Abstract
Objective
Careful characterization of how functional decline co-evolves with cognitive decline in older adults has yet to be well described. Most models of neurodegenerative disease postulate that cognitive decline predates and potentially leads to declines in everyday functional abilities; however, there is mounting evidence that subtle decline in instrumental activities of daily living (IADLs) may be detectable in older individuals who are still cognitively normal.
Methods
The present study examines how the relationship between change in cognition and change in IADLs are best characterized among older adults who participated in the ACTIVE trial. Neuropsychological and IADL data were analyzed for 2,802 older adults who were cognitively normal at study baseline and followed for up to 10 years.
Results
Findings demonstrate that subtle, self-perceived difficulties in performing IADLs preceded and predicted subsequent declines on cognitive tests of memory, reasoning, and speed of processing.
Conclusions
Findings are consistent with a growing body of literature suggesting that subjective changes in everyday abilities can be associated with more precipitous decline on objective cognitive measures and the development of mild cognitive impairment and dementia.
Keywords: IADL, everyday function, older adult, mild cognitive impairment, dementia, longitudinal
Introduction
Loss of autonomy/independence in everyday activities is a top concern among older adults (Prince et al., 2007). Previous studies have shown loss of independence in instrumental activities of daily living (IADLs) is associated with reduced quality of life (Andersen et al., 2004). It is also associated with economic burden as it necessitates the need for assistance by family or formal paid caregivers (Small et al., 2002). Although loss of IADL independence is a key feature of a dementia syndrome, it is also now well recognized that subtle changes in everyday function begin early in a neurodegenerative process, including at the stage of mild cognitive impairment (MCI) (Tomaszewski Farias et al., 2006; Nygård, 2006; Albert 2011) and even in those still considered cognitively normal but who later develop MCI or dementia (Tomaszewski Farias et al., 2013; Tomaszewski Farias et al., 2011; Lau et al., 2016). However, careful characterization of how functional decline develops in association with other clinical features, particularly cognitive decline, is still needed. Even among people who may never develop dementia, the dynamic interplay of functional and cognitive changes in older age has implications for disease management strategies in conditions such as depression (Kiosses & Alexopoulos, 2005).
A number of cross-sectional studies have shown that level of cognitive function is associated with everyday function (i.e., performance of IADL) as measured by self-report, informant-report, clinician-ratings, and performance-based tests (e.g., Aretouli et al., 2010; Burdick et al., 2005; Burton, Strauss, Hultsch, & Hunter, 2006; Jefferson, Paul, Ozonoff, & Cohen, 2006; Mortimer et al., 1992; Willis, 1996). Fewer studies have examined the relationship between cognition and everyday function using longitudinal designs; the results of these studies have shown mixed results. The results of several studies support the notion that cognitive decline may be detected on neuropsychological tests prior to decline in everyday function as measured by self-report, informant-report, or performance-based tests (e.g., Cahn-Weiner et al., 2007; Gross et al., 2011; see also Jack et al., 2011). However, other studies have shown that mild decline in everyday functional abilities, as measured by clinician ratings, informant-report, or performance-based tests, predict subsequent cognitive decline (Fong et al., 2015; Lau et al., 2016; Peres et al., 2008).
Previously, data from the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study have been used to investigate aspects of the complicated relationship between cognitive performance and IADLs. Specifically, Tucker-Drob (2011) found no relation between change in cognition and change in self-reported IADLs using parallel process models that related 5-year trajectories of change in cognitive performance with 5-year trajectories of change in IADL functioning. Further, Gross and colleagues (2011) examined whether baseline levels of cognitive function were associated with concurrent level and changes in everyday functioning in the ACTIVE cohort; memory accounted for the most variability in everyday functioning. That study, however, only examined cognition as a predictor of everyday tasks and did not evaluate bidirectional relationships between cognitive performance and everyday functioning, as we do in this study.
Our present study extends prior work by addressing whether initial levels of one process (i.e., cognition or IADL) are associated with subsequent changes in the other process using bivariate latent change score models. Specifically, the goal of the current study was to characterize the dynamic relationship between changes in cognitive performance and changes in self-reported IADL functioning. To do this we studied a group of older adults who were cognitively normal at study baseline to examine the changing relationship over a 10-year period between IADLs and three cognitive domains - memory, inductive reasoning, and speed of processing. Several models were tested in which each cognitive domain was evaluated separately. First, we tested a model in which there is no relationship between cognitive performance and IADLs. Next, as predicted by many disease models, we tested whether individual differences in cognitive performance are associated with subsequent changes in IADL functioning. Third, we examined an alternative model--the degree to which individual differences in IADL functioning precede and predict subsequent changes in cognitive function. There are a number of reasons to expect support for this alternative model. For example, cognitively normal older adults may be capable of detecting subtle declines in everyday functioning such that their self-reports may be more sensitive to early disease than traditional cognitive tests. Finally, a fourth model tested whether a dynamic, bidirectional relationship best explains the association between IADLs and cognition such that individual differences in both influence each other’s within-person changes over time. We hypothesized that the results would support the fourth model and that the bidirectional relation between cognition and self-reported function would explain the evidence for both model 1 (cognition predicting subsequent change in IADL) and model 2 (IADL predicting subsequent change in cognition) in the extant literature.
