Abstract
The transcription factor NRF2 (nuclear factor-E2-related factor 2) orchestrates major cellular defense mechanisms including phase-II detoxification, inflammatory signaling, DNA repair, and antioxidant response. Recent studies strongly suggest a protective role of NRF2-mediated gene expression in the suppression of cutaneous photodamage induced by solar UV (ultraviolet) radiation. The apocarotenoid bixin, a Food and Drug Administration (FDA)-approved natural food colorant (referred to as ‘annatto’) originates from the seeds of the achiote tree native to tropical America, consumed by humans since ancient times. Use of achiote preparations for skin protection against environmental insult and for enhanced wound healing has long been documented. We have recently reported that (i) bixin is a potent canonical activator of the NRF2-dependent cytoprotective response in human skin keratinocytes; that (ii) systemic administration of bixin activates NRF2 with protective effects against solar UV-induced skin damage; and that (iii) bixin-induced suppression of photodamage is observable in Nrf2+/+ but not in Nrf2−/− SKH-1 mice confirming the NRF2-dependence of bixin-induced antioxidant and anti-inflammatory effects. In addition, bixin displays molecular activities as sacrificial antioxidant, excited state quencher, PPAR (peroxisome proliferator-activated receptor) α/γ agonist, and TLR (Toll-like receptor) 4/NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) antagonist, all of which might be relevant to the enhancement of skin barrier function and environmental stress protection. Potential skin photoprotection and photochemoprevention benefits provided by topical application or dietary consumption of this ethno-pharmacologically validated phytochemical originating from the Americas deserves further preclinical and clinical examination.
Keywords: skin photodamage, skin barrier function, solar ultraviolet (UV), NRF2, PPARα, bixin, achiote
1. Introduction: Solar Radiation, Photodamage, Photoaging, and Skin Photocarcinogenesis
Exposure to solar ultraviolet (UV) radiation is a causative factor in acute skin photodamage, chronic photoaging, and photocarcinogenesis [1,2,3,4]. More recently, a causative role of solar photons in the visible and infrared spectral range contributing to skin photodamage has been substantiated [5,6,7,8]. Moreover, cutaneous exposure to other environmental stressors including combustion pollutants, heavy metals, metalloids, and ozone has been shown to contribute to skin damage and carcinogenesis. Remarkably, nonmelanoma skin cancer (NMSC; also referred to as keratinocyte cancers (KC)) is the most common malignancy in the United States, and skin cancer incidence is increasing rapidly, presenting a public health burden of considerable magnitude [9]. Even though sunscreen-based photoprotection is an effective component of a sun-safe strategy to reduce cumulative lifetime exposure to UV light, much effort has been directed towards the development of more effective molecular strategies acting through mechanisms different from (or synergistic with) photon absorption [9,10,11,12,13,14].
2. NRF2: A Master Regulator of Skin Barrier Function, Cellular Defense Mechanisms against Environmental Stress, and Solar Radiation Response
The redox-sensitive transcription factor NRF2 (nuclear factor-E2-related factor 2) orchestrates major cellular defense mechanisms including phase-II detoxification, inflammatory signaling, DNA repair, and antioxidant response, and recent experimental evidence supports an important role of NRF2 in skin barrier function. NRF2 has therefore emerged as a promising molecular target for the pharmacological prevention of human pathologies resulting from exposure to environmental toxicants including solar UV-induced damage and carcinogenesis [15,16,17,18]. Moreover, the potential of NRF2 for modulation of skin chronological and photodamage-associated aging has attracted considerable attention [9,19,20].
3. NRF2: Molecular Biology and Pharmacological Modulation
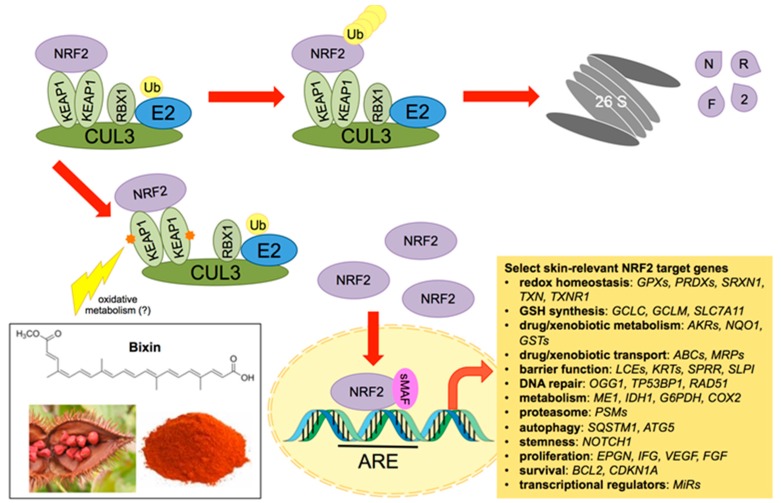
NRF2 is ubiquitously expressed in all tissues, including the skin, but its protein levels and consequently its activity are tightly regulated (Figure 1). Under basal (homeostatic) conditions, NRF2 resides in the cytosol, where it binds to its negative regulator Kelch-ECH associated protein 1 (KEAP1), a substrate adaptor for a cullin 3-RING box protein 1 (CUL3-RBX1) E3 ubiquitin ligase complex [21]. Thus, NRF2 is ubiquitylated and degraded by the 26 S proteasome [22]. However, upon exposure to reactive oxygen species (ROS) or to electrophilic compounds, key sensor cysteine residues in KEAP1 (cysteine 151 in particular) are chemically modified, causing a conformational change in KEAP1 that prevents degradation of NRF2, which remains complexed to KEAP1 [23,24]. This allows newly synthesized NRF2 to accumulate and translocate to the nucleus, where it heterodimerizes with small MAF (musculoaponeurotic fibrosarcoma) proteins and binds to the antioxidant response elements (AREs) in the regulatory regions of its downstream genes [25]. This mode of canonical NRF2 regulation has been extensively studied in the context of skin protection and pathogenesis. In addition, other modes of NRF2 regulation, such as the p62-dependent non-canonical pathway that activates NRF2 in an autophagy-dependent manner [26,27] or the GSK3-βTrCP (glycogen synthase kinase 3/β-transducin repeat containing protein) degradation pathway [28,29], have been described. However, the involvement of these other modes of NRF2 regulation in skin barrier function and environmental stress protection remain to be determined.
Figure 1.
The nuclear factor-E2-related factor 2 (NRF2) pathway with a focus on skin barrier function and environmental stress protection. The transcription factor NRF2 binds to Kelch-ECH associated protein 1 (KEAP1), the substrate adaptor protein for the cullin 3-RING box protein 1 (CUL3-RBX1) E3 ubiquitin ligase complex. Under basal conditions, NRF2 is ubiquitylated and degraded by the 26S proteasome. Upon modification of reactive cysteines in KEAP1 by reactive oxygen species (ROS) and electrophiles (including bixin), NRF2 is no longer ubiquitylated. This allows for newly synthesized NRF2 to accumulate, translocate to the nucleus, and activate the transcription of antioxidant response element (ARE)-containing target genes by dimerizing with small MAF (sMAF) proteins. Select skin-relevant NRF2 target genes are displayed according to the cellular function they perform. GPXs, glutathione peroxidases; PRDXs, peroxiredoxins; SRXN1, sulfiredoxin 1; TXN, thioredoxin; TXNR1, thioredoxin reductase 1; GCLC, glutamate cysteine ligase, catalytic subunit; GCLM, glutamate cysteine ligase, modifier subunit; SLC7A11, glutamate/cystine antiporter (xCT); AKRs, aldoketoreductases; NQO1, NAD(P)H:quinone oxidoreductase 1; GSTs, glutathione S-transferases; ABCs, ATP-binding cassette family proteins; MRPs, multidrug resistance-associated proteins; LCEs, late cornified envelope family members; KRTs, keratins; SPRR, small proline rich proteins; OGG1, 8-oxo-guanine glycosylase; TP53BP1, p53 binding protein 1; RAD51, DNA repair protein RAD51 homolog 1; ME1, malic enzyme; IDH1, isocitrate dehydrogenase 1; G6PDH, glucose-6-phosphate dehydrogenase; COX2, cytochrome c oxidase subunit 2; PSM, proteasome subunit proteins; SQSTM1, sequestosome 1 (p62); ATG5, autophagy-related gene 5; NOTCH1, Notch homolog 1, translocation-associated; EPGN, epigen; IGF, insulin-like growth factor; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; BCL2, B cell lymphoma 2; CDKN1A, cyclin dependent kinase inhibitor 1A (p21); MiR, microRNAs.
