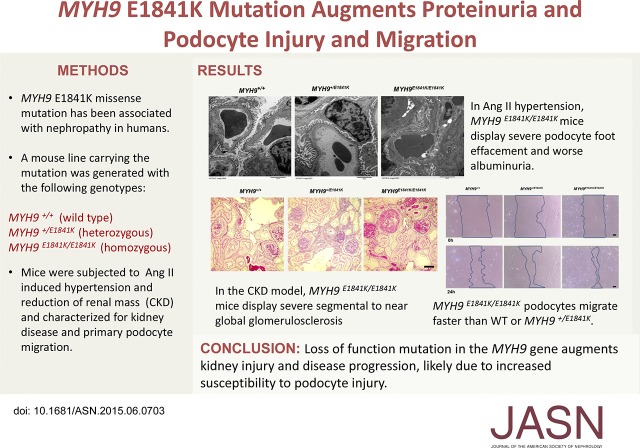
Abstract
Intronic variants of the MYH9 gene that encodes the nonmuscle myosin heavy chain IIA are associated with diabetic nephropathy in European Americans and with sickle cell disease–associated nephropathy. However, the causal functional variants of MYH9 have remained elusive. Rare missense mutations in MYH9 cause macrothrombocytopenia and are occasionally associated with development of nephropathy. The E1841K mutation is among the common MYH9 missense mutations and has been associated with nephropathy in some carriers. To determine the contribution of the E1841K mutation in kidney disease, we studied the effects of the E1841K mutation in mice subjected to high salt or angiotensin II (Ang II) as models of hypertension and in mice subjected to renal mass reduction as a model of CKD. Despite similar levels of BP among wild-type (MYH9+/+) mice and mice heterozygous (MYH9+/E1841K) and homozygous (MYH9E1841K/E1841K) for the mutation in each model, MYH9E1841K/E1841K mice exhibited mildly increased albuminuria in response to high salt; severe albuminuria, nephrinuria, FSGS, and podocyte foot effacement in Ang II–induced hypertension; and early mortality in the renal mass reduction model. Treatment with candesartan during Ang II–induced hypertension attenuated kidney disease development in MYH9E1841K/E1841K mice. In vitro, isolated primary podocytes from MYH9E1841K/E1841K mice exhibited increased lamellipodia formation and reorganization of F-actin stress fibers. Wound healing assays revealed that MYH9+/+ podocytes had the lowest migration rate, followed by MYH9+/E1841K then MYH9E1841K/E1841K podocytes. In conclusion, the MYH9 E1841K variant alters podocyte cytoskeletal structure and renders podocytes more susceptible to injury after a damaging stimulus.
Keywords: genetic renal disease, glomerulosclerosis, hypertension, MYH9, genetics and development, kidney disease
The MYH9 gene encodes the nonmuscle myosin heavy chain IIA (myosin II), a cytoskeletal contractile protein that is abundantly expressed within the glomerulus, in podocytes, and mesangial and endocapillary cells,1 and plays a role in cell adhesion and migration.2,3 Myosin II is considered to be a major component of the actin-myosin contractile apparatus in the podocyte foot processes (FPs). The contractile apparatus consists of F-actin, myosin II, α-actinin–4, and synaptopodin.4 The MYH9 E1 risk haplotype was thought to be a strong candidate gene that could explain the disproportionate susceptibility for ESRD in blacks,5,6 but this notion was later challenged by the discovery of stronger kidney disease associations of the functional variants of the APOL1 gene that are in linkage disequilibrium with MYH9 risk variants.7,8
Nevertheless, the MYH9 E1 risk haplotype is associated with diabetic nephropathy in European Americans in whom the APOL1 risks variants are very rare,9 and is also associated with sickle cell disease nephropathy even after accounting for the APOL1 risk variants.10 Moreover, rare autosomal-dominant missense mutations causing MYH9-related macrothrombocytopenia are commonly associated with the development of nephropathy.11 The E1841K mutation (a change from glutamine to lysine at the amino acid position 1841) in the human MYH9 gene is one of the three most common MYH9 missense mutations characterized by macrothrombocytopenia, and is associated with the development of kidney disease.12 It has been reported in at least 20 families with May–Hegglin anomaly (MHA), five of whom had members with nephropathy.13 The variability of kidney disease susceptibility in those with the same mutation suggests a second hit may be required or other factors may interact with the mutation for the manifestation of nephropathy. Here, we directly determined the contribution of the MYH9 E1841K mutation in kidney disease development in vivo, using hypertension and surgically induced loss of nephron mass as stress stimuli. We also assessed the effect of the MYH9 E1841K on the morphology and migratory phenotype of primary podocytes in vitro.
Results
Mice Homozygous for the MYH9 E1841K Mutation Have a Survival Disadvantage
The mouse line carrying the E1841K mutation was originally generated on an inbred C57BL/6 (B6) background at Duke University Medical Center.14 Because the B6 strain is generally resistant to glomerular injury,15,16 we generated mice on a mixed genetic background of 129S6×B6 by backcrossing the E1841K mutation onto the 129S6 (Taconic, Inc.) for two generations (G2). Herein, mice heterozygous for the E1841K mutation are MYH9+/E1841K and mice homozygous for the mutation are MYH9E1841K/E1841K. Wild-type mice are MYH9+/+. Only male mice were used in our phenotyping studies below. Matings of heterozygous 129S6 G2 mice over 4 years resulted in a total of 128 weaned male offspring (females were only genotyped when needed for breeding), of which the observed proportions were 38.3% MYH9+/+ and 48.4% MYH9+/E1841K. The proportion of MYH9 E1841K/E1841K mice was only 13.3% and is significantly less than the 25% expected (P<0.001 by chi-squared test). In addition, the 12% decreased proportion in MYH9 E1841K/E1841K mice did not result in an expected 8% increased proportion of MYH9+/E1841K mice. Thus, both MYH9+/E1841K and MYH9 E1841K/E1841K mice have a significant survival disadvantage.
Characterization of Mice with the E1841K Mutation at Baseline and during High-Salt Feeding
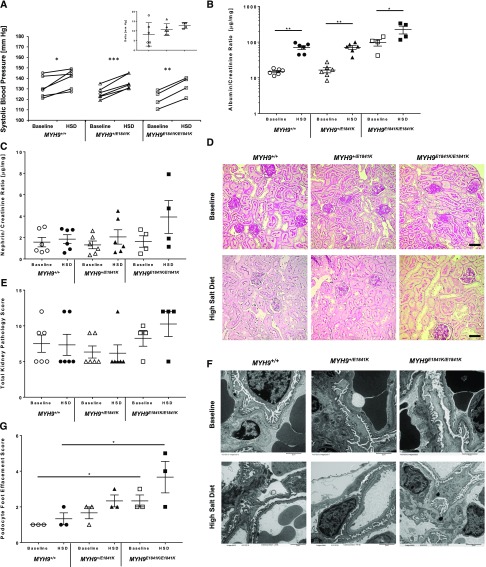
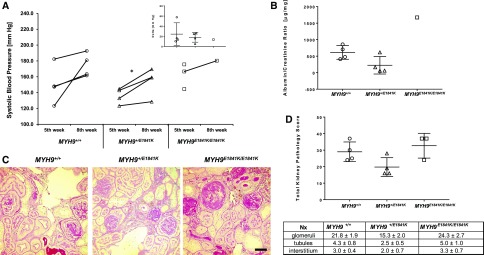
At baseline (normal chow), the E1841K mutation did not have any effect on systolic BP (SBP) (Figure 1A), but MYH9 E1841K/E1841K mice displayed a significant approximately five-fold higher in urinary albumin/creatinine (A/C) ratio (micrograms per milligram) compared with wild-type and heterozygous mice (MYH9 +/+ 14.9±1.1, MYH9 +/E1841K 16.5±2.7, MYH9E1841K/E1841K 98.0±20.7; P<0.001 [ANOVA]) (Figure 1B). After 2 weeks of high-salt diet (HSD), there was a significant increase in SBP within each group compared with baseline, but again no difference among groups (Figure 1A). Urinary A/C ratio also increased significantly within groups, and was approximately 2.5–3-fold higher in MYH9 E1841K/E1841K mice compared with the other two groups (MYH9 +/+ 70.6±8.8, MYH9 +/E1841K 71.2±8.7, MYH9E1841K/E1841K 224.2±55.3; P=0.002 [ANOVA]) (Figure 1B). The nephrin/creatinine (N/C) ratio, a marker of podocyte injury, was not different among groups at baseline, and increased only in MYH9E1841K/E1841K on HSD compared with baseline, but did not reach statistical significance (Figure 1C). Thus, homozygosity for the E1841K mutation increases susceptibility to albuminuria. However, by light microscopy, there were no significant differences in kidney pathology scores observed between groups or within groups at baseline and after HSD (Figure 1, D and E). It is possible that significant differences might be observed on longer duration of HSD. By electron microscopy, MYH9E1841K/E1841K mice had a statistically significant increased podocyte FP effacement at baseline when compared with the MYH9 +/+ group (*P<0.04 by two-way t test), and a trend for increased podocyte foot effacement after HSD (Figure 1, F and G).
