Abstract
Data indicate significant phenotypic and genotypic overlap, plus a common pathogenesis, between two groups of inherited disorders, autosomal dominant polycystic kidney diseases (ADPKD), a significant cause of ESRD, and autosomal dominant polycystic liver diseases (ADPLD), which result in significant PLD with minimal PKD. Eight genes have been associated with ADPKD (PKD1 and PKD2), ADPLD (PRKCSH, SEC63, LRP5, ALG8, and SEC61B), or both (GANAB). Although genetics is only infrequently used for diagnosing these diseases and prognosing the associated outcomes, its value is beginning to be appreciated, and the genomics revolution promises more reliable and less expensive molecular diagnostic tools for these diseases. We therefore propose categorization of patients with a phenotypic and genotypic descriptor that will clarify etiology, provide prognostic information, and better describe atypical cases. In genetically defined cases, the designation would include the disease and gene names, with allelic (truncating/nontruncating) information included for PKD1. Recent data have shown that biallelic disease including at least one weak ADPKD allele is a significant cause of symptomatic, very early onset ADPKD. Including a genic (and allelic) descriptor with the disease name will provide outcome clues, guide treatment, and aid prevalence estimates.
Keywords: ADPKD, cystic kidney, liver cysts, polycystic kidney disease
Clinical and Genetic Characteristics of ADPKD and ADPLD
Disease severity in autosomal dominant polycystic kidney disease (ADPKD) is highly variable.1 Whereas half of affected individuals reach ESRD by approximately 60 years,2,3 <1% of patients exhibit very early onset (VEO) disease, with a diagnosis made in utero or during infancy.4–6 At the other end of the spectrum, patients can live a normal lifespan without requiring RRT. Most patients with ADPKD develop liver cysts as they age, with severe Polycystic liver disease (PLD) requiring surgical intervention occurring in a small minority of patients.7,8 Autosomal dominant polycystic liver disease (ADPLD) is characterized as PLD, including severe, symptomatic disease that is indistinguishable from that found in ADPKD, but without (or only occasional) renal cysts.9
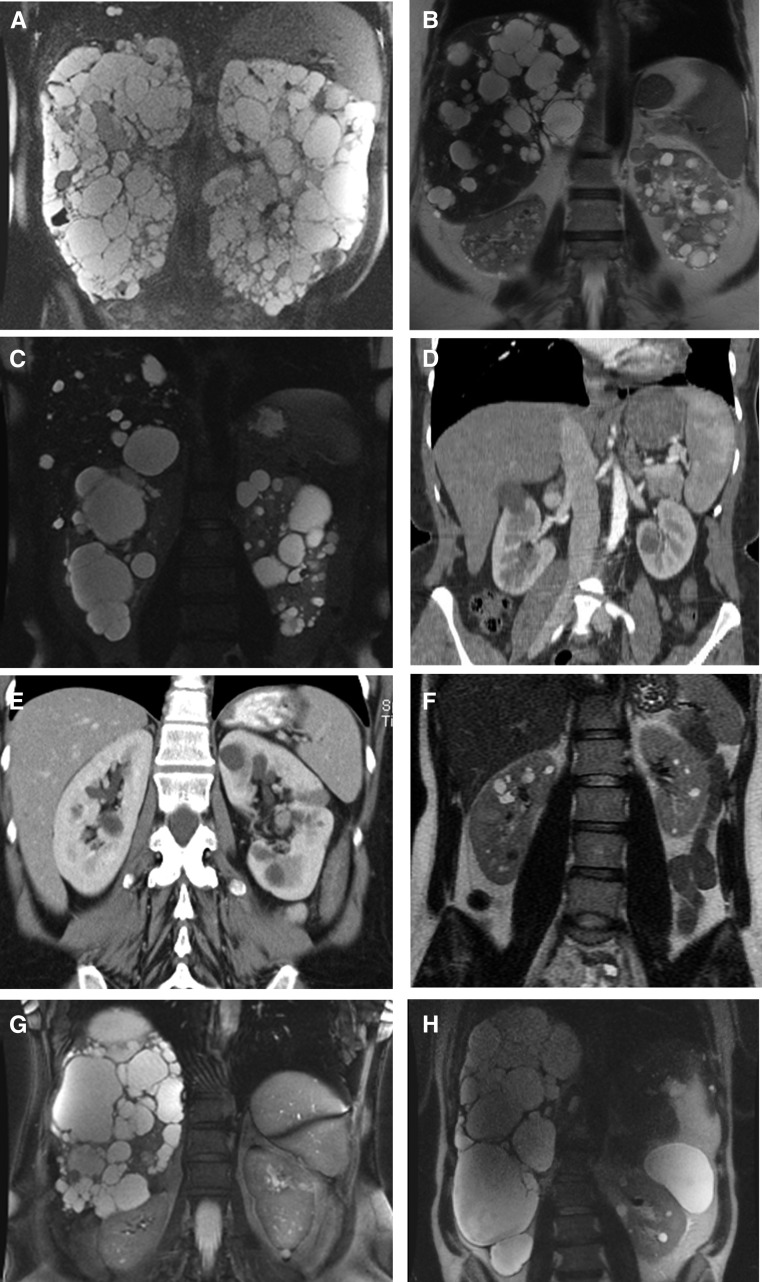
ADPKD is genetically heterogeneous, with two major genes, PKD1 (Chr. 16.p13.3; approximately 78% families) and PKD2 (4p21; approximately 15%), and a rare third locus, GANAB (11q12.3; approximately 0.3%), discovered last year.3,10–15 For ADPLD, PRKCSH (19p13.2; approximately 20%) and SEC63 (6q21; approximately 15%) are the major genes, but LRP5 (11q13.2), GANAB (approximately 2%), ALG8 (11q14.1; approximately 3%), and SEC61B (9q22.33; approximately 1%) have more recently been associated with ADPLD (Table 1).15–20 The difference in renal survival between PKD1 and PKD2 patients has been highlighted in multiple studies (Table 2).3,21 In addition, PKD1 patients have a larger height-adjusted total kidney volume (HtTKV; an early measure of the severity of renal disease in ADPKD) and lower eGFR than PKD2 patients.14,22 A further difference is the number of kidney cysts, with fewer in PKD2 than PKD1 (Figure 1, A and C), although the rate of growth of the kidneys does not differ between the groups.23 Occasionally, severe PLD can be the major clinical phenotype in ADPKD (Figure 1, B and G). PRKCSH and SEC63 have not been clinically differentiated and majorly consist of PLD without renal cysts, although a few kidney cysts have been described in 28%–35% of cases, and renal involvement may have been underestimated (Figure 1H).9 Missense mutations to LRP5 have been identified in four families, two with moderate-to-severe PLD without renal cysts, one with moderate PLD and three kidney cysts, and one with mild PLD but severe PKD in one family member.19 The renal prognosis in GANAB is consistently mild with progression to ESRD unlikely (Figure 1E), although all patients had some kidney cysts; predominant liver involvement was described in 4 of 11 families.15,20 Inactivating mutations to ALG8 were recently shown to cause ADPLD in five pedigrees, four with severe PLD and some kidney cysts, but one patient only had renal cysts.20 Mutations to SEC61B were identified in two families with mild PLD and no apparent renal phenotype.20
Table 1.
