Abstract
The bone-derived hormone fibroblast growth factor–23 (FGF-23) activates complexes composed of FGF receptors (FGFRs), including FGFR1, and α-Klotho in the kidney distal tubule (DT), leading to increased sodium retention and hypertension. However, the role of FGFR1 in regulating renal processes linked to hypertension is unclear. Here, we investigated the effects of selective FGFR1 loss in the DT. Conditional knockout (cKO) of FGFR1 in the DT (FGFR1DT-cKO mice) resulted in left ventricular hypertrophy (LVH) and decreased kidney expression of α-Klotho in association with enhanced BP, decreased expression of angiotensin converting enzyme 2, and increased expression of the Na+-K+-2Cl− cotransporter. Notably, recombinant FGF-23 administration similarly decreased the kidney expression of α-Klotho and induced LVH in mice. Pharmacologic activation of FGFR1 with a monoclonal anti-FGFR1 antibody (R1MAb1) normalized BP and significantly attenuated LVH in the Hyp mouse model of excess FGF-23, but did not induce a response in FGFR1DT-cKO mice. The hearts of FGFR1DT-cKO mice showed increased expression of the transient receptor potential cation channel, subfamily C, member 6 (TRPC6), consistent with cardiac effects of soluble Klotho deficiency. Moreover, administration of recombinant soluble Klotho lowered BP in the Hyp mice. Thus, FGFR1 in the DT regulates systemic hemodynamic responses opposite to those predicted by the actions of FGF-23. These cardiovascular effects appear to be mediated by paracrine FGF control of kidney FGFR1 and subsequent regulation of soluble Klotho and TRPC6. FGFR1 in the kidney may provide a new molecular target for treating hypertension.
Keywords: blood pressure, heart disease, hypertension, hypertrophy, renin angiotensin system, distal tubule
Hormonal fibroblast growth factor (FGF)-23 activates fibroblast growth factor receptors (FGFRs)/α-Klotho binary receptor complexes in the kidney tubules to regulate mineral homeostasis.1–5 FGFRs 3 and 4 mediate the effects of FGF-23 on vitamin D metabolism in the proximal tubule (PT),2,6,7 whereas FGFR1 plays a dominant role of mediating the effects of FGF-23 on phosphate transport in the PT and calcium reabsorption in the distal tubule (DT).8–12 Elevated FGF-23 is also associated with hypertension, left ventricular hypertrophy (LVH), and increased mortality in CKD.13–20 These cardiovascular effects are attributed, at least in part, to effects of FGF-23 activation of FGFRs/α-Klotho coreceptors in the kidney to enhance sodium (Na) reabsorption, and suppress angiotensin converting enzyme 2 (Ace2) and α-Klotho expression.13–17
FGF ligands produced in the kidney have paracrine/autocrine functions to regulate kidney development through heparin sulfate proteoglycan cofactor–dependent activation of FGF receptors.21–32 FGF signaling in the adult kidney may also lead to hypertension, as evidenced by genetic association studies linking FGF1, FGF2, and FGF5 to hypertension in humans.33–35 Local production renal FGF ligands regulate renal natriuretic peptide catabolism, the renin-angiotensin cascade, and Na handling.36–38 Surprisingly little is known about the specific role of FGFR1 in regulating renal processes linked to hypertension and LVH.
In this study, we examined the effects of the selective loss-of-FGFR1 in the DT on BP and LVH in mice. We found that mice with deletion of FGFR1 in the DT developed hypertension and LVH, whereas stimulation of FGFR1 with an activating antibody or the administration of recombinant soluble Klotho (sKl) decreased BP in the Hyp mouse model of FGF-23 excess. These findings are paradoxic to the reported cardiovascular effects of FGF-23, and suggest that FGFR1 in the kidney plays an important role in regulating BP and cardiovascular homeostasis.
Results
Selective Deletion of FGFR1 in the Kidney
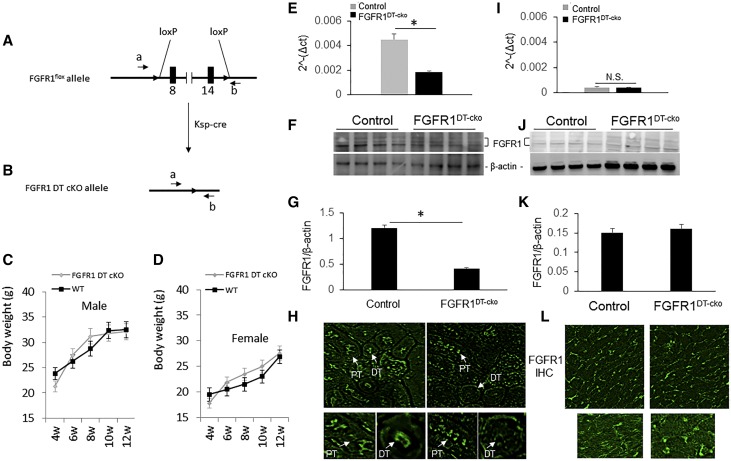
Deletion of FGFR1 in distal tubular segments of the kidney (FGFR1DT-cKO mice) was accomplished by crossing the Ksp-Cre transgenic mice with FGFR1flox/- mice.8,39,40 Deletion of FGFR1 in DT did not affect growth rate and body weight of FGFR1DT-cKO mice (Figure 1, A–D). We observed an approximately 60% reduction in FGFR1 message and approximately 65% total FGFR1 protein content of the whole kidney by quantitative RT-PCR and western blot analysis (Figure 1, E–G). FGFR1 was selectively deleted in distal tubular segments, but not the PT, in FGFR1DT-cKO mice (Figure 1H). Low levels of FGFR1 message and protein were detected in wild-type mouse hearts by quantitative RT-PCR, western blot analysis, and immunohistochemical staining (Figure 1, I–L). FGFR1DT-cKO mice exhibited no alterations in FGFR1 expression in the heart compared with controls (Figure 1, I–L), consistent with the lack of Ksp-Cre heart activity.8
Figure 1.
Specific deletion of FGFR1 in DT of kidney reduces renal FGFR1 expression in FGFR1DT cKO mice. (A and B) Schematic deletion of FGFR1 gene in DT of kidney by crossing FGFR1flox/flox female mouse with Ksp-Cre;FGFR1null/+ male mouse. (C and D) Growth charts of FGFR1DT-cKO male and female (4–12 weeks). (E) Quantitative PCR analysis of expression of FGFR1 in the kidney tissue of control and FGFR1DT cKO mice. (F) Western bolt analysis and (G) quantity of FGFR1 expression in the kidney of control and FGFR1DT cKO mice. (H) Immunofluorescence staining of kidney sections with PT (arrow) and DT (arrow) of control and FGFR1DT cKO mice (original magnification, ×40). (I) Quantitative PCR analysis, (J) western blot analysis, and (K) quantity of FGFR1 expression, and (L) immunohistochemical staining of heart sections of control and FGFR1DT cKO mice (original magnification, ×40). n=4–6/group. All values are shown as mean±SD. *P<0.05 versus controls by t test. IHC, Immunohistochemistry.