Figure 1.
MYH9 E1841K mutation increases susceptibility to proteinuria and podocyte foot effacement after high salt exposure. (A) Mean SBP at baseline and after HSD. SBPs were measured daily × ten measurements, averaged over a period of 2 weeks for each mouse. Mean of means of SBP (in mm Hg) for each genotype group at baseline are: MYH9 +/+ (n=6) 131.7±4.0, MYH9 +/E1841K (n=6) 125.9±2.3, MYH9E1841K/E1841K (n=4) 120.3±4.0; and after HSD: MYH9 +/+ 139.9±3.7, MYH9 +/E1841K 136.6±2.7, MYH9E1841K/E1841K 132.8±4.4; P=NS (ANOVA) between the three groups at baseline and after HSD. For comparison of HSD versus normal chow within group (by paired t test): P=0.02 for MYH9 +/+ (*), <0.001 for MYH9+/E1841K (***), and 0.001 for MYH9E1841K/E1841K (**). Insert shows mean change in SBP from baseline and after HSD, P=NS by ANOVA. (B) Mean urinary A/C ratio (micrograms per milligram, log scale) at baseline after 2 weeks of BP measurement: MYH9 +/+ 14.9±1.1, MYH9 +/E1841K 16.5±2.7, MYH9E1841K/E1841K 98.0±20.7; P<0.001 (ANOVA) among groups. After 2 weeks of HSD: MYH9 +/+ 70.6±8.8, MYH9 +/E1841K 71.2±8.7, MYH9E1841K/E1841K 224.2±55.3; P=0.002 (ANOVA) among groups. For comparison of HSD versus normal chow within groups (by paired t test), P≤0.002 for both MYH9 +/+ and MYH9 +/E1841K (**), and 0.05 for MYH9E1841K/E1841K (*). (C) Mean urinary N/C ratio (micrograms per milligram) at baseline: MYH9 +/+ 1.57±0.44, MYH9 +/E1841K 1.31±0.36, MYH9E1841K/E1841K 1.63±0.54; P=NS. After HSD: MYH9 +/+ 1.85±0.41, MYH9 +/E1841K 2.06±0.67, MYH9E1841K/E1841K 3.92±1.54; P=NS (ANOVA). (D) Representative periodic acid–Shiff staining of kidney sections (40×). Baseline: MYH9 +/+, MYH9 +/E1841K, and MYH9 E1841K/E1841K displayed similar renal histology. After HSD: MYH9 +/+, MYH9 +/E1841K, and MYH9 E1841K/E1841K, no observable changes in renal pathology were noted. Black scale bar=50 µm. (E) Mean total kidney pathology scores at baseline: MYH9 +/+ 7.5±1.2, MYH9 +/E1841K 6.3±0.8, MYH9E1841K/E1841K 8.3±1.1; P=NS. After HSD: MYH9 +/+ 7.3±1.5, MYH9 +/E1841K 6.2±1.2, MYH9E1841K/E1841K 10.3±1.8; P=NS. (F) Representative transmission EM images (6000×). Baseline: MYH9 +/+ mice showing normal podocyte FPs, MYH9 +/E1841K mice with rare FP effacement, MYH9 E1841K/E1841K with mild FP effacement. After HSD: MYH9 +/+ mice showing rare podocyte FP effacement, MYH9 +/E1841K mice mild FP effacement, and MYH9 E1841K/E1841K with a trend toward moderate FP effacement. (G) Mean podocytes FP effacement scores. Baseline (n=3 each): MYH9 +/+ 1.0±0.0, MYH9 +/E1841K 1.67±0.33, and MYH9E1841K/E1841K 2.33±0.33; MYH9E1841K/E1841K is significantly different from MYH9 +/+ mice; *P<0.02 by one-way ANOVA test. After HSD (n=3 each): MYH9 +/+ 1.33±0.33, MYH9 +/E1841K 2.33±0.33, MYH9E1841K/E1841K 3.67±0.88; P=NS (ANOVA).
Angiotensin II Infusion Augments Renal Injury in E1841K Mutant Mice
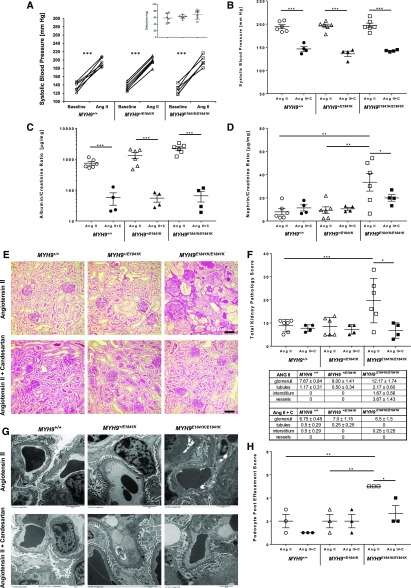
Because of a mild increase in SBP and only a modest elevation in urinary A/C and very modest kidney pathology after high salt feeding, we next assessed the effects of the E1841K mutation on SBP and urinary A/C and N/C in response to Ang II infusion via mini-osmotic pump. Despite similar increases in SBP after Ang II (Figure 2A), the E1841K mutation influenced albuminuria in an additive manner, with MYH9 E1841K/E1841K mice having the worst albuminuria (Figure 2C; MYH9 +/+ 741.2±101.6, MYH9 +/E1841K 1328.8±292.5, MYH9E1841K/E1841K 2247.5±300.9, P<0.001, ANOVA), and a significant increase in N/C ratio in MYH9E1841K/E1841K mice (P=0.004; Figure 2C) that is approximately ten-fold compared with baseline (see Figure 1C), suggesting severe podocyte injury. By light microscopy, Ang II–induced hypertension had only very mild effect on renal histopathology (Figure 2E) in MYH9 +/+ and MYH9 +/E1841K mice, as demonstrated by similar total kidney pathology scores (Figure 2F) compared with those at baseline (Figure 1D). However, in MYH9 E1841K/E1841K mice with Ang II–induced hypertension, FSGS and severe protein-filled renal microcysts were observed (Figure 2E), and total kidney pathology scores were significantly elevated (Figure 2F). By electron microscopy (Figure 2G), after 2 weeks of Ang II, MYH9 +/+ mice had mild-to-moderate podocyte pathology. Despite having a significantly higher degree of proteinuria, MYH9 +/E1841K mice had a similar degree of podocyte FP effacement as MYH9 +/+ mice (Figure 2, G and H). MYH9 E1841K/E1841K mice displayed >80% podocyte FP effacement/fusion ([**] P<0.01 versus MYH9 +/+ and versus MYH9 +/E1841K, by ANOVA). Thus, in Ang II–induced hypertension, the E1841K mutation increases susceptibility to severe podocyte foot effacement and kidney disease.
