Abstract
RATIONALE
Low-dose ketamine is a rapid-acting antidepressant, to which female rodents are more sensitive as compared to males. However, the mechanism mediating this sex difference in ketamine sensitivity remains elusive.
OBJECTIVES
We sought to determine whether male and female mice differ in their behavioral sensitivity to low doses of ketamine, and uncover how ovarian hormones influence females’ ketamine sensitivity. We also aimed to uncover some of the molecular mechanism(s) in mood-related brain regions that mediate sex differences in ketamine antidepressant effects.
METHODS
Male and female mice (freely-cycling, diestrus 1 [D1], proestrus [Pro], or D1 treated with an estrogen receptor (ER) α, ERβ, or progesterone receptor (PR) agonist) received ketamine (0, 1.5, or 3 mg/kg intraperitoneally) and were tested in the forced swim test (FST) 30 min later. Ketamine’s influence over synaptic plasticity markers in the prefrontal cortex (PFC) and hippocampus (HPC) of males, D1, and Pro females was quantified by Western blot 1 h post-treatment.
RESULTS
Males, freely-cycling females, D1 and Pro females exhibited antidepressant-like responses to 3 mg/kg ketamine. Pro females were the only group where ketamine exhibited an antidepressant effect at 1.5 mg/g. D1 females treated with an agonist for ERα and ERβ exhibited an antidepressant-like response to 1.5 mg/kg ketamine. Ketamine (3 mg/kg) increased synaptic plasticity-related proteins in the PFC and HPC of males, D1, and Pro females. Yet, Pro females exhibited an increase in p-Akt and p-CaMKIIα in response to 1.5 and 3 mg/kg ketamine.
CONCLUSION
Our results indicate that females’ enhanced sensitivity to ketamine during Pro is likely mediated through estradiol acting on ERα and ERβ, leading to greater activation of synaptic plasticity-related kinases within the PFC and HPC.
Keywords: estrogen, estrous cycle, forced swim test, ketamine, progesterone, sex differences
1. Introduction
The N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine, has shown great promise as a rapid-acting antidepressant in humans and rodents (Abdallah et al., 2015; Kavalali and Monteggia, 2015). In clinical studies, a single infusion of low-dose of ketamine alleviates symptoms of depression within hours (Berman et al., 2000; Zarate et al., 2006). This rapid effect is superior to other antidepressants (e.g., selective serotonin-reuptake inhibitors), which can take weeks to months to provide therapeutic benefit (Nutt, 2002; Rush et al., 2006). These exciting findings have burgeoned interest in uncovering the neural loci and molecular mechanisms of ketamine’s rapid antidepressant effects. Low doses of ketamine promote the expression of downstream signaling of synaptic plasticity-related pathways within mood-related brain regions, such as the prefrontal cortex (PFC) and hippocampus (HPC) (Björkholm and Monteggia, 2016; Duman et al., 2016). While there have been great advances in our understanding of some molecular mechanisms of ketamine’s rapid effects, the majority of these investigations have been conducted in males; creating a serious gap in our knowledge, since females experience a two-fold increased risk of depression as compared to males (Kessler, 2003; Van de Velde et al., 2010). In addition the disparity in risk of depression, there is evidence that pharmacodynamics and pharmacokinetics of antidepressant compounds may differ between the sexes, and can be influenced by a variety of factors including levels of ovarian hormones (Bigos et al., 2009). Ovarian hormones, estrogen (E2) and progesterone (P4), influence many common biochemical targets to promote synaptic plasticity and increase spine density in mood-related brain regions (Frick, 2015; Woolley and McEwen, 1992; Woolley et al., 1997). Thus, it is possible for peripherally- or centrally-mediated factors to influence females’ sensitivity to the antidepressant-like effects of ketamine.
Preclinical studies from our lab and others have shown female rodents exhibit behavioral sensitivity to doses of ketamine that are subthreshold for an antidepressant-like effect in males (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Sarkar and Kabbaj, 2016; Zanos et al., 2016). While most of these studies did not control for the stage of estrous, there is evidence that females’ sensitivity may be mediated by the cyclic fluctuations of the ovarian hormones, E2 and P4 (Carrier and Kabbaj, 2013; Sarkar and Kabbaj, 2016). Which is based upon evidence that surgical removal of the ovaries blocked females’ sensitivity and pharmacological replacement of E2/P4 restored females’ sensitivity to a male-subthreshold dose of ketamine (Carrier and Kabbaj, 2013). However, the molecular mechanism(s) mediating these sex differences remain elusive. In the first part of this work, we examined sensitivity to low doses of ketamine in male and female mice in which we did not control for the stage of estrous cycle (freely cycling). Then, to investigate the impact of estrous cycle on sensitivity to ketamine, we then evaluated sensitivity to ketamine in females experiencing a natural nadir (diestrus 1, D1) or a peak (proestrus, Pro) in ovarian hormone levels. To further determine which hormone receptor subtypes might be mediating ketamine high sensitivity in females, D1 females were treated with an estrogen receptor (ER) α, ERβ, or progesterone receptor (PR) agonist and tested 24h later in the FST. Finally, we examined sex/estrous stage differences and ketamine-induced activation of neurotrophic signaling pathways within the prefrontal cortex (PFC) and hippocampus (HPC) of males, D1, and Pro females to explore which one(s) may mediate sex differences in ketamine sensitivity.
2. Materials and Methods
2.1 Animals
One-hundred and eighty five adult male and female C57BL6/J mice (46 males and 139 females, mean weight at time of testing 23.7 and 18.8 g, respectively) from The Jackson Laboratory (Bar Harbor, Maine) aged 7 weeks at time of arrival were used in these experiments. Upon arrival, mice were pair-housed in same-sex pairs and maintained on a 12 h light:12 h dark cycle in a temperature-controlled vivarium in standard Plexiglass cages. Food and water were available ad libitum. Males and females were habituated to animal facilities and handling for one week prior to the start of behavioral testing. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conform to the NIH Guide for Care and Use of Laboratory Animals.
2.2 Drug Treatments
Mice received a single intraperitoneal injection of sterile saline (Veh), 1.5, or 3 mg/kg of racemic ketamine hydrochloride (Ketasthesia®, Henry Schein) and behavioral tests were conducted 30 min later. Females in D1 received a subcutaneous injection of either 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT, 0.5 mg/kg, Tocris Bioscience; ERα agonist), Diarylpropionitrile (DPN, 0.5 mg/kg, Tocris Bioscience; ERβ agonist) or progesterone (P4, 0.5 mg/kg, Sigma-Aldrich), 24 h prior to behavioral testing. This estrous stage was chosen because of the low levels of E2 and P4 during D1. The idea tested here is that during D1 ketamine at 1.5 mg/kg will have no antidepressant effects, however if we stimulate ERs or progesterone receptors during D1 (as to mimic what happens during Pro) ketamine may have an antidepressant effects. This experiment will also allow us to determine which receptors are critical in enhancing female sensitivity to ketamine antidepressant effects.
2.3 Vaginal Cytology
Female mice were gently handled and lavaged daily with 25 μl sterile 0.9% saline as described elsewhere (McLean et al., 2012) within the first 3 hours of the light cycle. For daily analysis of estrous stage, vaginal epithelial cells were deposited onto glass slides and immediately viewed with a light microscope. Degenerated, cornified epithelial cells and few stained leukocytes characterized the D1 estrous stage. The Pro stage was characterized by an abundance of rounded, nucleated epithelial cells. For crystal violet staining, slides were dried for 6 h at room temperature, stained with 0.1% crystal violet (Sigma-Aldrich) for 1 min, rinsed with DDH20 for 1 min ×2, sealed with 30 μl Permount (Sigma-Aldrich), and cover slipped. If a female remained in single estrous stage for more than 4 days, she was excluded from the study.
2.4 Forced swim test (FST)
The FST is a sensitive measure of behavioral effects of antidepressant treatments in rodents (Porsolt et al., 1977). Mice were placed into a 4-L Pyrex glass beaker containing 3 L of water at 24 ± 1ΔC and forced to swim for six minutes. After the FST, mice were returned to their home cage and placed in a holding room until sacrifice. The FST was recorded by a video camera and videos were saved for later analysis. Beakers were cleaned with 70% EtOH and filled with fresh water between each FST. Treatments were delivered in a completely randomized design and counterbalanced such that cage mates received different treatments, and were examined in the FST in parallel. An observer who was blind to experimental assignments when the FST was conducted and when immobility time was scored during the final four minutes of the FST. The final four minutes are commonly scored because mice are active during the onset of this test, and this activity may mask the effect of the treatment (Can et al., 2012). Immobility time was defined as the duration of time in which the mouse maintained this stationary posture and only made movements necessary to keep its head above water.