Methods
Participants
The ACTIVE study was a randomized, controlled trial of cognitive training for N=2,802 adults aged 65 and older who were cognitively healthy at baseline. Participants were randomized to receive training in memory, reasoning, or speed of processing, or to a no-contact control group. Each training intervention included 10 one-hour sessions over a 6-week period. Participants underwent follow-up cognitive testing immediately after training and one (N=2564), two (N=2326), three (N=2234), five (N=2101), and ten (N=1877) years after training. IADL functioning was not assessed at the immediate post-training visit, so we excluded that visit from latent change score analyses. As the primary goal of the present study was to examine the dynamic coupling of cognitive and IADL functioning and not to examine training effects on these couplings, we also excluded the baseline study visit to minimize the need to account for practice effects in cognitive testing which are pronounced in this sample (Gross et al., 2012). Participants were recruited from six metropolitan sites across the United States (University of Alabama at Birmingham, Johns Hopkins University, Wayne State University, Hebrew Rehabilitation Center for the Aged in Boston, Indiana University School of Medicine, and Pennsylvania State University). This research was completed in accordance with the Helsinki Declaration and approved by the Institutional Review Boards at each study site, including written informed consent.
Measures
We generated scores from tests in the ACTIVE neuropsychological battery representing memory, reasoning, and speed of processing from confirmatory factor analyses of each domain. The memory factor was constructed using immediate recall from the Hopkins Verbal Learning Test (Brandt, 1991), Auditory Verbal Learning Test (Rey, 1964), and paragraph recall from the Rivermead Behavioral Memory Test (Wilson et al., 1985). The reasoning factor was constructed from word series, letter series, and letter sets (Ekstrom et al., 1976; Gonda & Schaie, 1985; Thurstone & Thurstone, 1949; Willis, 1996). The speed of processing factor was composed of the second, third, and fourth trials of the Useful Field of View (UFOV) task (Owsley et al., 2002). Factor scores were estimated using maximum likelihood estimation with robust standard errors using the regression method. To evaluate model fit of measurement models for the cognitive factor scores, we could not use standard summary statistics (e.g., root mean square error of approximation, RMSEA, and comparative fit index, CFI) because they would be perfect as there were only three tests per domain. Thus, item-level fit was assessed using normalized residuals which characterize the difference, in standardized z-score units, between model-estimated correlations and sample correlations for each pairwise correlation in a factor (Bollen, 1989). All residuals were smaller than 2.0, indicating excellent fit to the data (Supplemental Table 1).
For self-reported IADL functioning, we derived a factor from 18 questions completed by participants assessing whether they were reporting difficulty with IADLs using the Minimum Dataset Home Care scale (see Table 1 for a list of IADLs assessed; MDS-HC; Morris et al., 1997). The validity and clinical utility of the MDS-HC has been previously established (Landi et al., 2000; Morris et al., 2004). This instrument was best represented by a single factor (see Supplemental Table 2 for comparison of a unidimensional verses bifactor model, the latter represented by a ‘cognitive’ and ‘physical’ factor, in addition to a IADL general factor). Because of restriction in range across the various item response options, from the original Likert scale we collapsed each item into a dichotomous rating of ‘any difficulty’ or ‘no difficulty’. The reference for each item is no difficulty. Higher scores indicate more IADL difficulty. This approach to scoring Minimum Data Set (MDS) IADL difficulty variable differs from that used in previous ACTIVE papers (e.g., Willis et al., 2006; Rebok et al., 2014). We chose the factor score over the MDS indicator to assess a wider range of IADL difficulty. The correlation between these two variables is r=0.88 and in a sensitivity analysis using the MDS indicator, no inferences changed.
Table 1.
