Abstract
Disrupted kynurenine pathway (KP) metabolism has been implicated in the progression of neurodegenerative disease, psychiatric disorders and cancer. Modulation of enzyme activity along this pathway may therefore offer potential new therapeutic strategies for these conditions. Considering their prominent positions in the KP, the enzymes indoleamine 2,3-dioxygenase, kynurenine 3-monooxygenase and kynurenine aminotransferase, appear the most attractive targets. Already, increasing interest in this pathway has led to the identification of a number of potent and selective enzyme inhibitors with promising pre-clinical data and the elucidation of several enzyme crystal structures provides scope to rationalize the molecular mechanisms of inhibitor activity. The field seems poised to yield one or more inhibitors that should find clinical utility.
Keywords: Kynurenine pathway; neurodegeneration; cancer; indoleamine 2,3-dioxygenase; kynurenine 3-monooxygenase; enzyme inhibitors
1. INTRODUCTION
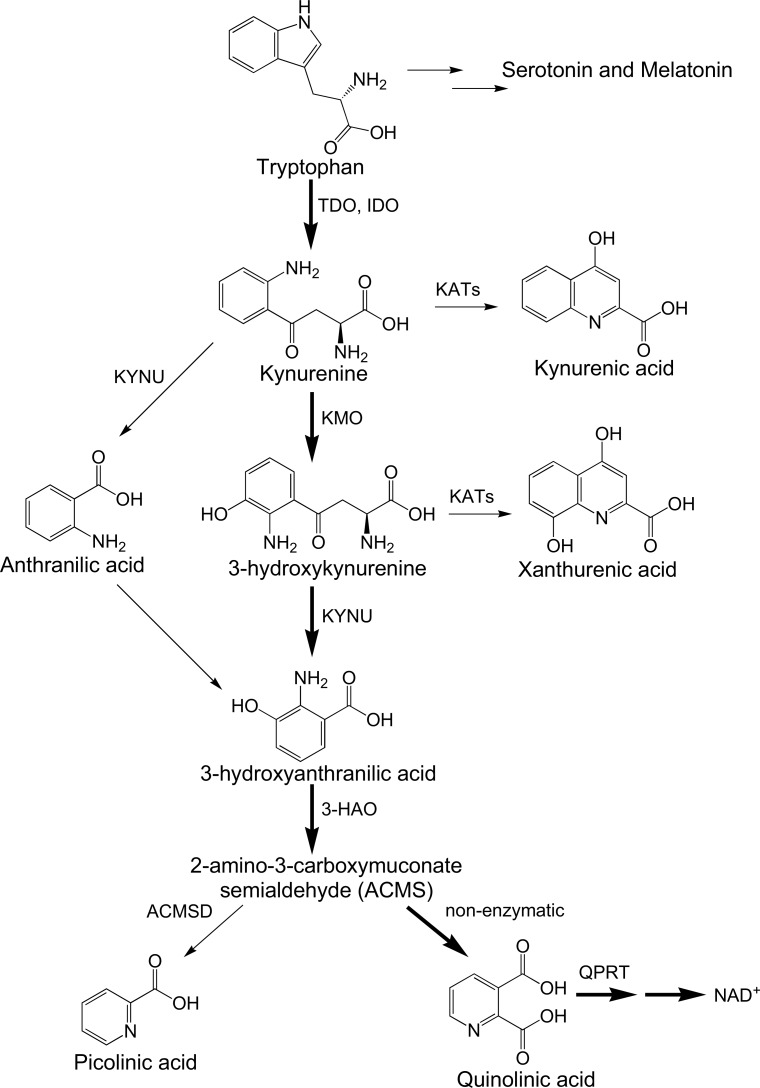
The kynurenine pathway (KP) (Fig. 1) is the major catabolic route of tryptophan (TRP) which ultimately leads to the de novo synthesis of the essential metabolic co-factor nicotinamide adenine dinucleotide (NAD+) [1]. Groundbreaking studies by Mellor and colleagues described that the first enzyme in the KP, indoleamine 2,3-dioxygenase (IDO-1) was essential for preventing foetal rejection by dampening the maternal immune response [2]. It was then hypothesized that IDO-1 may similarly facilitate tumor escape from immune system surveillance and several studies confirmed this link [3]. Small molecule inhibitors of IDO-1 have thus attracted attention as potential cancer chemotherapeutics with several progressing to clinical trial [4, 5]. More recently, it was shown that the IDO-1 product, kynurenine (KYN) interacts with the aryl hydrocarbon receptor (AhR) resulting in a cascade of events that leads to the suppression of immune cell proliferation and tumor growth [6].
Fig. (1).
Simplified diagram of the kynurenine pathway.
The KP also has a role in various neurodegenerative and psychiatric disorders where a variety of data show modulated KP enzyme activity and/or altered metabolite expression [7-11]. Induction of glutamatergic activity at the N-methyl-D-aspartate (NMDA) receptor by the KP metabolite quinolinic acid (QUIN) has been viewed as the central excitotoxic mechanism but at least six other neurotoxic mechanisms have been attributed to QUIN [12]. Aside from IDO-1, other major enzymes of the KP include kynurenine 3-monooxy-genase (KMO), kynurenine aminotransferase (KAT) and quinolinic acid phosphoribosyltransferase (QPRT) [13].
Considering the accumulating evidence implicating the KP in human disease, we comprehensively review the essential characteristics of enzymes and metabolites along this pathway together with the major classes of KP enzyme inhibitors, their key significant structural features, structure-activity relationships and future promise as therapeutics for cancer or neurodegenerative disease.
1.1. Indoleamine 2,3-Dioygenase (IDO) and Tryptophan Dioxygenase (TDO)
The enzymes that catalyze the first step of L-TRP catabolism are IDO-1, IDO-2 or TDO [14, 15]. These oxidatively cleave L-TRP to form N-formyl-L-kynurenine, which is hydrolyzed to the first stable metabolite of the KP, L-kynurenine (KYN). However, IDO-1, IDO-2 and TDO have different substrate specificities with the IDO enzymes capable of oxidizing both L- and D-TRP, whereas TDO shows a pronounced specificity for L-TRP, potentially reflecting the presence of a hydrogen bond between T342 and the substrate ammonium group in the L-TRP stereoisomer [16].
TDO is constitutively active in liver but is expressed in glioma and other cancer cells [13]. Increased TDO expression is correlated to tumor cell proliferation and malignancy [6]. TDO is inducible by TRP, tyrosine, histidine, glucocorticoids and KYN [17]. In comparison, IDO-1 is expressed in many cells including endothelial cells, smooth muscle cells, fibroblasts, astrocytes, macrophages, microglia and dendritic cells. It is up-regulated by certain cytokines and inflammatory molecules, such as lipopolysaccharides, amyloid peptides and HIV proteins [18, 19] but the most potent IDO-1 inducer is interferon gamma (IFN-γ) [20], which increases both gene expression and enzymatic activity [21]. IDO-2 is detected in human liver, small intestine, spleen, placenta, thymus, lung, brain, kidney, and colon [14, 15]. Similarly to TDO, IDO is expressed by a variety of tumor cells, with high IDO expression correlating to low survival rate in ovarian [22], hepatocellular [23] and endometrial cancers [24].
1.2. Kynurenine 3-Monoxygenase (KMO)
KMO is a flavin adenine dinucleotide (FAD)-dependent monooxygenase located on the outer mitochondrial membrane [25]. It catalyzes the incorporation of one atom of oxygen into KYN in the presence of NADPH resulting in the formation of 3-hydroxy-kynurenine (3-HK) and water [26].
KMO is primarily expressed in the liver and kidney. In the central nervous system, it is expressed by microglial cells and infiltrating macrophages but not by neurons or astrocytes [27].
KMO is positioned at a significant branch point in the KP, the neurotoxic metabolites 3-HK and QUIN (see sections 2.3 and 2.5 below, respectively) are produced one- and three-steps downstream respectively, but production of the neuroprotective metabolite kynurenic acid (KYNA; see section 2.2 below) is immediately upstream to KMO activity (Fig. 1). KMO inhibition may therefore favorably rebalance the KP by decreasing levels of neurotoxic metabolites while increasing neuroprotective KYNA. This hypothesis is supported by studies in KMO -/- mice which showed significantly reduced levels of 3-HK and QUIN coupled with increases in KYN, KYNA and anthranilic acid (AA) in brain, liver and plasma compared to wild type (WT) animals. Indeed, KMO inhibition in mouse models of Huntington’s and Alzheimer’s Disease showed a variety of beneficial effects [28].
While most interest in KMO centers on its roles in neurodegenerative diseases, emerging evidence suggests that excessive KMO activity stimulates tumor growth [29]. Most recently, KMO inhibition has been highlighted as protective against multiple organ dysfunction syndrome (MODS) in a rat model of acute pancreatitis [30].
1.3. Kynurenine Aminotransferase (KAT)
The KAT family contains 4 homologous pyridoxal phosphate (PLP) dependent enzymes KAT-I, II, III and IV which catalyze the cyclization of L-KYN to form KYNA [31]. In the brain, both KAT-I and KAT-II are expressed; however, KAT-II is the enzyme that predominantly produces KYNA [32-34]. KYNA has been shown to play a neuroprotective role in the brain, but accumulation of KYNA has been linked to psychiatric disorders including schizophrenia [35]. The KAT-II isozyme, in particular, has been identified as a promising neuropsychiatric target as it contains a number of unique structural features that may make it possible to develop an isozyme-specific inhibitor that targets KYNA production while leaving the remaining isoforms active [36].
1.4. Kynureninase (KYNU) and 3-Hydroxy-anthranilic Acid Oxygenase (3-HAO)
Kynureninase (KYNU) catalytically cleaves its substrates 3-HK or KYN into 3-hydroxyanthranillic acid (3-HAA) or anthranilic acid (AA), respectively [37]. In humans, KYNU more rapidly cleaves 3-HK and is therefore the major route of TRP catabolism [38]. As KYNU may also convert KYN into AA, which may then yield 3-HAA by the action of non-specific oxidases, KYNU may participate in a potential compensatory mechanism leading to the production of QUIN. 3-hydroxyanthranlic acid oxygenase (3-HAO) with an additional non-enzymatic step converts 3-HAA to QUIN. Interestingly, AA prevents synthesis of QUIN through inhibition of 3-HAO [39].
1.5. Quinolinic Acid Phosphoribosyltransferase (QPRT)
Quinolinic acid phosphoribosyltransferase (QPRT) catalytically converts QUIN into nicotinamide ribonucleotide (NANM), which is the first step of de novo NAD+ synthesis. QPRT exists at an important point in the KP, adjacent to NAD+ synthesis. Brain efflux or catabolism by QPRT are the only known methods by which QUIN is removed from the brain [40]. Cerebral neurons readily take up exogenous QUIN, but as QPRT becomes rapidly saturated, only a small quantity of QUIN can be metabolized, leading to a metabolic bottle-neck that results in QUIN accumulation [41]. Hence, QPRT has significant potential as a therapeutic target [42], as an activator of this enzyme could potentially reverse neurotoxic QUIN accumulation. Enhancement of QPRT activity would also result in an increase in NAD+ which has been hypothesized to promote cellular longevity [43].
2. BIOACTIVE METABOLITES OF THE KP
2.1. Kynurenine
KYN has been shown to cross the blood brain barrier (BBB) via the large neutral amino acid transporter [44]. Accordingly, increased peripheral KP activity can lead to an increase of KYN in the central nervous system (CNS) [44]. KYN has been described as an immunomodulator, it suppresses allogeneic T-cell proliferation and converts naïve T cells into regulatory T-cells (Treg) [45, 46]. More recently, KYN has also been shown to be an endogenous aryl hydrocarbon receptor (AhR) ligand [6]. The AhR was shown to have ligand-specific effects in promoting T-reg differentiation and T-helper (Th17) development [6, 47]. Activation of the AhR has been implicated in a number of cellular processes including inflammation and tumorigenesis. Essentially, when KYN binds to the AhR of an immune-cell, it is translocated to the nucleus where it binds dioxin responsive elements (DRE) in the promoter region of genes that suppress immune-cell proliferation and function thereby suppressing the immune response. The critical role of the TDO-AhR pathway was first shown in human brain tumors and was associated with both malignant progression and poor survival [6]. Other studies have since highlighted the role of the KYN-AhR interaction in breast cancer. D’Amato et al., described how the TDO-AhR signaling axis facilitated resistance to cell-death mechanisms and promoted metastasis in triple-negative breast cancer [48, 49].
2.2. Kynurenic Acid
KYNA plays an important role in the CNS, but does not cross the BBB and its presence in the brain is dependent upon its local production by astrocytes [33, 44]. KYNA acts as an antioxidant with the ability to scavenge hydroxyl and other reactive oxygen species (ROS) [50]. KYNA is a broad-spectrum endogenous antagonist of ionotropic excitatory amino acid (EAA) receptors which includes the NMDA, kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [51]. KYNA also inhibits α-7 nicotinic acetyl choline receptors (α7nAChR), resulting in the inhibition of glutamate release at the axonal terminals of the striatum thereby further reducing excitotoxicity [52]. However, other experiments showed that KYNA had little effect on α7nAChR in the sub micromolar range [53]. KMO inhibitors act to increase KYNA concentrations and have been shown to reduce glutamate concentration in the extra cellular space of the basal ganglia [54] and attenuate symptoms in some models of neurodegenerative disease [28, 55]. Conversely, both the NMDA and α7nAChR play important roles in psychosis and other cognitive phenomena. Increased levels of KYNA in the brain have been shown to lead to a decline in cognitive abilities in animal models whereas improvements were observed following acute reductions in hippocampal KYNA [56-58]. These studies highlight the dual physiological roles of KYNA in the brain, which should be considered in the development of therapeutic modulators of the KP.
2.3. 3-Hydroxykynurenine
It has been conventionally thought that 3-HK is an endogenous neurotoxin that leads to the formation of ROS including H2O2 and superoxide. Treatment of primary neuronal cell cultures with concentrations of 3-HK similar to those found at pathophysiological levels, led to apoptotic cell death [59, 60]. Conversely, 3-HK has been shown to be an effective scavenger of peroxyl radicals [61] and was shown to reduce several parameters of oxidative stress in both rat cerebral cortex and C6 glioma cells [62]. Other studies support the duality of 3-HK as both a pro- and anti-oxidant. When administered in vitro to isolated striatal slices, 3-HK was pro-oxidant at sub 20 µM concentrations but exhibited antioxidant activity at 100 µM. In other striatal slices, 3-HK did not induce mitochondrial dysfunction but attenuated the toxic effects of QUIN (see section 2.5) and the mitochondrial toxin 3-nitropropionic acid due to up regulation of glutathione S-transferase (GST) and superoxide dismutase (SOD). These authors also found that 3-HK increased the expression of the transcription factor and antioxidant regulator Nrf2 and related proteins, suggesting that 3-HK functions as a pro-oxidant under certain conditions, but that this then induces a redox modulatory activity that serves to limit cell damage [63]. Upon coordination to KMO, 3-HK also results in the NADPH induced reduction of FAD, resulting in the generation of H2O2 (see section 4.2 below).
2.4. 3-Hydroxyanthranilic Acid
Although 3-HAA was reported to lead to neuronal death as a result of oxidative stress, it was not as neurotoxic as 3-HK [60]. Other studies suggest that 3-HAA has anti-oxidant properties as 3-HAA suppressed glial cytokine and chemokine expression, reducing neuronal death [64]. It also induced the expression of the anti-inflammatory enzyme hemeoxygenase-1 (HO-1) in astrocytes and macrophages stimulated with INF-γ and lipopolysaccharide [64]. Inducible nitric oxide synthase, which can generate damaging nitric oxide and peroxynitrite radicals, had previously been shown to be down-regulated as a result of HO-1 induction by 3-HAA [65]. 3-HAA also inhibited the oxidation of low-density lipoprotein (LDL), a key process in the development of atherosclerosis, by acting as an oxidant scavenger [66]. Recent mechanistic studies show that while 3-HAA inhibited the Fenton reaction and scavenged ROS in a deoxyribose degradation assay, it was pro-oxidant in an iron (II) autoxidation assay [67]. Accordingly, 3-HAA may contribute to ROS-mediated disease processes or act as an inflammation defense mechanism in a milieu-specific way. 3-HAA also exhibits potent toxic effects on activated T-cells in the immune system by depleting intracellular glutathione (GSH) but not in resting T-cells [68]. In a range of diseases including Huntington’s disease, stroke and depression, the concentration of 3-HAA is decreased [69]. These authors speculate that the resulting decreased 3-HAA:AA ratio could be evidence of a ‘cleaning up’ effect whereby QUIN toxicity (see below) and ROS production is attenuated through inhibition of 3-HAO by AA. Given the ease by which 3-HAA can be oxidized by radical scavenging, lower 3-HAA concentrations may constitute a sensitive biomarker of ROS-mediated disease processes.
