery is still present and can be activated, even if morphological changes cannot be detected in flagellates examined under a microscope. It is notable that when Cryptocercus donor flagellates are transferred to recipient termites, they initiate an SE cycle during termite molt, but then degenerate, just as termite flagellates in the termite host usually do. The fact that these SE cycles abort, with resulting degeneration of the protists parallels precisely the behavior of the sexual protists after they are transferred to another cockroach host either more or less advanced in the molting cycle [44].
Table 2.
Evidence for sexual/encystment (SE) cycles in the flagellates of termites. In each case except Trichonympha magna in Porotermes adamsoni alates, the cycles abort and the flagellates die prior to molt.
| Flagellate | Termite Host | Reference | Notes |
|---|---|---|---|
| Deltotrichonympha | Mastotermes darwiniensis | [135] | Aspects of SE cycle similar to Barbulanympha and Trichonympha |
| Koruga bonita | Mastotermes darwiniensis | [136] | SE cycle similar to Deltotrichonympha |
| Mixotricha paradoxa | Mastotermes darwiniensis | [137] | Unique SE cycle |
| Trichonympha magna | Porotermes adamsoni | [138] | Studied in 3000 termites; in juvenile molt, all die; imaginal (alate) molt, a few survive. |
| Trichonympha turkestanica | Anacanthotermes ochraecus | [138] | Two instances of fertilization noted |
| Pseudotrichonympha sp. | Prorhinotermes simplex | [139] | SE cycle similar to Trichonympha magna |
| Pseudotrichonympha hertwigi | Coptotermes acinaciformis | [139] | Brief mention |
| Pseudotrichonympha parvipapillosa | Schedorhinotermes intermedius | [139] | Brief mention |
| Pseudotrichonympha-like | Archotermopsis | [133] | Brief mention |
The aborted SE cycles of termites during molt are a ‘smoking gun’ that supports Cleveland’s hypothesis: whatever hormonal mechanisms suppress or alter progressive maturational development of the termites also disrupts the SE cycle of termite protists. Flagellate disappearance prior to molt is not a programmed cell death, nor the result of starvation, dehydration or oxygen levels; it stems from an evolutionary change in the host hormonal regulatory network at molt. Recent support for the hypothesis comes from studies of termite physiology. Raina et al. [69] found that, prior to molt, a significant increase in JH titers coincided with the disappearance of the protists in the gut of Coptotermes formosanus, while ecdysteroids peaked a day later. Sen et al. [140] showed that juvenile hormone was associated with the down-regulation of protist genes in Reticulitermes flavipes, possibly indicating flagellate susceptibility to host hormones.
11. Exceptions to the ‘Rule’ in Termites
There are two exceptions to the ‘rule’ of flagellate loss during molt in termites. The first is that in some (but not all) termites, the smaller and simpler protists, specifically those in the genera Tricercomitus, Hexamastix, and Streblomastix, persist through the molting cycle of their host, and undergo unusual morphological modifications during this time [43,64,65,141,142,143]. These genera were not studied in detail by Cleveland, who concentrated his efforts on the large, cellulose-digesting taxa.
More intriguing from an evolutionary standpoint is that there are several reports that the symbiotic association with large, wood-digesting flagellates is often retained by the termite host during its final molt to the alate (adult, imaginal) stage. The imaginal molt differs from those previous in more than one aspect. It is a molt in which the termites acquire adult structures and reproductive competence, thus entailing a different hormonal regulatory network [44]; physiologically, it may be the only ‘normal’, cockroach-like, hemimetabolous molt experienced by termites [49]. This is also when alates prepare for their dispersal flight from the nest. During the imaginal molt much of the gut contents disappears, along with most of the protists; a representative sample of the whole is, however, retained within the sac-like shed intima and later released into the gut lumen of the imago [43,44,71,74,138,142,144]. Voiding of most of the gut contents prior to termite dispersal is thought to be a measure taken to make flight possible [138]. In Mastotermes darwiniensis, for example, the hindgut paunch at flight it is just 3% of alate wet weight, while it is typically 22% in two-month-old colonies [145].
Cleveland [133] reported sexual cycles in flagellates during this final molt (Trichonympha magna in Porotermes), and others report complex morphological transformations that suggest sexual phenomena [65,71,142]. There are a few reports of cysts or pseudocysts formed during the imaginal molt [65,71], but the consensus seems to be that the evidence for these is slim [72,74].
In one studied termite (Cryptotermes lamanianus), recently eclosed alates were found to be devoid of flagellates and therefore dependent on proctodeal feeding from nest mates to replenish their symbionts prior to flight from the nest [71]. There are also reports that Reticulitermes lucifugus retains its flagellates during the imaginal molt in some geographic locations but not others [65,71]. Currently, it appears that at least some termites retain their flagellate symbionts during their final molting cycle, the flagellates may undergo sexual processes during that period, but they probably do not encyst. How they survive the final molt to the alate stage is unknown, but the answer may lie with the flagellates of Cryptocercus that survive host molt, but for which there is no evidence of encystation.
Two Types of Encystment?
A sidetrack to the main arguments is in order here, because Dolan et al. [146] recently described cysts of the large hypermastigote Staurojoenia assimilis in the gut of the drywood termite Neotermes. These authors sampled ‘more than three dozen termites’, and found purported cysts (2% of cells) in a single termite of unreported developmental status. The cysts were mixed in among normal swimming cells of the same flagellate species. No cell division was observed, and the photographed cyst did not exhibit a space between the cyst wall and the cytoplasm, typical in encysted gut flagellates of Cryptocercus [96] and some other protists [130]. Moreover, it was not determined if the examined termite was in its molting period. These cysts, then, cannot be categorized with the flagellate cysts associated with molt in Cryptocercus, where nearly 100% of a given species in the host synchronously undergo an SE cycle during the host molting period [45,61,81]. It is fully possible, however, that the flagellates associated with the Cryptocercus-termite clade may have more than one type of resistant stage. A protist species can exhibit a variety of cysts: as a regular part of the life cycle (like the reproductive cysts in flagellates of Cryptocercus during molt), and those instigated by a variety of different environmental conditions (protective, or resting cysts) [147,148]. Those reported by Dolan et al. [146] may be an example of the latter. Support for the idea of more than one type of cyst comes from the finding that Cryptocercus punctulatus can be collected from frozen logs during winter in their Appalachian mountain environment [149]. During that time, their guts are a creamy white color (Nalepa, pers. obs.), indicating that they are not feeding on solid wood [150]. It would be of interest then, to determine how the gut flagellates of Cryptocercus are maintained during winter, as protective cysts are a distinct possibility. Cleveland [89] indicated that he observed Oxymonas encysted in the gut of Cryptocercus in winter, during the asexual phase of this flagellate’s life cycle.
12. Hypothesis I. Protists as Victim
The evidence to date suggests two evolutionary hypotheses regarding the death of termite flagellates during the host molting cycle, providing a basis for further studies. The fact that non-functional sexual cycles are still sometimes found in the flagellates of termite juveniles during molt (Table 2) more than 150 million years after their hosts became eusocial supports the idea that the loss of a functional SE cycle may have been imposed on the flagellates by the host.
In general, the partners in a vertically transmitted, obligate symbiosis are not equal. A loss of autonomy on the part of the smaller partner, in this case the flagellates, gives the host full control of the alliance, limiting the evolutionary pathways available to the symbionts. They can neither enter the system from the outside environment, nor re-evolve to free-living status. Eventually the smaller partner progresses to oblivion or to partial incorporation into the host [46,151,152,153]. Evolutionarily, the symbiosis with flagellates in the termite lineage did progress to oblivion: higher termites harbor only bacteria and archaea as gut symbionts [154,155]. Loss of the intestinal flagellates is the single definitive trait separating the higher termites from the lower termites [8], and may be the fourth, final step in the progression depicted in Figure 2. The pattern currently exhibited by flagellates in lower termites may be viewed as the penultimate step in this loss; they exemplify symbionts that lumber along, incompletely adapted to the present environment [151]. Cleveland et al. [84] argued that in flagellates of Cryptocercus, host ecdysone had been ‘captured by the protozoa’ and turned to their own ends. If so, perhaps it backfired on them when the hormonal regulation of host development changed.