2.5 Tissue Collection and Processing
Mice were sacrificed via rapid decapitation 24 min after the conclusion of the FST, which corresponds to 1-hour post-vehicle or ketamine treatment. This time point was chosen due to reports that ketamine can rapidly, yet transiently influence protein expression and phosphorylation of molecules implicated in plasticity (Autry et al., 2011; Li et al., 2010). Trunk blood was collected into tubes containing chilled 0.5 M EDTA (Sigma-Aldrich) and stored on ice. Plasma was extracted via refrigerated centrifugation (2800 rpm for 20 min, at 4°C), transferred to sterile pre-chilled microcentrifuge tubes, and stored at −80°. Brains were rapidly removed, flash frozen in methyl butane (Sigma-Aldrich) on dry ice at −20°C, and stored at −80°C. Brains underwent coronal sectioning at 100 μM at −20°C in a cryostat, tissue punches containing the PFC (prelimbic and infralimbic cortices) and HPC (CA1, CA3, and dentate gyrus) were collected in sterile microcentrifuge tubes, and stored at −80°C. Total protein was extracted from these punches using the Tri Reagent protocol (Molecular Research Center), and concentration was determined via Bradford Assay (Bio-Rad). Whole tissue was used for western blot examination as compared to synaptoneurosomes because of the limited volume of tissue that can be generated from specific regions of the mouse brain. Using whole tissue allowed for us to investigate many of the known targets of ketamine and of ovarian hormones within the same animals that underwent behavioral testing. Whole cell hippocampal extracts have also been used by other investigators to examine phosphorylation of molecular targets affected by ketamine (Autry et al., 2011; Choi et al., 2015; Gideons et al., 2014; Nosyreva et al., 2013).
2.6 Radioimmunoassay of Ovarian Hormones
Plasma levels of estradiol and P4 were examined in a subset of vehicle-treated females from the FST experiment, using radioimmunoassay (RIA) kits (Cat. No. 07-238102 and 07-270102, MP Biomedicals). Samples were run in duplicate and any sample with a coefficient of variation >10% was excluded from analysis.
2.7 Western Blot
Ten or 20 μg of protein per sample was resolved by a 12% acrylamide gel and subsequently transferred to a nitrocellulose membrane (Whatman, Protran BA83, GE Healthcare). Membranes were incubated with 5% milk in tris-buffered saline (TBS) for 1 h at room temperature then incubated overnight at 4°C with a primary antibody (full list found in Table 1). After incubation with the primary antibody, membranes were rinsed once with 0.2% TBS Tween and four times with TBS on a rocking platform at room temperature, then incubated for 45 min at room temperature with a donkey anti-rabbit or goat anti-mouse secondary antibody diluted 1:10,000 (Li-COR Biosciences), rinsed once with 0.2% TBST and four times with TBS on a rocking platform, and imaged using an Odyssey infrared imaging system (Li-COR). Quantification was performed using ImageJ software (NIH). Data are shown as the ratio to vehicle-treated male values. The amount of protein for CaMKIIα, GluR1, BDNF, MAPK, Akt, GSK-3β, and mTOR was calculated as the ratio of the optical density (OD) of the target band over the OD of the GAPDH band or from total protein. Levels of p-CaMKIIα, p-GluR1, p-MAPK, p-Akt, p-GSK-3β, and p-mTOR were calculated as a ratio of the OD of the phosphorylated band over the OD of the total band. Two lanes of each gel contained standard samples, which were each comprised of homogenized hippocampal protein from 8 male mice that received no drug treatment and were naïve to the FST. These standard samples were used to normalize protein quantities across membranes.
Table 1.
List of antibodies used in Western blot analysis of the prefrontal cortex and hippocampus.
| Primary Antibody | Host/Type | Manufacturer | Catalog Number | Dilution |
|---|---|---|---|---|
| GAPDH (D16H11) XP® | Rabbit, mAb | Cell Signaling | 5174 | 1:2000 |
| Phospho-CaMKII (Thr286) (D21E4) | Rabbit, mAb | Cell Signaling | 12716 | 1:2000 |
| CaMKII-α (6G9) | Mouse, mAb | Cell Signaling | 50049 | 1:2000 |
| AMPA Receptor (GluR 1) (D4N9V) | Rabbit, mAb | Cell Signaling | 13185 | 1:1000 |
| Phospho-AMPA Receptor (Ser831) | Rabbit, mAb | Abcam | ab109464 | 1:1000 |
| BDNF (N-20) | Rabbit, pAb | Santa Cruz | sc-546 | 1:500 |
| Phospho-p44/42 MAPK (Thr202/Thr204) | Rabbit, pAb | Cell Signaling | 9101 | 1:2000 |
| p44/42 MAPK | Mouse, pAb | Cell Signaling | 9102 | 1:2000 |
| Phospho-Akt (Ser473) (D9W9U) | Mouse, mAb | Cell Signaling | 12694 | 1:1000 |
| Akt | Rabbit, pAb | Cell Signaling | 9272 | 1:1000 |
| Phospho-GSK-3β (Ser9) (D85E12) XP® | Rabbit, mAb | Cell Signaling | 5558 | 1:2000 |
| GSK-3β (3D10) | Mouse, mAb | Cell Signaling | 9832 | 1:1000 |
| Phospho-mTOR (Ser2448) (D9C2) XP® | Rabbit, mAb | Cell Signaling | 5536 | 1:1000 |
| mTOR (L27D4) | Mouse, mAb | Cell Signaling | 4517S | 1:1000 |
mAb, monoclonal antibody; pAb, polyclonal antibody.
2.8 Statistics
Data are reported as mean + SEM. Two-way analysis of variance (ANOVA) was used to examine the effect of ketamine and sex/estrous stage. Unpaired t-tests were used to examine ovarian hormone plasma levels and to determine the effect of ketamine within each hormone receptor agonist condition. Western blot analysis of the effect of ketamine within each sex/estrous stage was examined using two-way ANOVA. When significant main effects or interaction of ketamine or of sex/estrous stage were identified in the two-way ANOVA, post hoc comparisons were made with Fisher’s LSD. Any value that was more than two standard deviations above the group mean was considered an outlier and was removed from the analysis. P-values of <0.05 were considered significant. Sample size was determined based upon previous studies conducted by our group and statistical computation with power analysis that indicated a need for a sample size of 8–10 animals per group in order to obtain power values of 0.8 and higher for p values of .05.
Results
3.1 Effect of sex (males versus freely cycling females) on behavioral sensitivity to the antidepressant-like effect of ketamine in the FST
Thirty min following treatment with ketamine, time spent immobile in the FST was examined in male and female mice (n=7–9/group) as shown in Figure 1. There was a significant effect of ketamine treatment (F(2,40)=5.54, p=.0007) and a significant effect of sex (F(1,40)=4.44, p=.041) to influence time spent immobile. There were however no interactions (F(2,40)=0.06, p=.94). Fisher’s post hoc analyses identified that 3 mg/kg ketamine significantly reduced males and females immobility (t(40)=2.22, p=.03; t(40)=2.49, p=.017, respectively).
Figure 1. Behavioral sensitivity to the antidepressant-like effect of ketamine is influenced by sex.
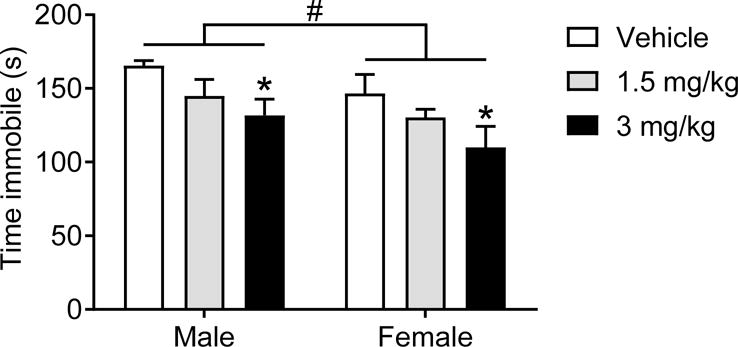
Ketamine at 3 mg/kg reduced immobility time in males and freely-cycling females. Overall, females exhibited lower immobility time as compared to males. *p<0.05 vs. vehicle, #p<0.05 males vs. females. Data are means + SEM. (males: n=7–8/group, females 8/group).