Figure 2.
MYH9 E1841K mutation causes severe proteinuria and podocyte foot effacement in Ang II induced hypertension. (A) Mean SBP at baseline (averaged daily for 2 weeks) and after Ang II infusions (averaged for 4 weeks) are shown for each mouse. Mean of means of SBP for the same genotyped group of mice (n=6) at baseline: MYH9 +/+ 135.8±4.4, MYH9 +/E1841K 133.0±3.1, MYH9E1841K/E1841K 128.5±4.5; P=NS among groups, by ANOVA. After Ang II infusion: MYH9 +/+ 194.3±4.3, MYH9 +/E1841K 195.8±4.0, MYH9E1841K/E1841K 197.2±5.0; P=NS among groups, by ANOVA. For comparison of SBP on Ang II alone versus baseline within group, paired t test was used: P<0.001 for all three genotypes (***). Insert shows change in mean SBP from baseline and after Ang II infusion. (B) Treatment with Candesartan while on Ang II infusion significantly improved SBP. n=4 for each separate group of mice. Mean of means of SBP (mm Hg) for Ang II + Candesartan treatment are: MYH9 +/+ 146.5±5.0, MYH9 +/E1841K 136.0±5.7, and MYH9 E1841K/E1841K 142.5±1.0; P<0.001 (***) for Ang II alone versus Ang II + Candesartan by two-sample t test. (C) Urinary A/C ratio (µg/mg) for each individual mouse is shown on log scale (24-hour urine collected at the end of treatment period). Mean urinary A/C ratio for Ang II infusion alone (n=6 each): MYH9 +/+ 741.2±101.6, MYH9 +/E1841K 1328.8±292.5, MYH9E1841K/E1841K 2247.5±300.9; Ang II + Candesartan (n=4): MYH9 +/+ 57.0±24.5, MYH9 +/E1841K 54.9±14.3, and MYH9 E1841K/E1841K 65.2±24.1; ***P<0.001 (ANOVA) for Ang II alone versus Ang II + Candesartan for each group. (D) Mean urinary N/C ratio (µg/mg) for Ang II alone: MYH9 +/+ 7.94±2.61, MYH9 +/E1841K 9.29±2.89, MYH9E1841K/E1841K 33.40±7.63; **P=0.004 (ANOVA); Ang II + Candesartan: MYH9 +/+ 11.30±2.66, MYH9 +/E1841K 10.48±1.22, and MYH9 E1841K/E1841K 19.89±2.80; *P=0.04 (ANOVA). In MYH9E1841K/E1841K, P=NS for Ang II alone versus Ang II + candesartan (by two-sample t test). (E) Representative periodic acid–Shiff staining of kidney sections (40×). After Ang II alone: MYH9 +/+ and MYH9 +/E1841K had only very mild changes from baseline such as rare casts, whereas MYH9 E1841K/E1841K had FSGS and severe protein-filled microcysts. Treatment with Ang II + Candesartan: MYH9 +/+ and MYH9 +/E1841K showed no detectable changes from baseline, but MYH9 E1841K/E1841K showed absence of FSGS and protein-filled microcysts. Black scale bar=50 µm. (F) Total kidney pathology score for each individual mouse is shown. Mean total kidney pathology scores for Ang II alone: MYH9 +/+ 8.8±1.1, MYH9 +/E1841K 8.5±1.6, MYH9E1841K/E1841K 19.7±3.9; ***P=0.001 (ANOVA); Ang II + Candesartan: MYH9 +/+ 7.8±0.8, MYH9 +/E1841K 7.3±1.0, MYH9E1841K/E1841K 6.8±1.7; P=NS. For Ang II + Candesartan versus Ang II alone for MYH9E1841K/E1841K mice: *P=0.01 (by two-sample t test). Table shows kidney pathology scores for glomerular, tubular, interstitial, and vascular injuries during Ang II infusion alone, and with treatment with candesartan. Glomerular injury includes hypercellularity; focal, segmental, and mesangial expansion; FSGS; and thickened membrane; tubular injury consists of fibrosis and tubular casts. Interstitial injuries represent chronic inflammation (focal and diffuse). Vascular injury represents injury to arteries and arterioles. (G) Representative transmission EM images for Ang II and Ang II + Candesartan. After Ang II alone: MYH9 +/+ and MYH9 +/E1841K have mild podocyte FP effacement, MYH9 E1841K/E1841K have very severe and >80% podocyte FP effacement/fusion. Treatment with Ang II + Candesartan: MYH9 +/+ have normal FPs, MYH9 +/E1841K with rare FP effacement, and MYH9 E1841K/E1841K show amelioration of FP effacement from very severe after Ang II to mild-to-moderate when treated with candesartan. Black scale bar under the picture represents 2 µm. (H) Mean podocyte FP effacement scores after Ang II infusion: MYH9 +/+ 2.00±0.58, MYH9 +/E1841K 2.00±0.58, and MYH9E1841K/E1841K 5.0±0.0; **P<0.01 by ANOVA. After Ang II + Candesartan: MYH9 +/+ 1.0±0.0, MYH9 +/E1841K 2.00±0.58, and MYH9E1841K/E1841K 2.67±0.67; P=NS (ANOVA). In MYH9E1841K/E1841K mice, candesartan significantly decreased Ang II–induced podocyte injury (5.0±0.0 versus 2.67±0.67; *P=0.03 [one-way ANOVA]). Ang II + C, Ang II + Candesartan.
Candesartan Ameliorates Kidney Injury Induced by Ang II Infusion in E1841K Mutant Mice
We next queried whether prevention of hypertension with candesartan, an angiotensin type 1 receptor (AT1R) inhibitor, during Ang II–induced hypertension would alter the kidney disease course of mice carrying the E1841K mutation. Treatment with candesartan clearly and significantly prevented Ang II–induced hypertension (Figure 2B) and albuminuria (Figure 2C) in all three genotypes, and significantly attenuated urinary N/C ratio (Figure 2D) in MYH9 E1841K/E1841K mice. Treatment with candesartan did not have any effect on N/C ratios and total kidney pathology scores of MYH9 +/+ and MYH9 +/E1841K mice (Figure 2, D–F). In MYH9 E1841K/E1841K mice, candesartan significantly prevented Ang II–induced development of FSGS and protein-filled renal microcysts and improved the total kidney pathology scores (Figure 2, D–F), and ameliorated podocyte FP effacement (Figure 2, G and H; *P<0.03). Although numerically decreased after candesartan, urine N/C ratio remained significantly elevated in MYH9 +/E1841K mice compared with the other two groups (Figure 2D; *P<0.04), and was not significantly different when compared with Ang II treatment alone.