Many natural chemopreventive compounds that have antioxidant properties exert their cytoprotective function through NRF2 activation. Classic examples of NRF2 inducers are sulforaphane (from cruciferous vegetables) [16], curcumin (from Curcuma longa) [30], cinnamaldehyde (from cinnamon) [31,32], and tanshinones (from Salvia miltiorrhiza) [33], among many others. These compounds are promiscuous electrophilic molecules that also react with cysteine 151 of KEAP1, induce NRF2, and confer protection against a number of chemical insults or radiation damage (including UV) observable in vitro and in vivo [34,35,36]. Recently, a synthetic triterpenoid NRF2 modulator and bardoxolone-derivative, RTA 408, has been tested for topical NRF2 activation in rat, murine, and human skin [37,38], but limited data on skin protection properties are available. Taken together, a significant opportunity for the development of cutaneous NRF2-dependent skin protection strategies using nutrient-derived molecular entities remains to be explored.
4. NRF2 Control of Skin Barrier Structure and Function
Recently, it has been shown that numerous genes encoding skin barrier structural and functional components are under NRF2 transcriptional control, including late cornified envelope 1 (LCE1) family members (LCE1B, LCE1C, LCE1E, LCE1G, LCE1H, LCE1M), keratins (KRT6A, KRT16, KRT17), small proline rich proteins (SPRR2D, SPRR2H), secretory leukocyte protease inhibitor (SLPI), and the EGF family member epigen (EPGN), some of which contain a validated ARE [39,40,41,42,43]. Moreover, a novel role of NRF2 in skin barrier and desmosome function has been attributed to transcriptional control of MiR-encoding genes (MIR29AB1 and MIR29B2C) in keratinocytes, substantiating a novel NRF2-miR29-DSC2 (desmocollin-2) axis in control of desmosome function and cutaneous homeostasis [44]. In addition, much research has substantiated a role of NRF2 in epidermal redox control, stress response regulation, terminal differentiation, and barrier homeostasis, and a crucial role of NRF2 in the control of a cytoprotective glutathione gradient throughout the epidermis has been demonstrated [13,35,40,41,45].
Additional functional implications of NRF2 relevant to skin barrier maintenance, repair, and rejuvenation have recently emerged, including a role in metabolic control and mitochondrial homeostasis, proteasomal function and autophagy, and stem cell renewal and pluripotency [46,47,48].
Moreover, abundant functional crosstalk exists between NRF2 and other cutaneous stress response pathways including AhR (arylhydrocarbon receptor) and NFκB [49,50,51]. For example, the co-occurrence of ARE- and xenobiotic response element- (XRE-)sequences in the promoter region of several AhR-controlled genes (including NQO1 (NAD(P)H quinone oxidoreductase 1) and GST (glutathione-S-transferase) indicates mechanistic crosstalk between NRF2 and AhR at the gene expression level [52]. Likewise, direct AhR binding to XREs located in the NRF2 promoter region has been confirmed by immunoprecipitation analysis, enabling AhR agonists to induce NRF2 expression at the mRNA and protein levels. It has also been demonstrated that protease-activated receptor-2 (PAR-2), an important mediator of inflammation and immune responses by serine proteinases, activates NQO1 via NRF2 stabilization in keratinocytes, suggesting that in addition to induction of inflammation, PAR-2 can play a cytoprotective role that depends on NRF2 [53].
5. NRF2 in Skin Pathology
A substantial body of experimental evidence indicates that NRF2 dysregulation, either due to insufficient adaptive activation in response to environmental stressors or due to constitutive hyperactivation as a result of genetic alterations that may also involve KEAP1, has detrimental effects compromising skin barrier function and stress responses. Seminal research has documented that constitutive epidermal NRF2 overactivation through permanent genetic deletion of KEAP1-caused hyperkeratosis in murine skin [54]. It has also been demonstrated that forced constitutive NRF2 overactivation causes chloracne-like skin disease characterized by acanthosis, hyperkeratosis, and cyst formation in mice [43]. Likewise, oncogenic NRF2 mutations have been detected in squamous cell carcinomas of the esophagus and skin [55,56,57]. In contrast to compromised skin structure and function that may originate from both impaired NRF2 activation as well as forced hyperactivation, NRF2 activation in healthy skin is transient and subject to extensive feedback regulation and modulatory crosstalk. Pharmacological modulation of NRF2 in skin aiming at a therapeutic, preventive, or regenerative benefit must therefore be performed without causing prolonged hyperactivation of the pathway as has been discussed before [56,58].
Wound healing. Recent research indicates that a glutathione-NRF2-thioredoxin cross-talk enables keratinocyte survival and wound repair through modulation of inflammation, apoptosis, and oxidative stress [59]. Importantly, substantial research has identified an essential role of NRF2 in diabetic wound healing, amenable to therapeutic intervention using small molecule NRF2 activators such as sulforaphane and cinnamaldehyde [32,60].
Psoriasis. In psoriasis, NRF2 is an important driver of keratinocyte proliferation with up-regulation of Keratin 6, Keratin 16, and Keratin 17 [61]. However, NRF2-directed intervention in psoriasis is efficacious since the anti-psoriatic drug monomethylfumarate increases NRF2 levels and induces aquaporin-3 mRNA and protein expression, important for keratinocyte differentiation [62].
Allergic dermatitis. NRF2 activation has been identified as a key event triggered by common skin sensitizers known be cysteine-directed electrophiles [63,64,65,66]. However, pharmacological NRF2 activation using ginger-derived 6-shogaol has shown efficacy in allergic dermatitis-like skin lesions through anti-inflammatory redox modulation [67].
Atopic dermatitis. Redox dysregulation is an emerging causative factor contributing to compromised skin barrier function in atopic dermatitis, and pharmacological intervention targeting NRF2 has shown promise targeting atopic dermatitis-like skin lesions in 2,4-dinitrochlorobenzene (DNCB)-sensitized and challenged mice [68,69].
Melanocytic dysfunction. It is now understood that NRF2 also plays an essential role in the maintenance of melanocyte responses to environmental stressors. NRF2 has been implicated in cutaneous pigmentation disorders resulting from redox alterations relevant to vitiligo and stress-induced and chronological hair greying [70,71,72,73]. Interestingly, recent evidence suggests that NRF2 plays a role in facilitating glutathione-dependent chemoresistance of malignant melanoma cells [74].