FGFR1DT-cKO Mice Have LVH and Hypertension
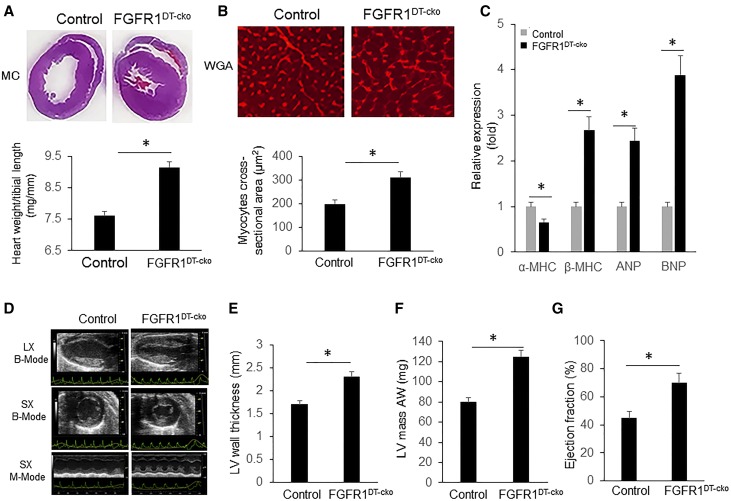
Because administration of rFGF-23 induces LVH in wild-type mice (Supplemental Figure 1),9,41 our a priori expectation was that loss-of-FGFR1 in the kidney would have opposite effects. Unexpectedly, we found that 12-week-old FGFR1DT-cKO mice displayed a significant increase in heart size (Figure 2A, top) and heart weight/tibia length ratio (Figure 2A, bottom) compared with control mice. Increased heart size was detected as early as 6 weeks of age in FGFR1DT-cKO mice, but not in FGFR1+/− (Supplemental Figure 2). Histologic analysis of heart cross-sections from 12-week-old animals found that FGFR1DT-cKO mice had greater cross-sectional area of individual cardiac myocytes (Figure 2B). In addition, FGFR1DT-cKO mice showed increased expression of biomarkers of heart failure, including atrial and brain natriuretic peptide (ANP and BNP) and β-myosin heavy chain (β-MHC) expression, compared with control mice (Figure 2C),42 Echocardiography showed that FGFR1DT-cKO had significant increases in LV wall thickness, LV mass, and systolic ejection fraction compared with control mice (Figure 2, D–G).
Figure 2.
Deletion of FGFR1 in DT of kidney results in LVH in FGFR1DT cKO mice. (A) Representative gross pathology of mid-chamber (MC) sections of the H&E stain (top, original magnification, ×4); FGFR1DT cKO mice showed increased ratio of heart weight to tibia length (bottom) (n=5–8/group). *P<0.05 versus controls by t test. (B) Wheat germ agglutinin (WGA)–stained sections from the left ventricular MC of WT and FGFR1DT cKO mice (top, original magnification, ×10); cross-sectional surface area of individual cardiomyoctes from the left ventricular MC of control and FGFR1DT cKO mice (bottom); (C) expression levels of hypertrophic markers (α-MHC, β-MHC, ANP, and BNP) (n=6–10/group). *P<0.05 versus controls by t test. (D) Compared with WT mice, FGFR1DT cKO mice developed LVH confirmed by echocardiography with (E) significant increase in LV wall thickness, (F) left ventricular mass (LVM), and (G) ejection fractions ratio. n=8–10/group. *P<0.05 versus controls by t test. All values are shown as mean±SD. AW, anterior wall; LV, left ventricular; LX, long axis; SX, short axis.
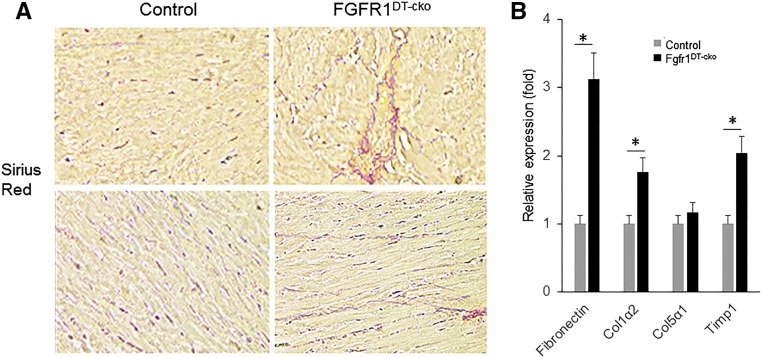
The LVH was not due to elevations of FGF-23 in FGFR1DT-cKO mice, because circulating FGF-23 concentrations were no different between FGFR1DT-cKO and control mice (Table 1). rFGF-23 administration (50 ng/g per mouse twice a day for 1 day) inhibited Ace2 and α-Klotho expression in wild-type mice (Table 2), consistent with prior reports.43 Additionally, we found histologic evidence for myocardial fibrosis (Figure 3A) and significantly increased expression levels of fibrotic markers fibronectin, collagen type 1 α 2 (Col1α2), and TIMP metallopeptidase inhibitor 1 (timp 1) in FGFR1DT-cKO mice compared with control mice (Figure 3B).
Table 1.
Serum and urine biochemistry
| Serum (n=6–8) | Control | FGFR1DT cKO | P Value |
|---|---|---|---|
| FGF23, pg/dl | 60.2±10.3 | 65.5±12.4 | 0.28 |
| Aldosterone, pg/ml | 157.8±7.8 | 150.0±16.1 | 0.65 |
| Na, mmol/L | 150.1±3.2 | 145.1±6.8 | 0.45 |
Data represent mean±SD from mice (n=5) of each genotype. P value versus controls was calculated by t test.
Table 2.
Kidney gene expression response to rFGF-23
| Gene | Control | FGFR1DT-cKO | ||
|---|---|---|---|---|
| Vehicle | rFGF23 | Vehicle | rFGF23 | |
| Ace2 | 1.00±0.32a | 0.54±0.16b | 0.45±0.08b | 0.28±0.06c |
| Klotho | 1.00±0.21a | 0.27±0.15c | 0.46±0.12b | 0.20±0.25c |
Data represent mean±SD from mice (n=5) of each genotype. One-way ANOVA analysis with post hoc test for multiple comparisons.
a–cValues not sharing the same superscript letter are significantly different at P<0.05.