2.5. Quinolinic Acid
3-HAA may then be converted to QUIN by 3-hydroxyanthranilic acid oxygenase (3-HAO) and an additional non-enzymatic step. The major sources of QUIN within the brain are activated microglia and infiltrating macrophages [33, 70, 71] but many other immune cells are capable of synthesizing or taking up QUIN including monocytes, dendritic cells, Langerhans cells, Kupffer cells, B cells, and T cells in the brain [72]. QUIN toxicity occurs by multiple mechanisms (for review see [12]) but its role as an endogenous agonist of the NMDA receptor is considered most important. QUIN acts as a competitive and specific agonist of the NR2A and NR2B subunits of the NMDA receptor, resulting in the influx of Ca2+ into neurons thereby activating nNOS and leading to the production of NO and other free radicals which cause DNA damage, decreased mitochondrial membrane potential leading to mitochondrial destabilization, activation of PARP, NAD+ depletion and finally cell death [73, 74].
Formation of redox enhancing QUIN-iron complexes, lipid peroxidation, disruption of the BBB, increased production of nitric oxide (NO) following induction of iNOS and increased phosphorylation of structural proteins including tau [12] have also been reported as mechanisms of QUIN toxicity. QUIN has also been shown to act as an immunomodulator and induces cell death via caspase 8 and cytochrome c release in Th1 cells and thymocytes [75].
2.6. Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD+) is the last product of the KP. Quinolinic acid phosphoribosyl transferase (QPRT) converts QUIN to nicotinic acid adenine ribonucleotide, which is further metabolized to NAD+ [76]. NAD+ is an essential cofactor for many enzyme-catalyzed oxidative reactions. It also serves as an electron transporter in ATP production and plays a key role in glutathione metabolism and the NADPH-dependent thioredoxin system, which is involved in antioxidant defense and detoxification. Further roles include DNA repair, gene silencing and cellular energy sensing [77]. Enhancing NAD+ synthesis may be a therapeutically effective strategy in pathologies where oxidative stress exacerbates progression.
3. SIGNIFICANCE OF THE KP IN HUMAN PATHOLOGY
3.1. Cancer
Tumor escape from immunosurveillance has been described as a fundamental characteristic of cancer [78]. Up regulation of IDO-1 leads to localized tryptophan depletion and inhibition of T-lymphocyte proliferation [79] and increased production of downstream KP metabolites KYN, 3-HK and 3-HAA, which act via the AhR, result in T-cell apoptosis. These metabolites have been shown to exert cytostatic and/or cytotoxic activities against a range of immune cells including T-lymphocytes, natural killer (NK) cells and invariant NKT cells [6, 46, 80, 81].
Malignant human tumors including prostatic, colorectal, pancreatic, cervical, endometrial, gastric carcinomas [3] and breast cancer [82] express IDO-1. These tumors escape immune detection [3, 83] in a manner reminiscent of the way IDO-1 producing trophoblasts allow the maternal immune system to tolerate the fetus [2].
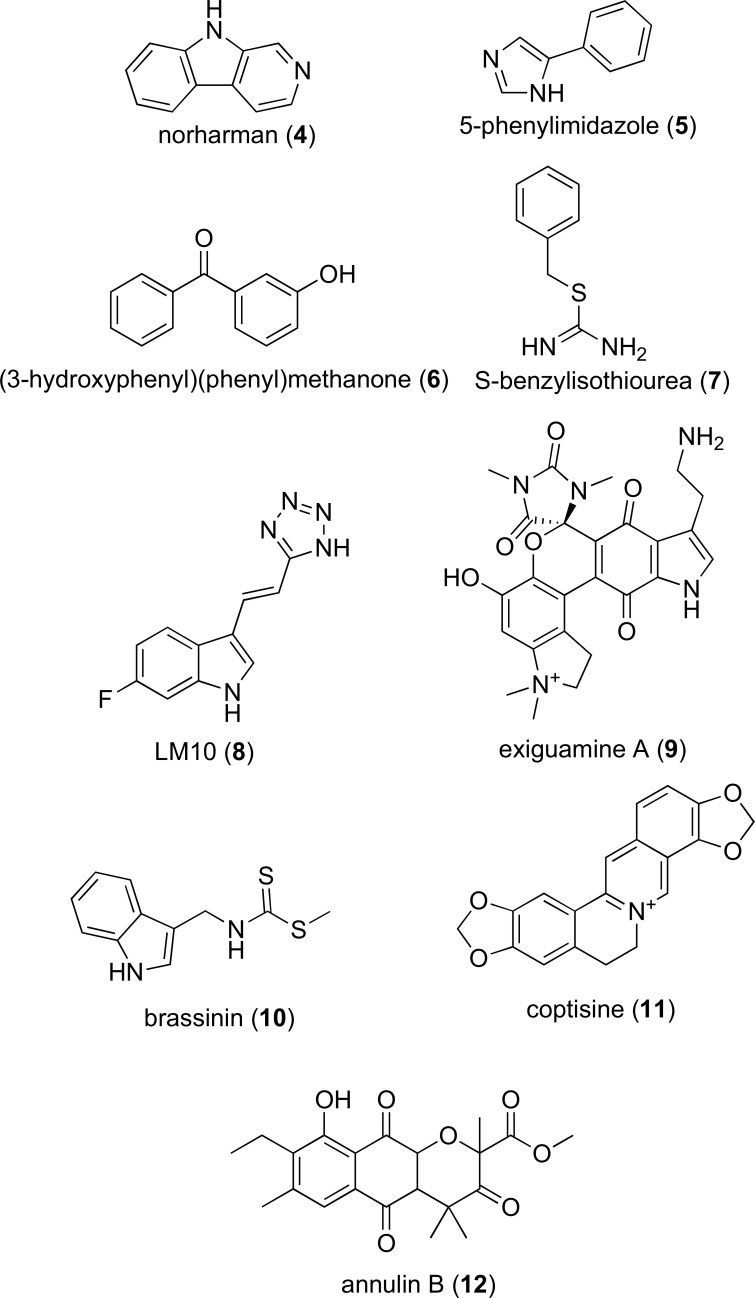
As with IDO-1, TDO is highly expressed in a range of human cancers including gliomas [84], bladder carcinoma, melanoma and hepatocarcinoma [13]. Systemic administration of TDO inhibitor LM10 (8, Fig. 3) promoted tumoral rejection in mouse models [13]. In a series of 104 human tumor cell lines 16% where shown to express genes for IDO1, 19% TDO2 and a further 15% expressed genes for both [13]. Therefore, targeting both IDO-1 and TDO may offer a synergistic approach to restoring immunosurveillance in cancer.
Fig. (3).
Representative structures of IDO-1 inhibitors scaffolds and TDO inhibitor LM10 which have not progressed to clinical trials.
The enzyme KMO may also promote cancer cell proliferation by several mechanisms. Recent studies showed that KMO inhibition may decrease the expression of the immunosuppressive KP metabolites 3-HK and 3-HAA, thereby enhancing anti-tumor immune function [85]. Additionally, increased KMO activity has the potential to increase levels of the cellular nutrient NAD + which may promote cancer cell growth by facilitating cellular energy production and DNA repair.
3.2. Neurodegenerative Disorders
Neuroinflammation resulting in over-activation of the KP is a feature present in all the major neurodegenerative diseases including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD) and Huntington’s disease (HD), where a common thread is the neurotoxicity of QUIN (see above for discussion of neurotoxic mechanisms).
In AD, increased expression of IDO-1 and TDO, resulting in QUIN accumulation, has been observed in the brain [7, 86, 87]. These enzymes were co-localized with pathological hallmarks of AD, Aβ deposits and tau [7, 41, 87]. Tau formation increased in a dose dependent manner following exposure to QUIN [41]. Furthermore, Aβ 1-42, a cleavage product of amyloid precursor protein, has been shown to induce IDO-1 resulting in a significant increase in the production of QUIN by macrophages and microglia [18]. The concentration of 3-HK was significantly increased in AD patient serum [8]. As 3-HK can readily cross the BBB, peripheral increases of 3-HK may result in increased 3-HK in the brain further contributing to neurotoxicity. No other changes to KP metabolite profiles were reported in AD serum highlighting the potential for 3-HK to be used as an AD biomarker [8]. Previously, in AD patient plasma, lower TRP and KYNA concentrations together with a marked increase of QUIN were reported [88].
In the brain and cerebral spinal fluid (CSF) of PD patients elevated 3-HK and decreased KYN and KYNA were reported [89, 90]. As PD is characterized by loss of dopamine producing neurons in the substantia nigra pars compacta [91], decreased KYNA may be particularly relevant to disease progression, as KYNA has been shown to protect dopaminergic neurons against QUIN mediated excitotoxicity [92].
Accumulation of QUIN is also evident in both CSF and serum of ALS patients [9], and as noted above, likely reflects the activation of microglia and infiltrating macrophages, a noted clinical feature of ALS [93]. These immune cells release inflammatory mediators including INF-γ which may ultimately lead to increased up regulation of IDO-1 and further accumulation of downstream KP
Similarly, increases in both QUIN and 3-HK are reported in early stage HD patient brain and mouse HD models [10, 94, 95]. Increased 3-HK:KYNA ratio is also observed in early stage HD and suggests a loss of balance between neurotoxic and neuroprotective KP metabolites, which may be indicative of the role of the KP in HD progression [95]. In contrast, changes in QUIN and 3-HK were not reported in advanced grade HD brain [10]. However, in end stage HD patients 3-HAA:AA ratio was significantly decreased [69].
Further evidence supporting the link between the KP and HD came from a genomic screen of yeast, following deletions of the KMO homologue BNA4 huntingtin induced toxicity was reduced [96]. Similar results were observed in a Drosophila melanogaster model of HD in which inhibition of both KMO and TDO led to neuroprotection following increased KYNA levels relative to 3-HK [97].
Accordingly, the KP is a well-rationalized therapeutic target to the treatment of neurodegenerative diseases and number of proof of concept studies have been conducted validating use of KP pathway modulators. KMO inhibitor JM6 (prodrug form of Ro-61-8048) was shown prevent spatial memory deficits, anxiety related behavior, synaptic loss and increase survival and prevent synaptic loss in AD and HD mouse models [28]. Similarly, KMO inhibitor CHDI-340246 dose dependently increased KYNA in HD rodent models resulting in restoration of normal excitability in striatal medium spiny neurons [98]. Most recently, the IDO-1 inhibitor, coptisine (11, Fig. 3), decreased the activation of microglia and astrocytes in AD mice. This prevented neuron loss, reduced amyloid plaque formation, and ameliorated impaired cognition [99].
3.3. Schizophrenia
Dopaminergic dysfunction was long believed to be responsible for schizophrenia, however observations that known NMDA antagonist, ketamine, produced schizophrenia like symptoms in normal individuals and exacerbated those of individuals with schizophrenia, implicated inhibition of the NMDA receptor in the development of disease [100, 101].
The up regulation of TDO [102, 103] in combination with decreased KMO gene expression and activity [35, 104] demonstrates KP unbalance in schizophrenia. This dysregulation results in increased metabolism of KYN by secondary branches of the KP leading to elevated levels of NMDA antagonist KYNA in the cortex [105] and increased KYNA and decreased QUIN:KYNA ratio in the CSF of schizophrenia patients [11, 106]. As KYNA acts to counteract QUIN induced excitotoxicity a shift in the balance of these metabolites is likely to have significant effects on brain chemistry [11]. Additionally, KYNA contributes to deficits in glutamate, dopamine and acetylcholine pathways as a result of α7nAChRs antagonism. These pathways have also been linked to both cognitive dysfunction and psychotic manifestations in humans [35].
An association between a KMO single nucleotide polymorphism (SNP) (rs1053230) and KYNA concentrations in the CSF of schizophrenia patients suggests a possible genetic component of the disease [107]. This mutation was later associated with a decrease in cognitive function [108]. The SNP encodes a change in the amino acid sequence from arginine to cysteine (KMO Arg452>Cys) in the active site of the enzyme, possibly resulting decreased turnover of the enzyme, shifting the KP towards KYNA. The same mutation is also associated with increased KYNA and psychotic features of bipolar disorder type 1 [109]. Therefore, normalization of the KP may prove to be an effective treatment strategy for schizophrenia and other psychiatric disorders.
3.4. Infections of the CNS
IDO-1 plays a seemingly contradictory role in defence mechanisms against various acute bacterial, viral and protozoa pathogens. In the event of acute infection IDO-1 is induced, resulting in localised TRP depletion and the accumulation of immune active metabolites limiting growth of pathogens and preventing an exaggerated immune response [110]. However, chronic upregulation of IDO-1 and production of immunosuppressive KYN results in T-cell exhaustion and the recruitment of T regulatory cells [46, 111, 112]. In the CNS, this also results in the accumulation of QUIN as has been linked to cognitive impairment in a range of infectious diseases including Human Immunodeficiency Virus (HIV) [113], cerebral malaria (CM) [114] and toxoplasmosis [115].
The KP is activated in HIV associated neurological disorder (HAND) and the severity of the neurological deficits are correlated with CSF concentrations of QUIN [116, 117]. Inhibition of IDO prevented neuronal abnormalities and damage in primary cell cultures [118]. More recently, a logistic regression model showed that lower KYN/TRP ratios in plasma were associated with cognitive impairments and major depressive disorder in an HIV+ group of patients although this observation was not statistically significant following Bonferroni correction [119].
Large increases in QUIN have also been observed in the CSF of patients with CM [120, 121]. In mouse models of CM, INF- γ dependent increases in IDO activity have been observed coupled with increased concentrations of KYN, 3-HK and QUIN [122]. Treatment with KMO inhibitor Ro-61-8048 protected against the development of neurological symptoms and extended life span in this model [123, 124].
Toxoplasma gondii is an intracellular parasite that is particularly susceptible to TRP depletion [125]. Murine hosts infected with T. gondii show early decreases in TRP and subsequent increases in KYN, KYNA, 3-HK and QUIN in the brain [126]. Recently, a series of studies have suggested the T. gondii infection increases the likelihood of psychiatric disorders include schizophrenia, depression and anxiety [127-129].
4. MODULATORS OF THE KYNURENINE PATHWAY
4.1. IDO Inhibitors
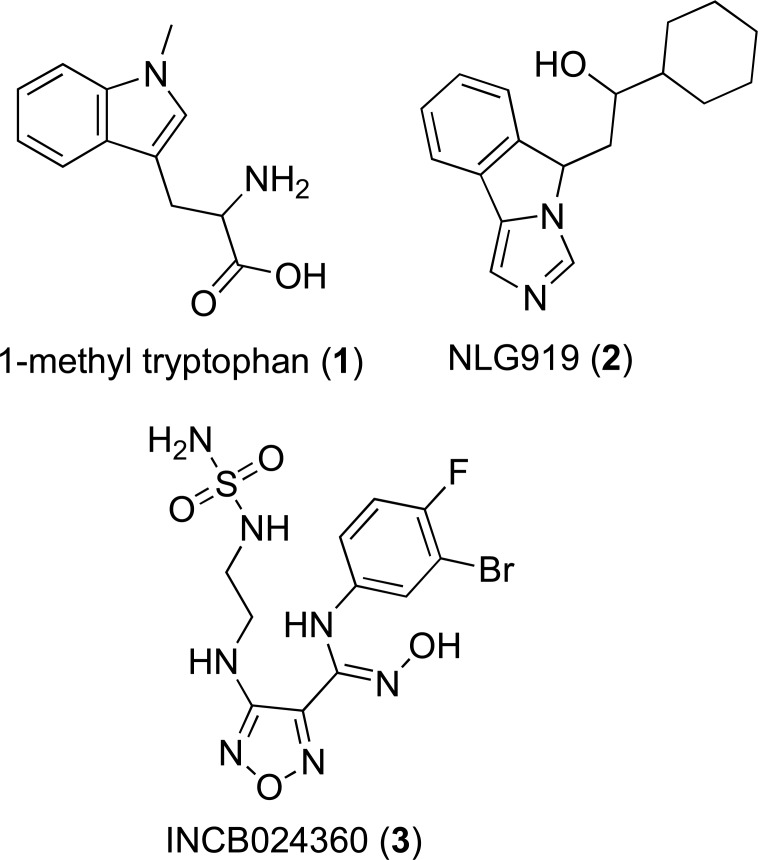
A diverse range of small molecules have been developed as IDO-1 inhibitors, with most as anti-cancer agents [130, 131]. To date, three IDO inhibitors (Fig. 2) have reached clinical trials as cancer therapies: 1-methyl-D-tryptophan (1, 1-D-MT, Indoxomod, Newlink Genetics), imidazole NLG919 (2, Newlink Genetics) and hydroxylamidine INCM024360 (3, Epacadostat, Incyte) [132].