Chronological aging and progeria. Increasing evidence indicates a role of NRF2 in the control of chronological cellular aging [75,76,77]. Recently, an unanticipated mechanistic role of NRF2 dysfunction as a key contributor to premature aging has been proposed in the genetic premature aging disorder Hutchinson-Gilford progeria syndrome (HGPS), attributed to increased chronic oxidative stress [78,79]. In HGPS, a de novo LMNA (lamin A/C) gene mutation encodes for progerin, a dysfunctional nuclear architectural protein variant of lamin A lacking 50 amino acids. Progerin formation is also observed during normal cellular aging, and chronic UVA exposure has been shown to induce progerin in cultured human dermal fibroblasts [80]. Recent experimental evidence suggests that progerin sequesters NRF2 and thereby causes its subnuclear mislocalization, resulting in impaired NRF2 transcriptional activity and consequently increased chronic oxidative stress. Importantly, reactivation of NRF2 activity in HGPS patient cells reverses progerin-associated nuclear aging defects, suggesting that progerin-dependent repression of NRF2-mediated antioxidant responses is a key factor underlying HGPS-type premature aging with potential relevance to chronological aging and UVA-induced photoaging.
NRF2 in skin photodamage. Recent studies strongly suggest a protective role of NRF2-mediated gene expression in the suppression of cutaneous photodamage induced by solar UV radiation (as evidenced by suppression of UV-induced apoptosis and inflammatory signaling), and NRF2 activation has been shown to protect cutaneous keratinocytes and fibroblasts against the cytotoxic effects of UVA and UVB [16,18,19,31,33,81,82,83,84,85,86,87,88]. Importantly, research performed in SKH-1 mice documents that genetic NRF2 activation protects mice against acute photodamage and photocarcinogenesis [36,89]. Therefore, pharmacological modulation of NRF2 has now attracted considerable attention as a novel approach to skin photoprotection, cancer photochemoprevention, and suppression of skin photoaging [13,33,34,86]. Indeed, protection of primary human keratinocytes from UVB-induced cell death by novel drug-like NRF2 activators has been reported, a photoprotective effect attributed in part to NRF2-dependent elevation of cellular glutathione levels [40,87,90].
Our own studies have demonstrated the photoprotective effects of pharmacological NRF2 activation in cultured human skin cells and reconstructed epidermal skin models [13,31,33]. Topical application of NRF2 inducers, e.g., the synthetic NRF2-activator TBE-31, has shown pronounced photoprotective and photochemopreventive activity in murine skin, and suppression of solar UV-induced human skin erythema was achieved by topical application of a standardized broccoli extract delivering the NRF2 inducer sulforaphane [36]. However, little research has explored the concept of cutaneous photoprotection and photochemoprevention achievable by systemic administration of NRF2 inducers [13,91].
6. Systemic Photoprotection by Dietary NRF2 Activators: Focus on the Apocarotenoid Bixin, an FDA-Approved Food Colorant and Spice Native to Tropical America
The dietary origin of numerous photochemopreventive factors suggests the possibility of achieving efficient skin delivery through oral systemic administration, an emerging concept referred to as ‘nutritional’ or ‘systemic photoprotection’ [9]. Indeed, clinical studies document feasibility of human skin photoprotection by dietary intake of lycopene from processed tomato and flavonoid-rich cocoa [10,92,93,94]. In an attempt to test for the first time the feasibility of NRF2-dependent systemic photoprotection by dietary constituents, we focused our photoprotection studies on the apocarotenoid bixin (Figure 1 and Figure 2), an FDA-approved natural food colorant from the seeds of the achiote tree (Bixa orellana) native to tropical America [13,95,96]. A native spice derived from the Americas, annatto is an orange-red condiment and food coloring used to impart a yellow or orange color to signature foods of Latin America and the Caribbean.
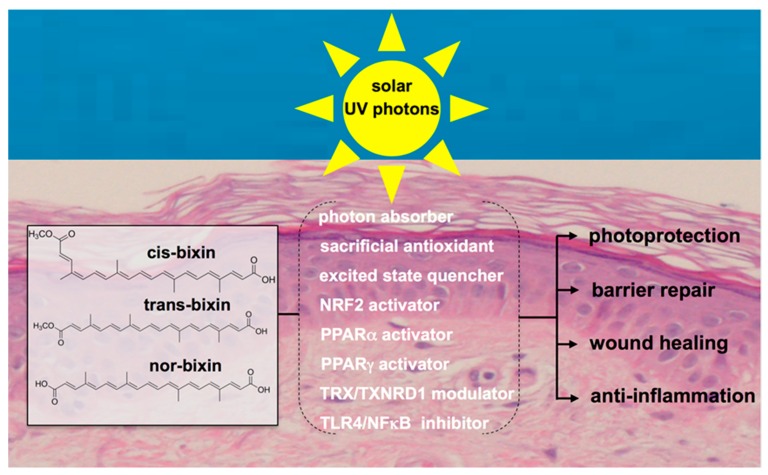
Figure 2.
Bixin for improved skin barrier function and photoprotection. Based on pleiotropic activities including direct chemical and NRF2-dependent antioxidant modulation, cis-bixin and its physiologically relevant derivatives trans-bixin and nor-bixin enhance skin barrier structure and function with photoprotective and potentially photochemopreventive efficacy; thioredoxin (TRX), thioredoxin reductase 1 (TXNRD1).
Consumed by human populations in the Americas since ancient times, this apocarotenoid, derived from lycopene through oxidative cleavage, is now used worldwide as a spice, food colorant, and cosmetic and pharmaceutical ingredient (referred to as ‘annatto’; E160b). Due to its unusual (linear/noncyclic) chemical structure, the apocarotenoid bixin displays characteristics different from all other carotenoids. Specifically, bixin is water soluble, does not display provitamin A activity, and is distinguished by an excellent safety record as well as established systemic bioavailability and pharmacokinetic profile upon oral administration as documented extensively in mice and humans [97,98,99]. Indeed, bixin is now one of the most consumed food colorants in the world distinguished by a long record of dietary and ethno-pharmacological use [95,96,100]. Chemical activities of bixin as sacrificial antioxidant, free radical scavenger, and efficient physical quencher of photoexcited states including singlet oxygen (surpassed only by lycopene) are documented [101]. Topical preparations of annatto extract have been in ethno-pharmacological use showing therapeutic efficacy for wound healing, mouth ulcers, and other pathologies associated with impaired epithelial barrier function [100,102]. It is also interesting that translational research documents the efficacy of bixin-loaded polycaprolactone nanofibers as an innovative delivery system accelerating wound healing and reducing scar tissue formation in diabetic mice [103]. Moreover, bixin-based systemic protection against environmental toxicants including methylmercury and carbon tetrachloride has been documented in vivo [104,105].
In prior studies, bixin has demonstrated antigenotoxic and antioxidant cytoprotective activities, and systemic availability of oral bixin and its demethylated metabolite norbixin has been documented in rodent studies and healthy human subjects [97,98,106,107]. In long term murine feeding experiments, supplementation levels up to 5% (w/w food) were well tolerated. Importantly, acceptable daily intake (ADI) over a lifetime without an appreciable health risk (http://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx) surpasses that of any other carotenoid approved as a food additive [ADI (bixin): 12 mg/kg body weight/day] [108].
