Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) was first identified in hypothalamus, based on its ability to elevate cyclic AMP in the anterior pituitary. PACAP has been identified as the adrenomedullary neurotransmitter in stress through a combination of ex vivo, in vivo, and in cellula experiments over the past two decades. PACAP causes catecholamine secretion, and activation of catecholamine biosynthetic enzymes, during episodes of stress in mammals. Features of PACAP signaling allowing stress transduction at the splanchnicoadrenomedullary synapse have yielded insights into the contrasting roles of acetylcholine and PACAP action as first messengers at the chromaffin cell, via differential release at different rates of splanchnic nerve firing, and different signaling pathways leading to catecholamine secretion and chromaffin cell gene transcription. Catecholamine secretion stimulated by PACAP, via calcium influx independent of action potential generation, is under active investigation in several laboratories, both at the chromaffin cell, and within autonomic ganglia of both the parasympathetic and sympathetic nervous systems. PACAP is a neurotransmitter important in stress transduction in the central nervous system as well, and is found at stress-transduction nuclei in brain including the paraventricular nucleus of hypothalamus, the amygdala and extended amygdalar nuclei, and the prefrontal cortex. The current status of PACAP as a ‘master regulator’ of stress signaling in the nervous system derives fundamentally from establishment of its role as the adrenomedullary transmitter in stress, and experimental elucidation of PACAP action at this synapse remains at the forefront of understanding its role in stress signaling throughout the nervous system.
Keywords: Acetylcholine, catecholamine, NCS-Rapgef2, PAC1, sympathoadrenal axis, sympathetic nervous system
Introduction
Coupland identified the features of the splanchnicoadrenomedullary synapse of the rat in 1965 (16)2. This synapse has, in the years following, been a paradigm, both by similarity and difference, for the study of chemical neurotransmission in the nervous system until today. In large part, this is because the purpose of the release of acetycholine from the splanchnic nerve to the chromaffin cell, like that of acetylcholine release from nerve to muscle at the neuromuscular junction (and unlike that of acetylcholine release at parasympathetic and sympathetic ganglia and in the brain), is transparently physiologically obvious: to effect a stimulus to the chromaffin cell resulting in expression of its primary function; to release catecholamines into the general circulation, analogous to the ability of acetylcholine released from the motor neuron to excite muscle contraction. In fact the naming of calcium-dependent release of catecholamines in response to acetylcholine as ‘stimulus-secretion coupling’ by Douglas (18) pays tribute to the discovery of acetylcholine-stimulated, calcium-dependent muscle contraction, called stimulus-contraction coupling (68). Following the characterization of the morphological features of the splanchnicoadrenomedullary synapse by Coupland, and of cholinergic neuroeffector junctions of the somatic nervous system (39), the mechanisms of acetylcholine-induced calcium influx at both cholinergic neuroeffector junctions and synapses proceeded synergistically and rapidly. Much of our knowledge about the action of acetylcholine released from the splanchnic nerve, acting through ionotropic nicotinic receptors, on the release of catecholamines from the adrenal medulla, has come from the laboratory of Antonio Garcia (32), to whom this volume of Pfluger’s Archive is dedicated.
As our understanding of cholinergic neurotransmission and its role in catecholamine release progressed (see (6) and references therein), three additional themes of synaptic transmission to the chromaffin cell came into view. One of these is that chromaffin cells also express muscarinic cholinergic receptors, which are metabotropic and allow calcium mobilization through Gq coupling (46), thus raising the question as to the relative roles of ionotropic and metabotropic cholinergic signaling in effecting catecholamine secretion. A second is that catecholamines are released from large dense core vesicles by the process of exocytosis, along with a cohort of proteins including the biosynthetic enzyme for catecholamine biosynthesis, dopamine beta-hydroxylase and the protein chromogranin A (which makes up 40% of the weight of the chromaffin granule, and 10% of the protein content of the chromaffin cell) ((21, 88, 89) and references therein) and later, a bevy of neuropeptides including enkephalins, NPY, galanin, and others (3, 27, 29, 30, 87). Importantly, the question was raised that if secretion exhausted an important set of proteins from the cell, there must be coupling not only between stimulus and secretion, but between stimulus and replenishment of the biosynthetic enzyme complement of the cell exhausted by protein exocytosis. The notion that a process of coupling between stimulus, secretion, and protein synthesis, termed by some ‘stimulus-secretion-synthesis coupling’, and ascribed to acetylcholine signaling not only to the cell membrane stimulating exocytosis, but also to the nucleus, stimulating gene transcription, emerged (1, 15). Finally, hints began to appear that, on the presynaptic side, a second neurotransmitter other than acetylcholine may be released from splanchnic nerve terminals (61). Early work by Ip and Zigmond, and Wakade and colleagues suggested that this second transmitter could be a peptide, augmenting cholinergic stimulation of secretion and regulating the production of catecholamine biosynthetic enzymes and other proteins secreted from the chromaffin cell ((74) and references therein). This review describes the discovery that the neuropeptide PACAP, itself first identified as a brain hypophysiotropic factor, is co-stored and released from the splanchnic nerve upon stimulation, and is in fact the principal neurotransmitter responsible for enhanced catecholamine release from the chromaffin cell during stress. It is dedicated, as are the other contributions to this issue of the journal to Antonio Garcia and his pioneering work to understand acetylcholine’s actions at the chromaffin cell.
PACAP as the adrenomedullary transmitter in stress
The pituitary adenylate cyclase-activating polypeptide (PACAP) was identified by Miyata and Arimura in 1989, as a 38-amino acid neuropeptide present in extracts of the ovine hypothalamus, causing elevation of cyclic AMP in perfused hemi-pituitary glands (60). In the years since, PACAP has been found in numerous deuterostome species, both chordate and echinoderm, and acts at three distinct receptors PAC1, VPAC1, and VPAC2 (the last two also activated by the related peptide VIP) all coupled to adenylate cyclase activation through heterotrimeric Gs-coupled receptors (4, 5). PACAP has been identified as the adrenomedullary neurotransmitter in stress through a combination of ex vivo, in vivo, and in cellula experiments over the past two decades (74, 77, 78).