13. Hypothesis II. Coordination of Life Cycles
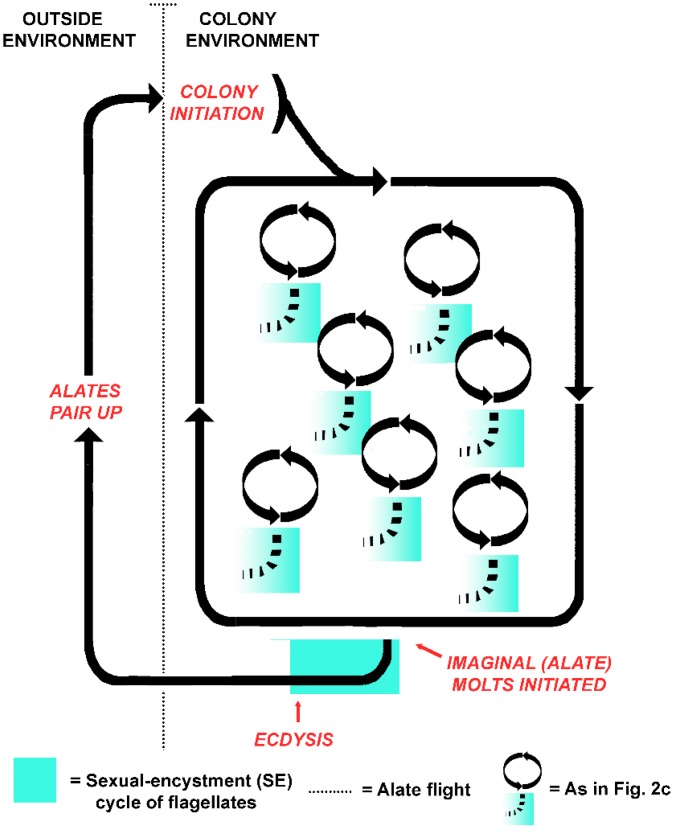
An alternative to the protist as victim hypothesis is that the life cycles of the termite colony and the flagellates became aligned when the host evolved eusociality. Instead of individual host-symbiont fitness, colony-symbiont fitness became coupled, with intermolt termites serving as flagellate reservoirs [51]. An intriguing possibility, then, is that the symbiotic flagellates alternate between sexual and asexual cycles in concert with the colony cycles of their superorganism host (Figure 4). In this model, both the termites and the flagellates are functionally asexual while within the colony, but sexual (in whatever form it takes) when they venture together into the outside environment. Two components of the holobiont became synchronized: the sexual cycles of the gut flagellates, and those of the termite colony, as alates are essentially the gametes of the superorganism. Rather than signaling that the termite flagellates are a victim of the hosts, then, the truncated SE cycles of flagellates in workers in a termite colony exist because the cycles are still functional and of value in termite alates. The crucial time for sexual processes occurs not at individual molt (Figure 2c), but with the founding of new colonies (Figure 4). This accords with the idea that symbiotic associations in social insects are best understood by considering the colony as the host, as the symbionts meet the needs of the colony and not necessarily the individuals that house them [156]. Why would flagellates have retained the mechanism for such an energetically costly process for eons were it merely a dead end?
Figure 4.
Coordination of the life cycle of a termite colony with the sexual/encystment (SE) cycle of the gut flagellates of individual colony members. During typical molts of colony members, the flagellates exhibit aborted SE cycles and die. During the imaginal molt to the alate stage, the flagellates undergo a functional SE cycle in concert with the reproductive maturation of the termites.
14. Future Prospects
Key to distinguishing between these hypotheses will be to determine the behavior of flagellates during the final, imaginal molt of their termite hosts. Survival and sexual activity of the flagellates should be the primary foci, as encystment may not be a requirement to withstand the host molting period. The behavior of the host at this time is of equal interest. Are alates fed hindgut fluids prior to their dispersal flight to assure an inoculum of symbionts? Or do they abstain because they have retained a representative sample of protists during their final molt, and flight weight is critical? Alates in three termite species studied by Heath [157] are reported to forego any form of nourishment from the time of their last molt until they have paired and begin establishing a new nest.
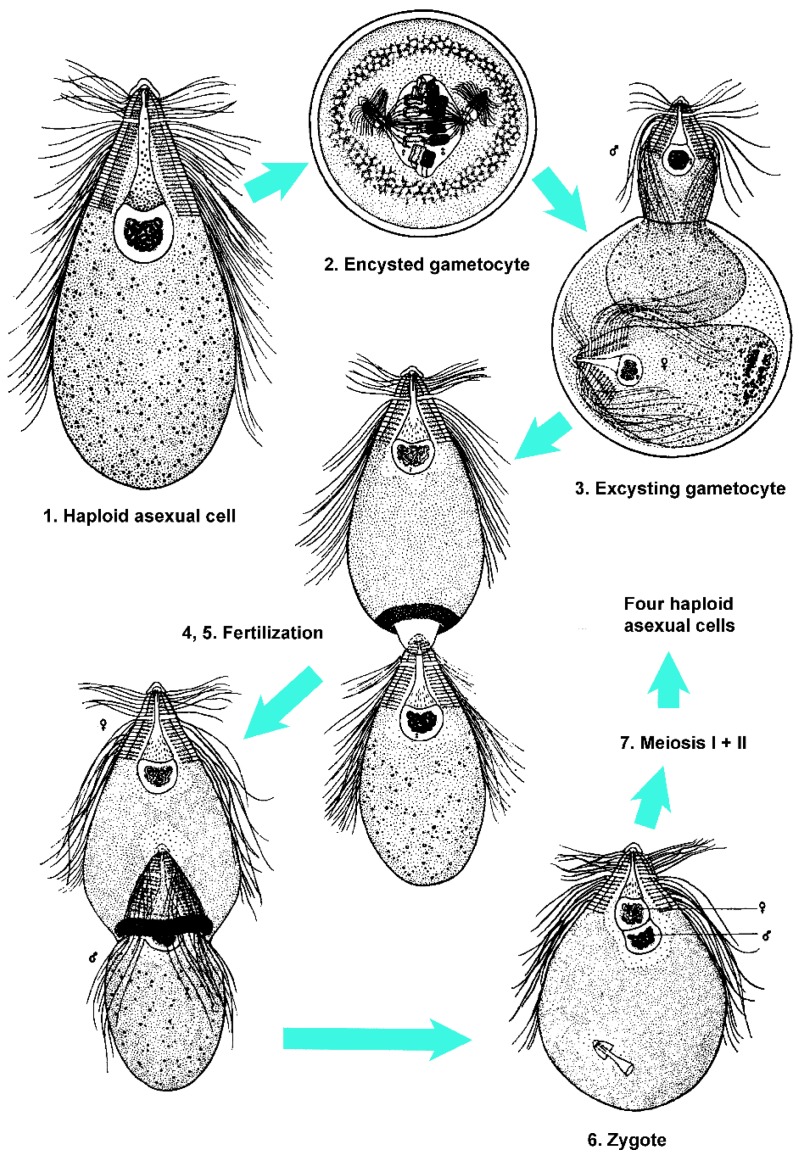
There is plenty of opportunity to address several pivotal aspects of this host-symbiont system. A good foundation lies in linking the regulation of developmental pathways in the host to the fate of the flagellates in its gut, and can be examined at a variety of levels. Cleveland’s (Figure 1) meticulous studies were based primarily on microscopic and film studies of the flagellates, and on transfaunation and hormone injection experiments. He cautioned that it is necessary to make in some cases several thousand slide preparations to find all stages of the SE cycle in a single genus of these flagellates, and made more than 20,000 slides during his studies of those in Cryptocercus [82,127]. To characterize the SE cycle of Trichonympha (Figure 5), he studied two complete sets of fixed and stained preparations at 15-min intervals from the beginning to the end of the sexual cycle (note: the cycle can last almost eight days—Figure 3). He additionally conducted extensive examinations of live Trichonympha using phase contrast, which he considered essential to rule out artifacts in the stained material [61].
Figure 5.
Sexual/encystment (SE) cycle in Trichonympha. After Figure A in Cleveland [167], with permission from John Wiley and Sons.