Classification of ADPKD and ADPLD including gene descriptor
| Designationa | Description | Phenotype | Example | ||
|---|---|---|---|---|---|
| PKD | ESRD Risk (Age) | PLD | |||
| ADPKDb | |||||
| ADPKD-PKD1T | ADPKD due to PKD1 truncating mutationc | Severe | Very high (55 yr) | Absent to severe | Figure 1A, Table 2 |
| ADPKD-PKD1NT | ADPKD due to PKD1 nontruncating mutationd | Mild to severe | High (67 yr) | Absent to severe | Figure 1, B and G, Table 2 |
| ADPKD-PKD1 ULP | ADPKD due to PKD1 ultra low penetrance allele | Extremely mild | None | Absent to severe | Figure 1D, Table 4 |
| ADPKD-PKD2 | ADPKD due to PKD2 mutation | Mild | Low (79 yr) | Absent to severe | Figure 1C, Table 2 |
| ADPKD-GANAB | ADPKD due to GANAB mutation | Very mild | None | Absent to severe | Figure 1E, Table 2 |
| ADPLDe | |||||
| ADPLD-PRKCSH | ADPKD due to PRKCSH mutation | Absent to very mild | None | Mild to severe | Figure 1H |
| ADPLD-SEC63 | ADPKD due to SEC63 mutation | Absent to very mild | None | Mild to severe | 18 |
| ADPLD-LRP5 | ADPKD due to LRP5 mutation | Absent to very mild | None | Mild to severe | 19 |
| ADPLD-GANAB | ADPLD due to GANAB mutation | Very mild | None | Mild to severe | Table 2 |
| ADPLD-ALG8 | ADPLD due to ALG8 mutation | Absent to very mild | None | Absent to severe | 20 |
| ADPLD-SEC61B | ADPLD due to SEC61B mutation | Absent to very mild | None | Mild to severe | 20 |
| Biallelicf | |||||
| ADPKD-PKD1a,b | Typical ADPKD due to two PKD1 alleles | Severe | High to very high | Absent to severe | Table 4 |
| ADPKDVEO-PKD1a,b | Symptomatic infantile ADPKD due two PKD1 alleles | Severe infantile | Extremely high | Absent to mild | Table 4 |
| ADPKDVEO-PKD2a,b | Symptomatic infantile ADPKD due two PKD2 alleles | Severe infantile | Extremely high | Absent to mild | Table 4 |
T, truncating; NT, nontruncating.
Examples are shown to illustrate the disease plus gene terminology but other combinations are possible.
ADPKD is defined as dominantly inherited disease with significant PKD,31,32 with or without significant PLD, and without phenotypes suggesting an alternative disease. Subjects with a proven pathogenic ADPKD allele, even if the renal disease does reach the imaging criteria, would be considered ADPKD.
Frameshifting deletion, duplication or insertion, nonsense, typical splicing mutation (disrupting the canonic intronic dinucleotides), or inframe deletion, duplication or insertion of ≥5 amino acids.
Missense, atypic splicing mutation (not disrupting the canonic intronic dinucleotides), or inframe deletion, duplication, or insertion of ≤4 amino acids.
ADPLD is defined as dominantly inherited disease with significant PLD without significant PKD.
PKD due to two mutations to the same ADPKD gene found on opposite alleles (in trans).
Table 2.
Age at ESRD in the different ADPKD subgroups
| Disease | Number of Patients (Number of Pedigrees) | Median Age at ESRD, yr | Reference, Year of Publication |
|---|---|---|---|
| ADPKD-PKD1T | 219 (54) | 54 | 59, 2002 |
| 701 (450) | 55.1 | 36, 2016 | |
| 249 (72) | 52.5 | 55, 2016 | |
| ADPKD-PKD1NT | 323 (228) | 65.8 | 36, 2016 |
| 152 (51) | 70.8a | 55, 2016 | |
| ADPKD-PKD2 | 291 (31) | 74 | 21, 1999 |
| 395 (71) | 72 | 85, 2003 | |
| 117 (23) | 70 | 101, 2009 | |
| 248 (172) | 77.8 | 36, 2016 | |
| 213 (57) | 80 | 55, 2016 | |
| 293 (203) | 77.8 | 97, 2017 | |
| ADPKD-GANAB | 22 (11) | No cases of ESRD described | 15, 2016 |
| 20, 2017 |
TGESP PKD1NT group included nonsynonymous missense mutations but excluded patients with inframe short deletion or insertion.
Figure 1.
These eight kidney and liver images illustrate the different genic and allelic forms of ADPKD and ADPLD, and the most frequent differential diagnosis. (A) ADPKD-PKD1T: MRI of a 45-year-old male patient with a truncating PKD1 mutation (c.4880_4883delATGT; p.Tyr1627fs), who has an eGFRCKDEPI=15.1 ml/min per 1.73 m2 and HtTKV=3853 ml/m. (B) ADPKD-PKD1NT: MRI of a 55-year-old woman with a PKD1 nontruncating mutation (c.2180T>C, p.Leu727Pro) with well preserved kidney function (eGFR=63.4 ml/min per 1.73 m2) and HtTKV=439 ml/m. Significant PLD is present. (C) ADPKD-PKD2: MRI of a 53-year-old man, with a PKD2 mutation (c.1057G>A, p.Glu353Lys), who has well preserved kidney function (eGFR=68.6 ml/min per 1.73 m2) and HtTKV=1245 ml/m. Note that a few large cysts in the right kidney largely explain the enlarged HtTKV. (D) ADPKD-PKD1 ULP: Contrast-enhanced CT-scan of a 48-year-old woman, with a ULP PKD1 nontruncating mutation (c.9829C>T, p.Arg3277Cys), who has a total of five cysts in the right kidney and one cyst in the left kidney, a normal eGFR, and nonenlarged kidneys. (E) ADPKD-GANAB: CT-scan of 45-year-old woman with a GANAB mutation (c.1914_1915delAG; p.Asp640fs) who has bilateral kidney cysts, a normal eGFR (104 ml/min) and HtTKV (318 ml/m), and without significant PLD. (F) A common differential diagnosis: ADTKD:HNF1B. MRI of 30-year-old woman with a large deletion of the entire HNF1B gene, who has preserved kidney function (75.5 ml/min per 1.73 m2) and approximately ten cysts per kidney (HtTKV=196.9 ml/m). (G) ADPKD-PKD1NT MRI of 54-year-old woman with severe PLD (HtTLV=4695 ml/min; liver resection performed after this MRI) and normal-sized kidneys, with a total of 12 bilateral small cysts. A PKD1NT allele was identified (c.3284A>G, p.Tyr1095Cys), with no mutation identified in other ADPLD genes. (H) ADPLD-PRKCSH MRI of a 49-year-old man with predominant PLD (HtTLV=7271 ml/min; liver resection was performed after this MRI) and mild PKD (eight cysts in the left kidney). A right nephrectomy was performed at 42 years (atrophic cystic kidney with suspected malignancy). The PRKCSH (c.1386T>G, p.Tyr462*) mutation was identified. MRI, magnetic resonance imaging; CT, computed tomography.
A loss-of-function genetic mechanism (due to a dosage/threshold or somatic second hit mechanism) has been established for these diseases.24 Studies of pathogenesis of ADPKD and ADPLD also show a commonality with all ADPLD proteins (except LRP5) involved in the glycosylation, quality control assessment, or translocation across the ER membrane of membrane/secreted proteins.15,20,25 A renal cystic phenotype is induced by inactivation of Prkcsh or Sec63 in the kidney, which can largely be rescued by Pkd1 overexpression.26 The action of these genes, and of GANAB, ALG8, and SEC61B, seems to be through inefficient maturation and trafficking of membrane/secreted proteins, with the PKD1 protein, polycystin-1 (PC1), particularly susceptible to dosage reduction of any of these proteins.15,20,26,27
An Increasing Role for Routine Genetic Analysis in ADPKD and ADPLD?