Following the identification of PACAP (60), and its availability as a chemically synthesized pharmacological reagent, several reports indicated PACAP’s ability to cause catecholamine secretion, via a PACAP receptor expressed by the chromaffin cell (reviewed in (38, 62)). In 2009 it was reported that the PACAP antagonist PACAP(6–38) inhibited maximum catecholamine secretion, measured by in situ amperometry, elicited by high-frequency electrical stimulation of the splanchnic nerve in mouse adrenal slices ex vivo, and was without effect on ‘basal’ catecholamine secretion elicited by low-frequency splanchnic nerve stimulation (40, 50). We reviewed the status of PACAP as an adrenomedullary neurotransmitter based on the existing evidence in 2012, and identified a series of experiments that, in our view, would establish PACAP’s status as the ‘stress transmitter’ at the splanchnicoadrenomedullary synapse (74). For example, the necessity for PACAP expression in an adrenomedullary response to insulin-induced hypoglycemia (catecholamine secretion; adrenomedullary tyrosine hydroxylase induction) adequate for survival due to compensatory glucogenesis was established in PACAP-deficient mice (37). However, evidence that the necessary PACAP was actually that released from the axon terminals of the splanchnic nerve innervating the chromaffin cell (14) was lacking. This evidence was later supplied in the form of ex vivo experiments performed in adrenal slices from wild-type and PACAP-deficient mice (78).
Establishing PACAP as the transmitter responsible for catecholamine secretion in stress at the mouse splanchnicoadrenomedullary synapse ex vivo, and for both catecholamine secretion and biosynthetic enzyme induction in vivo, was an important step in convincingly positing that PACAP, rather than acetylcholine, is the principal physiologically relevant transmitter for stress transduction at this endocrine gland in mammals. It may also have profound importance for our understanding of the basic physiology of the autonomic nervous system, long thought to be primarily regulated by only one neurotransmitter, acetylcholine, two neuroeffectors (acetylcholine and norepinephrine) and one hormone (epinephrine). The remainder of this review and commentary focuses on three questions that are begged by the demonstration of PACAP as the splanchnicoadrenomedullary transmitter in stress. How does PACAP cause catecholamine secretion from chromaffin cells? How does PACAP regulated gene transcription related to maintenance of the secretory competence of the chromaffin cell? Does PACAP have a neurotransmitter role in sympathetic ganglia similar to its actions at the splanchnicoadrenomedullary synapse? Finally, what is in fact the essential role of acetylcholine in controlling chromaffin cell function?
How does PACAP cause catecholamine secretion?
There is a plethora of studies that contribute to understanding the actions of PACAP on the electrical activity and secretory function of the chromaffin cell. These can be conceptually evaluated in a number of ways. First there are studies conducted in cell lines of adrenomedullary origin, such as the PC12 and NS-1 cell. These cells are conveniently manipulated in culture (‘in cellula’) so that hypotheses about the necessity for specific molecules of secretion and signaling for biosynthesis can be easily and robustly verified. However, generalizing these hypotheses to the chromaffin cell itself requires important caveats, not all of them obvious. Studies in chromaffin cells in culture of course beg the question of how generalizable experiments conducted using mouse, rat, bovine, etc. chromaffin cells actually are to other species. Here it is worth noting the meticulous contributions of the Garcia lab, and others, to establishing the variation in the type and abundance of the various voltage-gated ion channels across mouse, rat, cow and human adrenal chromaffin cells, and how important these studies have been in establishing a firm appreciation for species variation in calcium-dependent catecholamine secretion. These differences may reflect evolutionary flexibility in the use of specific molecules to solve the same biological problem (catecholamine secretion), or evolutionary differences in the fitness contribution of the adrenal medulla from one species to another. In studies conducted in chromaffin cells, special note must be made of those that examine the regulation of calcium per se, and those that examine the regulation of calcium-dependent downstream events that drive the professional activities of the cell. Many apparent conundrums centering on alternative mechanisms of calcium-dependent secretion are resolvable on the basis of whether or not a particular PACAP-dependent effect on calcium influx, mobilization, sequestration, or re-distribution actually affects catecholamine secretion or chromaffin cell gene regulation under conditions likely to obtain in vivo. For this reason we have focused here on studies in which secretion or gene regulation are examined under nominally physiological conditions.
It is well-established that acetylcholine causes cell depolarization, necessary for calcium entry and exocytosis, via action potentials generated by cation influx through the nicotinic receptor followed by activation of voltage-dependent sodium channels, cell depolarization, opening of voltage-gated calcium channels, and calcium-dependent large dense-core vesicle exocytosis (32, 67). Secretory effects of acetylcholine on chromaffin cells are absolutely dependent upon the opening of voltage-gated sodium channels, as evidenced by blockade of ACh-induced catecholamine release by tetrodotoxin (TTX), and absolutely dependent on calcium influx (57). It is equally well-established, though perhaps less well-appreciated, that PACAP-stimulated secretion does not depend upon opening of voltage-gated sodium channels (63), but only upon entry of extracellular calcium through a variety of voltage-gated calcium channels in cultured bovine chromaffin cells (63, 65), although some contributions to depolarization sufficient to cause opening of voltage-gated calcium channels (VGCC) and even action potentials under specialized circumstances, cannot be excluded (see Figure 1; and (82, 85). Kuri et al. have proposed a possible mechanism for PACAP’s effects on catecholamine secretion from rodent chromaffin cells in slice preparations, based on electrophysiological and pharmacological investigations featuring its dependence on a) cAMP, b) PKC (however see Vitale et al. ((86)) for data suggesting that stimulation of PKC does not affect calcium entry into chromaffin cells), c) T-type or other ‘low threshold’ calcium channel opening, and finally calcium influx through canonical (combination of L, P, N or Q) high-capacity voltage-gated calcium channels (50). The model is not dispositive for the specific components involved, but rather constrained by removal from the model of components (such as voltage-gated sodium channels and intracellular calcium mobilization rather than calcium influx) that are not required for PACAP-stimulated secretion, and the proposal of components with known properties consistent with inclusion in the model. Epac, for example, has been suggested to enhance depolarization-induced secretion from PC12 cells when expressed exogenously in them, however what percentage of PACAP-induced release in chromaffin cells occurs by this mechanism is not yet clearly defined (see Figure 1; (66, 70)). This model (incorporated into Figure 1, this report) clearly suggests that PACAP-induced catecholamine secretion from chromaffin cells does not require and is not accompanied by action potential generation, and the negative evidence supporting this is the TTX-insensitivity of PACAP-induced catecholamine and neuropeptide release from the chromaffin cell (50, 65, 82). It is worth noting that a cell depolarization of around 20 mV, in cells resting at −55 to −60 mV, would be sufficient to trigger sustained or ‘burst firing’ (36, 85) even in the absence of available Nav channels. Chromaffin cell in isolation have a variable resting potential between −80 and −50 mV (26). PACAP-evoked depolarization may be suprathreshold for action potential firing in those cells resting near the depolarized end of this range, and enhancement of burst firing, even if it does not occur in chromaffin cells, may be a mechanism of PACAP action in other excitable cell of the autonomic as well as the central nervous system.
