Abstract
Congenital anomalies of the kidneys and urinary tract (CAKUT) comprise a large spectrum of congenital malformations ranging from severe manifestations, such as renal agenesis, to potentially milder conditions, such as vesicoureteral reflux. CAKUT causes approximately 40% of ESRD that manifests within the first three decades of life. Several lines of evidence indicate that CAKUT is often caused by recessive or dominant mutations in single (monogenic) genes. To date, approximately 40 monogenic genes are known to cause CAKUT if mutated, explaining 5%–20% of patients. However, hundreds of different monogenic CAKUT genes probably exist. The discovery of novel CAKUT-causing genes remains challenging because of this pronounced heterogeneity, variable expressivity, and incomplete penetrance. We here give an overview of known genetic causes for human CAKUT and shed light on distinct renal morphogenetic pathways that were identified as relevant for CAKUT in mice and humans.
Keywords: monogenic disease, genetic kidney disease, congenital anomalies of the kidneys and urinary tract, CAKUT
Congenital anomalies of the kidneys and urinary tract (CAKUT) are common malformations with a potentially severe effect on health. CAKUT causes about 40% of cases of ESRD in patients who develop it within the first three decades of life.1,2 Manifestations of the CAKUT spectrum account for 20%–30% of congenital malformations3–7 and constitute a frequent cause of birth defects (approximately three to six per 1000 live births).4,8,9 CAKUT may occur either as an isolated condition or along with extrarenal manifestations as part of a syndromic disorder.10–13 To date, >200 clinical syndromes have been described that comprise features of CAKUT as part of their distinct phenotype.14
The term CAKUT summarizes a large variety of diverse congenital malformations that range from conditions of the upper urinary tract (e.g., renal agenesis and renal hypodysplasia) to phenotypes primarily affecting the lower urinary tract, such as vesicoureteral reflux (VUR), ureterovesical junction obstruction (UVJO), and posterior urethral valves (PUVs).3,8,11 In addition, abnormalities of kidney shape or anatomic position, such as horseshoe kidney or pelvic kidney, may occur. Malformations, such as UVJO, PUV, and VUR, may lead to anatomic (UVJO and PUV) or functional (VUR) stasis of urine flow, thereby increasing the risk of urinary tract infections, which may result in renal scarring and potentially, CKD (e.g., from reflux nephropathy). It is important to note that, although different manifestations from within the CAKUT spectrum may coexist in the same individual,3,15 unilateral CAKUT (e.g., renal agenesis) can also exist in the presence of an entirely unblemished contralateral urinary system.10
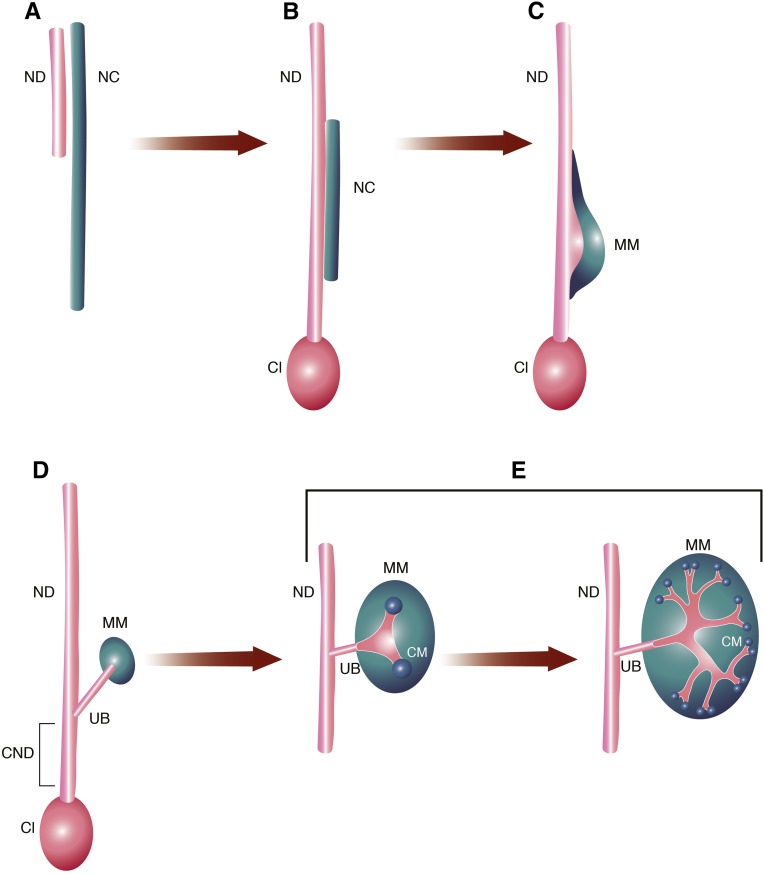
The diverse manifestations of CAKUT phenotypes are thought to result from disturbances at any point in renal morphogenesis (Figure 1).15,16 Most importantly, imbalances in the communication between the metanephric mesenchyme (MM) and the ureteric bud (UB) are believed to be central to the pathogenesis of CAKUT phenotypes (Figure 1, C–E).15 The developmental origin of PUVs, however, likely differs from other CAKUT manifestations and remains poorly understood.3,17,18
Figure 1.
Development of the kidneys and urinary tract. (A) The bilateral nephric ducts (NDs; alternatively mesonephric ducts or Wolffian ducts) and the nephric cords (NCs) are the precursor structures of the adult urinary system. Both originate from the embryonic intermediate mesoderm. The cells of the ND undergo an early mesenchymal to epithelial transition and assemble into epithelial tube–like structures. The associated NC retains characteristics of mesenchymal tissue.16,62 (B) As the embryo develops, the ND elongates caudally. At approximately E9.5, the most caudal portion of the ND fuses with the cloacal epithelium (Cl). The cloaca is the embryonic precursor of the bladder, and it is derived from cloacal endoderm.16,66 (C) The NC reorganizes and forms a morphologically distinct domain: the MM. Renal morphogenesis is initiated and maintained by reciprocal interactions between the epithelial ND and the MM. In mice, at embryonic day approximately E10–E10.5, signals from the MM induce the formation of a circumscribed, broad swelling of the ND at the level of the MM.15,16 (D) At E10.5 in mice and around the fifth week of human gestation, the UB emerges from the swollen portion of the ND and grows dorsally toward the MM.4,15,16,74 The caudal part of the ND, which is located between the UB and the insertion into the Cl, is referred to as the common nephric duct (CND). (E) Stimulated by MM-derived signals, the UB begins to branch repeatedly (branching morphogenesis) at approximately E11.5. Through continuous reciprocal induction, the MM is important for promoting and maintaining branching events of the UB. The UB branching continues for approximately 9–13 cycles (mice) and then slows down after approximately E15.5. Via branching morphogenesis, the UB gives rise to the renal collecting system consisting of collecting ducts and renal pelvis as well as the ureter. Reciprocally, signals from the UB also support development of MM cells. The MM that is in closest proximity to the UB tips condenses and forms the so-called cap mesenchyme (CM). Stimulated by signals from the UB, the CM undergoes a mesenchymal to epithelial transition. The epithelial cell population subsequently gives rise to structures of the nephron (glomerulus, the proximal tubule, and the distal tubule). Modified from refs. 16 and 62, with permission.
We have previously hypothesized that a high fraction of human CAKUT may be caused by single-gene defects. Monogenic diseases, also known as Mendelian disorders, are caused by mutations in a single causative gene.10 Supporting evidence for a monogenic etiology in the case of CAKUT comes from (1) the familial occurrence of CAKUT phenotypes (approximately 10%–15% of patients with CAKUT cases are familial), (2) the existence of monogenic syndromes with a distinct syndromic phenotype that includes manifestations from within the CAKUT spectrum (>200 to date), (3) the existence of monogenic mouse models that exhibit CAKUT (>180 to date), and (4) the fact that the development of the kidney and urinary tract is governed by distinct developmental genes.10,11,19,20
Approximately 40 different monogenic causes for human CAKUT (25 dominant and 15 recessive) have so far been identified (Table 1).12,13,21–51 Many established CAKUT genes encode transcription factors and follow a dominant pattern of inheritance.3,10 Some of the genes listed in Table 1, however, remain questionable in regard to their pathogenic effect and will require more support through functional studies in the future.52,53
Table 1.