Figure 3.
Deletion of FGFR1 in DT of kidney results in myocardial fibrosis in FGFR1DT cKO mice. (A) Picrosirius red staining from the left ventricular mid-chamber of WT and FGFR1DT cKO mice (original magnification, ×40) showed myocardial fibrosis. (B) Quantitative PCR analysis of heart tissue showed that expression level of myocardial fibrosis markers (fibronectin, collagen type 1 α 2 [col1α2], collagen type 5 α 1 [col5α1], and TIMP metallopeptidase inhibitor 1 [timp 1]) by real-time PCR of heart tissue from control and FGFR1DT cKO mice. n=6/group. *P<0.05 versus controls by t test.
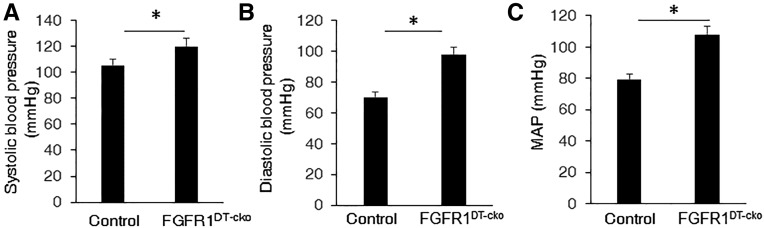
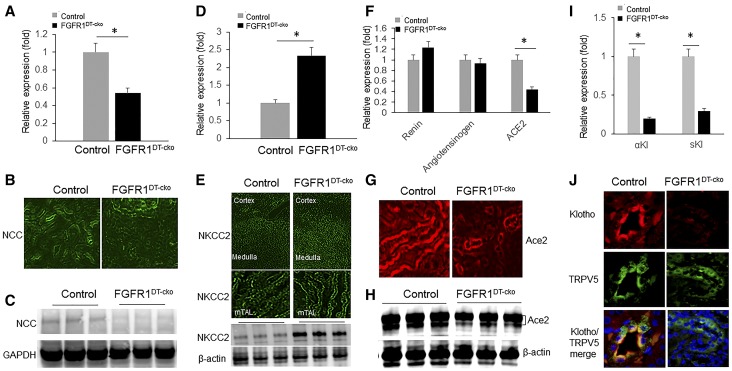
FGFR1DT-cKO mice exhibited significant increases in systolic, diastolic, and mean arterial pressure (Figure 4, A–C). Hypertension in FGFR1DT-cKO mice was associated with decreased expression of Na+-Cl− cotransporter (NCC) (Figure 5, A–C), increased Na+:K+:2Cl− cotransporter (NKCC2) that is expressed in the thick ascending limb (tAL) (Figure 5, D and E), without alterations in serum aldosterone (Table 1). We found no differences in expression of renin or angiotensinogen, but observed decreased expression of Ace2 in the kidney of FGFR1DT-cKO mice compared with controls (Figure 5, F–H).
Figure 4.
Deletion of FGFR1 in DT causes hypertension in FGFR1DT cKO mice. Compared with control mice, FGFR1DT cKO mice showed elevated (A) systolic BP, (B) diastolic BP, and (C) mean arterial pressure (MAP). n=6–8/group. *P<0.05 versus controls by t test. All values are shown as mean±SEM.
Figure 5.
Deletion of FGFR1 in DT of kidney downregulates Klotho expression in FGFR1DT cKO mice. Quantitative PCR analysis of kidney tissue showed that expression level of NCC was significantly downregulated (A) and NKCC2 was significantly upregulated (D) in FGFR1DT cKO mice compared with control mice (n=6–8/group; *P<0.05 versus controls by t test). Representative images of the kidney showing (B) NCC and (E) NKCC2 staining from control and FGFR1DT cKO mice. Original magnification, upper panel ×4, lower panel ×40. Western blot analysis of (C) NCC and (E, bottom) NKCC2 expression in the kidney tissue from control and FGFR1DT cKO mice. Expression of Ace2 was decreased in the kidney of FGFR1DT cKO mice as determined by (F) quantitative PCR (n=6–8/group; *P<0.05 versus controls by t test), (G) immunohistochemistry, and (H) western blot analysis. Quantitative PCR analysis of kidney tissue showed that expression levels of α-klotho and secreted klotho (sKl) were significantly decreased in FGFR1DT cKO mice compared with control mice (I) (n=6–8/group; *P<0.05 versus controls by t test). (J) Klotho staining, TRPV5 staining, and merged images from control and FGFR1DT cKO mice. β-actin served as loading control. All values are shown as mean±SD. α-Kl, α-klotho; mTAL, medullary thick ascending limb.
The expression of mRNA encoding the membrane-bound form of α-KL and the alternative spliced KL isoform was significantly decreased in FGFR1DT-cKO mice compared with controls (Figure 5I). Immunohistochemical staining for α-KL and TRPV5, a marker for the DT, on the kidney sections prepared from control and FGFR1DT-cKO mice revealed abundant α-KL immuno-reactivity in the DTs of control mice that colocalized with TRPV5 to the apical membranes (Figure 5J), but decreased α-KL and TRPV5 in the DT of FGFR1DT-cKO mice.