7. Bixin for NRF2-Dependent Systemic Skin Photoprotection
Bixin was identified as the result of a screen for diet-derived small molecule NRF2 activators targeting oxidative stress and redox dysregulation in epithelial cells [13,109]. Using activity guided fractionation and bio-analytical tools for the quantitative detection of bixin and other small molecule constituents in annatto extracts, we were able to demonstrate that bixin is the active molecular entity in annatto total organic extracts responsible for NRF2 activation. Recently, we have reported for the first time that (i) bixin is a potent activator of the NRF2-dependent cytoprotective response in cultured human skin keratinocytes; (ii) systemic administration of bixin activates cutaneous NRF2 with potent protective effects against solar UV-induced skin damage in SKH-1 mice; and (iii) bixin-induced suppression of photodamage is observable in Nrf2+/+ but not in Nrf2−/− SKH-1 mice confirming the NRF2-dependence of bixin-based antioxidant and anti-inflammatory cutaneous effects [13]. Based on its unique status as a FDA-approved food additive with an established safety profile and potent NRF2-inducing activity, we also have investigated and established efficacy of systemic NRF2 activation using intraperitoneal administration of bixin for lung protection against ventilation-induced oxidative stress [110]. Importantly, dietary carotenoids (including β-carotene, lycopene, lutein, 3,3′-dihydroxyisorenieratene, zeaxanthin, astaxanthin) and their biosynthetic precursor molecules (such as phytoene) have been under investigation for epithelial chemoprevention and cutaneous photoprotection before [10,92,111,112,113], and the systemic photoprotective activity of carotenoids, displayed only after dietary uptake and cutaneous accumulation, has largely been attributed to their activity as photon absorbers, sacrificial antioxidants, and excited state/singlet oxygen quenchers [101,113,114].
Interestingly, it has been shown that astaxanthin and its analogs (such as adonixanthin) activate NRF2, preventing light-induced ocular photoreceptor degeneration [115]. Moreover, fucoxanthin, another marine carotenoid from seaweed, has been shown to enhance the level of reduced glutathione via NRF2 in human keratinocytes [116]. Indeed, prior research has examined the specific mechanism of NRF2 activation by carotenoids, and oxidative metabolism leading to the generation of electrophilic unsaturated mono- and dialdehydes (such 10,10′-diapocarotene-10,10′-dial) has been identified as the mechanistic basis underlying upregulated antioxidant responses [117,118,119]. The specific structure-activity relationship of NRF2 upregulation by carotenoid-derived electrophilic metabolites has been explored before, and it is therefore likely that bixin-dependent NRF2 activation requires similar oxidative transformation to electrophilic intermediates, a subject of ongoing investigation. However, even though the concept of cutaneous photoprotection achieved by systemic administration of specific carotenoids and other phytochemicals has been explored in the past [10,92,111,112,120,121,122], prior to our own investigations, no research had investigated the NRF2-dependence of carotenoid-based systemic photoprotection [13]. However, the biological effects of prolonged cutaneous NRF2 activation as a consequence of oral/systemic delivery of a pharmacological molecular agent that may also affect NRF2 regulation in non-cutaneous tissue remain to be elucidated.
8. Other Molecular Targets of Bixin with Relevance to Skin Barrier Function and Protection
Beyond NRF2-directed activities, bixin has been demonstrated to cause specific modulation of the following molecular targets potentially relevant to skin barrier function and environmental stress responses (Figure 2).
8.1. PPARα and PPARγ
Interestingly, peroxisome proliferator-activated receptors (PPARs) have now been recognized as important determinants of keratinocyte responses to skin injury regulating skin homeostasis, epithelial repair, and morphogenesis [123,124]. Specifically, PPARα is a ligand-activated transcription factor that regulates the expression of genes involved in fatty acid oxidation.
Recently, it has been demonstrated that oral administration of bixin improves obesity-induced abnormalities of carbohydrate and lipid metabolism in mice, an affect attributed to PPARα activation confirmed by luciferase reporter assays [125]. Specifically, treatment with bixin- and norbixin-induced PPARα target gene expression upstream of fatty acid oxidation in PPARα-expressing HepG2 hepatocytes. Likewise, in obese KK-Ay mice, chronic nutritional supplementation using bixin suppressed the development of hyperlipidemia and hepatic lipid accumulation with improvement of hyperglycemia, hyperinsulinemia, and hypoadiponectinemia. This effect is consistent with upregulated mRNA expression levels of adiponectin (ADIPOQ), an adipocyte-derived adipokine with multiple beneficial effects such as anti-obesity and anti-insulin resistance roles as well as anti-apoptotic, anti-oxidative, and anti-inflammatory activities in skin [124]. Likewise, experimental evidence suggests that bixin also enhances adipocyte insulin sensitivity downstream of PPARγ activation [126]. It is therefore tempting to speculate that the documented beneficial effects of bixin on cutaneous barrier function and wound healing may be in part attributable to PPARα/γ-directed agonism operative in addition to NRF2 activation as discussed above. However, the effects of prolonged pharmacological PPARα- or γ-directed agonism on skin barrier function remain to be explored.
8.2. Thioredoxin/Thioredoxin Reductase
One of the key cellular antioxidant systems is regulated by the selenoproteins thioredoxin (TRX) and thioredoxin reductase (TXNRD1), which use NADPH as an electron donor to reduce oxidized substrates. TXNRD1 contains a very reactive selenocysteine in its active site that is prone to electrophilic or oxidative attack, making it another important sensor of the cellular redox state in addition to KEAP1 [127]. Thus, electrophilic compounds that typically activate NRF2 by KEAP1 cysteine modifications will also inhibit TXNRD1 [127]. The TRX/TXNRD1 system is essential for keratinocyte survival, UV protection, and wound healing [59]. Interestingly, one report indicates that at high (200 μM) concentrations bixin generates ROS, inhibiting both TRX and TXNRD1 with induction of cell death [128]. This could be due to an exacerbated redox imbalance caused by the inability of TRX/TXNRD1 to reduce their substrates, such as peroxiredoxins (PRX), as well as de-repression of proapoptotic proteins, such as apoptosis signaling kinase 1 (ASK1), apoptosis inducing factor (AIF), and caspase 3. Other important substrates of the TRX/TXNRD1 system are PTEN (phosphatase and tensin homolog), NF-κB, AP1 (activator protein 1), and p53 (tumor protein 53), with important implications for regulation of cell survival in response to TRX/TXNRD1 dysruption [129]. Interestingly, it has been proposed that the TRX/TXNRD1 system might reduce the oxidized cysteine residues in KEAP1 to restore its functionality [130]. Dual inactivation of these reactive proteins (KEAP1 and TRX/TXNRD1) could contribute to pronounced NRF2 activation achieved by bixin. However, since TRX and TXNRD1 are NRF2 target genes, reduced proteins might be restored by de novo synthesis and GSH synthesis.
8.3. TLR4/NFκB
It has been observed that nutrional bixin attenuates cardiac injury progression through inhibition of fibrosis, inflammation, and redox dysregulation, cytoprotective effects that were attributed to Toll-like receptor 4/nuclear factor kappa B (TLR4/NF-κB) antagonism in mice [131]. Likewise, bixin antagonized lipopolysaccharide (LPS)-induced pro-inflammatory cytokine over-expression in cultured cardiac muscle cells. Given the emerging importance of TLR4 signaling in skin inflammation and UV-induced photodamage, it is therefore tempting to speculate that nutritional bixin regimens may benefit human skin through TLR4 antagonism operative in addition to NRF2 activation [132,133].
9. Conclusions
The promising concept of achieving cutaneous solar protection through dietary intake of NRF2 activators remains largely unexplored, representing an innovative molecular strategy that deserves further exploration. Building on its excellent safety record as an FDA-approved natural food colorant and additive, its systemic availability upon oral administration in humans, and ability to activate NRF2 in skin, dietary consumption of bixin, an ethno-pharmacologically validated phytochemical originating from the Americas, warrants future preclinical and clinical evaluation for improved skin barrier function and photoprotection.