Fig. (2).
Chemical structures of 1-methyl tryptophan, imidazole NLG919 and the hydroxlyamidine INCM024360.
4.1.1. 1-Methyltryptophan
The enantiomeric molecule 1-methyl-tryptophan (1-MT) was identified as an IDO-1 inhibitor following investigation of TRP derivatives as potential IDO inhibitors [133]. It was subsequently concluded that the free from of the indole nitrogen was essential for enzymatic turnover [133]. With an inhibition constant (Ki) of 6.6 µM, 1-MT reversed maternal T-cell tolerance towards the allogenic foetus in mice [2] indicating an immunomodulatory function. This observation, together with the discovery of a role for IDO-1 in promoting tumor cell immune evasion [3], generated considerable interest in the potential of 1-MT as an anti-cancer immunomodulator. 1-MT was also found to enhance the activity of standard chemotherapy agents, where in combination with a sub-effective dose of paclitaxel, a 30% decrease in breast tumor volume in MMTV-Neu after two weeks of treatment was noted. Enhancement effects were also reported for cisplatin, cyclophosphamide and doxorubicin [130]. 1-MT restored immunosurveillance when acting as a single agent or in combination with established chemotherapeutics against gastric and bladder cancers [134], melanoma [135], lung [83] and ovarian cancer [136, 137]. In combination with temozolomide and radiation treatment 1-MT significantly increased the average life span of murine models of glioblastoma [138].
A recent study, however, suggested that 1-MT may have paradoxical tumor promoting activity. Opitz et al., found that D-1-MT increased production of KYN in human cancer cells and in cell free assays D-1-MT did not inhibit IDO-1 activity. Further experiments in cancer cells showed that D-1-MT induced the expression of IDO-1 mRNA and protein expression via p38 MAPK and JNK signaling. It was suggested that these off-target transcriptional effects may promote tumor growth [84].
One controversy surrounding 1-MT has been that D-1-MT stereoisomer shows higher anti-tumor activity in-vivo and is currently in clinical trials even though, biochemically, it is a weaker IDO inhibitor than L-1-MT. An explanation of this apparent anomaly may be that D-1-MT also inhibits IDO-2 [15], as it was shown that D-1-MT was more selective for IDO-2 than IDO-1, while L-1-MT more potently inhibited IDO-1 [15]. However, others have reported that IDO-2 is more efficiently inhibited by L-1-MT than by the D-1-MT [139].
Phase 1 clinical trials of 1-D-MT in combination with docetaxel in patients with metastatic solid tumors demonstrated the treatment was well tolerated and with 18% of patients seeing partial response, 4% remaining stable for more than 6 months and 41% stable for less than 6 months. From this study the highest D-1-MT dose of 1200 mg PO twice daily was recommended for further trials [140]. In a separate trial of D-1-MT alone 10% of patients remained stable for more than 6 months [141].
D-1-MT is currently in phase 2 clinical trials for treatment of malignant prostate (NCT01560923), metastatic melanoma (NCT02073123), metastatic pancreatic (NCT02077881) and metastatic breast cancer (NCT01792050) [142].
4.1.2. Hydroxylamidines
To date, the hydroxylamidine series are one of the most potent inhibitors of IDO-1 and were identified following high-throughput screening (HTS) of Incyte’s compound collection [143]. Optimization of the lead compound from this screen led to identification of a 3-chloro, 4-fluro substituted hydroxylamidine (4-amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadia-zole-3-carboximidamide) with an IC50 of 19 nM in HeLa cells. When the 3-chloro, 4-fluro substituted hydroxylamidine was administered to mice plasma concentrations of KYN were reduced by 60% [143]. SAR analysis of this compound revealed the hydroxylamidine moiety was essential for activity and substitution of the phenyl ring was limited to relatively small hydrophobic groups such as halogens and small alkyl chains. Based on the mode of binding of similar molecules, these molecules have been proposed to interact with IDO-1 by the interaction of the hydroxylaminidine oxygen with the IDO-1 heme moiety, through extension of the phenyl ring into the active sites hydrophobic pocket and hydrogen bonding between the amine of the furazan ring and the propionic acid arm of the heme moiety [143]. The furazan ring was predicted to extend towards the entrance of the IDO-1 active site allowing for further optimization in later SAR series and which was likely to have ultimately led to the identification of Incyte’s lead compound INCB024360 (3, Epacadostat, IC50 ~ 10 nM, Fig. 2).
INCB024360 was shown to inhibit CT26 colon carcinoma growth by 54% over a period of 24 days in Balb/c mice [4]. In co-culture systems of human allogeneic lymphocytes and dendritic cells (DCs) or tumor cells inhibition of IDO-1 by INCB024360 promoted growth of immune cells including T and natural killer (NK)–cell growth and following induction of IDO-1 by INF-γ reversed IDO-1 mediated DC apoptosis [144].
INCB024360 is currently in a number of phase 1 and 2 clinical trials as a monotherapy and in combination with other chemotherapeutic agents for the treatment of non-small cell lung cancer (NCT02298153), ovarian cancer and metastatic melanoma (NCT01-604889), stage III-IV melanoma (NCT01961115) [142]. Recently published results from the first-in-human phase 1 trial of INCB024360 in patients with advanced solid tumors showed the drug was well tolerated and stabilized disease in 13.5% of patients for 16 weeks or more [145]. Similarly, preliminary results from phase 1 trials of INCB024360 in combination of immune checkpoint inhibitor pembrolizumab showed promising objective response rates (58%) in patients with treatment-naive advanced melanoma (NCT021-78722) [142, 145].
4.1.3. Imidazoles
Fused imidazole derivatives were first patented as IDO-1 inhibitors by Newlink genetics in 2012 [146]. In preclinical trials second generation IDO-1 inhibitor, NLG919/GCD-0919/RG6078 (NewLink Genetics, Genentech and Roche respectively) led to a significant decrease in size of large established tumors. NLG919 showed enhanced immune activation and tumor regression when combined with Indoximod (1-MT) [5] and is currently in clinical trials for use both alone and in combination with Atezolizumab (MPDL3280A) for treatment of advanced solid tumors (NCT02048709 and NCT02471846) [142]. Similarly, in murine models of glioblastoma, NLG919, enhanced survival when combined with chemo-radiation therapy when compared to chemo-radiation therapy alone (temozolomide plus radiation) [138].
Most recently, the crystal structure of IDO-1, complexed with a NLG919 derivative was solved [147]. Unlike other available IDO-1 crystal structures complexed with relatively weak IDO inhibitors such as 4-phenylimidazole (PDB ID: 2D0T) [148], this structure highlighted a number of hydrogen bonds between the NLG919 derivative and the 7-propionic acid arm of the IDO heme moiety and the NH group of Ala264. Interestingly, a intramolecular hydrogen bond between the molecules isoindole nitrogen and hydroxyl group was also important for activity, the removal of which (via replacement of the hydroxyl group with a carbonyl) resulted in an approximately 12 fold decrease in potency (from an IC50 or 38 nM to 468 nM) [147]. The presence of these hydrogen bonds may be key to the potent activity observed for this series of imidazoles and suggests that conformational constraints may be a design clue relevant for other classes of IDO inhibitors.
4.1.4. Other IDO Inhibitors
Several other IDO inhibitors have also been identified based on the scaffolds of β-carboline (e.g. norharman, 4, IC50 90 µM) [149, 150], phenylimidazole (e.g. 5-phenylimidazole, 5, IC50 48 µM) [149, 151], quinone containing molecules including the natural products (exiguamine A, 9, Ki 41 nM); annulin B (12) [152-154] and brassinin (10, Ki 98 µM) [155], benzylisothiourea (7, IC50 2.2 µM) [156] and diaryl ketones (exemplified by 3-hydroxyphenyl(phenyl)methanone, 6, IC50 0.5 mM) [154]; structures shown in Fig. 3). Although some attempt has been made to characterize the structure-activity relationships of some classes of these molecules (e.g. brassinins and phenylimidazoles) these structures do not appear to have pharmacological profiles suitable to progression to clinical trials [132].
Common features of these molecules include a heteroatom that interacts with the IDO heme moiety, one or more hydrophobic groups that fit into the IDO hydrophobic pockets and in a number of cases polar groups for hydrogen bonding with the propionic arm of heme.
4.2. KMO Inhibitors
As discussed above, the enzyme kynurenine-3-monooxygenase (KMO) is a well-rationalized therapeutic target for a range of neurodegenerative conditions, as KMO inhibition decreases concentrations of neurotoxic QUIN but increases neuroprotective KYNA. However, while some IDO inhibitors have reached clinical trials, KMO inhibitors are still at the pre-clinical phase of development.
A potential drawback of KMO inhibition is the formation of H2O2 which occurs following NADPH-de- pendent reduction of FAD in the presence of 3-HK and some substrate-like molecules [157]. As H2O2 generation is potentially deleterious, this should be assessed during the course of new KMO inhibitor development.
4.2.1. Kynurenine Analogues
As the enzymes KMO and KAT both utilize the substrate KYN, there is the potential that KMO inhibitors structurally analogous to KYN, may inhibit both enzymes. This may be an important consideration in the development of highly selective KMO inhibitors.
4.2.1.1. 4-Phenyl-4-Oxobutanoic Acids
The first reported selective KMO inhibitor was the kynurenine analogue m-nitrobenzoyl alanine (m-NBA; IC50 = 0.9 µM, 13, Fig. 4) [158]. This compound selectively inhibited KMO relative to the enzyme KYNU (IC50 100 µM). When administered to rats m-NBA was shown to significantly increase levels of both KYN, and in turn, KYNA 13 and 5 fold respectively in the brain, 5 and 2.4 fold in the blood and 6 and 3.5 fold in the liver. This KYNA increase induced anticonvulsant and sedative effects. Treatment with m-NBA was also shown to decrease duration of maximal electroshock induced seizures [158].
Fig. (4).
Key KMO inhibitor structures.
The S(+)m-NBA stereoisomer was later shown to be the most active isomer with an IC50 of 0.5 µM in the presence of 100 µM kynurenine when compared to the R(-) stereoisomer which only demonstrated 60% inhibition under the same conditions [159]. Additionally, the S(+) stereoisomer showed activity towards KYNU whereas the R(-) was essentially inactive.
Structure activity relationship (SAR) studies showed that a 3,4-dicholoro substitution was optimal and led to the identification of inhibitor FCE 28833 (14, IC50 = 0.33 µM; Fig. 4) [160, 161]. Treatment of rats with a single oral doses of FCE 28833 led to a dose-dependent increase in endogenous KYN and KYNA in rat brain tissue, with a 400 mg/kg dose resulting in a 14-fold increase in KYN and 34 fold increase in KYNA [162]. Furthermore, extracellular hippocampal KYNA levels remained significantly higher than basal KYNA levels following both a single oral (10 fold increase) and intraperitoneal (80 fold increase) administration of FCE 28833 [162].
Other SAR study varied the benzoyl alanine portion of FCE 28833. The most potent analogues resulted from replacement of the amino group with either hydroxyl or benzyl moieties (IC50 values of 1.4 µM and IC50 2.9 µM, respectively). This SAR study demonstrated that the amino group was not essential for good activity and although these analogues were not as potent as the original scaffold, they more selectively inhibited KMO as they did not inhibit either KYNU or KAT [161].
The effect of limiting free-rotation of the FCE 28833 molecule was later examined through introduction of a double bond to the 4-oxobutanioc acid scaffold resulting in the synthesis of a FCE 28833 analogue with an IC50 of 1.0 µM [163].
The inhibitor UPF-648 (15, Fig. 4) was developed by adding a cyclopropyl linker to the benzoylalanine portion of FCE 28833, which further reduced the conformational freedom of the molecule and resulted in potent KMO inhibition (IC50 = 40 nM) [164]. Subsequently, UPF-648 was shown to selectively inhibit KMO compared to KAT in vitro (100% and 5% inhibition, respectively) [165]. When administered to gerbils, UPF-648 led to a dramatic and dose dependent increase of KYN and KYNA in both brain and plasma [166]. Studies in wild-type and KAT II knockout mice (mkat-2-/-), which have a pronounced KYNA deficit, showed that 30 mg/kg (i.p) of UPF-648 was shown to increase KYNA 14 fold in wild-type mice and 10 fold in mkat-2-/- mice. Consequently, the susceptibility of mkat-2-/-mice to QUIN toxicity in the striatum was reduced, as indicated by decreased QUIN-induced striatal lesions. WT mice did not show similar reductions in lesion volume. Notably, QUIN and 3-HK levels did not change, this further suggests that an increase KYNA to 3-HK/QUIN ratio is neuroprotective [55]. Treatment of rat pups with UPF-648 10 minutes after birth lead to substantial increase in KYN and KYNA and decreased QUIN and 3-HK in both brain and liver compared to controls [167].
The crystal structure of yeast KMO (S. Cerevisiae) was successfully elucidated in both its free form and when co-crystalized with UPF-648. Although yeast and human KMO do not share 100% sequence homology (38% identity and 51% similarity), all the residues involved in ligand binding are conserved (Arg 83, Tyr 97, Leu 212, Met 230, Ile 232, Leu 234, Phe 246, Pro 321 and Phe 322) between both species which provides a potential for comparing ligand-active site interactions. Indeed, the carboxylate group in UPF-648 was shown to interact with conserved residues Arg83 and Tyr97 whereas the aromatic moiety lay within the conserved hydrophobic pocket on the re-side of the FAD co-factor. The chemical similarity between UPF-648 and L-KYN permitted molecular modeling of L-KYN in the active site and suggested that both molecules bind to the enzyme in a similar manner [168]. However, UPF-648 coordination induced structural changes in the enzyme which included reorientation of the Pro321-Gln325 loop in order to accommodate the aromatic chlorine substituents not present in L-KYN. These structural perturbations may additionally affect KMO activity. The Pro321-Gln325 loop is also in close proximity to the postulated oxygen binding site on the re-side of the FAD cofactor, which is subsequently destroyed upon UPF-648 binding. A potential drawback of UPF-648 is the 20-fold increase in the production of H2O2 observed following UPF-648 coordination to KMO, which results from the destabilization of the C4a-hydroperoxide intermediate formed as part of the catalytic cycle of all flavin mono-oxygenases, including KMO [168].
4.2.1.2. Sulfonamides
The sulfonamides are among the most potent known KMO inhibitors. They are structurally analogous to the KMO substrate L-kynurenine and were developed following a SAR study based on previous observations that the amino moiety was not essential for activity and that carboxylic acid groups often reduce the bioavailability of molecules. In general, these molecules contain a FAD-binding benzene ring, a 5 or 6 membered aromatic ring as a linker, a sulfonamide as an amino acid bioisostere and a pendant benzene ring. The structure of the 5 or 6 membered ring leads to significant variation in inhibition with sulfonamides containing a thiazole linker being, by far, the most potent [169]. This class of molecules includes the well-known KMO inhibitor Ro-61-8048 (16, Fig. 4, IC50 = 37 nM). Originally described as a high-affinity inhibitor of KMO in vitro, Ro-61-8048 inhibited KMO in the gerbil brain under the same conditions more effectively than FCE 28833 (ED50 of 5.5 and 461 µmol/kg, respectively) [169]. Prolonged inhibition of KMO with Ro-61-8048 was shown to reduce development of levodopa-induced dyskinesia in Parkinsonian monkeys [170] and attenuated post-ischemic neural death [171]. More recently, Ro-61-8048 modulated KYNA levels in the brain, reducing the ability of tetrahydrocannibinol (THC) and other synthetic cannabinoids to stimulate dopamine release, in turn reducing the reward effects of marijuana and potentially providing an effective approach for the treatment of marijuana dependence [172].