Gene Expression Cardiac Signature Implicates sKl in the Pathogenesis of LVH in FGFR1DT-cKO Mice
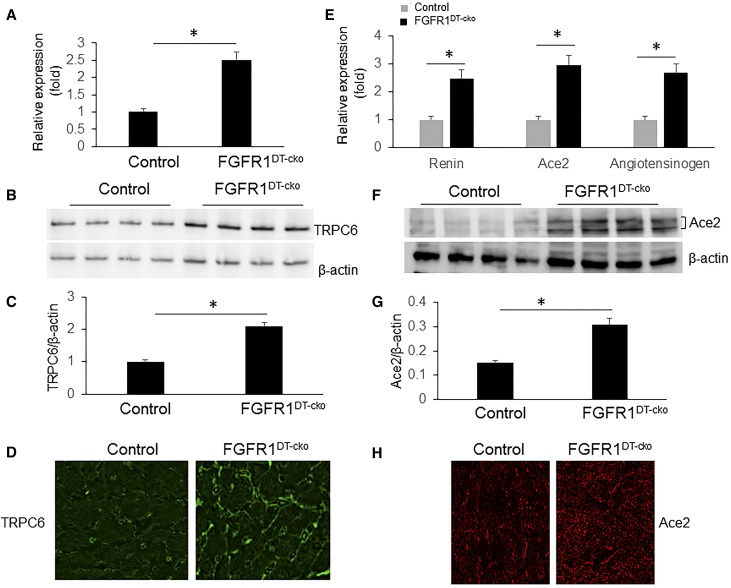
Xie et al.44 have shown that sKlotho protects TRPC6-induced cardiac hypertrophy by downregulation of TRPC6 expression and blocking TRPC6 exocytosis to the cell surface of cardiomyocytes. On the basis of this, we would expect that TRPC6 channels would be increased in the heart of FGFR1DT-cKO mice, if renal reductions in sKl are mediating the LVH. We found that TRPC6 message levels and protein expression were increased about two-fold determined by real-time PCR and western blot analysis in the heart of FGFR1DT-cKO mice compared with controls (Figure 6, A–C). TRPC6 staining was primarily detected on the cell membrane of cardiomyocytes of control mice (Figure 6D). We also found that expression of renin, angiotensinogen, and Ace2 was significantly increased in the heart of FGFR1DT-cKO compared with control mice, determined by RT-PCR, western blot analysis, and immunohistochemical staining (Figure 6, E–H).
Figure 6.
TRPC6 and Ace2 are overexpressed in the heart of FGFR1DT cKO mice. (A) Quantitative PCR analysis of heart tissue showed that expression level of TRPC6 was significantly decreased in FGFR1DT cKO mice compared with control mice (n=6–8/group; *P<0.05 versus controls by t test). (B and C) Western blot analysis of TRPC6 expression in the heart tissue from control and FGFR1DT cKO mice (n=4/group; *P<0.05 versus controls by t test). (D) Representative images of the heart showing TRPC6 staining from control and FGFR1DT cKO mice. Expression of renin, Ace2, and angiotensinogen was increased in the heart of FGFR1DT cKO mice as determined by (E) quantitative PCR (n=6–8/group; *P<0.05 versus controls by t test); elevated expression of Ace2 was confirmed by (F and G) western blot analysis (n=4/group; *P<0.05 versus controls by t test) and (H) immunohistochemistry. β-actin served as loading control. All values are shown as mean±SD.
Activation of FGFR1 or Administration of sKl Reduces BP in Hyp Mice
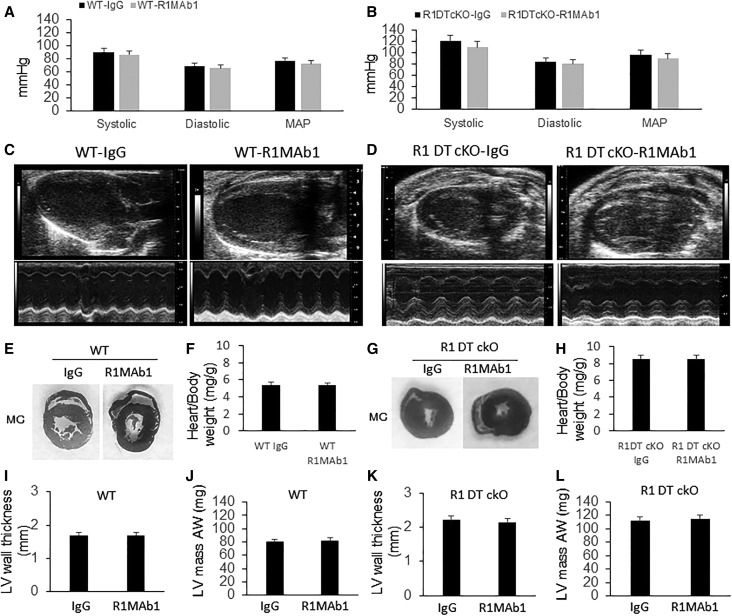
The presence of hypertension in FGFR1DT-cKO mice raises the possibility that activation of FGFR1 will lower BP. We tested this possibility by the administration of an FGFR1 activating antibody (R1MAb1) or Ig-G to wild-type C57BL/6, db/db diabetic hypertensive mouse model,45,46 FGFR1DT-cKO, and Hyp mice. We found that R1MAb1 (single 3 mg/kg intraperitoneally [ip]) did not affect BP in WT mice (Figure 7A). Notably, we found that FGFR1DT-cKO mice were hypertensive but refractory to R1MAb1-mediated reduction of BP (Figure 7B). R1MAb1 had no effect on the ratio of heart weight to body weight, LV mass, and LV wall thickness in WT or FGFR1DT-cKO mice (Figure 7, C–L). Treatment with R1MAb1 reduced arterial pressure (P=0.02), increased heart rate (P=0.02), and had no effect on body weight at 6 days after single mAb injection in db/db mice (Supplemental Figure 3, A–C). In addition, R1MAb1 reduced blood glucose (P<0.01) in db/db mice, reflecting effects of activation of FGFR1 in other tissues (Supplemental Figure 3D).47
Figure 7.
Deletion of FGFR1 in DT blocks effect of R1MAb1 on BP regulation. Wild type and FGFR1DT cKO (FGFR1flox/flox;Ksp-Cre) (4 weeks old) were treated with single injection of R1MAb1 (ip 3 mg/kg). Four weeks after R1MAb1 administration, we performed echocardiography and BP measurement. (A) R1MAb1 had no effect on BP in wild-type mice. (B) Compared with control mice, FGFR1DT cKO mice showed elevated systolic BP, diastolic BP, and mean arterial pressure (MAP). (B) R1MAb1 administration did not change the BP in FGFR1DT cKO mice. (C, E, F, I, and J) R1MAb1 administration had no effect on the heart including ratio of heart weight to body weight, LV mass, and LV wall thickness in WT mice. (D, G, H, K, and L) FGFR1DT cKO mice showed LVH and refractory to R1MAb1 treatment. n=6–8/group; *P<0.05 versus controls by t test. All values are shown as mean±SD. AW, anterior wall; LV, left ventricular; WT, wild type.