Molecular genetics has been performed routinely for several years in the management of a number of monogenic disorders, such as cystic fibrosis, where it is used for diagnostics and also recently to guide allelic-specific therapeutics.28,29 Genetic testing is part of either the diagnosis workup or the therapeutic decision-making algorithms of several kidney diseases, including steroid-resistant FSGS, atypic hemolytic uremic syndrome, and Alport syndrome.30 The introduction of next-generation sequencing (NGS) has allowed the development of cost- and time-efficient strategies, often including screening panels of candidate genes or whole-exome sequencing, improving diagnostics, providing prognostic value, and broadening the phenotypic spectrum of many kidney diseases.30
Genetics is presently rarely used for routine diagnostics and prognostics in ADPKD and ADPLD, with imaging much more commonly employed for these purposes.22,31–34 However, because new methods are revolutionizing molecular diagnostics, increasing availability, and reducing costs35—and the prognostic value is starting to be realized36—we propose that genetic information can play a more central role in the management of patients with ADPKD and ADPLD.
The Diagnosis Value of Molecular Genetics in ADPKD
Simple renal cysts (not considered to be of germline genetic origin) are commonly observed in the general population and increase in frequency with age.37,38 Consequently, age-related cyst number criteria have been determined for ultrasound (U/S), or the more sensitive MRI, to diagnose ADPKD, or to exclude a diagnosis in the setting of a positive family history.31,32 As an example, >10 total cysts detected by MRI are considered sufficient for a positive diagnosis in subjects <40 years. These criteria work well in the setting of a positive family history if multiple bilateral cysts (>10 per kidney) are detected, or if no cysts are detected in an older individual. However, if the family history is negative (10%–25% of ADPKD families39,40) and/or when few cysts are detected, the imaging-based diagnosis is less clear. This uncertainty is exemplified by GANAB, or patients with ADPKD due to an ultra-low penetrant (ULP) PKD1 allele (Figure 1, D and E), where only a small number (or even no) cysts might be detected, although genetically they are proven affected.15,41,42 It might be considered unimportant to diagnose these individuals because they are unlikely to develop renal insufficiency. However, the consequences of employing such a patient as a kidney donor in terms of their long-term renal function is unknown; these patients can have clinically significant extrarenal complications themselves or in offspring, such as PLD in GANAB,15,20 and evidence that biallelic disease, the coincidence of a ULP PKD1 allele in trans with a fully inactivating PKD1 allele, can result in early onset PKD (see later section), suggests that obtaining a firm diagnosis is important.4,41–45
Genetic Diagnostics in ADPKD and ADPLD: Prognostics, Complications, and Prospects
Genetic testing in the clinical management of these diseases has been overlooked for several reasons. First, analysis of PKD1 has been complicated due to its location in a segmentally duplicated region of the genome; three-quarters of the gene, 5′ to exon 1 to exon 33, is duplicated six times more proximally on chromosome 16, where they encode pseudogenes with high sequence similarity to PKD1.10,46,47 Hence, long-range PCR methods have been employed to specifically amplify PKD1 for molecular diagnostics by Sanger or NGS, with doubts that this gene is adequately covered by whole-exome sequencing methods, limiting the availability and increasing the costs of testing.39,48–51 However, the development of specific NGS protocols to screen PKD1 by hybridization capture methods and diversity in the groups offering clinical ADPKD molecular diagnostics is likely to increase its availability and reduce costs, even if specificity and sensitivity data are not yet widely available.52,53 Second, although both diseases are genetically heterogeneous (Table 1), because the majority of clinically significant renal patients are PKD1 the value of testing is perceived as limited. However, evidence of the incomplete penetrance of many nontruncating PKD1 alleles (not fully inactivating the protein and so associated with milder kidney disease, also termed hypomorphic) has shown potential prognostic value, and is changing this perception.3,14,41,54,55 Third, there is extreme allelic heterogeneity, with >1500 different PKD1, >250 PKD2, >30 PRKCSH, and >35 SEC63 pathogenic mutations described (with fewer for the other genes), necessitating analysis of the coding regions of all genes for comprehensive analysis.9,20,56 Sanger-based single gene approaches were hence costly and cumbersome, but with NGS panel approaches simultaneous screening of all genes is possible. Fourth, because 30%–35% of PKD1 pathogenic alleles are nontruncating (with significant levels at the other loci), it has proved a challenge to differentiate pathogenic from neutral changes, and their degree of penetrance.3,14 The development of better algorithms for predicting the pathogenicity and penetrance of nontruncating gene variants, collation of information on these variants (pathogenic to neutral), and more informative diagnostic reports will increase the value of molecular diagnostics in these disorders.14,56,57
Introducing a Disease, Plus Gene and Allelic Terminology for ADPKD and ADPLD
In autosomal dominant tubulointerstitial kidney disease (ADTKD), where there is also genetic and phenotypic heterogeneity, a terminology describing the disease and the genetic etiology has been adopted (i.e., ADTKD-MUC1, ADTKD-UMOD, ADTKD-HNF1B, and ADTKD-REN).58 We propose to introduce a similar nosology for ADPKD/ADPLD (see Table 1 for details). Patients without a genetic diagnosis (untested or unresolved) would simply be termed ADPKD or ADPLD, depending on the phenotype. This description of the disease phenotype and etiology immediately provides renal prognostic information (Table 1).
For most ADPKD and ADPLD genes there is no evidence of an allelic influence on the phenotype in the present small populations. However, there is strong evidence of allelic effects in ADPKD-PKD1. Although older data on relatively small populations suggested a role for PKD1 mutation position influencing renal disease severity,59 more recent data from larger studies have convincingly shown that mutation type is the key factor.3,14,55 The Genkyst study demonstrated that patients with PKD1 mutations predicted to truncate the protein experienced ESRD >12 years earlier than patients with nontruncating mutations (see Table 1 footnote for definitions and Table 2 for details).3 Because mutation type provides potential prognostic information, we propose to include this grouping in the nosology: ADPKD-PKD1T and ADPKD-PKD1NT (Figure 1, A and B, Table 1). The importance of mutation type was confirmed in a Canadian cohort, where ADPKD-PKD1T patients had larger htTKV, and in the HALT PKD clinical trial, where this group had a lower eGFR.14,55 The reason for this phenotypic effect is that some ADPKD-PKD1NT mutations have residual functional PC1.54,60,61 However, in ADPKD genic or allelic information less clearly define the development of severe PLD, leading to ADPKD-PKD1NT, ADPKD-PKD2, and ADPKD-GANAB patients with mild renal disease and severe PLD, where modifying effects beyond the disease-causing gene have a significant influence (Figure 1, B and G).8
VERY EARLY ONSET (VEO) ADPKD
When screening children at risk for ADPKD, renal cysts are detected in a majority of affected individuals, even in early childhood, but they do not necessarily implicate a more severe prognosis.62 Conversely, early diagnosis can be secondary to early symptoms, with “symptomatic” children diagnosed in utero or during childhood at risk of early hypertension, urologic events, reduced eGFR, and ESRD.4–6,63,64 We propose to use the term ADPKDVEO when the diagnosis is made in utero or before 18 months, and ADPKDEO when the diagnosis is made before 15 years, in both cases excluding asymptomatic individuals diagnosed by systematic familial screening or incidentally (see Table 3 for proposed diagnosis criteria).