Zwilling et al. described Ro-61-8048 as metabolically unstable and developed a slow-release prodrug form, JM6 (17, Fig. 4) [28]. Structurally, Ro-61-8048 and JM6 differ only in the addition of a 1-methylpiperidine substituent to the thiazole ring of the latter. It was hypothesized that under acidic conditions the N-methylenepiperidino moiety at C-5 of the isothiazole nucleus would be removed from JM6 leading to the formation of Ro-61-8048. Both molecules were found in the blood when administered orally but did not demonstrate significant ability to cross the blood brain barrier. Treatment of murine models of neurodegenerative disease with Ro-61-8048 still led to the peripheral inhibition of KMO in the blood, but also increased KYNA levels in the brain. As discussed previously, it was reported that in the APPtg mouse model of AD that this molecule prevented spatial memory deficits, anxiety related behavior and synaptic loss. When administered in the R6/2 mouse model of HD, JM6 was also able to prevent synaptic loss and attenuated microglial activation. These findings further supported the link between tryptophan metabolism in the blood and neurodegeneration and demonstrated that peripheral inhibition of KMO is sufficient to induce a neuroprotective effect [28]. Investigation into the metabolism and pharmacokinetics of JM6 and Ro-61-8048 by Beconi et al. proposed that the activity of JM6 did not result from an ability to act as a Ro-61-8048 prodrug but instead resulted from an impurity of Ro-61-8045 present in the JM6 preparations [173]. However, this finding is yet to be confirmed by others and potentially remains contentious.
4.2.1.3. Oxazolidinones
Most recently, the KYN like inhibitor GSK180 (IC50 6 nM, 22, Fig. 4) was developed by Mole et. al. as a KMO inhibitor with physiochemical properties suitable for i.v. drug administration. This series of propanoic acid heterocycles were designed to contain both a carboxylic acid and H-bond acceptor, which were deemed important for activity based on previous studies. The crystal structure of Pseudomonas fluorescens KMO-GSK180 complex highlighted interactions including ionic bonding between the carboxylate moiety and Arg84, H-bonding between the carboxylate and Tyr98 and Asn369, H-bonding between the oxazolidinone carbonyl and Tyr404 and hydrophobic interactions between the 5-chloro substituent and Phe238. GSK180 treatment reduced 3-HK and increased KYN and KYNA in both rat and mouse models of acute pancreatitis (AP). A 5.5 mg/kg/hr dose of GSK180 resulted in a plasma concentration of 600 µM over the course of the study and prevented multiple organ dysfunction syndrome (MODS) in a rat model of AP [30].
4.2.2. Other Synthetic KMO Inhibitors
4.2.2.1. Aryl Pyrimidines
The Cure Huntington’s Disease Initiative (CHDI) Foundation has patented a large number of phenylpyridine and phenylpyrimidine molecules as KMO inhibitors [174]. Most recently, Toledo-Sherman et. al. reported a number of pyrimidine carboxylic acids as competitive, potent and selective KMO inhibitors [175]. These compounds exhibited desirable pharmacokinetic profiles, making them promising molecules for further therapeutic development. These molecules were designed such that they would mimic key structural features of KYN, m-NBA and FCE-28833. Representative structures of this series include 6-(3-chlorophenyl)pyrimidine-4-carboxylic acid (18, Fig. 4) and its 3,4-dichloro substituted analogue with IC50 in the sub nanomolar range (IC50 0.5 nM and 0.6 nM respectively) [175]. When tested in stably transfected CHO cells, the 3,4-dichloro substituted molecule showed superior activity (IC50 33 nM), which may reflect increased ability to cross cell membranes. Replacement of the carboxylic acid moiety with carboxylic acid isosteres was also investigated to improve the potential to cross the BBB. Of the isosteres tested, tetrazole, oxadiazolone and acidic acyl sulfonamide substituted molecules had an IC50 of 2, 12, and 25 nM respectively. However, the remaining non-acidic isosteres showed little or no KMO inhibitory activity, highlighting the importance of an acidic functional group [175]. These observations are in line with the KMOs crystal structure and the predicted mode of binding of KYN-like molecules. In particular, the crystal structure highlighted ionic bonding between UPF-648s carboxylic acid moiety and a conserved arginine residue [168]. Pharmacokinetic analysis of the tetrazole and oxdiazolone substituted molecules revealed neither molecule appreciably crossed the BBB.
Optimization of the phenyl ring substitution led to the development of a molecule with 3-chloro, 4-cyclopropoxy substitution (CHDI-340246, IC50 of 0.5 nM, 19, Fig. 4) which was hypothesized to extend the molecule further into the postulated hydrophobic pocket binding site. Pharmacokinetic analysis of this molecule in non-fasted male Sprague-Dawley rats showed that in accordance with other acidic compounds its ability to cross the BBB was limited, however microdialysis studies in rat brain and CSF revealed concentrations of compound near the ED50 for rat KMO following a single oral 10 mg eq./kg dose [175]. KP metabolite analysis following administration of a single 10 mg/kg dose demonstrated significant elevations in plasma KYN, KYNA and AA and a decrease in 3-HK concentrations for 8-12 hours. Similarly, increases in KYN, KYNA and AA (approximately 6 fold, 8 fold and 15 fold respectively) were observed in the striatal dialysate. 3-HK levels increased only 2 fold. This relatively small increase in 3-HK following large increases of upstream metabolite KYN likely indicates some KMO inhibition in the brain [175].
CHDI’s lead KMO inhibitor, CHDI-340246 does not inhibit other KP enzymes and is currently in pre-clinical testing in non-human primates [53, 176]. The molecule was reported to have an IC50 of 0.5, 4.0 and 5.1 nM in human, rat and mouse live tissue lysate respectively, EC50 in human peripheral blood mononuclear cells (PBMC) and rat microglia were 82 and 72 nM respectively. CHDI-340246 dose dependently increased KYNA in the CSF of rodent HD models and re-established normal excitability to striatal medium spiny neurons [98]. Most recently, CHDI-340246 was shown to modulate the KP in both the periphery and CNS of murine HD models. Following a 10 mg/kg (p.o.) dose KYN, KYNA and AA concentrations increased approximately 15, 70 and 9 fold respectively in the striatum. Modest changes were also observed for 3-HK (2.5 fold) and QUIN (1.5 fold). Overall 3HK:KYN ratio decreased 22% in WT and 16% in Q175 heterozygotes, in contrast KYNA:KYN ratio increased 2.5 fold demonstrating a shift in KP metabolism away from 3-HK towards KYNA. Similar changes in metabolite ratios were not observed following a single dose of 30 mg/kg KYN suggesting shifts in KP metabolism resulted from KMO inhibition in the brain and not simply as a result from significant increases in KYN in the periphery. CHDI-340246 was shown to restore SNP membrane excitability in R6-2 mice. However, in this model, KMO inhibition did not significantly modify behavioral phenotype or ameliorate disease progression as judged by open field test, accelerated rotarod, grip strength and brain volumetric loss [53].
Several other KMO inhibitor scaffolds have been reported including the pyrroloquinolines, 3-oxo-propanitriles and tetrazoles. Little, if any work has been reported for these molecules in vivo, however they have been included for interest.
4.2.2.2. Pyrroloquinolines
Pyrroloquinolines are a class of moderately active KMO inhibitors with IC50 values ranging from 20-100 µM [177]. The most active of this series (7-Chloro-3-methyl-1H-pyrrolo [3,2-c]quinoline-4-carboxylic acid, 23, Fig. 4) showed selectivity towards KMO (IC50 24 µM in rat liver) in vitro compared to kynureninase (24% inhibition at 100 µM) and kynurenine aminotransferase (25% inhibition at 100 µM) [177].
4.2.2.3. 3-Oxo-Propanitriles
The 3-oxo-propanitriles are a series of molecules patented by Pharmacia, from this series one representative compound has been disclosed (PNU-168754 A, 24, Fig. 4) which was reported to have an IC50 of 40 nM [178].
4.2.2.4. Tetrazoles
Recently, a number of lead structures were identified by GlaxoSmithKline using High-Throughput RapidFire Mass Spectrometry [179]. A KMO enriched membrane preparation was used for a direct screen of approximately 78,000 compounds selected for low molecular weight (<300 kDa) and low lipophillicity. From this a number of hits were identified for further investigation, of this series, 5-(3-nitrobenzyl)-1H-tetrazole (21, Fig. 4) was reported to have an IC50 of 6.3 µM [179].
4.2.3. Natural Products
4.2.3.1. Marine Natural Product Ianthellamide A
Ianthellamide A (20, Fig. 4) was recently reported as a novel octopamine (naturally occurring neurotransmitter) derivative isolated from the Australian marine sponge Ianthella quadrangulata. Ianthellamide A selectively inhibited the activity of kynurenine 3-monooxygenase when compared to kynureninase at concentrations less than 125 µM (IC50 value of 1.5 µM). It also significantly increased the level of endogenous KYNA, 20 fold, in rat brain following administration of 200 mg/kg lanthellamide A and 100 mg/kg KYN [180].
4.2.4. KMO Inhibitors: Current Limitations
While KMO inhibitors have potential for the treatment of neurodegenerative conditions, to date none of these inhibitors have been shown to efficiently cross the BBB. As a result, the conversion of KYN to 3-HK, and ultimately QUIN, is not effectively inhibited. Although in vivo studies have demonstrated that peripheral inhibition of KMO successfully reduces symptoms in various AD and HD models, inhibition of KMO activity at the site of neuroinflammation can be expected to have a better therapeutic effect. A number of strategies may be used to achieve this. Current KMO inhibitors could be re-engineered to better comply with the requirements for CNS permeability or new KMO inhibitors could be developed that cross the BBB. Other strategies range from temporary disruption of the BBB, receptor- or cell -mediated delivery based on pro-drug development to a variety of nanoscience approaches including encapsulation in liposomes or polymeric nanoparticles [181].
4.3. KAT Inhibitors
As discussed, KYNA is an exogenous NMDA antagonist which counteracts QUIN induced excitotoxicity. However, increased brain KYNA has also been linked to a number of psychiatric diseases including schizophrenia and depression. A reduction in KYNA through the use of KAT inhibitors may prove an effective strategy for re-establishing KP metabolite balance for the treatment of these conditions. Of particular interest are KAT-I and KAT-II, the main isoforms expressed by astrocytes in the brain.
4.3.1. KAT (I)
KAT-I, also known as glutamine transaminase-K is inhibited by physiological concentrations of tryptophan, indole-3-pyruvic acid, glutamine, cysteine and phenylalanine [182]. In particular, cysteine and cysteine derivatives, were able to inhibit KAT-I and KAT-II in vitro and reduced KYNA in rat cortical slices [183]. The solved crystal structure of the enzyme was first reported by Rossi et. al, 2004 complexed with L-phenylalanine (PDB ID: 1W7L) [184] and later reported by Han et. al, 2009 containing known inhibitor indole-3-acetic acid (IAC; PDB ID: 3FVU) [185]. In each case the hydrophobic regions of the molecules were situated within KAT-Is hydrophobic pocket made up of Tyr 63, His 279, Phe 278, Tyr 101 and Phe 125 [184, 185]. In addition the carboxylic moiety of IAC formed extensive H-bond networks with Asn 185, Gly 36, gly 34, Arg 395, Gln 35 and Thr64 [185].
4.3.1.1. Tryptophan Derivatives
The complexed IAC/KAT-I crystal structure provided a logical starting point for the development of a small SAR series investigating both the indole and carboxylic moieties of IAC [185]. The series of molecules was designed such that they did not contain an α-keto or α-amino group which would prevent transamination of the molecule as seen with some other KAT-I inhibitors which act as both a substrate and inhibitor to the enzyme. The best inhibitor of the indole-containing molecules was indole-3-propionic acid with a reported IC50 of 140 µM [185].
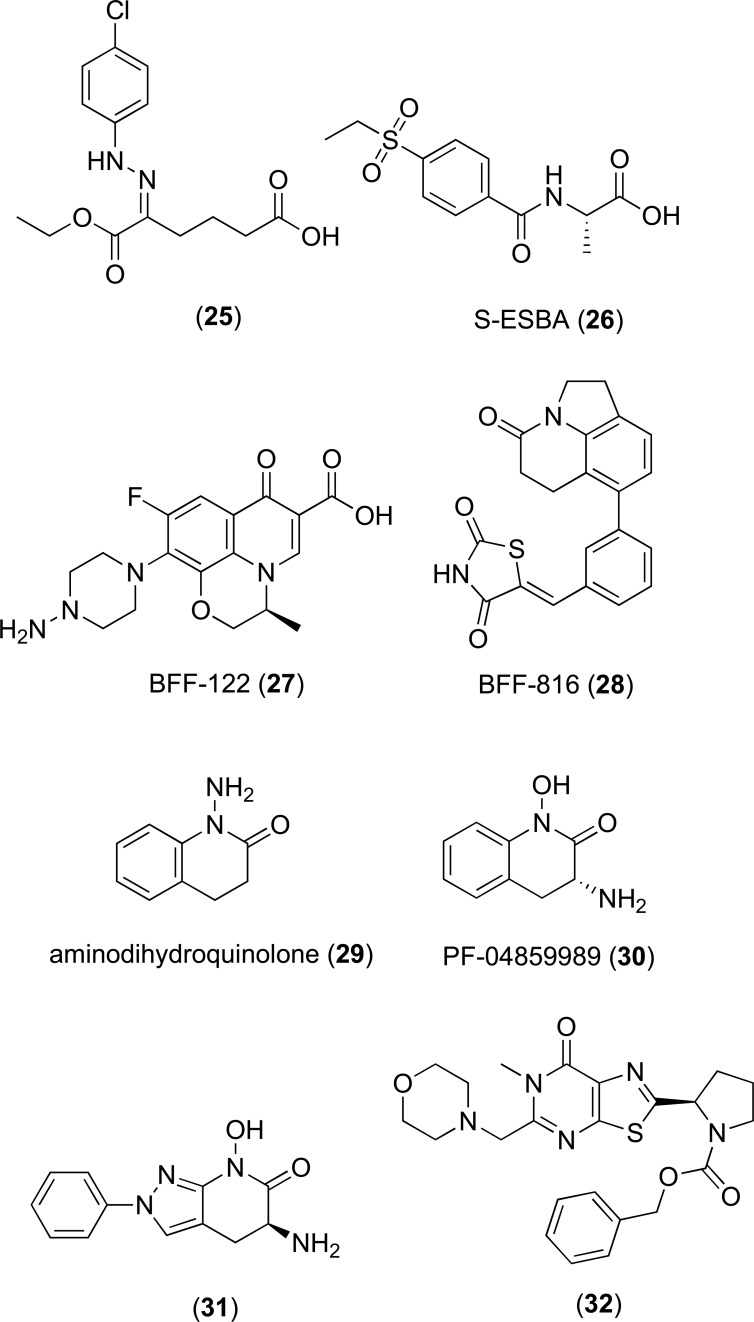
A number of other tryptophan derivatives were identified as KAT-I inhibitors by Akladois et al. with higher inhibitory activity relative to the indole acetic acids [31]. These included a series of phenylhydrazone hexanoic acids and indole derivatives, the most active of which 5-(2-(4-chlorophenyl) hydrazono)-6-ethoxy-6-oxohexanoic acid (25, Fig. 5) had a reported IC50 of 19.8 µM. A SAR study of the phenylhydrazone series demonstrated that phenyl ring substitutions significantly altered activity. Addition of electron withdrawing groups, such as nitrogen, led to a decrease in inhibitory activity when compared to addition of hydrophobic and/or electron donating groups, such as halogens, which led to an increased activity [31]. The specificity of these inhibitors towards KAT-I over KAT-II was not reported.
Fig. (5).
KAT inhibitor structures.
4.3.2. KAT (II)
As KAT-I has broad substrate specificity and is inhibited by physiological concentrations of glutamine and other amino acids, KAT-II may prove to be a more effective target for modulating KYNA synthesis. The crystal structure of KAT-II was reported by Rossi et. al, (PDB ID: 2VGZ) and revealed the protein exists as a functional dimer and therefore contains two active sites with coordinated PLP cofactors [36]. As the active sites for both KAT-I and KAT-II are structurally distinct, it may be possible to selectively target particular isoforms.
4.3.2.1. Kynurenine Derivatives
KAT-II inhibitor (S)-4-(ethylsulfonyl)benzoy-lala-nine (S-ESBA; 26, Fig. 5) [186] was originally developed for screening as a KMO inhibitor following SAR analysis of the benzoylalanine portion of KYN and with the knowledge that sulfonyl alky substituents offer a good hydrophobic/hydrophilic balance and have the ability to act as a H-bond acceptor. However, S-ESBA was not active towards KMO and KYNU and was shown to preferentially inhibit rat KAT-II over rat KAT-I with 97% and <5% inhibition at 1 mM respectively [187]. When infused by reverse dialysis in rat striatum S-ESBA significantly reduced extracellular KYNA by 32% after 1.5 hours. Increased dopamine was also observed, reaching a maximum of 265% of baseline level at 1.5 hours. To further investigate the link between KYNA and dopamine, rats were co-infused with 1 mM S-EBSA and 100 nM KYNA. In these animals, dopamine levels remained unchanged during the testing period. This demonstrated that KAT II and KYNA play an important role in dopaminergic function. This was hypothesized to reflect the ability of KYNA to act as an α-7nAChR antagonist, leading to a reduction in glutamate and a subsequent decrease in extracellular dopamine [188]. Additionally, infusion of 1 mM S-ESBA 1 hour prior to QUIN caused 3.7 fold increases in the size of QUIN induced striatal lesions. This effect was potentiated when co-administered with KYNA [187]. However, S-ESBA less potently inhibits human KAT-II compared to rat KAT-II (19% inhibition at 0.1 mM compared to 88% inhibition of rat KAT-II at the same concentration) which may indicate that S-ESBA is likely to be less effective in humans [189].