Fifteen recessive and 25 dominant genes that represent monogenic causes of human CAKUT if mutated
| Gene | Protein | Refs. |
|---|---|---|
| Autosomal recessive | ||
| ACE | Angiotensin I–converting enzyme | 38 |
| AGT | Angiotensinogen | 38 |
| AGTR1 | Angiotensin II receptor, type 1 | 38 |
| CHRM3 | Muscarinic acetylcholine receptor M3 | 39 |
| FRAS1 | ECM protein FRAS1 | 42 |
| FREM1 | FRAS1-related ECM protein 1 | 42 |
| FREM2 | FRAS1-related ECM protein 2 | 42 |
| GRIP1 | Glutamate receptor interacting protein 1 | 42 |
| HPSE2 | Heparanase 2 (inactive) | 43 |
| ITGA8 | Integrin-α8 | 44 |
| LRIG2 | Leucine-rich repeats and Ig-like domains 2 | 45 |
| REN | Renin | 38 |
| TRAP1 | Heat shock protein 75 (also known as TNF receptor–associated protein 1) | 46 |
| Autosomal dominant | ||
| BMP4 | Bone morphogenic protein 4 | 21 |
| CHD1L | Chromodomain helicase DNA binding protein 1 like | 22 |
| CRKL | CRK-like proto-oncogene, adaptor protein | 23 |
| DSTYK | Dual serine/threonine and tyrosine protein kinase | 24 |
| EYA1 | Eyes absent homolog 1 | 25 |
| GATA3 | GATA binding protein 3 | 26,188 |
| HNF1B | HNF homeobox B | 13 |
| MUC1 | Mucin 1 | 27 |
| NRIP1 | Nuclear receptor interacting protein 1 | 140 |
| PAX2 | Paired box 2 | 12 |
| PBX1 | PBX homeobox 1 | 53 |
| RET | Proto-oncogene tyrosine-protein kinase receptor Ret | 28 |
| ROBO2 | Roundabout, axon guidance receptor, homolog 2 (Drosophila) | 29,189 |
| SALL1 | Sal-like protein 1 (also known as spalt-like transcription factor 1) | 49 |
| SIX2 | SIX homeobox 2 | 21 |
| SIX5 | SIX homeobox 5 | 51 |
| SLIT2 | Slit homolog 2 | 29 |
| SOX17 | Transcription factor SIX-17 | 30 |
| SRGAP1 | SLIT-ROBO Rho GTPase activating protein 1 | 29 |
| TBX18 | T-box transcription factor TBX18 | 31 |
| TNXB | Tenascin XB | 32 |
| UMOD | Uromodulin | 33 |
| UPK3A | Uroplakin 3A | 34 |
| WNT4 | Protein Wnt-4 | 35–37 |
| X-linked recessive | ||
| KAL1 | Anosmin 1 | 47 |
Currently, at most, up to 18% of CAKUT cases can be explained by these established monogenic causes (Table 1).3,10 The percentage rate of molecularly “solved” cases, of note, is hereby likely dependent on the corresponding selection of patients and controls. Approximately 10%–15% of cases have additionally been described to occur due to copy number variations that predominantly involve either the HNFB1 locus on chromosome 17 or the Di-George/velocardiofacial syndrome region on chromosome 22.54
It is likely that hundreds of additional monogenic causes of human CAKUT have yet to be identified. Apart from the distinct heterogeneity, with 40 genes identified so far (Table 1), the identification of novel CAKUT-causing genes is complicated by variable expressivity and incomplete penetrance.10,11 These features are frequently encountered in dominant diseases and can result in the phenomenon that either individuals carrying a mutation in a CAKUT gene can present with a phenotype that differs from the phenotypic manifestation of other individuals with an identical mutation (variable expressivity) or alternatively, an individual carrying a mutation does not exhibit a CAKUT phenotype at all (incomplete penetrance).10
One theory proposed by Ichikawa et al.15 suggests that this phenotypic variability may occur as a result of (1) stochastic spatiotemporal differences during key events of renal morphogenesis, (2) gene dosage effects, and (3) redundancy of genes that belong to functionally related gene families.10,20 This hypothesis thereby provides an explanation for both intraindividual as well as interindividual (i.e., variable expressivity) differences in CAKUT phenotypes. Alternative hypotheses ascribe more importance to epigenetic or environmental influences (e.g., effects of malnutrition, vitamins, or drugs).3,55 Although epigenetic and environmental factors potentially provide a sufficient explanation for interindividual differences in CAKUT manifestations, they are less applicable in the context of intraindividual (left-right) dissimilarities. Particularly, environmental factors are extremely interesting to discover, because they may allow for a specific prophylaxis of CAKUT. However, despite large studies, to date, only little success has been achieved regarding the search for environmental factors with significant effect on the pathogenesis of CAKUT.3,56–60
Complex Genetics in CAKUT
Nowadays, modern study designs increasingly yield potential polygenic disease loci for a wide variety of human disease conditions.61 Despite the complicated etiology of CAKUT, which suggests an interplay of (epi-)genetic and environmental factors,3,62 to date, there is little evidence for the simultaneous contribution of more than one gene (di-, oligo-, or polygenic effects; i.e., complex genetics) to the pathogenesis of human CAKUT.63 Similar to monogenic research, the identification of polygenic causes of CAKUT is likely complicated by the vast heterogeneity of CAKUT. This results in very few affected patients with shared disease loci essential for the unequivocal identification of novel genetic regions. General awareness of the possibility of complex inheritance patterns in CAKUT is necessary in the genetic evaluation of affected individuals. Polygenic disease hypotheses, however, remain challenging to establish, test, and prove in human CAKUT.
Relevance of Mouse Candidate Genes
One of the reasons why it was hypothesized that a large fraction of human CAKUT may be of monogenic origin was the presence of a large number of monogenic mouse models of CAKUT; >180 monogenic causes of murine CAKUT have been described to date (Supplemental Table 1). These genes constitute promising candidates in the search for (novel) genetic causes of human CAKUT. In fact, many of the 40 established monogenic causes of human CAKUT (e.g., FRAS1, FREM1, FREM2, GRIP1, bone morphogenic protein 4 [BMP4], SIX2, Ret Proto-Oncogene [RET], SALL1, and UPK3A) were initially derived as candidate genes from observations in mouse models of CAKUT and subsequently screened for their prevalence in human disease cohorts.21,28,34,42,49 Controlled conditions in the evaluation of genetic mouse models allow for a careful assessment of subtle manifestations of underlying genotypes (e.g., mild phenotypes in single heterozygous carriers of recessive CAKUT genes). Mouse models furthermore provide the opportunity for a controlled evaluation of the contributions of multiple genes (i.e., complex inheritance) to the development of CAKUT. One example is the effect of the loss-of-function of the entire Hox11 paralogous group on renal morphogenesis in mice (complete loss of metanephric kidney induction) in comparison with an isolated loss-of-function of individual or two components of the paralogous group (i.e., Hoxa11, Hoxd11, and Hoxc11; no discernible kidney abnormalities or renal hypoplasia, respectively).64
The insights from mouse models, however, do not always directly translate to human genetics. Explanations for this discrepancy include potential species-specific differences in the development of the kidneys and urinary tract, alterations in the required gene dosage during developmental steps, functional compensation by redundant genes, incomplete penetrance, and likely, merely the rarity of human cases with mutations in a particular gene (due to distinct heterogeneity).
Molecular Pathways of Renal Morphogenesis
Despite challenges, mouse models of CAKUT have rendered it possible to identify many developmental pathways as relevant for renal morphogenesis and pathophysiologic events that underlie CAKUT.3,10,16,48 Most prominently, the glial cell–derived neurotrophic factor (Gdnf)-glial cell–derived neurotrophic factor family receptor α1 (Gfra1)-Ret pathway plays a decisive role in renal morphogenesis in general and specifically, the intricate regulation of the crosstalk between the UB and the MM (Figure 1, C–E).4,15,62,65 RET is a receptor tyrosine-kinase that is involved in a multitude of developmental processes.65,66 In the developing kidney, RET is predominantly expressed in nephric duct–derived structures (including UB) (Figure 1).65,67–69 During renal morphogenesis, RET requires the ligand GDNF as well as the coreceptor GFRA1 for its activation.65,66,70–73
Given the important role of RET signaling for renal morphogenesis, it is not surprising that many of the established causes of murine and human CAKUT (including PAX2, GATA3, EYA1, and SALL1) have been reported to function upstream and downstream of the GDNF-GFRA-RET regulatory pathway (summarized in Supplemental Table 2).4,16,62,65,74 Mechanistic details on the RET signaling pathway are beyond the scope of this review and have been reviewed in detail recently.4,16,62,65,74,75
However, various additional morphogenetic pathways have previously been implicated to be essential for proper renal morphogenesis. These signaling pathways include the BMP (TGFβ), NOTCH, Hedgehog-GLI, and FGF-related cascades as well as canonical WNT/β-catenin signaling that converge and partially modulate one another during the development of the kidneys and the urinary tract.76–78
In this review, we will place special emphasis on two pathways that appear interweaved with GDNF-RET signaling and are not commonly discussed in the context of CAKUT. These are (1) the role of extracellular matrix (ECM) proteins (Supplemental Table 3) at the epithelial-mesenchymal interface between UB and the MM (Figure 2), and (2) vitamin A/retinoic acid (RA) signaling (Figure 3). This latter pathway also constitutes the first molecular pathway potentially amenable to treatment. We furthermore briefly describe (3) the BMP signaling cascade as an example for a well established molecular pathway in renal morphogenesis (Figure 4, Supplemental Table 4). The BMP pathway represents an additional example of a signaling cascade, the genes and proteins of which have recently been identified as genetic causes of human and/or murine CAKUT.21,42,79–95
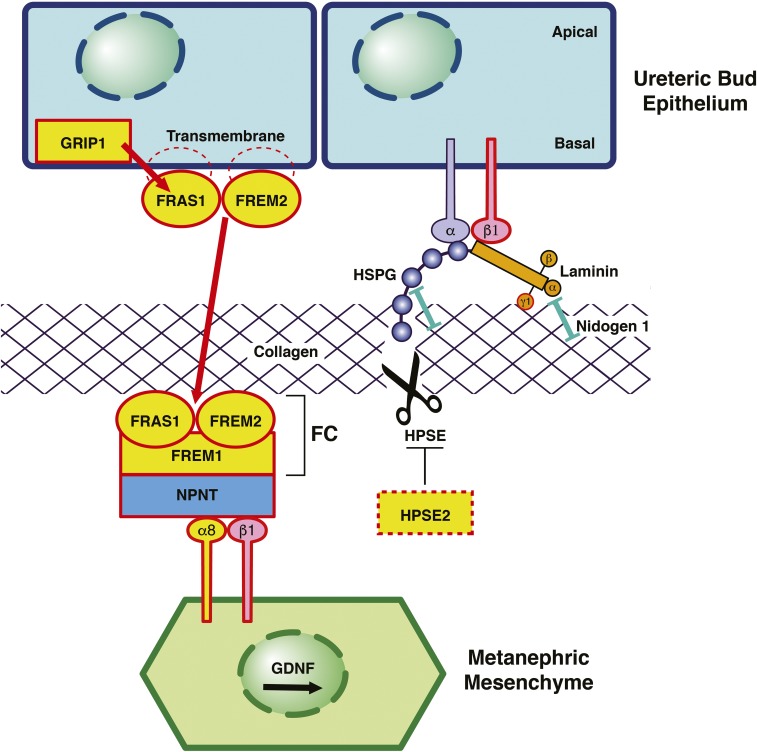
Figure 2.