Fig. 1. Schematic illustration of chromaffin cell signaling pathways leading to secretion of catecholamines by cholinergic and PACAPergic stimulation.
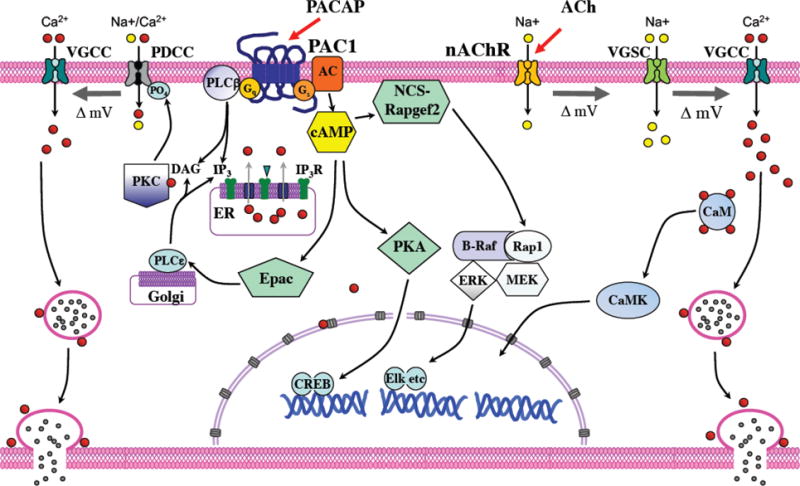
Signaling for secretion is indicated for each neurotransmitter as it is known or hypothesized to act on the mammalian chromaffin cell. PACAP-dependent signaling requires a phospho-dependent depolarizing cation conductance (PDCC) for activation of VGCC and secretion. The best characterized features of PDCC are that it requires phosphorylation by PKC for its activation and it is sensitive to inhibition by benzamil (50). The molecules most consistent with this pharmacological and functional profile are the sodium-calcium exchanger (50) and TRPP3 (17). As comprehensively as possible the authors have depicted the pathways for which the available evidence is strongest and most consensual across mammalian species, albeit features proven for some mammalian species may not be established for all, and especially may not be established clinically (i.e. in human; and see text). Scheme adapted from (62).
A cAMP/Epac-dependent signaling mechanism, whereby Epac activates a non-classical target, PLCepsilon leading to PKC activation has been reported in HEK293 cells (47, 69). Since these initial observations, Epac- and PKC-dependent signaling pathways for hormone secretion have been reported to play a role in glucose-induced insulin release (19) and glucagon-induced ghrelin release from stomach (31). Further investigation in this arena is of great importance for comprehensive understanding of the physiology of adrenomedullary function, and possibly of the autonomic nervous system in general (vide infra). We include Epac as a potential actor in PACAP-stimulated catecholamine secretion from chromaffin cells, as depicted in Figure 1.
How does PACAP regulate chromaffin cell gene transcription?
It has been long appreciated that activation of catecholamine secretion from the adrenal medulla following psychogenic, hypoglycemic and several other types of stress results in increased firing of the splanchnic nerve (48), and that prolonged stimulation of secretion (for up to six hours, the time of a prolonged episode of glucose deprivation) of catecholamines results in the secretion of the entire contents of the adrenal medulla (55). The fact that the adrenal medulla retains its full complement of catecholamine despite this high secretory rate means that the adrenal medulla is capable of re-synthesizing its entire catecholamine content during an episode of stress responding, and this in turn implies a dramatic up-regulation of the catecholamine biosynthetic capacity of the gland. This is accomplished by up-regulation of at least two biosynthetic enzymes for epinephrine production, tyrosine hydroxylase and phenylethanolamine N-methyltransferase ((75–78) and references therein). Thoenen, Mueller and Axelrod first remarked that up-regulation of TH in adrenal in vivo after stress (61) was abolished by splanchnic nerve transection, but not by nicotinic or muscarinic blockade, concluding that ‘it is possible that in the adrenal medulla either another cholinergic receptor or another neurotransmitter is involved in mediating the trans-synaptic increase in tyrosine hydroxylase.” Later Yp and Zigmond postulated that this peptide could be the vasoactive intestinal polypeptide (VIP) (42, 43), and indeed Wakade added support to this idea by demonstrating the release of VIP into adrenal perfusates upon splanchnic nerve stimulation, and the effects of VIP on catecholamine secretion from the rat adrenal (54, 56). Immunohistochemistry for PACAP using carefully validated antibodies (PACAP and VIP have similar structures and a propensity for immunological cross-reactivity) allowed the localization of PACAP, rather than VIP in mouse cholinergic splanchnic nerve terminals (37). The PACAP knockout mouse used to validate PACAP immunohistochemistry was also employed to show that PACAP is required not only for catecholamine secretion, but also for TH induction following hypoglycemic stress. Subsequent experiments demonstrated that both systemic and psychogenic stress (e.g. restraint stress) up-regulation of TH and PNMT mRNA in adrenal was PACAP-dependent (75, 77, 78).