The action of insect hormones and their precursors are considerably better known since Cleveland’s investigations, and molecular methods now allow a much deeper exploration of unseen underlying processes. Potentially productive directions for exploring the mechanistic basis of flagellate death at molt include characterizing the hormonal differences between young Cryptocercus and lower termite workers during their respective molting cycles; such data would help determine how the precise endocrine cascade to which the flagellates were adapted shifted when their host developed eusociality. Recent studies are beginning to lay the groundwork. Genes related to the synthesis, degradation, sequestration and reception of juvenile hormone have been detected in Cryptocercus punctulatus [158] and a common JH signaling pathway may regulate molting in this cockroach and in the termite Zootermopsis nevadensis [159]. A comparison of the endocrine underpinnings of the imaginal molt in this cockroach and lower termites would help illuminate responses of the gut flagellates during the final molting period.
Instead of relying on visual evaluation of whether a flagellate enters an SE cycle, molecular techniques now make it possible to detect both encystment and sexual processes. Every microbial cryptobiotic state is the direct result of the opening and closing of specific genes [129], and significant advances have been made in the molecular basis of encystation in pathogenic intestinal parasites. The pathway in the flagellate Giardia lamblia, for example, is well characterized, as cysts are a key virulence mechanism allowing it to survive in the environment and infect new hosts [102,160,161,162]. The encystment process is also well studied in ciliates [163,164,165,166].
Although Cleveland detected sexual cycles in flagellates of naturally molting termites, it took extraordinary effort on his part (Table 2). Molecular techniques now make the study of these events more tractable. The list of eukaryotic species that lack direct evidence for meiotic sex but appear sexual as suggested by the presence of meiosis-related genes is growing at a rapid rate [168]. Cryptic sex has been detected in dinoflagellates [169], Giardia [170], amoebae [171], and significantly, the parasite Trichomonas vaginalis [172], which is related to the parabasalids in Cryptocercus and lower termites. The genome of flagellates in Cryptocercus can be inventoried and used to develop a SE cycle ‘detection toolkit’ [173] to determine if these genes are conserved and expressed during molt in related flagellates of termites. This would allow characterization of the SE cycle during the early ‘conditioning’ period, when the process has been initiated, no visible changes are evident in the protists, but they are nonetheless vulnerable to hormonal shifts.
Because of the variation in SE cycles of the flagellates, efforts might best be directed to a single, well-studied flagellate genus such as Trichonympha, which predates the Cryptocercus-termite divergence [174]. There are seven species of Trichonympha in Cryptocercus, and the genus is found in at least 111 lower termite species [15,20,175]. It produces a well-formed cyst during the molting cycle of Cryptocercus, and all species in the cockroach go through precisely the same sexual cycle within the same time frame [15,61] (Figure 5). Moreover, Cleveland found traces of the SE cycle in two Trichonympha species from termites, including instances of T. magna surviving the imaginal molt in Porotermes (Table 2).
Symbiosis has entered a new era, with high throughput sequencing making it possible to analyze the capacities, functions and metabolism of each partner; analysis of the expression of mRNA or proteins further allow comparison of how symbiotic systems react when exposed to different environments [46]. These techniques will be crucial in uniting the functional and evolutionary biology of the Cryptocercus-termite lineage. In 1949, Cleveland [61] wrote that it would be a long time before an accurate evaluation could be made of the role of their hosts in initiating sexual cycles in their protozoa, in the alteration of the cycles once begun, and in protozoan evolution. Nearly 70 years later, it is time to revisit the problem.
Acknowledgments
I thank Pat Rand for advice on illustrations, and Gillian Gile for references. The comments of reviewers considerably improved the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Droop M.R. Algae and invertebrates in symbiosis. Symp. Soc. Gen. Microbiol. 1963;13:171–199. [Google Scholar]
- 2.Lo N., Eggleton P. Termite phylogenetics and co-cladogenesis with symbionts. In: Bignell D., Roisin Y., Lo N., editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht, The Netherlands: 2011. pp. 27–50. [Google Scholar]
- 3.Misof B., Liu S., Meusemann K., Peters R.S., Donath A., Mayer C., Frandsen P.B., Ware J., Flouri T., Beutel R.G., et al. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- 4.Lo N., Bandi C., Watanabe H., Nalepa C., Beninati T. Evidence for cocladogenesis between diverse dictyopteran lineages and their intracellular symbionts. Mol. Biol. Evol. 2003;20:907–913. doi: 10.1093/molbev/msg097. [DOI] [PubMed] [Google Scholar]
- 5.Inward D., Beccaloni G., Eggleton P. Death of an order: A comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol. Lett. 2007;3:331–335. doi: 10.1098/rsbl.2007.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ČEpička I., Dolan M., Gile G. Parabasalia. In: Archibald J.M., Simpson J.M., Slamovits C.H., editors. Handbook of the Protists. Springer; New York, NY, USA: 2016. pp. 1175–1218. [Google Scholar]
- 7.Yamin M.A. Flagellates of the orders Trichomonadida Kirby, Oxymonadida Grassi, and Hypermastigida Grassi and Foa reported from lower termites (Isoptera families Mastotermitidae, Kalotermitidae, Hodotermitidae, Termopsidae, Rhinotermitidae, and Serritermitidae) and from the wood-feeding roach Cryptocercus (Dictyoptera: Cryptocercidae) Sociobiology. 1979;4:1–120. [Google Scholar]
- 8.Bignell D.E. The role of symbionts in the evolution of termites and their rise to ecological dominance in the tropics. In: Hurst C., editor. The Mechanistic Benefits of Microbial Symbionts, Advances in Environmental Microbiology 2. Springer; Zurich, Switzerland: 2016. pp. 121–172. [Google Scholar]
- 9.Keeling P.J. Molecular phylogenetic position of Trichomitopsis termopsidis (Parabasalia) and evidence for the Trichomitopsiinae. Eur. J. Protistol. 2002;38:279–286. doi: 10.1078/0932-4739-00874. [DOI] [Google Scholar]
- 10.Noda S., Mantini C., Meloni D., Inoue J.I., Kitade O., Viscogliosi E., Ohkuma M. Molecular phylogeny and evolution of Parabasalia with improved taxon sampling and new protein markers of actin and elongation factor-1 alpha. PLoS ONE. 2012;7:e29938. doi: 10.1371/journal.pone.0029938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gile G.H., James E.R., Tai V., Harper J.T., Merrell T.L., Boscaro V., Husník F., Scheffrahn R.H., Keeling P.J. New species of Spirotrichonympha from Reticulitermes and the relationships among genera in Spirotrichonymphea (Parabasalia) J. Eukaryot. Microbiol. 2017 doi: 10.1111/jeu.12447. [DOI] [PubMed] [Google Scholar]