Albuminuria Correlates with BP
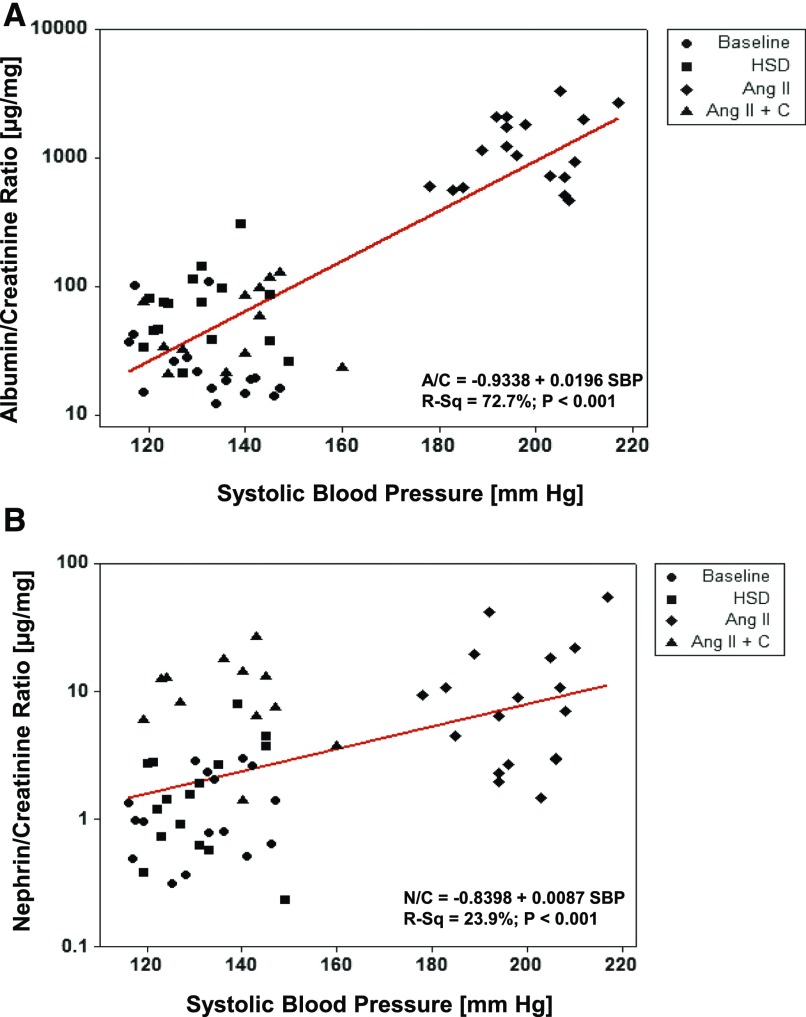
We assessed the strength of correlation between A/C ratio and N/C ratio with SBP for all mice in all experimental conditions. A/C ratio has a strong correlation with SBP (R2=72.7%, P<0.001; Figure 3A), whereas N/C ratio has a much weaker correlation (R2=23.9%, P<0.001; Figure 3B). This suggests that renal hemodynamics may influence albuminuria more than nephrinuria.
Figure 3.
Albuminuria significantly correlates with blood pressure. Correlation analysis shows that the degree of albuminuria is significantly determined by SBP (A), whereas nephrinuria only modestly correlated with SBP (B). Data are shown for all mice at all four different conditions: at baseline, after HSD, after angiotensin infusion (Ang II), and for candesartan treatment while on angiotensin infusion (Ang II + C). Urine was collected in metabolic cages for 24 hours at the end of each condition or treatment. R-Sq, R-squared.
Effect of E1841K Mutation in the Mouse Model of CKD
To evaluate the effect of E1841K mutation on kidney injury in a different model of kidney disease, we used the renal mass reduction or subtotal nephrectomy (Nx) model, a well established mouse model of CKD with cardinal features of hypertension, albuminuria, and glomerulosclerosis. We performed Nx on six MYH9 +/+, six MYH9 +/E1841K mice, and four MYH9 E1841K/E1841K mice. After 30 days, which is the usual recovery time for Nx surgery, the survival rate for this type of surgical model of CKD in our laboratory is usually between 80% and 90% for wild-type mice on 129S6 background. Here, the survival rate was 80% for MYH9 +/+, 67% for MYH9 +/E1841K, and 50% for MYH9 E1841K/E1841K at 30 days. At 60 days, the end of the experimental period, the survival rate for MYH9 +/+ and MYH9 +/E1841K mice did not change, whereas MYH9 E1841K/E1841K mice have a survival rate of only 25% (one out of four). Between 16 and 40 days after Nx, two of the six heterozygous mice and three out of the four homozygous mice for E1841K mutation became very sick and lost approximately 30% of their presurgery weight and had to be euthanized. Of note, at euthanization, all MYH9 E1841K/E1841K mice had extremely light-colored whole blood obtained from cardiac puncture, suggesting severe anemia or low hemoglobin level. Although the numbers are too small for statistical analysis because of poor survival in the MYH9 E1841K/E1841K mice, SBP was significantly elevated in all three groups (Figure 4A), compared with historical baseline or sham at fifth and eighth week after Nx. MYH9 E1841K/E1841K mice that had to be euthanized before and at day 60 demonstrated severe renal histopathology with increased total kidney pathology scores (Figure 4, B and C). There was, however, no statistical difference in the total kidney pathology scores among groups. This could be because of the effect of mixed genetic background, with more contribution from the genetically more susceptible 129 strain in the MYH9 +/+ group. However, it should be noted that the total kidney scores for MYH9 +/+ and MYH9 +/E1841K mice were from tissues obtained at day 60 after Nx, whereas in two out of three MYH9 E1841K/E1841K mice the tissues were obtained from before day 40 due to their moribund status requiring euthanasia.
Figure 4.
MYH9 E1841K mutation results in poor survival and severe FSGS in the subtotal nephrectomy model. (A) Mean of means for SBP at fifth week and eighth week. After 2 weeks of training, SBP was recorded daily during each specific week and averaged. Mean of means for SBP at fifth week (mm Hg): MYH9 +/+ (n=4) 150.2±12.1, MYH9 +/E1841K (n=4) 135.9±4.9, MYH9E1841K/E1841K (n=3) 162.3±9.2; at eighth week: MYH9 +/+ (n=4) 174.6±7.4, MYH9 +/E1841K (n=4) 154.1±8.8, MYH9E1841K/E1841K (n=1) 180.3. Insert shows differences between mean SBP at fifth and eighth week after Nx. P=NS among groups (ANOVA). Two moribund MYH9E1841K/E1841K mice were euthanized during the sixth and seventh week after Nx, and therefore their SBP could not be obtained, but their kidneys were preserved for histopathology scores which are included in the analysis below. (B) A/C ratio (log scale) for each individual mouse for 24-hour urine collected at the end of the eighth week after Nx is shown. Mean urinary A/C ratio (µg/mg): MYH9 +/+ 612.2±103, MYH9 +/E1841K 224.1±132.5, and one MYH9E1841K/E1841K mouse 1671.3. (C) Periodic acid–Shiff staining of kidney sections (40×) after subtotal Nx: MYH9 +/+ mice and MYH9 +/E1841K mice have similar moderate degree of FSGS, whereas MYH9 E1841K/E1841K have severe segmental to near global sclerosis in virtually all glomeruli. Black scale bar=50 µm. (D) Total pathology score is shown for each individual mouse. Two MYH9E1841K/E1841K mice euthanized earlier during the sixth and seventh week after Nx are included in the graph and in the calculation of means of total kidney pathology scores for each group of mice. MYH9 +/+ (n=4) 29.0±3.0, MYH9 +/E1841K (n=4) 19.8±2.8, and MYH9E1841K/E1841K (n=3) 32.7±4.3; P=0.06 (by ANOVA). Table shows kidney pathology scores for glomerular, tubular, and interstitial injuries in the chronic kidney model (after subtotal Nx). See description in Figure 2F above for pathologic features assessed.