Sample characteristics of the ACTIVE sample (N=2802)
| Variable | Mean (SD) or N (%) |
|---|---|
| Age, mean (SD) | 73.6 (5.9) |
| Sex, Female, n (%) | 2126 (75.9) |
| Race, White, n (%) | 2028 (72.4) |
| Years of Education, mean (SD) | 13.5 (2.7) |
| Self-rated health, n (%) | |
| Excellent | 252 (9.2) |
| Very Good | 953 (34.6) |
| Good | 1115 (40.5) |
| Fair | 403 (14.6) |
| Poor | 30 (1.1) |
| Intervention status, n (%) | |
| Memory | 702 (25.1) |
| Reasoning | 699 (24.9) |
| Speed | 702 (25.1) |
| Control | 698 (24.9) |
| SF-36 General health, mean (SD) | 69.0 (19.3) |
| Memory tests | |
| AVLT trial 1–5 sum, mean (SD) | 48.2 (10.3) |
| HVLT trial 1–3 sum, mean (SD) | 25.9 (5.5) |
| Rivermead score, mean (SD) | 6.3 (2.8) |
| Reasoning tests | |
| Word series, mean (SD) | 9.5 (4.9) |
| Letter series, mean (SD) | 10.0 (5.6) |
| Letter sets, mean (SD) | 5.8 (2.8) |
| Speed of processing tests | |
| UFOV Task 2, mean (SD) | 131.8 (122.9) |
| UFOV Task 3, mean (SD) | 319.5 (133.8) |
| UFOV Task 4, mean (SD) | 456.1 (69.1) |
| IADL any difficulty, n (%) | |
| Planning meals | 169 (6.0) |
| Setting out food & utensils | 74 (2.6) |
| Cooking | 197 (7.0) |
| Doing dishes, dusting, making beds | 379 (13.5) |
| Laundry | 211 (7.5) |
| Writing checks | 103 (3.7) |
| Bills paid on time | 118 (4.2) |
| Balancing a checkbook | 327 (11.7) |
| Keeping household expenses balanced | 142 (5.1) |
| Keeping track of doctor appointments | 109 (3.9) |
| Remembering to take medications | 223 (8.0) |
| Opening medicine bottles | 204 (7.3) |
| Giving self injections | 445 (15.9) |
| Looking up phone numbers | 171 (6.1) |
| Remembering often called numbers | 463 (16.5) |
| Answering phone calls | 44 (1.6) |
| Shopping | 257 (9.2) |
| Travel by vehicle | 135 (4.8) |
SD: standard deviation; AVLT: Auditory Verbal Learning Test; UFOV = Useful Field of View; IADL = Instrumental Activities of Daily Living.
Analysis Plan
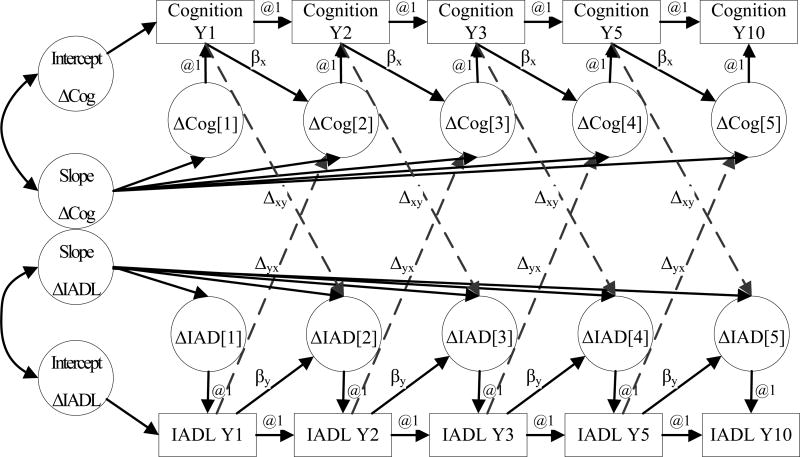
First, we derived factor scores for each of the three cognitive outcome and for IADL difficulty using confirmatory factor analysis. For each of the three cognitive outcomes of memory, reasoning, and speed, analyses followed a four-stage procedure. The Figure provides a generic structural equations model that describes the setup of a bivariate latent change score model. These analysis stages enabled us to test hypotheses for each cognitive outcome corresponding to (1) no relationship between cognitive performance and IADL functioning, (2) cognitive performance predicting changes in IADL functioning, (3) IADL functioning predicting changes in cognitive performance, and (4) a dynamic, bidirectional relationship between cognition and IADL function (e.g., .individual differences in both cognition and IALDs influence each other’s within-person changes over time). First, to describe changes in each cognitive and IADL outcome, we fit bivariate latent change score models that had no couplings between cognitive performance and IADL functioning (Figure 1, ignoring dashed arrows). Second, we examined the relationship between individual differences in cognitive performance and subsequent changes in IADL functioning by allowing couplings between the two (Figure 1, ignoring dashed arrows from cognitive functioning to IADL difficulty change). Coupling parameters represent the time-dependent effect of one latent variable on subsequent change in the other, and were held equal between each time point in models (Gerstorf et al., 2007). Third, we allowed couplings from IADL functioning to subsequent changes in cognitive performance (Figure 1, ignoring dashed arrows from IADL difficulty to cognitive change). Fourth, we allowed dual couplings between the processes (e.g., dashed arrows are present).
Figure 1.