3.2 Effect of estrous stage over behavioral sensitivity to the antidepressant-like effect of ketamine in the FST
To determine the effect of endogenous ovarian hormones to influence behavioral sensitivity to ketamine, time spent immobile in the FST was examined 30 min following ketamine treatment in males, D1, and Pro females (n=7–10/group) as shown in Figure 2. There was a significant effect of ketamine treatment (F(2,64)=10.87, p<.0001) and of sex/estrous stage to influence duration of immobility (F(2,64)=7.36, p=.001). There were however no interactions (F(4,64)=1.99, p=.10). Fisher’s post hoc analyses revealed that males and D1 females have significantly reduced immobility time following 3 mg/kg ketamine (males: t(64)=2.38, p=.02; D1: t(64)=3.295, p=.0016). In contrast, Pro females display significant reductions in immobility following both 1.5 mg/kg and 3 mg/kg ketamine (t(643)=2.897, p=.005; t(64)=2.346, p=.02, respectively). Fisher’s post hoc revealed that Pro females exhibited significantly less immobility as compared to D1 females in the vehicle condition (t(64)=2.016, p=.04). Similarly, Pro females exhibited significantly less immobility as compared to males and D1 females following treatment with 1.5 mg/kg ketamine (t(64)=3.58, p=.0007; t(64)=3.56, p=.0007; respectively).
Figure 2. Behavioral sensitivity to the antidepressant-like effect of ketamine is influenced by sex and by estrous stage.
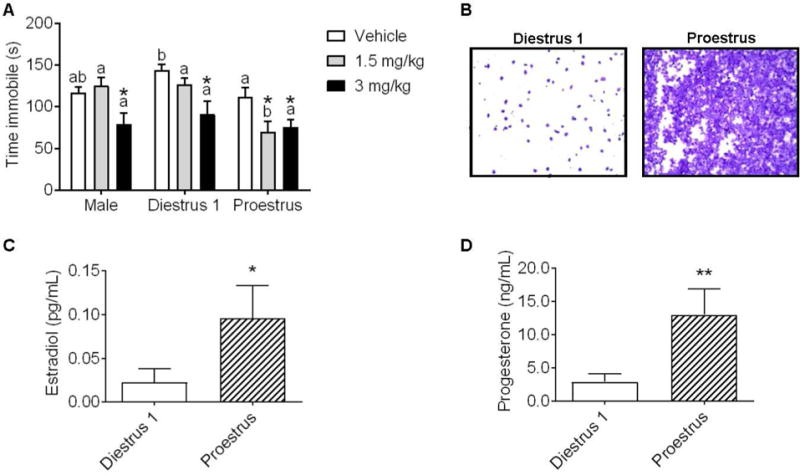
(A) Ketamine at 3 mg/kg reduced immobility time in males and diestrus 1 (D1) females, 1.5 and 3 mg/kg ketamine reduced immobility time in proestrus (Pro) females (*p<.05 vs. within-group vehicle). (B) Representative vaginal lavage samples from D1 and Pro females. (C) Plasma levels of estradiol (pg/mL) and levels of progesterone (ng/mL) were significantly lower in females in D1 vs. Pro females (*p<.05, ** p <.01). Data are means + SEM. Different letters indicate statistically significant differences within the treatment group. (males: n=8/group, D1: n=7–9/group, Pro: n=7–10/group; plasma: n=5–7)
3.3 Confirmation of estrous cycle via ovarian hormone assay
As shown in Figure 2B, D1 females exhibited significantly lower levels of plasma estradiol and P4 vs. Pro females (t(10)=2.03, p=.03; t(10)=2.87, p=.008; respectively).
3.4 Effect of agonists for ERα, ERβ, and PR over D1 females’ behavioral sensitivity to the antidepressant-like effect of ketamine in the FST
To determine which specific hormone receptor subtypes may influence females’ ketamine sensitivity, we examined D1 females’ behavior in the FST 24 h following treatment with an agonist for ERα (PPT), ERβ (DPN), or PR (P4), and 30 min following 0 or 1.5 mg/kg ketamine (n=7–10/group). As shown in Figure 3, two-way ANOVA revealed a significant main effect of vehicle/ketamine (F(1,58)=15.85, p=.0002) and a significant main effect of hormone treatment (F(3,58)=3.6, p=.019) to influence time spent immobile. There were however no interactions (F(3,58)=1.49, p=.22). Post hoc analysis revealed a significant effect of ketamine to reduce immobility time only when given in combination with PPT or DPN (t(58)=3.317, p=.0016; t(58=2.85, p=.006, respectively). PPT- or DPN-treated females exhibited less time immobile as compared to oil-treated females only when given in combination with 1.5 mg/kg ketamine (t(58)=2.719, p=.0086; t(58=2.805, p=.0068, respectively. The lack of effect of the ERα and ERβ agonists within the vehicle condition indicates that these doses of hormone receptor agonist are not sufficient to influence FST behavior on their own.
Figure 3. Behavioral sensitivity to the antidepressant-like effect of ketamine is influenced by agonists for ERα and ERβ.
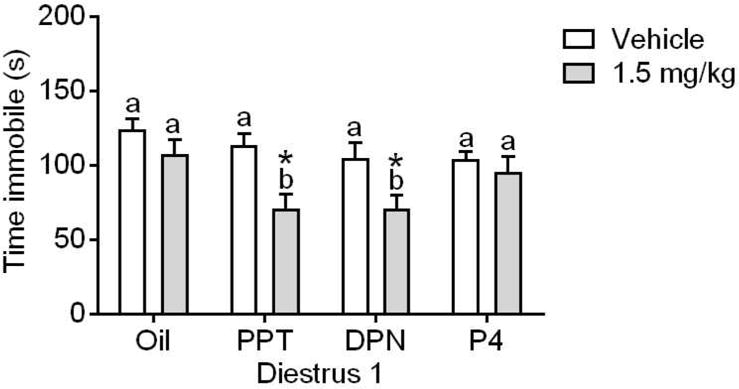
Ketamine at 1.5 mg/kg reduced immobility time for D1 females treated with PPT (ERα agonist) or DPN (ERβ agonist), but not progesterone (P4). *p<0.05 vs. within-group vehicle; Different letters indicate statistically significant differences within the treatment group. Data are means + SEM. (n=7–10/group)
3.5 Effect of sex/estrous stage and ketamine on PFC p-CaMKIIα levels
One hour following ketamine treatment, we examined the level of activation of synaptic plasticity markers p-CaMKIIα within the PFC (n=4–6 samples/group). There was no significant effect of ketamine or of sex/estrous stage to influence phosphorylation of CaMKIIα (F(2,38)=0.27, p=.76; F(2,38)=1.04, p=.36, respectively; Figure 4A). There were also no interactions (F(4,38)=1.45, p=.23).
Figure 4. Influence of sex/estrous stage on protein expression of synaptic plasticity markers within the prefrontal cortex (PFC) following treatment with vehicle or ketamine.