Acknowledgments
Funding through the following NIH grants contributed to this review: R03CA167580, R03CA212719, ES007091, ES006694.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen H., Weng Q.Y., Fisher D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014;134:2080–2085. doi: 10.1038/jid.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natarajan V.T., Ganju P., Ramkumar A., Grover R., Gokhale R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014;10:542–551. doi: 10.1038/nchembio.1548. [DOI] [PubMed] [Google Scholar]
- 3.Brash D.E. UV signature mutations. Photochem. Photobiol. 2015;91:15–26. doi: 10.1111/php.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S.L., Justiniano R., Williams J.D., Cabello C.M., Qiao S., Wondrak G.T. The Tryptophan-Derived Endogenous Aryl Hydrocarbon Receptor Ligand 6-Formylindolo(3,2-b)Carbazole Is a Nanomolar UVA Photosensitizer in Epidermal Keratinocytes. J. Investig. Dermatol. 2015;135:1649–1658. doi: 10.1038/jid.2014.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liebel F., Kaur S., Ruvolo E., Kollias N., Southall M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012;132:1901–1907. doi: 10.1038/jid.2011.476. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima Y., Ohta S., Wolf A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017;108:300–310. doi: 10.1016/j.freeradbiomed.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder P., Calles C., Benesova T., Macaluso F., Krutmann J. Photoprotection beyond ultraviolet radiation—Effective sun protection has to include protection against infrared A radiation-induced skin damage. Skin Pharmacol. Physiol. 2010;23:15–17. doi: 10.1159/000257259. [DOI] [PubMed] [Google Scholar]
- 8.Zastrow L., Groth N., Klein F., Kockott D., Lademann J., Renneberg R., Ferrero L. The missing link—Light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009;22:31–44. doi: 10.1159/000188083. [DOI] [PubMed] [Google Scholar]
- 9.Wondrak G.T. Sunscreen-Based Skin Protection Against Solar Insult: Molecular Mechanisms and Opportunities. In: Alberts D., Hess L.M., editors. Fundamentals of Cancer Prevention. Springer; Berlin/Heidelberg, Garmany: 2014. pp. 301–320. [Google Scholar]
- 10.Gonzalez S., Astner S., An W., Goukassian D., Pathak M.A. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003;121:399–405. doi: 10.1046/j.1523-1747.2003.12355.x. [DOI] [PubMed] [Google Scholar]
- 11.Wondrak G.T., Jacobson M.K., Jacobson E.L. Endogenous UVA-photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006;5:215–237. doi: 10.1039/B504573H. [DOI] [PubMed] [Google Scholar]
- 12.Nichols J.A., Katiyar S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao S., Park S.L., de la Vega M.R., Zhang D.D., Wondrak G.T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015;89:690–700. doi: 10.1016/j.freeradbiomed.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diffey B.L., Norridge Z. Reported sun exposure, attitudes to sun protection and perceptions of skin cancer risk: A survey of visitors to Cancer Research UK’s SunSmart campaign website. Br. J. Dermatol. 2009;160:1292–1298. doi: 10.1111/j.1365-2133.2009.09149.x. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Saw C.L., Huang M.T., Liu Y., Khor T.O., Conney A.H., Kong A.N. Impact of Nrf2 on UVB-induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 2011;50:479–486. doi: 10.1002/mc.20725. [DOI] [PubMed] [Google Scholar]
- 17.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer M., Werner S. Nrf2-A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Hirota A., Kawachi Y., Yamamoto M., Koga T., Hamada K., Otsuka F. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp. Dermatol. 2011;20:664–668. doi: 10.1111/j.1600-0625.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 20.Bosch R., Philips N., Suarez-Perez J.A., Juarranz A., Devmurari A., Chalensouk-Khaosaat J., Gonzalez S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants. 2015;4:248–268. doi: 10.3390/antiox4020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D.D., Lo S.C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baird L., Lleres D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell. Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 27.Lau A., Wang X.J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: Direct interaction between Keap1 and p62. Mol. Cell. Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Gao A., Jiang N., Liu Q., Liang B., Li R., Zhang E., Li Z., Zhu H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem. Photobiol. 2016;92:808–815. doi: 10.1111/php.12628. [DOI] [PubMed] [Google Scholar]
- 31.Wondrak G.T., Cabello C.M., Villeneuve N.F., Zhang S., Ley S., Li Y., Sun Z., Zhang D.D. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic. Biol. Med. 2008;45:385–395. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long M., Rojo de la Vega M., Wen Q., Bharara M., Jiang T., Zhang R., Zhou S., Wong P.K., Wondrak G.T., Zheng H., et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes. 2016;65:780–793. doi: 10.2337/db15-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao S., Justiniano R., Zhang D.D., Wondrak G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013;1:532–541. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun K.S., Kundu J., Kundu J.K., Surh Y.J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol. Lett. 2014;229:73–84. doi: 10.1016/j.toxlet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Mathew S.T., Bergstrom P., Hammarsten O. Repeated Nrf2 stimulation using sulforaphane protects fibroblasts from ionizing radiation. Toxicol. Appl. Pharmacol. 2014;276:188–194. doi: 10.1016/j.taap.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Knatko E.V., Ibbotson S.H., Zhang Y., Higgins M., Fahey J.W., Talalay P., Dawa R., Ferguson J., Huang J.T., Clarke R., et al. Nrf2 activation protects against solar-simulated ultraviolet radiation in mice and humans. Cancer Prev. Res. 2015;8:475–486. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reisman S.A., Goldsberry A.R., Lee C.Y., O’Grady M.L., Proksch J.W., Ward K.W., Meyer C.J. Topical application of RTA 408 lotion activates Nrf2 in human skin and is well-tolerated by healthy human volunteers. BMC Dermatol. 2015;15:10. doi: 10.1186/s12895-015-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagami Y., Masuda K. A novel Nrf2 activator from microbial transformation inhibits radiation-induced dermatitis in mice. J. Radiat. Res. 2016;57:567–571. doi: 10.1093/jrr/rrw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishitsuka Y., Huebner A.J., Rice R.H., Koch P.J., Speransky V.V., Steven A.C., Roop D.R. Lce1 Family Members Are Nrf2-Target Genes that Are Induced to Compensate for the Loss of Loricrin. J. Investig. Dermatol. 2016;136:1656–1663. doi: 10.1016/j.jid.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafer M., Farwanah H., Willrodt A.H., Huebner A.J., Sandhoff K., Roop D., Hohl D., Bloch W., Werner S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012;4:364–379. doi: 10.1002/emmm.201200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar V., Bouameur J.E., Bar J., Rice R.H., Hornig-Do H.T., Roop D.R., Schwarz N., Brodesser S., Thiering S., Leube R.E., et al. A keratin scaffold regulates epidermal barrier formation, mitochondrial lipid composition, and activity. J. Cell Biol. 2015;211:1057–1075. doi: 10.1083/jcb.201404147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huebner A.J., Dai D., Morasso M., Schmidt E.E., Schafer M., Werner S., Roop D.R. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev. Cell. 2012;23:1238–1246. doi: 10.1016/j.devcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schafer M., Willrodt A.H., Kurinna S., Link A.S., Farwanah H., Geusau A., Gruber F., Sorg O., Huebner A.J., Roop D.R., et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014;6:442–457. doi: 10.1002/emmm.201303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurinna S., Schafer M., Ostano P., Karouzakis E., Chiorino G., Bloch W., Bachmann A., Gay S., Garrod D., Lefort K., et al. A novel Nrf2-miR-29-desmocollin-2 axis regulates desmosome function in keratinocytes. Nat. Commun. 2014;5:5099. doi: 10.1038/ncomms6099. [DOI] [PubMed] [Google Scholar]