- 12.Boscaro V., James E.R., Fiorito R., Hehenberger E., Karnkowska A., del Campo J., Kolisko M., Irwin N.A.T., Mathur V., Scheffrahn R.H., et al. Molecular characterization and phylogeny of four new species of the genus Trichonympha (Parabasalia, Trichonymphea) from lower termite hindguts. Int. J. Syst. Evol. Microbiol. 2017 doi: 10.1099/ijsem.0.002169. [DOI] [PubMed] [Google Scholar]
- 13.Strassert J.F., Desai M.S., Brune A., Radek R. The true diversity of devescovinid flagellates in the termite Incisitermes marginipennis. Protist. 2009;160:522–535. doi: 10.1016/j.protis.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Inoue T., Kitade O., Yoshimura T., Yamaoka I. Symbiotic associations with protists. In: Abe T., Bignell D.E., Higashi M., editors. Termites: Evolution, Sociality, Symbioses, Ecology. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2000. pp. 275–288. [Google Scholar]
- 15.Cleveland L.R., Hall S.R., Sanders E.P., Collier J. The wood feeding roach Cryptocercus, its protozoa, and the symbiosis between protozoa and roach. Mem. Am. Acad. Arts Sci. 1934;17:185–342. doi: 10.2307/25058198. [DOI] [Google Scholar]
- 16.Kirby H. Host-parasite relations in the distribution of protozoa in termites. Univ. Calif. Publ. Zool. 1937;41:189–212. [Google Scholar]
- 17.Bourguignon T., Lo N., Cameron S.L., Sobotnik J., Hayashi Y., Shigenobu S., Watanabe D., Roisin Y., Miura T., Evans T.A. The evolutionary history of termites as inferred from 66 mitochondrial genomes. Mol. Biol. Evol. 2015;32:406–421. doi: 10.1093/molbev/msu308. [DOI] [PubMed] [Google Scholar]
- 18.Nalepa C.A., Bignell D.E., Bandi C. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Sociaux. 2001;48:194–201. doi: 10.1007/PL00001767. [DOI] [Google Scholar]
- 19.Ohkuma M., Noda S., Hongoh Y., Nalepa C.A., Inoue T. Inheritance and diversification of symbiotic trichonymphid flagellates from a common ancestor of termites and the cockroach Cryptocercus. Proc. R. Soc. 2009;276:239–245. doi: 10.1098/rspb.2008.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitade O. Comparison of symbiotic flagellate faunae between termites and a wood-feeding cockroach of the genus Cryptocercus. Microbes Environ. 2004;19:215–220. doi: 10.1264/jsme2.19.215. [DOI] [Google Scholar]
- 21.Noda S., Kitade O., Inoue T., Kawai M., Kanuka M., Hiroshima K., Hongoh Y., Constantino R., Uys V., Zhong J., et al. Cospeciation in the triplex symbiosis of termite gut protists (Pseudotrichonympha spp.), their hosts, and their bacterial endosymbionts. Mol. Ecol. 2007;16:1257–1266. doi: 10.1111/j.1365-294X.2006.03219.x. [DOI] [PubMed] [Google Scholar]
- 22.Radek R., Meuser K., Strassert J.F., Arslan O., Teßmer A., Šobotník J., Sillam-Dussès D., Nink R.A., Brune A. Exclusive gut flagellates of Serritermitidae suggest a major transfaunation event in lower termites: Description of Heliconympha glossotermitis gen. nov. spec. nov. J. Eukaryot. Microbiol. 2017 doi: 10.1111/jeu.12441. [DOI] [PubMed] [Google Scholar]
- 23.Tai V., James E.R., Nalepa C.A., Scheffrahn R.H., Perlman S.J., Keeling P.J. The role of host phylogeny varies in shaping microbial diversity in the hindguts of lower termites. Appl. Environ. Microbiol. 2015;81:1059–1070. doi: 10.1128/AEM.02945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potrikus C.J., Breznak J.A. Gut bacteria recycle uric acid nitrogen in termites: A strategy for nutrient conservation. Proc. Natl. Acad. Sci. USA. 1981;78:4601–4605. doi: 10.1073/pnas.78.7.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentley B.L. Nitrogen fixation in termites: Fate of newly fixed nitrogen. J. Insect Physiol. 1984;30:653–655. doi: 10.1016/0022-1910(84)90050-7. [DOI] [Google Scholar]
- 26.Brune A., Ohkuma M. Role of the termite gut microbiota in symbiotic digestion. In: Bignell D., Roisin Y., Lo N., editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht, The Netherlands: 2011. pp. 439–475. [Google Scholar]
- 27.Brune A., Stingl U. Prokaryotic symbionts of termite gut flagellates: Phylogenetic and metabolic implications of a tripartite symbiosis. In: Overmann J., editor. Molecular Basis of Symbiosis. Springer; Berlin, Germany: 2006. pp. 39–60. [DOI] [PubMed] [Google Scholar]
- 28.Berchtold M., Chatzinotas A., Schönhuber W., Brune A., Amann R., Hahn D., König H. Differential enumeration and in situ localization of microorganisms in the hindgut of the lower termite Mastotermes darwiniensis by hybridization with rRNA-targeted probes. Arch. Microbiol. 1999;172:407–416. doi: 10.1007/s002030050778. [DOI] [PubMed] [Google Scholar]
- 29.Ohkuma M. Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends Microbiol. 2008;16:345–352. doi: 10.1016/j.tim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Cleveland L.R., Grimstone A. The fine structure of the flagellate Mixotricha paradoxa and its associated micro-organisms. Proc. R. Soc. 1964;159:668–686. doi: 10.1098/rspb.1964.0025. [DOI] [Google Scholar]
- 31.Lee M.J., Schreurs P.J., Messer A.C., Zinder S.H. Association of methanogenic bacteria with flagellated protozoa from a termite hindgut. Curr. Microbiol. 1987;15:337–341. doi: 10.1007/BF01577591. [DOI] [Google Scholar]
- 32.Brugerolle G. Devescovinid features, a remarkable surface cytoskeleton, and epibiotic bacteria revisited in Mixotricha paradoxa, a parabasalid flagellate. Protoplasma. 2004;224:49–59. doi: 10.1007/s00709-004-0052-8. [DOI] [PubMed] [Google Scholar]
- 33.Stingl U., Radek R., Yang H., Brune A. “Endomicrobia”: Cytoplasmic symbionts of termite gut protozoa form a separate phylum of prokaryotes. Appl. Environ. Microbiol. 2005;71:1473–1479. doi: 10.1128/AEM.71.3.1473-1479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strassert J.F.H., Desai M.S., Radek R., Brune A. Identification and localization of the multiple bacterial symbionts of the termite gut flagellate Joenia annectens. Microbiology. 2010;156:2068–2079. doi: 10.1099/mic.0.037267-0. [DOI] [PubMed] [Google Scholar]
- 35.Noda S., Hongoh Y., Sato T., Ohkuma M. Complex coevolutionary history of symbiotic Bacteroidales bacteria of various protists in the gut of termites. BMC Evol. Biol. 2009;9:158. doi: 10.1186/1471-2148-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter K.J., Weber P.K., Davisson M.L., Pett-Ridge J., Haverty M.I., Keeling P.J. Correlated SEM, FIB-SEM, TEM, and NANOSIMS imaging of microbes from the hindgut of a lower termite: Methods for in-situ functional and ecological studies of uncultivable microbes. Microsc. Microanal. 2013;19:1490–1501. doi: 10.1017/S1431927613013482. [DOI] [PubMed] [Google Scholar]
- 37.Tamschick S., Radek R. Colonization of termite hindgut walls by oxymonad flagellates and prokaryotes in Incisitermes tabogae, I. marginipennis and Reticulitermes flavipes. Eur. J. Protistol. 2013;49:1–14. doi: 10.1016/j.ejop.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Sato T., Kuwahara H., Fujita K., Noda S., Kihara K., Yamada A., Ohkuma M., Hongoh Y. Intranuclear verrucomicrobial symbionts and evidence of lateral gene transfer to the host protist in the termite gut. ISME. 2014;8:1008. doi: 10.1038/ismej.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai V., Carpenter K.J., Weber P.K., Nalepa C.A., Perlman S.J., Keeling P.J. Genome evolution and nitrogen fixation in bacterial ectosymbionts of a protist inhabiting wood-feeding cockroaches. Appl. Environ. Microbiol. 2016;82:4682–4695. doi: 10.1128/AEM.00611-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breznak J. Biochemical aspects of symbiosis between termites and their intestinal microbiota. In: Anderson J.M., Rayner A.D.M., Walton D.W.H., editors. Invertebrate-Microbial Interactions. Symposium Series-British Mycological Society. Cambridge University Press; London, UK: 1984. pp. 173–203. [Google Scholar]