Table 3.
Suggested diagnosis criteria for VEO and early onset ADPKD
| Diagnostic Criteria |
|---|
| ADPKDVEO |
| In utero |
| Oligohydramnios |
| Hyperechoic enlarged kidneys (kidney length >2 SD) |
| Between birth and 18 mo of age |
| Enlarged palpable kidneys |
| Plus at least one of the following criteria |
| BP>95th percentile (or being under antihypertensive therapy) |
| GFRa<90 ml/min |
| Persistent and overt proteinuriab |
| ADPKDEO |
| Between 18 mo of age and 15 yr, at least one of the following criteria |
| Enlarged palpable kidneys |
| BP>95th percentile (or being under antihypertensive therapy) |
| GFRa<90 ml/min |
| Persistent and overt proteinuriab |
Calculated using the Bedside Schwartz Equation (2009).102
Urine excretion >4 mg/m2 per hour or spot proteinuria >0.5 g/g of creatinine, confirmed on at least two samples.
In a proportion of these cases a specific cause of biallelic disease has been demonstrated; for instance, coinheritance of the familial PKD1 mutation in trans with a second ULP PKD1 allele (Tables 1 and 4).4,41,43–45,48 Monoallelic ULP patients are unlikely to develop renal insufficiency but may development a small number of cysts in adulthood (Figure 1D). The best characterized such allele is PKD1: p.Arg3277Cys (Table 4).4,41,42,45,54 The combination of two ULP PKD1 alleles can also result in ADPKDVEO with an apparent negative family history (cysts undetected in the monoallelic parents—often with lower resolution U/S analysis), or adult onset ADPKD (Tables 1 and 4).41,42,45 This mechanism can involve PKD2; described as homozygosity of a PKD2 hypomorphic allele arising through uniparental disomy.65 Inference from mouse studies, and lack of described human patients, indicates that biallelism of fully inactivating PKD1 or PKD2 mutations is not compatible with life,66,67 showing that these ULP alleles have residual function and indicating that a dosage/threshold mechanism best explains ADPKD.24,54,68,69
Table 4.
Ultra low penetrant (ULP) alleles of PKD1 and PKD2 involved in biallelic ADPKD
| ULP Allelea | Other Mutant Allele | Phenotype Description, Age at Phenotype | Ref. |
|---|---|---|---|
| PKD1 c.9829C>T (p.Arg3277Cys) | None | Pedigree M34–6 relatives, 0–7 kidney cysts, 28–79 yrb | 41 |
| PKD1 c.9829C>T (p.Arg3277Cys) | Pedigree M34- ESRD in two siblings, 62 and 75 yr | 41 | |
| Pedigree PK10362- ESRD 76 yr | 36 | ||
| Pedigree PK11509- ESRD 48 yr | 36 | ||
| PKD1 ex1–5del | EHK, 22 pw (TOP) | 4 | |
| PKD1 c.6472C>T (p.Gln2158*) | EHK (>10 SD), at birth; HTN, 5 m; eGFR=56, 17 yr | 41 | |
| PKD1 c.6658C>T (p.Arg2220Trp)c | Two siblings EHK, RD; HTN 0 m, eGFR=70 or 65 at 1 or 8 yr | 42 | |
| PKD1 c.7483T>C (p.Cys2495Arg) | EHK, 17 pw, birth MEK, SC 2.1, 4 d | 45 | |
| PKD1 c.9563A>G (p.Asn3188Ser)c | PKD1 c.9563A>G (p.Asn3188Ser) | Father, BCK, Sibling 1, EHK, 22 pw, eGFR 67, 15.5 yr | 41 |
| Sibling 2, BCK 9 yr, eGFR 86, 15 yr | |||
| PKD1 c.5848G>A (p.Val1950Met)c | PKD1 c.8362_8363ins34 (p.Ser2788fs) | EHK, OH; double nephrectomy, RD, neonatal demise | 44 |
| PKD1 c.[3133C>G;4709C>T] (p.[Val1045Met;Thr1570Met]) | PKD1 c.[3133C>G;4709C>T] (p.[Val1045Met;Thr1570Met]) | Sibling 1- EHK, ESRD 8.5 yr | 42 |
| Sibling 2- EHK, 18 pw (TOP) | |||
| None | Grandfather: Few renal cysts at age? | ||
| PKD1 c.5305C > T (p.His1769Tyr) | PKD1 c.6727C > T (p.Gln2243*) | Sibling 1- MEK, ESRD 29 yr | 103 |
| Sibling 2- MEK, eGFR 91, 25 yr | |||
| None | Father: 7 small renal cysts, 58 yr | ||
| PKD1 c.6763C>T (p.Arg2255Cys) | PKD1 c.8259C>G (p.Tyr2753*) | EK, CRI, age? | 43 |
| None | Sibling 1- SIE, age?; Sibling 2- SIE, few cysts, age? | ||
| PKD1 c.12413G>A (p.Arg4138His)c | PKD1 c.4199del (p.Leu1400fs) | two siblings, MEK at birth, RD, HTN | 43 |
| PKD1 c.3828G>A (p.Val1274Met)c | PKD1 c.3828G>A (p.Val1274Met) | Sibling 1- EHK, neonatal, cyst, 8 yr, HTN, Sibling 2- EHK, multiple cysts, 7 yr, Sibling 3- EHK, multiple cysts, 17 m | 43 |
| PKD1 c.9884A>G (p.Asn3295Ser) | PKD1 c.10232G>A (p.Trp3411*) | EHK, 22 pw | 4 |
| PKD1 c.9548G>A (p.Arg3183Gln) | PKD1 c.11614G>T (p.Glu3872*) | EHK (6 SD), 22 pw | 4 |
| PKD1 c.12161C>T (p.Ser4054Phe) | PKD1 c.7978G>T (p.Asp2660Tyr) | EHK (4 SD), 26 pw | 4 |
| PKD1 c.11834C>T (p.Thr3945Met)c | PKD1 c.2180T>C (p.Leu727Pro) | EHK (14 SD), 15 pw (TOP) | 4 |
| PKD1 c.8129C>A (p.Thr2710Asn) | PKD1c.1010_1013dup (p.Leu339Glyfs*33) | EHK (3 SD), 17 pw | 4 |
| PKD1 c.5830G>A p.Gly1944Arg)c | PKD1c.5517G>T (p.Trp1839Cys) | EHK (2 SD), 25 pw | 4 |
| PKD1 c.6173G>A (p.Gln2058Arg)c | PKD1 c.9562A>G (p.Asn3188Asp) | EHK (14 SD), at birth; eGFR=56, 20 yr | 4 |
| PKD1 c.12074A>G (p.Glu4025Gly) | PKD1 c.2582G>A (p.Trp861*) | EHK (10 SD), 22 pw; CKD3a, 4 yr | 4 |
| PKD1 c.4831G>A (p.Val1611Ile) | PKD1 c.12503dup (p.Ser4169Leufs*41) | EHK (3 SD), 22 pw | 4 |
| PKD2 c.1967T>G (p.Leu656Trp)c,d,e | PKD2 c.1967T>G (p.Leu656Trp) | EHK (approximately 18 SD), at birth, HTN, 2m; eGFR=52, 18 yr | 65 |
EHK, enlarged hyperechoic kidneys; pw, pregnancy weeks; TOP, termination of pregnancy; HTN, hypertension; RD, respiratory distress; MEK, massively enlarged kidneys; BCK, bilateral cystic kidneys; OH, oligohydramnios; SIE, slight increased echogenicity.