The signaling pathways by which PACAP regulates catecholamine biosynthetic enzyme gene expression, like those activated for secretion, remain surprisingly ill-defined (2, 80, 81). Our own group has identified three separate sensors for cAMP elevated by PACAP in NS-1 and chromaffin cells responsible for gene activation: these are protein kinase A, Epac, and the novel cAMP sensor NCS-Rapgef2. Which of these pathways, if any, actually regulate TH and PNMT gene regulation associated with adrenal stress responding in vivo is unknown, although a PKA-independent pathway is suggested by experiments on TH and PNMT induction in cultured bovine chromaffin cells by PACAP (78). The possibility exists that Epac, PKA, and NCS-Rapgef2 are all involved in gene regulation by PACAP in chromaffin cells: this includes not only the catecholamine biosynthetic enzymes, but galanin and other neuropeptides that are significantly up-regulated in expression in adrenal medulla by stress (3, 28–30). The NCS-Rapgef2-mediated pathway has been associated with galanin gene regulation in chromaffin cells, through an ERK-dependent mechanism (22).
What is the role of ACh at the adrenomedullary synapse?
PACAP deficiency appears to completely block high-frequency splanchnic nerve-stimulated release of catecholamines ex vivo, and to attenuate it in vivo sufficiently to block catecholamine-dependent glucogenesis (and survival) after insulin shock. It is predicted that acetylcholine alone is released from splanchnic terminals at low splanchnic firing rats, and both acetylcholine and PACAP at high rates characteristic of stress responding (74). We have not adduced evidence for a co-transmitter role for acetylcholine in either the secretory or the gene regulatory actions of PACAP in chromaffin cells. However, it is difficult to approximate the actual concentrations of these two transmitters that reach their receptors at the splanchnicoadrenomedullary synapse. A perhaps overlooked role for acetylcholine in adrenomedullary function is the priming of adequate levels of stored catecholamines in the adrenal gland by the tonic low-frequency stimulation from the splanchnic nerve. Splanchnic innervation itself, even in the absence of stress leading to increased firing rate, does not appear to be required for maintenance of catecholamine levels (72). However, another role of acetylcholine-evoked calcium influx may be to maintain vesicle populations in a ‘ready-release’ mode (73) to allow the higher rates of CA release required for stress response. Finally, the role of tonic catecholamine release itself, even in the absence of stress, is likely to be of physiological significance, with the adrenal medulla and post-ganglionic sympathetic neurons supplying hormonal and synaptic catecholamines for cardiac and vascular regulation both at rest and during stress (49).
Does PACAP have a role in ganglionic transmission in sympathetic and parasympathetic neurons?
A critical unanswered question is whether or not PACAP functions as a stress-specific ganglionic transmitter in the sympathetic, and parasympathetic nervous systems, where it is in fact reported to be expressed. May and colleagues have adduced significant evidence, employing developing sympathetic neurons in culture, that PACAP has multiple roles in regulation of post-ganglionic sympathetic neurons in culture, including the regulation of biosynthesis and release of neuropeptides co-stored with norepinephrine in these cells (7, 8, 10, 11, 58). Zigmond and colleagues have likewise postulated a trophic role for PACAP in the developing sympathetic nervous system (90). Investigation of the role of PACAP in stress responding that is largely sympathetic rather than adrenal, for example cold stress (34, 35) would be of great interest. Parsons and colleagues have studied PACAPergic effects on cardiac tissue, i.e. on post-ganglionic parasympathetic neurons of the atrium (41, 59). What do the mechanisms of PACAP-evoked membrane currents have to tell us about PACAPergic secretory mechanisms? Can clearly defined effects of PAC1 receptor stimulation on after-hyperpolarization potential modulation in cardiac tissue, for example, inform PACAPergic signaling at the chromaffin cell, or is this signaling private to individual cell types in the parasympathetic and sympathetic nervous systems? In sum, it appears that the accumulating evidence justifies consideration of PACAP as the pre-ganglionic transmistter in stress at both sympathetic and parasympathetic ganglia based on cell culture and ex vivo experiments, with a final demonstration in vivo (in PACAP-deficient mice, for example) as the sine qua non for general acceptance of this concept (see Figure 2).
Fig. 2. The neurotransmitters of the autonomic nervous system.
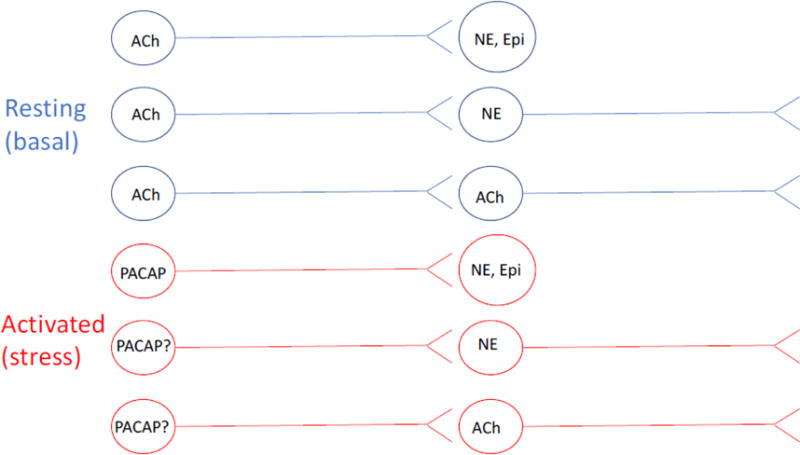
Neurotransmission under basal and activated (rest and stress) conditions are shown in blue and red, respectively. ACh, acetylcholine; Epi, epinephrine/adrenaline; NE, norepinephrine, noradrenaline; PACAP, pituitary adenylate cyclase-activating polypeptide. Neurotransmitters for which function is stress is hypothesized rather than demonstrated are indicated as‘X?’ Note that the PACAPergic component of the autonomic nervous system is most strongly supported by work in rodent species in vivo, and in the bovine adrenal medulla in culture, although there is a significant body of work supporting PACAPergic splanchnicoadrenomedullary neurotransmission for primate species as well (see text). Of course other non-adrenergic, non-cholinergic (NANC) neurotransmitters, including ATP, exist in the autonomic nervous system (see (12) for review).