- 41.Nakashima K., Watanabe H., Saitoh H., Tokuda G., Azuma J.-I. Dual cellulose-digesting system of the wood-feeding termite, Coptotermes formosanus Shiraki. Insect Biochem. Mol. Biol. 2002;32:777–784. doi: 10.1016/S0965-1748(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 42.Noirot C. From wood-to humus-feeding: An important trend in termite evolution; Proceedings of the 1st European Congress of Social Insects; Leuven, Belgium. 19–22 August 1991; Leuven, Belgium: Leuven University Press; 1991. [Google Scholar]
- 43.Kirby H. Systematic differentiation and evolution of flagellates in termites. Rev. Soc. Mex. Hist. Nat. 1949;10:57–79. [Google Scholar]
- 44.Nutting W. Reciprocal protozoan transfaunations between the roach, Cryptocercus, and the termite, Zootermopsis. Biol. Bull. 1956;110:83–90. doi: 10.2307/1538895. [DOI] [Google Scholar]
- 45.Cleveland L. Sex induced with ecdysone. Proc. Natl. Acad. Sci. USA. 1959;45:747–753. doi: 10.1073/pnas.45.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duperron S. Microbial Symbioses. Elsevier Ltd.; London, UK: 2017. [Google Scholar]
- 47.Cleveland L.R. Symbiosis among animals with special reference to termites and their intestinal flagellates. Q. Rev. Biol. 1926;1:51–59. doi: 10.1086/394236. [DOI] [Google Scholar]
- 48.Lin N., Michener C.D. Evolution of sociality in insects. Q. Rev. Biol. 1972;47:131–159. doi: 10.1086/407216. [DOI] [Google Scholar]
- 49.Nalepa C.A. Nourishment and the evolution of termite eusociality. In: Hunt J.H., Nalepa C.A., editors. Nourishment and Evolution in Insect Societies. Westview Press; Boulder, CO, USA: 1994. pp. 57–104. [Google Scholar]
- 50.Nalepa C.A. ‘Cost’of proctodeal trophallaxis in extant termite individuals has no relevance in analysing the origins of eusociality. Ecol. Entomol. 2016;41:27–30. doi: 10.1111/een.12276. [DOI] [Google Scholar]
- 51.Nalepa C.A. Origin of termite eusociality: Trophallaxis integrates the social, nutritional, and microbial environments. Ecol. Entomol. 2015;40:323–335. doi: 10.1111/een.12197. [DOI] [Google Scholar]
- 52.Roberts G. Cooperation through interdependence. Anim. Behav. 2005;70:901–908. doi: 10.1016/j.anbehav.2005.02.006. [DOI] [Google Scholar]
- 53.Van Nes E.H., Arani B.M., Staal A., van der Bolt B., Flores B.M., Bathiany S., Scheffer M. What do you mean, ‘tipping point’? Trends Ecol. Evol. 2016;31:902–904. doi: 10.1016/j.tree.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Ritter H. Defense of mate and mating chamber in a wood roach. Science. 1964;143:1459–1460. doi: 10.1126/science.143.3613.1459. [DOI] [PubMed] [Google Scholar]
- 55.Seelinger G., Seelinger U. On the social organization, alarm and fighting in the primitive cockroach Cryptocercus punctulatus. Z. Tierpsychol. 1983;61:315–333. doi: 10.1111/j.1439-0310.1983.tb01347.x. [DOI] [Google Scholar]
- 56.Nalepa C.A. Colony composition, protozoan transfer and some life history characteristics of the woodroach Cryptocercus punctulatus Scudder. Behav. Ecol. Sociobiol. 1984;14:273–279. doi: 10.1007/BF00299498. [DOI] [Google Scholar]
- 57.Park Y.-C., Grandcolas P., Choe J.C. Colony composition, social behavior and some ecological characteristics of the Korean wood-feeding cockroach (Cryptocercus kyebangensis) Zool. Sci. 2002;19:1133–1139. doi: 10.2108/zsj.19.1133. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg E., Sharon G., Atad I., Zilber-Rosenberg I. The evolution of animals and plants via symbiosis with microorganisms. Environ. Microbiol. Rep. 2010;2:500–506. doi: 10.1111/j.1758-2229.2010.00177.x. [DOI] [PubMed] [Google Scholar]
- 59.Yong E. I Contain Multitudes: The Microbes within Us and a Grander View of Life. Random House; New York, NY, USA: 2016. [Google Scholar]
- 60.Korb J., Thorne B. Sociality in termites. In: Rubenstein D., Abbot P., editors. Comparative Social Evolution. Cambridge University Press; Cambridge, UK: 2017. pp. 124–153. [Google Scholar]
- 61.Cleveland L.R. Hormone-induced sexual cycles of flagellates. I. Gametogenesis, fertilization, and meiosis in Trichonympha. J. Morphol. 1949;85:197–295. doi: 10.1002/jmor.1050850202. [DOI] [PubMed] [Google Scholar]
- 62.Bell W.J., Roth L.M., Nalepa C.A. Cockroaches: Behavior, Ecology and Evolution. The Johns Hopkins University Press; Baltimore, MD, USA: 2007. p. 230. [Google Scholar]
- 63.Cleveland L.R. The feeding habits of termite castes and its relation to their intestinal flagellates. Biol. Bull. 1925;48:295–306. doi: 10.2307/1536598. [DOI] [Google Scholar]
- 64.Andrew B.J. Method and rate of protozoan refaunation in the termite Termopsis angusticollis Hagen. Univ. Calif. Publ. Zool. 1930;33:449–470. [Google Scholar]
- 65.Grassé P.P. Role des flagellés symbiotique chez les blattes et les termites. Tijdschr. Entomol. 1952;95:70–80. [Google Scholar]
- 66.Cleveland L., Burke A. Modifications induced in the sexual cycles of the protozoa of Cryptocercus by change of host. J. Eukaryot. Microbiol. 1960;7:240–245. [Google Scholar]
- 67.To L.P., Margulis L., Chase D., Nutting W.L. The symbiotic microbial community of the Sonoran desert termite: Pterotermes occidentis. Biosystems. 1980;13:109–137. doi: 10.1016/0303-2647(80)90007-6. [DOI] [PubMed] [Google Scholar]
- 68.Lüscher M. Hormonal control of caste differentiation in termites. Ann. N. Y. Acad. Sci. 1960;89:549–563. doi: 10.1111/j.1749-6632.1960.tb27577.x. [DOI] [Google Scholar]
- 69.Raina A., Park Y.I., Gelman D. Molting in workers of the formosan subterranean termite Coptotermes formosanus. J. Insect Physiol. 2008;54:155–161. doi: 10.1016/j.jinsphys.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Xing L., Chouvenc T., Su N.-Y. Molting process in the formosan subterranean termite (Isoptera: Rhinotermitidae) Ann. Entomol. Soc. Am. 2013;106:619–625. doi: 10.1603/AN13007. [DOI] [Google Scholar]
- 71.Grassé P.-P., Noirot C. La transmission des flagelles symbiotiques et les aliments des termites. Biol. Bull. Fr. Belgium. 1945;79:273–297. [Google Scholar]
- 72.Radek R. Flagellates, bacteria, and fungi associated with termites: Diversity and function in nutrition—A review. Ecotropica. 1999;5:183–196. [Google Scholar]
- 73.Brugerolle G., Radek R. Symbiotic protozoa of termites. In: Konig H., Varma A., editors. Intestinal Microorganisms of Termites and Other Invertebrates. Springer; Berlin, Germany: 2006. pp. 243–269. Soil Biology 6. [Google Scholar]
- 74.Honigberg B.M. Protozoa associated with termites and their role in digestion. In: Krishna K., Weesner F.M., editors. Biology of Termites. Volume 2. Academic Press; New York, NY, USA: 1970. pp. 1–36. [Google Scholar]
- 75.Cleveland L.R. The effects of oxygenation and starvation on the symbiosis between the termite, Termopsis, and its intestinal flagellates. Biol. Bull. 1925;48:309–326. doi: 10.2307/1536599. [DOI] [Google Scholar]
- 76.Dropkin V.H. Host specificity relation of termite protozoa. Ecology. 1941;22:200–202. doi: 10.2307/1932217. [DOI] [Google Scholar]