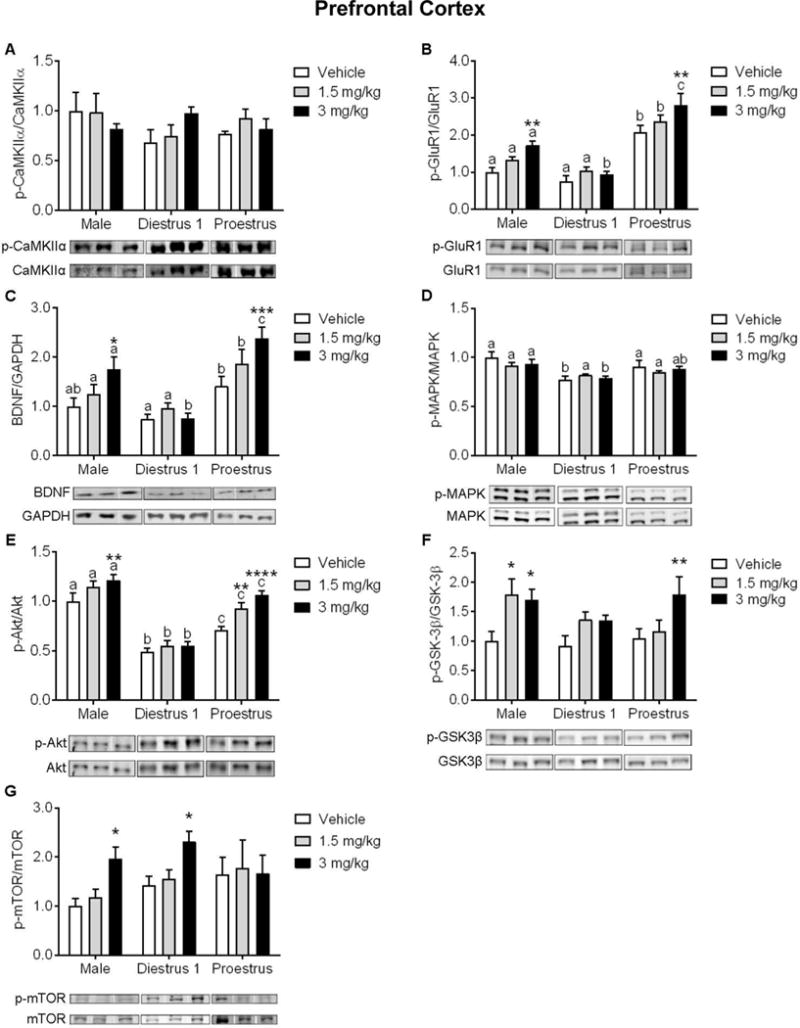
(A) Protein expression of p-CaMKIIα/CaMKIIα was not affected by ketamine nor by sex/estrous stage. (B) Males and Pro females exhibited an increased expression of p-GluR1/GluR1 and (C) increased expression of BDNF following 3 mg/kg ketamine. (D) Protein expression of p-MAPK/MAPK was not affected by ketamine, yet there was a main effect of sex/estrous stage. (E) Ketamine at 1.5 mg/kg increased expression of p-Akt/Akt in Pro females, and 3 mg/kg increased expression in males and Pro females. (F) Ketamine at 1.5 mg/kg increased expression of p-GSK-3β/GSK-3β in males, and in males and Pro females at 3 mg/kg. (G) Ketamine at 3 mg/kg increased p-mTOR/mTOR expression in males and D1 females. *p<.05, **p<.005, ***p<.0005, ****p<0.0001 vs. within-group vehicle. Different letters indicate statistically significant differences between sex/estrous stages within the vehicle treatment group. Data are means + SEM. (n=4–8/group)
3.6 Effect of sex/estrous stage and ketamine on PFC p-GluR1 levels
Two-way ANOVA indicated a main ketamine treatment effect (F(2,45)=9.667, p=.0003; n=4–7 samples/group; Figure 4B) and a main sex/estrous stage effects on the levels of p-GluR1 (F(2,45)=68.49, p<.0001; n=4–7 samples/group; Figure 4B). There were however no interactions (F(4,45)=1.33, p=.27). Post hoc examination revealed a significant effect of 3 mg/kg ketamine to increase p-GluR1 expression in males and Pro females (t(45)=3.38, p=.0015; t(45)=3.31, p=.0018, respectively). Within the vehicle and 1.5 mg/kg ketamine treatment conditions, Pro females exhibited higher levels of p-GluR1 as compared to males (t(45)=4.89, p<.0001, t(45)=4.19, p=.0001) and D1 females (t(45)=6.00, p<.0001, t(45)=5.41, p<.0001). Following treatment with 3 mg/kg ketamine, D1 females exhibited significantly lower p-GluR1 levels as compared to males and Pro females (t(45)=3.80, p=.0004; t(45)=8.78, p<.0001, respectively); Pro females exhibited higher levels of p-GluR1 as compared to males (t(45)=5.13, p<.0001).
3.7 Effect of sex/estrous stage and ketamine on PFC BDNF levels
Two-way ANOVA indicated a main ketamine treatment effect (F(2,44)=7.12, p=.002; n=4–7/group; Figure 4C) and a main sex/estrous stage effects on the levels of BDNF within the PFC ((2,44)=23.54, p<.0001; n=4–7/group; Figure 4C). There were however no interactions (F(4,44)=2.23, p=.08). Post hoc examination revealed a significant increase in BNDF levels following 3 mg/kg ketamine in males (t(44)=2.63, p=.01) and Pro females (t(44)=3.81, p=.0004). Pro females exhibited higher BDNF levels as compared to D1 females in all treatment conditions (vehicle: t(44)=2.62, p=.01; 1.5 mg/kg: t(44)=3.05, p=.004; 3 mg/kg: t(44)=6.39, p<.0001). Following treatment with 1.5 mg/kg and 3 mg/kg ketamine, Pro females exhibited higher BDNF levels as compared males (t(44)=2.08, p=.04; t(44)=2.57, p=.01, respectively). While males exhibited higher levels of BDNF as compared to D1 females in the 3 mg/kg ketamine treatment condition (t(44)=3.92, p=.0003).
3.8 Effect of sex/estrous stage and ketamine on PFC p-MAPK levels
There was no significant effect of ketamine treatment to influence p-MAPK levels within the PFC (F(2,47)=0.51, p=.6; n=5–7/group; Figure 4D), yet there was a significant main effect of sex/estrous stage (F(2,47)=14.68, p<.0001). There were however no interactions (F(4,47)=0.99, p=.42). Post hoc analysis revealed that in the vehicle condition, males and Pro females exhibited higher levels of p-MAPK as compared to D1 females (t(47)=4.47, p<.0001; t(47)=2.51, p=.01, respectively). Additionally, males exhibited higher p-MAPK levels following 3 mg/kg ketamine as compared to D1 female counterparts (t(47)=3.02, p=.004).
3.9 Effect of sex/estrous stage and ketamine on PFC p-Akt levels
Two-way ANOVA indicated a main ketamine treatment effect (F(2,53)=12.77, p<.0001; n=6–8/group; Figure 4E) and a main sex/estrous stage effects on the levels of p-Akt within the PFC (F(2,53)=99.16, p<.0001; n=6–8/group; Figure 4E). There were however no interactions (F(4,53)=2.19, p=.08). Post hoc analysis revealed a significant increase in p-Akt in males in response to 3 mg/kg ketamine (t(53)=2.96, p=.004), and in Pro females in response to 1.5 and 3 mg/kg ketamine (t(53)=2.8, p=.007; t(53)=4.69, p<.0001, respectively). In all treatment conditions, D1 females exhibited lower p-Akt as compared to males and Pro females (vehicle: t(53)=6.83, p<.0001; t(53)=2.89, p=.005; 1.5 mg/kg: t(53)=7.43, p<.0001; t(53)=4.94, p<.0001; 3 mg/kg: t(53)=9.98, p<.0001; t(53)=7.26, p<.0001, respectively). Additionally, males exhibited higher levels of p-Akt as compared to Pro females in all treatment conditions (vehicle: t(53)=3.69, p=.0005; 1.5 mg/kg: t(53)=2.64, p=.01; 3 mg/kg; t(53)=2.16, p=.03).
3.10 Effect of sex/estrous stage and ketamine on PFC p-GSK-3β levels
There was a significant main effect of ketamine treatment to influence levels of p-GSK-3β in the PFC (F(2,46)=7.75, p=.001; n=5–7/group; Figure 4F), yet no effect of sex/estrous stage (F(2,46)=1.47, p=.24). There were however no interactions (F(4,46)=1.24, p=.31). Post hoc examination revealed a significant effect of 1.5 mg/kg ketamine to increase p-GSK-3β in males (t(46)=2.65, p=.01), and a significant effect of 3 mg/kg ketamine to increase levels in males and Pro females (t(46)=2.35, p=.02; t(46)=2.72, p=.009, respectively).
3.11 Effect of sex/estrous stage and ketamine on PFC p-mTOR levels
There was a significant main effect of ketamine treatment to increase levels of PFC p-mTOR (F(2,52)=4.01, p=.02; n=5–8/group; Figure 4G), yet no effect of sex/estrous stage (F(2,52)=1.44, p=.25). There were however no interactions (F(4,52)=1.05, p=.39). Post hoc examination revealed a significant increase in p-mTOR in response to 3 mg/kg ketamine in males and D1 females (t(52)=2.36, p=.02; t(52)=2.34, p=.02, respectively). The effects of ketamine within the PFC are summarized in Table 2 and Figure 7A.
Table 2.
Summary of ketamine’s effects on activation of synaptic plasticity markers in the prefrontal cortex.
| Prefrontal Cortex | |||
|---|---|---|---|
| Male | Diestrus 1 | Proestrus | |
| p - CaMKIIα | ↔ | ↔ | ↔ |
| p - GluR1 | ↑ 3 mg/kg | ↔ | ↑ 3 mg/kg |
| BDNF | ↑ 3 mg/kg | ↔ | ↑ 3 mg/kg |
| p - MAPK | ↔ | ↔ | ↔ |
| p - Akt | ↑ 3 mg/kg | ↔ | ↑ 1.5 & 3 mg/kg |
| p - GSK - 3β | ↑ 1.5 & 3 mg/kg | ↔ | ↑ 3 mg/kg |
| p - mTOR | ↑ 3 mg/kg | ↑ 3 mg/kg | ↔ |
↔ no change from vehicle-treated samples, ↑ in levels as compared to vehicle-treated samples.