Latent change score model in which individual differences in IADL performance at time t predict subsequent change in cognitive performance between time t and t+1 (dotted arrows from IADL to change in cognition), and individual differences in cognition at time t predict subsequent change in IADL performance between time t and t+1 (dotted arrows from cognition to change in IADL). Models were estimated for memory, reasoning, and speed of processing. See Analysis Plan for the details of the model building procedure; the specific stage represented graphically here is shown in model stage 4 in Table 2 for each cognitive outcome.
We tested the significance of progressively relaxing model constraints using likelihood ratio tests. We adjusted the final models for age (in years), sex, years of education, race (white, nonwhite), and self-rated health (Excellent, Very Good, Good, Fair, Poor). We report absolute fit of the models using the root mean square error of approximation (RMSEA) and the comparative fit index (CFI). Lower values of the RMSEA and higher values of the CFI indicate better fit; RMSEA<0.1 and CFI>0.9 generally are indicative of acceptable model fit (Hu & Bentler, 1999). For the best-fitting final models for each cognitive outcome, we report means of latent variables and magnitudes of couplings.
We used Mplus statistical software (version 7.11, Muthén & Muthén, version 7.11, Muthén & Muthén, Los Angeles CA, 1998–2012). Models used a maximum likelihood estimator with the EM algorithm, which treats missing data in dependent variables as missing at random conditional on variables in the model.
Sensitivity analyses
To test for intervention group differences in the dynamic relationship between cognitive performance and IADL functioning, we freed couplings to vary across intervention group in sub-analyses at the second, third, and fourth stages listed earlier in the analysis plan. In a second sensitivity analysis, we reran models using only data from the first, fifth, and tenth annual ACTIVE study visits to be sure inferences were unaffected by different periods of follow-up between study visits. In a third sensitivity analysis, we estimated a bivariate latent change score model for a general cognitive performance factor score from a confirmatory factor analysis of all the cognitive tests combined; no inferences changed with respect to IADL difficulty when we used this score (Supplemental Table 3).
Results
Characteristics of the sample
Demographic characteristics of the ACTIVE sample are in Table 1. On average participants at baseline were in their early 70’s, about three quarters were female and white, and most participants had a high school or higher education. The sample varied in terms of general health status, but the majority of participants rated their health as at least “good.” By design, participants were selected to be cognitively normal as reflected by the mean MMSE of 27.3 (Table 1). Consistent with normal cognition, they also reported few to no IADL difficulties at study baseline.
The relationship between cognition and IADLs
The primary goal of the study was to better understand temporal associations between changes in cognition and change in IADLs (e.g., whether individual differences in cognition precede subsequent changes in IADLs, individual differences in IADLs precede subsequent changes in cognitive performance, or whether a bidirectional association between the two best explains their relationship). Because there are three primary cognitive measures - memory, reasoning and processing speed - three separate sets of models were used to examine the relationship between cognition and IADL difficulty.
Table 2 provides model fit information (e.g., RMSEA, CFI) for tests of constraints in latent change score models for memory, reasoning, and speed of processing with IADL functioning. For each cognitive score, absolute model fit statistics (RMSEA, CFI) were excellent and largely comparable across stages of constraint testing. The log-likelihood (LL) statistics are interpreted alongside the number of free parameters in the next column. The column of change in −2LL shows improvement in the log-likelihood statistic in χ2 units relative to model stage 1 (no coupling).
Table 2.
Model comparisons among alternative latent change score models of domain-specific cognitive performance and IADL functioning: Results from ACTIVE (N=2,802)
| Cognitive domain |
Model stage |
Model description | RMSEA | CFI | Log- likelihood |
Number of free parameters |
Change in - 2LL (df) |
|---|---|---|---|---|---|---|---|
| Memory performance | |||||||
| 1 | No coupling | 0.068 | 0.931 | −29510.602 | 28 | REF | |
| 2 | Memory → change IADL | 0.069 | 0.931 | −29509.842 | 29 | 1.520 (1) | |
| 3 | IADL → change Memory | 0.068 | 0.932 | −29502.117 | 29 | 16.970 (1) | |
| 4 | Dual coupling | 0.069 | 0.933 | −29500.766 | 30 | 19.672 (2) | |
| Reasoning performance | |||||||
| 1 | No coupling | 0.128 | 0.835 | −27824.813 | 28 | REF | |
| 2 | Reasoning → change IADL | 0.129 | 0.834 | −27824.74 | 29 | 0.146 (1) | |
| 3 | IADL → change Reasoning | 0.129 | 0.833 | −27708.008 | 29 | 233.610 (1) | |
| 4 | Dual coupling | 0.131 | 0.832 | −27708.008 | 30 | 233.610 (2) | |
| Speed of processing performance | |||||||
| 1 | No coupling | 0.104 | 0.791 | −31149.078 | 28 | REF | |
| 2 | Speed → change IADL | 0.105 | 0.79 | −31149.069 | 29 | 0.018 (1) | |
| 3 | IADL → change Speed | 0.107 | 0.781 | −31120.467 | 29 | 57.222 (1) | |
| 4 | Dual coupling | 0.109 | 0.78 | −31120.025 | 30 | 58.106 (2) | |
RMSEA: root mean squared error of approximation. CFI: comparative fit index. Df: degrees of freedom.