Other described examples where the high frequency of the ULP allele in normal individuals or in silico analysis makes the ULP allele an unlikely cause of the VEO PKD are not listed.
Detected by computed tomography or magnetic resonance imaging.
Identified alone in one parent, no cysts detected on ultrasound.
Homozygosity due to maternal isodisomy.
Likely ADPKDVEO but no details about presence or not of oligohydramnios and evolution after diagnosis (hypertension/kidney function/overt proteinuria).
Rarely, digenic disease, involving PKD1 and PKD2, has been described, resulting in more severe disease than for either variant monoallelically, but not VEO.61,70,71 Pathogenic alleles in other cystogenes may cause VEO PKD in combination with a PKD1 or PKD2 mutation, such as digenic disease involving a PKD1 and HNF1B allele,43 emphasizing the importance of comprehensive analysis of not only PKD1 and PKD2 in these cases. Occasionally, a contiguous gene syndrome (CGS) due to deletion of PKD1 and the tuberous sclerosis complex (TSC) gene TSC2 is associated with a more severe renal phenotype than TSC or ADPKD alone, often leading to renal insufficiency in childhood.72–74 CGS individuals with mosaicism can have milder, more typical ADPKD with TSC.74
Differential Diagnosis from Other Inherited Renal Cystic Diseases
A number of other diseases can appear similar to ADPKD or ADPLD, although are usually differentiated on clinical grounds. Autosomal recessive polycystic kidney disease (ARPKD), associated with biallelic PKHD1 mutations, although typically an infantile disease can manifest later in life with an ADPKD-like renal phenotype, but congenital hepatic fibrosis rather than PLD usually differentiates the two.75 Monoallelic mutations to PKHD1 have been described to cause increased kidney echogenicity and/or multiple small liver cysts (mimicking ADPLD) in approximately 15% of carriers.20,76 However, with a carrier rate of approximately 1 in 70 it seems that other genetic/environmental factors must be necessary to reveal the monoallelic cystic phenotype. The association of PKD with hyperinsulinic hypoglycemia was recently described in 11 pedigrees and linked to biallelic mutations (including a specific promoter mutation) of PMM2, encoding the N-linked glycosylation enzyme, phosphomannomutase 2, again linking glycosylation defects and PKD.77
ADTKD because of MUC1 or UMOD mutations can result in the development of small renal cysts late in the disease, but the lack of renal enlargement, and the parallel decline of kidney size and kidney function with advancing disease, usually differentiate them from ADPKD.78 ADTKD-HNF1B is associated with a highly heterogeneous phenotypic spectrum, including maturity onset diabetes of the young, a personal or familial history of urogenital malformations, early onset gout, and/or the presence of elevated liver enzymes or hypomagnesaemia, but dominant HNF1B mutations can sometimes phenocopy ADPKD (Figure 1F).58,79 Mutations to SEC61A1 have recently been identified in two families with ADTKD; glomerular cysts on renal biopsy and multiple bilateral renal cysts without renal enlargement were present in some family members.80 HANAC due to COL4A1 mutations can also result in renal cysts, although the additional features, such as hematuria, muscles cramps or elevated creatinine phosphokinase, tortuosity of the retinal artery, and brain small-vessel disease, usually allow for a differential diagnosis.81 Syndromic forms of PKD can sometimes prove challenging as a differential diagnosis from ADPKD. Oro-facial-digital syndrome type 1 is a rare X-linked dominant disorder with male prenatal lethality, where the monoallelic female renal presentation can be indistinguishable from ADPKD, although extrarenal manifestations should orient the diagnosis.82,83 TSC because of mutation to TSC1 or TSC2 (without the CGS) is phenotypically heterogeneous, with the development of benign tumors in many organs, but can occasionally present with kidney cysts with only discreet dermatologic features.84 In each of these cases, genetic analysis of a panel of PKD genes can better reveal the full etiology and aid diagnostics.
Other Influences on Phenotype, and Genetically Unresolved Cases
Accumulating data in ADPKD indicates that males tend to have more severe renal disease.3,14,21,36,59,85 Severe PLD associated with PKD1, PKD2, PRKCSH, and SEC63 mutation is predominantly a female disease, where growth moderates after menopause, suggesting a strong hormonal influence on the phenotype.8,9 It is unclear if there is a similar female bias associated with severe PLD and the rarer ADPLD genes.
The ADPKD-PKD1NT population encompasses a large spectrum, from fully inactivating mutations (similar to ADPKD-PKD1T) to ULP alleles associated with mild PKD (Figure 1D).86 The Canadian cohort suggested that PKD1 patients with inframe indels had renal severity intermediate between missense and PKD1T.55 In HALT PKD, ADPKD-PKD1NT patients were further classified into strongly or weakly predicted mutation strength groups (MSG2 or MSG3, respectively) using an in silico algorithm. MSG3, but not MSG2 patients, had significantly higher eGFR and lower HtTKV than ADPKD-PKD1T.14 However, no automated schema to assign MSG scores to PKD1NT variants is yet available and so at this stage we have not included these groups into the classification. However, as mutation screening becomes more widespread, variants are better collated, in silico tools are improved, and in vitro assays of PKD1 variants are developed, better classification of PKD1NT variants should be possible.14,56,87
Approximately 7% of ADPKD and 50% of ADPLD pedigrees remain genetically unresolved.3,9,14,20,55 Variants to the existing genes, including missense variants not considered pathogenic, deep intronic changes affecting splicing, or mutations in the promoter regions, could explain some of these cases. Mosaic, de novo mutations (mutation in the early embryo and so not present in all cells) are sometimes associated with a milder phenotype, and are often missed by conventional techniques due to their reduced dosage in leukocyte DNA.73,88–90 The recent identification of new ADPLD and ADPKD genes suggests that other genes encoding proteins involved in maturation and trafficking of PC1 are strong candidates.15,20
Estimating the Prevalence of ADPKD and ADPLD
Prevalence estimates can have important implications for drug development, with orphan diseases defined by a prevalence of <1 per 2000.91 Older population–based studies have estimated a lifetime risk of ADPKD at 1 per 1000, or even higher if autopsies—that likely included patients with few kidney and liver cysts and excellent renal prognoses plus other phenocopying disorders—are taken into consideration.92,93 More recent population-based minimum point prevalence estimates of 2.9 and 3.3 per 10,000, reflecting individuals with clinically significant disease, fall below the orphan threshold.94–96 ADPKD-PKD2 genetic prevalence in European individuals from the ExAC database was calculated at 1.64 per 10,000, suggesting a higher prevalence of the total ADPKD population.97 ADPLD prevalence, estimated in a single study at 1 per 158,000, is likely underestimated, because many affected individuals probably remain asymptomatic and undiagnosed.98 Conducting a combined analysis of clinical records, imaging, and genetic data in a geographically circumscribed area would be invaluable to refine prevalence estimates for the total population and genic/allelic groups.
Clinical Implications
Stratifying the ADPKD population is a prerequisite to the personalization of patient care. Combining genic and allelic information with the sex and clinical information (early hypertension and urinary tract infections), the PROPKD score, has shown strong renal prognostic value.36,99 Age-adjusted TKV is also a valuable prognostic marker.33 Multidisciplinary approaches involving clinical, imaging, and genetic data should now be favored as they help diagnostics, including the characterization of atypic clinical presentations, while predicting the evolution of patients with ADPKD, which is key to targeting therapies. Genic and allele information may also be important in the future as specific treatments proximal to the basic defect are developed, as is occurring in other common genetic diseases.100
Disclosures
None.