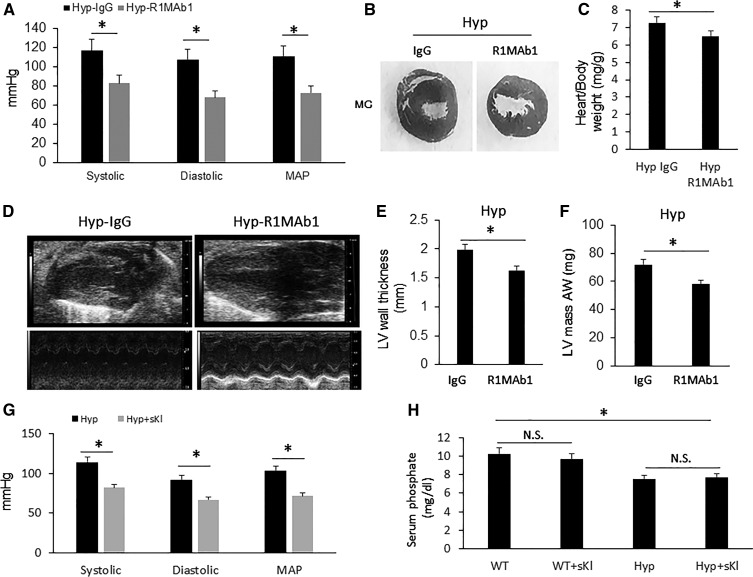
The cardiovascular response to FGF-23 activation of FGFR/αKl complexes in the DT of Hyp mice is opposite to loss-of-Fgfr1 function in FGFR1DT-cKO mice. To attempt to understand this apparent paradox in FGFR function, we examined the effect of administering R1MAb1 or recombinant Kl to Hyp mice. We found that administration of R1MAb1 significantly reduced BP in Hyp mice (Figure 8A). Furthermore, treatment with R1MAb1 measurably attenuated LVH in Hyp mice as demonstrated by significant decreases in ratio of heart weight to body weight, LV mass, and LV wall thickness compared with IgG-treated Hyp mice (Figure 8, B–F). Moreover, we found that the ip injection of sKl also normalized BP in Hyp mice, without affecting serum phosphorus concentration (Figure 8, G and H), suggesting that the reduction in Kl may be contributing to the cardiovascular effects of excess FGF-23 in Hyp mice.
Figure 8.
Activation of FGFR1 reduces BP and attenuates LVH in Hyp mice. Hyp mice (4 weeks old) were treated with single injection of R1MAb1 (ip 3 mg/kg). Four weeks after R1MAb1 administration, we performed echocardiography and BP measurement. (A) Hyp mice showed elevated BP, which was significantly reduced by treatment of R1MAb1 compared with IgG-treated control Hyp mice. (B and C) Hyp mice (IgG control) developed LVH, which was attenuated by R1MAb1 administration confirmed by (B) gross pathology of mid-chamber (MC) sections of the H&E stain (middle, original magnification, ×4), (C) ratio of heart weight to body weight, (D) echocardiography, (E) LV wall thickness, and (F) left ventricular mass (LVM). (G) Administration of sKl normalized BP in Hyp mice, (H) without affecting serum phosphorus concentration. n=6–8/group; *P<0.05 versus controls by t test. All values are shown as mean±SD. AW, anterior wall; LV, left ventricular; WT, wild type.
Discussion
We show that genetic deletion of FGFR1 in the kidney distal segments using Ksp-Cre leads to hypertension and LVH, whereas an FGFR1-activating antibody R1MAb1 lowers BP in a hypertensive mouse model, and reverses hypertension observed in the Hyp moue model of FGF-23 excess, through FGFR1-dependent renal tubular actions. Our effects are opposite to the effects of FGF-23 activation of FGFRs/α-Klotho binary complexes in renal tubules that causes hypertension and LVH9–11,43,48 or possible direct activation of FGFR4 in the heart.41,49 Selective inhibition of renal FGFR1 also differs from the effects of pan-FGFR inhibition that lead to inhibition of FGF-23 effects, hyperphosphatemia, and disturbed cardiovascular dysfunction.50 In addition, we observed alterations in gene expression in the DT of FGFR1DT cKO mice, but Ksp-Cre is also expressed in the collecting ducts and PTs and, consequently we cannot exclude additional contributions of loss-of-FGFR1 in other tubular segments.39,51 Thus, the hypertension and LVH induced by deletion of renal tubular FGFR1 is likely due to lack of FGFR1 in the DT, but definitive proof that other tubular segments are not contributing is lacking. Regardless, FGFR1 and FGFR/α-Klotho have unexpected paradoxic hemodynamic effects.
Our finding that FGFR1 in the kidney regulates BP is consistent with the observations that single nucleotide polymorphisms in FGF1 and FGFBP1 and elevation of FGF1 and FGFBP1 kidney expression are associated with familial susceptibility to hypertension in humans.37,38 Because activation of FGFR1 without α-Kl is characteristic of paracrine/autocrine FGF ligands, it is likely that locally produced FGFs are physiologic regulators of FGFR1’s hemodynamic effects. Indeed, several FGFs, including FGF1, 2, 9, 12, and 18, are known to be expressed in the adult kidney,5,52 and FGF2 is detected in the urine.53,54
The mechanisms underlying these apparent paradoxic cardiovascular effects of hormonal FGF-23/FGFR/α-Klotho and paracrine FGFR1 activation in the kidney are not clear. The renal expression of Ace2 was decreased and NKCC2 in the tAL was increased in FGFR1DT cKO mice. Both could contribute to higher systemic BP by respectively elevating levels of angiotensin II55 or increasing Na reabsorption,56 but we did not observe changes in serum aldosterone levels, as seen with FGF-23–induced Na reabsorption and hypertension.9 Increase in BP might also induce LVH due to pressure overload, which is associated with upregulation of local RAS expression in the heart.57
Our studies also do not determine the molecular mechanisms responsible for LVH observed in FGFR1DT cKO mice, which could be due to hypertension per se or to an alternative novel renal FGFR1–sKl–cardiac TRPC6 axis. Evidence that sKl is mediating the cardiac hypertrophy in FGFR1DT cKO mice is supported by decreased α-Klotho expression in the kidney, increased TRPC6 expression in the heart, and effects of recombinant sKl administration to rescue hypertension in Hyp mice. These findings are consistent with the observations that sKl has cardioprotective effects by downregulating TRPC6 channels in cardiomyocytes.44,51,58–60 In addition, kidney-specific deletion of α-Klotho causes salt-sensitive hypertension in mice,61 and administration of sKl inhibits RAS and normalizes BP in mouse models of kidney diseases, suggesting additional mechanisms of sKl actions.62 The cardioprotective effects of α-Klotho, however, are controversial, and transgenic mice with the global overexpression of transmembrane α-Klotho resulted in worsening of Ang II–induced cardiomegaly.58,63 The opposite cardiovascular effects of Klotho may reflect differences in the functions of transmembrane α-Klotho, acting as an FGFR cofactor, and the hormonal actions of sKl that are FGFR independent.64 Regardless, the respective pathogenic roles of hypertension and sKl-dependent upregulation of TRPC6 in the cardiomyocytes of FGFR1DT cKO mice remain to be determined.