Memory
We first examined a model in which individual differences in memory predicted change in IADLs (Table 2, stage 2 under memory). This model did not fit the data substantially better than a model in which there was no relationship between these two variables (Table 2, stage 1 under memory) (χ2=1.52, df=1). That is, neither a lack of relationship between memory and IADLs, or change in memory preceding change in IADLs appeared to explain the relationship between these two constructs. We next examined a model in which individual differences in IADLs were predicting change in subsequent memory (Table 2, stage 3 under memory); this model fit the data better than the model representing no association between change in the two variables (χ2=16.97, df=1). Finally, we examined a model in which change in IADLs and change in memory were allowed to be bidirectional (Table 2, stage 4 under memory); this model resulted in improved fit over the model with no couplings (χ2=19.67, df=2), but provided minimal gain relative to the model stage 3 that specified only associations between individual differences in IADLs and subsequent change in memory (χ2=19.672–16.97=2.70, df=1). Overall, these results suggest that preceding individual differences in IADLs appear to be driving subsequent memory change.
Reasoning
Next we examined a model where reasoning performance was a leading indicator of subsequent change in IADLs (Table 2, stage 2 under reasoning). Similar to memory, this model’s fit was not significantly different than a model in which the two variables were unrelated (χ2=0.15, df=1) (Table 2, stage 1 under reasoning). Neither no relationship, nor change in reasoning predicting subsequent change in IADLs explained the relationship between these two variables. We next examined a model in which IADLs were driving subsequent changes in reasoning (Table 2, stage 3 under reasoning); this model fit the data significantly better than the model representing no association between change in the two variables (χ2=233.61, df=1). The model with dynamic couplings between reasoning performance and IADLs fit better than model stage 1 (χ2=233.61, df=2), but was not statistically significantly better than model stage 3 (χ2=233.61–233.61=0.00, df=1). Thus, similar to memory, results suggest that IADLs are a leading indicator of subsequent changes in reasoning-based cognitive decline.
Speed of Processing
When modeling individual differences in the speed of processing factor as a predictor of subsequent change in IADLs (Table 2, stage 2 under speed), there was no improvement in fit over a model representing no association between these two variables (Table 2, stage 1 under speed) (χ2=0.02, df=1). As was the case for memory and reasoning outcomes, the model in which individual differences in IADLs predicted subsequent change in speed of processing provided optimal fit to the data (χ2=57.22, df=1), and the dual coupling model (stage 4 under speed) was not statistically significantly superior to that model (χ2=58.10–57.22=0.88, df=1). Thus, results suggest that declines in IADL difficulty drive reduced performance in speed of processing.
Table 3 shows latent variable means and couplings for each model adjusted for covariates. For each cognitive outcome and in each intervention condition, greater IADL difficulty was associated with a subsequently steeper rate of cognitive decline. The magnitude of the association was similar for each cognitive domain. The magnitude of these associations are on a standardized (mean 0, variance 1) scale and correspond to a small effect size (Cohen, 1988).
Table 3.
Parameter estimates from latent change score models with dynamic couplings between IADL functioning and cognitive performance: Results from ACTIVE (N=2,802)
| Memory performance (Higher is better) |
Reasoning performance (Higher is better) |
Speed of processing performance (Higher is worse) |
|
|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Latent variable means | |||
| Mean cognition | 0.01 (0.02) | −0.02 (0.02) | 0.00 (0.02) |
| Slope of change in cognition | 0.02* (0.00) | 0.02* (0.00) | 0.02* (0.00) |
| Mean IADL (higher is worse) | −0.12* (0.02) | −0.14* (0.02) | −0.10* (0.02) |
| Slope of change in IADL | 0.02* (0.01) | 0.02* (0.01) | 0.02* (0.01) |
| Coupling | |||
| IADL → change cognition | −0.11* (0.02) | −0.11* (0.01) | 0.09* (0.02) |
| Model fit | |||
| RMSEA | 0.066 | 0.081 | 0.060 |
| CFI | 0.917 | 0.910 | 0.910 |
Each column is from a different bivariate latent change score model for each cognitive process. Parameters for latent variable means are in standardized N(0,1) units, per baseline standard deviation units. The row of parameter coefficients for couplings represent the annual rate of change in cognitive functioning at time t + 1, in standardized N(0,1) units, per baseline standard deviation unit level of IADL difficulty at time t.