ECM proteins cause murine and human CAKUT. Schematic of the interface between the UB and the MM in renal development. Proteins encoded by genes that, if mutated, cause monogenic CAKUT in humans are highlighted in yellow. Red frames indicate proteins encoded by genes mutated in CAKUT in mice. (Left panel) FRAS1 and FREM2 localize in epithelial cells of the UB as transmembrane proteins with intracytoplasmic tail regions.41,98,103 The interaction with PDZ domains of the intracellular protein GRIP1 is essential for targeting to the basal surface of the UB cells and shedding of FRAS1 from the membrane.98,107 FREM1 is produced by the MM and secreted into the extracellular space.106,107 FRAS1, FREM2, and FREM1 assemble at the epithelial-mesenchymal interface to form the FC.107 Mutations in the genes encoding these proteins cause CAKUT (Supplemental Figure 2). Also, mutations in GRIP1 result in human and murine CAKUT. Nephronectin (NPNT) functions as an adaptor to interconnect the ternary FC with the ITGA8/integrin-β1 heterodimer on the surface of MM cells. ITGA8/integrin-β1 signaling leads to an increased expression of GDNF by the MM, thereby promoting renal morphogenesis.183 Mutations in ITGA8 in humans and mice cause CAKUT.44 Loss of FC integrity results in a significant decrease in GDNF expression in the MM, thereby hampering the interaction between the UB and the MM and consequentially, impeding renal morphogenesis.183 (Right panel) Laminins are cross-shaped ECM molecules that consist of three distinct chains (α, β, and γ).101 Laminins simultaneously interact with cell surface receptors (integrins), HSPG, collagen, and nidogen 1 (see box).184 Laminins that contain γ1-chains form high-affinity interactions with nidogen 1.101 Fourteen distinct laminin isoforms have been identified to date, of which ten possess the laminin-γ1 chain.101,185 A homozygous ablation of the nidogen binding site in laminin-γ1 has been shown to result in severe CAKUT phenotypes (including bilateral renal agenesis) in mice.101 Despite its important role in ECM assembly, targeted inactivation of nidogen 1 did not result in a CAKUT phenotype.101 Both nidogen 1 and Laminin are known interactors of HSPG. HPSE is one of the few enzymes with the ability to break down HSPG.115 Knockdown of neither HSPG nor HPSE has been reported to cause CAKUT phenotypes in mice.115,120,186,187 However, recessive mutations in HPSE2 have been identified in human patients with urofacial syndrome,43,93 with a similar phenotype in mice.43 Although HPSE2 has no detectable enzymatic activity itself, it has been shown to function as an endogenous inhibitor of HPSE (Supplemental Figure 3).116 Modified from refs. 107 and 184, with permission.
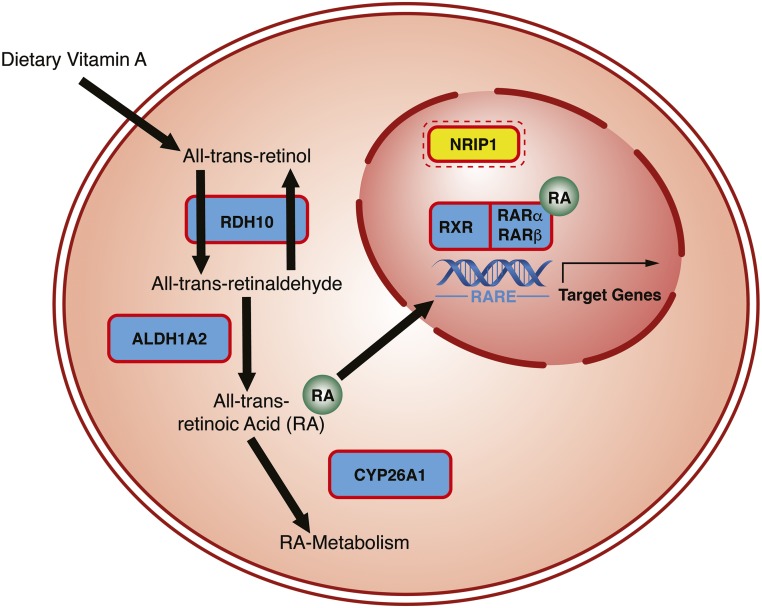
Figure 3.
RA signaling plays a central role for the development of the kidneys and urinary tract. Genes encoding the proteins highlighted by red frames have been implicated in murine cases of syndromic CAKUT (Supplemental Table 1). Genes highlighted in yellow, if mutated, constitute causes of human CAKUT. Mutations in genes encoding multiple proteins involved in intracellular RA processing and signaling have previously been identified to cause syndromic CAKUT in mice (Retinol Dehydrogenase 10 [Rdh10], Aldehyde Dehydrogenase 1 Family Member A2 [Aldh1a2], and Cytochrome P450 Family 26 [Cyp26], Rxr, Rarα, and Rarβ).138,150–152 RDH10150 catalyzes the reversible reaction from retinol to retinal. Retinal is then converted into the active metabolite all-trans RA via an irreversible reaction catalyzed by the enzyme ALD1A2.138 RA then either enters the nucleus or is metabolized via enzymes of the CYP26 family (e.g., CYP26A1)151,152 in the endoplasmic reticulum. In the nucleus, RA derivatives can serve as a ligand for two receptors: RXR (9-cis-RA and rexinoids) and RAR (all-trans-RA).123,142 Both receptor proteins have at least three subtypes and isoforms each. RXR and RAR heterodimers bind to retinoic acid–responsive elements (RAREs) of the nuclear DNA.121,141 RAREs are predominantly located in promoter regions of target genes. The presence of RA recruits coactivators and leads to enhanced binding to RAREs and expression of target genes.121,141 The transcriptional control of downstream genes is further modified by the binding of additional coregulators (coactivators and corepressors, including NRIP1) to RXR and RAR.121,141,153 We recently identified mutations in the corepressor NRIP1 (highlighted in yellow) as causing CAKUT in humans.140 NRIP1 may interact with both RXRs and RARs.153
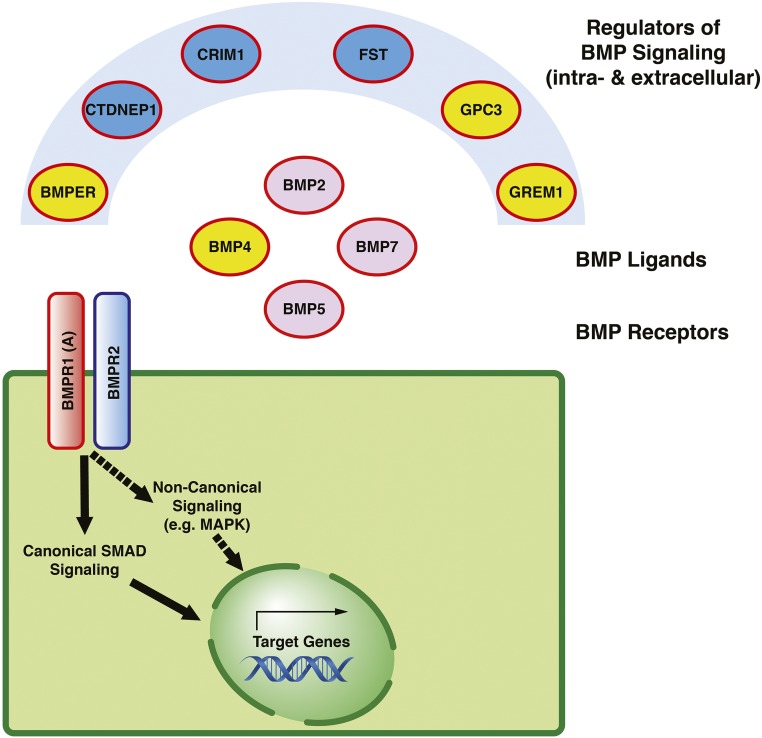
Figure 4.
Members of the BMP signaling cascade with a role in CAKUT. Proteins encoded by genes that, if mutated, cause murine CAKUT are outlined in red, and proteins highlighted in yellow constitute causes of isolated and/or syndromic manifestations of human CAKUT (Supplemental Table 4). BMPs act as ligands for two classes of transmembrane serine-threonine-kinase receptors on the surface of the corresponding effector cells (e.g., bone morphogenic protein receptor type 1A [BMPR1A] and BMPR2).155 After activated, these receptors initiate intracellular signaling cascades, including canonical SMAD signaling and noncanonical signaling (e.g., via mitogen-activated protein kinase [MAPK]), which eventually result in increased expression of distinct target genes in the cell nucleus.175,176 The cellular consequences of BMP signaling depend on the location and local concentration of the BMP ligand.177 The availability of ligands for receptor binding is regulated by diverse intra- and extracellular antagonists and agonists of BMP and includes, for example, Gremlin 1 (GREM1), Follistatin (FST), and bone morphogenic protein binding to the endothelial regulator (BMPER).178–180 Please note that, BMPR2, although depicted in the figure, has not been implicated in the pathogenesis of CAKUT to date. CRIM1, cysteine-rich transmembrane bone morphogenic protein regulator 1; CTDNEP1, CTD nuclear envelope phosphatase 1; GPC3, Glypican 3.