3.12 Effect of sex/estrous stage and ketamine on HPC p-CaMKIIα levels
Following the same timeline as the one described for the PFC, we examined the phosphorylation of synaptic plasticity markers within the HPC. As shown in Figure 5A, there were significant main effects of ketamine and of sex/estrous stage to influence levels of p-CaMKIIα (F(2,44)=9.54, p=.0004; F(2,44)=6.94, p=.002, respectively; n=5–6/group) as well as a significant interaction between the two (F(2,44)=2.59, p=.04). Post hoc analysis revealed that 3 mg/kg ketamine increased p-CaMKIIα levels in males (t(44)=3.22, p=.002), and both 1.5 and 3 mg/kg ketamine increased levels in Pro females (t(44)=3.04, p=.0039; t(44)=4.00, p=.0002, respectively). Post hoc analysis of sex/estrous stage effects revealed significantly higher levels of p-CaMKIIα in Pro females as compared to males and D1 females in response to treatment with 1.5 mg/kg ketamine (t(44)=6.17, p=.036; t(44)=2.64, p=.01, respectively). In contrast, D1 females exhibited lower levels of p-CaMKIIα as compared to males and Pro females following 3 mg/kg ketamine (t(44)=3.14, p=.003; t(44)=3.72, p=.0006, respectively).
Figure 5. Influence of sex/estrous stage on protein expression of synaptic plasticity markers within the hippocampus (HPC) following treatment with vehicle or ketamine.
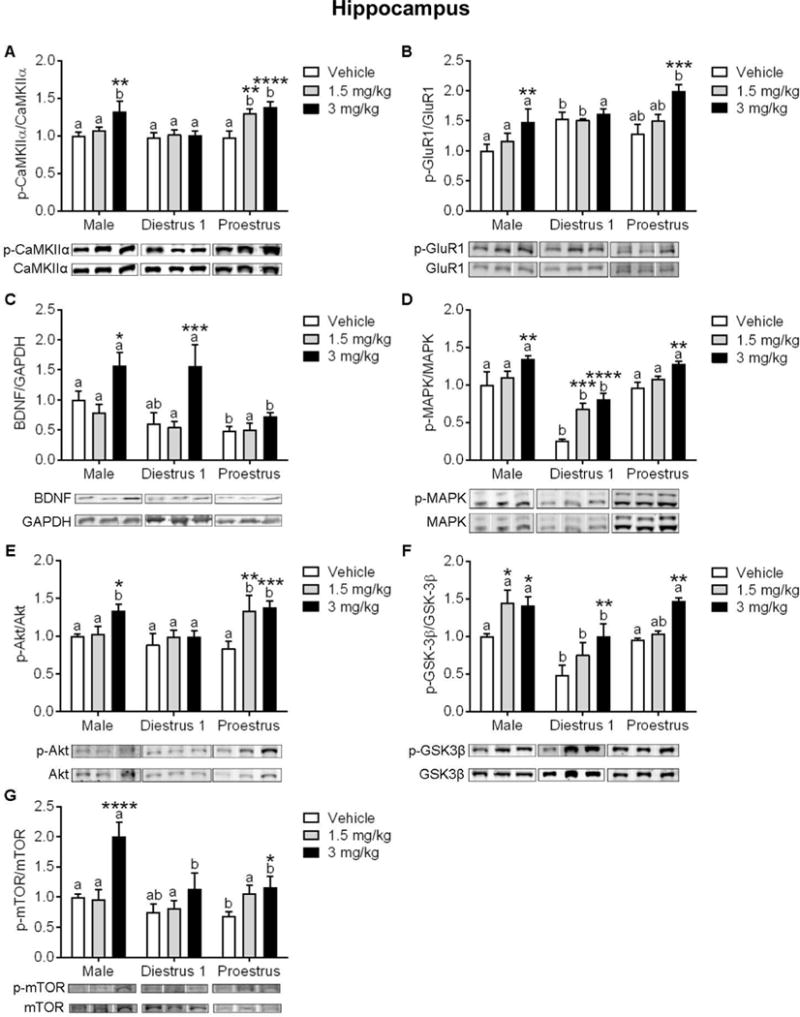
(A) Protein expression of p-CaMKIIα/CaMKIIα was increased by 1.5 mg/kg ketamine in Pro females, and in males and Pro females by 3 mg/kg. (B) Ketamine at 3 mg/kg increased expression of p-GluR1/GluR1 in males and Pro females, (C) and increased expression of BDNF in males and D1 females. (D) Protein expression of p-MAPK/MAPK was significantly lower in D1 females in all treatment conditions. Ketamine at 1.5 mg/kg increased expression of p-MAPK in D1 females, and in all groups at 3 mg/kg. (E) Ketamine at 1.5 mg/kg increased expression of p-Akt/Akt in Pro females, and 3 mg/kg increased expression in males and Pro females. (F) Ketamine at 1.5 mg/kg increased expression of p-GSK-3β/GSK-3β in males, and in all groups at 3 mg/kg. (G) Ketamine at 3 mg/kg increased p-mTOR/mTOR expression in males and D1 females. *p<.05, **p<.005, ***p<.0005, ****p<0.0001 vs. within-group vehicle. Different letters indicate statistically significant differences between sex/estrous stages within the vehicle treatment group. Data are means + SEM. (n=4–7/group)
3.13 Effect of sex/estrous stage and ketamine treatment on HPC p-GluR1
As shown in Figure 5B, there were significant main effects of ketamine and sex/estrous stage to influence levels of p-GluR1 within the HPC (F(2,43)=9.87, p=.0003; F(2,43)=8.88, p=.0006, respectively; n=4–7/group). There were however no interactions (F(4,43)=1.82, p=.14). Post hoc examination of the effect of ketamine revealed a significant increase in males and Pro females following 3 mg/kg (t(43)=2.82, p=.007; t(43)=4.15, p=.0002, respectively). Post hoc examination of the effect of sex/estrous stage revealed significantly higher levels of p-GluR1 in D1 females as compared to males in the vehicle condition and following 1.5 mg/kg ketamine (t(43)=3.18, p=.002; t(43)=2.04, p=0.04, respectively). In contrast, following 3 mg/kg ketamine Pro females exhibited higher p-GluR1 levels as compared to males and D1 females (t(43)=3.2, p=.002; t(43)=2.33, p=0.02, respectively).
3.14 Effect of sex/estrous stage and ketamine on HPC BDNF levels
There were significant main effects of ketamine and of sex/estrous stage to influence BDNF levels within the HPC (F(2,44)=13.31, p=.001; F(2,44)=7.48, p<.0001, respectively; n=5–7/group; Figure 5C). There were however no interactions (F(4,44)=1.79, p=.14). Post hoc analysis of the effect of ketamine revealed an increase in males and D1 females following 3 mg/kg ketamine (t(44)=2.25, p=.02; t(44)=3.94, p=.0003, respectively). Post hoc analysis of the effect of sex/estrous stage revealed significantly higher levels of BDNF in males as compared to Pro females in the vehicle condition (t(44)=2.12, p=.03), and significantly higher levels in males and D1 vs. Pro females following treatment with 3 mg/kg ketamine (t(44)=3.45, p=.001; t(44)=3.59, p=.0008, respectively).
3.15 Effect of sex/estrous stage and ketamine on HPC p-MAPK levels
For levels of HPC p-MAPK, there were significant main effects of ketamine and of sex/estrous stage (F(2,43)=22.51, p<.0001; F(2,43)=55.65, p<.0001, respectively; n=5–6/group; Figure 5D). There were however no interactions (F(4,43)=1.72, p=.16). Post hoc analysis of the main effect of ketamine revealed that 1.5 and 3 mg/kg ketamine increased p-MAPK levels in D1 females (t(43)=4.19, p=.0001; (t(43)=5.4, p<.0001, respectively) and 3 mg/kg increased levels in males and Pro females (t(43)=3.2, p=.003, t(43)=3.04, p=.004, respectively). Post hoc examination of the main effect of sex/estrous stage revealed significantly lower levels of p-MAPK in D1 females within all treatment conditions as compared to males (vehicle: t(43)=6.93, p<.0001; 1.5 mg/kg: t(43)=4.06, p=.0002; 3 mg/kg: t(43)=5.24, p<.0001) and vs. Pro females (vehicle: t(43)=6.93, p<.0001; 1.5 mg/kg: t(43)=3.69, p=.0006; 3 mg/kg: t(43)=4.57, p<.0001).
3.16 Effect of sex/estrous stage and ketamine on HPC p-Akt levels
There were significant main effects of ketamine treatment and of sex/estrous stage to influence protein expression of p-Akt in the HPC (F(2,41)=7.46, p=.0017; F(2,41)=3.49, p=.03, respectively; n=4–7/group; Figure 5E). There were however no interactions (F(4,41)=1.92, p=.12). Post hoc analysis of the main effect of ketamine revealed a significant increase in males following 3 mg/kg ketamine (t(44)=2.23, p=.03), and a significant increase in Pro females following 1.5 and 3 mg/kg (t(44)=3.08, p=.004; t(44)=3.9, p=.0004, respectively). Post hoc analysis of the main effect of sex/estrous stage revealed significantly higher levels of p-Akt in Pro females as compared to D1 females in the 1.5 mg/kg condition (t(44)=2.14, p=.04). Additionally, following treatment with 3 mg/kg ketamine, males and Pro females exhibited higher levels of p-Akt as compared to D1 counterparts (t(44)=2.04, p=.04; t(44)=2.47, p=.01, respectively).