Role of PACAP in stress signaling in the central nervous system
PACAP is required for the stress response originating in the central nervous system as well. PACAPergic nerve terminals heavily invest CRH-positive neurons of the paraventricular nucleus (PVN), which is the central final common pathway for activation of the hypothalamo-pituitary-adrenocortical (HPA) axis that is the hallmark, along with activation of the hypothalamo-sympathoadrenomedullary (HSA) axis, of the stress response (13, 71). However, it was initially observed that cortisol levels across the diurnal cycle, as well as CORT elevation accompanying hypoglycemic stress, were unaffected by PACAP deficiency (37). It was later learned, however, that PACAP does play an important role in regulation of the HPA axis in stress, but this role is restricted to psychogenic (allostatic) HPA axis activation, and not systemic (homeostatic) HPA axis activation (51, 64, 84). One to three hours of restraint stress causes an approximate doubling of CRH mRNA in the PVN, and this is completely abrogated in PACAP-deficient mice (79). Chronic psychological stress caused by either daily restraint, or daily social defeat for 14 days, results in elevated CORT, increased anxiety, and depressive behavior in the mouse: all of these effects of chronic psychogenic stress are greatly attenuated in PACAP-deficient mice (51, 64). An interesting feature of the PACAP dependence of CORT (cortisol in human; corticosterone in rodents) elevation attendant upon psychogenic stress responding is that graded (1, 2 or 3 hours) ‘doses’ of restraint stress reveal a dissociation between the effects of stress on CORT elevation, and on behavior (e.g. stress-induced appetite suppression). Thus, even a single episode of restraint, for one, two or three hours, results in acute CORT elevation and appetite suppression detected as significant weight loss after 24 hours. While both CORT elevation and appetite suppression elicited by two or three hours of restraint are blunted in PACAP-deficient mice, only appetite suppression, but not CORT elevation, is blunted in PACAP-deficient mice (44, 45). We have interpreted these findings as indicative of an effect of PACAP on CRH biosynthesis, via elevation of CRH mRNA, in PVN, but not on CRH secretion into the portal circulation to the pituitary gland (to release ACTH, thus elevating CORT). This implies that PACAP action at the CRH neuron, unlike that at the chromaffin cell, involves stimulus-transcription, but not stimulus-secretion coupling, in this cell type. A further implication is that PACAP has differential sites of action for stress-induced HPA axis activation than for stress-induced appetite suppression. In this case, PACAP is likely to mediate stimulus-secretion coupling, as triggering of behavior effects after only a single hour of restraint stress is unlikely to be mediated via a transcriptional regulatory mechanism alone, and that at this locus PACAP’s actions are more like those occurring at the chromaffin cell.
Future prospects based on work in chromaffin cells
The chromaffin cell has provided a neuroendocrine experimental system that has revealed the role of PACAP, at the cellular and organismic levels, in transduction of the stress response. Regarding the signaling mechanisms employed by this Gs-coupled GPCR for its secretory and biosynthetic activities, the chromaffin cell has also been a rich source for discovery of signaling components and pathways employed during PACAP signaling. Notably, the neuroendocrine-specific cAMP sensor NCS-Rapgef2, linking PACAP signaling through PAC1 and Gs to activation of the MAP kinase ERK was first identified in bovine chromaffin cells (22, 24). Equally fundamental to understanding the role of PACAP in stress signaling in the autonomic nervous system is elucidation of the mechanism(s) whereby PACAP, again through the PAC1 receptor, cause cell depolarization leading to calcium influx and secretion of catecholamines from the adrenal medulla and presumably also norepinephrine from post-ganglionic sympathetic neurons, and of acetylcholine from post-ganlgionic parasympathetic neurons (see Figure 2). Figure 1 summarizes the signaling pathways leading to catecholamine secretion from mammalian chromaffin cells. The figure prominently features the role of PDCCs activated by PKC after engagement of the PAC1 receptor in chromaffin cells. It is noteworthy that a clear exposition of whether or not Gq engagement or alternatively Gs engagement by PAC1 is the primary driver for PKC activation leading to activation presumably via phosphorylation of PDCCs in the chromaffin cell. Pharmacological evidence for a primary involvement of PKC in catecholamine secretion derives from the mouse adrenal ex vivo (50) the cultured bovine chromaffin cell (82) and, indirectly, from PMA pharmacological effects manifested as enhanced secretion of enkephalin from bovine chromaffin cells (20). A plethora of evidence adduced in the cardiac ganglion preparation ex vivo suggests that PACAP profoundly affects current input-response characteristics via a pathway involving cAMP and MAPK activation (83). Whether this is related to a general mechanism for PACAP’s secretogogue action, or whether these two cell types affect secretion by distinct mechanisms is an important question for full understanding of the neurochemistry and physiology of the autonomic nervous system. It has implications as well for PACAP signaling in stress at central synapses: current evidence suggests that PACAP acts as a secretogogue at some, as yet undefined central synapses, while in the paraventricular nucleus, PACAP, via the PAC1 receptor, appears to affect corticotropin-releasing factor synthesis (vide infra) without apparent secretogogue action.
Significant progress in understanding PACAP-dependent signaling to the nucleus in PC12 cells, and its high-content analysis-adapted variant, the NS-1 cell (22). In particular, the down-regulation of expression of specific signaling proteins using siRNA and CRISPR technology has been helpful in first identifying signaling proteins of interest by their deletion, and then development a pharmacology for them with high-content analytical screening (23, 25). However, it is somewhat remarkable that precise delineation of how this critical autonomic neurotransmitter causes catecholamine release from the chromaffin cell, either in culture or in vivo, is still incomplete. Such investigations are important not only in their own right, but to establish appropriate pharmacological tools with which to ascertain whether or not the mechanisms of PACAP-stimulated secretion are universal across multiple types of PACAP-receptor bearing neuroendocrine cells, or highly specific to the chromaffin cells, and even to chromaffin cells found in various mammalian species. In this regard, the work of Garcia and colleagues, over several decades (9, 33, 52, 53), in delineating the mechanisms and features of acetylcholine-induced catecholamine release from chromaffin cells is a worthy reminder that the chromaffin cell yields biological information perhaps slowly, but surely.