CAKUT and ECM Proteins
The ECM is a highly complex, three-dimensional network of proteins and proteoglycans.96,97 It is an essential component of all tissues and synthesized by embryonic cells starting at the earliest stages of development.96,97 Over the past decades, the understanding of the functional roles of the ECM has changed dramatically. Although the ECM was initially believed to merely provide structural support,97–99 the current understanding goes far beyond simple adhesion and space-filling properties and includes regulatory roles in cell-cell and cell matrix interactions.96
The pathophysiologic inter-relation of ECM components and renal morphogenesis has long been known from knockout animal models that display phenotypic features of the CAKUT spectrum (summarized in Supplemental Table 3). The relevance of ECM proteins for isolated human CAKUT, however, first came to attention when mutations in genes encoding members of the Fraser complex (FC) as well as the associated protein integrin-α8 (ITGA8) were identified in a significant proportion of patients with isolated human CAKUT phenotypes.42,44 Figure 2 depicts selected ECM components that have previously been shown to be causative of human (highlighted in yellow in Figure 2) as well as murine CAKUT (outlined in red in Figure 2).41–44,93,100–105
The FC is a ternary protein complex consisting of FRAS1, FREM2, and FREM1 (Figure 2).106 Although not participating in the complex formation itself, GRIP1 is a cytosolic protein essential for trafficking and proper targeting of the transmembrane protein FRAS1 to the basolateral surface of the UB cells.104 After they are assembled at the epithelial-mesenchymal interface, the members of the FC interact with nephronectin (NPNT), which serves as an adaptor for ITGA8 and integrin-β1 that are expressed by the cells of the MM (Figure 2).105,107,108 ITGA8 is an important upstream activator of GDNF, which, via interaction with RET, promotes renal organogenesis.105,107
Genes encoding members of the FC have previously only been known to be causative of the severe syndromic phenotype Fraser syndrome, a rare syndromic condition with phenotypic features including CAKUT as well as cryptophthalmos and cutaneous syndactyly (FRAS1, FREM1, and GRIP1).41,103,104,109,110 FREM1 mutations are also an established genetic cause for the phenotypically related conditions Manitoba-oculo-tricho-anal syndrome (OMIM 248450) and Bifid nose with or without anorectal and renal anomalies syndrome (OMIM 608990) (Supplemental Figures 1 and 2).111
Interestingly, the disease-causing mutations in FRAS1, FREM1, FREM2, and GRIP1 (Figure 2) in patients with FS or Manitoba-oculo-tricho-anal syndrome are almost exclusively recessive truncating variants41,42,103,110–112 (Supplemental Figure 2). Truncating mutations result in an abrogation and thereby, loss-of-function of the corresponding protein. Despite the interaction and a structural similarity, members of the FC do not exhibit functional redundancy, and an abrogation of any member of the FC and/or GRIP1 results in a failure of the complex to assemble.98,104,107 A consecutive epithelial-mesenchymal detachment is considered causative for distinct manifestations of FS (e.g., skin blebbing resulting in cutaneous syndactyly and cryptophthalmos).41
Conversely, we recently found that missense mutations in FRAS1, FREM1, FREM2, or GRIP1 result in isolated CAKUT phenotypes (Figure 2).42 These findings thereby indicate, that, in the case of the FC genes, the disease manifestation can vary from severe syndromic phenotypes on the basis of truncating mutations to isolated CAKUT on the basis of hypomorphic mutants (e.g., missense) (Supplemental Figure 2).
Another subgroup of genes that cause (predominantly murine) CAKUT, if mutated, coalesces around the proteoglycan component of the ECM (Supplemental Figure 3, Supplemental Table 3). Proteoglycans consist of a protein core coupled to unique, variably sulfated glycosaminoglycan chains (Supplemental Figure 3).97,113 Although there are various classes of glycosaminoglycan chains, heparan sulfate (HS) has previously been implicated to have the largest functional relevance within the developing kidney.113
The synthesis of heparan-sulfate proteoglycans (HSPGs) is very complicated and involves a large variety of enzymes (Supplemental Figure 3).114 In contrast, the breakdown is rather simple and predominantly carried out by the enzyme heparanase (HPSE) (Figure 2, Supplemental Figure 3).115 The protein HPSE2 is an inactive enzyme, but it has been reported to function as an endogenous regulator of HPSE activity.116 Recessive mutations in HPSE2 have been identified in humans and mice with urofacial syndrome (OMIM 236730), which includes manifestations from the CAKUT spectrum within its syndromic phenotype.43,93,100
Overall, the multitude of murine and human disease phenotypes that occur as result of disturbances in HSPG biosynthesis provides important insight into the mechanisms involved (Supplemental Figure 3, Supplemental Table 3).117,118 However, although for some HS-relevant enzymes, there are strong loss-of-function phenotypes in animal models, mouse models for other HS-related ECM components (e.g., mouse models with a knockout of HPSE function) do not result in overt defects, thereby suggesting a redundancy of certain HS enzymes and HSPGs in the ECM that allow for a functional compensation.96,119,120 This hypothesis, however, remains to be validated by additional functional studies in the future.
CAKUT and RA Signaling
Vitamin A is a fat-soluble compound that is derived from dietary sources.121 Vitamin A and its active metabolite, RA,121–123 play important roles in the regulation of a wide range of biologic processes, including differentiation, proliferation, and apoptosis.121,123–130 While exerting essential roles in adult tissues (e.g., vision, fertility, and immune response),131–133 vitamin A is also a well known teratogen.121,123,131,134 Interestingly, both the deficiency and the excess of vitamin A during pregnancy cause similar forms of an embryopathy known as vitamin A–deficient (VAD) syndrome.121,131,135 VAD syndrome affects many structures, including the ocular, cardiac, respiratory, and urinary systems, and is a cause of CAKUT.123,135,136 It is, therefore, recommended to carefully monitor vitamin A levels during pregnancy.121
Over the past decades, efforts, mainly led by Mendelsohn et al.,66,122,130,137–139 have broadened the understanding of the molecular mechanisms that underlie the well established interplay of vitamin A/RA signaling and renal morphogenesis in mice. Research by Chia et al.66 and Batourina et al.122 (among others) convincingly showed that RA is required for two key events during embryonic development of the urinary tract: the insertion of the nephric duct into the cloaca (approximately E9.5) (Figure 1B) as well as the branching morphogenesis of the UB (approximately E11.5–E15.5) (Figure 1, D and E). In both scenarios, the mechanism by which vitamin A/RA mainly exerts its effects seems to be due to control of expression levels of a key player of renal morphogenesis, Ret.66,122
Although the pathophysiologic relevance of vitamin A for embryologic development, including processes of renal morphogenesis, has long been known, very recently for the first time, a heterozygous mutation in a gene related to vitamin A function (Nuclear Receptor Interacting Protein 1 [NRIP1]) was discovered in one large kindred with isolated human CAKUT.140
RA plays an important role in the transcriptional regulation of developmentally relevant target genes.66,121 Retinoid X Receptor-α (RXR) and Retinoic Acid Receptor (RAR; are nuclear receptors that bind RA each has three subtypes, α, β, and γ, with different isoforms).121,141–143 RXR (5′) and RAR (3′) assemble to heterodimers and bind to RA-responsive elements in the DNA (Figure 3),121,123,141,142,144,145 thereby controlling transcription of functionally diverse target genes.121,141 The specific subtype and combination of RXRs-RARs thereby most likely permit a certain tissue selectivity, while at the same time, sustaining the pleiotropy of RA-mediated effects.123 RXR and RAR can additionally bind coregulators (coactivators and corepressors), which adds another level of complexity to the transcriptional regulation of RA-dependent genes in an RA-dependent manner.121,131,141,142,146–149 A potential contribution to the tissue specificity of RA-mediated effects via tissue-specific expression patterns of coregulators is likely.
Mutations in genes encoding enzymes that are involved in intracellular processing of RA, such as Retinol Dehydrogenase 10,150 Aldehyde Dehydrogenase 1 Family Member A2,138 and Cytochrome P450 Family 26 Subfamily A Member 1,151,152 as well as double mutants of specific isoforms within the RXR (Rxrα) and RAR family of proteins (Rarα and Rarβ)123 (Figure 3) have previously been shown to result in multisystemic phenotypes in mice that resemble VAD syndrome and present manifestations from within the CAKUT spectrum.
NRIP1 is a member of a group of ligand-inducible transcription factors, and it is involved in the coregulation of variety of biologic processes (e.g., estrogen and thyroid hormone signaling), including RA-mediated pathways.153,154 NRIP1 has been shown to have the capacity to interact with both RAR and RXR (Figure 3).153 Despite the large variety of biologic functions of NRIP1, in the examined family with seven affected individuals, the identified mutation resulted in an isolated CAKUT phenotype without any extrarenal manifestations.140
To the knowledge of the authors, this is the first time that a protein involved in RA signaling did not result in a syndromic CAKUT phenotype but rather, resulted in a selective CAKUT phenotype. This could potentially point toward a role for this coregulator in a select group of tissues as opposed to Retinol Dehydrogenase 10, Aldehyde Dehydrogenase 1 Family Member A2, and Cytochrome P450 Family 26 Subfamily A Member 1, RARs, and RXRs, which exhibit very broad expression patterns. Although this would have to be further elucidated, the possibility exists that, in the future, more regulators of RA-dependent transcriptional regulation may be identified in patients with cases with isolated features from within the syndromic VAD phenotype.
NRIP1 mutations as a cause of CAKUT are of particular interest, because they represent the first form of CAKUT that could be amenable to prevention treatment. It will have to be tested whether control of RA metabolism may be applicable in pregnant women with a family history of NRIP1-related cases of CAKUT. NRIP1 mutations as genetic cause of human CAKUT are very rare, however, with only one case of familial CAKUT to date.140
CAKUT and Signaling through BMPs
Bone morphogenic proteins (BMPs) constitute the largest family within the TGFβ superfamily of molecules.155 BMPs were originally identified as bone-derived proteins capable of inducing ectopic bone formation in vivo.156,157 Nowadays, the BMP pathway is renowned for its importance in general embryogenesis, with diverse roles depending on the spatial and temporal context.78 A large number of in vitro and in vivo studies underscore a crucial role of BMP signaling for renal morphogenesis in animal models (Figure 4, Supplemental Table 4).79–92,158–160 Also, the development of the human kidney and urinary tract seems to depend on intact BMP signaling. This is indicated by the finding of mutations in genes inter-related with BMP signaling in patients with isolated (BMP421 and GREM142) or syndromic (BMPER,94 BMP4,95 and GPC3161) manifestations of human CAKUT.
Details of the importance of BMP signaling for renal morphogenesis have been previously reviewed in detail78,155,162–164 and are continually being revealed. Experimental evidence suggests that the contribution of BMPs to renal morphogenesis is very complex. Research in the field of BMP signaling is generally challenged by the early embryonic lethality that often results from loss-of-function mutations in animal models of BMP-related genes (Supplemental Table 4). This complicates the investigation of respective contributions to the development of the kidneys and the urinary tract.85,165–167 Spatiotemporal differences in expression patterns of different components of the BMP signaling cascade indicate that proteins from the BMP family perform various functions for both UB and mesenchymal cells during development of the murine kidney.79,84,89,168–171 Tight regulation of these proteins seems to be critical in these processes. This is, for instance, exemplified by the finding of CAKUT phenotypes resulting from both mice with null mutations in distinct BMP genes as well as mice with a lack of inhibition of the respective BMP.78,84 However, it was shown that BMPs may be able to substitute one another during murine renal morphogenesis as previously shown for Bmp7 and Bmp4.172
Mechanisms of BMP signaling in the context of renal development are briefly outlined in Figure 4. Figure 4 depicts components of the signaling cascade that have previously been reported to result in manifestations of murine CAKUT if mutated (outlined in red in Figure 4).79–92,158–160 Some of these genes/proteins have additionally been identified in human forms of isolated (BMP421 and GREM142) or syndromic (BMPER,94 GPC3,161 and BMP495) CAKUT (highlighted in yellow in Figure 4).