3.17 Effect of sex/estrous stage and ketamine on HPC p-GSK-3β
There were significant main effects of ketamine and of sex/estrous stage to influence levels of p-GSK-3β in the HPC (F(2,43)=12.74, p<.0001; F(2,43)=17.24, p<.0001, respectively; n=5–7/group; Figure 5F). There were however no interactions (F(4,43)=1.14 p=.34). Post hoc analysis of the main effect of ketamine revealed a significant increase in males following 1.5 mg/kg and 3 mg/kg ketamine (t(43)=2.77, p=.008; t(43)=2.54, p=.01, respectively), as well as a significant increase in D1 and Pro females following 3 mg/kg (t(43)=3.07, p=.004; t(43)=3.12, p=.003, respectively). Post hoc analysis of the main effect of sex/estrous stage revealed significantly lower levels of p-GSK-3β in D1 females as compared to males and Pro females in the vehicle condition (t(43)=3.05, p=.004; t(43)=2.67, p=.01, respectively) and following 3 mg/kg ketamine (t(43)=2.51, p=.01; t(43)=2.98, p=.005, respectively). In response to 1.5 mg/kg ketamine, males exhibited higher levels of p-GSK-3β as compared to D1 and Pro females (t(43)=4.28, p=.0001; t(43)=2.43, p=.02, respectively).
3.17 Effect of sex/estrous stage and ketamine on HPC p-mTOR
There were significant main effects of ketamine and of sex/estrous stage to influence expression of p-mTOR in the HPC (F(2,41)=15.97, p<.0001; F(2,41)=13.4, p<.0001, respectively; n=4–6/group) as well as a significant interaction between the two (F(2,41)=4.73, p=.003; Figure 5G). Post hoc analysis of the ketamine effect revealed a significant increase in males and in Pro females following 3 mg/kg (t(41)=5.66, p<.0001; t(41)=2.04, p=.04, respectively). Post hoc analysis of the main effect of sex/estrous stage to influence p-mTOR revealed significantly higher levels in males as compared to Pro females treated with vehicle (t(41)=2.41, p=.02). Additionally, there were group differences in the 3 mg/kg ketamine condition, such that males exhibited higher levels as compared to all female counterparts (D1: t(41)=4.365, p<.0001; Pro: t(41)=6.34, p<.0001). The effects of ketamine within the HPC are summarized in Table 3 and Figure 7B.
Table 3.
Summary of ketamine’s effects on activation of synaptic plasticity markers in the hippocampus.
| Hippocampus | |||
|---|---|---|---|
| Male | Diestrus 1 | Proestrus | |
| p - CaMKIIα | ↑ 3 mg/kg | ↔ | ↑ 1.5 & 3 mg/kg |
| p - GluR1 | ↑ 3 mg/kg | ↔ | ↑ 3 mg/kg |
| BDNF | ↑ 3 mg/kg | ↑ 3 mg/kg | ↔ |
| p - MAPK | ↑ 3 mg/kg | ↑ 1.5 & 3 mg/kg | ↑ 3 mg/kg |
| p - Akt | ↑ 3 mg/kg | ↔ | ↑ 1.5 & 3 mg/kg |
| p - GSK3 - β | ↑ 1.5 & 3 mg/kg | ↑ 3 mg/kg | ↑ 3 mg/kg |
| p - mTOR | ↑ 3 mg/kg | ↔ | ↑ 3 mg/kg |
↔ no change from vehicle, ↑ in levels as compared to vehicle-treated samples.
4. Discussion
In this study, we demonstrate that when examined only by sex, male and female mice exhibit similar behavioral sensitivity to ketamine in the FST. In contrast, when stage of estrous is controlled for, D1 females and males exhibit similar ketamine sensitivity. While Pro females exhibit an antidepressant-like response to a dose of ketamine that is subthreshold for effect for the other groups (i.e., 1.5 mg/kg). Furthermore, we show that pharmacological activation of ERα or ERβ is sufficient to render D1 females sensitive to the Pro-effective dose of ketamine, which suggests that E2 action at these receptor subtypes may mediate females’ behavioral sensitivity to ketamine. Investigation into the molecular mechanism(s) that may mediate different ketamine sensitivity revealed that at behaviorally effective doses of ketamine (1.5 and 3 mg/kg), Pro females exhibited activation of CaMKIIα within the HPC, and of Akt in the PFC and HPC in response to a male- and D1-subthreshhold dose of ketamine.
Behavioral effects of ketamine and ovarian hormones in the FST
In the present study, we observed similar behavioral sensitivity to ketamine in males and freely cycling female mice in the FST when examined 30 min post-treatment. This was unexpected, due to previous reports in rats (Carrier and Kabbaj, 2013; Sarkar and Kabbaj, 2016) and in mice (Franceschelli et al., 2015; Zanos et al., 2016) which demonstrated that females respond to a male-subthreshold dose of ketamine. While unexpected, this difference is not unwarranted since when not controlling for the stage of estrous, the females within the test group may be comprised of more Pro vs. D1 females depending upon the group make up on the test day (Goldman et al., 2007). Furthermore, without assessing estrous cyclicity in females, it is impossible to determine if the females are experiencing natural fluctuations in ovarian hormones, experiencing a period of anestrus due to housing in an all-female environment (Cooper et al., 1993). To control for this potential confound, the goal of the present study was to examine females’ ketamine sensitivity during stages of the estrous cycle characterized by low and high ovarian hormone levels (D1 and Pro, respectively), because it is well known that ovarian hormones (E2 and P4) influence depression-like behavior (Frye and Walf, 2002; Mahmoud et al., 2016), can impact behavioral sensitivity to antidepressants (Carrier and Kabbaj, 2013; Estrada-Camarena et al., 2008; Kokras et al., 2015), as well as influence neurochemical responses to antidepressants (Allen et al., 2012; Kendall et al., 1981; Wilson et al., 1989). In line with the known antidepressant-like effects of E2 and P4 (Frye, 2011; Walf and Frye, 2009, 2010), we found that vehicle-treated D1 females exhibit higher indices of behavioral despair in the FST as compared to Pro counterparts. Males and D1 females exhibited similar sensitivity to ketamine in the FST, where both groups showed an antidepressant-like response 30 min following treatment with 3 mg/kg ketamine. These results are in line with a report by Autry et al., (2011) that demonstrated male mice exhibit sensitivity to 3 mg/kg ketamine at 30 min, 3 h, 24 h, and 1 week post-treatment (Autry et al., 2011), but see (Franceschelli et al., 2015; Zanos et al., 2016). In the present study, Pro females exhibited an antidepressant-like response to both 1.5 and 3 mg/kg ketamine, indicating enhanced behavioral sensitivity. To our knowledge, this is the first report of the effect of estrous stage to influence sensitivity to the antidepressant-like effects of ketamine. These findings are consistent with a recent report, which demonstrated females’ responsivity to ketamine’s reinforcing effects is dependent upon estrous stage (Wright et al., 2017). Our findings are also in agreement with findings that ovariectomized (OVX) females treated with E2/P4 exhibit behaviorally sensitivity to 2.5 mg/kg ketamine, which is subthreshold for effect in OVX females without E2/P4 and in males (Carrier and Kabbaj, 2013; Saland et al., 2016; Wright et al., 2017). Adding support to our findings in Pro females, when D1 females were treated with an agonist for ERα or ERβ, but not for PR, they exhibited an antidepressant-like response to 1.5 mg/kg ketamine. Taken together, these results suggest that endogenous ovarian hormones, specifically E2, can act at either ERα or ERβ subtypes to enhance females’ sensitivity to ketamine.
Neurotrophic effects of ketamine and ovarian hormones within the PFC and HPC
The present study aimed to elucidate potential molecular mechanisms that mediate sex/estrous stage differences in behavioral sensitivity to antidepressant doses of ketamine. The rapid antidepressant-like effects of ketamine are paralleled, and supported by, the expression and activation of neuroplasticity-related signaling pathways within the PFC and HPC (Björkholm and Monteggia, 2016; Duman et al., 2016). This includes increased expression and release of BDNF and activation of mTOR within mood-related brain regions. Ketamine, E2, and P4 share many biochemical targets, through which they support synaptic plasticity and increase spine density in mood-related brain regions (Frick, 2015; Woolley and McEwen, 1992; Woolley et al., 1997). Based upon this evidence we hypothesized that if females are experiencing a natural peak in levels of E2/P4, then enhanced activation of these shared plasticity-related signaling pathways may be one mechanism through which Pro females are more sensitive to ketamine as compared to males and D1 females.