Acknowledgments
This review was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant DK058816 and the Mayo Translational Polycystic Kidney Disease Center (DK090728). An American Society of Nephrology Foundation Kidney Research Fellowship supports E.C.L.G.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G; ERA-EDTA Working Group for Inherited Kidney Diseases : Autosomal dominant polycystic kidney disease: The changing face of clinical management. Lancet 385: 1993–2002, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audrézet MP, Corbiere C, Lebbah S, Morinière V, Broux F, Louillet F, Fischbach M, Zaloszyc A, Cloarec S, Merieau E, Baudouin V, Deschênes G, Roussey G, Maestri S, Visconti C, Boyer O, Abel C, Lahoche A, Randrianaivo H, Bessenay L, Mekahli D, Ouertani I, Decramer S, Ryckenwaert A, Cornec-Le Gall E, Salomon R, Ferec C, Heidet L: Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 722–729, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fick GM, Johnson AM, Strain JD, Kimberling WJ, Kumar S, Manco-Johnson ML, Duley IT, Gabow PA: Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1863–1870, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Zerres K, Rudnik-Schöneborn S, Deget F: German working group on paediatric nephrology: Childhood onset autosomal dominant polycystic kidney disease in sibs: Clinical picture and recurrence risk. J Med Genet 30: 583–588, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan MC, Abebe K, Torres VE, Chapman AB, Bae KT, Tao C, Sun H, Perrone RD, Steinman TI, Braun W, Winklhofer FT, Miskulin DC, Rahbari-Oskoui F, Brosnahan G, Masoumi A, Karpov IO, Spillane S, Flessner M, Moore CG, Schrier RW: Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol 13: 155–64.e6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chebib FT, Jung Y, Heyer CM, Irazabal MV, Hogan MC, Harris PC, Torres VE, El-Zoghby ZM: Effect of genotype on the severity and volume progression of polycystic liver disease in ADPKD. Nephrol Dial Transplant 31: 952–960, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cnossen WR, Drenth JP: Polycystic liver disease: An overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis 9: 69, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The European Polycystic Kidney Disease Consortium : The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77: 881–894, 1994 [DOI] [PubMed] [Google Scholar]
- 11.The International Polycystic Kidney Disease Consortium : Polycystic kidney disease: The complete structure of the PKD1 gene and its protein. Cell 81: 289–298, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millán JL, Gamble V, Harris PC: The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJM, Somlo S: PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Heyer CM, Sundsbak JL, Abebe KZ, Chapman AB, Torres VE, Grantham JJ, Bae KT, Schrier RW, Perrone RD, Braun WE, Steinman TI, Mrug M, Yu AS, Brosnahan G, Hopp K, Irazabal MV, Bennett WM, Flessner MF, Moore CG, Landsittel D, Harris PC; HALT PKD and CRISP Investigators : Predicted mutation strength of nontruncating PKD1 mutations aids genotype-phenotype correlations in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 2872–2884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Banales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrezet MP, Ferec C, Le Meur Y, Torres VE; Genkyst Study Group; HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease; Harris PC: Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 98: 1193–1207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drenth JP, te Morsche RH, Smink R, Bonifacino JS, Jansen JB: Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat Genet 33: 345–347, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Li A, Davila S, Furu L, Qian Q, Tian X, Kamath PS, King BF, Torres VE, Somlo S: Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet 72: 691–703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, Li A, Cai Y, Kamath PS, King BF, Azurmendi PJ, Tahvanainen P, Kääriäinen H, Höckerstedt K, Devuyst O, Pirson Y, Martin RS, Lifton RP, Tahvanainen E, Torres VE, Somlo S: Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet 36: 575–577, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Cnossen WR, te Morsche RH, Hoischen A, Gilissen C, Chrispijn M, Venselaar H, Mehdi S, Bergmann C, Veltman JA, Drenth JP: Whole-exome sequencing reveals LRP5 mutations and canonical Wnt signaling associated with hepatic cystogenesis. Proc Natl Acad Sci U S A 111: 5343–5348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besse W, Dong K, Choi J, Punia S, Fedeles SV, Choi M, Gallagher AR, Huang EB, Gulati A, Knight J, Mane S, Tahvanainen E, Tahvanainen P, Sanna-Cherchi S, Lifton RP, Watnick T, Pei YP, Torres VE, Somlo S: Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest 127: 1772–1785, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Harris PC, Bae K, Rossetti S, Torres VE, Grantham JJ, Chapman A, Guay-Woodford L, King BF, Wetzel LH, Baumgarten D, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang Q, Thompson PA, Zhu F, Miller JP, Consortium C: Cyst number but not the rate of cystic growth is associated with the mutated gene in ADPKD. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ong AC, Harris PC: A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int 88: 699–710, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen MJ, Waanders E, Woudenberg J, Lefeber DJ, Drenth JP: Congenital disorders of glycosylation in hepatology: The example of polycystic liver disease. J Hepatol 52: 432–440, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S: A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet 43: 639–647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedeles SV, Gallagher AR, Somlo S: Polycystin-1: A master regulator of intersecting cystic pathways. Trends Mol Med 20: 251–260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellani C, Massie J, Sontag M, Southern KW: Newborn screening for cystic fibrosis. Lancet Respir Med 4: 653–661, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL: From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol Biol Cell 27: 424–433, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokman MF, Renkema KY, Giles RH, Schaefer F, Knoers NV, van Eerde AM: The expanding phenotypic spectra of kidney diseases: Insights from genetic studies. Nat Rev Nephrol 12: 472–483, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei Y, Hwang YH, Conklin J, Sundsbak JL, Heyer CM, Chan W, Wang K, He N, Rattansingh A, Atri M, Harris PC, Haider MA: Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 26: 746–753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kline TL, Korfiatis P, Edwards ME, Bae KT, Yu A, Chapman AB, Mrug M, Grantham JJ, Landsittel D, Bennett WM, King BF, Harris PC, Torres VE, Erickson BJ; CRISP Investigators : Image texture features predict renal function decline in patients with autosomal dominant polycystic kidney disease [published online ahead of print May 20, 2017]. Kidney Int doi: 10.1016/j.kint.