In general, BMP ligands induce cellular responses by forming complexes with two major types of diverse membrane-bound serine-threonine kinase receptors: type 1 BMP receptors and type 2 BMP receptors.155 Both types 1 and 2 receptors are required for subsequent transduction of the BMP signal.173,174 The cellular responses, however, seem to be defined by the type 1 receptor.155 Downstream of ligand-receptor binding, BMPs activate either a canonical or a noncanonical signaling cascade through phosphorylation of downstream effectors. The canonical BMP pathway transmits signals via Smad proteins.175,176 Noncanonical BMP signaling includes the activation of the mitogen-activated protein kinase family of signaling molecules.175 Both signaling cascades eventually result in the increased transcription of corresponding target genes in the nucleus. The location and local concentration of the BMP ligand hereby determine the exact cellular consequences of BMP signaling.177 Intra- and extracellular agonists (e.g., BMPER) and antagonists (e.g., GREM1 and FST) of BMP tightly regulate the level of ligand-receptor interaction at the ligand or (more rarely) the receptor level,178–180 and they play an important role in renal development.
Conclusion
In conclusion, a long-standing hypothesis has recently found strong confirmation; it stated that CAKUT, to a large part, is caused by single-gene mutations in a high number of different monogenic CAKUT genes, involving different genes in different patients.
Whole-exome sequencing (WES) has facilitated discovery of these genes. Even the subhypotheses found confirmation, stating that monogenic CAKUT genes would often represent candidate genes from mouse models of CAKUT, renal developmental genes, or genes known to cause human clinical syndromes that have a CAKUT component.
The fraction of approximately 17% of patients with CAKUT, in whom a monogenic cause can be detected by WES, is lower than that in other causes of early-onset CKD, being approximately 30% in steroid-resistant nephrotic syndrome181 and 70% in renal cystic diseases.182 Monogenic CAKUT genes are more difficult to identify due to the high contribution of autosomal dominant renal developmental genes, which often exhibit features of incomplete penetrance (lack of genotype-phenotype correlation) and variable expressivity (differing organ involvement). Previous experience, however, strongly suggests that, before complex genetic mechanisms have to be evoked, such as polygenic inheritance or epigenetic mechanisms, hundreds of monogenic causative genes for CAKUT genes will still be identified.
In this context, the ability to represent a patient’s exact mutation in an animal model of CAKUT (e.g., mice or Xenopus) using CRISPR/Cas knockin techniques has strong potential to conclusively define genotype-phenotype relations for patients with CAKUT.
Identification of monogenic causes of CAKUT by WES will rapidly become common practice in pediatric nephrology and urology clinics due to reduction in cost for WES and continuous improvement of algorithms that define deleteriousness of mutant alleles.
Discovery of additional monogenic CAKUT genes will strongly aide our understanding of the complex pathogenesis of CAKUT. In addition, molecular genetic diagnostics using WES may offer important advances to clinical management for patients with CAKUT, who represent about 50% of all patients with ESRD before age 25 years old, because causative mutations will soon allow for prognostic conclusions, such as outcomes of reconstructive surgery, rate of progression into renal failure, and the likelihood for the development of extrarenal complications.
Disclosures
None.
Supplementary Material
Acknowledgments
A.T.v.d.V. is supported by postdoctoral research fellowship VE916/1-1 from the German Research Foundation (DFG). A.V. is supported by a Fulbright postdoctoral scholar award and grants from the Manton Center Fellowship Program, Boston Children’s Hospital, and the Mallinckrodt Research Fellowship. F.H. is the William E. Harmon Professor of Pediatrics. This work was supported by National Institutes of Health grant DK088767 (to F.H.). F.H. is supported by the Begg Family Foundation for Research into Reflux Nephropathy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017050561/-/DCSupplemental.
References
- 1.Chesnaye N, Bonthuis M, Schaefer F, Groothoff JW, Verrina E, Heaf JG, Jankauskiene A, Lukosiene V, Molchanova EA, Mota C, Peco-Antić A, Ratsch IM, Bjerre A, Roussinov DL, Sukalo A, Topaloglu R, Van Hoeck K, Zagozdzon I, Jager KJ, Van Stralen KJ; ESPN/ERA–EDTA registry : Demographics of paediatric renal replacement therapy in Europe: A report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 29: 2403–2410, 2014 [DOI] [PubMed] [Google Scholar]
- 2.(NAPRTCS) NAPRTCS : NAPRTCS 2008 Annual Report, Rockville, MD, The EMMES Corporation, 2008 [Google Scholar]
- 3.Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV: Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11: 720–731, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Schedl A: Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Loane M, Dolk H, Kelly A, Teljeur C, Greenlees R, Densem J; EUROCAT Working Group : Paper 4: EUROCAT statistical monitoring: Identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91[Suppl 1]: S31–S43, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Brown T, Mandell J, Lebowitz RL: Neonatal hydronephrosis in the era of sonography. AJR Am J Roentgenol 148: 959–963, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J: Malformations in newborn: Results based on 30,940 infants and fetuses from the Mainz congenital birth defect monitoring system (1990-1998). Arch Gynecol Obstet 266: 163–167, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman J, Edmonds LD, McClearn AB, Jensvold N, Shaw GM: Surveillance for and comparison of birth defect prevalences in two geographic areas--United States, 1983-88. MMWR CDC Surveill Summ 42: 1–7, 1993 [PubMed] [Google Scholar]
- 10.Vivante A, Hildebrandt F: Gentics of congenital anomalies of the kidneys and urinary tract. In: Congenital Anomalies of the Kidney and Urinary Tract, edited by Barakat A, Rushton H, Cham, Switzerland, Springer Nature, 2016, pp 303–322 [Google Scholar]
- 11.Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F: Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 29: 695–704, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR: Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9: 358–364, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Lindner TH, Njolstad PR, Horikawa Y, Bostad L, Bell GI, Sovik O: A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet 8: 2001–2008, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Limwongse C: Syndromes and malformations of the urinary tract. In: Pediatric Nephrology, 6th Ed., edited by Avner EDHW, Niaudet P, Yoshikawa N, Berlin, Springer, 2009, pp 122–138 [Google Scholar]
- 15.Ichikawa I, Kuwayama F, Pope JC 4th, Stephens FD, Miyazaki Y: Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int 61: 889–898, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Costantini F: Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol 1: 693–713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiaramonte C, Bommarito D, Zambaiti E, Antona V, Li Voti G: Genetic basis of posterior urethral valves inheritance. Urology 95: 175–179, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Nasir AA, Ameh EA, Abdur-Rahman LO, Adeniran JO, Abraham MK: Posterior urethral valve. World J Pediatr 7: 205–216, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Barratt TM, Avner ED, Harmon WE: Pediatric nephrology. In: Pediatric Nephrology, edited by Pine JW, Baltimore, MD, Lippinkott Williams & Wilkins, 1999, pp 3–30 [Google Scholar]
- 20.Davies JA: Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat (Basel) 156: 187–201, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knüppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD: SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol 19: 891–903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brockschmidt A, Chung B, Weber S, Fischer DC, Kolatsi-Joannou M, Christ L, Heimbach A, Shtiza D, Klaus G, Simonetti GD, Konrad M, Winyard P, Haffner D, Schaefer F, Weber RG: CHD1L: A new candidate gene for congenital anomalies of the kidneys and urinary tract (CAKUT). Nephrol Dial Transplant 27: 2355–2364, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, Yan Z, Mitrotti A, Martino J, Steers NJ, Fasel DA, Vukojevic K, Deng R, Racedo SE, Liu Q, Werth M, Westland R, Vivante A, Makar GS, Bodria M, Sampson MG, Gillies CE, Vega-Warner V, Maiorana M, Petrey DS, Honig B, Lozanovski VJ, Salomon R, Heidet L, Carpentier W, Gaillard D, Carrea A, Gesualdo L, Cusi D, Izzi C, Scolari F, van Wijk JA, Arapovic A, Saraga-Babic M, Saraga M, Kunac N, Samii A, McDonald-McGinn DM, Crowley TB, Zackai EH, Drozdz D, Miklaszewska M, Tkaczyk M, Sikora P, Szczepanska M, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Darlow JM, Puri P, Barton D, Casolari E, Furth SL, Warady BA, Gucev Z, Hakonarson H, Flogelova H, Tasic V, Latos-Bielenska A, Materna-Kiryluk A, Allegri L, Wong CS, Drummond IA, D’Agati V, Imamoto A, Barasch JM, Hildebrandt F, Kiryluk K, Lifton RP, Morrow BE, Jeanpierre C, Papaioannou VE, Ghiggeri GM, Gharavi AG, Katsanis N, Sanna-Cherchi S: Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med 376: 742–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, Choi M, Bodria M, Liu Y, Weng PL, Lozanovski VJ, Verbitsky M, Lugani F, Sterken R, Paragas N, Caridi G, Carrea A, Dagnino M, Materna-Kiryluk A, Santamaria G, Murtas C, Ristoska-Bojkovska N, Izzi C, Kacak N, Bianco B, Giberti S, Gigante M, Piaggio G, Gesualdo L, Vukic DK, Vukojevic K, Saraga-Babic M, Saraga M, Gucev Z, Allegri L, Latos-Bielenska A, Casu D, State M, Scolari F, Ravazzolo R, Kiryluk K, Al-Awqati Q, D’Agati VD, Drummond IA, Tasic V, Lifton RP, Ghiggeri GM, Gharavi AG: Mutations in DSTYK and dominant urinary tract malformations. N Engl J Med 369: 621–629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, König R, Vigneron J, Weissenbach J, Petit C, Weil D: Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet 6: 2247–2255, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH: Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet 11: 40–44, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Kirby A, Gnirke A, Jaffe DB, Barešová V, Pochet N, Blumenstiel B, Ye C, Aird D, Stevens C, Robinson JT, Cabili MN, Gat-Viks I, Kelliher E, Daza R, DeFelice M, Hůlková H, Sovová J, Vylet’al P, Antignac C, Guttman M, Handsaker RE, Perrin D, Steelman S, Sigurdsson S, Scheinman SJ, Sougnez C, Cibulskis K, Parkin M, Green T, Rossin E, Zody MC, Xavier RJ, Pollak MR, Alper SL, Lindblad-Toh K, Gabriel S, Hart PS, Regev A, Nusbaum C, Kmoch S, Bleyer AJ, Lander ES, Daly MJ: Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet 45: 299–303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ: Renal aplasia in humans is associated with RET mutations. Am J Hum Genet 82: 344–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang DY, Kohl S, Fan X, Vivante A, Chan S, Dworschak GC, Schulz J, van Eerde AM, Hilger AC, Gee HY, Pennimpede T, Herrmann BG, van de Hoek G, Renkema KY, Schell C, Huber TB, Reutter HM, Soliman NA, Stajic N, Bogdanovic R, Kehinde EO, Lifton RP, Tasic V, Lu W, Hildebrandt F: Mutations of the SLIT2-ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum Genet 134: 905–916, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM: Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat 31: 1352–1359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivante A, Kleppa MJ, Schulz J, Kohl S, Sharma A, Chen J, Shril S, Hwang DY, Weiss AC, Kaminski MM, Shukrun R, Kemper MJ, Lehnhardt A, Beetz R, Sanna-Cherchi S, Verbitsky M, Gharavi AG, Stuart HM, Feather SA, Goodship JA, Goodship TH, Woolf AS, Westra SJ, Doody DP, Bauer SB, Lee RS, Adam RM, Lu W, Reutter HM, Kehinde EO, Mancini EJ, Lifton RP, Tasic V, Lienkamp SS, Jüppner H, Kispert A, Hildebrandt F: Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet 97: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gbadegesin RA, Brophy PD, Adeyemo A, Hall G, Gupta IR, Hains D, Bartkowiak B, Rabinovich CE, Chandrasekharappa S, Homstad A, Westreich K, Wu G, Liu Y, Holanda D, Clarke J, Lavin P, Selim A, Miller S, Wiener JS, Ross SS, Foreman J, Rotimi C, Winn MP: TNXB mutations can cause vesicoureteral reflux. J Am Soc Nephrol 24: 1313–1322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ: Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39: 882–892, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins D, Bitner-Glindzicz M, Malcolm S, Hu CC, Allison J, Winyard PJ, Gullett AM, Thomas DF, Belk RA, Feather SA, Sun TT, Woolf AS: De novo Uroplakin IIIa heterozygous mutations cause human renal adysplasia leading to severe kidney failure. J Am Soc Nephrol 16: 2141–2149, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Biason-Lauber A, Konrad D, Navratil F, Schoenle EJA: A WNT4 mutation associated with Müllerian-duct regression and virilization in a 46,XX woman. N Engl J Med 351: 792–798, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Mandel H, Shemer R, Borochowitz ZU, Okopnik M, Knopf C, Indelman M, Drugan A, Tiosano D, Gershoni-Baruch R, Choder M, Sprecher E: SERKAL syndrome: An autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am J Hum Genet 82: 39–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B: Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 24: 550–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC: Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Weber S, Thiele H, Mir S, Toliat MR, Sozeri B, Reutter H, Draaken M, Ludwig M, Altmüller J, Frommolt P, Stuart HM, Ranjzad P, Hanley NA, Jennings R, Newman WG, Wilcox DT, Thiel U, Schlingmann KP, Beetz R, Hoyer PF, Konrad M, Schaefer F, Nürnberg P, Woolf AS: Muscarinic acetylcholine receptor M3 mutation causes urinary bladder disease and a Prune-Belly-like syndrome. Am J Hum Genet 89: 668–674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, Ornitz DM, Kopan R: FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22: 1191–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGregor L, Makela V, Darling SM, Vrontou S, Chalepakis G, Roberts C, Smart N, Rutland P, Prescott N, Hopkins J, Bentley E, Shaw A, Roberts E, Mueller R, Jadeja S, Philip N, Nelson J, Francannet C, Perez-Aytes A, Megarbane A, Kerr B, Wainwright B, Woolf AS, Winter RM, Scambler PJ: Fraser syndrome and mouse blebbed phenotype caused by mutations in FRAS1/Fras1 encoding a putative extracellular matrix protein. Nat Genet 34: 203–208, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Kohl S, Hwang DY, Dworschak GC, Hilger AC, Saisawat P, Vivante A, Stajic N, Bogdanovic R, Reutter HM, Kehinde EO, Tasic V, Hildebrandt F: Mild recessive mutations in six Fraser syndrome-related genes cause isolated congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 25: 1917–1922, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulum B, Özçakar ZB, Duman D, Cengiz FB, Kavaz A, Burgu B, Baskın E, Çakar N, Soygür T, Ekim M, Tekin M, Yalçınkaya F: HPSE2 mutations in urofacial syndrome, non-neurogenic neurogenic bladder and lower urinary tract dysfunction. Nephron 130: 54–58, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Humbert C, Silbermann F, Morar B, Parisot M, Zarhrate M, Masson C, Tores F, Blanchet P, Perez MJ, Petrov Y, Khau Van Kien P, Roume J, Leroy B, Gribouval O, Kalaydjieva L, Heidet L, Salomon R, Antignac C, Benmerah A, Saunier S, Jeanpierre C: Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet 94: 288–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuart HM, Roberts NA, Burgu B, Daly SB, Urquhart JE, Bhaskar S, Dickerson JE, Mermerkaya M, Silay MS, Lewis MA, Olondriz MB, Gener B, Beetz C, Varga RE, Gülpınar O, Süer E, Soygür T, Ozçakar ZB, Yalçınkaya F, Kavaz A, Bulum B, Gücük A, Yue WW, Erdogan F, Berry A, Hanley NA, McKenzie EA, Hilton EN, Woolf AS, Newman WG: LRIG2 mutations cause urofacial syndrome. Am J Hum Genet 92: 259–264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saisawat P, Kohl S, Hilger AC, Hwang DY, Yung Gee H, Dworschak GC, Tasic V, Pennimpede T, Natarajan S, Sperry E, Matassa DS, Stajić N, Bogdanovic R, de Blaauw I, Marcelis CL, Wijers CH, Bartels E, Schmiedeke E, Schmidt D, Märzheuser S, Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nöthen MM, Draaken M, Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A, van Rooij IA, Esposito F, Reutter HM, Hildebrandt F: Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int 85: 1310–1317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardelin JP, Levilliers J, del Castillo I, Cohen-Salmon M, Legouis R, Blanchard S, Compain S, Bouloux P, Kirk J, Moraine C, Chaussain J-L, Weissenbach J, Petit C: X chromosome-linked Kallmann syndrome: Stop mutations validate the candidate gene. Proc Natl Acad Sci U S A 89: 8190–8194, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W: Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet 18: 81–83, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM Jr., Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F: SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A 101: 8090–8095, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F: Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet 80: 800–804, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F: Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int 85: 1429–1433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heidet L, Morinière V, Henry C, De Tomasi L, Reilly ML, Humbert C, Alibeu O, Fourrage C, Bole-Feysot C, Nitschké P, Tores F, Bras M, Jeanpierre M, Pietrement C, Gaillard D, Gonzales M, Novo R, Schaefer E, Roume J, Martinovic J, Malan V, Salomon R, Saunier S, Antignac C, Jeanpierre C: Targeted exome sequencing identifies PBX1 as involved in monogenic congenital anomalies of the kidney and urinary tract [published online ahead of print May 31, 2017]. J Am Soc Nephrol doi:10.1681/ASN.2017010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG: Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91: 987–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woroniecki R, Gaikwad AB, Susztak K: Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol 26: 705–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu CW, Yamamoto KT, Henry RK, De Roos AJ, Flynn JT: Prenatal risk factors for childhood CKD. J Am Soc Nephrol 25: 2105–2111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dart AB, Ruth CA, Sellers EA, Au W, Dean HJ: Maternal diabetes mellitus and congenital anomalies of the kidney and urinary tract (CAKUT) in the child. Am J Kidney Dis 65: 684–691, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Parikh CR, McCall D, Engelman C, Schrier RW: Congenital renal agenesis: Case-control analysis of birth characteristics. Am J Kidney Dis 39: 689–694, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Tain YL, Luh H, Lin CY, Hsu CN: Incidence and risks of congenital anomalies of kidney and urinary tract in newborns: A population-based case-control study in Taiwan. Medicine (Baltimore) 95: e2659, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postoev VA, Grjibovski AM, Kovalenko AA, Anda EE, Nieboer E, Odland JO: Congenital anomalies of the kidney and the urinary tract: A murmansk county birth registry study. Birth Defects Res A Clin Mol Teratol 106: 185–193, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Dudbridge F: Polygenic epidemiology. Genet Epidemiol 40: 268–272, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Short KM, Smyth IM: The contribution of branching morphogenesis to kidney development and disease. Nat Rev Nephrol 12: 754–767, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Schild R, Knüppel T, Konrad M, Bergmann C, Trautmann A, Kemper MJ, Wu K, Yaklichkin S, Wang J, Pestell R, Müller-Wiefel DE, Schaefer F, Weber S: Double homozygous missense mutations in DACH1 and BMP4 in a patient with bilateral cystic renal dysplasia. Nephrol Dial Transplant 28: 227–232, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wellik DM, Hawkes PJ, Capecchi MR: Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 16: 1423–1432, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis TK, Hoshi M, Jain S: To bud or not to bud: The RET perspective in CAKUT. Pediatr Nephrol 29: 597–608, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M: Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 138: 2089–2097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain S: The many faces of RET dysfunction in kidney. Organogenesis 5: 177–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song R, El-Dahr SS, Yosypiv IV: Receptor tyrosine kinases in kidney development. J Signal Transduct 2011: 869281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parkash V, Goldman A: Comparison of GFL-GFRalpha complexes: Further evidence relating GFL bend angle to RET signalling. Acta Crystallogr Sect F Struct Biol Cryst Commun 65: 551–558, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sariola H, Saarma M: Novel functions and signalling pathways for GDNF. J Cell Sci 116: 3855–3862, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Keefe Davis T, Hoshi M, Jain S: Stage specific requirement of Gfrα1 in the ureteric epithelium during kidney development. Mech Dev 130: 506–518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enomoto H, Hughes I, Golden J, Baloh RH, Yonemura S, Heuckeroth RO, Johnson EM Jr., Milbrandt J: GFRalpha1 expression in cells lacking RET is dispensable for organogenesis and nerve regeneration. Neuron 44: 623–636, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM Jr.: Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol 158: 504–528, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Woolf AS, Davies JA: Cell biology of ureter development. J Am Soc Nephrol 24: 19–25, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Towers PR, Woolf AS, Hardman P: Glial cell line-derived neurotrophic factor stimulates ureteric bud outgrowth and enhances survival of ureteric bud cells in vitro. Exp Nephrol 6: 337–351, 1998 [DOI] [PubMed] [Google Scholar]