Effect of E1841K Mutation on the Cytoskeletal Structure of Primary Podocytes in Culture
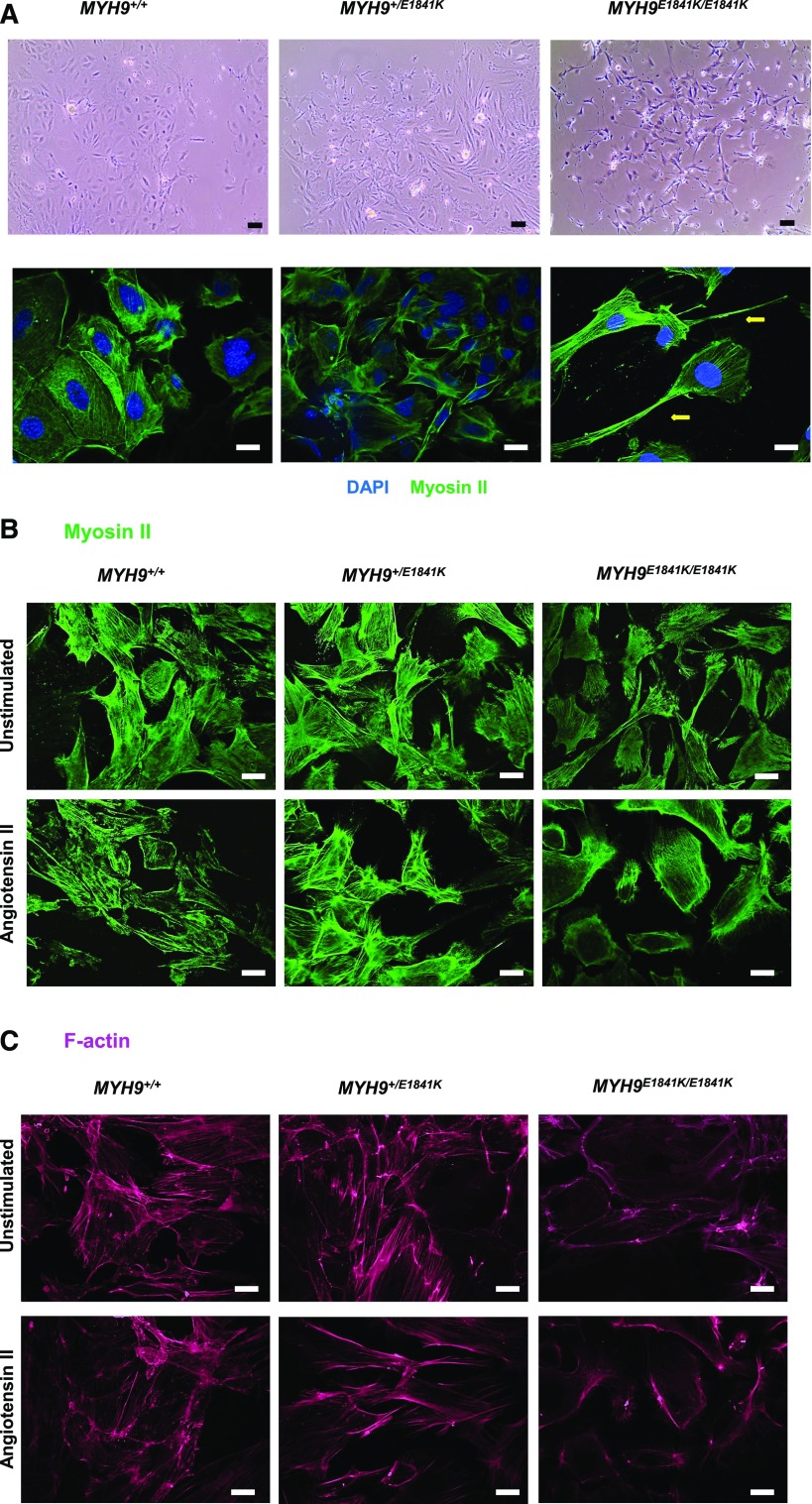
MYH9 (nonmuscle myosin heavy chain II-A) plays a role in cell adhesion and migration.2,3 It is highly expressed in the podocyte, where it has been shown to play a role in cytoskeletal structure organization in vitro.17 Therefore, we isolated primary podocytes from mice in all three genotypes to determine whether the MYH9 E1841K mutation affects the cytoskeletal structure and function of podocytes in vitro. Supplemental Figure 1 shows representative images of primary podocyte cells derived from glomeruli isolated from kidney tissues (top panel). Staining for DAPI (blue), F-actin (magenta), and synaptopodin (green) was performed (lower panel). In vitro, MYH9 +/+ podocytes display characteristic flattened and somewhat spherical cell bodies. MYH9 +/E1841K podocytes display mixed morphology between wild-type and double knock-in podocytes, and MYH9 E1841K/E1841K podocytes are irregularly shaped, less aggregated, and thinner, with elongated trailing tails (Figure 5A). Staining for myosin II-A (Figure 5, A and B) shows no obvious differences in MYH9 filaments among genotypes in unstimulated conditions, with the exception of occasional elongated trailing tails and large lamellipodia observed in MYH9 E1841K/E1841K podocytes. However, after stimulation with Ang II, MYH9 E1841K/E1841K podocytes appear to have more ruffled edges. Staining for F-actin (Figure 5C) shows strong F-actin stress fiber bundles in MYH9 +/+ and MYH9 +/E1841K podocytes, but primarily only cortical actin staining is generally seen in MYH9 E1841K/E1841K podocytes. After stimulation with Ang II (at 100 nM for 24 hours), F-actin is rearranged in podocytes characterized by ring formation or cortical actin and attenuation in stress fiber bundles in MYH9 +/+ and MYH9 +/E1841K podocytes, similar to a previous report.18 There was no detectable change in the cortical actin distribution in MYH9 E1841K/E1841K podocytes after Ang II stimulation compared with unstimulated condition.
Figure 5.
Primary podocytes from MYH9E1841K/E1841K mice exhibit increased lamellipodia formation and reorganization of F-actin stress fibers. (A) Upper panels (at 20× magnification) under phase contrast show primary podocytes cells in culture originating from glomeruli isolated from kidneys of the three genotypes. MYH9 +/+ podocytes display characteristic cobblestone morphology with flat and roundish cell bodies, MYH9 +/E1841K podocytes display mixed morphology between wild-type and double knock-in podocytes, and MYH9 E1841K/E1841K podocytes are irregularly shaped, thinner, and display elongated trailing tails and lamellipodia with ruffling activity. EVOSXL Core Cell Imaging System was used to take images of live cells. Black scale bar=50 µm. Lower panels (at 40×) show fixed podocytes stained with myosin II-A, depicting the main morphology of cells among the MYH9 genotypes. Yellow arrow points to a large lamellipodia in a MYH9 E1841K/E1841K podocyte. White scale bar=20 µm. (B) Staining for MYH9 (40×): Unstimulated condition (upper panels): No obvious differences are observed across genotypes, with the exception of occasional elongated tails in MYH9 E1841K/E1841K podocytes (yellow arrow). Angiotensin II stimulation (lower panels): There appears to be a mild decrease in staining of cytoplasmic MYH9 filaments in MYH9 E1841K/E1841K podocytes. White scale bar=20 µm. (C) Staining for F-actin (40×): Unstimulated condition (upper panels): MYH9 +/+ and MYH9 +/E1841K podocytes display strong staining for F-actin stress fiber bundles, whereas MYH9 E1841K/E1841K podocytes display primarily only cortical actin staining. Angiotensin II stimulation (lower panels): F-actin is rearranged in podocytes characterized by ring formation or cortical actin and attenuation in stress fiber bundles in MYH9 +/+ and MYH9 +/E1841K podocytes. There was no significant change in the primarily cortical actin distribution in MYH9 E1841K/E1841K podocytes after Ang II stimulation, compared with unstimulated condition. White scale bar=20 µm. DAPI, 4,6-Diamidino-2-phenylindole (nuclear staining).
E1841K Mutation Affects Podocyte Cell Migration
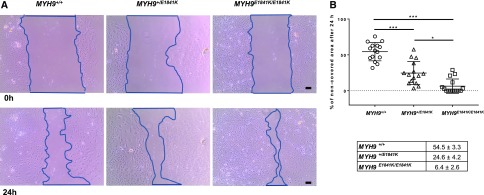
We next determined whether the MYH9 E1841K mutation influences the motility of podocytes by comparing cell migration rate of primary podocytes of the three MYH9 genotypes using scratch wound healing assay. Figure 6A shows representative scratch wounds induced at 0 hours and podocyte migration after 24 hours. Figure 6B shows the individual results of the scratch assays (n=15) for each MYH9 genotype group, and the averages of the percentage of noncovered area (red bars on graph and in table) after 24 hours. The percentages of remaining covered scratched areas are significantly larger in MYH9 +/+ podocytes compared with that of MYH9 +1K/E1841K podocytes, which are significantly larger than that of MYH9 E1841K/E1841K podocytes. The scratch assays demonstrate that the E1841K mutation significantly increases podocyte migratory phenotype in a dose dependent manner.
Figure 6.