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehm HL: Evolving health care through personal genomics. Nat Rev Genet 18: 259–267, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornec-Le Gall E, Audrézet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, Morin MP, Moal MC, Dantal J, Wehbe B, Perrichot R, Frouget T, Vigneau C, Potier J, Jousset P, Guillodo MP, Siohan P, Terki N, Sawadogo T, Legrand D, Menoyo-Calonge V, Benarbia S, Besnier D, Longuet H, Férec C, Le Meur Y: The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 942–951, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rule AD, Sasiwimonphan K, Lieske JC, Keddis MT, Torres VE, Vrtiska TJ: Characteristics of renal cystic and solid lesions based on contrast-enhanced computed tomography of potential kidney donors. Am J Kidney Dis 59: 611–618, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nascimento AB, Mitchell DG, Zhang XM, Kamishima T, Parker L, Holland GA: Rapid MR imaging detection of renal cysts: Age-based standards. Radiology 221: 628–632, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, Roy S, Bakkaloglu A, Komel R, Winearls CG, Harris PC: Mutation analysis of the entire PKD1 gene: Genetic and diagnostic implications. Am J Hum Genet 68: 46–63, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliuta IA, Kalatharan V, Wang K, Cornec-Le Gall E, Conklin J, Pourafkari M, Ting R, Chen C, Borgo AC, He N, Song X, Heyer CM, Senum SR, Hwang YH, Paterson AD, Harris PC, Khalili K, Pei Y: Polycystic kidney disease without an apparent family history. J Am Soc Nephrol 28: 2768–2776, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van’t Hoff WG, Niaudet P, Torres VE, Harris PC: Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75: 848–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Orndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC: Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 21: 1097–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergmann C, von Bothmer J, Ortiz Brüchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K: Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22: 2047–2056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert RD, Sukhtankar P, Lachlan K, Fowler DJ: Bilineal inheritance of PKD1 abnormalities mimicking autosomal recessive polycystic disease. Pediatr Nephrol 28: 2217–2220, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Gilbert RD, Evans H, Olalekan K, Nagra A, Haq MR, Griffiths M: Tolvaptan treatment for severe neonatal autosomal-dominant polycystic kidney disease. Pediatr Nephrol 32: 893–896, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loftus BJ, Kim U-J, Sneddon VP, Kalush F, Brandon R, Fuhrmann J, Mason T, Crosby ML, Barnstead M, Cronin L, Deslattes Mays A, Cao Y, Xu RX, Kang HL, Mitchell S, Eichler EE, Harris PC, Venter JC, Adams MD: Genome duplications and other features in 12 Mb of DNA sequence from human chromosome 16p and 16q. Genomics 60: 295–308, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Symmons O, Váradi A, Arányi T: How segmental duplications shape our genome: Recent evolution of ABCC6 and PKD1 Mendelian disease genes. Mol Biol Evol 25: 2601–2613, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Cornec-Le Gall E, Audrézet MP, Le Meur Y, Chen JM, Férec C: Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum Mutat 35: 1393–1406, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Phakdeekitcharoen B, Watnick TJ, Germino GG: Mutation analysis of the entire replicated portion of PKD1 using genomic DNA samples. J Am Soc Nephrol 12: 955–963, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Harris PC, Rossetti S: Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 197–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, Eckloff BW, Ward CJ, Winearls CG, Torres VE, Harris PC: Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol 23: 915–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trujillano D, Bullich G, Ossowski S, Ballarín J, Torra R, Estivill X, Ars E: Diagnosis of autosomal dominant polycystic kidney disease using efficient PKD1 and PKD2 targeted next-generation sequencing. Mol Genet Genomic Med 2: 412–421, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenberger T, Decker C, Hiersche M, Hamann RC, Decker E, Neuber S, Frank V, Bolz HJ, Fehrenbach H, Pape L, Toenshoff B, Mache C, Latta K, Bergmann C: An efficient and comprehensive strategy for genetic diagnostics of polycystic kidney disease. PLoS One 10: e0116680, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC: Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122: 4257–4273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang YH, Conklin J, Chan W, Roslin NM, Liu J, He N, Wang K, Sundsbak JL, Heyer CM, Haider M, Paterson AD, Harris PC, Pei Y: Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 1861–1868, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Autosomal dominant polycystic kidney disease mutation database, 2017. Available at: http://pkdb.mayo.edu. Accessed August 31, 2017
- 57.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee : Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O; Kidney Disease: Improving Global Outcomes : Autosomal dominant tubulointerstitial kidney disease: Diagnosis, classification, and management--A KDIGO consensus report. Kidney Int 88: 676–683, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Rossetti S, Burton S, Strmecki L, Pond GR, San Millán JL, Zerres K, Barratt TM, Ozen S, Torres VE, Bergstralh EJ, Winearls CG, Harris PC: The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Nephrol 13: 1230–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Cai Y, Fedeles SV, Dong K, Anyatonwu G, Onoe T, Mitobe M, Gao JD, Okuhara D, Tian X, Gallagher AR, Tang Z, Xie X, Lalioti MD, Lee AH, Ehrlich BE, Somlo S: Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest 124: 5129–5144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC: Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J Clin Invest 125: 607–620, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed B, Nobakht E, Dadgar S, Bekheirnia MR, Masoumi A, Belibi F, Yan XD, Cadnapaphornchai M, Schrier RW: Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am J Kidney Dis 56: 50–56, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Shamshirsaz AA, Reza Bekheirnia M, Kamgar M, Johnson AM, McFann K, Cadnapaphornchai M, Nobakhthaghighi N, Schrier RW: Autosomal-dominant polycystic kidney disease in infancy and childhood: Progression and outcome. Kidney Int 68: 2218–2224, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Nowak KL, Cadnapaphornchai MA, Chonchol MB, Schrier RW, Gitomer B: Long-term outcomes in patients with very-early onset autosomal dominant polycystic kidney disease. Am J Nephrol 44: 171–178, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Losekoot M, Ruivenkamp CA, Tholens AP, Grimbergen JE, Vijfhuizen L, Vermeer S, Dijkman HB, Cornelissen EA, Bongers EM, Peters DJ: Neonatal onset autosomal dominant polycystic kidney disease (ADPKD) in a patient homozygous for a PKD2 missense mutation due to uniparental disomy. J Med Genet 49: 37–40, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J: Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 17: 179–181, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S: Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Harris PC: What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 21: 1073–1076, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Gallagher AR, Germino GG, Somlo S: Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino GG, Parfrey P, Somlo S, St George-Hyslop P: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet 68: 355–363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pei Y, Lan Z, Wang K, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T: A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int 81: 412–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR: Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease--a contiguous gene syndrome. Nat Genet 8: 328–332, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Consugar MB, Wong WC, Lundquist PA, Rossetti S, Kubly VJ, Walker DL, Rangel LJ, Aspinwall R, Niaudet WP, Ozen S, David A, Velinov M, Bergstralh EJ, Bae KT, Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Sampson JR, Dawson BD, Harris PC; CRISP Consortium : Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int 74: 1468–1479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sampson JR, Maheshwar MM, Aspinwall R, Thompson P, Cheadle JP, Ravine D, Roy S, Haan E, Bernstein J, Harris PC: Renal cystic disease in tuberous sclerosis: Role of the polycystic kidney disease 1 gene. Am J Hum Genet 61: 843–851, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC: Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 85: 1–21, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Gunay-Aygun M, Turkbey BI, Bryant J, Daryanani KT, Gerstein MT, Piwnica-Worms K, Choyke P, Heller T, Gahl WA: Hepatorenal findings in obligate heterozygotes for autosomal recessive polycystic kidney