- 76.Gill PS, Rosenblum ND: Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle 5: 1426–1430, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Chi L, Zhang S, Lin Y, Prunskaite-Hyyryläinen R, Vuolteenaho R, Itäranta P, Vainio S: Sprouty proteins regulate ureteric branching by coordinating reciprocal epithelial Wnt11, mesenchymal Gdnf and stromal Fgf7 signalling during kidney development. Development 131: 3345–3356, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Reidy KJ, Rosenblum ND: Cell and molecular biology of kidney development. Semin Nephrol 29: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y: Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development 133: 4463–4473, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi M, Sharmin S, Taguchi A, Ohmori T, Fujimura S, Abe T, Kiyonari H, Komatsu Y, Mishina Y, Asashima M, Araki E, Nishinakamura R: The phosphatase Dullard negatively regulates BMP signalling and is essential for nephron maintenance after birth. Nat Commun 4: 1398, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Chiu HS, York JP, Wilkinson L, Zhang P, Little MH, Pennisi DJ: Production of a mouse line with a conditional Crim1 mutant allele. Genesis 50: 711–716, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A: Multiple defects and perinatal death in mice deficient in follistatin. Nature 374: 360–363, 1995 [DOI] [PubMed] [Google Scholar]
- 83.Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J: Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol 146: 255–264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A: Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131: 3401–3410, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Zhang H, Bradley A: Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122: 2977–2986, 1996 [DOI] [PubMed] [Google Scholar]
- 86.Hartwig S, Hu MC, Cella C, Piscione T, Filmus J, Rosenblum ND: Glypican-3 modulates inhibitory Bmp2-Smad signaling to control renal development in vivo. Mech Dev 122: 928–938, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA: The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell 71: 399–410, 1992 [DOI] [PubMed] [Google Scholar]
- 88.King JA, Marker PC, Seung KJ, Kingsley DM: BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol 166: 112–122, 1994 [DOI] [PubMed] [Google Scholar]
- 89.Dudley AT, Lyons KM, Robertson EJ: A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995 [DOI] [PubMed] [Google Scholar]
- 90.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G: BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820, 1995 [DOI] [PubMed] [Google Scholar]
- 91.Hartwig S, Bridgewater D, Di Giovanni V, Cain J, Mishina Y, Rosenblum ND: BMP receptor ALK3 controls collecting system development. J Am Soc Nephrol 19: 117–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu MC, Piscione TD, Rosenblum ND: Elevated SMAD1/beta-catenin molecular complexes and renal medullary cystic dysplasia in ALK3 transgenic mice. Development 130: 2753–2766, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Vivante A, Hwang DY, Kohl S, Chen J, Shril S, Schulz J, van der Ven A, Daouk G, Soliman NA, Kumar AS, Senguttuvan P, Kehinde EO, Tasic V, Hildebrandt F: Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol 28: 69–75, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Funari VA, Krakow D, Nevarez L, Chen Z, Funari TL, Vatanavicharn N, Wilcox WR, Rimoin DL, Nelson SF, Cohn DH: BMPER mutation in diaphanospondylodysostosis identified by ancestral autozygosity mapping and targeted high-throughput sequencing. Am J Hum Genet 87: 532–537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bakrania P, Efthymiou M, Klein JC, Salt A, Bunyan DJ, Wyatt A, Ponting CP, Martin A, Williams S, Lindley V, Gilmore J, Restori M, Robson AG, Neveu MM, Holder GE, Collin JR, Robinson DO, Farndon P, Johansen-Berg H, Gerrelli D, Ragge NK: Mutations in BMP4 cause eye, brain, and digit developmental anomalies: Overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet 82: 304–319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rozario T, DeSimone DW: The extracellular matrix in development and morphogenesis: A dynamic view. Dev Biol 341: 126–140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonnans C, Chou J, Werb Z: Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavlakis E, Chiotaki R, Chalepakis G: The role of Fras1/Frem proteins in the structure and function of basement membrane. Int J Biochem Cell Biol 43: 487–495, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Yurchenco PD, Patton BL: Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15: 1277–1294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JA, Voelckel MA, Fisher RB, Gu W, Xiong WC, Mei L, She JX, Wang CY: Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet 86: 957–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, Timpl R, Mayer U: Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development 129: 2711–2722, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Slavotinek AM, Baranzini SE, Schanze D, Labelle-Dumais C, Short KM, Chao R, Yahyavi M, Bijlsma EK, Chu C, Musone S, Wheatley A, Kwok PY, Marles S, Fryns JP, Maga AM, Hassan MG, Gould DB, Madireddy L, Li C, Cox TC, Smyth I, Chudley AE, Zenker M: Manitoba-oculo-tricho-anal (MOTA) syndrome is caused by mutations in FREM1. J Med Genet 48: 375–382, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jadeja S, Smyth I, Pitera JE, Taylor MS, van Haelst M, Bentley E, McGregor L, Hopkins J, Chalepakis G, Philip N, Perez Aytes A, Watt FM, Darling SM, Jackson I, Woolf AS, Scambler PJ: Identification of a new gene mutated in Fraser syndrome and mouse myelencephalic blebs. Nat Genet 37: 520–525, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Takamiya K, Kostourou V, Adams S, Jadeja S, Chalepakis G, Scambler PJ, Huganir RL, Adams RH: A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat Genet 36: 172–177, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Linton JM, Martin GR, Reichardt LF: The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development 134: 2501–2509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiyozumi D, Sugimoto N, Sekiguchi K: Breakdown of the reciprocal stabilization of QBRICK/Frem1, Fras1, and Frem2 at the basement membrane provokes Fraser syndrome-like defects. Proc Natl Acad Sci U S A 103: 11981–11986, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiyozumi D, Takeichi M, Nakano I, Sato Y, Fukuda T, Sekiguchi K: Basement membrane assembly of the integrin α8β1 ligand nephronectin requires Fraser syndrome-associated proteins. J Cell Biol 197: 677–689, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF: Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol 154: 447–458, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Slavotinek A, Li C, Sherr EH, Chudley AE: Mutation analysis of the FRAS1 gene demonstrates new mutations in a propositus with Fraser syndrome. Am J Med Genet A 140: 1909–1914, 2006 [DOI] [PubMed] [Google Scholar]
- 110.van Haelst MM, Maiburg M, Baujat G, Jadeja S, Monti E, Bland E, Pearce K, Hennekam RC, Scambler PJ; Fraser Syndrome Collaboration Group : Molecular study of 33 families with Fraser syndrome new data and mutation review. Am J Med Genet A 146A: 2252–2257, 2008 [DOI] [PubMed] [Google Scholar]
- 111.Nathanson J, Swarr DT, Singer A, Liu M, Chinn A, Jones W, Hurst J, Khalek N, Zackai E, Slavotinek A: Novel FREM1 mutations expand the phenotypic spectrum associated with Manitoba-oculo-tricho-anal (MOTA) syndrome and bifid nose renal agenesis anorectal malformations (BNAR) syndrome. Am J Med Genet A 161A: 473–478, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vogel MJ, van Zon P, Brueton L, Gijzen M, van Tuil MC, Cox P, Schanze D, Kariminejad A, Ghaderi-Sohi S, Blair E, Zenker M, Scambler PJ, Ploos van Amstel HK, van Haelst MM: Mutations in GRIP1 cause Fraser syndrome. J Med Genet 49: 303–306, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Steer DL, Shah MM, Bush KT, Stuart RO, Sampogna RV, Meyer TN, Schwesinger C, Bai X, Esko JD, Nigam SK: Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol 272: 310–327, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Lander AD, Selleck SB: The elusive functions of proteoglycans: In vivo veritas. J Cell Biol 148: 227–232, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rabelink TJ, van den Berg BM, Garsen M, Wang G, Elkin M, van der Vlag J: Heparanase: Roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat Rev Nephrol 13: 201–212, 2017 [DOI] [PubMed] [Google Scholar]
- 116.Levy-Adam F, Feld S, Cohen-Kaplan V, Shteingauz A, Gross M, Arvatz G, Naroditsky I, Ilan N, Doweck I, Vlodavsky I: Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J Biol Chem 285: 28010–28019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel VN, Pineda DL, Hoffman MP: The function of heparan sulfate during branching morphogenesis. Matrix Biol 57–58: 311–323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lamanna WC, Kalus I, Padva M, Baldwin RJ, Merry CL, Dierks T: The heparanome--the enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol 129: 290–307, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Holst CR, Bou-Reslan H, Gore BB, Wong K, Grant D, Chalasani S, Carano RA, Frantz GD, Tessier-Lavigne M, Bolon B, French DM, Ashkenazi A: Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS One 2: e575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP: Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One 4: e5181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Das BC, Thapa P, Karki R, Das S, Mahapatra S, Liu TC, Torregroza I, Wallace DP, Kambhampati S, Van Veldhuizen P, Verma A, Ray SK, Evans T: Retinoic acid signaling pathways in development and diseases. Bioorg Med Chem 22: 673–683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C: Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78, 2001 [DOI] [PubMed] [Google Scholar]
- 123.Mark M, Ghyselinck NB, Chambon P: Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 7: e002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kelley MW, Turner JK, Reh TA: Retinoic acid promotes differentiation of photoreceptors in vitro. Development 120: 2091–2102, 1994 [DOI] [PubMed] [Google Scholar]
- 125.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P: Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development 128: 1019–1031, 2001 [DOI] [PubMed] [Google Scholar]
- 126.Collop AH, Broomfield JA, Chandraratna RA, Yong Z, Deimling SJ, Kolker SJ, Weeks DL, Drysdale TA: Retinoic acid signaling is essential for formation of the heart tube in Xenopus. Dev Biol 291: 96–109, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin SC, Dollé P, Ryckebüsch L, Noseda M, Zaffran S, Schneider MD, Niederreither K: Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A 107: 9234–9239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Altucci L, Gronemeyer H: The promise of retinoids to fight against cancer. Nat Rev Cancer 1: 181–193, 2001 [DOI] [PubMed] [Google Scholar]