- 77.Nutting W.L., Cleveland L.R. Effects of glandular extirpations on Cryptocercus and the sexual cycles of its protozoa. J. Exp. Zool. 1958;137:13–37. doi: 10.1002/jez.1401370103. [DOI] [PubMed] [Google Scholar]
- 78.Bradbury P.C. Protozoan adaptations for survival. In: Henis Y., editor. Survival and Dormancy of Microorganisms. John Wiley & Sons; New York, NY, USA: 1987. pp. 267–299. [Google Scholar]
- 79.Gutiérrez J.C., Martin-Gonzalez A., Matsusaka T. Towards a generalized model of encystment (cryptobiosis) in ciliates: A review and a hypothesis. BioSystems. 1990;24:17–24. doi: 10.1016/0303-2647(90)90025-V. [DOI] [PubMed] [Google Scholar]
- 80.Chávez-Munguía B., Omaña-Molina M., González-Lázaro M., González-Robles A., Cedillo-Rivera R., Bonilla P., Martínez-Palomo A. Ultrastructure of cyst differentiation in parasitic protozoa. Parasitol. Res. 2007;100:1169–1175. doi: 10.1007/s00436-006-0447-x. [DOI] [PubMed] [Google Scholar]
- 81.Cleveland L.R., Nutting W.L. Suppression of sexual cycles and death of the protozoa of Cryptocercus resulting from change of hosts during molting period. J. Exp. Zool. 1955;130:485–513. doi: 10.1002/jez.1401300306. [DOI] [Google Scholar]
- 82.Cleveland L.R. Hormone induced sexual cycles of flagellates. XIV. Gametic meiosis and fertilization in Macrospironympha. Arch. Protistenkd. 1956;101:99–169. [Google Scholar]
- 83.Cleveland L. Correlation between the molting period of Cryptocercus and sexuality in its protozoa. J. Eukaryot. Microbiol. 1957;4:168–175. doi: 10.1111/j.1550-7408.1957.tb02504.x. [DOI] [Google Scholar]
- 84.Cleveland L.R., Burke A.W.J., Karlson P. Ecdysone induced modifications in the sexual cycles of the protozoa of Cryptocercus. J. Protozool. 1960;7:229–239. doi: 10.1111/j.1550-7408.1960.tb00735.x. [DOI] [Google Scholar]
- 85.Sonneborn T. Breeding systems, reproductive methods, and species problems in protozoa. In: Mayr E., editor. The Species Problem. AAS Publication #50; Washington, DC, USA: 1957. pp. 155–324. [Google Scholar]
- 86.Cheng T.C. Symbiosis. Organisms Living Together. Pegasus; New York, NY, USA: 1970. [Google Scholar]
- 87.Hawes R. The emergence of asexuality in protozoa. Q. Rev. Biol. 1963;38:234–242. doi: 10.1086/403859. [DOI] [Google Scholar]
- 88.Loidl J. Conservation and variability of meiosis across the eukaryotes. Ann. Rev. Genet. 2016;50:293–316. doi: 10.1146/annurev-genet-120215-035100. [DOI] [PubMed] [Google Scholar]
- 89.Cleveland L. Hormone-induced sexual cycles of flagellates II. Gametogenesis, fertilization, and one-division meiosis in Oxymonas. J. Morphol. 1950;86:185–213. doi: 10.1002/jmor.1050860110. [DOI] [PubMed] [Google Scholar]
- 90.Raikov I.B. Meiosis in protists: Recent advances and persisting problems. Eur. J. Protistol. 1995;31:1–7. doi: 10.1016/S0932-4739(11)80349-4. [DOI] [Google Scholar]
- 91.Cleveland L. Hormone-induced sexual cycles of flagellates. VI. Gametogenesis, fertilization, meiosis, oöcysts, and gametocysts in Leptospironympha. J. Morphol. 1951;88:199–243. doi: 10.1002/jmor.1050880203. [DOI] [PubMed] [Google Scholar]
- 92.Bengtsson B.O. Asex and evolution: A very large-scale overview. In: Schon I., Martens K., van Dijk P., editors. Lost Sex. Springer; Dordrecht, The Netherlands: 2009. pp. 1–19. [Google Scholar]
- 93.Lenormand T., Engelstädter J., Johnston S.E., Wijnker E., Haag C.R. Evolutionary mysteries in meiosis. Philos. Trans. R. Soc. B. 2016;371 doi: 10.1098/rstb.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mirzaghaderi G., Hörandl E. The evolution of meiotic sex and its alternatives. Proc. R. Soc. 2016 doi: 10.1098/rspb.2016.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Malley M.A., Simpson A.G.B., Roger A.J. The other eukaryotes in light of evolutionary protistology. Biol. Philos. 2013;28:299–330. doi: 10.1007/s10539-012-9354-y. [DOI] [Google Scholar]
- 96.Cleveland L. Hormone-induced sexual cycles of flagellates. VIII. Meiosis in Rhynchonympha in one cytoplasmic and two nuclear divisions followed by autogamy. J. Morphol. 1952;91:269–323. doi: 10.1002/jmor.1050910205. [DOI] [Google Scholar]
- 97.Wenrich D.H. In: Sex in protozoa: A comparative review, In Sex in Microorganisms: A Symposium. Wenrich D.H., editor. American Association for the Advancement of Science; Philadelphia, PA, USA: 1954. pp. 134–265. [Google Scholar]
- 98.Cleveland L. Hormone-induced sexual cycles of flagellates. IX. Haploid gametogenesis and fertilization in Barbulanympha. J. Morphol. 1953;93:371–403. doi: 10.1002/jmor.1050930206. [DOI] [Google Scholar]
- 99.Cleveland L. Hormone-induced sexual cycles of flagellates. VII. One-division meiosis and autogamy without cell division in Urinympha. J. Morphol. 1951;88:385–439. doi: 10.1002/jmor.1050880302. [DOI] [PubMed] [Google Scholar]
- 100.Cleveland L. Hormone-induced sexual cycles of flagellates. IV. Meiosis after syngamy and before nuclear fusion in Notila. J. Morphol. 1950;87:317–347. doi: 10.1002/jmor.1050870206. [DOI] [PubMed] [Google Scholar]
- 101.Cleveland L. Hormone-induced sexual cycles of flagellates. III. Gametogenesis, fertilization, and one-division meiosis in Saccinobaculus. J. Morphol. 1950;86:215–227. doi: 10.1002/jmor.1050860111. [DOI] [PubMed] [Google Scholar]
- 102.Krtková J., Thomas E.B., Alas G.C., Schraner E.M., Behjatnia H.R., Hehl A.B., Paredez A.R. Rac regulates Giardia lamblia encystation by coordinating cyst wall protein trafficking and secretion. mBio. 2016;7:e01003–e01016. doi: 10.1128/mBio.01003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weedall G.D., Hall N. Sexual reproduction and genetic exchange in parasitic protists. Parasitology. 2015;142:S120–S127. doi: 10.1017/S0031182014001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kolman J.A., Clopton R.E., Clopton D.T. Effects of developmental temperature on gametocysts and oocysts of two species of gregarines Blabericola migrator and Blabericola cubensis (Apicomplexa: Eugregarinida: Blabericolidae) parasitizing blaberid cockroaches (Dictyoptera: Blaberidae) J. Parasitol. 2015;101:651–657. doi: 10.1645/14-673. [DOI] [PubMed] [Google Scholar]
- 105.Clopton R.E., Steele S.M., Clopton D.T. Environmental persistence and infectivity of oocysts of two species of gregarines, Blabericola migrator and Blabericola cubensis (Apicomplexa: Eugregarinida: Blabericolidae), parasitizing blaberid cockroaches (Dictyoptera: Blaberidae) J. Parasitol. 2016;102:169–173. doi: 10.1645/15-934. [DOI] [PubMed] [Google Scholar]
- 106.Cleveland L. Induction and acceleration of gametogenesis in flagellates by the insect hormone ecdysone. Science. 1960;131:1317. [Google Scholar]
- 107.Nalepa C.A., Bandi C. Characterizing the ancestors: Paedomorphosis and termite evolution. In: Abe T., Bignell D.E., Higashi M., editors. Termites: Evolution, Sociality, Symbioses, Ecology. Kluwar Academic; Dordrecht, The Netherlands: 2000. pp. 53–75. [Google Scholar]
- 108.Nalepa C.A. Altricial development in wood-feeding cockroaches: The key antecedent of termite eusociality. In: Bignell D., Roisin Y., Lo N., editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht, The Netherlands: 2011. pp. 69–95. [Google Scholar]
- 109.Nalepa C.A., Maekawa K., Shimada K., Saito Y., Arellano C., Matsumoto T. Altricial development in subsocial wood-feeding cockroaches. Zool. Sci. 2008;25:1190–1198. doi: 10.2108/zsj.25.1190. [DOI] [PubMed] [Google Scholar]