- 45.Piao M.S., Park J.J., Choi J.Y., Lee D.H., Yun S.J., Lee J.B., Lee S.C. Nrf2-dependent and Nrf2-independent induction of phase 2 detoxifying and antioxidant enzymes during keratinocyte differentiation. Arch. Dermatol. Res. 2012;304:387–395. doi: 10.1007/s00403-012-1215-7. [DOI] [PubMed] [Google Scholar]
- 46.Jang J., Wang Y., Kim H.S., Lalli M.A., Kosik K.S. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2014;32:2616–2625. doi: 10.1002/stem.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmstrom K.M., Kostov R.V., Dinkova-Kostova A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins K.E., Joy S., Delhove J.M., Kotiadis V.N., Fernandez E., Fitzpatrick L.M., Whiteford J.R., King P.J., Bolanos J.P., Duchen M.R., et al. NRF2 Orchestrates the Metabolic Shift during Induced Pluripotent Stem Cell Reprogramming. Cell Rep. 2016;14:1883–1891. doi: 10.1016/j.celrep.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haarmann-Stemmann T., Abel J., Fritsche E., Krutmann J. The AhR-Nrf2 pathway in keratinocytes: On the road to chemoprevention? J. Investig. Dermatol. 2012;132:7–9. doi: 10.1038/jid.2011.359. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi N., Slocum S.L., Skoko J.J., Shin S., Kensler T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takei K., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara T., Furue M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015;234:74–80. doi: 10.1016/j.toxlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005;280:20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 53.Kim J.Y., Kim D.Y., Son H., Kim Y.J., Oh S.H. Protease-activated receptor-2 activates NQO-1 via Nrf2 stabilization in keratinocytes. J. Dermatol. Sci. 2014;74:48–55. doi: 10.1016/j.jdermsci.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D.R., et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y.R., Oh J.E., Kim M.S., Kang M.R., Park S.W., Han J.Y., Eom H.S., Yoo N.J., Lee S.H. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J. Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 56.Lau A., Villeneuve N.F., Sun Z., Wong P.K., Zhang D.D. Dual roles of Nrf2 in cancer. Pharmacol. Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X.J., Sun Z., Villeneuve N.F., Zhang S., Zhao F., Li Y., Chen W., Yi X., Zheng W., Wondrak G.T., et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harder B., Jiang T., Wu T., Tao S., Rojo de la Vega M., Tian W., Chapman E., Zhang D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015;43:680–686. doi: 10.1042/BST20150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Telorack M., Meyer M., Ingold I., Conrad M., Bloch W., Werner S. A Glutathione-Nrf2-Thioredoxin Cross-Talk Ensures Keratinocyte Survival and Efficient Wound Repair. PLoS Genet. 2016;12:e1005800. doi: 10.1371/journal.pgen.1005800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng H., Whitman S.A., Wu W., Wondrak G.T., Wong P.K., Fang D., Zhang D.D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang L., Fan X., Cui T., Dang E., Wang G. Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17. J. Investig. Dermatol. 2017;137:2168–2176. doi: 10.1016/j.jid.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Helwa I., Choudhary V., Chen X., Kaddour-Djebbar I., Bollag W.B. Anti-Psoriatic Drug Monomethylfumarate Increases Nuclear Factor Erythroid 2-Related Factor 2 Levels and Induces Aquaporin-3 mRNA and Protein Expression. J. Pharmacol. Exp. Ther. 2017;362:243–253. doi: 10.1124/jpet.116.239715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natsch A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—Functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol. Sci. 2010;113:284–292. doi: 10.1093/toxsci/kfp228. [DOI] [PubMed] [Google Scholar]
- 64.Delaine T., Niklasson I.B., Emter R., Luthman K., Karlberg A.T., Natsch A. Structure-activity relationship between the in vivo skin sensitizing potency of analogues of phenyl glycidyl ether and the induction of Nrf2-dependent luciferase activity in the KeratinoSens in vitro assay. Chem. Res. Toxicol. 2011;24:1312–1318. doi: 10.1021/tx200196s. [DOI] [PubMed] [Google Scholar]
- 65.Natsch A., Emter R. Nrf2 activation as a key event triggered by skin sensitisers: The development of the stable KeratinoSens reporter gene assay. Altern. Lab. Anim. 2016;44:443–451. doi: 10.1177/026119291604400513. [DOI] [PubMed] [Google Scholar]
- 66.El Ali Z., Delomenie C., Botton J., Pallardy M., Kerdine-Romer S. Dendritic cells’ death induced by contact sensitizers is controlled by Nrf2 and depends on glutathione levels. Toxicol. Appl. Pharmacol. 2017;322:41–50. doi: 10.1016/j.taap.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 67.Park G., Oh D.S., Lee M.G., Lee C.E., Kim Y.U. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol. Appl. Pharmacol. 2016;310:51–59. doi: 10.1016/j.taap.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Akram M., Shin I., Kim K.A., Noh D., Baek S.H., Chang S.Y., Kim H., Bae O.N. A newly synthesized macakurzin C-derivative attenuates acute and chronic skin inflammation: The Nrf2/heme oxygenase signaling as a potential target. Toxicol. Appl. Pharmacol. 2016;307:62–71. doi: 10.1016/j.taap.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 69.Choi J.H., Jin S.W., Han E.H., Park B.H., Kim H.G., Khanal T., Hwang Y.P., Do M.T., Lee H.S., Chung Y.C., et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053–1061. doi: 10.1016/j.phymed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 70.Marrot L., Jones C., Perez P., Meunier J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008;21:79–88. doi: 10.1111/j.1755-148X.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 71.Denat L., Kadekaro A.L., Marrot L., Leachman S.A., Abdel-Malek Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014;134:1512–1518. doi: 10.1038/jid.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jadkauskaite L., Coulombe P.A., Schafer M., Dinkova-Kostova A.T., Paus R., Haslam I.S. Oxidative stress management in the hair follicle: Could targeting NRF2 counter age-related hair disorders and beyond? Bioessays. 2017;39 doi: 10.1002/bies.201700029. [DOI] [PubMed] [Google Scholar]
- 73.Jian Z., Li K., Song P., Zhu G., Zhu L., Cui T., Liu B., Tang L., Wang X., Wang G., Gao T., Li C. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: A possible mechanism for melanocyte degeneration in vitiligo. J. Investig. Dermatol. 2014;134:2221–2230. doi: 10.1038/jid.2014.152. [DOI] [PubMed] [Google Scholar]