MYH9 E1841K mutation enhances podocyte migration in vitro. (A) Upper panel: Representative image of outlined scratch area of wound created by pipette tip in confluent layer of primary podocyte culture at time 0 hours. Lower panel: Representative image of outlined scratch area of wound 24 hours later showing podocytes migrating into wound. Pictures are taken by EVOSXL Core Cell Imaging System at 10× magnification. Black scale bar=100 µm. (B) Plotted data show percentage of noncovered area after 24 hours for each individual scratch, n=15 for each group. P<0.001 among groups (ANOVA). By two-sample t test: MYH9 +/+ versus MYH9 +/E1841K P<0.001 (***), MYH9 +/E1841K versus MYH9 E1841K/E1841K P=0.03 (*), and MYH9 +/+ versus MYH9 E1841K/E1841K P<0.001 (***). Table shows means of percentage of noncovered area after 24 hours, n=15 for each group from at least three separate experiments, P<0.001 (ANOVA).
Effect of E1841K Mutation on Expression of Small Rho GTPases in Kidney Tissue Lysate
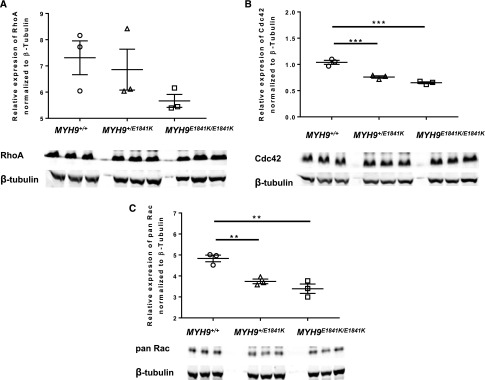
Rho GTPases play a central role in cell migration through their regulation of actin cytoskeleton dynamics. We next assessed whether the E1841K mutation influences the expression of RhoA, Cdc42, and Rac in kidney cortical tissue lysate. We tried to assess podocyte-specific expression of these Rho GTPases but the amount of protein obtained from primary podocytes was not adequate for analysis. In kidney cortical tissue, at baseline condition, there was a trend toward decreased protein expression of RhoA (Figure 7A) in MYH9 E1841K/E1841K. Cdc42 and pan Rac (Rac1, Rac 2, and Rac3) (Figure 7, B and C, respectively) were significantly decreased in both MYH9 +/E1841K and MYH9 E1841K/E1841K compared with WT mice.
Figure 7.
MYH9 E1841K mutation is associated with decreased protein abundance of small Rho GTPases. n=3 for each genotype. Intensities for RhoA, Cdc42, and pan Rac are normalized to β-tubulin. Western blots for each Rho GTPase for each genotype are illustrated below each graph. All three genotypes were run on the same gel. (A) RhoA expression, normalized to β-tubulin: MYH9 +/+ 7.311±0.646, MYH9 +/E1841K 6.858±0.781, and MYH9E1841K/E1841K 5.665±0.246; P=NS by ANOVA. (B) Cdc42 expression, normalized to β-tubulin: MYH9 +/+ 1.039±0.039, MYH9 +/E1841K 0.760±0.223, and MYH9E1841K/E1841K 0.650±0.0177; ***P<0.001 by ANOVA. (C) pan Rac (Rac1, Rac2, Rac3) expression, normalized to β-tubulin: MYH9 +/+ 4.83±0.16, MYH9 +/E1841K 3.74±0.11, and MYH9E1841K/E1841K 3.39±0.22; **P=0.002 by ANOVA.
Discussion
The human MYH9 E1841K mutation in MHA increases susceptibility to glomerular disease in the setting of Ang II–induced hypertension and Nx in the mouse. Our study demonstrates that the MYH9 E1841K mutation increases susceptibility to albuminuria and podocyte injury. The MYH9 E1841K/E1841K mice have mild podocyte foot effacement at baseline, but do not develop glomerular disease spontaneously, at least as has been determined up to 6 months of age. Our findings are similar to those reported by Holzman and associates, showing that podocyte-specific deletion of MYH9 predisposed mice to glomerulosclerosis only after injury had been induced in various models such as doxorubicin, HIV nephropathy, Adriamycin, and sheep nephrotoxic serum.19,20 It is not known whether these other models induce hypertension. The similar results observed from different models implicate a convergence of a common downstream pathway from the initial mode of injury. Although we did not detect worsened kidney pathology in MYH9 E1841K/E1841K mice treated with HSD, they displayed a trend toward increased podocyte foot effacement, and more kidney pathology might be uncovered with a longer duration of high salt exposure. It should also be noted that, in the Nx model, the lack of differences in total kidney pathology scores among groups is likely due to inclusion of scores from two out of three MYH9 E1841K/E1841K mice needing to be euthanized before day 40 instead of at day 60 as in the other two groups. However, we cannot rule out the possibility of more contributions from the 129 genetic background on kidney disease development in the MYH9 +/+ mice. All three models that were used in these studies result in increased renal oxidative stress, raising the possibility that the E1841K mutation may, in part, increase susceptibility of podocytes to oxidative stress.
Our in vitro studies demonstrate that the MYH9 E1841K mutation alters the morphology of the podocyte, resulting in prominent lamellipodia formation, that are associated with cortical actin reorganization, and increased cell motility. We show that the MYH9 E1841K mutation is associated with downmodulation of Rho-GTPases. However, our study is limited to analysis of Rho-GTPases in the kidney cortex rather than podocyte-specific expression. The precise role of Rho-GTPases in podocyte injury remains unclear; pharmacologic inhibition of the small GTPases Rac-1 and RhoA demonstrated protection from podocyte injury in the Nx model of CKD,21 whereas podocyte-specific deletion of Rac1 and Cdc42 promoted injury, depending on the injury model.22 It has been demonstrated that either over-activation or under-activation of RhoA can lead to podocyte FP effacement and proteinuria in vivo,23 suggesting a controlled balance of Rho GTPases is required for maintenance of podocyte health.24
In Ang II hypertension, the increased susceptibility to glomerular disease and podocyte foot effacement in MYH9 E1841K mutant mice in association with decreased abundance of Rac and Cdc42 is consistent with a previous study showing that podocyte-specific deletion of Rac1 exacerbated albuminuria and glomerulosclerosis in the UNX/DOCA-salt model of hypertension.22 Furthermore, this same study reported that podocyte-specific deletion of Cdc42 resulted in heavy proteinuria and early death, similar to the phenotype observed in MYH9 E1841K/E1841K in the CKD model.22 Taken together, the influence of Rho GTPases on podocytes may be disease context dependent. MYH9 is a downstream effector of Rho-GTPases; how the E1841K mutation in MYH9 downmodulates the protein abundance of Rho-GTPases is unclear. It is possible that the E1841K mutation indirectly influences Rho-GTPases, or that the diminished expression of Rac and Cdc42 associated with the E1841K mutation could be a compensatory mechanism yet to be defined.
In many disease models, stimuli that cause podocyte foot effacement in vivo also induce hypermotility of podocytes in vitro. There is a consensus that cortical actin reorganization and podocyte motility in vitro reflect podocyte foot effacement in vivo.24 Our findings are congruent with this notion. In this regard, even at baseline, MYH9 E1841K/E1841K mice developed mild podocyte foot effacement that became severe during Ang II–induced hypertension. In vitro, their podocytes exhibited cortical actin reorganization even in unstimulated condition. It is worth noting here that MYH9 +/E1841K mice displayed similar urinary N/C ratio and almost no podocyte foot effacement as MYH9 +/+ in vivo at baseline and during Ang II–induced hypertension, but their podocytes have a migratory phenotype in between that of MYH9 +/+ and MYH9 E1841K/E1841K mice. These findings are consistent with the notion that too little or too much podocyte motility both result in podocyte foot effacement,24 and suggest there may be a threshold at which hypermotility becomes detrimental.