4.3.2.2. Fluroquinolones
As S-ESBA was a relatively poor inhibitor of human KAT-II [189], a fluroquinolone BFF-122 (27, Fig. 5) was identified by in-house screening by Schwarcz and Kajii [190, 191] and patent protected. Unlike S-ESBA, BFF-122 more potently inhibited human KAT-II, with an IC50 in the low micromolar range and effectively reduced KYNA synthesis in rat brain [190]. The structure of BFF-122 co-crystalized with KAT-II (PDB ID: 2XH1) revealed covalent bonding between the primary amine and BFF-122 and the PLP cofactor of KAT-II, forming an irreversible adduct. The selectivity of the inhibitor was hypothesized to result from the bulkiness of the molecule which would likely encounter steric hindrance in coordinating to KAT-I, mitochondrial aspartate aminotransferase or other amino-transferases [190].
HTS and lead optimization resulted in the identification of orally active KAT-II inhibitor BFF-816 (IC50 13.4 µM, 28, Fig. 5). In male Sprague-Dawley a dose of 30 mg/kg p.o reduced extracellular KYNA by 30% and led to improvement in both spatial and contextual memory as assessed by the Morris water maze test [192].
4.3.2.3. Hydroxamate Derivatives
A novel KAT-II inhibitor scaffold was identified by Dounay et al, 2012 following a high-throughput screen of the Pfizer compound library [193]. Optimization of this scaffold yielded aminodihydroquinolone (29, Fig. 5) as a lead compound [193] and a potent group of bicyclic and tricyclic KAT-II inhibitors were subsequently patent protected by Claffey et. al [194]. Leading analogues from this series include (R)-3-amino-7-bromo-1-hydroxy-3,4-dihydroquinolin-2(1H)-one with a reported IC50 of 10.5 nM and (R)-3-amino-1-hydroxy-3,4-dihydroquinolin-2(1H)-one, known as PF-04859989 (30, Fig. 5) with an IC50 of 23 nM [194]. Importantly, PF-04859989 was shown to be more potent towards human KAT-II than rat KAT-II and could effectively cross the BBB. A single 10 mg/kg dose was shown to reduce brain KYNA by 50% [193] and restored nicotine induced glutamatergic activity in rat cortex [195]. Similarly, to BFF-122, X-ray crystallography and 13C NMR spectroscopy revealed the primary amine formed a covalent bond with the enzyme PLP co-factor, irreversibly inhibiting the enzyme [193]. Unfortunately, the pharmacokinetic properties of this molecule were not ideal, being poorly bioavailable and subject to O-glucuronidation at the hydroxamate moiety [196]. To improve these properties, a SAR study focused on modulating the hydroxamate moiety was conducted. A number of triazolinone and triazole hydroxamate replacements were utilized, resulting in analogues with improved pharmacokinetic properties, but less inhibitory activity, compared to the parent compound [196].
A number of other hydroxamate based KAT-II inhibitors were reported by Tuttle et al, based on the scaffold of PF-04859989. This relatively recent series of molecules includes a number of irreversible KAT (II) inhibitors with IC50 values in the range of 20-30 nM and inhibitory constants (Ki) of 0.0016-0.015 µM [197]. A SAR investigation of the hydroxamate scaffold revealed that hydrophobic substituents at C5 and C8 led to a decrease in potency while C6 and C7 substitution led to an increase. Further investigation of KAT (II) crystal structure revealed that this was likely to be due to C6 and C7 being oriented towards solvent exposed regions of the molecule while C5 and C8 are exposed to the walls of the binding pocket. Inhibitor activity was increased further following addition of a phenoxy group at C6 which consequently interacted with a previously unknown lipophilic pocket at the entrance of the active site. This series of molecules was shown to be weakly active towards isoforms KAT-I and KAT-III [197].
Pyrazole based KAT-II inhibitors were developed by Dounay et. al, 2013. The most potent molecule of this series (31, Fig. 5), had an IC50 value of 25 nM and improved lipophillicity when compared to the parent compound PF-04859989 [198]. The X-ray crystal structure of this compound bound to KAT-II showed covalent bonding between the primary amine and the PLP co-factor, hydrogen bonding between the hydroxamate functional group and Arg399 and Asn202 and interactions between the phenyl group and hydrophobic residues including Ile19, Leu40 and Tyr74 [198].
Most recently, a number of bi- and tricyclic heterocylic compounds were patent protected by Mitsubishi Tanabe Pharma Corporation, Japan for the treatment of KAT-II associated disorders [199]. A representative structure from this series (32, Fig. 5) was reported to have an IC50 of 0.61 µM.
4.4. Kynureninase (KYNU) Inhibitors
As a result of KYNs poor affinity for KYNU, its primary role in the KP is the conversion of 3-HK into 3-HAA. However, following inhibition of KMO, KYNU may play a role in compensatory mechanisms that ultimately lead to the production of QUIN. To date, few specific and potent inhibitors of this enzyme have been identified. A number of inhibitors that resemble the molecular transition state of a range of substrates have been developed including (4S)- and (4R)-dihydro-L-kynurenine [200], a series of S-aryl-L-cysteine S,S-dioxides [201] and a phosphinic acid [202] analogue of kynurenine which are all inhibitors of bacterial KYNU.
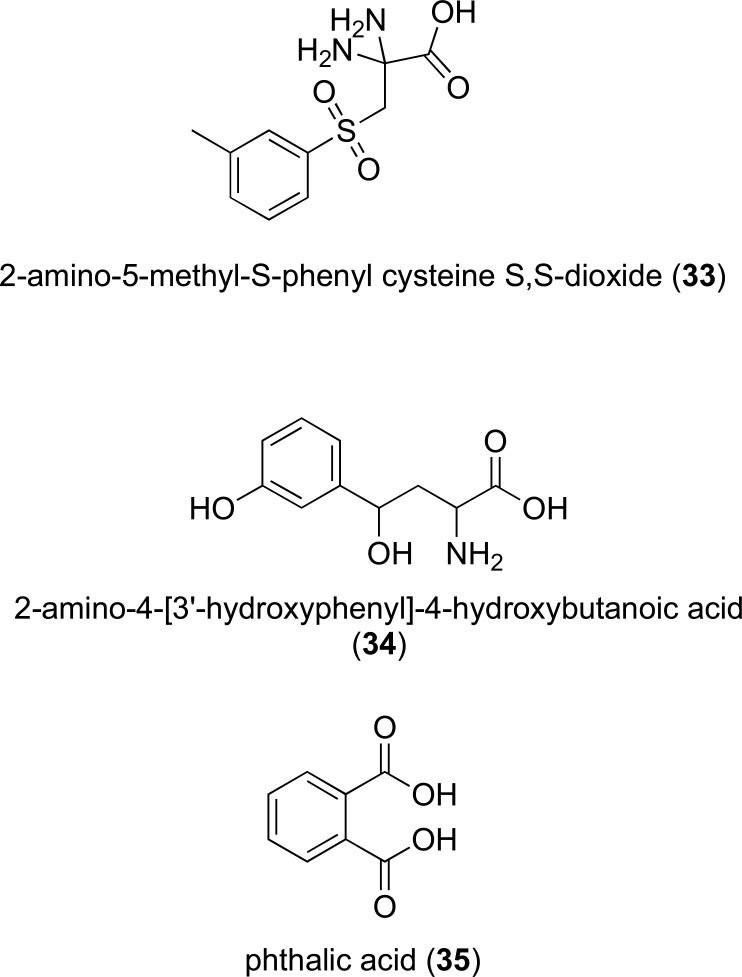
Further investigation into the S-aryl-L-cysteine S,S-dioxide scaffold resulted in the synthesis of 2-amino-5-methyl-S-phenyl cysteine S,S-dioxide (IC50 of 11 µM in rat liver KYNU, 33, Fig. 6). This molecule was shown to inhibit INF-γ induced QUIN synthesis in human macrophages [203].
Fig. (6).
KYNU and QPRT inhibitor structures.
4.4.1. Kynurenine Derivatives
A kynurenine derivative 2-amino-4-[3’-hydroxy-phenyl]-4-hydroxybutanoic acid (34, Fig. 6) was designed to include a hydroxyl moiety at C7 and C3 to mimic the substrates transition state and improve specificity towards mammalian KYNU respectively. This molecule was shown to have a Ki of 100 nM against with recombinant human KYNU [204] and was a mixed mode inhibitor, acting as a competitive inhibitor at low concentrations and non-competitive at higher concentrations [204]. This supported previous evidence that the enzyme has a second regulatory binding site [204, 205].
4.5. Quinolinic Acid Phosphoribosyltransferase (QPRT) Inhibitors
To date few modulators of QPRT have been identified, however as discussed above, QPRT enzyme acti-vators might have beneficial properties in neurodegenerative conditions. Conversely, QPRT has been proposed as a therapeutic target for the treatment of malignant gliomas [206]. QPRT expression is associated with poor prognosis in recurrent glioblastoma after radiochemotherapy [207] The inhibition of QPRT would result in a reduction of the synthesis of NAMN and in turn NAD, thereby limiting rapid cell growth [207, 208]. The crystal structure of human QPRT was determined in complex with its inhibitor phthalic acid (PDB ID: 4KWW), QUIN (PDB ID: 5AYY), NAMN (PDB ID: 5AYY) and in its free form (PDB ID: 5AYX) [208, 209], providing valuable information on QPRT structure and active site, which have utility in the development of new modulators of this enzyme.
QPRTs only reported inhibitor phthalic acid (35, Fig. 6) is a QUIN analogue which acts as a moderately potent competitive inhibitor of hQPRT with an inhibition constant of 2.8 µM [209]. Administration of phthalic acid to neurons and astrocytes has been shown to lead to a dose dependent decrease in QPRT activity which in turn correlated to a decrease in NAD+ in the cells and an increase in extracellular LDH activity [210].
CONCLUSION
Mounting evidence implicates disruption in kynurenine pathway metabolism in neurological disorders and cancer. As a result, enzymes of the KP pathway, in particular those responsible for the formation of neuroactive metabolites (such as KMO and KAT) or immune modulation (IDO) continue to be investigated as promising therapeutic targets. In recent years, elucidation of KP enzyme crystal structures and increased efforts to assess SAR of known inhibitors has resulted in the development potent inhibitors with increased selectivity and improved pharmacological profiles. Considering that a number of IDO inhibitors have already undergone clinical trials as cancer therapeutics, together with our increased understanding of the molecular interactions of KP metabolites with the AhR (and their downstream effects on tumor immunology), it seems almost inevitable that a KP enzyme inhibitor will soon be a part of cancer therapy. In neurodegenerative disease, KMO inhibitors in particular have emerged as potential therapeutics to limit the damaging disease-progressing effects of neuroinflammation. Despite this promise, lack of ability to permeate the BBB has retarded developmental progress and presents a focus of challenge for medicinal chemists.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012;13(7):465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 3.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 4.Koblish H.K., Hansbury M.J., Bowman K.J., Yang G., Neilan C.L., Haley P.J., Burn T.C., Waeltz P., Sparks R.B., Yue E.W., Combs A.P., Scherle P.A., Vaddi K., Fridman J.S. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol. Cancer Ther. 2010;9(2):489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 5.Nayak A., Hao Z., Sadek R., Vahanian N., Ramsey W.J., Kennedy E., Mautino M., Link C., Bourbo P., Dobbins R., Adams K., Diamond A., Marshall L., Munn D.H., Janik J., Khleif S.N. A Phase I study of NLG919 for adult patients with recurrent advanced solid tumors. J. Immunother. Cancer. 2014;2(Suppl. 3):250–P250. [Google Scholar]
- 6.Opitz C.A., Litzenburger U.M., Sahm F., Ott M., Tritschler I., Trump S., Schumacher T., Jestaedt L., Schrenk D., Weller M., Jugold M., Guillemin G.J., Miller C.L., Lutz C., Radlwimmer B., Lehmann I., von Deimling A., Wick W., Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 7.Wu W., Nicolazzo J.A., Wen L., Chung R., Stankovic R., Bao S.S., Lim C.K., Brew B.J., Cullen K.M., Guillemin G.J. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8(4):e59749. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz M.J., Guillemin G.J., Teipel S.J., Buerger K., Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(4):345–352. doi: 10.1007/s00406-012-0384-x. [DOI] [PubMed] [Google Scholar]
- 9.Guillemin G.J., Meininger V., Brew B.J. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2(3-4):166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 10.Guidetti P., Luthi-Carter R.E., Augood S.J., Schwarcz R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol. Dis. 2004;17(3):455–461. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Kegel M.E., Bhat M., Skogh E., Samuelsson M., Lundberg K., Dahl M.L., Sellgren C., Schwieler L., Engberg G., Schuppe-Koistinen I., Erhardt S. Imbalanced kynurenine pathway in schizophrenia. Int. J. Tryptophan Res. 2014;7:15–22. doi: 10.4137/IJTR.S16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillemin G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279(8):1356–1365. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 13.Pilotte L., Larrieu P., Stroobant V., Colau D., Dolušić E., Frédérick R., De Plaen E., Uyttenhove C., Wouters J., Masereel B., Van den Eynde B.J. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA. 2012;109(7):2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball H.J., Sanchez-Perez A., Weiser S., Austin C.J., Astelbauer F., Miu J., McQuillan J.A., Stocker R., Jermiin L.S., Hunt N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Metz R., Duhadaway J.B., Kamasani U., Laury-Kleintop L., Muller A.J., Prendergast G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67(15):7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 16.Capece L., Lewis-Ballester A., Batabyal D., Di Russo N., Yeh S.R., Estrin D.A., Marti M.A. The first step of the dioxygenation reaction carried out by tryptophan dioxygenase and indoleamine 2,3-dioxygenase as revealed by quantum mechani-cal/molecular mechanical studies. J. Biol. Inorg. Chem. 2010;15(6):811–823. doi: 10.1007/s00775-010-0646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor M.W., Feng G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5(11):2516–2522. [PubMed] [Google Scholar]
- 18.Guillemin G.J., Smythe G.A., Veas L.A., Takikawa O., Brew B.J. A beta 1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14(18):2311–2315. doi: 10.1097/00001756-200312190-00005. [DOI] [PubMed] [Google Scholar]
- 19.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated L-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005;338(1):12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta. 1989;1012(2):140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Dai W., Gupta S.L. Regulation of indoleamine 2,3-dioxygenase gene expression in human fibroblasts by interferon-gamma. Upstream control region discriminates between interferon-gamma and interferon-alpha. J. Biol. Chem. 1990;265(32):19871–19877. [PubMed] [Google Scholar]
- 22.Okamoto A., Nikaido T., Ochiai K., Takakura S., Saito M., Aoki Y., Ishii N., Yanaihara N., Yamada K., Takikawa O., Kawaguchi R., Isonishi S., Tanaka T., Urashima M. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res. 2005;11(16):6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 23.Pan K., Wang H., Chen M.S., Zhang H.K., Weng D.S., Zhou J., Huang W., Li J.J., Song H.F., Xia J.C. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2008;134(11):1247–1253. doi: 10.1007/s00432-008-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ino K., Yoshida N., Kajiyama H., Shibata K., Yamamoto E., Kidokoro K., Takahashi N., Terauchi M., Nawa A., Nomura S., Nagasaka T., Takikawa O., Kikkawa F. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br. J. Cancer. 2006;95(11):1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto H., Yamamoto S., Nozaki M., Hayaishi O. On the submitochondrial localization of l-kynurenine-3-hydroxylase. Biochem. Biophys. Res. Commun. 1967;26(3):309–314. doi: 10.1016/0006-291x(67)90123-4. [DOI] [PubMed] [Google Scholar]
- 26.Allegri G., Ragazzi E., Bertazzo A., Costa C. V. L. Enzyme activities along the kynurenine pathway in mice. 2003. [DOI] [PubMed]
- 27.Braidy N., Guillemin G., Grant R. Promotion of cellular NAD(+) anabolism: therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox. Res. 2008;13(3-4):173–184. doi: 10.1007/BF03033501. [DOI] [PubMed] [Google Scholar]
- 28.Zwilling D., Huang S-Y., Sathyasaikumar K.V., Notarangelo F.M., Guidetti P., Wu H-Q., Lee J., Truong J., Andrews-Zwilling Y., Hsieh E.W., Louie J.Y., Wu T., Scearce-Levie K., Patrick C., Adame A., Giorgini F., Moussaoui S., Laue G., Rassoulpour A., Flik G., Huang Y., Muchowski J.M., Masliah E., Schwarcz R., Muchowski P.J. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H., Zhang Y., You H., Tao X., Wang C., Jin G., Wang N., Ruan H., Gu D., Huo X., Cong W., Qin W. Prognostic significance of kynurenine 3-monooxygenase and effects on proliferation, migration, and invasion of human hepatocellular carcinoma. Sci. Rep. 2015;5:10466. doi: 10.1038/srep10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mole D.J., Webster S.P., Uings I., Zheng X., Binnie M., Wilson K., Hutchinson J.P., Mirguet O., Walker A., Beaufils B., Ancellin N., Trottet L., Bénéton V., Mowat C.G., Wilkinson M., Rowland P., Haslam C., McBride A., Homer N.Z., Baily J.E., Sharp M.G., Garden O.J., Hughes J., Howie S.E., Holmes D.S., Liddle J., Iredale J.P. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat. Med. 2016;22(2):202–209. doi: 10.1038/nm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akladios F.N., Nadvi N.A., Park J., Hanrahan J.R., Kapoor V., Gorrell M.D., Church W.B. Design and synthesis of novel inhibitors of human kynurenine aminotransferase-I. Bioorg. Med. Chem. Lett. 2012;22(4):1579–1581. doi: 10.1016/j.bmcl.2011.12.138. [DOI] [PubMed] [Google Scholar]
- 32.Han Q., Cai T., Tagle D.A., Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell. Mol. Life Sci. 2010;67(3):353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 2001;78(4):842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 34.Guidetti P., Okuno E., Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J. Neurosci. Res. 1997;50(3):457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Sathyasaikumar K.V., Stachowski E.K., Wonodi I., Roberts R.C., Rassoulpour A., McMahon R.P., Schwarcz R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011;37(6):1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi F., Garavaglia S., Montalbano V., Walsh M.A., Rizzi M. Crystal structure of human kynurenine aminotransferase II, a drug target for the treatment of schizophrenia. J. Biol. Chem. 2008;283(6):3559–3566. doi: 10.1074/jbc.M707925200. [DOI] [PubMed] [Google Scholar]