p<0.05, compared to 0 effect
Sensitivity analysis
Models all fit significantly better when parameters for any modeling stage were allowed to vary by intervention group (Supplemental Table 4). Supplemental Table 5 (for memory), 6 (for reasoning), and 7 (for speed of processing) show latent variable means and couplings (from IADL difficulty to subsequent change in cognition) from models with dynamic couplings between cognition and IADL difficulty, which were all allowed to vary by intervention group. The driver of intervention group differences was the post-training mean of the cognitive factor in the intervention group which trained that cognitive ability. That is, the mean cognitive score (representing post-training performance) was better in the intervention group that had been trained in that cognitive ability domain. For example, the mean memory score immediately post-training in the memory group was higher than the mean memory score in other groups. Dynamic couplings between cognition and IADL difficulty did not differ by intervention group.
In another sensitivity analysis, inferences were unchanged when we restricted analyses only to the first, fifth, and tenth study visits to balance the time between visits; see Supplemental Table 8.
Discussion
The primary goal of this study was to examine the temporal relationship between cognitive performance (memory, reasoning, and speed of processing) and self-reported IADL functioning in healthy older adults. Results suggested that subtle, self-perceived difficulties in performing IADLs preceded and predicted subsequent declines on cognitive tests of memory, reasoning, and speed of processing. Initially, such findings may seem to run counter to most traditional models of symptom development associated with neurodegenerative diseases of aging in which cognitive deterioration, measured by conventional neuropsychological tests of these abilities, are expected to be evident before deterioration in everyday functioning. Our results, however, are consistent with a growing body of research suggesting that older adults who are seemingly cognitively normal may be able to detect subtle changes in their everyday functional abilities (for example, taking longer to complete complex IADLs or being prone to making more errors) before changes are evident on objective tests of neuropsychological function (Tomaszewski Farias et al., 2013; McAlister & Schmitter-Edgecombe, 2016). Our results are consistent with other previous studies that have shown that when subjectively reported functional changes are present in individuals who still perform within the normal range on cognitive tests, it substantially increases the risk they will develop frank cognitive impairment (i.e., MCI) in subsequent years (Amariglio et al., 2015). It is important to note that our sample included healthy older adults. Our findings may depend on intact insight and recall to detect and reliably report subtle functional changes and may not generalize to self-reports from older adults with cognitive impairment that limits insight or recall.
In the present study, the greater sensitivity of self-perceived early changes in functional abilities as compared to objective cognitive testing may occur because some everyday tasks are cognitively complex compared to traditional tests of memory, reasoning, or processing speed. Alternatively, it may be that healthy older adults perform everyday tasks under more challenging conditions (e.g., while multitasking, under time pressure, with environmental distractions or interruptions) than the conditions under which laboratory-based cognitive tests are administered (e.g., in a silent, distraction-free room). This critique of cognitive tests has been made rather convincingly in the neuropsychological literature for decades (Eslinger et al., 1985; Shallice et al., 1991).
Our study’s primary purpose was not to examine the effect of cognitive training on the association between cognition and everyday function; we had no reason to hypothesize training would modify this coupling, and thus we did not design our main analysis to test this question fully. Because, however, the data were available in ACTIVE we conducted an extra analysis to ensure training did not affect this coupling. This analysis uncovered anticipated intervention group differences primarily on the post-training means for the cognitive test to which the participants were trained. For example, the group that received memory training showed a higher post-training memory cognitive test performance, compared to the non-memory trained groups. However, the relationships between self-reported IADLs changes and cognitive change were not affected by intervention assignment. This finding does not contrast with prior analyses from ACTIVE that have reported intervention group differences at five and ten year follow-up visits because those analyses focused on intervention group differences in IADL difficulty (Rebok et al., 2014; Willis et al., 2006).
Several distinct advantages of this study are the large, well-characterized ACTIVE sample with 10 years of longitudinal follow-up over six study visits. Contemporary modeling approaches used here enabled us to address the temporal ordering between changes in cognition and changes in IADLs. An important caveat of the study is the limited generalizability of the sample to high-functioning older adults who were rather homogenous in terms of education, sex, and ethnicity (although about 25% of the ACTIVE sample was African American); further research with other more diverse samples is needed to explore whether a similar temporal ordering of cognitive and IADL performance would be observed. In particular, previous studies have suggested the relationship between cognitive and functional changes may differ by diagnostic status (e.g., normal cognitive aging verses MCI verses dementia) (Rog et al., 2014). The cognitive measures used for this study were selected for the aims of the original ACTIVE study, which was designed to evaluate the effects of cognitive training on cognition over time. The specific domains of processing speed, memory, and reasoning were selected because they exhibit relatively early cognitive decline in older adults, they respond to cognitive intervention, and they are associated with IADL (Jobe et al., 2001; Tennsted & Unverzagt, 2013). The memory measure was limited to performance on immediate free recall trials, and a wide range of other cognitive domains, which may have yielded different results, were not included but should be explored in future work. Additionally, there are many other potential causes of functional dependence unrelated to cognition. Thus, the causal mechanism between IADL functioning and cognitive changes is likely multifactorial and could be studied more carefully in other samples with diagnostic heterogeneity with respect to dementia.