These findings have several clinical implications. First, our finding that loss-of-FGFR1 in the DT causes hypertension in mice provides a mechanism to explain unwanted hypertensive effects of FGFR antagonists being developed to treat certain types of cancers.50,65,66 Second, the antihypertensive effects of R1MAb1 suggest that activation of FGFR1 in the DT is a potential therapeutic target for treating hypertension. These antihypertensive effects were present even in the setting of FGFR-mediated elevations in FGF-23 concentrations.45,67 High levels of FGF-23 in Hyp mice are also not sufficient to prevent the antihypertensive effects of R1Mab1, indicating that the hemodynamic effects of R1Mab1 activation of FGFR1 predominate over FGF-23 activation of FGFR/α-Klotho complexes.
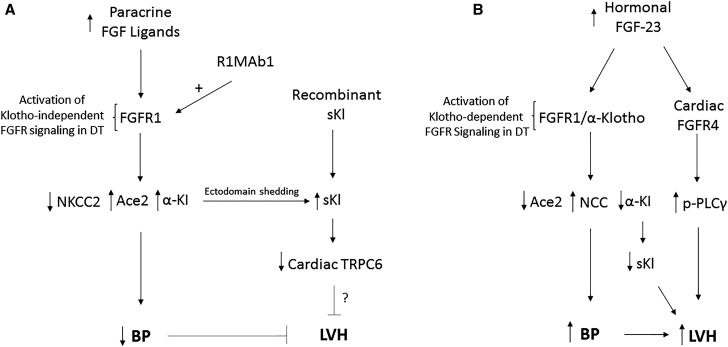
In summary, genetic deletion of FGFR1 in the kidney, likely due to loss of FGFR1 in the DT, leads to hypertension and LVH, whereas an FGFR1-activating antibody, R1MAb1, lowers BP in a hypertensive mouse model, and reverses hypertension observed in the Hyp mouse model of FGF-23 excess. Our findings suggest a new schema whereby FGFR1 in renal tubules plays a physiologic role in regulating systemic hemodynamics in response to paracrine FGFs in the kidney (Figure 9). Additional studies are needed to define the specific intrarenal FGF-ligands activating FGFR1, and how they are regulated and integrated with other networks controlling cardiovascular homeostasis. Also, the relative contributions of renal Na reabsorption, Ace2, and sKl in the cardiovascular responses need to be determined. Although the effects of FGFR1 to regulate BP through control of Na reabsorption and Ace2 are a possible mechanism to explain the cardiovascular effects of FGFR1 in the kidney, a more exciting and novel possibility is that paracrine activation of FGFR1 increases circulating sKl that exerts cardioprotective effects through inhibition of TRPC6 functions in the heart (i.e., a novel reno-cardiac axis) (Figure 9). Although the cardiac gene expression profile in FGFR1DT cKO mice is consistent with the reported direct actions of sKl to regulate cardiac hypertrophy, additional studies are needed to prove the importance of the proposed FGFR1-sKl–cardiac TRPC6 axis. Finally, the mechanisms mediating the opposite cardiovascular effects of paracrine FGFs and hormonal activation of FGFRs by FGF-23 in the kidney remain to be explained. Differential regulation of sKl by paracrine FGFR and FGFR/α-Klotho signaling might also account for these respective cardioprotective and cardiotoxic effects of FGFR1 activation and FGF-23. Regardless, this new schema suggests that developing therapeutic approaches to activate FGFR1-dependent pathways in the kidney might provide a novel way to treat hypertension and paradoxically prevent the adverse cardiovascular effects of excess FGF-23.
Figure 9.
Schematic showing opposite effects on hemodynamic response induced by paracrine FGF activation of FGFR1 in the renal distal tubule (DT) and FGF-23 activation of FGFR1/α-Klotho. (A) Novel scenario showing that paracrine FGF ligands released locally in the kidney, possibly from the glomerulus and/or renal tubules, in response to yet-to-be-defined stimuli (such as changes in hemodynamics or solute delivery) activate FGFR1, independently of α-Klotho, likely in the DT, leading to decreased tubular Na reabsorption (downregulation of NKCC2 expression), increased Ace2 expression that degrades Ang II, and elevated circulating sKl levels that alter TRPC6 expression in the heart. The cardioprotective effects could be due to reductions in BP via effects on Na reabsorption and Ace2 or through effects of sKl to regulate TRPC6 in the heart. (B) FGF-23 activates canonic FGFR1/α-Klotho complexes in the DT to increase NCC-dependent Na reabsorption, and to decrease Ace2 and sKI, leading to hypertension and LVH. FGF-23 is also purported to have direct effects on FGFR4 in the heart to stimulate LVH. The molecular mechanisms mediating the opposite cardiovascular effects of FGFR1 activation in the kidney by paracrine FGF ligands and activating FGFR1 antibodies (cardioprotective) and FGF-23 activation of FGFR/α-Klotho complexes in the kidney (cardiotoxic) remain to be defined.
Concise Methods
Animal Experiments
All animal research was conducted according to guidelines provided by the National Institutes of Health and the Institute of Laboratory Animal Resources, National Research Council. The University of Tennessee Health Science Center’s Animal Care and Use Committee or the Institutional Animal Care and Use Committee at Genentech reviewed and approved all animal studies (protocol number: 15–128.0 and #10–1033).
Animal Breeding and Genotyping
CMV-Cre mice were originally purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained on C57BL/6J background. Female db/db mice on BKS background were also purchased from the Jackson Laboratory. The floxed Fgfr1 mice (FGFR1 flox/flox) were obtained from Dr. Chuxia Deng as reported previously.8 Ksp-Cre39 mice were used to delete the floxed FGFR1 in kidney as described previously.8 Power calculations to determine minimal sample size were performed as previously described.68
Echocardiography and BP Measurement
Noninvasive ultrasound examination of the cardiovascular system was performed using a Vevo 2100 Ultrasound System (VisualSonics Inc., Toronto, Ontario, Canada) following standard procedures. BP was measured using the mouse tail-cuff technique (CODA, Kent Scientific), except for Ob/Ob mice, in which it was measured under anesthesia using Biopac BP collection equipment.