- 110.Nalepa C.A. Body size and termite evolution. Evol. Biol. 2011;38:243–257. doi: 10.1007/s11692-011-9121-z. [DOI] [Google Scholar]
- 111.Lüscher M. Environmental control of juvenile hormone (JH) secretion and caste differentiation in termites. Gen. Comp. Endocrinol. 1972;3:509–514. doi: 10.1016/0016-6480(72)90181-5. [DOI] [Google Scholar]
- 112.Noirot C., Pasteels J.M. Ontogenetic development and evolution of the worker caste in termites. Experientia. 1987;43:851–860. doi: 10.1007/BF01951642. [DOI] [Google Scholar]
- 113.Miura T. Morphogenesis and gene expression in the soldier-caste differentiation of termites. Insectes Sociaux. 2001;48:216–223. doi: 10.1007/PL00001769. [DOI] [Google Scholar]
- 114.Toga K., Yoda S., Maekawa K. The tunel assay suggests mandibular regression by programmed cell death during presoldier differentiation in the nasute termite Nasutitermes takasagoensis. Naturwissenschaften. 2011;98:801–806. doi: 10.1007/s00114-011-0825-9. [DOI] [PubMed] [Google Scholar]
- 115.Masuoka Y., Maekawa K. Ecdysone signaling regulates soldier-specific cuticular pigmentation in the termite Zootermopsis nevadensis. FEBS Lett. 2016;590:1694–1703. doi: 10.1002/1873-3468.12219. [DOI] [PubMed] [Google Scholar]
- 116.Di Cara F., King-Jones K. How clocks and hormones act in concert to control the timing of insect development. In: Rougvie A.E., O’Connor M.B., editors. Developmental Timing. Volume 105. Elsevier Science Bv; Amsterdam, The Netherlands: 2013. pp. 1–36. [DOI] [PubMed] [Google Scholar]
- 117.Miura T., Koshikawa S., Matsumoto T. Winged presoldiers induced by a juvenile hormone analog in Zootermopsis nevadensis: Implications for plasticity and evolution of caste differentiation in termites. J. Morphol. 2003;257:22–32. doi: 10.1002/jmor.10100. [DOI] [PubMed] [Google Scholar]
- 118.Elliott K.L., Stay B. Changes in juvenile hormone synthesis in the termite Reticulitermes flavipes during development of soldiers and neotenic reproductives from groups of isolated workers. J. Insect Physiol. 2008;54:492–500. doi: 10.1016/j.jinsphys.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Watanabe D., Shirasaki I., Maekawa K. Effects of juvenile hormone III on morphogenetic changes during a molt from each nymphal instar in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae) Appl. Entomol. Zool. 2010;45:377–386. doi: 10.1303/aez.2010.377. [DOI] [Google Scholar]
- 120.Miura T., Scharf M.E. Molecular basis underlying caste differentiation in termites. In: Bignell D., Roisin Y., Lo N., editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht, The Netherlands: 2011. pp. 211–253. [Google Scholar]
- 121.Masuoka Y., Yaguchi H., Suzuki R., Maekawa K. Knockdown of the juvenile hormone receptor gene inhibits soldier-specific morphogenesis in the damp-wood termite Zootermopsis nevadensis (Isoptera: Archotermopsidae) Insect Biochem. Mol. Biol. 2015;64:25–31. doi: 10.1016/j.ibmb.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 122.Abdou M., Peng C., Huang J., Zyaan O., Wang S., Li S., Wang J. Wnt signaling cross-talks with JH signaling by suppressing Met. and gce expression. PLoS ONE. 2011;6:e26772. doi: 10.1371/journal.pone.0026772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niwa Y.S., Niwa R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev. Growth Differ. 2016;58:94–105. doi: 10.1111/dgd.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jindra M., Palli S.R., Riddiford L.M. The juvenile hormone signaling pathway in insect development. Ann. Rev. Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 125.Hartfelder K., Emlen D. Endocrine control of insect polyphenism. In: Gilbert L.I., editor. Insect Endocrinology. Academic Press; London, UK: 2012. pp. 464–522. [Google Scholar]
- 126.De Loof A., Boerjan B., Ernst U.R., Schoofs L. The mode of action of juvenile hormone and ecdysone: Towards an epi-endocrinological paradigm? Gen. Comp. Endocrinol. 2013;188:35–45. doi: 10.1016/j.ygcen.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 127.Cleveland L.R. Induction and acceleration of gametogenesis in flagellates by the insect hormone ecdysone. In: Ludvík J., Lom J., Vavra J., editors. First International Congress on Protozoology. Academic Press; Prague, Czech Republic: 1963. pp. 289–291. [Google Scholar]
- 128.Gutiérrez J.C., Martin-González A., Callejas S. Nuclear changes, macronuclear chromatin reorganization and DNA modifications during ciliate encystment. Eur. J. Protistol. 1998;34:97–103. doi: 10.1016/S0932-4739(98)80018-7. [DOI] [Google Scholar]
- 129.Gutiérrez J., Callejas S., Borniquel S., Benítez L., Martín-González A. Ciliate cryptobiosis: A microbial strategy against environmental starvation. Int. Microbiol. 2001;4:151–157. doi: 10.1007/s10123-001-0030-3. [DOI] [PubMed] [Google Scholar]
- 130.Aguilar-Díaz H., Carrero J.C., Argüello-García R., Laclette J.P., Morales-Montor J. Cyst and encystment in protozoan parasites: Optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011;27:450–458. doi: 10.1016/j.pt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Einarsson E., Troell K., Hoeppner M.P., Grabherr M., Ribacke U., Svärd S.G. Coordinated changes in gene expression throughout encystation of Giardia intestinalis. PLoS Negl. Trop. Dis. 2016;10:e0004571. doi: 10.1371/journal.pntd.0004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bogdanov Y.F. Variation and evolution of meiosis. Russ. J. Genet. 2003;39:363–381. doi: 10.1023/A:1023345311889. [DOI] [Google Scholar]
- 133.Cleveland L.R. The centrioles of Trichonympha from termites and their functions in reproduction. J. Protozool. 1960;7:326–341. doi: 10.1111/j.1550-7408.1960.tb05979.x. [DOI] [Google Scholar]
- 134.Messer A.C., Lee M.J. Effect of chemical treatments on methane emission by the hindgut microbiota in the termite Zootermopsis angusticollis. Microb. Ecol. 1989;18:275–284. doi: 10.1007/BF02075814. [DOI] [PubMed] [Google Scholar]
- 135.Cleveland L. Fertilization in Deltotrichonympha. Arch. Protistenkd. 1966;109:15–17. [Google Scholar]
- 136.Cleveland L. Fertilization in Koruga. Arch. Protistenkd. 1966;109:24–25. [Google Scholar]
- 137.Cleveland L. Fertilization in Mixotricha. Arch. Protistenkd. 1966;109:37–39. [Google Scholar]
- 138.Cleveland L.R. Fertilization in Trichonympha from termites. Arch. Protistenkd. 1965;108:1–5. [Google Scholar]
- 139.Cleveland L.R. Fertilization in Pseudotrichonympha. Arch. Protistenkd. 1965;108:6–7. [Google Scholar]
- 140.Sen R., Raychoudhury R., Cai Y.P., Sun Y.J., Lietze V.U., Boucias D.G., Scharf M.E. Differential impacts of juvenile hormone, soldier head extract and alternate caste phenotypes on host and symbiont transcriptome composition in the gut of the termite Reticulitermes flavipes. BMC Genom. 2013;14:491. doi: 10.1186/1471-2164-14-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kirby H. Trichomonad flagellates from termites I. Tricercomitus gen. nov., and Hexamastix alexeieff. Univ. Calif. Publ. Zool. 1930;33:393–444. [Google Scholar]
- 142.May E. The behavior of the intestinal protozoa of termites at the time of the last ecdysis. Trans. Am. Microsc. Soc. 1941;60:281–292. doi: 10.2307/3222824. [DOI] [Google Scholar]