- 37.Lima S., Khristoforov R., Momany C., Phillips R.S. Crystal structure of Homo sapiens kynureninase. Biochemistry. 2007;46(10):2735–2744. doi: 10.1021/bi0616697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maitrani C., Phillips R.S. Substituents effects on activity of kynureninase from Homo sapiens and Pseudomonas fluorescens. Bioorg. Med. Chem. 2013;21(15):4670–4677. doi: 10.1016/j.bmc.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 39.Vescia A., Di Prisco G. Studies on purified 3-hydroxyanthranilic acid oxidase. J. Biol. Chem. 1962;237(7):2318–2324. [PubMed] [Google Scholar]
- 40.Foster A.C., Miller L.P., Oldendorf W.H., Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp. Neurol. 1984;84(2):428–440. doi: 10.1016/0014-4886(84)90239-5. [DOI] [PubMed] [Google Scholar]
- 41.Rahman A., Ting K., Cullen K.M., Braidy N., Brew B.J., Guillemin G.J. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009;4(7):e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster A.C., Schwarcz R. Characterization of quinolinic acid phosphoribosyltransferase in human blood and observations in Huntington’s disease. J. Neurochem. 1985;45(1):199–205. doi: 10.1111/j.1471-4159.1985.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 43.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS One. 2011;6(4):e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Fukui S., Schwarcz R., Rapoport S.I., Takada Y., Smith Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 45.Fallarino F., Grohmann U., You S., McGrath B.C., Cavener D.R., Vacca C., Orabona C., Bianchi R., Belladonna M.L., Volpi C., Santamaria P., Fioretti M.C., Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006;176(11):6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 46.Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196(4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 48.Bekki K., Vogel H., Li W., Ito T., Sweeney C., Haarmann-Stemmann T., Matsumura F., Vogel C.F. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic. Biochem. Physiol. 2015;120:5–13. doi: 10.1016/j.pestbp.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Amato N.C., Rogers T.J., Gordon M.A., Greene L.I., Cochrane D.R., Spoelstra N.S., Nemkov T.G., D’Alessandro A., Hansen K.C., Richer J.K. TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75(21):4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugo-Huitrón R., Blanco-Ayala T., Ugalde-Muñiz P., Carrillo-Mora P., Pedraza-Chaverrí J., Silva-Adaya D., Maldonado P.D., Torres I., Pinzón E., Ortiz-Islas E., López T., García E., Pineda B., Torres-Ramos M., Santamaría A., La Cruz V.P. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011;33(5):538–547. doi: 10.1016/j.ntt.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Perkins M.N., Stone T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 52.Konradsson-Geuken A., Wu H.Q., Gash C.R., Alexander K.S., Campbell A., Sozeri Y., Pellicciari R., Schwarcz R., Bruno J.P. Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience. 2010;169(4):1848–1859. doi: 10.1016/j.neuroscience.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaumont V., Mrzljak L., Dijkman U., Freije R., Heins M., Rassoulpour A., Tombaugh G., Gelman S., Bradaia A., Steidl E., Gleyzes M., Heikkinen T., Lehtimäki K., Puoliväli J., Kontkanen O., Javier R.M., Neagoe I., Deisemann H., Winkler D., Ebneth A., Khetarpal V., Toledo-Sherman L., Dominguez C., Park L.C., Munoz-Sanjuan I. The novel KMO inhibitor CHDI-340246 leads to a restoration of electrophysiological alterations in mouse models of Huntington’s disease. Exp. Neurol. 2016;282:99–118. doi: 10.1016/j.expneurol.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Moroni F., Cozzi A., Carpendo R., Cipriani G., Veneroni O., Izzo E. Kynurenine 3-mono-oxygenase inhibitors reduce glutamate concentration in the extracellular spaces of the basal ganglia but not in those of the cortex or hippocampus. Neuropharmacology. 2005;48(6):788–795. doi: 10.1016/j.neuropharm.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 55.Sapko M.T., Guidetti P., Yu P., Tagle D.A., Pellicciari R., Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Exp. Neurol. 2006;197(1):31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Chess A.C., Simoni M.K., Alling T.E., Bucci D.J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 2007;33(3):797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erhardt S., Schwieler L., Emanuelsson C., Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol. Psychiatry. 2004;56(4):255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Pocivavsek A., Wu H-Q., Potter M.C., Elmer G.I., Pellicciari R., Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36(11):2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okuda S., Nishiyama N., Saito H., Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc. Natl. Acad. Sci. USA. 1996;93(22):12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okuda S., Nishiyama N., Saito H., Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 61.Christen S., Peterhans E., Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. USA. 1990;87(7):2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leipnitz G., Schumacher C., Dalcin K.B., Scussiato K., Solano A., Funchal C., Dutra-Filho C.S., Wyse A.T., Wannmacher C.M., Latini A., Wajner M. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem. Int. 2007;50(1):83–94. doi: 10.1016/j.neuint.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Colín-González A.L., Maya-López M., Pedraza-Chaverrí J., Ali S.F., Chavarría A., Santamaría A. The Janus faces of 3-hydroxykynurenine: dual redox modulatory activity and lack of neurotoxicity in the rat striatum. Brain Res. 2014;1589:1–14. doi: 10.1016/j.brainres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 64.Krause D., Suh H-S., Tarassishin L., Cui Q.L., Durafourt B.A., Choi N., Bauman A., Cosenza-Nashat M., Antel J.P., Zhao M-L., Lee S.C. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am. J. Pathol. 2011;179(3):1360–1372. doi: 10.1016/j.ajpath.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh G-S., Pae H-O., Choi B-M., Chae S-C., Lee H-S., Ryu D-G., Chung H-T. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem. Biophys. Res. Commun. 2004;320(4):1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 66.Thomas S. R., Stocker R. Antioxidant activities and redox regulation of interferon-gamma-induced tryptophan metabolism in human mono-cytes and macrophages. 2000. [DOI] [PubMed]
- 67.Chobot V., Hadacek F., Weckwerth W., Kubicova L. Iron chelation and redox chemistry of anthranilic acid and 3-hydroxyanthranilic acid: A comparison of two structurally related kynurenine pathway metabolites to obtain improved insights into their potential role in neurological disease development. J. Organomet. Chem. 2015;782:103–110. doi: 10.1016/j.jorganchem.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S-M., Lee Y-S., Choi J-H., Park S-G., Choi I-W., Joo Y-D., Lee W-S., Lee J-N., Choi I., Seo S-K. Tryptophan metabolite 3-hydroxyanthranilic acid selectively induces activated T cell death via intracellular GSH depletion. Immunol. Lett. 2010;132(1-2):53–60. doi: 10.1016/j.imlet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Darlington L.G., Forrest C.M., Mackay G.M., Smith R.A., Smith A.J., Stoy N., Stone T.W. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. Int. J. Tryptophan Res. 2010;3:51–59. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heyes M.P., Achim C.L., Wiley C.A., Major E.O., Saito K., Markey S.P. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996;320(Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Espey M.G., Chernyshev O.N., Reinhard J.F., Jr, Namboodiri M.A., Colton C.A. Activated human microglia produce the excitotoxin quinolinic acid. Neuroreport. 1997;8(2):431–434. doi: 10.1097/00001756-199701200-00011. [DOI] [PubMed] [Google Scholar]
- 72.Moffett J.R., Namboodiri M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 73.Braidy N., Grant R., Adams S., Brew B.J., Guillemin G.J. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox. Res. 2009;16(1):77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 74.Michaels R.L., Rothman S.M. Glutamate neurotoxicity in vitro: antagonist pharmacology and intracellular calcium concentrations. J. Neurosci. 1990;10(1):283–292. doi: 10.1523/JNEUROSCI.10-01-00283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fallarino F., Grohmann U., Vacca C., Bianchi R., Orabona C., Spreca A., Fioretti M.C., Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9(10):1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 76.Bender D.A. Biochemistry of tryptophan in health and disease. Mol. Aspects Med. 1983;6(2):101–197. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- 77.Braidy N., Guillemin G., Grant R. Promotion of cellular NAD(+) anabolism: therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox. Res. 2008;13(3-4):173–184. doi: 10.1007/BF03033501. [DOI] [PubMed] [Google Scholar]
- 78.Prendergast G.C. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27(28):3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 79.Munn D.H., Shafizadeh E., Attwood J.T., Bondarev I., Pashine A., Mellor A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999;189(9):1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molano A., Illarionov P.A., Besra G.S., Putterman C., Porcelli S.A. Modulation of invariant natural killer T cell cytokine responses by indoleamine 2,3-dioxygenase. Immunol. Lett. 2008;117(1):81–90. doi: 10.1016/j.imlet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Della Chiesa M., Carlomagno S., Frumento G., Balsamo M., Cantoni C., Conte R., Moretta L., Moretta A., Vitale M. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108(13):4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 82.Isla Larrain M.T., Rabassa M.E., Lacunza E., Barbera A., Cretón A., Segal-Eiras A., Croce M.V. IDO is highly expressed in breast cancer and breast cancer-derived circulating microvesicles and associated to aggressive types of tumors by in silico analysis. Tumour Biol. 2014;35(7):6511–6519. doi: 10.1007/s13277-014-1859-3. [DOI] [PubMed] [Google Scholar]
- 83.Friberg M., Jennings R., Alsarraj M., Dessureault S., Cantor A., Extermann M., Mellor A.L., Munn D.H., Antonia S.J. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer. 2002;101(2):151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 84.Opitz C.A., Litzenburger U.M., Opitz U., Sahm F., Ochs K., Lutz C., Wick W., Platten M. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS One. 2011;6(5):e19823. doi: 10.1371/journal.pone.0019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adams S., Braidy N., Bessede A., Brew B.J., Grant R., Teo C., Guillemin G.J. The kynurenine pathway in brain tumor pathogenesis. Cancer Res. 2012;72(22):5649–5657. doi: 10.1158/0008-5472.CAN-12-0549. [DOI] [PubMed] [Google Scholar]
- 86.Widner B., Leblhuber F., Walli J., Tilz G.P., Demel U., Fuchs D. Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural Transm. (Vienna) 2000;107(3):343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- 87.Guillemin G.J., Brew B.J., Noonan C.E., Takikawa O., Cullen K.M. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol. Appl. Neurobiol. 2005;31(4):395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 88.Gulaj E., Pawlak K., Bien B., Pawlak D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 2010;55(2):204–211. doi: 10.2478/v10039-010-0023-6. [DOI] [PubMed] [Google Scholar]
- 89.Ogawa T., Matson W.R., Beal M.F., Myers R.H., Bird E.D., Milbury P., Saso S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology. 1992;42(9):1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- 90.Lewitt P.A., Li J., Lu M., Beach T.G., Adler C.H., Guo L. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov. Disord. 2013;28(12):1653–1660. doi: 10.1002/mds.25555. [DOI] [PubMed] [Google Scholar]
- 91.Braak H., Del Tredici K., Rüb U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 92.Miranda A.F., Boegman R.J., Beninger R.J., Jhamandas K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience. 1997;78(4):967–975. doi: 10.1016/s0306-4522(96)00655-0. [DOI] [PubMed] [Google Scholar]
- 93.Graves M.C., Fiala M., Dinglasan L.A., Liu N.Q., Sayre J., Chiappelli F., van Kooten C., Vinters H.V. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004;5(4):213–219. doi: 10.1080/14660820410020286. [DOI] [PubMed] [Google Scholar]
- 94.Guidetti P., Bates G.P., Graham R.K., Hayden M.R., Leavitt B.R., MacDonald M.E., Slow E.J., Wheeler V.C., Woodman B., Schwarcz R. Elevated brain 3-hydroxykynurenine and quinolinate levels in Huntington disease mice. Neurobiol. Dis. 2006;23(1):190–197. doi: 10.1016/j.nbd.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 95.Guidetti P., Reddy P.H., Tagle D.A., Schwarcz R. Early kynurenergic impairment in Huntington’s disease and in a transgenic animal model. Neurosci. Lett. 2000;283(3):233–235. doi: 10.1016/s0304-3940(00)00956-3. [DOI] [PubMed] [Google Scholar]
- 96.Giorgini F., Guidetti P., Nguyen Q., Bennett S.C., Muchowski P.J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat. Genet. 2005;37(5):526–531. doi: 10.1038/ng1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campesan S., Green E.W., Breda C., Sathyasaikumar K.V., Muchowski P.J., Schwarcz R., Kyriacou C.P., Giorgini F. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington’s disease. Curr. Biol. 2011;21(11):961–966. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dominguez C., Munoz-Sanjuan I. Foundation-directed therapeutic development in Huntington’s disease. J. Med. Chem. 2014;57(13):5479–5488. doi: 10.1021/jm4009295. [DOI] [PubMed] [Google Scholar]
- 99.Yu D., Tao B.B., Yang Y.Y., Du L.S., Yang S.S., He X.J., Zhu Y.W., Yan J.K., Yang Q. The IDO inhibitor coptisine ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015;43(1):291–302. doi: 10.3233/JAD-140414. [DOI] [PubMed] [Google Scholar]
- 100.Lahti A.C., Koffel B., LaPorte D., Tamminga C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 101.Anis N.A., Berry S.C., Burton N.R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br. J. Pharmacol. 1983;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller C.L., Llenos I.C., Dulay J.R., Barillo M.M., Yolken R.H., Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol. Dis. 2004;15(3):618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Miller C.L., Llenos I.C., Dulay J.R., Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 104.Wonodi I., Stine O.C., Sathyasaikumar K.V., Roberts R.C., Mitchell B.D., Hong L.E., Kajii Y., Thaker G.K., Schwarcz R. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch. Gen. Psychiatry. 2011;68(7):665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarcz R., Rassoulpour A., Wu H.Q., Medoff D., Tamminga C.A., Roberts R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50(7):521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 106.Erhardt S., Blennow K., Nordin C., Skogh E., Lindström L.H., Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001;313(1-2):96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 107.Holtze M., Saetre P., Engberg G., Schwieler L., Werge T., Andreassen O.A., Hall H., Terenius L., Agartz I., Jönsson E.G., Schalling M., Erhardt S. Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 2012;37(1):53–57. doi: 10.1503/jpn.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wonodi I., McMahon R.P., Krishna N., Mitchell B.D., Liu J., Glassman M., Hong L.E., Gold J.M. Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr. Res. 2014;160(1-3):80–87. doi: 10.1016/j.schres.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lavebratt C., Olsson S., Backlund L., Frisén L., Sellgren C., Priebe L., Nikamo P., Träskman-Bendz L., Cichon S., Vawter M.P., Osby U., Engberg G., Landén M., Erhardt S., Schalling M. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol. Psychiatry. 2014;19(3):334–341. doi: 10.1038/mp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacKenzie C.R., Heseler K., Müller A., Däubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr. Drug Metab. 2007;8(3):237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 111.Müller A., Heseler K., Schmidt S.K., Spekker K., Mackenzie C.R., Däubener W. The missing link between indoleamine 2,3-dioxygenase mediated antibacterial and immunoregulatory effects. J. Cell. Mol. Med. 2009;13(6):1125–1135. doi: 10.1111/j.1582-4934.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Routy J.P., Mehraj V., Vyboh K., Cao W., Kema I., Jenabian M.A. Clinical Relevance of Kynurenine Pathway in HIV/AIDS: An Immune Checkpoint at the Crossroads of Metabolism and Inflammation. AIDS Rev. 2015;17(2) [PubMed] [Google Scholar]
- 113.Kandanearatchi A., Brew B.J. The kynurenine pathway and quinolinic acid: Pivotal roles in HIV associated neurocognitive disorders. FEBS J. 2012;279(8):1366–1374. doi: 10.1111/j.1742-4658.2012.08500.x. [DOI] [PubMed] [Google Scholar]