The present findings have important implications for clinical practice and the development of intervention approaches. First, consistent with growing evidence from other studies, the present study suggests that older adults who perceive that their ability to perform high-level activities of daily living have diminished are an ‘at-risk’ group. At the very least, they should be followed with serial neuropsychological testing over time to monitor for the development of cognitive impairment. In addition, findings suggest that older adults with subtle changes in self-reported functional capacities may be candidates for behavioral interventions to prevent decline. Further, developing interventions that more explicitly target the maintenance of functional abilities (e.g., teaching compensatory strategies that help to promote continued independence) may prove to be an especially fruitful approach. In sum, there is mounting evidence that subjective cognitive and functional complaints constitute a risk for cognitive decline and eventual dementia, and the present study suggests that self-perceived diminished abilities to perform IADLs precede and predict subsequent cognitive decline. Such reports, when made by older adults without obvious cognitive impairment, should be taken seriously. They warrant additional subsequent monitoring and also offer a window of opportunity for the provision of support to bolster or maintain functional capacities.
Supplementary Material
Acknowledgments
The ACTIVE intervention trials were supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), the University of Alabama at Birmingham (U01AG14289), and the University of Florida (U01AG14276).ted by the Advanced Psychometrics Methods in Cognitive Aging Research (R13AG030995).
Footnotes
Authors have no disclosures of conflict of interest.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Snyder PJ. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Mormino EC, Pietras AC, Marshall GA, Vannini P, Johnson KA, Sperling RA, Rentz DM. Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CK, Wittrup-Jensen KU, Lolk A, Andersen K, Kragh-Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health and Quality of Life Outcomes. 2004;2:52. doi: 10.1186/1477-7525-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretouli E, Brandt J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. International Journal of Geriatric Psychiatry. 2010;25:224–233. doi: 10.1002/gps.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen K. Structural equations with latent variables. New York: John Wiley; 1989. [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. [Google Scholar]
- Burdick DJ, Rosenblatt A, Samus QM, Steele C, Baker A, Harper M, Rosenblatt A. Predictors of functional impairment in residents of assisted-living facilities: The Maryland Assisted Living Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2005;60:258–264. doi: 10.1093/gerona/60.2.258. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Hunter MA. Cognitive functioning and everyday problem solving in older adults. The Clinical Neuropsychologist. 2006;20:432–452. doi: 10.1080/13854040590967063. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner D, Tomaszewski Farias S, Julian L, Kramer JH, Reed BR, Mungas D, Chui H, Wetzel M. Cognitive and neuroimaging predictors of instrumental activities of daily living. Journal of International Neuropsychological Society. 2007;13:737–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation patient EVR. Neurology. 1985;35:1731–1731. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, Derman D. Kit of factor-referenced cognitive tests, revised. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Fong TG, Gleason LJ, Wong B, Habtemariam D, Jones RN, Schmitt EM, de Rooij SE, Saczynski JS, Gross AL, Bean JF, Brown CJ, Fick DM, Gruber-Baldini AL, O’Connor M, Tabloski PA, Marcantonio ER, Inouye SK Successful Aging after Elective Surgery Functional Measures Working Group. Cognitive and physical demands of activities of daily living in older adults: Validation of expert panel ratings. PMR. 2015 Feb 4; doi: 10.1016/j.pmrj.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Lovden M, Rocke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology. 2007;43:705–718. doi: 10.1037/0012-1649.43.3.705. [DOI] [PubMed] [Google Scholar]
- Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Palo Alto, CA: Consulting Psychologists Press; 1985. [Google Scholar]
- Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Cognitive predictors of everyday functioning in older adults: results from the ACTIVE Cognitive Intervention Trial. Journal of Gerontology, B Series Psychological Sciences and Social Sciences. 2011;66:557–66. doi: 10.1093/geronb/gbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Inouye SK, Rebok GW, Brandt J, Crane PK, Parisi JM, Tommet D, Brandeen-Roche L. Parallel but not equivalent: Challenges and solutions for repeated assessment of cognition over time. Journal of Clinical and Experimental Neuropsychology. 2012;34:758–772. doi: 10.1080/13803395.2012.681628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analtysis: conventional versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9(1):119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Paul RH, Ozonoff A, Cohen RA. Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs) Archives of Clinical Neuropsychology. 2006;21:311–320. doi: 10.1016/j.acn.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, Rebok GW, Morris JN, Helmers KF, Leveck MD, Kleinman K. ACTIVE: a cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses DN, Alexopoulos GS. IADL functions cognitive deficits, and severity of depression: A preliminary study. American Journal of Geriatric Psychiatry. 2005;13:244–249. doi: 10.1176/appi.ajgp.13.3.244. [DOI] [PubMed] [Google Scholar]
- Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, Gambassi G, Lattanzio F, Bernabei R SILVERNET-HC Study Group of Bergamo. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Medical Care. 2000;38:1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Lau KM, Parikh M, Harvey DJ, Huang CJ, Tomaszewski Farias S. Early Cognitively Based Functional Limitations Predict Loss of Independence in Instrumental Activities of Daily Living in Older Adults. JINS. 2016;21:688–689. doi: 10.1017/S1355617715000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister C, Schmitter-Edgecombe M. Everyday functioning and cognitive correlates in healthy older adults with subjective cognitive concerns. The Clinical Neuropsychologist. 2016;30:1087–1103. doi: 10.1080/13854046.2016.1190404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Steel K, Idegami N, Bernabei R, Carpenter GI, Gilgen R, Hirdes JP, Topinkova E. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. Journal of the American Geriatric Society. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- Morris JN, Jones RN, Fries BE, Hirdes JP. Convergent validity of minimum data set-based performance quality indicators in postacute care settings. American Journal of Medical Quality. 2004;19:242–247. doi: 10.1177/106286060401900603. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Ebbitt B, Jun SP, Finch MD. Predictors of cognitive and functional progression in patients with probable Alzheimer’s disease. Neurology. 1992;42:1689–1689. doi: 10.1212/wnl.42.9.1689. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus user’s guide: Seventh Edition. Los Angeles, CA: Muthen & Muthen; 1998–2012. [Google Scholar]
- Nygård L. Instrumental activities of daily living: a stepping-stone towards Alzheimer’s disease diagnosis in subjects with mild cognitive impairment? Acta Neurologica Scandinavica. 2003;107(s179):42–46. [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr, Ball K. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. The Gerontologist. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Pérès K, Helmer C, Amieva H, Orgogozo J-M, Rouch I, Dartigues J-F, Barberger-Gateau P. Natural History of Decline in Instrumental Activities of Daily Living Performance over the 10 Years Preceding the Clinical Diagnosis of Dementia: A Prospective Population-Based Study. Journal of the American Geriatrics Society. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- Prince D, Butler D. Clarity final report: aging in place in America. Nashville, TN: Prince Market Research; 2007. [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL. ACTIVE Study Group. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatric Society. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- Rog LA, Quitania Park L, Harvey DJ, Huang C-J, Mackin S, Tomaszewski Farias S. The independent contributions of cognitive impairment and neuropsychiatric symptoms to everyday function in older adults. The Clinical Neuropsychologist. 2014;28:215–236. doi: 10.1080/13854046.2013.876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice TIM, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Small GW, McDonnell DD, Brooks RL, et al. The impact of symptom severity on the cost of Alzheimer’s disease. Journal of the American Geriatric Society. 2002;50:321–327. doi: 10.1046/j.1532-5415.2002.50065.x. [DOI] [PubMed] [Google Scholar]
- Tennstedt SL, Unverzagt FW. The ACTIVE study: Study overview and major findings. Journal of Aging and Health. 2013;25:S3–S20. doi: 10.1177/0898264313518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone L, Thurstone T. Examiner manual for the SRA Primary Mental Abilities Test (Form 10–14) Chicago, IL: Science Research Associates; 1949. [Google Scholar]
- Tomaszewski Farias S, Chou E, Harvey JD, Mungas D, Reed B, DeCarli C, Quitania L, Beckett Park L. Longitudinal trajectories of everyday function by diagnostic status. Psychology of Aging. 2013;28:107–1075. doi: 10.1037/a0034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Mungas D, Hinton L, Haan M. Demographic, neuropsychological, and functional predictors of rate of longitudinal cognitive decline in Hispanic older adults. American Journal of Geriatric Psychiatry. 2011;19:440–50. doi: 10.1097/JGP.0b013e3181e9b9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, DeCarli C. MCI is associated with deficits in everyday functioning. Alzheimer disease and Associated Disorders. 2006;20:217. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Mugas D, Reed B, Harvey D, DeCarli C. Progression of Mild Cognitive Impairment to Dementia in clinic versus community-based cohorts. Archives of Neurology. 2009;66:1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25:368–77. doi: 10.1037/a0022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL. Everyday cognitive competence in elderly persons: Conceptual issues and empirical findings. The Gerontologist. 1996;36:595–601. doi: 10.1093/geront/36.5.595. [DOI] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E. ACTIVE Study Group. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Bury St. Edmunds, England: Thames Valley Test Company; 1985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.