Footnotes
Invited contribution to the PAEJ Special Issue Focus on chromaffin cells: from molecules to physiological functions (eds. R. Borges, E. Carbone and L. Gandia), in honour of Professor Antonio Garcia.
References for some earlier work relevant to this review can be found in the bibliographies of the reviews of the literature cited herein.
References
- 1.Affolter H-U, Giraud P, Hotchkiss AJ, Eiden LE. Stimulus-secretion-synthesis coupling: A model for cholinergic regulation of enkephalin secretion and gene transcription in adrenomedullary chromaffin cells. In: Fraioli F, editor. Opiate Peptides in the Periphery. Amsterdam: Elsevier; 1984. pp. 23–30. [Google Scholar]
- 2.Ait-Ali D, Samal B, Mustafa T, Eiden LE. Neuropeptides, growth factors and cytokines: A cohort of informational molecules whose expression is up-regulated by the stress-associated slow transmitter PACAP in chromaffin cells. Cell Mol Neurobiol. 2010;30:1441–1449. doi: 10.1007/s10571-010-9620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anouar Y, Eiden LE. Rapid and long-lasting increase in galanin mRNA levels in rat adrenal medulla following insulin-induced reflex splanchnic nerve stimulation. Neuroendocrinology. 1995;62:611–618. doi: 10.1159/000127057. [DOI] [PubMed] [Google Scholar]
- 4.Arimura A. Pituitary adenylate cyclase-activating polypeptide (PACAP): Discovery and current status of research. Regul Peptides. 1992;37:287–303. [PubMed] [Google Scholar]
- 5.Arimura A, Shioda S. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptors: neuroendocrine and endocrine interaction. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 6.Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. Int Rev Cytol. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [DOI] [PubMed] [Google Scholar]
- 7.Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- 8.Beaudet MM, Parsons RL, Braas KM, May V. Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci. 2000;20:7353–7361. doi: 10.1523/JNEUROSCI.20-19-07353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges R, Sala F, Garcia AG. Continuous monitoring of catecholamine release from perfused cat adrenals. J Neurosci Methods. 1986;16:289–300. doi: 10.1016/0165-0270(86)90054-3. [DOI] [PubMed] [Google Scholar]
- 10.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci. 1996;805:204–216. doi: 10.1111/j.1749-6632.1996.tb17484.x. [DOI] [PubMed] [Google Scholar]
- 11.Brandenburg CA, May V, Braas KM. Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple neuropeptide transcripts. J Neurosci. 1997;17:4045–4055. doi: 10.1523/JNEUROSCI.17-11-04045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 13.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 14.Chen Y, Hamelink C, Chen Y, Hallenbeck JM, Eiden LE. Mechanism for neuroprotective effects of PACAP in cerebral ischemic insult in PACAP-deficient mice. Washington, DC: Society for Neuroscience; 2002. Online Abstract Viewer/Itinerary Planner 2002. [Google Scholar]
- 15.Chuang D-M, Costa E. Biosynthesis of tyrosine hydroxylase in rat adrenal medulla after exposure to cold. Proc Natl Acad Sci USA. 1974;71:4570–4574. doi: 10.1073/pnas.71.11.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coupland RE. Electron microscopic observations on the structure of the rat adrenal medulla: II. Normal innervation. J Anat. 1965;99:255–272. [PMC free article] [PubMed] [Google Scholar]
- 17.Dai XQ, Ramji A, Liu Y, Li Q, Karpinski E, Chen XZ. Inhibition of TRPP3 channel by amiloride and analogs. Mol Pharmacol. 2007;72:1576–1585. doi: 10.1124/mol.107.037150. [DOI] [PubMed] [Google Scholar]
- 18.Douglas WW. Stimulus-secretion coupling: The concept and clues from chromaffin and other cells. Br J Pharmacol. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, Lu Y, Schwede F, Genieser HG, Smrcka AV, Holz GG. Phospholipase C-epsilon links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets. 2011;3:121–128. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eiden LE, Anouar Y, Hsu C-M, MacArthur L, Hahm SH. Transcription regulation coupled to calcium and protein kinase signaling systems through TRE- and CRE-like sequences in neuropeptide genes. Adv Pharmacol. 1998;42:264–269. doi: 10.1016/s1054-3589(08)60744-9. [DOI] [PubMed] [Google Scholar]
- 21.Eiden LE, Iacangelo A, Hsu C-M, Hotchkiss AJ, Bader M-F, Aunis D. Chromogranin A synthesis and secretion in chromaffin cells. J Neurochem. 1987;49:65–74. doi: 10.1111/j.1471-4159.1987.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 22.Emery A, Eiden MV, Mustafa T, Eiden LE. GPCR-Gs signaling to ERK is controlled by the cAMP-sensing guanine nucleotide exchange factor NCS/Rapgef2 in neuronal and endocrine cells. Science Signaling. 2013;6:ra51. doi: 10.1126/scisignal.2003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery AC, Alvarez RA, Eiden MV, Xu W, Simeon FG, Eiden LE. Differential Pharmacophore Definition of the cAMP Binding Sites of Neuritogenic cAMP Sensor-Rapgef2, Protein Kinase A, and Exchange Protein Activated by cAMP in Neuroendocrine Cells Using an Adenine-Based Scaffold. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.6b00462. [DOI] [PubMed] [Google Scholar]
- 24.Emery AC, Eiden LE. Signaling through the neuropeptide GPCR PAC1 induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J. 2012;26:3199–3211. doi: 10.1096/fj.11-203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emery AC, Eiden MV, Eiden LE. A new site and mechanism of action for the widely used adenylate cyclase inhibitor SQ22,536. Mol Pharmacol. 2013;83:95–105. doi: 10.1124/mol.112.081760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer-Colbrie R. Chromogranins A, B and C: widespread constituents of secretory vesicles. Ann N Y Acad Sci. 1987;493:120–134. doi: 10.1111/j.1749-6632.1987.tb27189.x. [DOI] [PubMed] [Google Scholar]
- 28.Fischer-Colbrie R, Diez-Guerra J, Emson PC, Winkler H. Bovine chromaffin granules: immunological studies with antisera against neuropeptide Y, [Met]enkephalin and bombesin. Neuroscience. 1986;18:167–174. doi: 10.1016/0306-4522(86)90185-5. [DOI] [PubMed] [Google Scholar]