- 114.Idro R., Marsh K., John C.C., Newton C.R. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr. Res. 2010;68(4):267–274. doi: 10.1203/PDR.0b013e3181eee738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kannan G., Pletnikov M.V. Toxoplasma gondii and cognitive deficits in schizophrenia: An animal model perspective. Schizophr. Bull. 2012;38(6):1155–1161. doi: 10.1093/schbul/sbs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heyes M.P., Brew B.J., Martin A., Price R.W., Salazar A.M., Sidtis J.J., Yergey J.A., Mouradian M.M., Sadler A.E., Keilp J. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: Relationship to clinical and neurological status. Ann. Neurol. 1991;29(2):202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- 117.Heyes M.P., Ellis R.J., Ryan L., Childers M.E., Grant I., Wolfson T., Archibald S., Jernigan T.L. Elevated cerebrospinal fluid quinolinic acid levels are associated with region-specific cerebral volume loss in HIV infection. Brain. 2001;124(Pt 5):1033–1042. doi: 10.1093/brain/124.5.1033. [DOI] [PubMed] [Google Scholar]
- 118.Kerr S.J., Armati P.J., Pemberton L.A., Smythe G., Tattam B., Brew B.J. Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology. 1997;49(6):1671–1681. doi: 10.1212/wnl.49.6.1671. [DOI] [PubMed] [Google Scholar]
- 119.Keegan M.R., Chittiprol S., Letendre S.L., Winston A., Fuchs D., Boasso A., Iudicello J., Ellis R.J. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int. J. Tryptophan Res. 2016;9:79–88. doi: 10.4137/IJTR.S36464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dobbie M., Crawley J., Waruiru C., Marsh K., Surtees R. Cerebrospinal fluid studies in children with cerebral malaria: an excitotoxic mechanism? Am. J. Trop. Med. Hyg. 2000;62(2):284–290. doi: 10.4269/ajtmh.2000.62.284. [DOI] [PubMed] [Google Scholar]
- 121.Medana I.M., Day N.P., Salahifar-Sabet H., Stocker R., Smythe G., Bwanaisa L., Njobvu A., Kayira K., Turner G.D., Taylor T.E., Hunt N.H. Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J. Infect. Dis. 2003;188(6):844–849. doi: 10.1086/377583. [DOI] [PubMed] [Google Scholar]
- 122.Sanni L.A., Thomas S.R., Tattam B.N., Moore D.E., Chaudhri G., Stocker R., Hunt N.H. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am. J. Pathol. 1998;152(2):611–619. [PMC free article] [PubMed] [Google Scholar]
- 123.Clark C.J., Mackay G.M., Smythe G.A., Bustamante S., Stone T.W., Phillips R.S. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect. Immun. 2005;73(8):5249–5251. doi: 10.1128/IAI.73.8.5249-5251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miu J., Ball H.J., Mellor A.L., Hunt N.H. Effect of indoleamine dioxygenase-1 deficiency and kynurenine pathway inhibition on murine cerebral malaria. Int. J. Parasitol. 2009;39(3):363–370. doi: 10.1016/j.ijpara.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 125.Oberdörfer C., Adams O., MacKenzie C.R., De Groot C.J., Däubener W. Role of IDO activation in anti-microbial defense in human native astrocytes. Adv. Exp. Med. Biol. 2003;527:15–26. doi: 10.1007/978-1-4615-0135-0_2. [DOI] [PubMed] [Google Scholar]
- 126.Notarangelo F.M., Wilson E.H., Horning K.J., Thomas M.A., Harris T.H., Fang Q., Hunter C.A., Schwarcz R. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr. Res. 2014;152(1):261–267. doi: 10.1016/j.schres.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fabiani S., Pinto B., Bonuccelli U., Bruschi F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. J. Neurol. Sci. 2015;351(1-2):3–8. doi: 10.1016/j.jns.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 128.Henriquez S.A., Brett R., Alexander J., Pratt J., Roberts C.W. Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation. 2009;16(2):122–133. doi: 10.1159/000180267. [DOI] [PubMed] [Google Scholar]
- 129.Hunt N. H., Too L. K., Khaw L. T., Guo J., Hee L., Mitchell A. J., Grau G. E., Ball H. J. The kynurenine pathway and parasitic infections that af-fect CNS function. 2017. [DOI] [PubMed]
- 130.Muller A.J., DuHadaway J.B., Donover P.S., Sutanto-Ward E., Prendergast G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005;11(3):312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 131.Wang D., Saga Y., Mizukami H., Sato N., Nonaka H., Fujiwara H., Takei Y., Machida S., Takikawa O., Ozawa K., Suzuki M. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int. J. Oncol. 2012;40(4):929–934. doi: 10.3892/ijo.2011.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vacchelli E., Aranda F., Eggermont A., Sautès-Fridman C., Tartour E., Kennedy E.P., Platten M., Zitvogel L., Kroemer G., Galluzzi L. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology. 2014;3(10):e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cady S.G., Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch. Biochem. Biophys. 1991;291(2):326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 134.Yang H.J., Yen M.C., Lin C.C., Lin C.M., Chen Y.L., Weng T.Y., Huang T.T., Wu C.L., Lai M.D. A combination of the metabolic enzyme inhibitor APO866 and the immune adjuvant L-1-methyl tryptophan induces additive antitumor activity. Exp. Biol. Med. (Maywood) 2010;235(7):869–876. doi: 10.1258/ebm.2010.010001. [DOI] [PubMed] [Google Scholar]
- 135.Hou D.Y., Muller A.J., Sharma M.D., DuHadaway J., Banerjee T., Johnson M., Mellor A.L., Prendergast G.C., Munn D.H. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 136.Inaba T., Ino K., Kajiyama H., Yamamoto E., Shibata K., Nawa A., Nagasaka T., Akimoto H., Takikawa O., Kikkawa F. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol. 2009;115(2):185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 137.Qian F., Villella J., Wallace P.K., Mhawech-Fauceglia P., Tario J.D., Jr, Andrews C., Matsuzaki J., Valmori D., Ayyoub M., Frederick P.J., Beck A., Liao J., Cheney R., Moysich K., Lele S., Shrikant P., Old L.J., Odunsi K. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69(13):5498–5504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 138.Li M., Bolduc A.R., Hoda M.N., Gamble D.N., Dolisca S-B., Bolduc A.K., Hoang K., Ashley C., McCall D., Rojiani A.M., Maria B.L., Rixe O., MacDonald T.J., Heeger P.S., Mellor A.L., Munn D.H., Johnson T.S. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J. Immunother. Cancer. 2014;2(1):21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qian F., Liao J., Villella J., Edwards R., Kalinski P., Lele S., Shrikant P., Odunsi K. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol. Immunother. 2012;61(11):2013–2020. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Soliman H.H., Jackson E., Neuger T., Dees E.C., Harvey R.D., Han H., Ismail-Khan R., Minton S., Vahanian N.N., Link C., Sullivan D.M., Antonia S. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5(18):8136–8146. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Soliman H.H., Minton S.E., Han H.S., Ismail-Khan R., Neuger A., Khambati F., Noyes D., Lush R., Chiappori A.A., Roberts J.D., Link C., Vahanian N.N., Mautino M., Streicher H., Sullivan D.M., Antonia S.J. A phase I study of indoximod in patients with advanced malignancies. Oncotarget. 2016;7(16):22928–22938. doi: 10.18632/oncotarget.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Trials.gov, C. Available at: https://clinicaltrials.gov/
- 143.Yue E.W., Douty B., Wayland B., Bower M., Liu X., Leffet L., Wang Q., Bowman K.J., Hansbury M.J., Liu C., Wei M., Li Y., Wynn R., Burn T.C., Koblish H.K., Fridman J.S., Metcalf B., Scherle P.A., Combs A.P. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J. Med. Chem. 2009;52(23):7364–7367. doi: 10.1021/jm900518f. [DOI] [PubMed] [Google Scholar]
- 144.Liu X., Shin N., Koblish H.K., Yang G., Wang Q., Wang K., Leffet L., Hansbury M.J., Thomas B., Rupar M., Waeltz P., Bowman K.J., Polam P., Sparks R.B., Yue E.W., Li Y., Wynn R., Fridman J.S., Burn T.C., Combs A.P., Newton R.C., Scherle P.A. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115(17):3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 145.Beatty G.L., O’Dwyer P.J., Clark J., Shi J.G., Bowman K.J., Scherle P.A., Newton R.C., Schaub R., Maleski J., Leopold L., Gajewski T.F. First-in-human phase i study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin. Cancer Res. 2017;23(13):3269–3276. doi: 10.1158/1078-0432.CCR-16-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mautino M., Kumar S., Waldo J., Jaipuri F., Kesharwani T. Preparation of fused imidazole derivatives as IDO inhibitors. WO2012142237A1 . 2012.
- 147.Peng Y-H., Ueng S-H., Tseng C-T., Hung M-S., Song J-S., Wu J-S., Liao F-Y., Fan Y-S., Wu M-H., Hsiao W-C., Hsueh C-C., Lin S-Y., Cheng C-Y., Tu C-H., Lee L-C., Cheng M-F., Shia K-S., Shih C., Wu S-Y. Important Hydrogen Bond Networks in Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Design Revealed by Crystal Structures of Imidazoleisoindole Derivatives with IDO1. J. Med. Chem. 2016;59(1):282–293. doi: 10.1021/acs.jmedchem.5b01390. [DOI] [PubMed] [Google Scholar]
- 148.Sugimoto H., Oda S., Otsuki T., Hino T., Yoshida T., Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. USA. 2006;103(8):2611–2616. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sono M., Cady S.G. Enzyme kinetic and spectroscopic studies of inhibitor and effector interactions with indoleamine 2,3-dioxygenase. 1. Norharman and 4-phenylimidazole binding to the enzyme as inhibitors and heme ligands. Biochemistry. 1989;28(13):5392–5399. doi: 10.1021/bi00439a012. [DOI] [PubMed] [Google Scholar]
- 150.Eguchi N., Watanabe Y., Kawanishi K., Hashimoto Y., Hayaishi O. Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by beta-carboline and indole derivatives. Arch. Biochem. Biophys. 1984;232(2):602–609. doi: 10.1016/0003-9861(84)90579-4. [DOI] [PubMed] [Google Scholar]
- 151.Kumar S., Jaller D., Patel B., LaLonde J.M., DuHadaway J.B., Malachowski W.P., Prendergast G.C., Muller A.J. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. J. Med. Chem. 2008;51(16):4968–4977. doi: 10.1021/jm800512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carr G., Chung M.K., Mauk A.G., Andersen R.J. Synthesis of indoleamine 2,3-dioxygenase inhibitory analogues of the sponge alkaloid exiguamine A. J. Med. Chem. 2008;51(9):2634–2637. doi: 10.1021/jm800143h. [DOI] [PubMed] [Google Scholar]
- 153.Pereira A., Vottero E., Roberge M., Mauk A.G., Andersen R.J. Indoleamine 2,3-dioxygenase inhibitors from the Northeastern Pacific Marine Hydroid Garveia annulata. J. Nat. Prod. 2006;69(10):1496–1499. doi: 10.1021/np060111x. [DOI] [PubMed] [Google Scholar]
- 154.Rohrig U.F., Awad L., Grosdidier A., Larrieu P., Stroobant V., Colau D., Cerundolo V., Simpson A.J., Vogel P., Van E.B., Zoete V., Michielin O. Design of Indoleamine 2,3-Dioxygenase Inhibitors. J. Med. Chem. 2010;53(3):1172–1189. doi: 10.1021/jm9014718. [DOI] [PubMed] [Google Scholar]
- 155.Gaspari P., Banerjee T., Malachowski W.P., Muller A.J., Prendergast G.C., DuHadaway J., Bennett S., Donovan A.M. Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 2006;49(2):684–692. doi: 10.1021/jm0508888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Matsuno K., Takai K., Isaka Y., Unno Y., Sato M., Takikawa O., Asai A. S-benzylisothiourea derivatives as small-molecule inhibitors of indoleamine-2,3-dioxygenase. Bioorg. Med. Chem. Lett. 2010;20(17):5126–5129. doi: 10.1016/j.bmcl.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 157.Crozier-Reabe K.R., Phillips R.S., Moran G.R. Kynurenine 3-monooxygenase from Pseudomonas fluorescens: substrate-like inhibitors both stimulate flavin reduction and stabilize the flavin-peroxo intermediate yet result in the production of hydrogen peroxide. Biochemistry. 2008;47(47):12420–12433. doi: 10.1021/bi8010434. [DOI] [PubMed] [Google Scholar]
- 158.Pellicciari R., Natalini B., Costantino G., Mahmoud M.R., Mattoli L., Sadeghpour B.M., Moroni F., Chiarugi A., Carpenedo R. Modulation of the kynurenine pathway in search for new neuroprotective agents. Synthesis and preliminary evaluation of (m-nitrobenzoyl)alanine, a potent inhibitor of kynurenine-3-hydroxylase. J. Med. Chem. 1994;37(5):647–655. doi: 10.1021/jm00031a015. [DOI] [PubMed] [Google Scholar]
- 159.Natalini B., Mattoli L., Pellicciari R., Carpenedo R., Chiarugi A., Moroni F. Synthesis and activity of enantiopure (S) (m-nitrobenzoyl) alanine, potent kynurenine-3-hydroxylase inhibitor. Bioorg. Med. Chem. Lett. 1995;5(14):1451–1454. [Google Scholar]
- 160.Speciale C., Cini M., Wu H.Q., Salvati P., Schwarcz R., Molinari A., Calabresi M., Varasi M. Kynurenic acid-enhancing and anti-ischemic effects of the potent kynurenine 3-hydroxylase inhibitor Fce 28833 in rodents. Adv. Exp. Med. Biol. 1996;398:221–227. doi: 10.1007/978-1-4613-0381-7_35. [DOI] [PubMed] [Google Scholar]
- 161.Giordani A., Pevarello P., Cini M., Bormetti R., Greco F., Toma S., Speciale C., Varasi M. 4-Phenyl-4-oxo-butanoic acid derivatives inhibitors of kynurenine 3-hydroxylase. Bioorg. Med. Chem. Lett. 1998;8(20):2907–2912. doi: 10.1016/s0960-894x(98)00517-4. [DOI] [PubMed] [Google Scholar]
- 162.Speciale C., Wu H-Q., Cini M., Marconi M., Varasi M., Schwarcz R. (R,S)-3,4-dichlorobenzoylalanine (FCE 28833A) causes a large and persistent increase in brain kynurenic acid levels in rats. Eur. J. Pharmacol. 1996;315(3):263–267. doi: 10.1016/s0014-2999(96)00613-9. [DOI] [PubMed] [Google Scholar]
- 163.Drysdale M.J., Hind S.L., Jansen M., Reinhard J.F. Jr Synthesis and SAR of 4-aryl-2-hydroxy-4-oxobut-2-enoic acids and esters and 2-amino-4-aryl-4-oxobut-2-enoic acids and esters: potent inhibitors of kynurenine-3-hydroxylase as potential neuroprotective agents. J. Med. Chem. 2000;43(1):123–127. doi: 10.1021/jm990396t. [DOI] [PubMed] [Google Scholar]
- 164.Pellicciari R., Amori L., Costantino G., Giordani A., Macchiarulo A., Mattoli L., Pevarello P., Speciale C., Varasi M. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents. Focus on kynurenine-3-hydroxylase. Adv. Exp. Med. Biol. 2003;527:621–628. doi: 10.1007/978-1-4615-0135-0_71. [DOI] [PubMed] [Google Scholar]
- 165.Amori L., Guidetti P., Pellicciari R., Kajii Y., Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009;109(2):316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Allegri G., Costa C.L., Ragazzi E., Steinhart H., Varesio L., Pellicciari R., Amori L., Costantino G., Giordani A., Macchiarulo A., Mattoli L., Pevarello P., Speciale C., Varasi M. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents. focus on kynurenine-3-hydroxylase. In: Developments in Tryptophan and Serotonin Metabolism, Springer US: . 2003;527:621–628. doi: 10.1007/978-1-4615-0135-0_71. [DOI] [PubMed] [Google Scholar]
- 167.Ceresoli-Borroni G., Guidetti P., Amori L., Pellicciari R., Schwarcz R. Perinatal kynurenine 3-hydroxylase inhibition in rodents: pathophysiological implications. J. Neurosci. Res. 2007;85(4):845–854. doi: 10.1002/jnr.21183. [DOI] [PubMed] [Google Scholar]
- 168.Amaral M., Levy C., Heyes D.J., Lafite P., Outeiro T.F., Giorgini F., Leys D., Scrutton N.S. Structural basis of kynurenine 3-monooxygenase inhibition. Nature. 2013;496(7445):382–385. doi: 10.1038/nature12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Röver S., Cesura A.M., Huguenin P., Kettler R., Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J. Med. Chem. 1997;40(26):4378–4385. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- 170.Grégoire L., Rassoulpour A., Guidetti P., Samadi P., Bédard P.J., Izzo E., Schwarcz R., Di Paolo T. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav. Brain Res. 2008;186(2):161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 171.Carpenedo R., Meli E., Peruginelli F., Pellegrini-Giampietro D.E., Moroni F. Kynurenine 3-mono-oxygenase inhibitors attenuate post-ischemic neuronal death in organotypic hippocampal slice cultures. J. Neurochem. 2002;82(6):1465–1471. doi: 10.1046/j.1471-4159.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 172.Justinova Z., Mascia P., Wu H-Q., Secci M.E., Redhi G.H., Panlilio L.V., Scherma M., Barnes C., Parashos A., Zara T., Fratta W., Solinas M., Pistis M., Bergman J., Kangas B.D., Ferré S., Tanda G., Schwarcz R., Goldberg S.R. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat. Neurosci. 2013;16(11):1652–1661. doi: 10.1038/nn.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Beconi M.G., Yates D., Lyons K., Matthews K., Clifton S., Mead T., Prime M., Winkler D., O’Connell C., Walter D., Toledo-Sherman L., Munoz-Sanjuan I., Dominguez C. Metabolism and pharmacokinetics of JM6 in mice: JM6 is not a prodrug for Ro-61-8048. Drug Metab. Dispos. 2012;40(12):2297–2306. doi: 10.1124/dmd.112.046532. [DOI] [PubMed] [Google Scholar]
- 174.Wityak J., Toledo-Sherman L. M., Leeds J., Dominguez C., Courtney S. M., Yarnold C. J., De A. P. P. C., Scheel A., Winkler D. Certain kynurenine-3-monooxygenase inhibitors, pharmaceutical compositions, and methods of use thereof. WO2010017179A1, 2010.