- 74.Rocha C.R., Kajitani G.S., Quinet A., Fortunato R.S., Menck C.F. NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells. Oncotarget. 2016;7:48081–48092. doi: 10.18632/oncotarget.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kubo E., Chhunchha B., Singh P., Sasaki H., Singh D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017;7:14130. doi: 10.1038/s41598-017-14520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruns D.R., Drake J.C., Biela L.M., Peelor F.F., 3rd, Miller B.F., Hamilton K.L. Nrf2 Signaling and the Slowed Aging Phenotype: Evidence from Long-Lived Models. Oxid. Med. Cell. Longev. 2015;2015:732596. doi: 10.1155/2015/732596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kubben N., Zhang W., Wang L., Voss T.C., Yang J., Qu J., Liu G.H., Misteli T. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorbunova V., Rezazadeh S., Seluanov A. Dangerous Entrapment for NRF2. Cell. 2016;165:1312–1313. doi: 10.1016/j.cell.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 80.Takeuchi H., Runger T.M. Longwave UV light induces the aging-associated progerin. J. Investig. Dermatol. 2013;133:1857–1862. doi: 10.1038/jid.2013.71. [DOI] [PubMed] [Google Scholar]
- 81.Hirota A., Kawachi Y., Itoh K., Nakamura Y., Xu X., Banno T., Takahashi T., Yamamoto M., Otsuka F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: Protective role in UVA-induced apoptosis. J. Investig. Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 82.Dinkova-Kostova A.T., Jenkins S.N., Fahey J.W., Ye L., Wehage S.L., Liby K.T., Stephenson K.K., Wade K.L., Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 83.Benedict A.L., Knatko E.V., Dinkova-Kostova A.T. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis. 2012;33:2457–2466. doi: 10.1093/carcin/bgs293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gruber F., Mayer H., Lengauer B., Mlitz V., Sanders J.M., Kadl A., Bilban M., de Martin R., Wagner O., Kensler T.W., et al. NF-E2-related factor 2 regulates the stress response to UVA-1-oxidized phospholipids in skin cells. FASEB J. 2010;24:39–48. doi: 10.1096/fj.09-133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian F.F., Zhang F.F., Lai X.D., Wang L.J., Yang L., Wang X., Singh G., Zhong J.L. Nrf2-mediated protection against UVA radiation in human skin keratinocytes. Biosci. Trends. 2011;5:23–29. doi: 10.5582/bst.2011.v5.1.23. [DOI] [PubMed] [Google Scholar]
- 86.Kalra S., Knatko E.V., Zhang Y., Honda T., Yamamoto M., Dinkova-Kostova A.T. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): Implications for protection against UV radiation during thiopurine therapy. Cancer Prev. Res. 2012;5:973–981. doi: 10.1158/1940-6207.CAPR-12-0041. [DOI] [PubMed] [Google Scholar]
- 87.Schafer M., Dutsch S., auf dem Keller U., Navid F., Schwarz A., Johnson D.A., Johnson J.A., Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010;24:1045–1058. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolfs F., Huber M., Kuehne A., Kramer S., Haertel E., Muzumdar S., Wagner J., Tanner Y., Bohm F., Smola S., et al. Nrf2 Activation Promotes Keratinocyte Survival during Early Skin Carcinogenesis via Metabolic Alterations. Cancer Res. 2015;75:4817–4829. doi: 10.1158/0008-5472.CAN-15-0614. [DOI] [PubMed] [Google Scholar]
- 89.Knatko E.V., Higgins M., Fahey J.W., Dinkova-Kostova A.T. Loss of Nrf2 abrogates the protective effect of Keap1 downregulation in a preclinical model of cutaneous squamous cell carcinoma. Sci. Rep. 2016;6:25804. doi: 10.1038/srep25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lieder F., Reisen F., Geppert T., Sollberger G., Beer H.D., auf dem Keller U., Schafer M., Detmar M., Schneider G., Werner S. Identification of UV-protective activators of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) by combining a chemical library screen with computer-based virtual screening. J. Biol. Chem. 2012;287:33001–33013. doi: 10.1074/jbc.M112.383430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dinkova-Kostova A.T., Fahey J.W., Benedict A.L., Jenkins S.N., Ye L., Wehage S.L., Talalay P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010;9:597–600. doi: 10.1039/b9pp00130a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sies H., Stahl W. Nutritional Protection Against Skin Damage From Sunlight. Annu. Rev. Nutr. 2004;24:173–200. doi: 10.1146/annurev.nutr.24.012003.132320. [DOI] [PubMed] [Google Scholar]
- 93.Heinrich U., Neukam K., Tronnier H., Sies H., Stahl W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J. Nutr. 2006;136:1565–1569. doi: 10.1093/jn/136.6.1565. [DOI] [PubMed] [Google Scholar]
- 94.Stahl W., Heinrich U., Wiseman S., Eichler O., Sies H., Tronnier H. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J. Nutr. 2001;131:1449–1451. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- 95.Ulbricht C., Windsor R.C., Brigham A., Bryan J.K., Conquer J., Costa D., Giese N., Guilford J., Higdon E.R., Holmes K., et al. An evidence-based systematic review of annatto (Bixa orellana L.) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2012;9:57–77. doi: 10.3109/19390211.2012.653530. [DOI] [PubMed] [Google Scholar]
- 96.Stohs S.J. Safety and efficacy of Bixa orellana (achiote, annatto) leaf extracts. Phytother. Res. 2014;28:956–960. doi: 10.1002/ptr.5088. [DOI] [PubMed] [Google Scholar]
- 97.Levy L.W., Regalado E., Navarrete S., Watkins R.H. Bixin and norbixin in human plasma: Determination and study of the absorption of a single dose of Annatto food color. Analyst. 1997;122:977–980. doi: 10.1039/a701304c. [DOI] [PubMed] [Google Scholar]
- 98.Junior A.C., Asad L.M., Oliveira E.B., Kovary K., Asad N.R., Felzenszwalb I. Antigenotoxic and antimutagenic potential of an annatto pigment (norbixin) against oxidative stress. Genet. Mol. Res. 2005;4:94–99. [PubMed] [Google Scholar]
- 99.World Health Organization Evaluation of certain food additives and contaminants. World Health Organ. Tech. Rep. Ser. 2013;983:1–75. [PubMed] [Google Scholar]
- 100.Vilar Dde A., Vilar M.S., de Lima e Moura T.F., Raffin F.N., de Oliveira M.R., Franco C.F., de Athayde-Filho P.F., Diniz Mde F., Barbosa-Filho J.M. Traditional uses, chemical constituents, and biological activities of Bixa orellana L.: A review. Sci. World J. 2014;2014:857292. doi: 10.1155/2014/857292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 102.Piva R.M., Johann A.C., Costa C.K., Miguel O.G., Rosa E.R., de Azevedo-Alanis L.R., Trevilatto P.C., Ignacio S.A., Bettega P.V., Gregio A.M. Bixin action in the healing process of rats mouth wounds. Curr. Pharm. Biotechnol. 2013;14:785–791. doi: 10.2174/1389201014666131227111026. [DOI] [PubMed] [Google Scholar]
- 103.Pinzon-Garcia A.D., Cassini-Vieira P., Ribeiro C.C., de Matos Jensen C.E., Barcelos L.S., Cortes M.E., Sinisterra R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:1938–1949. doi: 10.1002/jbm.b.33724. [DOI] [PubMed] [Google Scholar]
- 104.Barcelos G.R., Grotto D., Serpeloni J.M., Aissa A.F., Antunes L.M., Knasmuller S., Barbosa F., Jr. Bixin and norbixin protect against DNA-damage and alterations of redox status induced by methylmercury exposure in vivo. Environ. Mol. Mutagen. 2012;53:535–541. doi: 10.1002/em.21715. [DOI] [PubMed] [Google Scholar]