- 29.Fischer-Colbrie R, Eskay RL, Eiden LE, Maas D. Transsynaptic regulation of galanin, neurotensin, and substance P in the adrenal medulla: combinatorial control by second-messenger signaling pathways. J Neurochem. 1992;59:780–783. doi: 10.1111/j.1471-4159.1992.tb09440.x. [DOI] [PubMed] [Google Scholar]
- 30.Fischer-Colbrie R, Iacangelo A, Eiden LE. Neural and humoral factors separately regulate neuropeptide Y, enkephalin, and chromogranin A and B mRNA levels in rat adrenal medulla. Proc Natl Acad Sci USA. 1988;85:3240–3244. doi: 10.1073/pnas.85.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnon J, Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology. 2013;154:666–674. doi: 10.1210/en.2012-1994. [DOI] [PubMed] [Google Scholar]
- 32.Garcia AG, Garcia-De-Diego AM, Gandia L, Borges R, Garcia-Sancho J. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol Rev. 2006;86:1093–1131. doi: 10.1152/physrev.00039.2005. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Palomero E, Cuchillo-Ibanez I, Garcia AG, Renart J, Albillos A, Montiel C. Greater diversity than previously thought of chromaffin cell Ca2+ channels, derived from mRNA identification studies. FEBS Lett. 2000;481 doi: 10.1016/s0014-5793(00)01984-0. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein DS. Adrenal Responses to Stress. Cell Mol Neurobiol. 2010;30:1433–1440. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- 36.Guarina L, Vandael DH, Carabelli V, Carbone E. Low pHo boosts burst firing and catecholamine release by blocking TASK-1 and BK channels while preserving Cav1 channels in mouse chromaffin cells. J Physiol. 2017;595:2587–2609. doi: 10.1113/JP273735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee H-W, Eiden LE. Pituitary adenylate cyclase activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci USA. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamelink C, Weihe E, Eiden LE. PACAP: an ‘emergency response’ co-transmitter in the adrenal medulla. In: Vaudry H, Norwell Arimura A, editors. Pituitary Adenylate Cyclase-Activating Polypeptide. Massachusetts; Kluwer-Academic Press; 2003. pp. 227–250. [Google Scholar]
- 39.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill J, Chan SA, Kuri B, Smith C. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem. 2011;286:42459–42469. doi: 10.1074/jbc.M111.289389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoover DB, Girard BM, Hoover JL, Parsons RL. PAC receptors mediate positive chronotropic responses to PACAP-27 and VIP in isolated mouse atria. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip NY, Perlman RL, Zigmond RE. Acute transsynaptic regulation of tyrosine 3-monooxygenase activity in the rat superior cervical ganglion: Evidence for both cholinergic and noncholinergic mechanisms. Proc Natl Acad Sci USA. 1983;80:2081–2085. doi: 10.1073/pnas.80.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ip NY, Zigmond RE. Synergistic effects of muscarinic agonists and secretin or vasoactive intestinal peptide on the regulation of tyrosine hydroxylase activity in sympathetic neurons. J Neurobiol. 2000;42:14–21. [PubMed] [Google Scholar]
- 44.Jiang SZ, Eiden LE. Activation of the HPA axis and depression of feeding behavior induced by restraint stress are separately regulated by PACAPergic neurotransmission in the mouse. Stress. 2016;19:374–382. doi: 10.1080/10253890.2016.1174851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang SZ, Eiden LE. PACAPergic Synaptic Signaling and Circuitry Mediating Mammalian Responses to Psychogenic and Systemic Stressors. PACAP. 2016 [Google Scholar]
- 46.Kao SC, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem. 2001;276:18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 47.Keiper M, Stope MB, Szatkowski D, Bohm A, Tysack K, Vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, Schmidt M. Epac− and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 48.Klevans LR, Gebber GL. Comparison of differential secretion of adrenal catecholamines by splanchnic nerve stimulation and cholinergic agents. J Pharmacol Exp Ther. 1970;172:69–76. [PubMed] [Google Scholar]
- 49.Korthuis RJ. Regulation of vascular tone in skeletal muscle. San Rafael, CA: Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 50.Kuri BA, Chan SA, Smith CB. PACAP regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase C-dependent pathway. J Neurochem. 2009;110:1214–1225. doi: 10.1111/j.1471-4159.2009.06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2013;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez MG, Montiel C, Herrero CJ, Garcia-Palomero E, Mayorgas I, Hernandez-Guijo JM, Villarroya M, Olivares R, Gandia L, McIntosh JM, Olivera BM, Garcia AG. Unmasking the functions of the chromaffin cell alpha7 nicotinic receptor by using short pulses of acetylcholine and selective blockers. Proc Natl Acad Sci U S A. 1998;95:14184–14189. doi: 10.1073/pnas.95.24.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez MG, Villarroya M, Lara B, Martinez SR, Albillos A, Garcia AG, Gandia L. Q- and L-type channels dominate the control of secretion in bovine chromaffin cells. FEBS Lett. 1994;349:331–337. doi: 10.1016/0014-5793(94)00696-2. [DOI] [PubMed] [Google Scholar]
- 54.Malhotra RK, Blank M, Wakade TD, Wakade AR. Vasoactive intestinal polypeptide (VIP) serves as another neurotransmitter in the rat adrenal medulla. Regul Peptides. 1989;26:168–168. [Google Scholar]
- 55.Malhotra RK, Wakade TD, Wakade AR. Comparison of secretion of catecholamines from the rat adrenal medulla during continuous exposure to nicotine, muscarine or excess K. Neuroscience. 1988;26:313–320. doi: 10.1016/0306-4522(88)90147-9. [DOI] [PubMed] [Google Scholar]
- 56.Malhotra RK, Wakade TD, Wakade AR. Cross-communication between acetylcholine and VIP in controlling catecholamine secretion by affecting cAMP, inositol triphosphate, protein kinase C, and calcium in rat adrenal medulla. J Neurosci. 1989;9:4150–4157. doi: 10.1523/JNEUROSCI.09-12-04150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marley PD. Desensitization of the nicotinic secretory response of adrenal chromaffin cells. Trends Pharmacol Sci. 1988;9:102–107. doi: 10.1016/0165-6147(88)90177-0. [DOI] [PubMed] [Google Scholar]