Ketamine rapidly increases glutamate release and neuronal depolarization (Höflich et al., 2016; Li et al., 2016; Moghaddam et al., 1997). Preclinical evidence suggests that ketamine’s induction of neuronal plasticity-related functions likely underlies its rapid antidepressant-like effects (Lepack et al., 2015; Li et al., 2010; Li et al., 2011). Postsynaptic depolarization is accompanied by an influx of calcium, which promotes long-term potentiation and synaptic plasticity through its phosphorylation of CaMKIIα (Herring and Nicoll, 2016; Lisman et al., 2012). Previous reports have shown that ketamine can rapidly increase levels of p-CaMKIIα in the HPC of male rats (Choi et al., 2015), which we confirmed in the present investigation in male mice. As has been shown for ketamine, there is evidence that E2 also promotes the phosphorylation of CaMKIIα (Hasegawa et al., 2015). In line with this role of E2, we found that Pro females exhibited HPC CaMKIIα activation in a manner that was consistent with their behavioral ketamine sensitivity (i.e., increased by 1.5 and 3 mg/kg). Of the many functions carried out by p-CaMKIIα to support synaptic plasticity, one important task is the phosphorylation of the GluR1 subunit of AMPA receptors (Barria et al., 1997). This supports GluR1 insertion into the synapse and can enhance channel conductivity (Derkach et al., 2007). Previous reports posited that ketamine’s up-regulation of AMPA receptor expression may underlie enhanced glutamatergic sensitivity and synaptic plasticity (Kavalali and Monteggia, 2012; Li et al., 2010). Consistent with this effect of ketamine, we observed a significant increase in levels of p-GluR1 in the PFC and HPC of males and Pro females in 1 h following treatment with 3 mg/kg ketamine.
Antidepressant doses of ketamine rapidly increase BDNF levels in the PFC and HPC (Autry et al., 2011; Garcia et al., 2008; Lepack et al., 2015; Zhou et al., 2014b). In line with these previous reports in males, we observed this effect of ketamine in the male PFC and HPC. We extend previous reports and demonstrate an increase in BDNF within the PFC of Pro females, and in the HPC of D1 females. Consistent with the role of E2 and P4 to promote BDNF expression/release (Kaur et al., 2007; Scharfman et al., 2003; Segal and Murphy, 2001), Pro females exhibited higher levels of BDNF within the PFC as compared to D1 females in all treatment conditions. However, we observed no ovarian hormone-related difference in BDNF levels within the HPC. This may be due to our collection of the entire CA1/CA3 region, since the estradiol-related expression of BDNF exhibits subregion specificity (e.g., CA3) (Spencer-Segal et al., 2012).
There are many intracellular signaling pathways that are downstream of BDNF, and are activated in response to antidepressant doses of ketamine. Levels of phosphorylated MAPK have been shown to rapidly rise in response to antidepressant doses of ketamine in the PFC of male rodents (Li et al., 2010). Unexpectedly, we observed no effect of ketamine over activation of this kinase within the PFC. This lack of effect may be due to methodological differences between the studies such as rodent species (rat vs. mouse) or dose of ketamine (3 vs. 10 mg/kg). Additionally, protein extraction technique (synaptoneurosomal vs. total protein) may have impacted our ability to detect ketamine-induced phosphorylation of MAPK, as we used total protein and observed no effect of ketamine, consistent with another report (Réus et al., 2014). It is important to note that other studies have reported ketamine-induced elevations in p-MAPK within the HPC using total protein (Choi et al., 2015; Park et al., 2014), which we confirmed in the present study. E2/P4 exert neurotrophic functions through their ability to promote activation of MAPK within the HPC (Boonyaratanakornkit, 2011; Leonhardt et al., 2003; Orr et al., 2012; Tuscher et al., 2016). In line with these data, we demonstrate that D1 females exhibited lower levels of HPC p-MAPK as compared to Pro females. Interestingly, while 1.5 mg/kg ketamine was subthreshold for a behavioral effect in D1 females, this dose did significantly increase p-MAPK, which may be due to the low baseline levels of p-MAPK.
The phosphoinositide 3-kinase/protein kinase B (PI3K-Akt) pathway is another downstream intracellular signaling target of BDNF. Consistent with previous reports (Caffino et al., 2016; Li et al., 2010), we demonstrate that an antidepressant dose of ketamine increases levels of p-Akt within the PFC and HPC of male rodents. E2/P4 promote activation of the PI3K-Akt pathway and this is one mechanism through which they promote cell survival and neuronal plasticity (Briz and Baudry, 2014; Fortress et al., 2013; Singh, 2001). This is consistent with our finding that Pro females exhibit an increase in PFC and HPC p-Akt in a manner that directly mirrored their behavioral sensitivity to ketamine (i.e., 1.5 and 3 mg/kg). Following phosphorylation, p-Akt promotes synaptic plasticity via inhibition of the pro-apoptotic signaling molecule, GSK-3β (Beaulieu, 2012; Beurel et al., 2015). In line with the relationship between Akt and GSK-3β, ketamine promotes the phosphorylation of GSK-3β in the PFC and HPC of male rodents (Beurel et al., 2011; Zhou et al., 2014a), and this phosphorylation is required for ketamine’s rapid antidepressant-like effects (Beurel et al., 2011). Consistent with these reports we demonstrate that ketamine induced an increase in p-GSK-3β in the male and Pro PFC, as well as the HPC of all groups. It is important to note that in addition to the PI3K-Akt pathway, there are many ketamine-activated kinases that promote phosphorylation of GSK-3β (e.g., p70 S6 kinase) (Beurel et al., 2015). Thus, our finding that there was no effect of ketamine to affect phosphorylation of Akt in the HPC of D1 females, yet an effect of ketamine to influence levels of p-GSK-3β is somewhat surprising, but not unfounded.
mTOR is another neurotrophic signaling molecule that has garnered much attention in the field of antidepressant research, and is also downstream of BDNF and its associated signaling pathways (e.g., Akt and GSK-3β) (Beurel et al., 2011; Liu et al., 2013; Navé et al., 1999; Zoncu et al., 2011). Phosphorylation of mTOR and its ability to promote the translation of synaptic plasticity-related proteins in the PFC are required for the rapid antidepressant-like effects of ketamine in males (Li et al., 2010). Indeed, we found that a behaviorally effective dose of ketamine induced activation of mTOR in the PFC of males and D1 females, and in the HPC of males and Pro females.
The present study aimed to determine doses of ketamine that exert antidepressant-like effects in mice and to elucidate the contribution of endogenous ovarian hormones to mediate sex differences in ketamine sensitivity. We demonstrated that females experiencing a natural peak in levels of the ovarian hormones E2 and P4 exhibited heightened behavioral and biochemical sensitivity to ketamine. Taking this a step further, we demonstrate that pharmacological activation of ERα or ERβ was sufficient to render D1 females sensitive to a dose of ketamine that was only effective in Pro females. This finding indicates that action of E2 at either of these ER subtypes may mediate Pro females heightened behavioral sensitivity to ketamine. Investigation into the potential molecular mechanism(s) underlying Pro females’ ketamine sensitivity revealed some interesting clues. First, we confirmed that in male mice behaviorally effective dose of ketamine increased the expression and/or activation of many neurotrophic pathways within the PFC and HPC. Then, we identified ketamine-sensitive biochemical pathways in these same mood-related brain regions in D1 and Pro females. Overall, when compared to D1 counterparts, Pro females were more sensitive to the biochemical effects of ketamine in both the PFC and HPC, which is in line with evidence that both of these mood-related brain regions are robustly influenced by ovarian hormones and display estrous cycle-related changes in synaptogenesis and neuronal excitability (Scharfman and MacLusky, 2006). Furthermore, we found that Pro females exhibited activation of Akt and CaMKIIα within mood-related brain regions in response to 1.5 mg/kg ketamine; indicating that ovarian hormones may prime these signaling pathways to render females sensitive to male-subthreshold doses of ketamine. While these results provide some insight into the molecular mechanisms of ketamine within low and high ovarian hormone conditions, future studies need to examine the effects of ketamine in these hormone states in animals not exposed to an FST, as the acute stress associated with this test has the potential to influence expression of synaptic plasticity markers.