- 143.Honigberg B. Evolutionary and systematic relationships in the flagellate order Trichomonadida Kirby. J. Eukaryot. Microbiol. 1963;10:20–63. doi: 10.1111/j.1550-7408.1963.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 144.Kirby H. Relationships between certain protozoa and other animals. In: Calkins G., Summers F., editors. Protozoa in Biological Research. Columbia University Press; New York, NY, USA: 1941. pp. 890–1008. [Google Scholar]
- 145.Nalepa C., Miller L., Lenz M. Flight characteristics of Mastotermes darwiniensis (Isoptera, Mastotermitidae) Insectes Sociaux. 2001;48:144–148. doi: 10.1007/PL00001757. [DOI] [Google Scholar]
- 146.Dolan M.F., Wier A.M., Melnitsky H., Whiteside J.H., Margulis L. Cysts and symbionts of Staurojoenina assimilis Kirby from Neotermes. Eur. J. Protistol. 2004;40:257–264. doi: 10.1016/j.ejop.2004.01.005. [DOI] [Google Scholar]
- 147.Van Wagtendonk W.J. Encystment and excystment of protozoans. In: Hutner S., Lwoff A., editors. Biochemistry and Physiology of Protozoa. Volume II. Academic Press; New York, NY, USA: 1955. pp. 85–90. [Google Scholar]
- 148.Corliss J.O., Esser S.C. Comments on the role of the cyst in the life cycle and survival of free-living protozoa. Trans. Am. Microsc. Soc. 1974;93:578–593. doi: 10.2307/3225158. [DOI] [PubMed] [Google Scholar]
- 149.Hamilton R.L., Mullins D.E., Orcutt D.M. Freezing tolerance in the woodroach Cryptocercus punctulatus (Scudder) Experientia. 1985;41:1535–1536. doi: 10.1007/BF01964793. [DOI] [Google Scholar]
- 150.Scheffrahn R.H., Bourguignon T., Bordereau C., Hernandez-Aguilar R.A., Oelze V.M., Dieguez P., Šobotnik J., Pascual-Garrido A. White-gutted soldiers: Simplification of the digestive tube for a non-particulate diet in higher old world termites (Isoptera: Termitidae) Insectes Sociaux. 2017;64:525–533. doi: 10.1007/s00040-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boucher D.H., James S., Keeler K.H. The ecology of mutualism. Ann. Rev. Ecol. Syst. 1982;13:315–347. doi: 10.1146/annurev.es.13.110182.001531. [DOI] [Google Scholar]
- 152.McLaughlin G., Cain G. An inquiry into the nature of symbiosis. Evol. Theory. 1983;6:185–196. [Google Scholar]
- 153.Bulgheresi S. Microbial symbiont transmission: Basic principles and dark sides. In: Rosenberg E., Gophna U., editors. Beneficial Microorganisms in Multicellular Life Forms. Springer; Heidelberg, Germany: 2012. pp. 299–311. [Google Scholar]
- 154.Dietrich C., Köhler T., Brune A. The cockroach origin of the termite gut microbiota: Patterns in bacterial community structure reflect major evolutionary events. Appl. Environ. Microbiol. 2014;80:2261–2269. doi: 10.1128/AEM.04206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brune A., Dietrich C. The gut microbiota of termites: Digesting the diversity in the light of ecology and evolution. Ann. Rev. Microbiol. 2015;69:145–166. doi: 10.1146/annurev-micro-092412-155715. [DOI] [PubMed] [Google Scholar]
- 156.Wernegreen J.J., Wheeler D.E. Remaining flexible in old alliances: Functional plasticity in constrained mutualisms. DNA Cell Biol. 2009;28:371–382. doi: 10.1089/dna.2009.0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heath H. The habits of California termites. Biol. Bull. 1903;4:47–63. doi: 10.2307/1535553. [DOI] [Google Scholar]
- 158.Hayashi Y., Maekawa K., Nalepa C.A., Miura T., Shigenobu S. Transcriptome sequencing and estimation of DNA methylation level in the subsocial wood-feeding cockroach Cryptocercus punctulatus (Blattodea: Cryptocercidae) Appl. Entomol. Zool. 2017;52:643–651. doi: 10.1007/s13355-017-0519-7. [DOI] [Google Scholar]
- 159.Masuoka Y., Toga K., Nalepa C.A., Maekawa K. Molting in termites and woodroaches may be regulated by common JH signaling pathway. 2017. in preparation.
- 160.Luján H.D., Mowatt M.R., Nash T.E. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol. Mol. Biol. Rev. 1997;61:294–304. doi: 10.1128/mmbr.61.3.294-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lauwaet T., Davids B.J., Reiner D.S., Gillin F.D. Encystation of Giardia lamblia: A model for other parasites. Curr. Opin. Microbiol. 2007;10:554–559. doi: 10.1016/j.mib.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Samuelson J., Bushkin G.G., Chatterjee A., Robbins P.W. Strategies to discover the structural components of cyst and oocyst walls. Eukaryot. Cell. 2013;12:1578–1587. doi: 10.1128/EC.00213-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Benítez L., Gutiérrez J.C. Encystment-specific mRNA is accumulated in the resting cysts of the ciliate Colpoda inflata. IUBMB Life. 1997;41:1137–1141. doi: 10.1080/15216549700202221. [DOI] [PubMed] [Google Scholar]
- 164.Sogame Y., Asami H. Possible involvement of cAMP and protein phosphorylation in the cell signaling pathway for resting cyst formation of ciliated protozoan Colpoda cucullus. Acta Protozool. 2015;50:71–79. [Google Scholar]
- 165.Chen J., Gao X., Wang B., Chen F., Wu N., Zhang Y. Proteomic approach to reveal the proteins associated with encystment of the ciliate Euplotes encysticus. PLoS ONE. 2014;9:e97362. doi: 10.1371/journal.pone.0097362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao X., Chen F., Niu T., Qu R., Chen J. Large-scale identification of encystment-related proteins and genes in Pseudourostyla cristata. Sci. Rep. 2015;5:11360. doi: 10.1038/srep11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cleveland L. Brief accounts of the sexual cycles of the flagellates of Cryptocercus. J. Eukaryot. Microbiol. 1956;3:161–180. [Google Scholar]
- 168.Speijer D., Lukes J., Elias M. Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl. Acad. Sic. USA. 2015;112:8827–8834. doi: 10.1073/pnas.1501725112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chi J., Parrow M.W., Dunthorn M. Cryptic sex in Symbiodinium (Alveolata, Dinoflagellata) is supported by an inventory of meiotic genes. J. Eukaryot. Microbiol. 2014;61:322–327. doi: 10.1111/jeu.12110. [DOI] [PubMed] [Google Scholar]
- 170.Ramesh M.A., Malik S.B., Logsdon J.M. A phylogenomic inventory of meiotic genes: Evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr. Biol. 2005;15:185–191. doi: 10.1016/j.cub.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 171.Tekle Y.I., Wood F.C., Katz L.A., Cerón-Romero M.A., Gorfu L.A. Amoebozoans are secretly but ancestrally sexual: Evidence for sex genes and potential novel crossover pathways in diverse groups of amoebae. Genome Biol. Evol. 2017;9:375–387. doi: 10.1093/gbe/evx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malik S.-B., Pightling A.W., Stefaniak L.M., Schurko A.M., Logsdon J.M., Jr. An expanded inventory of conserved meiotic genes provides evidence for sex in Trichomonas vaginalis. PLoS ONE. 2008;3:e2879. doi: 10.1371/annotation/029c403b-10aa-47af-9e99-11451de76b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schurko A.M., Logsdon J.M. Using a meiosis detection toolkit to investigate ancient asexual “scandals” and the evolution of sex. Bioessays. 2008;30:579–589. doi: 10.1002/bies.20764. [DOI] [PubMed] [Google Scholar]
- 174.Carpenter K.J., Chow L., Keeling P.J. Morphology, phylogeny, and diversity of Trichonympha (Parabasalia: Hypermastigida) of the wood-feeding cockroach Cryptocercus punctulatus. J. Eukaryot. Microbiol. 2009;56:305–313. doi: 10.1111/j.1550-7408.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- 175.Kirby H. Flagellates of the genus Trichonympha in termites. Univ. Calif. Publ. Zool. 1932;37:349–376. [Google Scholar]