- 58.May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem. 2010;285:9749–9761. doi: 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in Guinea pig cardiac neuron excitability. J Neurosci. 2013;33:4614–4622. doi: 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 61.Mueller RA, Thoenen H, Axelrod J. Inhibition of neuronally induced tyrosine hydroxylase by nicotonic receptor blockade. Eur J Pharmacol. 1970;10:51–56. doi: 10.1016/0014-2999(70)90156-1. [DOI] [PubMed] [Google Scholar]
- 62.Mustafa T, Eiden LE. The Secretin Superfamily: PACAP, VIP and Related Peptides. In: Lim R, editor. Handbook of Neurochemistry and Molecular Neurobiology: XIII Neuroactive Peptides and Proteins. Heidelberg: Springer; 2006. pp. 1–36. [Google Scholar]
- 63.Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J Biol Chem. 2007;282:8079–8091. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, Eiden LE. Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress. 2015;18(4):408–418. doi: 10.3109/10253890.2015.1025044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mustafa T, Walsh J, Grimaldi M, Eiden LE. PAC1hop receptor activation facilitates catecholamine secretion selectively through 2-APB-sensitive Ca(2+) channels in PC12 cells. Cell Signal. 2010;22:1420–1426. doi: 10.1016/j.cellsig.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nature Cell Biology. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 67.Perez-Alvarez A, Albillos A. Key role of the nicotinic receptor in neurotransmitter exocytosis in human chromaffin cells. J Neurochem. 2007;103:2281–2290. doi: 10.1111/j.1471-4159.2007.04932.x. [DOI] [PubMed] [Google Scholar]
- 68.Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med. 1952;25:176–201. [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 70.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 71.Selye H. The evolution of the stress concept. American scientist. 1973;61:692–699. [PubMed] [Google Scholar]
- 72.Silver DM, Seaton JF, Harrison TS. Adrenal epinephrine and norepinephrine content following denervation and barbiturates. J Surg Res. 1968;8:177–181. doi: 10.1016/0022-4804(68)90081-4. [DOI] [PubMed] [Google Scholar]
- 73.Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- 74.Smith CB, Eiden LE. Is PACAP the Major Neurotransmitter for Stress Transduction at the Adrenomedullary Synapse? J Mol Neurosci. 2012;48:403–412. doi: 10.1007/s12031-012-9749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–1030. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stroth N, Hamelink CR, Eiden LE. PACAP-dependent cellular plasticity in the mouse adrenal gland. FASEB J. 2007;21 907.906/A1249-c-1250. [Google Scholar]
- 77.Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci. 2011;1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology. 2013;154:330–339. doi: 10.1210/en.2012-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stroth N, Liu Y, Aguilera G, Eiden LE. Pituitary adenylate cyclase-activating polypeptide (PACAP) controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol. 2011;23:944–955. doi: 10.1111/j.1365-2826.2011.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tai TC, Claycomb R, Siddall BJ, Bell RA, Kvetnansky R, Wong DL. Stress-induced changes in epinephrine expression in the adrenal medulla in vivo. J Neurochem. 2007;101:1108–1118. doi: 10.1111/j.1471-4159.2007.04484.x. [DOI] [PubMed] [Google Scholar]
- 81.Tai TC, Morita K, Wong DL. Role of Egr-1 in cAMP-dependent protein kinase regulation of the phenylethanolamine N-methyltransferase gene. J Neurochem. 2001;76:1851–1859. doi: 10.1046/j.1471-4159.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka K, Shibuya I, Nagamoto T, Yamasha H, Kanno T. Pituitary adenylate cyclase-activating polypeptide causes rapid Ca2+ release from intracellular stores and long lasting Ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinol. 1996;137:956–966. doi: 10.1210/endo.137.3.8603609. [DOI] [PubMed] [Google Scholar]
- 83.Tompkins JD, Parsons RL. Identification of intracellular signaling cascades mediating the PACAP-induced increase in guinea pig cardiac neuron excitability. J Mol Neurosci. 2008;36:292–298. doi: 10.1007/s12031-008-9086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsukiyama N, Saida Y, Kakuda M, Shintani N, Hayata A, Morita Y, Tanida M, Tajiri M, Hazama K, Ogata K, Hashimoto H, Baba A. PACAP centrally mediates emotional stress-induced corticosterone responses in mice. Stress. 2011;14:368–375. doi: 10.3109/10253890.2010.544345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vandael DH, Ottaviani MM, Legros C, Lefort C, Guerineau NC, Allio A, Carabelli V, Carbone E. Reduced availability of voltage-gated sodium channels by depolarization or blockade by tetrodotoxin boosts burst firing and catecholamine release in mouse chromaffin cells. J Physiol. 2015;593:905–927. doi: 10.1113/jphysiol.2014.283374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 87.Waschek JA, Pruss RM, Siegel RE, Eiden LE, Bader M-F, Aunis D. Regulation of enkephalin, VIP and chromogranin A biosynthesis in actively secreting chromaffin cells: multiple strategies for multiple peptides. Ann NY Acad Sci. 1987;493:308–323. doi: 10.1111/j.1749-6632.1987.tb27215.x. [DOI] [PubMed] [Google Scholar]
- 88.Winkler H. The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue. J Anat. 1993;183:237–252. [PMC free article] [PubMed] [Google Scholar]
- 89.Winkler H, Apps DK, Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986;18:261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- 90.Zigmond RE. Neuropeptide action in sympathetic ganglia. Evidence for distinct functions in intact and axotomized ganglia. Ann N Y Acad Sci. 2000;921:103–108. doi: 10.1111/j.1749-6632.2000.tb06955.x. [DOI] [PubMed] [Google Scholar]