Pharmacologic Interventions
Recombinant anti-FGFR1 R1MAb1 antibody and isotype control trastuzumab (anti–human Her2), which does not react with mouse Her2, were produced in CHO cells and purified to homogeneity in PBS by Genentech, as previously described.69 Female db/db mice received 3 mg/kg ip injection of R1MAb1 or the control trastuzumab. For studies using WT, FGFR1DT-cKO, or Hyp mice, mice (4 weeks old) received 3 mg/kg ip injection of R1MAb1 or the control IgG on day 0. On day 28, BP was measured using the mouse tail-cuff technique (CODA). For studies using Hyp, mice (4 weeks old) received 0.01 mg/kg ip injection of sKl (R&D Systems) or PBS on day 0 for 4 consecutive days,58 and then BP was measured. For recombinant FGF-23 (rFGF-23) administration, mice were injected ip with 50 ng/g rFGF23 (Amgen) or with vehicle (PBS) twice daily for 1 or 5 days.
Serum and Urine Biochemistry
Blood samples were collected by retro-orbital bleeding at the end point. Urine samples were collected overnight (from 6:00 pm to 6:00 am) from mice housed in metabolic cages. FGF-23 and phosphorus were measured as described previously.8 Na was measured using a Sanbio Sodium kit. Serum aldosterone was determined using an Aldosterone ELISA kit from Abcam.
Immunohistochemistry and Morphometry of Mouse Heart
Formalin-fixed heart and kidney samples were embedded in paraffin and immunohistochemical staining performed as described previously.8 Primary antibodies (Klotho antibody, R&D Laboratory, Minneapolis, MN; FGFR1, Cell Signaling Technology; TRPC6 and TRPV5 antibody, Alomone Labs, Jerusalem, Israel; ACE2 antibody, Abcam; NKCC2 antibody, EMD Millipore Corporation, CA) and secondary antibodies (Alexa Fluor 568 or 488, Life Technologies) were used in these studies. To visualize cellular borders, fixed tissue was stained with wheat germ agglutinin (WGA) conjugated to Alexa Fluor555 (Invitrogen) at 1 mg/ml in PBS containing 10 mm Na azide. ImageScope software was used to quantify cross-sectional area of 30 cells at four fields along the mid-chamber free wall on the basis of WGA-positive staining. To examine myocardial fibrosis, short-axis cardiac sections from mice were stained with picrosirius red solution (Sigma-Aldrich) for 1 hour.
Real Time Reverse Transcription–quantitative PCR and Western Blot Analysis of Gene Expression
Total RNA was isolated from whole kidney and heart of mice at 3 months of age using an RNeasy Mini Kit (Qiagen, Germany). Primers for quantitative real-time PCR of genes including FGFR1, Klotho, TRPC6, Ace2, renin, angiotensinogen, NCC, NKCC2, and GAPDH are listed in Supplemental Table 1.8 Primers used for quantitative real-time PCR of genes including α-MHC, β-MHC, ANP, BNP, fibronectin, col1α2, col5α1, and timp 1 were described previously.49 Relative expression values were evaluated with the 2−ΔΔCt or 2−ΔCt method using GAPDH as housekeeping gene. Western blot analysis of Klotho, TRPC6, Ace2, NCC, and NKCC2 was performed using the antibodies as described earlier.
Detailed experimental methods are presented in the Supplemental Material.
Statistical Analyses
Statistical analyses were performed using a two-tailed t test. All values are expressed as mean±SD. All statistical tests are performed with an α of 0.05 as the significance threshold. All computations were performed using GraphPad Prism5 (GraphPad Software Inc., La Jolla, CA).
Disclosures
J.R., G.K., and J.S. are present or former paid employees of Genentech/Roche. Part of the work was funded by Genentech, Inc. The authors have declared that no other conflict of interest exists. X.H., M.P., G.K., and L.D.Q. have no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Genentech colleagues for technical support including antibody production and mouse husbandry.
This work was supported by grant R01-AR045955 to L.D.Q. from the National Institutes of Health and by Genentech.
X.H. contributed to the experimental design, conducted and coordinated the study, analyzed data, and contributed to writing the paper. J.S. contributed to studies using FGFR1 activating antibody in db/db mice (Supplemental Figure 3), data analysis, and paper editing. J.R. and G.K. contributed to studies using FGFR1 activating antibody in db/db mice (Supplemental Figure 3). M.P. contributed to experiments using rFGF-23 (Supplemental Figure 1). G.K. contributed to klotho antibody and klotho immunohistochemical staining in kidney. L.D.Q. conceived the study, led the program, and wrote the paper.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017040412/-/DCSupplemental.
References
- 1.Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD: Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278: 37419–37426, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Quarles LD: Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest 112: 642–646, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cancilla B, Davies A, Cauchi JA, Risbridger GP, Bertram JF: Fibroblast growth factor receptors and their ligands in the adult rat kidney. Kidney Int 60: 147–155, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Martin A, David V, Quarles LD: Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab 300: E508–E517, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quarles LD: Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Yang J, Li L, Huang J, King G, Quarles LD: Conditional deletion of Fgfr1 in the proximal and distal tubule identifies distinct roles in phosphate and calcium transport. PLoS One 11: e0147845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG: FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6: 744–759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG: FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 33: 229–246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG: FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51: 621–628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quarles LD: FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab 285: E1–E9, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M: Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu HJ, Wu MS: Fibroblast growth factor 23: A possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337: 116–122, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Jean G, Bresson E, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant 24: 948–955, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Quarles LD. FGF23 from bench to bedside. Am J Physiol Renal Physiol 310: F1168–F1174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandenburg VM, Kleber ME, Vervloet MG, Tomaschitz A, Pilz S, Stojakovic T, Delgado G, Grammer TB, Marx N, März W, Scharnagl H: Fibroblast growth factor 23 (FGF23) and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 237: 53–59, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MS, DeRosa JT, Silverberg SJ, Mendez AJ, Dong C, Wright CB, Wolf M: Fibroblast growth factor 23 and cause-specific mortality in the general population: The Northern Manhattan Study. J Clin Endocrinol Metab 101: 3779–3786, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh N, Ornitz DM: Evolution of the Fgf and Fgfr gene families. Trends Genet 20: 563–569, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Eswarakumar VP, Lax I, Schlessinger J: Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16: 139–149, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Turner N, Grose R: Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer 10: 116–129, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Johnson DE, Lee PL, Lu J, Williams LT: Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol 10: 4728–4736, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahimi OA, Zhang F, Hrstka SC, Mohammadi M, Linhardt RJ: Kinetic model for FGF, FGFR, and proteoglycan signal transduction complex assembly. Biochemistry 43: 4724–4730, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Bates CM: Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol 301: F245–F251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancilla B, Ford-Perriss MD, Bertram JF: Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int 