- 129.Niles RM: Vitamin A and cancer. Nutrition 16: 573–576, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL: Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Shannon SR, Moise AR, Trainor PA: New insights and changing paradigms in the regulation of vitamin A metabolism in development [published online ahead of print February 16, 2017]. Wiley Interdiscip Rev Dev Biol doi:10.1002/wdev.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawai T, Yanaka N, Richards JS, Shimada M: De novo-synthesized retinoic acid in ovarian antral follicles enhances FSH-mediated ovarian follicular cell differentiation and female fertility. Endocrinology 157: 2160–2172, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH: Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314: 1157–1160, 2006 [DOI] [PubMed] [Google Scholar]
- 134.Vieux-Rochas M, Coen L, Sato T, Kurihara Y, Gitton Y, Barbieri O, Le Blay K, Merlo G, Ekker M, Kurihara H, Janvier P, Levi G: Molecular dynamics of retinoic acid-induced craniofacial malformations: Implications for the origin of gnathostome jaws. PLoS One 2: e510, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilson JG, Roth CB, Warkany J: An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat 92: 189–217, 1953 [DOI] [PubMed] [Google Scholar]
- 136.Wilson JG, Warkany J: Aortic-arch and cardiac anomalies in the offspring of vitamin A deficient rats. Am J Anat 85: 113–155, 1949 [DOI] [PubMed] [Google Scholar]
- 137.Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J: Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126: 1139–1148, 1999 [DOI] [PubMed] [Google Scholar]
- 138.Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C: Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137: 283–292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL: Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet 32: 109–115, 2002 [DOI] [PubMed] [Google Scholar]
- 140.Vivante A, Mann N, Yonath H, Weiss AC, Getwan M, Kaminski MM, Bohnenpoll T, Teyssier C, Chen J, Shril S, van der Ven AT, Ityel H, Schmidt JM, Widmeier E, Bauer SB, Sanna-Cherchi S, Gharavi AG, Lu W, Magen D, Shukrun R, Lifton RP, Tasic V, Stanescu HC, Cavaillès V, Kleta R, Anikster Y, Dekel B, Kispert A, Lienkamp SS, Hildebrandt F: A dominant mutation in nuclear receptor interacting protein 1 causes urinary tract malformations via dysregulation of retinoic acid signaling. J Am Soc Nephrol 28: 2364–2376, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rochette-Egly C, Germain P: Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl Recept Signal 7: e005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Niederreither K, Dollé P: Retinoic acid in development: Towards an integrated view. Nat Rev Genet 9: 541–553, 2008 [DOI] [PubMed] [Google Scholar]
- 143.Chambon P: A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954, 1996 [PubMed] [Google Scholar]
- 144.Khorasanizadeh S, Rastinejad F: Nuclear-receptor interactions on DNA-response elements. Trends Biochem Sci 26: 384–390, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Brelivet Y, Kammerer S, Rochel N, Poch O, Moras D: Signature of the oligomeric behaviour of nuclear receptors at the sequence and structural level. EMBO Rep 5: 423–429, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D: Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature 375: 377–382, 1995 [DOI] [PubMed] [Google Scholar]
- 147.Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D: Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature 378: 681–689, 1995 [DOI] [PubMed] [Google Scholar]
- 148.Egea PF, Rochel N, Birck C, Vachette P, Timmins PA, Moras D: Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J Mol Biol 307: 557–576, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Steinmetz AC, Renaud JP, Moras D: Binding of ligands and activation of transcription by nuclear receptors. Annu Rev Biophys Biomol Struct 30: 329–359, 2001 [DOI] [PubMed] [Google Scholar]
- 150.Rhinn M, Schuhbaur B, Niederreither K, Dollé P: Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci U S A 108: 16687–16692, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M: The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev 15: 226–240, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H: The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev 15: 213–225, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Farooqui M, Franco PJ, Thompson J, Kagechika H, Chandraratna RA, Banaszak L, Wei LN: Effects of retinoid ligands on RIP140: Molecular interaction with retinoid receptors and biological activity. Biochemistry 42: 971–979, 2003 [DOI] [PubMed] [Google Scholar]
- 154.L’Horset F, Dauvois S, Heery DM, Cavaillès V, Parker MG: RIP-140 interacts with multiple nuclear receptors by means of two distinct sites. Mol Cell Biol 16: 6029–6036, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cain JE, Hartwig S, Bertram JF, Rosenblum ND: Bone morphogenetic protein signaling in the developing kidney: Present and future. Differentiation 76: 831–842, 2008 [DOI] [PubMed] [Google Scholar]
- 156.Urist MR: Bone: Formation by autoinduction. Science 150: 893–899, 1965 [DOI] [PubMed] [Google Scholar]
- 157.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA: Novel regulators of bone formation: Molecular clones and activities. Science 242: 1528–1534, 1988 [DOI] [PubMed] [Google Scholar]
- 158.Wilkinson L, Gilbert T, Sipos A, Toma I, Pennisi DJ, Peti-Peterdi J, Little MH: Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney Int 76: 1161–1171, 2009 [DOI] [PubMed] [Google Scholar]
- 159.Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y: Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev 2: 134–141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I: Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Veugelers M, Cat BD, Muyldermans SY, Reekmans G, Delande N, Frints S, Legius E, Fryns JP, Schrander-Stumpel C, Weidle B, Magdalena N, David G: Mutational analysis of the GPC3/GPC4 glypican gene cluster on Xq26 in patients with Simpson-Golabi-Behmel syndrome: Identification of loss-of-function mutations in the GPC3 gene. Hum Mol Genet 9: 1321–1328, 2000 [DOI] [PubMed] [Google Scholar]
- 162.Nakamura J, Yanagita M: Bmp modulators in kidney disease. Discov Med 13: 57–63, 2012 [PubMed] [Google Scholar]
- 163.Katagiri T, Watabe T: Bone morphogenetic proteins. Cold Spring Harb Perspect Biol 8: a021899, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brazil DP, Church RH, Surae S, Godson C, Martin F: BMP signalling: Agony and antagony in the family. Trends Cell Biol 25: 249–264, 2015 [DOI] [PubMed] [Google Scholar]
- 165.Michos O, Gonçalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R: Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405, 2007 [DOI] [PubMed] [Google Scholar]
- 166.Winnier G, Blessing M, Labosky PA, Hogan BL: Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116, 1995 [DOI] [PubMed] [Google Scholar]
- 167.Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL: Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol 188: 235–247, 1997 [DOI] [PubMed] [Google Scholar]
- 168.Dudley AT, Robertson EJ: Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn 208: 349–362, 1997 [DOI] [PubMed] [Google Scholar]
- 169.Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P: Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology 136: 2652–2663, 1995 [DOI] [PubMed] [Google Scholar]
- 170.Martinez G, Loveland KL, Clark AT, Dziadek M, Bertram JF: Expression of bone morphogenetic protein receptors in the developing mouse metanephros. Exp Nephrol 9: 372–379, 2001 [DOI] [PubMed] [Google Scholar]
- 171.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND: Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol 231: 31–46, 2001 [DOI] [PubMed] [Google Scholar]
- 172.Oxburgh L, Dudley AT, Godin RE, Koonce CH, Islam A, Anderson DC, Bikoff EK, Robertson EJ: BMP4 substitutes for loss of BMP7 during kidney development. Dev Biol 286: 637–646, 2005 [DOI] [PubMed] [Google Scholar]
- 173.Liu F, Ventura F, Doody J, Massagué J: Human type II receptor for bone morphogenic proteins (BMPs): Extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 15: 3479–3486, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ruberte E, Marty T, Nellen D, Affolter M, Basler K: An absolute requirement for both the type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell 80: 889–897, 1995 [DOI] [PubMed] [Google Scholar]
- 175.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P: The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 277: 5330–5338, 2002 [DOI] [PubMed] [Google Scholar]
- 176.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J: TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014, 1992 [DOI] [PubMed] [Google Scholar]
- 177.Wharton KA, Ray RP, Gelbart WM: An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development 117: 807–822, 1993 [DOI] [PubMed] [Google Scholar]
- 178.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM: The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell 1: 673–683, 1998 [DOI] [PubMed] [Google Scholar]
- 179.Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N: Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci U S A 95: 9337–9342, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Connor MB, Umulis D, Othmer HG, Blair SS: Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133: 183–193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lovric S, Ashraf S, Tan W, Hildebrandt F: Genetic testing in steroid-resistant nephrotic syndrome: When and how? Nephrol Dial Transplant 31: 1802–1813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Braun DA, Schueler M, Halbritter J, Gee HY, Porath JD, Lawson JA, Airik R, Shril S, Allen SJ, Stein D, Al Kindy A, Beck BB, Cengiz N, Moorani KN, Ozaltin F, Hashmi S, Sayer JA, Bockenhauer D, Soliman NA, Otto EA, Lifton RP, Hildebrandt F: Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int 89: 468–475, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pitera JE, Scambler PJ, Woolf AS: Fras1, a basement membrane-associated protein mutated in Fraser syndrome, mediates both the initiation of the mammalian kidney and the integrity of renal glomeruli. Hum Mol Genet 17: 3953–3964, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hashmi S, Marinkovich MP: Molecular organization of the basement membrane zone. Clin Dermatol 29: 398–411, 2011 [DOI] [PubMed] [Google Scholar]
- 185.Libby RT, Champliaud MF, Claudepierre T, Xu Y, Gibbons EP, Koch M, Burgeson RE, Hunter DD, Brunken WJ: Laminin expression in adult and developing retinae: Evidence of two novel CNS laminins. J Neurosci 20: 6517–6528, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y: Perlecan is essential for cartilage and cephalic development. Nat Genet 23: 354–358, 1999 [DOI] [PubMed] [Google Scholar]
- 187.Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R: Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J 22: 236–245, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K: GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406: 419–422, 2000 [DOI] [PubMed] [Google Scholar]
- 189.Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL: Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet 80: 616–632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.