Although knockdown studies showed that MYH9 plays a role in podocyte cytoskeletal organization, it has also been shown to interact with many proteins, including those involved in the metabolic pathway of glycolysis, and in the anemia pathway.17 Interestingly, in the Nx CKD model, we noted that MYH9 E1841K/E1841K have very light-colored blood from cardiac puncture at euthanization, suggestive of severe anemia. This suggests that the MYH9 E1841K mutation may alter erythropoiesis, and impair proper interaction with other proteins that may play a protective role in stress response by podocytes. Alternatively, the anemia in MYH9 E1841K/E1841K may simply be due to severe renal failure.
It is noteworthy that the MYH9 E1841K mutation does not cause or worsen hypertension in our mouse models, but that hypertension is a stressor or a prerequisite for the development of kidney disease. Our data raise an important clinical question, of whether patients with MHA carrying the MYH9 E1841K mutation who develop nephropathy have worse hypertension compared with those without nephropathy. In addition, the Nx model suggests that the mutation impairs adaptive changes that occur in CKD. It remains to be determined how the renal effect of this mutation that results in significant structural and functional alteration of MYH9 protein12 contributes to our understanding of the common intronic MYH9 variants that have no known functional consequence but yet confer increased risks for diabetic kidney disease in European Americans9 and in nephropathy associated with sickle cell disease.10
The findings in our study raise additional questions. First, is the hypertension-associated development of glomerular disease seen in both the Ang II and Nx models specifically dependent on the upregulation of the renin-angiotensin system? Nx is also a model in which renal RAS is upregulated, despite suppressed plasma renin activity.25 Second, is the proinjury effect of Ang II on podocytes with the MYH9 E1841K mutation entirely mediated through AT1 receptor? Third, does altered MYH9 function in the mesangium contribute to the development of albuminuria and podocyte FP fusion and effacement? Finally, does the MYH9 mutation cause a defect in proximal tubular albumin reabsorption, because MYH9 is expressed in the brush border of the proximal tubule?1
In summary, our study suggests that the development of significant albuminuria or glomerular injury in the setting of loss-of-function mutations of MYH9 requires a second stimulus such as hypertension or loss of functioning nephrons.
Concise Methods
Mouse Strains
Mice carrying the E1841K mutation were generated using standard point mutation and homologous recombination techniques as previously reported.14
All mice were bred and maintained on a 12-hour light-dark cycle with free access to standard chow and water in the animal facility of the University of Virginia. Only male mice were used in our phenotyping studies. Experiments were carried out in accordance with local and National Institutes of Health guidelines.
Hypertension Models
HSD (6% NaCl) in pellets was purchased from Harlan Teklad (Madison, WI) and administered in place of normal chow for 2 weeks. Angiotensin II (Sigma-Aldrich, St. Louis, MO) was delivered at 1000 ng/kg per minute for 4 weeks via Alzet mini-osmotic pumps (model 2004; Durect Corporation, Cupertino, CA). Candesartan (a kind gift from Dr. Robert Carey, University of Virginia) was administered in drinking water at 10 mg/kg per day for the duration of Ang II infusion.
CKD Model
To induce CKD, mice were subjected to subtotal Nx: under 1.5% isoflurane anesthesia, the right kidney was removed, and the upper branch of the two main branches of the left renal artery were ligated to impede blood supply to the upper half of the kidney.
Mice were allowed to recover for 4 weeks before SBP measurements.
BP Measurements
SBP was measured via tail-cuff manometry using the MC4000 multichannel BP analysis system (Hatteras Instruments, Cary, NC). Mice were trained daily at the same time of the day for 2 weeks. After training, BP measurements were taken and recorded daily for another 2 weeks to obtain baseline readings (on regular chow), then (1) an additional 2 weeks for high salt treatment; (2) 3–4 weeks for Ang II; and (3) 3–4 weeks for Ang II + Candesartan treatments. Each day at least ten measurements for each mouse were recorded. At the end of the experiment, all measurements recorded during the treatment period were averaged for each mouse. Each mouse served as its own control for comparison of results in the experiments with high salt and Ang II treatments. For the experiment with Ang II + Candesartan, a separate group of mice was used.
Urine Measurements
Twenty-four-hour urine samples were collected from mice placed in individual metabolic cages at the end of each experiment (2 weeks of baseline; 2 weeks on HSD; 4 weeks of Ang II infusion; and 3 weeks of Ang II infusion + Candesartan in drinking water). Urinary albumin, nephrin, and creatinine were measured using the Albuwell M Murine ELISA kit, Nephrin ELISA kit, and Creatinine Companion kit (Exocell, Philadelphia, PA) as per the manufacturer’s instructions.
Histologic Analysis
For light microscopy, a center part of the kidney was cut in cross-section and fixed in 10% neutral-buffered formalin. Tissues were embedded in paraffin, sectioned, and stained with periodic acid–Schiff in the Research Histology Core of the University of Virginia. The pictures shown were taken at 40×. The slides were read and scored as previously described16 by a renal immunopathologist (P.R.) blinded to mouse genotypes and experimental conditions. Renal pathology was graded by standard methods for several morphologic features, including glomerular inflammation, proliferation, sclerosis (segmental or global), and mesangial changes. Tubular, interstitial, and vascular changes were also noted. Scores from 0 to 4 were assigned for each of the features, with 0 being normal, to 4 being most severe. Scores for inflammation were graded on the basis of both intensity and pattern of distribution (e.g., focal or diffuse). The scores were then added to yield a final kidney pathology score. For electron microscopy, a pie-shaped piece of kidney cortical tissue was cut and fixed in a solution containing 4% paraformaldehyde and 2.5% glutaraldehyde in PBS. Tissues were processed and pictures were taken at 6000× magnification at the Advance Microscopy Facility at the University of Virginia (JEOL 1230 with real-time digital imaging). The podocyte FP effacement was scored in a blinded manner by a renal pathologist (P.R.) on the basis of the following criteria: normal = no flattening of any podocytes = score 1; mild fusion = flattening of 0%–25% of podocytes = score 2; moderate fusion = flattening of 25%–50% of podocytes = score 3; severe fusion = flattening of 50%–75% of podocytes = score 4; and very severe fusion = flattening of >80% of podocytes = score 5.
Isolation of Glomeruli and Primary Podocyte Cell Culture
Protocol was modified from previously published papers.26,27 Mice were anesthetized by isoflurane and perfused with magnetic beads (6×108 beads/ml, Dynabeads; Invitrogen) diluted in 10 ml PBS through the heart. The kidneys were removed, minced on the sterile plate, and digested in collagenase A (1 mg/ml) at 37°C for 25 minutes. Digested tissues were gently pressed with a syringe pestle through a 100-µm cell strainer sitting on the 50-ml tube and washed with 5 ml PBS. The filtrate was passed one more time through another 100-µm cell strainer, and a second time through a 40-µm cell strainer, each time washed with 5 ml PBS. Isolated glomeruli were then washed out from the nylon mesh of the cell strainer with a prewarmed cell culture media. Finally, glomeruli containing magnetic beads were isolated by magnetic particle concentrator (Invitrogen) and washed with a prewarmed culture media two to three times. Glomeruli resuspended in culture media were placed in the six-well plate coated with rat tail type I collagen (or German coverslip coated with rat tail type I collagen placed on the bottom of well; Neuvitro) and incubated at 37°C. After 3–5 days the podocytes outgrowing from isolated glomeruli were observed under a light microscope. Cell culture media used was RPMI 1640 with L-glutamine supplemented with 10% FBS, 100 U/ml Pen/Strep, and 1% L-glutamine.