56: 2025–2039, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P: Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A 95: 5082–5087, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P: Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8: 3045–3057, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM: Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12: 390–397, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Wen X, Li X, Tang Y, Tang J, Zhou S, Xie Y, Guo J, Yang J, Du X, Su N, Chen L: Chondrocyte FGFR3 regulates bone mass by inhibiting osteogenesis. J Biol Chem 291: 24912–24921, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Vierthaler L, Tang W, Zhou J, Quarles LD: FGFR3 and FGFR4 do not mediate renal effects of FGF23. J Am Soc Nephrol 19: 2342–2350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N: Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 121: 2302–2309, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Tomaszewski M, Charchar FJ, Lynch MD, Padmanabhan S, Wang WY, Miller WH, Grzeszczak W, Maric C, Zukowska-Szczechowska E, Dominiczak AF: Fibroblast growth factor 1 gene and hypertension: from the quantitative trait locus to positional analysis. Circulation 116: 1915–1924, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB; Wellcome Trust Case Control Consortium : Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41: 666–676, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, Yin M, Coffin JD, Kong L, Kranias EG, Luo W, Boivin GP, Duffy JJ, Pawlowski SA, Doetschman T: Fibroblast growth factor 2 control of vascular tone. Nat Med 4: 201–207, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomaszewski M, Charchar FJ, Nelson CP, Barnes T, Denniff M, Kaiser M, Debiec R, Christofidou P, Rafelt S, van der Harst P, Wang WY, Maric C, Zukowska-Szczechowska E, Samani NJ: Pathway analysis shows association between FGFBP1 and hypertension. J Am Soc Nephrol 22: 947–955, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomaszewski M, Eales J, Denniff M, Myers S, Chew GS, Nelson CP, Christofidou P, Desai A, Büsst C, Wojnar L, Musialik K, Jozwiak J, Debiec R, Dominiczak AF, Navis G, van Gilst WH, van der Harst P, Samani NJ, Harrap S, Bogdanski P, Zukowska-Szczechowska E, Charchar FJ: Renal mechanisms of association between fibroblast growth factor 1 and blood pressure. J Am Soc Nephrol 26: 3151–3160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao X, Somlo S, Igarashi P: Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol 13: 1837–1846, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Olauson H, Lindberg K, Amin R, Jia T, Wernerson A, Andersson G, Larsson TE: Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23: 1641–1651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M: FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA: Myosin heavy chain gene expression in human heart failure. J Clin Invest 100: 2362–2370, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD: A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One 7: e44161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL: Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun 3: 1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu AL, Feng B, Chen MZ, Kolumam G, Zavala-Solorio J, Wyatt SK, Gandham VD, Carano RA, Sonoda J: Antibody-mediated activation of FGFR1 induces FGF23 production and hypophosphatemia. PLoS One 8: e57322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V: Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension 53: 387–392, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Hart AW, Baeza N, Apelqvist A, Edlund H: Attenuation of FGF signalling in mouse beta-cells leads to diabetes. Nature 408: 864–868, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Boudoulas KD, Triposkiadis F, Parissis J, Butler J, Boudoulas H: The cardio-renal interrelationship. Prog Cardiovasc Dis 59: 636–648, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C: Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab 22: 1020–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanochko GM, Vitsky A, Heyen JR, Hirakawa B, Lam JL, May J, Nichols T, Sace F, Trajkovic D, Blasi E: Pan-FGFR inhibition leads to blockade of FGF23 signaling, soft tissue mineralization, and cardiovascular dysfunction. Toxicol Sci 135: 451–464, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Östman Wernerson A, Lanske B, Olauson H, Larsson TE: The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 25: 2169–2175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Floege J, Hudkins KL, Eitner F, Cui Y, Morrison RS, Schelling MA, Alpers CE: Localization of fibroblast growth factor-2 (basic FGF) and FGF receptor-1 in adult human kidney. Kidney Int 56: 883–897, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Chodak GW, Hospelhorn V, Judge SM, Mayforth R, Koeppen H, Sasse J: Increased levels of fibroblast growth factor-like activity in urine from patients with bladder or kidney cancer. Cancer Res 48: 2083–2088, 1988 [PubMed] [Google Scholar]
- 54.Har R, Scholey JW, Daneman D, Mahmud FH, Dekker R, Lai V, Elia Y, Fritzler ML, Sochett EB, Reich HN, Cherney DZ: The effect of renal hyperfiltration on urinary inflammatory cytokines/chemokines in patients with uncomplicated type 1 diabetes mellitus. Diabetologia 56: 1166–1173, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Burrell LM, Johnston CI, Tikellis C, Cooper ME: ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 15: 166–169, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monette MY, Rinehart J, Lifton RP, Forbush B: Rare mutations in the human Na-K-Cl cotransporter (NKCC2) associated with lower blood pressure exhibit impaired processing and transport function. Am J Physiol Renal Physiol 300: F840–F847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwai N, Shimoike H, Kinoshita M: Cardiac renin-angiotensin system in the hypertrophied heart. Circulation 92: 2690–2696, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Hu MC, Shi M, Gillings N, Flores B, Takahashi M, Kuro-O M, Moe OW: Recombinant α-Klotho may be prophylactic and therapeutic for acute to chronic kidney disease progression and uremic cardiomyopathy. Kidney Int 91: 1104–1114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie J, Yoon J, An SW, Kuro-o M, Huang CL: Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26: 1150–1160, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN: TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Chen K, Lei H, Sun Z: Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26: 121–132, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, Liu Y: Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol 185: 3211–3223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Chen Y, McCoy CW, Zhao T, Quarles DL, Pi M, Bhattacharya SK, King G, Sun Y: Differential regulatory role of soluble klothos on cardiac fibrogenesis in hypertension. Am J Hypertens 29: 1140–1147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW, Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L, Huang CL: Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci U S A 114: 752–757, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touat M, Ileana E, Postel-Vinay S, Andre F, Soria JC. Targeting FGFR signaling in cancer. Clin Cancer Res 21: 2684–2694, 2015. [DOI] [PubMed] [Google Scholar]
- 66.Liang G, Chen G, Wei X, Zhao Y, Li X: Small molecule inhibition of fibroblast growth factor receptors in cancer. Cytokine Growth Factor Rev 24: 467–475, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD: Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One 9: e104154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charan J, Kantharia ND: How to calculate sample size in animal studies? J Pharmacol Pharmacother 4: 303–306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, Kates L, van Bruggen N, Leabman M, Wong A, West D, Stern H, Luis E, Kim HS, Yansura D, Peterson AS, Filvaroff E, Wu Y, Sonoda J: Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med 3: 113ra126, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.