disease. Mol Genet Metab 104: 677–681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cabezas OR, Flanagan SE, Stanescu H, García-Martínez E, Caswell R, Lango-Allen H, Antón-Gamero M, Argente J, Bussell AM, Brandli A, Cheshire C, Crowne E, Dumitriu S, Drynda R, Hamilton-Shield JP, Hayes W, Hofherr A, Iancu D, Issler N, Jefferies C, Jones P, Johnson M, Kesselheim A, Klootwijk E, Koettgen M, Lewis W, Martos JM, Mozere M, Norman J, Patel V, Parrish A, Pérez-Cerdá C, Pozo J, Rahman SA, Sebire N, Tekman M, Turnpenny PD, Hoff WV, Viering DHHM, Weedon MN, Wilson P, Guay-Woodford L, Kleta R, Hussain K, Ellard S, Bockenhauer D: Polycystic kidney disease with hyperinsulinemic hypoglycemia caused by a promoter mutation in phosphomannomutase 2. J Am Soc Nephrol 28: 2529–2539, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ekici AB, Hackenbeck T, Morinière V, Pannes A, Buettner M, Uebe S, Janka R, Wiesener A, Hermann I, Grupp S, Hornberger M, Huber TB, Isbel N, Mangos G, McGinn S, Soreth-Rieke D, Beck BB, Uder M, Amann K, Antignac C, Reis A, Eckardt KU, Wiesener MS: Renal fibrosis is the common feature of autosomal dominant tubulointerstitial kidney diseases caused by mutations in mucin 1 or uromodulin. Kidney Int 86: 589–599, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Verhave JC, Bech AP, Wetzels JF, Nijenhuis T: Hepatocyte nuclear factor 1β-associated kidney disease: More than renal cysts and diabetes. J Am Soc Nephrol 27: 345–353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolar NA, Golzio C, Živná M, Hayot G, Van Hemelrijk C, Schepers D, Vandeweyer G, Hoischen A, Huyghe JR, Raes A, Matthys E, Sys E, Azou M, Gubler MC, Praet M, Van Camp G, McFadden K, Pediaditakis I, Přistoupilová A, Hodaňová K, Vyleťal P, Hartmannová H, Stránecký V, Hůlková H, Barešová V, Jedličková I, Sovová J, Hnízda A, Kidd K, Bleyer AJ, Spong RS, Vande Walle J, Mortier G, Brunner H, Van Laer L, Kmoch S, Katsanis N, Loeys BL: Heterozygous loss-of-function SEC61A1 mutations cause autosomal-dominant tubulo-interstitial and glomerulocystic kidney disease with anemia. Am J Hum Genet 99: 174–187, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, Dracon M, Fardeau M, Van Agtmael T, Kerjaschki D, Antignac C, Ronco P: COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med 357: 2687–2695, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Prattichizzo C, Macca M, Novelli V, Giorgio G, Barra A, Franco B; Oral-Facial-Digital Type I (OFDI) Collaborative Group : Mutational spectrum of the oral-facial-digital type I syndrome: A study on a large collection of patients. Hum Mutat 29: 1237–1246, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Thauvin-Robinet C, Cossée M, Cormier-Daire V, Van Maldergem L, Toutain A, Alembik Y, Bieth E, Layet V, Parent P, David A, Goldenberg A, Mortier G, Héron D, Sagot P, Bouvier AM, Huet F, Cusin V, Donzel A, Devys D, Teyssier JR, Faivre L: Clinical, molecular, and genotype-phenotype correlation studies from 25 cases of oral-facial-digital syndrome type 1: A French and Belgian collaborative study. J Med Genet 43: 54–61, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Northrup H, Koenig MK, Pearson DA, Au KS: Tuberous sclerosis complex. In: GeneReviews, edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, Seattle, WA, University of Washington, 1993. Updated 2015 [Google Scholar]
- 85.Magistroni R, He N, Wang K, Andrew R, Johnson A, Gabow P, Dicks E, Parfrey P, Torra R, San-Millan JL, Coto E, Van Dijk M, Breuning M, Peters D, Bogdanova N, Ligabue G, Albertazzi A, Hateboer N, Demetriou K, Pierides A, Deltas C, St George-Hyslop P, Ravine D, Pei Y: Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14: 1164–1174, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Harris PC, Hopp K: The mutation, a key determinant of phenotype in ADPKD. J Am Soc Nephrol 24: 868–870, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Gainullin V, Heyer CM, Cornec-Le Gall E, Klein SLR, Torres VE, Harris PC: High-throughput, comparative variant profiling aids molecular diagnostics of ADPKD. J Am Soc Nephrol 27: 766A, 2016. 26376860 [Google Scholar]
- 88.Connor A, Lunt PW, Dolling C, Patel Y, Meredith AL, Gardner A, Hamilton NK, Dudley CR: Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am J Transplant 8: 232–237, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Reiterová J, Štekrová J, Merta M, Kotlas J, Elišáková V, Lněnička P, Korabečná M, Kohoutová M, Tesař V: Autosomal dominant polycystic kidney disease in a family with mosaicism and hypomorphic allele. BMC Nephrol 14: 59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan AY, Blumenfeld J, Michaeel A, Donahue S, Bobb W, Parker T, Levine D, Rennert H: Autosomal dominant polycystic kidney disease caused by somatic and germline mosaicism. Clin Genet 87: 373–377, 2015 [DOI] [PubMed] [Google Scholar]
- 91.Joly D, Béroud C, Grünfeld JP: Rare inherited disorders with renal involvement-approach to the patient. Kidney Int 87: 901–908, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Dalgaard OZ: Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl 328: 1–255, 1957 [PubMed] [Google Scholar]
- 93.Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT: Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935-1980. Am J Kidney Dis 2: 630–639, 1983 [DOI] [PubMed] [Google Scholar]
- 94.Neumann HP, Jilg C, Bacher J, Nabulsi Z, Malinoc A, Hummel B, Hoffmann MM, Ortiz-Bruechle N, Glasker S, Pisarski P, Neeff H, Krämer-Guth A, Cybulla M, Hornberger M, Wilpert J, Funk L, Baumert J, Paatz D, Baumann D, Lahl M, Felten H, Hausberg M, Zerres K, Eng C; Else-Kroener-Fresenius-ADPKD-Registry : Epidemiology of autosomal-dominant polycystic kidney disease: An in-depth clinical study for south-western Germany. Nephrol Dial Transplant 28: 1472–1487, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Patch C, Charlton J, Roderick PJ, Gulliford MC: Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: A population-based study. Am J Kidney Dis 57: 856–862, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, Czerwiec FS: Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol Dial Transplant 32: 1356– 1363, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cornec-Le Gall E, Audrézet MP, Renaudineau E, Hourmant M, Charasse C, Michez E, Frouget T, Vigneau C, Dantal J, Siohan P, Longuet H, Gatault P, Ecotière L, Bridoux F, Mandart L, Hanrotel-Saliou C, Stanescu C, Depraetre P, Gie S, Massad M, Kersalé A, Séret G, Augusto JF, Saliou P, Maestri S, Chen JM, Harris PC, Férec C, Le Meur Y: PKD2-related autosomal dominant polycystic kidney disease: Prevalence, clinical presentation, mutation spectrum, and prognosis. Am J Kidney Dis 70: 476–485, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Keimpema L, De Koning DB, Van Hoek B, Van Den Berg AP, Van Oijen MG, De Man RA, Nevens F, Drenth JP: Patients with isolated polycystic liver disease referred to liver centres: Clinical characterization of 137 cases. Liver Int 31: 92–98, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Cornec-Le Gall E, Blais J, Irazabal MV, Devuyst O, Gansevoort RT, Perrone R, Chapman A, Czerwiec FS, Ouyang J, Heyer CM, Senum SR, Le Meur Y, Torres VE, Harris PC: Can we further enrich ADPKD clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial. [published online ahead of print July 19, 2017]. Nephrol Dial Transpl doi: 10.1093/ndt/gfx188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffman LR, Ramsey BW: Cystic fibrosis therapeutics: The road ahead. Chest 143: 207–213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barua M, Cil O, Paterson AD, Wang K, He N, Dicks E, Parfrey P, Pei Y: Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 20: 1833–1838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ali H, Hussain N, Naim M, Zayed M, Al-Mulla F, Kehinde EO, Seaburg LM, Sundsbak JL, Harris PC: A novel PKD1 variant demonstrates a disease-modifying role in trans with a truncating PKD1 mutation in patients with autosomal dominant polycystic kidney disease. BMC Nephrol 16: 26, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]