- 175.Toledo-Sherman L.M., Prime M.E., Mrzljak L., Beconi M.G., Beresford A., Brookfield F.A., Brown C.J., Cardaun I., Courtney S.M., Dijkman U., Hamelin-Flegg E., Johnson P.D., Kempf V., Lyons K., Matthews K., Mitchell W.L., O’Connell C., Pena P., Powell K., Rassoulpour A., Reed L., Reindl W., Selvaratnam S., Friley W.W., Weddell D.A., Went N.E., Wheelan P., Winkler C., Winkler D., Wityak J., Yarnold C.J., Yates D., Munoz-Sanjuan I., Dominguez C. Development of a series of aryl pyrimidine kynurenine monooxygenase inhibitors as potential therapeutic agents for the treatment of Huntington’s disease. J. Med. Chem. 2015;58(3):1159–1183. doi: 10.1021/jm501350y. [DOI] [PubMed] [Google Scholar]
- 176.Mrzljak L. Development of kynurenine monooxygenase (KMO) inhibitor CHDI-340246 for the treatment of Huntington's disease: A progress update. In: CHDI Foundation 7th Annual HD Therapeutics Conference, Venice, Italy, Venice, Italy,; 2013. [Google Scholar]
- 177.Heidempergher F., Pevarello P., Pillan A., Pinciroli V., Della Torre A., Speciale C., Marconi M., Cini M., Toma S., Greco F., Varasi M. Pyrrolo[3,2-c]quinoline derivatives: A new class of kynurenine-3-hydroxylase inhibitors. Farmaco. 1999;54(3):152–160. doi: 10.1016/s0014-827x(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 178.Pevarello P., Varasi M., Amici R., Toma S., Speciale C. Preparation of 2-cyano-3-oxo-3-benz[g]indazole-propanamides and analogs as kynurenine-3-hydroxylase inhibitors. WO9916753A2, 1999.
- 179.Lowe D.M., Gee M., Haslam C., Leavens B., Christodoulou E., Hissey P., Hardwicke P., Argyrou A., Webster S.P., Mole D.J., Wilson K., Binnie M., Yard B.A., Dean T., Liddle J., Uings I., Hutchinson J.P. Lead discovery for human kynurenine 3-monooxygenase by high-throughput RapidFire mass spectrometry. J. Biomol. Screen. 2014;19(4):508–515. doi: 10.1177/1087057113518069. [DOI] [PubMed] [Google Scholar]
- 180.Feng Y., Bowden B.F., Kapoor V., Ianthellamide A. Ianthellamide A, a selective kynurenine-3-hydroxylase inhibitor from the Australian marine sponge Ianthella quadrangulata. Bioorg. Med. Chem. Lett. 2012;22(10):3398–3401. doi: 10.1016/j.bmcl.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 181.Jain K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine (Lond.) 2012;7(8):1225–1233. doi: 10.2217/nnm.12.86. [DOI] [PubMed] [Google Scholar]
- 182.Han Q., Li J., Li J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. Eur. J. Biochem. 2004;271(23-24):4804–4814. doi: 10.1111/j.1432-1033.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 183.Kocki T., Luchowski P., Luchowska E., Wielosz M., Turski W. A., Urbanska E. M. L-Cysteine sulphinate, endogenous sulphur-containing amino acid, inhibits rat brain kynurenic acid production via selective interference with kynurenine aminotransfer-ase II. 2003. [DOI] [PubMed]
- 184.Rossi F., Han Q., Li J., Li J., Rizzi M. Crystal structure of human kynurenine aminotransferase I. J. Biol. Chem. 2004;279(48):50214–50220. doi: 10.1074/jbc.M409291200. [DOI] [PubMed] [Google Scholar]
- 185.Han Q., Robinson H., Cai T., Tagle D.A., Li J. Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase K. J. Med. Chem. 2009;52(9):2786–2793. doi: 10.1021/jm9000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pellicciari R., Rizzo R.C., Costantino G., Marinozzi M., Amori L., Guidetti P., Wu H-Q., Schwarcz R. Modulators of the kynurenine pathway of tryptophan metabolism: synthesis and preliminary biological evaluation of (S)-4-(ethylsulfonyl)benzoylalanine, a potent and selective kynurenine aminotransferase II (KAT II) inhibitor. ChemMedChem. 2006;1(5):528–531. doi: 10.1002/cmdc.200500095. [DOI] [PubMed] [Google Scholar]
- 187.Amori L., Wu H. Q., Marinozzi M., Pellicciari R., Guidetti P., Schwarcz R. Specific inhibition of kynurenate synthesis enhances ex-tracellular dopamine levels in the rodent striatum. Neuroscience (Amsterdam, Neth.), . 2009;159(1):196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu H.Q., Rassoulpour A., Schwarcz R. Kynurenic acid leads, dopamine follows: A new case of volume transmission in the brain? J. Neural Transm. (Vienna) 2007;114(1):33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 189.Pellicciari R., Venturoni F., Bellocchi D., Carotti A., Marinozzi M., Macchiarulo A., Amori L., Schwarcz R. Sequence variants in kynurenine aminotransferase II (KAT II) orthologs determine different potencies of the inhibitor S-ESBA. ChemMedChem. 2008;3(8):1199–1202. doi: 10.1002/cmdc.200800109. [DOI] [PubMed] [Google Scholar]
- 190.Rossi F., Valentina C., Garavaglia S., Sathyasaikumar K.V., Schwarcz R., Kojima S., Okuwaki K., Ono S., Kajii Y., Rizzi M. Crystal structure-based selective targeting of the pyridoxal 5′-phosphate dependent enzyme kynurenine aminotransferase II for cognitive enhancement. J. Med. Chem. 2010;53(15):5684–5689. doi: 10.1021/jm100464k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schwarcz R., Kajii Y., Ono S. Preparation of aminopiperazinylquinolonecarboxylates as kynurenineaminotransferase inhibitors. WO2009064836A2. 2009.
- 192.Wu H.Q., Okuyama M., Kajii Y., Pocivavsek A., Bruno J.P., Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr. Bull. 2014;40(Suppl. 2):S152–S158. doi: 10.1093/schbul/sbt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dounay A.B., Anderson M., Bechle B.M., Campbell B.M., Claffey M.M., Evdokimov A., Evrard E., Fonseca K.R., Gan X., Ghosh S., Hayward M.M., Horner W., Kim J-Y., McAllister L.A., Pandit J., Paradis V., Parikh V.D., Reese M.R., Rong S., Salafia M.A., Schuyten K., Strick C.A., Tuttle J.B., Valentine J., Wang H., Zawadzke L.E., Verhoest P.R. Discovery of brain-penetrant, irreversible kynurenine aminotransferase II inhibitors for schizophrenia. ACS Med. Chem. Lett. 2012;3(3):187–192. doi: 10.1021/ml200204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Claffey M.M., Dounay A.B., Gan X., Hayward M.M., Rong S., Tuttle J.B., Verhoest P.R. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology. 2014;82:41–48. doi: 10.1016/j.neuropharm.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Koshy Cherian A., Gritton H., Johnson D.E., Young D., Kozak R., Sarter M. A systemically-available kynurenine aminotransferase II (KAT II) inhibitor restores nicotine-evoked glutamatergic activity in the cortex of rats. Neuropharmacology. 2014;82:41–48. doi: 10.1016/j.neuropharm.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Henderson J.L., Sawant-Basak A., Tuttle J.B., Dounay A.B., McAllister L.A., Pandit J., Rong S., Hou X., Bechle B.M., Kim J-Y., Parikh V., Ghosh S., Evrard E., Zawadzke L.E., Salafia M.A., Rago B., Obach R.S., Clark A., Fonseca K.R., Chang C., Verhoest P.R. Discovery of hydroxamate bioisosteres as KAT II inhibitors with improved oral bioavailability and pharmacokinetics. MedChemComm. 2012;4(1):125–129. [Google Scholar]
- 197.Tuttle J.B., Anderson M., Bechle B.M., Campbell B.M., Chang C., Dounay A.B., Evrard E., Fonseca K.R., Gan X., Ghosh S., Horner W., James L.C., Kim J-Y., McAllister L.A., Pandit J., Parikh V.D., Rago B.J., Salafia M.A., Strick C.A., Zawadzke L.E., Verhoest P.R. Structure-Based Design of Irreversible Human KAT II Inhibitors: Discovery of New Potency-Enhancing Interactions. ACS Med. Chem. Lett. 2012;4(1):37–40. doi: 10.1021/ml300237v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dounay A.B., Anderson M., Bechle B.M., Evrard E., Gan X., Kim J-Y., McAllister L.A., Pandit J., Rong S., Salafia M.A., Tuttle J.B., Zawadzke L.E., Verhoest P.R. PF-04859989 as a template for structure-based drug design: identification of new pyrazole series of irreversible KAT II inhibitors with improved lipophilic efficiency. Bioorg. Med. Chem. Lett. 2013;23(7):1961–1966. doi: 10.1016/j.bmcl.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 199.Okuyama M., Fukunaga K., Usui K., Hayashi N., Iijima D., Horiuchi H., Itagaki N. Novel bicyclic or tricyclic heterocyclic compounds and their pharmaceutical compositions for prophylactic and therapeutic treatment of KAT (kynurenine aminotransfer-ase) II-associated disorders. WO2015163339A1, 2015. 2015.
- 200.Phillips R.S., Dua R.K. Stereochemistry and mechanism of aldol reactions catalyzed by kynureninase. J. Am. Chem. Soc. 1991;113(19):7385–7388. [Google Scholar]
- 201.Dua R.K., Taylor E.W., Phillips R.S. S-Aryl-L-cysteine S,S-dioxides: Design, synthesis, and evaluation of a new class of inhibitors of kynureninase. J. Am. Chem. Soc. 1993;115(4):1264–1270. [Google Scholar]
- 202.Ross F.C., Botting N.P., Leeson P.D. Synthesis of phosphinic acid transition state analogues for the reaction catalysed by kynureninase. Bioorg. Med. Chem. Lett. 1996;6(22):2643–2646. [Google Scholar]
- 203.Drysdale M.J., Reinhard J.F. S-aryl cysteine S,S-dioxides as inhibitors of mammalian kynureninase. Bioorg. Med. Chem. Lett. 1998;8(2):133–138. doi: 10.1016/s0960-894x(97)10209-8. [DOI] [PubMed] [Google Scholar]
- 204.Walsh H.A., Leslie P.L., O’Shea K.C., Botting N.P. 2-Amino-4-[3′-hydroxyphenyl]-4-hydroxybutanoic acid; a potent inhibitor of rat and recombinant human kynureninase. Bioorg. Med. Chem. Lett. 2002;12(3):361–363. doi: 10.1016/s0960-894x(01)00758-2. [DOI] [PubMed] [Google Scholar]
- 205.Walsh H.A., Botting N.P. Purification and biochemical characterization of some of the properties of recombinant human kynureninase. Eur. J. Biochem. 2002;269(8):2069–2074. doi: 10.1046/j.1432-1033.2002.02854.x. [DOI] [PubMed] [Google Scholar]
- 206.Sahm F., Oezen I., Opitz C.A., Radlwimmer B., von Deimling A., Ahrendt T., Adams S., Bode H.B., Guillemin G.J., Wick W., Platten M. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013;73(11):3225–3234. doi: 10.1158/0008-5472.CAN-12-3831. [DOI] [PubMed] [Google Scholar]
- 207.Sahm F., Oezen I., Opitz C.A., Radlwimmer B., von Deimling A., Ahrendt T., Adams S., Bode H.B., Guillemin G.J., Wick W., Platten M. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013;73(11):3225–3234. doi: 10.1158/0008-5472.CAN-12-3831. [DOI] [PubMed] [Google Scholar]
- 208.Youn H.S., Kim T.G., Kim M.K., Kang G.B., Kang J.Y., Lee J.G., An J.Y., Park K.R., Lee Y., Im Y.J., Lee J.H., Eom S.H. Structural insights into the quaternary catalytic mechanism of hexameric human quinolinate phosphoribosyltransferase, a key enzyme in de novo NAD biosynthesis. Sci. Rep. 2016;6:19681. doi: 10.1038/srep19681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Malik S.S., Patterson D.N., Ncube Z., Toth E.A. The crystal structure of human quinolinic acid phosphoribosyltransferase in complex with its inhibitor phthalic acid. Proteins. 2014;82(3):405–414. doi: 10.1002/prot.24406. [DOI] [PubMed] [Google Scholar]
- 210.Braidy N., Guillemin G.J., Grant R. Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int. J. Tryptophan Res. 2011;4(2631):29–37. doi: 10.4137/IJTR.S7052. [DOI] [PMC free article] [PubMed] [Google Scholar]