The present study goes some way to determine the molecular mechanisms underlying sex differences in behavioral sensitivity to ketamine, yet it is important to note that other mechanisms may also contribute to the observed effects. Sex differences in pharmacokinetics and pharmacodynamics are well established (Bigos et al., 2009; Soldin and Mattison, 2009; Waxman and Holloway, 2009), and recent work by Zanos et al. (Zanos et al., 2016) demonstrated that female mice have three-fold higher levels of the ketamine metabolite, (2S,6;2R6R)hydroxynorketamine, up to one hour following treatment with ketamine. Taken together, these studies suggest that the females may take longer to clear metabolites of ketamine, which may explain sex differences in ketamine antidepressant-like effects. Furthermore, ovarian hormones can influence excitatory receptor expression and/or receptor sensitivity (Gazzaley et al., 1996; Woolley et al., 1997), which may influence sensitivity to the antidepressant effects of ketamine. Thus, further investigation into the impact of sex and hormonal status over sensitivity to the rapid-acting antidepressant ketamine is warranted.
Supplementary Material
Figure 6. Schematic representation of the hypothesized shared influence of ketamine and ovarian hormones within mood related brain regions.
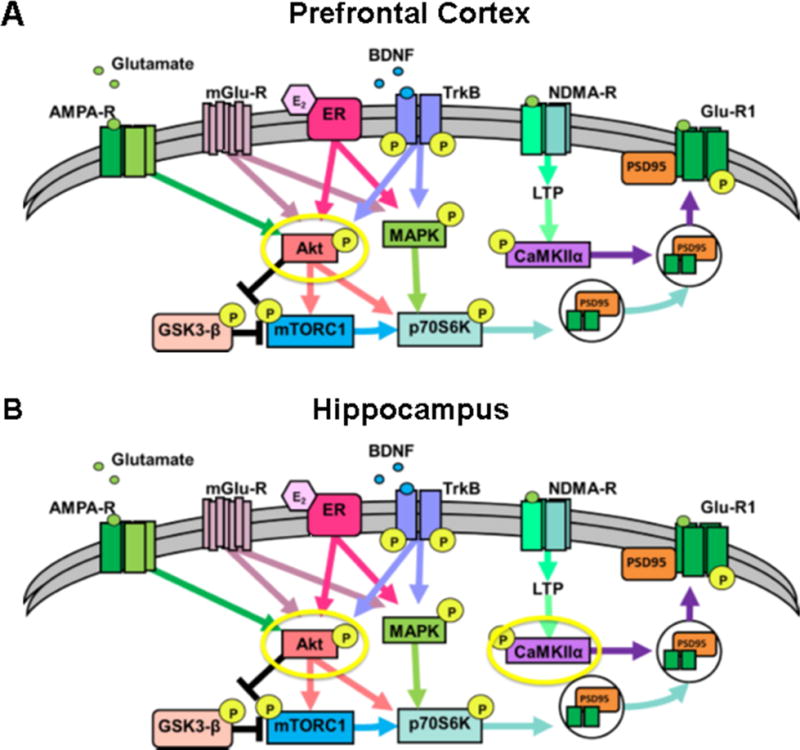
(A) A simplified schematic of the intracellular signaling pathways that are influenced by ketamine and ovarian hormones within the PFC and (B) and the HPC. Yellow circles indicate pathways activated by behaviorally-effective doses of ketamine in Pro females.
Males and diestrus 1 females exhibited antidepressant-like responses 3 mg/kg ketamine.
Females’ ketamine sensitivity was increased by ovarian hormones and by estrogen receptor agonists.
Proestrus behavioral ketamine sensitivity was mirrored by increased PFC activation of Akt and of HPC Akt and CaMKIIα.
Acknowledgments
We thank Dania Tawfiq for her outstanding assistance throughout the duration of the experiment.
Funding: This work was supported by the National Institute of Mental Health grant to M.K. (R01 MH087583 and R01 MH099085).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, D’Anci KE, Kanarek RB, Renshaw PF. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol Biochem Behav. 2012;101:588–601. doi: 10.1016/j.pbb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. [Google Scholar]
- Bigos KL, Pollock BG, Stankevich BA, Bies RR. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med. 2009;6:522–543. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011;76:877–884. doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Briz V, Baudry M. Estrogen Regulates Protein Synthesis and Actin Polymerization in Hippocampal Neurons through Different Molecular Mechanisms. Front Endocrinol (Lausanne) 2014;5:22. doi: 10.3389/fendo.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino L, Di Chio M, Giannotti G, Venniro M, Mutti A, Padovani L, Cheung D, Fumagalli GF, Yew DT, Fumagalli F, Chiamulera C. The modulation of BDNF expression and signalling dissects the antidepressant from the reinforcing properties of ketamine: Effects of single infusion vs. chronic self-administration in rats. Pharmacol Res. 2016;104:22–30. doi: 10.1016/j.phrs.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS, Kim YS, Duman RS, Son H. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A. 2015;112:15755–15760. doi: 10.1073/pnas.1513913112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Goldman J, Vandenbergh J. Monitoring of estrous cyclicity in the laboratory rodent by vaginal lavage. Academic Press; New York: 1993. [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Camarena E, Rivera NM, Berlanga C, Fernández-Guasti A. Reduction in the latency of action of antidepressants by 17 beta-estradiol in the forced swimming test. Psychopharmacology (Berl) 2008;201:351–360. doi: 10.1007/s00213-008-1291-8. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Progesterone attenuates depressive behavior of younger and older adult C57/BL6, wildtype, and progesterone receptor knockout mice. Pharmacol Biochem Behav. 2011;99:525–531. doi: 10.1016/j.pbb.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2002;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111:8649–8654. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015;1621:147–161. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Herring BE, Nicoll RA. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol. 2016;78:351–365. doi: 10.1146/annurev-physiol-021014-071753. [DOI] [PubMed] [Google Scholar]
- Höflich A, Hahn A, Küblböck M, Kranz GS, Vanicek T, Ganger S, Spies M, Windischberger C, Kasper S, Winkler D, Lanzenberger R. Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1291-0. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169:1150–1156. doi: 10.1176/appi.ajp.2012.12040531. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20C:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DA, Stancel GM, Enna SJ. Imipramine: effect of ovarian steroids on modifications in serotonin receptor binding. Science. 1981;211:1183–1185. doi: 10.1126/science.6258229. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C. Forced swim test: What about females? Neuropharmacology. 2015;99:408–421. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Leonhardt SA, Boonyaratanakornkit V, Edwards DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–770. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Demenescu LR, Colic L, Metzger CD, Heinze HJ, Steiner J, Speck O, Fejtova A, Salvadore G, Walter M. Temporal Dynamics of Antidepressant Ketamine Effects on Glutamine Cycling Follow Regional Fingerprints of AMPA and NMDA Receptor Densities. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Chaiton JA, Lieblich SE, Galea LA. Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology. 2016;107:278–293. doi: 10.1016/j.neuropharm.2016.01.033. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344(Pt 2):427–431. [PMC free article] [PubMed] [Google Scholar]
- Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013;33:6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17(Suppl 1):S1–12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- Orr PT, Rubin AJ, Fan L, Kent BA, Frick KM. The progesterone-induced enhancement of object recognition memory consolidation involves activation of the extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) pathways in the dorsal hippocampus. Horm Behav. 2012;61:487–495. doi: 10.1016/j.yhbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Lee JG, Seo MK, Lee CH, Cho HY, Lee BJ, Seol W, Kim YH. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int J Neuropsychopharmacol. 2014;17:1831–1846. doi: 10.1017/S1461145714000534. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz JR, Dal-Pizzol F, Quevedo J. MAPK signaling correlates with the antidepressant effects of ketamine. J Psychiatr Res. 2014;55:15–21. doi: 10.1016/j.jpsychires.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Saland SK, Schoepfer KJ, Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep. 2016;6:21322. doi: 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Murphy D. Estradiol induces formation of dendritic spines in hippocampal neurons: functional correlates. Horm Behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Tsuda MC, Mattei L, Waters EM, Romeo RD, Milner TA, McEwen BS, Ogawa S. Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Neuroscience. 2012;202:131–146. doi: 10.1016/j.neuroscience.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-Mediated Spine Changes in the Dorsal Hippocampus and Medial Prefrontal Cortex of Ovariectomized Female Mice Depend on ERK and mTOR Activation in the Dorsal Hippocampus. J Neurosci. 2016;36:1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Effects of two estradiol regimens on anxiety and depressive behaviors and trophic effects in peripheral tissues in a rodent model. Gend Med. 2009;6:300–311. doi: 10.1016/j.genm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Dwyer KD, Roy EJ. Direct effects of ovarian hormones on antidepressant binding sites. Brain Res Bull. 1989;22:181–185. doi: 10.1016/0361-9230(89)90040-3. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Strong CE, Addonizio MN, Brownstein NC, Kabbaj M. Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology (Berl) 2017;234:393–401. doi: 10.1007/s00213-016-4470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, Zhou ZQ. Akt mediates GSK-3β phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014a;21:183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014b;29:419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