- 105.Moreira P.R., Maioli M.A., Medeiros H.C., Guelfi M., Pereira F.T., Mingatto F.E. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biol. Res. 2014;47:49. doi: 10.1186/0717-6287-47-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Somacal S., Figueiredo C.G., Quatrin A., Ruviaro A.R., Conte L., Augusti P.R., Roehrs M., Denardin I.T., Kasten J., da Veiga M.L., et al. The antiatherogenic effect of bixin in hypercholesterolemic rabbits is associated to the improvement of lipid profile and to its antioxidant and anti-inflammatory effects. Mol. Cell. Biochem. 2015;403:243–253. doi: 10.1007/s11010-015-2354-x. [DOI] [PubMed] [Google Scholar]
- 107.Roehrs M., Figueiredo C.G., Zanchi M.M., Bochi G.V., Moresco R.N., Quatrin A., Somacal S., Conte L., Emanuelli T. Bixin and norbixin have opposite effects on glycemia, lipidemia, and oxidative stress in streptozotocin-induced diabetic rats. Int. J. Endocrinol. 2014;2014:839095. doi: 10.1155/2014/839095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.WHO (World Health Organization) Evaluation of Certain Food Additives and Contaminants. Thirty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives. Tech. Rep. Ser. 1990;789:1–48. [PubMed] [Google Scholar]
- 109.Long M., Tao S., Rojo de la Vega M., Jiang T., Wen Q., Park S.L., Zhang D.D., Wondrak G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev. Res. 2015;8:444–454. doi: 10.1158/1940-6207.CAPR-14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tao S., Rojo de la Vega M., Quijada H., Wondrak G.T., Wang T., Garcia J.G., Zhang D.D. Bixin protects mice against ventilation-induced lung injury in an NRF2-dependent manner. Sci. Rep. 2016;6:18760. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Astner S., Wu A., Chen J., Philips N., Rius-Diaz F., Parrado C., Mihm M.C., Goukassian D.A., Pathak M.A., Gonzalez S. Dietary lutein/zeaxanthin partially reduces photoaging and photocarcinogenesis in chronically UVB-irradiated Skh-1 hairless mice. Skin Pharmacol. Physiol. 2007;20:283–291. doi: 10.1159/000107576. [DOI] [PubMed] [Google Scholar]
- 112.Stahl W., Sies H. beta-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012;96:1179S–1184S. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez-Garcia E. Skin protection against UV light by dietary antioxidants. Food Funct. 2014;5:1994–2003. doi: 10.1039/C4FO00280F. [DOI] [PubMed] [Google Scholar]
- 114.Di Mascio P., Devasagayam T.P., Kaiser S., Sies H. Carotenoids, tocopherols and thiols as biological singlet molecular oxygen quenchers. Biochem. Soc. Trans. 1990;18:1054–1056. doi: 10.1042/bst0181054. [DOI] [PubMed] [Google Scholar]
- 115.Inoue Y., Shimazawa M., Nagano R., Kuse Y., Takahashi K., Tsuruma K., Hayashi M., Ishibashi T., Maoka T., Hara H. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017;134:147–157. doi: 10.1016/j.jphs.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 116.Zheng J., Piao M.J., Kim K.C., Yao C.W., Cha J.W., Hyun J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs. 2014;12:4214–4230. doi: 10.3390/md12074214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben-Dor A., Steiner M., Gheber L., Danilenko M., Dubi N., Linnewiel K., Zick A., Sharoni Y., Levy J. Carotenoids activate the antioxidant response element transcription system. Mol. Cancer Ther. 2005;4:177–186. [PubMed] [Google Scholar]
- 118.Linnewiel K., Ernst H., Caris-Veyrat C., Ben-Dor A., Kampf A., Salman H., Danilenko M., Levy J., Sharoni Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009;47:659–667. doi: 10.1016/j.freeradbiomed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 119.Linnewiel-Hermoni K., Khanin M., Danilenko M., Zango G., Amosi Y., Levy J., Sharoni Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015;572:28–35. doi: 10.1016/j.abb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 120.Vayalil P.K., Mittal A., Hara Y., Elmets C.A., Katiyar S.K. Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Investig. Dermatol. 2004;122:1480–1487. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 121.Gonzalez S., Gilaberte Y., Philips N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010;9:559–563. doi: 10.1039/b9pp00156e. [DOI] [PubMed] [Google Scholar]
- 122.Chen A.C., Damian D.L., Halliday G.M. Oral and systemic photoprotection. Photodermatol. Photoimmunol. Photomed. 2014;30:102–111. doi: 10.1111/phpp.12100. [DOI] [PubMed] [Google Scholar]
- 123.Icre G., Wahli W., Michalik L. Functions of the peroxisome proliferator-activated receptor (PPAR) alpha and beta in skin homeostasis, epithelial repair, and morphogenesis. J. Investig. Dermatol. Symp. Proc. 2006;11:30–35. doi: 10.1038/sj.jidsymp.5650007. [DOI] [PubMed] [Google Scholar]
- 124.Kim E.J., Lee D.H., Kim Y.K., Eun H.C., Chung J.H. Adiponectin Deficiency Contributes to Sensitivity in Human Skin. J. Investig. Dermatol. 2015;135:2331–2334. doi: 10.1038/jid.2015.150. [DOI] [PubMed] [Google Scholar]
- 125.Goto T., Takahashi N., Kato S., Kim Y.I., Kusudo T., Taimatsu A., Egawa K., Kang M.S., Hiramatsu T., Sakamoto T., et al. Bixin activates PPARalpha and improves obesity-induced abnormalities of carbohydrate and lipid metabolism in mice. J. Agric. Food Chem. 2012;60:11952–11958. doi: 10.1021/jf303639f. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi N., Goto T., Taimatsu A., Egawa K., Katoh S., Kusudo T., Sakamoto T., Ohyane C., Lee J.Y., Kim Y.I., et al. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARgamma activation. Biochem. Biophys. Res. Commun. 2009;390:1372–1376. doi: 10.1016/j.bbrc.2009.10.162. [DOI] [PubMed] [Google Scholar]
- 127.Cebula M., Schmidt E.E., Arner E.S. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015;23:823–853. doi: 10.1089/ars.2015.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tibodeau J.D., Isham C.R., Bible K.C. Annatto constituent cis-bixin has selective antimyeloma effects mediated by oxidative stress and associated with inhibition of thioredoxin and thioredoxin reductase. Antioxid. Redox Signal. 2010;13:987–997. doi: 10.1089/ars.2009.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang J., Li X., Han X., Liu R., Fang J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017;38:794–808. doi: 10.1016/j.tips.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Dinkova-Kostova A.T., Kostov R.V., Canning P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017;617:84–93. doi: 10.1016/j.abb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu Z., Kong X.Q. Bixin ameliorates high fat diet-induced cardiac injury in mice through inflammation and oxidative stress suppression. Biomed. Pharmacother. 2017;89:991–1004. doi: 10.1016/j.biopha.2017.02.052. [DOI] [PubMed] [Google Scholar]
- 132.Dickinson S.E., Wondrak G.T. TLR4-directed Molecular Strategies Targeting Skin Photodamage and Carcinogenesis. Curr. Med. Chem. 2017 doi: 10.2174/0929867324666170828125328. [DOI] [PubMed] [Google Scholar]
- 133.Janda J., Burkett N.B., Blohm-Mangone K., Huang V., Curiel-Lewandrowski C., Alberts D.S., Petricoin E.F., 3rd, Calvert V.S., Einspahr J., Dong Z., et al. Resatorvid-based Pharmacological Antagonism of Cutaneous TLR4 Blocks UV-induced NF-kappaB and AP-1 Signaling in Keratinocytes and Mouse Skin. Photochem. Photobiol. 2016;92:816–825. doi: 10.1111/php.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]