Immunofluorescent and Phalloidin Staining of Podocytes
Protocol was modified from previously published papers.28,29 Podocytes cultured on type I collagen–coated coverslips in six-well plate were washed twice with PBS and fixed with 3.7% methanol-free formaldehyde solution in PBS for 10 minutes at room temperature, followed by permeabilization with 0.2% Triton X-100 in PBS for 5 minutes. To reduce nonspecific background staining, the fixed cells were incubated with the Image-iT FX signal enhancer (Molecular Probe) for 30 minutes. After washing with PBS, cells were probed with (1) 1:50 dilution of Alexa Fluor 488-conjugated synaptopodin (P-19) (Santa Cruz Biotechnology) antibody for 30 minutes in the dark; or (2) with MYH9 (nonmuscle myosin heavy chain II-A antibody; Covance #PRB-440P; BioLegend) conjugated to Alexa Fluor 488, overnight at 4°C in the dark; and then in both cases followed by Alexa Fluor 647 phalloidin to stain F-actin (Molecular Probe). To visualize the nuclei, a 1:10,000 dilution of DAPI was used for 5 minutes at the end of the staining procedure. The cells on coverslips were air dried and permanently mounted on Superfrost Plus microscope slides with ProLong Gold reagent (Invitrogen). Images were obtained using an inverted fluorescent ApoTome microscope Axiovert 200M and analytic software Axio Vision (Zeiss).
Scratch Wound Healing Assay
The assay was performed following a published protocol30 on the primary cell culture of podocytes when they reached a confluent monolayer, approximately 7–9 days after glomeruli isolation. The mechanical scraping was performed with a 200-µl pipette tip from the top to the bottom of the well, creating a rectangular shape of wound in the podocyte layer. Images of podocytes with the scratched area were taken immediately after wound creation (0 hour) and after an additional 24 hours of incubation, using EVOS XL Core Cell Imaging System at 10× magnification. At least three independent experiments were performed for each phenotype studied. The images were analyzed using ImageJ software (1.48v; NIH), by measuring pixels of the outlined scratched area at 0 hour and after 24 hours (see Figure 6). The results are expressed as percentage of area that was not covered by migrating podocytes after 24 hours compared with the area created by scratching the confluent layer of podocytes at time zero (=area [in pixels] at 24 hours divided by area [in pixels] at 0 hour).
Electrophoresis and Western Blotting
Mouse kidney tissue was harvested and immediately put in cold Tris-Triton lysis buffer (10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate) containing Halt protease and phosphatase inhibitor cocktail (Thermo Scientific). Tissue was homogenized using a tissue lyser and stainless steel beads for 5 minutes at 50 Hz, then the homogenate was spun at 12,000 rpm, at 4°C for 20 minutes, and the supernatant was collected. Protein concentration was determined by the BCA assay (Pierce). Fifty micrograms of total protein was loaded onto 4%–12% SDS-PAGE gels and then transferred to nitrocellulose membranes per manufacturer instructions (X-Cell Blot Module; Invitrogen). The membrane was blocked using Odyssey PBS Blocking Buffer (LI-COR) for 1 hour at room temperature, then incubated at 4°C overnight with mouse monoclonal (Santa Cruz) primary antibody diluted in blocking buffer. Primary antibodies anti-RhoA (sc-418) and Cdc42 (sc-8401) were used at dilution 1:200; pan Rac (Rac1, Rac2, Rac3) (sc-514583) at 1:100; and β-tubulin (sc-9104, rabbit polyclonal antibody) at 1:500. Secondary antibody incubation was performed for 1 hour at room temperature with anti-rabbit IRDye 800CW and anti-mouse IRDye 680RD (LI-COR) antibodies diluted in blocking buffer at 1:15,000. Signals were detected with Odyssey Infrared Imaging System (LI-COR) and quantified by accompanying software.
Statistical Analyses
Data are expressed as the mean±SEM. Statistical analyses were performed using GraphPad Prism 7 software. Differences between matched samples were analyzed by paired t test. Unless otherwise stated, ANOVA was performed to test group differences and overall significant differences. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge and thank Adam Straub for help provided with several electron microscopic images.
This work was supported by internal funding from the Division of Nephrology, Department of Medicine, University of Virginia. The Research Histology Core at the Department of Medicine, University of Virginia, provided periodic acid-Schiff (PAS) staining of kidney tissues. The Advanced Microscopy Facility at Department of Medicine, University of Virginia, provided preparations of kidney tissue samples for electron microscopy. JEOL model JEM-1230 Transmission electron microscope, funded by National Institutes of Health grant 1S10RR021017-01, was used to take images.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060707/-/DCSupplemental.
References
- 1.Arrondel C, Vodovar N, Knebelmann B, Grünfeld JP, Gubler MC, Antignac C, Heidet L: Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol 13: 65–74, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS: Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279: 41263–41266, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR: Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS; Family Investigation of Nephropathy and Diabetes Research Group : MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke JN, Bostrom MA, Hicks PJ, Ng MC, Hellwege JN, Comeau ME, Divers J, Langefeld CD, Freedman BI, Bowden DW: Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant 27: 1505–1511, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ: MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 155: 386–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong F, Li S, Pujol-Moix N, Luban NL, Shin SW, Seo JH, Ruiz-Saez A, Demeter J, Langdon S, Kelley MJ: Genotype-phenotype correlation in MYH9-related thrombocytopenia. Br J Haematol 130: 620–627, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kelley MJ, Jawien W, Ortel TL, Korczak JF: Mutation of MYH9, encoding non-muscle myosin heavy chain A, in May-Hegglin anomaly. Nat Genet 26: 106–108, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Franke JD, Dong F, Rickoll WL, Kelley MJ, Kiehart DP: Rod mutations associated with MYH9-related disorders disrupt nonmuscle myosin-IIA assembly. Blood 105: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Conti MA, Malide D, Dong F, Wang A, Shmist YA, Liu C, Zerfas P, Daniels MP, Chan CC, Kozin E, Kachar B, Kelley MJ, Kopp JB, Adelstein RS: Mouse models of MYH9-related disease: Mutations in nonmuscle myosin II-A. Blood 119: 238–250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma LJ, Fogo AB: Model of robust induction of glomerulosclerosis in mice: Importance of genetic background. Kidney Int 64: 350–355, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Salzler HR, Griffiths R, Ruiz P, Chi L, Frey C, Marchuk DA, Rockman HA, Le TH: Hypertension and albuminuria in chronic kidney disease mapped to a mouse chromosome 11 locus. Kidney Int 72: 1226–1232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hays T, Ma’ayan A, Clark NR, Tan CM, Teixeira A, Teixeira A, Choi JW, Burdis N, Jung SY, Bajaj AO, O’Malley BW, He JC, Hyink DP, Klotman PE: Proteomics analysis of the non-muscle myosin heavy chain IIa-enriched actin-myosin complex reveals multiple functions within the podocyte. PLoS One 9: e100660, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, Schlatter E, Pavenstädt H: Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med (Berl) 86: 1379–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Johnstone DB, Zhang J, George B, Léon C, Gachet C, Wong H, Parekh R, Holzman LB: Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol 31: 2162–2170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnstone DB, Ikizler O, Zhang J, Holzman LB: Background strain and the differential susceptibility of podocyte-specific deletion of Myh9 on murine models of experimental glomerulosclerosis and HIV nephropathy. PLoS One 8: e67839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babelova A, Jansen F, Sander K, Löhn M, Schäfer L, Fork C, Ruetten H, Plettenburg O, Stark H, Daniel C, Amann K, Pavenstädt H, Jung O, Brandes RP: Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS One 8: e80328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M: Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, Ruiz P, Fields TA, Spurney RF: Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kistler AD, Altintas MM, Reiser J: Podocyte GTPases regulate kidney filter dynamics. Kidney Int 81: 1053–1055, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao W, Li A, Wang L, Zhou Z, Su Z, Bin W, Wilcox CS, Hou FF: A Salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol 26: 1619–1633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T: An improved method for primary culture of rat podocytes. Kidney Int 69: 2101–2106, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakairi T, Abe Y, Jat PS, Kopp JB: Cell-cell contact regulates gene expression in CDK4-transformed mouse podocytes. Am J Physiol Renal Physiol 299: F802–F809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Riahi R, Yang Y, Zhang DD, Wong PK: Advances in wound-healing assays for probing collective cell migration. J Lab Autom 17: 59–65, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.